Abstract
Long-term cotrimoxazole prophylaxis reduces mortality and morbidity in HIV infection but the mechanisms underlying these sustained clinical benefits are unclear. Here we investigate the impact of cotrimoxazole on systemic inflammation, an independent driver of HIV mortality. In HIV-positive Ugandan and Zimbabwan children receiving antiretroviral therapy, we show that plasma inflammatory markers were lower after randomization to continue (n=144) versus stop (n=149) cotrimoxazole. This was not explained by clinical illness, HIV progression or nutritional status. Since sub-clinical enteropathogen carriage and enteropathy can drive systemic inflammation, we explored the impact of cotrimoxazole on the gut microbiome and biomarkers of intestinal inflammation. Although global microbiome community composition was unchanged, viridans group Streptococci and streptococcal mevalonate pathway enzymes were lower among children who continued (n=36) versus stopped (n=36) cotrimoxazole. These changes were associated with lower fecal myeloperoxidase. To isolate direct effects of cotrimoxazole on immune activation from its antibiotic properties, we established in vitro models of systemic and intestinal inflammation. In vitro cotrimoxazole treatment had modest but consistent inhibitory effects on pro-inflammatory cytokine production by blood leukocytes from HIV-positive (n=16) and HIV-negative (n=8) U.K. adults. It also reduced IL-8 production by inflamed gut epithelial cell lines. Together, these data demonstrate that cotrimoxazole reduces systemic and intestinal inflammation both indirectly via antibiotic effects on the microbiome, and directly by blunting immune and epithelial cell activation. Synergy between these pathways may explain the clinical benefits of cotrimoxazole despite high antimicrobial resistance, providing further rationale for extending coverage among people living with HIV in sub-Saharan Africa.
Introduction
In 2017, 36.9 million people were living with HIV and 940,000 died from AIDS-related illnesses(1). To reduce mortality and morbidity(2, 3), World Health Organization (WHO) guidelines recommend long-term cotrimoxazole prophylaxis for all people living with HIV in areas with a high prevalence of malaria and/or severe bacterial infections(4). However, it is unclear how cotrimoxazole reduces mortality and morbidity, given the high rates of antimicrobial resistance and selection for resistant pathogens with long-term use(2). There is therefore a need to better understand the effect of cotrimoxazole on HIV pathogenesis.
Systemic inflammation is independently associated with mortality in HIV infection(5–7). Cotrimoxazole might plausibly confer benefits by reducing inflammation, either indirectly by targeting pathogens, or directly by modulating cells that produce pro-inflammatory mediators. Animal models suggest that antibiotics confer anti-inflammatory benefits(8), and observational studies of HIV-positive adults suggest that cotrimoxazole can reduce plasma inflammatory biomarkers(9, 10). Data from randomized trials and low-income settings are lacking and no studies have evaluated the effects of cotrimoxazole on pro-inflammatory pathways in HIV-positive individuals.
HIV drives a chronic enteropathy, characterized by loss of villous architecture, increased gut permeability, mucosal CD4+ T cell depletion(11), leukocyte infiltration(12–14), and microbial translocation(15, 16), accompanied by increased pathogen carriage and an altered microbiome(17, 18); together, these changes contribute to systemic inflammation.
Cotrimoxazole prophylaxis could influence intestinal inflammation through antibiotic effects on enteropathogens and/or the microbiome, or via direct effects on mucosal leukocytes and gut epithelial cells(19, 20). Among HIV-positive Ugandan adults cotrimoxazole had limited effects on the gut microbiome(21), however the effects of cotrimoxazole have not been assessed in a randomized trial or in children.
Cotrimoxazole comprises two folate pathway inhibitors, trimethoprim and sulfamethoxazole. The hypothesis that cotrimoxazole can directly inhibit pro-inflammatory immune cell activation was first posited in 1970, following the observation that intramuscular trimethoprim prolonged skin graft retention in mice(22). However, subsequent in vitro studies of the direct effects of cotrimoxazole on immune cells have yielded conflicting results(23–26) and none have assessed its anti-inflammatory effects in HIV-positive individuals. Cotrimoxazole treatment of rats impacts absorption across the gut epithelium(19), suggesting cotrimoxazole may influence gut barrier function, a critical regulator of cross-talk between the circulation and gut-resident microorganisms.
Thus, cotrimoxazole prophylaxis confers long-term clinical benefits in HIV infection, which are not entirely explained by its antibiotic effects(2, 3). Inconsistent evidence suggests that cotrimoxazole may have anti-inflammatory properties, but conclusive data are lacking. We therefore capitalized on a randomized trial of continuing versus stopping cotrimoxazole in HIV-positive children in sub-Saharan Africa, to test the hypothesis that cotrimoxazole reduces systemic inflammation. We then explored mechanistic pathways through which this may occur, using clinical data, stored specimens and in vitro models.
Results
Cotrimoxazole reduces systemic inflammation in HIV-positive children
We have previously shown that randomization to continue versus stop cotrimoxazole prophylaxis reduced hospitalization or death among HIV-positive children on long-term antiretroviral therapy (ART) in the ARROW trial in Uganda and Zimbabwe(27). Since the inflammatory biomarkers C-reactive protein (CRP) and interleukin (IL-) 6 were independently associated with mortality in ARROW(5), we hypothesized that the benefits of cotrimoxazole might be partly mediated through reductions in systemic inflammation. CRP, IL-6, soluble (s)CD14, and tumor necrosis factor (TNF)α) were quantified in longitudinal plasma samples from children randomized to continue (n=144) versus stop (n=149) cotrimoxazole (Fig. 1).
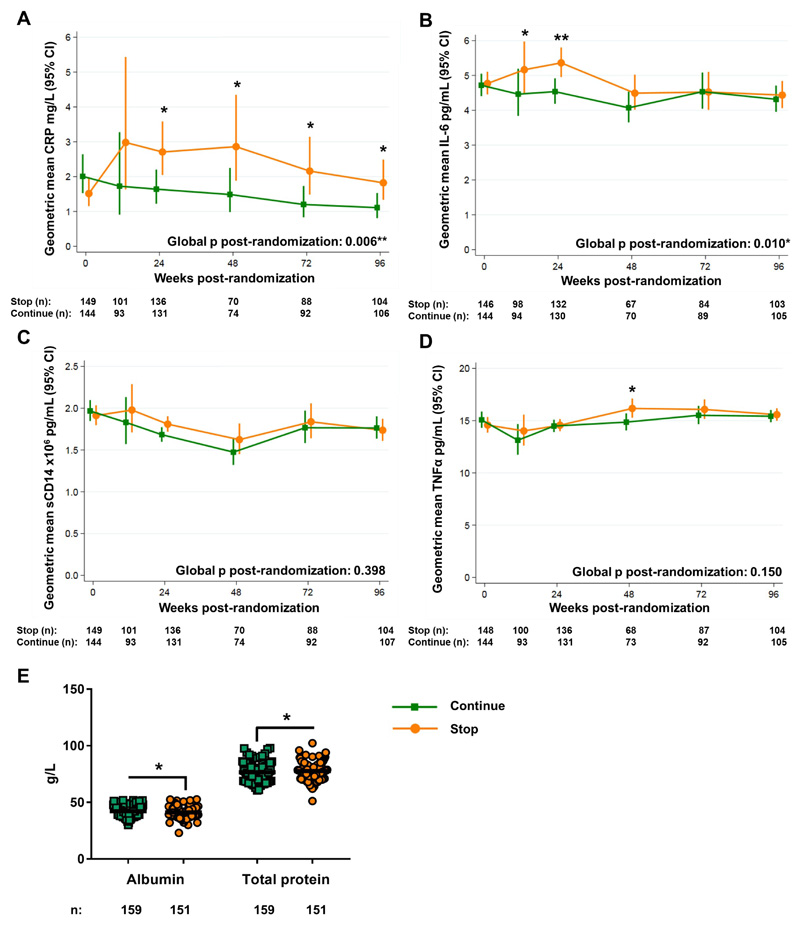
Figure 1. Systemic inflammation is lower among HIV-positive children randomized to continue daily oral cotrimoxazole prophylaxis.
Geometric mean concentrations of (A) CRP, (B) IL-6, (C) sCD14, and (D) TNFα in plasma of HIV-positive children who had been receiving ART and cotrimoxazole for ≥96 weeks and were then randomized to stop (orange circles) or continue (green squares) cotrimoxazole. Randomized groups were compared across timepoints using generalized estimating equations and at individual timepoints using standard regression models (normal distribution for log-transformed values), adjusted for center and baseline concentrations (global p; A-D); *p<0.05, **p<0.01 ***p<0.001. (E) Serum protein concentrations at week-48 post-randomization; horizontal bars indicate means. Comparisons between groups by Mann-Whitney U test; *p<0.05, **p<0.01 ***p<0.001.
Biomarkers were similar between groups at baseline (Fig. 1A-D), but subsequent CRP concentrations from week-24 until the end of follow-up were lower in children randomized to continue cotrimoxazole (global p: 0.006; Fig. 1A). IL-6 was also significantly lower among children continuing cotrimoxazole, particularly at early timepoints (Global p: 0.010; week-12 p: 0.014, week-24 p: 0.003; Fig. 1B). There was no evidence of global differences between groups in sCD14 (Fig. 1C) or TNFα (Fig. 1D). Serum albumin was significantly higher (median: 42 versus 41g/L, p=0.041) and total protein significantly lower (76 versus 78g/L, p=0.038) in children continuing cotrimoxazole at week-48 (Fig. 1E), consistent with less systemic inflammation. Collectively these results show that cotrimoxazole reduces systemic inflammation in HIV-positive children.
To estimate the clinical implications of these findings, we used relative risk estimates of adverse outcomes (death, new or recurrent WHO clinical stage 4 events, or poor immunological response to ART) associated with baseline (i.e. pre-ART) concentrations of CRP and IL-6 in ARROW(5). Stopping cotrimoxazole led to 1.65-fold higher CRP (stop, 2.71mg/L versus continue, 1.64mg/L; Fig. 1A) and 1.18-fold higher IL-6 (stop, 5.36pg/mL versus continue, 4.54pg/mL; Fig. 1B) at week-24, corresponding to an increased relative risk of adverse clinical outcomes among children stopping cotrimoxazole of 13% (95% CI: 4-24%) and 11% (95% CI: 4-18%), respectively, within 24 weeks(5). Relative differences in CRP peaked at week-48 (1.92-fold increase; stop, 2.86mg/L versus continue, 1.49mg/L; Fig. 1A), corresponding to an 18% (95% CI: 6-32%) increased risk of adverse clinical outcomes. Thus, differences in CRP and IL-6 with continued cotrimoxazole are important for long-term survival, health and immune restoration among HIV-positive children.
Reduced systemic inflammation is not solely due to less clinical disease
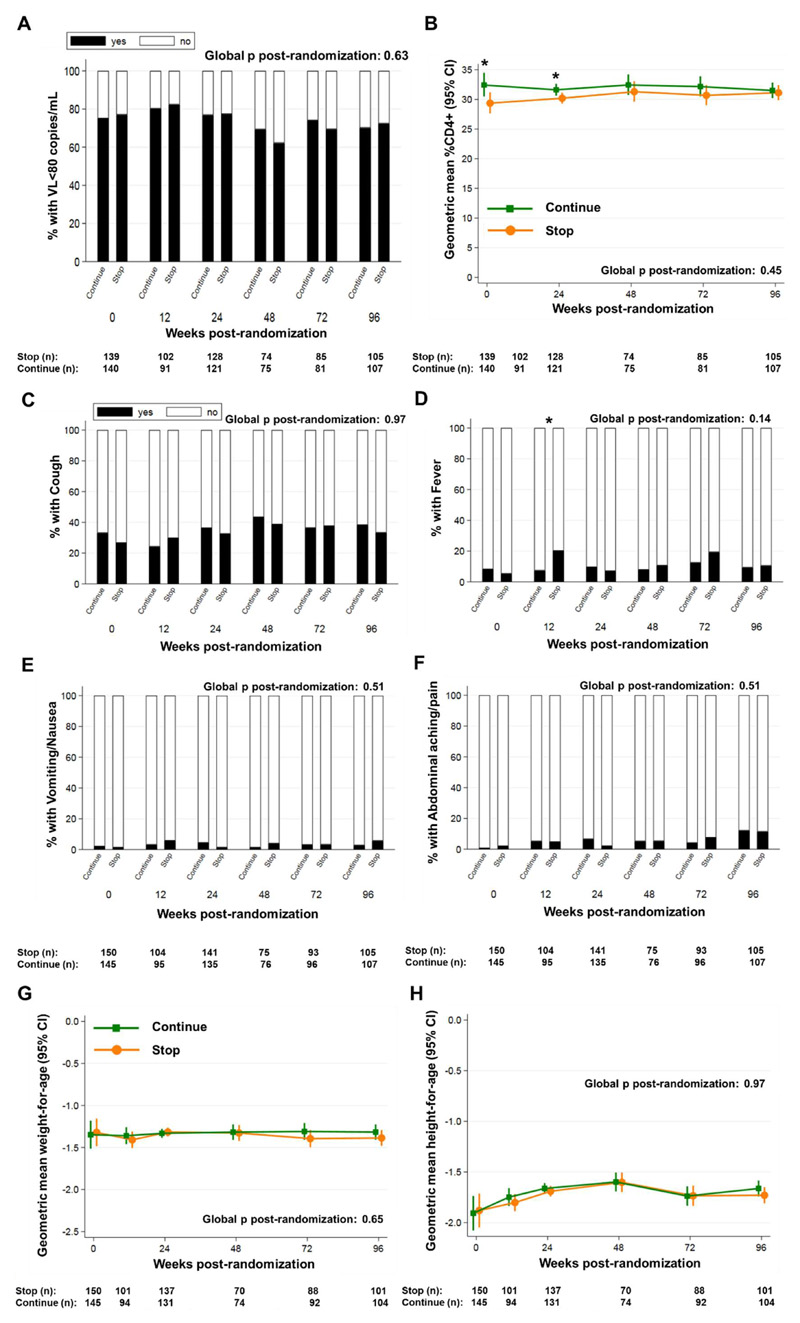
Lower systemic inflammation with long-term cotrimoxazole could be due to reductions in HIV disease progression or clinical illness(2, 27). However, there was no evidence of global differences in the proportion of children with viral suppression (<80 HIV RNA copies/mL; Fig. 2A) or in CD4+ T cell percentages (%CD4; Fig. 2B) between randomized groups. There was also no evidence for global differences in caregiver-reported cough (Fig. 2C), fever (Fig. 2D), nausea/vomiting (Fig. 2E) or abdominal pain (Fig. 2F). Too few children had persistent, bloody or moderate-to-severe diarrhea, difficult/fast breathing and/or weight loss for comparison between groups. HIV-positive children frequently have malnutrition; antibiotics (including cotrimoxazole) have been shown to improve growth(28) and slow weight-loss(29). We therefore compared anthropometry between randomized groups, reasoning that differences in systemic inflammation might be explained by underlying wasting or stunting. We found no evidence of differences in weight-for-age (Fig. 2G) or height-for-age Z-scores (Fig. 2H). Thus, effects of cotrimoxazole on systemic inflammation were not explained by differences in HIV disease progression, symptomatic infections or malnutrition between groups.
Figure 2. Cotrimoxazole effects on systemic inflammation are not solely due to differences in HIV disease progression, symptomatic infections, or nutritional status.
(A) Percentage of children with viral load <80 copies/mL; (B) geometric mean percentage CD4+ T cells; mean proportions of children with caregiver-reported (C) cough, (D) fever, (E) vomiting/nausea and (F) abdominal pain; geometric mean (G) weight-for-age and (H) height-for-age Z-scores in children randomized to continue versus stop cotrimoxazole prophylaxis (n per group shown under each graph). Randomized groups were compared by generalized estimating equations across timepoints (global p) and at individual timepoints using standard regression models (binomial distribution for viral load; normal distribution for log-transformed values) adjusted for recruitment center; *p<0.05, **p<0.01 ***p<0.001.
Cotrimoxazole alters circulating CD4+ T cell phenotypes in HIV-positive children
Although cotrimoxazole continuation had no impact on total CD4+ T cell counts, we hypothesized that CD4+ T cell phenotypes would differ between randomized groups because systemic inflammation is associated with T cell activation and proliferation(30, 31) (5). T cell immunophenotyping in a subset of Ugandan ARROW participants (stop n=48, continue n=47; fig. S1A) revealed no evidence of differences between randomized groups in the proportions of total CD4+ T cells expressing the activation marker HLA-DR or the proliferation marker Ki67 (fig. S1B and C). Children continuing cotrimoxazole had higher percentages of recent thymic emigrant-like cells (RTE, CD4+CD45RA+CD31+ T cells; an indicator of thymic output(32)) than children stopping prophylaxis (fig. S1B). There was no evidence of differences in proportions of naïve (CD4+CD45RA+CD31-) or effector-memory (CD4+CD45RA-CD31-) T cells or in the expression of HLA-DR on any CD4+ T cell sub-populations (fig. S1C). However, children continuing cotrimoxazole had lower percentages of proliferating (Ki67+) RTE and naïve T cells, particularly at later timepoints post-randomization (fig. S1D). Thus, cotrimoxazole continuation led to some changes in circulating T cells consistent with reduced systemic inflammation(5).
Cotrimoxazole suppresses abundance and function of gut-resident Streptococci
The gut microbiome is disrupted by HIV infection, which contributes to local and systemic inflammation(17, 33). We hypothesized that continuing cotrimoxazole would drive sustained sub-clinical differences in gut pathogens and commensals. We conducted whole metagenome shotgun sequencing of total fecal DNA from children randomized to continue (n=36 at week-84; n=33 at week-96) versus stop cotrimoxazole (n=36 at week-84; n=35 at week-96). Randomized groups did not differ in species-level diversity at week-84 or week-96 (Shannon indices: 13.1 continue versus 14.3 stop, p=0.27; and 13.5 continue versus 14.8 stop, p=0.72) or evenness (Pileou’s index: 0.59 continue versus 0.60 stop, p=0.605; and 0.60 continue versus 0.61 stop, p=0.883). Bacterial community composition was also similar between groups (Fig. 3A and B). However, false discovery rate (FDR)-adjusted zero-inflated beta regression analysis of individual microbiome characteristics identified 7 bacterial species (Alistipes onderdonkii, Eggerthella lenta, Clostridium bartlettii, Haemophilus parainfluenzae, Streptococcus mutans, Streptococcus parasanguinis and Streptococcus vestibularis; fig. S2) and 11 protein families (Pfam; fig. S3) mapping to Streptococcus parasanguinis, Streptococcus salivarius and Haemophilus parainfluenzae, that were consistently less abundant at both timepoints in the continue versus stop group (relative abundance ratio <1). The differentially abundant Streptococci are all within the viridans group (VGS), and largely fell in the quadrant of the NMDS ordination plot where the extremes of the treatment groups lay (Fig. 3A and B). The relative abundance of Enterobacteriaceae, which includes gastrointestinal pathogens (e.g. Salmonella, Escherichia coli, and Shigella) that are frequently resistant to cotrimoxazole(34, 35), was not affected by cotrimoxazole at week-84 (relative abundance ratio: 0.65, adjusted p=0.108) and was increased in those continuing versus stopping cotrimoxazole at week-96 (4.48, adjusted p<0.001).
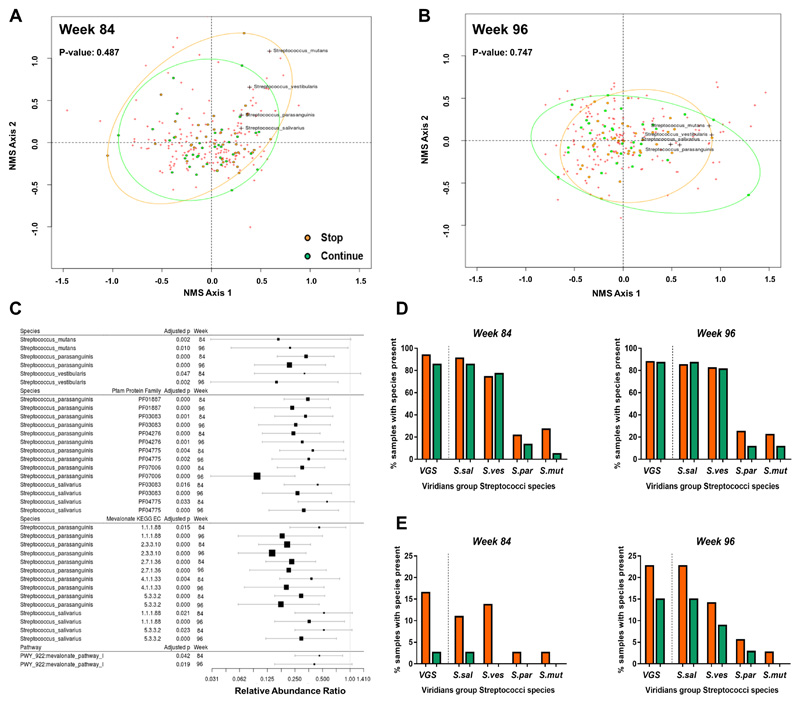
Figure 3. Continuation of cotrimoxazole suppresses the abundance and function of viridans group Streptococci in stool samples from HIV-positive children.
Non-metric multidimensional scaling plots of the Bray–Curtis dissimilarity index for stool samples from 72 HIV-positive Zimbabwean children randomized to stop (orange) versus continue (green) cotrimoxazole at (A) week-84 and (B) week-96 post-randomization. Red crosses indicate individual bacterial species irrespective of randomized group; VGS species that consistently differed between randomized groups are labelled. Randomized groups were compared by permutation tests. (C) Effect size plots of relative abundance ratios (±95% confidence interval) for all Streptococcus spp. and their protein families (Pfam) and mevalonate pathway-associated genes (KEGG EC), and metabolic pathways (all bacterial species) that significantly differed between randomized groups at both week-84 and week-96 in FDR-adjusted zero-inflated beta regression. Identities for Pfam and KEGG EC were established using HUMANn2 against the UniRef90 database. Relative abundance ratio <1.0 indicates lower relative abundance in children who continued versus stopped cotrimoxazole. Vertical line indicates null value. Size of square is inversely proportional to p-value. Percentage of samples positive for any of the four VGS or individual species according to (D) MetaPhlAn and (E) PanPhlAn analysis at week-84 (continue n=36, stop n=36) and week-96 (continue n=33, stop n=35)
To understand the effect of cotrimoxazole on microbiome function, we quantified the abundance of full sets of genes involved in metabolic pathways across bacterial taxa. Only mevalonate pathway I, which influences neutrophil and monocyte recruitment and function, was consistently different between groups at both timepoints. Mevalonate pathway-associated genes were significantly less abundant in stool samples from children continuing cotrimoxazole (week-84 adjusted p=0.042, and week-96 adjusted p=0.019; Fig. 3C). Of the enzyme-encoding genes within mevalonate pathway I, those with identity to Streptococcus parasanguinis (5 enzymes, KEGG EC: 1.1.1.88 (hydroxymethylglutaryl-CoA (HMG-CoA) reductase), 2.3.3.10 (HMG-CoA synthase), 2.7.1.36 (mevalonate kinase) and 4.1.1.33 (diphosphomevalonate decarboxylase) and 5.3.3.2 (isopentenyl-diphosphate Delta-isomerase); adjusted p<0.05 at both timepoints) and Streptococcus salivarius (2 enzymes, KEGG EC: 1.1.1.88 and 5.3.3.2; adjusted p<0.05 at both timepoints) were significantly less abundant in the continue group (Fig. 3C), suggesting that continuation of cotrimoxazole reduces VGS metabolic function in the gut.
To confirm this metagenomic signature of VGS suppression by cotrimoxazole, we conducted high-resolution mapping of metagenome sequencing reads to Streptococci pangenome datasets using PanPhlAn software, which has a lower false-positive rate for species identification and better discrimination between samples containing the same versus different bacterial genomes than MetaPhlAn(36). Of the 140 stool samples sequenced (both groups at week-84 and week-96), PanPhlAn identified 29 samples positive for any Streptococci (9 species present: S. salivarius, S. parasanguinis, S. mutans, S. vestibularis, S. australis, S. infantarius, S. oligofermentans, S. pasteurianus, and S. sanguinis) and, of these, 20 samples positive for at least one of the 4 VGS species identified using MetaPhlAn (7 at week-84 and 13 at week-96). PanPhlAn identified a lower percentage of VGS-positive samples on account of its higher species-level resolution (Fig. 3D and E). Six samples from children continuing and 14 samples from children stopping cotrimoxazole were confirmed VGS-positive across both timepoints, corroborating VGS suppression by cotrimoxazole. Individual VGS species were present less often in children continuing cotrimoxazole (Fig. 3E). Together, these findings show that continuing compared to stopping long-term cotrimoxazole does not affect global microbiome community composition, but does drive specific alterations in gut microbiome structure and function, with suppression of VGS and associated reductions in VGS mevalonate pathway genes.
Cotrimoxazole-induced changes in Streptococci reduce intestinal inflammation
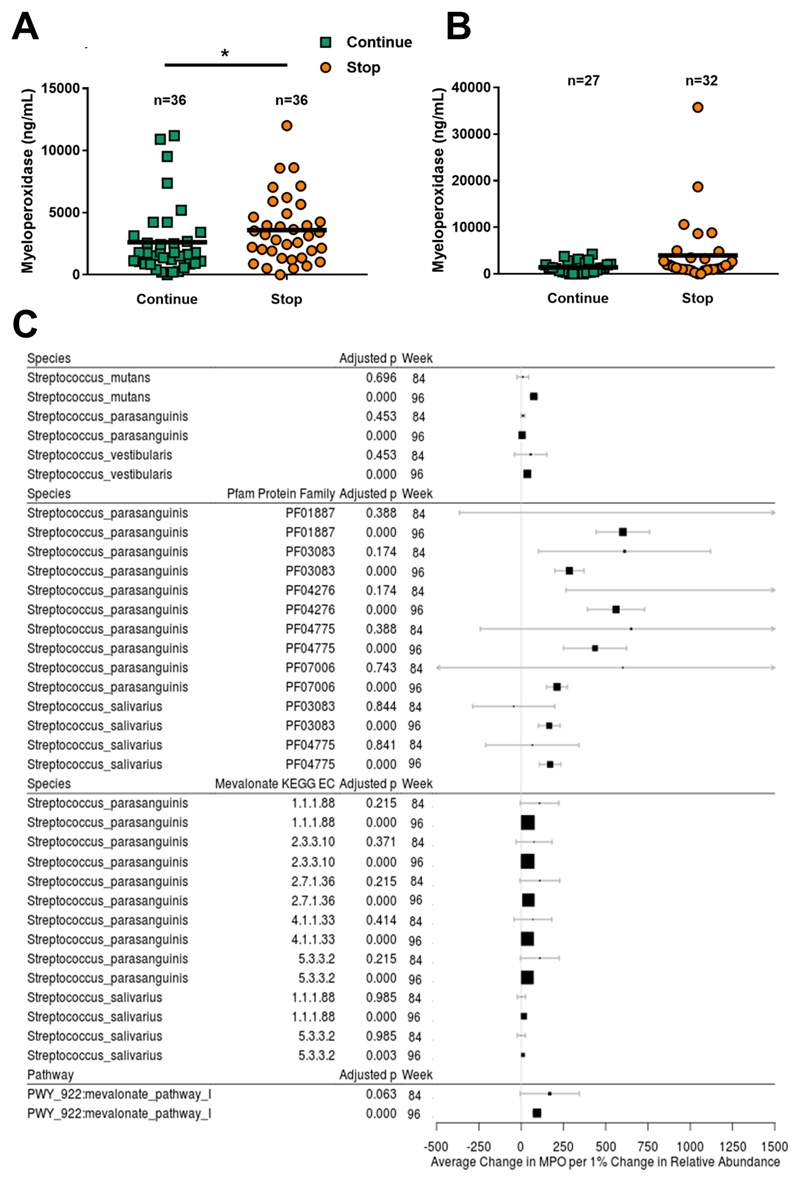
We next tested whether these microbiome changes influenced HIV enteropathy. We compared levels of fecal inflammatory markers at week-84 and week-96 post-randomization to continue (n=37) or stop (n=38) cotrimoxazole. At week-84, fecal myeloperoxidase was significantly lower in children continuing versus stopping cotrimoxazole (median: 1694ng/mL versus 3178ng/mL, p=0.022; Fig. 4A), but there was no evidence of differences in neopterin, alpha-1-antitrypsin, or REG1β between groups (p>0.15, fig. S4A). At week-96, myeloperoxidase did not significantly differ between randomized groups (1262 versus 1473ng/mL, p=0.093; Fig. 4B) and there was no evidence of differences in neopterin, alpha-1-antitrypsin or REG1β (p>0.15, fig. S4B). Since myeloperoxidase is an abundant peroxidase enzyme in monocytes and neutrophils that perpetuates granulocyte activation(37) and both cell types home to the gut mucosa during HIV infection(13, 14), these observations suggest that cotrimoxazole reduces innate immune cell activity in the gut.
Figure 4. Intestinal inflammation in HIV-positive children is associated with gut-resident viridans group Streptococci that are suppressed by continuation of cotrimoxazole.
Myeloperoxidase at (A) week-84 and (B) week-96 in stool samples from HIV-positive Zimbabwean children randomized to stop versus continue cotrimoxazole. Randomized groups compared by Mann-Whitney U test; *p<0.05, horizontal lines indicate median. (C) Effect size plots showing average change in myeloperoxidase per 1% change in relative abundance (±95% confidence interval) for all Streptococcus spp. and their protein families (Pfam) and mevalonate pathway-associated genes (KEGG EC), and metabolic pathways (all bacterial species) that significantly differed between randomized groups at both week-84 and week-96 in FDR-adjusted zero-inflated beta regression (Fig. 3C). Identities for Pfam and KEGG EC were established using HUMANn2 against the UniRef90 database. Average change >1.0 indicates increase in myeloperoxidase with increased abundance. Vertical line indicates null value. Size of square inversely proportional to p-value.
Of the bacterial species suppressed by cotrimoxazole, Streptococcus mutans, Streptococcus vestibularis, Streptococcus parasanguinis, and Haemophilus parainfluenzae were positively associated with myeloperoxidase levels at week-96 (Streptococcus spp. summarized in Fig. 4C; analysis of all species in fig. S5), after adjustment for age, sex, and cotrimoxazole group. Myeloperoxidase was also positively associated with Pfam that were differentially abundant according to cotrimoxazole treatment: 5 with identity to Streptococcus parasanguinis, 2 to Streptococcus salivarius, 2 to Haemophilus parainfluenzae, and 1 to Eubacterium bioforme at week-96 (Pfam with identify to Streptococcus spp. summarized in Fig. 4C; analysis of all Pfam in fig. S6). Overall mevalonate pathway I abundance was significantly associated with higher myeloperoxidase at week-96 (adjusted p<0.001, Fig. 4C). Of the mevalonate pathway I enzymes that differed between randomized groups, only those with identity to Streptococcus parasanguinis (5 enzymes, adjusted p<0.001) and Streptococcus salivarius (2 enzymes, adjusted p<0.01) had a significant positive association with myeloperoxidase (Fig. 4C). We therefore show that all VGS components suppressed by cotrimoxazole (Fig. 3C) were positively associated with myeloperoxidase (Fig. 4C), suggesting that reduced VGS abundance and function contribute to lower intestinal inflammation among children continuing cotrimoxazole.
Cotrimoxazole blunts pro-inflammatory cytokine responses in vitro
Having established that cotrimoxazole reduces both systemic and intestinal inflammation, we next investigated whether cotrimoxazole has direct immunomodulatory properties. To isolate any direct effects of cotrimoxazole on immune cells from its impact on enteropathy and the microbiome, we optimized an in vitro model of whole blood cytokine responses to bacterial and fungal antigens: heat-killed Salmonella typhimurium (HKST), which activates immune cells via Toll-like receptor (TLR) 2, 4 and 5; purified Escherichia coli lipopolysaccharide (LPS), which engages TLR4; and the Saccharomyces cerevisiae cell-wall component zymosan, which engages TLR2 and dectin-1. Antigens engaging pattern recognition receptors were chosen to reflect microbial translocation, which drives systemic inflammation and immune activation in HIV infection(7, 15, 18, 33). The cotrimoxazole dose was chosen to reflect maximum (high-dose; 8μg/mL trimethoprim and 200μg/mL sulfamethoxazole) and minimum (low-dose; 2μg/mL trimethoprim and 50μg/mL sulfamethoxazole) serum concentrations in HIV-positive patients taking cotrimoxazole(38). Laboratory cotrimoxazole preparations were confirmed to have antibiotic activity (fig. S7A) and doses did not reduce leukocyte viability in culture (fig. S7B-D).
Since the inflammatory milieu can affect immune cell responses, we obtained blood samples from three groups of U.K. adults (HIV-positive ART-treated (n=6), HIV-positive ART-naïve (n=10) and HIV-negative (n=8), table S1), with distinct baseline inflammatory profiles (fig. S8). There was no difference between groups in spontaneous cytokine production in 24h unstimulated cultures (Fig. 5).
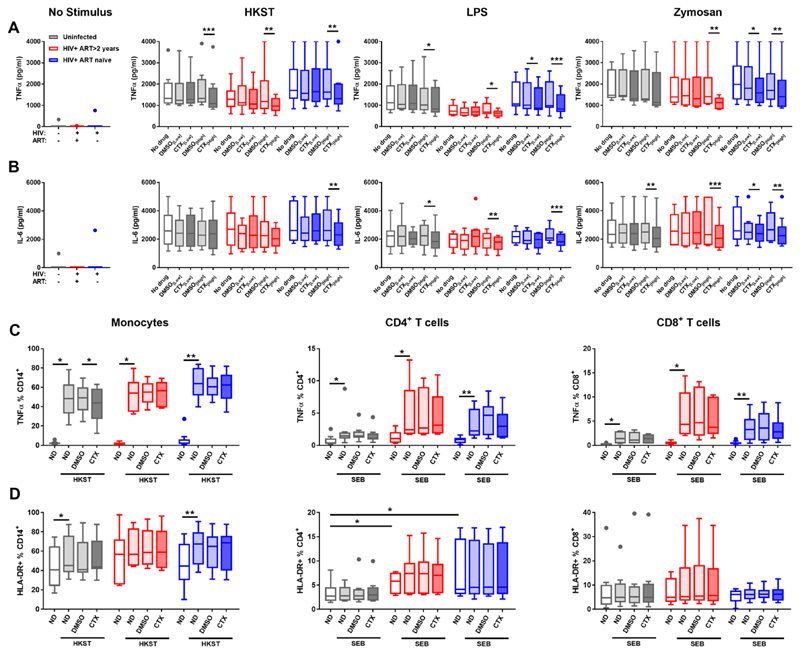
Figure 5. Cotrimoxazole inhibits in vitro pro-inflammatory cytokine responses to bacterial and fungal antigens.
Tukey boxplots of (A) TNFα and (B) IL-6 concentrations in supernatants from whole blood cultures without antigen (No Stimulus), with heat-killed Salmonella typhimurium (HKST), lipopolysaccharide (LPS); or zymosan. Cultures were treated with low-dose cotrimoxazole (CTX[Low]: 2 μg/mL trimethoprim, 50 μg/mL sulfamethoxazole), high-dose cotrimoxazole (CTX[High]: 8 μg/mL trimethoprim, 200 μg/mL sulfamethoxazole) or volume-matched controls (DMSO[Low], DMSO[High]). Proportions of monocytes (left), CD4+ (center) and CD8+ T-cells (C) producing TNFα and (D) expressing HLA-DR after 6h PBMC culture with HKST or staphylococcal enterotoxin B (SEB). Grey bars indicate HIV-negative (n=8); red indicate HIV-positive ART-treated (n=6); and blue indicate HIV-positive ART-naïve group (n=10). Cytokine concentrations in cotrimoxazole-treated cultures are indicated by darker shading. Drug treatments compared within groups by Freidman tests with post-hoc uncorrected Dunn’s tests; *p<0.05, **p<0.01, ***p<0.001.
High-dose cotrimoxazole significantly reduced HKST-, LPS- and zymosan-induced TNFα (Fig 5A) and IL-6 (Fig 5B) production relative to control treatment with drug diluent alone (dimethyl sulfoxide, DMSO) in ≥1 group. This was particularly evident for HKST- and LPS-induced TNFα and LPS- and zymosan-induced IL-6, which were significantly lower across all three clinical groups. LPS- and zymosan-induced TNFα and zymosan-induced IL-6 were also significantly reduced by low-dose cotrimoxazole in the HIV-positive ART-naïve group (Fig 5A and B). These observations confirm our hypothesis that cotrimoxazole directly modulates pro-inflammatory immune cell activation by pathogen antigens, both in HIV-positive and in HIV-negative individuals, independently of its effects on the microbiome or intestinal inflammation.
To determine the immune cell types modulated by cotrimoxazole, we evaluated intracellular TNFα production and surface expression of HLA-DR by monocytes and T cells during 6h PBMC culture with or without high-dose cotrimoxazole (gating strategy shown in fig. S9; antibodies in table S2). Cotrimoxazole reduced the proportion of TNFα+ monocytes after HKST stimulation relative to control-treated cultures in the HIV-negative group but not in the HIV-positive groups (Fig. 5C). Cotrimoxazole did not alter HKST-induced up-regulation of HLA-DR by monocytes (Fig. 5D). Cotrimoxazole also had no effect on the proportion of TNFα+ or HLA-DR+ CD4+ or CD8+ T cells after polyclonal stimulation with staphylococcal enterotoxin B (SEB; Fig. 5C and D). Thus, although cotrimoxazole reduces pro-inflammatory cytokine production by blood leukocytes and TNFα production by monocytes specifically, it did not directly reduce monocyte maturation or T cell activation.
Cotrimoxazole reduces IL-8 production by gut epithelial cells
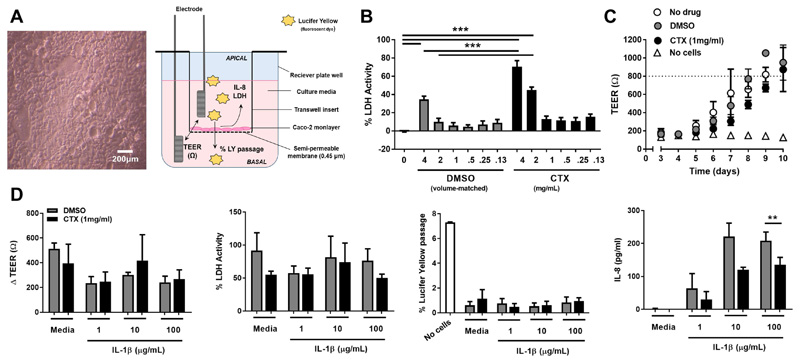
The gut epithelium provides a barrier between the microbiota and mucosal immune cells, responds to TLR ligands, and produces leukocyte chemoattractants under inflammatory conditions; direct effects of cotrimoxazole on epithelial cell function could contribute to its anti-inflammatory effects. To isolate direct effects of cotrimoxazole on the epithelial barrier from its impact on leukocytes or the microbiome, we used transwell cultures of the Caco-2 human colonic epithelial cell-line as a well-established model of gut epithelium. We induced epithelial inflammation with IL-1β and evaluated the effect of cotrimoxazole on four epithelial functions: epithelial integrity (trans-epithelial resistance, TEER), epithelial cell death (%Lactose dehydrogenase (LDH) activity), apical-to-basal translocation of a fluorescent dye (%Lucifer Yellow passage, a proxy for gut-to-circulation microbial translocation), and production of the neutrophil chemoattractant IL-8 (Fig. 6A). We used high cotrimoxazole concentrations for these experiments to reflect the concentration found in the gut lumen following oral dosing, after first titrating cotrimoxazole in Caco-2 cultures to identify a dose that did not differ in cytotoxicity from DMSO controls (1mg/mL; Fig. 6B).
Figure 6. Cotrimoxazole reduces in vitro IL-8 production by gut epithelial cells under inflammatory conditions.
(A) Light microscopy of confluent Caco-2 monolayer (200 μm scale bar) and diagram showing transwell culture model. (B) Percentage lactose dehydrogenase activity relative to lysed cells (%LDH) of Caco-2 cultured for 24h with titrated concentrations of cotrimoxazole (CTX; black bars) or volume-matched DMSO control (grey bars); %LDH compared to untreated controls and between volume-matched pairs of cotrimoxazole and DMSO by adjusted Tukey’s test; ***p<0.001. (C) Daily trans-epithelial resistance (TEER) in transwell Caco-2 cultures without drug (white circles), 1 mg/mL cotrimoxazole (black circles) or DMSO (grey circles) relative to transwells without Caco-2 (no cells; white triangles); mean ±SEM, n=3 separate experiments. Dotted line indicates culture confluence (TEER≥800Ω). (D) Epithelial cell functions (Δ TEER, % LDH, % apical-to-basal passage of Lucifer Yellow dye relative to transwells without Caco-2 cells, and IL-8 concentration in apical supernatants) of confluent Caco-2 monolayers treated with 1 mg/mL CTX or DMSO since seeding, then incubated with media alone (no stimulus) or IL-1β for 24h; mean ±SEM, n=3 separate experiments. Cotrimoxazole and DMSO-treatment compared by 2-tailed t-tests; *p<0.05, **p<0.01
Cotrimoxazole treatment throughout Caco-2 growth did not significantly alter the rate of monolayer confluence (mean TEER/plate >800Ω; Fig. 6C), ΔTEER, %LDH activity or %Lucifer yellow passage under inflammatory conditions (1, 10, or 100μg/mL IL-1β for 24h; Fig. 6D). However, cotrimoxazole-treated monolayers produced significantly less IL-8 than control-treated cultures when the inflammatory stimulus was highest (100μg/mL IL-1β, p=0.003, Fig. 6D). Taken together, these experiments suggest that cotrimoxazole directly inhibits IL-8 production by gut epithelial cells, which may contribute to reduced neutrophil recruitment to the intestinal mucosa under inflammatory conditions.
Discussion
Inflammation drives morbidity and mortality in HIV infection. There is therefore interest in using anti-inflammatory agents with ART to improve clinical outcomes(39–42). Long-term cotrimoxazole prophylaxis is recommended for children and adults living with HIV in settings with high prevalence of malaria or invasive bacterial infections, although global coverage remains poor(4, 43). We show here that cotrimoxazole reduces systemic inflammation in ART-treated children in sub-Saharan Africa, and demonstrate several underlying mechanisms, including antibiotic effects on the gut microbiome and direct anti-inflammatory effects on leukocytes and gut epithelial cells. Synergy between antibiotic and anti-inflammatory pathways may explain the sustained clinical benefits of cotrimoxazole(3, 27) and provides an additional rationale for increasing cotrimoxazole coverage in sub-Saharan Africa.
Using samples from the ARROW trial, we show definitively, using the randomized stop-versus-continue design, that systemic inflammatory biomarkers (CRP and IL-6) are reduced by cotrimoxazole. Pre-ART levels of CRP and IL-6, but not TNFα or sCD14, predicted mortality, WHO stage 4 clinical events, and poor CD4 reconstitution in ARROW; a 2-fold increase in CRP or IL-6 was independently associated with 19% and 54% increased risk, respectively(5). Based on these predictions, the reductions in CRP and IL-6 among children continuing cotrimoxazole would reduce the relative risk of adverse outcomes by 13% and 11%, respectively. HIV-positive children have lower absolute mortality risk after starting ART; however, our estimates highlight that the additive anti-inflammatory benefits of continuing cotrimoxazole are clinically meaningful. Previous studies suggest that Systemic inflammatory mediators are better predictors of poor clinical outcomes than T cell activation among HIV-positive people in resource-limited settings(44). Our assessment of circulating immune cell activation was limited to HLA-DR expression on CD4+ T cells, which did not differ between randomized groups. However, we observed lower percentages of proliferating naïve CD4+ T cells among children continuing cotrimoxazole, which we interpret as beneficial, since elevated CD4+ T cell proliferation without a corresponding increase in total counts leads to depletion of the naïve T-cell pool(45).
We went on to explore potential explanatory mechanisms. Systemic inflammation in HIV infection is partly driven by enteropathogen carriage and chronic enteropathy(11–13, 15). Using stool samples from a subset of ARROW children, we demonstrated that VGS were less abundant at week-84 and week-96 post-randomization in those continuing cotrimoxazole. Since speciation of VGS is challenging, we confirmed these differences using high-resolution mapping of metagenome sequencing reads to Streptococcal pangenomes databases(36). Cotrimoxazole effects on VGS are particularly striking because global microbiome community composition did not differ between randomized groups, likely because all children had received cotrimoxazole for median 2 years pre-randomization(27). VGS are a heterogeneous group of bacteria, which can be both commensal and pathogenic(46). They are found throughout the healthy human gut(47, 48) and are enriched in stool samples from children with stunting(49), a form of chronic malnutrition associated with systemic inflammation(50). VGS express several immune-stimulatory antigens that may drive intestinal inflammation, and potently trigger innate immune cell cytokine production in vitro(51). In contrast to changes in VGS, we found no evidence for suppression of Enterobacteriaceae, which include pathogens causing severe bacterial infections in sub-Saharan Africa(34, 35). Our microbiome analyses focused on later timepoints post-randomization, due to stool sample availability; there may plausibly be additional cotrimoxazole-driven changes at earlier timepoints and at other anatomic sites.
Children randomized to continue cotrimoxazole had lower fecal myeloperoxidase, an antimicrobial peroxidase enzyme abundant in neutrophils, and a biomarker of enteropathy(52). Of the cotrimoxazole-affected VGS, S. mutans, S. parasanguinis and S. vestibularis were positively associated with myeloperoxidase, suggesting that sub-clinical antibiotic effects of cotrimoxazole on VGS reduce intestinal inflammation. This does not appear to be a universal characteristic of antibiotic treatment since suppression of gut-resident gram-positive bacteria with vancomycin in rhesus macaques subsequently infected with SIV did not reduce IL-6 or CD4+ T cell activation in mesenteric lymph nodes(53). It is likely that timing of treatment, baseline microbiome, ART history, intercurrent infections, and antibiotic specificity influence the relationship between antibiotic prophylaxis, gut microbiome and enteropathy.
Functional analysis of ARROW stool samples identified a metagenomic signature of mevalonate metabolism, predominantly mapping to VGS, which was positively associated with fecal myeloperoxidase and suppressed by cotrimoxazole. The mevalonate pathway is one of two metabolic processes that produce isoprenoids, naturally-occurring organic precursors in eukaryote cholesterol and prokaryote cell wall peptidoglycan (a TLR2 ligand) synthesis (54). Several in vitro studies indicate that inhibition of mevalonate pathway enzymes impairs innate leukocyte recruitment and pro-inflammatory cytokine responses, providing a precedent for how inhibiting VGS mevalonate metabolism might influence HIV enteropathy. For example, inhibiting farnesyl pyrophosphate synthesis reduces neutrophil priming by IL-8(55), and inhibiting HMG-CoA reductase reduces monocyte IL-6 and IL-8(56) and neutrophil trans-epithelial migration(39, 42). HMG-CoA reductase with identity to Streptococcus parasanguinis and Streptococcus salivarius was among the cotrimoxazole-suppressed mevalonate pathway enzymes identified.
Leukocytes are an abundant source of pro-inflammatory cytokines. Levels of circulating microbial products that could trigger these pathways are elevated during HIV infection, including the TLR4 ligand LPS(10, 15). We developed an in vitro model of leukocyte activation by TLR ligands to isolate direct anti-inflammatory effects of cotrimoxazole from its antibiotic effects, using blood samples from HIV-negative and HIV-positive U.K. adults not receiving cotrimoxazole. Although this cohort differed in age, geographic location, likely HIV clade and co-morbidities compared to children in ARROW, these in vitro experiments provide proof-of-concept that physiologically-relevant cotrimoxazole doses consistently inhibited whole blood TNFα and IL-6 production elicited via TLR2, 4 and 5. Collectively, these findings suggest that modulation of innate pro-inflammatory cytokine production is a property of cotrimoxazole per se, affects multiple innate signaling pathways, and occurs independently of its antibiotic effects, HIV-driven inflammation or ART exposure. Intracellular cytokine staining suggested that monocyte rather than T cell cytokine production was most affected by cotrimoxazole. Our demonstration of direct modulation of pro-inflammatory cytokine production by human leukocytes clarifies a longstanding theory that cotrimoxazole modulates immune responses in mice via an undefined mode-of-action(22), for which subsequent in vitro models have yielded opposing conclusions for innate and adaptive immune cells (23–26). Although these immunomodulatory effects were quantitatively subtle, our relative risk estimates in ARROW indicate that even small reductions in inflammatory markers may improve clinical outcomes(5). The pharmacology of cotrimoxazole-mediated immunosuppression, its interaction with TLR signaling and potential therapeutic value in other inflammatory disorders are yet to be established.
Cotrimoxazole reduced production of the neutrophil chemoattractant IL-8 by gut epithelial cells in vitro. This is a putative pathway through which cotrimoxazole could directly contribute to reduced neutrophil recruitment and myeloperoxidase production in the gut mucosa. Cotrimoxazole did not alter epithelial characteristics associated with barrier function in vitro; however, it remains possible that cotrimoxazole alters these pathways in vivo by affecting gut barrier components such as mucus(19) and tight junction proteins(53), which we did not model. Primary epithelial cells and biopsies, which would better mimic trans-epithelial transport in vivo, were not available from ARROW. Since VGS express abundant TLR2 ligands and Caco-2 have limited TLR2 expression(57), alternative epithelial models are required to explore inter-relationships between cotrimoxazole, VGS metabolism and epithelial barrier function.
Our study raises the possibility that antibiotics other than cotrimoxazole may confer anti-inflammatory benefits that contribute to their impact at scale, including the recent finding of reduced child mortality following mass administration of azithromycin in sub-Saharan Africa(58). Accessory benefits from antibiotics are important considerations in the debate around antimicrobial stewardship, particularly in settings where antimicrobial resistance is already high and in conditions such as HIV, where chronic inflammation combines with intercurrent infection to exacerbate clinical outcomes. Whether cotrimoxazole has clinical benefits for HIV-positive people in high-income settings, where long-term cotrimoxazole prophylaxis is not currently recommended and ART alone does not fully prevent pathology, warrants further study. Recognition of its anti-inflammatory benefits should drive renewed efforts for universal cotrimoxazole coverage to improve clinical outcomes for all people living with HIV in sub-Saharan Africa.
Materials & Methods
Study design
The study objective was to determine whether cotrimoxazole has anti-inflammatory effects, and to elucidate underlying mechanisms. Experimental work comprised: 1) analysis of longitudinal blood samples (using ELISA and flow cytometry) and stool samples (using ELISA and whole metagenome sequencing) collected from HIV-positive Ugandan and Zimbabwean children randomized to continue versus stop open-label cotrimoxazole in the ARROW trial(27), until 16th March 2012; and, 2) in vitro cotrimoxazole treatment using blood samples from U.K. adults (ELISA and flow cytometry) and epithelial cell-line (Caco-2) cultures. Full details are in Supplementary Materials and Methods.
Within ARROW, children/adolescents (median age: 7.9 years, IQR: 4.6, 11.1) who had been receiving ART and once-daily cotrimoxazole prophylaxis (200mg of sulfamethoxazole and 40mg of trimethoprim, 400mg sulfamethoxazole/80mg trimethoprim, or 800mg sulfamethoxazole/160mg trimethoprim for body weight 5-15, 15-30, or >30 kg, respectively) for >96 weeks at four sites in Uganda and Zimbabwe, were randomized to stop (n=382) or continue (n=386) cotrimoxazole(27, 59). Children with a history of Pneumocystis jirovecii pneumonia were excluded(27). 98% of children enrolled into ARROW during the last 6 months of recruitment were also included in an immunology sub-study; additional assays were conducted for these children and for a random 23% sample of all remaining non-immunology sub-study children (5). The current analysis included children with available baseline plasma of sufficient volume to measure inflammatory biomarkers (stop n=149, continue n=144). Stool samples were collected at week-84 and week-96 post-randomization from a subgroup of children in Zimbabwe to assay intestinal inflammation. Total DNA was extracted from 150mg stool for whole metagenome sequencing (stop n=36, continue n=36).
Blood was collected from 8 HIV-uninfected adults, 6 HIV-positive adults on ART for ≥2 years, and 10 HIV-positive ART-naïve adults (table S1) who were not taking cotrimoxazole, for 24h whole blood culture and 6h PBMC culture with bacterial and fungal antigens. Pro-inflammatory cytokine responses were compared between parallel cultures treated with cotrimoxazole and volume-matched diluent without drug (DMSO).
Caco-2 monolayers were grown in transwell cultures as a gut epithelium model. Epithelial functions (integrity, cell death, translocation across the epithelium and chemokine production) were quantified after 24h stimulation with IL-1β and compared between cultures treated with cotrimoxazole or DMSO throughout growth, run in triplicate. Transwell cultures were repeated 3 times using separate Caco-2 passages. Data from individual transwells were excluded if monolayers were sub-confluent.
Ethics
ARROW (ISRCTN Registry# ISRCTN24791884) was approved by Research Ethics Committees in Uganda, Zimbabwe, and the U.K. Written informed consent from all caregivers and assent from participants (where appropriate) was obtained (27, 59). Approval for U.K. donor recruitment was provided by the National Health Service Research Authority (IRAS project ID: 209553; Research Ethics Council reference: 17/WM/0018) and the Research Ethics Committee of Queen Mary University of London. All participants provided written informed consent.
Statistical analysis
For ARROW data, fold-change in geometric means between randomized groups were compared for continuous variables at each timepoint using standard regression models and globally across all timepoints using generalized estimating equations (GEE; normal distribution for log-transformed values), both with adjustment for recruitment center and baseline values, and assuming variation in treatment effect by timepoint. Proportions of children with HIV viral load <80 copies/mL were compared between randomized groups at each timepoint using Exact tests and globally across all timepoints using GEE (binomial distribution) with adjustment for recruitment center and assuming variation in treatment effect by timepoint. Relative risk projections for CRP and IL-6 differences between randomized groups were calculated from the output of models based on enrolment (i.e. pre-ART and pre-cotrimoxazole) biomarker levels in the ARROW immunology sub-cohort (5). GEE and Exact tests were conducted in STATA version 15.1 (StataCorp LLC). Concentrations of fecal inflammatory markers and serum protein (Shapiro-Wilk test for normality, p<0.05) were compared between randomized groups using Mann-Whitney U test in Prism version 7.02 (GraphPad).
For microbiome sequencing data, differences in species relative abundance and diversity between randomized groups were evaluated at each timepoint by intention-to-treat analysis using linear regression models fitted against natural log-transformed inverse Shannon species-level alpha-diversity indices. Species-level beta diversity was evaluated using the Bray–Curtis dissimilarity index, and visualized using NMDS. Differences in relative abundance of species, Pfam, metabolic pathways, and enzymes (microbiome characteristics) were evaluated at each time point by intention-to-treat analysis using separate zero-inflated beta regression models fitted against relative abundances for each microbiome characteristic. Cotrimoxazole treatment effect was the ratio of relative microbiome characteristic abundance in continue versus stop groups. P-values were adjusted for multiple comparisons to maintain the FDR significance level (α=0.05)(60). Only differentially abundant microbiome characteristics with consistent significant differences between groups at both week-84 and week-96 were interpreted as causally related to cotrimoxazole continuation. Rank-based regression models were fitted against fecal myeloperoxidase concentration adjusted for age, sex, and randomized group, with FDR adjustment for multiple comparisons. Microbiome analyses were conducted in R version 3.3.2. VEGAN(61) was used to calculate Shannon diversity, Bray-Curtis dissimilarity and NMDS. Gamlss was used for zero-inflated beta regression(62). Rfit was used for rank-based regression(63).
For U.K. adults, continuous variables were compared between groups using unpaired Kruskall-Wallis tests. Comparisons between drug treatments were only conducted for responses that were significantly up-regulated in antigen-stimulated cultures without drug treatment versus un-stimulated cultures without drug treatment (paired Wilcoxon test, p<0.05). Comparisons between drug treatments used Freidman tests with post-hoc pair-wise comparisons via uncorrected Dunn’s test; post-hoc tests were only conducted where the global test was statistically significant. Caco-2 read-outs (TEER, ΔTEER, % LDH activity, % Lucifer Yellow passage, and IL-8; Shapiro-Wilk test for normality, p>0.05) were compared between cotrimoxazole-treated and DMSO-treated cultures using paired two-tailed t-tests. All analyses were conducted using Prism.
Supplementary Material
One Sentence Summary.
Cotrimoxazole reduces systemic and intestinal inflammation in HIV infection by suppressing gut-resident Streptococci and immune cell activation.
Acknowledgements
We thank participants, caregivers, and staff from all study centers; ViiV Healthcare/GlaxoSmithKline who donated ART and funded ARROW viral loads; Ministries for Health in Uganda and Zimbabwe who provided cotrimoxazole for ARROW; Margaret Govha, Sandra Rukobo, Macklyn Kihembo, Lydia Nakiire and Hannah Poulsom for laboratory work in ARROW; Anele Waters, Sarah Murphy and James Hand for U.K. participant recruitment; and Aine McKnight who hosted U.K. laboratory work.
Funding
Supported by Wellcome Trust (grant# 093768/Z/10/Z and 108065/Z/15/Z to A.J.P. with sub-contract to A.R.M; and 206225/Z/17/Z to C.D.B, co-funded by The Royal Society); Canadian Institutes of Health Research (grant to E.K.G.); Medical Research Council (MRC; grant# G0300400 to A.J.P., V.M., M.B-D., A.K., N.K., D.M.G., A.S.W. and G1001190 to A.J.P., V.M., M.B-D., A.K., N.K., D.M.G., A.S.W.); Department for International Development under MRC/DFID Concordat agreement and EDCTP2 programme supported by the European Union; MRC Clinical Trials Unit at UCL (A.J.S., M.J.S., D.M.G., A.S.W.; grant# MC_UU_12023/26).
Footnotes
This manuscript has been accepted for publication in Science Translational Medicine. This version has not undergone final editing. Please refer to https://stm.sciencemag.org/content/11/486/eaav0537 for the complete version. The manuscript may not be reproduced or used in any manner that does not fall within the fair use provisions of the Copyright Act without the prior written permission of AAAS.
Author Contributions
C.D.B. conceptualized and implemented in vitro work, analyzed data, prepared figures, recruited donors and led manuscript preparation; E.K.G. conceptualized and implemented microbiome analysis, prepared figures and led manuscript preparation; G.P., A.S. and C.B. implemented ARROW biomarker assays; L.T. recruited U.K. donors with oversight from J.R.D. and C.D.B.; L.B. and Y.K. assisted C.D.B. with whole blood culture and Caco-2 assays; N.C. supported Caco-2 experiments; D.M.G. and A.S.W. conceptualized and managed ARROW; N.K., A.J.P., A.S.W., D.M.G., M.J.S. and A.J.S. conceptualized and managed ARROW immunology work; M.B-D., V.M., J.L. and A.K. undertook ARROW clinical management; K.M. managed ARROW assays in Zimbabwe; M.G. and H.G. assisted E.K.G. with microbiome assays; C.P. supported U.K.-based experiments; T.J.E. and A.R.M. conducted PanPhlAn analysis; A.S.W. and A.J.S. conducted ARROW statistical analysis and prepared figures; A.J.P. and A.R.M. conceptualized the study and had primary responsibility for the manuscript. All authors read and contributed to the manuscript and approved submission. C.D.B. and E.K.G. contributed equally. A.R.M. and A.J.P. contributed equally.
Competing Interests
The authors declare no competing interests.
Data and Materials Availability
All data associated with this study are presented in the paper or Supplementary Materials.
References
- 1.UNAIDS. Fact sheet - Latest global and regional statistics on the status of the AIDS epidemic. 2018 Jul; [Google Scholar]
- 2.Church JA, Fitzgerald F, Walker AS, Gibb DM, Prendergast AJ. The expanding role of cotrimoxazole in developing countries. The Lancet Infectious Diseases. 2015;15:327–339. doi: 10.1016/S1473-3099(14)71011-4. [DOI] [PubMed] [Google Scholar]
- 3.Walker AS, Ford D, Gilks CF, Munderi P, Ssali F, Reid A, Katabira E, Grosskurth H, Mugyenyi P, Hakim J, Darbyshire JH, et al. Daily co-trimoxazole prophylaxis in severely immunosuppressed HIV-infected adults in Africa started on combination antiretroviral therapy: An observational analysis of the DART cohort. The Lancet. 2010;375:1278–1286. doi: 10.1016/S0140-6736(10)60057-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.WHO. Guidelines on post-exposure prophylaxis for HIV and the use of co-trimoxazole prophylaxis for HIV-related infections among adults, adolescents and childrenl: Recommendations for a public health approach - December 2014 supplement to the 2013 consolidated ARV guidelines. WHO Press; Geneva, Switzerland: 2014. p. 49. [PubMed] [Google Scholar]
- 5.Prendergast AJ, Szubert AJ, Berejena C, Pimundu G, Pala P, Shonhai A, Musiime V, Bwakura-Dangarembizi M, Poulsom H, Hunter P, Musoke P, et al. Baseline inflammatory biomarkers identify subgroups of HIV-infected African children with differing responses to antiretroviral therapy. Journal of Infectious Diseases. 2016;214:226–236. doi: 10.1093/infdis/jiw148. published online Epub05/1812/23/received04/04/accepted. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kuller LH, Tracy R, Belloso W. Inflammatory and coagulation biomarkers and mortality in patients with HIV infection. PLoS Medicine. 2008;5 doi: 10.1371/journal.pmed.0050203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sandler NG, Wand H, Roque A, Law M, Nason MC, Nixon DE, Pedersen C, Ruxrungtham K, Lewin SR, Emery S, Neaton JD, et al. Plasma levels of soluble CD14 independently predict mortality in HIV infection. Journal of Infectious Diseases. 2011;203:780–790. doi: 10.1093/infdis/jiq118. published online EpubMar 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Campbell JH, Burdo TH, Autissier P, Bombardier JP, Westmoreland SV, Soulas C, González RG, Ratai E-M, Williams KC. Minocycline Inhibition of Monocyte Activation Correlates with Neuronal Protection in SIV NeuroAIDS. PLoS ONE. 2011;6:e18688. doi: 10.1371/journal.pone.0018688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vesterbacka J, Nowak P, Barqasho B, Abdurahman S, Nyström J, Nilsson S, Funaoka H, Kanda T, Andersson L-M, Gisslèn M, Sönnerborg A. Kinetics of microbial translocation markers in patients on Efavirenz or Lopinavir/r based antiretroviral therapy. PLoS ONE. 2013;8:e55038. doi: 10.1371/journal.pone.0055038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Vesterbacka J, Barqasho B, Haggblom A, Nowak P. Effects of co-trimoxazole on microbial translocation in HIV-1-infected patients initiating antiretroviral therapy. AIDS research and human retroviruses. 2015;31:830–836. doi: 10.1089/aid.2014.0366. published online EpubAug. [DOI] [PubMed] [Google Scholar]
- 11.Brenchley JM, Schacker TW, Ruff LE. CD4+ T cell depletion during all stages of HIV disease occurs predominantly in the gastrointestinal tract. Journal of Experimental Medicine. 2004;200 doi: 10.1084/jem.20040874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mavigner M, Cazabat M, Dubois M, L’Faqihi F-E, Requena M, Pasquier C, Klopp P, Amar J, Alric L, Barange K, Vinel J-P, et al. Altered CD4+ T cell homing to the gut impairs mucosal immune reconstitution in treated HIV-infected individuals. Journal of Clinical Investigation. 2012;122:62–69. doi: 10.1172/JCI59011. published online Epub01/03/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Allers K, Fehr M, Conrad K, Epple H-J, Schürmann D, Geelhaar-Karsch A, Schinnerling K, Moos V, Schneider T. Macrophages accumulate in the gut mucosa of untreated HIV-infected patients. Journal of Infectious Diseases. 2013 doi: 10.1093/infdis/jit547. published online EpubOctober 16, 2013. [DOI] [PubMed] [Google Scholar]
- 14.Somsouk M, Estes JD, Deleage C, Dunham RM, Albright R, Inadomi JM, Martin JN, Deeks SG, McCune JM, Hunt PW. Gut epithelial barrier and systemic inflammation during chronic HIV infection. AIDS. 2015;29:43–51. doi: 10.1097/QAD.0000000000000511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Brenchley JM, Price DA, Schacker TW, Asher TE, Silvestri G, Rao S, Kazzaz Z, Bornstein E, Lambotte O, Altmann D, Blazar BR, et al. Microbial translocation is a cause of systemic immune activation in chronic HIV infection. Nature medicine. 2006;12:1365–1371. doi: 10.1038/nm1511. published online EpubDec. [DOI] [PubMed] [Google Scholar]
- 16.Kourtis AP, Ibegbu CC, Wiener J, King CC, Tegha G, Kamwendo D, Kumwenda J, Kaur SP, Flax V, Ellington S, Kacheche Z, et al. Role of intestinal mucosal integrity in HIV transmission to infants through breast-feeding: the BAN study. Journal of Infectious Diseases. 2013;208:653–661. doi: 10.1093/infdis/jit221. published online EpubAug 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lozupone CA, Li M, Campbell TB, Flores SC, Linderman D, Gebert MJ, Knight R, Fontenot AP, Palmer BE. Alterations in the gut microbiota associated with HIV-1 infection. Cell Host & Microbe. 2013;14 doi: 10.1016/j.chom.2013.08.006. 10.1016/j.chom.2013.1008.1006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dillon SM, Lee EJ, Kotter CV, Austin GL, Dong Z, Hecht DK, Gianella S, Siewe B, Smith DM, Landay AL, Robertson CE, et al. An altered intestinal mucosal microbiome in HIV-1 infection is associated with mucosal and systemic immune activation and endotoxemia. Mucosal immunology. 2014;7:983–994. doi: 10.1038/mi.2013.116. published online EpubJul. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dossou-Yovo F, Mamadou G, Soudy ID, Limas-Nzouzi N, Miantezila J, Desjeux J-F, Eto B. Metronidazole or cotrimoxazole therapy is associated with a decrease in intestinal bioavailability of common antiretroviral drugs. PLoS ONE. 2014;9:e89943. doi: 10.1371/journal.pone.0089943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pandrea I, Xu C, Stock JL, Frank DN, Ma D, Policicchio BB, He T, Kristoff J, Cornell E, Haret-Richter GS, et al. Antibiotic and anti-inflammatory therapy transiently reduces inflammation and hypercoagulation in acutely SIV-infected pigtailed macaques. PLoS Pathogens. 2016;12:e1005384. doi: 10.1371/journal.ppat.1005384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Monaco CL, Gootenberg DB, Zhao G, Handley SA, Ghebremichael MS, Lim ES, Lankowski A, Baldridge MT, Wilen CB, Flagg M, Norman JM, et al. Altered virome and bacterial microbiome in Human Immunodeficiency Virus-associated Acquired Immunodeficiency Syndrome. Cell Host & Microbe. 2016;19:311–322. doi: 10.1016/j.chom.2016.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ghilchik MW, Morris AS, Reeves DS. Immunosuppressive powers of the antibacterial agent trimethoprim. Nature. 1970;227:393–394. doi: 10.1038/227393a0. published online EpubJul 25. [DOI] [PubMed] [Google Scholar]
- 23.Anderson R, Grabow G, Oosthuizen R, Theron A, Van Rensburg AJ. Effects of sulfamethoxazole and trimethoprim on human neutrophil and lymphocyte functions in vitro: in vivo effects of cotrimoxazole. Antimicrobial agents and chemotherapy. 1980;17:322–326. doi: 10.1128/aac.17.3.322. published online EpubMar. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pos O, Stevenhagen A, Meenhorst PL, Kroon FP, Van Furth R. Impaired phagocytosis of Staphylococcus aureus by granulocytes and monocytes of AIDS patients. Clinical & Experimental Immunology. 1992;88:23–28. doi: 10.1111/j.1365-2249.1992.tb03033.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wolfish NM, Wassef N, Gonzalez H, Acharya C. Immunologic parameters of children with urinary tract infection: effects of trimethoprim-sulfamethoxazole. Canadian Medical Association journal. 1975;112:76–79. published online EpubJun 14. [PMC free article] [PubMed] [Google Scholar]
- 26.Gaylarde PM, Sarkany I. Suppression of thymidine uptake of human lymphocytes by co-trimoxazole. British medical journal. 1972;3:144–146. doi: 10.1136/bmj.3.5819.144. published online EpubJul 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bwakura-Dangarembizi M, Kendall L, Bakeera-Kitaka S, Nahirya-Ntege P, Keishanyu R, Nathoo K, Spyer MJ, Kekitiinwa A, Lutaakome J, Mhute T, Kasirye P, et al. Prendergast A randomized trial of prolonged co-trimoxazole in HIV-infected children in Africa. New England Journal of Medicine. 2014;370:41–53. doi: 10.1056/NEJMoa1214901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gough EK, Moodie EE, Prendergast AJ, Johnson SM, Humphrey JH, Stoltzfus RJ, Walker AS, Trehan I, Gibb DM, Goto R, Tahan S, et al. The impact of antibiotics on growth in children in low and middle income countries: systematic review and meta-analysis of randomised controlled trials. British medical journal. 2014;348:g2267. doi: 10.1136/bmj.g2267. published online EpubApr 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Prendergast A, Walker AS, Mulenga V, Chintu C, Gibb DM. Improved growth and anemia in HIV-infected African children taking cotrimoxazole prophylaxis. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America. 2011;52:953–956. doi: 10.1093/cid/cir029. published online EpubApr 1. [DOI] [PubMed] [Google Scholar]
- 30.Giorgi JV, Hultin LE, McKeating JA. Shorter survival in advanced human immunodeficiency virus type 1 infection is more closely associated with T lymphocyte activation than with plasma virus burden or virus chemokine coreceptor usage. Journal of Infectious Diseases. 1999;179 doi: 10.1086/314660. [DOI] [PubMed] [Google Scholar]
- 31.Liu Z, Cumberland WG, Hultin LE, Prince HE, Detels R, Giorgi JV. Elevated CD38 antigen expression on CD8+ T cells is a stronger marker for the risk of chronic HIV disease progression to AIDS and death in the Multicenter AIDS Cohort Study than CD4+ cell count, soluble immune activation markers, or combinations of HLA-DR and CD38 expression. Journal of acquired immune deficiency syndromes and human retrovirology : official publication of the International Retrovirology Association. 1997;16:83–92. doi: 10.1097/00042560-199710010-00003. published online EpubOct 01. [DOI] [PubMed] [Google Scholar]
- 32.Tanaskovic S, Fernandez S, Price P, Lee S, French MA. CD31 (PECAM-1) is a marker of recent thymic emigrants among CD4+ T-cells, but not CD8+ T-cells or γδ T-cells, in HIV patients responding to ART. Immunology & Cell Biology. 2010;88:321. doi: 10.1038/icb.2009.108. published online Epub01/12/online. [DOI] [PubMed] [Google Scholar]
- 33.Neff CP, Krueger O, Xiong K, Arif S, Nusbacher N, Schneider JM, Cunningham AW, Armstrong A, Li S, McCarter MD, Campbell TB, et al. Fecal microbiota composition drives immune activation in HIV-infected individuals. EBioMedicine. 2018;30:192–202. doi: 10.1016/j.ebiom.2018.03.024. published online EpubApr. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Powis KM, Souda S, Lockman S, Ajibola G, Bennett K, Leidner J, Hughes MD, Moyo S, van Widenfelt E, Jibril HB, Makhema J, et al. Cotrimoxazole prophylaxis was associated with enteric commensal bacterial resistance among HIV-exposed infants in a randomized controlled trial, Botswana. Journal of the International AIDS Society. 2017;20 doi: 10.1002/jia2.25021. e25021-n/a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mwansa J, Mutela K, Zulu I, Amadi B, Kelly P. Antimicrobial sensitivity in Enterobacteria from AIDS patients, Zambia. Emerging Infectious Diseases. 2002;8:92–93. doi: 10.3201/eid0801.010018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Scholz M, Ward DV, Pasolli E, Tolio T, Zolfo M, Asnicar F, Truong DT, Tett A, Morrow AL, Segata N. Strain-level microbial epidemiology and population genomics from shotgun metagenomics. Nature Methods. 2016;13:435. doi: 10.1038/nmeth.3802. published online Epub03/21/online https://www.nature.com/articles/nmeth.3802#supplementary-information. [DOI] [PubMed] [Google Scholar]
- 37.Lau D, Mollnau H, Eiserich JP, Freeman BA, Daiber A, Gehling UM, Brümmer J, Rudolph V, Münzel T, Heitzer T, Meinertz T, et al. Myeloperoxidase mediates neutrophil activation by association with CD11b/CD18 integrins. Proceedings of the National Academy of Sciences. 2005;102:431–436. doi: 10.1073/pnas.0405193102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chin TW, Vandenbroucke A, Fong IW. Pharmacokinetics of trimethoprim-sulfamethoxazole in critically ill and non-critically ill AIDS patients. Antimicrobial Agents and Chemotherapy. 1995;39:28–33. doi: 10.1128/aac.39.1.28. published online EpubJanuary 1, 1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Funderburg NT, Jiang Y, Debanne SM, Labbato D, Juchnowski S, Ferrari B, Clagett B, Robinson J, Lederman MM, McComsey GA. Rosuvastatin reduces vascular inflammation and T cell and monocyte activation in HIV-infected subjects on antiretroviral therapy. Journal of Acquired Immune Deficiency Syndromes. 2015;68:396–404. doi: 10.1097/QAI.0000000000000478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Paton NI, Goodall RL, Dunn DT, et al. Effects of hydroxychloroquine on immune activation and disease progression among HIV-infected patients not receiving antiretroviral therapy: A randomized controlled trial. Journal of the American Medical Association. 2012;308:353–361. doi: 10.1001/jama.2012.6936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Routy JP, Angel JB, Patel M, Kanagaratham C, Radzioch D, Kema I, Gilmore N, Ancuta P, Singer J, Jenabian MA. Assessment of chloroquine as a modulator of immune activation to improve CD4 recovery in immune nonresponding HIV-infected patients receiving antiretroviral therapy. HIV medicine. 2015;16:48–56. doi: 10.1111/hiv.12171. published online EpubJan. [DOI] [PubMed] [Google Scholar]
- 42.Moore RD, Bartlett JG, Gallant JE. Association between use of HMG CoA reductase inhibitors and mortality in HIV-infected patients. PLoS ONE. 2011;6:e21843. doi: 10.1371/journal.pone.0021843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.WHO. UNAIDS, Unicef, Global HIV/AIDS Epidemic update and health sector progress towards Universal Access. 2011 [Google Scholar]
- 44.Hunt PW, Lee SA, Siedner MJ. Immunologic biomarkers, morbidity, and mortality in treated HIV infection. Journal of Infectious Diseases. 2016;214:S44–S50. doi: 10.1093/infdis/jiw275. published online Epub09/13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hazenberg MD, Otto SA, van Rossum AMC, Scherpbier HJ, de Groot R, Kuijpers TW, Lange JMA, Hamann D, de Boer RJ, Borghans JAM, Miedema F. Establishment of the CD4+ T-cell pool in healthy children and untreated children infected with HIV-1. Blood. 2004;104:3513–3519. doi: 10.1182/blood-2004-03-0805. [DOI] [PubMed] [Google Scholar]
- 46.Doern CD, Burnham C-AD. It's not easy being green: the Viridans Group Streptococci, with a focus on pediatric clinical manifestations. Journal of Clinical Microbiology. 2010;48:3829–3835. doi: 10.1128/jcm.01563-10. published online EpubNovember 1, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Van den Bogert B, Boekhorst J, Herrmann R, Smid EJ, Zoetendal EG, Kleerebezem M. Comparative genomics analysis of Streptococcus isolates from the human small intestine reveals their adaptation to a highly dynamic ecosystem. PLoS ONE. 2014;8:e83418. doi: 10.1371/journal.pone.0083418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Li G, Yang M, Zhou K, Zhang L, Tian L, Lv S, Jin Y, Qian W, Xiong H, Lin R, Fu Y, et al. Diversity of duodenal and rectal microbiota in biopsy tissues and luminal contents in healthy volunteers. Journal of microbiology and biotechnology. 2015;25:1136–1145. doi: 10.4014/jmb.1412.12047. published online EpubJul. [DOI] [PubMed] [Google Scholar]
- 49.Vonaesch P, Morien E, Andrianonimiadana L, Sanke H, Mbecko JR, Huus KE, Naharimanananirina T, Gondje BP, Nigatoloum SN, Vondo SS, Kaleb Kandou JE, et al. Stunted childhood growth is associated with decompartmentalization of the gastrointestinal tract and overgrowth of oropharyngeal taxa. Proceedings of the National Academy of Sciences. 2018;115:E8489–e8498. doi: 10.1073/pnas.1806573115. published online EpubSep 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Prendergast AJ, Rukobo S, Chasekwa B, Mutasa K, Ntozini R, Mbuya MNN, Jones A, Moulton LH, Stoltzfus RJ, Humphrey JH. Stunting is characterized by chronic inflammation in Zimbabwean infants. PLoS ONE. 2014;9:e86928. doi: 10.1371/journal.pone.0086928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.van den Bogert B, Meijerink M, Zoetendal EG, Wells JM, Kleerebezem M. Immunomodulatory properties of Streptococcus and Veillonella isolates from the human small intestine microbiota. PLoS ONE. 2014;9:e114277. doi: 10.1371/journal.pone.0114277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Harper KM, Mutasa M, Prendergast AJ, Humphrey J, Manges AR. Environmental enteric dysfunction pathways and child stunting: A systematic review. PLoS Neglected Tropical Diseases. 2018;12:e0006205. doi: 10.1371/journal.pntd.0006205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ortiz AM, Flynn JK, DiNapoli SR, Vujkovic-Cvijin I, Starke CE, Lai SH, Long ME, Sortino O, Vinton CL, Mudd JC, Johnston L, et al. Experimental microbial dysbiosis does not promote disease progression in SIV-infected macaques. Nature medicine. 2018;24:1313–1316. doi: 10.1038/s41591-018-0132-5. published online EpubSep. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Heuston S, Begley M, Gahan CG, Hill C. Isoprenoid biosynthesis in bacterial pathogens. Microbiology (Reading, England) 2012;158:1389–1401. doi: 10.1099/mic.0.051599-0. published online EpubJun. [DOI] [PubMed] [Google Scholar]
- 55.Elmore MA, Daniels RH, Hill ME, Finnen MJ. Inhibition of neutrophil priming by inhibitors of farnesyl transferase. Biochemical Society transactions. 1997;25:253s. doi: 10.1042/bst025253s. published online EpubMay. [DOI] [PubMed] [Google Scholar]
- 56.Terkeltaub R, Solan J, Barry M, Jr, Santoro D, Bokoch GM. Role of the mevalonate pathway of isoprenoid synthesis in IL-8 generation by activated monocytic cells. Journal of leukocyte biology. 1994;55:749–755. doi: 10.1002/jlb.55.6.749. published online EpubJun. [DOI] [PubMed] [Google Scholar]
- 57.Furrie E, Macfarlane S, Thomson G, Macfarlane GT, Microbiology, G. Gut Biology. Tayside T, Tumour B. Toll-like receptors-2, -3 and -4 expression patterns on human colon and their regulation by mucosal-associated bacteria. Immunology. 2005;115:565–574. doi: 10.1111/j.1365-2567.2005.02200.x. published online Epub11/25/received04/20/revised04/21/accepted. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Keenan JD, Bailey RL, West SK, Arzika AM, Hart J, Weaver J, Kalua K, Mrango Z, Ray KJ, Cook C, Lebas E, et al. Azithromycin to reduce childhood mortality in Sub-Saharan Africa. New England Journal of Medicine. 2018;378:1583–1592. doi: 10.1056/NEJMoa1715474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kekitiinwa A, Cook A, Nathoo K, Mugyenyi P, Nahirya-Ntege P, Bakeera-Kitaka S, Thomason M, Bwakura-Dangarembizi M, Musiime V, Munderi P, Naidoo-James B, et al. Routine versus clinically driven laboratory monitoring and first-line antiretroviral therapy strategies in African children with HIV (ARROW): a 5-year open-label randomised factorial trial. The Lancet. 2013;381:1391–1403. doi: 10.1016/s0140-6736(12)62198-9. published online EpubApr 20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Benjamini Y, Hochberg Y. Controlling the False Discovery Rate: a practical and powerful approach to multiple testing. Journal of the Royal Statistical Society. Series B (Methodological) 1995;57:289–300. [Google Scholar]
- 61.Dixon P, Palmer MW. VEGAN, a package of R functions for community ecology. Journal of Vegetation Science. 2003;14:927–930. doi: 10.1658/1100-9233(2003)014[0927:VAPORF]2.0.CO;2. published online Epub2003/12/01. [DOI] [Google Scholar]
- 62.Rigby RA, Stasinopoulos DM. Generalized additive models for location, scale and shape. Journal of the Royal Statistical Society: Series C (Applied Statistics) 2005;54:507–554. doi: 10.1111/j.1467-9876.2005.00510.x. [DOI] [Google Scholar]
- 63.JD K, McKean J. Rfit: Rank-based estimation for linear models. R Journal. 2012;4:57–64. [Google Scholar]
- 64.Antiretroviral therapy for HIV infection in infants and children: towards universal access: Recommendations for a public health approach. 2006. [PubMed] [Google Scholar]
- 65.Bwakura-Dangarembizi M, Musiime V, Szubert AJ, Prendergast AJ, Gomo ZA, Thomason MJ, Musarurwa C, Mugyenyi P, Nahirya P, Kekitiinwa A, Gibb DM, et al. Prevalence of lipodystrophy and metabolic abnormalities in HIV-infected African children after 3 years on first-line antiretroviral therapy. The Pediatric infectious disease journal. 2015;34:e23–31. doi: 10.1097/inf.0000000000000491. published online EpubFeb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Truong DT, Franzosa EA, Tickle TL, Scholz M, Weingart G, Pasolli E, Tett A, Huttenhower C, Segata N. MetaPhlAn2 for enhanced metagenomic taxonomic profiling. Nature Methods. 2015;12:902–903. doi: 10.1038/nmeth.3589. published online EpubOct. [DOI] [PubMed] [Google Scholar]
- 67.Abubucker S, Segata N, Goll J, Schubert AM, Izard J, Cantarel BL, Rodriguez-Mueller B, Zucker J, Thiagarajan M, Henrissat B, White O, et al. Metabolic reconstruction for metagenomic data and its application to the human microbiome. PLoS Computational Biology. 2012;8:e1002358. doi: 10.1371/journal.pcbi.1002358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Finn RD, Bateman A, Clements J, Coggill P, Eberhardt RY, Eddy SR, Heger A, Hetherington K, Holm L, Mistry J, Sonnhammer EL, et al. Pfam: the protein families database. Nucleic acids research. 2014;42:D222–230. doi: 10.1093/nar/gkt1223. published online EpubJan. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.