Abstract
Background:
We examined the association between sarcopenia and posttransplant mortality in acutely ill inpatients with cirrhosis who underwent urgent liver transplantation.
Methods:
Included were inpatients at 4 centers who were urgently listed as non-Status 1 and transplanted from 2005–17 with an abdominal computed tomography scan <90 days prior. Skeletal muscle index (SMI) = total skeletal muscle cross sectional area the L3 vertebral level, normalized to height. Cox regression associated SMI with posttransplant mortality. Optimal search identified SMI cutoffs to detect survival.
Results:
Of 126 inpatients: 63% were male, MELD-Na was 32, and follow up was 5.1 years.
Among men: 23% died. Median SMI was lower in men who died versus survived (45 versus 51 cm2/m2). SMI was associated with posttransplant mortality (HR=0.96 per cm2/m2, 95%CI 0.92–0.99). Patients with SMI ≤ versus >48 cm2/m2 experienced higher rates of death at 1- (86% versus 95%) and 3-years (73% versus 95%) (logrank P = 0.01). In MELD-adjusted analysis, sarcopenia was strongly associated with posttransplant mortality (HR=4.39, 95%CI 1.49–12.97).
Among women: 35% died. Median SMI was similar in women who died versus survived (45 versus 44 cm2/m2). SMI was not associated with posttransplant mortality (HR=1.02, 95%CI 0.96–1.09). Optimal search did not identify any SMI cutoff that predicted posttransplant mortality.
Conclusion:
Among patients who underwent urgent inpatient evaluation and liver transplantation, we identified an SMI cut-off of 48 cm2/m2 to predict posttransplant mortality in men. Our data support the use of SMI as a tool to capture the impact of muscle depletion on posttransplant mortality in acutely ill men with cirrhosis undergoing urgent liver transplantation.
INTRODUCTION
In patients with cirrhosis, physical frailty – a construct representing an individual’s physiologic reserve to withstand health stressors1 – is a critical determinant of adverse health outcomes, including hospitalizations, resource utilization, and death.2–8 In the liver transplant setting, performance-based measures of physical frailty, such as the Liver Frailty Index, may be particularly useful given their objectivity and ability to enhance mortality risk stratification beyond that provided by the Model for End-Stage Liver Disease (MELDNa) score or clinician assessments alone.2,9 However, studies evaluating performance-based metrics of physical frailty have largely included liver transplant candidates in the outpatient setting, but there remains a unmet need for tools to assess those who present for the first time to a liver transplant center seeking urgent evaluation (and liver transplantation) as an inpatient.10 In these situations, decisions to proceed with liver transplantation must be made urgently when the patient is acutely ill, and performance-based tests may not accurately represent an individual’s underlying “steady state” physiologic reserve.
Sarcopenia, or loss of muscle mass, is a highly prevalent complication of cirrhosis that is associated with increased mortality both before and after transplantation.11–13 While frailty is a multi-dimensional construct, sarcopenia is the dominant domain of frailty in patients with cirrhosis. Therefore, we hypothesized that skeletal muscle mass could serve as a surrogate marker for physiologic reserve in patients who are acutely ill (and unable to fully engage in performance-based tests of physical frailty) – and predict outcomes after liver transplantation. In this study, we aimed to evaluate this association specifically in acutely-ill inpatients with cirrhosis undergoing urgent evaluation and liver transplantation.
METHODS
Study population and setting
Data were collected from four North American liver transplant centers: University of California-San Francisco (n = 47), University of Pittsburgh (n = 40), University of Alberta (n = 23), Mayo Clinic Scottsdale (n = 16).
Included were adult inpatients who underwent urgent evaluation and liver transplantation within 30 days of listing during the same hospitalization from January 1, 2005 through December 31, 2017, and who had an abdominal CT scan capturing L3 within 90 days prior to transplant. Excluded were those who underwent liver transplantation for fulminant hepatic failure. Characteristics of both the transplant recipients and the donors were retrospectively collected from the electronic health records of each patient; data collectors were blinded to the skeletal muscle results of the patients. We ascertained the following outcomes: posttransplant length of stay and days in the intensive care unit (ICU), posttransplant discharge location (categorized as home, transfer to other acute hospital, acute rehabilitation facility, skilled nursing facility), death, retransplantation, reoperations (other than retransplantation), re-hospitalizations within 6 months of transplant, episodes of acute cellular rejection (biopsy proven), and infections (defined as positive microbial culture within 6 months of transplant).
Measurement of muscle mass
Quantification of muscle mass was performed as has previously been described,11 by trained personnel who were blinded to all clinical patient data. Briefly, skeletal muscle area in cm2 (including psoas, paraspinal, rectus abdominus, transverse abdominis, and internal/external obliques) was semi-automatically quantified from the CT image at the level of third lumbar vertebra using the image analysis software application General Electric Advanced Workstation 4.6 (GE-AW 4.6). The L3 skeletal muscle area was then normalized to height to calculate the SMI: SMI (cm2/m2) = (total abdominal skeletal muscle area in cm2)/(height in m2).
Statistical Analysis
Baseline characteristics were reported as medians [interquartile ranges (IQR)] or numbers (percentages) and compared by gender using Wilcoxon ranksum or chi-square tests as appropriate. Cox regression was used to assess associations between SMI and other potential predictors with the primary outcome of all-cause posttransplant mortality. All patients were followed until death after transplant or, for patients who did not die, were censored between March 15, 2018-May 18, 2018. All factors that were associated with posttransplant mortality in univariable analysis with a P-value of 0.20 were evaluated for inclusion in the final multivariable model. The final multivariable model was developed using backwards elimination of variables until all variables included were associated with a P-value of <0.05.
Optimal search method was used to identify potential cutoffs of SMI that best predicted mortality after liver transplantation. Given the well-known differences in muscle mass between men and women, our analyses were stratified by gender. Specifically, a cutoff SMI value was estimated based on a grid search guided by log-rank and Wilcoxon test statistics that identified values of SMI that separated patients into two groups according to survival. The final SMI cutoff was selected based on greatest statistical significance (lowest P-value <0.05).
Statistical analysis was performed with Stata, version 15.1 (StataCorp, College Station, TX). The study was approved by the Institutional Review Boards of each center prior to data collection. Clinical data and images were shared under the provisions of a multi-center Data Use Agreement among the participating institutions, who are all members of the Fitness, Life Enhancement, and Exercise in Liver Transplantation (FLEXIT) Consortium.11
Results
Baseline characteristics of the patients
A total of 126 patients from four North American transplant centers was included. Baseline characteristics are shown in Table 1. Median age was 53 years and 63% were male. The majority of patients were Non-Hispanic Whites (71%); chronic HCV (31%) and alcoholic liver disease (25%) were the two most common etiologies. Median MELD and MELDNa on admission were 30 and 33, respectively, and at transplant were 32 and 32, reflecting the high acuity of this inpatient-only cohort. Only 11% underwent simultaneously liver/kidney transplantation. The median time from abdominal CT scan to transplant was 14 days; median posttransplant time to discharge was 16 days.
Table 1.
Baseline characteristics of 126 men and women with cirrhosis who underwent urgent evaluation and liver transplantation within the same hospitalization
| Characteristic* | All (n = 126) | Women n = 46 (37%) |
P-value | |
|---|---|---|---|---|
| Age, years | 53 (46–59) | 54 (46–60) | 0.32 | |
| Race | Non-Hispanic white | 87 (71) | 32 (71) | 0.33 |
| Black | 6 (5) | 2 (4) | ||
| Hispanic | 17 (14) | 5 (11) | ||
| Asian | 7 (6) | 2 (4) | ||
| Other | 5 (4) | 4 (9) | ||
| Etiology of liver disease | HCV | 39 (31) | 12 (26) | 0.02 |
| Alcohol | 31 (25) | 8 (17) | ||
| NASH/NAFLD | 9 (7) | 5 (11) | ||
| AIH/PBC/PSC | 16 (13) | 11 (24) | ||
| HBV | 12 (10) | 2 (4) | ||
| Other | 19 (15) | 8 (17) | ||
| HCC | 16 (13) | 5 (11) | 0.67 | |
| Diabetes | 23 (18) | 11 (25) | 0.4 | |
| Coronary Artery Disease | 5 (4) | 1 (2) | 0.5 | |
| Weight, kg | 81 (69–95) | 77 (67–87) | 0.02 | |
| BMI, kg/m2 | 28 (24–33) | 27 (24–32) | 0.38 | |
| Admit MELD | 30 (22–36) | 30 (24–37) | 0.60 | |
| Admit MELDNa | 33 (25–38) | 33 (26–38) | 0.78 | |
| Laboratories at transplant | MELD | 32 (25–37) | 31 (25–36) | 0.23 |
| MELDNa | 32 (26–38) | 32 (26–37) | 0.17 | |
| Sodium | 137 (133–141) | 138 (135–141) | 0.07 | |
| Bilirubin | 16.9 (6.3–31.2) | 18.5 (7.4–31.2) | 0.88 | |
| INR | 2.3 (1.7–3.1) | 2.3 (1.7–3.0) | 0.97 | |
| Creatinine | 1.8 (1.0–3.0) | 1.5 (0.9–2.2) | 0.04 | |
| Albumin (g/dL) | 3.2 (2.9–3.7) | 3.4 (3.1–4.0) | 0.01 | |
| Ascites | None | 25 (20) | 7 (16) | 0.56 |
| Mild/moderate | 56 (44) | 20 (44) | ||
| Severe | 44 (35) | 18 (40) | ||
| Hepatic encephalopathy | Absent | 45 (36) | 12 (26) | 0.19 |
| Altered Mood/Confusion | 47 (38) | 21 (46) | ||
| Markedly Confused/Comatose | 33 (26) | 13 (28) | ||
| Simultaneous liver/kidney transplant | 9 (11) | 3 (10) | 0.82 | |
| Time from abdominal CT scan to transplant, days | 14 (7–30) | 19 (9–35) | 0.04 | |
| Posttransplant follow-up time, years | 5.1 (2–9.8) | 4.2 (2–8.9) | 0.31 | |
Median (IQR) or n (%)
Association between SMI, sex, and mortality
Overall median (IQR) SMI was 47 cm2/m2 (42–53 cm2/m2). The median SMI for men was 49 cm2/m2 (44–55) and for women was 44 cm2/m2 (40–51). Median SMI did not differ significantly by center for either men (P = 0.5) or women (P = 0.09). At a median posttransplant follow-up of 5.1 years, 99 (79%) were alive and 27 (21%) had died. Men who died had a significantly lower SMI than those who lived (51 versus 45 cm2/m2; P = 0.04) (Table 2). There was no significant difference in SMI among women who died versus lived (44 versus 45 cm2/m2; P = 0.64) (Table 2). In men, each unit increase in SMI was associated with lower post-liver transplant mortality in univariable Cox regression (HR 0.96 per cm2/m2, 95%CI 0.92–0.99; P = 0.03). In women, SMI was not associated with posttransplant mortality (HR 1.02; 95%CI 0.96–1.08; P = 0.51).
Table 2.
Relationship between SMI and mortality
| Alive (n = 92; 73%) |
Dead (n = 34; 27%) |
P-value | |
|---|---|---|---|
| SMI, cm2/m2 | 48 (42–53) | 45 (41–51) | 0.14 |
| Males SMI, cm2/m2 | 51 (45–56) | 45 (42–48) | 0.04 |
| Females SMI, cm2/m2 | 44 (40–51) | 45 (40–52) | 0.64 |
Establishing cutoff values for sarcopenia
We sought to establish SMI cutoffs by starting with the relationship between posttransplant outcomes and SMI stratified by gender. For men, SMI cutoffs between 47–50 cm2/m2 were statistically significantly associated with posttransplant mortality (Table 3). Chi-square test statistic values from Wilcoxon and Log-rank analyses for both men and women are represented in Figures S1a and S1b. Each potential cutoff for men was evaluated to maximize statistical significance while maintaining a sufficient number of events to detect differences in outcomes. SMI cutoff of 48 cm2/m2 met these criteria. Of the 80 men in our cohort, 37 (46%) were sarcopenic as defined as SMI<48 cm2/m2; men with sarcopenia, compared to those without sarcopenia, had a significantly higher rates of posttransplant mortality at 1-year (86% versus 95%) and 3-years (73% versus 95%) (logrank P = 0.01). In univariable analysis, sarcopenia was strongly associated with an increased risk of posttransplant mortality among men (HR 3.65; 95% CI, 1.29–10.28; P = 0.01). Other covariates that were associated with posttransplant mortality in univariable analysis at a p-value <0.20 were MELD score (HR per point 1.06; P = 0.03), BMI (HR 0.94; P = 0.17), alcoholic liver disease (HR 0.29; P = 0.12), presence of diabetes (HR 2.01; P = 0.09), and severe hepatic encephalopathy (HR 2.69; P = 0.14). In multivariable analysis, the association between sarcopenia (SMI<48 cm2/m2) and posttransplant mortality remained significant after adjustment for MELD etiology of liver disease (HR, 4.39; P = 0.007) (Table 4). Unadjusted Kaplan-Meier posttransplant survival curves for men with and without sarcopenia as defined by SMI<48 cm2/m2 are shown in Figure 1.
Table 3.
Evaluation of potential SMI cutoffs for (A) men and (B) women
| Cutoff SMI, cm2/m2 | n (%) | Hazard Ratio (95% CI) | P-value | |
| ≤44 | 17 (21) | 1.49 (0.53–4.17) | 0.45 | |
| ≤45 | 23 (29) | 2.23 (0.86–5.66) | 0.09 | |
| ≤46 | 28 (35) | 1.94 (0.77–4.89) | 0.16 | |
| ≤47 | 34 (43) | 3.18 (1.19–8.49) | 0.02 | |
| ≤48 | 37 (46) | 3.65 (1.29–10.28) | 0.01 | |
| ≤49 | 41 (51) | 2.82 (1.00–7.92) | 0.05 | |
| ≤50 | 43 (54) | 2.53 (0.90–7.11) | 0.08 | |
| ≤51 | 46 (58) | 2.21 (0.79–6.21) | 0.13 | |
| ≤52 | 52 (65) | 1.98 (0.65–6.02) | 0.23 | |
| ≤53 | 54 (68) | 1.74 (0.57–5.29) | 0.33 | |
| ≤54 | 59 (74) | 1.20 (0.39–3.64) | 0.75 | |
| ≤55 | 60 (75) | 1.64 (0.48–5.68) | 0.43 | |
| Cutoff SMI, cm2/m2 | n (%) | Hazard Ratio (95%CI) | P-value | |
| ≤39 | 10 (22) | 1.27 (0.41–3.97) | 0.68 | |
| ≤40 | 12 (26) | 0.96 (0.31–2.98) | 0.93 | |
| ≤41 | 15 (33) | 0.83 (0.29–2.42) | 0.74 | |
| ≤42 | 17 (37) | 0.71 (0.24–2.03) | 0.51 | |
| ≤43 | 19 (41) | 0.60 (0.21–1.74) | 0.35 | |
| ≤44 | 21 (46) | 0.75 (0.27–2.07) | 0.58 | |
| ≤45 | 24 (52) | 1.01 (0.38–2.70) | 0.98 | |
| ≤46 | 27 (59) | 1.07 (0.40–2.88) | 0.89 | |
| ≤47 | 29 (63) | 1.07 (0.40–2.88) | 0.89 | |
| ≤48 | 30 (65) | 0.96 (0.35–2.64) | 0.93 | |
| ≤49 | 32 (70) | 1.02 (0.35–2.93) | 0.97 | |
| ≤50 | 33 (72) | 0.95 (0.33–2.72) | 0.92 | |
| ≤51 | 35 (76) | 0.71 (0.25–2.05) | 0.53 | |
Table 4.
Univariable and Multivariable Cox regression for the outcome of posttransplant mortality in men
| Variable | Unadjusted HR (95% CI) P-value |
Adjusted* HR (95% CI) P-value |
|---|---|---|
| Sarcopenic (SMI <48) | 3.65 (1.29–10.28) 0.01 |
4.39 (1.49–12.97) 0.007 |
| MELD | 1.06 (1.01–1.11) 0.03 |
1.08 (1.02–1.13) 0.005 |
| Age | 1.03 (0.98–1.08) 0.29 |
|
| BMI | 0.94 (0.87–1.03) 0.17 |
|
| HCC | 2.02 (0.66–6.12) 0.21 |
|
| Diabetes | 2.01 (0.94–4.47) 0.09 |
|
| Etiology of Liver Disease | ||
| HCV | Reference | |
| Alcohol | 0.29 (0.06–1.36) 0.12 |
|
| NASH/NAFLD | 0.99 (0.12–7.90) 0.99 |
|
| AIH/PSC/PBC | 1.07 (0.23–5.04) 0.94 |
|
| HBV | 0.67 (0.14–3.16) 0.61 |
|
| Other | 0.89 (0.24–3.37) 0.87 |
|
| Hepatic Encephalopathy | ||
| Absent | Reference | |
| Altered Mood/Confusion | 1.89 (0.51–7.07) 0.34 |
|
| Markedly Confused/Comatose | 2.69 (0.72–10.05) 0.14 |
|
| Ascites | ||
| Absent | Reference | |
| Mild/Moderate | 1.09 (0.32–3.62) 0.89 |
|
| Severe | 0.55 (0.12–2.45) 0.43 |
|
| Donor age | 0.98 (0.94–1.02) 0.38 |
All variables associated with a P-value <0.2 in univariable analysis were evaluated for inclusion in the final multivariable model. The final multivariable model was developed using backwards elimination and included only those variables that were associated with a P <0.05.
Figure 1.
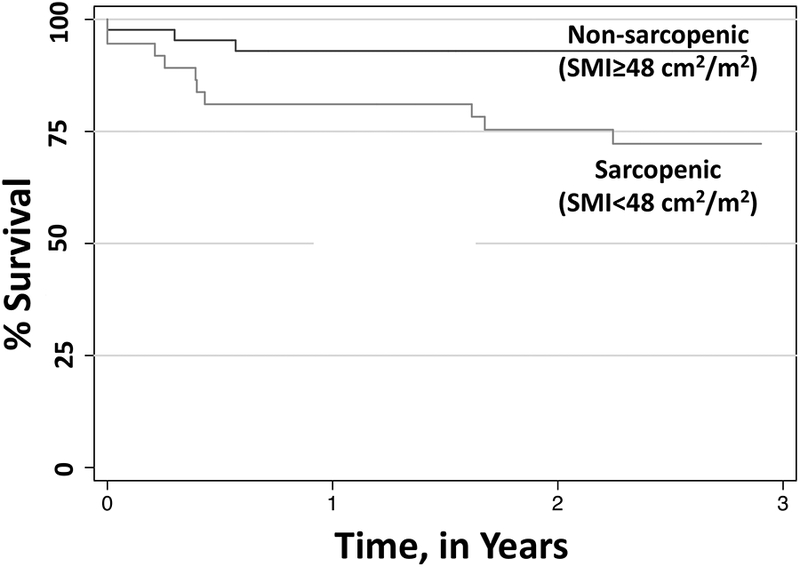
Kaplan-Meier survival curves in sarcopenic men using SMI cutoff of 48 cm2/m2.
For women, no cutoff of SMI was significantly associated with posttransplant mortality.
DISCUSSION
Transplant clinicians are frequently faced with the clinical dilemma of how to assess risk in the acutely decompensated patient with cirrhosis presenting to our liver transplant centers as inpatients for the very first time. They all look very ill and unwell – but is that merely a reflection of their acute on chronic liver failure, in which case this vulnerability should easily reverse with liver transplantation? Or is it really a reflection of long-standing, chronic illness that will not reverse quickly after liver transplantation and importantly, negatively impact post-operative recovery despite having a new liver?14 While prior studies have shown that sarcopenia is a useful metric to capture physiologic reserve – and therefore vulnerability to poor outcomes after liver transplantation – these studies have largely included stable outpatients who were previously listed and with relatively lower MELD scores.12,13,15,16 It is not known whether these data on stable outpatients are generalizable to the acutely ill, high MELD patient undergoing urgent inpatient evaluation and liver transplantation.
In our multicenter study including this select population of inpatients at 4 North American liver transplant centers, we identified SMI 48 cm2/m2 as a cutoff to predict posttransplant mortality in men, but our analyses did not identify an optimal SMI cutoff for women. Compared to our prior multicenter study that used the same methods to quantify sarcopenia for the outcome of pre-transplant mortality, the median MELD score of our population was higher (33 versus 15), but the median SMI was similar (47 versus 48 cm2/m2).11 It is interesting to note that the SMI cut-off that we identified in this subpopulation of acutely-ill men was quite similar to the SMI cut-off that we previously identified to predict pre-transplant mortality (<50 cm2/m2), supporting the general scientific premise that low muscle mass is a surrogate for underlying factors that contribute to mortality.
Our analyses add to the current body of work focused on the impact of sarcopenia in the liver transplant setting by drawing attention to the lack of association between sarcopenia and posttransplant mortality in women. Other studies investigating sarcopenia and posttransplant outcomes reported an overall association (for both men and women together) but did not stratify their results by gender.12,16 The one study that did stratify their results by gender reported a similar finding where muscle mass only predicted posttransplant mortality in men but not for women; however, this study included a heterogeneous cohort of liver transplant recipients (presumably both outpatient and inpatient) with a median MELD score of 20.15 Our study underscores the importance of gender-stratification in analyses involving sarcopenia to better understand whether this gender-based difference in the association between sarcopenia and posttransplant mortality is due to small sample sizes of women or whether it is a true non-association.
It is worth noting that there were relatively few patients with a diagnosis of NASH/NAFLD in our cohort (7%). This may have been due to the fact that this cohort dates back to 2005 when diagnosis of NASH/NAFLD was less frequent or could be due to the fact that we only recruited patients who presented as inpatients (whereas a patient with NASH cirrhosis might more commonly present as outpatients). Given the reported associations between NAFLD and sarcopenia,17,18 future studies with a larger number of patients with NASH/NAFLD should focus more specifically on the potential interaction NAFLD and sarcopenia on the outcome of posttransplant mortality.
We acknowledge several limitations to our study. Because we were interested in studying a highly a selected population of acutely-ill patients undergoing urgent evaluation and liver transplantation, our sample size was relatively small, increasing the likelihood of a type II statistical error with respect to our findings in women. We conducted our search for SMI cut-points using standard optimal search methodology using univariable logistic regression – which is well-known to be vulnerable to Type I statistical error.19 However, Figure 1A illustrates how the search clustered around optimal SMI cut-point we identified, increasing confidence in our cut-point. Another limitation is that we included only patients with an abdominal CT scan within 3 months prior to transplant, raising the possibility of selection bias in our cohort. However, the median SMI in our cohort was similar to that reported in our other study that included an entirely different population of outpatients with a median MELDNa of 14 suggesting that this population is fairly representative of liver transplant recipients (aside from being acutely ill at the time of evaluation).11 Furthermore, the inclusion of patients from four liver transplant centers enhances the generalizability of these findings to the broader high-MELD liver transplant population as a whole. Lastly, we only measured SMI at a single time point. While the median time from SMI measurement to transplant was only 14 days, skeletal muscle wasting can occur within a few days in critically ill patients.20 The degree to which such wasting occurred – and impacted posttransplant outcomes in this population – remains to be investigated.
Despite these limitations, our findings have important implications for the liver transplant community as a whole, as this is the first study to investigate the effects of sarcopenia in this specific subpopulation of inpatient liver transplant candidates. Although the concept of physiologic reserve is more traditionally operationalized by multidimensional measures of physical frailty, sarcopenia is likely the dominant component of the frail phenotype in patients with cirrhosis – and traditional measures of physical frailty have limited utility in acutely ill patients who cannot reliably comply with performance-based tests or answer self-reported questions accurately. Our determination of an SMI cutoff for acutely ill men with cirrhosis undergoing urgent evaluation and liver transplantation fills a clinical need for an objective metric of muscle mass among patients in whom traditional measures of physical frailty are not feasible or reliable. While larger studies are necessary to confirm our findings and our cutpoints, our study provides important data to justify incorporation of objective muscle mass measurement, such as with CT-scan, into the evaluation of the critically-ill liver transplant candidates who are presenting to a liver transplant center for the first time without any corroborating evidence of their functional status prior to acute hepatic decompensation. For a patient without any other co-morbidities or risk factors for poor posttransplant outcome, the presence of sarcopenia may guide the decision regarding donor quality – such as avoidance of a marginal quality liver.21 For a patient with multiple other co-morbidities, the presence of sarcopenia may be indicative of the long-standing effects of cirrhosis and other co-morbidities that make it unlikely that he or she will be able to recover fully functional independence after liver transplantation.
Supplementary Material
Financial disclosure:
This study was funded by K23AG048337 (Paul B. Beeson Career Development Award in Aging Research). These funding agencies played no role in the analysis of the data or the preparation of this manuscript.
Abbreviations:
- HR
hazard ratio
- IQR
interquartile range
- MELD
Model for End Stage Liver Disease
- SMI
skeletal muscle index
Footnotes
Conflicts of interest: The authors of this manuscript have no conflicts of interest to disclose as described by Transplantation.
REFERENCES
- 1.Fried LP, Tangen CM, Walston J, et al. Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci Med Sci. 2001;56(3):146–156. [DOI] [PubMed] [Google Scholar]
- 2.Lai JC, Covinsky KE, Dodge JL, et al. Development of a novel frailty index to predict mortality in patients with end-stage liver disease. Hepatology. 2017;66(2):564–574. doi: 10.1002/hep.29219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lai JC, Feng S, Terrault NA, et al. Frailty predicts waitlist mortality in liver transplant candidates. Am J Transplant. 2014;14(8):1870–1879. doi: 10.1111/ajt.12762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lai JC, Dodge JL, Sen S, et al. Functional decline in patients with cirrhosis awaiting liver transplantation: results from the Functional Assessment in Liver Transplantation (FrAILT) Study. Hepatology. 2016;(63):574–580. doi: 10.1002/hep.28316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dunn MA, Josbeno DA, Tevar AD, et al. Frailty as tested by gait speed is an independent risk factor for cirrhosis complications that require hospitalization. Am J Gastroenterol. 2016;111(12):1768–1775. doi: 10.1038/ajg.2016.336. [DOI] [PubMed] [Google Scholar]
- 6.Sinclair M, Poltavskiy E, Dodge JL, et al. Frailty is independently associated with increased hospitalisation days in patients on the liver transplant waitlist. World J Gastroenterol. 2017;23(5):899–905. doi: 10.3748/wjg.v23.i5.899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tandon P, Reddy KR, O’Leary JG, et al. A Karnofsky performance status-based score predicts death after hospital discharge in patients with cirrhosis. Hepatology. 2017;65(1):217–224. doi: 10.1002/hep.28900. [DOI] [PubMed] [Google Scholar]
- 8.Tandon P, Tangri N, Thomas L, et al. A rapid bedside screen to predict unplanned hospitalization and death in outpatients with cirrhosis: a prospective evaluation of the clinical frailty scale. Am J Gastroenterol. 2016;111(12):1759–1767. doi: 10.1038/ajg.2016.303. [DOI] [PubMed] [Google Scholar]
- 9.Lai JC, Covinsky KE, McCulloch CE, et al. The liver frailty index improves mortality prediction of the subjective clinician assessment in patients with cirrhosis. Am J Gastroenterol. 2018;113(2):235–242. doi: 10.1038/ajg.2017.443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.OPTN Database. Accessed February 2016. https://optn.transplant.hrsa.gov/data/view-data-reports/national-data/.
- 11.Carey EJ, Lai JC, Wang CW, et al. A multicenter study to define sarcopenia in patients with end-stage liver disease. Liver Transpl. 2017;23(5):625–633. doi: 10.1002/lt.24750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Englesbe MJ, Patel SP, He K, et al. Sarcopenia and mortality after liver transplantation. J Am Coll Surg. 2010;211(2):271–278. doi: 10.1016/j.jamcollsurg.2010.03.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Montano-Loza AJ. Skeletal muscle abnormalities and outcomes after liver transplantation. Liver Transpl. 2014;20(11):1293–1295. doi: 10.1002/lt.23995. [DOI] [PubMed] [Google Scholar]
- 14.Lai JC. A framework to determine when liver transplantation is futile. Clin Liver Dis (Hoboken). 2016;8(6):137–139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.DiMartini A, Cruz RJ Jr, Dew MA, et al. Muscle mass predicts outcomes following liver transplantation. Liver Transpl. 2013;19(11):1172–1180. doi: 10.1002/lt.23724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hamaguchi Y, Kaido T, Okumura S, et al. Impact of quality as well as quantity of skeletal muscle on outcomes after liver transplantation. Liver Transpl. 2014;20(11):1413–1419. doi: 10.1002/lt.23970. [DOI] [PubMed] [Google Scholar]
- 17.Koo BK, Kim D, Joo SK, et al. Sarcopenia is an independent risk factor for non-alcoholic steatohepatitis and significant fibrosis. J Hepatol. 2017;66(1):123–131. doi: 10.1016/j.jhep.2016.08.019. [DOI] [PubMed] [Google Scholar]
- 18.Tovo CV, Fernandes SA, Buss C, et al. Sarcopenia and non-alcoholic fatty liver disease: is there a relationship? A systematic review. World J Hepatol. 2017;9(6):326–332. doi: 10.4254/wjh.v9.i6.326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Williams BA, Mandrekar JA, Mandrekar SJ, et al. Finding optimal cutpoints for continuous covariates with binary and time-to-event outcomes. Rochester, MN, Mayo Foundation, Technical Report Series 79, 2006. [Google Scholar]
- 20.Puthucheary ZA, Rawal J, McPhail M, et al. Acute skeletal muscle wasting in critical illness. JAMA. 2013;310(15):1591–1600. doi: 10.1001/jama.2013.278481. [DOI] [PubMed] [Google Scholar]
- 21.Lai JC. Transplant for the very sick: no limitations in donor quality? Liver Transpl. 2017;23(S1):S40–S43. doi: 10.1002/lt.24824. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


