Abstract
Background:
This study aimed to perform a network meta-analysis to evaluate the therapeutic effect and safety of various modalities in treating advanced hepatocellular carcinoma (HCC). Typically, the modalities of interest were comprised of sorafenib, transarterial chemoembolization (TACE), sorafenib combined with TACE, TACE combined with traditional Chinese medicine (TCM), and sorafenib combined with hepatic arterial infusion chemotherapy (HAIC).
Methods:
Potentially eligible studies were systemically retrieved from the electronic databases (including PubMed and Cochrane Library) up to September 2018. The overall survival (OS) associated with the 5 modalities of interest enrolled in this study was compared by means of network meta-analysis. Meanwhile, major adverse events (AEs) were also evaluated.
Results:
The current network meta-analysis enrolled 7 published randomized controlled trials (RCTs), and the pooled results indicated that the TACE-TCM regimen displayed the highest efficacy in treating advanced HCC, followed by HAIC-sorafenib. By contrast, the TACE alone and sorafenib alone regimens had the least efficacy. Relative to other regimens of interest, the TACE-TCM regimen was associated with less incidence of treatment-associated AEs.
Conclusion:
The TACE-TCM regimen was associated with higher treatment responses in advanced HCC patients than those of the other regimens of interest.
Keywords: advanced HCC, network meta-analysis, RCT, survival analysis
1. Introduction
Hepatocellular carcinoma (HCC) ranks the 6th place in terms of its morbidity, which is also the 3rd-leading cause of cancer-related death in the world.[1] The at-risk patients are monitored, but many patients are diagnosed at an advanced stage of the disease.[2,3]
Sorafenib, an oral multikinase inhibitor, has become the preferred treatment for advanced HCC, since it had been shown to markedly improve the overall survival (OS) compared with that of placebo in the SHARP trial.[4–6] However, dose reduction or discontinuation of sorafenib is frequently necessary due to the occurrence of adverse events (AEs).[5–7] Transarterial therapies are the common treatments for intermediate-stage HCC. Typically, recent studies indicate that transarterial chemoembolization (TACE), transarterial radioembolization (TARE), and repetitive hepatic arterial infusion chemotherapy (HAIC) have achieved favorable outcomes in advanced HCC. However, many patients fail to respond to the transarterial treatment, even though the optimal technique is adopted.[8] Notably, traditional Chinese medicine (TCM) has been developed and tested in different trials, which has displayed promising benefits to HCC.[9,10] Besides, As2O3 has been approved by the State Food and Drug Administration of China to be used to treat HCC since 2004.[11] Additionally, ginsenoside Rg3 has multiple antitumor activities, which can downregulate vascular endothelial growth factor (VEGF) expression, promote apoptosis, and inhibit the proliferation and invasion abilities through several signaling pathways.[12–16] Moreover, the rational combinations of the above therapies have also been developed. Chow et al[17] and Vilgrain et al[2] found OS did not differ significantly between TARE and sorafenib; Hu et al[11] concluded TACE-TCM (As2O3) prolonged OS compared with TACE alone, and same result came to TACE-TCM (ginsenoside Rg3) by Zhou et al.[18] In Meyer et al[3] and Kudo et al's[19] studies, no improvement in OS was observed when adding sorafenib to TACE; HAIC-sorafenib yielded favorable OS when compared sorafenib alone in Ikeda et al's study.[20]
Therefore, it is helpful to evaluate the therapeutic effect and safety of various treatment modalities. On this account, a multiarm trial is required to compare these various agents; nonetheless, evidence at such level is lacking at present. Fortunately, network meta-analysis can help to simultaneously compare 2 or more treatments.
Cucchetti et al performed a network meta-analysis of phase III randomized controlled trials (RCTs) to compare the efficacy and safety of novel drugs instead of treatment strategies for advanced HCC.[21] Tian et al conducted network meta-analysis to compare and rank different treatment strategies for HCC. However, they included cohort and RCT studies regardless of tumor stage.[22] In this study, a systemic review and network meta-analysis was carried out based on RCTs, so as to compare the therapeutic effects of 5 different therapeutic modalities (sorafenib, TACE, TACE-sorafenib, TACE-TCM, and HAIC-sorafenib) in advanced HCC and to rank these interventions for clinical consideration.
2. Methods
2.1. Literature retrieval strategy
Potentially eligible studies published before September 2018 were systemically retrieved in electronic databases, including PubMed and Cochrane Library. Typically, the PubMed database was retrieved using the keywords below: (((Barcelona Clinic Liver Cancer C) OR Barcelona staging C) OR BCLC C) OR ((((hepatoma) OR liver cell carcinomas) OR hepatocellular carcinoma) OR liver cancer) AND ((advanced) AND Randomized Controlled Trial[ptyp]). Meanwhile, the Cochrane database was retrieved using the strategies below: #1(Barcelona Clinic Liver Cancer C):ti,ab,kw OR (BCLC C):ti,ab,kw OR (Barcelona staging C):ti,ab,kw; #2 (hepatoma):ti,ab,kw OR (liver cell carcinomas):ti,ab,kw OR (hepatocellular carcinoma):ti,ab,kw OR (liver cancer):ti,ab,kw; #3 (advanced):ti,ab,kw; #4 #2 and #3; #5 #1 or #4; #6 (randomized controlled trial):pt; #7 #5 and #6. Additionally, titles of all of the references associated with the enrolled studies were manually examined, so as to obtain other potentially eligible studies.
2.2. Study inclusion and exclusion criteria
The study inclusion criteria were as follows: studies with the design of RCTs; studies that included patients with proven advanced HCC; studies with complete data on methodology, patient characteristics, AEs, and OS; studies comparing 2 arms and above, which consisted of the modalities of interest, including sorafenib, TACE (TARE), TACE-sorafenib, TACE-TCM, and HAIC-sorafenib. TARE seemed to result in similar complications and survival rates compared with TACE, and it had been regarded as a safe replacement therapy to the latter[23]; therefore, TACE and TARE were considered as one therapy in this study. Moreover, the study exclusion criteria were as follows: letters to the editor, study protocols, conference abstracts, case reports, non-RCTs, animal studies, editorials, and posters. Besides, only English literature was enrolled in this study.
2.3. Literature screening
All titles and abstracts were evaluated by 2 reviewers independently. Besides, the full texts of relevant studies and citations that could not be judged based on the abstract were obtained. To avoid the same study appeared in different publications, the institutions of authorship, treatments, study time, and study populations were identified to prevent duplicate study, and only the latest article was retained to rule of the possibility of duplication. Any disagreements among them were settled by consensus and the opinion of 3rd reviewers.
2.4. Data extraction and quality assessment
Data, including the name of first author, publication year, population characteristics, number of patients in every treatment arm, and outcomes (including OS and AEs), were extracted and summarized in an Excel file.
Afterwards, the Revman tool (version 5.3) was utilized to evaluate the quality of the enrolled studies. Specifically, each study was evaluated with the judgment system below: low risk of bias, high risk of bias, or unclear (including data insufficiency or uncertainty of bias).
2.5. Statistical analysis
In this study, a network meta-analysis was carried out, so as to directly (head-to-head) and indirectly compare the treatment outcomes among various enrolled studies on advanced HCC. Moreover, the existing treatment modalities were compared using frequentist network meta-analysis in 1 single analytical framework.[24] The Stata 14.0 (StataCorp., College Station, TX) was employed to carry out the network meta-analysis; at the same time, direct and indirect therapeutic schemes were simultaneously synthesized by the “network” command and routines, and all potential comparisons between different schemes were summarized as a league table. The surface under the cumulative ranking curve (SUCRA),[25] which uses the rank probabilities from rankograms, represents a relative ranking measure, and a larger SUCRA value had indicated better rank for a certain therapy among the various therapeutic schemes available. In this study, the inconsistency was assessed in the network meta-analysis, but the results suggested that direct comparisons were lacking, and no inconsistency could be formally detected in this model,[24,26,27] as shown in Figure 1. In addition, the common heterogeneity variance for the network (tau [τ]) was employed to assess the degree of heterogeneity in all network analyses, and the values of τ < 0.5 were considered as reasonable. Finally, the network meta-analysis was presented according to the revised PRISMA guidelines of network meta-analyses.[26] That confidence interval (CI) included 1 was considered as not significant in survival analysis.
Figure 1.
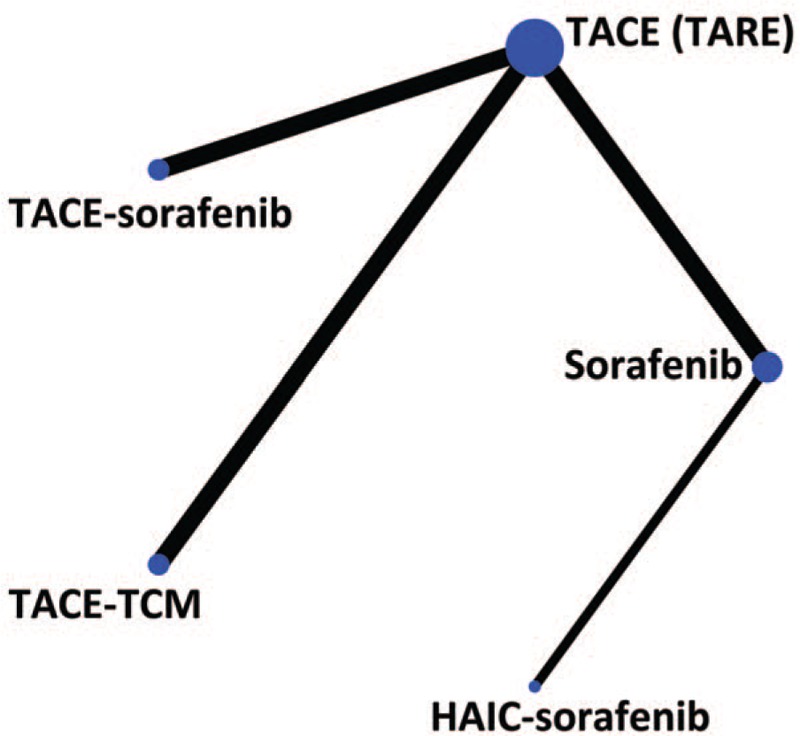
Network of included studies with the available direct comparisons. The size of the nodes and the thickness of the edges are weighted according to the number of studies evaluating each treatment and direct comparison, respectively. HAIC = hepatic arterial infusion chemotherapy, TACE = transarterial chemoembolization, TARE = transarterial radioembolization, TCM = traditional Chinese medicine.
2.6. Ethical review
Ethical approval was not necessary, because this article is a meta-analysis and it does not involve the participation of ethics committee.
3. Results
3.1. Characteristics and quality assessment of the enrolled studies
Altogether 1255 related articles were identified based on the comprehensive retrieval. After removing the duplicates, the titles and abstracts of all studies were reviewed, and the full texts of 149 studies were further assessed. Subsequently, 126 noneligible studies were excluded, and a total of 7 studies meeting the inclusion criteria were finally enrolled in this network meta-analysis.[2,3,11,17–20] The study characteristics are presented in Table 1, while a flow chart of literature screening is displayed in Figure 2, and the risk of bias among those 7 enrolled articles is illustrated in Figure 3. In summary, all enrolled articles seemed to be at low to moderate risks of bias.
Table 1.
Characteristics of included studies.
Figure 2.
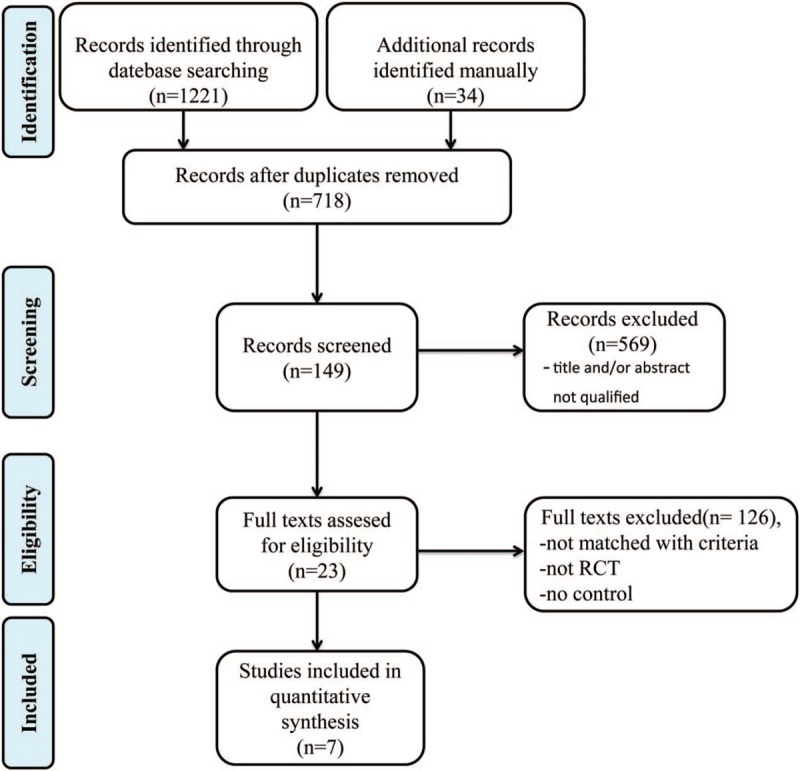
Flow chart diagram of searching strategy.
Figure 3.
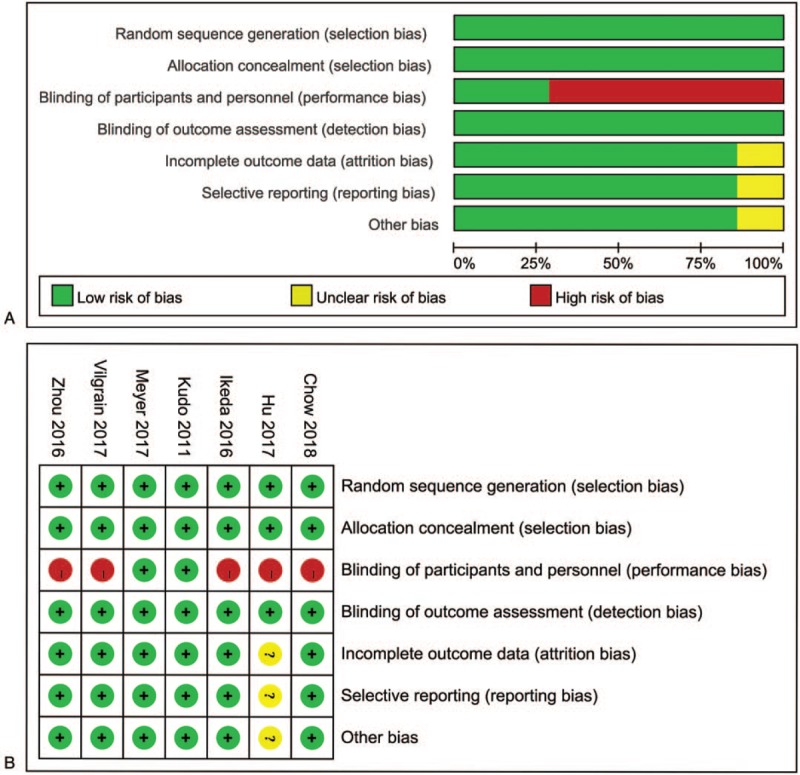
Quality assessment of included studies: (A) overall and (B) study-level risk of bias.
3.2. Network meta-analysis of efficacy
Pairwise meta-analysis on the different treatments for advanced HCC is shown in Figure 4, and Table 2 compares the therapeutic effects of all therapeutic modalities on advanced HCC with regard to the survival rate. As could be obviously seen in the as-generated ranking table, TACE-TCM had the highest therapeutic effect on advanced HCC patients. Besides, network meta-analysis on therapeutic effect was also carried out, in which TACE (TARE) was used as the comparator, and the results suggested that TACE-TCM had shown marked OS benefits for patients ([hazard ratio{HR}0.5-year = 2.34; 95% CI: 1. 08–5.10], [HR1-year = 2.13; 95% CI: 1.30–3.51], [HR2-year = 3.14; 95% CI: 1.26–7.86]); however, no significant benefit could be observed on TACE-sorafenib ([HR0.5-year = 1.24; 95% CI: 0.64–2.40], [HR1-year = 0.82; 95% CI: 0.58–1.18], [HR2-year = 1.12; 95% CI: 0.80–1.57]).
Figure 4.
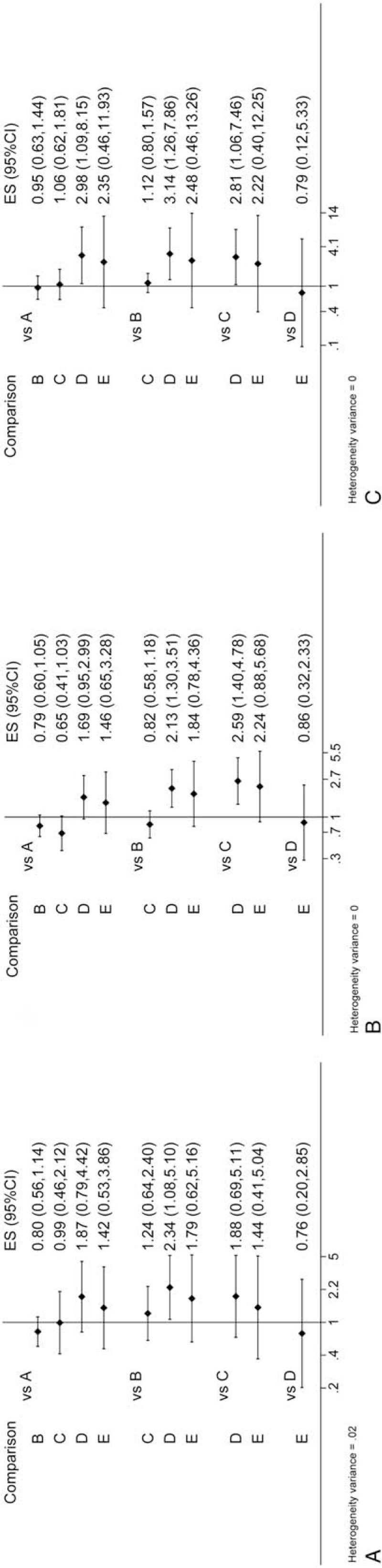
Interval plot: (A) 0.5-year survival rate; (B) 1-year survival rate; (C) 2-year survival rate. A: sorafenib; B: TACE (TARE); C: TACE-sorafenib; D: TACE-TCM; E: HAIC-sorafenib. HAIC = hepatic arterial infusion chemotherapy, TACE = transarterial chemoembolization, TARE = transarterial radioembolization, TCM = traditional Chinese medicine.
Table 2.
Comparisons of efficacy in terms of overall survival in advanced HCC.
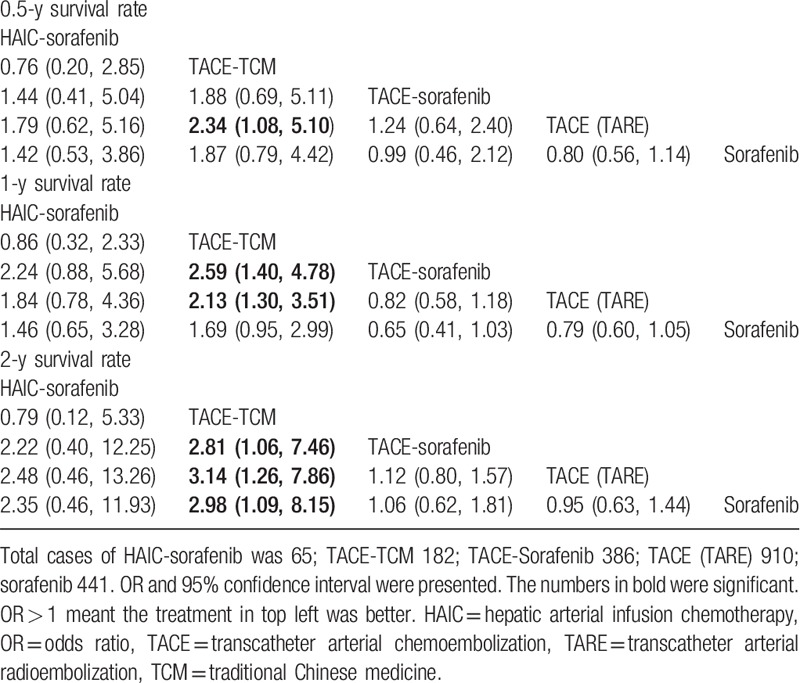
In addition, differences in the OS benefits of TACE (TARE) ([HR0.5-year = 0.80; 95% CI: 0.56–1.14], [HR1-year = 0.76; 95% CI: 0.60–1.05], [HR2-year = 0.95; 95% CI: 0.63–1.44]), TACE-sorafenib ([HR0.5-year = 0.99; 95% CI: 0.46–2.12], [HR1-year = 0.65; 95% CI: 0.41–1.03], [HR2-year = 1.06; 95% CI: 0.62–1.81]) and HAIC-sorafenib ([HR0.5-year = 1.42; 95% CI: 0.53–3.86], [HR1-year = 1.46; 95% CI: 0.65–3.28], [HR2-year = 2.35; 95% CI: 0.46–11.93]) were not statistically significant compared with that of sorafenib.
With regard to the 2-year survival rate, results of the current network meta-analysis demonstrated that 3 therapies, including TACE-sorafenib (HR = 2.81; 95% CI: 1.06–7.46), TACE (TARE) alone (HR = 3.14; 95% CI: 1.26–7.86), and sorafenib (HR = 2.98; 95% CI: 1.09–8.15), had remarkable effects on reducing the survival rate compared with that of TACE-TCM.
In addition, low heterogeneity was detected for the 0.5-, 1-, and 2-year OSs (τ < 0.1), and no inconsistency could be detected.
3.3. Rank test
As could be observed, it was most probable that TACE combined with TCM was the best treatment considering OS, which had ranked the first in hypothetical cases, followed by HAIC-sorafenib (Fig. 5). On the contrary, TACE (TARE) alone was predicted as the worst treatment for 0.5- and 2-year survivals, while TACE-sorafenib had a lower 1-year survival rate. Besides, the SUCRA values of the 5 investigated regimens were shown as follows: TACE-TCM0.5-year = 0.891, TACE-TCM1-year = 0.895, TACE-TCM2-year = 0.866; HAIC-sorafenib0.5-year = 0.729, HAIC-sorafenib1-year = 0.768, HAIC-sorafenib2-year = 0.666; TACE-sorafenib0.5-year = 0.381, TACE-sorafenib1-year = 0.056, TACE-sorafenib2-year = 0.405; sorafenib0.5-year = 0.298, sorafenib1-year = 0.533, sorafenib2-year = 0.432; and TACE (TARE)0.5-year = 0.202, TACE (TARE)1-year = 0.248, TACE (TARE)2-year = 0.132. The possibilities as the optimal treatment with regard to the OS are presented in the rankogram in Figure 6.
Figure 5.
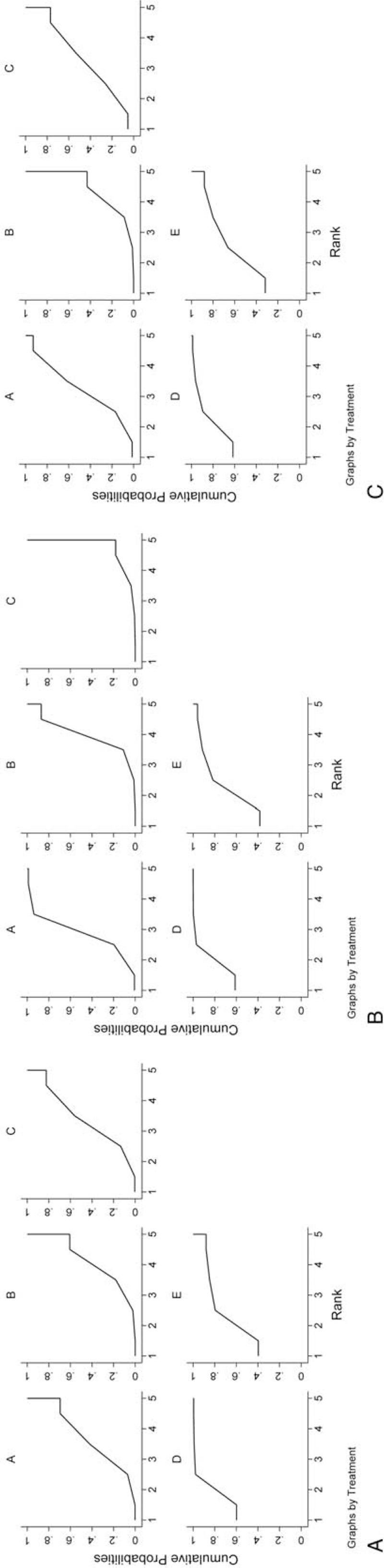
SUCRA of different regimens: (A) 0.5-year survival rate; (B) 1-year survival rate; (C) 2-year survival rate. A: sorafenib; B: TACE (TARE); C: TACE-sorafenib; D: TACE-TCM; E: HAIC-sorafenib. HAIC = hepatic arterial infusion chemotherapy, SUCRA = surface under the cumulative ranking curve, TACE = transarterial chemoembolization, TARE = transarterial radioembolization, TCM = traditional Chinese medicine.
Figure 6.
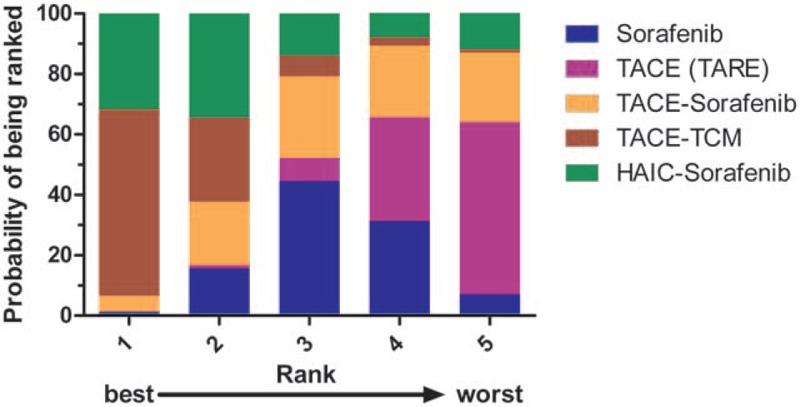
Rankogram of interested treatment modality.
3.4. Analysis of safety
As for analysis of safety, all included studies were described merely under the condition that the authors reported AEs in different manners. For instance, Chow et al[17] reported 437 AEs in TARE group and 1031 in sorafenib group; whereas Vilgrain et al[2] witnessed 1297 AEs in their TARE group and 2837 in sorafenib group. Additionally, Meyer et al's study suggested that, adding sorafenib seemed not to increase the TACE-associated toxicity.[3] Typically, the major differences were related to the well-known sorafenib-associated toxicities, including stomatitis, diarrhea, hand-foot skin reaction, rash, and bleeding. Similarly, Kudo et al's study[19] showed 100% incidence of AEs in TACE-sorafenib group and 61% in TACE alone group, respectively, and most AEs were mild to moderate as expected. Moreover, it seemed to be safe to add TCM to TACE, and the difference in the incidence of AEs was not statistically significant between the 2 groups.[11,18] Besides, AEs were more frequently seen in HAIC-sorafenib group than in sorafenib group.[20]
4. Discussion
To our knowledge, this study first carried out a network meta-analysis aiming to evaluate the therapeutic effect and safety of different treatment modalities for advanced HCC patients based on all available data from the included RCTs. Notably, the pooled results demonstrated that TACE-TCM displayed the highest therapeutic effect on advanced HCC patients. Compared with TACE alone group, the TACE combined with TCM group was associated with a higher survival rate, which could not be observed in the TACE combined with sorafenib group. Moreover, sorafenib showed no marked difference compared with TACE (TARE) in terms of patient survival, and the addition of TACE or HAIC to sorafenib could not prolong patient survival.
Sorafenib is recommended in the BCLC guidelines to be the preferred 1st-line systemic treatment for advanced HCC patients. Specifically, sorafenib has been shown to extend the survival of advanced HCC patients; however, its high cost, frequent AEs, and unsatisfactory efficacy have greatly restricted its application.[28] Specifically, TACE is an alternative option for patients with liver-confined disease, preserved hepatic function, and favorable performance status but are not appropriate for curative therapies. However, TACE is limited by its high recurrence rate, as a result of the over-expression of angiogenic and inflammatory factors, including insulin-like growth factor 2 and VEGF, which has thereby promoted the proliferation and metastasis of the residual tumor cells.[29,30] It has been explored for the feasibility of applying sorafenib following TACE as an adjuvant treatment. TACE is shown to induce acute hypoxia, which will thereby upregulate VEGF and may facilitate revascularization. Nonetheless, TACE is recommended to be used in combination with sorafenib, so as to suppress revascularization and inhibit tumor proliferation. Noteworthily, the combination of sorafenib with TACE appears to be the optimal option for advanced HCC, but its poor tolerance and high financial burden remain the urgent problems to be solved.[31,32] Kudo et al reported that 73% sorafenib-treated patients required dose reductions and 91% required interruptions.[19] Moreover, patients in the sorafenib group discontinued TACE treatments earlier.[33] These could contribute to the lack of difference in OS when adding sorafenib to TACE. HAIC can locally increase the anticancer agent concentrations in tumor and reduce the systemic distribution of these agents; besides, it is also expected to display stronger antitumor activity while less systemic AEs. Furthermore, additional sorafenib may synergistically enhance the anticancer activity.[34] Some studies indicate that TCM plays an important role in the course of terminal stage liver cancer.[35–38] For example, Sadaf et al's results had clearly suggested that As2O3 could markedly restrict the growth and induce the apoptosis of liver cancer cells compared with normal cells.[39] Ginsenoside Rg3 possesses multiple antitumor activities, which can downregulate the hypoxia-induced VEGF expression, promote apoptosis, and inhibit the proliferation and invasion abilities of human cancer cells.[12,14] Thus, the combination of TCM with TACE may synergistically affect the advanced HCC.
In this study, the conventional meta-analysis was compared with this network meta-analysis. In the systematic review and meta-analysis conducted by Liu et al, the therapeutic effect of TACE was compared with that of HAIC, and the results suggested that TACE was associated with higher favorable survival and response rates than HAIC in intermediate or advanced HCC patients.[40] However, TACE-sorafenib and HAIC-sorafenib had not been directly compared in previous studies, but it was suggested in this study that TACE-sorafenib appeared to be equally effective to HAIC-sorafenib. In addition, in the meta-analysis conducted by Cai et al, the therapeutic effect and safety for TACE-sorafenib were assessed in advanced HCC patients. Their results suggested that the combined group exhibited marked improvements compared with TACE alone group; besides, and the AEs rates related to combined therapy were increased compared with that of TACE treatment alone.[41] However, no significant survival benefit of TACE-sorafenib over sorafenib was found in our study. Actually, Cai's study only contained 2 RCTs in English language, and the study population in 1 study was the intermediate stage HCC. Similar to our results, Wang et al had conducted a meta-analysis and systematic review, which suggested that the combined therapy could not improve the OS.[42] As respected, the combined therapy might also markedly increase the risks of incidence of AEs.[43] Therefore, more RCTs are required to further examine the clinical benefits of TACE-sorafenib for advanced HCC. Moreover, Lv et al indicated in a meta-analysis that the adjuvant As2O3 therapy combined with TACE could attain superior therapeutic effects over TACE alone.[44]
The data of rank test demonstrated that among all the 5 investigated therapies, TACE-TCM ranked top with regard to its efficacy in improving the 0.5-, 1-, and 2-year survival rates. Furthermore, the combination of HAIC with sorafenib took the 2nd place as for the 0.5-, 1-, and 2-year survival rates. Therefore, our results suggested that the combination therapies, especially for TACE-TCM, had the greatest efficacy in terms of the prognosis for advanced HCC patients.
However, there were some limitations in this study. Firstly, the basic characteristics of all the studies enrolled might affect the possible heterogeneity as well as the final results. Secondly, estimates in this study were merely based on indirect studies since few direct comparative studies were available, which might warrant lower confidence than estimates based on direct and indirect comparative studies. Thirdly, TACE and TARE were deemed as one kind of therapy in this study to establish a network, since no significant difference was reported between TACE and TARE.[23,45] Consequently, findings of the current network meta-analysis might not completely conform to the clinical practice to some extent.
Taken together, it is revealed in the current network meta-analysis that TACE-TCM is the best therapeutic option for advanced HCC in terms of improving the OS outcomes. Nonetheless, future multicenter and high-quality RCTs with large sample sizes are warranted to confirm the advantages of the combined therapy for HCC.
Acknowledgments
The authors thank Ms Yue-Ming Du for providing continuous encouragement to Dr Qi-Feng Chen to pursue his career in medicine.
Author contributions
Conceptualization: Qi-Feng Chen, Wang Li.
Data curation: Qi-Feng Chen, Pei-Hong Wu, Tao Huang, Lu-Jun Shen.
Formal analysis: Qi-Feng Chen, Tao Huang, Lu-Jun Shen, Zi-Lin Huang.
Methodology: Qi-Feng Chen, Wang Li.
Supervision: Zi-Lin Huang, Wang Li.
Validation: Qi-Feng Chen, Pei-Hong Wu, Tao Huang, Zi-Lin Huang.
Writing – original draft: Qi-Feng Chen, Wang Li.
Writing – review & editing: Qi-Feng Chen, Pei-Hong Wu, Tao Huang, Lu-Jun Shen, Zi-Lin Huang.
Footnotes
Abbreviations: AE = adverse event, BCLC = Barcelona Clinic Liver Cancer, CI = confidence interval, HAIC = hepatic arterial infusion chemotherapy, HCC = hepatocellular carcinoma, HR = hazard ratio, OS = overall survival, RCT = randomized controlled trial, SUCRA = surface under the cumulative ranking curve, TACE = transarterial chemoembolization, TARE = transarterial radioembolization, TCM = traditional Chinese medicine, VEGF = vascular endothelial growth factor.
How to cite this article: Chen QF, Wu PH, Huang T, Shen LJ, Huang ZL, Li W. Efficacy of treatment regimens for advanced hepatocellular carcinoma. Medicine. 2019;98:40(e17460).
Funding: This study was supported by the National Natural Science Foundation of China, No. 81801804.
The authors have no conflicts of interest to disclose.
References
- [1].Torre LA, Bray F, Siegel RL, et al. Global cancer statistics, 2012. CA Cancer J Clin 2015;65:87–108. [DOI] [PubMed] [Google Scholar]
- [2].Vilgrain V, Pereira H, Assenat E, et al. Efficacy and safety of selective internal radiotherapy with yttrium-90 resin microspheres compared with sorafenib in locally advanced and inoperable hepatocellular carcinoma (SARAH): an open-label randomised controlled phase 3 trial. Lancet Oncol 2017;18:1624–36. [DOI] [PubMed] [Google Scholar]
- [3].Meyer T, Fox R, Ma YT, et al. Sorafenib in combination with transarterial chemoembolisation in patients with unresectable hepatocellular carcinoma (TACE 2): a randomised placebo-controlled, double-blind, phase 3 trial. Lancet Gastroenterol Hepatol 2017;2:565–75. [DOI] [PubMed] [Google Scholar]
- [4].Abou-Alfa GK, Schwartz L, Ricci S, et al. Phase II study of sorafenib in patients with advanced hepatocellular carcinoma. J Clin Oncol 2006;24:4293–300. [DOI] [PubMed] [Google Scholar]
- [5].Cheng AL, Kang YK, Chen Z, et al. Efficacy and safety of sorafenib in patients in the Asia-Pacific region with advanced hepatocellular carcinoma: a phase III randomised, double-blind, placebo-controlled trial. Lancet Oncol 2009;10:25–34. [DOI] [PubMed] [Google Scholar]
- [6].Llovet JM, Ricci S, Mazzaferro V, et al. Sorafenib in advanced hepatocellular carcinoma. New Engl J Med 2008;359:378–90. [DOI] [PubMed] [Google Scholar]
- [7].Pinter M, Sieghart W, Graziadei I, et al. Sorafenib in unresectable hepatocellular carcinoma from mild to advanced stage liver cirrhosis. Oncologist 2009;14:70–6. [DOI] [PubMed] [Google Scholar]
- [8].Golfieri R, Renzulli M, Mosconi C, et al. Hepatocellular carcinoma responding to superselective transarterial chemoembolization: an issue of nodule dimension? J Vasc Interv Radiol 2013;24:509–17. [DOI] [PubMed] [Google Scholar]
- [9].Yang Z, Liao X, Lu Y, et al. Add-on therapy with traditional chinese medicine improves outcomes and reduces adverse events in hepatocellular carcinoma: a meta-analysis of randomized controlled trials. Evid Based Complement Alternat Med 2017;2017:3428253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Xu L, Wang S, Zhuang L, et al. Jian Pi Li Qi decoction alleviated postembolization syndrome following transcatheter arterial chemoembolization for hepatocellular carcinoma: a randomized, double-blind, placebo-controlled trial. Integr Cancer Ther 2016;15:349–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Hu HT, Yao QJ, Meng YL, et al. Arsenic trioxide intravenous infusion combined with transcatheter arterial chemoembolization for the treatment of hepatocellular carcinoma with pulmonary metastasis: long-term outcome analysis. J Gastroenterol Hepatol 2017;32:295–300. [DOI] [PubMed] [Google Scholar]
- [12].Kim JW, Jung SY, Kwon YH, et al. Ginsenoside Rg3 inhibits endothelial progenitor cell differentiation through attenuation of VEGF-dependent Akt/eNOS signaling. Phytother Res 2012;26:1286–93. [DOI] [PubMed] [Google Scholar]
- [13].Chen QJ, Zhang MZ, Wang LX. Gensenoside Rg3 inhibits hypoxia-induced VEGF expression in human cancer cells. Cell Physiol Biochem 2010;26:849–58. [DOI] [PubMed] [Google Scholar]
- [14].Wang JH, Nao JF, Zhang M, et al. 20(s)-ginsenoside Rg3 promotes apoptosis in human ovarian cancer HO-8910 cells through PI3K/Akt and XIAP pathways. Tumour Biol 2014;35:11985–94. [DOI] [PubMed] [Google Scholar]
- [15].Kim DG, Jung KH, Lee DG, et al. 20(S)-Ginsenoside Rg3 is a novel inhibitor of autophagy and sensitizes hepatocellular carcinoma to doxorubicin. Oncotarget 2014;5:4438–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Zhang C, Liu L, Yu Y, et al. Antitumor effects of ginsenoside Rg3 on human hepatocellular carcinoma cells. Mol Med Rep 2012;5:1295–8. [DOI] [PubMed] [Google Scholar]
- [17].Chow PKH, Gandhi M, Tan SB, et al. SIRveNIB: selective internal radiation therapy versus sorafenib in Asia-Pacific patients with hepatocellular carcinoma. J Clin Oncol 2018;36:1913–21. [DOI] [PubMed] [Google Scholar]
- [18].Zhou B, Yan Z, Liu R, et al. Prospective study of transcatheter arterial chemoembolization (TACE) with ginsenoside Rg3 versus TACE ALone for the treatment of patients with advanced hepatocellular carcinoma. Radiology 2016;280:630–9. [DOI] [PubMed] [Google Scholar]
- [19].Kudo M, Imanaka K, Chida N, et al. Phase III study of sorafenib after transarterial chemoembolisation in Japanese and Korean patients with unresectable hepatocellular carcinoma. Eur J Cancer 2011;47:2117–27. [DOI] [PubMed] [Google Scholar]
- [20].Ikeda M, Shimizu S, Sato T, et al. Sorafenib plus hepatic arterial infusion chemotherapy with cisplatin versus sorafenib for advanced hepatocellular carcinoma: randomized phase II trial. Ann Oncol 2016;27:2090–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Cucchetti A, Piscaglia F, Pinna AD, et al. Efficacy and safety of systemic therapies for advanced hepatocellular carcinoma: a network meta-analysis of phase III trials. Liver Cancer 2017;6:337–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Tian G, Yang S, Yuan J, et al. Comparative efficacy of treatment strategies for hepatocellular carcinoma: systematic review and network meta-analysis. BMJ Open 2018;8:e021269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Lobo L, Yakoub D, Picado O, et al. Unresectable hepatocellular carcinoma: radioembolization versus chemoembolization: a systematic review and meta-analysis. Cardiovasc Intervent Radiol 2016;39:1580–8. [DOI] [PubMed] [Google Scholar]
- [24].Fallone CA. Epidemiology of the antibiotic resistance of Helicobacter pylori in Canada. Can J Gastroenterol 2000;14:879–82. [DOI] [PubMed] [Google Scholar]
- [25].Salanti G, Ades AE, Ioannidis JP. Graphical methods and numerical summaries for presenting results from multiple-treatment meta-analysis: an overview and tutorial. J Clin Epidemiol 2011;64:163–71. [DOI] [PubMed] [Google Scholar]
- [26].Hutton B, Salanti G, Caldwell DM, et al. The PRISMA extension statement for reporting of systematic reviews incorporating network meta-analyses of health care interventions: checklist and explanations. Ann Intern Med 2015;162:777–84. [DOI] [PubMed] [Google Scholar]
- [27].Jones SD, Drake DJ. Case series of undetected intranasal impression material in patients with clefts. Br J Oral Maxillofac Surg 2013;51:e34–6. [DOI] [PubMed] [Google Scholar]
- [28].Pinter M, Hucke F, Graziadei I, et al. Advanced-stage hepatocellular carcinoma: transarterial chemoembolization versus sorafenib. Radiology 2012;263:590–9. [DOI] [PubMed] [Google Scholar]
- [29].Strebel BM, Dufour JF. Combined approach to hepatocellular carcinoma: a new treatment concept for nonresectable disease. Expert Rev Anticancer Ther 2008;8:1743–9. [DOI] [PubMed] [Google Scholar]
- [30].Sergio A, Cristofori C, Cardin R, et al. Transcatheter arterial chemoembolization (TACE) in hepatocellular carcinoma (HCC): the role of angiogenesis and invasiveness. Am J Gastroenterol 2008;103:914–21. [DOI] [PubMed] [Google Scholar]
- [31].Choi GH, Shim JH, Kim MJ, et al. Sorafenib alone versus sorafenib combined with transarterial chemoembolization for advanced-stage hepatocellular carcinoma: results of propensity score analyses. Radiology 2013;269:603–11. [DOI] [PubMed] [Google Scholar]
- [32].Bai W, Wang YJ, Zhao Y, et al. Sorafenib in combination with transarterial chemoembolization improves the survival of patients with unresectable hepatocellular carcinoma: a propensity score matching study. J Dig Dis 2013;14:181–90. [DOI] [PubMed] [Google Scholar]
- [33].Lencioni R, Llovet JM, Han G, et al. Sorafenib or placebo plus TACE with doxorubicin-eluting beads for intermediate stage HCC: The SPACE trial. J Hepatol 2016;64:1090–8. [DOI] [PubMed] [Google Scholar]
- [34].Kudo M. Treatment of advanced hepatocellular carcinoma with emphasis on hepatic arterial infusion chemotherapy and molecular targeted therapy. Liver Cancer 2012;1:62–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Ling CQ, Yue XQ, Ling C. Three advantages of using traditional Chinese medicine to prevent and treat tumor. J Integr Med 2014;12:331–5. [DOI] [PubMed] [Google Scholar]
- [36].Konkimalla VB, Efferth T. Evidence-based Chinese medicine for cancer therapy. J Ethnopharmacol 2008;116:207–10. [DOI] [PubMed] [Google Scholar]
- [37].Ling CQ, Wang LN, Wang Y, et al. The roles of traditional Chinese medicine in gene therapy. J Integr Med 2014;12:67–75. [DOI] [PubMed] [Google Scholar]
- [38].Chen FP, Kung YY, Chen YC, et al. Frequency and pattern of Chinese herbal medicine prescriptions for chronic hepatitis in Taiwan. J Ethnopharmacol 2008;117:84–91. [DOI] [PubMed] [Google Scholar]
- [39].Sadaf N, Kumar N, Ali M, et al. Arsenic trioxide induces apoptosis and inhibits the growth of human liver cancer cells. Life Sci 2018;205:9–17. [DOI] [PubMed] [Google Scholar]
- [40].Liu X, Wang Z, Chen Z, et al. Efficacy and safety of transcatheter arterial chemoembolization and transcatheter arterial chemotherapy infusion in hepatocellular carcinoma: a systematic review and meta-analysis. Oncol Res 2018;26:231–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Cai R, Song R, Pang P, et al. Transcatheter arterial chemoembolization plus sorafenib versus transcatheter arterial chemoembolization alone to treat advanced hepatocellular carcinoma: a meta-analysis. BMC Cancer 2017;17:714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Wang G, Liu Y, Zhou SF, et al. Sorafenib combined with transarterial chemoembolization in patients with hepatocellular carcinoma: a meta-analysis and systematic review. Hepatol Int 2016;10:501–10. [DOI] [PubMed] [Google Scholar]
- [43].Zhang L, Hu P, Chen X, et al. Transarterial chemoembolization (TACE) plus sorafenib versus TACE for intermediate or advanced stage hepatocellular carcinoma: a meta-analysis. PloS One 2014;9:e100305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Lv XH, Wang CH, Xie Y. Arsenic trioxide combined with transarterial chemoembolization for primary liver cancer: a meta-analysis. J Gastroenterol Hepatol 2017;32:1540–7. [DOI] [PubMed] [Google Scholar]
- [45].Chang J, Charalel R, Noda C, et al. Liver-dominant breast cancer metastasis: a comparative outcomes study of chemoembolization versus radioembolization. Anticancer Res 2018;38:3063–8. [DOI] [PubMed] [Google Scholar]


