Supplemental Digital Content is available in the text
Keywords: arthroscopic knee surgery, meloxicam, postoperative analgesia, preoperative analgesia
Abstract
Background:
This study aimed to investigate the efficacy and safety between early preoperative administration and postoperative administration of oral meloxicam in patients underwent arthroscopic knee surgery (AKS).
Methods:
Totally 296 patients with the intention to undergo AKS were recruited and randomly allocated as 1:1 ratio into early preoperative analgesia (EPA) group and postoperative analgesia (POA) group. Pain visual analog scale (VAS) score and severity (at rest and at flexion), patient global assessment (PGA) score, the consumption of rescue analgesia (pethidine), and adverse events were evaluated during the perioperation. And knee range of motion (ROM), International Knee Documentation Committee (IKDC) score, and Lysholm score were assessed at baseline and at 3 months after AKS.
Results:
Both pain VAS score and severity (at rest and at flexion) were decreased at 4, 8, and 12 hours, but similar at −24, −2, 24, 36, and 48 hours after AKS in EPA group compared with POA group. Besides, PGA score was lower at 4, 8, 12, and 24 hours, but similar at −24, −2, 36, and 48 hours after AKS in EPA group compared with POA group. As to the consumption of pethidine in perioperative period, it was decreased in EPA group compared with POA group. No difference was observed in knee ROM, IKDC score, Lysholm score, and adverse effects between EPA group and POA group.
Conclusion:
Early preoperative administration of meloxicam was a superior approach in pain control compared with postoperative administration in treating patients underwent AKS.
1. Introduction
Arthroscopic knee surgery (AKS) is accepted as an efficient method for various diagnostic and therapeutic purposes among patients with knee diseases including removal of debris, debridement of meniscal tears and cartilage flaps, recontouring of cartilage flaps, arthroscopic reconstruction of ligaments, transplantation of the meniscus, and so on.[1,2] Evidence indicates that AKS help to improve the knee range of motion (ROM) and the mid-term functional outcomes such as: Lysholm score and the International Knee Documentation Committee (IKDC) score.[3] However, estimated 80% of the patients underwent AKS report the severe pain from surgery, which prevents them from early rehabilitation after surgery, especially those who are sensitive to pain, contributing to low quality of patient recovery as well as unfavorable knee function.[4] Therefore, it is essential to investigate effective and safe analgesia for patients receiving AKS, and adequate pain relief is also a key concern for the mobilization, rehabilitation, and discharge period of the patients underwent AKS.
Meloxicam, as an enol-carboxamide nonsteroidal anti-inflammatory drug (NSAID), blocks the production of prostaglandins through selectively inhibiting the isoforms of cyclooxygenase-2, thus, it retains the properties of analgesic, antipyretic, and anti-inflammatory with less unfavorable gastric effects such as: gastrointestinal irritation compared with other nonselective NSAIDs.[5] Meloxicam is used in the treatment of acute and chronic pain disorders, such as: rheumatoid arthritis, dental pain, and multiple pain syndromes of skeletomuscular origin.[6,7] Additionally, meloxicam has been investigated previously to effectively control the knee osteoarthritis pain.[8] Regarding perioperative pain control, previous studies indicate that both preoperative and postoperative oral meloxicam is effective in pain relief in surgeries such as: inguinal hernia repair and dental surgeries.[7,9,10] However, up to date, there is no study illustrating the superiority between preoperative and postoperative administration of meloxicam in pain relief and knee functional recovery in patients underwent AKS. Therefore, in this present study, we compared the efficacy and safety between early preoperative administration and postoperative administration of oral meloxicam in treating patients underwent AKS.
2. Methods
2.1. Study design
This was a randomized, controlled study, and the Consolidated Standards of Reporting Trials guidelines was followed.
2.2. Participants
In this randomized, controlled study, 296 patients with the intention to undergo AKS in Handan Central Hospital from January 2016 to December 2017 were consecutively recruited. Patients were eligible if they
-
(1)
intended to receive AKS for clinical indications, such as ligament reconstruction, meniscectomy, synovectomy, intra-articular fractures reduction, or other knee joint diseases,
-
(2)
aged above 18 years,
-
(3)
were able to understand the research completely, and
-
(4)
had no difficulty in independently filling the assessment scales used in the present study.
And patients were excluded if they
-
(1)
were unsuitable for undergoing AKS due to clinical status or concurrent diseases,
-
(2)
had contraindications to meloxicam or other drugs used in the study,
-
(3)
treated with analgesics within 1 week, corticosteroid within 3 months or intra-articular hyaluronic acid injections within 9 months before the enrollment,
-
(4)
history of neurologic disease, knee surgery, chronic pain or consumption of daily analgesics,
-
(5)
were lactating or pregnant women.
2.3. Ethics statement
The present study protocol was approved by the Ethics Committee of Handan Central Hospital with institutional review board number of 182777195 and was carried out in accordance with the Declaration of Helsinki. Written informed consents were collected from all participants on the enrollment.
2.4. Sample size calculation
The calculation of sample size was performed on the PASS V11.0 software (NCSS, Kaysville, UT), which was estimated on the prediction: mean pain-rest visual analog scale (VAS) score at 12 hours of 3.5 (standard deviation [SD] = 1.5) in early preoperative analgesia (EPA) group and 4.5 (SD = 2.0) in postoperative analgesia (POA) group; mean pain-flexion VAS score at 12 hours of 4.0 (SD = 2.0) in EPA group and 5.0 (SD = 2.5) in POA group. Based on the prediction of mean pain-rest VAS score at 12 hours, using a 2-sided 2-sample t test, 5% level of significance (α) and a power of 0.90, the required simple size of each group was 67; while based on the prediction of mean pain-flexion VAS score at 12 hours, using a 2-sided 2-sample t test, 5% level of significance (α) and a power of 0.90, the required simple size of each group was 109. To ensure a power of 0.90 in the analysis, the minimum sample size should be 109 in each group. Moreover, in consideration of about 25% attrition rate, finally, the simple size was expanded to 148 patients per group in the present study.
2.5. Randomization and grouping
After confirmation of patient eligibility, random assignment was performed as 1:1 ratio with the use of blocked randomization method (block size was 4). Random allocation list was created using the SAS 9.0 software (SAS Institute, Inc., Cary, NC), and assignment of patients was performed by a nurse who was blind to study design. Finally, 148 patients were randomly allocated to EPA group, correspondingly, 148 patients were randomly assigned to POA group.
2.6. Interventions
For patients in the EPA group (N = 148), meloxicam was administered as follows: 15 mg orally at 24 hours before the AKS, followed by 7.5 mg orally at 1 hour before the AKS, then 7.5 mg orally at 24 hours post-AKS. As for patients in the POA group (N = 148), meloxicam was administered as follows: 15 mg orally at 4 hours after AKS, and 7.5 mg orally at 24 hours after AKS. Besides, during the perioperative period, which was defined as the time interval from the 24 hours before AKS (−24 hours) to the 48 hours after AKS (48 hours), rescue analgesia by pethidine was administered to the patients who suffered from intolerable pain, and the consumption of rescue pethidine was required to be documented.
2.7. Clinical and surgical features collection
Patients’ demographic information, such as age, gender, and body mass index (BMI), was registered after enrollment, and the surgical characteristics including surgery type and operative time were recorded after AKS.
2.8. Efficacy assessment
VAS for pain at rest and at flexion was applied to assess the efficacy of pain relief, and the patient global assessment (PGA) scale was used to assess patients’ global status. All scales were independently filled by the patients at 24 hours before AKS (−24 hours), 2 hours before AKS (−2 hours), 4 hours post AKS (4 hours), 8 hours post AKS (8 hours), 12 hours post AKS (12 hours), 24 hours post AKS (24 hours), 36 hours post AKS (36 hours), and 48 hours post AKS (48 hours), respectively. According to the pain VAS score, pain severity was classified as: 0, no pain; 1 to 3, mild pain; 4 to 6, moderate pain; 7 to 10, severe pain. Moreover, knee ROM, IKDC score and Lysholm score were assessed at −24 hours (which was defined as baseline) and at 3 months after AKS. The knee ROM was measured using goniometer. The IKDC score was evaluated by use of IKDC Subjective Knee Form, which was originally designed to measure the symptoms and functional limitations in sports activities caused by various knee impairments, with a total score ranging from 0 to 100, and high IKDC scores indicated a low level of symptoms and a high level of function.[11] The Lysholm score was assessed using the Lysholm knee scale, in which, a total score of 0 to 100 was calculated on 8 domains: limp, locking, pain, stair climbing, support, instability, swelling, and squatting, and the higher Lysholm score indicated the better the knee function.[12,13]
2.9. Safety evaluation
Adverse events occurred during perioperative period was documented for assessing the safety of meloxicam, which included nausea, constipation, vomiting, dizziness, and drowsiness.
2.10. Study endpoints
The primary endpoints were pain VAS score at rest, pain VAS score at flexion, PGA score, and adverse events during the perioperation. The secondary endpoints were knee ROM, IKDC score, and Lysholm score at 3 months post AKS.
2.11. Statistical analysis
All 296 patients were included in the final analysis based on the intention-to-treat (ITT) principle with the last observation carried forward method for the missing data. Normality of continuous variables was determined by the Shapiro–Wilk test, due to most continuous variables were normally distributed or approximately normally distributed, all continuous variables in the present study were described as mean value ± SD. For the repeatedly measured continuous variable, 2-way repeated measures of analysis of variance was used to analyze the within-subject effect (time effect), between-subject effect (treatment effect), and interaction between time and treatment. And at the same time point, according to the homogeneity of variance test, the comparisons of the continuous variables between 2 groups were determined by the Student t test or separate variance estimation t test (as appropriate). Categorical variables were displayed as count (percentage), and the comparison between 2 groups was determined by Chi-square test. All repeated comparisons between 2 groups at different time points were corrected by the Bonferroni method. SPSS 24.0 statistical software (SPSS Inc, Chicago, IL) was used for data analysis, and GraphPad Prism 7.02 software (GraphPad Software Inc., San Diego, CA) was applied to plot graphs. All tests were 2-sided with a P value of less than .05 threshold for significance.
3. Results
3.1. Study flow
In the present study, 406 patients who were about to undergo AKS were initially invited, while 36 of them were excluded because they refused to attend pre-screening procedure (Fig. 1). And the remained 370 patients were further screened for eligibility, while 74 were excluded (including 47 patients excluded for not meeting inclusion criteria, and 27 patients who disagreed to sign informed consents). The remained 296 patients were recruited in the present study and randomized as 1:1 ration into EPA group (N = 148) and POA group (N = 148). In both groups, pain VAS score at rest/flexion, PGA score and adverse events were assessed during perioperation, and knee ROM, IKDC score, and Lysholm score were assessed at 3 months post-AKS. In EPA group, 9 patients withdrew (including 2 violating protocol within 48 hours and 7 lost follow-up) and the remaining 139 (93.9%) patients completed the study. In POA group, 10 patients withdrew (including 1 patient violating protocol within 48 hours and 9 lost follow-up), and the remaining 138 (93.2%) patients completed the study. Totally 148 patients in EPA group and 148 patients in POA group were included in final analysis based on ITT principle.
Figure 1.
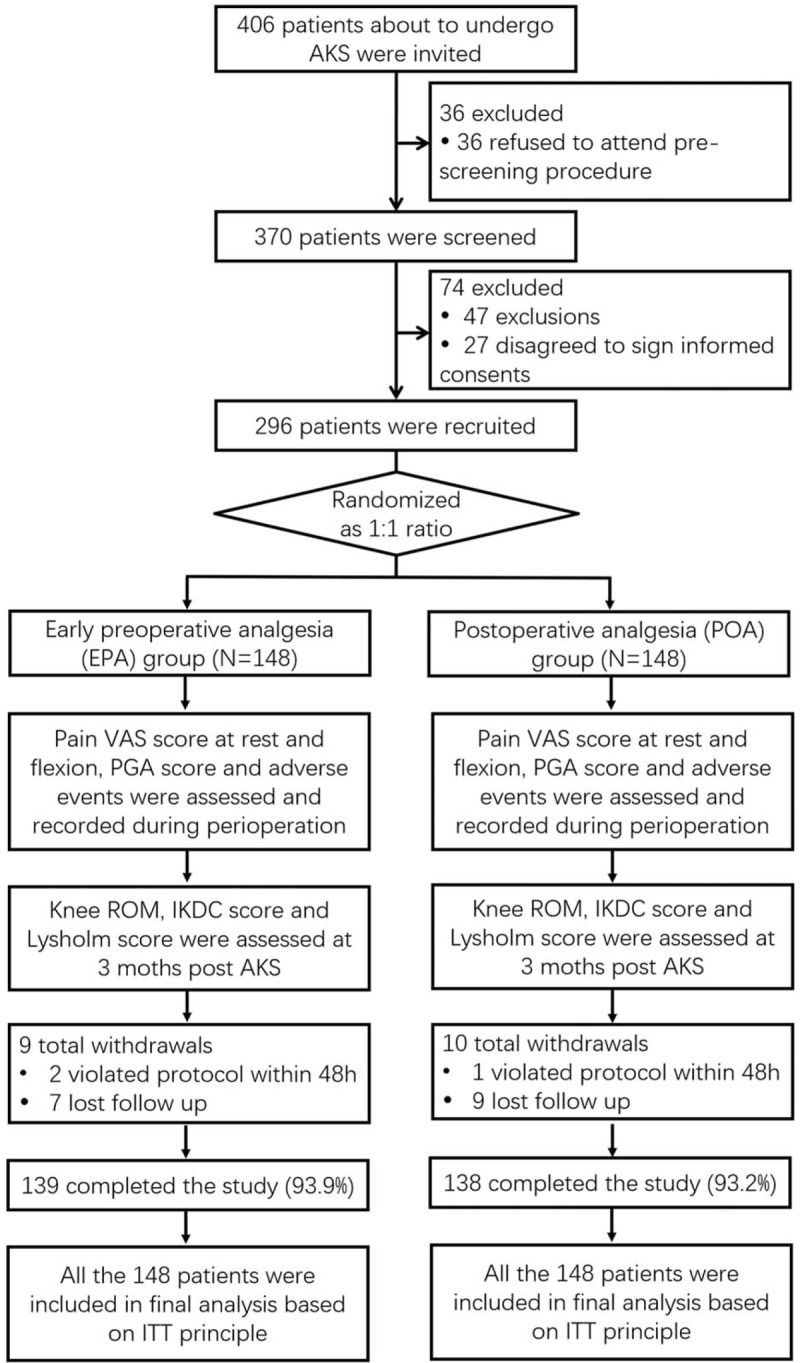
Study flow. AKS = arthroscopic knee surgery, IKDC = International Knee Documentation Committee, ITT = intention-to-treat, PGA = patient global assessment, ROM = range of motion, VAS = visual analog scale.
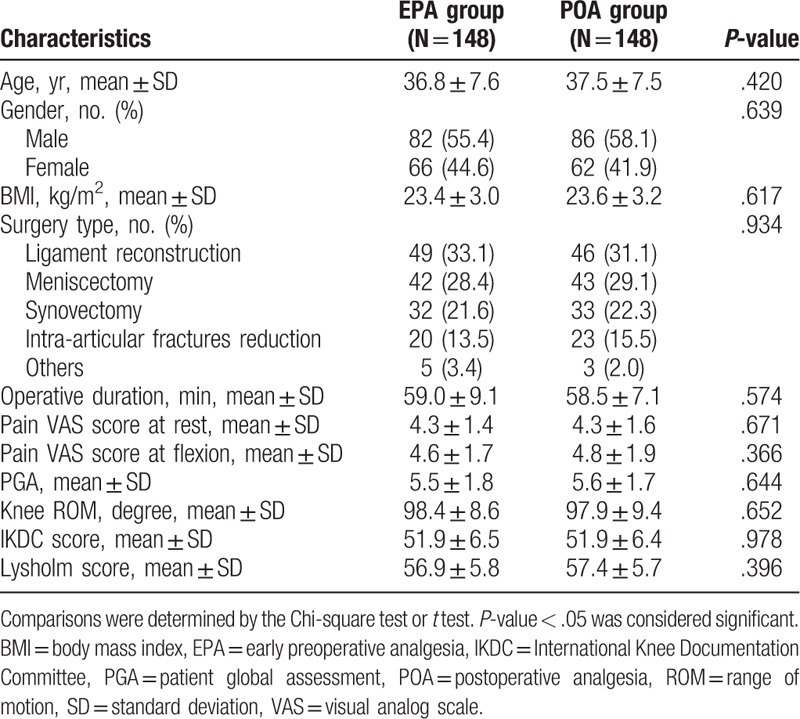
3.2. Baseline characteristics
There was no difference of age, gender, BMI, surgery type, operative duration, pain VAS score at rest/flexion, PGA, knee ROM, IKDC score, and Lysholm score between 2 groups at baseline (all P > .05) (Table 1). The mean age of EPA group and POA group were 36.8 ± 7.6 and 37.5 ± 7.5, respectively. There were 82 (55.4%) males and 66 (44.6%) females included in EPA group, and as to POA group, there were 86 (58.1%) males and 62 (41.9%) females. And the detailed information of baseline characteristics between EPA group and POA group were listed in Table 1.
Table 1.
Baseline characteristics of patients.
3.3. Comparison of pain VAS score and severity
For the pain VAS score at rest, the main effect of the treatment (F = 10.036, P = .001), the main effect of time (F = 223.037, P < .001) and the interaction between treatment and time effect (F = 6.599, P < .001) was significant (Supplementary Table 1). The pain VAS score at rest was lower at 4 hours (P < .01), 8 hours (P < .001), and 12 hours (P < .001), while similar at −24, −2, 24, 36, and 48 hours in EPA group compared to POA group (all P > .05) (Fig. 2A, Supplementary Table 2). And the percentage of patients with moderate-severe pain at rest was reduced at 4 hours (P < .01), 8 hours (P < .001), and 12 hours (P < .05), but similar at −24, −2, 24, 36, and 48 hours in EPA group compared to POA group (all P > .05) (Fig. 2B, Supplementary Table 3). As for the pain VAS score at flexion, the main effect of the treatment (F = 11.875, P = .001), the main effect of time (F = 215.373, P < .001), and the interaction between treatment and time effect (F = 5.102, P = .001) was significant (Supplementary Table 4). The pain VAS score at flexion was decreased at 4 hours (P < .01), 8 hours (P < .001), and 12 hours (P < .001), while similar at −24, −2, 24, 36, and 48 hours in EPA group compared with POA group (all P > .05) (Fig. 2C, Supplementary Table 5). The percentage of patients with moderate-severe pain at flexion was reduced at 4 hours (P < .05), 8 hours (P < .001), and 12 hours (P < .01), but similar at −24, −2, 24, 36, and 48 hours in EPA group compared to POA group (all P > .05) (Fig. 2D, Supplementary Table 6). These data indicated that EPA was more effective in reducing pain during first 12 hours after AKS.
Figure 2.
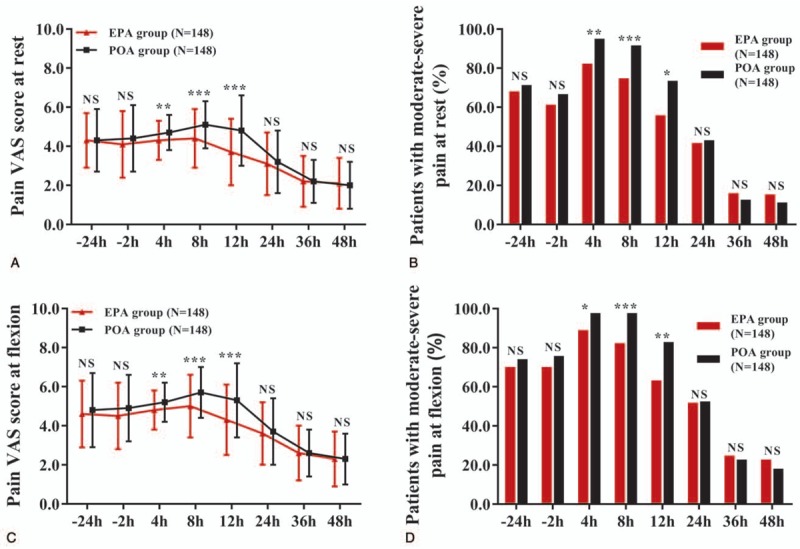
Pain VAS score and severity in EPA group and POA group. EPA group presented lower pain VAS score at rest at 4, 8, and 12 h, but similar at −24, −2, 24, 36, and 48 h compared with POA group (A). EPA group exhibited decreased percentage of patients with moderate-severe pain at rest at 4, 8, and 12 h, while similar at −24, −2, 24, 36, and 48 h compared with POA group (B). EPA group presented lower pain VAS score at flexion at 4, 8, and 12 h, but similar at −24, −2, 24, 36, and 48 h compared with POA group (C). EPA group exhibited decreased percentage of patients with moderate-severe pain at flexion at 4, 8, and 12 h, while similar at −24, −2, 24, 36, and 48 h compared with POA group (D). Comparison between 2 groups was determined by the t test or Chi-square test. All repeated comparisons between 2 groups at different time points were corrected by the Bonferroni method. P < .05 was considered significant. ∗∗∗P < .001, ∗∗P < .01. AKS = arthroscopic knee surgery, EPA = early preoperative analgesia, NS = nonsignificant, POA = postoperative analgesia, VAS = visual analog scale.
3.4. Comparison of PGA score
For the PGA score, the main effect of the treatment (F = 15.049, P < .001), the main effect of time (F = 234.359, P < .001), and the interaction between treatment and time effect (F = 4.450, P = .001) was significant (Supplementary Table 7). The PGA score was reduced at 4 hours (P < .05), 8 hours (P < .001), 12 hours (P < .001), and 24 hours (P < .05), but similar at −24, −2, 36, and 48 hours (all P > .05) in EPA group compared to POA group, which indicated that EPA reduced PGA score more effectively compared with POA during the first 24 hours after AKS (Fig. 3, Supplementary Table 8).
Figure 3.
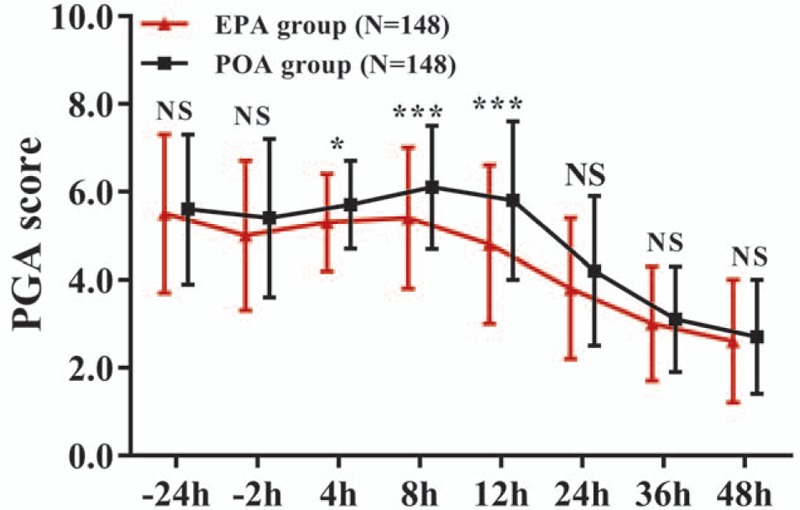
PGA score in EPA group and POA group. EPA group exhibited lower PGA score at 4, 8, 12, and 24 h, but similar PGA score at −24, −2, 36, and 48 h compared with POA group. Comparison between 2 groups was determined by the t test. All repeated comparisons between 2 groups at different time points were corrected by the Bonferroni method. P < .05 was considered significant. ∗P < .05, ∗∗∗P < .001. EPA = early preoperative analgesia, NS = nonsignificant, PGA = patient global assessment, POA = postoperative analgesia.
3.5. The comparison of pethidine consumption
The consumption of pethidine was recorded during the perioperative period when patients suffered from intolerable pain, which observed that EPA group (23.0 ± 18.3 mg) consumed less pethidine compared with POA group (27.7 ± 19.2 mg) (P = .031) (Fig. 4, Supplementary Table 9).
Figure 4.
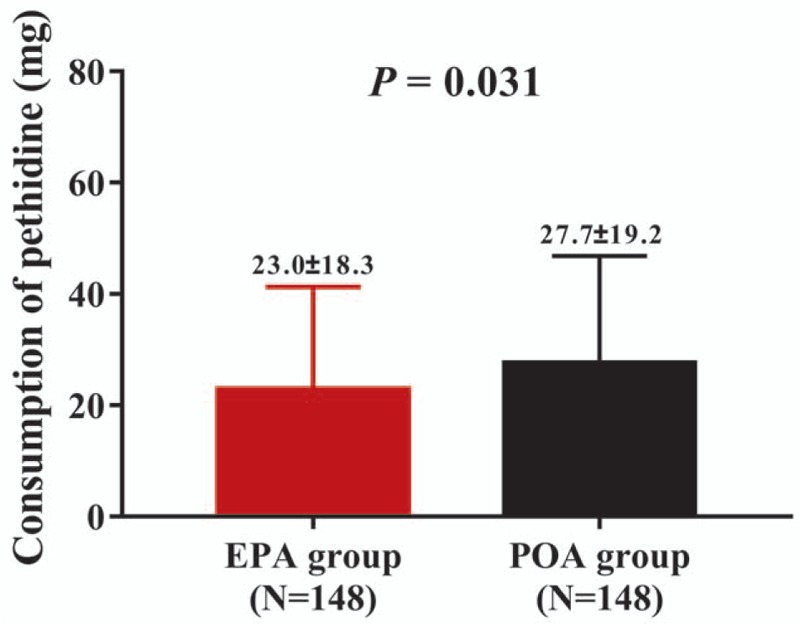
Consumption of pethidine in EPA group and POA group. EPA group presented decreased consumption of pethidine compared with POA group. Comparison between 2 groups was determined by the t test. All repeated comparisons between 2 groups at different time points were corrected by the Bonferroni method. P < .05 was considered significant. EPA = early preoperative analgesia, POA = postoperative analgesia.
3.6. Comparisons of knee ROM, IKDC score, and Lysholm score
Knee ROM, IKDC score, and Lysholm score were assessed before AKS and at 3 months post-AKS (Fig. 5, Supplementary Table 9). There was no difference of knee ROM, IKDC score, and Lysholm score between EPA group and POA group (all P > .05). These data suggested that EPA and POA had similar effects on knee functional recovery in patients underwent AKS.
Figure 5.
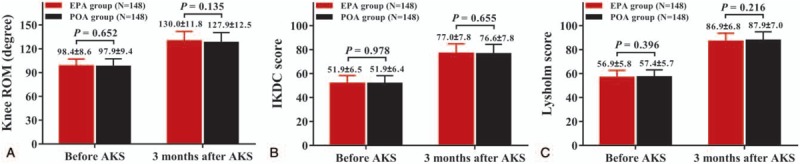
Knee ROM, IKDC score, and Lysholm score in EPA group and POA group. EPA group presented similar knee ROM compared with POA group before AKS and at 3 months after AKS (A). EPA group exhibited similar IKDC score compared with POA group before AKS and at 3 months after AKS (B). EPA group exhibited similar Lysholm score compared with POA group before AKS and at 3 months after AKS (C). Comparison between 2 groups was determined by the t test. All repeated comparisons between 2 groups at different time points were corrected by the Bonferroni method. P < .05 was considered significant. EPA = early preoperative analgesia, IKDC = International Knee Documentation Committee, POA = postoperative analgesia, ROM = range of motion.
3.7. Comparison of adverse events during perioperation
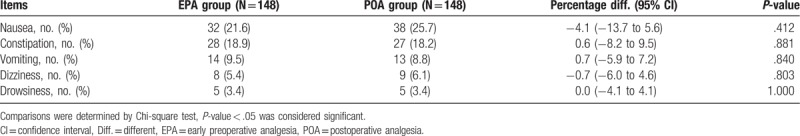
The incidence of nausea, constipation, vomiting, dizziness and drowsiness were 21.6%, 18.9%, 9.5%, 5.4%, and 3.4% in EPA group and 25.7%, 18.2%, 8.8%, 6.1%, and 3.4% in POA group (Table 2). No difference in the incidence of adverse effects exists between 2 groups (all P > .05). More detailed information on adverse events was shown in Table 2.
Table 2.
Adverse events during perioperation.
4. Discussion
In this present randomized, controlled study, 296 patients with the intention to undergo AKS were recruited and randomized as 1:1 ration into EPA group and POA group to compare VAS score, PGA score, consumption of rescue analgesia, adverse events. And the reasons why these scores were selected were as follows: VAS was used as a common instrument to measure intensity or severity of self-reported pain, which enabled clinicians make clinical decision for pain management; PGA score was developed to capture patients’ overall situation in clinical trials. Additionally, it only contained 1 question, thus, it was easy and flexible to measure in many randomized clinical trials; in addition, consumption of rescue analgesia and adverse effect were often used to detect the analgesia effect and safety in some clinical randomized trials.[14–16]
In the present study, we found that pain VAS score and severity were decreased within 12 hours but similar at 24, 36, and 48 hours in EPA group compared with POA group, meanwhile PGA score was reduced within 24 hours after AKS, but similar after 24 hours post AKS in EPA group compared with POA group. Besides, patients presented no difference of adverse effects and knee function recovery in EPA group compared with POA group. These suggested that EPA of meloxicam was more effective in pain relief compared with POA, while presented the same tolerability and long-term knee function recovery as POA.
Meloxicam, as a selective NSAID, is a potent inhibitor of prostaglandin biosynthesis in pleural and peritoneal exudate, which had weaker inhibitory effects on gastric tract as well as kidney compared with other nonselective NSAIDs.[17] The use of oral meloxicam is widely applied to treat several postoperative pains.[7,18,19] One randomized double-blind study reveals that the oral administration of meloxicam before abdominal hysterectomy reduces pain at rest, on movement and on coughing during the first 24 hours after surgery.[20] In another study, preoperative administration of meloxicam is effective in relieving pain after separator placement, and it is regarded as a useful analgesic in orthodontic pain control.[21] In addition, 1 randomized controlled crossover study examines the effect of postoperative meloxicam administration in patients underwent lower third molar removal, which exhibits that postoperative regimen of meloxicam effectively controls pain as well as swelling.[10] These previous studies have indicated the analgesia effect of preoperative or postoperative meloxicam administration in pain relief during the operation of abdominal hysterectomy, orthodontic placement, and molar removal, while the study comparing the efficacy of preoperative and postoperative application of meloxicam in pain relief during AKS has not been carried out before. Thus, we conducted this study and observed that EPA of meloxicam reduced pain more effectively within 12 hours but had similar effects on pain relief after 12 hours post-AKS compared with POA. The possible explanations were as follows:
-
(1)
Evidence exhibited that when patients were in fasted state, maximum meloxicam plasma concentration of oral administration (15 mg) was achieved after approximately 10 hours, thus meloxicam plasma concentration remains at a higher level in EPA group compared with POA group within 12 hours after AKS, leading to more effective pain control.
-
(2)
Preemptive analgesia, as a prophylactical approach of pain control, reduced painful stimulus by inactivating neuronal sensitization and preventing the transmission of nerve impulse to the central nervous system before the surgery.[22]
-
(3)
Moreover, preoperative administration of meloxicam might give patients psychological comfort, leading to a lower perception of pain subjectively.
Therefore, EPA of meloxicam reduced pain more effectively compared with POA within 12 hours after AKS. As AKS was a minimally invasive surgery and the pain might gradually disappear after 12 hours post the surgery, therefore no difference of pain control was observed in EPA group and POA group after 12 hours postoperation.
PGA is widely used to provide the patient's perspective on their overall health status in inflammatory joint disorder, allowing a more holistic assessment of disease beyond objective measures of inflammation or structural damage.[2,22] In our study, we found that patients receiving EPA of meloxicam presented reduced PGA score within 24 hours post-AKS, but similar after 24 hours post-AKS compared with POA, and no difference of knee function was observed between patients receiving EPA and OPA of meloxicam. These indicated that patients with EPA of meloxicam displayed higher overall status compared with POA in a short period after the AKS; however, EPA and POA of meloxicam had no effect on the long-term knee function recovery in patients underwent AKS. The possible reason might be that the main factors influencing knee function were the contribution of AKS and the early rehabilitation after surgery, but not the short-term analgesic effect in the perioperative period, therefore no difference of knee function was observed between patients received preoperative and postoperative administration of meloxicam at 3 months after AKS.
The main concern of using analgesia is their undesirable side effects, which contribute to longer discharge time and unfavorable quality of recovery, hence, studies focusing on the safety of pain control are necessary.[23] Emerging evidence suggested that meloxicam is commonly associated with gastrointestinal adverse events.[21,24] For example, 1 clinical study reveals that pre-procedural administration of meloxicam is correlated with gastric side effects including: perforation, ulceration, and bleeding.[21] However, the safety and tolerability of meloxicam have not been explored in patients underwent AKS. In our study, we found that the adverse effects by meloxicam included nausea, constipation, vomiting, dizziness, and drowsiness, which was consistent with the previous evidence, and there was no difference between EPA and POA group in the adverse events. These data revealed that the meloxicam was tolerable as analgesia in AKS. The possible reasons might include:
-
(1)
It was widely accepted that gastrointestinal adverse events commonly occur after the usage of NSAIDs, which was consistent with our founding that nausea, constipation, vomiting were seen in both EPA and POA group.[25]
-
(2)
Additionally, evidence indicated that meloxicam at a common dose (7.5 mg/15 mg) had low incidence of adverse events, and there were only 3 times (15, 7.5, 7.5 mg) and 2 times (15, 7.5 mg) of meloxicam administration in EPA group and POA group, therefore no difference of adverse events was observed between EPA group and POA group.[7]
The present study was the first study which compared the efficacy and safety of preoperative and postoperative administration of meloxicam in patients underwent AKS. However, there were still some limitations in our study as follows:
-
(1)
Pain VAS score is used to measure intensity or severity of self-reported pain using a unidimensional approach, and it was suggested that patients’ features and ethnicities influenced variations in pain perception and expression, leading to subjectivity bias in the assessment.[26]
-
(2)
Although pain and functional incapacity were the main drivers of PGA, while PGA was also influenced by other factors including psychological condition and societal status, which were not included in the consideration.[27]
-
(3)
This study was not a blinded randomized controlled study, which might lead to the bias of clinician's assessments and patients’ self-reported evaluation.
-
(4)
Due to that our study was a non-double-blind trial rather than a double-blind trial, the assessment bias by researchers and patients might exist.
-
(5)
Although the clinicians-in-charge did not change during the study, while patients might receive AKS by different surgeons, which might lead to the compounding factor.
5. Conclusions
In conclusion, early preoperative administration of meloxicam was a superior approach in pain control compared with postoperative administration in treating patients underwent AKS.
Author contributions
Conceptualization: Liping Yang.
Data curation: Junde Hou.
Formal analysis: Junde Hou, Wei Li, Yongxue Chen, Liying Li, Lu Zhao.
Investigation: Junde Hou, Wei Li, Yongxue Chen.
Methodology: Wei Li, Yongxue Chen, Liying Li.
Resources: Liping Yang, Liying Li.
Validation: Lu Zhao.
Visualization: Liping Yang.
Writing – original draft: Yongxue Chen, Liying Li.
Writing – review & editing: Liping Yang, Lu Zhao.
Supplementary Material
Footnotes
Abbreviations: AKS = arthroscopic knee surgery, BMI = body mass index, EPA = early preoperative analgesia, IKDC = International Knee Documentation Committee, NSAID = nonsteroidal anti-inflammatory drug, PGA = patient global assessment, POA = postoperative analgesia, ROM = range of motion, SD = standard deviation, VAS = visual analog scale.
How to cite this article: Hou J, Li W, Chen Y, Yang L, Li L, Zhao L. Early preoperative versus postoperative administration of meloxicam in pain control, patient global status improvement, knee function recovery of arthroscopic knee surgery. Medicine. 2019;98:40(e17133).
The authors have no conflicts of interest to disclose.
Supplemental Digital Content is available for this article.
References
- [1].Uribe AA, Arbona FL, Flanigan DC, et al. Comparing the efficacy of IV ibuprofen and ketorolac in the management of postoperative pain following arthroscopic knee surgery. A randomized double-blind active comparator pilot study. Front Surg 2018;5:59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Zhou F, Du Y, Huang W, et al. The efficacy and safety of early initiation of preoperative analgesia with celecoxib in patients underwent arthroscopic knee surgery: a randomized, controlled study. Medicine (Baltimore) 2017;96:e8234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Liu Z, Li Y, Sun P, et al. All-arthroscopic release for treating severe knee extension contractures could improve the knee range of motion and the mid-term functional outcomes. Knee Surg Sports Traumatol Arthrosc 2019;27:724–30. [DOI] [PubMed] [Google Scholar]
- [4].Hayashi K, Kako M, Suzuki K, et al. Associations among pain catastrophizing, muscle strength, and physical performance after total knee and hip arthroplasty. World J Orthop 2017;8:336–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Jackson LM, Hawkey CJ. COX-2 selective nonsteroidal anti-inflammatory drugs: do they really offer any advantages? Drugs 2000;59:1207–16. [DOI] [PubMed] [Google Scholar]
- [6].Furst DE, Kolba KS, Fleischmann R, et al. Dose response and safety study of meloxicam up to 22.5 mg daily in rheumatoid arthritis: a 12 week multicenter, double blind, dose response study versus placebo and diclofenac. J Rheumatol 2002;29:436–46. [PubMed] [Google Scholar]
- [7].Bekker A, Kloepping C, Collingwood S. Meloxicam in the management of post-operative pain: narrative review. J Anaesthesiol Clin Pharmacol 2018;34:450–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Turck D, Roth W, Busch U. A review of the clinical pharmacokinetics of meloxicam. Br J Rheumatol 1996;35Suppl 1:13–6. [DOI] [PubMed] [Google Scholar]
- [9].Kurukahvecioglu O, Karamercan A, Ege B, et al. Effect of meloxicam on postoperative pain relief after inguinal hernia repair with local anaesthesia. West Indian Med J 2007;56:530–3. [PubMed] [Google Scholar]
- [10].Calvo AM, Sakai VT, Giglio FP, et al. Analgesic and anti-inflammatory dose-response relationship of 7.5 and 15 mg meloxicam after lower third molar removal: a double-blind, randomized, crossover study. Int J Oral Maxillofac Surg 2007;36:26–31. [DOI] [PubMed] [Google Scholar]
- [11].Irrgang JJ, Anderson AF, Boland AL, et al. Development and validation of the international knee documentation committee subjective knee form. Am J Sports Med 2001;29:600–13. [DOI] [PubMed] [Google Scholar]
- [12].Lysholm J, Gillquist J. Evaluation of knee ligament surgery results with special emphasis on use of a scoring scale. Am J Sports Med 1982;10:150–4. [DOI] [PubMed] [Google Scholar]
- [13].Tegner Y, Lysholm J. Rating systems in the evaluation of knee ligament injuries. Clin Orthop Relat Res 1985;43–9. [PubMed] [Google Scholar]
- [14].Nie Y, Tu W, Shen X, et al. Dexmedetomidine added to sufentanil patient-controlled intravenous analgesia relieves the postoperative pain after cesarean delivery: a prospective randomized controlled multicenter study. Sci Rep 2018;8:9952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Castera L, Negre I, Samii K, et al. Patient-administered nitrous oxide/oxygen inhalation provides safe and effective analgesia for percutaneous liver biopsy: a randomized placebo-controlled trial. Am J Gastroenterol 2001;96:1553–7. [DOI] [PubMed] [Google Scholar]
- [16].Minkowitz HS, Rathmell JP, Vallow S, et al. Efficacy and safety of the fentanyl iontophoretic transdermal system (ITS) and intravenous patient-controlled analgesia (IV PCA) with morphine for pain management following abdominal or pelvic surgery. Pain Med 2007;8:657–68. [DOI] [PubMed] [Google Scholar]
- [17].Engelhardt G. Pharmacology of meloxicam, a new non-steroidal anti-inflammatory drug with an improved safety profile through preferential inhibition of COX-2. Br J Rheumatol 1996;35Suppl 1:4–12. [DOI] [PubMed] [Google Scholar]
- [18].Ahmed M, Khanna D, Furst DE. Meloxicam in rheumatoid arthritis. Expert Opin Drug Metab Toxicol 2005;1:739–51. [DOI] [PubMed] [Google Scholar]
- [19].Altman R, Hochberg M, Gibofsky A, et al. Efficacy and safety of low-dose SoluMatrix meloxicam in the treatment of osteoarthritis pain: a 12-week, phase 3 study. Curr Med Res Opin 2015;31:2331–43. [DOI] [PubMed] [Google Scholar]
- [20].Thompson JP, Sharpe P, Kiani S, et al. Effect of meloxicam on postoperative pain after abdominal hysterectomy. Br J Anaesth 2000;84:151–4. [DOI] [PubMed] [Google Scholar]
- [21].Zarif Najafi H, Oshagh M, Salehi P, et al. Comparison of the effects of preemptive acetaminophen, ibuprofen, and meloxicam on pain after separator placement: a randomized clinical trial. Prog Orthod 2015;16:34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Goncalves de Freitas AT, Lemonica L, De Faveri J, et al. Preemptive analgesia with acupuncture monitored by c-Fos expression in rats. J Acupunct Meridian Stud 2016;9:16–21. [DOI] [PubMed] [Google Scholar]
- [23].Sun QB, Liu SD, Meng QJ, et al. Single administration of intra-articular bupivacaine in arthroscopic knee surgery: a systematic review and meta-analysis. BMC Musculoskelet Disord 2015;16:21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Singla N, Bindewald M, Singla S, et al. Efficacy and safety of intravenous meloxicam in subjects with moderate-to-severe pain following abdominoplasty. Plast Reconstr Surg Glob Open 2018;6:e1846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Zeidler H, Kaltwasser JP, Leonard JP, et al. Prescription and tolerability of meloxicam in day-to-day practice: postmarketing observational cohort study of 13,307 patients in Germany. J Clin Rheumatol 2002;8:305–15. [DOI] [PubMed] [Google Scholar]
- [26].Ham OK, Kang Y, Teng H, et al. Consistency and accuracy of multiple pain scales measured in cancer patients from multiple ethnic groups. Cancer Nurs 2015;38:305–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Nikiphorou E, Radner H, Chatzidionysiou K, et al. Patient global assessment in measuring disease activity in rheumatoid arthritis: a review of the literature. Arthritis Res Ther 2016;18:251. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


