Abstract
Aims
Solid papillary breast carcinoma resembling the tall cell variant of papillary thyroid neoplasms (BPTC), also known as solid papillary carcinoma with reverse polarity, is a rare histologic type of breast cancer that morphologically resembles the tall cell variant of papillary thyroid carcinoma. BPTCs are characterized by IDH2 R172 hotspot somatic mutations or mutually exclusive TET2 somatic mutations, concurrently with mutations affecting PI3K pathway-related genes. We sought to characterize their histology, and investigate the frequency of IDH2 and PIK3CA mutations in an independent cohort of BPTCs, as well as in conventional solid papillary carcinomas (SPCs).
Methods and Results
Six BPTCs, not previously analyzed molecularly, and 10 SPCs were centrally reviewed. Tumor DNA was extracted from microdissected histological sections, and subjected to Sanger sequencing of the IDH2 R172 hotspot locus and exons 9 and 20 of PIK3CA. All six BPTCs were characterized by solid, papillary and follicular architecture with circumscribed, invasive tumor nodules composed of epithelial cells with reverse polarity. IDH2 mutations were identified in all six BPTCs (3 R172S, 2 R172T, and 1 R172G), four of which also harbored PIK3CA mutations (2 H1047R, 1 Q546K, and 1 Q546R). By contrast, all SPCs lacked IDH2 mutations, whilst 1/10 harbored a PIK3CA mutation (H1047R).
Conclusion
We validated the presence of IDH2 R172 hotspot mutations and PIK3CA hotspot mutations in 100% and 67% BPTCs tested, respectively, and documented absence of IDH2 R172 mutations in SPCs. These findings confirm the genotypic-phenotypic correlation previously reported in BPTC, which constitutes an entity distinct from conventional SPC.
INTRODUCTION
Solid papillary breast carcinoma resembling the tall cell variant of papillary thyroid neoplasms (BPTC),1 also known as breast tumor resembling the tall cell variant of papillary thyroid carcinoma,2,3 or solid papillary carcinoma with reverse polarity,4 is a rare and distinctive subtype of invasive breast cancer. First recognized because of striking similarities with papillary thyroid carcinomas (PTCs),3 including nuclear diagnostic features (e.g., nuclear grooves and intra-nuclear pseudo-inclusions), BPTCs were further catalogued under different terminologies, all of which are synonyms. Despite histologic similarities to papillary thyroid neoplasms, clinical, immunohistochemical and genetic data indicate that BPTCs are primary breast tumors, given lack of expression of thyroid-specific markers, including TTF1 and thyroglobulin,3,5,6 and somatic genetic alterations typically found in PTCs, including those affecting RET/PTC and BRAF.3,6–8
Histologically, BPTCs are characterized by circumscribed tumor nodules lacking a peripheral layer of myoepithelial cells growing in solid, papillary and/ or follicular architectural patterns.1,5,6 The term ‘reverse polarity’ stems from the tumor cells harboring nuclei at the apical rather than the basal pole, thereby creating the impression of reverse polarization.4 The polarization of the cells, however, is preserved, with expression of MUC1 being found at the apical luminal border.4 To date, forty such tumors have been reported.1–6 BPTCs are mostly of triple-negative phenotype, with a third being weakly ER-positive/ HER2-negative.1,3–6 Clinically, the majority of BPTCs display an indolent biological behavior with an excellent prognosis; only two reported cases displayed axillary lymph node metastasis.1,3,5,6
We previously demonstrated that BPTCs constitute a discrete subtype of breast cancer underpinned by highly recurrent IDH2 R172 hotspot somatic mutations or TET2 loss-of-function somatic mutations, alongside PI3K-pathway related genes.4 As in leukemias,9 IDH2 and TET2 mutations were found to be mutually exclusive in BPTCs,4 functioning as alternative genetic drivers. In vitro studies demonstrated that expression of IDH2 mutations in non-malignant breast epithelial cells results in a phenotype closely recapitulating that of BPTCs. Whilst, Bhargava et al. recently described IDH2 R172 mutations in two of three additional BPTCs,2 to the best of our knowledge, IDH2 R172 mutations, which are common in hematological malignancies and brain tumors,10 have not been described in other breast cancer subtypes.11–13 Here, we sought to characterize the unique histology of BPTCs further and investigate the frequency of IDH2 and PIK3CA hotspot mutations in an independent series of BPTCs, as well as in conventional solid papillary carcinomas (SPCs) to define their potential genetic relatedness.
METHODS
Cohort
After obtaining approval by the IRBs and the local research ethics committees from the authors’ institutions, six BPTCs, not previously molecularly analyzed, and ten SPCs were retrieved, centrally reviewed by three breast pathologists (F.C.G., F.P., and E.B.), and anonymized prior to analysis. Three cases were previously reported by Foschini et al.1 (BPTC14, BPTC15, BPTC17).
Microdissection and DNA extraction
Representative sections of formalin-fixed paraffin-embedded BPTCs and SPCs were subjected to microdissection to ensure >80% tumor content, as previously described,14,15 and DNA was extracted.
Immunohistochemistry (IHC)
IHC data (cytokeratin (CK) 5/6, p63 and/or calponin) from BPTC14, BPTC15, BPTC17 was retrieved from Foschini et al.1 Representative histological sections of BPTC18, BPTC19, BPTC20 were analyzed for CK5/6, p63, and/or smooth muscle myosin heavy chain, as previously described16 (Supplementary Table S1). Positive and negative controls were included in each slide run. ER, PR and HER2 IHC data were retrieved from original pathology reports for all cases.
Sanger sequencing analysis
The IDH2 R172 hotspot residue and exons 9 and 20 of PIK3CA were investigated in the cohort of BPTCs and SPCs by Sanger sequencing, as previously described (Supplementary Table S2).15,17
RESULTS
Clinicopathologic characteristics of BPTC
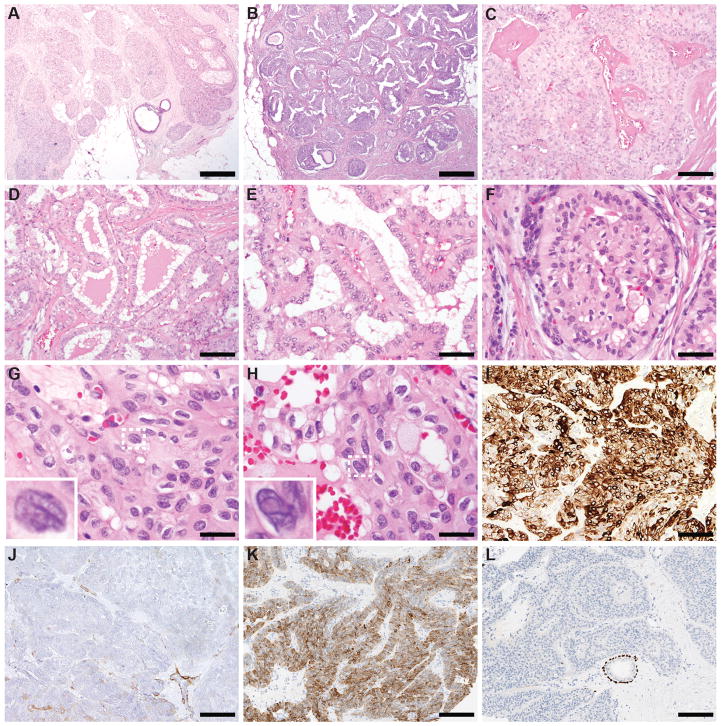
The six BPTCs occurred in female patients, with a median age at diagnosis of 60 years (range 58–85 years). Tumor size ranged from 0.6 cm to 5.0 cm. The histologic features were consistent across all cases (Figure 1, Supplementary Figure S1). All BPTCs were multi-lobulated and characterized by solid and papillary architecture, with circumscribed and invasive tumor nodules composed of epithelial cells displaying reverse polarity. Follicle-like structures with colloid-like secretion were also focally present. The nuclei displayed grade 2 pleomorphism, with occasional grooves and intra-nuclear pseudo-inclusions. The six BPTCs were of histologic grade 1 and of triple-negative phenotype (Supplementary Table S3), expressed CK5/6 diffusely, and lacked a myoepithelial cell layer (Figure 1I–L; Supplementary Table S3).
Figure 1. Morphological features of solid papillary breast carcinomas resembling the tall cell variant of papillary thyroid neoplasms (BPTCs).
A, B) Low-power magnification of BPTC18 (A) and BPTC17 (B), displaying lobulated contours, without an overt infiltrative pattern (20x magnification, scale bar 1mm). C) Solid and papillary architecture, with evident fibrovascular cores, often with perivascular hyalinized stroma, characterized all BPTCs included in this study (BPTC14, 100x magnification, scale bar 200μm). D) Diffuse or focal follicle-like structures with colloid-like secretion were also found in all cases (BPTC15, 100x magnification, scale bar 200μm). E, F) Reverse polarity characterized by apical localization of the nuclei and enhanced cytoplasmic granularity in the basal part, was also observed in all cases (E, BPTC15; F, BPTC19; both 200x magnification, scale bar 100μm). G, H) All cases displayed grade 2 nuclear atypia, with irregularities of the nuclear membrane, nuclear grooves (G, BPTC19, 400x magnification, scale bar 50μm) and intra-nuclear pseudo-inclusions (H, BPTC19, 400x magnification, scale bar 50μm). In G and H, the highlighted nuclei with dashed lines are shown in the inset. I, J) BPTC19 diffusely expressed CK5/6 (I, 100x magnification, scale bar 200μm) and lacked a myoepithelial cell layer as shown by immunohistochemistry for smooth muscle myosin heavy chain (J, note a vessel wall as internal positive control, 100x magnification, scale bar 200μm). K, L) BPTC20 diffusely expressed CK5/6 (K, 100x magnification, scale bar 200μm) and lacked a myoepithelial cell layer as shown by immunohistochemistry for p63 (J, note the normal duct as internal positive control, 100x magnification, scale bar 200μm).
BPTCs harbor highly recurrent mutations affecting IDH2 and PIK3CA
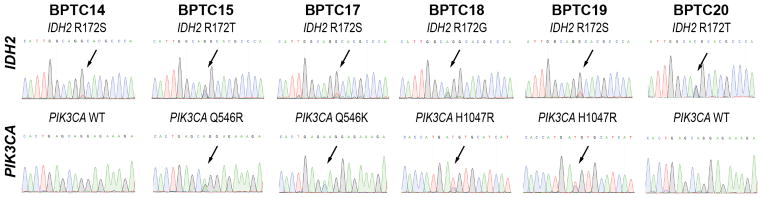
Given that our previous study4 identified highly recurrent mutations affecting the IDH2 R172 hotspot residue and PIK3CA hotspots in BPTCs, we sought to define the frequencies of these mutations in an independent set of BPTCs. Sanger sequencing analysis revealed high mutation frequencies in both genes (Figure 2). IDH2 R172 hotspot mutations were found in 100% of BPTCs (6/6; 3 R172S, 2 R172T, and 1 R172G). PIK3CA hotspot mutations were found in 67% of BPTCs (4/6; 2 H1047R, 1 Q546K, and 1 Q546R). These findings are consistent with our previous study, which identified IDH2 R172 hotspot and PIK3CA hotspot mutations in 77% and 69% of BPTCs, respectively, and concurrent IDH2 R172 hotspot and PIK3CA mutations in 62% of BPTCs.4 In contrast, all conventional SPCs lacked IDH2 R172 hotspot mutations (0/10 versus 6/6 BPTCs, p<0.001, Fisher’s exact test), and 10% harbored a PIK3CA hotspot mutation (H1047R; 1/10 versus 4/6 BPTCs, p=0.035, Fisher’s exact test; Supplementary Figure S2).
Figure 2. IDH2 R172 hotspot and PIK3CA hotspot mutations identified by Sanger sequencing analysis in solid papillary breast carcinomas resembling the tall cell variant of papillary thyroid neoplasms (BPTCs).
Representative sequence electropherograms of the IDH2 R172 hotspot and exons 9 and 20 of PIK3CA identified by Sanger sequencing in the six BPTCs studied.
DISCUSSION
Here we confirm that BPTC constitutes a rare histologic type of breast cancer, with unique morphologic features and underpinned by highly recurrent IDH2 R172 mutations alongside PIK3CA mutations. Yet to be recognized as a special type of breast cancer by the WHO classification of breast tumors,18 our previous4 and current findings, together with those by Bhargava et al.2, establish BPTC as a distinct histologic type of invasive breast carcinoma, driven by mutually exclusive genetic alterations affecting the IDH2 R172 hotspot residue or TET2, in conjunction with mutations in PI3K pathway-related genes. Using in vitro studies, we have previously shown that expression of mutant IDH2 in PIK3CA-mutant breast epithelial cells results in the characteristic phenotype of BPTC.4 Whilst PI3K pathway-related gene mutations are common in breast cancer,11–13 IDH2 mutations are vanishingly rare or non-existent in other breast tumors.11–13 Indeed, the conventional SPCs analyzed here lacked IDH2 R172 mutations, consistent with their driver role in the pathogenesis of BPTCs, and with the notion that BPTC should not be perceived as a histologic variant of SPC.
The mechanism by which IDH2 contributes to tumorigenesis has yet to be fully understood. IDH2 mutations result in loss of catalytic activity and production of α-ketoglutarate (α-KG) and gain of new activity with production of the oncometabolite R-2-hydroxylglutarate (R-2-HG).19–22 R-2-HG competitively inhibits activity of α-KG-dependent dioxygenases, altering genome-wide histones and DNA methylation and resulting in abnormal epigenetic regulation, cell differentiation, and tumorigenesis.21,22 Notably, IDH2-mutant BPTCs display a hypermethylated profile and higher expression levels of H3K27me3 compared to conventional forms of invasive breast cancer.4 Importantly, however, the mechanism by which IDH2 R172 hotspot mutations or TET2 inactivating mutations alongside PI3K-pathway-related gene mutations result in phenotypic features similar to those found in BPTC remains to be defined.
The identification of BPTC in clinical practice carries important implications. BPTCs ought to be distinguished from conventional ER-positive SPCs, but still recognized as a primary invasive neoplasm of the breast requiring complete surgical excision. Whilst its infiltrative nature may not be best ascertained with immunohistochemistry,1 its immunoprofile with CK5/6 expression and non-homogeneous cytomorphology may be interpreted as evidence of a hyperplastic process.2 Interestingly, a link between BPTC and infiltrating epitheliosis, a complex sclerosing lesion with overt, CK5/6-positive epithelial proliferation,16 has been hypothesized due to similarities in immunoprofile.2 Whilst recurrent PIK3CA mutations have been documented in infiltrating epitheliosis, IDH2 gene status has yet to be investigated.16 Furthermore, BPTCs ought to be distinguished from breast secretory carcinomas, which display a similar triple-negative phenotype and CK5/6 expression. Finally, although the number of BPTCs with adequate follow-up is limited, their outcome appears to be excellent despite the triple-negative or weakly ER-positive phenotype.1,2 Hence, patients with BPTCs are likely adequately managed without adjuvant systemic therapy. Given that IDH2 R172 mutations are likely pathognomonic of BPTC in the context of breast carcinomas, their identification may be used as a confirmatory molecular finding.
This study has several limitations. First, given the rarity of BPTC, the number of cases analyzed is small. Despite this, we validated the high frequency of IDH2 and PIK3CA mutations. Second, our results are limited to the selected IDH2 and PIK3CA hotspot residues, hence we cannot exclude the possibility of additional concurrent genetic alterations in BPTCs, such as a concurrent mutation in a PI3K-pathway related gene in the PIK3CA wild-type cases. Finally, further analyses of larger cohorts are warranted to define the driver genetic alterations of BPTCs lacking IDH2 or TET2 mutations.
Despite these limitations, our study confirmed the histologic and genetic features that characterize BPTCs, validating the high frequencies of mutations affecting IDH2 and PIK3CA previously reported4, and demonstrating the genotypic-phenotypic correlation that characterizes this unique breast cancer subtype of favorable prognosis. As somatic mutations affecting IDH2 R172 are vanishingly rare in other breast tumors, they may be employed as ancillary molecular markers in the diagnosis of BPTC.
Supplementary Material
Acknowledgments
Research reported in this paper was supported in part by the Breast Cancer Research Foundation, a Cancer Center Support Grant of the National Institutes of Health/National Cancer Institute (grant No P30CA008748), and by the National Research, Development and Innovation Office (Hungary) grant GINOP-2.3.2-15-2016-00020. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Competing Interests:
The authors declare no competing financial interests.
AUTHORS’ CONTRIBUTIONS
F.C.G., E.B., B.W., and J.S.R.-F. conceived the study. P.Q., G.C., M.P.F., E.A.R., E.B. and D.C.G. provided tissue samples and clinical data. F.C.G., F.P., and E.B. performed pathology review. J.R.L., T.B.O., F.C.G., B.A., A.C.P and R.G.M. performed experiments. J.R.L., T.B.O., and F.C.G. analyzed and interpreted the data. J.R.L., F.C.G., and J.S.R.-F. wrote the first manuscript, which was reviewed by all co-authors.
CONFLICTS OF INTEREST
The authors have no conflicts of interest to declare.
References
- 1.Foschini MP, Asioli S, Foreid S, et al. Solid papillary breast carcinomas resembling the tall cell variant of papillary thyroid neoplasms: A unique invasive tumor with indolent behavior. Am J Surg Pathol. 2017;41:887–895. doi: 10.1097/PAS.0000000000000853. [DOI] [PubMed] [Google Scholar]
- 2.Bhargava R, Florea AV, Pelmus M, et al. Breast tumor resembling tall cell variant of papillary thyroid carcinomaa solid papillary neoplasm with characteristic immunohistochemical profile and few recurrent mutations. Am J Clin Pathol. 2017;147:399–410. doi: 10.1093/ajcp/aqx016. [DOI] [PubMed] [Google Scholar]
- 3.Eusebi V, Damiani S, Ellis IO, Azzopardi JG, Rosai J. Breast tumor resembling the tall cell variant of papillary thyroid carcinoma: Report of 5 cases. Am J Surg Pathol. 2003;27:1114–1118. doi: 10.1097/00000478-200308000-00008. [DOI] [PubMed] [Google Scholar]
- 4.Chiang S, Weigelt B, Wen HC, et al. Idh2 mutations define a unique subtype of breast cancer with altered nuclear polarity. Cancer research. 2016;76:7118–7129. doi: 10.1158/0008-5472.CAN-16-0298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Colella R, Guerriero A, Giansanti M, Sidoni A, Bellezza G. An additional case of breast tumor resembling the tall cell variant of papillary thyroid carcinoma. Int J Surg Pathol. 2015;23:217–220. doi: 10.1177/1066896914536222. [DOI] [PubMed] [Google Scholar]
- 6.Masood S, Davis C, Kubik MJ. Changing the term “breast tumor resembling the tall cell variant of papillary thyroid carcinoma” to “tall cell variant of papillary breast carcinoma”. Adv Anat Pathol. 2012;19:108–110. doi: 10.1097/PAP.0b013e318249d090. [DOI] [PubMed] [Google Scholar]
- 7.Hameed O, Perry A, Banerjee R, Zhu X, Pfeifer JD. Papillary carcinoma of the breast lacks evidence of ret rearrangements despite morphological similarities to papillary thyroid carcinoma. Mod Pathol. 2009;22:1236–1242. doi: 10.1038/modpathol.2009.91. [DOI] [PubMed] [Google Scholar]
- 8.Cameselle-Teijeiro J, Abdulkader I, Barreiro-Morandeira F, et al. Breast tumor resembling the tall cell variant of papillary thyroid carcinoma: A case report. Int J Surg Pathol. 2006;14:79–84. doi: 10.1177/106689690601400116. [DOI] [PubMed] [Google Scholar]
- 9.Figueroa ME, Abdel-Wahab O, Lu C, et al. Leukemic idh1 and idh2 mutations result in a hypermethylation phenotype, disrupt tet2 function, and impair hematopoietic differentiation. Cancer Cell. 2010;18:553–567. doi: 10.1016/j.ccr.2010.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dang L, Yen K, Attar EC. Idh mutations in cancer and progress toward development of targeted therapeutics. Ann Oncol. 2016;27:599–608. doi: 10.1093/annonc/mdw013. [DOI] [PubMed] [Google Scholar]
- 11.Cancer Genome Atlas Network. Comprehensive molecular portraits of human breast tumours. Nature. 2012;490:61–70. doi: 10.1038/nature11412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ciriello G, Gatza ML, Beck AH, et al. Comprehensive molecular portraits of invasive lobular breast cancer. Cell. 2015;163:506–519. doi: 10.1016/j.cell.2015.09.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pereira B, Chin SF, Rueda OM, et al. The somatic mutation profiles of 2,433 breast cancers refines their genomic and transcriptomic landscapes. Nat Commun. 2016;7:11479. doi: 10.1038/ncomms11479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Piscuoglio S, Ng CK, Murray M, et al. Massively parallel sequencing of phyllodes tumours of the breast reveals actionable mutations, and tert promoter hotspot mutations and tert gene amplification as likely drivers of progression. J Pathol. 2016;238:508–518. doi: 10.1002/path.4672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Guerini-Rocco E, Piscuoglio S, Ng CK, et al. Microglandular adenosis associated with triple-negative breast cancer is a neoplastic lesion of triple-negative phenotype harbouring tp53 somatic mutations. J Pathol. 2016;238:677–688. doi: 10.1002/path.4691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Eberle CA, Piscuoglio S, Rakha EA, et al. Infiltrating epitheliosis of the breast: Characterization of histological features, immunophenotype and genomic profile. Histopathology. 2016;68:1030–1039. doi: 10.1111/his.12897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lozada JR, Burke KA, Maguire A, et al. Myxoid fibroadenomas differ from conventional fibroadenomas: A hypothesis-generating study. Histopathology. 2017 doi: 10.1111/his.13258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lakhani SR, Ellis IO, Schnitt SJ, Tan PH, van de Vijver MJ. Who classification of tumours of the breast. Lyon, France: IARC Press; 2012. [Google Scholar]
- 19.Dang L, White DW, Gross S, et al. Cancer-associated idh1 mutations produce 2-hydroxyglutarate. Nature. 2009;462:739–744. doi: 10.1038/nature08617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ward PS, Patel J, Wise DR, et al. The common feature of leukemia-associated idh1 and idh2 mutations is a neomorphic enzyme activity converting alpha-ketoglutarate to 2-hydroxyglutarate. Cancer Cell. 2010;17:225–234. doi: 10.1016/j.ccr.2010.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yang H, Ye D, Guan K-L, Xiong Y. Idh1 and idh2 mutations in tumorigenesis: Mechanistic insights and clinical perspectives. Clin Cancer Res. 2012;18:5562–5571. doi: 10.1158/1078-0432.CCR-12-1773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ye D, Xiong Y, Guan K-L. The mechanisms of idh mutations in tumorigenesis. Cell Res. 2012;22:1102–1104. doi: 10.1038/cr.2012.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.