Abstract
Obesity and dyslipidemia can be associated with cellular senescence, and may impair kidney function. However, whether senescence contributes to renal dysfunction in these conditions remains unclear. Quercetin is an abundant dietary flavonoid that selectively clears senescent cells by inhibiting PI3K/AKT and p53/p21/serpines and inducing apoptosis. We hypothesized that high-fat-diet-induced obesity causes renal senescence, which would be mitigated by quercetin.
Methods
C57BL/6J mice fed either standard chow or high-fat diets were treated with quercetin (50mg/kg) or vehicle 5-days biweekly via oral gavage for 10-weeks. Subsequently, renal function was studied in vivo using magnetic resonance imaging, and renal senescence and histology were evaluated ex vivo.
Results
Mice fed with a high-fat diet developed obesity and hypercholesterolemia, whereas renal size remained unchanged. Murine obesity impaired renal function and cortical oxygenation, and induced glomerulomegaly. Renal markers of senescence (e.g., expression of p16, p19, and p53) and its secretory phenotype were upregulated in the obese hypercholesterolemic compared to lean mice in renal tubular cells, but attenuated in quercetin-treated murine kidneys, as was renal fibrosis. Quercetin treatment also increased renal cortical oxygenation and decreased plasma creatinine levels in obese mice, whereas body weight and cholesterol levels were unaltered. Therefore, murine obesity and dyslipidemia induce renal tissue senescence and impairs kidney function, which is alleviated by chronic senolytic treatment. These findings implicate senescence in loss of kidney function in murine dyslipidemia and obesity, and support further studies of senolytic therapy in obesity.
Keywords: high-fat diet, kidney, senescence, senolytic, quercetin
Introduction
Obesity is a known risk factor for development of kidney failure,1 but the underlying mechanisms are yet to be fully elucidated. Obesity and hypercholesterolemia both induce cellular senescence, an irreversible arrest of cell cycle progression evoked in response to stress and damage.2 Recent studies demonstrate that many cell types in adipose tissue, aorta, pancreas, and liver, including endothelial cells and pre-adipocytes, show increased senescence markers in obesity and dyslipidemia, such as senescence-associated β-Galactosidase (SA-β-Gal) activity and p16, p19, p21, and p53 gene expression.2–4 However, whether cellular senescence is triggered in the kidney in obesity and contributes to renal dysfunction remains to be clarified.
Senescent cells activate pro-survival pathways that endow resistance to apoptosis. These persistent senescent cells can express the senescence-associated secretory phenotype (SASP), producing inflammatory, growth-promoting, and remodeling factors.5 The SASP consolidates senescence and exerts noxious effects on neighboring cells, contributing to pathogenesis in aging and chronic diseases.5 Identification of senescent cell anti-apoptotic pathways (SCAPs) led to discovery of several senolytic drugs, such as dasatinib, quercetin, navitoclax, and fisetin,6, 7 which transiently inhibit SCAPs and selectively clear senescent cells at doses insufficient to affect normal cells.8 Among these, quercetin is an abundant dietary flavonoid that selectively kills senescent cells by inhibiting PI3K, other kinases, and serpines, and inducing their apoptosis.8 In vitro, it is effective in eliminating senescent human umbilical vein endothelial cells and mouse bone marrow-derived mesenchymal stromal/stem cells.8 Quercetin treatment combined with dasatinib also improves cardiac function and vascular phenotypes in vivo with aging and hypercholesterolemia.9 However, the effects of senescent cell clearance using quercetin on kidney tissue in obese mice have yet to be tested.
We therefore tested the hypothesis that high-fat diet (HFD) induces kidney cellular senescence, which would be mitigated by chronic quercetin treatment.
Materials and Methods
Thirty male C57BL/6J mice (Jackson Lab, Bar Harbor, ME, 11 weeks of age) were randomly assigned to consume standard chow or HFD (60%). After 6 months, the mice started treatment with either vehicle (100μL) or quercetin (50mg/kg) 5-days biweekly via oral gavage for another 10-weeks along with continued diet (n=6–8 each). Subsequently, renal function was studied in vivo using 16.4T magnetic resonance imaging (MRI). After in vivo studies, urine samples were collected via cystocentesis, and mice euthanized by terminal cardiac blood sampling. The kidneys were harvested, weighed, and cut into two halves to be fresh frozen or preserved in formalin. Senescence markers, including activity of SA-β-Gal, gene expression of p16, p19, p21, p53, and of the SASP components Tnf-α and Il-6, were measured in kidney tissues. Renal sections were stained with Masson’s trichrome (MT) and Periodic acid-Schiff (PAS). All animal procedures were approved by the Mayo Clinic Institutional Animal Care and Use Committee.
In vivo renal oxygenation
MRI experiments were performed to assess renal oxygenation. Mice were anesthetized with 2% isoflurane and maintained with 1.0–2.0% isoflurane in the supine position. Warm air was blown onto mice to maintain body temperature at approximately 36°C. ECG, respiration, and body temperature were monitored using a physiological monitoring system (SA Instruments, Stony Brook, NY).
MRI studies were performed on a vertical 16.4T animal scanner equipped with a 38mm inner diameter birdcage coil (Bruker, Billerica, MA). Renal volume was measured from coronal images acquired using a respiration-gated 3D Fast Imaging with Steady Precession (3D-FISP) sequence with the following parameters: TR 14ms; TE 2.1ms; flip angle 20°; FOV 5.12×2.56×1.28cm3; matrix size 256×128×64; number of averages 2. Renal oxygenation was assessed by blood oxygen level dependent (BOLD) MRI using a respiration-gated 3D multiecho gradient echo (MGE) sequence,10, 11 with the following imaging parameters: TR 200ms; TE 3.5~24.5ms; echo number 8; flip angle 25°; slab thickness 1mm; FOV 2.56×2.56 or 3.0×3.0cm2; matrix size 128×128×8; number of averages 2. A total of 30 images were acquired with sampling delays after inversion between 40 and 7900ms.
MRI image analysis
Renal volumes were quantified from the 3D-FISP images using ANALYZE™ (version 12.0, Biomedical Imaging Resource, Mayo Clinic, MN). The total kidney volume was calculated from regions of interest manually traced on all frames where kidney was observed.11
All other MRI images were reconstructed and analyzed off-line using in-house developed software packages in Matlab® (The Mathworks, Natick, MA). For BOLD, 8 images were reconstructed from the MGE data using 3D inverse Fourier transform after the k-space data were zero-filled from 128×128 to 256×256. The magnitude of all 8 images was added to generate a single 1mm 2D image. T2* was quantified by pixel-wise mono-exponential curve fitting on signal intensity over echo times. R2* (1/T2*) was used as an index of tissue hypoxia in cortex and medulla. Renal cortical and medullary segmentation was performed on the calculated perfusion map, where a good contrast was observed. The generated masks were propagated to the quantification of other MRI indices.
Systemic measurements
Blood pressure (BP) was measured shortly before euthanasia by tail-cuff, using a XBP1000 system (Kent Scientific, Torrington, CT).12 Plasma cholesterol levels were determined at the Mayo Immunochemistry Laboratory using an automatic chemical analyzer with enzymatic assay kits. The level of plasma creatinine was assayed using the DetectX® Serum Creatinine kit (Arbor assays, Ann Arbor, MI).11 Standards or samples were pipetted into a microtiter plate with an assay diluent, and the color generating reaction was initiated with the DetectX® Creatinine Reagent. Urinary microalbumin was measured by the Mouse Microalbumin ELISA Kit (Kamiya Biomedical Company, Seattle, WA)13 according to the manufacturer’s instructions.
Renal senescence
SA-β-Gal activity was studied using a Staining Kit (#9860, Cell Signaling, Boston, MA) according to the manufacturer’s protocol, with minor modifications. Kidney tissue embedded in OCT was sectioned at 10μm thickness, air-dried, rehydrated in PBS, and fixed with 1X fixative solution (2% formaldehyde and 0.2% glutaraldehyde in PBS, from the kit) at room temperature for 15 minutes. Freshly prepared 5-bromo-4-chloro-3-indolyl-b-D-galactopyranoside (X-Gal) staining solution (0.1% X-gal in 40mM citric acid/sodium phosphate buffer (pH 6.0) containing 5mM K3Fe[CN]6, 5mM K4Fe [CN]6, 2mM MgCl2, and 150mM NaCl) was added, and sections incubated for 16 hours at 37°C.14 Then sections were counterstained with eosin, dehydrated, and mounted. The degree of senescence (blue color) was quantified in 10 randomly chosen fields per section using AxioVision© (Carl Zeiss SMT, Oberkochen, Germany) and expressed as an average of percent SA-β-Gal–positive to total field area.15
Senescence and SASP genes were studied by real time PCR. About 5mg of frozen kidney tissue were homogenized in 400μl of ice cold lysis buffer, supplied by mirVana PARIS total RNA isolation kit (Thermo Fisher Scientific Inc., Waltham, MA). Total RNA was isolated from each homogenized sample and measured by a NanoDrop Spectrophotometer (Thermo Fisher Scientific). 50μl of the RNA samples were then treated with DNase to remove possible DNA contamination. First-strand cDNA was produced from 240ng of total RNA using SuperScript VILO cDNA Synthesis kit (Thermo Fisher Scientific). Relative quantitative PCR was performed using Taqman assays, containing 10ng of cDNA products. Taqman probes (Thermo Fisher Scientific) used in the relative quantitative PCRs were: p16 (mm00494449), p19 (mm00486943), p21 (mm00432448), p53 (mm01731290), Tnf-α (mm00443258), Il-1α (mm00439620), Il-6 (mm00446190), MCP-1 (mm00441242), PAI-1 (mm00436753), and TATA-box binding protein (TBP, mm01277042) as an internal control. Negative controls with no cDNA were cycled in parallel with each run. PCR analysis was done on Applied Biosystems ViiA7 Real-Time PCR systems using the following conditions: 50°C for 2 minutes, 95°C for 10 minutes, and 40 cycles of 95°C for 15 seconds and 60°C for 1 minute. Fold changes of each target gene in the experimental groups relative to control group were calculated using the 2-ΔΔCT method.16
The percentage of p19-positive cells was studied on paraffin-embedded renal sections with p19 immunohistochemistry staining (LS-C49180, LSBio, WA).17 The number of intranuclear p19-positive cells was manually counted in 8–10 fields per slide (ZEN; Carl Zeiss SMT, Oberkochen, Germany), and classified by location and morphology as tubular or intraglomerular cells. Their fraction out of 1000 counted respective cells was then calculated.
Histologic analysis
To detect apoptotic signals, paraffin-embedded renal sections were stained using the Dead-End Fluorimetric Terminal deoxynucleotidyl transferase dUTP nick end-labeling (TUNEL) System (Promega, Madison, WI), and with an anti-active caspase-3 antibody (AF835, Novus Biologicals, CO), respectively. In the fragmented DNA of apoptotic cells, fluorescein-12-dUTP was catalytically incorporated at 3´-OH DNA-ends using the recombinant Terminal Deoxynucleotidyl Transferase. The degree of apoptosis was expressed as the average of manually counted TUNEL-positive per 1000 cells, and active caspase-3-positive area percent, respectively, in 10–15 fields per section. Active caspase-3+ area percent was calculated as the ratio of pixels representing active caspase-3+ to total renal tissue using Matlab®. Glomerular area was assessed on paraffin-embedded PAS-stained sections. Images of 8–10 fields per slide were digitized using ZEN©. Glomeruli containing visible vascular pole were sampled. Glomerular tufts were traced, the areas calculated using AxioVision©, and their mean calculated.18 Mesangial expansion was characterized by an increase in PAS positive mesangial area.19 Paraffin-embedded renal tissue was sectioned at 5μm thickness for trichrome staining. The degree of interstitial fibrosis in the renal cortex was semi-automatically quantified in 10 fields using a masking algorithm based on color thresholding and edge detection in AxioVision© and expressed as an average of total field area.20 Additional staining for interstitial fibrosis was performed using anti-collagen-I antibody (ab34710, Abcam, MA), and positive area percent calculated as for caspase-3. Slides were examined in a blinded manner.
Statistical analysis
Statistical analysis was performed using JMP software version 13.0 (SAS Institute Inc., Cary, NC). Results are presented as mean ± standard deviation for normally distributed variables, and as median (interquartile range) for data that did not conform to a normal distribution. Parametric (one-way analysis of variance (ANOVA) followed by Student’s t-test) and non-parametric (Kruskal-Wallis followed by Wilcoxon) test were used for comparisons among groups. A P value ≤ 0.05 was considered statistically significant.
Results
Systemic characteristics
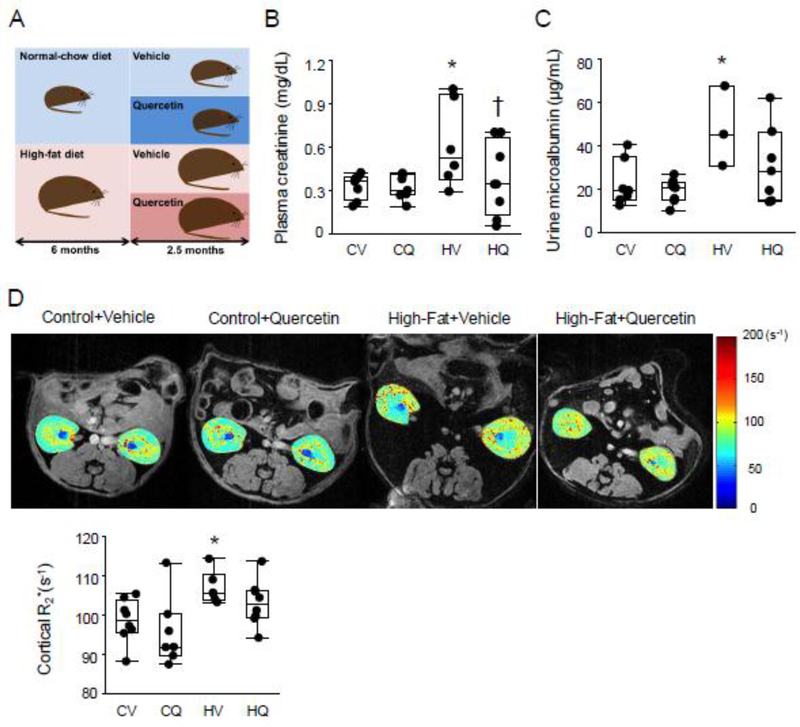
HFD-fed mice were markedly heavier than control groups (all P≤0.004), whereas kidney weights (P=0.30) and systolic BP (P=0.86) were not significantly different among the 4 groups (Table 1). Plasma creatinine and urine microalbumin levels were elevated in HFD+Vehicle compared with Control+Vehicle (both P=0.01). Quercetin treatment restored creatinine levels in obese mice (P=0.01 vs. HFD+Quercetin) and urinary microalbumin levels were no longer higher than in controls (P=0.08 vs. HFD+Quercetin) (Figure 1B, C). Plasma total cholesterol levels were higher in both HFD diet groups than in Control+Vehicle (both P≤0.008) (Table 1).
Table 1.
Characteristics of mice fed a standard or high-fat diet and treated with quercetin or vehicle (n=6–8 each)
| Control+Vehicle | Control+Quercetin | High-fat+Vehicle | High-fat+Quercetin | |
|---|---|---|---|---|
| Body weight (g) | 34.5 (32.7–35.5) | 35.0 (33.7–35.2) | 56.0 (56.0–61.0)* | 58.0 (54.5–61.0)* |
| Kidney weight (mg) | 212.7±33.9 | 206.0±24.2 | 231.7±11.6 | 215.4±20.8 |
| Systolic blood pressure (mmHg) | 88.2±25.7 | 99.2±18.8 | 89.0±31.0 | 94.8±35.1 |
| Plasma total cholesterol (mg/dL) | 72.5 (64.2–80.0) | 76.0 (71.2–79.2) | 288.5 (204.7–307.7)* | 217.0 (187.0–232.2)* |
Data are expressed as median (interquartile range) or mean±standard deviation.
P≤0.05 vs. Control+Vehicle
P≤0.05 vs. High-fat+Vehicle
Body weight, plasma total cholesterol, Wilcoxon test; Kidney weight, Systolic blood pressure, two-tailed Student’s t-tests.
Figure 1.
Renal function in obese dyslipidemic mice. A. Mice consumed either standard chow or high fat diet, and at 6 months started adjunct treatment with quercetin or vehicle for another 10 weeks. B, C. Plasma creatinine and urine microalbumin levels were elevated in High fat+Vehicle (HV) compared with Control+Vehicle (CV), but quercetin treatment restored creatinine levels. CV: n=7–8, CQ: n=8, HV: n=3–6, HQ: n=7–8; Wilcoxon test. D. In vivo assessment of renal oxygenation by blood oxygen-level-dependent MRI. Renal cortical hypoxia (R2*) was elevated in HV vs. CV mice, but not in High fat+Quercetin (HQ) or Control+Quercetin (CQ) mice. CV n=8, CQ n=7, HV n=6, HQ n=8; two-tailed Student’s t-tests. *P≤0.05 vs. CV, †P≤0.05 vs. HV.
In vivo renal oxygenation
Renal cortical hypoxia was elevated in HFD+Vehicle (P=0.02), but not in HFD+Quercetin (P=0.16) compared with in Control+Vehicle mice (Figure 1D).
Renal senescence
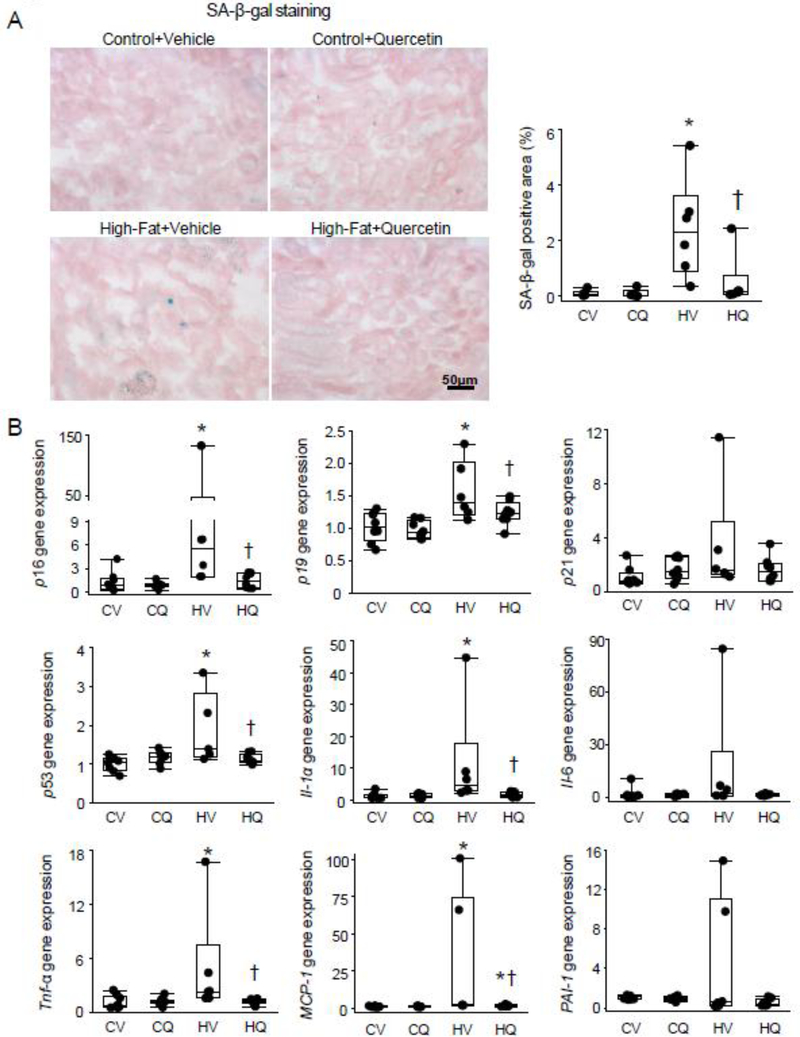
On renal cryosections, the percentage of SA-β-Gal positive area was markedly elevated in HFD+Vehicle mice compared to controls (P=0.005) (Figure 2A). After quercetin treatment, the percentage of SA-β-Gal+ area was decreased in HFD mice (P=0.02 vs. HFD+Vehicle), although not fully normalized (P=0.09 vs. Control+Vehicle). By PCR, renal gene expression of p16, p19, and p53 was upregulated in HFD+Vehicle vs. Control+Vehicle (P=0.01, 0.0006, and 0.008, respectively), but not in HFD+Quercetin, and was lower in HFD+Quercetin than in HFD+Vehicle (P=0.03, 0.02, and 0.02, respectively) (Figure 2B). p21 gene expression remained unchanged among the groups (P=0.12). Renal gene expression of Tnf-α and IL-1α was increased in HFD+Vehicle vs. Control+Vehicle (P=0.02 and 0.008, respectively), and normalized in HFD+Quercetin (P=0.002 and 0.005 vs. HFD+Vehicle, respectively), whereas Il-6 and PAI-1 gene expression remained unchanged (P=0.16 and 0.12, respectively). MCP-1 gene expression was higher in HFD+Vehicle than Control+Vehicle (P=0.002) and lower in HFD+Quercetin than HFD+Vehicle (P=0.03) but not fully normalized.
Figure 2.
Renal senescence in obese dyslipidemic mice. A. Representative renal senescence-associated β-galactosidase (SA-β-Gal) staining. SA-β-Gal+ area (blue) was larger in both High fat+Vehicle (HV) and High fat+Quercetin (HQ) groups than in controls, but lower in HQ. n=6 each; Wilcoxon test. B. Renal p16, p19, and p53 gene expression was upregulated in HV compared with Control+Vehicle (CV), and decreased in HQ. Renal gene expression of IL-1α, Tnf-α, and MCP-1 was increased in HV compared with CV and decreased in HQ compared with in HV. CV n=8, CQ n=8, HV n=6, HQ n=8; Wilcoxon test. *P≤0.05 vs. CV, †P≤0.05 vs. HV.
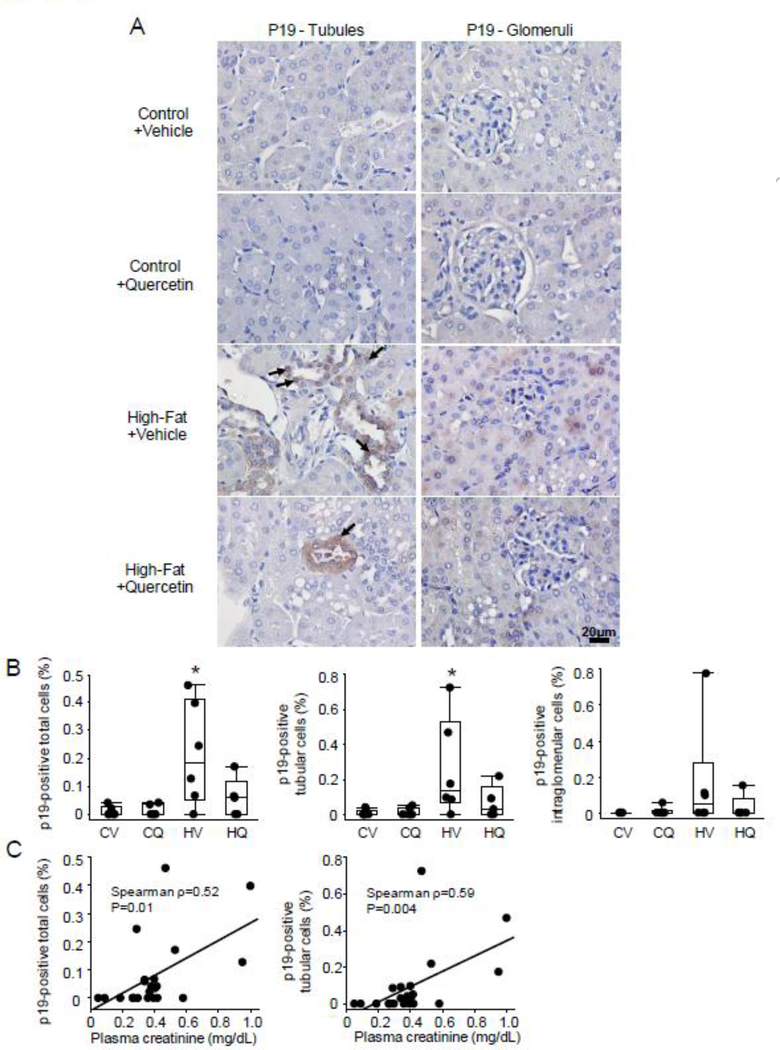
Renal staining for p19 showed that a greater percentage of overall p19-positive cells in the HFD+Vehicle group than in controls (P=0.02), but not in quercetin-treated HFD mice (P=0.19 vs. Control+Vehicle) (Figure 3A–B). The number of p19-positive tubular cells was increased only in HFD+Vehicle compared with controls (P=0.02), and p19-positive intraglomerular cells tended to increase as well (P=0.07 vs. Control+Vehicle). The number of p19-positive total and tubular cells correlated directly with plasma creatinine levels (Figure 3C).
Figure 3.
Representative p19 immunohistochemistry staining in the kidney. A-B. The percentage of total as well as tubular p19-positive (arrow) was greater in High fat+Vehicle (HV) than in Control+Vehicle (CV), but not in High fat+Quercetin (HQ) mice. CV n=6, CQ n=6, HV n=6, HQ n=5; Wilcoxon test. *P≤0.05 vs. CV. C. The number of these cells also correlated with plasma creatinine levels.
Renal damage
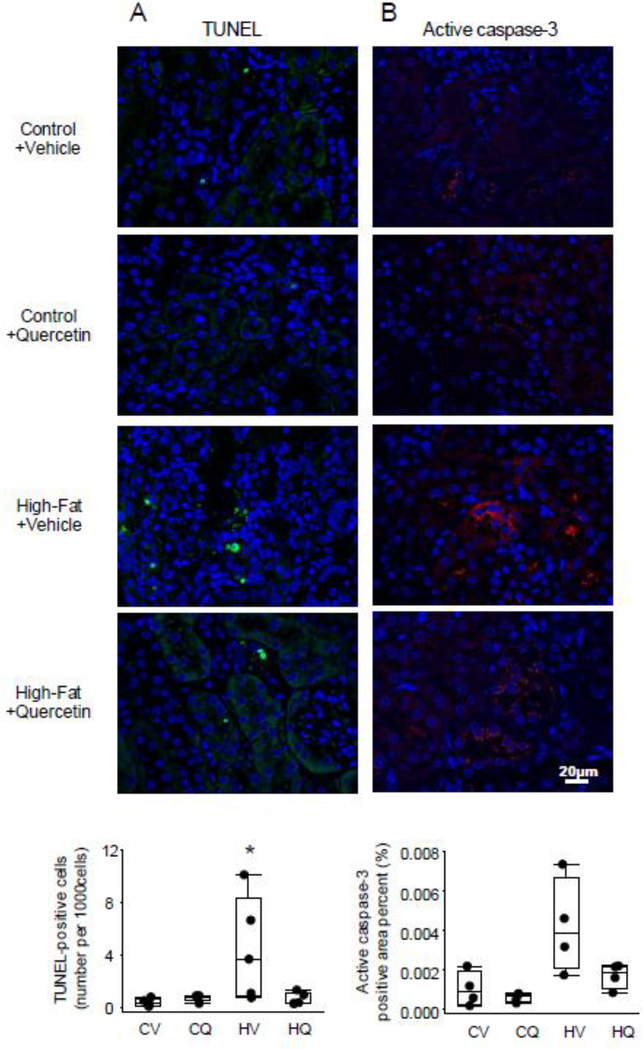
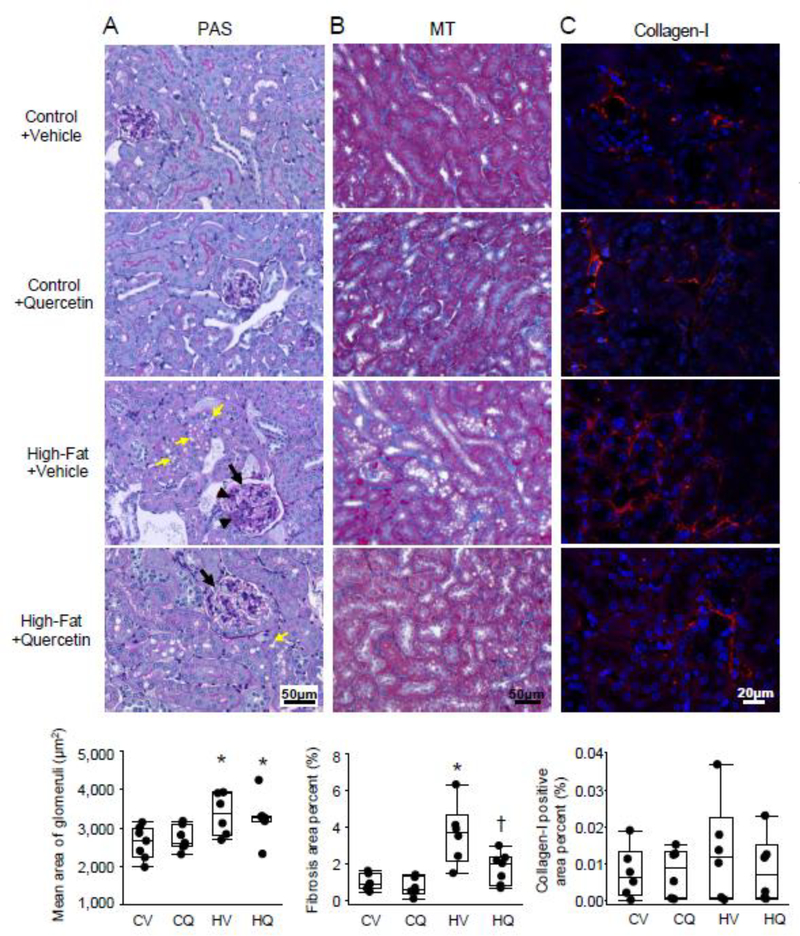
TUNEL-positive apoptotic cells were increased in HFD+Vehicle (P=0.02 vs. Control+Vehicle), but not in HFD+Quercetin (P=0.06 vs. HFD+Vehicle) (Figure 4A), and appeared to be mostly tubular epithelial cells. Active caspase-3 positive area percent strongly tended to increase in HFD+Vehicle compared with Control+Vehicle (P=0.06) (Figure 4B). PAS staining revealed glomerulomegaly and expansion of mesangial matrix in obese kidneys, with no evident glomerulosclerosis (Figure 5A). It also showed abundant vacuoles localized primarily to proximal tubular cells of HFD mice, whereas their brush borders were relatively intact. Mean glomerular area was elevated in both HFD+Vehicle and HFD+Quercetin mice compared with Control+Vehicle (P=0.009 and 0.01, respectively). Cortical trichrome staining showed higher interstitial fibrosis in HFD+Vehicle than in Control+Vehicle (p=0.005), which was significantly attenuated in HFD+Quercetin (p=0.03 vs. HFD+Vehicle) (Figure 5B), whereas collagen-I positive area percent was not different among the groups (figure 5C).
Figure 4.
Renal apoptotic signals in obese dyslipidemic mice. A. Representative renal Terminal deoxynucleotidyl-transferase dUTP nick end labeling (TUNEL) staining. The number of TUNEL-positive cells per 1000 cells was increased in High fat+Vehicle (HV) compared with Control+Vehicle (CV). n=5 each; Wilcoxon test. B. Active caspase-3 staining in the kidneys. Active caspase-3 positive area (red) percent tended to be increased in HV compared with CV. n=4 each; Wilcoxon test. *P≤0.05 vs. CV.
Figure 5.
Representative renal Periodic acid-Schiff (PAS), Masson’s trichrome (MT), and collagen-I staining. A. PAS staining illustrated that compared to Control+Vehicle (CV), high-fat diet (HV) led to glomerulomegaly (black arrow) and mesangial matrix expansion (black arrowhead), which were unaffected by quercetin (HQ). In addition, abundant vacuoles (yellow arrow) localized mostly to proximal tubular cells of HV mice, whereas their brush borders were relatively intact. CV n=7, CQ n=7, HV n=6, HQ n=6; two-tailed Student’s t-tests. B. Cortical trichrome staining showed significantly lower interstitial fibrosis (blue stain) in HQ than in HV. CV n=7, CQ n=6, HV n=6, HQ n=7; Wilcoxon test. C. In renal collagen-I staining, collagen-I positive area (red) percent was not significantly different among the groups. n=6 each; Wilcoxon test. *P≤0.05 vs. CV, †P≤0.05 vs. HV.
Discussion
This study shows that murine high-fat diet-induced obesity induces renal cellular senescence and impairs kidney function and cortical oxygenation. Chronic clearance of senescent cells using quercetin improved renal functional indices and alleviated fibrosis. These findings implicate senescence in HFD-related renal injury and support further studies of senolytic therapy in obese and dyslipidemic subjects.
Our HFD-fed mice showed functional and structural renal damage, although hypertension was yet to develop. In early obesity, renal vasodilation involving mainly the afferent arteriole increases renal plasma flow and glomerular filtration rate, resulting in microalbuminuria and glomerulomegaly.21, 22 Insulin resistance and increased proximal reabsorption of sodium likely play an important role in hyperfiltration associated with obesity, and leads to tubular hypertrophy.23–25 Sustained hyperfiltration burdens glomerular capillaries, resulting in renal hypoxia and consequent insufficiency,26 as we observed in our mouse model. This model was likely beyond the phase of hyperfiltration, because renal size was not elevated whereas plasma creatinine was. Deleterious changes in mesangial cells, podocytes, and proximal tubular cells may be also secondary to ectopic deposition of lipids in the kidney.27
Recent studies implicate cellular senescence in pathological conditions related to obesity in various organs,2–4 but its link to obesity-related renal injury has yet to be fully elucidated. We found that HFD induced kidney cellular senescence, evidenced by increased expression of the cyclin-dependent kinase inhibitors p16, p19, and p53 and SA-β-Gal activity. The mechanisms by which HFD leads to renal senescence may be multifactorial, as both dyslipidemia and obesity may contribute to this process. Obesity prompts insulin resistance and potential aberrant glucose metabolism, which may induce cellular senescence in insulin target-tissues like adipose tissue,3, 28 as well as in the kidney.29 In type-2 diabetic nephropathy, expression of p16INK4a and SA-β-Gal increase in the kidney, and tubular SA-β-Gal activity is directly associated with body mass index.30 The trigger for cellular senescence in this setting may also include increased cholesterol levels, lipid peroxidation,31 increased oxidative stress, and mitochondrial dysfunction. Reactive oxygen species can induce senescence through either p53-mediated induction of p21 or activation of p16INK4a through p38-MAPK-mediated mitochondrial dysfunction.32, 33 Furthermore, adipokines can directly induce cellular senescence,34 implicating obesity in cellular senescence in our model.
We found that HFD-induced murine renal senescence involved primarily activation of p16, p19, and p53. In many cell lines p21 activation is transient, and falls after cell-cycle arrest.35 Congruently, our study showed that p21 expression was not different among the groups despite upregulated expression of p19 and p53 in untreated obese mice. Importantly, markers of cellular senescence correlated with renal function, suggesting a link between the two, and were particularly evident in vivo in renal tubular epithelial cells, implicating this process in HFD-related tubular injury. Accordingly, increased staining for lipofuscin, a marker of senescence, was recently observed in proximal tubular epithelial cells in HFD-fed mice, potentially related to lipid peroxidation.36 Additionally, cellular senescence may be accompanied by upregulated expression of SASP markers, which can lead to renal fibrosis. Indeed, we observed upregulated gene expression of MCP-1, Tnf-α, and IL-1α in obese kidneys, although Il-6 and PAI-1 expression remained unaltered due to high variability.
Quercetin, a member of the flavonoid family, targets PI3K/AKT and p53/p21/serpines SCAPs.37 However, its effect on renal senescence has not been established. Our results demonstrated that quercetin decreased cellular senescence in vivo in renal tubular epithelial and possibly other cells. This might have been mediated by improving senescence-related mitochondrial dysfunction38 or a decrease in the SASP, thereby blunting the proinflammatory and profibrotic microenvironment.14 Pertinently, renal gene expression of apoptotic factors like Tnf-α was normalized in quercetin-treated mice, possibly resulting in decreased prevalence of apoptotic cells in the kidneys, which were likely not senescent. While quercetin induces apoptosis of senescent cells, their relatively small numbers may not have counterbalanced the decreased apoptosis of non-senescent cells. Quercetin also decreased expression of IL-1α and MCP-1 in obese mice, but not in control mice. Furthermore, quercetin decreased renal fibrosis and serum creatinine levels in HFD-fed mice. Conversely, quercetin did not affect intraglomerular senescent cells or restore glomerular size, which may be attributable to hemodynamic or irreversible effects of obesity or early structural changes in the kidney. Notably, the effects of quercetin include both direct senolysis (elimination of senescent cells) and prevention of senescence induction,39 as it may indirectly prevent further spread of senescence by clearance of noxious SASP-producing senescent cells. Given that quercetin had no effect on body weight or plasma cholesterol levels, it likely blunted downstream pathways, such as adipokines, cytokines, or oxidized lipids.
In this study, relatively young animals were used to distinguish the effects of HFD from aging on senescence, but might also be more resistant to high-fat diet-related effects on BP. One HV mouse appeared to be an outlier in some senescence/SASP gene expressions, but was not phenotypically different from other mice in the group, and its removal did not affect the significance of our results. Therefore, it was included in the analysis. Some analyses were not performed in all samples because of availability, as indicated in Figure Legends. Quercetin might act through other mechanisms besides SCAPs, including off-target effects observed during chronic continuous dietary intake.40, 41 However, we used intermittent quercetin treatment, which is consistent with senolytic effects since quercetin has an elimination half-life of <6 hrs, and is unlikely to have acted by being continuously present to bind a receptor, or targeting an enzyme.42, 43 Although p16 gene expression was upregulated many-fold in our model, we stained the kidneys for p19 because murine tissue does not easily lend itself to staining with an anti-p16 antibody, and because p16 and p19 often change in the same direction.44
In conclusion, our study demonstrates that murine high-fat diet triggers renal cellular senescence, suggesting a potentially important role in the progression of renal injury. Chronic intermittent quercetin treatment alleviates renal senescence in high-fat diet and improves renal function. Therefore, senescent cell clearance may be an effective strategy to improve high-fat diet-related renal injury. Our results support novel intervention strategies and targeted senolytic therapy in high-fat diet, thereby alleviating renal dysfunction and delaying renal injury.
Brief commentary.
Background
Obesity and dyslipidemia is a risk factor for development of kidney failure and can be associated with senescence. However, whether senescence contributes to renal dysfunction in these conditions remains unclear. Quercetin selectively clears senescent cells by targeting their upregulated anti-apoptosis, yet its renal impact in high-fat-diet-induced obese mice is unknown.
Translational Significance
This manuscript shows that obesity and dyslipidemia triggers renal senescence, suggesting a potential role in the progression of renal injury. Quercetin improves renal senescence and function in obesity and dyslipidemia. These observations reveal new mechanisms that contribute to the pathogenesis of high-fat-diet-induced obesity-related renal injury, and support novel intervention strategies and targeted senolytic therapy in obesity and dyslipidemia.
Acknowledgements
The authors wish to thank Mariya Sweetwyne, PhD, and Stuart J. Shankland M.D., MBA, for sharing p19 IHC staining methods.
This work was partly supported by NIH grants numbers DK120292, HL123160, DK104273, DK102325, DK109134, and AG013925, the Connor Group and Robert J. and Theresa W. Ryan, and the Noaber Foundation. The authors are grateful for support from the Histology Core Facility (Mayo Clinic Arizona) and Mayo Clinic Pathology Research Core (Rochester, MN).
Abbreviations
- BOLD
blood oxygen level-dependent
- BP
blood pressure
- BF
bright field
- FISP
Fast Imaging with Steady Precession
- HFD
high-fat diet
- MGE
multi-echo gradient echo
- MRI
magnetic resonance imaging
- MT
Masson’s trichrome
- PAS
Periodic acid-Schiff
- SA-β-Gal
senescence-associated β-Galactosidase
- SASP
senescence-associated secretory phenotype
- SCAPs
senescent cell anti-apoptotic pathways
- TUNEL
Terminal deoxynucleotidyl transferase dUTP nick-end labeling
- X-Gal
5-bromo-4-chloro-3-indolyl-b-D-galactopyranoside
Footnotes
All authors have read the journal’s authorship agreement and policy on disclosure of potential conflicts of interest, and the manuscript has been reviewed by and approved by all named authors.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Ejerblad, Fored, Lindblad, et al. Obesity and risk for chronic renal failure. J Am Soc Nephrol. 2006;17:1695–702. [DOI] [PubMed] [Google Scholar]
- [2].Tchkonia Morbeck, Zglinicki Von, et al. Fat tissue, aging, and cellular senescence. Aging cell. 2010;9:667–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Minamino, Orimo, Shimizu, et al. A crucial role for adipose tissue p53 in the regulation of insulin resistance. Nat Med. 2009;15:1082–7. [DOI] [PubMed] [Google Scholar]
- [4].Zhang, Zhou, Strakovsky, Zhang, Pan. Hepatic cellular senescence pathway genes are induced through histone modifications in a diet-induced obese rat model. Am J Physiol Gastrointest Liver Physiol. 2012;302:G558–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Tchkonia, Zhu, van Deursen, Campisi, Kirkland. Cellular senescence and the senescent secretory phenotype: Therapeutic opportunities. J Clin Invest. 2013;123:966–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Zhu, Doornebal, Pirtskhalava, et al. New agents that target senescent cells: The flavone, fisetin, and the bcl-xl inhibitors, a1331852 and a1155463. Aging (Albany NY). 2017;9:955–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Eirin, Zhu, Puranik, et al. Mesenchymal stem cell-derived extracellular vesicles attenuate kidney inflammation. Kidney Int. 2017;92:114–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Zhu, Tchkonia, Pirtskhalava, et al. The achilles’ heel of senescent cells: From transcriptome to senolytic drugs. Aging Cell. 2015;14:644–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Roos, Zhang, Palmer, et al. Chronic senolytic treatment alleviates established vasomotor dysfunction in aged or atherosclerotic mice. Aging cell. 2016;15:973–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Ebrahimi, Crane, Knudsen, et al. Evolution of cardiac and renal impairment detected by high-field cardiovascular magnetic resonance in mice with renal artery stenosis. J Cardiovasc Magn Reson. 2013;15:98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Jiang, Ferguson, Ebrahimi, et al. Noninvasive assessment of renal fibrosis with magnetization transfer mr imaging: Validation and evaluation in murine renal artery stenosis. Radiology. 2017;283:77–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Cheng, Zhou, Warner, et al. Temporal analysis of signaling pathways activated in a murine model of two-kidney, one-clip hypertension. Am J Physiol Renal Physiol. 2009;297:F1055–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Yu, Fang, Liu, et al. The balance of beneficial and deleterious effects of hypoxia-inducible factor activation by prolyl hydroxylase inhibitor in rat remnant kidney depends on the timing of administration. Nephrol Dial Transplant. 2012;27:3110–9. [DOI] [PubMed] [Google Scholar]
- [14].Baker, Childs, Durik, et al. Naturally occurring p16(ink4a)-positive cells shorten healthy lifespan. Nature. 2016;530:184–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Melk, Kittikowit, Sandhu, et al. Cell senescence in rat kidneys in vivo increases with growth and age despite lack of telomere shortening. Kidney Int. 2003;63:2134–43. [DOI] [PubMed] [Google Scholar]
- [16].Park, Herrmann, Saad, et al. Circulating and renal vein levels of micrornas in patients with renal artery stenosis. Nephrol Dial Transplant. 2015;30:480–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Sweetwyne, Pippin, Eng, et al. The mitochondrial-targeted peptide, ss-31, improves glomerular architecture in mice of advanced age. Kidney Int. 2017;91:1126–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Serra, Romero, Lopez, et al. Renal injury in the extremely obese patients with normal renal function. Kidney Int. 2008;73:947–55. [DOI] [PubMed] [Google Scholar]
- [19].Yakala, van der Heijden, Molema, et al. Beneficial effects of an alternating high- fat dietary regimen on systemic insulin resistance, hepatic and renal inflammation and renal function. PLoS One. 2012;7:e45866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Korsmo, Ebrahimi, Eirin, et al. Magnetic resonance elastography noninvasively detects in vivo renal medullary fibrosis secondary to swine renal artery stenosis. Investigative radiology. 2013;48:61–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Valensi, Assayag, Busby, et al. Microalbuminuria in obese patients with or without hypertension. Int J Obes Relat Metab Disord. 1996;20:574–9. [PubMed] [Google Scholar]
- [22].Chakkera, Chang, Thomas, et al. Obesity correlates with glomerulomegaly but is not associated with kidney dysfunction early after donation. Transplant Direct. 2015;1:1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Hayashi, Fujiwara, Oka, et al. Effects of insulin on rat renal microvessels: Studies in the isolated perfused hydronephrotic kidney. Kidney Int. 1997;51:1507–13. [DOI] [PubMed] [Google Scholar]
- [24].Vallon Richter, Blantz, Thomson, Osswald. Glomerular hyperfiltration in experimental diabetes mellitus: Potential role of tubular reabsorption. J Am Soc Nephrol. 1999;10:2569–76. [DOI] [PubMed] [Google Scholar]
- [25].Vallon, Blantz, Thomson. Glomerular hyperfiltration and the salt paradox in early [corrected] type 1 diabetes mellitus: A tubulo-centric view. J Am Soc Nephrol. 2003;14:530–7. [DOI] [PubMed] [Google Scholar]
- [26].Zhang, Lerman. The metabolic syndrome and chronic kidney disease. Transl Res. 2017;183:14–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Kiss, Kranzlin, Wagenblabeta, et al. Lipid droplet accumulation is associated with an increase in hyperglycemia-induced renal damage: Prevention by liver x receptors. Am J Pathol. 2013;182:727–41. [DOI] [PubMed] [Google Scholar]
- [28].Aguayo-Mazzucato, van Haaren, Mruk, et al. Beta cell aging markers have heterogeneous distribution and are induced by insulin resistance. Cell Metab. 2017;25:898–910 e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Homayounfar, Jeddi-Tehrani, Cheraghpour, Ghorbani, Zand. Relationship of p53 accumulation in peripheral tissues of high-fat diet-induced obese rats with decrease in metabolic and oncogenic signaling of insulin. Gen Comp Endocrinol. 2015;214:134–9. [DOI] [PubMed] [Google Scholar]
- [30].Verzola, Gandolfo, Gaetani, et al. Accelerated senescence in the kidneys of patients with type 2 diabetic nephropathy. Am J Physiol Renal Physiol. 2008;295:F1563–73. [DOI] [PubMed] [Google Scholar]
- [31].Liao, Yang, Liu, Zheng. Glp-1 and ghrelin attenuate high glucose/high lipid-induced apoptosis and senescence of human microvascular endothelial cells. Cell Physiol Biochem. 2017;44:1842–55. [DOI] [PubMed] [Google Scholar]
- [32].Passos, Nelson, Wang, et al. Feedback between p21 and reactive oxygen production is necessary for cell senescence. Mol Syst Biol. 2010;6:347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Freund, Patil, Campisi. P38mapk is a novel DNA damage response-independent regulator of the senescence-associated secretory phenotype. EMBO J. 2011;30:1536–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Zhao, Dong, Zhang, et al. Leptin changes differentiation fate and induces senescence in chondrogenic progenitor cells. Cell Death Dis. 2016;7:e2188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Romanov, Pospelov, Pospelova. Cyclin-dependent kinase inhibitor p21(waf1): Contemporary view on its role in senescence and oncogenesis. Biochemistry (Mosc). 2012;77:575–84. [DOI] [PubMed] [Google Scholar]
- [36].Kuwahara, Hosojima, Kaneko, et al. Megalin-mediated tubuloglomerular alterations in high-fat diet-induced kidney disease. J Am Soc Nephrol. 2016;27:1996–2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Kirkland, Tchkonia. Cellular senescence: A translational perspective. EBioMedicine. 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Ogrodnik, Miwa, Tchkonia, et al. Cellular senescence drives age-dependent hepatic steatosis. Nat Commun. 2017;8:15691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Xu, Pirtskhalava, Farr, et al. Senolytics improve physical function and increase lifespan in old age. Nat Med. 2018;24:1246–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Jeong, Kang, Choi, Kim, Kim. Quercetin ameliorates hyperglycemia and dyslipidemia and improves antioxidant status in type 2 diabetic db/db mice. Nutr Res Pract. 2012;6:201–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Kobori, Masumoto, Akimoto, Oike. Chronic dietary intake of quercetin alleviates hepatic fat accumulation associated with consumption of a western-style diet in c57/bl6j mice. Mol Nutr Food Res. 2011;55:530–40. [DOI] [PubMed] [Google Scholar]
- [42].Kirkland, Tchkonia, Zhu, Niedernhofer, Robbins. The clinical potential of senolytic drugs. J Am Geriatr Soc. 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Graefe, Wittig, Mueller, et al. Pharmacokinetics and bioavailability of quercetin glycosides in humans. J Clin Pharmacol. 2001;41:492–9. [DOI] [PubMed] [Google Scholar]
- [44].Mikawa, Suzuki, Baskoro, et al. Elimination of p19(arf) -expressing cells protects against pulmonary emphysema in mice. Aging cell. 2018;17:e12827. [DOI] [PMC free article] [PubMed] [Google Scholar]