Abstract
The modulatory effects of baclofen on the sensitivity of peripheral afferent endings to mechanical stimulation were investigated using anin vitro ferret gastroesophageal vagal afferent preparation. Changes in sensitivity of three types of gastroesophageal vagal afferent endings previously categorized as mucosal, tension, and tension–mucosal (TM) receptors according to their mechanoreceptive field characteristics were investigated. Baclofen (30–200 μm) dose dependently reduced responses of mucosal afferents to mucosal stroking with calibrated von Frey hairs (10–1000 mg). This was reversed by the GABAB receptor antagonist SCH50911 (1 μm). TM afferent responses to mucosal stroking (10–1000 mg) were unaffected by baclofen (30–200 μm). However, baclofen (30–200 μm) significantly inhibited the response of 11 of 18 TM afferents to circumferential tension. This was reversed by SCH50911 (1 μm). Baclofen (100 and 200 μm) significantly inhibited the response of all tension receptor afferents to circumferential tension in the lower range (1–3 gm) but not in the higher range (4–7 gm). This inhibition was reversed by SCH50911 (1 μm; n = 3). This study provides the first direct evidence for the inhibitory modulation of peripheral mechanosensory endings by the G-protein-coupled GABABreceptor. Inhibition was dose-dependent, pharmacologically reversible, and selective to certain aspects of mechanosensitivity. These findings have important relevance to strategies for selective reduction of sensory input to the CNS at a peripheral site.
Keywords: GABAB receptors, vagal afferents, ferret, visceral afferents, esophagus, mechanoreceptors
Since its discovery in the brain nearly 50 years ago (Awapara et al., 1950; Roberts and Frankel, 1950;Udenfriend, 1950), GABA has been well characterized as an inhibitory neurotransmitter within the CNS (Curtis and Johnston, 1974). GABA has also been detected in a variety of peripheral organs (Tanaka, 1985), being found in large quantities within the myenteric plexus of the gut (Erdö et al., 1982), most likely within myenteric neurons (Jessen et al., 1986).
GABA exerts its effects through two ligand-gated channels, GABAA and GABAC receptors, and a third recently cloned receptor, GABAB(Bowery, 1993; Bettler et al., 1998), which acts through G-proteins to regulate potassium and calcium channels. Activation of GABAA receptors classically produces postsynaptic inhibition (Nicoll et al., 1990). The role of GABAB receptors is broader. Both presynaptic and postsynaptic inhibition have been demonstrated (Bowery et al., 1980;Newberry and Nicoll, 1985). In addition to slow postsynaptic inhibition (Brooks et al., 1992), GABAB receptors are found presynaptically at a variety of central synapses, including primary afferent terminals of muscle spindle afferents (Capaday, 1995) within the spinal cord. At least two effects of the agonist baclofen have been attributed to the activation of GABAB receptors within the spinal cord: muscle relaxation and antinociception. The main therapeutic use of baclofen relates to its antispastic ability, achieved by inhibiting neurotransmitter release from these primary afferent terminals, as well as from spinal interneurons (Davidoff and Sears, 1974; Knutsson et al., 1974; Kato et al., 1978). Recent studies have revealed that baclofen also inhibits transient lower esophageal sphincter (LES) relaxation and thereby gastroesophageal reflux in humans (Lidums et al., 1999) and ferrets (Blackshaw et al., 1999). A possible explanation for this finding is that baclofen acts on inhibitory presynaptic GABAB receptors on vagal afferent terminals in the dorsal medulla oblongata (Bowery and Pratt, 1992) from which it inhibits neurotransmitter release.
In the periphery, there is evidence that GABABreceptor activation inhibits transmitter release from primary afferents directly onto the effector tissue (Green and Cottrell, 1988), such as in the airways (Belvisi et al., 1989). Evidence indicates therefore that GABA receptors are functionally expressed at peripheral and central endings of primary afferents. However, there is no direct information on the effect of GABA or its analogs on mechanotransduction at sensory receptive fields.
We have developed a gastroesophageal vagal afferent preparation that enables us to study accurately different populations of mechanosensory afferent fibers and their receptive fields in vitro while precisely controlling their mechanical environment (Page and Blackshaw, 1998). Using this preparation, we investigated the effect of the GABAB agonist baclofen on the sensitivity of gastroesophageal vagal afferents to mechanical stimulation. This involved investigation of changes in sensitivity of three distinct populations of gastroesophageal vagal afferents previously identified using this preparation (Page and Blackshaw, 1998), which respond to mucosal tactile stimulation, circumferential strain, or both mucosal and strain stimuli.
MATERIALS AND METHODS
General. All studies were performed in accordance with the guidelines of the Animal Ethics Committees of the Royal Adelaide Hospital and Institute for Medical and Veterinary Sciences (Adelaide, Australia).
Ferrets (0.4–1.0 kg body weight) were deeply anesthetized with sodium pentobarbitone (50 mg/kg, i.p.), and the thorax and abdomen were opened by a midline incision. The ferrets were then exsanguinated by cardiac puncture. The stomach and esophagus with attached vagal nerves, heart, and lungs were removed and placed in a modified Krebs’ solution of the following composition (mm): NaCl 118.1, KCl 4.7, NaHCO3 25.l, Na2PO4 1.3, MgCl 1.2, CaCl2 1.5, citric acid 1.0, and glucose 11.1, bubbled with 95% O2–5% CO2. The temperature was maintained at 4°C during dissection to preserve the tissue and prevent metabolic degradation. The heart, lungs, and major blood vessels were removed, and the vagus nerve was cleared of connective tissue. The diaphragm was also cleared from around the lower esophageal sphincter. The preparation was then opened longitudinally along the esophagus and greater curve of the stomach (2 cm length of stomach) and pinned out flat, mucosa side up, in a perspex chamber and perfused at a rate of 12 ml/min with Krebs’ bicarbonate buffer solution maintained at 34°C. The vagus nerve (free length of 3.0 cm) was drawn through a small hole into an isolated recording chamber filled with paraffin oil. Under a dissecting microscope, a small longitudinal incision was made in the nerve sheath. Using fine forceps, nerve fibers were teased back onto a platinum recording electrode.
Characterization of esophageal vagal afferent properties.Location of receptive fields of all types of vagal afferent fibers was determined by mechanical stimulation throughout the preparation with a brush and then more accurately with a blunt glass rod. Accurate quantification of mechanical responsivity was performed differently according to the primary adequate stimulus for the type of fiber. Mechanical thresholds of all types were determined using calibrated von Frey hairs. Mucosal receptors showed rapidly adapting responses to maintained pressure on the receptive field with a von Frey hair. This type of responsiveness was also seen in responses of tension–mucosal (TM) receptors to low-level mucosal mechanical stimulation. The most reproducible, stimulus-dependent, and probably physiological responses of these afferents to mucosal stimuli were evoked when the probe was moved at a rate of 5 mm/sec across the receptive field rather than being static. Because receptive fields of these afferents are small (1–3 mm2), a single test at each intensity is prone to missing the center of the receptive field on occasions. Therefore, we minimized experimenter error by measuring the mean response to the middle 8 of 10 standard strokes given at 1 sec intervals. Because the von Frey hair was bent throughout the stroking stimulus, the receptive field was subjected to an even force as the hair passed over it. This protocol was found to give highly reproducible data and was therefore used to assess effects of GABAB receptor ligands on vagal afferents. Tension response curves were also obtained for all afferent fibers, which were used in combination with von Frey thresholds to determine whether the receptive fields of fibers were located in the mucosa, the muscle layer, or both. Tension stimuli were applied via a thread attached to an unpinned point adjacent to the mechanoreceptive field. The thread was attached to a cantilever via a pulley close to the preparation. Reference standard weights were then placed on the opposite end of the cantilever (Page and Blackshaw, 1998). Each weight was applied as a step and maintained for 1 min, and the response was measured as the mean discharge evoked over this period. Because all responses to tension were similarly slowly adapting, this method of assessment was considered representative of physiological responsiveness. The tension–response curves were produced by randomly applying weights to the cantilever system in the range of 0.5–7 gm. A maximum of 7 gm was applied because this has been shown previously to evoke maximal responses without causing tissue damage (Page and Blackshaw, 1998). A recovery period of at least 1 min was allowed between each tension stimulus.
Forty-nine mechanically sensitive vagal afferent fibers were recorded and characterized as either myelinated or unmyelinated according to their conduction velocity calculated from the time and distance between a stimulating (1 Hz, 20 V, 0.1 msec duration) electrode placed over the receptive field and the recording electrodes. Afferent fibers with conduction velocities below 2.0 m/sec were classified as C-fibers and those between 2 and 25 m/sec as Aδ-fibers. Aβ-fibers were not encountered during these experiments.
Effects of GABAB receptor agonist and antagonist on mechanical sensitivity of vagal afferents. After mechanical sensitivity of the gastroesophageal vagal afferents had been established, the effect of the GABAB receptor agonist baclofen on mechanical sensitivity was determined. Baclofen (30 μm) was added to the superfusing Krebs’ solution and was allowed to equilibrate for 20 min, after which time the tension–response and stroke–response curves were redetermined. This equilibration period was observed so as to ensure penetration of the drug into all layers of the tissue. Pilot experiments observing time course of the effect of baclofen showed no evidence of diminution over time and therefore no desensitization. This procedure was repeated for baclofen at increasingly higher doses (100 and 200 μm). Only three doses of baclofen were used so that emphasis could be placed on accurate quantification of mechanical responsiveness at each dose. These doses were comparable with those previously used to characterize peripheral GABAB receptors (Santicioli et al., 1991; Minocha and Galligan, 1993). Time control experiments were also performed in which there was no significant change in the mechanical responses over a comparable duration. In a separate series of experiments, the GABAB receptor antagonist SCH50911 (1 μm) (Bolser et al., 1995) was used to reverse the effect of baclofen (100 μm); after mechanical sensitivity of the gastroesophageal vagal afferents had been established, baclofen (100 μm) was added to the superfusing Krebs’ solution, and 20 min was allowed for equilibration. Mechanical response curves were then reestablished. SCH50911 (1 μm) was then added to the superfusing Krebs’ solution along with baclofen (100 μm), and again this was allowed to equilibrate for 20 min before mechanical response relationships were redetermined.
Data recording and analysis. Afferent impulses were amplified with a biological amplifier (BA.1; JRAK, Melbourne, Australia) and scaling amplifier (SA.1; JRAK) filtered (F1 filter; JRAK) and monitored using an oscilloscope (DL 1200A; Yokogawa, Tokyo, Japan). Single units were discriminated on the basis of action potential shape, duration, and amplitude on-line using a JRAK window discriminator (WD1) and also off-line using Spike II software (Cambridge Electronic Design, Cambridge, UK). All data were recorded on magnetic tape and analyzed off-line using a personal computer (Power Macintosh 7300/200). Peristimulus time histograms and discharge traces were displayed using Spike II analysis.
All data are represented as the mean ± SEM. Differences in conduction velocities between types of afferent fibers were assessed using a Kruskal–Wallis test, because these were not normally distributed (Fig. 1). Statistical differences between stimulus–response curves were evaluated using two-way ANOVA. If this showed significance, one-way ANOVA on individual mechanical stimuli was performed. A Dunnett’s or Tukeypost hoc test was used to determine significance between control and treated or between all the points tested, respectively. Differences were considered significant ifp ≤ 0.05.
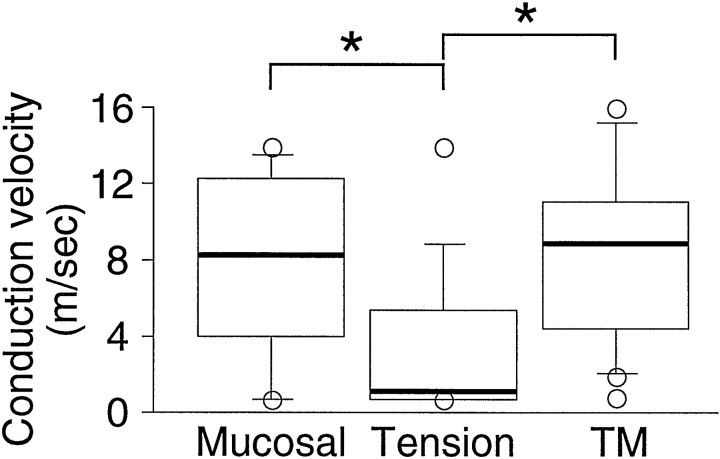
Fig. 1.
Conduction velocities of gastroesophageal vagal afferent fibers. The bars denote the interquartile range of the mucosal (n = 12), TM (n = 18), and tension (n = 13) receptor fiber conduction velocity. The bold line within the bar represents the median. The circles are individual values outside the interquartile range. Conduction velocities of tension receptor fibers were significantly less than those of mucosal and TM receptor fibers. *p < 0.05; Kruskal–Wallis test.
Drugs. Stock solutions of all drugs were kept frozen and diluted to their final concentration in Krebs’ solution on the day of the experiment. Baclofen was obtained from Research Biochemicals (Natick, MA), and SCH50911 was obtained from Tocris Cookson (Bristol, UK).
RESULTS
Mechanosensory properties of gastroesophageal vagal afferent fibers
Three types of mechanosensitive fiber were observed using thisin vitro preparation: those responding to circumferential tension but not to low-intensity mucosal stimuli (tension receptors;n = 17), those responding only to mucosal stroking (mucosal receptors; n = 14), and those responding to both mucosal stroking and circumferential tension, which we have previously termed TM receptors (n = 18) (Page and Blackshaw, 1998).
The conduction velocities for mucosal, TM, and tension afferents were in the C and Aδ range and are illustrated in Figure 1, which shows median data because they did not form a normal gaussian distribution. Conduction velocities of tension receptor afferents were significantly less than those of mucosal and TM afferents. Twenty-five percent of mucosal receptors, 11% of TM receptors, and 62% of tension receptors were classified as C-fibers (<2.0 m/sec). The remainder of the fibers tested comprised Aδ-fibers.
All the mucosal afferents observed were located in the esophagus. Fourteen TM receptor afferents were found in the esophagus, and four were found on or very close to the LES. In total, we recorded from 17 tension receptor afferents; 12 were located in the esophagus, one was on or very close to the LES, and four had receptive fields within the gastric wall.
Effect of baclofen on the mechanical sensitivity of gastroesophageal vagal afferents
Baclofen (30–200 μm) did not significantly affect the basal discharge of vagal gastroesophageal mucosal (data not illustrated), tension (Fig.2B), or TM (Fig.2D) receptor afferents.
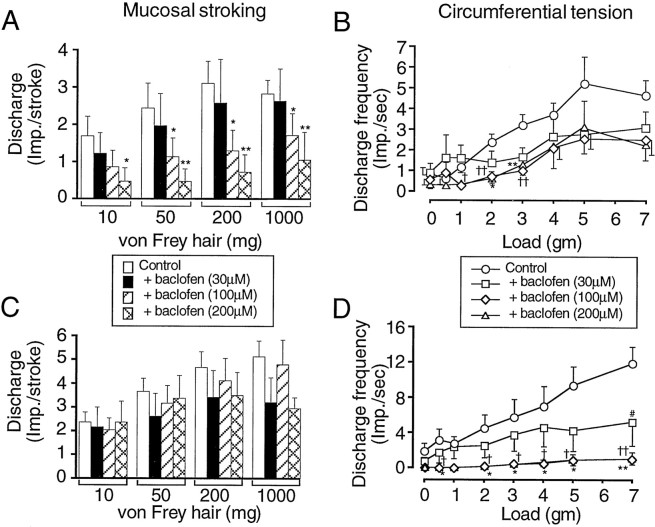
Fig. 2.
Group data for responses of mucosal (A), tension (B), and TM (C, D) gastroesophageal vagal afferents to mechanical stimulation. The effects of baclofen are shown on the responses to mucosal stroking [impulses (imp.) per stroke, mean of eight strokes] of mucosal (A) and TM (C) afferents. The effects of baclofen are shown also on the response to circumferential tension (average impulses per second over stimulus duration of 1 min) of tension (B) and TM (D) afferents. The graphs and histograms show the mean ± SE (n ≥ 5). Significant differences between treated and control (assessed using one-way ANOVA with a Dunnett’s post hoc test) are shown nearest each treated curve or histogram. *p < 0.05, **p < 0.01, control versus baclofen-treated (200 μm) responses; †p < 0.05, ††p < 0.01, control versus baclofen-treated (100 μm) responses; #p < 0.05, control versus baclofen-treated (30 μm) responses.
The effect of baclofen on mucosal, TM, and tension receptor afferent sensitivity to mechanical stimulation is illustrated in Figures3-5and is summarized in Figure 2. A typical response of mucosal afferents to mucosal stroking is illustrated in Figure 3. Using a two-way ANOVA baclofen significantly reduced the response curve of mucosal afferents to mucosal stroking with calibrated von Frey hairs (p < 0.0001). Using one-way ANOVA and a Dunnett’s post hoc test, baclofen (100–200 μm) significantly reduced the mucosal afferent response to mechanical stimulation with calibrated von Frey hairs throughout the range of stimuli (10–1000 mg) (Figs.2A, 3). The extent of inhibition was related to increasing dose (Fig. 2A), with baclofen (30 μm) having no effect on mechanical sensitivity.
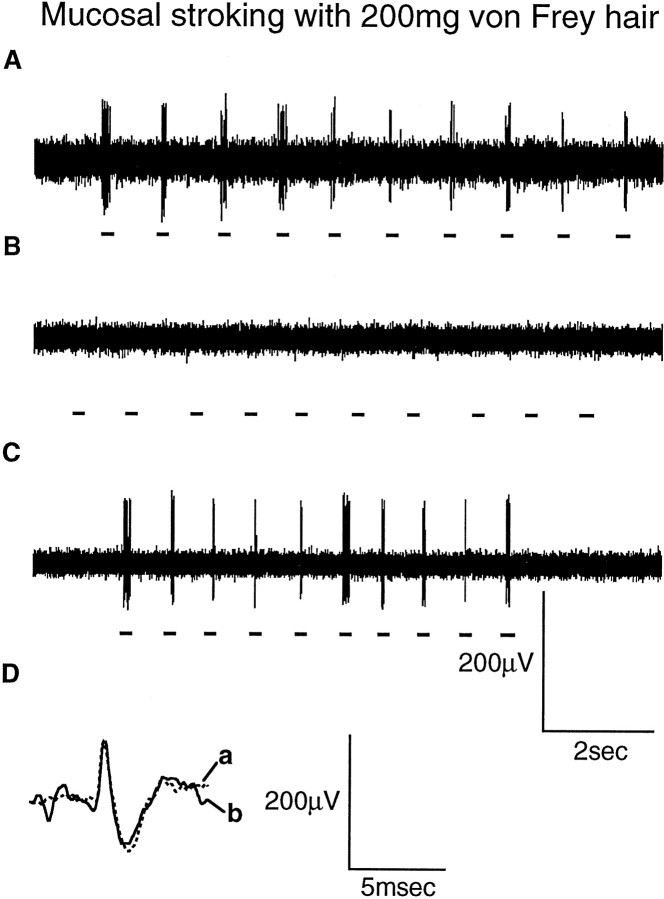
Fig. 3.
Typical response of a mucosal afferent to mucosal stroking with a calibrated von Frey hair (200 mg). The responses were obtained in the absence of baclofen (A), the presence of baclofen (100 μm) (B), and the presence of baclofen (100 μm) with SCH50911 (1 μm) (C). Dillustrates the average spike shape of the mucosal unit in response to mucosal stroking with a calibrated von Frey hair in the absence (a) and presence (b) of baclofen (100 μm) and SCH50911 (1 μm), showing that both responses were obtained from the same unit. Thehorizontal bars below the raw traces indicate where mucosal stroking occurred.
Fig. 4.
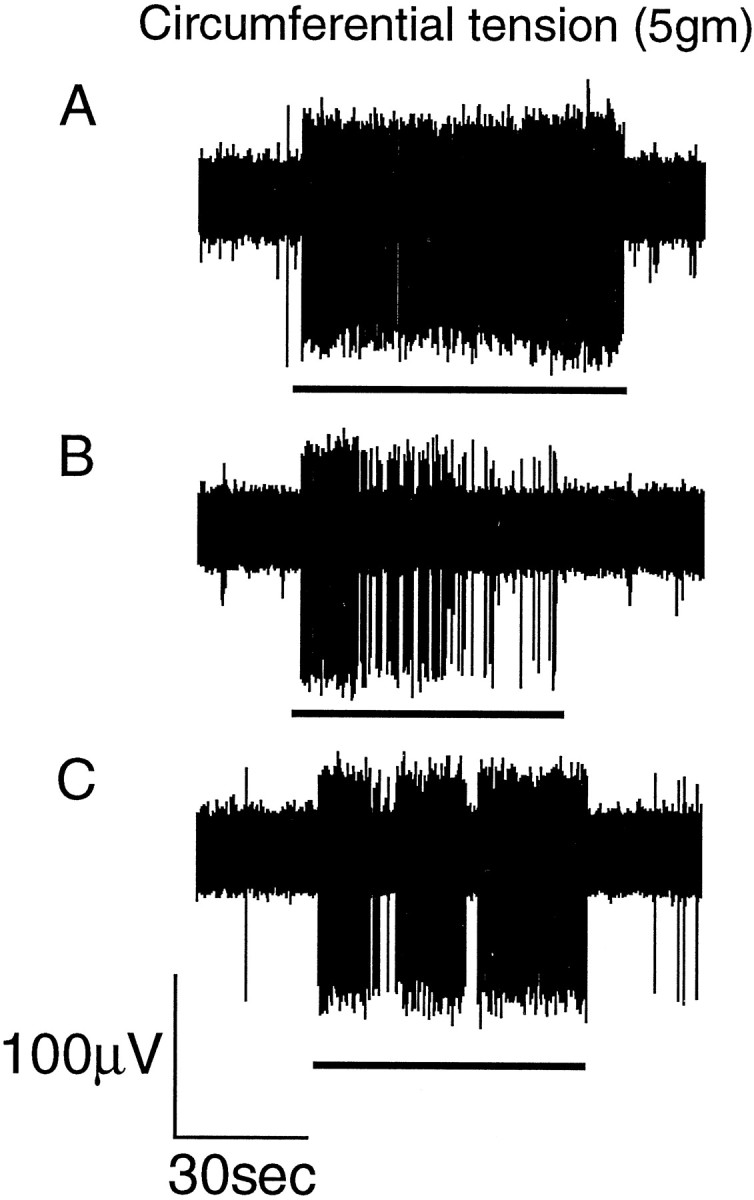
Typical response of a tension receptor afferent to circumferential tension (5 gm). The responses were obtained in the absence of baclofen (A), the presence of baclofen (100 μm) (B), and the presence of baclofen (100 μm) and SCH50911 (1 μm) (C). The horizontal bar below the raw traces indicate when the tension stimulus was applied. Inhibition by baclofen is most obvious during the static phase of the slowly adapting response. See Figure 2 for mean group data.
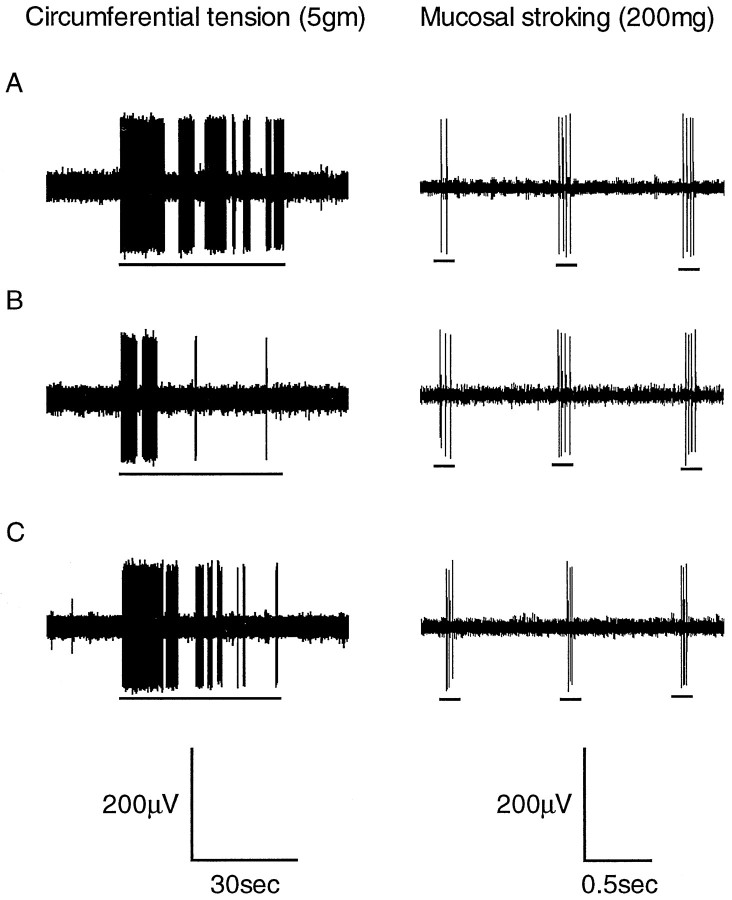
Fig. 5.
Typical response of a tension–mucosal afferent to circumferential tension (5 gm) and mucosal stroking (200 mg). The responses were obtained in the absence of baclofen (A), the presence of baclofen (100 μm) (B), and the presence of baclofen (100 μm) with SCH50911 (1 μm) (C). The horizontal bars below the raw traces indicate when the mechanical stimuli were applied. Although responses to circular tension are reversibly reduced by baclofen, no effect occurs on the response of this fiber to mucosal stroking.
The effect of baclofen on tension receptor afferents is summarized in Figure 2B. A typical response to circumferential tension is illustrated in Figure 4. Using a two-way ANOVA, baclofen significantly (p < 0.001) reduced the mean response of tension-sensitive afferents to circumferential tension. A one-way ANOVA with a Dunnett’s post hoc test revealed that this was principally attributable to the effect of baclofen (100 and 200 μm) on the responses to low-intensity stimuli (1–3 gm). Baclofen (30 μm) had no significant effect on the response to circumferential tension at any stimulus intensity.
Whereas all mucosal and tension receptors were affected by baclofen, only 11 of 18 of the TM receptor afferents had reduced mechanosensory function. The effect of baclofen (30–200 μm) on these is summarized in Figure 2, C and D, and is illustrated in Figure 5. Using a two-way ANOVA, baclofen significantly (p < 0.0001) affected the response curve to circumferential tension. One-way ANOVA, followed by a Dunnett’spost hoc test, revealed that baclofen (100–200 μm) significantly inhibited the response of TM afferents to circumferential tension throughout the range of stimuli (0.5–7 gm) (Fig. 2D). The response of TM receptor afferents to mucosal stroking with calibrated von Frey hairs (10–1000 mg) was completely unaffected by baclofen (30–200 μm) (Figs. 2C, 5).
Effect of the GABAB receptor antagonist SCH50911
Addition of SCH50911 (1 μm) to baclofen (100 μm) did not significantly affect basal discharge of mucosal, tension, or TM receptor afferents (Fig.3-6).
Fig. 6.
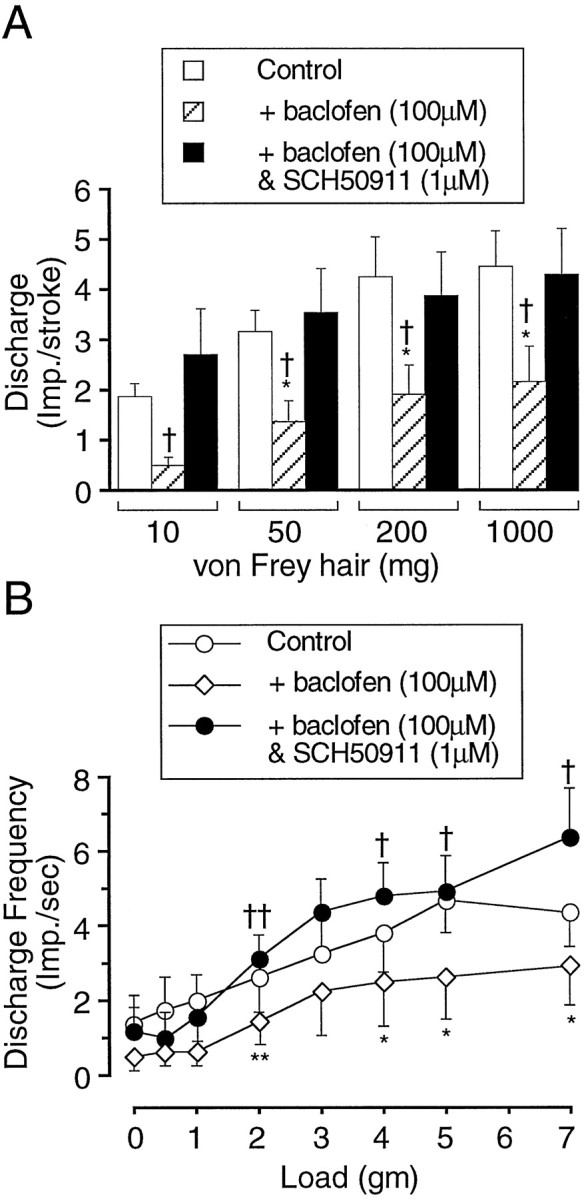
Group data for responses of mucosal afferents to mucosal stroking (impulses per stroke, mean 8 strokes) (A) and tension–mucosal afferents to circumferential tension (average impulses per second over stimulus time of 1 min) (B). The graphs and histograms show data mean ± SE (n ≥ 5) obtained in the presence of baclofen (100 μm) and baclofen (100 μm) plus SCH50911 (1 μm). Significant differences were assessed using one-way ANOVA with a Tukey post hoc test. *p < 0.05, **p < 0.01, control versus baclofen (100 μm); †p < 0.05; ††p < 0.01, baclofen (100 μm)-treated versus baclofen (100 μm) and SCH50911 (1 μm)-treated.
The reversal of the effect of baclofen by the GABAB receptor antagonist SCH50911 (1 μm) on mucosal and tension receptor afferents is illustrated in Figure 6, A and B, respectively. SCH50911 (1 μm) significantly reversed baclofen (100 μm)-induced inhibition of the response of mucosal afferents to mucosal stroking with calibrated von Frey hairs (10–1000 mg) (Fig. 6A). The inhibitory effect of baclofen (100 μm) on TM receptor afferent responses to circumferential tension was also significantly reversed by SCH50911 (1 μm) (Figs. 5,6B). SCH50911(1 μm) had no significant affect on mucosal sensitivity of TM receptor afferents in the presence of baclofen (100 μm; data not shown).
Although statistical evaluation was not performed, SCH50911 (1 μm) was observed to reverse the effect of baclofen (100 μm) on the response of tension receptor afferents to circumferential tension in three of three cases (Fig. 4).
DISCUSSION
The present study provides the first evidence that GABAB receptors inhibit mechanotransduction in primary sensory endings. This was elicited by the selective GABAB receptor agonist baclofen and reversed by the potent and selective antagonist SCH50911 (Bolser et al., 1995), confirming the involvement of GABAB receptors in inhibitory modulation at the sensory receptive field. Inhibition was seen in all mucosal and tension receptors recorded from the ferret upper gastrointestinal tract but was confined to a subgroup of tension–mucosal afferents in which only selective aspects of mechanosensitivity were affected. The selectivity and potency we have observed indicate a complexity and potential therapeutic benefit for modulation of G-protein-coupled receptors on sensory endings.
GABAB receptor agonists have been shown to inhibit somatomotor (Capaday, 1995), respiratory (Trippenbach, 1995), cardiovascular (Silva Carvalho et al., 1995), and gastrointestinal (Andrews et al., 1987) reflexes. They were thought to do this by inhibiting transmitter release from central afferent terminals in the spinal cord or medullary vagal nuclei (Brooks et al., 1992; Capaday, 1995). In addition to central GABAB receptors, there is also evidence for the existence of GABABreceptors in the periphery. These may have inhibitory effects on transmitter release from intrinsic neurons in the myenteric plexus of the gut (Kleinrok and Kilbinger, 1983; Minocha and Galligan, 1993) or from peripheral axon collaterals of extrinsic primary afferent fibers (Santicioli et al., 1991). Therefore, there is already an abundance of information on the effects of GABAB receptors on transmitter release from sensory fibers, yet this study provides the first direct evidence for a selective effect of GABAB receptors on mechanotransduction at sensory receptive fields.
Apart from our discovery of GABAB receptor actions on primary afferent transduction, evidence is sparse elsewhere for direct G-protein-coupled receptor-mediated inhibition of this kind.Su et al. (1994) have demonstrated a direct pharmacological inhibition of the sensitivity of a single population of visceral afferents by showing an inhibitory action of κ-opioid receptor agonists on the sensitivity of colonic pelvic afferents. Morphine has been shown to desensitize nerve endings in pacinian corpuscles of the cat by increasing the firing threshold (Chelyshev and Gataullin, 1983). Somatostatin inhibits the mechanosensitivity to noxious movements of articular afferents in normal and inflamed knee joints of the rat (Heppelmann and Pawlak, 1997).
Although our study is the first to demonstrate electrophysiologically a direct selective effect of GABAB receptors on primary afferent mechanotransduction, there is previous indirect evidence for effects of GABA and its analogs on sensitivity of other populations of afferents (Bolser et al., 1994). For instance, the site of action of GABAB agonists to inhibit the cough reflex may be within the CNS, but a peripheral site of action is also indicated because some GABAB agonists with limited penetration into the CNS are effective. Furthermore, these compounds inhibit the cough reflex in guinea pigs after topical administration to the airways by inhalation of aerosols (Bolser et al., 1994). The in vitro preparation used in this study (Page and Blackshaw, 1998) has enabled us to substantiate such observations through direct recordings of vagal afferent sensitivity without the uncertainty about complication of possible central effects of GABA agonists. There are potentially therapeutic correlates of actions we have demonstrated at the cellular level also in the whole animal. These are reflected in the inhibition by GABAB agonists of triggering of transient lower esophageal sphincter relaxation after gastric distension. These relaxations are known to be mediated by a vagal pathway and are the major target for novel treatments for gastroesophageal reflux disease. (Blackshaw et al., 1999; Lidums et al., 1999).
A peculiar observation in this study was that GABAB receptor inhibition of mechanosensitivity in TM afferents was restricted to their responses to tension, with no effect on their response to mucosal stroking. This finding contrasts with the observation that sensitivity of mucosal afferents to mucosal stroking was potently inhibited by baclofen. Therefore, differences in the effects of baclofen were seen both between and within groups of afferents, indicating that they are not artifactual. These differences raise several questions about the fundamental transduction mechanisms underlying them. Our data do not provide direct evidence to support a particular mechanism. However, we would speculate that differential sensitivity may be related to two possible factors. GABAB receptor inhibition may be manifested only within a small part of the dynamic range of TM receptors to mucosal stimuli that was undetectable with the range of stimulus levels that we used. Alternatively, a selective distribution of GABAB receptors may occur toward those regions of the TM receptor ending responsive to tension and away from those responsive to mucosal stroking, in a way not unlike the differential distribution of neurochemical features to peripheral and central endings of sensory fibers suggested by circumstantial evidence (Bakhle and Bell, 1995). To verify differential distribution in the periphery using combined retrograde tracing and immunohistochemistry would be frustrated by the twisted paths that afferents often take when approaching their terminations (Berthoud and Powley, 1992), so it is difficult to determine whether several endings arise from one or several parent axons. Our electrophysiological observations are therefore likely to have revealed greater complexity than that which may be corroborated by future anatomical studies.
In conclusion, the present study has provided the first direct evidence for the inhibitory modulation of primary afferent mechanotransduction by the G-protein-coupled GABAB receptor. The study also confirms the existence of the three types of vagal afferent documented previously (Page and Blackshaw, 1998) and shows differential sensitivity to baclofen among them.
Footnotes
This project was supported by Astra Pharmaceuticals and the National Health and Medical Research Council of Australia.
Correspondence should be addressed to Dr. Amanda Page, Nerve–Gut Research Laboratory, Department of Gastrointestinal Medicine, Royal Adelaide Hospital, North Terrace, Adelaide SA5000, Australia.
REFERENCES
- 1.Andrews PLR, Bingham S, Wood KL. Modulation of the vagal drive to the intramural cholinergic and non-cholinergic neurones in the ferret stomach by baclofen. J Physiol (Lond) 1987;388:25–39. doi: 10.1113/jphysiol.1987.sp016599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Awapara J, Landua AJ, Fuerst R, Seale B. Free γ-aminobutyric acid in brain. J Biol Chem. 1950;187:35–39. [PubMed] [Google Scholar]
- 3.Bakhle YS, Bell C. Neurokinin A and substance P vary independently in different regions of rat sensory neurons. Neuropeptides. 1995;28:237–241. doi: 10.1016/0143-4179(95)90027-6. [DOI] [PubMed] [Google Scholar]
- 4.Belvisi MG, Ichinose M, Barnes PJ. Modulation of non-adrenergic, non-cholinergic neural bronchoconstriction in guinea-pig airways via GABAB-receptors. Br J Pharmacol. 1989;97:1225–1231. doi: 10.1111/j.1476-5381.1989.tb12582.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Berthoud H-R, Powley TL. Vagal afferent innervation of the rat fundic stomach: morphological characterization of the gastric tension receptor. J Comp Neurol. 1992;319:261–276. doi: 10.1002/cne.903190206. [DOI] [PubMed] [Google Scholar]
- 6.Bettler B, Kaupmann K, Bowery N. GABAB receptors: drugs meet clones. Curr Opin Neurobiol. 1998;8:345–350. doi: 10.1016/s0959-4388(98)80059-7. [DOI] [PubMed] [Google Scholar]
- 7.Blackshaw LA, Staunton E, Lehmann A, Dent J (1999) Inhibition of transient LES relaxations and reflux in ferrets by GABABreceptor agonists. Am J Physiol, in press. [DOI] [PubMed]
- 8.Bolser C, Blythin DJ, Chapman RW, Egan RW, Hey JA, Rizzo C, Kuo S-C, Kreutner W. The pharmacology of SCH50911: a novel, orally-active GABA-B receptor antagonist. J Pharmacol Exp Ther. 1995;274:1393–1398. [PubMed] [Google Scholar]
- 9.Bolser DC, DeGennaro FC, O’Reilly S, Chapman RW, Kreutner W, Egan RW, Hey JA. Peripheral and central sites of action of GABA-B agonists to inhibit the cough reflex in the cat and guinea-pig. Br J Pharmacol. 1994;113:1344–1348. doi: 10.1111/j.1476-5381.1994.tb17145.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bowery NG. GABAB receptor pharmacology. Annu Rev Pharmacol Toxicol. 1993;33:109–147. doi: 10.1146/annurev.pa.33.040193.000545. [DOI] [PubMed] [Google Scholar]
- 11.Bowery NG, Pratt GD. GABAB receptors as targets for drug action. Drug Res. 1992;42:215–223. [PubMed] [Google Scholar]
- 12.Bowery NG, Hill DR, Hudson AL, Doble A, Middlemiss DN, Shaw J, Turnbull M. (−)Baclofen decreases neurotransmitter release in the mammalian CNS by an action at a novel GABA receptor. Nature. 1980;283:92–94. doi: 10.1038/283092a0. [DOI] [PubMed] [Google Scholar]
- 13.Brooks PA, Glaum SR, Miller RJ, Spyer KM. The actions of baclofen on the neurones and synaptic transmission in the nucleus tractus solitarii of the rat in vitro. J Physiol (Lond) 1992;457:115–129. doi: 10.1113/jphysiol.1992.sp019367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Capaday C. The effects of baclofen on the stretch reflex parameters of the cat. Exp Brain Res. 1995;104:287–296. doi: 10.1007/BF00242014. [DOI] [PubMed] [Google Scholar]
- 15.Chelyshev YA, Gataullin RR. Effect of morphine and leucine–enkephalin on the electrophysiological responses of pacinian corpuscles of the cat. Neurosci Lett. 1983;39:165–168. doi: 10.1016/0304-3940(83)90071-x. [DOI] [PubMed] [Google Scholar]
- 16.Curtis DR, Johnston GAR. Aminoacid transmitters in the mammalian central nervous system. Rev Physiol Biochem Exp Pharamacol. 1974;69:97–188. doi: 10.1007/3-540-06498-2_3. [DOI] [PubMed] [Google Scholar]
- 17.Davidoff RA, Sears E. The effects of Lioresal on synaptic activity in the isolated spinal cord. Neurology. 1974;24:957–963. doi: 10.1212/wnl.24.10.957. [DOI] [PubMed] [Google Scholar]
- 18.Erdö SL, Rosdy B, Szporny C. Higher GABA concentrations in fallopian tube than in brain of the rat. J Neurochem. 1982;38:1174–1176. doi: 10.1111/j.1471-4159.1982.tb05368.x. [DOI] [PubMed] [Google Scholar]
- 19.Green KA, Cottrell GA. Actions of baclofen on components of the Ca-current in rat and mouse DRG neurones in culture. Br J Pharmacol. 1988;94:235–245. doi: 10.1111/j.1476-5381.1988.tb11520.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Heppelmann B, Pawlak M. Inhibitory effect of somatostatin on the mechanosensitivity of articular afferents in normal and inflamed knee joints of the rat. Pain. 1997;73:377–382. doi: 10.1016/S0304-3959(97)00124-3. [DOI] [PubMed] [Google Scholar]
- 21.Jessen KR, Hills JM, Saffery MJ. Immunohistochemical demonstration of GABAergic neurons in the enteric nervous system. J Neurosci. 1986;6:1628–1634. doi: 10.1523/JNEUROSCI.06-06-01628.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kato M, Waldmann U, Murakami S. Effects of baclofen on spinal neurones of cats. Neuropharmacology. 1978;17:827–833. doi: 10.1016/0028-3908(78)90071-0. [DOI] [PubMed] [Google Scholar]
- 23.Kleinrok A, Kilbinger H. γ-Aminobutyric acid and cholinergic transmission in the guinea-pig ileum. Naunyn Schmiedebergs Arch Pharmacol. 1983;322:216–220. doi: 10.1007/BF00500768. [DOI] [PubMed] [Google Scholar]
- 24.Knutsson E, Lindblom U, Martensson A. Plasma and cerebrospinal fluid levels of baclofen (Lioresal) at optimal therapeutic responses in spastic paresis. J Neurol Sci. 1974;23:473–484. doi: 10.1016/0022-510x(74)90163-4. [DOI] [PubMed] [Google Scholar]
- 25.Lidums I, Lehmann A, Checklin H, Dent J, Holloway RH (1999) The GABAB agonist baclofen inhibits transient lower oesophageal sphincter relaxations and gastroesophageal reflux in normal human subjects. Gastroenterology, in press. [DOI] [PubMed]
- 26.Minocha A, Galligan JJ. Excitatory and inhibitory responses mediated by GABAA and GABAB receptors in guinea distal colon. Eur J Pharmacol. 1993;230:187–193. doi: 10.1016/0014-2999(93)90801-n. [DOI] [PubMed] [Google Scholar]
- 27.Newberry NR, Nicoll RA. Comparison of the action of baclofen with γ-aminobutyric acid on rat hippocampal pyramidal cells in vitro. J Physiol (Lond) 1985;360:161–185. doi: 10.1113/jphysiol.1985.sp015610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nicoll RA, Malenka RC, Kauer JA. Functional comparison of neurotransmitter receptor subtypes in mammalian central nervous system. Physiol Rev. 1990;70:513–565. doi: 10.1152/physrev.1990.70.2.513. [DOI] [PubMed] [Google Scholar]
- 29.Page AJ, Blackshaw LA. An in vitro study of the properties of vagal afferent fibres innervating the ferret oesophagus and stomach. J Physiol (Lond) 1998;512:907–916. doi: 10.1111/j.1469-7793.1998.907bd.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Roberts E, Frankel S. γ-Aminobutyric acid in brain: its formation from glutamic acid. J Biol Chem. 1950;187:55–63. [PubMed] [Google Scholar]
- 31.Santicioli P, Tramontana M, Del Bianco E, Maggi CA, Geppetti P. GABAA and GABAB receptors modulate the K+-evoked release of sensory CGRP from the guinea-pig urinary bladder. Life Sci. 1991;48:169–172. doi: 10.1016/0024-3205(91)90188-h. [DOI] [PubMed] [Google Scholar]
- 32.Silva Carvalho L, Dawid Milner MS, Spyer KM. The pattern of excitatory inputs to the nucleus tractus solitarii evoked on stimulation in the hypothalamic defence area of the cat. J Physiol (Lond) 1995;487:727–737. doi: 10.1113/jphysiol.1995.sp020913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Su X, Sengupta JN, Gebhart GF. Effects of κ opioid receptor-selective agonists on responses of pelvic nerve afferents to noxious colorectal distension. J Neurophysiol. 1997;78:1003–1012. doi: 10.1152/jn.1997.78.2.1003. [DOI] [PubMed] [Google Scholar]
- 34.Tanaka C. γ-Aminobutyric acid in peripheral tissues. Life Sci. 1985;37:2221–2235. doi: 10.1016/0024-3205(85)90013-x. [DOI] [PubMed] [Google Scholar]
- 35.Trippenbach T. Baclofen-induced block of the Hering-Breuer expiratory-promoting reflex in rats. Can J Physiol Pharmacol. 1995;73:706–713. doi: 10.1139/y95-091. [DOI] [PubMed] [Google Scholar]
- 36.Udenfriend S. Identification of γ-aminobutyric acid in brain by the isotope derivative method. J Biol Chem. 1950;187:65–69. [PubMed] [Google Scholar]