Abstract
To investigate the functional connectome alterations in cerebral small-vessel disease (CSVD) patients with thalamus lacunes and its relation to cognitive impairment.
This case-control study was approved by the local research ethics committee, and all participants provided informed consent. There were 14 CSVD patients with thalamus lacunes (CSVDw.), 27 without (CSVDwo.), and 34 healthy controls (HC) recruited matched for age, sex, and education to undergo a 3T resting-state functional MR examination. The whole-brain functional connectome was constructed by thresholding the Pearson correlation matrices of 90 brain regions, and the topologic properties were analyzed by using graph theory approaches. Networks were compared between CSVD patients and HC, and associations between network measures and cognitive function were tested.
Compared with HC, the functional connectome in CSVDw. patients showed abnormalities at the global level and at the nodal level (P < .05, false discovery rate corrected). The network-based statistics method identified a significantly altered network consisting 6 nodes and 13 connections. Among all the 13 connections, only two connections had significant correlation with episodic memory (EM) and processing speed (PS) respectively (P < .05). The CSVDwo. patients showed no significant network alterations relative to controls (P > .05).
The configurations of brain functional connectome in CSVDw. patients were perturbed but not obvious for those without, and correlated with the mild cognitive impairment, especially for EM and PS. This study suggested that lacunes on thalamus played a vital role in mediating the neural functional changes of CSVD patients.
Keywords: cerebral small vessel disease, cognitive impairment, functional connectivity, resting-state fMRI, thalamus
1. Introduction
Cerebral small vessel disease (CSVD) is the most common vascular cause of dementia, which included several types of neuroimaging features on magnetic resonance imaging (MRI): recent small subcortical infarcts, white matter hyperintensities (WMH), lacunes, prominent perivascular spaces, cerebral microbleeds, and atrophy.[1] Among all those features, WMH and lacunes have consistently been shown to be associated with covert neurological and cognitive symptoms in previous studies.[2,3] Especially, previous studies have indicated that the spatial distribution of lacunes played a more important role in determining cognitive outcome than lesion load (eg, number and volume).[4,5] The underlying mechanism may be due to the disruption of several cortical-subcortical pathways which connecting the networks that underlie cognitive processes.[4]
The thalamus is a complex structure possessing unique connectional properties with prefrontal, temporal, occipital, and insular area.[6] Thalamic lacunes have been determined as “strategic” lacunes and associated with impaired cognitive performance, irrelevant of WMH.[4,7] Although small infarcts on thalamus have been reported to interrupt fiber projections and associate with cognitive decline,[8–10] lacunes on the thalamus has not been separated from those without in CSVD patients, especially those with very mild cognitive impairment.
The recent development of resting-state functional MRI (rs-fMRI) has provided valuable insights into the pathomechanism of cognitive impairment and allowed us to detect intrinsic brain activity noninvasively. Conjunction with the graph-based network theory, brain networks have been described as graphs in which anatomic brain regions are defined as nodes linked by edges and direct noninvasive characterization of brain network topologic organization has been described.[11] This method has shown good performance in determining the cognitive impairment in patients with type 2 diabetes,[12] Parkinson disease,[13] and Alzheimer's disease.[14] In CSVD patients, intrinsic brain connectivity at rest or functional connectome has been analyzed using rs-fMRI in previous studies.[5,15–17] However, those studies have not separated CSVD patients with thalamus lacunes from those without, which may result in a confusion of the functional disruption. The alteration of large-scale functional brain networks in CSVD patients with thalamus lacunes, especially those with mild cognitive impairment, have not been fully elucidated and associated with cognitive symptoms.
In this cross-sectional study, CSVD patients with mild cognitive impairment, were classified into those with thalamus lacunes and those without, which have been matched with the extent of WMH. We aimed to investigate the functional connectome alterations in CSVD patients with or without thalamus lacunes respect to healthy controls (HC). The hypothesis of this study was that the brain functional connectome in CSVD patients with thalamus lacunes was perturbed, but not so much for those without, and the alterations have been correlated with cognitive impairment.
2. Materials and methods
2.1. Participants
This cross-sectional study was approved by the Ethics Committee of Tongji hospital, and written informed consent was obtained from all participants before enrollment.
We enrolled patients diagnosed with CSVD from March 2014 to January 2016 from the outpatient. Patient inclusion criteria were as follows:
-
(1)
moderate to severe WMH (Fazekas rating score ≥3) with lacunes (3–15 mm in diameter);
-
(2)
not demented based on the criteria of the Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition; clinical dementia rating (CDR) global score ≤0.5 and total score of 3 CDR domains (home and hobbies, community affairs, and personal care) reflecting activities of daily living ≤1.5
-
(3)
mini-mental state examination (MMSE) ≥20 (primary school) or ≥24 (junior school or above).
The CSVD patients were further subdivided into 2 groups whether the thalamus had lacunes or not according to their MRI findings.
Exclusion criteria included:
-
(1)
any infarct with a diameter >20 mm on T1 weighted images (T1WI), cortical infarcts, cardioembolic stroke, cerebral hemorrhage;
-
(2)
a history of head trauma, Parkinson's disease, epilepsy, inherited small vessel disease, multiple sclerosis, hypoxic-ischemic encephalopathy, leukodystrophy, and/or psychiatric diseases;
-
(3)
use of medications that may affect cognitive function, alcohol or drug abuse;
-
(4)
subjects who presented severe aphasia, visual or hearing loss, claustrophobia or insufficient cooperation with study procedures for other reasons;
-
(5)
Hamilton depression scale score >17.
In addition, 35 HC subjects without lacunes or moderate to severe WMH were recruited. The control subjects also underwent a brief clinical interview and MMSE to confirm that they satisfied the criteria.
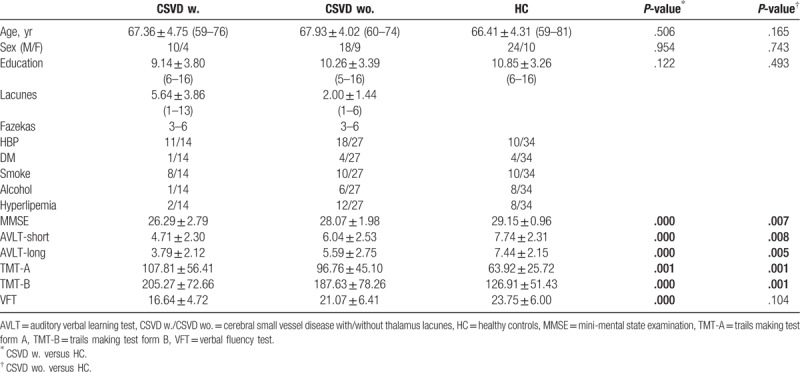
Subjects included in further analyses had to satisfy the criteria for head movement (less than 2 mm translation in any axis and less than 2° angular rotation about any axis) during rs-fMRI scanning. Two CSVD participants who had excessive head motion during scanning and 1 healthy subject who did not have a complete set of MRI data due to technical problems were excluded, resulting in a sample of 14 CSVD patients with thalamus lacunes, 27 CSVD patients without thalamus lacunes and 34 HC. The demographic and psychological characteristics of the samples are summarized in Table 1.
Table 1.
Demographic and clinical characteristics of participants.
2.2. Neuropsychological tests
All subjects were right-handed and able to perform the neuropsychological tests. These participants were administered a battery of neuropsychological assessments, including 4 cognitive domains: MMSE, episodic memory (EM) (the auditory verbal learning test short and long delay recall [AVLT]), processing speed (PS) (the trails making test form A [TMT-A]), executive function (the trails making test form B [TMT-B]; and the verbal fluency test [VFT]). The neuropsychological tests were conducted by a neuropsychologist with 10 years’ experience (W. Z).
2.3. Data acquisition and preprocessing
MRI data were acquired using a 3.0T MR scanner (Discovery MR750; GE Healthcare, Milwaukee, WI) with 32-channel head array coil. The neuroimaging protocol included T1WI, T2-weighted images (T2WI), T2-fluid-attenuated inversion recovery (T2FLAIR), high-resolution 3D T1-weighted images, and rs-fMRI sequences. T2-FLAIR images were acquired using repetition time (TR) = 8000 ms, echo time (TE) = 160 ms, inversion time (TI) = 2100 ms, flip angle (FA) = 111°, slice thickness = 5.0 mm, slice gap = 1.5 mm, data matrix = 512 × 512, and field of view (FOV) = 240 × 240 mm2. High-resolution 3D T1-weighted images were obtained using a sagittal brain volume sequence with TR = 8.16 ms, TE = 3.18 ms, TI = 450 ms, FA = 12°, number of slices = 188, slice thickness = 1.0 mm, data matrix = 256 × 256, and FOV = 256 × 256 mm2. Rs-fMRI was obtained using an axial gradient echo-planar imaging sequence with TR = 2000 ms, TE = 35 ms, FA = 90°, number of slices = 36, slice thickness = 3.0 mm, slice gap = 1.0 mm, data matrix = 64 × 64, FOV = 220 × 220 mm2, scan time = 7 minutes, and 210 volumes for each subject. Subjects were asked to relax with their eyes closed without falling asleep during the scan. Foam pads and earplugs were used to minimize head movement and scanner noise.
We used software statistical parametric mapping 8 (http://www.fil.ion.ucl.ac.uk/spm/) to perform image preprocessing of rs-fMRI data. The first 10 volumes for each subject were discarded. Head motion parameters were estimated, and each volume was realigned to the mean whole volume map to correct for geometrical displacements using a 6-parameter rigid-body transformation. Data were excluded from further analysis if their maximum displacement in any of the orthogonal directions (x, y, z) was greater than 2 mm or if there was a maximum rotation (x, y, z) greater than 2.0°. Individual functional images were spatially normalized to the Montreal Neurological Institute space. The functional images were then re-sampled into a voxel size of 3 × 3 × 3 mm3 and spatially smoothed with a 6-mm full width at half-maximum Gaussian kernel. Finally, the datasets were bandpass filtered with frequencies ranging from 0.01 to 0.08 Hz, and several nuisance covariates (6 motion parameters and average blood oxygen level-dependent signals of the ventricular and white matter) were regressed out from the data.
2.4. Assessment of WMH and lacunes
The extent of WMH was visually rated on T2-FLAIR images according to the Fazekas rating scale. The final score was the sum of the subcortical WMH score (0-absent, 1-punctuate foci, 2-beginning confluence of foci, 3-large confluent areas) and the periventricular WMH score (0-absent, 1-caps or pencil lining, 2-smooth halo, 3-irregular periventricular hyperintensities extending into deep white matter).[1,18] Moderate or severe WMH was defined as a rating score of three or higher.[18] Lacunes were counted using the combination of T1WI, T2WI, and T2-FLAIR, with the definition of a round or ovoid, subcortical, fluid-filled cavity (signal similar to cerebrospinal fluid) of between 3 mm and 15 mm in diameter.[1] The evaluation of WMH and lacunes were conducted by 2 neuroradiologists with 8- and 5-years’ experiences respectively (C. L and Z. W).
2.5. Network construction and analysis
Functional brain networks were constructed by calculating pairwise Pearson Correlation Coefficients among 90 regions of interest in terms of the anatomical automatic labeling atlas. A sparsity threshold S was then used to convert each of the resulting correlation matrices into a series of weighted networks (0.05 ≤ S ≤ 0.5, interval = 0.05). For the brain networks at each sparsity level, we calculated both global and nodal network metrics and then the area under the curve over the sparsity range. The global network metrics were of 2 kinds: small-world parameters (for definitions see Watts and Strogatz[19]) including the clustering coefficient, characteristic path length, normalized clustering coefficient (γ), normalized characteristic path length (λ), and small-worldness (σ); and network efficiency parameters (for definitions see Latora and Marchiori[20]) including the local efficiency and global efficiency. The nodal centrality metrics were the nodal degree,[11] nodal efficiency,[21] and nodal betweenness.[22]
To locate the specific pairs of brain regions with altered functional connectivity, we identified region pairs that exhibited between-group differences in nodal characteristics and then used the network-based statistics method (http://www.nitrc.org/projects/nbs/)[23] to define a set of suprathreshold significant changes between any connected regions (P < .05, threshold T = 2.02). Statistical significance was estimated by using the nonparametric permutation method (10,000 permutations).
2.6. Statistical analysis
The analyses of demographic, clinical and MRI data were performed with software (SPSS version 16.0). Normality was checked using the Kolmogorov–Smirnov test. The group differences were explored by using Student t test if normally distributed, and Mann–Whitney U test if not (eg, lacunes numbers and Fazekas scores). Categorical data (gender) were analyzed with a χ2 test. To address the problem of multiple comparisons of nodal metrics, we adopted a Benjamini–Hochberg false discovery rate correction method at a significance value of 0.05. Finally, in CSVD patients with thalamus lacunes, Pearson correlations were computed to examine relationships between these values and MMSE, AVLT (short and long delay recall), TMT-A, TMT-B, and VFT.
3. Results
3.1. Demographic and clinical comparisons
Demographic and clinical data from 41 CSVD patients (14 with and 27 without thalamus lacunes) and 34 HC participants are summarized in Table 1. Age, sex, and education did not differ significantly between the patients and the HC group (P > .05). The CSVD subjects, with thalamus lacunes or without, both exhibited reduced cognitive performance (MMSE, EM, PS, and executive function) relative to HC (P < .05). More interestingly, though there was no significant difference for the extent of WMH (P = .067) between 2 CSVD patient groups, the total number of lacunes in the group with thalamus lacunes were larger than the group without (P = .001).
3.2. Global topologic organization of the functional connectome
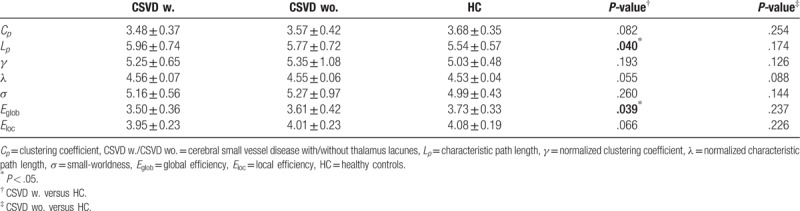
In the defined threshold range, both patient group and HC participants showed small world topologic organization in the brain functional connectome. Compared with HC, CSVD patients with thalamus lacunes showed a significant decrease in the global efficiency (P = .039), and an increase in the characteristic path length (P = .040), with no significant differences in local efficiency (P = .066), clustering coefficient (P = .082) (Fig. 1 and Table 2), normalized clustering coefficient γ (P = .193), normalized characteristic path length λ (P = .055), or small-worldness σ (P = .260). CSVD patients without thalamus lacunes exhibited no significant changes in all the indexes relative to HC (P > .05) but showed a similar tendency as patients with thalamus lacunes.
Figure 1.
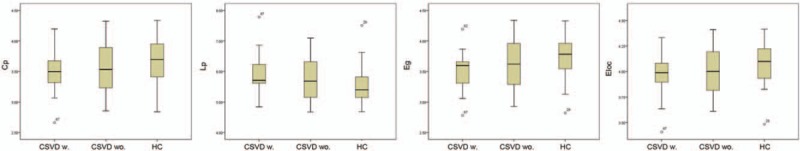
Box-and-whisker plots show differences in topologic properties of brain functional connectome among CSVD w., CSVD wo., and HC participants. Compared with HC participants, CSVD w. showed a significant decrease in the global efficiency (Eg, P = .039), and an increase in the characteristic path length (Lp, P = .040), with no significant differences in local efficiency (Eloc, P = .066), clustering coefficient (Cp, P = .082); CSVD wo. exhibited no significant changes in all the indexes relative to HC (P > .05). CSVD = cerebral small-vessel disease, CSVD w. = CSVD patients with thalamus lacunes, CSVD wo. = CSVD patients without thalamus lacunes, HC = healthy control.
Table 2.
Comparisons of global topologies.
3.3. Regional topologic organization of the functional connectome
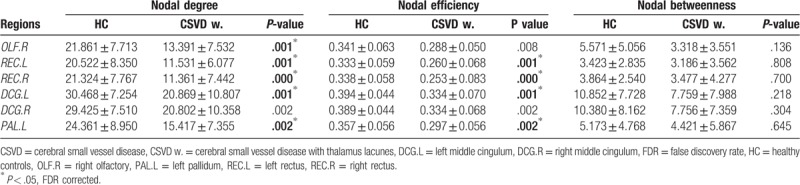
Among all the nodal centrality metrics, only the node degree and node efficiency showed significant between-group differences after multiple comparison correction (P < .05, false discovery rate (FDR) corrected). Specifically, compared with HC participants, CSVD patients with thalamus lacunes showed decreased node degree in the right olfactory, the left rectus, the right rectus, the left middle cingulum, and the left pallidum (Fig. 2 and Table 3), as well as decreased nodal efficiency in the left rectus, the right rectus, the left middle cingulum, and the left pallidum (P < .05, FDR corrected), with no significant differences in nodal betweenness (P > .05). There were no significant changes in any nodal metric between CSVD patients without thalamus lacunes and HC group.
Figure 2.
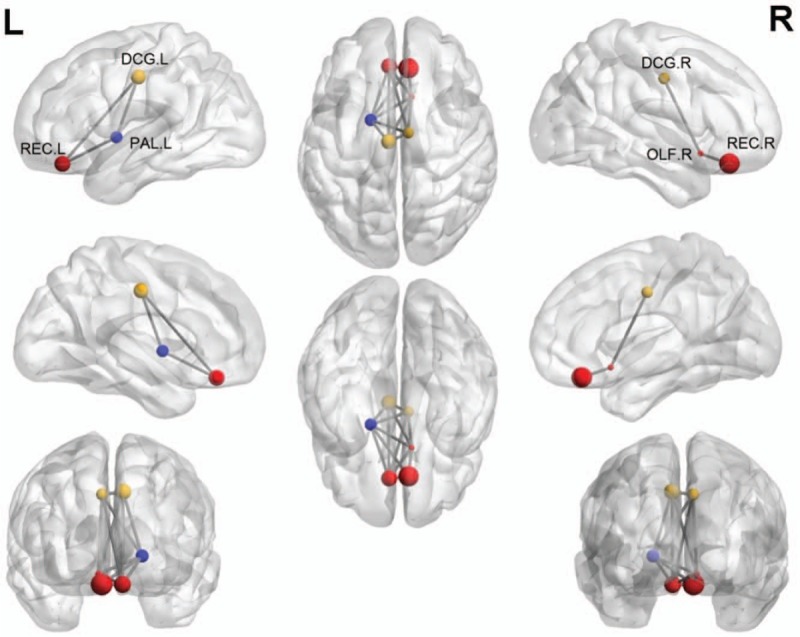
Schematic of significantly decreased nodal centralities of brain functional connectome in CSVD patients with thalamus lacunes relative to HC. Every node denotes a brain region, and every line denotes a connection. Different-color nodes represent different brain regions: red, prefrontal; yellow, frontal; blue, subcortical pallidum area. These connections formed a single connected network with 6 nodes and 13 connections (P < .05, network-based statistics). CSVD = cerebral small-vessel disease, DCG.L = left middle cingulum, DCG.R = right middle cingulum, OLF.R = right olfactory, PAL.L = left pallidum, REC.L = left rectus, REC.R = right rectus.
Table 3.
Regions showing decreased area under the curve values of nodal centralities in CSVD group with thalamus lacunes compared with HC participants.
3.4. CSVD-related alterations in functional connectivity
The network-based statistics method identified a significantly altered network in CSVD patients with thalamus lacunes (P < .05, threshold T = 2.02). This network had 6 nodes and 13 connections (Fig. 2), mainly involved in limbic, paralimbic (prefrontal and frontal, red and yellow), and subcortical (blue) regions. Within this network, all the connections were decreased in patients compared with HC participants. No significant differences were identified between CSVD patients without thalamus lacunes and HC group.
3.5. Correlation analysis
Within the group of CSVD patients with thalamus lacunes, no significant correlation was found for any nodal metrics (P > .05). Among all the 13 connections, only 2 connections had significant correlation with EM and PS, respectively (Fig. 3). Specifically, the connectivity strength of the right olfactory – right rectus was negatively correlated with AVLT-long delay recall (A: r = −0.562; P = .037, representing EM). The connectivity strength of the right olfactory – left pallidum was negatively correlated with AVLT-short delay recall (B: r = −0.674; P = .008, representing EM), and positively correlated with TMT-A (C: r = 0.586; P = .028, representing PS).
Figure 3.
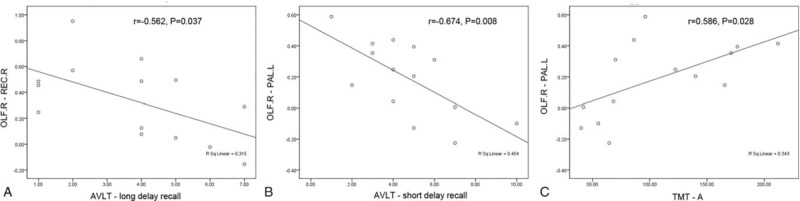
Plots show the relationship between the connectivity strength and clinical characteristics of CSVD patients with thalamus lacunes: (A, B) AVLT-long and short delay recall, representing EM; (C) TMT-A, representing PS. AVLT = auditory verbal learning test, CSVD = cerebral small-vessel disease, EM = episodic memory, OLF.R = right olfactory, PAL.L = left pallidum, PS = processing speed, REC.R = right rectus, TMT-A = trails making test form A.
4. Discussion
In this study, we used rs-fMRI and network analysis to explore the topological organization of functional brain networks in CSVD patients with mild cognitive impairment. Compared with HC, CSVD patients with thalamus lacunes had increased characteristic path length and decreased global efficiency, implying a disturbance of the normal global integration of whole-brain network. At the nodal level, decreased nodal degree and nodal efficiency in several brain regions have been detected, involving the limbic, paralimbic, and subcortical pallidum regions. Furthermore, networks were less densely connected in CSVD patients with thalamus lacunes, with the connectivity strength of the olfactory – rectus in right hemisphere and the right olfactory – left pallidum significantly related to cognitive impairment. However, CSVD patients without thalamus lacunes showed no significant alterations relative to HC at global, nodal or edge level. These findings provide insights into the neurobiology of CSVD patients from a network perspective and aid development of new biomarkers of disease detection at early stage.
In the framework of graph theory, a network is declared to be small-world when it attains a balance between local specialization (indexed by a high clustering coefficient) and global integration (indexed by low characteristic path length).[19] In our study, the brain networks of both patients with CSVD and HC participants exhibited small worldness. Regarding global topologic properties, global efficiency was decreased whereas characteristic path length was increased in CSVD patients with thalamus lacunes compared with HC participants, but not for those without thalamus lacunes, indicating that the brain networks may fail to maintain global integration as the lacunes involved thalamus particularly. The location of lacunes within subcortical grey matter, in particular the thalamus, has been reported to be a determinant of cognitive impairment.[7] However, our study also found that CSVD patients with thalamus lacunes had higher total number of lacunes. Since the effect of lacune location may also partly combined with the potential influence of the number of lacunes, a much larger sample with better-balanced groups would be required to demonstrate if thalamic lacunes play some specific role in cognitive impairment through specifics of connections in future work. The decreased global network efficiency may be the consequence of CSVD-related disruptions of interregional coordination, as demonstrated by our nodal efficiency and functional connectivity analyses and also as reported in recent studies.[15] In addition, the global efficiency was significantly decreased in CSVD patients with thalamus lacunes but no significant decrease in local efficiency, indicating that lacunes on thalamus may had more impact on long-range connection (mainly related to global efficiency) than short-range connection.
Other than these altered global topologic properties, CSVD patients with thalamus lacunes also showed impaired nodal centralities of several regions in the functional connectome, including limbic, paralimbic (prefrontal and frontal), and subcortical pallidum regions. Decreased nodal centralities in the limbic and paralimbic regions were in accordance with the mild cognitive impairment of CSVD patients. Abnormalities in white matter integrity and specific functional network alterations have been reported in patients with thalamus lacunes.[4] Resting-state thalamus functional connectivity with a set of brain regions have been disrupted in CSVD patients.[5] Particularly, left thalamic infarction can cause frontal dysfunction through the “diaschisis.”[24] Our observation of decreased nodal centralities in the prefrontal regions is congruent with neuropathologic observations that thalamus lacunes may disrupt the cortical-subcortical-thalamus circuit, which involved the cognitive process closely.[25]
We identified a CSVD-related subnetwork composed of 6 brain regions with 13 connections, mainly involving the prefrontal – subcortical pathways. These regions all showed decreased connectivity strength in patients with thalamus lacunes. Specifically, we observed a significant negative association between the connectivity strength of the right olfactory – right rectus and AVLT-long delay recall score, as well as between the connectivity strength of the right olfactory – left pallidum and AVLT-short delay recall score, implying lower connectivity strength of those regions in patients with worse EM. We also observed a significant positive association between the connectivity strength of right olfactory – left pallidum and TMT-A score implying higher connectivity strength in patients with a better performance on measures of PS. Our observations were consistent with previous task-based and rs-fMRI studies which reported abnormal frontal-subcortical circuit in patients with CSVD.[26] Therefore, our findings suggested that the mild cognitive impairment in CSVD could be explained by the imbalance between the limbic and frontal networks, and the frontal-subcortical circuit may be at the root of the pathogenesis of CSVD with thalamus lacunes.
The present study has several limitations. First, we only enrolled CSVD patients that not demented. The very mild cognitive impairment in CSVD patients without thalamus lacunes may resulted the negative results relative to HC. Second, the number of patients with thalamus lacunes was relatively small. Since the recruited CSVD patients were heterogeneous in terms of the lacune locations outside the thalamus as well as the total number of lacunes, we did not compare the two-patient group directly. Theoretically, further studies should subdivide CSVD patients into several subgroups according to different location of lacunes, which need much larger database. Third, severe WMH have been associated with cognitive dysfunction. Although we matched the extent of WMH in the 2 groups of CSVD patients, subtle influence from WMH could not be fully excluded. Fourth, for graph-based brain network studies, there is still not an optimum method for generating brain parcellations for node definition. Further studies are needed to determine which brain parcellation strategy or spatial scale is most appropriate for the characterization of network topology in CSVD patients. Fifth, physiologic noise, including respiratory and cardiac fluctuations, might have compromised our results.
In summary, the functional connectome of CSVD patients with mild cognitive impairment, especially those with thalamus lacunes, shows a shift toward “regularization,” supporting the idea that this is a general pattern in CSVD. It is of much interest that the functional connectivity strength of frontal-limbic pathways in CSVD patients with thalamus lacunes was associated with worse cognitive performance than those without. The results of our study indicate that thalamus lacune was a pessimistic marker of cognitive performance in CSVD patients, which might help to define early interventions to attenuate adverse brain development in the non-demented CSVD patients.
Author contributions
Data curation: Wenhao Zhu, Zhenxiong Wang.
Formal analysis: Wenhao Zhu.
Funding acquisition: Yuanyuan Qin.
Investigation: Wenhao Zhu.
Methodology: Chengxia Liu.
Project administration: Zhenxiong Wang, Wenzhen Zhu.
Resources: Zhenxiong Wang.
Software: Chengxia Liu, Zhenxiong Wang.
Supervision: Wenzhen Zhu.
Visualization: Chengxia Liu.
Writing – original draft: Yuanyuan Qin.
Writing – review & editing: Chengxia Liu, Wenzhen Zhu.
Yuanyuan Qin orcid: 0000-0002-0673-3200.
Footnotes
Abbreviations: AVLT = auditory verbal learning test, CDR = clinical dementia rating, CSVD = cerebral small-vessel disease, EM = episodic memory, FA = flip angle, FOV = field of view, HC = healthy controls, MMSE = mini-mental state examination, MRI = magnetic resonance imaging, PS = processing speed, rs-fMRI = resting state functional MRI, T1WI = T1 weighted images, T2FLAIR = T2-fluid-attenuated inversion recovery, TE = echo time, TI = inversion time, TMT-A = trails making test form A, TMT-B = trails making test form B, TR = repetition time, VFT = verbal fluency test, WMH = white matter hyperintensities.
How to cite this article: Qin Y, Zhu W, Liu C, Wang Z, Zhu W. Functional brain connectome and its relation to mild cognitive impairment in cerebral small vessel disease patients with thalamus lacunes. Medicine. 2019;98:40(e17127).
This work was supported by the National Natural Science Foundation of China (No. 81873890, 81401389).
The authors have no conflicts of interest to disclose.
References
- [1].Wardlaw JM, Smith EE, Biessels GJ, et al. Neuroimaging standards for research into small vessel disease and its contribution to ageing and neurodegeneration. Lancet Neurol 2013;12:822–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Roseborough A, Ramirez J, Black SE, et al. Associations between amyloid beta and white matter hyperintensities: a systematic review. Alzheimers Dement 2017;13:1154–67. [DOI] [PubMed] [Google Scholar]
- [3].Zeestraten EA, Lawrence AJ, Lambert C, et al. Change in multimodal MRI markers predicts dementia risk in cerebral small vessel disease. Neurology 2017;89:1869–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Benjamin P, Lawrence AJ, Lambert C, et al. Strategic lacunes and their relationship to cognitive impairment in cerebral small vessel disease. NeuroImage Clin 2014;4:828–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Zhou X, Hu X, Zhang C, et al. Aberrant functional connectivity and structural atrophy in subcortical vascular cognitive impairment: relationship with cognitive impairments. Front Aging Neurosci 2016;8:14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Schmahmann JD. Vascular syndromes of the thalamus. Stroke 2003;34:2264–78. [DOI] [PubMed] [Google Scholar]
- [7].Benisty S, Gouw AA, Porcher R, et al. Location of lacunar infarcts correlates with cognition in a sample of non-disabled subjects with age-related white-matter changes: the LADIS study. J Neurol Neurosurg Psychiatry 2009;80:478–83. [DOI] [PubMed] [Google Scholar]
- [8].Biesbroek JM, Weaver NA, Biessels GJ. Lesion location and cognitive impact of cerebral small vessel disease. Clin Sci (Lond) 2017;131:715–28. [DOI] [PubMed] [Google Scholar]
- [9].Swartz RH, Stuss DT, Gao F, et al. Independent cognitive effects of atrophy and diffuse subcortical and thalamico-cortical cerebrovascular disease in dementia. Stroke 2008;39:822–30. [DOI] [PubMed] [Google Scholar]
- [10].Swartz RH, Black SE. Anterior-medial thalamic lesions in dementia: frequent, and volume dependently associated with sudden cognitive decline. J Neurol Neurosurg Psychiatry 2006;77:1307–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Rubinov M, Sporns O. Complex network measures of brain connectivity: uses and interpretations. NeuroImage 2010;52:1059–69. [DOI] [PubMed] [Google Scholar]
- [12].Cui Y, Li SF, Gu H, et al. Disrupted brain connectivity patterns in patients with type 2 diabetes. AJNR Am J Neuroradiol 2016;37:2115–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Galantucci S, Agosta F, Stefanova E, et al. Structural brain connectome and cognitive impairment in Parkinson disease. Radiology 2017;283:515–25. [DOI] [PubMed] [Google Scholar]
- [14].He Y, Chen Z, Gong G, et al. Neuronal networks in Alzheimer's disease. Neuroscientist 2009;15:333–50. [DOI] [PubMed] [Google Scholar]
- [15].Schaefer A, Quinque EM, Kipping JA, et al. Early small vessel disease affects frontoparietal and cerebellar hubs in close correlation with clinical symptoms – a resting-state fMRI study. J Cereb Blood Flow Metab 2014;34:1091–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Sun YW, Qin LD, Zhou Y, et al. Abnormal functional connectivity in patients with vascular cognitive impairment, no dementia: a resting-state functional magnetic resonance imaging study. Behav Brain Res 2011;223:388–94. [DOI] [PubMed] [Google Scholar]
- [17].Liu C, Li C, Yin X, et al. Abnormal intrinsic brain activity patterns in patients with subcortical ischemic vascular dementia. PloS One 2014;9:e87880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Prins ND, Scheltens P. White matter hyperintensities, cognitive impairment and dementia: an update. Nat Rev Neurol 2015;11:157–65. [DOI] [PubMed] [Google Scholar]
- [19].Watts DJ, Strogatz SH. Collective dynamics of 'small-world’ networks. Nature 1998;393:440–2. [DOI] [PubMed] [Google Scholar]
- [20].Latora V, Marchiori M. Efficient behavior of small-world networks. Phys Rev Lett 2001;87:198701. [DOI] [PubMed] [Google Scholar]
- [21].Achard S, Bullmore E. Efficiency and cost of economical brain functional networks. PLoS Comput Biol 2007;3:e17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Zhang J, Wang J, Wu Q, et al. Disrupted brain connectivity networks in drug-naive, first-episode major depressive disorder. Biol Psychiatry 2011;70:334–42. [DOI] [PubMed] [Google Scholar]
- [23].Zalesky A, Fornito A, Bullmore ET. Network-based statistic: identifying differences in brain networks. NeuroImage 2010;53:1197–207. [DOI] [PubMed] [Google Scholar]
- [24].Meguro K, Akanuma K, Ouchi Y, et al. Vascular dementia with left thalamic infarction: neuropsychological and behavioral implications suggested by involvement of the thalamic nucleus and the remote effect on cerebral cortex. The Osaki-Tajiri project. Psychiatry Res 2013;213:56–62. [DOI] [PubMed] [Google Scholar]
- [25].Metzger CD, van der Werf YD, Walter M. Functional mapping of thalamic nuclei and their integration into cortico-striatal-thalamo-cortical loops via ultra-high resolution imaging-from animal anatomy to in vivo imaging in humans. Front Neurosci 2013;7:24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Righart R, Duering M, Gonik M, et al. Impact of regional cortical and subcortical changes on processing speed in cerebral small vessel disease. NeuroImage Clin 2013;2:854–61. [DOI] [PMC free article] [PubMed] [Google Scholar]


