Abstract
Background:
Aberrations in both neural reward processing and stress reactivity are associated with increased risk for mental illness; yet, how these two factors relate to each other remains unclear. Several studies suggest that stress exposure impacts reward function, thus increasing risk for psychopathology. However, the alternative hypothesis, in which reward dysfunction impacts stress reactivity, has been rarely examined. The current study aimed to test both hypotheses using a longitudinal design.
Methods:
Participants were 38 children (23 females, 61%) from a prospective cohort study. A standard stress-exposure measure was collected at age 7. Children performed a well-validated imaging reward paradigm at age 10, and a standardized acute psychological stress laboratory protocol was administered both at age 10 and 13. Structural equation modeling was used to examine bidirectional associations between stress and neural response to reward anticipation.
Results:
Higher exposure to stressful life events at age 7 predicted lower neural response to reward anticipation in regions of the basal ganglia at age 10, which included ventral caudate, nucleus accumbens, putamen and globus pallidus. Lower response to reward anticipation in medial prefrontal and anterior cingulate cortex predicted higher stress reactivity at age 13.
Conclusions:
Our findings provide support for bidirectional associations between stress and reward processing, in that, stress may impact on reward anticipation, but also reduced reward anticipation may increase susceptibility to stress.
Keywords: reward, stress, imaging, longitudinal, children, depression
Introduction
Aberrations in both reward processing and stress reactivity occur in mental illness (1–3), but the nature of relations between these two factors remains unclear. Evidence derived mostly from adult samples suggests that stress exposure impacts reward function, which increases risk for psychopathology (4). However, an alternative theory might be that reward dysfunction affects stress sensitivity, increasing the risk for stress-related disorders. This hypothesis has been rarely examined. Such neglect is surprising since reward dysfunction is a risk factor for psychiatric disorders, particularly depression (5). Here, we leverage a prospective pediatric cohort to evaluate both models. To do so, we utilize data from a standard stress-exposure measure collected at age 7, a well-validated imaging paradigm performed at age 10, and a standardized acute psychological stress laboratory protocol administered both at age 10 and 13.
It has been suggested that the effects of stress on depression arise through mediating effects on the brain reward circuit (2–4, 6). Support for this model comes from both cross-sectional and prospective studies examining the impact of stress on neural reward processing. Longitudinal studies relate early life stress exposure to later reward dysfunction. Overall, these studies find early stress exposure to predict decreased responses to reward cues and outcomes in the basal ganglia, including nucleus accumbens, caudate, putamen, and globus pallidus (7–11). To a lesser extent, some studies find associations between early life stress and lower response in the insula, anterior cingulate cortex (ACC), and medial prefrontal cortex (mPFC) (7, 12). These findings suggest that exposure to early adversity during sensitive periods impacts the development of reward-related regions, especially in the striatum.
Cross-sectional studies find acute or chronic psychological stress to impact reward processing. However, whereas considerable evidence links current stress to altered reward processing, findings vary based on types of stress and phases of reward. For example, under conditions of acute stress, some studies find decreased response in the striatum (13) or the mPFC (14) during reward anticipation, whereas others find increased striatal response (15). Reward feedback responses under acute stress are consistently blunted in the striatum (15–17). Conversely, chronic stress has been associated with decreased response of the mPFC during reward feedback (18), and with low positive affect in those with decreased striatum response to reward feedback (19).
Several brain mechanisms have been suggested to mediate the effects of stress on reward signaling (for reviews, see (2, 3)). Human and animal studies have shown that stress exposure impacts on dopamine (DA) release from the ventral tegmental area (VTA) towards the NAcc and mPFC. In addition, the amygdala, a region involved in stress and fear conditioning, also receives DA from VTA and modulates stress responses and DA release in the NAcc and mPFC (20, 21). Indeed, increased connectivity between these regions has been linked to higher stress and depressive symptoms, including anhedonia (22–24). Moreover, the neuropeptide corticotropin-releasing factor (CRF), a key component of the hypothalamic pituitary adrenal (HPA) axis functioning, is released by the amygdala projecting to VTA and NAcc and seems to impact DA release in these regions, especially following stress (25, 26). Recent studies have also implicated CRF within the mPFC with impairing cognitive function and frontostriatal signaling (27, 28), which might have an impact on reward-based learning (29). Other factors, such as opioid receptors or inflammatory markers have been also implicated in stress-induced reward dysfunction (2, 3).
In summary, there is substantial evidence to suggest that stress is related to brain-reward dysfunction. However, an alternative model might be that the presence of brain-reward dysfunction can increase stress sensitivity and the likelihood of exposure to stress, which in turn would increase the risk for depression (4, 30, 31). Surprisingly, this alternative model has not been tested, though the original framework is not new. Nearly thirty years ago Hammen (32) showed that women with remitted depression were more likely to generate interpersonal stressors that actually predicted new depressive episodes. Since then, research has implicated stress generation in depression pathogenesis among adolescents (33) and adults (34). While several predictors of stress generation have been studied (35–37), reward processing is not one of them, even though altered reward processing is a key mechanism in the prediction of depression onset in adolescents (38–40). In addition, some evidence suggests that exposure to rewarding environments can buffer the effects of stress exposure (41). Therefore, it is plausible that the opposite might also be true; that is, that brain-reward dysfunction may increase sensitivity to stress.
In the current study, we employed a prospective cohort design to examine whether past stress exposure predicted alterations in reward anticipation processing in children, and whether reward anticipation processing in children predicted stress reactivity to a social stressor in early adolescence. To test both hypotheses within a single model, we used structural equation modeling (SEM), and accounted for past, current and prospective associations.
Methods and Materials
Participants
Participants were 38 children (23 females, 61%) drawn from a longitudinal study of temperament’s effect on social development throughout infancy and childhood. The original cohort consisted of 291 children (134 boys) that were selected at 2 years of age based on 4-month temperament (for a complete description of the recruitment and selection process see (42) and (43)). Parent-reported stress measures were collected when participants were 7 years old. On two separate days at age 10 (Mean = 10.5, SD = 0.4, Range = 9.6-11.6), participants completed an imaging reward paradigm and a standardized laboratory stress paradigm. At 13 (Mean = 13.2, SD = 0.6, Range = 12.3-14.6), they repeated the stress paradigm. Participants were 68% white (n = 26), 13% Black or African American (n = 5), 5% Asian (n = 2), 5% multiple races (n = 2), and 8% (n = 3) did not report race. All participants provided informed assent and the participants’ guardians provided informed consent. The study was approved by the Institutional Review Boards of the National Institute of Mental Health and the University of Maryland, College Park. The design and time course of the study is depicted in Figure 1.
Figure 1.
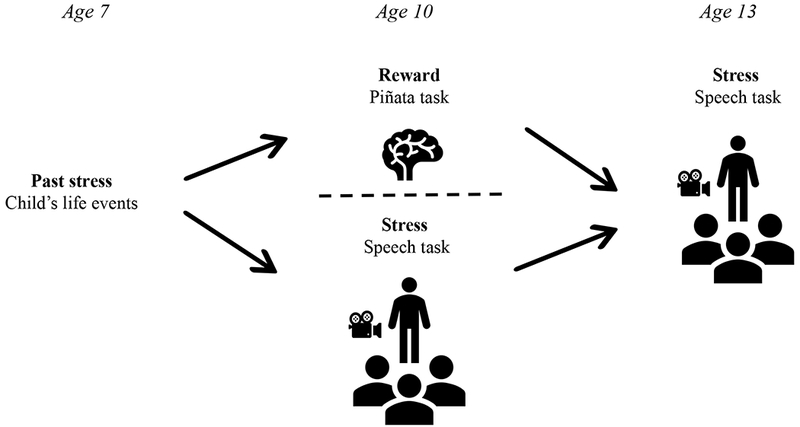
Time course and design of the current study. Past stress information was collected at age 7, brain reward function was assessed at age 10, and stress reactivity was assessed at ages 10 and 13.
Past exposure to stress
Parents completed the Child’s Life Events Inventory (44) when participants were 7 years old. The Child’s Life Events Inventory contains 31 items covering events including changes in family composition, upheavals in living arrangements, sickness or death of people close to the child, sickness or injury experienced by the child, school changes, legal problems in the child’s family, child’s exposure to violence, and family accomplishments. The parents answered yes or no to whether each event occurred in the child’s life. For the current study, we employed the total number of life events reported as a measure of past stress exposure.
Reward paradigm
At age 10, participants completed a child-friendly version of the Monetary Incentive Delay (MID) fMRI task (45) called the Piñata task (42, 46) (see supplemental information for a description). In this task, participants were told to press a button as fast as possible after a piñata appeared on screen in order to “hit” the piñata and earn stars inside (0 stars, 1 star, 2 stars, or 4 stars) (Figure S1). Participants were told that a larger number of stars earned would correspond to a larger monetary reward at the end of the task. Participants were not informed of the payment structure but received a base payment of $3, plus an additional $3 for every 47 stars earned, up to $15.
Acute stress laboratory paradigm
At ages 10 and 13, participants completed a Speech task (see supplemental information for a description), an adapted version of the Trier Social Stress Test (TSST), a widely-used laboratory paradigm for generating acute psychological stress (47).
Participants perceived-stress levels during the task were assessed by self-report. Specifically, participants rated their stress levels on a scale of 0-10 at three-time points: at baseline (prior to receiving task instructions), after the two-minute preparation period (before giving the speech), and immediately following the speech. For each participant, we calculated the area under the curve with respect to ground (AUCg) (Figure S2) for the self-report measures collected during the task (48) using the following formula,
| (1) |
with m1 representing the value of the self-report measure collected at baseline, prior to the task explanation, m2 representing the measure collected after the preparation period immediately prior to the speech, m3 representing the measure collected immediately post-speech, and t1 and t2 representing the length of time between the baseline and pre-speech measures, and between the pre-speech and post-speech measures, respectively.
Image Acquisition
Participants were scanned in a General Electric (Waukesha, WI, USA) Signa 3 Tesla magnet. Task stimuli were displayed via back-projection from a head-coil mounted mirror to a screen at the foot of the scanner bed. Foam padding was used to constrain head movement. Behavioral data were recorded using a hand-held two-button response box. Forty-seven sagittal slices (3.0 mm thickness) per volume were obtained using a T2*-weighted echo planar sequence (echo time, 25 ms; flip angle, 50°; 96 × 96 matrix; field of view, 240 mm; in-plane resolution, 2.5 mm × 2.5 mm; repetition time was 2300 ms). A total of 77 volumes were collected in each run. To improve the localization of activations, a high-resolution structural image was also collected from each participant during the same scanning session using a T1-weighted standardized magnetization prepared spoiled gradient recalled echo sequence with the following parameters: 124 1.2-mm axial slices; repetition time, 8100 ms; echo time, 32 ms; flip angle, 15°; 256 × 256 matrix; field of view, 240 mm; in-plane resolution, 0.86 mm × 0.86 mm; NEX, 1; bandwidth, 31.2 kHz.
Image Processing and Analysis
Analysis of fMRI data was performed using Analysis of Functional and Neural Images (AFNI) software version 17.3.05 (49). Echo-planar images (EPI) were visually inspected to confirm image quality and minimal movement. Standard pre-processing of EPI data included slice-time correction, motion correction and spatial smoothing with a 6-mm full width half-maximum Gaussian smoothing kernel. Each subject’s data were transformed to a percent signal change using each subject’s voxel-wise time series mean blood oxygen level dependent (BOLD) activity as a baseline. Subjects who had more than 25% of the data censored based on average motion per TR greater than 0.8mm and/or an outlier limit of 0.1mm were excluded (42). However, only two subjects had data censored above 20%. Images were analyzed using an event-related design. Time series data for each individual were analyzed using multiple regression (50). The entire trial was modeled using a gamma-variate basis function, including five anticipation events, composed by the times of cue presentation and anticipation period with no cue visible (i.e., 0 star cues, 1 star cues, 2 star cues, 4 star cues and cues from premature response trials), one target event, and all feedback events. The model also included six nuisance variables modeling the effects of residual translational (motion in the x, y and z planes) and rotational motion (roll, pitch, and yaw). Baseline was the non-modelled phases. Our regressor of interest was the anticipation stage for each reward cue. Specifically, we analyzed the contrast composed by the difference of anticipating a reward (i.e., average of 1 star, 2 stars and 4 stars) versus anticipating no reward (i.e. star 0).
A region-of-interest (ROI) approach was used to test our hypotheses (i.e. past exposure to stress impacts reward function and reward function impacts future stress reactivity). We extracted average beta values from anatomical ROIs defined by Talairach anatomical boundaries provided by AFNI. These included left and right of nucleus accumbens (NAcc), ventral and dorsal caudate, putamen, globus pallidus, insula, amygdala, medial prefrontal cortex (mPFC), and anterior cingulate cortex (ACC). For clarity and simplicity, and since correlations between left and right ROIs ranged r=0.70-0.94 (all p<0.0001), we decided to generate single bilateral ROIs by averaging beta values across sides. Since we were testing nine different ROIs, a Benjamini-Hochberg correction for False Discovery Rate (FDR) was applied to all tests (q=0.015).
In addition, as an exploratory approach, we also examined our hypotheses by performing whole-brain analyses. The statistical threshold for individual voxels was set to p < 0.005, uncorrected. Correction for multiple comparisons was applied following Monte Carlo simulations to determine appropriate cluster thresholds of a minimum cluster size of 110 voxels for the whole brain to achieve a corrected alpha level of p < 0.05. Full results of whole-brain analyses unadjusted and adjusted for average motion are shown in the supplemental material (Table S1 and S2, Figures S3 and S4)
Data analysis
Analyses were performed in MPlus v7.4 using Full Information Maximum Likelihood (FIML) to account for missing data. Specifically, six participants had no information on past stressful life events, and stress reactivity was not collected for three participants at age 10 and five participants at age 13. Maximum likelihood uses all available data to generate parameter estimates without discarding cases or filling in data prior to analysis. Methodologists regard maximum likelihood estimation as an outstanding missing data technique (51) because it requires less strict assumptions about the mechanisms that led to missing data and produces more accurate estimates than other traditional missing-data-handling techniques (e.g., discarding cases).
Stress reactivity to the Speech task.
To examine associations across time in stress reactivity we used regression analysis with reported stress at age 10 as predictor of interest and reported stress at age 13 as outcome. Repeated measure ANOVA was used to examine changes in reported stress across the procedure (i.e., baseline, prior speech, after speech) at ages 10 and 13.
Association between early life stress, brain reward function, and future stress reactivity.
To test our hypotheses, we took advantage of SEM and tested a path analysis in which both brain reward function and stress reactivity at ages 10 and 13 were regressed on past stress; stress reactivity at age 13 was additionally regressed on reward function and stress reactivity at age 10, with the latter two allowed to correlate (see Figure 2). All variables were regressed on sex and age, using these as covariates. We performed these analyses for each extracted anatomical ROI.
Figure 2.
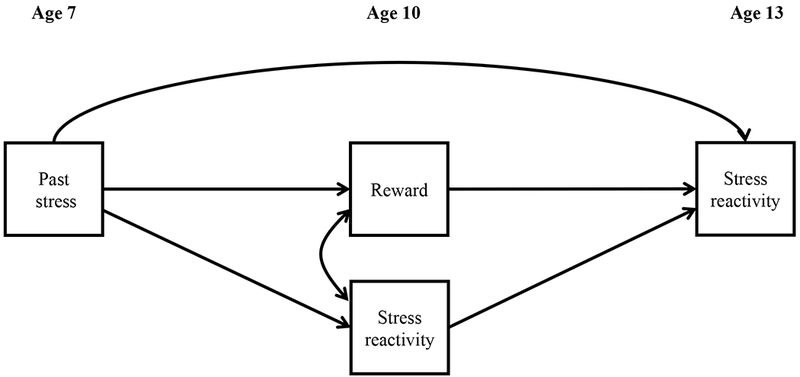
Path analysis employed to test the past stress on reward function and the effect of reward function on future stress reactivity.
All ROI models were re-analyzed adding the following covariates: maternal depressive symptoms collected when the child was 2 years old as measured with the Beck’s Depression Inventory. peer rejection at age 10 as measured with Children’s Loneliness Scale, depressive-like symptoms at age 10 as measured with the withdrawn/depressed subscale of the Child Behavior Checklist, and average motion.
Results
Stress reactivity to the Speech tasks.
Repeated measures ANOVA with self-reported measures at age 10 revealed a main effect of time, F(1.9,65.8)=6.12, p = 0.004, η2 = 0.15. Stress levels significantly increased between baseline and after speech preparation (p=0.001), and significantly decreased between prior and after speech delivery (p=0.022). No differences were found between baseline and post-speech delivery (p=0.466) (Figure 3). At age 13, there was also a main effect of time, F(1.9,60.7) =8.00, p = 0.001, η2 = 0.20. Likewise, stress levels significantly increased between baseline and after speech preparation (p<0.001), and significantly decreased between prior and after speech delivery (p=0.028). No differences were found between baseline and after speech delivery (p=0.129) (Figure 3). Self-reported stress at age 10 was significantly associated with self-reported stress at age 13 (β=0.58, p<0.001, R2=0.35).
Figure 3.
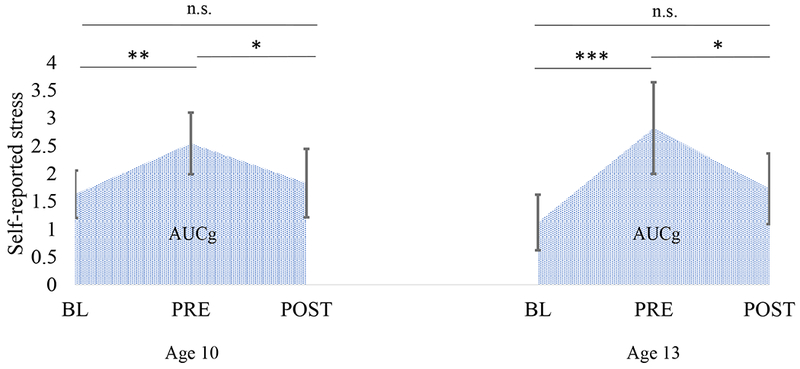
Self-reported stress during the Speech task at ages 10 and 13. Bars are 95% confidence intervals. BL, baseline; PRE, prior speech delivery; POST, after speech delivery. *p<0.05, **p<0.01, ***p<0.001
Association between past stress, brain reward function, and future stress reactivity
Reported stressful life events ranged 1 to 10 (Mean=4.6, SD=2.4). Higher exposure to past stress predicted lower response to anticipation of reward in ventral caudate (β=−0.49, p<0.001) (Figure 4A), NAcc (β=−0.40, p=0.003), globus pallidus (β=−0.51, p<0.001), and putamen (β=−0.39, p=0.006). Other areas showed significant associations that did not survive our FDR correction. These comprised the amygdala (β=−0.35, p=0.017), the dorsal caudate (β=−0.34, p=0.022), the mPFC (β=−0.31, p=0.048), and the ACC (β=−0.31, p=0.048). Results with anatomical ROIs were similar to those from whole brain analysis (Figure 4B, Table S1) in which a single cluster in the basal ganglia showing decreased activation survived thresholding correction (even when adjusting for motion, Figure S3, Table S2), encompassing parts of the left ventral caudate, left putamen, left and right lentiform nucleus, and right thalamus.
Figure 4.
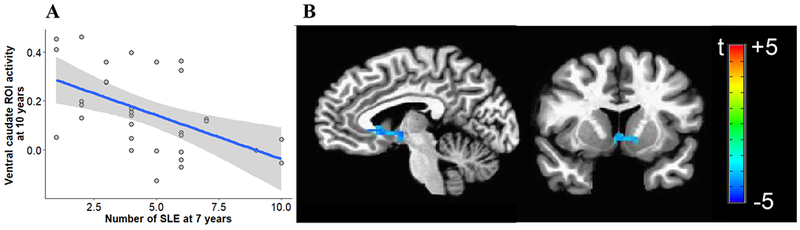
(A) Scatter plot of the association between exposure to stress at age 7 and neural activity in the ventral caudate during anticipation of reward at age 10. Shaded area represents 95% confidence intervals. SLE, stressful life events. (B) Voxel-wise t-test maps for the effect of past stress on reward anticipation, corrected at cluster-level with a threshold of p<0.05 and a minimal cluster size of 110 voxels, k=240, xyz=+5 −7 −2 (Note: R is L).
At age 10, the only region cross-sectionally associated with stress reactivity in the Speech task was the mPFC (r=−0.39, p=0.008). However, this analysis did not survive our FDR correction.
Lower response to reward anticipation in the mPFC (β=−0.54, p<0.001) (Figure 5A) and the ACC (β=−0.49, p=0.001) (Figure 5B) at age 10 predicted higher stress reactivity in the Speech task at age 13, after accounting for baseline stress reactivity. Whole-brain analysis also showed a negative association between neural activity in prefrontal regions and future stress reactivity (Figure 5C, Table S1), which was a trend when covarying for motion (Figure S4, Table S2). Other ROIs that showed significant associations but did not survive our FDR correction were the NAcc (β=−0.40, p=0.007), and the ventral caudate (β=−0.39, p=0.018).
Figure 5.
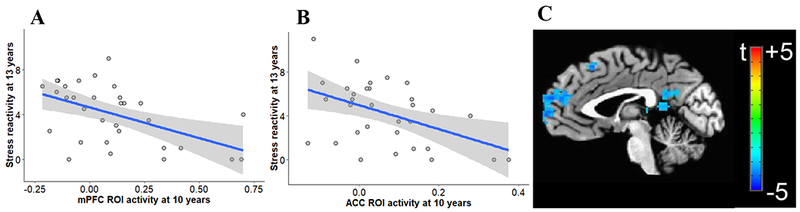
Scatter plot of the association between neural activity in the mPFC (A) and ACC (B) during reward anticipation at age 10 and self-reported stress reactivity during the Speech task at age 13. Shaded area represents 95% confidence intervals. (C) Voxel-wise t-test maps for the association between neural activity during reward anticipation at age 10 and self-reported stress reactivity at age 13, corrected at cluster-level with a threshold of p<0.05 and a minimal cluster size of 110 voxels, xyz=−2 −56 20.
Figure 6 summarizes the associations with self-reported stress reactivity. Whereas past exposure to stress had a small effect on prefrontal areas (PFC), this was largest and significant in the basal ganglia (BG: R2=0.28, p<0.029). Interestingly, although the activation in prefrontal areas and basal ganglia was highly correlated (r=0.61, p<0.001), only the activation in prefrontal areas was associated with stress reactivity at both ages 10 and 13. Higher past exposure to stress was associated with lower stress reactivity at age 13 only in the full model, but not direct association was found neither with stress reactivity at age 10 (β=−0.04, p=0.844) nor with stress reactivity at age 13 (β=−0.03, p=0.851).
Figure 6.
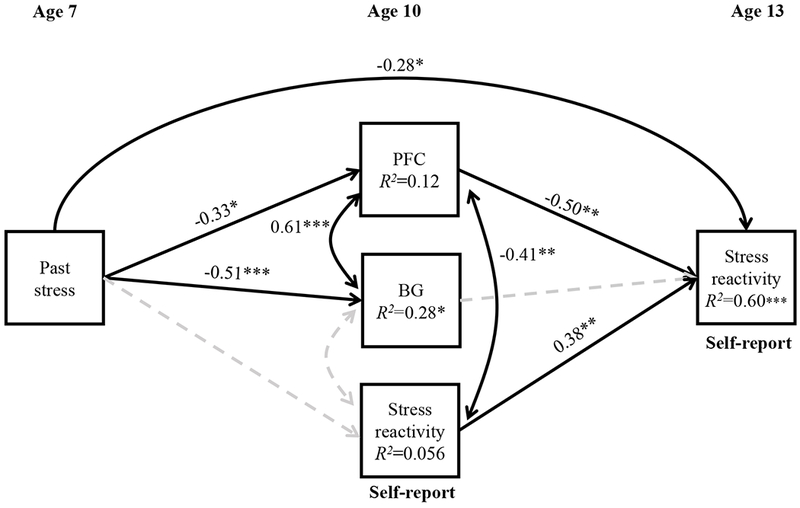
Path analysis of the relationship between past stress at age 7, response to reward anticipation at age 10 in prefrontal areas (PFC: average of mPFC and ACC), basal ganglia (BG: average of ventral caudate, NAcc, putamen and globus pallidus) and self-reported stress reactivity to the Speech task at ages 10 and 13. Significant paths with standardized coefficients (β) and correlations (r) are presented as straight and curved continuous lines respectively. Dashed and faded lines represent non-significance. R2, proportion of variance explained. *p<0.05, **p<0.01, ***p<0.001
In terms of covariates, peer rejection at age 10 was associated with stress reactivity at age 10 (r=0.48, p=0.002); maternal depressive symptoms were associated with neural response to reward anticipation in the mPFC (β=−34, p=0.020); and motion was associated with neural response to reward anticipation in the mPFC (r=0.42, p=0.008) and the amygdala (r=0.42, p=0.009), as well as stress reactivity at age 13 (β=−0.52, p<0.001). Regardless of these significant associations, the results of the ROI analyses adjusted for key covariates remained practically unchanged (Tables S3 and S4).
Discussion
This study tested two hypotheses relating stress and reward processing, namely 1) previous stress exposure impacts future reward processing and 2) altered reward processing impacts future stress reactivity. Two findings emerged. First, higher prior exposure to stress predicted decreased neural activity during reward anticipation, especially in the basal ganglia. Second, decreased neural activity during reward anticipation, especially in prefrontal regions, predicted higher stress reactivity three years later, independent of baseline stress reactivity. These results emerged even when considering several confounding factors. Therefore, results supported both hypotheses; however, brain regions involved in each case differed.
Our study replicates previous findings linking past stress exposure to blunted reward-related neural responses (2, 3), particularly in regions of the basal ganglia (7, 8, 10, 52). Moreover, the current study demonstrates such associations in late childhood, prior to the age when stress-related disorders exhibit peak onset (53, 54). Such findings add to the few studies examining associations between early stress and later reward processing in young people (9, 11). Certainly, more studies are needed in this area.
Most importantly, we provide evidence for a theoretically plausible, yet long-neglected hypothesis: that altered reward function predicts future stress reactivity (4, 30). The reasons for this relationship remain unclear, since several unmeasured factors might be intervening. However, difficulties in recognizing rewarding social cues, along with decreased motivation to seek such cues, could make youth more likely to withdraw from potential rewarding social experiences. Eventually, this could lead to interpersonal stress and heightened stress reactivity in response to anticipated social cues. In line with reinforcement-based theories (55), this also could lead to decreased response-contingent positive reinforcement and increased aversive experiences, eventually leading to stress-related disorders, such as depression. This would be in line with cross-sectional data linking depressive symptoms to low motivation to obtain social approval (56) and blunted response to social cues (57). Indeed, blunted sensitivity to social reward has also been found in youth at risk of depression (58).
Whereas several potential neurobiological mechanisms have been proposed to examine the impact of stress on neural reward processing (2, 3), the mechanisms that mediate between impaired reward processing and future stress remain unclear. Preliminary animal studies suggest that impaired mesocorticolimbic DA pathways as a result of stress exposure might sensitize such pathways to future stressors (59). In our sample, the association between blunted reward response and future stress reactivity was particularly strong for the mPFC and the ACC, implicating these regions in stress susceptibility and resilience. Prior work links the ACC to stress controllability (60) and reactivity (61). In animal studies, dysfunction of the mPFC has been linked to maladaptive behavioral responses to stress (62). In humans, altered function of frontal regions has been associated with rumination (63, 64), which has been associated with higher reactivity to stressful events (65, 66) and might play a role in the generation of interpersonal stress (67), consequently increasing the likelihood of depression and anxiety.
Interestingly, we found different regions involved depending on the timing of predictions and type of stress. Some studies suggest that the reward related regions affected by stress might differ depending on which developmental period the stress exposure takes place (10, 12, 52). Moreover, different types of stress might have different effects. For example, whereas exposure to previous adversity has been consistently linked to altered function in the striatum (7–9), cross-sectional studies examining the effects of acute experimental and chronic stress have shown mixed results (13–15, 18). Therefore, our finding will require replication in samples that examine distinct developmental periods and types of stress.
If altered reward function proves to be a predecessor of increased stress reactivity, this can have important implications for treatment and prevention. There is some indirect evidence from animal and human studies suggesting that exposure to rewarding environments and stimuli might confer resilience to stress exposure (41, 68, 69). Most of these studies are cross-sectional and none of them examine neural responses to reward or test the effects on long-term stress. However, these findings along with our results, suggest that targeting reward processing with reinforcement-based treatment, such as behavioral activation or even neurofeedback, might protect against the noxious effects of stress and consequently decrease the likelihood of illness onset in those at high risk, or even decrease the likelihood of relapse in those with depression.
The strengths of this study include the prospective design and data collection, and the use of well-validated experimental procedures to measure reward and stress reactivity. However, our results should be considered in the light of some limitations. First, the sample of this study was small, and our findings will need replication in a larger sample. A larger sample would also allow us to examine sex differences, which might be relevant given the sex differences in stress reactivity and rates of stress-related disorders, especially in adolescence. Second, we could not test the stress generation model originally proposed by Hammen because we did not have specific measures of depression and interpersonal stressors as outcome. Instead, we examined the prediction of stress sensitivity to an interpersonal stressor; though related, it is not the same construct. However, this same cohort will have specific depression measures in future waves of data collection, which will allow us to test mediation effects with both hypotheses in a single sequential model. Future studies should incorporate measures of interpersonal stressors as outcome and test the role of reward dysfunction in generating stress in depressed populations. A third limitation of this study is that, whereas the stress generator model seems to be specific for interpersonal stress, the current study examined reward processing using monetary-like stimuli. It would be interesting to examine whether responses to social reward yield the same results. Fourth, future studies examining temporal relations between reward processing and stress should incorporate physiological measures of stress, such as cortisol changes or heart rate. Finally, it is possible that frontal regions were associated with the type of stress in our sample (i.e., stress reactivity to social evaluative threat), as opposed to future stress specifically. Future prospective studies will need to examine the effects of past stress reactivity to social evaluative tasks on reward function to answer this question. Moreover, it remains to be seen whether reward dysfunction is predictive of stress reactivity in non-social situations.
In summary, our findings suggest that the relations between stress and reward processing are bidirectional, with stress exposure impacting future reward processing, and reward processing impacting future stress reactivity. Further studies with larger samples will be needed to replicate these findings.
Supplementary Material
Acknowledgements:
This study was supported by NIMH-Intramural Research Program Project ZIAMH002782. Results of the Piñata task for this sample have been reported in Lahat et al (2016).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures:
All authors report no biomedical financial interests or potential conflicts of interest.
References:
- 1.Hagele C, Schlagenhauf F, Rapp M, Sterzer P, Beck A, Bermpohl F, Stoy M, Strohle A, Wittchen HU, Dolan RJ, Heinz A. Dimensional psychiatry: reward dysfunction and depressive mood across psychiatric disorders. Psychopharmacology. 2015;232:331–341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Stanton CH, Holmes AJ, Chang SWC, Joormann J. From Stress to Anhedonia: Molecular Processes through Functional Circuits. Trends in neurosciences. 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ironside M, Kumar P, Kang MS, Pizzagalli DA. Brain mechanisms mediating effects of stress on reward sensitivity. Current opinion in behavioral sciences. 2018;22:106–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Auerbach RP, Admon R, Pizzagalli DA. Adolescent depression: stress and reward dysfunction. Harvard review of psychiatry. 2014;22:139–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Keren H, O’Callaghan G, Vidal-Ribas P, Buzzell GA, Brotman MA, Leibenluft E, Pan PM, Meffert L, Kaiser A, Wolke S, Pine DS, Stringaris A. Reward Processing in Depression: A Conceptual and Meta-Analytic Review Across fMRI and EEG Studies. The American journal of psychiatry. 2018:appiajp201817101124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Luking KR, Nelson BD, Infantolino ZP, Sauder CL, Hajcak G. Ventral Striatal Function Interacts With Positive and Negative Life Events to Predict Concurrent Youth Depressive Symptoms. Biological psychiatry : cognitive neuroscience and neuroimaging. 2018;3:937–946. [DOI] [PubMed] [Google Scholar]
- 7.Boecker R, Holz NE, Buchmann AF, Blomeyer D, Plichta MM, Wolf I, Baumeister S, Meyer-Lindenberg A, Banaschewski T, Brandeis D, Laucht M. Impact of early life adversity on reward processing in young adults: EEG-fMRI results from a prospective study over 25 years. PloS one. 2014;9:e104185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dillon DG, Holmes AJ, Birk JL, Brooks N, Lyons-Ruth K, Pizzagalli DA. Childhood adversity is associated with left basal ganglia dysfunction during reward anticipation in adulthood. Biological psychiatry. 2009;66:206–213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mehta MA, Gore-Langton E, Golembo N, Colvert E, Williams SC, Sonuga-Barke E. Hyporesponsive reward anticipation in the basal ganglia following severe institutional deprivation early in life. Journal of cognitive neuroscience. 2010;22:2316–2325. [DOI] [PubMed] [Google Scholar]
- 10.Hanson JL, Albert D, Iselin AM, Carre JM, Dodge KA, Hariri AR. Cumulative stress in childhood is associated with blunted reward-related brain activity in adulthood. Social cognitive and affective neuroscience. 2016;11:405–412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Goff B, Gee DG, Telzer EH, Humphreys KL, Gabard-Durnam L, Flannery J, Tottenham N. Reduced nucleus accumbens reactivity and adolescent depression following early-life stress. Neuroscience. 2013;249:129–138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Casement MD, Guyer AE, Hipwell AE, McAloon RL, Hoffmann AM, Keenan KE, Forbes EE. Girls’ challenging social experiences in early adolescence predict neural response to rewards and depressive symptoms. Developmental cognitive neuroscience. 2014;8:18–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Oei NY, Both S, van Heemst D, van der Grond J. Acute stress-induced cortisol elevations mediate reward system activity during subconscious processing of sexual stimuli. Psychoneuroendocrinology. 2014;39:111–120. [DOI] [PubMed] [Google Scholar]
- 14.Ossewaarde L, Qin S, Van Marle HJ, van Wingen GA, Fernandez G, Hermans EJ. Stress-induced reduction in reward-related prefrontal cortex function. NeuroImage. 2011;55:345–352. [DOI] [PubMed] [Google Scholar]
- 15.Kumar P, Berghorst LH, Nickerson LD, Dutra SJ, Goer FK, Greve DN, Pizzagalli DA. Differential effects of acute stress on anticipatory and consummatory phases of reward processing. Neuroscience. 2014;266:1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Porcelli AJ, Lewis AH, Delgado MR. Acute stress influences neural circuits of reward processing. Frontiers in neuroscience. 2012;6:157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lincoln SH, Pisoni A, Bondy E, Kumar P, Singleton P, Hajcak G, Pizzagalli DA, Auerbach RP. Altered reward processing following an acute social stressor in adolescents. PloS one. 2019;14:e0209361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Treadway MT, Buckholtz JW, Zald DH. Perceived stress predicts altered reward and loss feedback processing in medial prefrontal cortex. Frontiers in human neuroscience. 2013;7:180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nikolova YS, Bogdan R, Brigidi BD, Hariri AR. Ventral striatum reactivity to reward and recent life stress interact to predict positive affect. Biological psychiatry. 2012;72:157–163. [DOI] [PubMed] [Google Scholar]
- 20.Jackson ME, Moghaddam B. Amygdala regulation of nucleus accumbens dopamine output is governed by the prefrontal cortex. J Neurosci. 2001;21:676–681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Stevenson CW, Gratton A. Basolateral amygdala modulation of the nucleus accumbens dopamine response to stress: role of the medial prefrontal cortex. The European journal of neuroscience. 2003;17:1287–1295. [DOI] [PubMed] [Google Scholar]
- 22.Olson EA, Kaiser RH, Pizzagalli DA, Rauch SL, Rosso IM. Anhedonia in Trauma-Exposed Individuals: Functional Connectivity and Decision-Making Correlates. Biological psychiatry : cognitive neuroscience and neuroimaging. 2018;3:959–967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hanson JL, Knodt AR, Brigidi BD, Hariri AR. Heightened connectivity between the ventral striatum and medial prefrontal cortex as a biomarker for stress-related psychopathology: understanding interactive effects of early and more recent stress. Psychological medicine. 2017:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Grandjean J, Azzinnari D, Seuwen A, Sigrist H, Seifritz E, Pryce CR, Rudin M. Chronic psychosocial stress in mice leads to changes in brain functional connectivity and metabolite levels comparable to human depression. NeuroImage. 2016;142:544–552. [DOI] [PubMed] [Google Scholar]
- 25.Lemos JC, Wanat MJ, Smith JS, Reyes BA, Hollon NG, Van Bockstaele EJ, Chavkin C, Phillips PE. Severe stress switches CRF action in the nucleus accumbens from appetitive to aversive. Nature. 2012;490:402–406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bryce CA, Floresco SB. Perturbations in Effort-Related Decision-Making Driven by Acute Stress and Corticotropin-Releasing Factor. Neuropsychopharmacology. 2016;41:2147–2159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hupalo S, Martin AJ, Green RK, Devilbiss DM, Berridge CW. Prefrontal Corticotropin-Releasing Factor (CRF) Neurons Act Locally to Modulate Frontostriatal Cognition and Circuit Function. J Neurosci. 2019;39:2080–2090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hupalo S, Berridge CW. Working Memory Impairing Actions of Corticotropin-Releasing Factor (CRF) Neurotransmission in the Prefrontal Cortex. Neuropsychopharmacology. 2016;41:2733–2740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Collins AGE, Albrecht MA, Waltz JA, Gold JM, Frank MJ. Interactions Among Working Memory, Reinforcement Learning, and Effort in Value-Based Choice: A New Paradigm and Selective Deficits in Schizophrenia. Biological psychiatry. 2017;82:431–439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pizzagalli DA. Depression, Stress, and Anhedonia: Toward a Synthesis and Integrated Model. 2014;10:393–423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Post RM. Transduction of psychosocial stress into the neurobiology of recurrent affective disorder. The American journal of psychiatry. 1992;149:999–1010. [DOI] [PubMed] [Google Scholar]
- 32.Hammen C Generation of stress in the course of unipolar depression. Journal of abnormal psychology. 1991;100:555–561. [DOI] [PubMed] [Google Scholar]
- 33.Rudolph KD. Developmental influences on interpersonal stress generation in depressed youth. Journal of abnormal psychology. 2008;117:673–679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hammen C, Shih JH, Brennan PA. Intergenerational transmission of depression: test of an interpersonal stress model in a community sample. Journal of consulting and clinical psychology. 2004;72:511–522. [DOI] [PubMed] [Google Scholar]
- 35.Auerbach RP, Bigda-Peyton JS, Eberhart NK, Webb CA, Ho MH. Conceptualizing the prospective relationship between social support, stress, and depressive symptoms among adolescents. Journal of abnormal child psychology. 2011;39:475–487. [DOI] [PubMed] [Google Scholar]
- 36.Auerbach RP, Eberhart NK, Abela JR. Cognitive vulnerability to depression in Canadian and Chinese adolescents. Journal of abnormal child psychology. 2010;38:57–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Auerbach RP, Ho MH. A cognitive-interpersonal model of adolescent depression: the impact of family conflict and depressogenic cognitive styles. Journal of clinical child and adolescent psychology : the official journal for the Society of Clinical Child and Adolescent Psychology, American Psychological Association, Division 53. 2012;41:792–802. [DOI] [PubMed] [Google Scholar]
- 38.Stringaris A, Vidal-Ribas Belil P, Artiges E, Lemaitre H, Gollier-Briant F, Wolke S, Vulser H, Miranda R, Penttila J, Struve M, Fadai T, Kappel V, Grimmer Y, Goodman R, Poustka L, Conrod P, Cattrell A, Banaschewski T, Bokde AL, Bromberg U, Buchel C, Flor H, Frouin V, Gallinat J, Garavan H, Gowland P, Heinz A, Ittermann B, Nees F, Papadopoulos D, Paus T, Smolka MN, Walter H, Whelan R, Martinot JL, Schumann G, Paillere-Martinot ML. The Brain’s Response to Reward Anticipation and Depression in Adolescence: Dimensionality, Specificity, and Longitudinal Predictions in a Community-Based Sample. The American journal of psychiatry. 2015;172:1215–1223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nelson BD, Perlman G, Klein DN, Kotov R, Hajcak G. Blunted Neural Response to Rewards as a Prospective Predictor of the Development of Depression in Adolescent Girls. The American journal of psychiatry. 2016;173:1223–1230. [DOI] [PubMed] [Google Scholar]
- 40.Bress JN, Foti D, Kotov R, Klein DN, Hajcak G. Blunted neural response to rewards prospectively predicts depression in adolescent girls. Psychophysiology. 2013;50:74–81. [DOI] [PubMed] [Google Scholar]
- 41.Dutcher JM, Creswell JD. The role of brain reward pathways in stress resilience and health. Neuroscience and biobehavioral reviews. 2018;95:559–567. [DOI] [PubMed] [Google Scholar]
- 42.Lahat A, Benson BE, Pine DS, Fox NA, Ernst M. Neural responses to reward in childhood: relations to early behavioral inhibition and social anxiety. Social cognitive and affective neuroscience. 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hane AA, Fox NA, Henderson HA, Marshall PJ. Behavioral reactivity and approach-withdrawal bias in infancy. Developmental psychology. 2008;44:1491–1496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Monaghan JH, Robinson JO, Dodge JA. The children’s Life Events Inventory. Journal of psychosomatic research. 1979;23:63–68. [DOI] [PubMed] [Google Scholar]
- 45.Knutson B, Westdorp A, Kaiser E, Hommer D. FMRI visualization of brain activity during a monetary incentive delay task. NeuroImage. 2000;12:20–27. [DOI] [PubMed] [Google Scholar]
- 46.Helfinstein SM, Kirwan ML, Benson BE, Hardin MG, Pine DS, Ernst M, Fox NA. Validation of a child-friendly version of the monetary incentive delay task. Social cognitive and affective neuroscience. 2013;8:720–726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Dickerson SS, Kemeny ME. Acute stressors and cortisol responses: a theoretical integration and synthesis of laboratory research. Psychological bulletin. 2004;130:355–391. [DOI] [PubMed] [Google Scholar]
- 48.Pruessner JC, Kirschbaum C, Meinlschmid G, Hellhammer DH. Two formulas for computation of the area under the curve represent measures of total hormone concentration versus time-dependent change. Psychoneuroendocrinology. 2003;28:916–931. [DOI] [PubMed] [Google Scholar]
- 49.Cox RW. AFNI: software for analysis and visualization of functional magnetic resonance neuroimages. Computers and biomedical research, an international journal. 1996;29:162–173. [DOI] [PubMed] [Google Scholar]
- 50.Neter J, Kutner MH, Nachtsheim CJ, Wasserman W: Applied linear statistical models, Irwin Chicago; 1996. [Google Scholar]
- 51.Schafer JL, Graham JW. Missing data: our view of the state of the art. Psychological methods. 2002;7:147–177. [PubMed] [Google Scholar]
- 52.Goff B, Tottenham N. Early-life adversity and adolescent depression: mechanisms involving the ventral striatum. CNS spectrums. 2015;20:337–345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Merikangas KR, He JP, Burstein M, Swanson SA, Avenevoli S, Cui L, Benjet C, Georgiades K, Swendsen J. Lifetime prevalence of mental disorders in U.S. adolescents: results from the National Comorbidity Survey Replication--Adolescent Supplement (NCS-A). Journal of the American Academy of Child and Adolescent Psychiatry. 2010;49:980–989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lewinsohn PM, Clarke GN, Seeley JR, Rohde P. Major Depression in Community Adolescents: Age at Onset, Episode Duration, and Time to Recurrence. Journal of the American Academy of Child & Adolescent Psychiatry. 1994;33:809–818. [DOI] [PubMed] [Google Scholar]
- 55.Lewinsohn PM: A behavioral approach to depression in Psychology of depression: Contemporary theory and research. Edited by Friedman RJ, Katz MM. Oxford, England, Wiley; 1974. pp. 157–178. [Google Scholar]
- 56.Brinkmann K, Franzen J, Rossier C, Gendolla GHE. I don’t care about others’ approval: Dysphoric individuals show reduced effort mobilization for obtaining a social reward. Motivation and Emotion. 2014;38:790–801. [Google Scholar]
- 57.Chan RC, Li Z, Li K, Zeng YW, Xie WZ, Yan C, Cheung EF, Jin Z. Distinct processing of social and monetary rewards in late adolescents with trait anhedonia. Neuropsychology. 2016;30:274–280. [DOI] [PubMed] [Google Scholar]
- 58.Olino TM, Silk JS, Osterritter C, Forbes EE. Social Reward in Youth at Risk for Depression: A Preliminary Investigation of Subjective and Neural Differences. Journal of child and adolescent psychopharmacology. 2015;25:711–721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Cuadra G, Zurita A, Lacerra C, Molina V. Chronic stress sensitizes frontal cortex dopamine release in response to a subsequent novel stressor: reversal by naloxone. Brain research bulletin. 1999;48:303–308. [DOI] [PubMed] [Google Scholar]
- 60.Amat J, Baratta MV, Paul E, Bland ST, Watkins LR, Maier SF. Medial prefrontal cortex determines how stressor controllability affects behavior and dorsal raphe nucleus. Nature neuroscience. 2005;8:365–371. [DOI] [PubMed] [Google Scholar]
- 61.Dedovic K, D’Aguiar C, Pruessner JC. What stress does to your brain: a review of neuroimaging studies. Canadian journal of psychiatry Revue canadienne de psychiatrie. 2009;54:6–15. [DOI] [PubMed] [Google Scholar]
- 62.Wang M, Perova Z, Arenkiel BR, Li B. Synaptic modifications in the medial prefrontal cortex in susceptibility and resilience to stress. J Neurosci. 2014;34:7485–7492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Cooney RE, Joormann J, Eugene F, Dennis EL, Gotlib IH. Neural correlates of rumination in depression. Cognitive, affective & behavioral neuroscience. 2010;10:470–478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Burkhouse KL, Jacobs RH, Peters AT, Ajilore O, Watkins ER, Langenecker SA. Neural correlates of rumination in adolescents with remitted major depressive disorder and healthy controls. Cognitive, affective & behavioral neuroscience. 2017;17:394–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Michl LC, McLaughlin KA, Shepherd K, Nolen-Hoeksema S. Rumination as a mechanism linking stressful life events to symptoms of depression and anxiety: longitudinal evidence in early adolescents and adults. Journal of abnormal psychology. 2013;122:339–352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Ruscio AM, Gentes EL, Jones JD, Hallion LS, Coleman ES, Swendsen J. Rumination predicts heightened responding to stressful life events in major depressive disorder and generalized anxiety disorder. Journal of abnormal psychology. 2015;124:17–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.McLaughlin KA, Nolen-Hoeksema S. Interpersonal stress generation as a mechanism linking rumination to internalizing symptoms in early adolescents. Journal of clinical child and adolescent psychology : the official journal for the Society of Clinical Child and Adolescent Psychology, American Psychological Association, Division 53. 2012;41:584–597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Creswell JD, Pacilio LE, Denson TF, Satyshur M. The effect of a primary sexual reward manipulation on cortisol responses to psychosocial stress in men. Psychosomatic medicine. 2013;75:397–403. [DOI] [PubMed] [Google Scholar]
- 69.Sherman DK, Bunyan DP, Creswell JD, Jaremka LM. Psychological vulnerability and stress: the effects of self-affirmation on sympathetic nervous system responses to naturalistic stressors. Health psychology : official journal of the Division of Health Psychology, American Psychological Association. 2009;28:554–562. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


