Abstract
Fructose intake is known to induce obesity, insulin resistance, metabolic syndrome and non-alcoholic fatty liver disease (NAFLD). We aimed to evaluate the effects of fructose drinking on gut leakiness, endotoxemia, and NAFLD and study the underlying mechanisms in rats, mice, and T84 colon cells. The levels of ileum junctional proteins, oxidative stress markers and apoptosis-related proteins in rodents, T84 colonic cells and human ileums were determined by immunoblot, immunoprecipitation, and immunofluorescence analyses. Fructose drinking caused microbiome change, leaky gut, and hepatic inflammation/fibrosis with increased levels of nitroxidative stress marker proteins cytochrome P450–2E1 (CYP2E1), inducible nitric oxide synthase, and nitrated proteins in small intestine and liver of rodents. Fructose drinking significantly elevated plasma bacterial endotoxin levels likely resulting from decreased levels of intestinal tight junction (TJ) proteins (ZO-1, occludin, claudin-1, and claudin-4), adherent junction (AJ) proteins (β-catenin and E-cadherin), and desmosome plakoglobin along with α-tubulin in wild-type rodents but not in the fructose-exposed Cyp2e1-null mice. Consistently, decreased intestinal TJ/AJ proteins and increased hepatic inflammation with fibrosis were observed in autopsied obese people compared to lean individuals. Furthermore, histological and biochemical analyses showed markedly elevated hepatic fibrosis markers proteins in fructose-exposed rats compared to controls. Immunoprecipitation followed by immunoblot analyses revealed that intestinal TJ proteins were nitrated and ubiquitinated, leading to their decreased levels in the fructose-exposed rats.
Conclusion: These results showed for the first time that fructose intake causes protein nitration of intestinal TJ and AJ proteins, resulting in increased gut leakiness, endotoxemia and steatohepatitis with liver fibrosis, at least partly, through a CYP2E1-dependent manner.
Keywords: fructose drinking, leaky gut, endotoxemia, nonalcoholic steatohepatitis, CYP2E1, oxidative and nitrative stress
Non-alcoholic fatty liver disease (NAFLD) is a hepatic manifestation of metabolic syndrome and the increased incidence of NAFLD is a major public health concern in the western, developed countries.(1) Indeed, NAFLD encompasses a spectrum of liver disease ranging from simple steatosis to nonalcoholic steatohepatitis (NASH), advanced fibrosis/cirrhosis, and progression to hepatic cancer in some patients.(2) Recent studies suggest that over-consumption of western-style high fat diet and/or high-fructose corn syrup, primarily in the form of soft-drinks, is a major causal factor for the development of NAFLD/NASH.(2, 3) Increased fructose intake causes fatty liver in animals along with the increased fat mass and obesity,(2, 3) de novo lipogenesis,(3) and inflammation(2) and induces insulin resistance and metabolic syndrome,(4) particularly in overweight animals and individuals.(2)
Recent studies in both rodent models and people of NAFLD/NASH have shown that dietary habits can influence gut flora, leading to increased gut permeability (leaky gut) and circulating endotoxin levels.(5, 6) An n-6 fatty acid-enriched high-fat and high fructose-containing diet offers a potential explanation for the increased prevalence of intestinal bacterial overgrowth in patients with cirrhosis, leading to NAFLD/NASH.(7) In fact, Bergheim and colleagues previously reported that fructose alone either in drinking water or solid diet can cause insulin resistance, obesity and NAFLD with inflammation. (8–10) These conditions accompanied with leaky gut, contribute to increased levels of serum bacterial endotoxin lipopolysaccharide (LPS) and advanced liver disease. However, in our opinion, the underlying mechanisms of fructose-mediated gut leakiness are poorly understood with respect to the role of increased oxidative and nitrative (nitroxidative) stress in stimulating protein modifications of intestinal proteins, including various tight junction (TJ) and adherent junction (AJ) proteins, and apoptosis of enterocytes.
Therefore, this study was aimed to investigate the roles of the intestinal and hepatic ethanol-inducible cytochrome P450–2E1 (CYP2E1) and inducible nitric oxide synthase (iNOS) in promoting nitroxidative stress, contributing to leaky gut and inflammatory liver disease in fructose-exposed rats and mice. We further studied the roles of microbiome change, apoptosis of intestinal enterocytes and protein modifications of gut TJ/AJ proteins in increased gut leakiness and advanced liver disease in rats and WT versus Cyp2e1-null mice. The effects of fructose on the levels of cell death and TJ proteins were also investigated in T84 colonic cells as a model to strengthen our rodent results and conduct the mechanistic studies on fructose-mediated epithelial barrier dysfunction. Finally, we analyzed the ileums and livers from autopsied obese people with NASH compared to those of non-obese individuals to validate the results observed with the experimental models.
Material and Methods
Human subjects
This study with the protocol to collect ileums and body fluids from autopsied people was approved by the Human Subjects Institutional Review Board of the Seoul National University Medical College, Seoul, Korea. The clinical characteristics of the autopsied obese and non-obese individuals are presented in Table 1.
Table 1.
Clinical data of autopsied human subjects with NASH.
| Sample No. | Gender | Age | BMI (kg/m2) | Group* | Liver Histology |
|---|---|---|---|---|---|
| 1 | Female | 26 | 17.8 | Non-obese | normal |
| 2 | Male | 74 | 17.8 | Non-obese | normal |
| 3 | Female | 46 | 15.9 | Non-obese | normal |
| 4 | Male | 29 | 21.8 | Non-obese | normal |
| 5 | Male | 60 | 16.7 | Non-obese | normal |
| 6 | Male | 58 | 18.9 | Non-obese | normal |
| 7 | Female | 61 | 25.5 | Obese | NASH/fibrosis |
| 8 | Male | 62 | 30.8 | Obese | NASH/fibrosis |
| 9 | Male | 57 | 29.1 | Obese | NASH/fibrosis |
| 10 | Male | 54 | 30.7 | Obese | Not analyzed |
| 11 | Male | 50 | 29.7 | Obese | Not analyzed |
Classification of obesity as recommended by the Asia-Pacific Task Force.
BMI: body mass index.
Animal treatments
All animal experimental procedures were carried out by following the National Institutes of Health (NIH) guidelines for small animal experiments and approved by the NIAAA Institutional Animal Care and Use Committee, as described.(11) Age-matched 7-weeks old female Fischer 344 wild-type (WT) rats (n≥8/group) as well as 6~8 weeks old female WT and Cyp2e1-null mice on Svj129 background (n=6/group)(11) were exposed to tap water (control) or 30% (w/v) fructose in drinking water for 8 weeks ad libitum. The food intake, weight gain, and liver weight in rats or mice are listed in Supporting Table S1. However, we do not know the reason(s) why fructose exposure significantly increased the liquid intake and decreased the chow intake in rats but not in mice.
Histological analysis and measurements of plasma ALT and endotoxin
After exposure to fructose or regular tap water (control) for 8 weeks, each rat or mouse was briefly sedated to carbon dioxide gas followed by decapitation to immediately collect trunk blood, small intestine and liver. Paraffin-embedded blocks of formalin-fixed individual liver or small intestine sections were cut at 4 microns, stained with hematoxylin/eosin (H/E) or Sirius red by American Histolabs, Inc. (Gaithersburg, MD). In addition, the plasma ALT and endotoxin levels were determined by using the standard end-point colorimetric assay kit (TECO Diagnostics, Anaheim, CA) and the commercially available endpoint LAL Chromogenic Endotoxin Quantitation Kit with a concentration range of 0.015–1.2 EU/mL (Thermo Fisher Scientific, Waltham, MA), respectively, as previously described.(11)
Triglyceride determination in liver
Hepatic triglyceride (TG) levels were assessed by using a commercially available kit (Thermo Fisher Scientific), as described.(11)
STASTISTICAL ANALYSIS
The experiments were conducted at least twice, unless otherwise stated. Statistical significance was determined by using two-tailed t-test. ANOVA and Dunnet’s multiple comparison post-test were used to compare the means of multiple groups by using GraphPad Prism 7.0 (GraphPad Software Inc., La Jolla, CA).
Please see Supporting Material and Methods for details.
Results
Increased endotoxin, oxidative stress proteins with decreased intestinal TJ and AJ proteins, and liver fibrosis in autopsied obese people with NASH
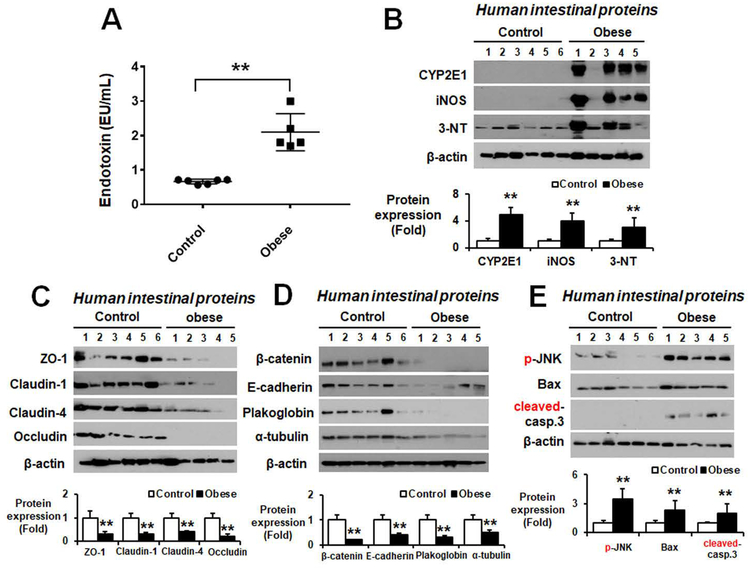
Recent studies suggest that excessive consumption of fructose is linked to the development and severity of NAFLD/NASH, especially in overweight individuals.(3) In addition, high blood LPS levels are found in fructose- and/or western-style high fat diet-induced obesity. Therefore, we evaluated the LPS levels in body fluids of autopsied obese versus non-obese people, whose body mass index (BMI) and other characteristics, including NAFLD/NASH and fibrosis, are summarized Table 1. Liver histology revealed that obese people (BMI≥25) had significantly greater levels of hepatic fat and inflammatory cells (Supporting Fig. S1) with fibrosis (Supporting Fig. S2) than the non-obese individuals (BMI<25). The LPS levels were significantly elevated in the body fluids of obese people with NASH (Fig. 1A).
Figure 1. Increased levels of endotoxin and oxidative stress marker proteins with decreased intestinal TJ and AJ proteins in autopsied obese people.
(A) Plasma endotoxin levels and (B) the amounts of immunoreactive CYP2E1, iNOS, or nitrated proteins detected by anti-3-NT antibody in gut homogenates and densitometric quantitation for each protein relative to β-actin are shown for the indicated groups. (C) Immunoblot results of the intestinal TJ proteins ZO-1, claudin-1, claudin-4, and occludin, (D) the intestinal AJ proteins (β-catenin, E-cadherin), desmosome plakoglobin, and α-tubulin, and (E) apoptosis-associated proteins and their amounts relative to β-actin are presented for the indicated groups. **P < 0.01.
Several reports showed that hepatic CYP2E1 is increased in NASH patients compared with normal individuals.(12) Increased levels of nitroxidative stress, as reflected by elevated levels of CYP2E1, iNOS, and nitrated proteins (Fig. 1B), were observed in ileums of obese people with NASH compared to the non-obese controls. The amounts of the indicated intestinal TJ (Fig. 1C), AJ proteins and plakoglobin (Fig. 1D) were markedly decreased in the obese people with NASH. In addition, apoptosis marker proteins such as p-JNK, Bax, and cleaved caspase-3 were significantly increased in the obese people (Fig. 1E). All these results suggest elevated leaky gut and endotoxemia, resulting in hepatic inflammation (NASH) and fibrosis in obese people.
Increased plasma endotoxin, intestinal CYP2E1 and iNOS proteins in fructose-exposed rats
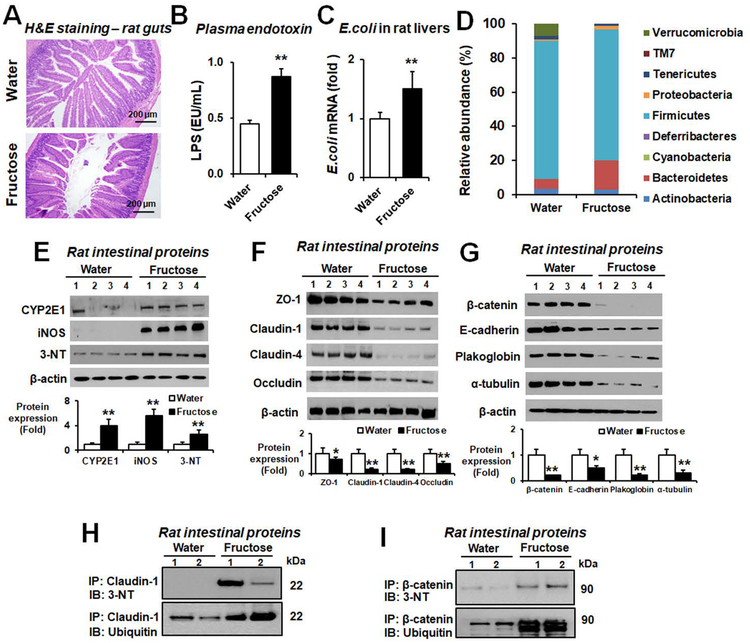
We also evaluated the histological change in small intestines and plasma endotoxin levels in fructose-exposed rats, since fructose was shown to cause NASH through leaky gut and endotoxemia.(8, 13) Histological analysis revealed markedly increased loss, blebbing of the laminar propria, and inflammatory cell infiltration/activation in small intestines and colons in fructose-exposed rats (Fig. 2A and Supporting Fig. S3). Consistently the levels of plasma endotoxin (Fig. 2B) and E. coli mRNA in fresh liver tissues (Fig. 2C) were elevated in fructose-exposed rats compared to water controls. Sequencing analyses of fecal DNA revealed that nine gut bacteria phyla were predominantly detected in water or fructose-exposed rats (Fig. 2D). The increased abundance of Bacteroidetes and Proteobacteria in the fructose-exposed rats were mainly explained by elevated levels of Bacteroides and Escherichia (Supporting Fig. S4). In addition, the decreased abundance of Firmicutes and Verrucomicrobia in the fructose-exposed group could be explained by the reduced amounts of Lactobacillus and Akkermansia (Supporting Fig. S4). These gut microbiota changes in the fructose-exposed group might also contribute to leaky gut, as similar to the earlier report on gut microbiome status in NASH patients.(14)
Figure 2. Fructose drinking stimulated gut leakiness with elevated plasma endotoxin and intestinal CYP2E1 and other oxidative/nitrative stress markers in rats.
Age-matched Fischer F344 female rats were exposed to water or 30% (w/v) fructose in drinking water for 8 weeks (n≥8/group). (A) Representative H&E staining of formalin-fixed small intestine sections, (B) plasma LPS levels, and (C) amounts of E. coli mRNA transcripts in the fresh liver extracts determined by real-time RT-PCR are presented for the indicated rat groups. (D) Comparison of the taxonomic abundance bacteria phyla. (E) Immunoblot results for CYP2E1, iNOS, or nitrated proteins detected by anti-3-NT antibody in gut homogenates and densitometric quantitation for each protein relative to β-actin, used as a loading control, are shown from the indicated groups. (F) Immunoblot results of the intestinal TJ proteins ZO-1, claudin-1, Claudin-4, and occludin and (G) the intestinal AJ proteins (β-catenin, E-cadherin), desmosome plakoglobin, and α-tubulin and their amounts relative to β-actin are presented. (H-I) Same amounts of intestinal proteins from the water control or fructose-exposed rats were immunoprecipitated with the specific antibody to each target protein claudin-1 or β-catenin and then subjected to immunoblot analysis with the specific antibody to 3-NT or ubiquitin. *P < 0.05, **P < 0.01.
CYP2E1 is critically important in causing alcoholic liver injury through increased oxidative stress and gut leakiness in WT mice since Cyp2e1-null mice were protected despite the extreme doses of binge alcohol.(11) Consistently, the amounts of CYP2E1 and iNOS proteins, markers of oxidative and nitrative stress, respectively, were significantly elevated in small intestines of fructose-exposed rats (Fig. 2E).
Role of nitration and ubiquitin-dependent protein degradation of intestinal TJ and AJ proteins in fructose-exposed rats
The amounts of intestinal TJ proteins ZO-1, occludin, claudin-1, and claudin-4, critically important in the integrity and function of the intestinal barrier, were significantly decreased in fructose-exposed rats compared to water controls (Fig. 2F). The levels of AJ proteins (β-catenin and E-cadherin), desmosomes plakoglobin, and α-tubulin were also significantly decreased in fructose-exposed rats (Fig. 2G). Additionally, apoptosis marker proteins such as p-JNK, Bax, cleaved caspase-3, and caspase-3 activity were significantly increased in fructose-exposed rats (Supporting Fig. S5). Immunoprecipitation with the specific antibody to the selected TJ or AJ protein followed by immunoblot analysis with anti-3-NT or anti-ubiquitin antibody revealed that nitration and ubiquitin-conjugation of claudin-1 (Fig. 2H) or β-catenin (Fig. 2I) were significantly increased in fructose-exposed rats, suggesting ubiquitin-dependent proteolytic degradation of the nitrated TJ and AJ proteins, contributing to leaky gut, endotoxemia and inflammatory liver disease.
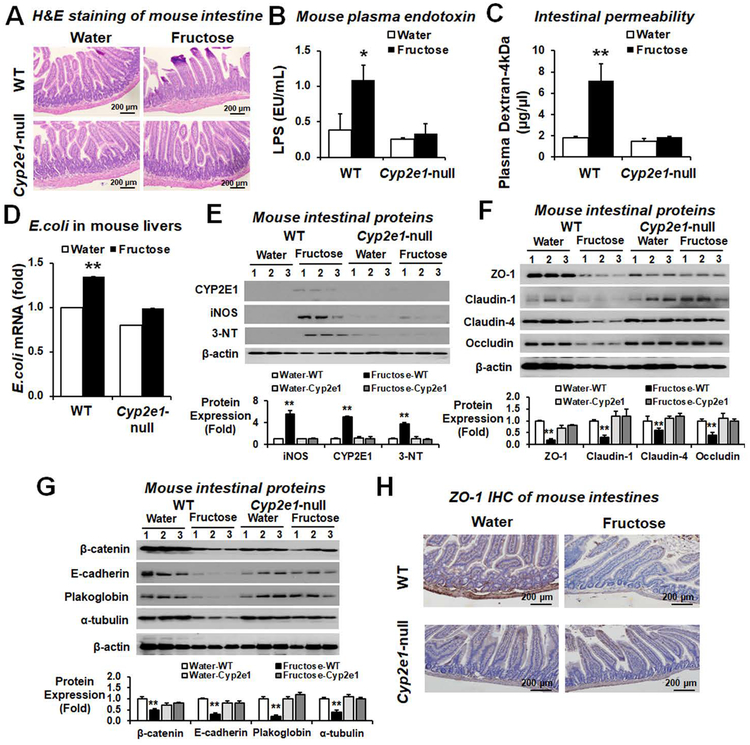
Role of CYP2E1 and oxidative stress in decreased gut junctional proteins in fructose-exposed mice
We further determined the critical role of CYP2E1 in fructose-mediated intestinal barrier dysfunction and endotoxemia in fructose-exposed WT and Cyp2e1-null mice. Histological analysis revealed markedly increased loss, blebbing of the laminar propria, and inflammatory cell infiltration in small intestines and colons in fructose-exposed WT but not in the corresponding Cyp2e1-null mice (Fig. 3A and Supporting Fig. S6). Consistently, the levels of plasma endotoxin (Fig. 3B), in vivo permeability (Fig. 3C), and the E. coli mRNA transcripts in the liver (Fig. 3D) were significantly elevated in fructose-exposed WT but not in the corresponding Cyp2e1-null mice. In addition, the amounts of intestinal CYP2E1, iNOS and nitrated proteins were markedly elevated in fructose-exposed WT (Fig. 3E). Consistently, the amounts of immunoreactive intestinal TJ proteins ZO-1, occludin, claudin-1 and claudin-4 were markedly decreased in fructose-exposed WT mice (Fig. 3F). The levels of intestinal AJ proteins, desmosome plakoglobin and α-tubulin were also markedly decreased in the fructose-exposed WT (Fig. 3G). Immunohistochemistry (IHC) of ZO-1 also showed decreased levels in fructose-exposed WT compared to the water controls or the fructose-exposed Cyp2e1-null mice (Fig. 3H). Additionally, apoptosis marker proteins such as p-JNK, Bax, cleaved caspase-3 and caspase 3 activity (Supporting Fig. S7A,B) as well as TUNEL-positive apoptotic enterocytes (Supporting Fig. S7C) were significantly increased in fructose-exposed WT mice. These results indicate the similar mechanisms of fructose-induced gut leakiness in rats and WT mice through decreased gut TJ/AJ proteins with increased apoptosis of intestinal enterocytes in a CYP2E1-dependent manner. Significantly elevated plasma ethanol concentrations with little changes in the uric acid concentrations were observed in fructose-exposed rats and mice (Supporting Fig. S8A,B, respectively). The levels of plasma reactive oxygen species (ROS) were significantly increased in fructose-drinking rats and WT mice compared to those of the water controls or fructose-exposed Cyp2e1-null mice (Supporting Fig. S9). Furthermore, the levels of decreased TJ and AJ proteins appeared to be correlated with those of the circadian clock proteins CLOCK and PER-2 and their respective mRNAs in fructose-exposed rats and WT mice (Supporting Fig. S10). In contrast, the amounts of CLOCK and PER-2 proteins and their mRNA transcripts were unchanged in the fructose-exposed Cyp2e1-null mice (Supporting Fig. S10C,D, respectively).
Figure 3. Fructose drinking increased endotoxemia, and nitroxidative stress proteins with decreased TJ and AJ proteins in WT compared to Cyp2e1-null mice.
The levels of (A) Representative H&E staining (B) plasma endotoxin, (C) intestinal permeability (D) E. coli mRNA transcripts in the liver extracts assessed by real-time RT-PCR, (E) gut nitroxidative stress marker proteins, (F) gut TJ proteins, and (G) AJ and associated proteins, and a loading control β-actin for the indicated mouse groups (n≥6/group) are presented. *P < 0.05, **P < 0.01. (H) Representative IHC staining for ZO-1 in formalin-fixed small intestine sections of the indicated mouse groups.
Disruption of TJ proteins with elevated apoptosis signals in fructose-exposed T84 colon cells
To further study the underlying mechanisms by which fructose causes intestinal barrier dysfunction, T84 colon cells were used as a model and treated with fructose at 0, 2.5, and 5 mM for 24 h. The amounts of ZO-1 were significantly decreased in fructose-exposed T84 cells (Supporting Fig. S11A). The levels of apoptosis-related marker proteins (phosphorylated-JNK, Bax, and cleaved-caspase 3) were increased in fructose-treated T84 cells (Supporting Fig. S11B). In addition, fructose exposure significantly reduced trans-epithelial electrical resistance (TEER) and increased permeation of FITC-labeled 4-kDa dextran (FITC-D4) (Supporting Fig. S12A, B, respectively). However, co-treatment with the specific CYP2E1 inhibitor chlormethiazole (CMZ) or the specific iNOS inhibitor 1400W efficiently prevented epithelial cell permeability in fructose-exposed T84 cells while these inhibitors significantly decreased the levels of CYP2E1 and iNOS, respectively (Supporting Fig. S12C). These biochemical and confocal image analysis results clearly support an important role of nitroxidative stress in redistribution of ZO-1 and increased apoptosis of fructose-exposed T84 colon cells, contributing to epithelial cell barrier dysfunction.
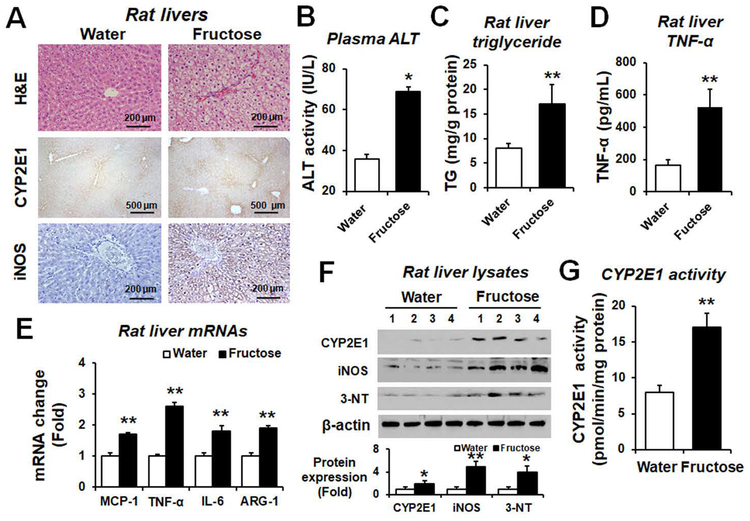
Increased amounts of hepatic fat, proinflammatory cytokines, CYP2E1, and iNOS proteins in fructose-exposed rats
Liver histology and IHC revealed ballooned hepatocytes with increased fat accumulation, CYP2E1 and iNOS contents in fructose-exposed rats compared to water controls (Fig. 4A). Fructose drinking significantly increased the levels of plasma ALT (Fig. 4B), hepatic TG (Fig. 4C), and hepatic TNF-α (Fig. 4D). Additionally, real-time RT-PCR analyses showed increased amounts of hepatic MCP-1, TNF-α, IL-6, and arginase-1 mRNA transcripts in fructose-drinking rats compared to water controls (Fig. 4E). The hepatic CYP2E1 contents (Fig. 4F) and activity (Fig. 4G) as well as the levels of iNOS and nitrated proteins (Fig. 4F) were significantly elevated in fructose-exposed rats. These results suggest that fructose drinking significantly increased the proinflammatory cytokines and hepatic contents of nitroxidative stress marker proteins CYP2E1, iNOS and nitrated proteins in rats. Consistently, the hepatic levels of these oxidative stress marker proteins were significantly increased in the fructose-exposed WT mice (Supporting Fig. S13).
Figure 4. Fructose drinking elevated fatty liver, oxidative stress, and hepatic inflammation in rats.
(A) Representative H&E staining and IHC staining for CYP2E1 or iNOS in formalin-fixed liver sections of the indicated rat groups. (B) Plasma ALT levels, (C) Hepatic triglyceride (TG) levels, and (D) the amounts of hepatic TNF-α measured by ELISA (n≥6/group) are shown for the indicated groups. (E) Relative amounts of the mRNA transcripts of hepatic inflammatory markers as determined by real-time RT-PCR and (F) the immunoreactive levels of hepatic CYP2E1, iNOS, and nitrated proteins detected with anti-3-NT antibody are shown. Densitometric quantitation of each protein relative to β-actin, used as a loading control, is shown. (G) CYP2E1 activity in each indicated group was measured by hydroxylation of p-nitrophenol and expressed as pmol/min/mg of protein. *P < 0.05, **P < 0.01.
Role of CYP2E1 in elevated hepatic fat contents, proinflammatory cytokines, and macrophages infiltration in fructose-exposed mice
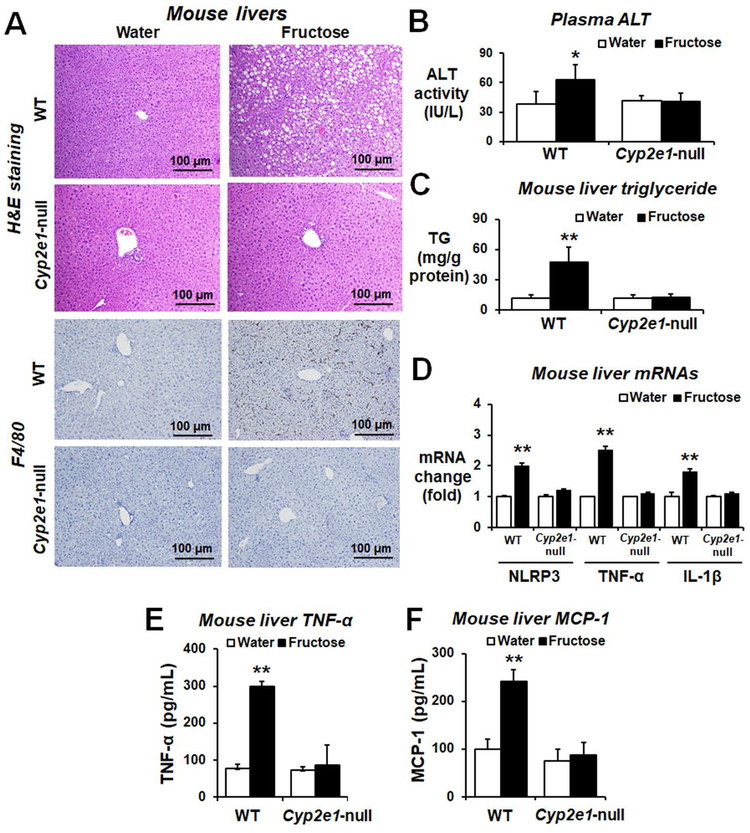
Histological analysis showed numerous lipid droplets, necrotic hepatocytes and infiltration/activation of macrophages determined by F4/80 marker in the liver (Fig. 5A and Supporting Fig. S14). The plasma ALT and hepatic total TG levels (Fig. 5B,C, respectively) were significantly elevated in fructose-exposed WT mice compared to the corresponding Cyp2e1-null mice or water controls. Fructose drinking increased the mRNA amounts of hepatic NLRP3, TNF-α, and IL-1β in WT mice (Fig. 5D). The amounts of hepatic cytokine (TNF-α) and chemokine (MCP-1) proteins were significantly increased in fructose-exposed WT (Fig. 5E,F, respectively). These results suggest that fructose intake increased the hepatic levels of lipids, proinflammatory cytokines, and infiltration of macrophages in WT mice, reflecting NAFLD/NASH in a CYP2E1-dependent manner.
Figure 5. Fructose drinking significantly increased the levels of inflammatory marker proteins in WT mice.
(A) Representative H&E staining and F4/80 immunohistochemistry image of formalin-fixed mouse liver for the indicated groups (n≥6/group). The levels of (B) plasma ALT, (C) hepatic triglyceride (TG) and (D) relative levels of inflammatory marker gene NLRP3, TNF-α, or IL-1β in the fructose-exposed WT or Cyp2e1-null mice and their respective water controls are shown. **P < 0.01. (E-F) The amounts of the TNF-α or MCP-1 protein in the indicated mouse groups. *P < 0.05 **P < 0.01.
Increased liver fibrosis in fructose-exposed rats
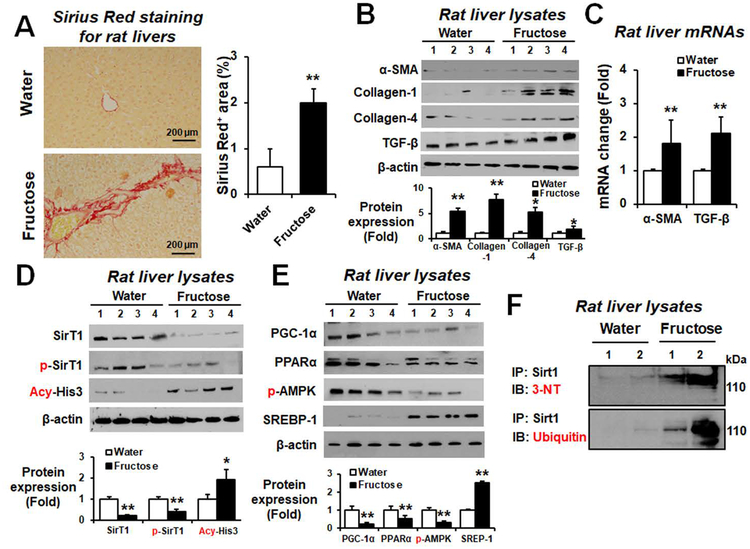
Sirius Red staining showed greater areas of liver fibrosis in fructose-exposed rats compared to the water controls, suggesting that fructose can promote hepatic fibrosis in rats (Fig. 6A). Consistently, fructose exposure significantly elevated the hepatic amounts of α-smooth muscle actin (α-SMA), collagen-1, collagen-4, and TGF-β in rats (Fig. 6B). In addition, the expressed levels of hepatic α-SMA and TGF-β mRNA transcripts were significantly elevated in fructose-exposed rats (Fig. 6C).
Figure 6. Fructose drinking elevated the hepatic fibrosis markers in rats.
(A) Representative Sirius red staining of formalin-fixed liver sections for the indicated rat groups and densitometric quantitation are shown. (B) Immunodetection of the hepatic fibrosis marker proteins (α-SMA, collagen-1, collagen-4, and TGF-β) from the indicated groups. Densitometric quantitation of each protein relative to β-actin, used as a loading control, is shown. (C) Relative amounts of the mRNA transcripts of hepatic fibrosis markers (α-SMA or TGF-β) were determined by real-time RT-PCR and are shown for the indicated rat groups. (D) Immunoblot results for hepatic Sirt1, p-Sirt1, or acetylated-Histone3 (Acy-His3) and (E) hepatic PGC1-α, PPARα, p-AMPK, and SREBP-1 relative to β-actin are shown. (F) Same amounts of liver lysates from the rats exposed to water or fructose were immunoprecipitated with the specific antibody to Sirt1 antibody and then subjected to immunoblot analysis with the antibody to 3-NT or ubiquitin protein, as indicated. *P < 0.05, **P < 0.01.
Hepatic sirtuin-1 (Sirt1), a cytosolic NAD+-dependent protein deacetylase, plays a major role in ameliorating steatosis and inhibiting fibrosis in AFLD.(15) The Levels of hepatic Sirt1 and p-Sirt1 proteins were significantly decreased while acetyl-histone-3 (Acy-His3; Fig. 6D) (were) significantly elevated in fructose-exposed rats. Furthermore, the hepatic amounts of PGC1-α, PPARα, phosphorylated-AMPK proteins were significantly decreased while the SREBP-1 levels were increased in fructose-drinking rats (Fig. 6E). Similarly, the hepatic levels of PGC1-α and PPARα mRNA transcripts were significantly decreased in fructose-exposed rats (Supporting Fig. S15). Immunoprecipitation with the specific antibody to Sirt1 protein followed by immunoblot analysis with anti-3-NT or anti-ubiquitin antibody revealed that nitration and ubiquitination of Sirt-1 were markedly increased in fructose-exposed rats (Fig. 6F). Taken together, the fructose-drinking stimulated liver fibrosis at least partly through the decreased amount of Sirt1 in rats.
However, Sirius Red staining did not show obvious signs of liver fibrosis in fructose-exposed WT and Cyp2e1-null mice (Supporting Fig. S16A). Consistently, the amounts of hepatic α-SMA, collagen-1, and TGF-β proteins were not significantly increased in fructose-exposed WT and Cyp2e1-null mice compared to their respective water controls (Supporting Fig. S16B). These results showed that fructose-drinking only stimulated hepatic fibrosis in rats, but not in mice, as previously reported.(8)
CYP2E1-dependent activation of hepatic stellate cells by LPS
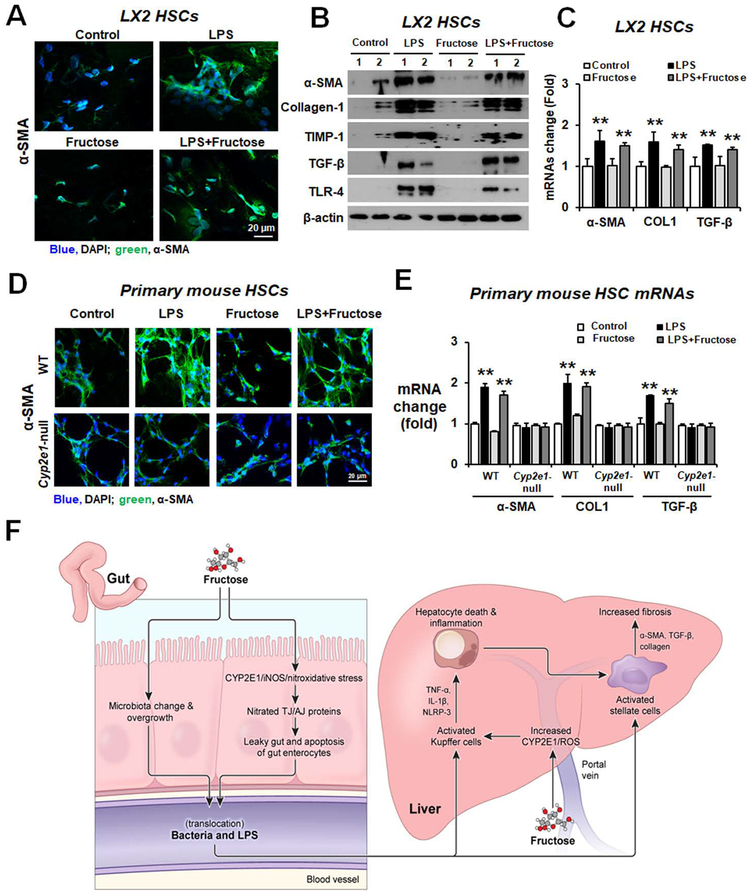
Increased gut permeability with elevated LPS can activate hepatic stellate cells (HSCs), contributing to liver fibrosis.(16) Therefore, we determined whether LPS or fructose can activate LX2 human HSCs and primary mouse HSCs. Confocal microscope analysis showed that α-SMA protein expression was markedly increased in LPS-treated LX2 cells or primary mouse HSCs compared to those treated with fructose or water control (Fig. 7A,D, respectively). The amounts of α-SMA, collagen-1, tissue inhibitor of metalloproteinase-1 (TIMP-1), TGF-β, and TLR-4 proteins were significantly elevated in LPS-treated LX2 cells (Fig. 7B). The expressed levels of α-SMA, collagen-1 (COL1), and TGF-β mRNAs were also increased in LPS-treated LX2 cells (Fig. 7C) or primary mouse HSCs (Fig. 7E). However, fructose alone did not increase the expression of these fibrosis-marker proteins and their mRNA transcripts in HSCs. In addition, the liver fibrosis marker proteins in primary mouse HSCs isolated from Cyp2e1-null mice were unchanged by the same LPS treatment (data not shown). These results strongly indicate that the elevated LPS, resulting from fructose-induced leaky gut but not fructose per se, is likely to activate HSCs, contributing to liver inflammation/fibrosis in a CYP2E1-dependent manner, emphasizing the critical roles of CYP2E1, gut leakiness and endotoxemia in promoting inflammatory liver disease through the gut-liver axis.
Figure 7. LPS activated the hepatic stellate cells.
(A) LX2 cells were treated with water, LPS, fructose (2.5 mM), or LPS+fructose for 24 h. The confocal image shows upregulation of α-SMA in LPS-exposed LX2 cells. Nuclei of LX2 cells were counterstained with DAPI. (B) Immunoblot results for fibrosis marker proteins α-SMA, collagen-1, TIMP-1, TGF-β and TLR-4 in LX2 cells. (C) Real-time PCR results for fibrosis marker mRNAs α-SMA, collagen-1 (COL1), and TGF-β in LX2 cells. (D) The primary HSCs from WT or Cyp2e1-null mice were treated with LPS, fructose, or LPS+fructose for 24 h. The confocal image shows increased α-SMA in LPS-exposed mouse primary HSCs from WT mice. Nuclei of mouse primary HSCs were counterstained with DAPI. (E) Real-time PCR results for fibrosis marker mRNAs α-SMA, COL1, and TGF-β in mouse primary HSCs. **P < 0.01. (F) The proposed mechanisms of fructose-induced gut leakiness and liver fibrosis. LPS, lipopolysaccharide.
Discussion
Consumption of soft drinks in the United States has been increased by >40 fold since 1990s and has been implicated as a major risk factor of the development NAFLD/NASH, (17, 18) which can also be caused by a variety of agents including a high-fat western diet containing n-6 fatty acids and cholesterol. Several randomized trials of sugar-containing soft drinks versus low-calorie or calorie-free beverages show that either sugar, 50% of which is fructose, or fructose alone increased serum triglycerides, body weight, visceral adipose tissue, muscle fat, and liver fat in humans.(17) Thus, our experimental models exposed to fructose and mechanistic studies are potentially implicated in human settings where many people, who consume fructose-containing soft drinks, show signs of obesity and NAFLD/NASH.
Recent reports showed that fructose drinking or solid diets can induce inflammatory hepatic injury (NAFLD/NASH) directly or indirectly through alterations in gut microbiota, barrier dysfunction, and loss of TJ proteins with elevated serum LPS,(13) which activates the hepatic TLR4.(19) In fact, Bergheim and colleagues reported that the dietary fructose decreased the amounts of ZO-1 and occludin in the mouse duodenums.(5) However, the roles of CYP2E1 and increased nitroxidative stress in fructose-induced gut leakiness and advanced liver disease and the underlying mechanisms for gut leakiness have been poorly understood. To our knowledge, the contributing roles of CYP2E1, protein modifications of TJ and/or AJ proteins and apoptosis of enterocytes in intestinal permeability and inflammatory liver disease in fructose or other nonalcoholic substances have not been studied systematically. Thus, this study was aimed to evaluate whether fructose drinking can induce liver inflammation and fibrosis through elevated plasma endotoxin resulting from the loss of TJ and AJ protein integrity with elevated apoptosis of enterocytes in rats, mice, T84 colon cells and autopsied human ileum specimens. In our experimental conditions, fructose [30% (w/v) in drinking water] raised the intestinal CYP2E1 levels with elevated oxidative and nitrative stress, which can decrease the amounts of TJ proteins (e.g., ZO-1, claudin-1, claudin-4 and occludin), AJ proteins (e.g., β-catenin and E-cadherin), desmosomes plakoglobin, and α-tubulin with increased apoptosis of gut enterocytes, contributing to increased levels of blood LPS and steatohepatitis (NASH) in rats, mice, and autopsied obese people with NASH and/or fibrosis. The decreased levels of intestinal TJ/AJ proteins in fructose-exposed rats and WT mice were likely caused by nitration followed by ubiquitin-dependent protein degradation,(20, 21) leading to elevated leaky gut. In contrast, Cyp2e1-null mice were resistant to these changes despite the same amount of fructose exposure, confirming an important role of CYP2E1 in gut leakiness and hepatic inflammation (NASH), similar to the intestinal and hepatic injury caused by binge alcohol exposure(11, 22) and western-style high-fat diet-mediated gut leakiness and NASH.(23) Consistent with the results with rodent models, in vitro mechanistic studies with T84 colonic cells also showed disorganization or disruption of ZO-1 TJ protein and elevated apoptosis-related marker proteins after fructose exposure. In this case, the effects of oxidative and nitrative stress on fructose-mediated epithelial barrier dysfunction, determined by TEER and FITC-D4 transport, were significantly blocked by the treatment with the specific inhibitor of CYP2E1 or iNOS. Although we have not studied nitration and ubiquitin conjugation of many TJ and AJ proteins, it is likely that other junctional complex proteins might undergo similar types of modifications followed by proteolytic degradations, resulting in their decreased levels. Nonetheless, these results indicate that both apoptosis of intestinal enterocytes and decreased TJ/AJ proteins contribute to epithelial cell barrier dysfunction and leaky gut, leading to inflammatory liver disease. Furthermore, our results with cultured HSC cells underscore a contributing role of gut leakiness (with elevated endotoxins) in stimulating liver fibrosis, since fructose alone did not activate human and mouse HSCs, while these cells were activated by LPS.
We observed that fructose did not cause additional weight gain in WT mice compared to controls, similar to the previous report of little body weight change in fructose-exposed mice compared to the control mice.(8) However, as expected, fructose markedly increased the levels of plasma ALT and hepatic TG, which is reflected by ballooned hepatocytes with numerous lipid droplets possibly through de novo lipogenesis.(3) Recent report suggested that TNF-α plays a casual role in the onset of fructose-induced NAFLD/NASH and insulin resistance in mice.(10) Consistent to the histological and biochemical data, the levels of hepatic TNF-α and MCP-1 were significantly elevated in fructose-exposed rats and WT mice but not in the corresponding Cyp2e1-null counterparts.
Miele et al. demonstrated that gut dysbiosis can cause intestinal barrier dysfunction, leading to the development of obesity-related NAFLD/NASH, and patients with NAFLD/NASH were known to have intestinal bacterial overgrowth and increased intestinal permeability.(7) Our study also revealed unique composition, ecological diversity, and enterotyping patterns of gut microbiomes in the fructose-exposed rats possibly through adaptation, as reviewed.(24) Furthermore, the patterns of the intestinal bacterial composition in the fructose-exposed rats were similar to the changes in gut microbiome in NASH patients.(14) For instance, the increased abundance of Bacteroides(25, 26) and Escherichia(14) with decreased levels of Lactobacillus- and Akkermansia(27) in the fructose-exposed rats may also stimulate intestinal permeability change, contributing to leaky gut, liver inflammation and/or fibrosis.
The gut microbiome changes in the fructose-exposed rodents may also be responsible for the endogenous ethanol production, although we do not know how fructose exposure would alter the gut microbiomes. Altered nuclear farnesoid and G-protein coupled receptors with decreased enterohepatic circulation of bile acids are known to regulate gut microbiome population, which in turn modulates the rates of bile acid production.(28) In addition, Schnabl and colleagues recently reported that chronic alcohol exposure disrupted the enterohepatic circulation of bile acids and reduced the intestinal farnesoid X receptor (FXR)/fibroblast growth factor 15/19 (FGR15/19) axis, which could in turn change the gut microbiome in mice with alcoholic liver disease.(29) Whether the altered bile acids and FXR/FGR15 pathway are also responsible for the gut microflora changes in fructose-exposed rodents need further investigations.
Keshavarzian and colleagues recently reviewed that CYP2E1 is the major mediator of alcohol-induced gut leakiness.(30) In this report, elevated CYP2E1 following alcohol exposure was suggested to regulate the circadian clock proteins CLOCK and PER-2, both of which are important in alcohol-induced epithelial barrier dysfunction and leaky gut in ethanol-exposed Caco-2 colon cells and mice, respectively, through increased oxidative and nitrative stress. Suppression of CYP2E1 function with a specific siRNA or treatment with an antioxidant decreased the expression of CLOCK and PER-2 proteins, leading to normalization of the intestinal permeability and liver injury. Interestingly, our results showed increased CYP2E1 in the intestines and livers of fructose-exposed rats and WT mice possibly attributed to enhanced protein stabilization in the presence of elevated plasma ethanol concentration.(31) Consistent with the previous report with the alcohol-exposed models,(30) our quantitative real-time RT-PCR and immunoblot analyses showed significantly elevated mRNA and protein amounts of CLOCK and PER-2 in fructose-exposed rats or WT mice. These results indicate that increased oxidative stress is likely to regulate the circadian rhythm proteins CLOCK and PER-2, which also play a contributing role in fructose-induced leaky gut and inflammatory liver injury in both rats and WT mice in a CYP2E1-dependent manner.
Increased hepatic CYP2E1 is known to directly cause fatty liver disease by elevating oxidative/nitrative stress and various forms of post-translational modification of cellular proteins, contributing to endoplasmic reticulum stress, mitochondrial dysfunction and apoptosis of hepatocytes.(32) Furthermore, intestinal CYP2E1 can cause liver inflammation indirectly through promoting post-translational modifications of the TJ/AJ proteins and apoptosis of enterocytes, contributing to leaky gut and endotoxemia, as demonstrated in binge alcohol-exposed rodents.(11) Based on these facts, it is likely that both intestinal and hepatic CYP2E1 increased in fructose-exposed rats, WT mice and T84 cells could regulate the circadian proteins, elevate oxidative/nitrative stress, and promote post-translational modifications of the junctional complex proteins and apoptosis of enterocytes, resulting in epithelial barrier dysfunction. Consequently, elevated LPS accelerates inflammatory liver disease in a CYP2E1-dependent manner, since Cyp2e1-null mice were resistant to all these changes.
In present study, IHC and immunoblot analyses showed that hepatic amounts of CYP2E1, iNOS, nitrated proteins and CYP2E1 activity were significantly elevated in fructose-exposed rats and WT mice compared to their respective controls. These results are consistent with the earlier reports that hepatic CYP2E1 levels were increased in NASH patients(33) and in animals fed a high-fat diet containing n-6 fatty acids(34) or fructose.(35) CYP2E1 induction or activation is usually associated with an increased production of ROS, as observed in the fructose-exposed rats and WT mice compared to the corresponding Cyp2e1-null mice or the water controls. Elevated ROS and reactive nitrogen species via increased iNOS could be involved in the upregulation of the intestinal circadian clock proteins and nitration of TJ/AJ proteins, leading to the fructose-mediated leaky gut and liver disease, similar to those observed in alcohol-exposed cells or rodents.(30) In fact, several studies indicate that iNOS also plays a critical role in the progression of AFLD(36) and NAFLD, since iNOS-null mice were protected from AFLD and NAFLD/NASH(37) possibly through preventing the endotoxin and TLR-4 interactions, as shown in fructose-induced NAFLD/NASH.(38) In this study, we also demonstrated the important role of CYP2E1 in fructose-mediated gut leakiness and NASH by comparing the results between WT and Cyp2e1-null mice as well as using a specific inhibitor of CYP2E1 in T84 cells. We believe that fructose-mediated epithelial barrier dysfunction and leaky gut are likely caused by CYP2E1-mediated oxidative and nitrative stress.
In NAFLD/NASH patients, daily fructose ingestion is associated with reduced hepatic steatosis but increased hepatic fibrosis.(39) In present study, we found that classical fibrosis marker proteins α-SMA, collagen-1, collagen-4, and TGF-β were elevated in fructose-exposed rats. Similar results were reported for the mice with hepatic fibrosis after exposure to a diet containing saturated fat, fructose, and cholesterol (HFCD).(40) Although liver injury was associated with significantly increased mucosal inflammation, TJ disruption, and intestinal epithelial permeability to bacterial endotoxins, the underlying mechanisms of decreased TJ proteins and apoptosis of enterocytes were not studied. Our current study not only confirmed the contributing role of leaky gut in advanced liver inflammation/fibrosis but also demonstrated the important role of CYP2E1 in causing intestinal barrier dysfunction in fructose-exposed WT rodents and T84 colon cells. Furthermore, we demonstrated the underlying mechanisms of the decreased amounts of several TJ/AJ proteins as well as other desmosome proteins associated with the junctional complex(41) by protein nitration followed by ubiquitin conjugation.
Recently, sirtuin isoforms (Sirts 1–7) have been shown to play important roles in the pathophysiology of various metabolic diseases including NAFLD.(42) Sirt1 has received great attention because it increases expression of antioxidant proteins and decreases apoptosis and inflammation. Indeed, liver-specific deletion of Sirt1 or Sirt1 downregulation worsened hepatic steatosis, inflammation and endoplasmic reticulum stress in mouse models.(43) The amounts of hepatic Sirt1 were also decreased in fructose-exposed rats.(44) We further demonstrated a mechanism of decreased levels of Sirt1 through nitration and ubiquitin conjugation in fructose-exposed rats. The current study also suggests that fructose-mediated Sirt1 inhibition can directly regulate the PGC1-α signaling, which is critically important in mitochondrial biogenesis and other functions.(45) Taken together, decreased Sirt1 is also likely to contribute to the development of steatohepatitis and hepatic fibrosis in fructose-exposed rats.
In summary, our data demonstrate for the first time that fructose intake causes gut microbiome change, apoptosis of intestinal enterocytes, and tyrosine nitration of intestinal TJ and AJ proteins through increased gut CYP2E1 and nitroxidative stress. These changes result in elevated intestinal barrier dysfunction and endotoxemia, eventually contributing to the activation of hepatic TLR4 and development of NAFLD/NASH with hepatic fibrosis in rats. Most of the results of increased CYP2E1, nitroxidative stress markers, apoptosis-associated proteins, epithelial cell barrier dysfunction with decreased gut TJ/AJ complex proteins, and elevated liver inflammation were also observed in fructose-exposed WT mice (and T84 cells) but not in the corresponding Cyp2e1-null mice. These results support the important role of CYP2E1 in promoting leaky gut and the development of NASH.(11, 22, 23, 30) Finally, the results observed in experimental models were also consistently observed in the ileums of autopsied obese people, suggesting the existence of conserved mechanisms of the gut-liver interactions among different species.
Supplementary Material
Acknowledgments:
We thank Dr. Klaus Gawrisch for supporting our experiments. We are also grateful to Dr. Thomas Hennessey and colleagues at Omega Bioservices Inc. for cecal bacterial DNA sequencing and bioinformatics analyses.
Financial support:
This study was supported by the Intramural Research Program of the National Institute on Alcohol Abuse and Alcoholism. In addition, this research was partially supported by the Korean Biomedical Scientist Fellowship Program Award (to Y.E. Cho) provided by Korean Research Institute of Bioscience and Biotechnology.
Abbreviations:
- AFLD
alcoholic fatty liver disease
- AJ
adherent junction
- ALT
alanine aminotransferase
- BMI
body mass index
- Col-1
collagen-1
- CYP2E1
ethanol-inducible cytochrome P450–2E1
- FITC-D4
FITC-labeled 4-kDa dextran
- HSC
hepatic stellate cell
- IHC
immunohistochemistry
- iNOS
inducible nitric oxide synthase
- LPS
lipopolysaccharide
- NAFLD
non-alcoholic fatty liver disease
- NASH
nonalcoholic steatohepatitis
- α-SMA
α-smooth muscle actin
- SREBP
sterol regulatory element-binding protein
- TEER
trans-epithelial electrical resistance
- TIMP-1
tissue inhibitor of metalloproteinase-1
- TJ
tight junction
- WT
wild-type
- PGC1-α
proliferator-activated receptor-γ coactivator
- PPARα
peroxisome proliferator activated receptor alpha
- TGF-β1
Transforming growth factor beta 1
Footnotes
Conflicts of interest statement: All authors declare that they have no competing financial interests with respect to this manuscript.
References
- 1.Clark JM, Brancati FL, Diehl AM. Nonalcoholic fatty liver disease. Gastroenterology 2002;122:1649–1657. [DOI] [PubMed] [Google Scholar]
- 2.Lim JS, Mietus-Snyder M, Valente A, Schwarz JM, Lustig RH. The role of fructose in the pathogenesis of NAFLD and the metabolic syndrome. Nat Rev Gastroenterol Hepatol 2010;7:251–264. [DOI] [PubMed] [Google Scholar]
- 3.Softic S, Gupta MK, Wang GX, Fujisaka S, O’Neill BT, Rao TN, Willoughby J, et al. Divergent effects of glucose and fructose on hepatic lipogenesis and insulin signaling. J Clin Invest 2017;127:4059–4074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Balakumar M, Raji L, Prabhu D, Sathishkumar C, Prabu P, Mohan V, Balasubramanyam M. High-fructose diet is as detrimental as high-fat diet in the induction of insulin resistance and diabetes mediated by hepatic/pancreatic endoplasmic reticulum (ER) stress. Mol Cell Biochem 2016;423:93–104. [DOI] [PubMed] [Google Scholar]
- 5.Sellmann C, Priebs J, Landmann M, Degen C, Engstler AJ, Jin CJ, Garttner S, et al. Diets rich in fructose, fat or fructose and fat alter intestinal barrier function and lead to the development of nonalcoholic fatty liver disease over time. J Nutr Biochem 2015;26:1183–1192. [DOI] [PubMed] [Google Scholar]
- 6.Spruss A, Kanuri G, Wagnerberger S, Haub S, Bischoff SC, Bergheim I. Toll-like receptor 4 is involved in the development of fructose-induced hepatic steatosis in mice. Hepatology 2009;50:1094–1104. [DOI] [PubMed] [Google Scholar]
- 7.Miele L, Valenza V, La Torre G, Montalto M, Cammarota G, Ricci R, Masciana R, et al. Increased intestinal permeability and tight junction alterations in nonalcoholic fatty liver disease. Hepatology 2009;49:1877–1887. [DOI] [PubMed] [Google Scholar]
- 8.Bergheim I, Weber S, Vos M, Kramer S, Volynets V, Kaserouni S, McClain CJ, et al. Antibiotics protect against fructose-induced hepatic lipid accumulation in mice: role of endotoxin. J Hepatol 2008;48:983–992. [DOI] [PubMed] [Google Scholar]
- 9.Jegatheesan P, Beutheu S, Ventura G, Nubret E, Sarfati G, Bergheim I, De Bandt JP. Citrulline and Nonessential Amino Acids Prevent Fructose-Induced Nonalcoholic Fatty Liver Disease in Rats. J Nutr 2015;145:2273–2279. [DOI] [PubMed] [Google Scholar]
- 10.Kanuri G, Spruss A, Wagnerberger S, Bischoff SC, Bergheim I. Role of tumor necrosis factor alpha (TNFalpha) in the onset of fructose-induced nonalcoholic fatty liver disease in mice. J Nutr Biochem 2011;22:527–534. [DOI] [PubMed] [Google Scholar]
- 11.Cho YE, Yu LR, Abdelmegeed MA, Yoo SH, Song BJ. Apoptosis of enterocytes and nitration of junctional complex proteins promote alcohol-induced gut leakiness and liver injury. J Hepatol 2018;69:142–153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Weltman MD, Farrell GC, Hall P, Ingelman-Sundberg M, Liddle C. Hepatic cytochrome P450 2E1 is increased in patients with nonalcoholic steatohepatitis. Hepatology 1998;27:128–133. [DOI] [PubMed] [Google Scholar]
- 13.Spruss A, Bergheim I. Dietary fructose and intestinal barrier: potential risk factor in the pathogenesis of nonalcoholic fatty liver disease. J Nutr Biochem 2009;20:657–662. [DOI] [PubMed] [Google Scholar]
- 14.Zhu L, Baker SS, Gill C, Liu W, Alkhouri R, Baker RD, Gill SR. Characterization of gut microbiomes in nonalcoholic steatohepatitis (NASH) patients: a connection between endogenous alcohol and NASH. Hepatology 2013;57:601–609. [DOI] [PubMed] [Google Scholar]
- 15.You M, Jogasuria A, Taylor C, Wu J. Sirtuin 1 signaling and alcoholic fatty liver disease. Hepatobiliary Surg Nutr 2015;4:88–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bataller R, Brenner DA. Liver fibrosis. J Clin Invest 2005;115:209–218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bray GA. Energy and fructose from beverages sweetened with sugar or high-fructose corn syrup pose a health risk for some people. Adv Nutr 2013;4:220–225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nseir W, Nassar F, Assy N. Soft drinks consumption and nonalcoholic fatty liver disease. World J Gastroenterol 2010;16:2579–2588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sharifnia T, Antoun J, Verriere TG, Suarez G, Wattacheril J, Wilson KT, Peek RM Jr., et al. Hepatic TLR4 signaling in obese NAFLD. Am J Physiol Gastrointest Liver Physiol 2015;309:G270–278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Abdelmegeed MA, Moon KH, Chen C, Gonzalez FJ, Song BJ. Role of cytochrome P450 2E1 in protein nitration and ubiquitin-mediated degradation during acetaminophen toxicity. Biochem Pharmacol 2010;79:57–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ciechanover A The unravelling of the ubiquitin system. Nat Rev Mol Cell Biol 2015;16:322–324. [DOI] [PubMed] [Google Scholar]
- 22.Abdelmegeed MA, Banerjee A, Jang S, Yoo SH, Yun JW, Gonzalez FJ, Keshavarzian A, et al. CYP2E1 potentiates binge alcohol-induced gut leakiness, steatohepatitis, and apoptosis. Free Radic Biol Med 2013;65:1238–1245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Abdelmegeed MA, Choi Y, Godlewski G, Ha SK, Banerjee A, Jang S, Song BJ. Cytochrome P450–2E1 promotes fast food-mediated hepatic fibrosis. Sci Rep 2017;7:39764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Payne AN, Chassard C, Lacroix C. Gut microbial adaptation to dietary consumption of fructose, artificial sweeteners and sugar alcohols: implications for host-microbe interactions contributing to obesity. Obes Rev 2012;13:799–809. [DOI] [PubMed] [Google Scholar]
- 25.Henao-Mejia J, Elinav E, Jin C, Hao L, Mehal WZ, Strowig T, Thaiss CA, et al. Inflammasome-mediated dysbiosis regulates progression of NAFLD and obesity. Nature 2012;482:179–185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Swidsinski A, Weber J, Loening-Baucke V, Hale LP, Lochs H. Spatial organization and composition of the mucosal flora in patients with inflammatory bowel disease. J Clin Microbiol 2005;43:3380–3389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Caesar R, Tremaroli V, Kovatcheva-Datchary P, Cani PD, Backhed F. Crosstalk between Gut Microbiota and Dietary Lipids Aggravates WAT Inflammation through TLR Signaling. Cell Metab 2015;22:658–668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fiorucci S, Distrutti E. Bile Acid-Activated Receptors, Intestinal Microbiota, and the Treatment of Metabolic Disorders. Trends Mol Med 2015;21:702–714. [DOI] [PubMed] [Google Scholar]
- 29.Hartmann P, Hochrath K, Horvath A, Chen P, Seebauer CT, Llorente C, Wang L, et al. Modulation of the intestinal bile acid/farnesoid X receptor/fibroblast growth factor 15 axis improves alcoholic liver disease in mice. Hepatology 2018;67:2150–2166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Forsyth CB, Voigt RM, Keshavarzian A. Intestinal CYP2E1: A mediator of alcohol-induced gut leakiness. Redox Biol 2014;3:40–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Roberts BJ, Song BJ, Soh Y, Park SS, Shoaf SE. Ethanol induces CYP2E1 by protein stabilization. Role of ubiquitin conjugation in the rapid degradation of CYP2E1. J Biol Chem 1995;270:29632–29635. [DOI] [PubMed] [Google Scholar]
- 32.Song BJ, Akbar M, Abdelmegeed MA, Byun K, Lee B, Yoon SK, Hardwick JP. Mitochondrial dysfunction and tissue injury by alcohol, high fat, nonalcoholic substances and pathological conditions through post-translational protein modifications. Redox Biol 2014;3:109–123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chalasani N, Gorski JC, Asghar MS, Asghar A, Foresman B, Hall SD, Crabb DW. Hepatic cytochrome P450 2E1 activity in nondiabetic patients with nonalcoholic steatohepatitis. Hepatology 2003;37:544–550. [DOI] [PubMed] [Google Scholar]
- 34.Abdelmegeed MA, Banerjee A, Yoo SH, Jang S, Gonzalez FJ, Song BJ. Critical role of cytochrome P450 2E1 (CYP2E1) in the development of high fat-induced non-alcoholic steatohepatitis. J Hepatol 2012;57:860–866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pooranaperundevi M, Sumiyabanu MS, Viswanathan P, Sundarapandiyan R, Anuradha CV. Insulin resistance induced by a high-fructose diet potentiates thioacetamide hepatotoxicity. Singapore Med J 2010;51:389–398. [PubMed] [Google Scholar]
- 36.McKim SE, Gabele E, Isayama F, Lambert JC, Tucker LM, Wheeler MD, Connor HD, et al. Inducible nitric oxide synthase is required in alcohol-induced liver injury: studies with knockout mice. Gastroenterology 2003;125:1834–1844. [DOI] [PubMed] [Google Scholar]
- 37.Nozaki Y, Fujita K, Wada K, Yoneda M, Kessoku T, Shinohara Y, Imajo K, et al. Deficiency of iNOS-derived NO accelerates lipid accumulation-independent liver fibrosis in non-alcoholic steatohepatitis mouse model. BMC Gastroenterol 2015;15:42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Spruss A, Kanuri G, Uebel K, Bischoff SC, Bergheim I. Role of the inducible nitric oxide synthase in the onset of fructose-induced steatosis in mice. Antioxid Redox Signal 2011;14:2121–2135. [DOI] [PubMed] [Google Scholar]
- 39.Abdelmalek MF, Suzuki A, Guy C, Unalp-Arida A, Colvin R, Johnson RJ, Diehl AM, et al. Increased fructose consumption is associated with fibrosis severity in patients with nonalcoholic fatty liver disease. Hepatology 2010;51:1961–1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rahman K, Desai C, Iyer SS, Thorn NE, Kumar P, Liu Y, Smith T, et al. Loss of Junctional Adhesion Molecule A Promotes Severe Steatohepatitis in Mice on a Diet High in Saturated Fat, Fructose, and Cholesterol. Gastroenterology 2016;151:733–746 e712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Neunlist M, Van Landeghem L, Mahe MM, Derkinderen P, des Varannes SB, Rolli-Derkinderen M. The digestive neuronal-glial-epithelial unit: a new actor in gut health and disease. Nat Rev Gastroenterol Hepatol 2013;10:90–100. [DOI] [PubMed] [Google Scholar]
- 42.Nassir F, Ibdah JA. Sirtuins and nonalcoholic fatty liver disease. World J Gastroenterol 2016;22:10084–10092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Purushotham A, Schug TT, Xu Q, Surapureddi S, Guo X, Li X. Hepatocyte-specific deletion of SIRT1 alters fatty acid metabolism and results in hepatic steatosis and inflammation. Cell Metab 2009;9:327–338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sodhi K, Puri N, Favero G, Stevens S, Meadows C, Abraham NG, Rezzani R, et al. Fructose Mediated Non-Alcoholic Fatty Liver Is Attenuated by HO-1-SIRT1 Module in Murine Hepatocytes and Mice Fed a High Fructose Diet. PLoS One 2015;10:e0128648. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 45.Alcendor RR, Gao S, Zhai P, Zablocki D, Holle E, Yu X, Tian B, et al. Sirt1 regulates aging and resistance to oxidative stress in the heart. Circ Res 2007;100:1512–1521. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.