Abstract
The master clock of the biological rhythm, located in the suprachiasmatic nucleus of the anterior hypothalamus, synchronizes the molecular biological clock found in every cell of most peripheral tissues. The human circadian rhythm is largely based on the light-dark cycle. In night shift workers, alteration of the cycle and inversion of the sleep-wake rhythm can result in disruption of the biological clock and induce adverse health effects. This paper offers an overview of the main physiological mechanisms that regulate the circadian rhythm and of the health risks that are associated with its perturbation in shift and night workers. The Occupational Physician should screen shift and night workers for clinical symptoms related to the perturbation of the biological clock and consider preventive strategies to reduce the associated health risks.
Keywords: Circadian rhythm, Biological clocks, Shift work schedule, Rotating shift work, Night shift work, Chronobiology disorders, Desynchronization of circadian rhythms, Health survey
Introduction
Life on Earth has adapted to the planet’s 24-h rotation; the human biological rhythm is also circadian (Latin, circa diem). The 2017 Nobel Prize in Medicine and Physiology was awarded to Hall, Rosbash, and Young for their discoveries on the biological mechanisms that regulate the circadian clock. In the 1980s, they isolated in Drosophila melanogaster a gene (period) that regulates the daily biological rhythm. The gene encodes a protein (PER) that is produced during biological daytime and is subsequently degraded1, 2).
In humans, nearly all cells of the body contains its own molecular circadian clock3). The entrainment of each of these clocks to the 24-h day is based on the signal received from a master clock located in the suprachiasmatic nucleus (SCN) of the anterior hypothalamus. The master clock consists of ca. 10,000 neurons and is itself synchronized to 24-h by information on environmental light received from intrinsically photosensitive retinal ganglion cells (ipRGCs) that are not involved in vision, which contain the photopigment melanopsin4, 5). The function of the endogenous biological clock is to generate the circadian rhythms by entraining them to the external light-dark cycle. Accordingly, cortisol secretion and the rise in core body temperature take place in daytime, whereas melatonin secretion and sleep take place during the night6). These patterns occur when the circadian system is normally entrained to the 24-h light-dark cycle and are altered or not present in shift workers, those who travel across time zones, or in blind individual who have lost ipRGC signaling7, 8).
Functioning of the Biological Clock
The light-dark cycle is the main zeitgeber (German, time giver) acting on the SCN9, 10). However, the circadian periodicity is ensured even in the absence of environmental light cues (free-running rhythm)11, 12). The ability of the biological clock to generate self-sustaining rhythms relies on the presence in cells of genes called “clock genes” which generate an oscillation of the duration of about 24 h by transcription/translation feedback loops11, 13). The main loop consists of two transcriptional activators, CLOCK (Circadian Locomotor Output Cycles Kaput) and BMAL (Brain and Muscle ARNT-Like protein), and the target genes period and cryptochrome, which code for the expression of their own protein products, respectively PER1, PER2, and PER3 and CRY1 and CRY2. Notably, CLOCK and BMAL1 accumulate in the cytoplasm and form CLOCK-BMAL1 heterodimers which translocate to the nucleus, where they bind the E-box sequences of period and cryptochrome genes, activating their expression. During the night, PER and CRY proteins accumulate in the cytoplasm and translocate to the nucleus, inhibiting the CLOCK-BMAL1 complex. In the early morning, their degradation by casein kinase suppresses the inhibition, enabling the resumption of CLOCK and BMAL1 transcription, and allowing a new cycle to begin. Another feedback loop regulates bmal1 gene transcription via REV-ERBα and RORα, which act respectively as a repressor and an activator13, 14).
The clock genes are not localized exclusively to the SCN, but are also found in the cells of nearly all peripheral tissues (peripheral clock genes, PCGs), where they regulate the expression of several clock-controlled genes (CCGs)15).
Outputs of the Biological Clock
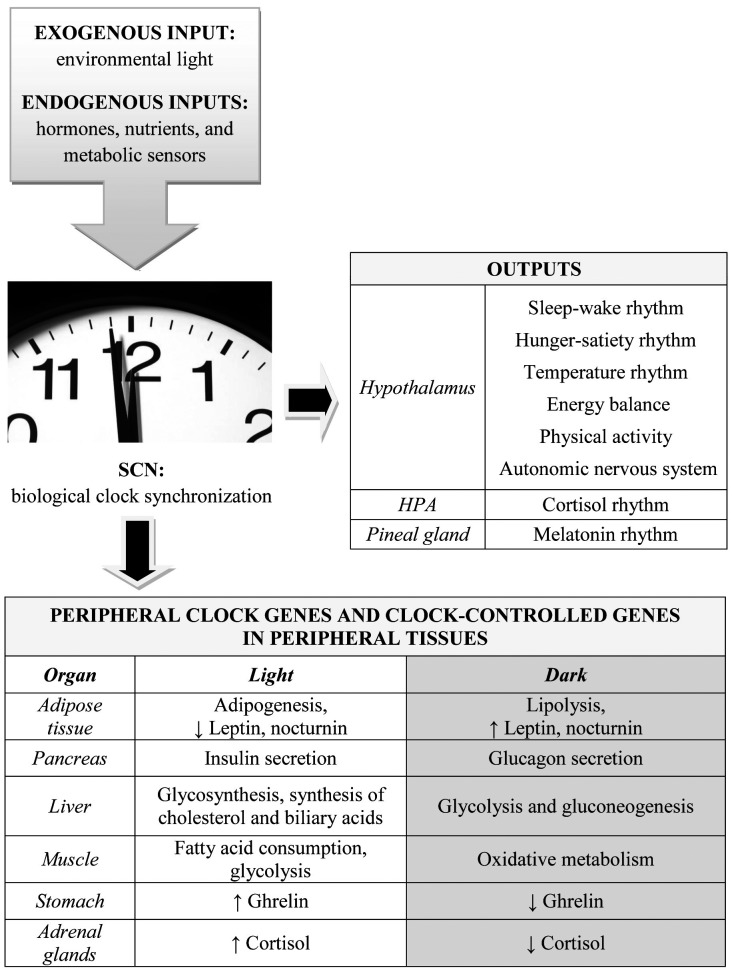
The main function of the biological clock is to regulate the circadian rhythms by integrating with other cerebral and extracerebral nervous centers, to ensure system homeostasis (Fig. 1). In humans there are two main circadian cycles, the sleep-wake and the hunger-satiety cycle, which are closely integrated because the awake phase coincides with feeding and the sleep phase coincides with satiety and fasting.
Fig. 1.
The suprachiasmatic nucleus (SCN) receives exogenous environmental luminosity information through the retinohypothalamic tract as well as endogenous information from hormones (insulin, ghrelin, leptin), nutrients (amino acids, glucose, fatty acids) and metabolic sensors (NAD+/SIRT1/AMPK). The SCN provides several outputs from the hypothalamic nuclei, HPA axis, and pineal gland. It also regulates metabolism, energy expenditure, and hormones in peripheral tissues though hierarchical control of peripheral clock genes and clock-controlled genes.
Central outputs: the sleep-wake cycle
The sleep-wake cycle is determined by a process that gauges the need for sleep as a proportion of the duration of wake (process S) and by a circadian process (process C) that regulates the temporal distribution of wake and sleep independently of the duration of wake. Sleep in process S is ensured by “time windows” of sleep propensity between 9:30 p.m. and 11:30 p.m. (main window) and between 2 p.m. and 4 p.m. (secondary window)16). This rhythm of sleep propensity is ensured by the reciprocal transmission of data and information between the SCN and extra-SCN regions that play important roles in circadian control17,18,19,20,21,22):
• hypothalamic nuclei in the lateral area (LHA) which produce orexin, a neuropeptide that is critical for the wake phase23),
• nuclei making up the ascending reticular formation between the midbrain and the medulla oblongata, which also participate in wake and alertness24),
• the dorsomedial hypothalamic nucleus, which through the ventrolateral preoptic nucleus regulates the sleep phase18, 25).
The SCN exchanges information with the hypothalamic-pituitary-adrenal (HPA) axis and regulates the circadian expression patterns of cortisol26), thyroid-stimulating hormone, somatotropic hormone, and other hormones27) through the paraventricular hypothalamic nucleus (PVN). Finally, its anatomical and functional relationship with the pineal gland allows the SCN to regulate its circadian melatonin production28).
Box 1. Assessment of the clinical symptoms related to sleep-wake cycle perturbation
When assigning night shifts:
• workers should be assessed for chronic sleep disturbances being treated with hypnotics, anxiolytics, or antidepressants,
• night workers should be assessed for excessive daytime sleepiness due to lack of sleep.Diagnostic tests include polysomnography29, 30) or actigraphy31,32,33) and psychometric tests such as:
• the Stanford Sleepiness Scale34), which is commonly used to evaluate short-term changes in sleepiness;
• the Leeds Sleep Evaluation Questionnaire35), which has been developed specifically to assess the effects on sleep of psychoactive drugs in general and of sedative hypnotic agents in particular;
• the Sleep Disorders Questionnaire36), to diagnose sleep disturbances and their severity in the previous six months;
• the Pittsburgh Sleep Quality Index37), a self-rating scale developed to provide a reliable, sound and standardized measure of sleep quality. This tool distinguishes between “good” and “bad” sleepers and has clinical use in evaluating the different aspects of sleep that can compromise rest;
• the Epworth Sleepiness Scale38), which measures the level of daytime sleepiness.Assessment of the circadian rhythm type (or chronotype) is important for clinicians. The Morningness Eveningness Questionnaire39) is the most widely used self-rating tool to establish the circadian type of adults.
Central outputs: the hunger-satiety cycle
Humans eat their meals at regular that characterize the daily schedules of organizations and families alike. A zeitgeber role has been advanced for food nutrients, which would provide a sort of input to the peripheral clocks: some researchers have suggested the existence of a “food-entrainable oscillator” residing in the brain, whereas others hold that it is an integral part of the biological clock found in the SCN40, 41).
A third loop, characterized by activation of the gene nampt by the CLOCK-BMAL1 complex, has recently been described in the biological clock42,43,44). Nampt controls the biosynthesis of nicotinamide adenine dinucleotide (NAD+), which in turn regulates the activity of the protein sirtuin 1 (SIRT1), which affects clock and bmal1 transcription. NAD+ and SIRT1, together with AMPK (activated protein kinase), act as metabolic sensors, gauging cellular energy and nutrient requirements and sending their signals to the SCN through the hypothalamic arcuate nucleus (ARC)45), to anticipate energy and nutrient depletion46).
During the night the increase of leptin induces an anorexigenic action deactivating neurons that synthesize anorexigenic neuropeptides. Leptin also suppresses melanocyte concentrating hormone, an anorexigenic hormone produced by LHA hypothalamic nuclei40). Conversely, the morning ebb of leptin secretion results in immediate activation of the neurons producing the orexigenic neuropeptides45).
Further mechanisms are involved in feeding patterns besides leptin signaling:
• the PVN, which is anatomically and functionally related with the ARC and the SCN, is responsible for the central control of feeding requirements through the measurement of the energy used by muscle activity18); it also regulates metabolic and energy activity in peripheral tissues through its direct connection to the autonomic nervous system47, 48) and controls HPA axis activity via direct innervation27),
• the ventromedial hypothalamic nucleus is also connected to the ARC and regulates food intake and glycemic homeostasis48).
Box 2. Assessment of the clinical symptoms related to hunger-satiety cycle perturbation
The possible effects of hunger-satiety cycle perturbation in shift workers is related to the interaction of two factors49,50,51):
• changes in meal times
• the hunger experienced during night shifts, which leads to food intake.
Qualitative and quantitative information on the eating habits of shift workers can be collected by direct interviews or through a food diary52). Deviations from healthy eating can be corrected by formulating diets appropriate to the shift work or by asking companies to provide healthier food in canteens and vending machines53,54,55).
Unhealthy eating habits can promote overweight, obesity, and cardiovascular diseases (CVDs) like type 2 diabetes (T2D), dyslipidemia, metabolic syndrome (MS) and myocardial infarction, and further affect the poor sleep patterns of shift workers56, 57).
Central outputs: metabolic and energy control
The information reviewed above highlights the important role played by the SCN, LHA, and ARC in controlling food intake and sleep-wake patterns. The ARC integrates short- and long-term hunger and satiety signals45). The expression of metabolic hormone receptors (e.g. insulin, leptin, and ghrelin) is peripheral information sensed by the ARC and relayed to the central nervous system (CNS). Signals from nutrients and energy sensors (NAD+, SIRT1, and AMPK), located in peripheral cells and controlled by clock genes, trigger the demand for food and indicate which processes affecting the energy balance should be activated40, 45, 47, 48). The circadian alignment of food intake and physical activity is essential for the control of body weight11, 58). The orexin neurons in the LHA are involved both in alertness and in glucose metabolism23).
Box 3. Assessment of the clinical symptoms related to perturbation of metabolic and energy control
Metabolic control can be adversely affected by night work and can promote conditions such as overweight and obesity, T2D, dyslipidemia and MS. Periodic assessment of parameters such as weight, abdominal circumference, body mass index (BMI), fasting glycemia, glycosylated hemoglobin, triglycerides and total, HDL and LDL cholesterol enables swift balance restoration in case of pathological changes56, 59, 60).
The amount of energy used by basal metabolism and physical activity can change in individuals with poor eating habits, especially if they are associated with a sedentary lifestyle, as described in shift workers61, 62).
Energy use and other parameters (e.g. sleep duration) can be monitored by a metabolic Holter monitor worn for a few days63, 64).
Central outputs: core body temperature
The SCN is connected to the medial preoptic region, where the centers that control core body temperature ensure the equilibrium of heat production and dissipation65). Core body temperature also oscillates according to a circadian rhythm in relation to environmental light66). Its trend subsumes all cortisol-activated ergotropic functions, including metabolic activity; values tend to rise in the morning, they peak at 4–5 p.m., and decline again, especially during the biological night, with a trough at 2–5 a.m. that ensures adequate night rest65, 66). The night-time temperature reduction is due to a greater peripheral heat dissipation through the skin and to a low metabolic rate, related to reduced energy requirements65, 67).
Central outputs: hormone secretion
The SCN is connected to the pineal gland through the sympathetic nervous system. Light inhibits signal transmission to the gland, hence melatonin production, until the night, when it peaks around 2–4 a.m.68). Melatonin has been defined as a key that opens the doors to sleep16, 69>). It is involved in a number of cell functions, exerting antioxidant, antiestrogenic and immunomodulatory activities28, 70, 71>). The wake state coincides with HPA axis activation by the SCN and production of the ergotropic hormone cortisol27). The SCN controls, via the PVN, the secretion of corticotropin releasing hormone, which acts on the pituitary by inducing the release of adrenocorticotropic hormone (ACTH)26); in turn, ACTH stimulates the adrenal cortex, where PCGs control cortisol production72). Cortisol secretion peaks at 6–8 a.m. and declines during the evening and night. Insulin, produced by the pancreas, reaches a postprandial acrophase 60 min after meals, then declines. Blood glucose and insulin show SCN-dependent circadian oscillations, insulin reaching its acrophase around 5 p.m.40), CCGs in the pancreas regulate insulin production73). Leptin, which is also produced according to a circadian rhythm in white adipose tissue74), exhibits low levels during the day and high nocturnal levels (acrophase around 1 a.m.). Leptin is also involved in the sleep-wake cycle, since sleep restriction induces leptin reduction75, 76).
Peripheral outputs: peripheral clock genes
Although the function of PCGs is still largely unclear, they are likely to have a role in guiding the local rhythms of each tissue15). PCGs are hierarchically subordinated to the SCN and synchronized with its rhythm46). However, they have been demonstrated to have also an autonomous rhythm, as they respond to local, tissue-specific requirements with local outputs (Fig. 1) that modulate the metabolic activity of pancreas, liver, muscle, and adipose tissue according to a circadian pattern40, 77, 78). Studies that decoupled the meal timing cycle from the light-dark cycle showed that some PCGs respond more to food than to light79, 80). PCGs in the liver participate in glucose metabolism according to a circadian rhythm81, 82). PCGs in the pancreas (β-cell clock) are involved in the secretion of insulin; those located in the gut regulate nutrient adsorption; adipose tissue PCGs control fat accumulation through lipid synthesis, and muscle PCGs regulate glucose metabolism40, 48, 73, 83).
Peripheral outputs: clock-controlled genes
CCGs, which are not capable of generating a self-sustaining circadian rhythm, are nonetheless expressed according to a circadian pattern through the hierarchical control of the SCN. CCGs, like PCGs, are involved in the circadian regulation of several processes such as cell metabolism, the control of cellular DNA growth and damage, and the response to substances like xenobiotics, medications, and alcohol77, 84). A CCG with an important role in cell metabolism is nocturnin (NOC), whose 24-h oscillation is under the control of the CLOCK-BMAL1 complex. Whereas most deadenylases are arrhythmic or have narrow rhythms that peak during the day, NOC shows high-amplitude rhythms with nocturnal peaks in mice85). The NOC gene, though not belonging to the circadian clock, regulates the circadian cycles and adjusts them to the metabolic rhythms; since it is involved in lipid metabolism, adipogenesis, glucose homeostasis, and inflammation, it could have a role in promoting metabolic alterations86,87,88). Mice whose gene has been silenced are resistant to obesity and hepatic steatosis, probably due to changes in lipid absorption by the gut or in their utilization in alternative metabolic cycles, simultaneously increasing glucose tolerance and insulin sensitivity89).
The human genome is physiologically subject to damage caused by the products of normal metabolism (radicals, products of lipid peroxidation, alkylating agents), exogenous chemicals, and physical agents90, 91). The worst damage is caused by reactive chemical species, which have been divided into those reactive to oxygen (ROS) and to nitrogen. Reactive chemical species induce DNA injury via oxidation, alkylation, nitration, and halogenation. The human organism can repair such damage in various ways. Base excision repair (BER) system identifies and removes 8-oxoguanine, the most widely investigated DNA oxidation lesion, which can induce DNA mutation90, 91). According to a recent study by our group91), only OGG1 of all BER system genes (APEX1, XRCC1, and PARP1) displays a circadian oscillation characterized by a peak at 8 a.m. and a trough at 8 p.m. Moreover, it correlates positively with period and cryptochrome and negatively with BMAL1 and REV-ERBα mRNA levels. The repair activity of the enzyme OGG1 also follows a circadian oscillation pattern; as a result, OGG1 downregulation during the night involves a slower night-time repair and enables DNA damage accumulation over time.
Box 4. Assessment of the circadian rhythms
The circadian rhythms can be assessed and any deviation identified by melatonin and cortisol dosage and by monitoring body temperature. Melatonin and cortisol can be measured in saliva, blood or urine92, 93). The major metabolite of melatonin is the 6-sulphatoxymelatonin, it can be measured in urine and is useful to assess the overnight production of melatonin94). Body temperature data can be collected with metabolic Holters or small dataloggers. Body temperature measurement should be combined with the recording of physical activity using the metabolic Holter6, 95, 96).
Peripheral clock gene and CCGs expression is a useful approach to circadian rhythm assessment and can be measured in various matrices (blood lymphocytes, hair bulb)6, 97, 98).
Perturbation of the Biological Clock
Circadian rhythm changes are determined by discrepancies between the endogenous circadian rhythm and the exogenous rhythm, which cannot be compensated for by the biological clock. The misalignment of the sleep-wake cycle due to the exogenous rhythms imposed by life commitments result in fatigue, poor job performance, and sleep disturbances, especially difficulty in falling asleep or in waking at the desired hour. Jet lag, sleep disturbances, aging, and shift and night work are major causes of circadian rhythm disruption99,100,101,102,103).
External perturbation: shift and night work
It is well established that shift and night work, its duration, and worker age affect the risk of developing some chronic disorders. Night work has been associated with abdominal adiposity, obesity, T2D, MS, CVD, cancer, and disturbed sleep59, 104,105,106,107,108,109,110,111,112,113,114,115,116,117). Lack of sleep disrupts the circadian clock, disconnecting the environmental cycle (dark-light) from the biological cycle (sleep-wake). The chief consequence is a greater frequency of sleep disturbances in shift workers99, 118), not only because they fail to rest during the biological night, but also because even shifts beginning at 6 a.m. prevent the completion of the last phase of sleep. Sleep restriction is associated with weight gain, which may be compounded by a sedentary lifestyle, and with abnormal ghrelin and leptin blood levels76, 119), which can foster a greater food intake. Alterations in clock gene are associated with obesity and MS48). The clock gene bmal is implicated in controlling adipogenesis and lipid metabolism in adipocytes120), some variants are associated with a greater sensitivity to arterial hypertension and T2D121). Changes in per2 gene expression are associated with obesity58). NOC can also promote weight gain, since its higher levels during the biological night stimulate fat absorption in the gut via chylomicrons86). Sleep-wake cycle perturbation may foster nocturnin elevation, hence greater fat absorption by enterocytes, promoting the development of obesity and metabolic disturbances. OGG1-related DNA repair may also be affected, due to a slower removal of damaged bases. In a study by our group, nurses involved in shift work showed significant OGG1 underexpression compared with day nurses91).
Exposure to artificial light during the night reduces melatonin secretion, which is quickly restored by a 24-h rest97, 122). Body temperature is controlled by the medial preoptic region, which receives SCN outputs65). Exposure to light affects thermoregulation66): during the night, increased heat dissipation through the skin induces a reduction in core body temperature, whereas the more limited dissipation during the day raises it. Heat dissipation is also an important factor in the circadian regulation of core body temperature65). Measurement of 24-h peripheral temperature found a significantly greater dissipation from 10 a.m. to 1 p.m. and from 8 to 10 p.m. in shift workers compared with day workers6), due to limited ergotropic activation associated with low cortisol values in the former and to an anticipation of the phase of sleep propensity in the latter. Desynchronization of the circadian rhythms has also been implicated in neoplastic disease (e.g. breast cancer) and CVD113, 114, 123,124,125).
The demonstration of a cause-effect relationship between shift work and chronic disease is hampered by the non-univocal definition of shift and night work and by the need for long-term studies126, 127). Moreover, a number of variables, like smoking habits, a high BMI, dietary habits, and sleep duration, affect results. Shift and night work duration and shift type are further, equally important variables. In 2007, the IARC defined shift work that induces circadian rhythm disruption as probably carcinogenic to humans (group 2A)113). In 2016, the National Toxicology Program (NTP) convened an expert panel at a public workshop entitled “Shift Work at Night, Artificial Light at Night, and Circadian Disruption” and conducted a literature-based health hazard assessment128). Persistent night shift work that causes circadian disruption is now listed as “known to be a human carcinogen” in the draft of Report on Carcinogens Monograph on Night Shift Work and Light at Night129).
Several experimental investigations have stressed that high melatonin production during the biological night is a powerful anticancer stimulus that can protect normal cells against the disease28, 69). Its suppression due to night-time exposure to artificial light has the potential to foster breast cancer development in night workers10, 112, 128, 130), also in relation to higher β-estradiol levels detected in women shift workers97). Other studies have found an association between night work and a greater CVD risk. A study of a population of more than 189,000 North-American shift woman nurses has found that greater job seniority in night work involves a greater risk of coronary heart disease. Specifically, in the younger cohort (mean age at baseline, 34.8 yr) a seniority of up to 5 yr involved a 12% higher risk, a seniority between 5 and 9 yr involved a 19% increase, and a seniority greater than 10 yr involved a 27% higher risk114).
Internal perturbation: aging
Shift and night workers who have been exposed to disruption of the circadian rhythms for years may experience accelerated aging and be at higher risk of disease. The biological clock undergoes a number of age-related changes, but whereas until recently they had been considered as part of the normal aging process, there is mounting evidence that circadian system dysfunctions can accelerate aging131, 132). The adaptability of the biological clock declines with age. Sleep becomes increasingly fragmented, with frequent awakenings and a shortening of stages 3, 4 and REM sleep133). Over the years the circadian rhythms tend to lose their temporal structure, while the oscillation amplitude of their outputs (core body temperature, melatonin and cortisol secretion) diminishes and shows phase advance134). These changes are due less to the age-related reduction in CNS neurons than to changes in their properties, which include a reduction of resting membrane potential and transmission ability135). In addition, aging is associated with a reduction in CNS dendritic spines and dendrite shortening, resulting in poorer neuron connectivity and synchronization with other neurons in the network, increasingly fragmented production of the circadian rhythm, and loss of rhythm amplitude135).
Recent evidence shows that in subjects aged more than 60 yr the circadian oscillation of period genes in the brain shows decreased amplitude and a phase advance of 4–6 h, whereas CRY1 expression becomes increasingly arrhythmic compared with individuals aged less than 40 yr136). Neuropeptides VIP, AVP, and GABA, which play an important role in CNS cell rhythm synchronization, undergo an age-related reduced production that impairs CNS communication and synchronization abilities134). Such changes are due partly to intrinsic degeneration of the biological clock and partly to its altered responsiveness to environmental light. A study of human retina has shown that melanopsin-containing retinal ganglion cells, which mediate the synchronization of environmental light and CNS activity, diminish with age, reducing circadian rhythm outputs and giving rise to decreased oscillations and to phase advance137). In D. melanogaster a number of genes called “late-life cyclers” (LLC), which are part of the circadian clock, undergo rhythmic activation in the final phases of life or at times of intense stress138). LLC genes seem to be activated by and to respond to common age-related stimuli such as cellular and molecular damage, oxidative stress, and some pathological states. As age advances they become increasingly active, preventing the build-up of defective proteins; however, in a biological clock disrupted by chronic changes during the individual’s working life their action could be altered and actually induce an acceleration of aging.
Health Surveillance and Preventive Strategies
The Occupational Physician should assess shift workers for clinical symptoms related to the perturbation of the sleep-wake cycle, such as chronic sleep disturbance and excessive daytime drowsiness due to lack of sleep (Box 1). The sensation of hunger, experienced during night shifts, may induce changes in meal times that disturb the hunger-satiety cycle, resulting in overweight, obesity, type 2 diabetes, dyslipidemia, and metabolic syndrome (Box 2). Periodic assessment of parameters such as weight, abdominal circumference, body mass index (BMI), fasting glycemia, glycosylated hemoglobin, triglycerides and total, HDL and LDL cholesterol enables swift correction in case of pathological changes (Box 3). The assessment of specific parameters related to the biological clock like cortisol, melatonin, and body temperature and the expression of peripheral clock genes and CCGs is important in studies involving groups of workers; where individual workers are concerned, these tests may be prescribed for a higher level check-up (Box 4). With regard to the increasing evidence for an association between shift and night work and breast cancer, the Occupational Physician should suggest screening tests such as mammography139,140,141). Screening mammography is generally recommended after the age of 50 yr, but it should be performed sooner (between 40 and 49 yr) in women who have often worked night shifts and have done so from an early age.
The Occupational Physician should also advise workers on the strategies that can help limit the circadian desynchronization induced by shift work. Workers should try to preserve regular resting hours and avoid cutting the total number of hours of sleep, for instance by sleeping a few hours in the afternoon before a night shift and by go to bed earlier the evening before a morning shift. Meal times should be maintained as much as possible, taking the evening meal before the night shift. Exposure to intense light with a strong blue component before going to sleep should be avoided.
Shift work organization should follow some criteria that should be suggested to the employer142,143,144,145). Fast rotation, i.e. limitation of the number of consecutive night shifts, and clockwise rotation should be preferred. Shifts should be at least 11 h apart. The morning shift should not begin too early, and shift duration should be commensurate to its difficulty. Work schedules should envisage a rest after a night shift and pauses during it. Ensuring the largest possible number of free Saturdays and Sundays would help preserve workers’ social life and integration. Similarly, shifts should be scheduled in advance, they should be flexible, and workers should be able to exchange them.
Conclusions
Evidence continues to accumulate regarding the critical importance of circadian rhythms to health. This paper offers an overview of the physiological mechanisms regulating circadian processes and the health risks associated with disruption of rhythms. The Occupational Physician must take into careful consideration the circadian rhythms in research and clinical practice towards shift and night workers.
References
- 1.Bargiello TA, Jackson FR, Young MW. (1984) Restoration of circadian behavioural rhythms by gene transfer in Drosophila. Nature 312, 752–4. [DOI] [PubMed] [Google Scholar]
- 2.Zehring WA, Wheeler DA, Reddy P, Konopka RJ, Kyriacou CP, Rosbash M, Hall JC. (1984) P-element transformation with period locus DNA restores rhythmicity to mutant, arrhythmic Drosophila melanogaster. Cell 39, 369–76. [DOI] [PubMed] [Google Scholar]
- 3.Buhr ED, Takahashi JS. (2013) Molecular components of the Mammalian circadian clock. Handb Exp Pharmacol 217, 3–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Do MT, Yau KW. (2010) Intrinsically photosensitive retinal ganglion cells. Physiol Rev 90, 1547–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kofuji P, Mure LS, Massman LJ, Purrier N, Panda S, Engeland WC. (2016) Intrinsically Photosensitive Retinal Ganglion Cells (ipRGCs) are necessary for light entrainment of peripheral clocks. PLoS One 11, e0168651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bracci M, Ciarapica V, Copertaro A, Barbaresi M, Manzella N, Tomasetti M, Gaetani S, Monaco F, Amati M, Valentino M, Rapisarda V, Santarelli L. (2016) Peripheral skin temperature and circadian biological clock in shift nurses after a day off. Int J Mol Sci 17, 17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mirick DK, Bhatti P, Chen C, Nordt F, Stanczyk FZ, Davis S. (2013) Night shift work and levels of 6-sulfatoxymelatonin and cortisol in men. Cancer Epidemiol Biomarkers Prev 22, 1079–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Quera Salva MA, Hartley S, Léger D, Dauvilliers YA. (2017) Non-24-hour sleep-wake rhythm disorder in the totally blind: diagnosis and management. Front Neurol 8, 686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Roenneberg T, Kantermann T, Juda M, Vetter C, Allebrandt KV. (2013) Light and the human circadian clock. Handb Exp Pharmacol 217, 311–31. [DOI] [PubMed] [Google Scholar]
- 10.Stevens RG, Zhu Y. (2015) Electric light, particularly at night, disrupts human circadian rhythmicity: is that a problem? Philos Trans R Soc Lond B Biol Sci 370, 370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Takahashi JS, Hong HK, Ko CH, McDearmon EL. (2008) The genetics of mammalian circadian order and disorder: implications for physiology and disease. Nat Rev Genet 9, 764–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Aschoff J. (1965) Circadian rhythms in man. Science 148, 1427–32. [DOI] [PubMed] [Google Scholar]
- 13.Lowrey PL, Takahashi JS. (2011) Genetics of circadian rhythms in Mammalian model organisms. Adv Genet 74, 175–230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Reppert SM, Weaver DR. (2002) Coordination of circadian timing in mammals. Nature 418, 935–41. [DOI] [PubMed] [Google Scholar]
- 15.Cermakian N, Boivin DB. (2009) The regulation of central and peripheral circadian clocks in humans. Obes Rev 10 Suppl 2, 25–36. [DOI] [PubMed] [Google Scholar]
- 16.Borbély AA, Daan S, Wirz-Justice A, Deboer T. (2016) The two-process model of sleep regulation: a reappraisal. J Sleep Res 25, 131–43. [DOI] [PubMed] [Google Scholar]
- 17.Abrahamson EE, Leak RK, Moore RY. (2001) The suprachiasmatic nucleus projects to posterior hypothalamic arousal systems. Neuroreport 12, 435–40. [DOI] [PubMed] [Google Scholar]
- 18.Berk ML, Finkelstein JA. (1981) An autoradiographic determination of the efferent projections of the suprachiasmatic nucleus of the hypothalamus. Brain Res 226, 1–13. [DOI] [PubMed] [Google Scholar]
- 19.Saeb-Parsy K, Lombardelli S, Khan FZ, McDowall K, Au-Yong IT, Dyball RE. (2000) Neural connections of hypothalamic neuroendocrine nuclei in the rat. J Neuroendocrinol 12, 635–48. [DOI] [PubMed] [Google Scholar]
- 20.Saper CB, Scammell TE, Lu J. (2005) Hypothalamic regulation of sleep and circadian rhythms. Nature 437, 1257–63. [DOI] [PubMed] [Google Scholar]
- 21.Vrang N, Mikkelsen JD, Larsen PJ. (1997) Direct link from the suprachiasmatic nucleus to hypothalamic neurons projecting to the spinal cord: a combined tracing study using cholera toxin subunit B and Phaseolus vulgaris-leucoagglutinin. Brain Res Bull 44, 671–80. [DOI] [PubMed] [Google Scholar]
- 22.Daan S, Beersma DG, Borbély AA. (1984) Timing of human sleep: recovery process gated by a circadian pacemaker. Am J Physiol 246, R161–83. [DOI] [PubMed] [Google Scholar]
- 23.Sakurai T. (2005) Roles of orexin/hypocretin in regulation of sleep/wakefulness and energy homeostasis. Sleep Med Rev 9, 231–41. [DOI] [PubMed] [Google Scholar]
- 24.Horn AK. (2006) The reticular formation. Prog Brain Res 151, 127–55. [DOI] [PubMed] [Google Scholar]
- 25.Huang W, Ramsey KM, Marcheva B, Bass J. (2011) Circadian rhythms, sleep, and metabolism. J Clin Invest 121, 2133–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chan S, Debono M. (2010) Replication of cortisol circadian rhythm: new advances in hydrocortisone replacement therapy. Ther Adv Endocrinol Metab 1, 129–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tsang AH, Astiz M, Friedrichs M, Oster H. (2016) Endocrine regulation of circadian physiology. J Endocrinol 230, R1–11. [DOI] [PubMed] [Google Scholar]
- 28.Hill SM, Belancio VP, Dauchy RT, Xiang S, Brimer S, Mao L, Hauch A, Lundberg PW, Summers W, Yuan L, Frasch T, Blask DE. (2015) Melatonin: an inhibitor of breast cancer. Endocr Relat Cancer 22, R183–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hirshkowitz M. (2016) Polysomnography challenges. Sleep Med Clin 11, 403–11. [DOI] [PubMed] [Google Scholar]
- 30.Jafari B, Mohsenin V. (2010) Polysomnography. Clin Chest Med 31, 287–97. [DOI] [PubMed] [Google Scholar]
- 31.Acebo C, LeBourgeois MK. (2006) Actigraphy. Respir Care Clin N Am 12, 23–30, viii [DOI] [PubMed] [Google Scholar]
- 32.Morgenthaler T, Alessi C, Friedman L, Owens J, Kapur V, Boehlecke B, Brown T, Chesson A, Jr, Coleman J, Lee-Chiong T, Pancer J,, Swick TJ, Standards of Practice CommitteeAmerican Academy of Sleep Medicine (2007) Practice parameters for the use of actigraphy in the assessment of sleep and sleep disorders: an update for 2007. Sleep 30, 519–29. [DOI] [PubMed] [Google Scholar]
- 33.Sadeh A. (2011) The role and validity of actigraphy in sleep medicine: an update. Sleep Med Rev 15, 259–67. [DOI] [PubMed] [Google Scholar]
- 34.Hoddes E, Zarcone V, Smythe H, Phillips R, Dement WC. (1973) Quantification of sleepiness: a new approach. Psychophysiology 10, 431–6. [DOI] [PubMed] [Google Scholar]
- 35.Parrott AC, Hindmarch I. (1980) The Leeds Sleep Evaluation Questionnaire in psychopharmacological investigations—a review. Psychopharmacology (Berl) 71, 173–9. [DOI] [PubMed] [Google Scholar]
- 36.Douglass AB, Bornstein R, Nino-Murcia G, Keenan S, Miles L, Zarcone VP, Jr, Guilleminault C, Dement WC. (1994) The Sleep Disorders Questionnaire. I: creation and multivariate structure of SDQ. Sleep 17, 160–7. [DOI] [PubMed] [Google Scholar]
- 37.Buysse DJ, Reynolds CF, 3rd, Monk TH, Berman SR, Kupfer DJ. (1989) The Pittsburgh Sleep Quality Index: a new instrument for psychiatric practice and research. Psychiatry Res 28, 193–213. [DOI] [PubMed] [Google Scholar]
- 38.Johns MW. (1991) A new method for measuring daytime sleepiness: the Epworth sleepiness scale. Sleep 14, 540–5. [DOI] [PubMed] [Google Scholar]
- 39.Horne JA, Ostberg O. (1976) A self-assessment questionnaire to determine morningness-eveningness in human circadian rhythms. Int J Chronobiol 4, 97–110. [PubMed] [Google Scholar]
- 40.Green CB, Takahashi JS, Bass J. (2008) The meter of metabolism. Cell 134, 728–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pendergast JS, Yamazaki S. (2018) The mysterious food-entrainable oscillator: insights from mutant and engineered mouse models. J Biol Rhythms 33, 458–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Nakahata Y, Kaluzova M, Grimaldi B, Sahar S, Hirayama J, Chen D, Guarente LP, Sassone-Corsi P. (2008) The NAD+-dependent deacetylase SIRT1 modulates CLOCK-mediated chromatin remodeling and circadian control. Cell 134, 329–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Nakahata Y, Sahar S, Astarita G, Kaluzova M, Sassone-Corsi P. (2009) Circadian control of the NAD+ salvage pathway by CLOCK-SIRT1. Science 324, 654–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ramsey KM, Yoshino J, Brace CS, Abrassart D, Kobayashi Y, Marcheva B, Hong HK, Chong JL, Buhr ED, Lee C, Takahashi JS, Imai S, Bass J. (2009) Circadian clock feedback cycle through NAMPT-mediated NAD+ biosynthesis. Science 324, 651–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yi CX, van der Vliet J, Dai J, Yin G, Ru L, Buijs RM. (2006) Ventromedial arcuate nucleus communicates peripheral metabolic information to the suprachiasmatic nucleus. Endocrinology 147, 283–94. [DOI] [PubMed] [Google Scholar]
- 46.Lamia KA, Storch KF, Weitz CJ. (2008) Physiological significance of a peripheral tissue circadian clock. Proc Natl Acad Sci USA 105, 15172–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bailey SM, Udoh US, Young ME. (2014) Circadian regulation of metabolism. J Endocrinol 222, R75–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bass J, Takahashi JS. (2010) Circadian integration of metabolism and energetics. Science 330, 1349–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Santa Cecília Silva AA, Lopes TDVC, Teixeira KR, Mendes JA, de Souza Borba ME, Mota MC, Waterhouse J, Crispim CA. (2017) The association between anxiety, hunger, the enjoyment of eating foods and the satiety after food intake in individuals working a night shift compared with after taking a nocturnal sleep: a prospective and observational study. Appetite 108, 255–62. [DOI] [PubMed] [Google Scholar]
- 50.Grant CL, Dorrian J, Coates AM, Pajcin M, Kennaway DJ, Wittert GA, Heilbronn LK, Vedova CD, Gupta CC, Banks S. (2017) The impact of meal timing on performance, sleepiness, gastric upset, and hunger during simulated night shift. Ind Health 55, 423–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Souza RV, Sarmento RA, de Almeida JC, Canuto R. (2019) The effect of shift work on eating habits: a systematic review. Scand J Work Environ Health 45, 7–21. [DOI] [PubMed] [Google Scholar]
- 52.Waterhouse J, Buckley P, Edwards B, Reilly T. (2003) Measurement of, and some reasons for, differences in eating habits between night and day workers. Chronobiol Int 20, 1075–92. [DOI] [PubMed] [Google Scholar]
- 53.Nea FM, Kearney J, Livingstone MB, Pourshahidi LK, Corish CA. (2015) Dietary and lifestyle habits and the associated health risks in shift workers. Nutr Res Rev 28, 143–66. [DOI] [PubMed] [Google Scholar]
- 54.Lowden A, Moreno C, Holmbäck U, Lennernäs M, Tucker P. (2010) Eating and shift work—effects on habits, metabolism and performance. Scand J Work Environ Health 36, 150–62. [DOI] [PubMed] [Google Scholar]
- 55.Keogh K. (2014) Shift work and vending machines to blame for poor workplace diet. Nurs Stand 29, 14–5. [DOI] [PubMed] [Google Scholar]
- 56.Brum MC, Filho FF, Schnorr CC, Bottega GB, Rodrigues TC. (2015) Shift work and its association with metabolic disorders. Diabetol Metab Syndr 7, 45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ferri GM, Cavone D, Intranuovo G, Macinagrossa L. (2017) Healthy diet and reduction of chronic disease risks of night shift workers. Curr Med Chem 24, 1–19. [DOI] [PubMed] [Google Scholar]
- 58.Scott EM, Carter AM, Grant PJ. (2008) Association between polymorphisms in the Clock gene, obesity and the metabolic syndrome in man. Int J Obes 32, 658–62. [DOI] [PubMed] [Google Scholar]
- 59.Copertaro A, Bracci M, Barbaresi M, Santarelli L. (2008) Assessment of cardiovascular risk in shift healthcare workers. Eur J Cardiovasc Prev Rehabil 15, 224–9. [DOI] [PubMed] [Google Scholar]
- 60.Kawada T, Otsuka T. (2014) Effect of shift work on the development of metabolic syndrome after 3 years in Japanese male workers. Arch Environ Occup Health 69, 55–61. [DOI] [PubMed] [Google Scholar]
- 61.Quatela A, Callister R, Patterson A, MacDonald-Wicks L. (2016) The energy content and composition of meals consumed after an overnight fast and their effects on diet induced thermogenesis: a systematic review, meta-snalyses and meta-regressions. Nutrients 8, 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Panahi S, Tremblay A. (2018) Sedentariness and health: is sedentary behavior more than just physical inactivity? Front Public Health 6, 258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ainsworth BE. (2009) How do I measure physical activity in my patients? Questionnaires and objective methods. Br J Sports Med 43, 6–9. [DOI] [PubMed] [Google Scholar]
- 64.Plasqui G. (2017) Smart approaches for assessing free-living energy expenditure following identification of types of physical activity. Obes Rev 18 Suppl 1, 50–5. [DOI] [PubMed] [Google Scholar]
- 65.Tansey EA, Johnson CD. (2015) Recent advances in thermoregulation. Adv Physiol Educ 39, 139–48. [DOI] [PubMed] [Google Scholar]
- 66.te Kulve M, Schellen L, Schlangen LJ, van Marken Lichtenbelt WD. (2016) The influence of light on thermal responses. Acta Physiol (Oxf) 216, 163–85. [DOI] [PubMed] [Google Scholar]
- 67.Taylor NA, Tipton MJ, Kenny GP. (2014) Considerations for the measurement of core, skin and mean body temperatures. J Therm Biol 46, 72–101. [DOI] [PubMed] [Google Scholar]
- 68.Plano SA, Casiraghi LP, García Moro P, Paladino N, Golombek DA, Chiesa JJ. (2017) Circadian and metabolic effects of light: implications in weight homeostasis and health. Front Neurol 8, 558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Blask DE, Hill SM, Dauchy RT, Xiang S, Yuan L, Duplessis T, Mao L, Dauchy E, Sauer LA. (2011) Circadian regulation of molecular, dietary, and metabolic signaling mechanisms of human breast cancer growth by the nocturnal melatonin signal and the consequences of its disruption by light at night. J Pineal Res 51, 259–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Reiter RJ, Mayo JC, Tan DX, Sainz RM, Alatorre-Jimenez M, Qin L. (2016) Melatonin as an antioxidant: under promises but over delivers. J Pineal Res 61, 253–78. [DOI] [PubMed] [Google Scholar]
- 71.Pandi-Perumal SR, Srinivasan V, Maestroni GJ, Cardinali DP, Poeggeler B, Hardeland R. (2006) Melatonin: nature’s most versatile biological signal? FEBS J 273, 2813–38. [DOI] [PubMed] [Google Scholar]
- 72.Oster H, Damerow S, Kiessling S, Jakubcakova V, Abraham D, Tian J, Hoffmann MW, Eichele G. (2006) The circadian rhythm of glucocorticoids is regulated by a gating mechanism residing in the adrenal cortical clock. Cell Metab 4, 163–73. [DOI] [PubMed] [Google Scholar]
- 73.Perelis M, Marcheva B, Ramsey KM, Schipma MJ, Hutchison AL, Taguchi A, Peek CB, Hong H, Huang W, Omura C, Allred AL, Bradfield CA, Dinner AR, Barish GD, Bass J. (2015) Pancreatic β cell enhancers regulate rhythmic transcription of genes controlling insulin secretion. Science 350, aac4250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Park HK, Ahima RS. (2015) Physiology of leptin: energy homeostasis, neuroendocrine function and metabolism. Metabolism 64, 24–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Copinschi G, Leproult R, Spiegel K. (2014) The important role of sleep in metabolism. Front Horm Res 42, 59–72. [DOI] [PubMed] [Google Scholar]
- 76.Taheri S, Lin L, Austin D, Young T, Mignot E. (2004) Short sleep duration is associated with reduced leptin, elevated ghrelin, and increased body mass index. PLoS Med 1, e62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Bozek K, Relógio A, Kielbasa SM, Heine M, Dame C, Kramer A, Herzel H. (2009) Regulation of clock-controlled genes in mammals. PLoS One 4, e4882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Brown SA, Azzi A. (2013) Peripheral circadian oscillators in mammals. Handb Exp Pharmacol 217, 45–66. [DOI] [PubMed] [Google Scholar]
- 79.Damiola F, Le Minh N, Preitner N, Kornmann B, Fleury-Olela F, Schibler U. (2000) Restricted feeding uncouples circadian oscillators in peripheral tissues from the central pacemaker in the suprachiasmatic nucleus. Genes Dev 14, 2950–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Wehrens SMT, Christou S, Isherwood C, Middleton B, Gibbs MA, Archer SN, Skene DJ, Johnston JD. (2017) Meal timing regulates the human circadian system. Curr Biol 27, 1768–1775.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Storch KF, Lipan O, Leykin I, Viswanathan N, Davis FC, Wong WH, Weitz CJ. (2002) Extensive and divergent circadian gene expression in liver and heart. Nature 417, 78–83. [DOI] [PubMed] [Google Scholar]
- 82.Ueda HR, Chen W, Adachi A, Wakamatsu H, Hayashi S, Takasugi T, Nagano M, Nakahama K, Suzuki Y, Sugano S, Iino M, Shigeyoshi Y, Hashimoto S. (2002) A transcription factor response element for gene expression during circadian night. Nature 418, 534–9. [DOI] [PubMed] [Google Scholar]
- 83.Shostak A, Meyer-Kovac J, Oster H. (2013) Circadian regulation of lipid mobilization in white adipose tissues. Diabetes 62, 2195–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Mazzoccoli G, Pazienza V, Vinciguerra M. (2012) Clock genes and clock-controlled genes in the regulation of metabolic rhythms. Chronobiol Int 29, 227–51. [DOI] [PubMed] [Google Scholar]
- 85.Douris N, Green CB. (2008) NOC out the fat: a short review of the circadian deadenylase Nocturnin. Ann Med 40, 622–6. [DOI] [PubMed] [Google Scholar]
- 86.Douris N, Kojima S, Pan X, Lerch-Gaggl AF, Duong SQ, Hussain MM, Green CB. (2011) Nocturnin regulates circadian trafficking of dietary lipid in intestinal enterocytes. Curr Biol 21, 1347–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Hee SW, Tsai SH, Chang YC, Chang CJ, Yu IS, Lee PC, Lee WJ, Yun-Chia Chang E, Chuang LM. (2012) The role of nocturnin in early adipogenesis and modulation of systemic insulin resistance in human. Obesity (Silver Spring) 20, 1558–65. [DOI] [PubMed] [Google Scholar]
- 88.Stubblefield JJ, Terrien J, Green CB. (2012) Nocturnin: at the crossroads of clocks and metabolism. Trends Endocrinol Metab 23, 326–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Green CB, Douris N, Kojima S, Strayer CA, Fogerty J, Lourim D, Keller SR, Besharse JC. (2007) Loss of Nocturnin, a circadian deadenylase, confers resistance to hepatic steatosis and diet-induced obesity. Proc Natl Acad Sci USA 104, 9888–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Boiteux S, Coste F, Castaing B. (2017) Repair of 8-oxo-7,8-dihydroguanine in prokaryotic and eukaryotic cells: properties and biological roles of the Fpg and OGG1 DNA N-glycosylases. Free Radic Biol Med 107, 179–201. [DOI] [PubMed] [Google Scholar]
- 91.Manzella N, Bracci M, Strafella E, Staffolani S, Ciarapica V, Copertaro A, Rapisarda V, Ledda C, Amati M, Valentino M, Tomasetti M, Stevens RG, Santarelli L. (2015) Circadian modulation of 8-oxoguanine DNA damage repair. Sci Rep 5, 13752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.de Almeida EA, Di Mascio P, Harumi T, Spence DW, Moscovitch A, Hardeland R, Cardinali DP, Brown GM, Pandi-Perumal SR. (2011) Measurement of melatonin in body fluids: standards, protocols and procedures. Childs Nerv Syst 27, 879–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Gatti R, Antonelli G, Prearo M, Spinella P, Cappellin E, De Palo EF. (2009) Cortisol assays and diagnostic laboratory procedures in human biological fluids. Clin Biochem 42, 1205–17. [DOI] [PubMed] [Google Scholar]
- 94.Graham C, Cook MR, Kavet R, Sastre A, Smith DK. (1998) Prediction of nocturnal plasma melatonin from morning urinary measures. J Pineal Res 24, 230–8. [DOI] [PubMed] [Google Scholar]
- 95.Kańtoch E, Augustyniak P, Markiewicz M, Prusak D. (2014) Monitoring activities of daily living based on wearable wireless body sensor network. Conf Proc IEEE Eng Med Biol Soc 2014, 586–9. [DOI] [PubMed] [Google Scholar]
- 96.Kamišalić A, Fister I, Jr, Turkanović M, Karakatič S. (2018) Sensors and functionalities of non-invasive wrist-wearable devices: a review. Sensors (Basel) 18, 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Bracci M, Manzella N, Copertaro A, Staffolani S, Strafella E, Barbaresi M, Copertaro B, Rapisarda V, Valentino M, Santarelli L. (2014) Rotating-shift nurses after a day off: peripheral clock gene expression, urinary melatonin, and serum 17-β-estradiol levels. Scand J Work Environ Health 40, 295–304. [DOI] [PubMed] [Google Scholar]
- 98.Braun R, Kath WL, Iwanaszko M, Kula-Eversole E, Abbott SM, Reid KJ, Zee PC, Allada R. (2018) Universal method for robust detection of circadian state from gene expression. Proc Natl Acad Sci USA 115, E9247–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Costa G. (2015) Sleep deprivation due to shift work. Handb Clin Neurol 131, 437–46. [DOI] [PubMed] [Google Scholar]
- 100.Khan S, Duan P, Yao L, Hou H. (2018) Shiftwork-mediated disruptions of circadian rhythms and sleep homeostasis cause serious health problems. Int J Genomics 2018, 8576890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Copertaro A .(2013) Lavoro a turni e notturno: valutazione del rischio e sorveglianza sanitaria. Booksprint, Romagnano al Monte. [Google Scholar]
- 102.Sack RL, Blood ML, Lewy AJ. (1992) Melatonin rhythms in night shift workers. Sleep 15, 434–41. [DOI] [PubMed] [Google Scholar]
- 103.Touitou Y, Motohashi Y, Reinberg A, Touitou C, Bourdeleau P, Bogdan A, Auzéby A. (1990) Effect of shift work on the night-time secretory patterns of melatonin, prolactin, cortisol and testosterone. Eur J Appl Physiol Occup Physiol 60, 288–92. [DOI] [PubMed] [Google Scholar]
- 104.Copertaro A, Bracci M, Barbaresi M, Santarelli L. (2008) [Role of waist circumference in the diagnosis of metabolic syndrome and assessment of cardiovascular risk in shift workers]. Med Lav 99, 444–53. [PubMed] [Google Scholar]
- 105.Costa G. (2010) Shift work and health: current problems and preventive actions. Saf Health Work 1, 112–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Fenga C. (2016) Occupational exposure and risk of breast cancer. Biomed Rep 4, 282–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Folkard S, Minors DS, Waterhouse JM. (1985) Chronobiology and shift work: current issues and trends. Chronobiologia 12, 31–54. [PubMed] [Google Scholar]
- 108.Kecklund G, Axelsson J. (2016) Health consequences of shift work and insufficient sleep. BMJ 355, i5210. [DOI] [PubMed] [Google Scholar]
- 109.Kecklund G, Di Milia L, Axelsson J, Lowden A, Åkerstedt T. (2012) 20th International Symposium on Shiftwork and Working Time: biological mechanisms, recovery, and risk management in the 24-h society. Chronobiol Int 29, 531–6. [DOI] [PubMed] [Google Scholar]
- 110.Knutsson A. (2003) Health disorders of shift workers. Occup Med (Lond) 53, 103–8. [DOI] [PubMed] [Google Scholar]
- 111.Meloni M, Setzu D, Del Rio A, Campagna M, Cocco P. (2013) QTc interval and electrocardiographic changes by type of shift work. Am J Ind Med 56, 1174–9. [DOI] [PubMed] [Google Scholar]
- 112.Stevens RG, Brainard GC, Blask DE, Lockley SW, Motta ME. (2014) Breast cancer and circadian disruption from electric lighting in the modern world. CA Cancer J Clin 64, 207–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Straif K, Baan R, Grosse Y, Secretan B, El Ghissassi F, Bouvard V, Altieri A, Benbrahim-Tallaa L,, Cogliano V, WHO International Agency For Research on Cancer Monograph Working Group (2007) Carcinogenicity of shift-work, painting, and fire-fighting. Lancet Oncol 8, 1065–6. [DOI] [PubMed] [Google Scholar]
- 114.Vetter C, Devore EE, Wegrzyn LR, Massa J, Speizer FE, Kawachi I, Rosner B, Stampfer MJ, Schernhammer ES. (2016) Association between rotating night shift work and risk of coronary heart disease among women. JAMA 315, 1726–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Wang XS, Armstrong ME, Cairns BJ, Key TJ, Travis RC. (2011) Shift work and chronic disease: the epidemiological evidence. Occup Med (Lond) 61, 78–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Yuan X, Zhu C, Wang M, Mo F, Du W, Ma X. (2018) Night shift work increases the risks of multiple primary cancers in women: a systematic review and meta-analysis of 61 articles. Cancer Epidemiol Biomarkers Prev 27, 25–40. [DOI] [PubMed] [Google Scholar]
- 117.Scheer FA, Hilton MF, Mantzoros CS, Shea SA. (2009) Adverse metabolic and cardiovascular consequences of circadian misalignment. Proc Natl Acad Sci USA 106, 4453–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Ramin C, Devore EE, Wang W, Pierre-Paul J, Wegrzyn LR, Schernhammer ES. (2015) Night shift work at specific age ranges and chronic disease risk factors. Occup Environ Med 72, 100–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Chaput JP, Després JP, Bouchard C, Tremblay A. (2007) Short sleep duration is associated with reduced leptin levels and increased adiposity: results from the Quebec family study. Obesity (Silver Spring) 15, 253–61. [DOI] [PubMed] [Google Scholar]
- 120.Shimba S, Ishii N, Ohta Y, Ohno T, Watabe Y, Hayashi M, Wada T, Aoyagi T, Tezuka M. (2005) Brain and muscle Arnt-like protein-1 (BMAL1), a component of the molecular clock, regulates adipogenesis. Proc Natl Acad Sci USA 102, 12071–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Woon PY, Kaisaki PJ, Bragança J, Bihoreau MT, Levy JC, Farrall M, Gauguier D. (2007) Aryl hydrocarbon receptor nuclear translocator-like (BMAL1) is associated with susceptibility to hypertension and type 2 diabetes. Proc Natl Acad Sci USA 104, 14412–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Bracci M, Copertaro A, Manzella N, Staffolani S, Strafella E, Nocchi L, Barbaresi M, Copertaro B, Rapisarda V, Valentino M, Santarelli L. (2013) Influence of night-shift and napping at work on urinary melatonin, 17-β-estradiol and clock gene expression in pre-menopausal nurses. J Biol Regul Homeost Agents 27, 267–74. [PubMed] [Google Scholar]
- 123.Bøggild H, Knutsson A. (1999) Shift work, risk factors and cardiovascular disease. Scand J Work Environ Health 25, 85–99. [DOI] [PubMed] [Google Scholar]
- 124.Leonardi GC, Rapisarda V, Marconi A, Scalisi A, Catalano F, Proietti L, Travali S, Libra M, Fenga C. (2012) Correlation of the risk of breast cancer and disruption of the circadian rhythm (Review). Oncol Rep 28, 418–28. [DOI] [PubMed] [Google Scholar]
- 125.Schernhammer ES, Laden F, Speizer FE, Willett WC, Hunter DJ, Kawachi I, Colditz GA. (2001) Rotating night shifts and risk of breast cancer in women participating in the nurses’ health study. J Natl Cancer Inst 93, 1563–8. [DOI] [PubMed] [Google Scholar]
- 126.Knutsson A. (2004) Methodological aspects of shift-work research. Chronobiol Int 21, 1037–47. [DOI] [PubMed] [Google Scholar]
- 127.Stevens RG, Hansen J, Costa G, Haus E, Kauppinen T, Aronson KJ, Castaño-Vinyals G, Davis S, Frings-Dresen MH, Fritschi L, Kogevinas M, Kogi K, Lie JA, Lowden A, Peplonska B, Pesch B, Pukkala E, Schernhammer E, Travis RC, Vermeulen R, Zheng T, Cogliano V, Straif K. (2011) Considerations of circadian impact for defining ‘shift work’ in cancer studies: IARC Working Group Report. Occup Environ Med 68, 154–62. [DOI] [PubMed] [Google Scholar]
- 128.Lunn RM, Blask DE, Coogan AN, Figueiro MG, Gorman MR, Hall JE, Hansen J, Nelson RJ, Panda S, Smolensky MH, Stevens RG, Turek FW, Vermeulen R, Carreón T, Caruso CC, Lawson CC, Thayer KA, Twery MJ, Ewens AD, Garner SC, Schwingl PJ, Boyd WA. (2017) Health consequences of electric lighting practices in the modern world: a report on the National Toxicology Program’s workshop on shift work at night, artificial light at night, and circadian disruption. Sci Total Environ 607-608, 1073–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.National Toxicology Program, U.S. Department of Health and Human Services, RoC Review of Shift Work at Night, Light at Night, and Circadian Disruption 2018. https://ntp.niehs.nih.gov/pubhealth/roc/listings/shiftwork/. Accessed December 24, 2018.
- 130.Davis S, Mirick DK, Stevens RG. (2001) Night shift work, light at night, and risk of breast cancer. J Natl Cancer Inst 93, 1557–62. [DOI] [PubMed] [Google Scholar]
- 131.Kondratov RV. (2007) A role of the circadian system and circadian proteins in aging. Ageing Res Rev 6, 12–27. [DOI] [PubMed] [Google Scholar]
- 132.Belancio VP, Blask DE, Deininger P, Hill SM, Jazwinski SM. (2015) The aging clock and circadian control of metabolism and genome stability. Front Genet 5, 455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Dijk DJ, Duffy JF, Czeisler CA. (2000) Contribution of circadian physiology and sleep homeostasis to age-related changes in human sleep. Chronobiol Int 17, 285–311. [DOI] [PubMed] [Google Scholar]
- 134.Hood S, Amir S. (2017) The aging clock: circadian rhythms and later life. J Clin Invest 127, 437–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Banks G, Nolan PM, Peirson SN. (2016) Reciprocal interactions between circadian clocks and aging. Mamm Genome 27, 332–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Chen CY, Logan RW, Ma T, Lewis DA, Tseng GC, Sibille E, McClung CA. (2016) Effects of aging on circadian patterns of gene expression in the human prefrontal cortex. Proc Natl Acad Sci USA 113, 206–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Esquiva G, Lax P, Pérez-Santonja JJ, García-Fernández JM, Cuenca N. (2017) Loss of melanopsin-expressing ganglion cell subtypes and dendritic degeneration in the aging human retina. Front Aging Neurosci 9, 79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Kuintzle RC, Chow ES, Westby TN, Gvakharia BO, Giebultowicz JM, Hendrix DA. (2017) Circadian deep sequencing reveals stress-response genes that adopt robust rhythmic expression during aging. Nat Commun 8, 14529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Altobelli E, Rapacchietta L, Angeletti PM, Barbante L, Profeta FV, Fagnano R. (2017) Breast cancer screening programmes across the WHO European region: differences among countries based on national income level. Int J Environ Res Public Health 14, 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Siu AL.U.S. Preventive Services Task Force (2016) Screening for breast cancer: U.S. preventive services task force recommendation statement. Ann Intern Med 164, 279–96. [DOI] [PubMed] [Google Scholar]
- 141.Winters S, Martin C, Murphy D, Shokar NK. (2017) Breast cancer epidemiology, prevention, and screening. Prog Mol Biol Transl Sci 151, 1–32. [DOI] [PubMed] [Google Scholar]
- 142.Costa G .(2013) Lavoro a turni e notturno. SEE, Firenze. [Google Scholar]
- 143.Folkard S, Tucker P. (2003) Shift work, safety and productivity. Occup Med (Lond) 53, 95–101. [DOI] [PubMed] [Google Scholar]
- 144.Harrington JM. (1994) Shift work and health—a critical review of the literature on working hours. Ann Acad Med Singapore 23, 699–705. [PubMed] [Google Scholar]
- 145.Neil-Sztramko SE, Pahwa M, Demers PA, Gotay CC. (2014) Health-related interventions among night shift workers: a critical review of the literature. Scand J Work Environ Health 40, 543–56. [DOI] [PubMed] [Google Scholar]