Abstract
Axonal regeneration is normally limited within myelinated fiber tracts in the CNS of higher vertebrates. Numerous studies suggest that CNS myelin contains inhibitors that may contribute to abortive axonal growth. In contrast to the evidence of myelin-associated neurite inhibitors, embryonic neurons transplanted into the CNS can regenerate extensively within myelinated tracts in vivo. It has been speculated that embryonic neurons do not yet express the appropriate receptors for myelin-associated inhibitors. Recently, however, extensive regeneration from transplanted adult neurons has also been reported within myelinated tracts of the CNS, casting doubt on the role myelin-associated inhibitors play in abortive regeneration. The present study reexamined the potential of white matter to support neurite growth in vitro. By the use of Neurobasal medium, neurons were cultured onto unfixed cryostat sections of mature rat CNS tissue. As documented previously, robust neuronal attachment and neurite outgrowth occurred on gray matter but these neurites were sharply inhibited by white matter. In addition, however, increased rates of neuronal attachment directly to white matter occurred with neurite outgrowth comparable in length with that on gray matter but limited to directions parallel to the fiber tract. Frequently, the same section of white matter was found to inhibit neurite outgrowth from neurons on gray matter while supporting parallel neurite outgrowth from neurons on white matter. These results suggest that whether white matter supports or inhibits axonal growth depends on the geometric relationship between the axon and the fiber tract; more specifically, white matter supports parallel growth but inhibits nonparallel growth.
Keywords: axonal regeneration, myelin, white matter, inhibition, neurite outgrowth, tissue section culture, cryoculture, Neurobasal medium, sympathetic, fasciculation, neuronal attachment, corpus callosum, optic tract, spinal cord, geometry, glial fibrillary acidic protein (GFAP), vital dye
Axonal regeneration is usually abortive in the mature CNS of higher vertebrates (Ramon y Cajal, 1928). This abortive regeneration does not seem to be attributable to intrinsic limitations on the ability of mature CNS neurons to grow axons but, rather, to properties of the mature CNS making it nonpermissive for axonal growth (David and Aguayo, 1981). These nonpermissive properties may include potent neurite growth-inhibiting factors in CNS myelin (Schwab and Thoenen, 1985; Caroni and Schwab, 1988a,b; Schwab and Caroni, 1988; Schnell and Schwab, 1990; Schwab et al., 1993b; McKerracher et al., 1994; Mukhopadhyay et al., 1994;Filbin, 1995) and in glial scars (Snow et al., 1990; McKeon et al., 1991; Bovolenta et al., 1992; Pindzola et al., 1993; Davies et al., 1997, 1999).
Some of the earliest evidence of the existence of neurite growth-inhibiting factors in white matter came from studies in which neurons were cultured on unfixed cryostat sections of neural tissue showing robust neuronal attachment and neurite outgrowth on gray matter or peripheral nerve but little or no attachment or neurite outgrowth on white matter (Carbonetto et al., 1987; Sandrock and Matthew, 1987;Crutcher, 1989; Crutcher and Privitera, 1989; Savio and Schwab, 1989;Watanabe and Murakami, 1989, 1990). Moreover, neurites extending on gray matter or peripheral nerve were sharply inhibited from extension onto white matter (Carbonetto et al., 1987; Sandrock and Matthew, 1987;Crutcher, 1989; Savio and Schwab, 1989).
However, despite the compelling evidence, both from in vitroand in vivo studies, that CNS myelin contains potent neurite growth-inhibiting factors, white matter can support axonal growth from transplants of either embryonic (Wictorin et al., 1989, 1990a,b, 1991,1992; Tønder et al., 1990; Davies et al., 1993, 1994; Humpel et al., 1994; Lehman et al., 1998) or adult (Davies et al., 1997, 1999) neuronsin vivo. Where assessed, the direction of axonal growth in these cases was in parallel with the longitudinal axis of the tract.
The present study assessed the potential of CNS white matter to support neuronal attachment and neurite outgrowth under conditions that augment survival of sympathetic neurons. More specifically, survival of sympathetic neurons is greatly enhanced when neurons are cultured in Neurobasal medium (Pettigrew and Crutcher, 1996), and this approach was used to culture sympathetic neurons on cryostat sections of adult rat forebrain or spinal cord. Consistent with the previous studies, robust neuronal attachment and extensive neurite outgrowth occurred on gray matter, and these neurites were sharply inhibited at borders with white matter. However, neuronal attachment also occurred to white matter, in which case the neurites were comparable in length with those on gray matter but generally limited to directions parallel to the longitudinal axis of the tract. In fact, the same white matter region in the same tissue sections supported long neurite outgrowth from neurons attached directly to it while sharply inhibiting neurites extending to it from gray matter. These results suggest that CNS white matter can support long axonal growth providing that the axons extend along a parallel trajectory.
MATERIALS AND METHODS
Preparation of substrata. Adult Sprague Dawley rats (maintained in the University of Cincinnati vivarium in accordance with the National Institutes of Health guide for the care of research animals) were deeply anesthetized using pentobarbital sodium solution (4 μl/gm, i.p.; Abbott Labs, Irving, TX) and decapitated. The brains were rapidly removed and frozen at −80°C. Brains were cut coronally using a cryostat, and 10- or 16-μm-thick sections were thaw-mounted onto untreated 35-mm-diameter plastic culture dishes (catalog #1008; Fisher Scientific, Houston, TX; five sections per dish) and kept at −20°C until plating 2–4 hr later. Horizontal sections of cervical spinal cord were prepared in the same manner (for review, see Crutcher, 1993).
Tissue culture. Lumbar sympathetic chain ganglia were dissected from embryonic day 10 Leghorn chicken embryos (Spafas, Boston, MA) in Ham’s F12 medium (Sigma, St. Louis, MO). In some cases, the sympathetic chain ganglia were further dissected into explants (area, 3,000–45,000 μm2) using a Bard-Parker scalpel fitted with a no. 10 blade and seeded directly onto the prepared tissue sections. In other cases, the sympathetic chain ganglia were incubated with 0.25% trypsin (Sigma) for 20 min at 37°C. Trypsinization was subsequently blocked by exposure to 100% heat-inactivated fetal bovine serum (Harlan Bioproducts for Science, Indianapolis, IN) for 5 min, and the tissue was washed three times with serum-free Ham’s F12 medium. The tissue was then dissociated by gentle trituration using flamed Pasteur pipets (catalog #13-678-6A; Fisher Scientific), and the cell suspension was seeded onto the prepared tissue sections. All cultures were established in serum-free Neurobasal medium (2 ml per dish) supplemented with B27 [Life Technologies, Gaithersburg, MD; 50:1 (v/v)] and 0.5 mml-glutamine (Sigma) and subsequently transferred to either fresh Neurobasal medium on the third day or Ham’s F12 medium supplemented with 20 nm progesterone (Sigma), 100 μm putrescine (Sigma), 30 nm selenium (Sigma), and 100 μg/ml human apotransferrin (Sigma) after 23 hr. Some cultures were established with 2.5 ng/ml nerve growth factor (NGF; product code BT-5017; Harlan Bioproducts for Science). Cultures were grown for 2–8 d in a humidified environment at 37°C and 6% CO2.
Evaluation of neurite growth and glial fibrillary acidic protein immunohistochemistry. Neurite outgrowth and cell attachment were assessed using a dye for living cells (vital dye). Specifically, each dish was treated with 400 μl (15 ng/ml) of 5-carboxy-fluorescein diacetate AM (Molecular Probes, Eugene, OR) for 45–90 min at 37°C. In some cases, to label astrocytes within the tissue section substrata, we treated cultures simultaneously with a Cy3-conjugated monoclonal antibody raised against mouse glial fibrillary acidic protein (GFAP; catalog #C9205; Sigma; final working dilution, 1:200 or 1:400). Subsequently, all media were removed and replaced with 1.5 ml per dish of Ham’s F12 medium. The cultures were then visualized using a Nikon (Garden City, NY) Diaphot fluorescent microscope with a fluorescein (vital dye) or rhodamine (Cy3-conjugated anti-GFAP) filter and a 4× or 10× objective. Digitized images were captured using a video camera attached to a Power Macintosh microcomputer with a Data Translation frame-grabber card and electronically enhanced to increase contrast. Alternatively, cultures were scanned using a Molecular Dynamics (Sunnyvale, CA) 2010 confocal microscope and a 10× objective. Separate confocal micrographs (488 and 568 nm wavelengths) of the same field were captured to a Silicon Graphics (Mountain View, CA) Indy workstation and then superimposed to produce a composite micrograph.
Neurite length from explants was quantified using NIH Image 1.60 software by measuring the radial extent of the neuritic halo. The radius of the neuritic halo was defined as the linear distance between the perimeter of the explant core (central region containing cell somata) and the point where the majority of the neurites ended. In some cases, individual neurites extended beyond this point (these individual neurites were excluded from analysis), but the majority of the neurites fell within the defined perimeter.
In cases in which explants were attached to gray matter, the neuritic halo was measured in four orthogonal directions and averaged. In cases in which explants were attached to white matter, the neuritic halo was measured in two directions parallel to the longitudinal axis of the white matter tract and in two perpendicular directions and averaged separately. Because it was difficult to assess the longitudinal axis of lateral portions of the corpus callosum, only explants attached to medial portions of the corpus callosum were used in the analysis. Explants were included in the analysis only if their halos were confined to the area of interest, i.e., the medial corpus callosum, hippocampus, or neocortex. Explants were excluded from analysis if their halos overlapped with adjacent explant halos. All statistical comparisons were made using a two-tailed, unpaired Student’st test. Photomicrographs were captured with a Nikon 35 mm camera.
RESULTS
Technical considerations
Explants were clearly discernible using phase-contrast optics (see Figs. 5D, 6D for examples). However, under these conditions neurites and dissociated neurons were difficult to visualize against the tissue section background. Labeling neurons and their neurites with a fluorescent vital dye resulted in a detectable signal under epifluorescent illumination. The tissue sections contained no living components and consequently provided a low background signal against which the neurons and their growing neurites could be visualized clearly. In some figures epifluorescence was combined with phase-contrast optics to make anatomical landmarks visible. In other cases, in which phase contrast made visualization of the fluorescent signal difficult, fluorescence and phase-contrast micrographs are presented in tandem. Because the vital dye stains all living cells, this dye also makes it possible to assess the presence of non-neuronal cells. Occasionally, small cells could be seen within the explant outgrowth halos, but the majority of the neurites were not associated with these cells. Those neurites that were associated with these cells appeared to be advancing well ahead of them.
Fig. 5.
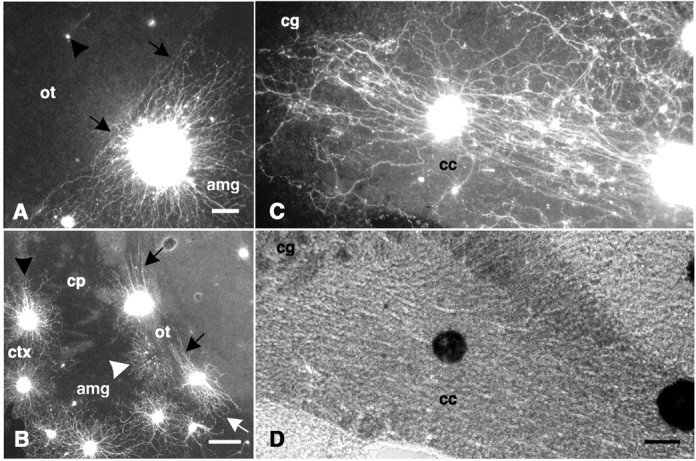
Explants on coronal forebrain sections.A, A fluorescence-labeled explant is shown attached to the amygdala (amg) with its neurite halo sharply inhibited at the border (black arrows) with the optic tract (ot). A single neuron is shown (black arrowhead) with the neurite extending on, and in parallel with, the optic tract. B, Fluorescence-labeled explants are shown attached directly to the optic tract with extensive outgrowth on, and in parallel with, the tract (black arrows). Outgrowth from explants on the optic tract is comparable in length with that of explants on neocortex (ctx; black arrowhead). Neurite outgrowth on portions of the optic tract sectioned transversely is more mixed in orientation (white arrow). The outgrowth halo of a detached explant (white arrowhead) is shown having grown on the amygdala but inhibited by the adjacent optic tract. C, A fluorescence-labeled explant is shown attached to the corpus callosum (cc) with extensive outgrowth extending on, and in parallel with, the tract. As neurites approach the cingulum (cg), they become increasingly mixed in orientation. D, The same field shown in C is shown with phase-contrast optics.cp, Caudate-putamen. Scale bars: A, 100 μm; B, 250 μm; C, D, 100 μm.
Fig. 6.
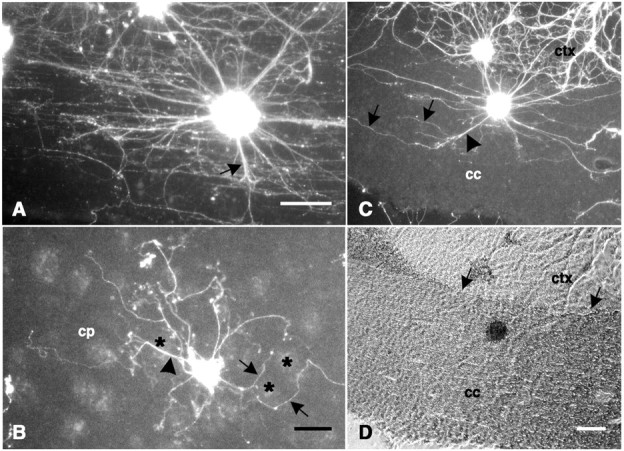
Explants on CNS tissue sections. A,A horizontal section of cervical spinal cord showing a fluorescence-labeled explant attached to the lateral funiculus with extensive neurite outgrowth on, and predominantly in parallel with, the fiber tract. A few neurites are oriented in nonparallel directions (black arrow) but heavily fasciculated.B, A coronal section of forebrain showing a fluorescence-labeled explant attached to the caudate-putamen (cp). Some neurites (black arrowhead) cross directly over white matter (black asterisks), but these are generally fasciculated, whereas nonfasciculated neurites (black arrows) generally avoid white matter.C, A coronal section of forebrain showing a fluorescence-labeled explant attached to the corpus callosum (cc) with some fascicles (black arrowhead) oriented nonparallel to the fiber tract. Defasciculation (black arrows) is coincident with reorientation in the parallel direction. D, The same field shown in C with phase-contrast optics showing the border (black arrows) between the neocortex (ctx) and the corpus callosum. Scale bars:A, 150 μm; B, 100 μm; C, D, 100 μm.
To analyze the substrate properties of the tissue sections apart from the influence of the culture dish, we mounted sections on untreated plastic. Explants or dissociated neurons often attached near the edge of sections of forebrain, but neurites from these neurons did not extend onto the surrounding untreated tissue culture plastic (data not shown). No attachment occurred to tissue culture plastic when sections of forebrain were used, indicating that the untreated plastic does not support attachment or neurite growth (Crutcher, 1989, 1993; Crutcher and Privitera, 1989; Pettigrew and Crutcher, 1997). In contrast, as has been reported previously, some attachment and neurite outgrowth occurred on untreated plastic in the presence of spinal cord sections (Crutcher, 1989).
In previous studies using tissue section culture, both dissociated neurons and explants frequently attached to CNS gray matter but rarely attached to CNS white matter (Carbonetto et al., 1987; Crutcher, 1989;Savio and Schwab, 1989; Watanabe and Murakami, 1989, 1990). Culture in Neurobasal medium led to a greater, more reliable incidence of attachment to white matter regions of the tissue sections. This permitted assessment of the extent to which neurite outgrowth will occur on white matter tracts.
Attachment and outgrowth of dissociated neurons
In Neurobasal medium, as in previous studies using other media, greater attachment of neurons occurred to gray matter than to white matter. The neocortex and caudate-putamen supported high numbers of attached neurons compared with the corpus callosum (Fig.1A), as did the hippocampal formation (see Fig. 3A). However, culture in Neurobasal medium allowed sympathetic neurons to attach near the midline of the corpus callosum in appreciable numbers. Interestingly, lateral portions of the corpus callosum still supported little or no attachment of sympathetic neurons. In fact, the distribution of sympathetic neurons on the corpus callosum appeared to correspond to the underlying organization of the fiber tract. The majority of fibers composing the corpus callosum at the midline pass within the plane of section. In contrast, many of the fibers more laterally in the corpus callosum pass in and out of the plane of section. This distribution of neurons on the corpus callosum was consistent from section to section at this anatomical level, and in general, it appeared that the portion of the tract that passes within the plane of section is a more conducive substrate for attachment than is white matter sectioned transversely. Similarly, there was little neuronal attachment to the transversely sectioned cingulum (see Fig.3A,B). The contrasting geometry of the medial and lateral corpus callosum is evident using phase-contrast optics (Fig. 1B).
Fig. 1.
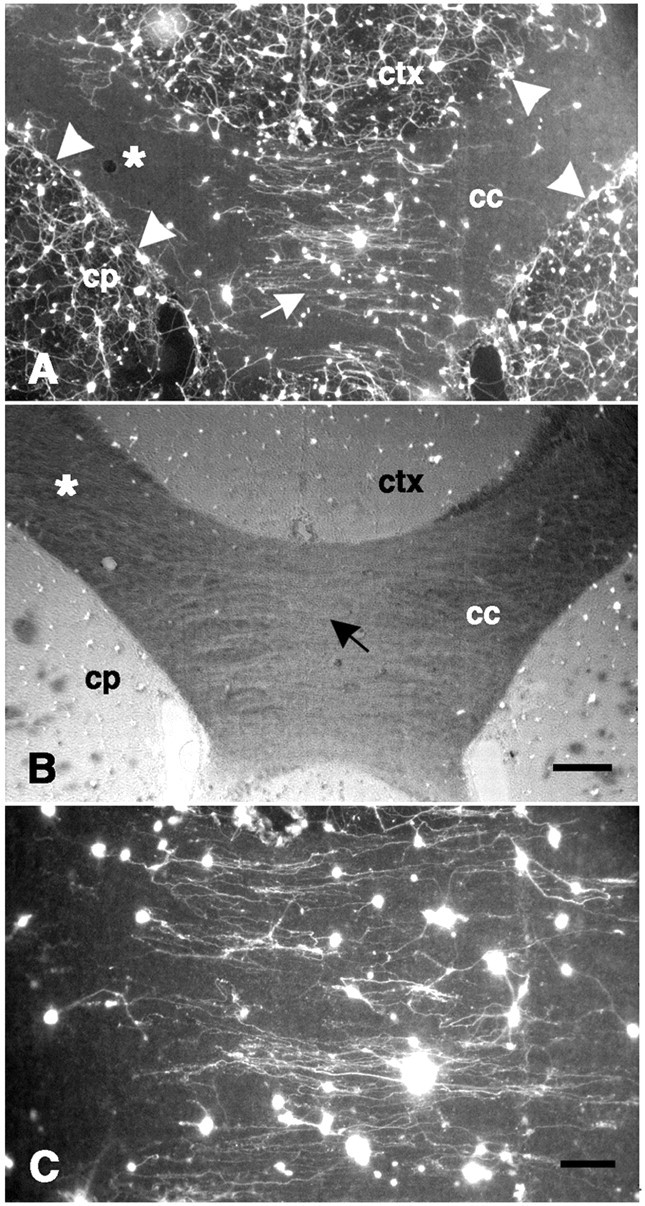
Dissociated neurons on a coronal forebrain section. A, The density of fluorescence-labeled neurons is greatest on gray matter, such as the neocortex (ctx) and caudate-putamen (cp); intermediate levels of attachment are found near the corpus callosum (cc) midline (white arrow); and virtually no neurons are attached to more lateral portions of the corpus callosum (white asterisk). The dense plexus of neurites on gray matter is sharply inhibited at the border with the corpus callosum (white arrowheads). From neurons attached to the corpus callosum, neurite outgrowth is oriented in a direction parallel to the longitudinal axis of the tract (see C) in contrast to the complex pattern of neurite outgrowth on gray matter (see also Fig. 2A). B, The same field shown in A is shown with fluorescence in combination with phase-contrast optics. Note the low optical density (black arrow) near the midline of the corpus callosum corresponding to the relatively parallel orientation of the fibers here, in contrast to the darker regions (white asterisk) found laterally where fibers run more obliquely.C, Higher power photomicrograph of the center of fieldA showing neurite outgrowth that is primarily in parallel with the underlying fiber tract is shown. Scale bars:A, B, 300 μm; C, 100 μm.
Fig. 3.
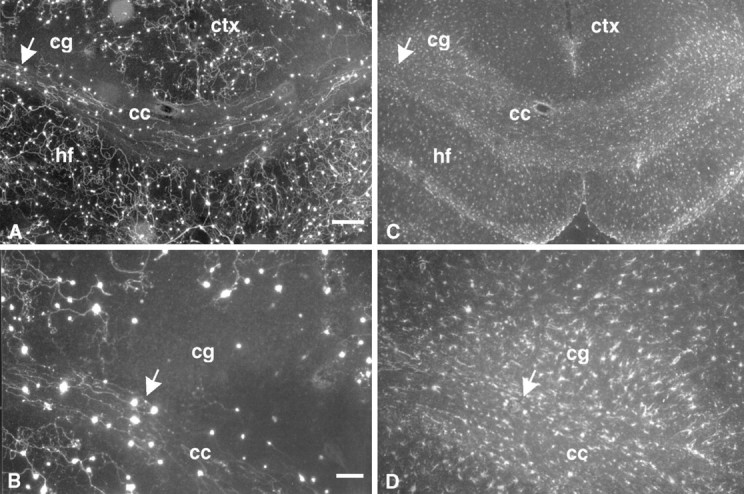
Dissociated neurons on GFAP-stained sections.A, Many fluorescence-labeled neurons are attached to the corpus callosum (cc) with neurite outgrowth oriented in parallel with the longitudinal axis of the tract. Few neurons, in contrast, are attached to the transversely sectioned cingulum (cg). B, Higher power photomicrograph of an area indicated by the white arrows inA and C is shown. C, GFAP immunoreactivity is densely represented in all regions of the forebrain. Within the corpus callosum, GFAP-immunoreactive processes are primarily oriented in parallel with the longitudinal axis of the tract. D, Area corresponding to B shows GFAP staining. ctx, Neocortex; hf,hippocampal formation. Scale bars: A, C, 300 μm;B, D, 100 μm.
Neurite outgrowth from dissociated sympathetic neurons on gray matter occurred in all directions with no preference in orientation. For example, outgrowth from dissociated neurons was heavily fasciculated but radiated in almost all directions on gray matter portions of the caudate-putamen (Fig.2A). A similar pattern of neurite outgrowth, although less fasciculated, occurred from dissociated neurons on neocortex (Fig. 1A) and on hippocampus (Fig. 3A). Neurite outgrowth from dissociated neurons on gray matter generally did not extend across white matter borders, consistent with previous studies (Savio and Schwab, 1989).
Fig. 2.
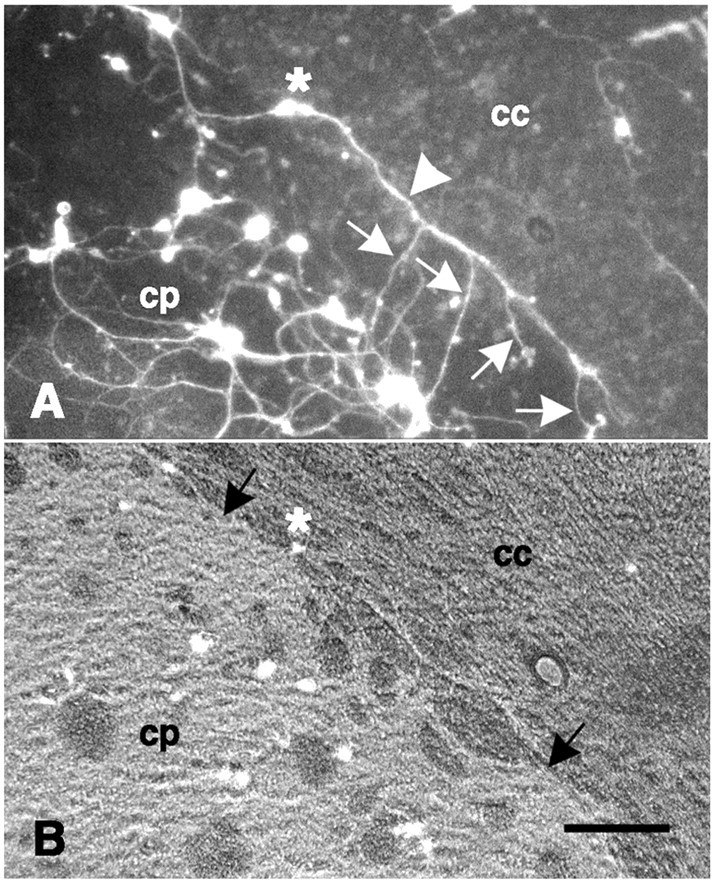
Dissociated neurons on a coronal forebrain section. A, Neurite outgrowth from fluorescence-labeled neurons attached to the caudate-putamen (cp) shows no preferred orientation. A single neuron (white asterisk) is attached near the border between the caudate-putamen and the corpus callosum (cc) with a neurite (white arrowhead) extending parallel to, but not crossing onto, the corpus callosum. Several neurites (white arrows) extend on the caudate-putamen but not on the corpus callosum.B, The same field with epifluorescence in combination with phase-contrast optics shows the border (black arrows) between the caudate-putamen and the corpus callosum and the location of the neuron featured in A (white asterisk). Scale bar, 100 μm.
The dense plexus of neurites found on neocortex or caudate-putamen was, in most cases, sharply limited at the border of the corpus callosum (Fig. 1A). Neurite outgrowth from dissociated neurons attached to the corpus callosum near the midline, in contrast, was long and mostly confined to a direction parallel to the longitudinal axis of the tract (Fig. 1C). Constrained to this parallel trajectory, these neurites rarely encountered gray matter. Thus, the same section of corpus callosum inhibited neurites originating on gray matter but supported long neurite outgrowth from neurons on white matter. When attachment of neurons occasionally occurred to more lateral portions of the corpus callosum, neurite outgrowth was generally more variable but still appeared to correspond to the orientation of the underlying anatomy of the tract (data not shown). It was also in this region that neurites were more likely to pass in either direction across white matter–gray matter borders.
Neurites growing near white matter borders generally either grew alongside and parallel to the tract or were directed away from it. For example, a neurite is shown extending along the border between the lateral corpus callosum and caudate-putamen, with additional processes extending on the caudate-putamen (Fig. 2A). It is not clear whether these processes are collateral branches of the neurite extending along the border or neurites originating from neurons located on the caudate-putamen. Irrespective of their origin, however, it is apparent that they are inhibited from extending onto the corpus callosum.
To determine whether astrocyte processes within the tissue sections formed a preferred substrate for the neurites, we double-labeled live cultures with the vital dye and with a fluorescent antibody raised against GFAP. GFAP immunoreactivity was found to be densely represented in all brain regions, and within white matter, GFAP-immunopositive processes were primarily oriented in parallel with the longitudinal axis of the tract (Fig. 3C,D). To determine the extent to which neurites were associated with astrocytic processes, we analyzed some of the cultures using confocal microscopy. Neurites were rarely colocalized with GFAP immunoreactivity (Fig. 4).
Fig. 4.
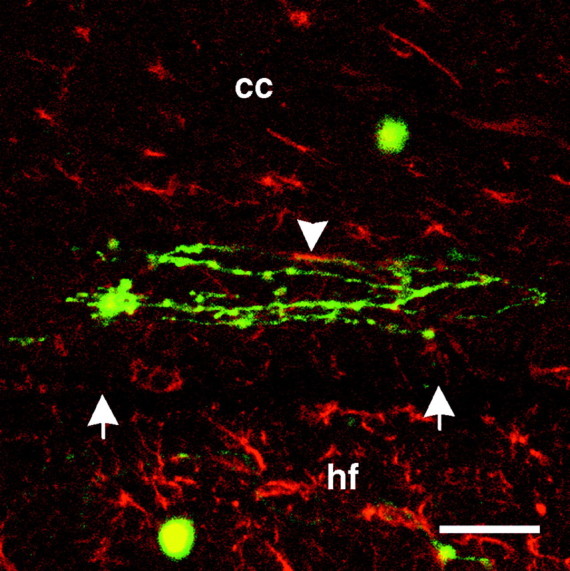
Composite confocal photomicrograph of dissociated neurons on GFAP-labeled sections. Dissociated neurons and neurites (green) on corpus callosum (cc) near the border (white arrows) with the underlying hippocampal formation (hf) are shown. GFAP immunoreactivity is shown in red. Colocalization of neurites and GFAP immunoreactivity is limited (white arrowhead). Scale bar, 50 μm.
Attachment and outgrowth of explants
Explants attached to gray matter gave rise to dense halos radiating neurites in all directions. However, these outgrowth halos were sharply interrupted by white matter regions such as the optic tract (Fig. 5A). In fact, explant outgrowth halos rarely crossed white matter borders from gray matter, as has been reported (Carbonetto et al., 1987; Sandrock and Matthew, 1987; Crutcher, 1989; Savio and Schwab, 1989). Neurites near the white matter border appeared to be reoriented in a direction parallel to, and alongside, the white matter tract (Fig.5A). However, this same section of optic tract did support neurite growth from a dissociated neuron attached directly to it, oriented in parallel with the longitudinal axis of the tract (Fig.5A).
As was the case with dissociated cultures of sympathetic neurons, Neurobasal medium significantly increased the attachment rate of sympathetic explants directly to white matter. Under conditions in which explants attached to white matter, long neurite outgrowth occurred on, and in parallel with, fiber tracts such as the optic tract (Fig. 5B), the corpus callosum (Fig. 5C), or the lateral funiculus of the spinal cord (Fig.6A). Neurites extended on the optic tract along its longitudinal axis as it turns along its dorsocaudal trajectory toward the lateral geniculate nucleus and were comparable in length with those on neocortex within the same section (Fig. 5B). Ventrally, where the optic tract is sectioned more transversely, a more mixed and unoriented halo is evident (Fig.5B). In contrast, neurites from a detached explant on the adjacent amygdala were inhibited from crossing onto the same section of optic tract that otherwise supported extensive outgrowth from explants attached directly to it (Fig. 5B).
Neurite outgrowth from an explant attached directly to the medial corpus callosum, sectioned in the same plane as the fibers in the tract, was primarily oriented in parallel with the longitudinal axis of the tract (Fig. 5C). Laterally, as the neurites approached the cingulum, sectioned here transversely, the pattern of neurite outgrowth was less isotropic.
In a few cases, neurite outgrowth from explants occurred in directions nonparallel to the longitudinal axis of the fiber tract. In these cases, however, the outgrowth often consisted of fasciculated neurites such that the majority of fibers appeared to be using the fascicle as the substrate. Subsequent defasciculation was coincidental with a reorientation of the neurites in a direction parallel to the longitudinal axis of the tract. For example, sparse radial neurite outgrowth of heavily fasciculated neurites could be seen from explants attached to the lateral funiculus of the spinal cord (Fig.6A). These fasciculated neurites gave rise to individual neurites oriented in parallel with the longitudinal axis of the tract. On the caudate-putamen, which consists of a mixture of gray and white matter, outgrowth was sparse and often fasciculated (Fig.6B). In some cases, fasciculated bundles of neurites could be seen directly crossing white matter, which in these sections was cut in cross section. In other cases, nonfasciculated neurites could be seen circuitously avoiding white matter. On the corpus callosum, explants occasionally gave rise to sparse, fasciculated bundles of neurites oriented obliquely to the tract (Fig.6C). After defasciculation, individual neurites were reoriented in parallel with the longitudinal axis of the tract.
Effects of nerve growth factor
Because NGF is well established as a tropic factor for sympathetic neurites, some cultures were established in the presence of exogenous NGF (2.5 ng/ml) to determine its effect on neurites growing on or approaching white matter. NGF had no observable effect on neuronal attachment rate, either on gray matter or white matter. Also, NGF did not appear to make white matter less inhibitory to neurites growing on gray matter and did not alter the direction of neurite growth on white matter.
Neurite growth rate: white matter versus gray matter
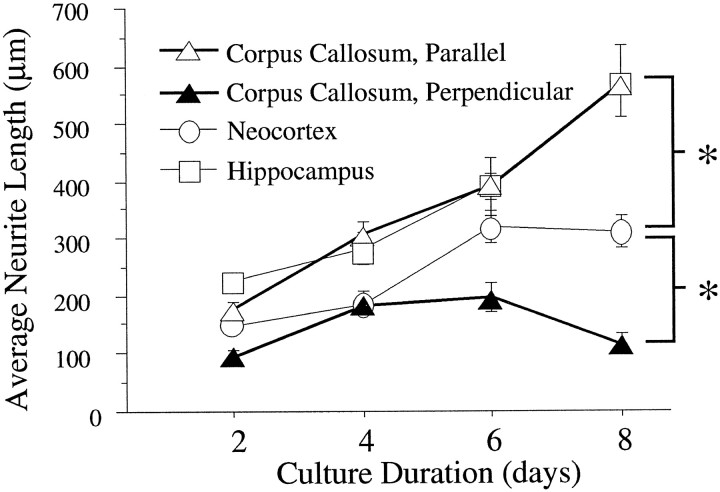
To determine whether the rate of neurite outgrowth was slower on white matter, we compared the extent of neurite outgrowth on the corpus callosum and representative gray matter regions (cultures were not treated with NGF). Not surprisingly, neurite outgrowth on neocortex was significantly greater than that on the corpus callosum measured perpendicular to its longitudinal axis (Fig.7). However, parallel to its longitudinal axis, the corpus callosum supported significantly greater neurite outgrowth than that on neocortex. Moreover, sympathetic neurite outgrowth parallel to the corpus callosum was comparable in length with that on the hippocampal formation, which has been documented previously as a highly permissive substrate for sympathetic neurite growth in tissue section culture (Crutcher, 1989, 1993; Pettigrew and Crutcher, 1997).
Fig. 7.
Rate of neurite outgrowth on corpus callosum, assessed both in parallel and perpendicular to the longitudinal axis, and on representative gray matter regions. After 8 d in culture, parallel neurite growth on the corpus callosum (white triangles) is comparable with that on the hippocampus (white squares) and significantly greater than neurite growth on neocortex (white circles). Neurite outgrowth on neocortex, in turn, is significantly greater than growth on the corpus callosum, assessed perpendicular to the longitudinal axis (black triangles). Values are expressed as mean ± SEM. Asterisks indicate statistical significance after 8 d in culture (p < 0.001).
DISCUSSION
Tissue section culture has been used previously to demonstrate that CNS white matter is nonpermissive for neurite outgrowth from various neuronal types (Sandrock and Matthew, 1987; Savio and Schwab, 1989; Watanabe and Murakami, 1989, 1990) including embryonic chick sympathetic neurons (Carbonetto et al., 1987; Crutcher, 1989; Crutcher and Privitera, 1989). Nevertheless, embryonic or adult neurons transplanted within gray matter with access to myelinated tracts, or directly within white matter in a manner that minimizes disruption of the fiber tract organization and glial scarring, can regenerate long axons within myelinated tracts (Wictorin et al., 1989, 1990a,b, 1991,1992; Tønder et al., 1990; Davies et al., 1993, 1994, 1997,1999; Humpel et al., 1994; Lehman et al., 1998). The direction of axonal growth, when assessed in these studies, was mostly oriented in parallel with the longitudinal axis of the tract.
Because of the evidence that myelinated tracts of the CNS can support axonal growth in vivo, the present study sought to determine whether white matter can support neurite outgrowth in vitrounder conditions that improve survival of cultured neurons. Neurobasal culture medium (Brewer et al., 1993) promotes greater survival of embryonic chick sympathetic neurons than do other media such as Ham’s F12, even when the latter is supplemented with 5% horse serum or nerve growth factor up to 500 ng/ml (Pettigrew and Crutcher, 1996), and was used to culture these neurons on cryostat sections of adult rat CNS.
White matter inhibits orthogonal neurite outgrowth
Neurobasal medium did not mask the inhibitory properties of white matter. Although neuronal attachment to white matter was increased compared with that of previous studies, the density of attachment to gray matter was greater than that to white matter, and as reported previously, neurite outgrowth on gray matter was extensive but inhibited by white matter (Carbonetto et al., 1987; Sandrock and Matthew, 1987; Crutcher, 1989; Savio and Schwab, 1989). For example, neurites growing on the caudate-putamen or neocortex did not cross the corpus callosum border. Similarly, neurites growing on the amygdala were sharply inhibited at the optic tract. In many cases, neurites that reached these borders were reoriented in a direction parallel to, and alongside, them. Neurites rarely crossed onto white matter from gray matter in nonparallel directions. This is consistent with the lack of nonparallel neurite growth from neurons on white matter.
White matter supports parallel neurite outgrowth
Considering the evidence that CNS myelin contains potent neurite growth inhibitors (Schwab and Thoenen, 1985; Caroni and Schwab, 1988a,b; Schwab and Caroni, 1988; Schnell and Schwab, 1990; Schwab et al., 1993b; McKerracher et al., 1994; Mukhopadhyay et al., 1994;Filbin, 1995) and that neurite outgrowth was clearly inhibited here by white matter, one might have expected that outgrowth from neurons attached directly to white matter would be prevented or, at least, reduced. It is, therefore, remarkable that neurite outgrowth not only occurred on the corpus callosum but, when assessed parallel to the fiber tract, was comparable in rate with that on gray matter. In fact, the corpus callosum was among the most permissive substrates in the forebrain for neurite growth, comparable with the hippocampus and significantly exceeding the neocortex.
That the rate of outgrowth on the corpus callosum was comparable with that of other permissive regions emphasizes the limiting role of initial attachment in detecting such growth potential. However, this growth, unless fasciculated, was primarily limited to directions parallel to the longitudinal axis of the tract, and when assessed perpendicular to the longitudinal axis, the corpus callosum was among the least permissive substrates for neurite growth. Long parallel outgrowth was also observed on the optic tract and spinal cord white matter. Interestingly, this neurite outgrowth is similar in morphology to that reported on cryostat sections of peripheral nerve (Carbonetto et al., 1987; Sandrock and Matthew, 1987; Savio and Schwab, 1989; Bedi et al., 1992; Shewan et al., 1993; Anand et al., 1996).
Successful neurite growth on white matter did not seem to be mediated by non-neuronal cells derived from the explants. The vital dye used to label neurons labels all living cells, and in fact, small cells were occasionally visible within the outgrowth halos of the explants. However, the majority of growing neurites on white matter were not associated with these cells. Moreover, if such cells could mediate attachment to an otherwise inhibitory substrate, one would have expected them to mediate at least some attachment to bare plastic in these culture dishes. In cultures using sections of forebrain, virtually no attachment to bare plastic occurred.
One concern with tissue section culture is that by sectioning the tissue to be used as a substrate, intracellular molecules are exposed to which neurons and their neurites would not ordinarily have access. Such intracellular molecules may provide a substrate conducive for attachment and neurite growth. However, the same caveat applies to previous studies using tissue section culture. In those studies, exposure of these molecules was not sufficient to mediate neuronal attachment to white matter.
Embryonic chick sympathetic neurons detect inhibitors in white matter
These results are not likely an artifact of culture in Neurobasal medium. Neuronal attachment to white matter has been observed using other media (Carpenter et al., 1994), and long, parallel neurite outgrowth also occurred (Crutcher, 1993), although too infrequently and unreliably for systematic study. Therefore, the limiting factor in successful neurite outgrowth on white matter seems to be neuronal attachment, and in the event of attachment, long, parallel outgrowth is possible.
Embryonic chick sympathetic neurons were selected because Neurobasal medium augments their viability in culture. It has been speculated that early embryonic neurons can escape the influence of myelin-associated inhibitors, perhaps because the appropriate receptors are not yet expressed (Wictorin et al., 1990a, 1991, 1992; Shewan et al., 1995;Varga et al., 1995). However, embryonic chick sympathetic neurons have been shown to be inhibited by both rat and human white matter (Carbonetto et al., 1987; Crutcher, 1989, 1993; Crutcher and Privitera, 1989), which also suggests that these results are not likely a result of species differences between the neurons and the substrata. In fact, one monoclonal antibody raised against the myelin-associated inhibitor Nogo can neutralize the inhibitory effects of CNS myelin from at least seven species, suggesting that the corresponding inhibitory antigen is well conserved and its inhibitory properties are not species specific (Schnell and Schwab, 1990; Kapfhammer et al., 1992; Schwab et al., 1993a; Lang et al., 1995; Rubin et al., 1995; Spillmann et al., 1997). In the present study, embryonic chick sympathetic neurites were clearly inhibited by white matter, indicating their appropriateness for assessing outgrowth on white matter.
Implications for understanding axonal regeneration in the CNS
White matter supported mostly parallel outgrowth and often simultaneously inhibited neurites extending from gray matter, suggesting a general model of neurite growth vis-à-vis white matter. Successful neurite outgrowth on white matter depends on the geometric relationship between the tract and the neurite; specifically, white matter supports parallel outgrowth but inhibits nonparallel outgrowth. Consistent with this hypothesis, transversely sectioned white matter did not support neurite growth, and neurites approaching white matter from gray matter often extended in parallel along the border.
Several possible mechanisms may limit neurite outgrowth on white matter to parallel directions. This may reflect haptotactic interactions between the neurites and the mechanical properties of the substrate (Harrison, 1910; Crutcher, 1993). Alternatively, a permissive substrate, arranged in parallel with the fiber tract, such as astrocytes, may offset the inhibitory influence of myelin (Wictorin et al., 1990b; Fawcett et al., 1992; Davies et al., 1993, 1994, 1997;Goldberg and Barres, 1998). However, although occasional colocalization of neurites with GFAP-immunoreactive processes was observed, neurites were not usually associated with such processes. Regardless of the substrate on which neurites grow, other factors may mediate the constraint of neurites to a parallel direction. For example, parallel neurite growth may reflect the longitudinal organization of neurite inhibitors associated with myelin, which is organized in parallel with the tract. Neurites may be able to avoid these inhibitors by growing within the interfascicular spaces between myelinated axons [also suggested by Goldberg and Barres (1998)]. However, these same myelinated axons would pose a barrier to orthogonal growth. Additional studies will be necessary to determine which factors mediate the geometric constraints on neurite outgrowth on white matter.
Irrespective of the mechanism, if successful neurite outgrowth on white matter depends on geometry, successful outgrowth should depend on the integrity of the white matter, and any injury disrupting its organization may alter the permissiveness of the tract for neurite growth. Herein lies a possible reconciliation of the contrasting lines of evidence concerning white matter support of axonal regeneration. Most of the studies providing evidence of myelin-associated inhibitory activity have altered the organization of the myelinated tract. For example, isolated CNS myelin or myelinating oligodendrocytes used as culture substrates inhibit neurite outgrowth (Caroni and Schwab, 1988a,b; Schwab and Caroni, 1988; McKerracher et al., 1994;Mukhopadhyay et al., 1994). However, this abolishes the normal organization of glia and their processes, including those that form myelin. In other cases, fiber tracts were transected in vivo, and the extent of regeneration was assessed in response to treatment with inhibitor-neutralizing antibodies (Schnell and Schwab, 1990; Cadelli and Schwab, 1991; Weibel et al., 1994). In still other cases, fragments of optic nerve were excised and studied as a substrate for growth in vitro (Schwab and Thoenen, 1985). It is likely that the organization of these tracts was significantly disrupted at the site of transection, and in the case of the optic nerve cultures, oligodendrocytes were reported to migrate out of the optic nerve fragment forming a field of unorganized inhibitory activity surrounding the explant.
Such alterations, in view of the present data, may have decreased the permissiveness of the white matter, either by disrupting the organization of permissive substrates or of inhibitory factors or both. Studies in which regeneration was successful within myelinated tracts depended on techniques that minimized disruption of the fiber tract organization and formation of a glial scar (see discussion above). Traumatic injuries to CNS fiber tracts inevitably disrupt their organization. The present data suggest that whether regeneration within myelinated tracts is ultimately successful may depend on the geometric organization of the tract. Strategies for promoting regeneration within the injured brain and spinal cord may benefit from consideration of the relevant geometry.
Footnotes
This work was supported by the Mayfield Education and Research Foundation. The technical and intellectual contributions of Dr. Molly Bailey, Tonya Hines, Krissy Klosowski, Dr. Marcos Marques, and John-Andrews McQuade are gratefully acknowledged. The assistance of Dr. Mark Sussman and Angela Walker with confocal microscopy is also gratefully acknowledged. Parts of this paper have been published previously in abstract form at the 1998 meeting of the Society for Neuroscience.
Correspondence should be addressed to Dr. Keith A. Crutcher, Department of Neurosurgery, University of Cincinnati School of Medicine, ML 0515, Cincinnati, Ohio 45267-0515.
REFERENCES
- 1.Anand U, McMahon SB, Cohen J. Preferential growth of neonatal rat dorsal root ganglion cells on homotypic peripheral nerve substrates in vitro. Eur J Neurosci. 1996;8:649–657. doi: 10.1111/j.1460-9568.1996.tb01250.x. [DOI] [PubMed] [Google Scholar]
- 2.Bedi KS, Winter J, Berry M, Cohen J. Adult rat dorsal root ganglion neurons extend neurites on predegenerated but not on normal peripheral nerves in vitro. Eur J Neurosci. 1992;4:193–200. doi: 10.1111/j.1460-9568.1992.tb00867.x. [DOI] [PubMed] [Google Scholar]
- 3.Bovolenta P, Wandosell F, Nieto-Sampedro M. CNS glial scar tissue: a source of molecules which inhibit central neurite outgrowth. Prog Brain Res. 1992;94:367–379. doi: 10.1016/s0079-6123(08)61765-3. [DOI] [PubMed] [Google Scholar]
- 4.Brewer GJ, Torricelli JR, Evege EK, Price PJ. Optimized survival of hippocampal neurons in B27-supplemented Neurobasal, a new serum-free medium combination. J Neurosci Res. 1993;35:567–576. doi: 10.1002/jnr.490350513. [DOI] [PubMed] [Google Scholar]
- 5.Cadelli D, Schwab ME. Regeneration of lesioned septohippocampal acetylcholinesterase-positive axons is improved by antibodies against the myelin-associated neurite growth inhibitors NI-35/250. Eur J Neurosci. 1991;3:825–832. doi: 10.1111/j.1460-9568.1991.tb00093.x. [DOI] [PubMed] [Google Scholar]
- 6.Carbonetto S, Evans D, Cochard P. Nerve fiber growth in culture on tissue substrata from central and peripheral nervous systems. J Neurosci. 1987;7:610–620. doi: 10.1523/JNEUROSCI.07-02-00610.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Caroni P, Schwab ME. Two membrane protein fractions from rat central myelin with inhibitory properties for neurite growth and fibroblast spreading. J Cell Biol. 1988a;106:1281–1288. doi: 10.1083/jcb.106.4.1281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Caroni P, Schwab ME. Antibody against myelin-associated inhibitor of neurite growth neutralizes nonpermissive substrate properties of CNS white matter. Neuron. 1988b;1:85–96. doi: 10.1016/0896-6273(88)90212-7. [DOI] [PubMed] [Google Scholar]
- 9.Carpenter MK, Hassinger TD, Whalen LR, Kater SB. CNS white matter can be altered to support neuronal outgrowth. J Neurosci Res. 1994;37:1–14. doi: 10.1002/jnr.490370103. [DOI] [PubMed] [Google Scholar]
- 10.Crutcher KA. Tissue sections from the mature rat brain and spinal cord as substrates for neurite outgrowth in vitro: extensive growth on gray matter but little growth on white matter. Exp Neurol. 1989;104:39–54. doi: 10.1016/0014-4886(89)90007-1. [DOI] [PubMed] [Google Scholar]
- 11.Crutcher KA. Tissue sections as culture substrates: overview and critique. Hippocampus. 1993;3:157–164. [PubMed] [Google Scholar]
- 12.Crutcher KA, Privitera M. Axonal regeneration on mature human brain tissue sections in culture. Ann Neurol. 1989;26:580–583. doi: 10.1002/ana.410260414. [DOI] [PubMed] [Google Scholar]
- 13.David S, Aguayo AJ. Axonal elongation into peripheral nervous system “bridges” after central nervous system injury in adult rats. Science. 1981;214:931–933. doi: 10.1126/science.6171034. [DOI] [PubMed] [Google Scholar]
- 14.Davies SJA, Field PM, Raisman G. Long fibre growth by axons of embryonic mouse hippocampal neurons microtransplanted into the adult rat fimbria. Eur J Neurosci. 1993;5:95–106. doi: 10.1111/j.1460-9568.1993.tb00474.x. [DOI] [PubMed] [Google Scholar]
- 15.Davies SJA, Field PM, Raisman G. Long interfascicular axon growth from embryonic neurons transplanted into adult myelinated tracts. J Neurosci. 1994;14:1596–1612. doi: 10.1523/JNEUROSCI.14-03-01596.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Davies SJA, Fitch MT, Memberg SP, Hall AK, Raisman G, Silver J. Regeneration of adult axons in white matter tracts of the central nervous system. Nature. 1997;390:680–683. doi: 10.1038/37776. [DOI] [PubMed] [Google Scholar]
- 17.Davies SJA, Goucher DR, Doller C, Silver J. Robust regeneration of adult sensory axons in degenerating white matter of the adult rat spinal cord. J Neurosci. 1999;19:5810–5822. doi: 10.1523/JNEUROSCI.19-14-05810.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fawcett JW, Fersht N, Housden L, Schachner M, Pesheva P. Axonal growth on astrocytes is not inhibited by oligodendrocytes. J Cell Sci. 1992;103:571–579. doi: 10.1242/jcs.103.2.571. [DOI] [PubMed] [Google Scholar]
- 19.Filbin MT. Myelin-associated glycoprotein: a role in myelination and in the inhibition of axonal regeneration? Curr Opin Neurobiol. 1995;5:588–595. doi: 10.1016/0959-4388(95)80063-8. [DOI] [PubMed] [Google Scholar]
- 20.Goldberg JL, Barres BA. Neural regeneration: extending axons from bench to brain. Curr Biol. 1998;8:R310–R312. doi: 10.1016/s0960-9822(98)70195-2. [DOI] [PubMed] [Google Scholar]
- 21.Harrison RG. The outgrowth of the nerve fiber as a mode of protoplasmic movement. J Exp Zool. 1910;9:787–846. doi: 10.1002/jez.1401420103. [DOI] [PubMed] [Google Scholar]
- 22.Humpel C, Bygdeman M, Olson L, Strömberg I. Human fetal neocortical tissue grafted to rat brain cavities survives, leads to reciprocal nerve fiber growth, and accumulates host IgG. J Comp Neurol. 1994;340:337–348. doi: 10.1002/cne.903400305. [DOI] [PubMed] [Google Scholar]
- 23.Kapfhammer JP, Schwab ME, Schneider GE. Antibody neutralization of neurite growth inhibitors from oligodendrocytes results in expanded pattern of postnatally sprouting retinocollicular axons. J Neurosci. 1992;12:2112–2119. doi: 10.1523/JNEUROSCI.12-06-02112.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lang DM, Rubin BP, Schwab ME, Stuermer CAO. CNS myelin and oligodendrocytes of the Xenopus spinal cord—but not optic nerve—are nonpermissive for axon growth. J Neurosci. 1995;15:99–109. doi: 10.1523/JNEUROSCI.15-01-00099.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lehman MN, Lesauter J, Silver R. Fiber outgrowth from anterior hypothalamic and cortical xenografts in the third ventricle. J Comp Neurol. 1998;391:133–145. doi: 10.1002/(sici)1096-9861(19980202)391:1<133::aid-cne11>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 26.McKeon RJ, Schreiber RC, Rudge JS, Silver J. Reduction of neurite outgrowth in a model of glial scarring following CNS injury is correlated with the expression of inhibitory molecules on reactive astrocytes. J Neurosci. 1991;11:3398–3411. doi: 10.1523/JNEUROSCI.11-11-03398.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.McKerracher L, David S, Jackson DL, Kottis V, Dunn RJ, Braun PE. Identification of myelin-associated glycoprotein as a major myelin-derived inhibitor of neurite growth. Neuron. 1994;13:805–811. doi: 10.1016/0896-6273(94)90247-x. [DOI] [PubMed] [Google Scholar]
- 28.Mukhopadhyay G, Doherty P, Walsh FS, Crocker PR, Filbin MT. A novel role for myelin-associated glycoprotein as an inhibitor of axonal regeneration. Neuron. 1994;13:757–767. doi: 10.1016/0896-6273(94)90042-6. [DOI] [PubMed] [Google Scholar]
- 29.Pettigrew DB, Crutcher KA. Neurobasal medium promotes greater survival of embryonic chick sympathetic neurons than Ham’s F12 medium. Soc Neurosci Abstr. 1996;22:299.15. [Google Scholar]
- 30.Pettigrew DB, Crutcher KA. No difference in chick sympathetic neurite outgrowth on young and aged rat brain tissue sections. Soc Neurosci Abstr. 1997;23:779.7. [Google Scholar]
- 31.Pindzola RR, Doller C, Silver J. Putative inhibitory extracellular matrix molecules at the dorsal root entry zone of the spinal cord during development and after root and sciatic nerve lesions. Dev Biol. 1993;156:34–48. doi: 10.1006/dbio.1993.1057. [DOI] [PubMed] [Google Scholar]
- 32.Ramon y Cajal S. Degeneration and regeneration of the nervous system. Oxford UP; London: 1928. [Google Scholar]
- 33.Rubin BP, Spillmann AA, Bandtlow CE, Hillenbrand R, Keller F, Schwab ME. Inhibition of PC12 cell attachment and neurite outgrowth by detergent solubilized CNS myelin proteins. Eur J Neurosci. 1995;7:2524–2529. doi: 10.1111/j.1460-9568.1995.tb01052.x. [DOI] [PubMed] [Google Scholar]
- 34.Sandrock AW, Matthew WD. Identification of a peripheral nerve neurite growth-promoting activity by development and use of an in vitro bioassay. Proc Natl Acad Sci USA. 1987;84:6934–6938. doi: 10.1073/pnas.84.19.6934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Savio T, Schwab ME. Rat CNS white matter, but not gray matter, is nonpermissive for neuronal cell adhesion and fiber outgrowth. J Neurosci. 1989;9:1126–1133. doi: 10.1523/JNEUROSCI.09-04-01126.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Schnell L, Schwab ME. Axonal regeneration in the rat spinal cord produced by an antibody against myelin-associated neurite growth inhibitors. Nature. 1990;343:269–272. doi: 10.1038/343269a0. [DOI] [PubMed] [Google Scholar]
- 37.Schwab ME, Caroni P. Oligodendrocytes and CNS myelin are nonpermissive substrates for neurite growth and fibroblast spreading in vitro. J Neurosci. 1988;8:2381–2393. doi: 10.1523/JNEUROSCI.08-07-02381.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Schwab ME, Thoenen H. Dissociated neurons regenerate into sciatic but not optic nerve explants in culture irrespective of neurotrophic factors. J Neurosci. 1985;5:2415–2423. doi: 10.1523/JNEUROSCI.05-09-02415.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Schwab ME, Bandtlow CE, Nicholls J. Developmental expression of myelin-associated neurite growth inhibitors correlates with the loss of regeneration after spinal cord lesions in the opossum. Soc Neurosci Abstr. 1993a;19:283.19. [Google Scholar]
- 40.Schwab ME, Kapfhammer JP, Bandtlow CE. Inhibitors of neurite growth. Annu Rev Neurosci. 1993b;16:565–595. doi: 10.1146/annurev.ne.16.030193.003025. [DOI] [PubMed] [Google Scholar]
- 41.Shewan D, Berry M, Bedi K, Cohen J. Embryonic optic nerve tissue fails to support neurite outgrowth by central and peripheral neurons in vitro. Eur J Neurosci. 1993;5:809–817. doi: 10.1111/j.1460-9568.1993.tb00932.x. [DOI] [PubMed] [Google Scholar]
- 42.Shewan D, Berry M, Cohen J. Extensive regeneration in vitro by early embryonic neurons on immature and adult CNS tissue. J Neurosci. 1995;15:2057–2062. doi: 10.1523/JNEUROSCI.15-03-02057.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Snow DM, Lemmon V, Carrino DA, Caplan AI, Silver J. Sulfated proteoglycans in astroglial barriers inhibit neurite outgrowth in vitro. Exp Neurol. 1990;109:111–130. doi: 10.1016/s0014-4886(05)80013-5. [DOI] [PubMed] [Google Scholar]
- 44.Spillmann AA, Amberger VR, Schwab ME. High molecular weight protein of human central nervous system myelin inhibits neurite outgrowth: an effect which can be neutralized by the monoclonal antibody IN-1. Eur J Neurosci. 1997;9:549–555. doi: 10.1111/j.1460-9568.1997.tb01631.x. [DOI] [PubMed] [Google Scholar]
- 45.Tønder N, Sørensen T, Zimmer J. Grafting of fetal CA3 neurons to excitotoxic, axon-sparing lesions of the hippocampal CA3 area in adult rats. Prog Brain Res. 1990;83:391–409. doi: 10.1016/s0079-6123(08)61264-9. [DOI] [PubMed] [Google Scholar]
- 46.Varga ZM, Bandtlow CE, Erulkar SD, Schwab ME, Nicholls JG. The critical period for repair of CNS of neonatal opossum (Monodelphis domestica) in culture: correlation with development of glial cells, myelin and growth inhibitory molecules. Eur J Neurosci. 1995;7:2119–2129. doi: 10.1111/j.1460-9568.1995.tb00634.x. [DOI] [PubMed] [Google Scholar]
- 47.Watanabe E, Murakami F. Preferential adhesion of chick central neurons to the gray matter of the central nervous system. Neurosci Lett. 1989;97:69–74. doi: 10.1016/0304-3940(89)90141-9. [DOI] [PubMed] [Google Scholar]
- 48.Watanabe E, Murakami F. Cell attachment to and neurite outgrowth on tissue sections of developing, mature and lesioned brain: the role of inhibitory factor(s) in the CNS white matter. Neurosci Res. 1990;8:83–99. doi: 10.1016/0168-0102(90)90061-i. [DOI] [PubMed] [Google Scholar]
- 49.Weibel D, Cadelli D, Schwab ME. Regeneration of lesioned rat optic nerve fibers is improved after neutralization of myelin-associated neurite growth inhibitors. Brain Res. 1994;642:259–266. doi: 10.1016/0006-8993(94)90930-x. [DOI] [PubMed] [Google Scholar]
- 50.Wictorin K, Simerly RB, Isacson O, Swanson LW, Björklund A. Connectivity of striatal grafts implanted into the ibotenic acid-lesioned striatum. III. Efferent projecting graft neurons and their relation to host afferents within the grafts. Neuroscience. 1989;30:313–330. doi: 10.1016/0306-4522(89)90256-x. [DOI] [PubMed] [Google Scholar]
- 51.Wictorin K, Brundin P, Gustavii B, Lindvall O, Björklund A. Reformation of long axon pathways in adult rat central nervous system by human forebrain neuroblasts. Nature. 1990a;347:556–558. doi: 10.1038/347556a0. [DOI] [PubMed] [Google Scholar]
- 52.Wictorin K, Clarke DJ, Bolam JP, Björklund A. Fetal striatal neurons grafted into the ibotenate lesioned adult striatum: efferent projections and synaptic contacts in the host globus pallidus. Neuroscience. 1990b;37:301–315. doi: 10.1016/0306-4522(90)90401-o. [DOI] [PubMed] [Google Scholar]
- 53.Wictorin K, Lagenaur CF, Lund RD, Björklund A. Efferent projections to the host brain from intrastriatal striatal mouse-to-rat grafts: time course and tissue-type specificity as revealed by a mouse specific neuronal marker. Eur J Neurosci. 1991;3:86–101. doi: 10.1111/j.1460-9568.1991.tb00814.x. [DOI] [PubMed] [Google Scholar]
- 54.Wictorin K, Brundin P, Sauer H, Lindvall O, Björklund A. Long distance directed axonal growth from human dopaminergic mesencephalic neuroblasts implanted along the nigrostriatal pathway in 6-hydroxydopamine lesioned adult rats. J Comp Neurol. 1992;323:475–494. doi: 10.1002/cne.903230403. [DOI] [PubMed] [Google Scholar]