Abstract
Junctions that connect the endoplasmic reticulum (ER) and the plasma membrane (PM) are unique yet ubiquitous subcellular compartments. Giordano et al. now report that extended synaptotagmins (E-Syts) promote their formation, providing fundamental insight into the molecular machinery controlling ER and plasma membrane crosstalk.
An important concept in cell biology is that proteins and phospholipids in the plasma membrane organize into specialized regions that facilitate and specify cell signaling and other cellular processes. Endoplasmic reticulum (ER)-plasma membrane (PM) junctions are small circular or spatially extended compartments in which the ER and PM membranes are stably kept at a distance of about 10 to 20 nm. Contacts between the ER and plasma membrane were first described in muscle in the 1950s (Porter and Palade, 1957). Although these and similar connections have been recognized for several decades, it is only recently that functional roles of ER-PM junctions have started to emerge. Calcium (Ca2+) homeostasis is the best-characterized cellular function of ER-PM junctions, although nonvesicular lipid transfer has also been investigated (Carrasco and Meyer, 2011). In skeletal muscle, voltage-dependent Ca2+ channels in the plasma membrane and ryanodine receptor channels in the sarcoplasmic reticulum can bind to each other at such ER-PM junctions to generate the increase in cytosolic Ca2+ concentration needed for contraction. The discovery of stromal interaction molecule (STIM) proteins as the ER-localized lumenal sensors for store-operated Ca2+ entry and their translocation into puncta near the plasma membrane as the ER Ca2+ stores are emptied provided a unique inside-out signaling mechanism from the lumen of the ER to the PM (Lewis, 2011; Liou et al., 2005). Further work has established that ER-localized STIM1 can directly interact with PM PI(4,5)P2 lipids to enhance the formation of the ER-PM junction as well as with PM-localized Orai Ca2+ channels to induce store-operated Ca2+ influx into cells (Carrasco and Meyer, 2011; Lewis, 2011; Walsh et al., 2010).
In this issue of Cell, Giordano et al. (2013) describe a new role for the members of the extended synaptotagmins (E-Syts) family of proteins in tethering the ER to the plasma membrane. The molecular functions of the three human homologs of these proteins have thus far been elusive. As their name implies, the sequences of E-Syts are similar to those of synaptotagmins, which are best known for their role in exocytosis (Pang and Südhof, 2010). Synaptotagmins, as well as the three E-Syts, contain multiple C2 domains, which often target proteins to the plasma membrane in a Ca2+-dependent manner, as exemplified by conventional protein kinase C (PKC) and synaptotagmin-1. In other cases, C2 domains are Ca2+ insensitive or can be targeted to internal membranes. Despite these structural similarities, Giordano et al. (2013) now show that the proteins differ in fundamental ways and uncover a unique function for E-Syts. The study, built on previous light microscopy analysis that characterized the subcellular localization of E-Syts by overexpression, showed that E-Syt1 localizes to intracellular compartments, whereas E-Syt2 and E-Syt3 colocalize with the plasma membrane (Min et al., 2007). The authors now determine that all E-Syts closely colocalize with ER markers and that the reported plasma membrane localization of E-Syt2 and E-Syt3 is in fact due to an E-Syt-mediated tethering of sections of the ER to the PM. A potential role of E-Syts in promoting the formation of ER-PM junctions is also supported by recent studies in yeast showing that tricalbins, homologs of E-Syts, have a similar role in tethering the ER to the plasma membrane (Manford et al., 2012; Toulmay and Prinz, 2012).
Analogous to the ER-PM junction targeting of STIM1 protein (Walsh et al., 2010), the interaction of E-Syt2 and E-Syt3 with the plasma membrane depended on PI(4,5)P2 because induced depletion of plasma membrane PI(4,5)P2 reversed the association of E-Syt2 and E-Syt3 with the plasma membrane. Mutations and deletions in E-Syt2 and E-Syt3 established the role of the C2C domain in targeting the proteins to the plasma membrane through binding to PI(4,5)P2 (Figure 1). In contrast to E-Syt2 and E-Syt3, E-Syt1 localized to intracellular ER but translocated to ER-PM junctions only after an increase in intracellular Ca2+ concentration. The authors further show that the plasma membrane association of E-Syt1 occurs through a Ca2+-dependent binding of the C2C domain to PI(4,5)P2 (Figure 1). Nevertheless, as a note of caution, discerning the subcellular localization of endogenous E-Syts has thus far been hampered by the difficulties of producing specific antibodies for immunocytochemistry (Giordano et al., 2013; Min et al., 2007).
Figure 1. ER-PM Tethering.
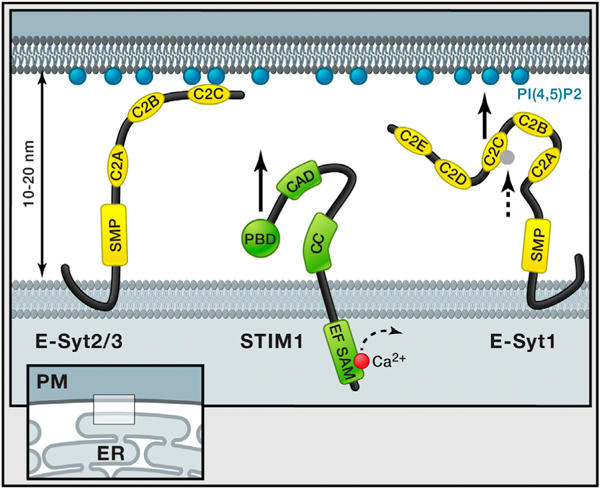
ER-PM junctions are ubiquitous cell compartments In which the two membranes are stably kept at a distance of 10 to 20 nm. Giordano et al. (2013) show that three ER-localized proteins, E-Syts, play an important role in tethering the ER to the plasma membrane. Overexpression of E-Syt2 and E-Syt3 connects large regions of the ER to the plasma membrane mediated by E-Syt C2C domains that bind plasma membrane PI(4,5)P2 independent of Ca2+. In contrast, E-Syt1 is at basal Ca2+ levels not associated with the plasma membrane, but, as the intracellular Ca2+ level increases, the C2C domain will bind Ca2+ (dashed arrow), resulting in binding to PIP2 in the plasma membrane (black arrow) and trapping of E-Syt1 at the ER-PM junction. This is very different from the regulation of STIM1, for which previous studies showed that STIM1 translocation to these same ER-PM junctions is mediated by lowering of lumenal ER Ca2+ levels (illustrated with dashed arrow), which triggers its oligomerization and the exposure of a PI(4,5)P2 binding and an Orai interaction region (black arrow). Giordano et al. (2013) further show that the previously predicted transmembrane region at the N terminus of E-Syts forms a hairpin insertion in the ER membrane, making both N and C termini accessible to the cytosol. All E-Syts have a cytosolic SMP domain, whereas E-Syt1 has five C2 domains compared to three for E-Syt2 and E-Syt3. Domains depicted on STIM1; EF, EF hand; SAM, sterile α motif; CC, coiled-coil; CAD, CRAC activation domain; and PBD, polybasic domain.
An attractive hypothesis for the function of E-Syts is that they stabilize ER-PM junctions throughout the cell to enable STIM-ORAI interaction and store operated Ca2+ influx. However, this does not seem to be the case because small interfering RNA (siRNA) knockdown of all three E-Syts does not have a significant effect on Ca2+ influx. Thus, although E-Syts—and possibly also STIM proteins—may have a role in supporting the tethering of the ER to the plasma membrane, there are likely still additional components that have a complementary tethering function. Such alternative tethering mechanisms may not be restricted to interactions of ER proteins with PI(4,5)P2, as overexpressed STIM1 proteins can connect to the plasma membrane either through binding to PI(4,5)P2 lipids or by binding to PM-localized Orai, suggesting that protein-protein interaction between other ER and PM proteins could contribute to the formation of ER-PM junctions. Given the structural organization of E-Syts that includes conserved regions not required for tethering, it is also likely that E-Syts may have yet-unknown signaling roles at ER-PM junctions.
In conclusion, the study of Giordano et al. (2013) shows that three ER-localized E-Syts play an important role in tethering the ER to the plasma membrane in a PI(4,5)P2- and—in the case of E-Syt1—a Ca2+-dependent manner. This adds a key piece to the puzzle of how ER-PM junctions are created and maintained and how they might be regulated over time. At the same time, the study renews the question as to whether these ER-PM junctions have additional roles beyond Ca2+ signaling, with the possibility that E-Syts are founding members of ER-PM junction-localized mediators of alternative signaling processes.
ACKNOWLEDGMENTS
T.M. was supported by NIH grant GM030179, and S.M. was supported by the Swedish Society for Medical Research (SSMF) Postdoctoral Fellowship.
REFERENCES
- Carrasco S, and Meyer T (2011). Annu. Rev. Biochem. 80, 973–1000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giordano F, Saheki Y, Idevall-Hagren O, Colombo S, Pirruccello M, Milosevic I, Gracheva EO, Bagriantsev SN, Borgese N, and De Camilli P (2013). Cell 153, this issue, 1494–1509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis RS (2011). Cold Spring Harb. Perspect. Biol. 3, a003970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liou J, Kim ML, Heo WD, Jones JT, Myers JW, Ferrell JE, and Meyer T (2005). Curr. Biol. 15, 1235–1241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manford AG, Stefan CJ, Yuan HL, Macgurn JA, and Emr SD (2012). Dev. Cell 23, 1129–1140. [DOI] [PubMed] [Google Scholar]
- Min SW, Chang WP, and Südhof TC (2007). Proc. Natl. Acad. Sci. USA 104, 3823–3828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pang ZP, and Südhof TC (2010). Curr. Opin. Cell Biol. 22, 496–505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porter KR, and Palade GE (1957). J. Biophys. Biochem. Cytol. 3, 269–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toulmay A, and Prinz WA (2012). J. Cell Sci. 125, 49–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walsh CM, Chvanov M, Haynes LP, Petersen OH, Tepikin AV, and Burgoyne RD (2010). Biochem. J. 425, 159–168. [DOI] [PMC free article] [PubMed] [Google Scholar]


