Abstract
Since the advent of organ-on-a-chip, many researchers have tried to mimic the physiology of human tissue on an engineered platform. In the case of brain tissue, structural connections and cell–cell interactions are important factors for brain function. The recent development of brain-on-a-chip is an effort to mimic those structural and functional aspects of brain tissue within a miniaturized engineered platform. From this perspective, we provide an overview of trace of brain-on-a-chip development, especially in terms of complexity and high-content/high-throughput screening capabilities, and future perspectives on more in vivo-like brain-on-a-chip development.
I. ORGAN-ON-A-CHIP (OoC) AS AN ALTERNATIVE PLATFORM FOR DRUG DISCOVERY
With the advent of an aging society, the disease incidence rate is increasing, and the cost of drug development and disease treatment is expanding exponentially.1,2 According to the World Health Organization (WHO), nearly one billion people in the world suffer from neurodegenerative diseases such as Alzheimer's (AD) and Parkinson's diseases.3 Despite decades of research on neurodegenerative diseases by many biologists and pharmaceutical companies, the underlying mechanism of their onset and progression is still largely unknown. The resolution of these diseases has a long way to go, and such steps are limited due to the lack of a suitable in vitro model system for mechanism study and drug development. In particular, the complex tissue structures and cell–cell interactions of the in vivo system make it challenging to unravel the underlying mechanism of the diseases and to predict the efficacy of clinical medicine. This is why FDA approval rates are low, and some of them are even withdrawn after commercialization.
Traditionally, two-dimensional (2D) culture models and animal models have been used for mechanism research and drug development. However, 2D models, such as Petri dishes and culture flasks, cannot mimic the physiology of human tissue in terms of number of cell types,4 mechanical properties,5 chemokine-mediated cross talk,6 and fluidic conditions.7–11 Therefore, cellular behaviors and drug responses have been frequently over- or underestimated, and the results provide biased information. In the case of animal models, there have been unavoidable discrepancies, such as genetic heterogeneity and loss of immune systems.12 For these reasons, animal models cannot precisely reflect human physiology either and, thus, have not been able to accurately predict in vivo responses upon drug treatment. In addition, the complex physiology of animal models in vivo makes it challenging to distinguish the exact causal relationship in the in vivo response that occurs during drug treatment. For example, a recent review article indicated that the success rate of anticancer drugs in humans, which exhibited good responses in animal experimentation, was below 8%.13 In parallel with the success rate of translation, the animal model always carries ethical issues with it. In this regard, many industries have been looking for and developing a new platform to replace animal models or flask cell-culture models, and recently, organs-on-a-chip (OoCs) have risen as an alternative candidate for cell experiments and drug screening.
II. CHARACTERISTICS OF THE BRAIN AND THE NEED FOR BRAIN-ON-A-CHIP (BoC)
The brain is the most complex organ in the human body, comprising the central nervous system (CNS) along with the spinal cord. As the upper backbone of CNS, the brain processes, integrates, and coordinates the received information, and then makes decisions, in order to organize the activities of individual body parts. The brain comprises numerous neurons that unidirectionally communicate with each other via synapses, where the axon terminal of one cell contacts the dendrites of another in a specific direction.14 These neurons also communicate with nonneuronal cells such as astrocytes, microglia, and oligodendrocytes.15 The functions of the brain are maintained by passing electrical or chemical signals between neurons through the synapses, and if this is not done correctly, it can become a neurodegenerative disease. Huntington's disease (HD), which is a heritable neurodegenerative disorder, results in the death of brain cells, according to animal studies, and has a substantial effect on synaptic alterations in the corticostriatal network.16,17 However, these results, obtained from animal studies, are an end point assay, and thus, while HD is progressing, it is not clear how it progresses and through which path.
Alzheimer's disease (AD), which is a neurodegenerative disorder, is known to be associated with neuronal cell death and neuroinflammation, as well as deposition of neurotoxic protein plaques, which have been observed in human postmortem and animal studies.18 The AD drugs developed so far have shown good efficacy in the treatment of AD mouse models but consecutively failed in phase III clinical trials, while raising concerns about using animal models, which are biologically different with human models, in mechanism studies and drug screening.19
Here, we review the recent advancements in brain-on-a-chip (BoC), focusing on the biomimicry of the neural circuitry and blood–brain barrier (BBB) in the engineered platform. Depending on their complexity and high-content/high-throughput screening ability, we classified the current BoCs into five cases.
III. DEVELOPMENT OF BoC
Structurally, the brain comprises oriented multiple layers in which numerous neuronal, glial, and immunological cells interact and are functionally protected by the skull from mechanical stress and by the tight BBB from the toxicants. At present, the recapitulation of the full structure and function of the brain in an engineered system is unavailable due to the limited technique, and, thus, recent studies have focused on the recapitulation of the specific parts of brain tissue, such as unidirectional neural network, functionally tight BBB, myelination process, and structure of the spinal cord.
Since the development of the in vitro cell-culture technique by Harrison et al. in 1907,20 neuronal cells have been cultured in an open environment, such as glass substrates or Petri dishes. The introduction of soft lithography by Whitesides et al. enabled cell culture in a physically confined microenvironment such as microchannels.21 Later, microchannels with different heights and widths for a single BoC can be fabricated using the multistep lithography technique, making it possible to perform compartmentalized cultures, physically separating the soma and axon. By fabricating high- and low-height microchannels within a single BoC, the axon and soma of a neuron can be separated, enabling only the axon to pass through the shallow channels22,23 [Fig. 1(a)]. This “compartmentalization” technique allows neuroscientists to study the characteristics of the axon itself, treat drugs only in the axonal region, or study the regeneration of the axon after axotomy.24 In addition, the geometry of shallow channels in which only the axon can pass through can be modified, allowing unidirectional axon growth (i.e., axon diode) to be induced.25,26 In addition, by constructing three or more channels within BoC, the interconnection of various neurons, through axons alone, can be demonstrated.27 These BoCs also allowed the study of axon myelination in real time by visualizing it under a microscope. Myelination is a process in which oligodendrocytic feet sheath the axon. Myelination plays a critical role in the propagation of action potential by electrically shielding the axon from the environment. If demyelination occurs due to autoimmune responses or a traumatic brain injury, neurodegenerative brain diseases, such as multiple sclerosis, may be developed. If the myelination process can be recapitulated in BoC, this platform can act as an optical window for the fundamental studies of myelination. For example, recent studies have visualized the step of myelination using the compartmentalized BoC system.28 Furthermore, the effectiveness of optical and electrical stimulations in myelination was evaluated using the compartmentalized BoC system, showing enhanced oligodendrocyte differentiation and wrapping in the presence of optical and electrical stimulations.29,30 However, these microchannel-mediated BoCs pose several limitations, such as 2D cell adhesion and the much stiffer mechanical properties of glass substrates. Furthermore, this monolayer system cannot be used to form a transport barrier, such as BBB.
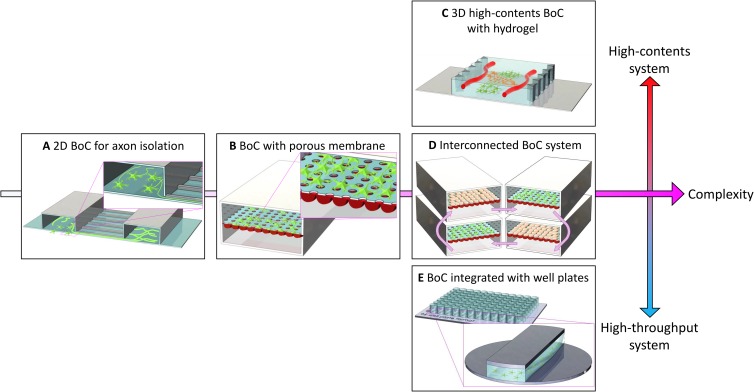
FIG. 1.
Development process of BoCs. (a) A BoC that enables compartmentalized cell culture. Cells are cultured on a 2D glass with the intention of isolating only the axonal part of the neurons in shallow channels. (b) A BoC that mimics the BBB. A porous membrane is located between the two microchannels. Neural and endothelial cells are attached on either side of the membrane. (c) A BoC that aims at high-content screening. Tight cell–cell interactions within the 3D microenvironment are possible by patterning the hydrogel into BoC. This method attempts to mimic the neural tissue more closely. (d) An interconnected multichip system. Culture media can be circulated using a pump to investigate the interactions between various cells through the connection of several BoCs. (e) A BoC developed for high-throughput screening. The BoC can be miniaturized to be fit into conventional cell-culture ware, such as a 96-well plate; this platform is compatible with a conventional high-throughput screening apparatus.
BBB is a unique blood vessel that only appears in the brain, performing a highly effective and selective semipermeable border. It not only regulates the transport of nutrients and wastes but also prevents the influx of pathogens, neurotoxicants, and large hydrophilic molecules. This selective transport ability allows the brain to maintain normal function.31 Many drugs developed for disease treatment have a large molecular size or hydrophilic molecules that are usually restricted from entering the brain tissue through BBB. For this reason, many drug candidates have frequently failed in drug screening stages, and therefore, the commercialization of newly developed drugs has been hindered.32
To address the issue of transport across the cell layer, a porous-membrane-based microfluidic model has been developed33 [Fig. 1(b)]. In this model, the porous membrane is sandwiched between two microchannels and various cells are attached on either side of the membrane. In particular, these cells can communicate with each other through the pores in a cytokine-mediated manner. Such BoC is useful in mimicking the BBB structure. Although the layer-by-layer structure is similar to a Transwell, this type of BoC allows a flow that cannot be obtained in a Transwell. In addition, a three-dimensional (3D) cell-culture environment can be established by introducing a neural cell-laden hydrogel on one side of the membrane.34 It is also possible to measure the transendothelial electrical resistance of BBB by inserting an electrode in each channel.35 However, the cell-culture environment can be regarded as a 2D culture rather than a 3D culture, in that it adheres to the surface of a porous membrane. Moreover, cell–cell interactions are limited because interactions between different cells are made only through a cytokine-mediated long-range communication without physical contact. Nevertheless, endothelial cells and astrocytes can make direct contact in some areas through the pores, allowing for another form of cell–cell interaction.36 Such direct contact-mediated interaction increases with the pore density, and the cell activity increases with intercellular interaction.37
In addition to BoC, as part of CNS, a spinal cord-on-a-chip has been recently developed.38 Sances et al. seeded the induced pluripotent stem cell (iPSC)-derived ventral spinal neurons and cocultured them with brain endothelial cells in the porous-membrane-based BoC. When the spinal cord neurons were cocultured with brain microvascular endothelial cells in BoC, the gene expression, such as SST, was close to that of the fetus compared to the monoculture and 2D culture cases, showing the importance of endothelial cell–neuron interaction and a 3D culture environment.
Recent developments in BoCs can be divided into three categories depending on their high-throughput or high-content screening abilities: (1) 3D high-content systems [Fig. 1(c)], which mimic the 3D brain tissue environment in terms of materials, cell types, and physiological stimulation; (2) interconnected multichip systems [Fig. 1(d)], which simulate cell-to-cell and organ-to-organ interactions; and (3) high-throughput systems, which can massively screen various experimental conditions by making them compatible with conventional well plate-based assay systems [Fig. 1(e)].
The goal of a 3D high-content system lies in the establishment of an in vivo-like tissue microenvironment on an engineered platform to guide the cells to behave similarly to those in vivo. Three-dimensional high-content systems are being developed in various ways: modeling of a 3D neural circuit, modeling of a 3D BBB, and merging a neural circuit and BBB into one BoC. A significant topic in the modeling of 3D neural circuits is the alignment of axons in a 3D environment.39,40 In initial 2D BoCs, the axons were aligned by limiting the growth area of the axon to the microchannel wall. However, in recent 3D BoCs, the neuronal circuit is formed by engineering the orientation of the hydrogel fibrils. Owing to the “contact guidance,” growing axons follow the ready-aligned fibers. The modeling of a 3D BBB aims to form a perfusable vascular network within the hydrogel.41–44 As described earlier, previous BBB models were developed using a porous membrane, but in the recent BoC-adapted hydrogel channels, vascular transport barriers have been established. These vascular networks are structurally and functionally similar to in vivo capillaries because endothelial cells spontaneously form the vascular network in a 3D environment. In addition, cell–cell interaction was promoted because of the direct contact between the brain microvasculature and neural cells. Some studies have attempted to merge a neural circuit and BBB into a single 3D BoC.38,45 When the neural circuit was cultured in the presence of a vascular network, axonal growth was promoted, and the frequency, amplitude, and synchrony of the neuronal electrical activity were increased.
Interconnected multichip systems are also being developed to simulate interactions between various neural cells or between the brain and other organs. These systems connect several membrane-type BoCs, and culture media can be circulated to facilitate cytokine-mediated interactions. Recently, a system for studying the interactions between various cells forming the brain has been developed.46 This BoC system connects three membrane-type BoCs and circulates the culture media. Researchers have separated the flows of artificial blood and cerebral spinal fluid by separating blood vessels and brain mesenchymal regions using membranes. By using this system, they could reveal changes in the metabolism via the interactions of the various cells that formed the neurovascular unit. These systems are used to study not only intercellular connections in the brain but also interactions between the brain and various other organs. A system linking the brain to the heart, liver, and muscles and that connecting the brain with the intestines, liver, kidneys, and muscles have been developed to show the reactions of the connected organs to different drugs.47–49
For high-throughput screening purposes, the BoC system must be concise enough for mass production and must be compatible with high-throughput screening imaging systems. To address this second issue, attempts have been made to tailor BoCs to be compatible with traditional cell-culture platforms, such as a 96-well plate. For the past few years, BoCs have been miniaturized to be assembled with well plates for brain cell culture.50–52 By merging a traditional platform with a BoC, a high-throughput quantification of cell responses in the BoC can be achieved. As the well plate is fabricated with polystyrene (PS), it would potentially be beneficial to fabricate a high-throughput BoC model with PS instead of polydimethylsiloxane (PDMS). Compared to PDMS-based BoC, PS-based BoC has several advantages such as reliability, mass production, and long-term storage, which are suitable for commercial production. However, PDMS-based BoC is still valid for biological studies and drug screening because it has a wide range of tunability for laboratory-level studies. Therefore, it is expected that these two materials can be used in parallel to meet goal-specific needs.53,54 Further attempts have been made to automate the experimental process using BoC to perform the ultimate high-throughput experiment. Compared to the manually fabricated PDMS-based BoCs, injection-molded, PS-based BoCs have potential in terms of time efficiency and cost-effectiveness. By merging the injection-molded BoC with an automated liquid supply system and a high-throughput imaging system, the drug screening efficiency can be enhanced dramatically.55–57 The details described above can be found in Table I that summarizes the features and applications of various types of BoCs.
TABLE I.
Summary of classification and features of developed BoCs.
| Type of BoC | Features | Applications | Reference |
|---|---|---|---|
| (A) 2D BoC for axon isolation |
|
Axon-specific drug testing | 22 and 23 |
| Axon regeneration after axotomy | 24 | ||
| Unidirectional axonal growth | 25 and 26 | ||
| Myelination on the axon | 28–30 | ||
| (B) BoC with porous membrane |
|
Cell–cell communication via cytokine-mediated manners | 33 and 34 |
| Cell–cell communication via direct contact | 36 | ||
| Measure of transendothelial electrical resistance | 35 | ||
| (C) 3D high-content BoC with hydrogel |
|
3D neural circuit formation | 39 and 40 |
| Recapitulation of 3D BBB structure and functions | 41–45 | ||
| (D) Interconnected BoC system |
|
Interactions of various cells of neurovascular unit | 46 |
| Interactions between brain and other organs | 47 and 48 | ||
| (E) BoC integrated with well plate |
|
Compatibility with traditional cell-culture platforms | 50–52 |
| Imaging of BoC in a high-throughput manner | 55–57 |
IV. FUTURE OF BoC
Although many BoC models have been developed, the following additional factors need to be considered to precisely mimic the structure and physiology of the brain tissue: (1) cell sources, (2) cell–cell interactions, and (3) cell–extracellular matrix (ECM) interactions.
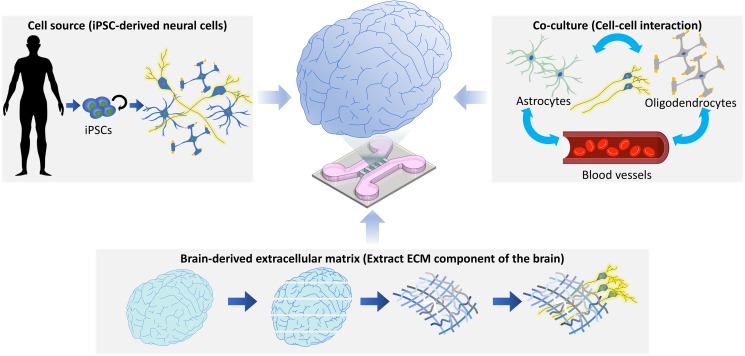
First, human cell sources are required for recapitulating the human brain physiology and developing personalized medicine. As described previously, animal-originated cells differ from human cells in terms of their genetics, and cell lines frequently lose key functions. Furthermore, human-originated primary cells are difficult to acquire. In this regard, the induced pluripotent stem cell (iPSC) technology, introduced in 2006, is a good candidate for supplying human cells.58 iPSCs can be obtained by delivering key factors to stromal cells, which are then induced to differentiate into any cell, especially neurons that are difficult to isolate from the human brain. Furthermore, it allows the development of personalized medicine, including patient-specific cocktail drug designs,59 because the genetic information is preserved during the induction of the pluripotent state and the differentiation process. Recently, patient-specific BBB models have been introduced.36,38,60,61 In these pioneering papers by Vatine et al., Park et al., and Sances et al., the BBB models were fabricated using iPSCs-derived cells and demonstrated the potential of engineered BBB models for personalized medicine applications.
Second, cell–cell interactions toned should be closely investigated to understand brain diseases better. The on-chip approach is useful for studying cell–cell interactions because it can simplify various complex interactions between cells and identify how the disease initiates and progresses through cell–cell communication. In case of AD, the microglia were activated by amyloid-beta, as the representative marker, which accelerated neuronal cell death through interaction with neurons via secreted neurotoxic inflammatory factors and microglial activators.62 As another example, the synapse, which is usually a function for passing electrical or chemical signals between neurons, can be a path for the propagation of neurodegenerative diseases. The representative marker, the phosphorylated tau, was delivered through synapses to other neurons, which meant the propagation and progression of neurodegenerative diseases through intercellular interactions.63 In addition, the on-chip research simplifies the cell population and allows one to monitor the response of each cell type to the developed drugs and, thus, will enable commercializing by selecting the active drugs and minimizing the undesired side-effects.
Finally, it is necessary to determine which ECM component should be used for in vitro culture, because the ECM not only functions in simplistic physical adhesion but also acts as a signaling activator to cells.64 As shown in Fig. 2, the decellularized brain ECM can be used in the culture of neurons produced by direct reprogramming.65 The neurons cultured in the decellularized brain ECM were differentiated better than those cultured in the traditionally used 2D culture and those in the collagen-based 3D culture, ensuring not only structural integrity but also functional maturation, such as calcium signals. These differences were shown through yes-associated protein (YAP) signaling, which is involved in a central stem cell fate-regulating pathway induced by mechanical cues. These results suggest that cell–ECM interactions contribute significantly to the growth and functions of neuronal cells.66 As another example, astrocytes—which have various functions in the brain, such as glutamate recycling, modulation of inflammation, and circulation of cerebrospinal fluid—are usually in an inactivated state. When exposed to physical damage, they become an activate, forming a glial scar to protect the intact brain.67 However, even if astrocytes are not exposed to physical images, especially when culturing in vitro in stiff substrates68 or a less stress-relaxing hydrogel, astrocytes enter an activated state. Such an unintended activation of astrocytes is not desirable for the modeling of the healthy brain tissue. Considering these factors, the preparation of a suitable matrix for culturing brain cells is a prerequisite for mimicking in vivo-like cell functions on an engineered platform.
FIG. 2.
Future of BoCs. For BoCs to mimic normal and disease states of brain tissue on an engineered platform, it is beneficial to use human-originated cells. In this regard, the preparation of patient-derived iPSCs and their differentiation into various neuronal cells are good options for reflecting patient-specific genetic information in an in vitro model. Furthermore, the coculturing of various neuronal cells (e.g., astrocytes, oligodendrocytes, microglia, and blood vessels) can help unravel the underlying mechanism of brain diseases. The consideration of the composition and mechanical properties of ECM is also important to facilitate the reconstitution of in vivo-like cell behaviors and functions in BoCs.
V. CONCLUSIONS
From this perspective, we have summarized the path of BoC development in terms of complexity and high-throughput/high-content screening ability. Various BoCs are still widely used for individual research purposes, such as axon-specific responses, cell–cell interactions, and high-throughput screening. Despite advances in BoC development, the consideration of cell sources, cell–cell interactions, and cell–matrix interactions must be pursued to precisely mimic brain physiology on an engineered platform.
ACKNOWLEDGMENTS
This work was supported by the National Research Foundation of Korea (NRF) grant funded by the Korean Government (MSIT) (Grant No. 2018R1A2A3075013) and by the Brain Research Program through the NRF funded by the Ministry of Science and ICT (Grant Nos. NRF-2016M3C7A1913845 and NRF-2018M3C7A1056896). This research was also supported by the Technology Innovation Program (Grant No. 10067787) funded by the Ministry of Trade, Industry & Energy (MOTIE, Korea) and by the KIST Institutional Program (Grant Nos. 2E29200 and 2V07460).
Contributor Information
Nakwon Choi, Email: .
Hong Nam Kim, Email: .
REFERENCES
- 1.Jin K., Simpkins J. W., Ji X., Leis M., and Stambler I., Aging Dis. 6(1), 1 (2015). 10.14336/AD.2014.1210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cummings J., Reiber C., and Kumar P., Alzheimers Dement (N Y) 4, 330–343 (2018). 10.1016/j.trci.2018.04.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.World Health Organization, Neurological Disorders: Public Health Challenges (World Health Organization, 2006). [Google Scholar]
- 4.Koh I. and Kim P., Biochip J. 13(1), 1–7 (2019). 10.1007/s13206-018-3102-6 [DOI] [Google Scholar]
- 5.Kim H. N. and Choi N., BioChip J. 13(1), 8–19 (2019). 10.1007/s13206-018-3101-7 [DOI] [Google Scholar]
- 6.Boussommier-Calleja A., Li R., Chen M. B., Wong S. C., and Kamm R. D., Trends Cancer 2(1), 6–19 (2016). 10.1016/j.trecan.2015.12.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lu H., Koo L. Y., Wang W. M., Lauffenburger D. A., Griffith L. G., and Jensen K. F., Anal. Chem. 76(18), 5257–5264 (2004). 10.1021/ac049837t [DOI] [PubMed] [Google Scholar]
- 8.Miller J. S., Stevens K. R., Yang M. T., Baker B. M., Nguyen D. H., Cohen D. M., Toro E., Chen A. A., Galie P. A., Yu X., Chaturvedi R., Bhatia S. N., and Chen C. S., Nat. Mater. 11(9), 768–774 (2012). 10.1038/nmat3357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Moya M. L., Hsu Y. H., Lee A. P., Hughes C. C., and George S. C., Tissue Eng. Part C Methods 19(9), 730–737 (2013). 10.1089/ten.tec.2012.0430 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hsu Y. H., Moya M. L., Hughes C. C., George S. C., and Lee A. P., Lab Chip 13(15), 2990–2998 (2013). 10.1039/c3lc50424g [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Paek J., Park S. E., Lu Q., Park K. T., Cho M., Oh J. M., Kwon K. W., Yi Y. S., Song J. W., Edelstein H. I., Ishibashi J., Yang W., Myerson J. W., Kiseleva R. Y., Aprelev P., Hood E. D., Stambolian D., Seale P., Muzykantov V. R., and Huh D., ACS Nano 13(7), 7627–7643 (2019). 10.1021/acsnano.9b00686 [DOI] [PubMed] [Google Scholar]
- 12.Rämer P. C., Chijioke O., Meixlsperger S., Leung C. S., and Münz C., Immunol. Cell Biol. 89(3), 408–416 (2011). 10.1038/icb.2010.151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mak I. W., Evaniew N., and Ghert M., Am. J. Trans. Res. 6(2), 114 (2014). [PMC free article] [PubMed] [Google Scholar]
- 14.Swanson L. W. and Lichtman J. W., Annu. Rev. Neurosci. 39, 197–216 (2016). 10.1146/annurev-neuro-071714-033954 [DOI] [PubMed] [Google Scholar]
- 15.Jäkel S. and Dimou L., Front. Cell. Neurosci. 11, 24 (2017). 10.3389/fncel.2017.00024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Saudou F. and Humbert S., Neuron 89(5), 910–926 (2016). 10.1016/j.neuron.2016.02.003 [DOI] [PubMed] [Google Scholar]
- 17.Lage O. M., Ramos M. C., Calisto R., Almeida E., Vasconcelos V., and Vicente F., Mar. Drugs 16(8), 279 (2018). 10.3390/md16080279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Masters C. L., Bateman R., Blennow K., Rowe C. C., Sperling R. A., and Cummings J. L., Nat. Rev. Dis. Primers 1, 15056 (2015). 10.1038/nrdp.2015.56 [DOI] [PubMed] [Google Scholar]
- 19.Franco R. and Cedazo-Minguez A., Front. Pharmacol. 5, 146 (2014). 10.3389/fphar.2014.00146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Harrison R. G., Greenman M. J., Mall F. P., and Jackson C. M., Anat. Rec. 1(5), 116–128 (1907). 10.1002/ar.1090010503 [DOI] [Google Scholar]
- 21.Whitesides G. M., Ostuni E., Takayama S., Jiang X., and Ingber D. E., Annu. Rev. Biomed. Eng. 3(1), 335–373 (2001). 10.1146/annurev.bioeng.3.1.335 [DOI] [PubMed] [Google Scholar]
- 22.Taylor A. M., Blurton-Jones M., Rhee S. W., Cribbs D. H., Cotman C. W., and Jeon N. L., Nat. Methods 2(8), 599–605 (2005). 10.1038/nmeth777 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Park J., Koito H., Li J., and Han A., Biomed. Microdevices 11(6), 1145–1153 (2009). 10.1007/s10544-009-9331-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tong Z. Q., Segura-Feliu M., Seira O., Homs-Corbera A., Del Rio J. A., and Samitier J., RSC Adv. 5(90), 73457–73466 (2015). 10.1039/C5RA11522A [DOI] [Google Scholar]
- 25.Peyrin J. M., Deleglise B., Saias L., Vignes M., Gougis P., Magnifico S., Betuing S., Pietri M., Caboche J., Vanhoutte P., Viovy J. L., and Brugg B., Lab Chip 11(21), 3663–3673 (2011). 10.1039/c1lc20014c [DOI] [PubMed] [Google Scholar]
- 26.Na S., Kang M., Bang S., Park D., Kim J., Sim S. J., Chang S., and Jeon N. L., Technology 4(04), 240–248 (2016). 10.1142/S2339547816500102 [DOI] [Google Scholar]
- 27.Dauth S., Maoz B. M., Sheehy S. P., Hemphill M. A., Murty T., Macedonia M. K., Greer A. M., Budnik B., and Parker K. K., J. Neurophysiol. 117(3), 1320–1341 (2017). 10.1152/jn.00575.2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kerman B. E., Kim H. J., Padmanabhan K., Mei A., Georges S., Joens M. S., Fitzpatrick J. A., Jappelli R., Chandross K. J., August P., and Gage F. H., Development 142(12), 2213–2225 (2015). 10.1242/dev.116517 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lee H. U., Nag S., Blasiak A., Jin Y., Thakor N., and Yang I. H., ACS Chem. Neurosci. 7(10), 1317–1324 (2016). 10.1021/acschemneuro.6b00157 [DOI] [PubMed] [Google Scholar]
- 30.Lee H. U., Blasiak A., Agrawal D. R., Loong D. T. B., Thakor N. V., All A. H., Ho J. S., and Yang I. H., PLoS One 12(7), e0179642 (2017). 10.1371/journal.pone.0179642 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Daneman R., Ann. Neurol. 72(5), 648–672 (2012). 10.1002/ana.23648 [DOI] [PubMed] [Google Scholar]
- 32.Pardridge W. M., Alzheimers Dement. 5(5), 427–432 (2009). 10.1016/j.jalz.2009.06.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Booth R. and Kim H., Lab Chip 12(10), 1784–1792 (2012). 10.1039/c2lc40094d [DOI] [PubMed] [Google Scholar]
- 34.Brown J. A., Pensabene V., Markov D. A., Allwardt V., Neely M. D., Shi M., Britt C. M., Hoilett O. S., Yang Q., Brewer B. M., Samson P. C., McCawley L. J., May J. M., Webb D. J., Li D., Bowman A. B., Reiserer R. S., and Wikswo J. P., Biomicrofluidics 9(5), 054124 (2015). 10.1063/1.4934713 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wang Y. I., Abaci H. E., and Shuler M. L., Biotechnol. Bioeng. 114(1), 184–194 (2017). 10.1002/bit.26045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Vatine G. D., Barrile R., Workman M. J., Sances S., Barriga B. K., Rahnama M., Barthakur S., Kasendra M., Lucchesi C., Kerns J., Wen N., Spivia W. R., Chen Z., Van Eyk J., and Svendsen C. N., Cell Stem Cell 24(6), 995–1005.E6 (2019). 10.1016/j.stem.2019.05.011 [DOI] [PubMed] [Google Scholar]
- 37.Hayashi Y., Nomura M., Yamagishi S. I., Harada S. I., Yamashita J., and Yamamoto H., Glia 19(1), 13–26 (1997). [DOI] [PubMed] [Google Scholar]
- 38.Sances S., Ho R., Vatine G., West D., Laperle A., Meyer A., Godoy M., Kay P. S., Mandefro B., Hatata S., Hinojosa C., Wen N., Sareen D., Hamilton G. A., and Svendsen C. N., Stem Cell Rep. 10(4), 1222–1236 (2018). 10.1016/j.stemcr.2018.02.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bang S., Na S., Jang J. M., Kim J., and Jeon N. L., Adv. Healthcare Mater. 5(1), 159–166 (2016). 10.1002/adhm.201500397 [DOI] [PubMed] [Google Scholar]
- 40.Kim S. H., Im S. K., Oh S. J., Jeong S., Yoon E. S., Lee C. J., Choi N., and Hur E. M., Nat. Commun. 8, 14346 (2017). 10.1038/ncomms14346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bang S., Lee S. R., Ko J., Son K., Tahk D., Ahn J., Im C., and Jeon N. L., Sci. Rep. 7(1), 8083 (2017). 10.1038/s41598-017-07416-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Campisi M., Shin Y., Osaki T., Hajal C., Chiono V., and Kamm R. D., Biomaterials 180, 117–129 (2018). 10.1016/j.biomaterials.2018.07.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Adriani G., Ma D., Pavesi A., Kamm R. D., and Goh E. L., Lab Chip 17(3), 448–459 (2017). 10.1039/C6LC00638H [DOI] [PubMed] [Google Scholar]
- 44.Xu H., Li Z., Yu Y., Sizdahkhani S., Ho W. S., Yin F., Wang L., Zhu G., Zhang M., Jiang L., Zhuang Z., and Qin J., Sci. Rep. 6, 36670 (2016). 10.1038/srep36670 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Osaki T., Sivathanu V., and Kamm R. D., Sci. Rep. 8(1), 5168 (2018). 10.1038/s41598-018-23512-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Maoz B. M., Herland A., FitzGerald E. A., Grevesse T., Vidoudez C., Pacheco A. R., Sheehy S. P., Park T. E., Dauth S., Mannix R., Budnik N., Shores K., Cho A., Nawroth J. C., Segre D., Budnik B., Ingber D. E., and Parker K. K., Nat. Biotechnol. 36(9), 865–874 (2018). 10.1038/nbt.4226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Oleaga C., Bernabini C., Smith A. S., Srinivasan B., Jackson M., McLamb W., Platt V., Bridges R., Cai Y., Santhanam N., Berry B., Najjar S., Akanda N., Guo X., Martin C., Ekman G., Esch M. B., Langer J., Ouedraogo G., Cotovio J., Breton L., Shuler M. L., and Hickman J. J., Sci. Rep. 6, 20030 (2016). 10.1038/srep20030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Vernetti L., Gough A., Baetz N., Blutt S., Broughman J. R., Brown J. A., Foulke-Abel J., Hasan N., In J., and Kelly E., Sci. Rep. 7, 42296 (2017). 10.1038/srep42296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sung J. H., Koo J., and Shuler M. L., Biochip J. 13(2), 115–126 (2019). 10.1007/s13206-019-3201-z [DOI] [Google Scholar]
- 50.Wevers N. R., van Vught R., Wilschut K. J., Nicolas A., Chiang C., Lanz H. L., Trietsch S. J., Joore J., and Vulto P., Sci. Rep. 6, 38856 (2016). 10.1038/srep38856 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wevers N. R., Kasi D. G., Gray T., Wilschut K. J., Smith B., van Vught R., Shimizu F., Sano Y., Kanda T., Marsh G., Trietsch S. J., Vulto P., Lanz H. L., and Obermeier B., Fluids Barriers CNS 15(1), 23 (2018). 10.1186/s12987-018-0108-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lee S. R., Hyung S., Bang S., Lee Y., Ko J., Lee S., Kim H. J., and Jeon N. L., Biofabrication 11(3), 035013 (2019). 10.1088/1758-5090/ab1402 [DOI] [PubMed] [Google Scholar]
- 53.Berthier E., Young E. W., and Beebe D., Lab Chip 12(7), 1224–1237 (2012). 10.1039/c2lc20982a [DOI] [PubMed] [Google Scholar]
- 54.Tsao C. W., Micromachines 7(12), 225 (2016). 10.3390/mi7120225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lee S., Lim J., Yu J., Ahn J., Lee Y., and Jeon N. L., Lab Chip 19(12), 2071–2080 (2019). 10.1039/C9LC00148D [DOI] [PubMed] [Google Scholar]
- 56.Kane K. I. W., Moreno E. L., Hachi S., Walter M., Jarazo J., Oliveira M. A. P., Hankemeier T., Vulto P., Schwamborn J. C., Thoma M., and Fleming R. M. T., Sci. Rep. 9(1), 1796 (2019). 10.1038/s41598-018-34828-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Peel S., Corrigan A. M., Ehrhardt B., Jang K. J., Caetano-Pinto P., Boeckeler M., Rubins J. E., Kodella K., Petropolis D. B., Ronxhi J., Kulkarni G., Foster A. J., Williams D., Hamilton G. A., and Ewart L., Lab Chip 19(3), 410–421 (2019). 10.1039/C8LC00829A [DOI] [PubMed] [Google Scholar]
- 58.Takahashi K. and Yamanaka S., Cell 126(4), 663–676 (2006). 10.1016/j.cell.2006.07.024 [DOI] [PubMed] [Google Scholar]
- 59.Singh V. K., Kalsan M., Kumar N., Saini A., and Chandra R., Front. Cell Dev. Biol. 3, 2 (2015). 10.3389/fcell.2015.00002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Vatine G. D., Al-Ahmad A., Barriga B. K., Svendsen S., Salim A., Garcia L., Garcia V. J., Ho R., Yucer N., Qian T., Lim R. G., Wu J., Thompson L. M., Spivia W. R., Chen Z., Van Eyk J., Palecek S. P., Refetoff S., Shusta E. V., and Svendsen C. N., Cell Stem Cell 20(6), 831–843 e835 (2017). 10.1016/j.stem.2017.04.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Park T. E., Mustafaoglu N., Herland A., Hasselkus R., Mannix R., FitzGerald E. A., Prantil-Baun R., Watters A., Henry O., Benz M., Sanchez H., McCrea H. J., Goumnerova L. C., Song H. W., Palecek S. P., Shusta E., and Ingber D. E., Nat. Commun. 10(1), 2621 (2019). 10.1038/s41467-019-10588-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Park J., Wetzel I., Marriott I., Dréau D., D’Avanzo C., Kim D. Y., Tanzi R. E., and Cho H., Nat. Neurosci. 21(7), 941 (2018). 10.1038/s41593-018-0175-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Takeda S., Wegmann S., Cho H., DeVos S. L., Commins C., Roe A. D., Nicholls S. B., Carlson G. A., Pitstick R., Nobuhara C. K., Costantino I., Frosch M. P., Muller D. J., Irimia D., and Hyman B. T., Nat. Commun. 6, 8490 (2015). 10.1038/ncomms9490 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Boudreau N. and Bissell M. J., Curr. Opin Cell Biol. 10(5), 640–646 (1998). 10.1016/S0955-0674(98)80040-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Jin Y., Lee J. S., Kim J., Min S., Wi S., Yu J. H., Chang G. E., Cho A. N., Choi Y., Ahn D. H., Cho S. R., Cheong E., Kim Y. G., Kim H. P., Kim Y., Kim D. S., Kim H. W., Quan Z., Kang H. C., and Cho S. W., Nat. Biomed. Eng. 2(7), 522–539 (2018). 10.1038/s41551-018-0260-8 [DOI] [PubMed] [Google Scholar]
- 66.Stukel J. M. and Willits R. K., Tissue Eng. Part B Rev. 22(3), 173–182 (2016). 10.1089/ten.teb.2015.0380 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Silver J. and Miller J. H., Nat. Rev. Neurosci. 5(2), 146–156 (2004). 10.1038/nrn1326 [DOI] [PubMed] [Google Scholar]
- 68.Wilson C. L., Hayward S. L., and Kidambi S., RSC Adv. 6(41), 34447–34457 (2016). 10.1039/C5RA25916A [DOI] [PMC free article] [PubMed] [Google Scholar]