Abstract
To examine the current situation of patient delay and to identify factors associated with patient delay among women with breast cancer in China.
A total of 283 women, aged 23 to 83 years old and with histologically confirmed breast cancer, were investigated in this cross-sectional study. The women were recruited from seven selected hospitals in Sichuan Province, China. Face-to-face interviews using a structured questionnaire were performed.
Among the 283 participants, the range of patient delay was 0.2 to 900 days with a median patient delay of 50 days. A total of 35.8% of patients waited ≥90 days to access medical treatment after symptom onset. Binary logistic regression analysis showed that the main predictors of patient delay were knowledge of breast cancer symptoms (OR = 0.716, 95%CI:0.637–0.804, P = .000), external health locus of control (OR = 1.173, 95%CI:1.087–1.266, P = .000), breast self-examination/physical examination (OR = 0.065, 95%CI: 0.007–0.590, P = .015), perceived health competence (OR = 0.873, 95%CI:0.808–0.944, P = .000), family support (OR = 0.911,95%CI:0.847–0.981, P = .013), pain stimulation (OR = 0.191, 95%CI:0.046–0.792, P = .023) and age (OR = 1.028, 95%CI:1.000–1.058, P = .049).
These factors explained 41.0% of the variance.
Information on the current situation and predictors of patient delay in Chinese women with breast cancer might provide meaning insights into the early diagnosis of breast cancer. The results of this study may help health professionals develop specific clinical practice strategies to reduce patient delay of initial treatment as a way to improve outcomes for women with breast cancer.
Keywords: breast cancer, cancer, oncology, patient delay
1. Introduction
According to the GLOBOCAN 2012 statistics, breast cancer has the highest incidence among all cancers and is the leading cause of cancer death for women worldwide.[1] China accounts for 12.2% of all newly diagnosed breast cancers worldwide, and breast cancer remains the most common cancer among Chinese women.[2] The most recent incidence and mortality rates showed an upward trend, as indicated by the National Central Cancer Registry of China (NCCR) database.[3] Early diagnosis of breast cancer is the most crucial factor for reducing mortality. However, additional delays between the discovery of symptoms and seeking medical care are likely to postpone an early diagnosis.
There is strong evidence that long delays are associated with lower survival.[4–6] Richards conducted a meta-analysis and found that the 5-year survival rate of patients with delays of 3 to 6 months was lower than that of patients with delays of <3 months.[5] In developing countries, patient delay is a major contributor to reduced survival.[7] Based on these results, several cancer prevention and treatment organizations regard reducing delayed patient presentation as equal in importance to breast self-examination (BSE) and screening for controlling breast cancer globally.[8]
Delayed presentation can be categorized into patient delay and provider delay.[4,9] Patient delay refers to a prolonged interval from the onset of symptoms to the initial presentation for a clinical appointment.[10,11] Typically, a patient delay ≥90 days after the onset of possible symptoms of the disease is considered to be the criterion.[12,13]
A large number of studies have focused on patient delay. However, little attention has been given to Chinese women with breast cancer. To our knowledge, the importance of early detection and timely seeking of medical treatment are not fully recognized in Chinese women, especially older women.[14,15]
The aims of this study were to identify the current situation of patient delay and the predictive factors that affect Chinese women with breast cancer. The present study, conducted in seven hospitals in China, cannot only improve our understanding of the current extent of and factors associated with patient delay, but can be of great help for developing specific clinical practice strategies to reduce patient delay as a way to improve outcomes for women with breast cancer in China.
2. Methods
2.1. Participants and procedures
A cross-sectional descriptive research design was used, and a total of 312 Chinese women with a histologically confirmed diagnosis of breast cancer were recruited by a convenience sampling strategy from January 2017 to June 2017. The participants were consecutively enrolled from seven hospitals (six tertiary grade A general hospitals and one tertiary grade A specialized cancer hospital) from five regions of central Chengdu in Sichuan Province, which is located in Southwest China: West China Hospital; Sichuan Academy of Medical Sciences & Sichuan Provincial People's Hospital; General Hospital of Chengdu Military Region; Chengdu First People's Hospital; Chengdu Second People's Hospital; The Third People's Hospital of Chengdu, and Sichuan Cancer Hospital & Institute. The inclusion criteria were as follows: patients who
-
1.
had pathologically diagnosed breast cancer;
-
2.
had normal linguistic and comprehension abilities;
-
3.
were older than 18 years of age; and
-
4.
agreed to participate in the study.
The study exclusion criteria were as follows:
-
1.
a history of mental illness,
-
2.
cognitive disorders,
-
3.
other comorbid cancers,
-
4.
relapsed breast cancer and
-
5.
a history of drug addiction.
2.2. Measures
Data were obtained via face-to-face interviews. All participants were asked to complete the structured questionnaires independently after giving their consent. A total of five instruments were used to collect the participants’ socio-demographic and clinical-related data and to measure the three dependent variables. The questionnaires include a socio-demographic characteristics form, a clinical-related characteristics form, the Breast Cancer Symptom Knowledge (BCSK) scale, the Revised Health Hardiness Inventory (RHHI-24) and the Perceived Social Support from Family scale (PSS-Fa).
2.2.1. Socio-demographic and clinical-related characteristics
A socio-demographic characteristics questionnaire based on a literature review was self-designed to collect the participants’ socio-demographic information, including age, ethnicity, religion, marital status, place of residence, education level, household income, and medical payment method. In addition, we designed a clinical-related characteristics questionnaire reflecting medical-treatment-seeking behavior, including pathological classification, clinical stage, symptom type, method of symptom discovery, when the symptoms were first discovered, and when the patient sought medical treatment for the first time.
2.2.2. Breast cancer symptom knowledge
The 15-item BCSK scale[16] was used to assess the patients’ knowledge regarding breast cancer symptoms and presentation, including the danger signs of breast cancer endorsed by the American Cancer Society. The range of total scores is from 0 to 15. The scale shows excellent reliability and validity among English-speaking and Spanish-speaking populations in America.[17] In the present study, the Cronbach's α of the scale was 0.87, and the content validity index was 0.84.
2.2.3. Health hardiness
Patients’ health hardiness was assessed by the RHHI-24.[18] The RHHI-24 was developed based on the Health-Related Hardiness scale (HRHS).[19] The RHHI-24 comprises 24 items and four dimensions: health values, internal health locus of control, external health locus of control, and perceived health competence. Responses to this self-report scale are given using a 5-point Likert scale from 1 = “completely disagree” to 5 = “completely agree.” The Cronbach's α of each subscale ranges from 0.66 to 0.79.[18] The Cronbach's α of the Chinese version ranges from 0.77 to 0.83.[20] In the present study, the Cronbach's α of the four dimensions ranged from 0.67 to 0.87.
2.2.4. Family support
Participants’ family support was assessed by the PSS-Fa, developed by Procidano and Heller. The reliability coefficient of the PSS-Fa was 0.90, showing good construct validity in three validation studies.[21] The Chinese version of the scale comprises 15 items with a “yes” or “no” response format that measure support from family members. The total scale scores range from 0 to 15, with higher scores indicating higher levels of family support. The reliability coefficient of the scale, as tested with the Kuder–Richardson 21 (KR-21) formula, was 0.95.[22] In the present study, the Cronbach's α of the scale was 0.83.
2.3. Ethics considerations
Ethics approval to conduct the study was obtained from the West China Hospital Sichuan University Biomedical Ethics Committee (approval number: 201712).
2.4. Statistical analysis
All data analyses were performed using SPSS, version 22.0 (Chicago, IL). Descriptive statistics were calculated for the socio-demographic and clinical-related characteristics data and the magnitude of patient delay. Categorical variables between the groups were compared using the non-parametric Mann–Whitney U or Kruskal–Wallis H tests. Correlations were calculated using the Spearman rank correlation analysis. Multivariate analyses were performed using binary logistic regression to identify the predictor variables for patient delay. For all analyses, a P value < .05 (two-tailed) was considered statistically significant.
3. Results
A total of 312 questionnaires were distributed, and 283 were returned. Twenty-nine questionnaires were excluded because the participants did not remember the exact time of the onset of their breast disease symptoms. The return rate of valid questionnaires was 90.71%.
3.1. The characteristics of the study population
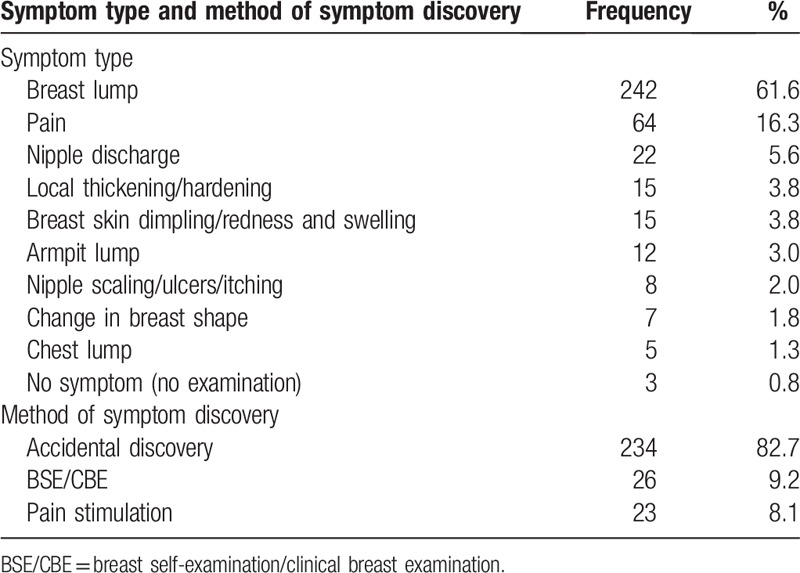
Table 1 shows the socio-demographic and clinical characteristics of the study population (n = 283). The mean age of all patients was 47 years (range: 23–83 years). All the participants were Han Chinese, and 266 (94%) patients had no religious beliefs. The majority of the patients, 223 (78.8%), came from an urban setting. A total of 258 (91.2%) patients were married. A total of 92 (32.5%) of the surveyed patients paid their own medical fees. Additionally, 94.3% of the patients had invasive breast cancer, and 25.8% of patients had stage II breast cancer. Table 2 shows the symptom type and method of symptom discovery of the participants. The first symptom noticed by 242 (61.6%) of the patients was a breast lump. Pain in the breast was reported by 64 participants (16.3%). The main method of symptom discovery was “accidental,” accounting for 82.7%.
Table 1.
The characteristics of the study population (n = 283).
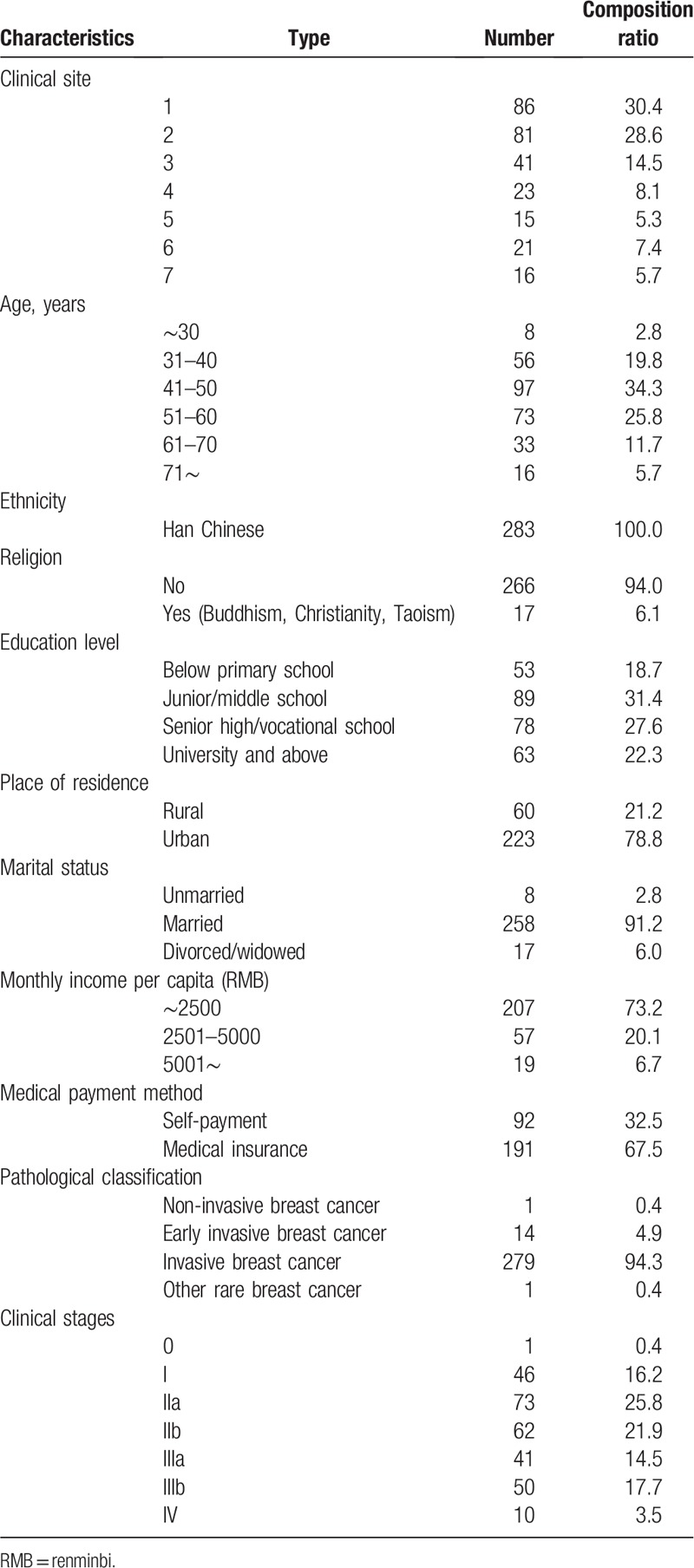
Table 2.
Symptom type and method of symptom discovery of the participants (n = 283).
3.2. Patient delay in seeking medical treatment
The frequency distribution of patient delay is shown in Table 3. The median patient delay in our setting was 50 days (range: 0.2–900 days). The interquartiles for patient delay were 170 days. For 45.9% (130/283) of the patients, a consultation was sought within 29 days after the patients noticed symptoms related to breast cancer. The remaining 35.8% (101/283) of patients delayed treatment by more than 90 days, while 11.8% (33/283) of patients sought medical treatment after more than 366 days.
Table 3.
Frequency distribution of patient delay (n = 283, unit: days).

3.3. Factors influencing patient delay
According to the normality test results, patient delay showed a non-normal distribution (Kolmogorov–Smirnov Z test, P < .05). Therefore, patient delay levels in different categories were compared using the non-parametric Mann–Whitney U or Kruskal–Wallis H test. Patient delay was significant in the following subgroups: age (Z = −2.200, P = .028), place of residence (Z = −2.485, P = .013), and method of symptom discovery (χ2 = 23.667, P = .013). The results of the Spearman rank correlation showed that education level (r = −0.214, P < .003), monthly income per capita (r = −0.148, P < .010), knowledge of breast cancer symptoms (r = −0.447, P < .000), family support (r = −0.140, P = .019), health values (r = −0.287, P < .000), internal health locus of control (r = −0.180, P < .002), external health locus of control (r = 0.408, P < .000), and perceived health competence (r = −0.333, P < .000) were correlated with patient delay.
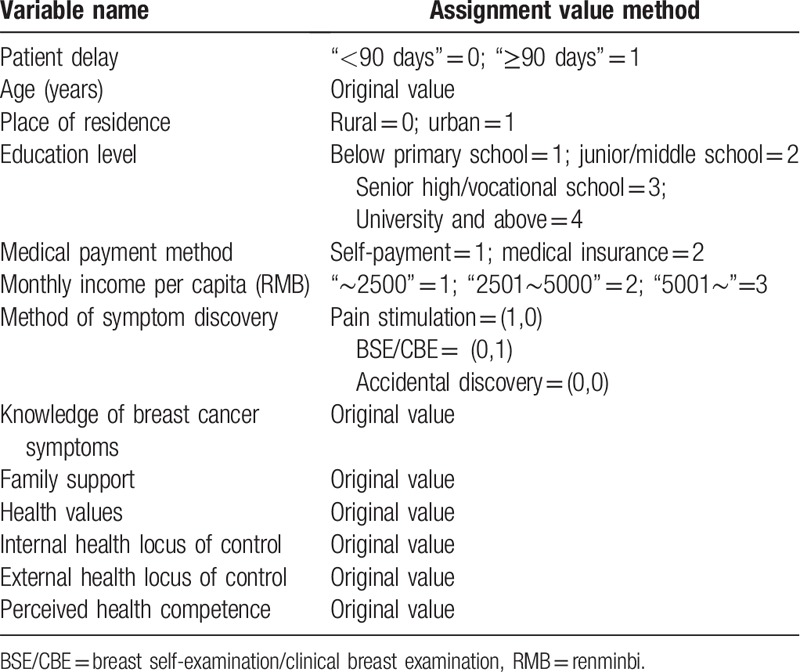
Multivariate analyses using patient delay as a dependent variable after transformation to a binary variable (“<90 days” = 0 and“≥90 days” = 1) were performed. Combining the significant variables from the univariate analysis and the results of the literature review, the following variables were used as independent variables: age, place of residence, education level, monthly income per capita, medical payment method, method of symptom discovery, knowledge of breast cancer symptoms, family support, health values, internal health locus of control, external health locus of control, and perceived health competence. The defined values of all the independent variables and the dependent variable are shown in Table 4. The partial maximum likelihood estimation forward method (forward: LR) was used to establish the binary logistic regression model and verify the affected factors of patient delay.
Table 4.
Assignment values of the variables included in the logistic regression analyses.
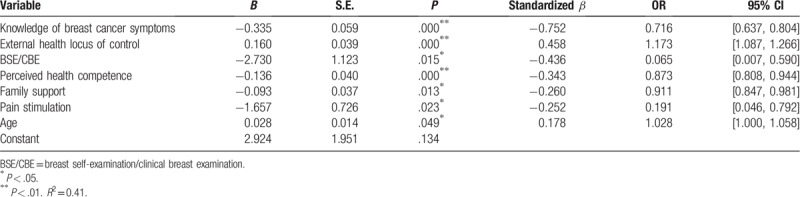
Binary logistic regression analysis revealed that the main predictors of patient delay were knowledge of breast cancer symptoms, external health locus of control, BSE/clinical breast examination (CBE), perceived health competence, family support, pain stimulation, and age (Table 5). These variables explained 41% of the variance in patient delay among breast cancer patients. Knowledge of breast cancer symptoms (standardized β = −0.752, OR = 0.716, 95%CI:0.637–0.804, P = .000), BSE/CBE (standardized β = −0.436, OR = 0.065, 95%CI:0.007–0.590, P = .015), perceived health competence (standardized β = −0.343, OR = 0.873, 95%CI:0.808–0.944, P = .000), family support (standardized β = −0.260, OR = 0.911, 95%CI:0.847–0.981, P = .013), and pain stimulation (standardized β = −0.252, OR = 0.191, 95%CI:0.046–0.792, P = .023) were protective factors of patient delay in breast cancer patients. However, age (standardized β = 0.178, OR = 1.028, 95%CI:1.000–1.058, P = .049) and external health locus of control (standardized β = 0.458, OR = 1.173, 95%CI:1.087–1.266, P = .000) were risk factors of patient delay in breast cancer patients (Table 5).
Table 5.
The result of the multiple logistic regression analysis for patient delay (n = 283).
4. Discussion
The results show that the median patient delay for the recruited sample was 50 days, and the rate of patient delay (length of delay ≥ 90 days) was 35.8%. Compared with the results in studies from other developing countries, the rate of delay in the present study is moderate. In Uganda, 89% of patients had a delay of ≥3 months after the first discovery of symptoms or anomalies, and the reported median delay was 13 months.[23] Among Nigerian breast cancer patients, 81.6% delayed seeking professional help for more than 3 months.[24] Compared with studies from developed countries, our study showed a slightly more severe patient delay. A survey conducted in the United States by George reported that 26.4% of African American patients and 17.5% of white patients with early-stage breast cancer delayed treatment by ≥3 months.[25] Forbes reported that 8.4% of breast cancer patients in England delayed seeking medication for more than 3 months.[26]
We found that knowledge of breast cancer symptoms is the most predictive of all the factors that affect patient delay (OR = 0.716, 95%CI:0.637–0.804). Similar results were reported in other studies from different nations, namely, that a lack of specific BCSK is an important factor influencing delayed presentation for treatment.[25–29] Better BCSK might help individuals identify cancer symptoms and reduce possible patient delay. Smith reported that incorrect identification of symptoms may be the main cause of patient delay.[30] In China, women need to improve their awareness and behavior of early detection of breast cancer.[31] Sichuan Province is located in Southwest China, and residents have a relatively low socioeconomic level, inadequate BCSK, and lack of awareness regarding the early discovery of breast cancer symptoms.
Consistent with other studies, our study identified that age is an important factor related to patient delay. In the regression analysis, older age is the only socio-demographic factor that is associated with patient delay (OR = 1.028, 95%CI:1.000–1.058). This result is consistent with those of studies from several other countries.[11,32] A systematic review showed strong evidence that older age is associated with longer delays in seeking medical treatment.[11] Burgess reported that older women are more likely to delay presentation with breast cancer.[33] In the present study, some participants believed that seeking medical treatment would only cause trouble for others. In China, many older women live with their children and often tend to the needs of other family members rather than their own.[34] Therefore, they do not seek medical treatment immediately after discovering symptoms and choose to wait and/or overlook their symptoms. The present study observed that some older patients who delayed presenting for medical treatment tended to neglect breast cancer symptoms, usually believing that they were natural changes after the onset of menopause. The elderly women attributed these symptoms to aging instead of viewing them as an early warning sign.
External health locus of control refers to attributing health to external events such as chance and fate. In our study, patients with an external health locus of control are more likely to delay seeking medical help (OR = 1.173, 95%CI:1.087–1.266), whereas perceived health competence reduces patient delay (OR = 0.873, 95%CI:0.808–0.944). These results are consistent with the study by Tromp et al regarding head and neck cancer patients.[35] In addition, our results showed that health value and internal health locus of control do not affect delays in seeking medical treatment, which is consistent with the findings of other studies.[35,36] The reason for these results might be that when facing a life-threatening disease, such as cancer, individual differences in health value are very small, as people generally attach great importance to health.[36,37]
The results of the multivariate analysis in this study revealed that patients with less family support reported a long patient delay (OR = 0.911, 95%CI:0.847–0.981). One study showed that most patients sought medical treatment after persuasion and encouragement from their families.[38] When individuals disclose their symptoms to someone close to them, they might receive encouragement as well as assistance to seek medical treatment and are thus less likely to delay. A previous study suggested that female patients who have a high level of support are less likely to have a long patient delay.[39] In the present study, family support is associated with the length of patient delay. High levels of family support can help patients to confide in others, encouraging them to disclose their symptoms and providing encouragement as well as assistance to seek medical treatment.
In this study, 6.0% (17/283) of the participants discovered their symptoms through BSE, and only 3.2% (9/283) of the participants discovered their symptoms through CBE. Compared with participants who discovered their symptoms by accident, those who discovered their symptoms through BSE/CBE were less likely to delay seeking medical treatment (OR = 0.065, 95%CI:0.007–0.590). BSE and/or CBE can both be effectively used to identify and improve early detection to increase the chances of disease treatment at an early stage.[40–42] Although the association of breast cancer screening with reduced breast cancer mortality has not been adequately demonstrated,[43–45] early detection of breast cancer has been included in international cancer control programs as the cornerstone of efforts to control breast cancer and support breast health services in low- and middle-income resource settings.[46,47]
According to the multivariate analysis, pain stimulation promotes early seeking of medical treatment (OR = 0.191, 95%CI:0.046–0.792), which is consistent with the results of Nosarti's study.[12] Lumley reviewed the psychological literature from the last decade and found that strong pain is related to emotional stress.[48] Forbes noted that pain could increase an individual's emotional stress and thus motivate him/her to seek early medical treatment or increase his/her sensitivity to symptoms.[49] Therefore, pain stimulation might prompt individuals to take a positive action to reduce delay.
Some limitations of this study should be noted. First, the selection criteria excluded patients with cognitive disorders, including mild cognitive impairment, which can introduce selection bias. Future studies of the Chinese breast cancer population with mild cognitive impairment should be considered. Second, the representativeness of the sample may be undermined by the convenience sampling method used in the present study. Third, cancer may persist over several years and can become a chronic disease, and the risk of possible recall bias in self-reported questionnaires cannot be excluded in studies of patient delay. Fourth, the distance between the place of residence and the consulting hospital may be associated with the patient delay. Unfortunately, we did not collect these data, which warrants further measurements in future investigations.
5. Conclusions
The results in this study show that patient delays are relatively common, with 35.8% of patients in Sichuan Province, China, having at least a 90-day delay in seeking medical treatment. The findings support that appropriate interventions are needed to reduce the length of patient delay among breast cancer patients, such as increasing knowledge about breast cancer symptoms to improve women's health competence in the identification of symptoms; guiding women in BSE and encouraging women, especially elderly ones, to participate in physical examinations; and providing health information to women. Additionally, family support is critical in encouraging women to seek medical help in a timely manner and in helping them to access care after they discover breast abnormalities.
The findings in the present study suggest that future efforts should focus on providing health education regarding the early detection of breast cancer to increase individuals’ health consciousness and knowledge of symptoms and facilitate earlier medical presentation. Such efforts should include the development of clinical interventions to improve women's health competence and to reduce the delay in medical treatment, particularly by providing guidance for high-risk groups in China.
Acknowledgments
The authors thank all the participants in our study and the medical staff of the breast cancer department who facilitated the study. Special thanks to the directors of Sichuan Province People's Hospital, West China Hospital and the other selected hospitals for their organizational support of the project.
Author contributions
Data curation: Guorong Wang, Ying Lu.
Investigation: Huaguo Zhang, Guorong Wang.
Methodology: Huaguo Zhang.
Project administration: Huaguo Zhang, Guorong Wang, Xiaolian Jiang.
Writing – original draft: Huaguo Zhang.
Writing – review & editing: Huaguo Zhang.
Footnotes
Abbreviations: 95%CI = 95% confidence interval, BCSK = breast cancer symptom knowledge, BSE = breast self-examination, CBE = clinical breast examination, HRHS = Health-Related Hardiness Scale, OR = odds ratio, PSS-Fa = Perceived Social Support from Family Scale, RHHI = Revised Health Hardiness Inventory.
How to cite this article: Zhang H, Wang G, Zhang J, Lu Y, Jiang X. Patient delay and associated factors among Chinese women with breast cancer. Medicine. 2019;98:40(e17454).
HZ and GW are the co-first authors.
The authors have no funding and conflicts of interest to disclose.
References
- [1].Torre LA, Bray F, Siegel RL, et al. Global cancer statistics, 2012. CA Cancer J Clin 2015;65:87–108. [DOI] [PubMed] [Google Scholar]
- [2].Fan L, Strasser-Weippl K, Li JJ, et al. Breast cancer in China. Lancet Oncol 2014;15:e279–89. [DOI] [PubMed] [Google Scholar]
- [3].Chen W, Zheng R, Baad PD, et al. Cancer statistics in China, 2015. CA Cancer J Clin 2016;66:115–32. [DOI] [PubMed] [Google Scholar]
- [4].Burgess CC, Ramirez AJ, Richards MA, et al. Who and what influences delayed presentation in breast cancer? Br J Cancer 1998;77:1343–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Richards MA, Westcombe AM, Love SB, et al. Influence of delay on survival in patients with breast cancer: a systematic review. Lancet 1999;353:1119–26. [DOI] [PubMed] [Google Scholar]
- [6].Harirchi I, Ghaemmaghami F, Karbakhsh M, et al. Patient delay in women presenting with advanced breast cancer: an Iranian study. Public Health 2005;119:885–91. [DOI] [PubMed] [Google Scholar]
- [7].Anderson BO, Jakesz R. Breast cancer issues in developing countries: an overview of the Breast Health Global Initiative. World J Surg 2008;32:2578–85. [DOI] [PubMed] [Google Scholar]
- [8].NHS Executive L N. Referral guidelines for suspected cancer. 2000. [Google Scholar]
- [9].Facione NC. Delay versus help seeking for breast cancer symptoms: a critical review of the literature on patient and provider delay. Soc Sci Med 1993;36:1521–34. [DOI] [PubMed] [Google Scholar]
- [10].Pack GT, Gallo JS. The culpability for delay in the treatment of cancer. Am J Cancer 1938;33:443–62. [Google Scholar]
- [11].Ramirez AJ, Westcombe AM, Burgess CC, et al. Factors predicting delayed presentation of symptomatic breast cancer: a systematic review. Lancet 1999;353:1127–31. [DOI] [PubMed] [Google Scholar]
- [12].Nosarti C, Crayford T, Roberts JV, et al. Delay in presentation of symptomatic referrals to a breast clinic: patient and system factors. Br J Cancer 2000;82:742–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Bish A, Ramirez A, Burgess C, et al. Understanding why women delay in seeking help for breast cancer symptoms. J Psychosom Res 2005;58:321–6. [DOI] [PubMed] [Google Scholar]
- [14].Leung D, Leung A, Chi I. Breast and colorectal cancer screening and associated correlates among Chinese older women. Asian Pac J Cancer Prev 2012;13:283. [DOI] [PubMed] [Google Scholar]
- [15].Liu LY, Wang F, Yu LX, et al. Breast cancer awareness among women in Eastern China: a cross-sectional study. BMC Public Health 2014;14:1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Facione NC, Dodd MJ, Holzemer W, et al. Helpseeking for self-discovered breast symptoms. Implications for cancer early detection. Cancer Pract 1997;5:220–7. [PubMed] [Google Scholar]
- [17].Facione NC, Miaskowski C, Dodd MJ, et al. The self-reported likelihood of patient delay in breast cancer: new thoughts for early detection. Prev Med 2002;34:397–407. [DOI] [PubMed] [Google Scholar]
- [18].Gebhardt WA, van der Doef MP, Paul LB. The Revised Health Hardiness Inventory (RRHI-24): psychometric properties and relationship with self-reported health and health behavior in two Dutch samples. Health Educ Res 2001;16:579–92. [DOI] [PubMed] [Google Scholar]
- [19].Pollock SE, Duffy ME. The Health-Related Hardiness Scale: development and psychometric analysis. Nurs Res 1990;39:218–22. [PubMed] [Google Scholar]
- [20].Wang JF. Verification of the Health-Related Hardiness Scale: cross-cultural analysis. Holist Nurs Pract 1999;13:44–52. [DOI] [PubMed] [Google Scholar]
- [21].Procidano ME, Heller K. Measures of perceived social support from friends and from family: three validation studies. Am J Community Psychol 1983;11:1–24. [DOI] [PubMed] [Google Scholar]
- [22].Zhang H, Tatsana YM. Relationship between family social support and selfcare behaviors in breast cancer patients. J Nurs Sci 1999;14:195–6. [Google Scholar]
- [23].Odongo J, Makumbi T, Kalungi S, et al. Patient delay factors in women presenting with breast cancer in a low income country. BMC Res Notes 2015;8:467–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Ibrahim NA, Oludara MA. Socio-demographic factors and reasons associated with delay in breast cancer presentation: a study in Nigerian women. Breast 2012;21:416–8. [DOI] [PubMed] [Google Scholar]
- [25].George, Chandwani S, Gabel M, et al. Diagnosis and surgical delays in African American and White women with early-stage breast cancer. J Womens Health (Larchmt) 2015;24:209–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Forbes LJ, Warburton F, Richards MA, et al. Risk factors for delay in symptomatic presentation: a survey of cancer patients. Br J Cancer 2014;111:581–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Unger-Saldana K, Infante-Castaneda CB. Breast cancer delay: a grounded model of help seeking behaviour. Soc Sci Med 2011;72:1096–104. [DOI] [PubMed] [Google Scholar]
- [28].Taib NA, Yip CH, Low WY. Recognising symptoms of breast Cancer as a reason for delayed presentation in Asian women-the psycho-socio-cultural model for breast symptom appraisal: opportunities for intervention. Asian Pac J Cancer Prev 2011;12:1601–8. [PubMed] [Google Scholar]
- [29].Rastad H, Khanjani N, Khandani BK. Causes of delay in seeking treatment in patients with breast cancer in Iran: a qualitative content analysis study. Asia Pac J Cancer Prev 2012;13:4511–5. [DOI] [PubMed] [Google Scholar]
- [30].Smith LK, Pope C, Botha JL. Patients’ help-seeking experiences and delay in cancer presentation. Lancet 2005;366:825–31. [DOI] [PubMed] [Google Scholar]
- [31].Weijia S, Dandan C, Yue Z, et al. The study of breast cancer screening awareness, behavior and its influencing factors. Maternal Child Health Care China 2018;33:242–5. [Google Scholar]
- [32].Arndt V, Sturmer T, Stegmaier C, et al. Patient delay and stage of diagnosis among breast cancer patients in Germany – a population based study. Br J Cancer 2002;86:1034–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Burgess CC, Potts HW, Hamed H, et al. Why do older women delay presentation with breast cancer symptoms. Psychooncology 2006;15:962–8. [DOI] [PubMed] [Google Scholar]
- [34].Chang ES, Simon MA, Dong XQ. Using community-based participatory research to address Chinese older women's health needs: toward sustainability. J Women Aging 2016;28:276–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Tromp DM, Brouha XD, Hordijk GJ, et al. Medical care-seeking and health-risk behavior in patients with head and neck cancer: the role of health value, control beliefs and psychological distress. Health Edu Res 2005;20:665–75. [DOI] [PubMed] [Google Scholar]
- [36].Keinan G, Carmil D, Rieck M. Predicting women's delay in seeking medical care after discovery of a lump in the breast: the role of personality and behavior patterns. Behav Med 1991;17:177–83. [DOI] [PubMed] [Google Scholar]
- [37].Smith MS, Wallston KA, Smith CA. The development and validation of the perceived health competence scale. Health Educ Res 1995;10:51–64. [DOI] [PubMed] [Google Scholar]
- [38].de Nooijer J, Lechner L, de Vries H. Social psychological correlates of paying attention to cancer symptoms and seeking medical help. Soc Sci Med 2003;56:915–20. [DOI] [PubMed] [Google Scholar]
- [39].Pedersen AF, Olesen F, Hansen RP, et al. Social support, gender and patient delay. Br J Cancer 2011;104:1249–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Thomas DB, Gao DL, Ray RM, et al. Randomized trial of breast self-examination in Shanghai: final results. J Natl Cancer Inst 2002;94:1445–57. [DOI] [PubMed] [Google Scholar]
- [41].Miller AB. Practical applications for clinical breast examination (CBE) and breast self-examination (BSE) in screening and early detection of breast cancer. Breast Care (Basel) 2008;3:17–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Miller AB, Baines CJ. The role of clinical breast examination and breast self-examination. Prev Med 2011;53:118–20. [DOI] [PubMed] [Google Scholar]
- [43].Miller AB, To T, Baines CJ, et al. Canadian National Breast Screening Study-2:13-year results of a randomized trial in women aged 50-59 years. J Natl Cancer Inst 2000;92:1490–9. [DOI] [PubMed] [Google Scholar]
- [44].Croswell JM, Ransohoff DF, Kramer BS. Principles of cancer screening: lessons from history and study design issues. Semin Oncol 2010;37:202–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Duffy SW, Tabar L, Vitak B, et al. Tumor size and breast cancer detection: what might be the effect of a less sensitive screening tool than mammography? Breast J 2006;12Suppl 1:S91–95. [DOI] [PubMed] [Google Scholar]
- [46].World Health Organization. Breast cancer: prevention and control; 2014. http://www.who.int/cancer/detection/breastcancer/en/ Accessed May 23, 2014. [Google Scholar]
- [47].Union for International Cancer Control, NIH. National Cancer Institute. Breast Cancer Initiative 2.5 (BCI2.5). Knowledge summaries-comprehensive breast cancer control; 2017. http://www.fredhutch.org/en/labs/phs/projects/breast-cancer-initiative_2-5/knowledge-summaries.html Accessed March 15, 2017. [Google Scholar]
- [48].Lumley MA, Cohen JL, Borszcz GS, et al. Pain and emotion: a biopsychosocial review of recent research. J Clin Psychol 2011;67:942–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Forbes LJ, Warburton F, Richards MA, et al. Pain and Emotion: new research directions. J Clin Psychol 2011;57:587–607. [DOI] [PubMed] [Google Scholar]


