Abstract
PURPOSE:
To demonstrate rapid improvement of recalcitrant cystoid macular edema (CME) and perivascular leakage, in a patient with non-paraneoplastic autoimmune retinopathy (npAIR) and autoimmune optic neuropathy (AON) after treatment with sarilumab, a human anti-interleukin-6 (IL-6) receptor antibody.
METHODS:
Observational case report.
RESULTS:
A 29-year-old woman was diagnosed with npAIR and AON and followed over 1.5 years. She had recalcitrant cystoid macular edema despite local corticosteroid and immunosuppressive therapy that included azathioprine and adalimumab. Subcutaneous Sarilumab, was initiated at a dose of 200mg every two weeks. CME significantly decreased after two injections and resolved after four injections with associated improvement in visual acuity as well as significant improvement in perivascular leakage on fluorescein angiography. There was sustained visual and anatomical improvement at 6 months along with mild improvement in electroretinogram responses. The patient tolerated the medication with no side effects.
CONCLUSION:
Management of CME in npAIR is challenging and long-term immunosuppression is often employed with varying degrees of success. The improvement in refractory CME and perivascular leakage in this case supports the potential role of an IL-6 inhibitor to treat CME associated with npAIR suggesting the role.
Keywords: Anti-IL-6 receptor antibody, non-paraneoplastic autoimmune retinopathy, macular edema, sarilumab, uveitis
SUMMARY STATEMENT
We demonstrate rapid improvement of recalcitrant cystoid macular edema and perivascular leakage, with improvement in vision, after initiation of subcutaneous sarilumab, a human anti-interleukin-6 (IL-6) receptor antibody in a patient with non-paraneoplastic autoimmune retinopathy and autoimmune optic neuropathy, that was refractory to prior local therapy and systemic immunosuppression.
Introduction
Non-paraneoplastic autoimmune retinopathy (npAIR) is characterized by presence of antiretinal antibodies, visual field deficits, and photoreceptor dysfunction in the setting of progressive unexplained vision loss. 1 The diagnosis and management of npAIR is challenging, due to lack of standardized clinical and laboratory diagnostic criteria. 1 As no standardized diagnostic criteria exist, there are no consensus guidelines for the management of npAIR or associated cystoid macular edema (CME), which is observed frequently in npAIR.
Herein, we report a case of npAIR with recalcitrant CME that resolved after the initiation of sarilumab, a recombinant human monoclonal IL-6 receptor antibody.
Case Report
A 29-year-old healthy woman presented with bilateral vision loss of unknown etiology. She reported that vision had been poor in the left eye for over a year and had more recently noted a change in her right eye over the last 6 weeks. Her history was negative for malignancy, systemic inflammation or infection, prior ocular surgery, ocular trauma or drug abuse. On examination, her best-corrected visual acuity was 20/70 in the right eye and count fingers at 2 feet in the left eye.
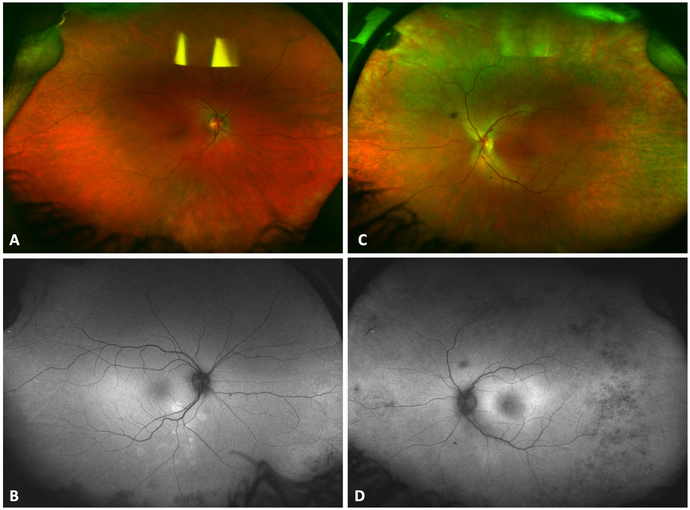
On ophthalmoscopy, her fundus was unremarkable except for macular edema in both eyes (Figure 1 A and C). On optical coherence tomography (OCT), there was bilateral CME with extensive loss of outer retinal layers and disorganization of retinal inner layers in the left eye. Fundus autofluorescence showed mild parafoveal hyperautofluorescence in the left eye and a few areas of peripheral hypoautofluorescence in both eyes (Figure 1 B and D).
Figure 1.
Color fundus photograph of the right (Figure 1 A) and left (Figure 1 C) eye. Fundus autofluorescence showed mild parafoveal hyperautofluorescence in the left eye (Figure 1 D) and a few areas of peripheral hypoautofluorescence in both eyes (Figure 1 B).
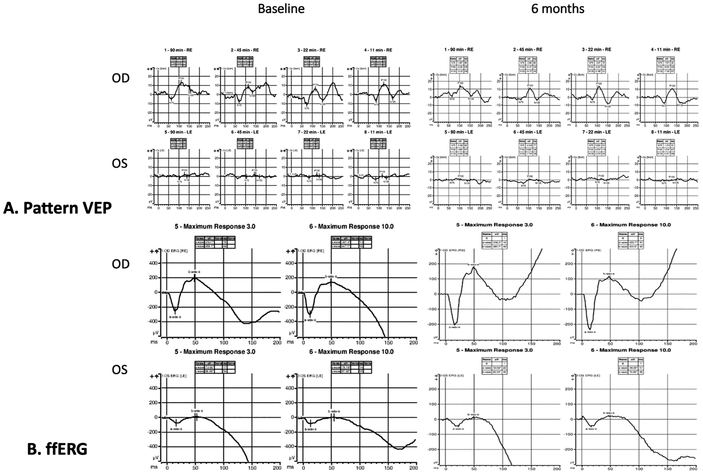
On Western blot, she had serum antiretinal autoantibodies against carbonic anhydrase II and enolase proteins and on immunohistochemistry the photoreceptor cell layer stained. She also had anti-optic nerve antibodies against the 42, 46 and 58 kDa proteins. In addition to routine cancer screening, she underwent a neoplastic workup including a computerized tomography scan of the chest, abdomen and pelvis and magnetic resonance imaging of the brain which were unremarkable. Goldman visual fields showed a markedly constricted left eye visual field and moderately decreased right eye sensitivity. Pattern visual evoked potentials showed normal amplitude in the right eye and a markedly reduced amplitude in the left eye (Figure 2 A). Full-field electroretinography showed an electronegative waveform with marked reduction of all responses in the left eye greater than the right eye (Figure 2 B). Based on these findings, she was diagnosed with npAIR.
Figure 2.
Pattern visual evoked potentials showed normal amplitude in the right eye and a markedly reduced amplitude in the left eye (Figure 2 A). At 6 months follow up (right panel) there is stable amplitude in both eyes with slightly improvement at 45 min arc in the left eye. Full-field electroretinography showed an electronegative waveform with marked reduction of all responses in the left eye greater than the right eye (Figure 2 B) with slight improvement in both eyes at 6 months (right panel).
The CME was refractory to treatment with topical prednisolone acetate and ketorolac and did not improve after multiple posterior subtenon triamcinolone acetonide injections. Given the progressive disease and refractory CME, she was started on immunosuppressive therapy beginning with azathioprine titrated up to 225 mg/day with no response at 3 months. Other antimetabolite agents such as mycophenolate mofetil were avoided since the patient was considering trying to conceive at that time. Later on, she decided to delay trying to conceive until the CME was better controlled. She had progressively worsening CME and was then started on adalimumab 40mg subcutaneous every two weeks with no improvement and also developed an injection site rash. Given progressive decline in visual acuity to 20/80 in the right eye, an intravitreal triamcinolone acetonide injectable suspension injection (2mg/0.05mL) was administered which resulted in resolution of the CME and improvement in vision to 20/50 but she developed corticosteroid-induced elevated intraocular pressure to 42 mmHg that was not controlled with topical medications or oral acetazolamide and required a glaucoma tube shunt drainage device. The intravitreal corticosteroid injection also caused progression of her posterior subcapsular cataract. Two months following the triamcinolone acetonide injection she had recurrent CME with reduction in visual acuity to 20/70.
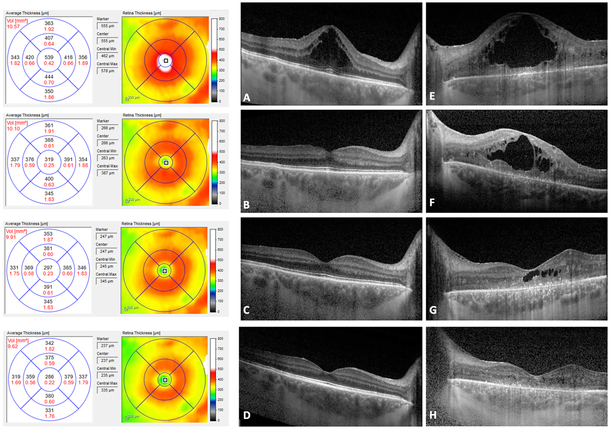
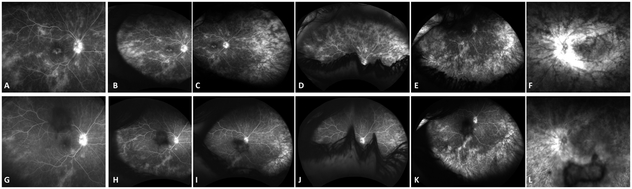
At this time, Sarilumab (Regeneron Pharmaceuticals, Inc.; Tarrytown, NY, USA), 200 mg subcutaneously given every 2 weeks was added to the patient’s regimen (Figure 3, A and E). Within two weeks of initiation of sarilumab she had marked improvement in CME (Figure 3, B and F) and vision improved to 20/50 in the right eye. After six weeks on Sarilumab, there was complete resolution of CME in the right eye with improvement in vision to 20/40 (limited by the posterior subcapsular cataract) and significant improvement in the CME in the left eye (Figure 3, C and G) as well with subjective improvement in left eye vision which was limited by the optic neuropathy and severe loss of the outer retinal layers. There was also significant improvement in perivascular leakage on fluorescein angiography (Figure 4, top row, A-F prior to initiation of sarilumab and bottom row G-L, six weeks following sarilumab). At her most recent follow up visit at 6 months, vision had improved to 20/32 in the right eye and there was further subjective improvement in the left eye. There was further reduction in retinal thickening in both eyes with complete resolution of CME (Figure 3 D and H) along with a slight improvement in ERG amplitudes at 6 months (Figure 2). Goldmann visual field remained stable in the right eye and was improved slightly in the left eye.
Figure 3.
Optical Coherence Tomography (OCT) showing Cystoid Macular Edema (CME) at baseline in the right eye (A) and left eye with extensive loss of the outer retinal layers and disorganization of retinal inner layers (E). Following two weeks on Sarilumab subcutaneous injections, there is marked improved in CME in both eyes (B and F) and at 6 weeks there is complete resolution of CME in the right eye (C) and near resolution in the left eye (G). At 6 months there is continued resolution of CME in the right eye with further improvement in non-cystic thickening (D) along with complete resolution of CME in the left eye (H).
Figure 4.
Fluorescein angiogram showing disc leakage, parafoveal leakage and mid peripheral perivascular leakage in the right eye (A) and more peripheral perivascular leakage on temporal (B), nasal (C), superior (D) and inferior (E) steered widefield images. Following six weeks of Sarilumab therapy, there is significant improvement of leakage in the posterior pole (G) as well as improvement in peripheral leakage on temporal (H), nasal (I), superior (J) and inferior (K) steered images. The left eye (F and L) also showed a similar improvement in leakage.
The patient has tolerated the medication with no systemic side effects. She continues treatment with sarilumab injections every two weeks, and her disease has remained stable. The azathioprine is being tapered down. Because of the recalcitrant nature of her disease before sarilumab initiation, we plan to continue the treatment for at least 2 years.
Discussion
The management of npAIR remains a challenge and due to lack of consensus of treatment protocols and inconsistent patient outcomes regarding the effectiveness of treatment in npAIR, immunosuppression is often empirical due to the presumed autoimmune pathogenesis. 1 Corticosteroids, cyclosporine, mycophenolate, intravenous immunoglobulin, and rituximab all have been used with varying degrees of success in npAIR to limit disease progression and/or to improve visual acuity. 2
CME is seen frequently in npAIR and although its development is a poorly understood process, it is often seen in younger patients in whom it may cause severe visual disability and impact productivity and quality of life. We have recently shown that CME is a biomarker of more severe and more progressive disease in npAIR and is associated with decreased ERG amplitudes and a greater velocity of ellipsoid zone loss. 3 Eyes with CME at initial presentation also have a more severe form of disease. 3 It is therefore important to treat CME aggressively in npAIR.
Recent evidence suggests that interleukin (IL)-6 may play a central role in posterior segment uveitis. 4 IL-6 is a pleiotropic cytokine that regulates the immune and inflammatory response. It is essential to T helper 17 cell differentiation and these cells play a key role in several autoimmune diseases. Vitreous and aqueous IL-6 levels are elevated in eyes with uveitis. 5 Sarilumab is a recombinant human monoclonal antibody directed against the alpha subunit of the IL-6 receptor complex, and thereby blocks the IL-6 binding and disrupts the IL-6 signaling cascade.
The recent phase 2 SATURN study (SARIL-NIU: Sarilumab for the Treatment of Posterior Segment Non-Infectious Uveitis), demonstrated the efficacy and safety of inhibition of IL-6 signaling with sarilumab 200 mg given subcutaneously every 2 weeks for 16 weeks resulting in improved vitreous haze, CME, and visual acuity. 6
Efficacy and safety of sarilumab for the treatment of rheumatoid arthritis have been investigated in three pivotal phase III clinical trials.7-9Sarilumab administered as mono or combination therapy improved signs, symptoms, and physical function in rheumatoid arthritis patients with inadequate response or intolerance to methotrexate or tumor necrosis factor inhibitors. Based on these findings, sarilumab was approved by the US Food and Drug Administration (May, 2017) for treatment of adult patients with moderately to severely active rheumatoid arthritis with an inadequate response or intolerance to one or more disease-modifying antirheumatic drugs.
We report the first use of sarilumab in a patient with npAIR with rapid and successful treatment of refractory CME and reduction in leakage on fluorescein angiogram, with improved visual acuity following subcutaneous administration of the drug. In a recent case report, we have also shown that another humanized monoclonal antibody IL-6 inhibitor, tocilizumab, given as an intravenous infusion (Genentech, South San Francisco, CA) was effective to treat CME associated with npAIR, at an dose of 8mg/kg every 4 weeks. 10
Although npAIR remains poorly understood and difficult to treat, this report of rapid and successful treatment of a patient with refractory CME and npAIR adds to evidence that IL-6 antagonists may be useful to manage CME associated with npAIR. A larger series with longer follow-up is needed to determine whether early administration of IL-6 inhibitors to selected patients with CME associated with npAIR may lead to better functional results and long-term control in this challenging recalcitrant disease.
Acknowledgments
Financial Support: NIH Core Grant for Vision Research EY005722 and the Unrestricted RPB Grant from Research to Prevent Blindness Inc. (both to Duke University Department of Ophthalmology).
Footnotes
Conflict of Interest: No conflicting relationship exists for any author. None of the authors have a proprietary interest.
REFERENCES
- 1.Sen HN, Grange L, Akanda M, Fox A. Autoimmune Retinopathy: Current Concepts and Practices (An American Ophthalmological Society Thesis). Transactions of the American Ophthalmological Society. 2017;115:T8. [PMC free article] [PubMed] [Google Scholar]
- 2.Davoudi S, Ebrahimiadib N, Yasa C, et al. Outcomes in Autoimmune Retinopathy Patients Treated With Rituximab. American journal of ophthalmology. 2017;180:124–132. [DOI] [PubMed] [Google Scholar]
- 3.Finn AP, Thomas AS, Stinnett SS, Keenan RT, Grewal DS, Jaffe GJ. The role of cystoid macular edema as a marker in the progression of non-paraneoplastic autoimmune retinopathy. Graefe’s archive for clinical and experimental ophthalmology = Albrecht von Graefes Archiv fur klinische und experimentelle Ophthalmologie. 2018;256(10):1867–1873. [DOI] [PubMed] [Google Scholar]
- 4.Mesquida M, Molins B, Llorenc V, de la Maza MS, Adan A. Targeting interleukin-6 in autoimmune uveitis. Autoimmunity reviews. 2017;16(10):1079–1089. [DOI] [PubMed] [Google Scholar]
- 5.Perez VL, Papaliodis GN, Chu D, Anzaar F, Christen W, Foster CS. Elevated levels of interleukin 6 in the vitreous fluid of patients with pars planitis and posterior uveitis: the Massachusetts eye & ear experience and review of previous studies. Ocular immunology and inflammation. 2004;12(3):193–201. [DOI] [PubMed] [Google Scholar]
- 6.Heissigerova J, Callanan D, de Smet MD, et al. Efficacy and Safety of Sarilumab for Noninfectious Uveitis of Posterior Segment: Outcomes From the Phase 2 SATURN Trial. Ophthalmology. 2018. [DOI] [PubMed] [Google Scholar]
- 7.Burmester GR, Lin Y, Patel R, et al. Efficacy and safety of sarilumab monotherapy versus adalimumab monotherapy for the treatment of patients with active rheumatoid arthritis (MONARCH): a randomised, double-blind, parallel-group phase III trial. Annals of the rheumatic diseases. 2017;76(5):840–847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fleischmann R, van Adelsberg J, Lin Y, et al. Sarilumab and Nonbiologic Disease-Modifying Antirheumatic Drugs in Patients With Active Rheumatoid Arthritis and Inadequate Response or Intolerance to Tumor Necrosis Factor Inhibitors. Arthritis & rheumatology (Hoboken, NJ). 2017;69(2):277–290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Genovese MC, Fleischmann R, Kivitz AJ, et al. Sarilumab Plus Methotrexate in Patients With Active Rheumatoid Arthritis and Inadequate Response to Methotrexate: Results of a Phase III Study. Arthritis & rheumatology (Hoboken, NJ). 2015;67(6):1424–1437. [DOI] [PubMed] [Google Scholar]
- 10.Finn AP, Keenan RT, Jaffe GJ. Reconstitution Of The Ellipsoid Zone With Tocilizumab In Autoimmune Retinopathy. Retinal cases & brief reports. 2018. [DOI] [PubMed] [Google Scholar]