Abstract
Substantial growth in the biosensor research has enabled novel, sensitive and point-of-care diagnosis of human diseases in the last decade. This paper presents an overview of the research in the field of biosensors that can potentially predict and diagnosis of common placental pathologies. A survey of biomarkers in maternal circulation and their characterization methods is presented, including markers of oxidative stress, angiogenic factors, placental debris, and inflammatory biomarkers that are associated with various pathophysiological processes in the context of pregnancy complications. Novel biosensors enabled by microfluidics technology and nanomaterials is then reviewed. Representative designs of plasmonic and electrochemical biosensors for highly sensitive and multiplexed detection of biomarkers, as well as on-chip sample preparation and sensing for automatic biomarker detection are illustrated. New trends in organ-on-a-chip based placental disease models are highlighted to illustrate the capability of these in vitro disease models in better understanding the complex pathophysiological processes, including mass transfer across the placental barrier, oxidative stress, inflammation, and malaria infection. Biosensor technologies that can be potentially embedded in the placental models for real time, label-free monitoring of these processes and events are suggested. Merger of cell culture in microfluidics and biosensing can provide significant potential for new developments in advanced placental models, and tools for diagnosis, drug screening and efficacy testing.
Keywords: Placental pathology, biomarker, biosensor, microfluidics, nanomaterials, placenta-on-a-chip
INTRODUCTION
Human placenta is a transient organ of vital importance in control and regulation of maternal-fetal communications(1). It allows selective transport of nutrients, wastes, hormones and neurotransmitters, as well as a barrier to toxins and infectious organisms, which are required for healthy growth and survival of fetus and maintenance of pregnancy. Dysfunctional or insufficient placental development may result in morbidity and mortality of both mother and fetus. This review is focused on a discussion on the biosensor technologies and their applications in detection of biomarkers for prediction and diagnosis of common placental pathologies due to maternal health complications. We intend to highlight the possible merger between the circulating biomarkers and engineering techniques towards minimally invasive detection of placental pathologies.
Common placental pathologies.
Among common placental pathologies, preeclampsia (PE) is a unique human condition due to placental dysfunction, which releases substances in the maternal circulation causing endothelium dysfunction and symptoms of hypertension and proteinuria. It is one of the most common and life-threatening disorders during pregnancy(2). A fetal adverse outcome is intrauterine growth restriction (IUGR). It occurs when the fetus fails to reach its expected growth potential, characterized by insufficient transfer of nutrients and oxygen to the fetus and impaired growth of fetal organs and tissues. IUGR may result from maternal under-nutrition, medical conditions, as well as infection. Malaria infection during pregnancy is characterized by sequestration of infected erythrocytes in placenta, known as placental malaria (PM). It is an established risk factor for small-for-gestational-age births, and highly associated with IUGR in comparison to the undemutrition(3). PM is also characterized by intervillous inflammation with infiltration of mononuclear immune cells and fibrin deposits, and receiving antimalarial treatment in the previous month was significantly protective against IUGR(3). Another IUGR condition opposite to small-for-gestational-age births is large-for-gestational age, which can occur in gestational diabetes mellitus (GDM). GDM is the onset of impaired glucose tolerance during pregnancy, due to increased insulin resistance and inadequate β-cell compensation, partially caused by insufficient production of placental hormones such as placental lactogen (4).
Placental hormones.
Human placenta is an important endocrine organ and secrets numerous important hormones, which are vital to support the development of pregnancy and fetus, as well as to drive the maternal physiological changes from gestation to beyond childbirth. These placental hormones are released into maternal circulation during pregnancy. Many of these hormones have been extensively studied and correlations between their alterations and certain pregnancy complications have been established. The results suggest that they are potentially useful biomarkers for prediction and diagnosis of pregnancy complications. It was reported that the concentrations of the steroids (progesterone and estrogen) are associated with hypertensive disorders in pregnancy(1), and increased in women with GDM(2, 3) but decreased in the case of PE(4). Placental lactogens is a polypeptide hormone, mainly synthesized by syncytiotrophoblast and are detected in both maternal and fetal circulations. It plays a critical role for materno-fetal energy homeostasis by regulating maternal lipid and carbohydrate metabolism. Placental lactogen was found to be low in GDM, which causes the insufficient adaption of the maternal beta cells to the decreased insulin sensitivity induced by pregnancy(7). Another example is human placental growth hormone, which reduces maternal insulin sensitivity to promote nutrient availability for fetal development (5). It was found that its maternal serum concentration is significantly increased in women with PE(6). Comprehensive analysis and discussion regarding the role of these placental hormones in mediating maternal adaptations through pregnancy, parturition to lactation, as well as their associations with common pregnancy pathologies can be found in reviews by Napso et al. (8) and by Costa (9), respectively.
Biosensor technology.
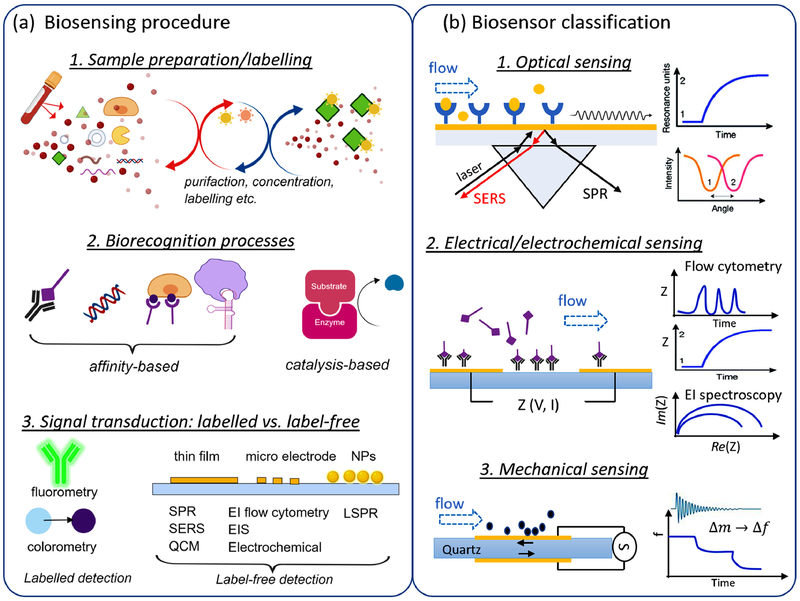
A biosensor is a self-contained integrated receptor-transducer device, which is capable of providing specific quantitative or semi-quantitative analytical information using a biological recognition element(5). Three critical components of a biosensor device are analyte of interest, bioreceptor element that recognizes a specific analyte, and a transducer element that provides a measurable chemical, optical, or electrical signal (Fig. 1a). In development of minimally invasive diagnosis tools for placental pathologies, the analytes may include various circulating biological elements in maternal blood and amniotic fluid (6). An affinity-based biorecognition events may be antigen-antibody binding, nucleic acid hybridization, specific binding of cell-surface receptor to immobilized ligand, or aptamer binding to target cells. A catalysis-based biorecognition may be catalysis production from enzymic reaction (7). Correspondingly, an immunosensor exploits the specific antigen–antibody binding with high sensitivity and selectivity, and either component can be used as a biorecognition element (8). A nucleic acid-based biosensor employs hybridization between a surface immobilized nucleic acid probe sequence (DNA oligomers or its modified forms) and its complementary target sequence(9). A cell-based biosensor utilizes the constituted bioreceptor layer of live cells to detect functional information of biologically active analytes, changes in intracellular and extracellular microenvironments, or cellular response to external stimuli(10). Aptamers are synthetic strands of nucleic acid but behave more like antibodies (11, 12). An enzyme-based biosensor applies catalytic action and binding capabilities for a specific detection(13).
Fig 1.
(a) Illustration of biosensing procedure: sample preparation and labeling, which separates, concentrate and label the analytes of interest; biorecognition process associated with antigen-antibody binding, nucleic acid hybridization, specific binding of cell-surface receptor to ligand, aptamer binding to target cells, and catalysis production from enzymic reaction. (b) Classification of biosensors where the biorecognition events are transduced into optical signals such as SERS and SPR, electrical signals such as EI-based flow cytometry, analyte-substrate impedance, and EI spectroscopy, and mechanical signals such as changes in quartz resonance frequency.
Based on the transduction mechanism, a biosensor can be optical, electrochemical/electrical, or mechanical (Fig. 1b). Optical transduction is the most common method and can be based on colorimetry or fluorometry. The colorimetric method is developed based upon color changes using chemo-responsive dyes or surface modified nanoparticles for the detection of analyte(14, 15). A well-known example of colorimetric biosensor is enzyme-linked immunosorbent assay (ELISA), which has been the primary tool for detection of analytes of interest in biological samples and are widely used in life science research and clinical diagnostics. Fluorometric biosensors utilize fluorescent mechanisms for signal transduction, which are more complex and costly comparing to colorimetric sensors. Other optical technologies reply on illuminating noble metal using incident laser (ultraviolet-visible). The highly active field of research has been focused on the signals of surface plasmon resonance (SPR) and surface-enhanced Raman scattering (SERS). SPR operates using incident light to collectively excite electrons of a conduction band in a metal film or surfaces of metal nanoparticles (NPs), such as gold, silver, copper and aluminum (16). SPR technique has been used in concentration analysis of analytes for clinical diagnostics(17, 18). SERS is a powerful vibrational spectroscopy by illuminating a plasmonic substrate material, such as colloidal or metal NPs with Raman laser(19). SERS signals can rely on the direct adsorption or binding of an analyte molecule to the substrate or via a reporter molecule(20). SERS-based immunoassays have been demonstrated to be effective in detection and quantification of biomarkers of cardiovascular diseases in vitro(21–23) and in vivo (24). Fundamentals, techniques for improved SERS performance, and applications have been discussed in several in-depth reviews (25–27).
In an electrochemical biosensor, biorecognition element is kept in direct contact with the transduction electrode, where the electrical signal is produced by physical changes during the biorecognition process or electrochemical reaction (oxidation and reduction processes)(7). Depending on the type of electrical signals, electrochemical biosensors can be generally categorized into amperometric/voltammetric(28), potentiometric(29), conductometric(30), and electrical impedance (EI) sensors (31). EI sensors can be based on Coulter principle, impedance spectroscopy of cell suspension, or electric cell-substrate impedance sensing, offering single particle analysis, dielectric characterization of cells in suspension, and cells adherent to substrate electrodes, respectively (32). EI-based sensors can operate on a broad range of frequencies that span from α-dispersion (Hz – kHz), β-dispersion (kHz – MHz), to γ-dispersion (10 GHz and higher), to provide measurements from single-particle volume, surface and interior characteristics of cells, to kinetics of cell spreading, proliferation, barrier function, cell junctions, and cell motility etc. Depending on the configuration, impedance of single particles can be measured via a microfluidic channel in EI-based flow cytometry (33, 34) or via a size-tunable pore in tunable resistive pulse sensing (TRPS) system (35, 36). Both EI-based flow cytometry and TRPS are historically used for measuring microparticles but with the advances in nanofabrication, they can be used to measure particles of submicron range down to nanoscale, such as colloids, biomolecular analytes, and extracellular vesicles (37, 38). The electrode materials are selected from gold, carbon, platinum, silver/silver chloride according to the specific electrical signal. Electrochemical transduction is widely used to detect various biorecognition and biochemical reactions for disease diagnosis and monitoring (39, 40) with a great effort in development of portable electrochemical immunosensors (41, 42). Recent advances in electrochemical immunosensors can be found in a review by Koncki (43).
Mechanical biosensors depend on mechanical transduction, where the biorecognition process causes a change in the mass on the transducer surface, which is related to the change in resonant frequency. Surface acoustic wave sensors and quartz crystal microbalance (QCM) are mostly investigated. In a surface acoustic wave sensor, a mass loading to an immobilized biorecognition layer (44) on the piezoelectric surface is related to the changes in the velocity of surface acoustic waves (SAW) that are excited at the resonant frequency by patterned electrodes(45). In QCM, difference in resonating frequency of a piezoelectric quartz crystal transducer is tracked. QCM-based biosensors was employed as high sensitivity method to study interaction between biomolecules (46). The higher center frequencies in SAW-based sensors (40-200 MHz) than QCM based sensors (5–20 MHz) contributes to the increased sensitivity of mass loading (47).
To improve the biosensing performance, many labeling and immobilization techniques can be employed to enhance signal-to-noise ratio and sensitivity of measurements. Labeling with fluorescence signals, particles and ions to the biorecognition element can enhance the detection of analytes from background interferences. The immobilization methods are separated based on different chemical and physical properties of protein and electrode (41). Proteins (antibody and antigens), can be immobilized using physical, covalent and oriented methods or can be absorbed onto surface of the transduction element via noncovalent interactions such as ionic bonds, electrostatic force and hydrophobic interface, which can be further enhanced by physical entrapment of proteins using matrices of polymer (48) or iridium oxide thin film (49). Recent advances in immobilization techniques enabled by novel nanomaterials, such as gold NPs, carbon nanotubes, and graphene have further improved the sensitivity and performance of enzyme-based biosensors(50). Some label-free biosensors may involve reduced sensitivity due to nonspecific adsorption of components in a complex sample but can be improved by appropriate sample preparation processes.
BIOMARKERS OF PLACENTAL PATHOLOGIES AND CHARACTERIZATION METHODS
Table 1 is a brief summary of some common biomarkers associated with abnormal pathophysiological processes in common pregnancy complications. These biomarkers are coarsely categorized into four major classes, based on the pathological processes, including oxidative stress markers, angiogenic factors, circulating placental residue/particles, and inflammatory biomarkers. They have potential values in prediction and diagnosis of common placental pathologies, such as PE, IUGR, PM and GDM. We use this table as a basis for subsequent discussion on the characterization methods currently employed in clinical and laboratory researches as well as the innovation in biosensing approaches.
Table 1.
Maternal circulating biomarkers in placental pathologies and their characterization
| Biomarkers | Placental Pathologies | Characterization Method |
|---|---|---|
| MDA, protein carbonyls, Ox-LDL, superoxide dismutase, Catalase, oxidized DNA |
PE: (51–56) IUGR: (57–60) PM: (61) GDM: (59, 62–65) |
ELISA(51, 53, 57, 59, 61, 66), chromatography(67), UV spectrophotometry (63), HPLC(68), colorimetric biosensors (55, 56, 61), electrochemical assay (69, 70), chemiluminescence (71–73), electrochemical impedance spectroscopy (74), SPR(75), SERS(76) |
| sFlt-1, sENG, PlGF, hCG, VEGF, PP-13, PAPP-A |
PE: (64, 77–84) IUGR: (81, 85–90) PM: (91, 92) GDM: (93–97) |
ELISA(60, 81, 91, 98), colorimetric assay(99–102), automated biochemical analyzers (94), electrochemical impedance spectroscopy (103), electrochemical aptasensor (104), electrochemical assay(105–110), impedimetric assay (111), chemiluminescence(112), SPR(113–116), SERS(117), acoustic wave sensor (118) |
| Exosomes; Mitochondrial DNA |
PE: (119, 120) IUGR: (121) PM: (122) GDM: (123, 124) |
Nanoparticle tracking analysis (125), fluorescent NTA(126); nano-plasmonic sensor(127), fluorometry-based PCR(120, 121) |
| TNF-α and its receptors TNF-R1, TNF-R2; IL-1, IL-6, IL-10, IFN-γ; C-reactive protein |
PE: (128–134) IUGR: (135–137) PM: (138–141) GDM: (142–145) |
ELISA(130, 139, 143, 144), chemiluminescence(146), electrochemical assay(147–153), SPR-based sensor(154, 155), deflection cantilever sensor(156–158), QCM(159), acoustic wave sensor (160) |
Oxidative stress markers.
Oxidative stress (OS) is the imbalance between oxidants and antioxidants (161). It causes a disruption of redox signaling and control and/or molecular damage. OS can be detected based on assessment of markers of lipid peroxidation, such as malondialdehyde (MDA), delta-aminolevulinate dehydratase, and other classic OS biomarkers such as vitamin C, catalase (CAT), and thiols. Activities of delta-aminolevulinate dehydratase and classic OS biomarkers were found to be significantly lower in PE(162) and GDM(163), comparing to normal pregnancies. MDA is usually assessed using thiobarbituric acid reactive substances. Higher MDA levels were found in PE and GDM. Typically, concentrations of protein and non-protein thiol levels, Catalase activity, and vitamin C levels in plasma are determined by spectrophotometry at specific wavelengths(164–166). By-products of tissue oxidation, such as protein carbonyls was found to be increased in maternal serum of PE(52), GDM(62), and IUGR(58). Measurement of protein carbonyls can be performed using ELISA and Pierce BCA Protein Assay kit. Oxidized DNAs such as 8-oxo-7, 8 dihydro-2 deoxyguanosine, are detected in GDM(64, 65) and IUGR(60). OS-induced lipid, such as oxidized low-density lipoproteins (Ox-LDLs), can be used as a biomarker in woman with PE(53), GDM(59), and IUGR(63). Its plasma level can be measured by the methods of spectrophotometry and ELISA methods(53, 167). Determining the level of fetal cellular mRNA, such as soluble vascular endothelial growth factor (VEGF) receptor −1 (sFlt-1), and endoglin (sENG), as well as the pro-angiogenic factors such as placental growth factor (PlGF) in blood, can be another way of OS measurement (168). The serum level of sFlt-1 was found to be higher in PE(77), IUGR (85), GDM(93), but lower(91) or higher (92, 169) in primigravida and associated with PM. ELISA and Mann–Whitney U-test methods were used to measure this biomarker value (91, 93). ENG serum level was found to be higher in PE(77) and IUGR(85). PlGF was found to vary among in PE(77), IUGR(86) and GDM(94). More comprehensive discussion on the cellular sources and biomarkers of OS and production of free radicals in human and animal models of placental pathologies can be found in the review by Aouache (170).
Although the OS role in PM is still unclear, some studies claimed the relations between OS biomarker and PM, where superoxide dismutase, Catalase levels were found to be lower in women with PM. ELISA-based methods are the most commonly used for detection of these biomarkers(51, 53, 57, 59, 61, 66). Other standard methods for analyzing these biomarkers include chromatography(67), HPLC(68), electrochemical assay (69, 70), chemiluminescence (71–73), UV spectrophotometry for thiobarbituric acid reactive substances (63), and automated biochemical analyzer(94). New sensors based on SPR(75), SERS(76), electrochemical impedance (74), and colorimetry (55, 56, 61) have also been developed.
Angiogenic factors.
Placental pathologies are associated with abnormal profile of angiogenic factors, including PlGF, sFlt1, and sENG as well as abnormal variations in placental proteins and hormones, such as pregnancy associated plasma protein A (PAPP-A), placental tissue protein 13 (PP-13), human chorionic gonadotropin (hCG), and VEGF. A single biomarker may not be specific to a disease, but the combinational profile of these markers seems to differ among different diseases. PE was found to be associated with higher levels of sFlt-1(171), sENG(172), and angiotensin fragment 1-7 (Ang 1-7) (173) but lower concentrations of Angiotensin I and angiotensin II(174), comparing to normal pregnancy. The ratio between sFlt-1/PlGF showed descent diagnostic accuracy for PE but remained insufficient for clinical application (171). In GDM, angiotensin II cord blood level (82) was higher than normal pregnancy and preeclamptic pregnancy, but Ang1–7 level was decreased(89). Levels of sFlt-1 was found to be increased in IUGR(85). The elevated levels of plasma sENG were associated with other pregnancy complications, such as preterm premature rupture of membranes(175), which makes this marker nonspecific to a disease. The level of PP-13 decreases in first trimester but elevates in second and third trimester in women with PE (79) and IUGR(176). In GDM, PP-13 level increases in second trimester (93) but decreases in third trimester(177). It was found that serum PAPP-A level in the first trimester declined in PE(83, 84), IUGR(90), and GDM(96, 97). Different studies showed higher concentration of hCG serum level in women with IUGR(88) and with PE in the second trimester(78, 178). High concentration of nesfatin-1 and vaspin as biomarker was reported in women with GDM (179). Lower concentration of VEGA in serum has been reported in PE (60, 80), PM(91) and GDM(95) pregnancy disorders.
Immunoaffinity-based colorimetry, such as ELISA, remains as the gold standard to characterize the levels and concentrations of these biomarkers(60, 81, 91, 98). Other related approaches employed electrochemical impedance spectroscopy(103) and electrochemical aptasensor(104) to measure sENG levels. Various sensor techniques, such as electrochemical assay(105–107), SPR(113), SERS(117) and chemiluminescence(112), were applied for hCG detection. In detection of VEGF, besides ELISA, colorimetric assay(101, 102), and electrochemical assay(109, 110), SPR(114, 115) and impedimetric assay (111) were also employed. Novel sensing approaches, such as acoustic wave(118) and SPR (116) were applied to detect PP-13 and PAPP-A levels.
Placental debris, circulating extracellular vesicles and subcellular fragments.
Excessive OS in placental cells elevates the release of placental debris, vesicles, and subcellular fragments (e.g. cell free DNA, RNA, and miRNA) into maternal circulation. Extracellular vesicles (EVs) can be categorized by their size into microvesicles (0.1-1 μm), exosomes (~100 nm), apoptotic bodies (0.8-5 μm) and nanovesicles (40 - 120 nm)(180). These EVs carry active molecules as markers for tracing their origins and evaluation of placental pathogenesis. Exosome concentration level is increased in PE(119) and GDM(123). Common techniques to characterize size and concentration of EVs are based optical detection, such as nanoparticle tracking analysis (125, 126, 181–183), dynamic light scattering (184–186), TRPS(187–189), and single-EV analysis by fluorescent antibody staining(190). Extensive methodology guidelines for the study of EVs refers to reference (191). For analysis of individual EVs, nanoscale flow cytometry is a powerful technique. By optimizing the configuration of the commercially available BD influx flow cytometer, the detection and analysis of fluorescent cell-derived vesicles of ~100 nm was achieved(192). Exosomes were successfully differentiated from microparticle/microvesicles by distinguishing exosomal markers using nanoscale flow cytometry(193). Multiplex nanoscale flow cytometry is suggested to be a solution for the comprehensive assessment of concentration, size, and composition of EVs, considering their complex profile and variable subsets. Among these emerging high-resolution nanoscale flow cytometric techniques, NanoFACs developed by Morales-Kastresana et al.(194) utilizes multiparametric scattered light and fluorescence measurements along with sample enumeration for comprehensive evaluation of various types and subsets of EVs by evaluating multiple labels on individual cells, which are not assessible using other methods (195). A more in-depth review on the multiplex nanoscale flow cytometry technology and its applications in the study of placental EVs is made by Morgan(196). Further analysis of the lipid composition of exosomes may provide potential markers of certain pathologies. Serum level of lipid may exhibit abnormal profile, in terms of high-density lipoprotein cholesterol (HDL-C), non-HDL-C, and triglyceride, through the trimesters of pregnancy that is complicated by PE (197–199). The triglyceride level is measured by using the enzymatic colorimetric method (198). Anand et al. investigated lipid biomarkers for PE by mass spectrometer(199). Subcellular fragments, such as mitochondrial DNA released into maternal circulation can be assessed using real-time quantitative polymerase chain reaction (PCR) (120, 121, 124) and nano-plasmonic sensor(127). Maternal blood mitochondrial DNA level was found to be significantly higher in PE(120), about 3 fold higher in women with IUGR (121), but lower in GDM (124). In addition, a linear trend (P for trend=0.03) was found in higher odds of mitochondrial DNA copy number with increasing quartiles of preeclamptic pregnancy comparing to normal pregnancy (120).
Inflammatory biomarkers.
Placental EVs in blood can interact with maternal endothelial cells and potentially transfer their contents, leading to transcriptome alterations and inflammation. Inflammatory biomarkers are classified into four groups (200, 201) , including cytokines (TNF-α, IL-6, IFN-γ), chemokines (CCL2, CCL3), cell adhesion molecules (ICAM1, ICAM3), and acute phase protein. TNF-α was found to be significantly higher in PE(128, 129), IUGR(135) and GDM(142). C-reactive protein serum concentration level was found to increase in PE(133, 134) and GDM(142) but no significant difference in IUGR(137), comparing to normal pregnancy. IL-6 serum level was elevated in PE(131, 132), IUGR(135), PM(202), and GDM(90). Higher blood level of IFN-γ, IL-4 and IL-10 were found in women with PM (139). These markers are typically measured by ELISA method (142). Studies also indicated that soluble TNF receptors (TNF-R1 and TNF-R2), IL-10 and G-CSF may represent markers of PM-related placental dysfunction (140, 141). It was suggested that a combination of multiple biomarkers involved in different pathophysiological pathways of PM may increase specificity for disease diagnosis(92). Characterization for inflammatory biomarkers can be performed using standard assays such as ELISA(130, 139, 143, 144), chemiluminescence(146), QCM(159), and electrochemical assay(147–153), as well as novel biosensing techniques, such as deflection cantilever beam(156–158), acoustic wave (160), and SPR (154, 155).
INNOVATIONS IN BIOSENSING TECHNOLOGIES
To reduce the interferences from background, biosensors can benefit significantly from a series of sample preparation processes. Microfluidics is a technology that deals with the behavior, control and manipulation of fluids in engineered, microscale environment. Unique phenomena in microfluidics, such as laminar flow, diffusion, inertial focusing due to the scaling down have enabled various novel strategies for transport, control, and manipulate bioparticles in fluids of ultra-small volumes. Microfluidics can be either standalone or integrated with transduction components on-chip for detection and quantitation of analytes in a biological or biochemical complex.
On-chip sample preparation and detection.
Circulating biomarkers of placental pathologies exist in complex bodily fluids. Detection of those biomarkers using current technologies in healthcare diagnostics often require large sample volumes of milliliters and high cost for preparation prior to analysis. On-chip sample preparation using microfluidics strategies have shown promising opportunities to selectively extract, preconcentrate and label target analytes automatically from a complex mixture of low sample volume and at low cost (203).
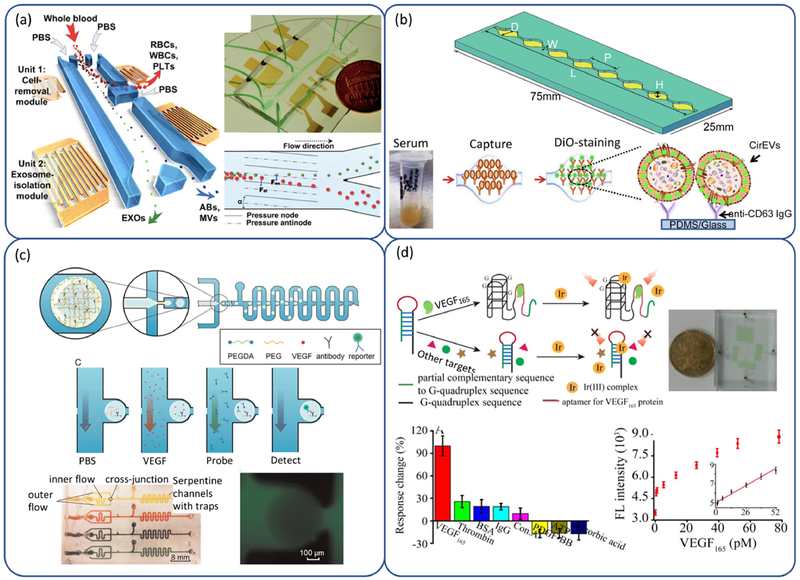
A major obstacle to use exosomes as a predictive and diagnostic marker of placental pathologies, lies in a lack of isolation and characterization assays that can provide both cell- and size-specificity(196). Current methods for isolation of exosomes are largely based on the differences in size and density between exosomes and other EVs and cells, using ultracentrifugation, precipitation, filtration, size-exclusion chromatography. They do not meet the nanoscale resolution needed for exosome isolation due to a lack of isolation efficiency, recovery rate, and yield. Some residuals from precipitation matrix in commercial kits are likely influence the biological activities and characteristics of collected exosomes(204, 205). To overcome the size limitation in isolation of exosomes, engineered, nanoscale features of comparable size to exosomes of interest were integrated into microfluidic channel to physically trap the exosomes(206–208). They often yield uniform capture of exosome subpopulations given the sophisticated fabrication process. Recovery of captured exosomes usually require slow dissolving the nanoscale features(206) and result in low yield rate and slow processing(208). Considering the non-continuous isolation limit of size-based exosome separation designs, there has been a growing interest in high throughput separation in continuous flow. A novel integration of acoustics to microfluidics provided a label-free method to isolate exosome subpopulations in a cascade manner(209). The device consists of a microscale cell-removal module and an exosome-isolation module (Fig 2a). The first module removes larger blood components with a 99% yield of 110 nm particles, followed by EV subgroup separation in the second module that isolates exosomes with a purity of 98.4%. Capability of this device was demonstrated by isolation of exosomes and EVs from whole blood as well as from a primary human trophoblast-derived mixture.
Fig 2.
(a) Schematic illustration and mechanisms underlying integrated acoustofluidic device for isolating exosomes (209). (b) The features of a single channel and illustration depicting the scheme of exosomes capture and analysis procedure used in (212). (c) Schematic of the detection system including microgel droplet generation and positioning and on-chip protein assay, and prototype of the array scalability. (Reprinted from Ref. (233) with permission of Elsevier.) (d) Schematic illustration of VEGF165 assay based on G-quadruplex probe and the analysis of VEGF165 based on DNAG1’ and complex 1. (Reprinted from Ref. (238) with permission of Elsevier.)
For isolation of placenta specific exosomes, immunoaffinity-based approach can be integrated to microfluidics. A unique placental protein-specific marker is placental alkaline phosphatase, an integral membrane protein (enzyme) (210, 211). It was also found that multiple biomarkers such as syncytiotrophoblast-specific RNAs and proteins (miRNAs, pro-apoptotic molecules FasL and TRAIL, and TGFB) can be detected in placental alkaline phosphatase positive exosomes in maternal peripheral blood and urine. These biomarkers may be used to coat the microchannel walls for isolation of placental exosomes by immunoaffinity, similar to the schemes (212, 213) for capture of exosomes from serum and cell culture medium via the selective binding to anti-CD63-coated microfluidic channel surface (Fig 2b). Incorporation of antibody labelled magnetic beads into the microfluidic chip allowed isolation of exosome subpopulations with a ultra-small volume (30 μL) plasma samples (214). Miniaturization of downstream processes and analyses components, such as chemical lysis and chemifluorescene assays, enabled an integrated on-chip immunoassay for protein analysis of the isolated tumor exosomes (215). The same technique may also be used for isolation of placenta-derived exosomes.
Fetomaternal transfer probably occurs in all pregnancies and the migrated fetal cells during pregnancy persist long in maternal circulation (216). Circulating nucleated cells, such as fetal nucleated red blood cells (fnRBC) and trophoblast cells provide a source of genetic information about the fetus, which can be used as biomarkers of multiple placental pathologies. For example, fetal cellular mRNA expression of anti-angiogenic and pro-angiogenic factors indicates the OS level. Since fnRBCs are extremely rare in maternal blood (one per millimeter), enrichment of fnRBCs is challenging. Microfluidic approaches were employed in combination with immunoaffmity method to effectively isolate fnRBCs from maternal blood. A microfluidic platform “Cell Reveal”, was developed by Huang et al. (217). It allows capture of rare fnRBCs and circulating trophoblasts using the specific antibodies immobilized on the Si nanostructure with a porous morphology. The captured fnRBC and human extravillous trophoblasts in every 4 mL of maternal blood were about 14–22 cells and 1–44 cell, respectively.
Several techniques have been developed to improve the immobilization of antibody and the interaction efficiency between fnRBCs and the immobilized antibodies. Wei et al.(218) designed a multifunctional NP-based microfluidic device for enhanced isolation and release of nfRBCs. The device utilizes a curved fluidic channel and herringbone structures to create chaotic mixing for enhanced interaction between fnRBCs and immobilized antibodies. In addition, the microchannel was coated by anti-CD147 conjugated gelatin NPs, which enhances antibody immobilization for nfRBC capture, and importantly, allows gentle release of the captured fnRBCs by dissolving the gelatin NPs with enzyme for subsequent off-chip analysis. This method provided high capture efficiency (> 80%) and release efficiency (~89%) with a considerable purity (~85%) and a high cell viability (>90%). The fetal origin of the isolated target cells was confirmed by detecting different fetal chromosomal aneuploidy cases including Trisomy 21 syndrome, Trisomy 13 syndrome, Klinefelter’s syndrome and XXX syndrome using fluorescence in situ hybridization technique. Other microfabricated features, such as rotated triangular microposts arranged with an offset from previous row has been utilized (219) to enhance the interaction frequency between fnRBCs and immobilized antibodies. In this design, particles larger than the critical diameter would continually contact the antibody-coated microposts in different streamlines while the smaller particles would remain in the first streamline and flow through channel. The platform was able to detect fnRBCs in as little as 2 mL peripheral blood as early as 7 weeks after conception at a capture efficiency of 90%. A unique method to amplify the sensing signal is to use oriented immobilization, in which the capture probes are immobilized with their recognition sites arranged and exposed to the sample solution, such as in the case of affinity ligand to the N/C-terminus of chimeric antigen (220).
Increased number of large fragments of syncytiotrophoblast, which is formed by the fusion of progenitor cytotrophoblasts into a continuous cell layer, is observed in pregnancy complicated by PE(221) and IUGR(222), comparing to healthy pregnancies(223). Similar to fnRBCs, the number of circulating trophoblasts in maternal blood is as low as 1-5 trophoblasts/mL(224). The large size of trophoblastic cells provides a mechanism for isolation by microfluidic approaches. Winter et al.(225) developed a slanted microfluidic platform for efficient isolation of trophoblastic cells from maternal peripheral blood. The slanted microchannel has a trapezoidal cross-section, with which the operation of the device only needs a single inlet in contrast to the rectangular-cross-sectioned channels that require a sheath flow when the particle concentration is high. Along with a high throughput of processing 20 mL blood in less than 45 min, a high recovery efficiency (> 79%) was achieved using a trophoblastic cell line and further a maternal blood sample. Diagnosis of fetal trisomy 21 was achieved using fluorescence in situ hybridization with automated laser scanning cytometry.
Placental hormone hCG may serve a potential role in the diagnostic evaluation of pregnancy disorders (226). Detection of hCG is typically achieved by antibody-based immunoassays. A new capacitive immunosensor was developed via covalently coupling anti-hCG to epoxysilane-modified glassy carbon electrode, which allows the label-free quantitative detection of hCG in serum (227). A piezoelectric quartz-based immunosensor was developed by combining self-assembled monolayer based-antibody immobilization technique and a 2x5 piezoelectric microarray(228). This sensor allows for simultaneous detection of hCG in serum or urine sample in the range of 2.5–500 mIU/ml with coefficient of variability less than 5.0% within 40 min. Another immunosensor design was developed based on the electrochemical sensing of the reaction between the primary antibody that was immobilized on the screen-printed carbon strips and the hCG in the gold NP-labelled hCG immunocomplex (229). The use of disposable electrode strips allowed for cost-effective detection of hCG in a small volume of antigen sample (2 μL). Graphene oxide-peptide-based SPR biosensor was developed for label-free detection of hCG in pregnancy disorder (113). In this method, modified peptide aptamer was attached to the graphene oxide surface for an enhanced target of the hCG protein assay using graphene oxidie-peptide specific interactions. To overcome the limitation in the detection sensitivity due to the randomly immobilized antibodies, Chung et al. (230) developed a method to properly orient the anti-hCG via a nondenaturing linker specific to the Fc fragment so that the ligand binding sites are densely packed and exposed to the hCG solution for maximum sensitivity of detection. Other microfluidic strategies were employed to separate plasma from whole blood for improved sensitivity of hCG detection, such as sheath flow (231) and inertial focusing (232).
An example of microfluidic enhanced VEGF detection using capture anti-VEGF antibody immobilized porous poly(ethylene glycol) diacrylate hydrogel microspheres was demonstrated by Zhao et al. (233). The processes of antibody encapsulation, trapping, and flow perfusion were incorporated in a single device under fluorescence microscopy and the fluorescent intensities were analyzed (Fig 2c). Compared to conventional technologies, the detection sensitivity was as high as 0.9 pg/mL and the incubation time was only 1+ hours. Nucleic acid aptamers that bind specific molecular targets are also applicable in VEGF detection. Since the protein-nucleic acid interaction happens shortly once a DNA/RNA conformational transition has been promoted by protein molecules (234, 235), a high resolution of millisecond or even microsecond (236, 237) is required for the detection approach. This remains a huge challenge by the existing methods. A novel method was developed by Lin et al. (238) for detecting aptamers-VEGF interaction in different transient states during kinetics process through microfluidic hydrodynamic focusing and diffusive mixing. A combination of microfluidic techniques and G-quadruplex luminescent probe was proved to be an effective way for quantitative analysis of VEGF165 from cell metabolism, especially when the reagents or samples are limited. In the presence of VEGF165, the hairpin DNA was opened, and G-quadruplex structure would be formed. Then the special Irdium complex 1 would bind to G-quadruplex structure and generate to strong luminescence. In the absence of VEGF165, hairpin structure can not be opened, resulting in weak luminescence (Fig 2d).
Multi-biomarker characterization.
Significant heterogeneity in biomarkers of various pregnancy complications exists. Profile of theses markers vary among patients and through the duration of pregnancy. In PE, PlGF was found as the best biomarker for prediction of severe early-onset PE; a combination model of multiple biomarkers together with clinical measures performed better in PE prediction(239) and late onset of PE(240). Current methods for multianalyte detection are largely based on molecular methods such as multiplex ligation-dependent probe amplification(241), DNA microarray for gene expression or SNP detection assays(242), protein microarray(243), and commercially available immunoassays(240). In this respect, directed immobilization of pre-fabricated protein- or antibody-nucleic acid complexes combined with electrochemical detection in multiplexed microelectrode array biochips(244, 245) might be especially uselul for high-throughput analyses of multiple antigens present in samples. Microfluidic design supports integrated analytical steps, parallel operation, and repeatable sample manipulation. Araz et al.(246) achieved multiplexed analyte detection in a single channel by patterning biotin and biotinylated capture reagents in discrete regions along the axis of the microchannel, resulting in a barcode-like pattern of reagents and spacers. With only a single inlet and a single outlet, immunoglobulin specific for HCV-clOOp, HCV-NS3 and HIV-I p24 were detected simultaneously for diseases with high coinfection rates in significantly reduced time frame. Microfluidic devices that couple multiple channels together in a single chip have also been adopted for biomarker detection. For example, Zhou et al.(247) designed a multichannel microfluidic chip embedded with high-sensitivity plasmonic nanocave array sensors for simultaneous detection of multiple biomarkers of pancreatic carcinoma. Several new biosensors were developed that require small sample volume and minimum sample processing(248), such as Micro-Nuclear Magnetic Resonance (NMR)(249, 250), SPR-based sensor(251), and Integrated Magnetic-Electrochemical Exosome (iMEX) Sensor(248). NMR sensor measures the transverse relaxation rate of water molecules in target cells. Improved sensitivity, as few as 2 cancer cells in 1 μL sample volumes of unprocessed fine-needle aspirates of tumors, was achieved using magnetic NPs to label target cancer cells(252, 253). Microfluidics-based NMR showed higher sensitivity for detection of extracellular vesicle biomarkers in comparison to conventional ELISA method(254). Another example is an integrated magnetic-electrochemical sensor, which consists of magnetic selection and electrochemical detection and is capable of profiling of multiple protein markers simultaneously within an hour for analysis of exosomes(255).
Point-of-care biosensors.
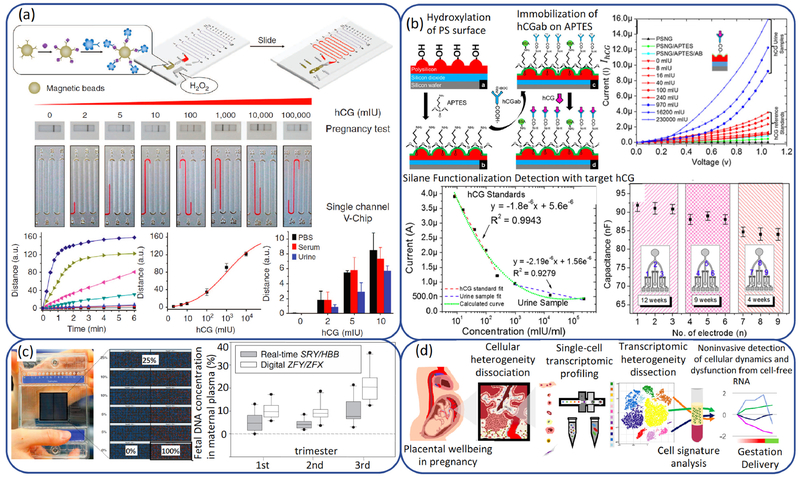
Microfluidics-based point-of-care system, which involves miniaturization and integration of sample preparation processes and sensing functions to achieve multiplexing, automation, and high-throughput analysis, can provide a consistent and economic way for potential early detection and improved care of pregnancy complications. The well-known example of hCG test was invented in 1980s for home pregnancy test (256). On-chip hCG test is normally achieved by immunoaffinity-based colorimetric or electrochemical methods. A multiplexed volumetric bar-chart chip (V-Chip) was developed to detect hCG at high detection sensitivity of 2 mIU/mL (257). The quantitative measure of analyte concentration, which is proportional to the oxygen production by catalase in the chip was visualized by the correlated change in the ink color (Fig 3a). Another example is an on-chip electrochemical immunosensor with a ultra-low detection of 0.28 mIU/mL (258). This sensor utilizes polysilicon nanogap electrodes, which has a 25-nm nanogap to minimize the effect of electrode polarization and achieve a wide linear range of detection (Fig 3b).
Fig 3.
(a) V-Chip hCG detection scheme and results. (Reprinted from Ref. (257) with permission of Nature.) (b) Schematic illustration of chemical functionalization on the PSNG surface, DC amperometric detection of hCG and reproducibility test of signal transduction on PSNG electrodes. (Reprinted from Ref. (258) with permission of PLOS.) (c) Frontal view of a microfluidics digital array with the digital readout of the accuracy experiment and box plots of male fetal-DNA percentages measured with the nondigital real-time SRY/HBB assay and the digital ZFY/ZFX assay. (Reprinted from Ref. (259) with permission of AACC.) (d) Schematic diagram showing single-cell transcriptomic profiling and the dissection of the cellular heterogeneity of human placenta. (Reprinted from Ref. (263) with permission of PNAS.)
DNA-based diagnosis involves sophisticated processes of PCR, electrophoresis and fluorescence scan by trained personnel in well-quipped laboratory. This limits the wide use of DNA-based diagnostics. Microfluidics provides an excellent platform for integration of these processes onto a single chip towards the point-of-care DNA diagnosis. Digital PCR can overcome the low throughout in conventional PCR (one reaction per sample) by separating target nucleic acid across a large number of termed partitions, so that different reactions are carried out in each partition individually. Lun et al. (259) compared the capacity of different PCR techniques in measuring circulating fetal DNA in maternal plasma (Fig 3c). The results demonstrated that digital PCR is about 3.1 times more precise than real-time PCR. Moreover, comparing to the assays based on real-time PCR and mass spectrometry, microfluidic digital PCR revealed the least bias in measuring the concentration of fetal DNA. Other than fetal DNA, selected placenta-specific RNA transcripts have also been reported in the maternal plasma of women with PE (260, 261) and restricted fetal growth(262). Droplet-based single-cell digital transcriptomic profiling is a novel technology for sequencing RNA at single-cell resolution by encapsulating individual cells in microdroplets with hydrogel beads that contain reverse transcription and template-switching oligonucleotides. The RNA molecules in each droplet are tagged by the specific oligonucleotides. By using this technique, Tsang et al.(263) analyzed more than 24,000 nonmarker-selected cells from full-term and early PE placentas, and defined individual cell-type-specific gene signatures (Fig 3d). These results enabled identification of various cellular subtypes in the human placenta as well as the cellular dysfunction of extravillous trophoblast in early PE placentas.
In this respect, a just-published novel Tandem Oligonucleotide Repeat Cascade Amplification assay with an attomolar detection limit, appropriate for detection of specific DNA/cDNA molecules(264), might be a promising and attractive alternative to PCR methods or RNA-Seq. It can detect any specific DNA/cDNA in a complex nucleic acid mixture, is comparable to qPCR in sensitivity, does not require complex instrumentation, and proposed to be significantly cheaper, amenable to multiplexing, and field-friendly with a potential for development of a hand-held device.
NPs-based biosensors.
NPs have high surface-to-volume ratio, which can increase the protein freedom for high reaction activity. Most commonly used NPs include carbon nanotubes (265, 266), gold NPs (267, 268), and carbon nano-composites (269–271). Signal amplification technique using functional nanomaterials have shown to improve the biosensing response besides enzyme catalysis and biology reaction (272). NPs are commonly used to improve the immobilization of bioreceptors. Single walled carbon nanotubes (SWNTs) was used to immobilize PAPP-A on glassy carbon electrode in an electrochemical immunosensor (58). Kim et al. (107) developed sandwich type electrochemical immunoassay platform with NPs to detect the VEGF. Gold NPs were used to immobilize the VEGF antibodies (109). Sezgintürk et al. developed a different type of sensor based on fourth-generation poly (amido amine) PAMAM dendrimers to detect VEGF(273). In a field-effect transistor sensor, carboxylated polypyrrole nanotubes was used to increase the transducer performance in detection of VEGF(274). Rosales-Rivera et al. (275) used Carboxylic-ended bipodal alkanethiol to immobilize the antigen on the surface. Bio-functionalized gold NPs have shown excellent optical properties, ion-exchange ability, stability and biocompatibility to improve the performance of electrochemical (26, 276) and optical immunosensors(113, 277). In addition, metal NPs-based variation of SPR, known as localized SPR, has shown more compact sensing volume and high spatial resolution, limited only by the size of NPs (subwavelength), thus it is more sensitive to molecular binding and requires less complex optical hardware. A newly developed SPR-based sensor, nPLEX, which comprises arrays of periodic nanoholes patterned in a metal film, has shown capability in label-free detection and molecular profiling of exosomes (127). Optimized nanoholes geometry were investigated(127), which makes nPLEX more accurate than ELISA. Many other sophisticated biosensors can be potentially used for detection of biomarkers of placental pathologies. An example is a recently developed SERS-based microfluidic sensor, which can provide automated, reliable detection of anthrax biomarker, poly-γ-D-glutamic acid (PGA) in solution(278). Analysis was based on the competitive reaction between PGA and PGA-conjugated gold NPs with anti-PGA-immobilized magnetic beads. The limit of detection for PGA in serum was as low as 100 pg/mL.
ON-CHIP SENSING IN BIOENGINEERED PLACENTAL MODELS
Organ-on-a-chip technology is a relatively new field in which the properties of microfluidics are utilized within a biomimetic cell culture model. Merger of microfluidics and cell culture have generated miniaturized organ-on-a-chips for the study of various biological and pathophysiological processes of human, including brain(279, 280), heart(281, 282), liver(283, 284), lung(285), and multi-organs(286, 287). These organ-on-a-chip devices showed great promise and potential in pre-clinical drug testing and toxicology applications, as well as fundamental pathophysiological research. Research regarding “placcnta-on-a-chip” has been few. In addition, biosensors and organ-on-a-chips remain distinct fields yet. They start to overlap recently, which will offer promising solutions for real time detection of the onset, progression of pathological processes in human placenta, as well as a means to monitor and quantify the placental responses to various external stimuli and therapeutic treatment.
Mass transfer across placental barrier.
The essential function of the placenta is to serve as the interface between the maternal and fetal circulations and regulates the transfer of oxygen, nutrients, and waste products. When endogenous and exogenous substances are present in the maternal blood, the effect of this exposure on the fetus depends on the transport processes of these substances through the placental barrier (288, 289). Some placental pathologies such as PE are associated with the abnormal changes of the barrier structure (290, 291). The basic structure of placental barrier is a thin interstitium with syncytiotrophoblast on one side and the fetal capillary endothelium on the other side (Fig 4a). To recapitulate the architecture and physiological microenvironment critical to placental barrier function, a common technique is to co-culture trophoblast cells and endothelial cells on a semipermeable membrane in a microfluidic environment.
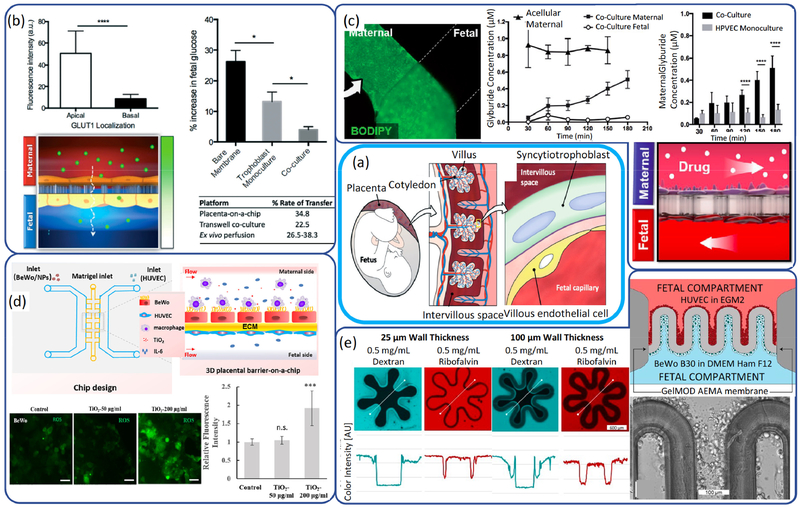
Fig 4.
(a) features of the human placenta and multilayered membranous structure of placental barrier (295). (b) Glucose transporter expression and quantitative analysis of glucose transport in the placenta-on-a-chip system (295). (c) The transporter-mediated efflux of glyburide and the transport of maternal glyburide. (Reprinted from Ref. (296) with permission of RSC.) (d) Schematic diagram showing design of 3D placental barrier-on-a-chip microdevice, and response of 3D placental barrier exposed to NPs. (Reprinted from Ref. (297) with permission of Elsevier.) (e) Schematic illustration of two-photon polymerization structured placental barrier model, and quantitative study of semi-permeability of hydrogel membranes produced with two-photon polymerization techniques. (Reprinted from Ref. (298) with permission of Whioce.)
Miura et al.(292) created a placenta-on-a-chip by culturing confluent layer of BeWo cells and human umbilical vein cells on each side of a collagen vitrigel membrane for the study of the transcellular compound transport in placenta. It was verified that fluid shear stress serves as a trigger for microvilli formation in human placental trophoblastic cells through activating vanilloid family type-6 calcium ion channel (293). Lee et al.(294) used trophoblasts (JEG-3) as the maternal cells, seeded onto one side of the collagen vitrigel membrane and human umbilical vein cells seeded on the other side, and cultured under dynamic flow conditions to form confluent epithelial and endothelial layers in close apposition. Glucose transport across the trophoblast-endothelial interface over time was tested by a glucose analyzer. The transfer rates measured in this model ranged from 22.6 to 33.9%, corresponding to the metabolic consumption rate from 66.1 to 77.4%.
In more recent researches, commercial semipermeable polycarbonate membrane containing submicron to microscale pores were adopted as a replacement for collagen vitrigel membrane to simplify the fabrication without affecting the functions. Blundell et al. (295) used the co-culture model to induce progressive fusion of trophoblast cells and to form a syncytialized epithelium that resembles the syncytiotrophoblast (Fig 4b). In this model, dense microvilli were successfully formed under dynamic flow. Furthermore, the expression of membrane transport proteins such as glucose transporters were reconstituted, which is critical to the barrier function. The measured glucose transfer rate was close to that measured in an ex vivo human placenta model, demonstrating the capability of this co-culture model to recapitulate native function of the placental barrier. Importantly, it provides a new approach to screen and predict the effects of the maternally administered drugs on the developing fetus. Blundell et al.(296) used the gestational diabetes chug glyburide as a model compound to study the native function of efflux transporters and to mimic the limited placental transfer of a maternally administered drug (Fig 4c). The results showed that the transport rate of glyburide was very close to that measured in ex vivo human placenta methods.
Recently, fabrication of interface closer to the in vivo barrier than a single flat membrane has attracted the researchers’ attention. Yin et al.(297) designed and fabricated a microdevice for the construction of in vivo-like three-dimensional (3D) placental barrier (Fig 4d). The maternal channel and fetal channel in this design are arranged in parallel and separated by a 3D extracellular matrix channel that serves as a near-physiological scaffold for the growth of placental cells. With this improved model, they further explored complicated placental responses to NPs exposure. With the exposure to titanium dioxide NPs, a series of placental responses such as OS, cell apoptosis, barrier permeability, and maternal immune cell behavior were investigated. NPs accumulated in the maternal side and finally transported into the matrix in the middle channel. It was also observed that inflammatory-related cytokine transported from maternal side into fetal side. It was demonstrated that placental barrier integrity and maternal immune cells were greatly influenced even with low concentrations of NPs. Mandt et al.(298) produced the microstructure of the membrane by using a 3D two-photon polymerization printing method (Fig 4e). The structures can reach to a resolution as high as a few microns. Furthermore, complex membrane structures can be achieved to better mimic the membranous structure of the placental membrane. Transcellular transport processes were investigated with the placenta model and the results showed that small molecules in the size of glucose can diffuse across the barrier while larger molecules did not permeate.
In all the existing placental mass transfer studies, the transfer of molecules was detected by fluorescence staining and microscopic image analysis. For example, BODIPY-conjugated glyburide was introduced into the upper maternal channel and the fluorescence of perfusate retrieved from the fetal compartment was monitored to investigate glyburide transport (210, 211). Exceptionally, glucose transport was measured by glucose analyzer with the collected outflows. Some novel techniques regarding on-chip glucose sensing can be embedded to the existing placental glucose transfer models. A widely-adopted mechanism for quantifying glucose concentration is by measuring the concentration of hydrogen peroxide as a product of the glucose-oxidase reaction. Hydrogen peroxide can be measured in an electrochemical assay patterned as thin-film electrodes on the microchannel substrate (299), or recognized by color change after integrating with colorizing agent (300). A more recent example was reported by Wang et al. (301), which provides a non-enzymatic and cost-effective way for on-chip sensing of glucose in placenta model. The novel flexible electrochemical sensor was made of copper and graphene, and the copper nanoflower structure ensures the high sensitivity and selectivity in glucose sensing.
Trophoblast migration, OS, and placental inflammation.
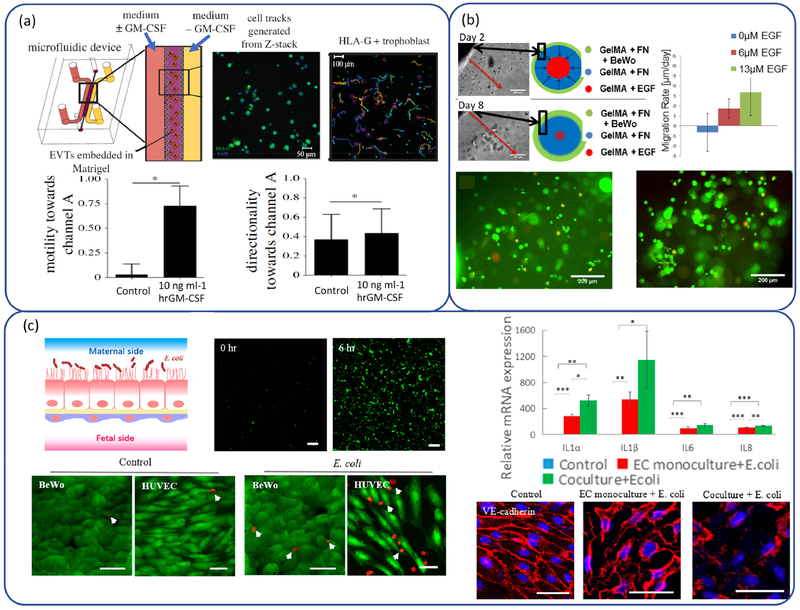
In PE, cytotrophoblast invasion of the interstitial uterine compartment is often shallow. The exact pathobiology of PE remains poorly understood, largely due to a lack of effective experimental models. Adapting from an assay of fibrosarcoma cancer cell migration, Abbas et al.(302) performed the study of the migration of primary human trophoblast cells. The device consists of a central human extravillous trophoblasts-containing matrigel channel and two medium channels distributed symmetrically aside (Fig 5a). Gradient of granulocyte–macrophage colony-stimulating factor was generated by the medium channels to increase trophoblast migration. Dynamic readouts on cell migration such as directionality, motility and velocity were tracked in the 3D microenvironment by real-time, high-resolution imaging. Compared to previous work, this assay adds greater quantification on granulocyte–macrophage colony-stimulating factor as a chemoattractant for human extravillous trophoblasts migration. A smartphone-based imaging platform was developed by Yang et al. (303)for performing cell migration assay. The processes of imaging and data analysis were accomplished by the smartphone app without the need for microscope or computer.
Fig 5.
(a) Schematic illustration of model for trophoblast invasion and the migration of human extravillous trophoblasts. (Reprinted from Ref. (302) with permission of The Royal Society.) (b) Model design of trophoblast migration and the migration rate of BeWo cells under different concentrations of EGF. (Reprinted from Ref. (304) with permission of ACS.) (c) Schematic of bacterial infection in placenta and placental inflammatory responses to E. coli infection. (Reprinted from Ref. (307) with permission of ACS.)
Kuo et al.(304) developed a 3D, bioengineered placenta model to study and quantify cell migration, mimicking biophysical properties, geometry of the biomaterials, and the chemotactic gradients in native tissue (Fig 5b). The effect of epidermal growth factor on the migratory behavior of trophoblast and human mesenchymal stem cells were evaluated by the simplified bioengineered placenta model, which is based on a cylindrical gelatin methacrylate hydrogel. It was found that BeWo cell migration rate increases in response to higher epidermal growth factor, indicating the sophisticated 3D placenta model could be utilized as a powerful tool to test and develop treatments for PE.
Inflammatory processes in placenta are complicated and tightly associated with micro-organisms and host immune responses to non-replicating antigens. Conventional cell culture-based and animal models are not efficient in the study of the underlying mechanisms of placental inflammation (305, 306). Microfluidic co-culture models provide a useful tool for the study of placental inflammatory responses. A pioneer study was to study placental inflammatory responses to bacterial infection(307). E. coli was selected as the model bacteria and applied to the maternal side in order to induce the acute inflammation of the placental membranes(308). Inflammatory responses were tracked by examining inflammatory cytokines at mRNA level in trophoblast cells. The results demonstrated that bacterial infection adversely affected both maternal responses and matemal-fetal communications (Fig 5c).
On-chip sensing of inflammatory cytokines has been of interest since the emerging of microfluidic techniques. Placenta inflammatory cytokines TNF-α and IFN-γ were successfully detected by aptamer-based electrochemical assays (309, 310). By imbedding this technique with placenta model, high-throughput anti-inflammatory drug screening platform can be established in the future. In this respect, many established EI-based biosensors (311) that have been used for cell migration assays, inflammatory response(312, 313) and barrier functions(314) can be potentially integrated in the placental pathology models. The principle of electrical impedance is label-free and real time. It can limit the interferences to the biophysiological processes in placenta model. An example is to measure changes in electrical impedance as endothelial cells attach to and spread on gold microelectrodes, as well as invasion of endothelial cells by malignant tumor cells (315). Impedance signal was correlated to the attacked endothelial junctions by tumor cell invasion, retraction of endothelial layer and replacement by tumor cells. Similarly, impedance signal may also be used to monitor placental inflammatory responses.
Placental malaria.
Pathological basis of PM is sequestration of infected erythrocytes and consequent inflammation in the placental villi(316). The interface between maternal blood and villi interior is “multinuclcar giant cell” syncytiotrophoblast where nearly the entire matemofetal exchange takes place(317), as well as infected erythrocytes sequester during PM (318). It is assumed at present that only one member of the Plasmodium falciparum Erythrocyte Membrane Protein 1 (PfEMP1) family of adhesins, VAR2CSA, which binds to placental receptor chondroitin sulfate A (CSA) that expresses on the surface of syncytiotrophoblast, is playing a crucial role in PM (319–321). Several studies clearly demonstrated that cytoadhesion directly affects vascular inflammation (322, 323). This, in turn, contributes to disease severity (324), plays key role in placental and cerebral malaria, and is characterized by induction of adhesion molecules (like ICAM1) and pro-inflammatory cytokines, and vascular permeability caused by disruption of endothelial cell barrier integrity (325–327). Further, sequestration of Plasmodium falciparum infected erythrocytes (IEs) on this surface can lead to macrophage infiltration into the intervillous space, local inflammation, and pathological changes in the syncytiotrophoblast (316, 318). The localized inflammatory processes induced by IE sequestration (318, 328) are implicated in LBW and other poor outcomes during PM (reviewed in (329, 330)). Incidence of PM in malaria endemic areas decreases with parity (331), as specific antibodies (Abs), that prevent IE sequestration in the placental villi (332, 333) through chondroitin sulfate A (CSA) (334–336), develop. This, in turn, reduces placental inflammation and positively correlates with birth weight (332).
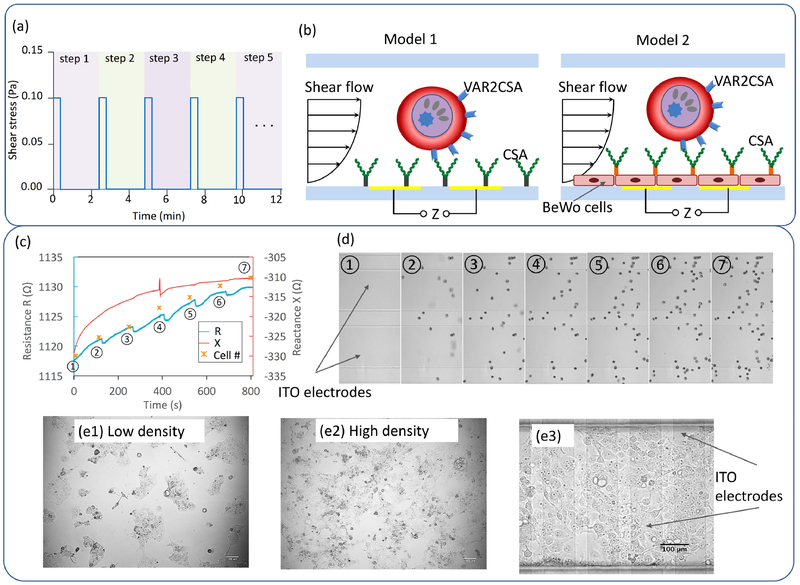
Despite the intensive prior work, the precise molecular details of placental pathology in PM are still poorly understood. We developed a microfluidics-based placenta model with embedded microelectrodes to mimic and quantify the processes that takes place during PM, including interaction of IEs with the CSA receptors (Fig 6). The apical surface of the syncytiotrophoblast has microvilli (brush-border) expanding its surface to ~12.5 m2 for extensive molecular exchange (337). Clearly, at present, it is very difficult to recapitulate this tissue architecture in full in in vitro models. Therefore, some simplification in the models is expected. To model syncytiotrophoblast – maternal blood interface, we used the villous cytotrophoblast BeWo cell line, which is the most extensively used in vitro model of placental functions. It reveals most of the characteristics of cytotrophoblast cells, including expression of normal receptors and syncytial fusion (338). Our own results have demonstrated expression of megalin and Dab2 on the plasma membrane of these cells, two highly abundant proteins of the term placenta, suggested to play an important role in matemo-fetal exchange (339). Other cytotrophoblast cells have also been used to model syncytiotrophoblast (338). Cytotrophoblast BeWo cells were primarily seeded into the collagen pre-coated microfluidic channel and incubated for 24 hours to form a monolayer, which expresses CSA to mimic the surface of the intervillous space of the placenta. With precisely controlled shear flow, IEs were introduced into the microchannel at a physiologically appropriate flow rate, simulating the blood flow in vivo (340). Comparison between the simultaneously recorded electrical impedance and microscopic imaging of cell transportation and adhesion events in the developed placental model allowed us to study the placental malaria in a real-time, quantitative way. The strength of the interactions between the IEs and the receptors can be measured by quantitation of the applied variable shear stress. The capability of screening for anti-adhesion drug of this impedimetric platform was demonstrated by treating the attached red blood cells with the anti-adhesion compounds, which we characterized previously in a static model(341).
Fig 6.
(a) Schematic illustration of stepwise control of shear flow. Shear flow was controlled by a programmable syringe pump as repeated 20 seconds of a constant flow rate corresponding a wall shear stress of 0.1 Pa followed by a stopped flow for 2 min. (b) Schematic illustration of impedimetric sensing of adhesive interaction of IEs and CSA receptor or BeWo cells under flow conditions. (c) Impedance measurement of the IE attachment on CSA coating. Increased resistance (the real part of impedance, R) and reactance (the imaginary part of impedance, X) were observed as the accumulation of attached IEs; (d) time sequence microscopic images of IE attachment. (e) BeWo cell culture (e1, e2) in flask and (e3) in microchannel.
Sequestration of IE on this surface can lead to macrophage infiltration into the intervillous space, local inflammation, and pathological changes in the syncytiotrophoblast including lesions (316, 318). These processes, in turn, may lead to reduced matemofetal exchange and reduced fetal growth during gestation, and finally to low-birth-weight and other poor outcomes including premature birth and PE (329, 342, 343). These pathological changes link PM to other placental pathologies, described above. At the molecular level, recent insights into mechanisms of the placental pathological processes during PM suggest involvement of various pathways, including angiogenesis (344, 345), insulin-like growth factor axis (346), mammalian target for rapamycin (347)(all extensively reviewed in (316, 330)), and megalin-Dab2 axis (339). This diverse set of involved pathways implicates not only affected nutrient exchange but also affected signaling between mother and fetus. Nevertheless, how changes to these pathways, induced by IE sequestration and local inflammation, contribute to the placental and fetal dysfunction during PM are still poorly understood. Our placenta-on-a-chip model, which recapitulates some of the placental structures and PM processes, may contribute to a better understanding of these pathological processes and to potential treatment.
CONCLUSION
Enormous innovations have been made in biosensing tools for detection of circulating biomarkers in bodily fluids for disease diagnosis, with a great effort being made in cancer. Many biomarkers are present in various pathophysiological processes, such as EVs, exosomes, inflammatory factors, and angiogenic factors, the corresponding biosensing designs can be adapted to detect those biomarkers of common placental pathologies. Some important developments have been made in microfluidics and nanomaterials, which have shown great potentials for sensitive measurements of multiplexed analyte detection from small volume and low concentration complex samples. Important trends include integration of biosensing transduction into microfluidics platforms which provides automatic sample preparation, separation and labelling on the same sensor platform, utilization of NPs- or nanofeatures for improved sensitivity and performance all integrated on a single chip, as well as point-of-care solutions for monitoring biomarker profile during the pregnancy. Additionally, a new trend is to develop placenta-on-a-chip devices by coculturing cell lines in well-controlled microfluidic platform to create various placenta disease models for a better understanding of complex interferences between placental pathologies and physiological processes. In this respect, recent advances into differentiation of pluripotent stem cells into trophoblastic cells (348, 349) may further expand a pool of and improve on placental models and application of biosensors and placenta-on-a-chip to study human placental pathologies. Significant efforts have been made in the study of trophoblast migration, placental inflammatory response, oxidative stress and mass transfer across the placental barrier. A limitation in current research field is the off-line measurement of analytes and processes using conventional optical and electrochemical sensing tools. Otherwise, detection of some biological processes relies on live-cell imaging techniques. An attractive method is to integrate label-free biosensing functions, such as electrochemical, EI, SERS or SPR-based techniques into the placenta-on-a-chip systems for real time, minimally invasive monitoring of the pathophysiological placental processes.
ACKNOWLEDGEMENTS
Research reported in this paper was supported by the Eunice Kennedy Shriver National Institute of Child Health and Human Development under award 5R21HD092779 and by the National Institute of Allergy and Infectious Diseases under award 1R21AI137721. All authors have read the journal’s policy on the disclosure of potential conflicts of interest and have none to declare. All authors have read the journal’s authorship agreement and that the manuscript has been reviewed by and approved by all named authors.
Abbreviations:
- PE
Preeclampsia
- GDM
Gestational Diabetes Mellitus
- IUGR
Intra-uterine growth restriction
- PM
Placental malaria
- EV
Extracellular vesicle
- ELISA
Enzyme-linked immunosorbent assay
- SPR
Surface Plasmon Resonance
- SERS
Surface Enhanced Plasmon Resonance
- NPs
nanoparticles
- EI
Electrical impedance
- TRPS
tunable resistive pulse sensing
- QCM
Quartz Crystal Microbalance
- OS
Oxidative stress
- MDA
Malondialdehyde
- PlGF
placental growth factor
- sFlt-1
Soluble VEGF receptor - 1
- sENG
Soluble endoglin
- PAPP-A
Pregnancy associated plasma protein A
- PP-13
placental tissue protein 13
- hCG
human chorionic gonadotropin
- VEGF
Vascular endothelial growth factor
- fnRBC
fetal nucleated red blood cells
- CSA
chondroitin sulfate A
- IE
P. falciparum-infected erythrocytes
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Linzer DI, Fisher SJ. The placenta and the prolactin family of hormones: regulation of the physiology of pregnancy. Molecular Endocrinology. 1999;13(6):837–40. [DOI] [PubMed] [Google Scholar]
- 2.Odegard RA, Vatten LJ, Nilsen ST, Salvesen KA, Austgulen R. Preeclampsia and fetal growth. Obstet Gynecol. 2000;96(6):950–5. [PubMed] [Google Scholar]
- 3.Landis S, Lokomba V, Ananth C, Atibu J, Ryder R, Flartmann K, et al. Impact of maternal malaria and under-nutrition on intrauterine growth restriction: a prospective ultrasound study in Democratic Republic of Congo. Epidemiology & Infection. 2009;137(2):294–304. [DOI] [PubMed] [Google Scholar]
- 4.Zhang H, Zhang J, Pope CF, Crawford LA, Vasavada RC, Jagasia SM, et al. Gestational diabetes mellitus resulting from impaired beta-cell compensation in the absence of FoxMl, a novel downstream effector of placental lactogen. Diabetes. 2010;59(1): 143–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Thévenot DR, Toth K, Durst RA, Wilson GS. Electrochemical biosensors: recommended definitions and classification. Analytical Letters. 2001;34(5):635–59. [DOI] [PubMed] [Google Scholar]
- 6.Yoon J-Y. Spectrophotometry and optical biosensor Introduction to Biosensors: Springer; 2013. p. 121–39. [Google Scholar]
- 7.Perumal V, Hlashim UJJoab. Advances in biosensors: Principle, architecture and applications. 2014; 12(1): 1–15. [Google Scholar]
- 8.Borisov SM, Wolfbeis OSJCr. Optical biosensors. 2008; 108(2):423–61. [DOI] [PubMed] [Google Scholar]
- 9.Simeonov A, Nikiforov TTJNar. Single nucleotide polymorphism genotyping using short, fluorescently labeled locked nucleic acid (LNA) probes and fluorescence polarization detection. 2002;30(17):e91–e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Liu Q, Wang P. Cell-based biosensors: principles and applications: Artech House; 2009. [Google Scholar]
- 11.Turner A Approaches to allergy detection using aptasensors. 2007. [Google Scholar]
- 12.Schneider H-J, Lim S, Strongin RM. Biomimetic synthetic receptors as molecular recognition elements Recognition Receptors in Biosensors: Springer; 2010. p. 777–816. [Google Scholar]
- 13.Morrison DW, Dokmeci MR, Demirci U, Khademhosseini A. Clinical applications of micro-and nanoscale biosensors: John Wiley & Sons, Inc.: Hoboken, NJ, USA; 2007. [Google Scholar]
- 14.Preechaburana P, Suska A, Filippini DJTib. Biosensing with cell phones. 2014;32(7):351–5. [DOI] [PubMed] [Google Scholar]
- 15.Abbaspour A, Khajehzadeh A, Ghaffarinejad AJA. A simple and cost-effective method, as an appropriate alternative for visible spectrophotometry: development of a dopamine biosensor. 2009; 134(8): 1692–8. [DOI] [PubMed] [Google Scholar]
- 16.Unser S, Bruzas I, He J, Sagle L. Localized surface plasmon resonance biosensing: current challenges and approaches. Sensors. 2015;15(7): 15684–716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mahmoudpour M, Dolatabadi JEN, Torbati M, Homayouni-Rad AJB, Bioelectronics. Nanomaterials based surface plasmon resonance signal enhancement for detection of environmental pollutions. 2018. [DOI] [PubMed] [Google Scholar]
- 18.Pimková K, Bocková M, Hegnerová K, Suttnar J, Čermák J, Homola J, et al. Surface plasmon resonance biosensor for the detection of VEGFR-1—a protein marker of myelodysplastic syndromes. Aialytical and bioanalytical chemistry. 2012;402(1):381–7. [DOI] [PubMed] [Google Scholar]
- 19.Khansili N, Rattu G, Krishna PM. Label-free optical biosensors for food and biological sensor applications. Sensors and Actuators B: Chemical. 2018. [Google Scholar]
- 20.Laing S, Jamieson LE, Faulds K, Graham D. Surface-enhanced Raman spectroscopy for in vivo biosensing. Nat Rev Chem. 2017; 1 (8). [Google Scholar]
- 21.Noble J, Attree S, Horgan A, Knight A, Kumarswami N, Porter R, et al. Optical Scattering Artifacts Observed in the Development of Multiplexed Surface Enhanced Raman Spectroscopy Nanotag Immunoassays. Anal Chem. 2012;84(19): 8246–52. [DOI] [PubMed] [Google Scholar]
- 22.Chon H, Lee S, Yoon SY, Lee EK, Chang SI, Choo J. SERS-based competitive immunoassay of troponin I and CK-MB markers for early diagnosis of acute myocardial infarction. Chem Commun. 2014;50(9): 1058–60. [DOI] [PubMed] [Google Scholar]
- 23.Zhang D, Huang L, Liu B, Ni HB, Sun LD, Su EB, et al. Quantitative and ultrasensitive detection of multiplex cardiac biomarkers in lateral flow assay with core-shell SERS nanotags. Biosens Bioelectron. 2018;106:204–11. [DOI] [PubMed] [Google Scholar]
- 24.Tian FR, Conde J, Bao CC, Chen YS, Curtin J, Cui DX. Gold nanostars for efficient in vitro and in vivo real-time SERS detection and drug delivery via plasmonic-tunable Raman/FTIR imaging. Biomaterials. 2016;106:87–97. [DOI] [PubMed] [Google Scholar]
- 25.Shen M-Y, Li B-R, Li Y-K. Silicon nanowire field-effect-transistor based biosensors: From sensitive to ultra-sensitive. Biosensors and Bioelectronics. 2014;60:101–11. [DOI] [PubMed] [Google Scholar]
- 26.Wu L, Xiong E, Zhang X, Zhang X, Chen J. Nanomaterials as signal amplification elements in DNA-based electrochemical sensing. Nano Today. 2014;9(2): 197–211. [Google Scholar]
- 27.Moore TJ, Moody AS, Payne TD, Sarabia GM, Daniel AR, Sharma B. In Vitro and In Vivo SERS Biosensing for Disease Diagnosis. Biosensors-Basel. 2018;8(2). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Luo X, Davis JJJCSR. Electrical biosensors and the label free detection of protein disease biomarkers. 2013;42(13):5944–62. [DOI] [PubMed] [Google Scholar]
- 29.Liu S, Guo XJNAM. Carbon nanomaterials field-effect-transistor-based biosensors. 2012;4(8):e23. [Google Scholar]
- 30.Park J-W, Kallempudi SS, Niazi JH, Gurbuz Y, Youn B-S, Gu MBJB, et al. Rapid and sensitive detection of Nampt (PBEF/visfatin) in human serum using an ssDNA aptamer-based capacitive biosensor. 2012;38(1):233–8. [DOI] [PubMed] [Google Scholar]
- 31.McGuinness RP, Verdonk EJL-fbt, applications. 1 1 Electrical impedance technology applied to cell-based assays. 2009:251. [Google Scholar]
- 32.Xu YC, Xie XW, Duan Y, Wang L, Cheng Z, Cheng J. A review of impedance measurements of whole cells. Biosens Bioelectron. 2016;77:824–36. [DOI] [PubMed] [Google Scholar]
- 33.Du E, Ha S, Diez-Silva M, Dao M, Suresh S, Chandrakasan AP. Electric impedance microflow cytometry for characterization of cell disease states. Lab Chip. 2013;13(19):3903–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Liu J, Qiang YH, Alvarez O, Du E. Electrical impedance microflow cytometry with oxygen control for detection of sickle cells. Sensor Actuat B-Chem. 2018;255:2392–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sowerby SJ, Broom MF, Petersen GB. Dynamically resizable nanometre-scale apertures for molecular sensing. Sensor Actuat B-Chem. 2007;123(1):325–30. [Google Scholar]
- 36.Vogel R, Willmott G, Kozak D, Roberts GS, Anderson W, Groenewegen L, et al. Quantitative Sizing of Nano/Microparticles with a Tunable Elastomeric Pore Sensor. Anal Chem. 2011;83(9):3499–506. [DOI] [PubMed] [Google Scholar]
- 37.Akers JC, Ramakrishnan V, Nolan JP, Duggan E, Fu C-C, Hochberg FH, et al. Comparative analysis of technologies for quantifying extracellular vesicles (EVs) in clinical cerebrospinal fluids (CSF). 2016; 1 l(2):e0149866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Momen-Heravi F, Balaj L, Alian S, Tigges J, Toxavidis V, Ericsson M, et al. Alternative methods for characterization of extracellular vesicles. Front Physiol. 2012;3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Fang C, He J, Chen ZJS, Chemical AB. A disposable amperometric biosensor for determining total cholesterol in whole blood. 2011; 155(2):545–50. [Google Scholar]
- 40.Wang Y, Xu H, Zhang J, Li GJS. Electrochemical sensors for clinic analysis. 2008;8(4):2043–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Grieshaber D, MacKenzie R, Voeroes J, Reimhult EJS. Electrochemical biosensors-sensor principles and architectures. 2008;8(3): 1400–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Monošík R, Stred’anský M, Šturdík EJJocla. Application of electrochemical biosensors in clinical diagnosis. 2012;26(1):22–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Koncki RJAca. Recent developments in potentiometric biosensors for biomedical analysis. 2007;599(1):7–15. [DOI] [PubMed] [Google Scholar]
- 44.Länge K, Rapp BE, Rapp MJA, chemistry b. Surface acoustic wave biosensors: a review. 2008;391(5): 1509–19. [DOI] [PubMed] [Google Scholar]
- 45.Arruda DL, Wilson WC, Nguyen C, Yao QW, Caiazzo RJ, Talpasanu I, et al. Microelectrical sensors as emerging platforms for protein biomarker detection in point-of-care diagnostics. Expert Rev Mol Diagn. 2009;9(7):749–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Vigneshvar S, Sudhakumari C, Senthilkumaran B, Prakash H. Recent advances in biosensor technology for potential applications–an overview. Frontiers in bioengineering and biotechnology. 2016;4:11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kabir K, Ippolito SJ, Sabri YM, Harrison CJ, Matthews G, Bhargava SK. A comparison of Surface Acoustic Wave (SAW) and Quartz Crystal Microbalance (QCM) based sensors for portable, online mercury vapour sensing. Chemeca 2014: Processing excellence; Powering our future. 2014:1402. [Google Scholar]
- 48.Dai Z, Yan F, Chen J, Ju HJAc. Reagentless amperometric immunosensors based on direct electrochemistry of horseradish peroxidase for determination of carcinoma antigen-125. 2003;75(20):5429–34. [DOI] [PubMed] [Google Scholar]
- 49.Wilson MS, Rauh RDJB, Bioelectronics. Novel amperometric immunosensors based on iridium oxide matrices. 2004;19(7):693–9. [DOI] [PubMed] [Google Scholar]
- 50.Putzbach W, Ronkainen NJ. Immobilization Techniques in the Fabrication ofNanomaterial-Based Electrochemical Biosensors: A Review. Sensors-Basel. 2013;13(4):4811–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Fruscalzo A, Londero A, Biasizzo J, Bortolotti N, Bertozzi S, Curcio F, et al. Second trimester amniotic fluid retinol in patients developing preeclampsia. Archives of gynecology and obstetrics. 2015;291(4):831–6. [DOI] [PubMed] [Google Scholar]
- 52.Zusterzeel PLM, Mulder TPJ, Peters WHM, Wiseman SA, Steegers EAP. Plasma protein carbonyls in nonpregnant, healthy pregnant and preeclamptic women. Free Radical Res. 2000;33(5):471–6. [DOI] [PubMed] [Google Scholar]
- 53.Mehmetoglu I, Kurban S, Toker A, Annagur A, Altunhan H, Erbay E, et al. Serum ischemia-modified albumin and oxidized LDL in cord blood and serum of neonates bom to pre- eclamptic mothers. Pediatrics International. 2015;57(3):422–6. [DOI] [PubMed] [Google Scholar]
- 54.Hsieh Ts-Ta, Chen S-F, Lo L-M, Li M-J, Yeh Y-L, Hung T-H. The association between maternal oxidative stress at mid-gestation and subsequent pregnancy complications. Reproductive sciences. 2012; 19(5):505–12. [DOI] [PubMed] [Google Scholar]
- 55.Wang Y, Walsh SW. Increased superoxide generation is associated with decreased superoxide dismutase activity and mRNA expression in placental trophoblast cells in pre-eclampsia. Placenta. 2001;22(2–3):206–12. [DOI] [PubMed] [Google Scholar]
- 56.Megnekou R, Djontu JC, Bigoga JD, Medou FM, Tenou S, Lissom A. Impact of Placental Plasmodium falciparum Malaria on the Profile of Some Oxidative Stress Biomarkers in Women Living in Yaounde, Cameroon. Plos One. 2015; 10(8). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Karowicz-Bilinska A, Kęziora-Komatowska K, Bartosz G. Indices of oxidative stress in pregnancy with fetal growth restriction. Free radical research. 2007;41(8):870–3. [DOI] [PubMed] [Google Scholar]
- 58.Saker M, Mokhtari NS, Merzouk SA, Merzouk H, Belarbi B, Narce M. Oxidant and antioxidant status in mothers and their newborns according to birthweight. Eur J Obstet Gyn R B. 2008; 141(2):95–9. [DOI] [PubMed] [Google Scholar]
- 59.Aydemir B, Baykara O, Cinemre FBS, Cinemre H, Tuten A, Kiziler AR, et al. LOX-1 gene variants and maternal levels of plasma oxidized LDL and malondialdehyde in patients with gestational diabetes mellitus. Archives of gynecology and obstetrics. 2016;293(3):517–27. [DOI] [PubMed] [Google Scholar]
- 60.Suman P, Gandhi S, Kumar P, Garg K. Prospects of electrochemical immunosensors for early diagnosis of preeclampsia. American Journal of Reproductive Immunology. 2017;77(1):el2584. [DOI] [PubMed] [Google Scholar]
- 61.Megnekou R, Djontu JC, Bigoga JD, Medou FM, Tenou S, Lissom A. Impact of placental Plasmodium falciparum malaria on the profile of some oxidative stress biomarkers in women living in Yaounde, Cameroon. PloS one. 2015; 10(8):e0134633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Gelisgen R, Gene H, Kayali R, Oncul M, Benian A, Guralp O, et al. Protein oxidation markers in women with and without gestational diabetes mellitus: A possible relation with paraoxonase activity. Diabetes Res Clin Pr. 2011;94(3):404–9. [DOI] [PubMed] [Google Scholar]
- 63.Maisonneuve E, Delvin E, Ouellet A, Morin L, Dube J, Boucoiran I, et al. Oxidative conditions prevail in severe IUGR with vascular disease and Doppler anomalies. The Journal of Maternal-Fetal & Neonatal Medicine. 2015:28(12): 1471–5. [DOI] [PubMed] [Google Scholar]
- 64.Sahai K, Saraswathy S, Yadav TP, Arora D, Krishnan M. Pre-eclampsia: Molecular events to biomarkers. Medical Journal Armed Forces India. 2017:73(2): 167–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Qiu C, Hevner K, Abetew D, Enquobahrie DA, Williams MA. Oxidative DNA damage in early pregnancy and risk of gestational diabetes mellitus: a pilot study. Clinical biochemistry. 2011. ;44(10–11):804–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Qiu CF, Hevner K, Abetew D, Enquobahrie DA, Williams MA. Oxidative DNA damage in early pregnancy and risk of gestational diabetes mellitus: A pilot study. Clin Biochem. 2011. ;44(10–11):804–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Yagi K A simple fluorometric assay for lipoperoxide in blood plasma. Biochemical medicine. 1976;15(2):212–6. [DOI] [PubMed] [Google Scholar]
- 68.Moselhy HF, Reid RG, Yousef S, Boyle SP. A specific, accurate, and sensitive measure of total plasma malondialdehyde by HPFC. Journal of lipid research. 2013;54(3): 852–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Wang J, Jiang Z, Xie F, Fiu M, Yuan Y. Determination of the activity of superoxide dismutase using a glassy carbon electrode modified with ferrocene imidazolium salts and hydroxy-functionalized graphene. Microchimica Acta. 2017; 184(1):289–96. [Google Scholar]
- 70.Santharaman P, Das M, Singh SK, Sethy NK, Bhargava K, Claussen JC, et al. Fabel-free electrochemical immunosensor for the rapid and sensitive detection of the oxidative stress marker superoxide dismutase 1 at the point-of-care. Sensors and Actuators B: Chemical. 2016;236:546–53. [Google Scholar]
- 71.Nam S, Fee W-Y. Electrogenerated chemiluminescence of lucigenin at mesoporous platinum electrode and its biosensing application to superoxide dismutase. Journal of Electroanalytical Chemistry. 2018;808:59–64. [Google Scholar]
- 72.Saqib M, Bashir S, Fi H, Wang S-s, Jin Y. Fucigenin-tris (2-carboxyethyl) phosphine chemiluminescence for selective and sensitive detection of TCEP, superoxide dismutase, mercury (II), and dopamine. Anal Chem. 2019. [DOI] [PubMed] [Google Scholar]
- 73.Saqib M, Qi F, Hui P, Nsabimana A, Halawa MI, Zhang W, et al. Development of luminol-N-hydroxyphthalimide chemiluminescence system for highly selective and sensitive detection of superoxide dismutase, uric acid and Co2+. Biosensors and Bioelectronics. 2018;99:519–24. [DOI] [PubMed] [Google Scholar]
- 74.Cao H, Yu F, Zhao Y, Scianmarello N, Fee J, Dai W, et al. Stretchable electrochemical impedance sensors for intravascular detection of lipid-rich lesions in New Zealand White rabbits. Biosensors and Bioelectronics. 2014;54:610–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Gaus K, Hall EA. Detection of oxidized low-density lipoproteins using surface plasmon resonance. Analytical chemistry. 1999;71(13):2459–67. [DOI] [PubMed] [Google Scholar]
- 76.Zhang D, Haputhanthri R, Ansar SM, Vangala K, De Silva HI, Sygula A, et al. Ultrasensitive detection of malondialdehyde with surface-enhanced Raman spectroscopy. Analytical and bioanalytical chemistry. 2010;398(7–8):3193–201. [DOI] [PubMed] [Google Scholar]
- 77.Widmer M, Cuesta C, Khan KS, Conde-Agudelo A, Carroli G, Fusey S, et al. Accuracy of angiogenic biomarkers at< 20 weeks’ gestation in predicting the risk of pre-eclampsia: A WHO multicentre study. Pregnancy Hypertension: An International Journal of Women’s Cardiovascular Health. 2015;5(4):330–8. [DOI] [PubMed] [Google Scholar]
- 78.Reis FM, D’antona D, Petraglia F. Predictive value of hormone measurements in maternal and fetal complications of pregnancy. Endocrine reviews. 2002;23(2):230–57. [DOI] [PubMed] [Google Scholar]
- 79.Wortelboer E, Koster M, Cuckle H, Stoutenbeek P, Schielen P, Visser G. First- trimester placental protein 13 and placental growth factor: markers for identification of women destined to develop early- onset pre- eclampsia. B JOG: An International Journal of Obstetrics & Gynaecology. 2010; 117(11): 1384–9. [DOI] [PubMed] [Google Scholar]
- 80.Staff AC. Circulating predictive biomarkers in preeclampsia. Pregnancy Hypertension: An International Journal of Women’s Cardiovascular Health. 2011; 1(1):28–42. [DOI] [PubMed] [Google Scholar]
- 81.Burger O, Pick E, Zwickel J, Klayman M, Meiri H, Slotky R, et al. Placental protein 13 (PP-13): effects on cultured trophoblasts, and its detection in human body fluids in normal and pathological pregnancies. Placenta. 2004;25(7):608–22. [DOI] [PubMed] [Google Scholar]
- 82.Zhang F, Xiao X, Fiu D, Dong X, Sun J, Zhang X. Increased cord blood angiotensin II concentration is associated with decreased insulin sensitivity in the offspring of mothers with gestational diabetes mellitus. Journal of Perinatology. 2013;33(1):9. [DOI] [PubMed] [Google Scholar]
- 83.D’antonio F, Rijo C, Thilaganathan B, Akolekar R, Khalil A, Papageourgiou A, et al. Association between first- trimester maternal serum pregnancy- associated plasma protein- A and obstetric complications. Prenatal diagnosis. 2013;33(9):839–47. [DOI] [PubMed] [Google Scholar]
- 84.Yliniemi A, Nurkkala M-M, Kopman S, Korpimaki T, Kouru H, Ryynanen, et al. First trimester placental retinol-binding protein 4 (RBP4) and pregnancy-associated placental protein A (PAPP-A) in the prediction of early-onset severe pre-eclampsia. Metabolism. 2015;64(4):521–6. [DOI] [PubMed] [Google Scholar]
- 85.Borras D, Perales-Puchalt A, Ruiz Sacedon N, Perales A. Angiogenic growth factors in maternal and fetal serum in pregnancies complicated with intrauterine growth restriction. Journal of Obstetrics and Gynaecology. 2014;34(3):218–20. [DOI] [PubMed] [Google Scholar]
- 86.Joó JG, Rigó J Jr, Börzsönyi B, Demendi C, Komya F. Placental gene expression of the placental growth factor (PlGF) in intrauterine growth restriction. The Journal of Maternal-Fetal & Neonatal Medicine. 2017;30(12): 1471–5. [DOI] [PubMed] [Google Scholar]
- 87.Audibert F, Benchimol Y, Benattar C, Champagne C, Frydman R. Prediction of preeclampsia or intrauterine growth restriction by second trimester serum screening and uterine Doppler velocimetry. Fetal diagnosis and therapy. 2005;20(1):48–53. [DOI] [PubMed] [Google Scholar]
- 88.Androutsopoulos G, Gkogkos P, Decavalas G. Mid-trimester maternal serum HCG and alpha fetal protein levels: clinical significance and prediction of adverse pregnancy outcome. International journal of endocrinology and metabolism. 2013; 11(2): 102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Nogueira AI, Santos RAS, e Silva ACS, Cabral ACV, Vieira RLP, Drumond TC, et al. The pregnancy-induced increase of plasma angiotensin-(1-7) is blunted in gestational diabetes. Regulatory peptides. 2007;141(1-3):55–60. [DOI] [PubMed] [Google Scholar]
- 90.Abdel Moety GAF, Almohamady M, Sherif NA, Raslana AN, Mohamed TF, El Moneam FIMA, et al. Could first-trimester assessment of placental functions predict preeclampsia and intrauterine growth restriction? A prospective cohort study. The Journal of Maternal-Fetal & Neonatal Medicine. 2016;29(3):413–7. [DOI] [PubMed] [Google Scholar]
- 91.Conroy AL, Liles WC, Molyneux ME, Rogerson SJ, Kain KC. Performance characteristics of combinations of host biomarkers to identify women with occult placental malaria: a case-control study from Malawi. PloS one. 2011;6(12):e28540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Gueneuc A, Deloron P, Bertin GI. Usefulness of a biomarker to identify placental dysfunction in the context of malaria. Malaria journal. 2017; 16(1): 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Zhao B, Han X, Meng Q, Luo Q. Early second trimester maternal serum markers in the prediction of gestational diabetes mellitus. Journal of diabetes investigation. 2018;9(4):967–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Syngelaki A, Kotecha R, Pastides A, Wright A, Nicolaides KH. First-trimester biochemical markers of placentation in screening for gestational diabetes mellitus. Metabolism. 2015:64(11): 1485–9. [DOI] [PubMed] [Google Scholar]
- 95.Meng Q, Shao L, Luo X, Mu Y, Xu W, Gao L, et al. Expressions of VEGF-A and VEGFR-2 in placentae from GDM pregnancies. Reproductive Biology and Endocrinology. 2016; 14(1):61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Beneventi F, Simonetta M, Locatelli E, Cavagnoli C, Badulli C, Lovati E, et al. Temporal variation in soluble human leukocyte antigen- G (sHLA- G) and pregnancy- associated plasma protein A (PAPP- A) in pregnancies complicated by gestational diabetes mellitus and in controls. American Journal of Reproductive Immunology. 2014;72(4):413–21. [DOI] [PubMed] [Google Scholar]
- 97.Lovati E, Beneventi F, Simonetta M, Laneri M, Quarleri L, Scudeller L, et al. Gestational diabetes mellitus: including serum pregnancy-associated plasma protein-A testing in the clinical management of primiparous women? A case-control study. Diabetes Res Clin Pr. 2013;100(3):340–7. [DOI] [PubMed] [Google Scholar]
- 98.Diab AE, El- Behery MM, Ebrahiem MA, Shehata AE. Angiogenic factors for the prediction of pre- eclampsia in women with abnormal midtrimester uterine artery Doppler velocimetry. International Journal of Gynecology & Obstetrics. 2008;102(2):146–51. [DOI] [PubMed] [Google Scholar]
- 99.Wang W, Zou Y, Yan J, Liu J, Chen H, Li S, et al. Ultrasensitive colorimetric immunoassay for hCG detection based on dual catalysis of Au@ Pt core–shell nanoparticle functionalized by horseradish peroxidase. Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy. 2018; 193:102–8. [DOI] [PubMed] [Google Scholar]
- 100.Chang C-C, Chen C-Y, Chen C-P, Lin C-W. Facile colorimetric detection of human chorionic gonadotropin based on the peptide-induced aggregation of gold nanoparticles. Analytical Methods. 2015;7(1):29–33. [Google Scholar]
- 101.Zhu X, Kou F, Xu H, Lin L, Yang G, Lin Z. A highly sensitive aptamer-immunoassay for vascular endothelial growth factor coupled with portable glucose meter and hybridization chain reaction. Sensors and Actuators B: Chemical. 2017;253:660–5. [Google Scholar]
- 102.Xu H, Kou F, Ye H, Wang Z, Huang S, Liu X, et al. Highly sensitive antibody-aptamer sensor for vascular endothelial growth factor based on hybridization chain reaction and pH meter/indicator. Talanta. 2017; 175:177–82. [DOI] [PubMed] [Google Scholar]
- 103.Heras JY, Pallarola D, Battaglini F. Electronic tongue for simultaneous detection of endotoxins and other contaminants of microbiological origin. Biosensors and Bioelectronics. 2010;25(11):2470–6. [DOI] [PubMed] [Google Scholar]
- 104.Kim S-E, Su W, Cho M, Lee Y, Choe W-S. Harnessing aptamers for electrochemical detection of endotoxin. Analytical biochemistry. 2012;424(1): 12–20. [DOI] [PubMed] [Google Scholar]
- 105.Viet NX, Chikae M, Ukita Y, Maehashi K, Matsumoto K, Tamiya E, et al. Gold-linked electrochemical immunoassay on single-walled carbon nanotube for highly sensitive detection of human chorionic gonadotropinhormone. Biosensors and Bioelectronics. 2013;42:592–7. [DOI] [PubMed] [Google Scholar]
- 106.Li R, Wu D, Li H, Xu C, Wang H, Zhao Y, et al. Label-free amperometric immunosensor for the detection of human serum chorionic gonadotropin based on nanoporous gold and graphene. Analytical biochemistry. 2011. ;414(2): 196–201. [DOI] [PubMed] [Google Scholar]
- 107.Kerman K, Nagatani N, Chikae M, Yuhi T, Takamura Y, Tamiya E. Label-free electrochemical immunoassay for the detection of human chorionic gonadotropin hormone. Anal Chem. 2006;78(15):5612–6. [DOI] [PubMed] [Google Scholar]
- 108.Li J, Liu G, Zhang W, Cheng W, Xu H, Ding S. Competitive detection of pregnancy-associated plasma protein-A in serum using functional single walled carbon nanotubes/chitosan-based electrochemical immunosensor. Journal of Electroanalytical Chemistry. 2013;708:95–100. [Google Scholar]
- 109.Kim G-I, Kim K-W, Oh M-K, Sung Y-M. Electrochemical detection of vascular endothelial growth factors (VEGFs) using VEGF antibody fragments modified Au NPs/ITO electrode. Biosensors and Bioelectronics. 2010;25(7): 1717–22. [DOI] [PubMed] [Google Scholar]
- 110.Pan L-H, Kuo S-H, Lin T-Y, Lin C-W, Fang P-Y, Yang H-W. An electrochemical biosensor to simultaneously detect VEGF and PSA for early prostate cancer diagnosis based on graphene oxide/ssDNA/PLLA nanoparticles. Biosensors and Bioelectronics. 2017;89:598–605. [DOI] [PubMed] [Google Scholar]
- 111.Sezginturk MK, Uygun ZO. An impedimetric vascular endothelial growth factor biosensor-based PAMAM/cysteamine-modified gold electrode for monitoring of tumor growth. Anal Biochem. 2012;423(2):277–85. [DOI] [PubMed] [Google Scholar]
- 112.Park J-M, Jung H-W, Chang YW, Kim H-S, Kang M-J, Pyun J-C. Chemiluminescence lateral flow immunoassay based on Pt nanoparticle with peroxidase activity. Analytica chimica acta. 2015;853:360–7. [DOI] [PubMed] [Google Scholar]
- 113.Chiu N-F, Kuo C-T, Lin T-L, Chang C-C, Chen C-Y. Ultra-high sensitivity of the non-immunological affinity of graphene oxide-peptide-based surface plasmon resonance biosensors to detect human chorionic gonadotropin. Biosensors and Bioelectronics. 2017;94:351–7. [DOI] [PubMed] [Google Scholar]
- 114.Cennamo N, Pesavento M, Lunelli L, Vanzetti L, Pederzolli C, Zeni L, et al. An easy way to realize SPR aptasensor: A multimode plastic optical fiber platform for cancer biomarkers detection. Talanta. 2015;140:88–95. [DOI] [PubMed] [Google Scholar]
- 115.Chen FI, Flou Y, Qi F, Zhang J, Koh K, Shen Z, et al. Detection of vascular endothelial growth factor based on rolling circle amplification as a means of signal enhancement in surface plasmon resonance. Biosensors and Bioelectronics. 2014;61:83–7. [DOI] [PubMed] [Google Scholar]
- 116.Bocková M, Song XC, Gedeonová E, Levová K, Kalousová M, Zima T, et al. Surface plasmon resonance biosensor for detection of pregnancy associated plasma protein A2 in clinical samples. Analytical and bioanalytical chemistry. 2016;408(26):7265–9. [DOI] [PubMed] [Google Scholar]
- 117.Ma L, Wen G, Ye L, Lu Z, Luo Y, Liang A, et al. SERS quantitative detection of trace human chorionic gonadotropin using a label- free Victoria blue B as probe in the aggregated immunonanogold sol substrate. Luminescence. 2015;30(6):790–7. [DOI] [PubMed] [Google Scholar]
- 118.Mitsakakis K, Gizeli E. Detection of multiple cardiac markers with an integrated acoustic platform for cardiovascular risk assessment. Analytica chimica acta. 2011;699(1): 1–5. [DOI] [PubMed] [Google Scholar]
- 119.Goswami D, Tannetta D, Magee L, Fuchisawa A, Redman C, Sargent I, et al. Excess syncytiotrophoblast microparticle shedding is a feature of early-onset pre-eclampsia, but not normotensive intrauterine growth restriction. Placenta. 2006;27(1):56–61. [DOI] [PubMed] [Google Scholar]
- 120.Qiu C, Flevner K, Enquobahrie DA, Williams MA. A case-control study of maternal blood mitochondrial DNA copy number and preeclampsia risk. International journal of molecular epidemiology and genetics. 2012;3(3):237. [PMC free article] [PubMed] [Google Scholar]
- 121.Colleoni F, Lattuada D, Garretto A, Massari M, Mandò C, Somigliana E, et al. Maternal blood mitochondrial DNA content during normal and intrauterine growth restricted (IUGR) pregnancy. American journal of obstetrics and gynecology. 2010;203(4):365 el–e6. [DOI] [PubMed] [Google Scholar]
- 122.Oktavianthi S, Fauzi M, Trianty L, Trimarsanto FI, Bowolaksono A, Noviyanti R, et al. Placental mitochondrial DNA copy number is associated with reduced birth weight in women with placental malaria. Placenta. 2019. [DOI] [PubMed] [Google Scholar]
- 123.Salomon C, Scholz-Romero K, Sarker S, Sweeney E, Kobayashi M, Correa P, et al. Gestational diabetes mellitus is associated with changes in the concentration and bioactivity of placenta-derived exosomes in maternal circulation across gestation. Diabetes. 2016;65(3):598–609. [DOI] [PubMed] [Google Scholar]
- 124.Crovetto F, Lattuada D, Rossi G, Mangano S, Somigliana E, Bolis G, et al. A role for mitochondria in gestational diabetes mellitus? Gynecological Endocrinology. 2013;29(3):259–62. [DOI] [PubMed] [Google Scholar]
- 125.Pillay P, Maharaj N, Moodley J, Mackraj IJP. Placental exosomes and pre-eclampsia: maternal circulating levels in normal pregnancies and, early and late onset pre-eclamptic pregnancies. 2016;46:18–25. [DOI] [PubMed] [Google Scholar]
- 126.Dragovic R, Collett G, Flole P, Ferguson D, Redman C, Sargent I, et al. Isolation of syncytiotrophoblast microvesicles and exosomes and their characterisation by multicolour flow cytometry and fluorescence Nanoparticle Tracking Analysis. 2015;87:64–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Im FI, Shao FI, Park YI, Peterson VM, Castro CM, Weissleder R, et al. Label-free detection and molecular profiling of exosomes with a nano-plasmonic sensor. Nature biotechnology. 2014;32(5):490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Ferguson KK, McElrath TF, Chen YH, Mukherjee B, Meeker JD. Longitudinal profiling of inflammatory cytokines and C- reactive protein during uncomplicated and preterm pregnancy. American journal of reproductive immunology. 2014;72(3):326–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Silva DMCe, Marreiro DdN, Moita Neto JM, Brito JA, Neta EAdS, Matias JP, et al. Oxidative stress and immunological alteration in women with preeclampsia. Flypertension in pregnancy. 2013;32(3):304–11. [DOI] [PubMed] [Google Scholar]
- 130.Kumar A, Begum N, Prasad S, Agarwal S, Sharma S. IL-10, TNF-alpha & IFN-gamma: Potential early biomarkers for preeclampsia. Cell Immunol. 2013;283(1-2):70–4. [DOI] [PubMed] [Google Scholar]
- 131.Pinheiro MB, Martins-Filho OA, Mota APL, Alpoim PN, Godoi LC, Silveira AC, et al. Severe preeclampsia goes along with a cytokine network disturbance towards a systemic inflammatory state. Cytokine. 2013;62(1): 165–73. [DOI] [PubMed] [Google Scholar]
- 132.Daneva AM, Fladzi-Lega M, Stefanovic M. Correlation of the system of cytokines in moderate and severe preeclampsia. Clin Exp Obstet Gynecol. 2016;43(2):220–4. [PubMed] [Google Scholar]
- 133.Tuuri A, Jauhiainen M, Tikkanen M, Kaaja R. Systolic blood pressure and fatty acid-binding protein 4 predict pregnancy-induced hypertension in overweight nulliparous women. Placenta. 2014;35(10):797–801. [DOI] [PubMed] [Google Scholar]
- 134.van Rijn BB, Veerbeek JH, Scholtens LC, Uiterweer EDP, Koster MP, Peeters LL, et al. C-reactive protein and fibrinogen levels as determinants of recurrent preeclampsia: a prospective cohort study. Journal of hypertension. 2014;32(2):408–14. [DOI] [PubMed] [Google Scholar]
- 135.Elfayomy A, Habib F, Almasry S, Safwat M, Eldomiaty M. Serum levels of adrenomedullin and inflammatory cytokines in women with term idiopathic intrauterine growth restriction. Journal of Obstetrics and Gynaecology. 2013;33(2): 135–9. [DOI] [PubMed] [Google Scholar]
- 136.Chandrasiri UP, Randall LM, Saad AA, Bashir AM, Rogerson SJ, Adam I. Low antibody levels to pregnancy-specific malaria antigens and heightened cytokine responses associated with severe malaria in pregnancy. The Journal of infectious diseases. 2013;209(9): 1408–17. [DOI] [PubMed] [Google Scholar]
- 137.Boutsikou T, Mastorakos G, Kyriakakou M, Margeli A, Hassiakos D, Papassotiriou I, et al. Circulating levels of inflammatory markers in intrauterine growth restriction. Mediators of inflammation. 2010;2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Chandrasiri UP, Randall LM, Saad AA, Bashir AM, Rogerson SJ, Adam I. Low antibody levels to pregnancy-specific malaria antigens and heightened cytokine responses associated with severe malaria in pregnancy. J Infect Dis. 2014;209(9): 1408–17. [DOI] [PubMed] [Google Scholar]
- 139.Bayoumi NK, Bakhet KH, Mohmmed AA, Eltom AM, Elbashir MI, Mavoungou E, et al. Cytokine profiles in peripheral, placental and cord blood in an area of unstable malaria transmission in eastern Sudan. Journal of tropical pediatrics. 2008;55(4):233–7. [DOI] [PubMed] [Google Scholar]
- 140.Wilson NO, Bythwood T, Solomon W, Jolly P, Yatich N, Jiang Y, et al. Elevated levels of IL-10 and G-CSF associated with asymptomatic malaria in pregnant women. Infectious diseases in obstetrics and gynecology. 2010;2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Thevenon AD, Zhou JA, Megnekou R, Ako S, Leke RG, Taylor DW. Elevated levels of soluble TNF receptors 1 and 2 correlate with Plasmodium falciparum parasitemia in pregnant women: potential markers for malaria-associated inflammation. The Journal of Immunology. 2010; 185(11):7115–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Syngelaki A, Visser GH, Krithinakis K, Wright A, Nicolaides KH. First trimester screening for gestational diabetes mellitus by maternal factors and markers of inflammation. Metabolism. 2016:65(3): 131–7. [DOI] [PubMed] [Google Scholar]
- 143.Gene S, Kusku-Kiraz Z, Dervisoglu E, Oztop N, Dinccag N, Gurdol F. The Relation of Oxidative Stress Biomarkers with Proinflammatory Cytokines in Gestational Diabetes. Clinical Investigation. 2017;7(2):43–8. [Google Scholar]
- 144.SIDDIQUI S WAGHDHARE S. PANDA M. DUBEY S. JHA S Association of IL-6 and CRP Levels with Gestational Diabetes Mellitus. Am Diabetes Assoc; 2018. [Google Scholar]
- 145.Wolf M, Sandler L, Hsu K, Vossen-Smimakis K, Ecker JL, Thadhani R. First-trimester C-reactive protein and subsequent gestational diabetes. Diabetes care. 2003;26(3):819–24. [DOI] [PubMed] [Google Scholar]
- 146.Luo L, Zhang Z, Ma L. Determination of recombinant human tumor necrosis factor-α in serum by chemiluminescence imaging. Analytica chimica acta. 2005;539(1-2):277–82. [Google Scholar]
- 147.Mazloum-Ardakani M, Hosseinzadeh L, Khoshroo A. Label-free electrochemical immunosensor for detection of tumor necrosis factor abased on fullerene-functionalized carbon nanotubes/ionic liquid. Journal of Electroanalytical Chemistry. 2015;757:58–64. [Google Scholar]
- 148.Liu Y, Zhou Q, Revzin A. An aptasensor for electrochemical detection of tumor necrosis factor in human blood. Analyst. 2013;138(15):4321–6. [DOI] [PubMed] [Google Scholar]
- 149.Bettazzi F, Enayati L, Sanchez IC, Motaghed R, Mascini M, Palchetti I. Electrochemical bioassay for the detection of TNF-α using magnetic beads and disposable screen-printed array of electrodes. Bioanalysis. 2013;5(1): 11–9. [DOI] [PubMed] [Google Scholar]
- 150.Kongsuphol P, Ng HH, Pursey JP, Arya SK, Wong CC, Stulz E, et al. ElS-based biosensor for ultra-sensitive detection of TNF-α from non-diluted human serum. Biosensors and Bioelectronics. 2014;61:274–9. [DOI] [PubMed] [Google Scholar]
- 151.Bryan T, Luo X, Bueno PR, Davis JJ. An optimised electrochemical biosensor for the label-free detection of C-reactive protein in blood. Biosensors and Bioelectronics. 2013;39(1):94–8. [DOI] [PubMed] [Google Scholar]
- 152.Songjaroen T, Feeny RM, Mensack MM, Laiwattanapaisal W, Henry CS. Label-free detection of C-reactive protein using an electrochemical DNA immunoassay. Sensing and bio-sensing research. 2016;8:14–9. [Google Scholar]
- 153.Zhang J, Zhang W, Guo J, Wang J, Zhang Y. Electrochemical detection of C-reactive protein using Copper nanoparticles and hybridization chain reaction amplifying signal. Analytical biochemistry. 2017;539:1–7. [DOI] [PubMed] [Google Scholar]
- 154.Jung S-H, Jung J-W, Suh I-B, Yuk JS, Kim W-J, Choi EY, et al. Analysis of C-Reactive Protein on Amide-Linked N-Hydroxysuccinimide– Dextran Arrays with a Spectral Surface Plasmon Resonance Biosensor for Serodiagnosis. Anal Chem. 2007;79(15):5703–10. [DOI] [PubMed] [Google Scholar]
- 155.Hu W-P, Hsu H-Y, Chiou A, Tseng K, Lin H-Y, Chang G-L, et al. Immunodetection of pentamer and modified C-reactive protein using surface plasmon resonance biosensing. Biosensors and Bioelectronics. 2006;21(8): 1631–7. [DOI] [PubMed] [Google Scholar]
- 156.van den Hurk R, Evoy S. Deflection cantilever detection of interferon gamma. Sensors and Actuators B: Chemical. 2013;176:960–5. [Google Scholar]
- 157.Chen C-H, Hwang R-Z, Huang L-S, Lin S-M, Chen H-C, Yang Y-C, et al. A wireless bio-MEMS sensor for C-reactive protein detection based on nanomechanics. IEEE Transactions on Biomedical Engineering. 2009;56(2):462–70. [DOI] [PubMed] [Google Scholar]
- 158.Lee JH, Yoon KH, Hwang KS, Park J, Ahn S, Kim TS. Label free novel electrical detection using micromachined PZT monolithic thin film cantilever for the detection of C-reactive protein. Biosensors and Bioelectronics. 2004;20(2):269–75. [DOI] [PubMed] [Google Scholar]
- 159.Bahk YK, Kim HH, Park D-S, Chang S-C, Go JS. A new concept for efficient sensitivity amplification of a QCM based immunosensor for TNF-alpha by using modified magnetic particles under applied magnetic field. Bulletin of the Korean Chemical Society. 2011;32(12):4215–20. [Google Scholar]
- 160.Krishnamoorthy S, Iliadis AA, Bei T, Chrousos GP. An interleukin-6 ZnO/Si02/Si surface acoustic wave biosensor. Biosensors and Bioelectronics. 2008;24(2):313–8. [DOI] [PubMed] [Google Scholar]
- 161.Schoots MH, Gordijn SJ, Scherjon SA, van Goor H, Hillebrands JL. Oxidative stress in placental pathology. Placenta. 2018;69:153–61. [DOI] [PubMed] [Google Scholar]
- 162.de Lucca L, Rodrigues F, Jantsch LB, Kober H, Neme WS, Gallarreta FMP, et al. Delta-aminolevulinate dehydratase activity and oxidative stress markers in preeclampsia. Biomed Pharmacother. 2016;84:224–9. [DOI] [PubMed] [Google Scholar]
- 163.Rodrigues F, de Lucca L, Neme WS, Goncalves TD. Influence of gestational diabetes on the activity of delta-aminolevulinate dehydratase and oxidative stress biomarkers. Redox Rep. 2018;23(1):63–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Jacques-Silva MC, Nogueira CW, Broch LC, Flores EMM, Rocha JBT. Diphenyl diselenide and ascorbic acid changes deposition of selenium and ascorbic acid in liver and brain of mice. Pharmacol Toxicol. 2001;88(3): 119–25. [DOI] [PubMed] [Google Scholar]
- 165.Galley HF, Davies MJ, Webster NR. Ascorbyl radical formation in patients with sepsis: Effect of ascorbate loading (vol 20, pg 139. 1996). Free Radical Bio Med. 1996;20(3):494-. [DOI] [PubMed] [Google Scholar]
- 166.Aebi Η [13] Catalase in vitro Method Enzymol. 105: Elsevier; 1984. p. 121–6. [DOI] [PubMed] [Google Scholar]
- 167.Ghaneei A, Yassini S, Ghanei ME, Shojaoddiny-Ardekani A. Increased serum oxidized low-density lipoprotein levels in pregnancies complicated by gestational diabetes mellitus. Iranian journal of reproductive medicine. 2015; 13(7):421. [PMC free article] [PubMed] [Google Scholar]
- 168.Schoots MH, Gordijn SJ, Scherjon SA, van Goor H, Elillebrands J-L. Oxidative stress in placental pathology. Placenta. 2018;69:153–61. [DOI] [PubMed] [Google Scholar]
- 169.Muehlenbachs A, Mutabingwa TK, Edmonds S, Fried M, Duffy PE. Elypertension and matemal–fetal conflict during placental malaria. PLoS medicine. 2006;3(11):e446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Aouache R, Biquard L, Vaiman D, Miralles F. Oxidative stress in preeclampsia and placental diseases. International journal of molecular sciences. 2018; 19(5): 1496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Liu Y, Zhao Y, Yu A, Zhao B, Gao Y, Niu El. Diagnostic accuracy of the soluble Fms-like tyrosine kinase-1/placental growth factor ratio for preeclampsia: a meta-analysis based on 20 studies. Archives of gynecology and obstetrics. 2015;292(3): 507–18. [DOI] [PubMed] [Google Scholar]
- 172.Kenny LC, Black MA, Poston L, Taylor R, Myers JE, Baker PN, et al. Early pregnancy prediction of preeclampsia in nulliparous women, combining clinical risk and biomarkers: the Screening for Pregnancy Endpoints (SCOPE) international cohort study. Elypertension. 2014;64(3):644–52. [DOI] [PubMed] [Google Scholar]
- 173.Merrill DC, Karoly M, Chen K, Ferrario CM, Brosnihan KB. Angiotensin-(1-7) in normal and preeclamptic pregnancy. Endocrine. 2002;18(3):239–45. [DOI] [PubMed] [Google Scholar]
- 174.Langer B, Bader A-M, Schlaeder G, Imbs J-L. Plasma active renin, angiotensin I, and angiotensin II during pregnancy and in preeclampsia. Obstetrics & Gynecology. 1998;91(2): 196–202. [DOI] [PubMed] [Google Scholar]
- 175.Nergiz Avcioğlu S, Demircan Sezer S, Küçük M, Zafer E, Yüksel H, Akcan B, et al. Maternal serum concentrations of s-Endoglin and IL-6 in pregnancy complicated by preterm premature membrane rupture. The Journal of Maternal-Fetal & Neonatal Medicine. 2016;29(12):1957–62. [DOI] [PubMed] [Google Scholar]
- 176.Chafetz I, Kuhnreich I, Sammar M, Tal Y, Gibor Y, Meiri H, et al. First-trimester placental protein 13 screening for preeclampsia and intrauterine growth restriction. American journal of obstetrics and gynecology. 2007;197(1):35 el–e7. [DOI] [PubMed] [Google Scholar]
- 177.Unverdorben L, Huttenbrenner R, Knabl J, Jeschke U, Hutter S. Galectin-13/PP-13 expression in term placentas of gestational diabetes mellitus pregnancies. Placenta. 2015;36(2): 191–8. [DOI] [PubMed] [Google Scholar]
- 178.Olsen RN, Woelkers D, Dunsmoor-Su R, LaCoursiere DY. Abnormal second-trimester serum analytes are more predictive of preterm preeclampsia. American journal of obstetrics and gynecology. 2012;207(3):228 el–e7. [DOI] [PubMed] [Google Scholar]
- 179.Mierzyński R, Poniedziałek-Czajkowska E, Dłuski D, Patro-Małysza J, Kimber-Trojnar Ż, Majsterek M, et al. Nesfatin-1 and Vaspin as Potential Novel Biomarkers for the Prediction and Early Diagnosis of Gestational Diabetes Mellitus. International journal ofmolecular sciences. 2019;20(1): 159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Tannetta D, Collett G, Vatish M, Redman C, Sargent I. Syncytiotrophoblast extracellular vesicles - Circulating biopsies reflecting placental health. Placenta. 2017;52:134–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Sokolova V, Ludwig A-K, Homung S, Rotan O, Horn PA, Epple M, et al. Characterisation of exosomes derived from human cells by nanoparticle tracking analysis and scanning electron microscopy. Colloids and Surfaces B: Biointerfaces. 2011. ;87(1): 146–50. [DOI] [PubMed] [Google Scholar]
- 182.Théry C, Amigorena S, Raposo G, Clayton A. Isolation and characterization of exosomes from cell culture supernatants and biological fluids. Current protocols in cell biology. 2006;30(1):3.22. 1-3.. 9. [DOI] [PubMed] [Google Scholar]
- 183.Hakulinen J, Sankkila L, Sugiyama N, Lehti K, Keski- Oja J. Secretion of active membrane type 1 matrix metalloproteinase (MMP- 14) into extracellular space in microvesicular exosomes. Journal of cellular biochemistry. 2008;105(5): 1211–8. [DOI] [PubMed] [Google Scholar]
- 184.Lawrie A, Albanyan A, Cardigan R, Mackie I, Harrison P. Microparticle sizing by dynamic light scattering in fresh- frozen plasma. Vox sanguinis. 2009;96(3):206–12. [DOI] [PubMed] [Google Scholar]
- 185.Xu Y, Nakane N, Maurer- Spurej E. Novel test for microparticles in platelet- rich plasma and platelet concentrates using dynamic light scattering. Transfusion. 2011;51(2):363–70. [DOI] [PubMed] [Google Scholar]
- 186.Sitar S, Kejžar A, Pahovnik D, Kogej K, Tušek-Žnidarič M, Lenassi M, et al. Size characterization and quantification of exosomes by asymmetrical-flow field-flow fractionation. Anal Chem. 2015;87(18):9225–33. [DOI] [PubMed] [Google Scholar]
- 187.Coumans FA, van der Pol E, Böing AN, Hajji N, Sturk G, van Leeuwen TG, et al. Reproducible extracellular vesicle size and concentration determination with tunable resistive pulse sensing. Journal of extracellular vesicles. 2014;3(1):25922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Akers JC, Ramakrishnan V, Nolan JP, Duggan E, Fu C-C, Hochberg FH, et al. Comparative analysis of technologies for quantifying extracellular vesicles (EVs) in clinical cerebrospinal fluids (CSF). PLoS One. 2016; 11(2):e0149866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Maas SL, Broekman ML, de Vrij J. Tunable resistive pulse sensing for the characterization of extracellular vesicles Exosomes and Microvesicles: Springer; 2017. p. 21–33. [DOI] [PubMed] [Google Scholar]
- 190.Lee K, Fraser K, Ghaddar B, Yang K, Kim E, Balaj L, et al. Multiplexed profiling of single extracellular vesicles. ACS nano. 2018; 12(1):494–503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Coumans FAW, Brisson AR, Buzas El, Dignat-George F, Drees EEE, El-Andaloussi S, et al. Methodological Guidelines to Study Extracellular Vesicles. Circulation research. 2017; 120(10): 1632–48. [DOI] [PubMed] [Google Scholar]
- 192.Van Der Vlist EJ, Nolte EN, Stoorvogel W, Arkesteijn GJ, Wauben MH. Fluorescent labeling of nano-sized vesicles released by cells and subsequent quantitative and qualitative analysis by high-resolution flow cytometry. Nature protocols. 2012;7(7): 1311. [DOI] [PubMed] [Google Scholar]
- 193.Padda RS, Deng FK, Brett SI, Biggs CN, Durfee PN, Brinker CJ, et al. Nanoscale flow cytometry to distinguish subpopulations of prostate extracellular vesicles in patient plasma. The Prostate. 2019. [DOI] [PubMed] [Google Scholar]
- 194.Morales-Kastresana A, Telford B, Musich TA, McKinnon K, Claybome C, Braig Z, et al. Labeling extracellular vesicles for nanoscale flow cytometry. Scientific reports. 2017;7(1): 1878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Bobrie A, Colombo M, Krumeich S, Raposo G, Tilery C. Diverse subpopulations of vesicles secreted by different intracellular mechanisms are present in exosome preparations obtained by differential ultracentrifugation. Journal of extracellular vesicles. 2012; 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Morgan TK. Cell- and size-specific analysis of placental extracellular vesicles in maternal plasma and pre-eclampsia. Translational research : the journal of laboratory and clinical medicine. 2018;201:40–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Spracklen CN, Smith CJ, Saftlas AF, Robinson JG, Ryckman KK. Maternal hyperlipidemia and the risk of preeclampsia: a meta-analysis. American journal of epidemiology. 2014; 180(4):346–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Siddiqui I Maternal Serum Lipids in Women with Pre- eclampsia. Annals of medical and health sciences research. 2014;4(4):638–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Anand S, Young S, Esplin MS, Peaden B, Tolley HD, Porter TF, et al. Detection and confirmation of serum lipid biomarkers for preeclampsia using direct infusion mass spectrometry. 2016;57(4):687–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Black KD, Horowitz JAJNr. Inflammatory Markers and Preeclampsia: A Systematic Review. 2018;67(3):242–51. [DOI] [PubMed] [Google Scholar]
- 201.Rodrigo N, Glastras SJJocm. The emerging role of biomarkers in the diagnosis of gestational diabetes mellitus. 2018;7(6): 120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Chandrasiri UP, Randall LM, Saad AA, Bashir AM, Rogerson SJ, Adam I. Low Antibody Levels to Pregnancy-specific Malaria Antigens and Heightened Cytokine Responses Associated With Severe Malaria in Pregnancy. J Infect Dis. 2014;209(9): 1408–17. [DOI] [PubMed] [Google Scholar]
- 203.Sonker M, Sahore V, Woolley AT. Recent advances in microfluidic sample preparation and separation techniques for molecular biomarker analysis: A critical review. Analytica chimica acta. 2017;986:1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Gámez-Valero A, Monguió-Tortajada M, Carreras-Planella L, Beyer K, Borras FE. Size-exclusion chromatography-based isolation minimally alters extracellular vesicles’ characteristics compared to precipitating agents. Scientific reports. 2016;6:33641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Paolini L, Zendrini A, Di Noto G, Busatto S, Lottini E, Radeghieri A, et al. Residual matrix from different separation techniques impacts exosome biological activity. Scientific reports. 2016;6:23550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Wang Z, Wu H-j, Fine D, Schmulen J, Hu Y, Godin B, et al. Ciliated micropillars for the microfluidic-based isolation of nanoscale lipid vesicles. Lab Chip. 2013;13(15):2879–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Huang LR, Cox EC, Austin RH, Sturm JC. Continuous particle separation through deterministic lateral displacement. Science. 2004;304(5673):987–90. [DOI] [PubMed] [Google Scholar]
- 208.Santana SM, Antonyak MA, Cerione RA, Kirby BJ. Microfluidic isolation of cancer-cell-derived microvesicles from hetergeneous extracellular shed vesicle populations. Biomedical microdevices. 2014; 16(6):869–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Wu M, Ouyang Y, Wang Z, Zhang R, Huang P-H, Chen C, et al. Isolation of exosomes from whole blood by integrating acoustics and micro fluidics. Proceedings of the National Academy of Sciences. 2017; 114(40): 10584–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Hedlund M, Stenqvist A-C, Nagaeva O, Kjellberg L, Wulff M, Baranov V, et al. Human placenta expresses and secretes NKG2D ligands via exosomes that down-modulate the cognate receptor expression: evidence for immunosuppressive function. The Journal of Immunology. 2009;183(1):340–51. [DOI] [PubMed] [Google Scholar]
- 211.Mincheva- Nilsson L, Baranov V. Placenta- derived exosomes and syncytiotrophoblast microparticles and their role in human reproduction: immune modulation for pregnancy success. American journal of reproductive immunology. 2014;72(5):440–57. [DOI] [PubMed] [Google Scholar]
- 212.Kanwar SS, Dunlay CJ, Simeone DM, Nagrath S. Microfluidic device (ExoChip) for on-chip isolation, quantification and characterization of circulating exosomes. Lab Chip. 2014;14(11):1891–900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Chen C, Skog J, Hsu C-H, Lessard RT, Balaj L, Wurdinger T, et al. Microfluidic isolation and transcriptome analysis of serum microvesicles. Lab Chip. 2010;10(4):505–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Zhao Z, Yang Y, Zeng Y, He M. A microfluidic ExoSearch chip for multiplexed exosome detection towards blood-based ovarian cancer diagnosis. Lab Chip. 2016;16(3):489–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.He M, Crow J, Roth M, Zeng Y, Godwin AK. Integrated immunoisolation and protein analysis of circulating exosomes using microfluidic technology. Lab Chip. 2014;14(19):3773–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Dawe GS, Tan XW, Xiao Z-C. Cell migration from baby to mother. Cell adhesion & migration. 2007;l(1):19–27. [PMC free article] [PubMed] [Google Scholar]
- 217.Huang C-E, Ma G-C, Jou H-J, Lin W-H, Lee D-J, Lin Y-S, et al. Noninvasive prenatal diagnosis of fetal aneuploidy by circulating fetal nucleated red blood cells and extravillous trophoblasts using silicon-based nanostructured microfluidics. Molecular cytogenetics. 2017; 10(1):44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Wei X, Cai B, Chen K, Cheng L, Zhu Y, Wang Z, et al. Enhanced isolation and release of fetal nucleated red blood cells using multifunctional nanoparticle-based micro fluidic device for non-invasive prenatal diagnostics. Sensors and Actuators B: Chemical. 2019;281:131–8. [Google Scholar]
- 219.Zhang H, Yang Y, Li X, Shi Y, Hu B, An Y, et al. Frequency-enhanced transferrin receptor antibody-labelled microfluidic chip (FETAL-Chip) enables efficient enrichment of circulating nucleated red blood cells for non-invasive prenatal diagnosis. Lab Chip. 2018; 18(18):2749–56. [DOI] [PubMed] [Google Scholar]
- 220.Feng B, Luo Y, Ge F, Wang L, Huang L, Dai YJS, et al. Site- oriented immobilization of fusion antigen directed by an affinity ligand, and its validation in an immunoassay. 2011;43(10): 1304–10. [Google Scholar]
- 221.Schmidt M, Hoffmann B, Beelen D, Gellhaus A, Winterhager E, Kimmig R, et al. Detection of circulating trophoblast particles in peripheral maternal blood in preeclampsia complicated pregnancies. Hypertension in pregnancy. 2008;27(2):131–42. [DOI] [PubMed] [Google Scholar]
- 222.Holzgreve W, Zhong XY, Troeger C, Haari I, Kiefer V, Hahn S, editors. Fetal cells in maternal Blood An Overview of the Basel Experience. Fetal Cells and Fetal DNA in Maternal Blood: New Developments for a New Millennium: 11th Fetal Cell Workshop, Basel, April 15, 2000; 2001: Karger Medical and Scientific Publishers. [Google Scholar]
- 223.Coleman S, Gerza F, Jones C, Sibley C, Aplin J, Heazell A. Syncytial nuclear aggregates in normal placenta show increased nuclear condensation, but apoptosis and cytoskeletal redistribution are uncommon. Placenta. 2013;34(5):449–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Vestergaard EM, Singh R, Schelde P, Hatt F, Ravn K, Christensen R, et al. On the road to replacing invasive testing with cell- based NIPT: Five clinical cases with aneuploidies, microduplication, unbalanced structural rearrangement, or mosaicism. Prenatal diagnosis. 2017:37(11):1120–4. [DOI] [PubMed] [Google Scholar]
- 225.Winter M, Hardy T, Rezaei M, Nguyen V, Zander- Fox D, Ebrahimi Warkiani M, et al. Isolation of circulating fetal trophoblasts using inertial microfluidics for noninvasive prenatal testing. Advanced Materials Technologies. 2018;3(7):1800066. [Google Scholar]
- 226.Choi J, Smitz J. Futeinizing hormone and human chorionic gonadotropin: distinguishing unique physiologic roles. Gynecological Endocrinology. 2014:30(3): 174–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Fiao J-Y. Detection of human chorionic gonadotrophin hormone using a label-free epoxysilane-modified capacitive immunosensor. Applied microbiology and biotechnology. 2007;74(6): 1385–91. [DOI] [PubMed] [Google Scholar]
- 228.Zhang B, Mao Q, Zhang X, Jiang T, Chen M, Yu F, et al. A novel piezoelectric quartz micro-array immunosensor based on self-assembled monolayer for determination of human chorionic gonadotropin. Biosensors and Bioelectronics. 2004; 19(7):711–20. [DOI] [PubMed] [Google Scholar]
- 229.Idegami K, Chikae M, Kerman K, Nagatani N, Yuhi T, Endo T, et al. Gold Nanoparticle- Based Redox Signal Enhancement for Sensitive Detection of Human Chorionic Gonadotropin Hormone. Electroanalysis: An International Journal Devoted to Fundamental and Practical Aspects of Electroanalysis. 2008;20(1): 14–21. [Google Scholar]
- 230.Chung NN, Hamlin RE, Zwang TJ, Johal MS. Human chorionic gonadotropin interactions with immobilized anti- hCG studied by quartz- crystal microbalance with dissipation monitoring. Journal of Biomedical Materials Research Part A. 2012; 100(6): 1600–4. [DOI] [PubMed] [Google Scholar]
- 231.Hung M-S, Chang H-Y. A simple microfluidics for real-time plasma separation and hCG detection from whole blood. Journal of the Chinese Institute of Engineers. 2015;38(6):685–91. [Google Scholar]
- 232.Tripathi S, Kumar YB, Agrawal A, Prabhakar A, Joshi SS. Microdevice for plasma separation from whole human blood using bio-physical and geometrical effects. Scientific reports. 2016;6:26749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Zhao Z, Al-Ameen MA, Duan K, Ghosh G, Eo JF. On-chip porous microgel generation for microfluidic enhanced VEGF detection. Biosensors and Bioelectronics. 2015;74:305–12. [DOI] [PubMed] [Google Scholar]
- 234.Hofmann H, Hillger F, Pfeil SH, Hoffmann A, Streich D, Haenni D, et al. Single-molecule spectroscopy of protein folding in a chaperonin cage. Proceedings of the National Academy of Sciences. 2010;107(26):11793–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Fipman EA, Schuler B, Bakajin O, Eaton WA. Single-molecule measurement of protein folding kinetics. Science. 2003;301(5637): 1233–5. [DOI] [PubMed] [Google Scholar]
- 236.Vahidi S, Stocks BB, Eiaghati-Mobarhan Y, Konermann E. Submillisecond protein folding events monitored by rapid mixing and mass spectrometry-based oxidative labeling. Anal Chem. 2013;85(18):8618–25. [DOI] [PubMed] [Google Scholar]
- 237.Zhang J, Zhou R, Inoue J, Mikawa T, Ha T. Single molecule analysis of Thermus thermophilus SSB protein dynamics on single-stranded DNA. Nucleic acids research. 2013;42(6):3821–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Lin X, Leung K-H, Lin L, Lin L, Lin S, Leung C-H, et al. Determination of cell metabolite VEGF 165 and dynamic analysis of protein-DNA interactions by combination of micro fluidic technique and luminescent switch-on probe. Biosensors and Bioelectronics. 2016;79:41–7. [DOI] [PubMed] [Google Scholar]
- 239.Wu P, van den Berg C, Alfirevic Z, O’Brien S, Rothlisberger M, Baker PN, et al. Early Pregnancy Biomarkers in Pre-Eclampsia: A Systematic Review and Meta-Analysis. Int J Mol Sci. 2015;16(9):23035–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Park HJ, Kim SH, Jung YW, Shim SS, Kim JY, Cho YK, et al. Screening models using multiple markers for early detection of late-onset preeclampsia in low-risk pregnancy. BMC pregnancy and childbirth. 2014;14(1):35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Sorensen KM, El- Segaier M, Femlund E, Errami A, Bouvagnet P, Nehme N, et al. Screening of congenital heart disease patients using multiplex ligation- dependent probe amplification: Early diagnosis of syndromic patients. American Journal of Medical Genetics Part A. 2012;158(4):720–5. [DOI] [PubMed] [Google Scholar]
- 242.Lee S, Yoo NC, Choi J, Yoo NC, Yoo SM, Choi JH. Applications of DNA microarray in disease diagnostics. Journal of microbiology and biotechnology. 2009;19(7):635–46. [PubMed] [Google Scholar]
- 243.Hanash S Disease proteomics. Nature. 2003;422(6928):226. [DOI] [PubMed] [Google Scholar]
- 244.Oleinikov AV, Gray MD, Zhao J, Montgomery DD, Ghindilis AL, Dill K. Self-assembling protein arrays using electronic semiconductor microchips and in vitro translation. Journal of proteome research. 2003;2(3):313–9. [DOI] [PubMed] [Google Scholar]
- 245.Dill K, Montgomery D, Ghindilis A, Schwarzkopf K, Ragsdale S, Oleinikov A. Immunoassays based on electrochemical detection using microelectrode arrays. Biosensors and Bioelectronics. 2004;20(4):736–42. [DOI] [PubMed] [Google Scholar]
- 246.Araz MK, Apori AA, Salisbury CM, Herr AE. Microfluidic barcode assay for antibody-based confirmatory diagnostics. Lab Chip. 2013;13(19):3910–20. [DOI] [PubMed] [Google Scholar]
- 247.Zhou J, Tao F, Zhu J, Lin S, Wang Z, Wang X, et al. Portable tumor biosensing of serum by plasmonic biochips in combination with nanoimprint and microfluidics. Nanophotonics. 2018;8(2):307–16. [Google Scholar]
- 248.Shao H, Im H, Castro CM, Breakefield X, Weissleder R, Lee HJCr. New technologies for analysis of extracellular vesicles. 2018; 118(4): 1917–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Shao H, Yoon T-J, Liong M, Weissleder R, Lee HJBjon. Magnetic nanoparticles for biomedical NMR-based diagnostics. 2010;1(1): 142–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Shao H, Min C, Issadore D, Liong M, Yoon T-J, Weissleder R, et al. Magnetic nanoparticles and microNMR for diagnostic applications. 2012;2(1):55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.Brolo AGJNP. Plasmonics for fiiture biosensors. 2012;6(11):709. [Google Scholar]
- 252.Lee H, Sun E, Ham D, Weissleder RJNm. Chip-NMR biosensor for detection and molecular analysis of cells. 2008;14(8):869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253.Lee H, Yoon T-J, Figueiredo J-L, Swirski FK, Weissleder RJPotNAoS. Rapid detection and profiling of cancer cells in fine-needle aspirates. 2009; 106(30): 12459–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254.Shao H, Chung J, Balaj L, Charest A, Bigner DD, Carter BS, et al. Protein typing of circulating microvesicles allows real-time monitoring of glioblastoma therapy. 2012;18(12): 1835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255.Jeong S, Park J, Pathania D, Castro CM, Weissleder R, Lee HJAn. Integrated magneto-electrochemical sensor for exosome analysis. 2016; 10(2): 1802–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256.Leuvering JH, Thai P, Waart Mvd, Schuurs A. Sol particle immunoassay (SPIA). Journal of immunoassay. 1980; 1(1): 77–91. [DOI] [PubMed] [Google Scholar]
- 257.Song Y, Zhang Y, Bernard PE, Reuben JM, Ueno NT, Arlinghaus RB, et al. Multiplexed volumetric bar-chart chip for point-of-care diagnostics. Nature communications. 2012;3:1283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258.Balakrishnan S, Hashim U, Gopinath SC, Poopalan P, Ramayya H, Omar MI, et al. A point-of-care immunosensor for human chorionic gonadotropin in clinical urine samples using a cuneated polysilicon nanogap lab-on-chip. PLoS One. 2015; 10(9):e0137891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259.Lun FM, Chiu RW, Chan KA, Leung TY, Lau TK, Lo YD. Microfluidics digital PCR reveals a higher than expected fraction of fetal DNA in maternal plasma. Clinical chemistry. 2008;54(10):1664–72. [DOI] [PubMed] [Google Scholar]
- 260.Enquobahrie DA, Meller M, Rice K, Psaty BM, Siscovick DS, Williams MA. Differential placental gene expression in preeclampsia. American journal of obstetrics and gynecology. 2008; 199(5):566 e1–e11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261.Okazaki S, Sekizawa A, Purwosunu Y, Farina A, Wibowo N, Okai T. Placenta-derived, cellular messenger RNA expression in the maternal blood of preeclamptic women. Obstetrics & Gynecology. 2007; 110(5): 1130–6. [DOI] [PubMed] [Google Scholar]
- 262.Pang WW, Tsui MH, Sahota D, Leung TY, Lau TK, Lo YD, et al. A strategy for identifying circulating placental RNA markers for fetal growth assessment. Prenatal diagnosis. 2009;29(5):495–504. [DOI] [PubMed] [Google Scholar]
- 263.Tsang JC, Vong JS, Ji L, Poon LC, Jiang P, Lui KO, et al. Integrative single-cell and cell-free plasma RNA transcriptomics elucidates placental cellular dynamics. Proceedings of the National Academy of Sciences. 2017; 114(37):E7786–E95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 264.Ghindilis AL, Chesnokov O, Ngasala B, Smith MW, Smith K, Martensson A, et al. Detection of sub-microscopic blood levels of Plasmodium falciparum using Tandem Oligonucleotide Repeat Cascade Amplification (TORCA) assay with an attomolar detection limit. Sci Rep-Uk. 2019;9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265.Malhotra R, Patel V, Vaque JP, Gutkind JS, Rusling JFJAc. Ultrasensitive electrochemical immunosensor for oral cancer biomarker IL-6 using carbon nanotube forest electrodes and multilabel amplification. 2010;82(8):3118–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 266.Munge BS, Fisher J, Millord LN, Krause CE, Dowd RS, Rusling JFJA. Sensitive electrochemical immunosensor for matrix metalloproteinase-3 based on single-wall carbon nanotubes. 2010; 135(6): 1345–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 267.Liu G, Liu J, Davis TP, Gooding JJJB, Bioelectronics. Electrochemical impedance immunosensor based on gold nanoparticles and aryl diazonium salt functionalized gold electrodes for the detection of antibody. 2011;26(8):3660–5. [DOI] [PubMed] [Google Scholar]
- 268.Chullasat K, Kanatharana P, Limbut W, Nunmuam A, Thavarungkul PJB, Bioelectronics. Ultra trace analysis of small molecule by label-free impedimetric immunosensor using multilayer modified electrode. 2011;26(11):4571–8. [DOI] [PubMed] [Google Scholar]
- 269.Kong F-Y, Xu M-T, Xu J-J, Chen H-YJT. A novel lable-free electrochemical immunosensor for carcinoembryonic antigen based on gold nanoparticles-thionine-reduced graphene oxide nanocomposite film modified glassy carbon electrode. 2011;85(5):2620–5. [DOI] [PubMed] [Google Scholar]
- 270.Leng C, Wu J, Xu Q, Lai G, Ju H, Yan FJB, et al. A highly sensitive disposable immunosensor through direct electro-reduction of oxygen catalyzed by palladium nanoparticle decorated carbon nanotube label. 2011;27(1):71–6. [DOI] [PubMed] [Google Scholar]
- 271.Tang J, Hu R, Wu Z-S, Shen G-L, Yu R-QJT. A highly sensitive electrochemical immunosensor based on coral-shaped AuNPs with CHITs inorganic-organic hybrid film. 2011;85(1):117–22. [DOI] [PubMed] [Google Scholar]
- 272.Liu Y, Liu Y, Qiao L, Liu Y, Liu B. Advances in signal amplification strategies for electrochemical biosensing. Current Opinion in Electrochemistry. 2018. [Google Scholar]
- 273.Sezginttirk MK, Uygun ZO. An impedimetric vascular endothelial growth factor biosensor-based PAMAM/cysteamine-modified gold electrode for monitoring of tumor growth. Analytical biochemistry. 2012;423(2):277–85. [DOI] [PubMed] [Google Scholar]
- 274.Kwon OS, Park SJ, Jang J. A high-performance VEGF aptamer functionalized polypyrrole nanotube biosensor. Biomaterials. 2010;31(17):4740–7. [DOI] [PubMed] [Google Scholar]
- 275.Rosales-Rivera L, Acero-Sanchez J, Lozano-Sanchez P, Katakis I, O’Sullivan CJB, Bioelectronics. Electrochemical immunosensor detection of antigliadin antibodies from real human serum. 2011. ;26(11):4471–6. [DOI] [PubMed] [Google Scholar]
- 276.You M, Yang S, Tang W, Zhang F, He P-G. Ultrasensitive electrochemical detection of glycoprotein based on boronate affinity sandwich assay and signal amplification with functionalized Si02@ Au nanocomposites. ACS applied materials & interfaces. 2017;9(16)il3855–64. [DOI] [PubMed] [Google Scholar]
- 277.Feng B, Zhu R, Xu S, Chen Y, Di J. A sensitive LSPR sensor based on glutathione-functionalized gold nanoparticles on a substrate for the detection of Pb 2+ ions. RSC Advances. 2018;8(8):4049–56. [Google Scholar]
- 278.Gao R, Ko J, Cha K, Jeon JH, Rhie G-e, Choi J, et al. Fast and sensitive detection of an anthrax biomarker using SERS-based solenoid micro fluidic sensor. Biosensors and Bioelectronics. 2015;72:230–6. [DOI] [PubMed] [Google Scholar]
- 279.Kilic O, Pamies D, Lavell E, Schiapparelli P, Feng Y, Hartung T, et al. Brain-on-a-chip model enables analysis of human neuronal differentiation and chemotaxis. Lab Chip. 2016;16(21):4152–62. [DOI] [PubMed] [Google Scholar]
- 280.Park J, Lee BK, Jeong GS, Hyun JK, Lee CJ, Lee S-H. Three-dimensional brain-on-a-chip with an interstitial level of flow and its application as an in vitro model of Alzheimer’s disease. Lab Chip. 2015; 15(1): 141–50. [DOI] [PubMed] [Google Scholar]
- 281.Zhang YS, Arneri A, Bersini S, Shin S-R, Zhu K, Goli-Malekabadi Z, et al. Bioprinting 3D microfibrous scaffolds for engineering endothelialized myocardium and heart-on-a-chip. Biomaterials. 2016;110:45–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 282.Marsano A, Conficconi C, Lernme M, Occhetta P, Gaudiello E, Votta E, et al. Beating heart on a chip: a novel microfluidic platform to generate functional 3D cardiac microtissues. Lab Chip. 2016;16(3):599–610. [DOI] [PubMed] [Google Scholar]
- 283.Bhise NS, Manoharan V, Massa S, Tamayol A, Ghaderi M, Miscuglio M, et al. A liver-on-a-chip platform with bioprinted hepatic spheroids. Biofabrication. 2016;8(1):014101. [DOI] [PubMed] [Google Scholar]
- 284.Lee K-H, Lee J, Lee S-H. 3D liver models on a microplatform: well-defined culture, engineering of liver tissue and liver-on-a-chip. Lab Chip. 2015;15(19):3822–37. [DOI] [PubMed] [Google Scholar]
- 285.Stucki AO, Stucki JD, Hall SR, Felder M, Mermoud Y, Schmid RA, et al. A lung-on-a-chip array with an integrated bio-inspired respiration mechanism. Lab Chip. 2015;15(5): 1302–10. [DOI] [PubMed] [Google Scholar]
- 286.Maschmeyer I, Lorenz AK, Schimek K, Hasenberg T, Ranime AP, Htibner J, et al. A four-organ-chip for interconnected long-term co-culture of human intestine, liver, skin and kidney equivalents. Lab Chip. 2015;15(12):2688–99. [DOI] [PubMed] [Google Scholar]
- 287.Mateme E-M, Ranime AP, Terrasso AP, Serra M, Alves PM, Brito C, et al. A multi-organ chip co-culture of neurospheres and liver equivalents for long-term substance testing. Journal of biotechnology. 2015;205:36–46. [DOI] [PubMed] [Google Scholar]
- 288.Lager S, Powell TL. Regulation of nutrient transport across the placenta. Journal of pregnancy. 2012;2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 289.Syme MR, Paxton JW, Keelan JA. Drug transfer and metabolism by the human placenta. Clinical pharmacokinetics. 2004;43(8):487–514. [DOI] [PubMed] [Google Scholar]
- 290.Sibley C Understanding placental nutrient transfer-why bother? New biomarkers of fetal growth. The Journal of physiology. 2009;587(14):3431–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 291.Spencer R, Carr D, David A. Treatment of poor placentation and the prevention of associated adverse outcomes–what does the future hold? Prenatal diagnosis. 2014;34(7):677–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 292.Miura S, Morimoto Y, Takeuchi S, editors. Multi-layered placental barrier structure integrated with microfluidic channels. 2013 IEEE 26th International Conference on Micro Electro Mechanical Systems (MEMS); 2013: IEEE. [Google Scholar]
- 293.Miura S, Sato K, Kato-Negishi M, Teshima T, Takeuchi S. Fluid shear triggers microvilli formation via mechanosensitive activation of TRPV6. Nature communications. 2015;6:8871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 294.Lee JS, Romero R, Han YM, Kim HC, Kim CJ, Hong J-S, et al. Placenta-on-a-chip: a novel platform to study the biology of the human placenta. The Journal of Maternal-Fetal & Neonatal Medicine. 2016;29(7):1046–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 295.Blundell C, Tess ER, Schanzer AS, Coutifaris C, Su EJ, Parry S, et al. A microphysiological model of the human placental barrier. Lab Chip. 2016; 16(16):3065–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 296.Blundell C, Yi YS, Ma L, Tess ER, Farrell MJ, Georgescu A, et al. Placental drug transport- on- a- chip: A microengineered in vitro model of transporter- mediated drug efflux in the human placental barrier. Advanced healthcare materials. 2018;7(2): 1700786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 297.Yin F, Zhu Y, Zhang M, Yu H, Chen W, Qin J. A 3D human placenta-on-a-chip model to probe nanoparticle exposure at the placental barrier. Toxicology in Vitro. 2019;54:105–13. [DOI] [PubMed] [Google Scholar]
- 298.Mandt D, Gruber P, Markovic M, Tromayer M, Rothbauer M, Kratz SRA, et al. Fabrication of biomimetic placental barrier structures within a microfluidic device utilizing two-photon polymerization. International Journal of Bioprinting. 2018;4(2). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 299.Hsieh Y-C, Zahn JD. On-chip microdialysis system with flow-through glucose sensing capabilities. Journal of diabetes science and technology. 2007; l(3):375–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 300.Jung D, Jung D, Kong S. A Lab-on-a-Chip-Based Non-Invasive Optical Sensor for Measuring Glucose in Saliva. Sensors. 2017; 17(11):2607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 301.Wang B, Wu Y, Chen Y, Weng B, Li C. Flexible paper sensor fabricated via in situ growth of Cu nanoflower on RGO sheets towards amperometrically non-enzymatic detection of glucose. Sensors and Actuators B: Chemical. 2017;238:802–8. [Google Scholar]
- 302.Abbas Y, Oefiier CM, Polacheck WJ, Gardner L, Farrell L, Sharkey A, et al. A microfluidics assay to study invasion of human placental trophoblast cells. Journal of The Royal Society Interface. 2017;14(130):20170131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 303.Yang K, Wu J, Peretz-Soroka H, Zhu L, Li Z, Sang Y, et al. Mkit: A cell migration assay based on microfluidic device and smartphone. Biosensors and Bioelectronics. 2018;99:259–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 304.Kuo C-Y, Eranki A, Placone JK, Rhodes KR, Aranda-Espinoza H, Fernandes R, et al. Development of a 3D printed, bioengineered placenta model to evaluate the role of trophoblast migration in preeclampsia. ACS Biomaterials Science & Engineering. 2016;2(10): 1817–26. [DOI] [PubMed] [Google Scholar]
- 305.Liu NQ, Kaplan AT, Lagishetty V, Ouyang YB, Ouyang Y, Simmons CF, et al. Vitamin D and the regulation of placental inflammation. The Journal of Immunology. 2011;186(10):5968–74. [DOI] [PubMed] [Google Scholar]
- 306.Srinivas SK, Ma Y, Sammel MD, Chou D, McGrath C, Parry S, et al. Placental inflammation and viral infection are implicated in second trimester pregnancy loss. American journal of obstetrics and gynecology. 2006; 195(3):797–802. [DOI] [PubMed] [Google Scholar]
- 307.Zhu Y, Yin F, Wang H, Wang L, Yuan J, Qin J. Placental barrier-on-a-chip: modeling placental inflammatory responses to bacterial infection. ACS Biomaterials Science & Engineering. 2018;4(9):3356–63. [DOI] [PubMed] [Google Scholar]
- 308.Abrahams VM, Bole-Aldo P, Kim YM, Straszewski-Chavez SL, Chaiworapongsa T, Romero R, et al. Divergent trophoblast responses to bacterial products mediated by TLRs. The Journal of Immunology. 2004;173(7):4286–96. [DOI] [PubMed] [Google Scholar]
- 309.Chen Y, Pui TS, Kongsuphol P, Tang KC, Arya SK. Aptamer-based array electrodes for quantitative interferon-γ detection. Biosensors and Bioelectronics. 2014;53:257–62. [DOI] [PubMed] [Google Scholar]
- 310.Liu Y, Matharu Z, Rahimian A, Revzin A. Detecting multiple cell-secreted cytokines from the same aptamer-functionalized electrode. Biosensors and Bioelectronics. 2015;64:43–50. [DOI] [PubMed] [Google Scholar]
- 311.Picollet-D’hahan N Live cell analysis: when electric detection interfaces microfluidics. Journal of Biochips and Tissue Chips S. 2011; 1:001. [Google Scholar]
- 312.Ge Y, Deng T, Zheng X. Dynamic monitoring of changes in endothelial cell-substrate adhesiveness during leukocyte adhesion by microelectrical impedance assay. Acta biochimica etbiophysica Sinica. 2009;41(3):256–62. [DOI] [PubMed] [Google Scholar]
- 313.Sedgwick JB, Menon I, Gem JE, Busse WW. Effects of inflammatory cytokines on the permeability of human lung micro vascular endothelial cell monolayers and differential eosinophil transmigration. The Journal of allergy and clinical immunology. 2002; 110(5):752–6. [DOI] [PubMed] [Google Scholar]
- 314.Sun T, Swindle EJ, Collins JE, Holloway JA, Davies DE, Morgan H. On-chip epithelial barrier function assays using electrical impedance spectroscopy. Lab Chip. 2010; 10(12): 1611–7. [DOI] [PubMed] [Google Scholar]
- 315.Rahim S, Uren A. A real-time electrical impedance based technique to measure invasion of endothelial cell monolayer by cancer cells. Journal of visualized experiments : JoVE. 2011(50). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 316.Kidima WB. Syncytiotrophoblast Functions and Fetal Growth Restriction during Placental Malaria: Updates and Implication for Future Interventions. Biomed Res Int. 2015;2015(10):451735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 317.Benirschke K, Kauffmann P. Pathology of the human placenta. 4th edition ed: Springer; 2000. [Google Scholar]
- 318.Walter PR, Garin Y, Blot P. Placental pathologic changes in malaria. A histologic and ultrastmctural study. Am J Pathol. 1982;109(3):330–42. [PMC free article] [PubMed] [Google Scholar]
- 319.Duffy MF, Maier AG, Byrne TJ, Marty AJ, Elliott SR, O’Neill MT, et al. VAR2CSA is the principal ligand for chondroitin sulfate A in two allogeneic isolates of Plasmodium falciparum. Mol Biochem Parasitol. 2006; 148(2): 117–24. Epub 2006 Apr 7. [DOI] [PubMed] [Google Scholar]
- 320.Salanti A, Staalsoe T, Lavstsen T, Jensen AT, Sowa MP, Amot DE, et al. Selective upregulation of a single distinctly structured var gene in chondroitin sulphate A-adhering Plasmodium falciparum involved in pregnancy-associated malaria. Mol Microbiol. 2003;49(1): 179–91. [DOI] [PubMed] [Google Scholar]
- 321.Viebig NK, Levin E, Dechavanne S, Rogerson SJ, Gysin J, Smith JD, et al. Disruption of var2csa gene impairs placental malaria associated adhesion phenotype. PLoS One. 2007;2(9):e910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 322.Jenkins N, Wu Y, Chakravorty S, Kai O, Marsh K, Craig A. Plasmodium falciparum intercellular adhesion molecule-1-based cytoadherence-related signaling in human endothelial cells. J Infect Dis. 2007;196(2):321–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 323.Yipp BG, Robbins SM, Resek ME, Baruch DI, Looareesuwan S, Ho M. Src-family kinase signaling modulates the adhesion of Plasmodium falciparum on human microvascular endothelium under flow. Blood. 2003;101(7):2850–7. [DOI] [PubMed] [Google Scholar]
- 324.Cunnington AJ, Walther M, Riley EM. Piecing together the puzzle of severe malaria. Sci Transl Med. 2013;5(211):211psl8. [DOI] [PubMed] [Google Scholar]
- 325.Francischetti IM, Seydel KB, Monteiro RQ. Blood coagulation, inflammation, and malaria. Microcirculation. 2008;15(2):81–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 326.Kim H, Higgins S, Liles WC, Kain KC. Endothelial activation and dysregulation in malaria: a potential target for novel therapeutics. Current opinion in hematology. 2011;18(3): 177–85. [DOI] [PubMed] [Google Scholar]
- 327.van der Heyde HC, Nolan J, Combes V, Gramaglia I, Grau GE. A unified hypothesis for the genesis of cerebral malaria: sequestration, inflammation and hemostasis leading to microcirculatory dysfunction. Trends in parasitology. 2006;22(11):503–8. [DOI] [PubMed] [Google Scholar]
- 328.Galbraith RM, Fox H, Hsi B, Galbraith GM, Bray RS, Faulk WP. The human matemo-foetal relationship in malaria. II. Histological, ultrastmctural and immunopathological studies of the placenta. Trans R Soc Trop Med Hyg. 1980;74(1):61–72. [DOI] [PubMed] [Google Scholar]
- 329.Fried M, Duffy PE. Maternal malaria and parasite adhesion. J Mol Med (Berl). 1998;76(3–4): 162–71. [DOI] [PubMed] [Google Scholar]
- 330.Umbers AJ, Aitken EH, Rogerson SJ. Malaria in pregnancy: small babies, big problem. Trends in parasitology. 2011;27(4): 168–75. [DOI] [PubMed] [Google Scholar]
- 331.McGregor IA. Thoughts on malaria in pregnancy with consideration of some factors which influence remedial strategies. Parassitologia. 1987;29(2–3): 153–63. [PubMed] [Google Scholar]
- 332.Duffy PE, Fried M. Antibodies that inhibit Plasmodium falciparum adhesion to chondroitin sulfate A are associated with increased birth weight and the gestational age of newborns. Infect Immun. 2003. ;71(11):6620–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 333.Fried M, Nosten F, Brockman A, Brabin BJ, Duffy PE. Maternal antibodies block malaria. Nature. 1998;395(6705):851–2. [DOI] [PubMed] [Google Scholar]
- 334.Fried M, Duffy PE. Adherence of Plasmodium falciparum to chondroitin sulfate A in the human placenta. Science. 1996;272(5267): 1502–4. [DOI] [PubMed] [Google Scholar]
- 335.Rogerson SJ, Chaiyaroj SC, Ng K, Reeder JC, Brown GV. Chondroitin sulfate A is a cell surface receptor for Plasmodium falciparum-infected erythrocytes. J Exp Med. 1995; 182(1): 15–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 336.Salanti A, Dahlback M, Turner L, Nielsen MA, Barfod L, Magistrado P, et al. Evidence for the involvement of VAR2CSA in pregnancy-associated malaria. J Exp Med. 2004;200(9): 1197–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 337.Castelucci M, Kaufman P. Basic Structure of the Villous Trees In: Benirschke K, Kaufmann P, Baergen RN, editors. Pathology of the Human Placenta. 5th ed. New York: Springer-Verlag; 2006. p. 50–120. [Google Scholar]
- 338.Orendi K, Kivity V, Sammar M, Grimpel Y, Gonen R, Meiri H, et al. Placental and trophoblastic in vitro models to study preventive and therapeutic agents for preeclampsia. Placenta. 2011:32 Suppl:S49–54. [DOI] [PubMed] [Google Scholar]
- 339.Lybbert J, Gullingsrud J, Chesnokov O, Turyakira E, Dhorda M, Guerin PJ, et al. Abundance of megalin and Dab2 is reduced in syncytiotrophoblast during placental malaria, which may contribute to low birth weight. Sci Rep. 2016;6(24508):24508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 340.Wang Y, editor Vascular biology of the placenta Colloquium Series on Integrated Systems Physiology: from Molecule to Function; 2010: Morgan & Claypool Life Sciences. [PubMed] [Google Scholar]
- 341.Gullingsrud J, Milman N, Saveria T, Chesnokov O, Williamson K, Srivastava A, et al. High-Throughput Screening Platform Identifies Small Molecules That Prevent Sequestration of Plasmodium falciparum-infected Erythrocytes. The Journal of infectious diseases. 2014:211(7): 1134–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 342.Chua CL, Brown G, Hamilton JA, Rogerson S, Boeuf P. Monocytes and macrophages in malaria: protection or pathology? Trends in parasitology. 2013;29(1):26–34. [DOI] [PubMed] [Google Scholar]
- 343.Menendez C, Ordi J, Ismail MR, Ventura PJ, Aponte JJ, Kahigwa E, et al. The impact of placental malaria on gestational age and birth weight. J Infect Dis. 2000:181(5): 1740–5. [DOI] [PubMed] [Google Scholar]
- 344.Silver KL, Zhong K, Leke RG, Taylor DW, Kain KC. Dysregulation of angiopoietins is associated with placental malaria and low birth weight. PLoS One. 2010;5(3):e9481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 345.Ataide R, Murillo O, Dombrowski JG, Souza RM, Lima FA, Lima GF, et al. Malaria in Pregnancy Interacts with and Alters the Angiogenic Profiles of the Placenta. PLoS Negl Trop Dis. 2015;9(6):e0003824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 346.Umbers AJ, Boeuf P, Clapham C, Stanisic DI, Baiwog F, Mueller I, et al. Placental malaria-associated inflammation disturbs the insulin-like growth factor axis of fetal growth regulation. J Infect Dis. 2011;203(4):561–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 347.Dimasuay KG, Aitken EH, Rosario F, Njie M, Glazier J, Rogerson SJ, et al. Inhibition of placental mTOR signaling provides a link between placental malaria and reduced birthweight. BMC medicine. 2017; 15(1): 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 348.Wang JL, Anguera MC. In Vitro Differentiation of Human Pluripotent Stem Cells into Trophoblastic Cells. Jove-J Vis Exp. 2017(121). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 349.Jiang B, Yan L, Wang X, Li E, Murphy K, Vaccaro K, et al. Concise Review: Mesenchymal Stem Cells Derived from Human Pluripotent Cells, an Unlimited and Quality-Controllable Source for Therapeutic Applications. Stem cells. 2019;37(5):572–81. [DOI] [PubMed] [Google Scholar]