Abstract
Rumination is an involuntary cognitive process theorized to prolong arousal and inhibit proper emotion regulation. Most available research has examined individual differences in cognitive dispositions to ruminate about stress as a risk marker for psychopathology and other health problems. This intensive longitudinal study extended previous research by examining day-to-day associations of rumination about stress with objectively-measured actigraph-based sleep and diurnal salivary cortisol activity. Sixty-one healthy participants (Mage = 20.91) completed up to five ecological momentary assessments (EMA) each day and wore actigraph wristwatches for eight days (N = 488). On three of these days, participants provided five saliva samples assayed for cortisol (N = 910). On average, greater daily stress levels were associated with shorter sleep duration and higher waking cortisol levels. In day-to-day analyses, greater daily stress levels, when combined with ruminating about daily stress more than usual, was associated with higher waking cortisol levels the following morning. Ruminating more than usual about daily stress, in the context of low-stress days, was also associated with flatter diurnal cortisol slopes the next day. These findings highlight the potential influences of daily stress, and rumination about stress, on sleep and diurnal cortisol activity – two important markers of health and well-being.
Keywords: Rumination, sleep, diurnal cortisol, actigraphy, daily diaries
Rumination involves repetitive, unwanted, pastoriented thoughts about negative content (NolenHoeksema, Wisco, & Lyubomirsky, 2008). Individuals who ruminate more often are at greater risk for affective disorders (Watkins, 2008) and somatic symptoms (Brosschot, Gerin, & Thayer, 2006). Rumination is theorised to maintain arousal after stressful experiences by prolonging physiological stress responses (Brosschot et al., 2006), and thus may help to explain one pathway through which daily life stress leads to psychopathology and other physical health problems (e.g. cardiovascular disease, compromised immune function). Sleep and hypothalamic–pituitary–adrenal (HPA) axis activity are biobehavioral markers of regulation that are sensitive to daily stress (Arbel, Shapiro, Timmons, Moss, & Margolin, 2017; Doane & Thurston, 2014), but available studies have primarily focused on whether dispositional differences in rumination relate to average indicators of sleep and HPA axis activity. In order to more closely consider the daily dynamics of rumination about stress, sleep, and diurnal cortisol, the present study adopted a time-lagged daily diary approach to examine whether interactions between perceived daily stress and ruminating about stress of the day (i.e. day-to-day, within-person differences) predicted (1) differences in sleep that night, and (2) diurnal cortisol patterns the following day.
Brosschot and colleagues (2006) identified perseverative cognitions as a common trend in the disparate literatures on worry, anticipatory stress, rumination, and other related cognitive processes. The Perseverative Cognition Hypothesis suggests that repeated or chronic cognitions about stress, both in advance of and following stressors, exacerbate the health consequences of stressors via prolonged activation of physiological stress response systems (Brosschot et al., 2006). Yet the majority of studies that have examined the potential health consequences of perseverative cognition have relied on surveys that assess dispositional (i.e. “trait”) tendencies to ruminate based on a single assessment or measure “state” worry in controlled laboratory experiments (see Brosschot et al., 2006; Zoccola & Dickerson, 2012, for reviews). Such methods may be limited in terms of more fully capturing the process of repeatedly focusing on the problems or stress of daily life, and further, how daily rumination relates to sleep problems (Lancee, Eisma, van Zanten, & Topper, 2017) and diurnal cortisol activity (Zoccola & Dickerson, 2012). To address this limitation, rumination researchers have advanced experience sampling or daily process designs (i.e. ecological momentary assessment, daily diaries) that have revealed dynamic within-person associations of momentary (i.e. everyday) instances of rumination with more negative affect and less positive affect in daily life (e.g. Brans, Koval, Verduyn, Lim, & Kuppens, 2013; Kircanski, Thompson, Sorenson, Sherdell, & Gotlib, 2018; Moberly & Watkins, 2008). In the present study, we extended this approach to assess daily rumination about stress in relation to sleep and diurnal cortisol activity.
Up to 60% of young adults may suffer from poor quality sleep (e.g. trouble falling asleep, waking up at night; Lund, Reider, Whiting, & Prichard, 2010), and greater night-to-night variability in sleep schedules has been associated with greater internalising problems (Doane, GressSmith, & Breitenstein, 2015b). Poor emotion regulation (e.g. ruminating more frequently) may result in sleep problems via heightened cognitive and physiological arousal (Baglioni, Spiegelhalder, Lombardo, & Riemann, 2010). Self-report survey research has shown that a greater tendency to ruminate is associated with more intrusive thoughts before sleep, lower self-reported sleep quality, and delayed sleep timing (Guastella & Moulds, 2007; Nota & Coles, 2015). Spending more time thinking back on a stressful task was associated with taking more time to fall asleep that night (reported retrospectively the following morning; Zoccola, Dickerson, & Lam, 2009).
In one of few studies to use objective actigraph based measures of sleep in coordination with a daily diary approach, greater daily stress than usual (i.e. within-person increase) was associated with sleeping less that night among high school students, based on three days (Doane & Thurston, 2014). In another seven-day diary study of college students at risk for depression, pre-sleep rumination (reported retrospectively the following morning) was associated with greater actigraph-detected sleep onset latency (time to fall asleep; Pillai, Steenburg, Ciesla, Roth, & Drake, 2014). No studies have yet examined whether rumination about stress of the day might amplify how daily stress levels are related to objective indicators of sleep duration and onset latency that night.
The HPA axis hormonal product cortisol is released in a typical diurnal pattern characterised by relatively high waking levels, an increase about 30 min later (cortisol awakening response, CAR), and a general decrease thereafter with lowest levels around midnight (Adam & Kumari, 2009). Alterations to this normative diurnal cortisol rhythm, such as “flatter” (less negative) slopes from waking to bedtime, serve as risk markers for mental health disorders and other physical health problems (Adam et al., 2017). Although theory suggests that rumination prolongs neuroendocrine stress responses (Brosschot et al., 2006), results have been mixed regarding how rumination is generally associated with diurnal cortisol activity (Zoccola & Dickerson, 2012, for review). Ruminating more than others (i.e. between-person difference) has been associated with reduced (Cropley, Rydstedt, Devereux, & Middleton, 2015), increased (Schlotz, Hellhammer, Schulz, & Stone, 2004; Zoccola, Dickerson, & Yim, 2011), or no differences in the CAR (Hilt, Sladek, Doane, & Stroud, 2017), with results potentially varying based on differences in methods (e.g. adherence to sample timing taken into account). A greater tendency to ruminate has also been associated with greater overall diurnal cortisol output (Huffziger et al., 2013), greater evening cortisol levels (McCullough, Orsulak, Brandon, & Akers, 2007; Rydstedt, Cropley, Devereux, & Michalianou, 2009), and flatter diurnal cortisol slopes (Hilt et al., 2017).
Substantial variance in diurnal cortisol, particularly morning values, can be attributed to day-to-day fluctuations rather than stable individual differences (Doane, Chen, Sladek, Van Lenten, & Granger, 2015a). In one of few related studies to collect three days of matched diary and saliva collection, CARs were greater the morning after days of worrying more than usual for females (Arbel et al., 2017), but daily stress levels were not assessed. Day-to-day differences in rumination (focusing on problems/stress) were not associated with diurnal cortisol patterns in another study of adolescent girls when adjusting for adherence to sample timing and perceived stress levels before bedtime (Hilt et al., 2017). As the rigour of this methodological approach improves, there remains considerable room to continue exploring the daily dynamics of stress, rumination about this stress, and multiple aspects of the diurnal cortisol rhythm using short burst longitudinal methods. Capitalising on the strengths afforded by a daily process design (Affleck, Zautra, Tennen, & Armeli, 1999), the present study examined whether rumination about stress one day was associated with typical cortisol patterns the next day. Theory and empirical research suggest that examining cross-day “spillover” effects from one day to the next helps to decompose direction of effects. For example, research has identified associations between stress and next-day negative affect for individuals high on depressive symptoms (Gunthert, Cohen, Butler, & Beck, 2007; Peeters, Nicolson, Berkhof, Delespaul, & deVries, 2003). Theoretical frameworks focused on physiological stress processes suggest that HPA axis changes occur in response to social and emotional experiences over multiple time courses (Adam, 2012), including day-to-day variation in anticipated or experienced stress and subsequent changes in physiological or emotional states. Such daily spillover effects have been identified by several research teams regarding associations between negative emotions or rumination in the evening and typical physiological stress patterns the next day (e.g. Adam, Hawkley, Kudielka, & Cacioppo, 2006; Cropley et al., 2015; Doane & Adam, 2010; Zoccola et al., 2011).
Guided by the Perseverative Cognition Hypothesis (Brosschot et al., 2006), we theorised that daily deviations in rumination would help to identify when and how naturally occurring perceptions of stress contribute to acute changes in typical sleep and diurnal cortisol patterns. Based on this theoretical framework, rumination about stress/problems of the day was tested as a moderator of how daily stress levels predict subsequent sleep and daily cortisol patterns. The current study featured several methodological strengths to provide unique contributions to available literature: (1) participants reported daily stress levels and rumination about stress for eight days using web-enabled smartphones, (2) participants wore actigraph wristwatches during this same period to assess multiple objective sleep indices, and (3) participants provided five saliva samples each day for three of these days. For sleep outcomes, we focused on common indices available from actigraphy data, including sleep onset latency (i.e. time between getting in bed and falling asleep) and sleep duration (i.e. time spent asleep at night; Sadeh, 2011). For diurnal cortisol outcomes, we focused on multiple aspects of the next-day pattern, including waking levels, the CAR, and diurnal slope (i.e. rate of decline from waking to bedtime; Adam & Kumari, 2009).
First, we examined whether day-to-day (withinperson) differences in daily perceived stress, rumination about daily stress, and their interaction predicted night-to-night variation in sleep onset latency and duration (up to eight days per participant). Based on prior studies (e.g. Pillai et al., 2014; Zoccola et al., 2009), we expected that greater stress in combination with ruminating more than usual would be related to longer sleep onset latency. Studies have generally not found associations between rumination and sleep duration (Tang & Harvey, 2004; Zoccola et al., 2009), and thus we considered this outcome exploratory. Second, we examined whether day-to-day (withinperson) differences in stress, rumination, and their interaction predicted day-to-day variation in next-day waking cortisol levels, CAR, and diurnal slope (up to three days per participant). We expected that greater daily stress would prolong physiological arousal specifically under the conditions of ruminating more than usual about this stress (Brosschot et al., 2006), resulting in greater cortisol levels the following morning and thereby alterations to the observed diurnal rate of decline (slope). Prior findings are mixed for the CAR (Arbel et al., 2017; Cropley et al., 2015; Zoccola et al., 2011), particularly when examining adherence to morning sample timing (Hilt et al., 2017), and thus we treated the CAR as an exploratory outcome.
Method
Participants
Eighty-two healthy participants were initially recruited for a longitudinal study of the transition from high school into college (see Doane et al., 2015a, 2015b). The current analyses focus on 61 participants who completed intensive ecological momentary assessment (EMA) three years later (25% male; Mage =20.91, SD = 0.36). Participants were 51% White/European American, 29% Hispanic/Latino, 5% Black/African American, 5% Asian American/Pacific Islander, and 10% multiracial. The sample was socioeconomically diverse: 7% of participants’ parents completed some high school, 16% high school diploma, 25% some college, 26% associate’s degree, 11% bachelor’s degree, and 15% graduate degree.
Procedure
Procedures were approved by the university Institutional Review Board. Study personnel collected signed consent forms and explained EMA procedures. For eight days starting on Thursday evening through the following Friday morning of a typical week (i.e. normal class or work schedule), participants completed diary reports of stress, rumination, and health behaviours (e.g. eating, caffeine use) using webenabled smartphones (M = 31.26 diaries out of 33 possible; SD = 3.42; 49% completed 33 reports, 77% completed at least 30). Participants wore the Actiwatch Score (Phillips Respironics, Inc.), an actigraph wrist-based accelerometer, on their non-dominant wrist. Of these eight days, participants provided saliva samples five times per day (upon waking, 30 min later, approximately three and eight hours after waking, and bedtime) for three typical weekdays (M = 14.92 samples out of 15 total, SD = 0.76). Participants were instructed not to eat, drink, or brush their teeth 30 min prior to providing a saliva sample. Participants were compensated.
Measures
Objective sleep
Actigraph-detected sleep data were cross-checked with diary-reported bed and wake times to identify significant outliers and equipment malfunction (Doane et al., 2015b). After removing 11 outlier nights of sleep, 52 participants had valid actigraphy data for all eight nights, eight had data for seven nights, and one had data for five nights. Three to five nights of actigraphy data are considered sufficient to estimate regular sleep (Acebo et al., 1999). Outcome measures included sleep onset latency (i.e. minutes between getting in bed and falling asleep) and sleep duration (i.e. time asleep at night, in hours, excluding wake periods). Actigraph sleep estimates have been validated with polysomnography (Sadeh, 2011).1 Due to positive skew, sleep onset latency minutes were transformed using the square root function.
Salivary cortisol
Upon return to the laboratory, saliva samples were stored at −20C until sent by courier on dry ice over three days to Biochemisches Labor at the University of Trier, Germany, for assay. Cortisol samples were assayed in duplicate using a solid phase time-resolved fluorescence immunoassay with fluorometric endpoint detection (DELFIA; Dressendörfer, Kirschbaum, Rohde, Stahl, & Strasburger, 1992). The intra-assay coefficients of variation ranged from 4.0% to 6.7%, and inter-assay coefficients of variation ranged from 7.1% to 9.0%. Due to positive skew, cortisol values (nmol/L) were log transformed. Self-reported sample times were checked with actigraph-detected wake times and electronic monitoring devices (MEMS 6™ Aardex track caps). Following prior work to reduce sampling time bias (Doane et al., 2015a; Hilt et al., 2017), waking samples were considered compliant if track cap times were within 15 min of actigraph wake times, and second samples were considered compliant if track cap times were between 23 and 37 min after waking samples. In total, 6.2% of samples were deemed non-compliant and treated as missing data.
Daily perceived stress level
Coinciding with the saliva sampling schedule (even on non-sampling days), participants were prompted to briefly describe the most stressful situation that occurred in the last hour and rate its severity (0 = not at all stressful to 4 = very stressful; Sladek, Doane, Luecken, & Eisenberg, 2016). Participants were asked to complete four diary reports on non-sampling days and five diary reports to match the five saliva samples on sampling days. These experience sampling ratings were averaged for each day to create a daily stress level score. Similar to prior work (Sladek et al., 2016), stress levels and variability in stress were present across time of day (Waking: M = 1.13, SD =0.86, range = 0.00–4.00; +30Min: M = 1.52, SD = 0.77, range: 0.00–3.67; +3Hours: M = 1.51, SD = 0.76, range: 0.33–4.00; +8Hours: M = 1.54, SD = 0.72, range: 0.00–3.33; Bedtime: M = 1.56, SD = 0.83, range: 0.00–4.00).
Daily rumination
Participants responded to one question at bedtime, “Overall today, how much did you focus on your problems/stress?”, from 1 (not at all) to 4 (a lot; Moberly & Watkins, 2008). This item has been used successfully in other diary studies, demonstrating associations with trait survey measures of rumination and daily negative affect ratings (Hilt et al., 2017; Moberly & Watkins, 2008). Although one-item measures carry certain limitations, this approach was necessary to reduce participant burden and avoid introducing participant fatigue as a study confound, particularly because multiple assessments of biological data were also included (see Harari et al., 2016). In the present study, daily rumination scores were positively correlated with the Ruminative Responses Scale (Treynor, Gonzalez, & Nolen-Hoeksema, 2003), a survey measure of trait rumination, r = .44, p < .01.
Covariates
Covariates for sleep outcomes were selected based on observed associations in prior research (Doane & Thurston, 2014; Galambos, Vargas Lascano, Howard, & Maggs, 2013) and/or correlations with sleep in the present sample (see below), including participants’ sex, average diary-reported stress (throughout the week), and weekday vs. weekend night. Covariates for diurnal cortisol outcomes were also selected based on associations in prior research (Adam & Kumari, 2009; Doane et al., 2015a) and/or correlations with cortisol in the present sample (see below), including participant’s sex, race/ethnicity, average diary reported stress, daily actigraph wake time, momentary diary reports of negative affect (PANAS; Watson, Clark, & Tellegen, 1988), and reports of eating in the hour before sampling. As a robustness check to acknowledge the role of potential confounds, other variables were also introduced as covariates in alternative models although they were not significantly associated with sleep or cortisol: individual differences in rumination, depressive symptoms (Radloff, 1977), oral contraceptive use, and caffeine or other medication use.
Analytic plan
A series of multilevel models were fit separately for each sleep outcome (sleep onset latency, duration; N = 488 days nested within 61 individuals). Aside from dummy coding for dichotomous variables, continuous Level 1 (day-level; L1) predictors were centred within-person (i.e. individual’s average of available scores subtracted from each daily score), and Level 2 (person-level; L2) variables were grandmean centred (Kreft, De Leeuw, & Aiken, 1995). Thus, L1 coefficients can be interpreted as estimated differences in sleep associated with a 1-unit increase in the predictor relative to an individual’s average or typical level (i.e. when perceiving more stress than usual). The product of within-person centred daily stress and rumination was added to the model to test the statistical interaction.
Three-level growth models were fit to address sample (L1), daily (L2), and individual (Level 3; L3) variation in cortisol (N = 910 samples within 184 days and 61 individuals). Included at L1 were the time of sampling occasion from day and person-specific wake time (i.e. linear growth term), this growth parameter squared to represent curvilinear trend, and a dummy code (1 = second sample, 0 = not second sample) to represent the cortisol awakening response as a deviation from the diurnal cortisol rhythm (Adam & Kumari, 2009; Sladek et al., 2016). Similar to sleep models, daily stress and rumination (time-lagged from the prior day) were centred within-person, and the product of these centred variables was added to test the statistical interaction.
Multilevel models were fit in Mplus 7.4 (Muthén & Muthén, 1998–2012) using maximum likelihood estimation with robust standard errors. Significant interactions were probed using simple slopes techniques for multilevel modelling (Preacher, Curran, & Bauer, 2006). Simple slopes were estimated for associations between daily perceived stress and outcomes at the within-person mean of daily rumination (i.e. when an individual reported ruminating at their own average level for the week) and either 1 or 1.5 SD above and below this mean.
Results
Descriptive statistics and bivariate correlations are presented in Table 1. Based on bivariate correlations between person-level aggregated variables (i.e. individual averages for repeated measures), greater average daily stress was positively correlated with cortisol levels three hours after waking and average diaryreported rumination. Males tended to have shorter sleep duration and lower average diary-reported rumination compared to females. Bedtime cortisol levels were lower for non-Hispanic White participants compared to non-White participants, on average. Depressive symptoms were not correlated with any sleep or cortisol indicators, and thus was only included as a covariate in post-hoc alternative models as a robustness check. At the within-person level, daily stress and rumination were slightly positively correlated, r = .27, p < .01.
Table 1.
Bivariate correlations and descriptive statistics.
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1. Sleep onset latency (min) | - | |||||||||||
| 2. Sleep duration (hours) | .10 | - | ||||||||||
| 3. Waking cortisol | −.14 | .02 | - | |||||||||
| 4. 30 min post-waking cortisol | −.09 | .06 | .54* | - | ||||||||
| 5. 3 h post-waking cortisol | .02 | −.17 | .09 | .22† | - | |||||||
| 6. 8 h post-waking cortisol | .28* | .11 | .17 | .14 | .39* | - | ||||||
| 7. Bedtime cortisol | .11 | −.03 | −.05 | .07 | .09 | .36* | - | |||||
| 8. Average diary stress | −.12 | −.18 | .10 | .12 | .32* | .05 | −.05 | - | ||||
| 9. Average diary rumination | −.13 | −.05 | .19 | .04 | .24† | −.04 | .01 | .30* | - | |||
| 10. Male | .07 | −.27* | .22† | .13 | −.04 | .08 | −.12 | −.14 | −.25* | - | ||
| 11. White race/ethnicity | −.12 | .11 | .25† | .09 | −.11 | −.09 | −.35* | .13 | .18 | −.05 | - | |
| 12. Depressive symptoms | −.08 | .001 | .14 | .01 | .02 | −.001 | .09 | .39* | .25† | −.01 | .09 | - |
| Ma | 6.63 | 6.01 | 7.43 | 11.76 | 5.77 | 3.56 | 1.65 | 1.39 | 2.61 | 0.25 | 0.51 | 14.28 |
| SD | 5.88 | 0.85 | 3.46 | 5.25 | 3.41 | 2.31 | 1.48 | 0.49 | 0.62 | - | - | 8.62 |
| Minimum | 0.13 | 3.87 | 0.60 | 0.79 | 1.01 | 0.74 | 0.16 | 0.33 | 1.31 | - | - | 0.00 |
| Maximum | 39.75 | 7.88 | 17.22 | 27.35 | 17.03 | 12.57 | 6.84 | 3.14 | 4.25 | - | - | 36.00 |
Note. N = 61. Averages of raw cortisol values (nmol/L) presented for descriptive purposes. Average stress and rumination = person-level averages of diary reports. Male: 1 = male, 0 = female. White race/ethnicity: 1 = White, 0 = non-White.
p < .10.
p < .05.
Mean for continuous variables and percentage of study sample for dichotomous variables.
In two-level models for sleep (Table 2), average between-person differences in diary-reported stress were significantly associated with less sleep duration, γ01 = −0.41, p = .01 (L2), such that 1-unit more stress (e.g. moving from not at all to a little, or a little to somewhat) predicted approximately 24.6 min less sleep each night, on average. There were no significant within-person associations of diary-reported stress (p = .84), rumination (p = .41), or their interaction (p = .34) with sleep duration. There was a trend indicating a potential interaction for sleep-onset latency, γ30 = 0.30, p = .099. Given the theorised role of daily rumination as a moderator of this stress-sleep association, we explored this trend further by probing the simple slopes. This post-hoc analysis revealed a positive within-person association of daily stress with sleep onset latency specifically on days when participants ruminated at least 1 SD above their own rumination mean, b = 0.42, p = .05 (16.3% of days), but not on days when they ruminated at their own mean, b =0.14, p = .30, or 1 SD below their own mean, b = −0.10, p = .60. On these days of ruminating more than usual, reporting 1-unit more stress than usual was associated with taking approximately 1.52 more minutes to fall asleep that night. Overall, these results presented in Table 2 were highly similar when fitting alternative models with additional covariates, including caffeine use before bed, between person differences in diary-reported rumination, and depressive symptoms.
Table 2.
Fixed effects estimates from multilevel models predicting sleep from diary-reported stress and rumination.
| Sleep Onset Latency (square root of minutes) | Sleep Duration (hours) | |||
|---|---|---|---|---|
| Fixed effects | Est. | SE | Est. | SE |
| L2: Intercept, Y00 | 1.84* | 0.11 | 6.05* | 0.14 |
| Average diary stress, Y01 | −0.33† | 0.20 | −0.41* | 0.16 |
| Male, Y02 | −0.14 | 0.28 | −0.57* | 0.22 |
| L1: Daily stress, y10 | 0.13 | 0.13 | −0.02 | 0.10 |
| Daily rumination, Y20 | −0.05 | 0.08 | 0.06 | 0.07 |
| Daily stress x daily rumination, Y30 | 0.30† | 0.18 | 0.11 | 0.12 |
| Weekend, Y40 | 0.36* | 0.15 | ||
Note. 488 days nested within 61 individuals. Sleep-onset latency = time to fall asleep (square root of minutes to correct skewed distribution); Sleep duration = time asleep (in hours); Continuous level 1 predictors centred within-person; continuous level 3 predictors grand-mean centred. Male: 1 = Male, 0 = Female; Weekend: 1 = Friday or Saturday night, 0 = all other nights; Est. = partial regression coefficient estimate (unstandardised); SE = robust standard error.
p < .10.
p < .05.
Three-level models for diurnal cortisol revealed the expected average pattern, with cortisol levels relatively high upon waking (5.21 nmol/L), an approximate 97.4% increase 30 min after waking (cortisol awakening response; CAR),2 an approximate 6.8% decrease per hour estimated at waking (diurnal slope), and a significant change in this linear pattern across the day (quadratic; Table 3). Average between-person differences in diary-reported stress were significantly associated with higher waking cortisol levels, γ001 = 0.30, p = .01, such that 1-unit more stress predicted approximately 1.35 nmol/L higher waking cortisol, on average. Regarding day-to-day (within-person) associations, there were significant interactions of daily stress and rumination predicting next-day waking cortisol level, γ030 = 0.16, p = .04, and diurnal cortisol slope (linear decline), γ230 = −0.02, p = .03, but not the CAR, γ130 = −0.23, p = .13. Overall, these results presented in Table 3 were highly similar when fitting an alternative model with additional covariates, including between-person differences in diary-reported rumination, depressive symptoms, and oral contraceptive use, as well as caffeine use in the hour prior to sampling.
Table 3.
Fixed effects estimates from 3-level growth model predicting diurnal cortisol from previous day stress and rumination.
| Fixed effects | Est. | SE |
|---|---|---|
| L1: Intercept (waking cortisol level), Y000 | 1.65* | 0.10 |
| L2: Day-before stress, Y010 | 0.05 | 0.08 |
| L2: Day-before rumination, Y020 | −0.03 | 0.03 |
| L2: Day-before stress x day-before rumination, Y030 | 0.16* | 0.08 |
| L2: Actigraph wake time, Y040 | 0.06 | 0.04 |
| L3: Average diary stress, Y001 | 0.30* | 0.12 |
| L3: Male, Y002 | 0.29* | 0.11 |
| L3: White race/ethnicity, Y003 | 0.10 | 0.13 |
| L1: Cortisol awakening response, Y100 | 0.68* | 0.10 |
| L2: Day-before stress, y110 | −0.07 | 0.12 |
| L2: Day-before rumination, Y120 | −0.03 | 0.06 |
| L2: Day-before stress x day-before rumination, Y130 | −0.23 | 0.15 |
| L2: Actigraph wake time, Y140 | −0.08 | 0.05 |
| L3: Average diary stress, y101 | −0.14 | 0.09 |
| L3: Male, Y102 | −0.13 | 0.12 |
| L3: White race/ethnicity, Y103 | −0.09 | 0.11 |
| L1: Time since waking (diurnal slope), Y200 | −0.07* | 0.01 |
| L2: Day-before stress, Y210 | −0.01 | 0.01 |
| L2: Day-before rumination, Y220 | 0.01† | 0.01 |
| L2: Day-before stress x day-before rumination, Y230 | −0.02* | 0.01 |
| L2: Actigraph wake time, Y240 | −0.02* | 0.004 |
| L3: Average diary stress, Y201 | −0.004 | 0.01 |
| L3: Male, Y202 | −0.02 | 0.02 |
| L3: White race/ethnicity, Y203 | −0.03* | 0.01 |
| L1: Time since waking2 (quadratic), Y300 | −0.15* | 0.02 |
| L1: Negative affect in last hour, Y400 | 0.20* | 0.08 |
| L1: Ate in last hour, Y500 | 0.14* | 0.01 |
Note. N = 910 samples nested within 184 days and 61 individuals. Cortisol values (nmol/L) log transformed. Continuous level 1 and level 2 predictors centred within-person; continuous level 3 predictors grand-mean centred. Male: 1 = Male, 0 = Female; White race/ethnicity: 1 = non-Hispanic White, 0 = non-White; Ate in last hour: 1 = ate, 0 = did not eat.
p < .10.
p < .05.
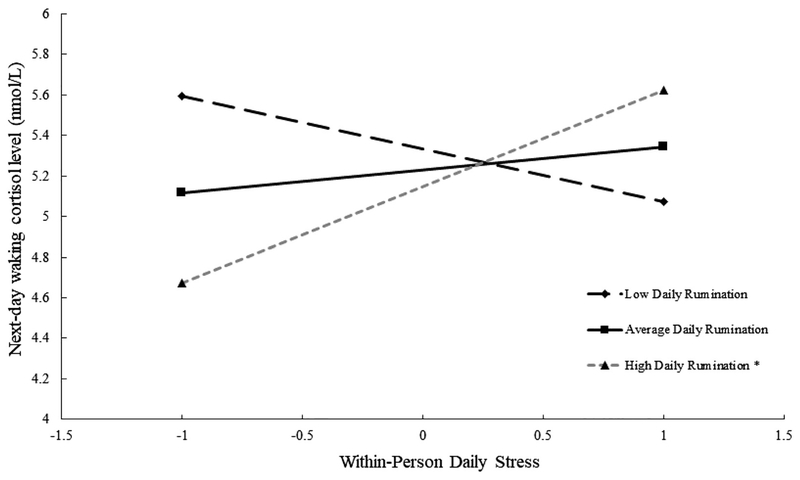
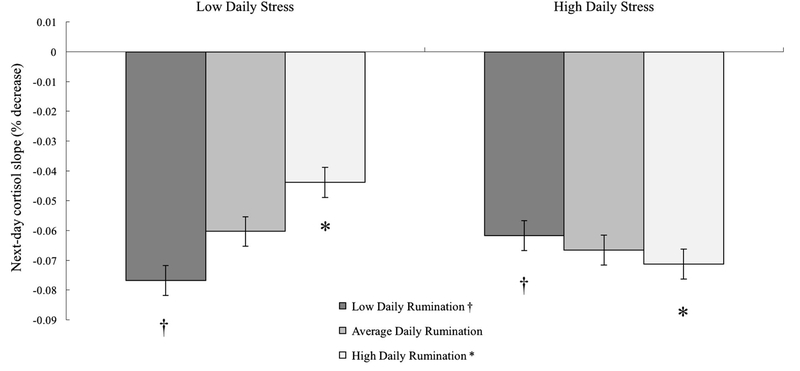
Probing interactions revealed a significant within person association of daily stress with next-day waking cortisol level following days when participants ruminated 1.5 SD above their own rumination mean, b = 0.24, p = .049, but not following days at their own mean, b = 0.05, p = .53, or 1.5 SD below their own mean, b = −0.11, p = .30 (Figure 1). Following these days of ruminating more than usual (7.9% of days), reporting 1-unit more daily stress than usual was associated with approximately 23.6% higher waking cortisol the next day. Similarly, daily stress was associated with a more negative (steeper) next-day diurnal slope at 1 SD above the within-person rumination mean, b = −0.03, p = .048, but not at the mean, b =−0.01, p = .48, or 1 SD below the mean, b = 0.02, p = .10 (Figure 2). Following days of ruminating more than usual, 1-unit less daily stress than usual was associated with approximately 2.0% less decline in cortisol per hour (flatter slope, estimated at waking) the next day.
Figure 1.
Note. Simple slopes by daily stress (1 SD above and below within-person mean) plotted at low (−1.5 SD below within-person mean), average (within-person mean), and high (+1.5 SD above within-person mean) daily rumination. *p < .05.
Figure 2.
Note. Simple slopes for next-day diurnal cortisol slope (% decrease from waking) by daily stress (1 SD above and below within-person mean) plotted at low (−1 SD below within-person mean), average (within-person mean), and high (+1 SD above within-person mean) daily rumination. Less negative rates of decline reflect “flatter” slopes. †p = .10. *p < .05.
Discussion
Prior studies have identified individual differences in rumination tendencies as a risk marker for poor sleep quality (Guastella & Moulds, 2007) and physiological dysregulation (Zoccola et al., 2011). The present intensive eight-day longitudinal study extended this available literature by examining day-to-day (within-person) differences in perceived stress levels and rumination about stress, and how these daily cognitive transactions were associated with objectively-measured sleep that night and diurnal cortisol activity the following day. Between-person analyses showed that greater daily perceived stress throughout the week was associated with sleeping less and with higher waking cortisol levels, on average. Analyses at the day-to-day (within-person) level showed that cortisol levels were higher upon waking following higher stress days when individuals also ruminated about daily problems more than usual. Diurnal cortisol slopes were flatter (i.e. less negative) following days of ruminating about problems more than usual, but only in the context of low-stress days.
Individuals who reported 1-unit greater daily stress via EMA throughout the week tended to sleep approximately 25 min less at night, on average. However, no significant within-person (day-to-day) findings emerged for sleep duration. Similarly, several prior studies have not found relations between rumination and sleep duration (Tang & Harvey, 2004; Zoccola et al., 2009), suggesting that rumination may be more closely linked with subjective sleep quality than activity-based measures of sleep quantity (Guastella & Moulds, 2007; Zoccola et al., 2009). Post-hoc analyses exploring a trend-level finding suggested that the combination of greater daily stress levels and greater rumination may be related to slightly longer actigraph-detected sleep onset latency, but further research is necessary to replicate and clarify this trend finding. Following diary studies that have examined daily stress and rumination separately (Doane & Thurston, 2014; Pillai et al., 2014), our study was among the first to consider perceptions of stress (reported at several occasions throughout the day) and rumination about daily stress (reported at bedtime) in an interactive framework to predict sleep. Other strategies to cope with daily stress, such as the nonjudgmental attentiveness of mindfulness (Howell, Digdon, Buro, & Sheptycki, 2008) or benefiting from social connections with others (Sladek & Doane, 2015), have been related to better sleep and may provide useful stress-responsive alternatives to rumination.
Consistent with our hypothesis, greater daily stress levels and greater rumination interacted to predict higher cortisol levels upon waking the following morning. This finding provides support for the Perseverative Cognition Hypothesis (Brosschot et al., 2006) in daily life and complements prior studies showing associations between rumination and heightened cortisol responses to laboratory stressors (e.g. Zoccola, Quas, & Yim, 2010). Focusing back on one’s stress or problems of the day may maintain physiological arousal throughout the sleep period (Brosschot et al., 2006). Thus, greater waking levels of cortisol may represent a “spillover” effect of continued physiological arousal from the previous night. On the other hand, it is possible that these relatively small elevations in waking cortisol could signify adaptive changes, such as how the CAR may provide a “boost” to prepare for the upcoming day (Adam et al., 2006) and support enhanced cognitive performance (Shi et al., 2018). However, our sampling and modelling approach allowed us to examine waking levels and the magnitude of the CAR simultaneously and separately in this study, showing that the specific combination of greater stress and rumination from the previous day was related to higher levels of cortisol at waking and not the size of the CAR. Based on prior mixed findings (Zoccola & Dickerson, 2012), we did not form specific expectations for the CAR. Our stringent criteria for assessing sampling compliance was consistent with recent expert consensus guidelines (Stalder et al., 2016). With these criteria applied in the present study to avoid time-biased estimates of the CAR, neither daily stress, rumination, nor their interaction predicted day-to-day differences in the CAR. Future studies should continue to emphasise the importance of morning sample timing in examination of the CAR, as well as addressing the potential confounding of cognitive constructs like rumination and completion of intensive study protocols.
Regarding the diurnal cortisol slope, ruminating about daily stress more than usual was associated with a flatter slope (i.e. less rapid rate of decline) following days perceived as less stressful than usual. Flatter diurnal cortisol slopes have most consistently been identified as a risk marker for an array of health problems (e.g. compromised immune function, depression; Adam et al., 2017), suggesting that spending more time focusing on problems may be maladaptive specifically within the context of low-stress days. The concordance between stressor severity and cognitive responses to the stressor (i.e. “goodness of fit”) has been theorised to play a key role in determining the adaptive nature of stress responses (DeLongis & Holtzman, 2005; Forsythe & Compas, 1987). Following these goodness of fit theories and the Perseverative Cognition Hypothesis (Brosschot et al., 2006), how much one attends to or perseverates over stressful content helps to determine the resulting level of distress. Following this transactional framework, ruminating about problems of the day in the absence of reported stress may reflect a more maladaptive response than a relatively better fit between stress and response, potentially contributing to physiological dysregulation following this mismatch. However, this finding was not expected and to our knowledge is the first such test of this daily interaction with respect to cortisol and thus warrants continued attention in healthy and clinical study samples. The day-to-day (withinperson) finding from the present study extends prior research that has identified between-person associations of trait rumination with greater diurnal cortisol output (Huffziger et al., 2013) and flatter diurnal cortisol slopes (Hilt et al., 2017).
Limitations of this study include a modest sample size with a higher proportion of females, precluding tests of moderation by gender. Future work should consider how associations among stress, rumination, sleep, and cortisol may differ by gender. Daily stress and rumination were each measured with a single item, given the needfor brevity to reduce participant burden inthis type of intensive repeated measures design. Future research should explore measuring stress and rumination about stress with greater precision to continue identifying the specific role of perseverative cognitions in the stress process. For example, future research might consider past- and future-oriented focus on stress in daily life, a distinction that has implications for depression and anxiety (Olatunji, Naragon-Gainey, & WolitzkyTaylor, 2013) but was not measured in this study. Future research should also consider additional emotion regulation strategies that individuals employ throughout the day (Lennarz, Hollenstein, Lichtwarck Aschoff, Kuntsche, & Granic, 2019), such as active coping efforts, which have been related to diurnal cortisol activity in prior work (Sladek et al., 2016). Despite our longitudinal EMA approach that allowed for temporal ordering (e.g. stress and rumination preceding sleep and cortisol), these daily processes likely influence each other reciprocally over longer periods of time. Prior studies have found that actigraphy may overestimate sleep duration and underestimate sleep latency in comparison to polysomnography, the gold standard for estimating objective sleep (McCall & McCall, 2012; see review by Sadeh, 2011). However, these over- and under-estimates in actigraphy measurement have been demonstrated in clinical samples, such as individuals with insomnia or ADHD (e.g. Hvolby, Jorgensen, & Bilenberg, 2008; Vallieres & Morin, 2003), rather than healthy community samples; further validation studies may help to clarify concordance between multiple assessments of sleep. Finally, sleep and diurnal stress physiology are interconnected (e.g. Van Lenten & Doane, 2016; Vargas & Lopez-Duran, 2014); we did not have sufficient power to test a mediation model with our daily variables, but we did control for objectively measured wake time in the cortisol model and future research should explore this potential pathway further. Limitations aside, the current study identified associations among daily stress, rumination, and multiple objectively-recorded health-relevant markers, furthering evidence for daily stress and rumination as potential risk factors. Future studies should consider adopting this process-oriented approach to further identify the complexity of daily stress, rumination, and corresponding acute alterations to sleep and diurnal HPA patterns.
Supplementary Material
Funding
M.R.S. is now affiliated with Harvard Graduate School of Education. This research was supported by the Arizona State University College of Liberal Arts and Sciences NS-SS-GRG Research Seed Grant. This research was also conducted with the support of the National Science Foundation Graduate Research Fellowship Program under grant number DGE-1311230 to M.R.S., the Eunice Kennedy Shriver National Institute of Child Health and Human Development of the National Institutes of Health under Award Number R01HD079520 to L.D.D, and a William T. Grant Foundation Scholar Award to L.D.D. Any opinion, findings, and conclusions expressed in this material are those of the authors and do not necessarily reflect views of funding agencies. Thanks to participants and research assistants of the ASU Transition to College Study, Andrea Gierens at Biochemisches Labor at the University of Trier for technical assistance with salivary assays, Kevin Grimm for comments on a previous version of the manuscript, and Saul Castro and HyeJung Park for assistance with data cleaning.
Footnotes
The current study scored sleep using the Phillips Actiware 6 program and the validated Sadeh algorithm to measure sleep (Oakley, 1997; Sadeh, Hauri, Kripke, & Lavie, 1995; Sadeh, Sharkey, & Carskadon, 1994): A = E−2(1/25) + E−1(1/5) + E + E + 1(1/5) + E + 2(1/25), where A denotes activity counts and E denotes epoch. Within the Sadeh algorithm, activity counts (A) within each epoch (E) were calculated based on activity levels during the adjacent 2 min period. The threshold in the algorithm and model was set to 40, with a range of 20–80. We calculated sleep parameters based on 1-minute epochs and significant movement after at least 10 min of inactivity.
Because cortisol values were log transformed, the effect sizes can be interpreted as a percent change per 1-unit change in the predictor after using the formula: % change = [(eb) − 1].
Disclosure statement
No potential conflict of interest was reported by the authors.
Supplemental data for this article can be accessed http://dx.doi.org/10.1080/09500340.2019.1601781
References
- Acebo C, Sadeh A, Seifer R, Tzischinsky O, Wolfson AR, & Hafer A (1999). Estimating sleep patterns with activity monitoring in children and adolescents: How many nights are necessary for reliable measures? Sleep, 22, 95–103. [DOI] [PubMed] [Google Scholar]
- Adam EK (2012). Emotion—cortisol transactions occur over multiple time scales in development: Implications for research on emotion and the development of emotional disorders. Monographs of the Society for Research in Child Development, 77, 17–27. [Google Scholar]
- Adam EK, Hawkley LC, Kudielka BM, & Cacioppo JT (2006). Day-to-day dynamics of experience–cortisol associations in a population-based sample of older adults. Proceedings of the National Academy of Sciences, 103, 17058–17063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adam EK, & Kumari M (2009). Assessing salivary cortisol in large-scale, epidemiological research. Psychoneuroendocrinology, 34, 1423–1436. [DOI] [PubMed] [Google Scholar]
- Adam EK, Quinn ME, Tavernier R, McQuillan MT, Dahlke KA, & Gilbert KE (2017). Diurnal cortisol slopes and mental and physical health outcomes: A systematic review and meta-analysis. Psychoneuroendocrinology, 83, 25–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Affleck G, Zautra A, Tennen H, & Armeli S (1999). Multilevel daily process designs for consulting and clinical psychology: A preface for the perplexed. Journal of Consulting and Clinical Psychology, 67, 746–754. [DOI] [PubMed] [Google Scholar]
- Arbel R, Shapiro LS, Timmons AC, Moss IK, & Margolin G (2017). Adolescents’ daily worry, morning cortisol, and health symptoms. Journal of Adolescent Health, 60, 667–673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baglioni C, Spiegelhalder K, Lombardo C, & Riemann D (2010). Sleep and emotions: A focus on insomnia. Sleep Medicine Reviews, 14, 227–238. [DOI] [PubMed] [Google Scholar]
- Brans K, Koval P, Verduyn P, Lim YL, & Kuppens P (2013). The regulation of negative and positive affect in daily life. Emotion, 13, 926–939. [DOI] [PubMed] [Google Scholar]
- Brosschot JF, Gerin W, & Thayer JF (2006). The perseverative cognition hypothesis: A review of worry, prolonged stressrelated physiological activation, and health. Journal of Psychosomatic Research, 60, 113–124. [DOI] [PubMed] [Google Scholar]
- Cropley M, Rydstedt LW, Devereux JJ, & Middleton B (2015). The relationship between work-related rumination and evening and morning salivary cortisol secretion. Stress and Health, 31, 150–157. [DOI] [PubMed] [Google Scholar]
- DeLongis A, & Holtzman S (2005). Coping in context: The role of stress, social support, and personality in coping. Journal of Personality, 73(6), 1633–1656. [DOI] [PubMed] [Google Scholar]
- Doane LD, & Adam EK (2010). Loneliness and cortisol: Momentary, day-to-day, and trait associations. Psychoneuroendocrinology, 35, 430–441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doane LD, Chen FR, Sladek MR, Van Lenten SA, & Granger DA (2015a). Latent trait cortisol (LTC) levels: Reliability, validity, and stability. Psychoneuroendocrinology, 55, 21–35. [DOI] [PubMed] [Google Scholar]
- Doane LD, Gress-Smith JL, & Breitenstein RS (2015b). Multimethod assessments of sleep over the transition to college and the associations with depression and anxiety symptoms. Journal of Youth and Adolescence, 44, 389–404. [DOI] [PubMed] [Google Scholar]
- Doane LD, & Thurston EC (2014). Associations among sleep, daily experiences, and loneliness in adolescence: Evidence of moderating and bidirectional pathways. Journal of Adolescence, 37, 145–154. [DOI] [PubMed] [Google Scholar]
- Dressendörfer RA, Kirschbaum C, Rohde W, Stahl F, & Strasburger CJ (1992). Synthesis of a cortisol-biotin conjugate and evaluation as a tracer in an immunoassay for salivary cortisol measurement. The Journal of Steroid Biochemistry and Molecular Biology, 43, 683–692. [DOI] [PubMed] [Google Scholar]
- Forsythe CJ, & Compas BE (1987). Interaction of cognitive appraisals of stressful events and coping: Testing the goodness of fit hypothesis. Cognitive Therapy and Research, 11, 473–485. [Google Scholar]
- Galambos NL, Vargas Lascano DI, Howard AL, & Maggs JL (2013). Who sleeps best? Longitudinal patterns and covariates of change in sleep quantity, quality, and timing across four university years. Behavioral Sleep Medicine, 11, 8–22. [DOI] [PubMed] [Google Scholar]
- Guastella AJ, & Moulds ML (2007). The impact of rumination on sleep quality following a stressful life event. Personality and Individual Differences, 42, 1151–1162. [Google Scholar]
- Gunthert KC, Cohen LH, Butler AC, & Beck JS (2007). Depression and next-day spillover of negative mood and depressive cognitions following interpersonal stress. Cognitive Therapy and Research, 31, 521–532. [Google Scholar]
- Harari GM, Lane ND, Wang R, Crosier BS, Campbell AT, & Gosling SD (2016). Using smartphones to collect behavioral data in psychological science: Opportunities, practical considerations, and challenges. Perspectives on Psychological Science, 11, 838–854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hilt LM, Sladek MR, Doane LD, & Stroud CB (2017). Daily and trait rumination: Diurnal cortisol patterns in adolescent girls. Cognition and Emotion, 31, 1757–1767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howell AJ, Digdon NL, Buro K, & Sheptycki AR (2008). Relations among mindfulness, well-being, and sleep. Personality and Individual Differences, 45(773e), 7. [Google Scholar]
- Huffziger S, Ebner-Priemer U, Zamoscik V, Reinhard I, Kirsch P, & Kuehner C (2013). Effects of mood and rumination on cortisol levels in daily life: An ambulatory assessment study in remitted depressed patients and healthy controls. Psychoneuroendocrinology, 38, 2258–2267. [DOI] [PubMed] [Google Scholar]
- Hvolby A, Jorgensen J, & Bilenberg N (2008). Actigraphic and parental reports of sleep difficulties in children with attentiondeficit/hyperactivity disorder. Archives of Pediatric Adolescent Medicine, 162, 323–329. [DOI] [PubMed] [Google Scholar]
- Kircanski K, Thompson RJ, Sorenson J, Sherdell L, & Gotlib IH (2018). The everyday dynamics of rumination and worry: Precipitant events and affective consequences. Cognition and Emotion, 32, 1424–1436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreft IG, De Leeuw J, & Aiken LS (1995). The effect of different forms of centering in hierarchical linear models. Multivariate Behavioral Research, 30(1), 1–21. [DOI] [PubMed] [Google Scholar]
- Lancee J, Eisma MC, van Zanten KB, & Topper M (2017). When thinking impairs sleep: Trait, daytime and nighttime repetitive thinking in insomnia. Behavioral Sleep Medicine, 15, 53–69. [DOI] [PubMed] [Google Scholar]
- Lennarz HK, Hollenstein T, Lichtwarck-Aschoff A, Kuntsche E,& Granic I (2019). Emotion regulation in action: Use, selection, and success of emotion regulation in adolescents’ daily lives. International Journal of Behavioral Development, 43(1), 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lund HG, Reider BD, Whiting AB, & Prichard JR (2010). Sleep patterns and predictors of disturbed sleep in a large population of college students. Journal of Adolescent Health, 46, 124–132. [DOI] [PubMed] [Google Scholar]
- McCall C, & McCall WV (2012). Comparison of actigraphy with polysomnography and sleep logs in depressed insomniacs. Journal of Sleep Research, 21, 122–127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCullough ME, Orsulak P, Brandon A, & Akers L (2007). Rumination, fear, and cortisol: An in vivo study of interpersonal transgressions. Health Psychology, 26, 126–132. [DOI] [PubMed] [Google Scholar]
- Moberly NJ, & Watkins ER (2008). Ruminative self-focus and negative affect: An experience sampling study. Journal of Abnormal Psychology, 117, 314–323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muthén LK, & Muthén BO (1998–2012). Mplus User’s Guide (7th ed.). Los Angeles, CA: Muthén & Muthén. Nolen-Hoeksema, S., Wisco, B. E., & Lyubomirsky, S. (2008). Rethinking rumination. Perspectives on Psychological Science, 3, 400–424. [DOI] [PubMed] [Google Scholar]
- Nota JA, & Coles ME (2015). Duration and timing of sleep are associated with repetitive negative thinking. Cognitive Therapy and Research, 39(2), 253–261. [Google Scholar]
- Oakley NR (1997). Validation with polysomnography of the Sleepwatch sleep/wake scoring algorithm used by the Actiwatch activity monitoring system. Bend: Mini Mitter, Cambridge Neurotechnology. [Google Scholar]
- Olatunji BO, Naragon-Gainey K, & Wolitzky-Taylor KB (2013). Specificity of rumination in anxiety and depression: A multimodal meta-analysis. Clinical Psychology: Science and Practice, 20, 225–257. [Google Scholar]
- Peeters F, Nicolson NA, Berkhof J, Delespaul P, & deVries M (2003). Effects of daily events on mood states in major depressive disorder. Journal of Abnormal Psychology, 112, 203–211. [DOI] [PubMed] [Google Scholar]
- Pillai V, Steenburg LA, Ciesla JA, Roth T, & Drake CL (2014). A seven day actigraphy-based study of rumination and sleep disturbance among young adults with despressive symptoms. Journal of Psychosomatic Research, 77, 70–75. [DOI] [PubMed] [Google Scholar]
- Preacher KJ, Curran PJ, & Bauer DJ (2006). Computational tools for probing interaction effects in multiple linear regression, multilevel modeling, and latent curve analysis. Journal of Educational and Behavioral Statistics, 31, 437–448. [Google Scholar]
- Radloff LS (1977). The CES-D scale: A self-report depression scale for research in the general population. Applied Psychological Measurement, 1, 385–401. [Google Scholar]
- Rydstedt LW, Cropley M, Devereux JJ, & Michalianou G (2009). The effects of gender, long-term need for recovery and trait inhibition-rumination on morning and evening saliva cortisol secretion. Anxiety, Stress, & Coping, 22, 465–474. [DOI] [PubMed] [Google Scholar]
- Sadeh A (2011). The role and validity of actigraphy in sleep medicine: An update. Sleep Medicine Reviews, 15, 259–267. [DOI] [PubMed] [Google Scholar]
- Sadeh A, Hauri PJ, Kripke DF, & Lavie P (1995). The role of actigraphy in the evaluation of sleep disorders. Sleep, 18, 288–302. [DOI] [PubMed] [Google Scholar]
- Sadeh A, Sharkey KM, & Carskadon MA (1994). Activitybased sleep-wake identification: An empirical test of methodological issues. Sleep, 17, 201–207. [DOI] [PubMed] [Google Scholar]
- Schlotz W, Hellhammer J, Schulz P, & Stone AA (2004). Perceived work overload and chronic worrying predict weekend–weekday differences in the cortisol awakening response. Psychosomatic Medicine, 66, 207–214. [DOI] [PubMed] [Google Scholar]
- Shi X, Sun X, Yao Z, Yuan Y, Wu J, & Clow A (2018). The cortisol awakening response predicts response inhibition in the afternoon of the same day. Psychoneuroendocrinology, 89, 23–29. [DOI] [PubMed] [Google Scholar]
- Sladek MR, & Doane LD (2015). Daily diary reports of social connection, objective sleep, and the cortisol awakening response during adolescents’ first year of college. Journal of Youth and Adolescence, 44, 298–316. [DOI] [PubMed] [Google Scholar]
- Sladek MR, Doane LD, Luecken LJ, & Eisenberg N (2016). Perceived stress, coping, and cortisol reactivity in daily life: A study of adolescents during the first year of college. Biological Psychology, 117, 8–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stalder T, Kirschbaum C, Kudielka BM, Adam EK, Pruessner JC, Wüst S, … Clow A (2016). Assessment of the cortisol awakening response: Expert consensus guidelines. Psychoneuroendocrinology, 63, 414–432. [DOI] [PubMed] [Google Scholar]
- Tang NK, & Harvey AG (2004). Effects of cognitive arousal and physiological arousal on sleep perception. Sleep, 27, 67–78. [DOI] [PubMed] [Google Scholar]
- Treynor W, Gonzalez R, & Nolen-Hoeksema S (2003). Rumination reconsidered: A psychometric analysis. Cognitive Therapy and Research, 27(3), 247–259. [Google Scholar]
- Vallieres A, & Morin CM (2003). Actigraphy in the assessment of insomnia. Sleep, 26, 902–906. [DOI] [PubMed] [Google Scholar]
- Van Lenten SA, & Doane LD (2016). Examining multiple sleep behaviors and diurnal salivary cortisol and alpha-amylase: Within-and between-person associations. Psychoneuroendocrinology, 68, 100–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vargas I, & Lopez-Duran N (2014). Dissecting the impact of sleep and stress on the cortisol awakening response in young adults. Psychoneuroendocrinology, 40, 10–16. [DOI] [PubMed] [Google Scholar]
- Watkins ER (2008). Constructive and unconstructive repetitive thought. Psychological Bulletin, 134, 163–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson D, Clark LA, & Tellegen A (1988). Development and validation of brief measures of positive and negative affect: The PANAS scales. Journal of Personality and Social Psychology, 54, 1063–1070. [DOI] [PubMed] [Google Scholar]
- Zoccola PM, & Dickerson SS (2012). Assessing the relationship between rumination and cortisol: A review. Journal of Psychosomatic Research, 73(1), 1–9. [DOI] [PubMed] [Google Scholar]
- Zoccola PM, Dickerson SS, & Lam S (2009). Rumination predicts longer sleep onset latency after an acute psychosocial stressor. Psychosomatic Medicine, 71, 771–775. [DOI] [PubMed] [Google Scholar]
- Zoccola PM, Dickerson SS, & Yim IS (2011). Trait and state perseverative cognition and the cortisol awakening response. Psychoneuroendocrinology, 36, 592–595. [DOI] [PubMed] [Google Scholar]
- Zoccola PM, Quas JA, & Yim IS (2010). Salivary cortisol responses to a psychosocial laboratory stressor and later verbal recall of the stressor: The role of trait and state rumination. Stress, 13, 435–443. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.