Abstract
Rationale:
Occupational exposures at the WTC site after September 11, 2001 have been associated with several presumably inflammatory lower airway diseases. Pulmonary arterial enlargement, as suggested by an increased ratio of the diameter of the pulmonary artery to the diameter of the aorta (PAAr) has been reported as a computed tomographic (CT) scan marker of adverse respiratory health outcomes, including WTC-related disease. In this study, we sought to utilize a novel quantitative CT (QCT) measurement of PAAr to test the hypothesis that an increased ratio is associated with FEV1 below each subject’s statistically determined lower limit of normal (FEV1<LLN).
Methods:
In a group of 1,180 WTC workers and volunteers, we examined whether FEV1<LLN was associated with an increased QCT-measured PAAr, adjusting for previously identified important covariates.
Results:
Unadjusted analyses showed a statistically significant association of FEV1<LLN with PAAr (35.3% vs. 24.7%, p=0.0001), as well as with height, body mass index, early arrival at the WTC disaster site, shorter WTC exposure duration, posttraumatic stress disorder checklist (PCL) score, wall area percent, and evidence of bronchodilator response. The multivariate logistic regression model confirmed the association of FEV1<LLN with PAAr (OR 1.63, 95% CI 1.21, 2.20, p=0.0015) and all the unadjusted associations, except for PCL score.
Conclusions:
In WTC workers, FEV1<LLN is associated with elevated PAAr which, although likely multifactorial, may be related to distal vasculopathy, as has been hypothesized for chronic obstructive pulmonary disease.
Trial registration:
ClinicalTrials.gov identifier .
Keywords: multidetector computed tomography, Computer-Assisted Image Processing, smoke inhalation injury, spirometry, Pulmonary artery, Occupational Medicine, World Trade Center Attack, 2001
Introduction
Pulmonary arterial enlargement, as suggested by an increased ratio of the diameter of the pulmonary artery to the diameter of the aorta (PAAr) has been reported as a computed tomographic (CT) scan marker of adverse respiratory health outcomes, as a reflection of disease-related distal pulmonary vascular abnormalities. A study reported that increased PAAr predicted severe exacerbations among chronic obstructive pulmonary disease patients1. A variety of chronic lower airway diseases has been associated with occupational exposures to dust, gases and fumes during the rescue, search, and recovery efforts at the World Trade Center (WTC) disaster site in 2001–20022,3. A recent case-control study among New York City firefighters reported that increased PAAr was an independent predictor of the reduction of first-second forced expiratory volume (FEV1) below the individuals’ lower limit lower of normal (FEV1<LLN), even after adjustment for WTC exposure, body mass index, and age at the time of the CT4. Interestingly, an FEV1<LLN was in turn associated with serum biomarkers predictive of vasculopathy in that study4. Quantitative CT (QCT) measurements have emerged as powerful research tools in the noninvasive evaluation of the airway, pulmonary parenchymal, and vascular and other thoracic structures, allowing further phenotypical characterization of a variety of lung diseases5.
In this study, we sought to utilize a novel QCT measurement of PAAr to test the hypothesis that an increased ratio is associated with FEV1<LLN in with a substantially larger group of similarly exposed WTC workers and volunteers, also adjusting for a larger number of very important predictors of adverse respiratory health effects in this patient group6.
Methods
Subjects and clinical data acquisition:
All subjects participated in the screening, surveillance, and clinical programs of the World Trade Center (WTC) Clinical Center of Excellence at Mount Sinai Medical Center, in New York City, and were also part of the subcohort (n=1,641) evaluated by the WTC Pulmonary Evaluation Unit (WTC PEU), who underwent chest computed tomography (CT) scanning between 2003 and 2012, as part of their diagnostic evaluation. The study was approved by the Mount Sinai Program for the Protection of Human Subjects (HS12–00925). Details on subject recruitment, eligibility criteria, and screening and surveillance protocols have been previously reported7. In brief, participants were all workers and volunteers who performed rescue, recovery, and service restoration duties at the WTC disaster site from September 11, 2001 to June 2002. This cohort includes all occupational groups, except firefighters8. Beginning in July 2002, all subjects underwent a baseline screening evaluation, which included questionnaires on respiratory symptoms, pre-WTC- and WTC-related occupational exposures, laboratory testing, and spirometry. Subsequent (“monitoring”) health surveillance visits included a similar evaluation at 12- to 18-month intervals, and clinical services were offered for individualized diagnostic and treatment services2,9.
CT imaging procedures:
All CT studies were obtained at Mount Sinai in General Electric® or Siemens® multidetector row chest CT scanners. Chest CT studies were performed using a protocol10,11 with a radiation dose at 120 kVp, and a mean of 146 (SD 69) mAs, with subjects in the supine position. CT scans were obtained from the lung apices to the bases in a single breath hold at maximum inspiration. All deidentified and coded chest CT images were stored and catalogued from 2012 to 2017 in the WTC PEU Chest CT Image Archive (ClinicalTrials.gov identifier )11.
Inclusion criteria and QCT systems:
Inclusion into this study required that the WTC workers had (1) adequate quality study for quantitative chest CT scan (QCT) measurements of their pulmonary artery and aortic diameters, and wall area percent (WAP) performed with the Simba system (http://www.via.cornell.edu/simba/simba) 12,13, (2) at least two screening and surveillance spirometries, and (3) complete data for all covariates of interest. After excluding 17 subjects with missing data on other variables, and imputing missing data for WAP (described in Statistical Analyses), a total of 1,180 subjects met inclusion criteria (Figure 1). None of the included subjects had interstitial lung disease, infectious or primary pulmonary neoplastic processes, and other disorders. QCT measurements were performed blinded to any and all identifiers and clinical information.
Figure 1.
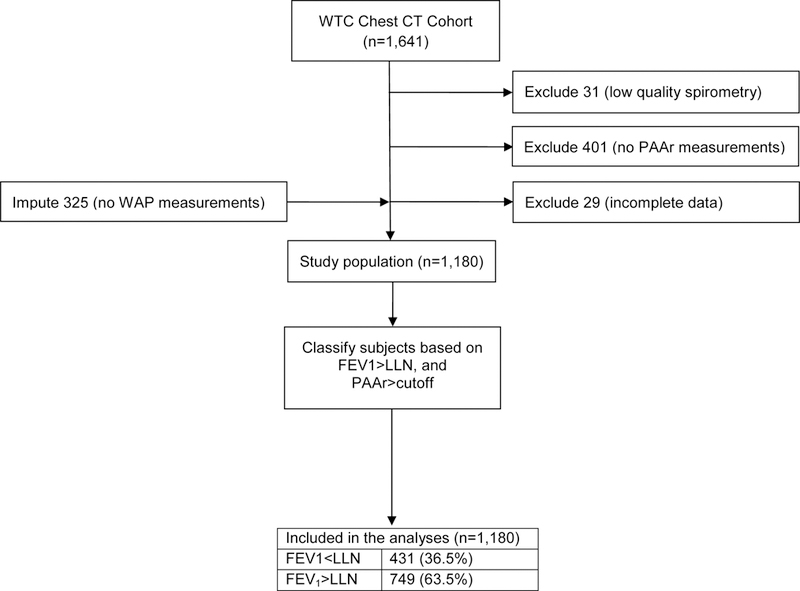
Study flow chart.
Spirometry:
we selected the spirometry performed on the date closest to the date of the chest CT scan in which PAAr was measured by QCT (median interval 0.5 years). Spirometry was performed using the EasyOne® portable flow device (ndd, Zurich, Switzerland), selected for its accuracy, and quality feedback14,15. Bronchodilator response (BDR) was assessed at least once (and most often at the baseline visit) by repeating spirometry 15 minutes after the administration of 180 mcg of albuterol via metered dose inhaler. BDR was defined as both a percent change ≥12% and an absolute increment ≥200 ml in either FEV1 or the forced vital capacity (FVC) after bronchodilator administration. Predicted values for spirometric measurements were calculated for all subjects’ acceptable tests, based on reference equations from the third National Health and Nutrition Examination Survey (NHANES III)16, and all testing, quality assurance, ventilatory impairment pattern definitions, and interpretative approaches followed American Thoracic Society recommendations17,18. Spirometries in this study were selected if deemed acceptable, and also had a good quality grade (computer quality grade A or B, or grade C with at least 5 trials)18,19.
Measurements:
Our outcome of interest was the occurrence of an FEV1 value below each subject’s statistically determined lower limit of normal (FEV1<LLN). Our main dichotomous predictor was an increased PAAr, measured by QCT as previously described13. Briefly, pulmonary artery diameter was segmented and measured automatically in the axial plane between the heart and the artery bifurcation (instead of the traditional manual measurement at the level of the latter20, see Figure 2). The aortic diameter was determined at the level of the pulmonary artery bifurcation.
Figure 2.
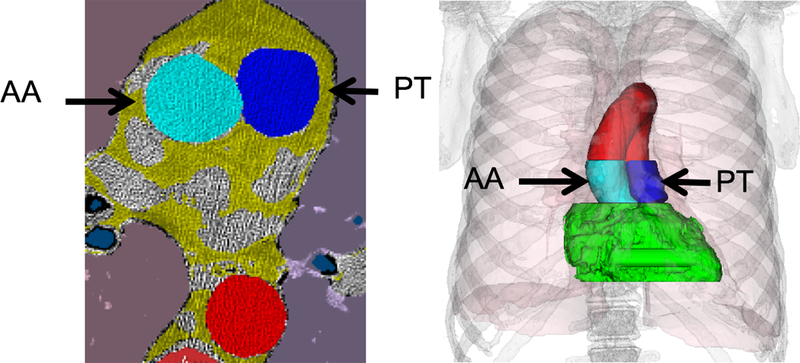
Pulmonary artery and aortic diameter are measured from a tridimensional volume, with the regions in dark and light blue indicating where the pulmonary trunk and the aorta measurements are made, respectively. To obtain a robust measure from the largest possible number of data points, the diameter is estimated from a 3D cylinder model matched to the blue pixels.
Covariates of interest included age on the date of the CT study, gender, height, race/ethnicity (grouped as Latino, non-Latino white, and non-Latino of all other races), body mass index (BMI, expressed in kg/m2) at baseline evaluation, evidence of bronchodilator response (BDR) at one or more visits, spirometric forced exhalatory time (FET, dichotomously, with a 6-second cutoff), QCT measurement of WAP, post-traumatic stress disorder checklist (PCL) score, diagnosis of obstructive sleep apnea by nocturnal polysomnography, baseline smoking status, and WTC occupational exposure indicators.
Smoking status was assessed at the baseline examination. A subject was considered a lifetime nonsmoker if (s)he had smoked less than 20 packs of cigarettes (or 12 oz. of tobacco) in their lifetime, or less than 1 cigarette/day (or 1 cigar/week) for one year. A minimum of 12 months without tobacco use was required to deem a subject a former smoker21.
WTC occupational exposure relied on two self-reported dichotomous variables, assessed at the baseline examination: arrival at the WTC site within 48 hours of the terrorist attack, and cumulative WTC exposure duration exceeding 60 days2. For descriptive purposes, an occupational physician (RED) recoded, grouped, and labeled occupations into the following 6 categories: (1) management, business, science, arts, service, sales, or office occupations (“management/services”); (2) construction trades, maintenance, and natural resources (“construction trades/maintenance”); (3) construction and demolition laborers, asbestos removers, and building cleaners (“laborers/cleaners/asbestos removal”)22; (4) production, transportation, and material moving (“transportation”); (5) law enforcement specific and military (“law enforcement”); and (6) unemployed, retired, or unknown (“unemployed/retired”).
WAP was measured by QCT in the 3rd bronchial generation of the right upper lobe5, using the Simba system12,23. The automated process starts with identification of the airways and their branch points on inspiratory scans. Airways can be followed out up to 5 generations, depending on the resolution of the images. Based primarily on density differences between the luminal air, airway wall, and surrounding parenchyma, the airway lumen area (Ai), total airway area (Ao), and airway wall area (Aaw) are measured. These cross-sectional area measurements are averaged along the length of the bronchus. WAP is calculated as (Ao − Ai)/Ao × 100%, and was averaged over all measurable airways. An increase in WAP suggests airway wall thickening, in relation to the lumen, which is in turn suggestive of airway inflammatory changes.
Symptoms of chronic posttraumatic stress disorder were assessed with the PTSD Checklist (PCL) questionnaire, a validated scale24, where probable PTSD is defined by having a score equal to or greater than 44 points. This score has been used in a number of previous studies of the WTC25 and other cohorts, and is strongly correlated with a clinical diagnosis of PTSD24. We also adjusted for a diagnosis of obstructive sleep apnea, in most cases by in-laboratory nocturnal polysomnogram.
For post hoc descriptive purposes, we classified subjects with FEV1<LLN according to the presence or absence of evidence of lower airway disease (LAD), as follows: (1) LAD: both abnormal spirometry, revealing at any time either fixed or reversible obstruction, low FVC, or bronchodilator response, and reported shortness of breath with a score of 3 or more on the Medical Research Council breathlessness scale (MRC, “I walk slower than people of the same age on the level because of breathlessness or have to stop for breath when walking at my own pace on the level”)26,27; and (2) No LAD: normal or low FVC spirometry without associated significant breathlessness.
Statistical analyses:
Descriptive statistics included mean and standard deviation (SD), and median and interquartile ranges (IQR) for normally and non-normally distributed continuous variables, respectively, and counts and proportions for categorical variables. Unadjusted bivariate analyses included t-test or Chi square test, as appropriate. We initially determined sex-specific PAAr cutoff values that maximized the Youden J index (sensitivity+specificity−1) in bivariate analyses with our outcome of interest, FEV1<LLN28. We then classified subjects dichotomously according to whether or not their PAAr exceeded their sex-specific cutoff value, and used a logistic regression model to estimate the odds of having a FEV1<LLN, adjusting for important covariates6. Although some of the predictors were correlated, significant multicollinearity was excluded by the variance inflation factor method. The discrimination of the logistic regression model was evaluated by means of the c statistic. After excluding 17 cases with more than 1 missing variable, WAP remained as the only variable with a considerable amount of missing data in the sample. We employed a multiple imputation (MI) procedure with monotone missing pattern method to account for missingness in our regression model. The results with the MI and the complete case data set were essentially identical, so only the former will be presented. The SAS program, version 9.4 (SAS Institute, Cary, NC), and a two-sided significance level of α=0.05 were used for all analyses.
Results
The study group consisted of 1,180 subjects. Subjects were predominantly (82.4%) male, with mean age at the time of the CT scan of 49.8 (SD 9.3) years (Table 1). The leading occupational categories of the study subjects were those of laborers/building cleaners and law enforcement, with a significantly higher proportion of the latter (26.9% vs. 19.4%) having experienced our outcome of interest, FEV1<LLN. Subjects had their chest CT scan at a median of 7.5 (IQR 6–9) years after September 11, 2001. The spirometry closest to the chest CT scan was performed at a median of 0.54 (IQR 0.21–1.09) years either preceding (n=394) or following (n=786) the CT scan, and a median of 7.6 (IQR 5.78–9.13) years after 11-September-2001. The sex-specific PAAr cutoff for our outcome of interest was determined to be 0.87 for men and 0.91 for women. As expected, the prevalence of LAD was much higher among subjects with than those without FEV1<LLN (71.6% vs. 20.1%). Figure 1 presents a study flow diagram, and Table E1 presents the comparison of the included and excluded subjects (n=461 subjects). The two groups were very similar: the only statistically significant difference was that, compared to excluded subjects, included subjects were more likely to have been diagnosed with obstructive sleep apnea.
Table 1.
Characteristics of the subjects in this study, unadjusted and adjusted analyses.
| All subjects (n=1180) | FEV1>LLN (n=749) | FEV1<LLN (n=431, 36.5%) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| n or mean | % or SD | n or mean | % or SD | n or mean | % or SD | p | ORadj | 95%CI | p | |||
| Increased PAAr | ||||||||||||
| Yes | 337 | 28.6 | 185 | 24.7 | 152 | 35.3 | 0.0001 | 1.63 | 1.21 | 2.20 | 0.0015 | |
| No | 843 | 71.4 | 564 | 75.3 | 279 | 64.7 | ref | |||||
| Sex | ||||||||||||
| Male | 972 | 82.4 | 611 | 81.6 | 361 | 83.8 | 0.3433 | ref | ||||
| Female | 208 | 17.6 | 138 | 18.4 | 70 | 16.2 | 1.38 | 0.89 | 2.15 | 0.1537 | ||
| Age (years) | 49.8 | 9.34 | 49.8 | 9.43 | 49.8 | 9.20 | 0.9907 | 1.01 | 0.99 | 1.02 | 0.3973 | |
| Height (cm) | 171.6 | 9.78 | 170.6 | 9.82 | 173.2 | 9.48 | <.0001 | 1.05 | 1.03 | 1.07 | <0.0001 | |
| Race/Ethnicity | ||||||||||||
| Non-Latino White | 630 | 53.4 | 409 | 54.6 | 221 | 51.3 | 0.2621 | ref | ||||
| Non-Latino Non-White | 139 | 11.8 | 80 | 10.7 | 59 | 13.7 | 1.41 | 0.92 | 2.15 | 0.1150 | ||
| Latino | 411 | 34.8 | 260 | 34.7 | 151 | 35.0 | 1.89 | 1.35 | 2.66 | 0.0002 | ||
| Body mass index, kg/m2 | 29.82 | 5.14 | 29.30 | 4.70 | 30.72 | 5.73 | <.0001 | 1.04 | 1.01 | 1.07 | 0.0077 | |
| Smoking status | ||||||||||||
| Never | 627 | 53.1 | 400 | 53.4 | 227 | 52.7 | 0.9417 | - | - | - | 0.7619 | |
| Former | 329 | 27.9 | 209 | 27.9 | 120 | 27.8 | - | - | - | - | ||
| Current | 224 | 19.0 | 140 | 18.7 | 84 | 19.5 | - | - | - | - | ||
| Occupational category | ||||||||||||
| Management/service | 214 | 18.1 | 113 | 15.1 | 101 | 23.4 | <.0001 | - | - | - | - | |
| Construction trades/maintenance | 215 | 18.2 | 122 | 16.3 | 93 | 21.6 | - | - | - | - | ||
| Laborer/cleaner/asbestos removal | 385 | 32.6 | 306 | 40.8 | 79 | 18.3 | - | - | - | - | ||
| Transportation | 69 | 5.9 | 36 | 4.8 | 33 | 7.7 | - | - | - | - | ||
| Law enforcement/military | 261 | 22.1 | 145 | 19.4 | 116 | 26.9 | - | - | - | - | ||
| Unemployed/retired/unknown | 36 | 3.1 | 27 | 3.6 | 9 | 2.1 | - | - | - | - | ||
| WTC arrival | ||||||||||||
| <48 hours | 582 | 49.3 | 319 | 42.6 | 263 | 61.0 | <.0001 | 1.71 | 1.29 | 2.28 | 0.0002 | |
| >48 hours | 598 | 50.7 | 430 | 57.4 | 168 | 39.0 | ref | |||||
| WTC exposure duration | ||||||||||||
| <60 days | 476 | 40.3 | 275 | 36.7 | 201 | 46.6 | 0.0008 | 1.44 | 1.09 | 1.90 | 0.0099 | |
| 60+ days | 704 | 59.7 | 474 | 63.3 | 230 | 53.4 | ref | |||||
| Wall area percent (WAP)** | 62.32 | 7.78 | 61.23 | 7.73 | 64.22 | 7.51 | <.0001 | 1.05* | 1.02 | 1.08 | 0.0044 | |
| Sleep apnea | ||||||||||||
| Yes | 479 | 40.6 | 308 | 41.1 | 171 | 39.7 | 0.6262 | 0.77 | 0.58 | 1.03 | 0.0794 | |
| No | 701 | 59.4 | 441 | 58.9 | 260 | 60.3 | ref | |||||
| Bronchodilator response (BDR) | ||||||||||||
| Yes | 268 | 22.7 | 89 | 11.9 | 179 | 41.5 | <.0001 | 5.37 | 3.91 | 7.38 | <0.0001 | |
| No | 912 | 77.3 | 660 | 88.1 | 252 | 58.5 | ref | |||||
| PTSD Checklist (PCL) score | 44.4 | 19.13 | 45.5 | 18.85 | 42.6 | 19.48 | 0.0080 | 1.00 | 0.99 | 1.00 | 0.3107 | |
| FET >= 6 sec | ||||||||||||
| Yes | 708 | 60.0 | 462 | 61.7 | 246 | 57.1 | 0.7799 | 0.78 | 0.59 | 1.03 | 0.0772 | |
| No | 472 | 40.0 | 287 | 38.3 | 185 | 42.9 | ref | |||||
per each 5% units of WAP.
Imputed
Table 1 shows the results of the analysis of the relation between PAAr (and other covariates of interest) and an FEV1<LLN. In the unadjusted analysis, subjects with an increased PAAr were significantly more likely to have an FEV1<LLN than those without an increase PAAr (35.3% vs. 24.7%, p=0.0001). In this unadjusted analysis, covariates significantly associated with an FEV1<LLN included height, BMI, early arrival at the WTC disaster site, shorter WTC exposure duration, PCL score, WAP, and BDR. The multivariable logistic regression analysis adjusting for sex, race/ethnicity, WTC exposure, and other covariates, PAAr was significantly associated with 1.63 increased odds of an FEV1<LLN (95% confidence interval for odds ratio= 1.21 to 2.20, p=0.0015). The c statistic for this multivariable model was 0.76.
Discussion
We have demonstrated that pulmonary arterial enlargement, as indicated by the PAAr, is associated with an FEV1 below the predicted lower limit of normal, even after adjustment for several known predictors of lung function6, such as bronchodilator hyperresponsiveness, wall area percent, baseline BMI, arrival at the WTC within 48 hours and shorter WTC occupational exposure duration, and smoking status.
A previous study of WTC firefighters of 34 cases with FEV1%predicted within one standard deviation of the lowest observed (77%) at a subspecialty unit, and 63 control subjects, reported an association with an elevated PAAr (≥0.92)4. Whereas PAAr was manually measured in that study, we utilized highly accurate automated measurements13 that can be readily deployed to a large imaging dataset like ours. Moreover, we measured the PA diameter between the heart and the PA bifurcation, instead of the traditional manual measurement at the level of the latter20). This automated method was developed to make PAAr measurements perform more reliably on low-radiation dose chest CT scans, and thus be applicable to a diverse range of chest CT scan protocols. In further contrast to the previous study in WTC firefighters, we assessed the FEV1<LLN instead of FEV1 %predicted, as FEV1<LLN is a more informative and generalizable outcome.
The association between an elevated PAAr and an FEV1<LLN may be related to distal vasculopathy, as previously suggested4, and could be similar to what has been described in smokers with or without significant emphysema29. Although that could result from toxicant induced injury, as has been proposed for tobacco related chronic obstructive pulmonary disease30, another potential explanation is that increased PAAr reflects obesity-31 (with or without metabolic syndrome32) associated pulmonary hypertension, and a post hoc analysis showed that PAAr was significantly associated with obesity (data not shown). Our studies in this cohort have demonstrated the adverse effect of obesity and weight gain on longitudinal follow up6, the prevalence of obesity33,34 and the metabolic syndrome is known to be substantial in this cohort35, and a case-control study among WTC firefighters demonstrated associations of metabolic syndrome biomarkers with adverse respiratory outcomes (FEV1% less than predicted lower limit of normal)36.
We selected a substantial number of important covariates and potential confounders. WAP is an indicator of proximal airway wall thickening, which can result from inflammatory changes, and be a common feature to the different WTC-related lower airway diseases2,9, and we previously demonstrated that WAP was significantly associated with adverse respiratory outcomes in this WTC occupational cohort6. To the extent that significant BDR also reflects bronchial inflammation, it would also be expected to be associated with adverse expiratory flow outcomes, and BDR has been associated with increased susceptibility to tobacco-smoke pulmonary toxicity37. The negative impact of obesity (more highly prevalent in the WTC occupational cohorts (more than 80%33,34 than in the U.S. population) on lung function38 and pulmonary vasculature31 is well known, and we previously demonstrated in this cohort that weight gain and loss were associated with accelerated expiratory flow decline or gain, respectively6. Although most research has focused on adverse effects of obesity on asthma39,40, our studies suggest that WTC-related inflammatory lower airway diseases are also similarly impacted by obesity6. Several studies have identified the association of early arrival at the WTC disaster site2,41,42 with adverse respiratory health outcomes. Although a large study reported an association between prolonged exposure at the disaster site (defined as >90 days) and incident asthma42, our study suggests an inverse relation. One possible explanations for this finding could be the well-known markedly lower decreased dust levels a few days after the collapse of the WTC towers43. Another possible and not mutually exclusive explanation could be that subjects with adverse respiratory outcomes may have shortened their recovery work assignments at the disaster site. Sleep apnea is highly prevalent (and mostly untreated) in the WTC cohorts, and known to be associated with pulmonary hypertension. We found an unadjusted association of baseline smoking status with FEV1<LLN, but this variable was correlated with multiple covariates in our models, and was kept in the multivariable model for adjustment purposes.
The strengths of this study relate to the richness of the patient population, the amount of data available for co-variates of interest, the availability of imaging data from the largest established WTC chest CT archive to date, and of a QCT tool that allows fast measurements in large imaging data sets of an increasingly recognized predictor of adverse health outcomes. This study also has some limitations. We lacked comparison QCT imaging data from a well-defined control group of occupationally and WTC unexposed, totally asymptomatic subjects, with normal spirometry and chest radiograph. Our study relied on retrospective chest CT imaging data, which were subject to variations in protocols over time. However, most studies were performed in a very small number of scanners at a single tertiary care institution, with an intended technical consistency, and quality control was exerted to exclude a priori studies that did not meet technical standards for QCT. We recently published the findings on systematic readings of the CT scans11, and noted the paucity of interstitial lung disease abnormalities. Indeed, the present study group did not include any subject with that type of disease. Despite the richness of our data and the good overall performance of our model, we lacked information on other factors that can relate to airway disease outcomes, like atopy, pre-WTC occupational exposures, smoking intensity, and smoking status after baseline, or on pulmonary vascular disease, such as metabolic syndrome components other than obesity32. Previous studies, however, have not suggested an association between atopy and WTC lower airway disease44, and occupational airway disease45,46, and periodic cross-sectional assessments of smoking status in this cohort do not suggest increasing group smoking rates (data not presented).
In summary, our study demonstrates that PAAr is an independent predictor of adverse expiratory flow outcome, independently from several important and previously identified predictors of adverse respiratory outcomes in this and other patient populations. The findings also demonstrate the usefulness of QCT in the investigation and characterization of the different WTC-related lower airway diseases2,3, and the potential application of PAAr QCT measurement to future investigations of pulmonary vasculature in pulmonary diseases.
Supplementary Material
Acknowledgements
This work was supported by grants U01 OH011300 (AN, PI), and U01 OH010401 (RED, PI), and contract 200-2017-93325 (WTC General Responders Cohort Data Center) from the Centers for Disease Control and Prevention/National Institute for Occupational Safety and Health (CDCP/NIOSH). The authors had no other relevant financial conflict of interest. The contents of this article are the sole responsibility of the authors and do not necessarily represent the official views of the CDCP/NIOSH. ClinicalTrials.gov identifier . The authors would like to thank all participants in this study, and the staff of the Mount Sinai WTC Health Program Clinical Center of Excellence, and the WTC GRC Data Center. We also acknowledge the able support of Lilliam Tirado, Raymond Mathews, and Horacio Romero as research coordinators.
References
- 1.Wells JM, Washko GR, Han MK, et al. Pulmonary arterial enlargement and acute exacerbations of COPD. N Engl J Med 2012;367(10):913–921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.de la Hoz RE, Shohet MR, Chasan R, et al. Occupational toxicant inhalation injury: the World Trade Center (WTC) experience. Int Arch Occup Environ Health 2008;81(4):479–485. [DOI] [PubMed] [Google Scholar]
- 3.de la Hoz RE. Occupational lower airway disease in relation to World Trade Center inhalation exposure. Curr Opin Allergy Clin Immunol 2011;11(2):97–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Schenck EJ, Echevarria GC, Girvin FG, et al. Enlarged pulmonary artery is predicted by vascular injury biomarkers and is associated with WTC-lung injury in exposed fire fighters: a case–control study. BMJ Open 2014;4(9):e005575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.San José Estépar R, Reilly JJ, Silverman EK, Washko GR. Three-dimensional airway measurements and algorithms. Proc Am Thorac Soc 2008;5(9):905–909. [DOI] [PubMed] [Google Scholar]
- 6.de la Hoz RE, Liu X, Doucette JT, et al. Increased airway wall thickness is associated with adverse longitudinal first-second forced expiratory volume trajectories of former World Trade Center workers. Lung 2018;196(4):481–489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Herbert R, Moline J, Skloot G, et al. The World Trade Center disaster and the health of workers: five-year assessment of a unique medical screening program. Environ Health Perspect 2006;114(12):1853–1858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Woskie SR, Kim H, Freund A, et al. World Trade Center disaster: assessment of responder occupations, work locations, and job tasks. Am J Ind Med 2011;54(9):681–695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.de la Hoz RE. Occupational asthma and lower airway disease in former World Trade Center workers and volunteers. Curr Allergy Asthma Rep 2010;10(4):287–294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mendelson DS, Roggeveen M, Levin SM, Herbert R, de la Hoz RE. Air trapping detected on end-expiratory high-resolution computed tomography in symptomatic World Trade Center rescue and recovery workers. J Occup Environ Med 2007;49(8):840–845. [DOI] [PubMed] [Google Scholar]
- 11.de la Hoz RE, Weber J, Xu D, et al. Chest CT scan findings in World Trade Center workers. Arch Environ Occup Health 2018;[in press, doi: 10.1080/19338244.2018.1452712]. [DOI] [PMC free article] [PubMed]
- 12.Lee J, Reeves AP, Fotin SV, Apananosovich TV, Yankelevitz DF. Human airway measurement from CT Images. In: SPIE International Symposium on Medical Imaging (February 2008) Vol 6915 Bellingham, WA: SPIE; 2008:691518 [Google Scholar]
- 13.Xie Y, Liang M, Yankelevitz DF, Henschke CI, Reeves AP. Automated measurement of pulmonary artery in low-dose non-contrast chest CT images. Proc SPIE 9414, Medical Imaging 2015: Computer-Aided Diagnosis 2015;9414:94141G. [Google Scholar]
- 14.Walters JA, Wood-Baker R, Walls J, Johns DP. Stability of the EasyOne ultrasonic spirometer for use in general practice. Respirology 2006;11(3):306–310. [DOI] [PubMed] [Google Scholar]
- 15.Pérez-Padilla R, Vázquez-García JC, Márquez MN, et al. The long-term stability of portable spirometers used in a multinational study of the prevalence of chronic obstructive pulmonary disease. Respir Care 2006;51(10):1167–1171. [PubMed] [Google Scholar]
- 16.Hankinson JL, Odencratz JR, Fedan KB. Spirometric reference values from a sample of the general U.S. population. Am J Respir Crit Care Med 1999;159(1):179–187. [DOI] [PubMed] [Google Scholar]
- 17.Miller MR, Hankinson J, Brusasco V, et al. Standardisation of spirometry. Eur Respir J 2005;26(2):319–338. [DOI] [PubMed] [Google Scholar]
- 18.Enright PL, Skloot GS, Cox-Ganser JM, Udasin IG, Herbert R. Quality of spirometry performed by 13,599 participants in the World Trade Center Worker and Volunteer Medical Screening Program. Respir Care 2010;55(3):303–309. [PubMed] [Google Scholar]
- 19.Ferguson GT, Enright PL, Buist AS, Higgins MW. Office spirometry for lung health assessment in adults: a consensus statement from the National Lung Health Education Program. Chest 2000;117(4):1146–1161. [DOI] [PubMed] [Google Scholar]
- 20.Edwards PD, Bull RK, Coulden R. CT measurement of main pulmonary artery diameter. The British journal of radiology 1998;71(850):1018–1020. [DOI] [PubMed] [Google Scholar]
- 21.Ferris BG. Epidemiology standardization project (American Thoracic Society). Am Rev Respir Dis 1978;118(6 Pt 2):1–120. [PubMed] [Google Scholar]
- 22.de la Hoz RE, Hill S, Chasan R, et al. Health care and social issues of immigrant rescue and recovery workers at the World Trade Center site. J Occup Environ Med 2008;50(12):1329–1334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lee J, Reeves AP, Yankelevitz DF, Henschke CI. Bronchial segment matching in low-dose lung CT scan pairs. In: SPIE Medical Imaging Bellingham, WA: SPIE; 2009. [Google Scholar]
- 24.Blanchard EB, Jones-Alexander J, Buckley TC, Forneris CA. Psychometric properties of the PTSD checklist (PCL). Behav Res Ther 1996;34(8):669–673. [DOI] [PubMed] [Google Scholar]
- 25.American Thoracic Society. Standardization of spirometry, 1994 update. Am J Respir Crit Care Med 1995;152(3):1107–1136. [DOI] [PubMed] [Google Scholar]
- 26.Fletcher CM. The clinical diagnosis of pulmonary emphysema - an experimental study. Proc R Soc Med 1952;45(9):577–584. [PubMed] [Google Scholar]
- 27.Stenton C The MRC breathlessness scale. Occup Med 2008;58(3):226–227. [DOI] [PubMed] [Google Scholar]
- 28.Fluss R, Faraggi D, Reiser B. Estimation of the Youden Index and its associated cutoff point. Biom J 2005;47(4):458–472. [DOI] [PubMed] [Google Scholar]
- 29.San José Estépar R, Kinney GL, Black-Shinn JL, et al. Computed tomographic measures of pulmonary vascular morphology in smokers and their clinical implications. Am J Respir Crit Care Med 2013;188(2):231–239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wouters EFM, Franssen FM. COPD: shifting the paradigm to the vasculature [Editorial]. Am J Respir Crit Care Med 2018;[in press]. [DOI] [PubMed]
- 31.Friedman SE, Andrus BW. Obesity and pulmonary hypertension: a review of pathophysiologic mechanisms. J Obes 2012;2012:505274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Willson C, Watanabe M, Tsuji-Hosokawa A, Makino A. Pulmonary vascular dysfunction in metabolic syndrome. J Physiol 2018;[in press]. [DOI] [PMC free article] [PubMed]
- 33.de la Hoz RE, Jeon Y, Miller GE, Wisnivesky JP, Celedón JC. Post-traumatic stress disorder, bronchodilator response, and incident asthma in World Trade Center rescue and recovery workers. Am J Respir Crit Care Med 2016;194(11):1383–1391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Webber MP, Lee R, Soo J, et al. Prevalence and incidence of high risk for obstructive sleep apnea in World Trade Center-exposed rescue/recovery workers. Sleep Breath 2011;15(3):283–294. [DOI] [PubMed] [Google Scholar]
- 35.Moline JM, McLaughlin MA, Sawit ST, et al. The prevalence of metabolic syndrome among law enforcement officers who responded to the 9/11 World Trade Center attacks. Am J Ind Med 2016;59(9):752–760. [DOI] [PubMed] [Google Scholar]
- 36.Naveed B, Weiden MD, Kwon S, et al. Metabolic syndrome biomarkers predict lung function impairment: a nested case-control study. Am J Respir Crit Care Med 2011;185(4):392–399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Celedón JC, Speizer FE, Drazen JM, et al. Bronchodilator responsiveness and serum total IgE levels in families of probands with severe early-onset COPD. Eur Respir J 1999;14(5):1009–1014. [DOI] [PubMed] [Google Scholar]
- 38.Jones RL, Nzekwu MM. The effects of body mass index on lung volumes. Chest 2006;130(3):827–833. [DOI] [PubMed] [Google Scholar]
- 39.Dixon AE, Holguin F, Sood A, et al. An official American Thoracic Society workshop report: obesity and asthma. Proceedings of the American Thoracic Society 2010;7(5):325–335. [DOI] [PubMed] [Google Scholar]
- 40.Khalid F, Holguin F. A review of obesity and asthma across the life span. J Asthma 2018;[in press]:1–15. [DOI] [PubMed]
- 41.Prezant DJ, Weiden M, Banauch GI, et al. Cough and bronchial responsiveness in firefighters at the World Trade Center site. N Engl J Med 2002;347(11):806–815. [DOI] [PubMed] [Google Scholar]
- 42.Wheeler K, McKelvey W, Thorpe L, et al. Asthma diagnosed after September 11, 2001 among rescue and recovery workers: findings from the World Trade Center Health Registry. Environ Health Perspect 2007;115 (11):1584–1590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Geyh AS, Chillrud S, Williams DL, et al. Assessing truck driver exposure at the World Trade Center disaster site: personal and area monitoring for particulate matter and volatile organic compounds during October 2001 and April 2002. J Occup Environ Hyg 2005;2(3):179–193. [DOI] [PubMed] [Google Scholar]
- 44.de la Hoz RE, Shohet MR, Wisnivesky JP, Bienenfeld LA, Afilaka AA, Herbert R. Atopy and upper and lower airway disease among former World Trade Center workers and volunteers. J Occup Environ Med 2009;51(9):992–995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tarlo SM, Broder I. Irritant-induced occupational asthma. Chest 1989;96:297–300. [DOI] [PubMed] [Google Scholar]
- 46.Demir A, Joseph L, Becklake MR. Work-related asthma in Montreal, Quebec: population attributable risk in a community-based study. Can Respir J 2008;15(8):406–412. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


