Supplemental Digital Content is available in the text
Keywords: antidiabetics, cancer, carcinogenicity, drug toxicity, long-term drug exposure, medicine, observational health data, pharmacoepidemiology, safe drugs
Abstract
Antidiabetic medications are commonly used around the world, but their safety is still unclear. The aim of this study was to investigate whether long-term use of insulin and oral antidiabetic medications is associated with cancer risk.
We conducted a well-designed case–control study using 12 years of data from Taiwan's National Health Insurance Research Database and investigated the association between antidiabetic medication use and cancer risk over 20 years. We identified 42,500 patients diagnosed with cancer and calculated each patient's exposure to antidiabetic drugs during the study period. We matched cancer and noncancer subjects matched 1:6 by age, gender, and index date, and used Cox proportional hazard regression and conditional logistic regression, adjusted for potential confounding factors, that is, medications and comorbid diseases that could influence cancer risk during study period.
Pioglitazone (adjusted odds ratio [AOR], 1.20; 95% confidence interval [CI], 1.05–1.38); and insulin and its analogs for injection, intermediate or long acting combined with fast acting (AOR, 1.22; 95% CI, 1.05–1.43) were significantly associated with a higher cancer risk. However, metformin (AOR, 1.00; 95% CI, 0.93–1.07), glibenclamide (AOR, 0.98; 95% CI, 0.92–1.05), acarbose (AOR, 1.06; 95% CI, 0.96–1.16), and others do not show evidence of association with cancer risk. Moreover, the risk for specific cancers among antidiabetic users as compared with nonantidiabetic medication users was significantly increased for pancreas cancer (by 45%), liver cancer (by 32%), and lung cancer (by 18%).
Antidiabetic drugs do not seem to be associated with an increased cancer risk incidence except for pioglitazone, insulin and its analogs for injection, intermediate or long acting combined with fast acting.
Key Message
Several studies have reported that the metformin, the most commonly prescribed antidiabetic drug, has anti-cancer properties. Metformin therapy has been associated with reduced colorectal and pancreatic cancer risk. Insulin dose-related cancer incidence has also been reported with glargine, but not with human insulin
What are the new findings?
There is not only an association of pioglitazone and higher cancer risk was found in a Taiwanese population but also insulin and its analogues for injection, intermediate or long-acting combined with fast-acting are associated with a higher cancer risk.
Investigated the association in consideration of length of exposure, defined daily dose, other confounding factors such as co-medications and comorbid diseases.
It demonstrates whether long-term use of insulin and oral anti-diabetic medications are associated with cancer risk incidence.
How might these results change the focus of research or clinical practice?
Glipizide may act in a protective way against cancer, motivating further investigation.
There is a clear need to further assess a pharmacological activity of anti-diabetic drugs for cancer risk on alternative populations.
1. Introduction
Antidiabetic drugs are the most commonly used drugs among the 347 million individuals diagnosed with diabetes globally.[1] The majority of diabetics are between 40 and 59 years old, and the number is increasing day-by-day, especially those with type 2 diabetes. The World Health Organization estimated that the number of diabetic people will increase by 55% by the year 2035.[2] Similarly, the International Diabetes Federation (IDF) projects that the number of diabetics will increase from 382 to 592 million by 2035, with 80% of cases being from low- and middle-income countries.[3] Despite these predictions and the fact that diabetes is the 4th leading cause of death in developing countries, diabetes is a treatable disease with a number of currently available medications.
Several studies have reported that the metformin, the most commonly prescribed antidiabetic drug, has anti-cancer properties. For example, metformin therapy has been associated with reduced colorectal and pancreatic cancer risk.[4,5] On the contrary, a different antidiabetic drug, rosiglitazone, has been reported to increase the risk of myocardial infarction and death from cardiovascular conditions.[6] Recent study, however, found that rosiglitazone does not appear to increase the risk of death.[7]
Additional research suggests that insulin, which many diabetics take multiple times a day, may also increase cancer risk due to its mitogenic properties, which may promote certain types of cancers.[8] The long-acting insulin, glargine, reportedly increased the risk of breast cancer in Swedish women.[9] In contrary, another study reported that short-term use did not carry any increased cancer risk, while long-term use was associated with breast cancer.[10] Insulin dose-related cancer incidence has also been reported with glargine, but not with human insulin.[11] It has also been reported that patients who have solid tumors are more likely to have been taking insulin or insulin secret agogues as compared to oral hypoglycemic drugs.[4] It is still unclear whether diabetes itself or antidiabetic drugs are carcinogenic in nature.[12]
Several pharmacologic classes of antidiabetic drugs are on the market, but it is unclear how safe they are in terms of their carcinogenicity. Different antidiabetic drugs have different pharmacokinetics and potencies, which further vary among individuals. The Food and Drug Administration's (FDA) preclinical studies are usually done before the drug is marketed, but further evaluation for long-term use studies normally not taken into account due to high costs of clinical trials and long-term follow-up. Fortunately, a pharmacoepidemiologic approach makes it possible to assess the carcinogenic profile of the long-term antidiabetic drugs use retrospectively.
The aim of our study was to evaluate whether antidiabetic medication use in diabetic patients is associated with cancer risk in the Taiwanese population. Specifically, we investigated whether the length of exposure (LOE) and defined daily dose (DDD) of the antidiabetic oral and injectable medications affected the risk for the most common cancers. We investigated the carcinogenic risk of individual drugs as well as of pharmacologic classes of antidiabetic drugs.
2. Methods
2.1. Data source
In this study, we used the Taiwan National Health Insurance Research Database (NHIRD), which was established by the Taiwan's National Health Research Institute. The NHIRD consists of detailed claim data for more than 23 million enrollees, covering 99% of Taiwanese residents.[13] From this population-wide database, we randomly selected a 2 million sample covering years 1998 to 2009, inclusively.[14] The data were anonymized and de-identified prior to our analysis; hence, this type of study did not require the Institutional Review Board approval.
2.2. Study population
We conducted a retrospective case–control study using incident cases by retrieving all individuals who were 1st diagnosed with any cancer between January 1, 2001 and December 31, 2009 as identified by codes 104 to 208 as per the International Classification of Disease, Clinical Modification, Ninth Revision (ICD-9-CM). These 42,500 subjects served as cases in this study, with cancer being the outcome of interest. The date of cancer diagnosis was taken as the index date (see Supplemental S1). Patients without any cancer during the 12 years study period served as controls. For each case, we randomly selected 6 matched controls in the sample population, resulting on 255,000 control subjects. Covariates included for the matching were: sex, age, at cancer diagnosis for the case, and the year of diagnosis. Controls were assigned an index date identical to the date of diagnosis for the corresponding case. We excluded subjects with cancer who were <20 years of age.
2.3. Antidiabetic medications exposure
Information regarding patients’ medications exposure was retrieved from the pharmacy prescription database. Antidiabetic drugs were determined by their Anatomical Therapeutic Chemical (ATC) drug classification codes. The classes analyzed were: biguanides (A10BA), sulfonylureas (A10BB), combinations of oral blood glucose lowering drugs (A10BD), alpha glucosidase inhibitors (A10BF), thiazolidinediones (A10BG), dipeptidyl peptidase 4 (DPP-4) inhibitors (A10BH), other blood glucose lowering drugs: insulins (A10BX), insulins and analogs for injection, fast acting (A10AB), insulins and analogs for injection, intermediate acting (A10AC), insulins and analogs for injection, intermediate or long acting combined with fast acting (A10AD), insulins and analogs for injection, long acting (A10AE) (see Supplemental Table S1). For each filled prescription of each study participant, we recorded drug codes, drug names, dispensing data, and the total daily dose (i.e., the assumed average maintenance dose per day). Antidiabetic medication doses were analyzed for DDD per day in the following categories: 0.00 (reference), 0.10 to 0.65, 0.66 to 1.30, and >1.30.
The antidiabetic medication exposure was analyzed only before the cancer diagnosis/index date. We considered whether individuals have ever been exposed to antidiabetic medications or not. Therefore, patients who had antidiabetic medications prescribed at least 2 months during the study period were classified as antidiabetic medication users. Exposure to these drugs was categorized according to exposure duration (i.e., 61–180 days, 181–1 year, and over 1 years) before the index date. An additional category, “no use,” was created for patients who had never been on an antidiabetic drug for at least two months.[15]
2.4. Covariate assessment
We matched for age, gender, diagnosis year but adjusted for comorbid diseases, confounding drugs, region, and socioeconomic status (SES) done to estimate the probabilities of a patient being classified into the cancer (case) or noncancer (control) groups, as shown in Table 1. The following drugs were considered in the study as potential confounders, the drugs known or suspected of modifying the risk of some cancers[16]: aspirin (ATC codes: B01AC06, N02BA01, N02BA51), nonsteroidal anti-inflammatory drugs (NSAIDs) (M01A, excluding M01AX), statins (C10AA), and angiotensin-II antagonists (C09C and C09D). Exposure to these confounder drugs was defined as positive if they were dispensed at least twice a year within 3 years of the cancer diagnosis date.
Table 1.
Baseline characteristics of cancer cases and controls.
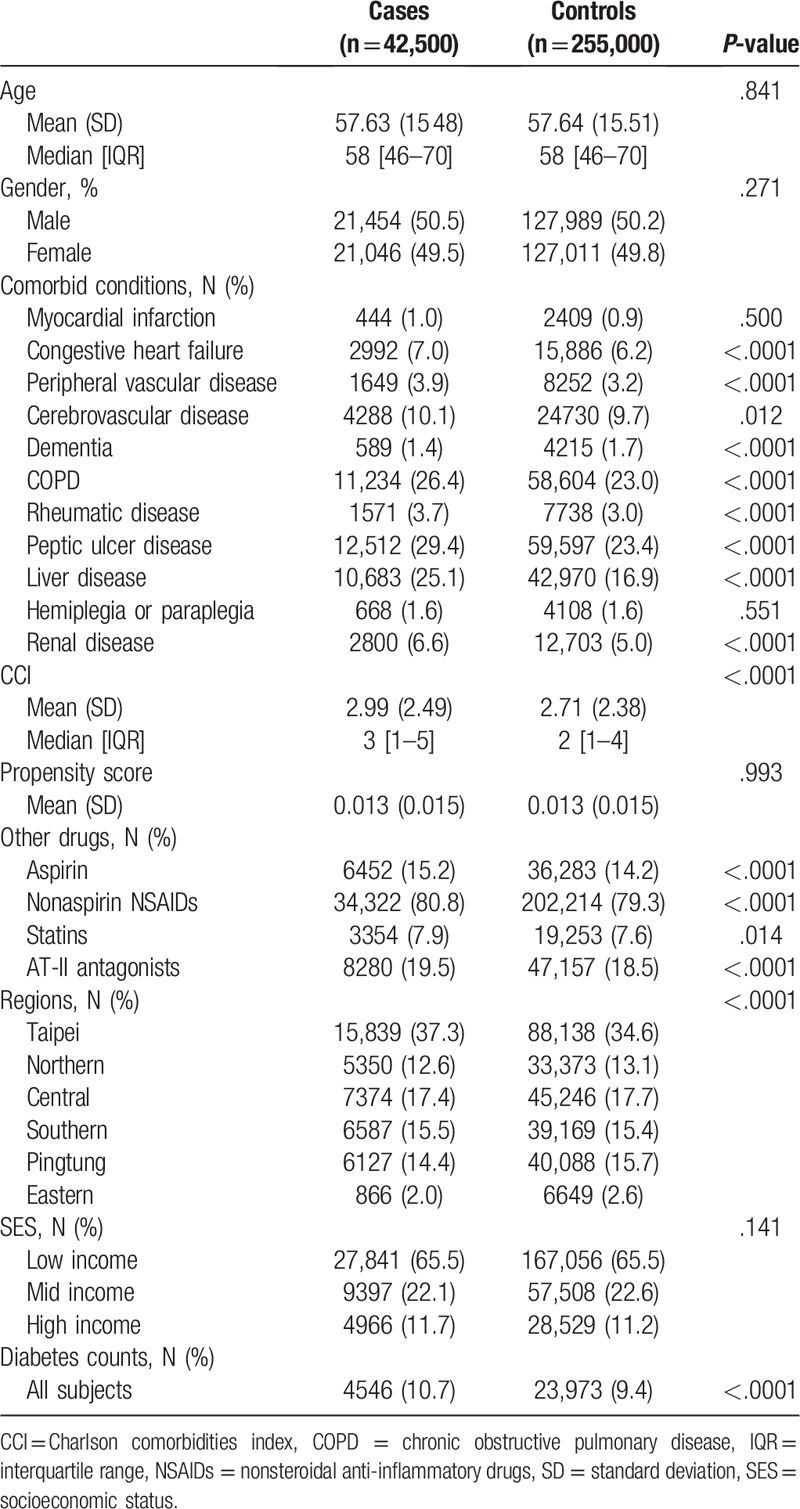
Since the chance of cancer can be confounded by competing risks, we identified comorbidities that may be associated with mortality based on diagnostic codes from outpatient data sets prior to the outcome of interest. All diseases were included in the Charlson comorbidity index (CCI) and analyzed, except for human immunodeficiency virus.[17]
Additionally, other confounding factors could influence the risk of some cancers, such as location (i.e., Regions) and SES (i.e., based on the total amounts of payment to Taiwan's National Health Insurance), so these were also accounted for in our study.
2.5. Data analysis
We analyzed the data through a classic case–control study design, with cases being cancer affected and controls being cancer-free subjects. All antidiabetic drugs’ use was measured in both cases and controls 3 years before the date of diagnosis/index date. Conditional logistic regression was applied to investigate the association between exposure to the different drugs and risk of cancer. Our interest was to identify individual antidiabetics drugs or antidiabetic drug classes that were associated with cancer incidence. The results were expressed as adjusted odds ratios (AORs) with 95% confidence intervals (CIs).
We used SPSS v.20·0 (IBM Corp, Armonk, NY) to perform data analysis, and results were expressed as the estimated numbers together with 95% CI. Statistical power was estimated over 0.9, under type I error rate at 0.05, and the minimum detectable odds ratio of 1.10.
2.6. Ethical approval
The ethical approval was not required as we used anonymous data. This type of study did not require the Institutional Review Board approval according to the policies of the National Health Research Institutes which provides large computerized deidentified data (http://nhird.nhri.org.tw/en/). This study contained unidentifiable living individual medical information, that the informed consent is not needed.
3. Results
3.1. Study sample
Among the 297,500 patients in the study, 42,500 patients had cancer, whereas 255,000 patients did not, during the study period. Baseline patient characteristics are shown in Table 1. The mean (standard deviation) of their CCI was 2.99 (2.49) for the cases and 2.71 (2.38) for the controls, respectively. The prevalence of comorbidities and other drugs used in the cases were significantly higher than in the controls group except for myocardial infarction, cerebrovascular disease, hemiplegia/paraplegia, and the statins use. The difference in prevalence of diabetes in cases as compared to controls was 1.30%. Even though this difference is significant, it does not appear to be practically relevant. The distribution of diabetics among cases and controls across different cancers is shown in Table 1, also confirming that proportions of diabetics among cases and controls are rather balanced.
3.2. Antidiabetic drugs use and cancer risk
We found that the use of pioglitazone and insulin and its analogs for injection, intermediate or long-acting combined with fast-acting insulin significantly increased cancer risk compared to diabetics who did not use them. These results held no matter how long a patient was exposed to the drugs within the 3 years before their cancer diagnosis. In contrast, both adjusted hazard ratios and AORs provide no evidence that metformin (AOR [95% CI], 1.00 [0.93–1.07]), glibenclamide (AOR [95% CI], 0.98 [0.92–1.05]), glipizide (AOR [95% CI], 0.92 [0.85–0.99]), and insulins and analogs for injection, fast acting (AOR [95% CI], 1.04 [0.89–1.22]) were associated with an elevated cancer risk compared to nonusers are shown in Table 2. Notably, based on the AOR, there is significant evidence of glipizide acting as protective against cancer which, however, requires further investigation. Overall, it appears that pioglitazone and insulin and its analogs for injection, intermediate or long acting combined with fast-acting insulin drive the elevated risk of cancer when all antidiabetics were combined for the analysis.
Table 2.
Antidiabetic drugs associated with cancer risk.
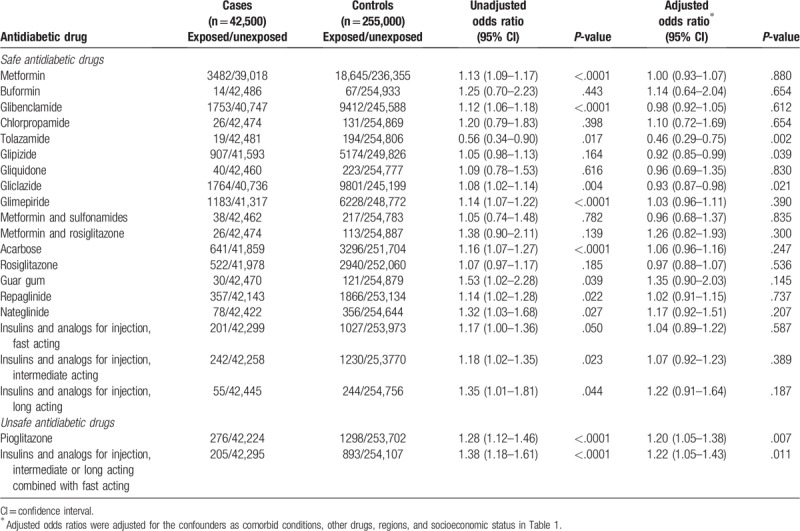
Figure 1 presents further detailed risks for specific cancers that are associated with antidiabetic medications exposure. We observed a high risk for pancreatic (AOR, 1.45; 95% CI, 1.08–1.95), liver (AOR, 1.32; 95% CI, 1.19–1.47), and lung (AOR, 1.18; 95% CI, 1.05–1.33) among antidiabetic medication users. However, cervical cancer (AOR, 0.77; 95% CI, 0.61–1.98) were observed not associated with antidiabetic drugs exposure. We did not find any significant association for ovarian (AOR, 0.98; 95% CI, 0.67–1.44), stomach (AOR, 1.04; 95% CI, 0.86–1.26), and renal cancer (AOR, 1.14; 95% CI, 0.92–1.41). Again, the increased risk of individual cancers can be driven by specific medications, such as pioglitazone as documented above with respect to all cancers, rather than by the entire group of considered antidiabetic medications.
Figure 1.

Overall antidiabetic drugs and their association with specific cancer risk.
3.3. Antidiabetic drug exposure, dose, and cancer risk
We calculated the LOE to individual drugs as well as classes of antidiabetic drugs as shown in Appendix file (see Supplemental Tables S2 and S3). Antidiabetic medication use made the risk of all cancers 1.10 times greater for cases as compared to controls (AOR, 1.10; 95% CI, 1.06–1.14).
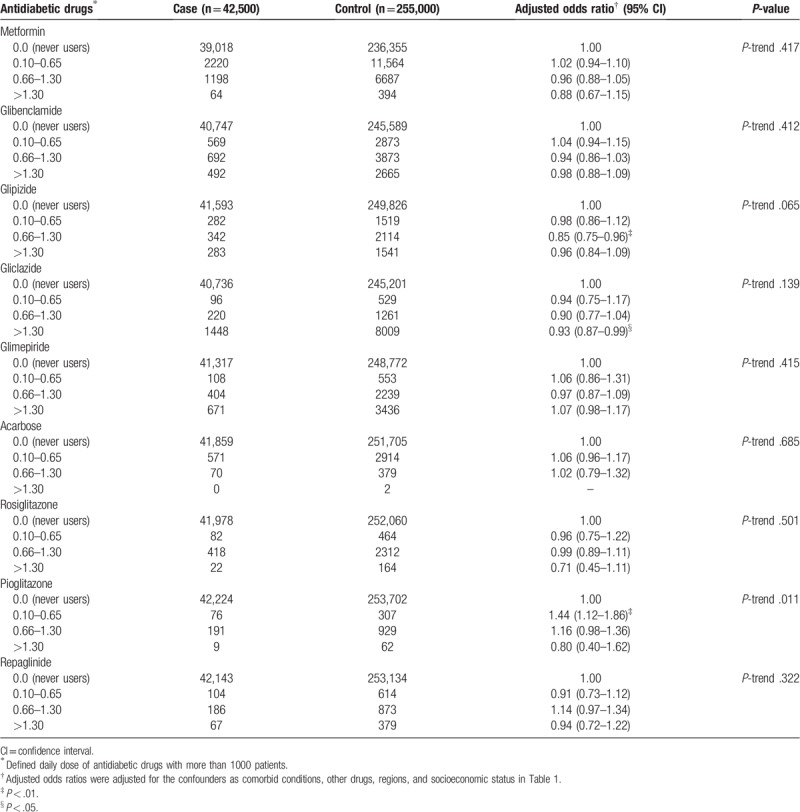
In addition, the multivariate-AORs for metformin accordingly to the DDD, as compared with no antidiabetic medication use were 1.02 (95% CI, 0.94–1.10) for a dose 0.10 to 0.65 DDD, 0.96 (95% CI, 0.88–1.05) for 0.66 to 1.30 DDD, and 0.88 (95% CI, 0.67–1.15) for higher than 1.30 DDD (P-trend = 0.417), as shown in Table 3.
Table 3.
The classification of define daily dose for antidiabetic drugs.
4. Discussion
We reported the evidence that overall antidiabetic drugs are associated with an increased cancer risk based on the case–control study. We evaluated both oral and injectable antidiabetic drugs individually and as pharmacologic classes (also accounting for both LOE and DDD) with respect to carcinogenicity. The results have been obtained on the basis of a single Taiwanese population and further studies on alterative populations would be advisable for a valid generalization. We observed that the long-term use of antidiabetic drugs was not significantly associated with increased cancer risk except for 2 drugs: Pioglitazone (AOR [95% CI], 1.20 [1.05–1.38]), and the drugs classified as insulins and analogs for injection, intermediate or long acting combined with fast acting (AOR [95% CI], 1.22 [1.05–1.43]) (Table 2). There is evidence that glipizide may act as protective against cancer (AOR [95% CI], 0.92 [0.85–0.99]). This study is important in assessing associations between antidiabetic medications and the risk of cancer using a national database.
Although the results of an observational study should be interpreted with caution, LOE and dose-dependent association provide evidence in favor of a true association between prescription of antidiabetic drugs and cancer risk. While the results from observational studies are not as robust compared to randomized control trials, the evidence obtained from an appropriately designed observational studies should not be underestimated and can be used as a guide for both further scientific investigation and clinical practice.[13,18,19]
Overall, we found that most antidiabetic drugs are not associated with cancer risk. Libby and colleagues[20] reported that metformin use was associated with a reduced cancer risk. Evans et al[21] reported that there might be a biologically plausible mechanism for the dose–response relationship between metformin use and cancer risk reduction, which is the fact that, many clinical and preclinical studies have shown that metformin has the potential to inhibit the growth of cancer cells growth in vitro and in vivo.[22,23] However, we did not find any significant reduction in cancer risk for long term use of metformin.
A striking finding in our study was that pioglitazone from the thiazolidinediones drug class and insulin's and analogs for injection, intermediate or long acting combined with fast-acting drug class were both associated with an elevated cancer incidence. Ferrara et al,[24] Koro et al,[25] and Dormandy et al[26] reported no association between pioglitazone and cancer risk. These studies findings contradict with our study findings as we observed that pioglitazone is associated with a 53% increase in cancer risk in the Taiwanese population. Our findings supported by Ramos-Nino et al,[27] Chen et al,[28] and Piccinni et al[29] who all reported similar findings.
The clinical trials evidence regarding insulin use and cancer risk is also inconclusive.[30] Hemkens et al[11] in a cohort study from Germany reported that there is a dose–response relationship between the synthetic insulin glargine and cancer risk compared to human insulin. Currie et al[4] and his colleagues study from United Kingdom reported a 40% higher risk of all cancers was associated with the use of insulin-based regimens. The studies done by Jonasson et al[31] on Swedish and by Colhoun[32] in a Scottish population reported no significant association between insulin glargine alone or in combination with other drugs and cancer risk. Yang et al[33] observed a decreased cancer risk with insulin use in the Chinese population. The US FDA[34] has also cautioned clinicians not to respond rapidly to the trial results mentioned above, stating to the public that further analysis is required to evaluate the safety. We observed in the Taiwanese population that only 1 insulin (i.e., insulins and analogs for injection, intermediate or long acting combined with fast acting) among other insulins is significantly associated with cancer risk. Based on our study, it is possible that insulins and their analogs may be associated with increased risk of cancer in Taiwanese population; however, further evidence is needed before any definitive conclusion can be drawn.
Overall, the exposure to antidiabetic medications raised the overall cancer risk up to 52%, even though these association appears to be driven by pioglitazone and insulin and its analogs for injection, intermediate or long acting combined with fast-acting insulin. Broken down by specific cancers, antidiabetic medications raised the risk of liver cancer (AOR, 1.32), of lung cancer (AOR, 1.18), and of pancreatic cancer (AOR, 1.45) (Fig. 1). Interestingly, we found (AOR, 0.77), a reduced risk for cervical cancer in our study. No significant effects were observed for bladder, esophagus, and prostate cancer. The observed pattern of odds ratios for various cancers could be attributable to an expected normal distribution, and the large sample size provides an opportunity to find significant results. However, these findings are very important since several studies reported contradictory results and randomized control trails have failed to define clear associations. A retrospective study from the United Kingdom by Currie and colleagues[4] reported a lower risk of colon and pancreatic cancer risk with metformin use but observed no effect on breast and prostate cancer. However, they also observed that insulin or insulin secretagogues users were more likely to develop cancers compared to metformin users. Moreover, in this study they also observed that the use of insulin analogs was not associated with increased cancer risk when compared to human insulin. A meta-analysis by DeCensi et al[35] epidemiologic studies reported an inverse association between pancreatic and hepatocellular cancer and no significance for colon, breast, and prostate cancer. This contradicts with our findings that there is a 60% greater risk for pancreatic cancer and 57% greater risk for liver cancer; however, it is supported by Li et al[36] that insulin or insulin secretagogue use associated with increased pancreatic cancer risk. Murtola et al[37] reported decreased prostate cancer risk with an overall or ever use of any antidiabetic drugs however, we did not find any association for prostate cancer with antidiabetic drugs. We suggest that the therapeutic effectiveness and pharmacologic mechanism of antidiabetic drugs should be monitored closely in relation to multiple medications and diseases for carcinogenicity on a large population.
We aim to properly investigate the documented association of pioglitazone and insulin analogs vs specific cancer in further study. As for now, the presented findings could facilitate clinicians to inform their patient about overall risk for cancer with respect to specific drugs and implement prevention measures.
Together with the strengths of our population-wide study, we have several limitations, such as residual bias, although NHIRD data are now used for both billing and quality purpose which could persist owing to unmeasured or imprecisely measured potential confounding factors (e.g., life style, family history, smoking status, etc). Moreover, observational studies tend to be less robust compared to randomized control trial studies, but it still contains real-world data on prescriptions and diagnoses for the whole Taiwanese population where all prescriptions are continuously documented, allowing unbiased exposure assessment (i.e., no recall bias). There is significant difference between case and control. We knew that the indication is the important confounder for this study but we cannot find without diabetes subjects using diabetic drugs. Therefore, this is only association study and the drug might increase or decrease the cancer risk. We found association only but not causality. For causality, we need to have randomized control trials; however, the significant association provide hint for researchers to re-evaluate with trials. Several approaches have been proposed to prevent immortal time bias in observational studies, and we controlled it matching by year and we only assigned the date of cancer diagnosis to control group.
We do not have hemoglobin A1c levels of patients which is the limitation of our database and we were also not able to access some drugs, such as DDP-4, as it has been added recently in 2011 to NHI Taiwan. However, Elashoff and colleagues[38] examined the US FDA's database of adverse events and found that sitagliptin or exenatide, which is a DPP-4 inhibitor antidiabetic drug, increases the risk for specific cancers. Moreover, a dose–response effect is not always evident. The drug exposure days are provided for reference only, which might not provide accurate reflection of drugs taken by patients as directed. In this study, we observed antidiabetic drugs exposure and evidence of association, but not their mechanism and metabolism related to cancer, which could be also be seen as a limitation. Therefore, further animal or cellular model are needed to help in identifying a possible biologic mechanism linking antidiabetic drugs with cancer risk. Finally, in the reported study, we assessed only the specific antidiabetic drugs (i.e., 24 different drugs) vs overall risk of cancer (i.e., 10 different cancers combined) and exposure to any of antidiabetic drugs (24 drugs combined) vs specific cancers. We did not look into each specific drug vs each specific cancer as it would generate 24 × 10 = 240 association estimates that are problematic to present and interpret clearly in this study. Moreover, the associations of multiple drugs and cancers were performed and corrections for multiple comparisons were not performed, resulting in a potential for some apparent associations to be due to chance.
5. Conclusion
We found evidence that the only antidiabetic drugs associated with cancer risk were pioglitazone and insulin analogs for injection, intermediate or long acting combined with fast acting among all antidiabetic drugs. We observed that glipizide may act in a protective way against cancer.
Findings from this study provide incremental evidence regarding the long-term influence of antidiabetic medications on cancer risk as measured by DDD and LOE to individual drugs as well as antidiabetic drug classes.
Randomized controlled trials are always expensive and sometimes impractical because of cost, study duration, or ethical concerns, but it is important to investigate further the pharmacologic activity of antidiabetic drugs for cancer risk. We conducted the retrospective observational study as a plausible alternative to a randomized controlled trial, documenting objective evidence and providing ground for further scientific investigation.
Author contributions
Conceptualization: Yi-Chun Liu, Min-Huei Hsu, Yu-Chuan (Jack) Li, Wen-Shan Jian, Usman Iqbal.
Data curation: Yi-Chun Liu, Phung-Anh Nguyen, Shuo-Chen Chien, Rahma Novita Asdary, Shabbir Syed-Abdul, Min- Huei Hsu, Yun Yen, Yu-Chuan (Jack) Li, Wen-Shan Jian.
Formal analysis: Phung-Anh Nguyen, Shuo-Chen Chien, Hsuan- Chia Yang, Min-Huei Hsu, Yun Yen, Usman Iqbal.
Funding acquisition: Yu-Chuan (Jack) Li, Wen-Shan Jian, Usman Iqbal.
Investigation: Phung-Anh Nguyen, Ayesha Humayun, Hsuan-Chia Yang, Min-Huei Hsu, Yun Yen, Usman Iqbal.
Methodology: Wen-Shan Jian, Phung-Anh Nguyen, Usman Iqbal, Yu-Chuan (Jack) Li.
Project administration: Wen-Shan Jian, Usman Iqbal.
Resources: Rahma Novita Asdary, Usman Iqbal.
Software: Hsuan-Chia Yang, Usman Iqbal, Yu-Chuan (Jack) Li.
Supervision: Yu-Chuan (Jack) Li, Usman Iqbal, Wen-Shan Jian, Min-Huei Hsu, Yun Yen.
Validation: Phung-Anh Nguyen, Max Moldovan, Yu-Chuan (Jack) Li, Usman Iqbal.
Visualization: Hsuan-Chia Yang, Max Moldovan, Wen-Shan Jian, Usman Iqbal.
Writing – original draft: Usman Iqbal, Phung-Anh Nguyen.
Writing – review & editing: Yi-Chun Liu, Hsuan-Chia Yang, Rahma Novita Asdary, Shabbir Syed-Abdul, Min-Huei Hsu, Max Moldovan, Yu-Chuan (Jack) Li, Wen-Shan Jian.
Rahma Novita Asdary orcid: 0000-0001-9444-040X.
Supplementary Material
Footnotes
Abbreviations: AOR = adjusted odds ratio, ATC = anatomical therapeutic chemical, CCI = Charlson comorbidity index, CI = confidence interval, DDD = defined daily dose, FDA = Food and Drug Administration, ICD-9-CM = International Classification of Disease, Clinical Modification, Ninth Revision, IDF = International Diabetes Federation, LOE = length of exposure, NHIRD = National Health Insurance Research Database, NSAIDs = nonsteroidal anti-inflammatory drugs.
How to cite this article: Liu YC, Nguyen PA, Humayun A, Chien SC, Yang HC, Asdary RN, Syed-Abdul S, Hsu MH, Moldovan M, Yen Y, Li YC, Jian WS, Iqbal U. Does long-term use of antidiabetic drugs changes cancer risk? Medicine. 2019;98:40(e17461).
YCL, WSJ, and UI are equal co-corresponding authors.
This research is in part of supported by Ministry of Science of Technology project number MOST107-2218-E-038-004-MY and as a part of Taipei Medical University project number TMU105-AE1-B54 and YUAN's hospital project number 107YGH-TMU-10.
The authors have no conflicts of interest to disclose.
Supplemental Digital Content is available for this article.
References
- [1].Emami-Riedmaier A, Schaeffeler E, Nies AT, et al. Stratified medicine for the use of antidiabetic medication in treatment of type II diabetes and cancer: where do we go from here? J Intern Med 2015;277:235–47. [DOI] [PubMed] [Google Scholar]
- [2].IDF DIABETES ATLAS Sixth edition 2013. Available at: https://www.idf.org/sites/default/files/EN_6E_Atlas_Full_0.pdf Accessed October 20th, 2016. [Google Scholar]
- [3].Shi Y, Hu FB. The global implications of diabetes and cancer. Lancet (London, England) 2014;383:1947–8. [DOI] [PubMed] [Google Scholar]
- [4].Currie CJ, Poole CD, Gale E. The influence of glucose-lowering therapies on cancer risk in type 2 diabetes. Diabetologia 2009;52:1766–77. [DOI] [PubMed] [Google Scholar]
- [5].Bodmer M, Becker C, Meier C, et al. Use of antidiabetic agents and the risk of pancreatic cancer: a case–control analysis. Am J Gastroenterol 2012;107:620–6. [DOI] [PubMed] [Google Scholar]
- [6].Nissen SE, Wolski K. Effect of rosiglitazone on the risk of myocardial infarction and death from cardiovascular causes. N Engl J Med 2007;356:2457–71. [DOI] [PubMed] [Google Scholar]
- [7].Simons C, Schouten L, Godschalk R, et al. Body size, physical activity, genetic variants in the insulin-like growth factor pathway, and colorectal cancer risk. Carcinogenesis 2015;36:971–81. [DOI] [PubMed] [Google Scholar]
- [8].Call R, Grimsley M, Cadwallader L, et al. Insulin–carcinogen or mitogen? Preclinical and clinical evidence from prostate, breast, pancreatic, and colorectal cancer research. Postgrad Med 2010;122:158–65. [DOI] [PubMed] [Google Scholar]
- [9].Ljung R, Talbäck M, Haglund B, et al. Insulin glargine use and short-term incidence of breast cancer - a four-year population-based observation. Acta Oncol 2012;51:400–2. [DOI] [PubMed] [Google Scholar]
- [10].Suissa S, Azoulay L, Dell’Aniello S, et al. Long-term effects of insulin glargine on the risk of breast cancer. Diabetologia 2011;54:2254–62. [DOI] [PubMed] [Google Scholar]
- [11].Hemkens L, Grouven U, Bender R, et al. Risk of malignancies in patients with diabetes treated with human insulin or insulin analogues: a cohort study. Diabetologia 2009;52:1732–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Li C, Zhao G, Okoro CA, et al. Prevalence of diagnosed cancer according to duration of diagnosed diabetes and current insulin use among US adults with diagnosed diabetes findings from the 2009 Behavioral Risk Factor Surveillance System. Diabetes Care 2013;36:1569–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Hsing AW, Ioannidis JA. Nationwide population science: lessons from the taiwan national health insurance research database. JAMA Intern Med 2015;175:1527–9. [DOI] [PubMed] [Google Scholar]
- [14].Lu J-FR, Hsiao WC. Does universal health insurance make health care unaffordable? Lessons from Taiwan. Health Aff 2003;22:77–88. [DOI] [PubMed] [Google Scholar]
- [15].Iqbal U, Nguyen P-A, Syed-Abdul S, et al. Is long-term use of benzodiazepine a risk for cancer? Medicine 2015;94:e483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Pottegård A, Friis S, Andersen M, et al. Use of benzodiazepines or benzodiazepine related drugs and the risk of cancer: a population-based case-control study. Br J Clin Pharmacol 2013;75:1356–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Charlson ME, Pompei P, Ales KL, et al. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis 1987;40:373–83. [DOI] [PubMed] [Google Scholar]
- [18].Anglemyer A, Horvath HT, Bero L. Healthcare outcomes assessed with observational study designs compared with those assessed in randomized trials The Health well: Systematic Reviews, Cochrane Reviews; 2014. Available at: http://www.thehealthwell.info/node/763371 Accessed: September 10th, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Concato J, Shah N, Horwitz RI. Randomized, controlled trials, observational studies, and the hierarchy of research designs. N Engl J Med 2000;342:1887–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Libby G, Donnelly LA, Donnan PT, et al. New users of metformin are at low risk of incident cancer - a cohort study among people with type 2 diabetes. Diabetes Care 2009;32:1620–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Evans JMM, Donnelly LA, Emslie-Smith AM, et al. Metformin and reduced risk of cancer in diabetic patients. BMJ 2005;330:1304–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Sahra IB, Laurent K, Loubat A, et al. The antidiabetic drug metformin exerts an antitumoral effect in vitro and in vivo through a decrease of cyclin D1 level. Oncogene 2008;27:3576–86. [DOI] [PubMed] [Google Scholar]
- [23].Buzzai M, Jones RG, Amaravadi RK, et al. Systemic treatment with the antidiabetic drug metformin selectively impairs p53-deficient tumor cell growth. Cancer Res 2007;67:6745–52. [DOI] [PubMed] [Google Scholar]
- [24].Ferrara A, Lewis JD, Quesenberry CP, et al. Cohort study of pioglitazone and cancer incidence in patients with diabetes. Diabetes Care 2011;34:923–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Koro C, Barrett S, Qizilbash N. Cancer risks in thiazolidinedione users compared to other anti-diabetic agents. Pharmacoepidemiol Drug Saf 2007;16:485–92. [DOI] [PubMed] [Google Scholar]
- [26].Dormandy JA, Charbonnel B, Eckland DJ, et al. Secondary prevention of macrovascular events in patients with type 2 diabetes in the PROactive Study (PROspective pioglitAzone Clinical Trial In macroVascular Events): a randomised controlled trial. Lancet 2005;366:1279–89. [DOI] [PubMed] [Google Scholar]
- [27].Ramos-Nino ME, MacLean CD, Littenberg B. Association between cancer prevalence and use of thiazolidinediones: results from the Vermont Diabetes Information System. BMC Med 2007;5:17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Chen S-W, Tsan Y-T, Chen J-D, et al. Use of thiazolidinediones and the risk of colorectal cancer in patients with diabetes - a nationwide, population-based, case-control study. Diabetes Care 2013;36:369–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Piccinni C, Motola D, Marchesini G, et al. Assessing the association of pioglitazone use and bladder cancer through drug adverse event reporting. Diabetes Care 2011;34:1369–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Home P, Lagarenne P. Combined randomised controlled trial experience of malignancies in studies using insulin glargine. Diabetologia 2009;52:2499–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Jonasson J, Ljung R, Talbäck M, et al. Insulin glargine use and short-term incidence of malignancies—a population-based follow-up study in Sweden. Diabetologia 2009;52:1745–54. [DOI] [PubMed] [Google Scholar]
- [32].Colhoun H. Use of insulin glargine and cancer incidence in Scotland: a study from the Scottish Diabetes Research Network Epidemiology Group. Diabetologia 2009;52:1755–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Yang X, Ko GT, So WY, et al. Associations of hyperglycemia and insulin usage with the risk of cancer in type 2 diabetes: the Hong Kong diabetes registry. Diabetes 2010;59:1254–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Early communication about safety of Lantus (inslin glargine): US Food and Drug Administration; 2009. Available at: http://www.fda.gov/Drugs/ Accessed October 24th, 2015. [Google Scholar]
- [35].DeCensi A, Puntoni M, Goodwin P, et al. Metformin and cancer risk in diabetic patients: a systematic review and meta-analysis. Cancer Prev Res 2010;3:1451–61. [DOI] [PubMed] [Google Scholar]
- [36].Li D, Yeung SCJ, Hassan MM, et al. Antidiabetic therapies affect risk of pancreatic cancer. Gastroenterology 2009;137:482–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Murtola TJ, Tammela TL, Lahtela J, et al. Antidiabetic medication and prostate cancer risk: a population-based case-control study. Am J Epidemiol 2008;168:925–31. [DOI] [PubMed] [Google Scholar]
- [38].Elashoff M, Matveyenko AV, Gier B, et al. Pancreatitis, pancreatic, and thyroid cancer with glucagon-like peptide-1-based therapies. Gastroenterology 2011;141:150–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


