Abstract
Cognitive fatigue (CF) impairs ability to perform daily activities, is a common complaint of aging and a symptom of multiple neurological conditions. However, knowledge of the neural basis of CF is limited. This is partially because CF is difficult to systematically modulate in brain imaging experiments. The most common approach has been to scan brain activity during effortful cognitive tasks. Consequently, neural correlates of CF tend to be task-specific and may vary across tasks. This makes it difficult to know how results generalize across studies and is outside the subjective experience of CF which tends to be similar in different tasks. It has been hypothesized that the subjective experience of CF might arise from domain general systems monitoring and acting on energy depletion in task specific circuits. Direct supporting neural evidence is lacking. By repeatedly scanning aging individuals undertaking four different tasks using functional Magnetic Resonance Imaging and referencing scans to detailed CF self-ratings taken before and after scanning, we sought task-general correlates of CF. We ran a data-driven representational similarity analysis, treating each brain region as a candidate CF functional connectivity hub, and correlating inter-participant differences in hub-based connectivity patterns with inter-participant differences in self-rated CF-profiles (a pattern of ratings across 18 questions). Both right insula and right putamen-based network connectivity patterns reflected CF across all tasks and could underpin subjective experience of CF.
Introduction
Cognitive fatigue, also known as mental fatigue, refers to the subjective sensation of a lack of mental as opposed to physical energy. Fatigue can be persistent (trait/chronic fatigue, Krupp et al. 1989) or short-term as induced by mentally demanding tasks (state fatigue, Lee et al. 1991). Our focus is on the latter, state cognitive fatigue, which shall be referred to as CF throughout. CF is a daily experience to many individuals and impairs ability to work, drive and operate machinery (e.g. Arnedt et al. 2005). Perhaps more importantly, CF is a common complaint of aging (Eldadah 2011) often resulting in the failure of older adults to self-initiate effortful behavior, and is a symptom of neurological conditions including multiple sclerosis (Krupp 2006), Parkinson’s disease (Friedman et al. 2007; Kluger et al. 2016), stroke (Lerdal et al. 2009), and traumatic brain injury (Bushnik et al. 2008). Despite this we still have only a limited understanding of how CF is represented in brain activity.
One reason for our limited knowledge of the neural basis of CF is that CF is difficult to systematically study with brain imaging. This is because CF builds slowly and is difficult to induce in a controlled way. Blain et al. (2016) overcame this by having participants undertake executive control (working memory and task switching) tasks for six hours, and scanning brain activity at the start, middle and end of this period. However most work has examined how neural activation changes as people fatigue whilst undertaking cognitive tasks during scanning (e.g. Cook et al. 2007, Lim et al. 2010, Tajima et al. 2010, Persson et al. 2013, Rocca et al. 2016; Wang et al. 2016, Wylie et al. 2017, Spiteri et al. 2017, Dobryakova et al. 2018, Dimitrakopoulos et al. 2018) and/or compared differences between patients with diseases that often accompany CF with controls (incl. brain structure Calabrese et al. 2010 and resting state functional connectivity Hampson et al. 2015).
Tasks used to induce CF in experiments (CF-manipulation tasks) have either tended to be mentally effortful or reward oriented, the latter because CF is associated with diminishing self-control / a desensitization to task outcome (e.g. Blain et al. 2016, Dobryakova et al. 2013). Mentally effortful tasks have included psychophysical tests that require sustained attention to complete and/or tax working memory or cognitive control processes (e.g. Lim et al. 2010, Lin et al. 2014; Persson et al. 2013, Wang et al. 2016, Wylie et al. 2017, Spiteri et al. 2017), and also more natural driving simulations (e.g. Dimitrakopoulos et al. 2018). Reward-oriented tests have employed gambling paradigms (e.g. Dobryakova et al. 2013 and Dobryakova et al. 2018). In analysis, behavioral measures such as reaction times or gambling performance during different time intervals are typically used as a proxy for CF (i.e. performance fatigability as per Kluger et al.’s 2013 taxonomy). Then these metrics are linked to changes in task specific brain activity.
Although studies grounded on task-specific performance measures and task-specific neural activity have been critical to gaining an initial understanding of the neural bases of fatigue, it is unclear how well they can generalize to different tasks that activate different brain regions and depend upon different performance metrics. Even though fatigue in one task can influence subsequent performance on a different task (e.g. Inzlicht and Schmeichel, 2012, Blain et al. 2016), task performance measures often end up uncorrelated with the subjective experience of cognitive fatigue (e.g. Dobryakova et al. 2013, Lin et al. 2014) which tends to be similar across tasks (e.g. Müller and Apps (2018). Subjective experience of CF can be measured behaviorally through questionnaires that measure how fatigued participants feel at the current time of assessment (e.g. the Visual Analogue Scale of Fatigue, Lee et al. 1991). Our current interest is in testing for neural correlates of CF that are common to different cognitive task types.
In their recent review Müller and Apps (2018) argue that cognitive (and physical fatigue) arises in circuits involved in task performance, and that a “domain general” system comprising dorsal anterior cingulate cortex, dorsolateral prefrontal cortex and anterior insula “monitors” the internal state of (and fatigue in) cognitive/physical task performing regions and chooses whether to continue exerting effort. This echoes Kluger et al. (2013) who hypothesized that frontal lobes and basal ganglia “act as a central governor to limit energy utilization and avoid energetic collapse”.
We take as our central hypothesis that there exist centers/hubs in the brain that reflect CF in a task-general fashion. To test this hypothesis, we scanned participants’ brain activity using functional Magnetic Resonance Imaging (fMRI) as they undertook four different cognitive tasks. Two of the tasks were classic executive function tasks that required sustained attention and as such were mentally effortful. These were a dual N-back task: more specifically a 1-back task, chosen to place demands on attention and working memory, but not be so challenging (> 1 back) that aging participants might give up. Also, a Stroop task, which places demands on cognitive control. We also examined two commonly used gambling tasks, because of the association between CF and diminished self-control/outcome desensitization. Both test financial decision making, and more specifically how well participants implicitly learn task-related risks/payoffs through experience. However, the two tasks differ in ideal gambling strategies. The Iowa Gambling Task (IGT) favors participants who make safe choices and learn to avoid initially attractive risky options. Conversely the Balloon Analogue Risk Task (BART) favors participants who make riskier but potentially more lucrative choices. We estimated CF using a comprehensive questionnaire (Lee et al. 1991) completed both before and after scanning so as to estimate CF arising during the scanning period. This contained 13 questions relating to cognitive fatigue, and 5 relating to energy levels. Participants were all healthy agers with no cognitive, behavioral, neurological, or psychiatric complications known to interfere with CF. Agers were tested in part because older individuals are especially susceptible to CF, and in part to provide a foundation for future tests on aging clinical populations.
To test for task-general neural correlates of fatigue, we selected a task-general (i.e. stimulus independent) measure of brain activity and computed functional connectivity separately for each task. The importance of deriving a stimulus independent neural measure here is to make precisely the same computational methods suitable for reapplication to neural data recorded under any circumstances. This includes cases when there is no explicit task or when there is a complex cognitive task for which there is no good stimulus model to use to reveal task specific activity. We anticipated that CF would be marked by a decrease in connectivity between putative CF-hubs that monitor task-related activity and neural systems that undertake it, possibly brought about by fatigue-related demotivation/inhibition. Because different combinations of brain regions are engaged by different cognitive tasks and therefore CF may be reflected by different CF-hub centered connectivity networks in different tasks, we designed our analysis to accommodate this.
We adopted a data-driven (brain region agnostic) approach to reveal CF sensitive brain regions: We treated each region in turn as a candidate CF-hub and represented that region as a vector of its functional connectivity coefficients with each other region (see Figure 1). To test for a relationship between regional functional connectivity vectors and CF self-rating profiles (18 questions) we used representational similarity analysis (RSA) (Kriegeskorte et al. 2008). This approach correlates inter-participant differences in hub-centric connectivity vectors with inter-participant differences in CF self-rating profiles, and thus detects when participants with similar hub-connectivity profiles also have similar CF profiles. Importantly, even if CF impacts different combinations of brain regions for different tasks, as long as those regions are functionally connected to a central hub then CF will be revealed by significant RSA correlations for that hub across all four tasks.
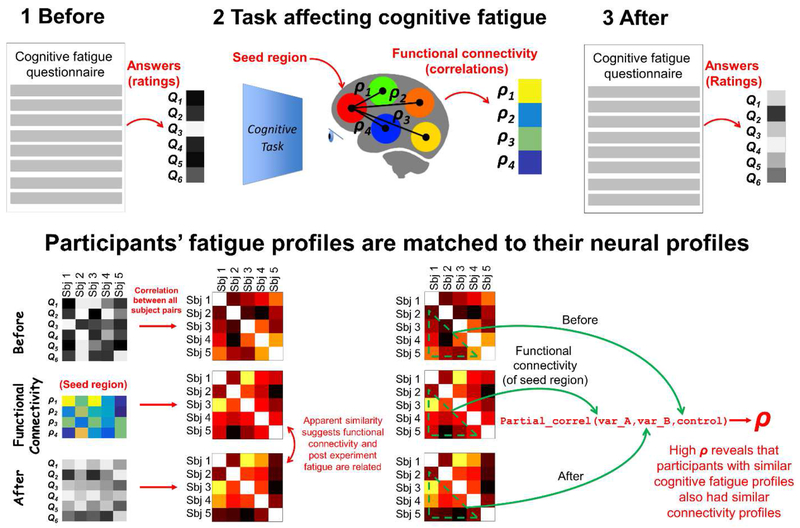
Figure 1.
Testing whether individual differences in task-associated CF match to individual differences in the FC of a “seed” brain region. The RSA in the bottom row was repeated many times treating each brain region in turn as a seed region. The analytical procedure was conducted for four CF-manipulation tasks separately.
Methods:
We report how we determined our sample size, all data exclusions, all inclusion/exclusion criteria, whether inclusion/exclusion criteria were established prior to data analysis, all manipulations, and all measures in the study.
Participants:
Fifty-two older adults with normal cognition (mean age = 71 (SD = 5.12), 69.2% were female, mean years of education = 17 (SD = 2.81)) indexed by Montreal Cognitive Assessment ≥ 26 and Rey Auditory Verbal Learning Test delayed recall ≥6, were recruited. Sample size was estimated via a power analysis undertaken in NIH R21 application: AG053193. Participants were required to have intact capacity to give consent (indexed by an intact score in the UCSD Brief Assessment of Capacity to Consent), to have adequate visual and auditory acuity for testing measured by self-report, to be free from major depression (indexed by the Geriatric Depression Scale, and a 15 item score of less than 6), not to have a sleep disorder (indexed by the Pittsburg Sleep Quality Inventory and a global sleep quality score of less than 14), and chronic fatigue (indexed by a score of less than 20 on the general fatigue subscale of the 20-item Multidimensional Fatigue Inventory), to be a minimum of 60 years of age, English-speaking, and community-dwelling. Exclusion criteria included (1) disease or medication potentially confounded with fatigue symptoms: neurologic or vascular disorders (e.g., multiple sclerosis, stroke, transient ischemic attack, heart attack, or traumatic brain injury within the past five years, severe cerebrovascular disease, Parkinson’s disease); an episode of a diagnosed and active psychiatric disorder (i.e., major depression, anxiety, bipolar disorder) within the past five years; schizophrenia (regardless of the time since the last episode); a clinical diagnosis of mild cognitive impairment or dementia as defined by DSM-5; change in medication or beta-blocker dosage within the past three months; and (2) MRI contraindications (e.g., pacemaker, metallic implant, claustrophobia). The study was approved by the university’s research subject review board.
General fMRI task protocol:
General fMRI task protocol: There were two MRI related study visits, occurring during a controlled morning window (8am-12pm) to avoid a potential diurnal fluctuation in the CF indices. Participants were instructed to eat breakfast but to avoid nicotine, caffeine, and exercise for at least 2 hours before their arrival. Participants rested for 5 minutes upon arrival and then rated their current state of pre-task CF (CF “before” rating) on an 18-item questionnaire (see next section). The MRI scan started with T1-weighted sequence and was followed by BOLD fMRI sequences for two CF-manipulation tasks that were scanned back to back for 15mins each with a brief pause in between for task switching. These were either two executive function-related tasks or two gambling tasks. Finally, after the two tasks had been completed, participants rated their post-task CF (CF “after” rating) immediately after they left the scanner.
The order that participants undertook the two gambling tasks or the two executive function tasks during each visit was pseudorandomized. This was such that for the gambling tasks 50% of participants undertook IGT first and 50% BART first, and for the executive function tasks 50% of participants undertook N-Back first and 50% Stroop first. 50% of participants performed the 2 gambling tasks during their first scanning visit and then the 2 executive function tasks during their second visit (and vice versa for the other 50%).
Each participant’s two visits were 2-weeks apart to reduce any cognitive or fatigue effects from the prior visit.
18-item state CF questionnaire:
To measure CF we used the questionnaire introduced by Lee et al. (1991) where participants rate their current state (how they felt “RIGHT NOW”) on a scale based on terms related to fatigue and energy (e.g. not at all tired/extremely tired, keeping my eyes open is not/extremely difficult, concentrating is/is not effortful, not at all/extremely energetic). However, instead of using a “visual analogue scale” to score responses to each question, we used a Likert scale ranging from 0 (“not at all”) to 10 (“very much”), and participants marked an X in the corresponding box. Participants were first shown an example, then given the scale to complete themselves. The complete questionnaire is in Supplementary Table 1.
Out of the 52 participants, one participant’s data was deemed invalid because they answered 0 for each question, and in so doing gave contradictory responses for questions anticipating a high value for fatigue (Q1 to Q5, and Q11 to Q18) and those anticipating a high value for “energy” (Q6 to Q10). This participant was consequently excluded from the entire analysis. This decision was made after data collection and the discovery of their contradictory responses. There were occasional cases of missing/invalid answers (10 entries in total). These missing entries were filled in by averaging across that same participant’s other answers. If the missing answer related to a question directly testing fatigue (Q1 to Q5 and Q11 to Q18) the other answers from that set of 13 questions were averaged. If the missing answer related to a question directly testing “energy” (Q6 to Q10) the other answers from that set of 5 questions were averaged.
CF-manipulation task paradigms:
Each participant completed two scans, one of which tested executive function, while the other tested gambling strategy. Each scan involved two 15-minute tasks, randomized in order, with a total scanning duration of 30 minutes. Executive function was assessed utilizing the Stroop and Dual 1-back tasks to evaluate inhibitory control and working memory, respectively. During the Stroop trials, participants were presented with the name of a single color (either “red” or “green”) as text on a black background; the font color was either red or green. Participants were instructed to identify the font color, regardless of the actual color name, as quickly and accurately as possible. Participants selected the font color by pressing one of two buttons and received feedback after each response, indicating whether the response was correct.
During the Dual 1-back trials, participants were presented with a series of capitalized letters from the English alphabet, one at a time, displayed in different locations on the screen. Each stimulus combination (type of letter and location) was selected randomly from a set of 21 letters and 15 different locations; lowercase letters and numbers were excluded from the stimulus set. Participants were instructed to judge whether the current stimulus matched the previous stimulus one screen prior in the sequence, as quickly and accurately as possible. Matched trials were identical in both letter type and location; mismatched trials differed in letter, location, or both variables. Participants indicated their judgment by pressing one of two buttons.
For both executive function tasks, each trial consisted of a 0.5-second fixation period (white dot), followed by a 4-second response window and a 1.5-second feedback display (“correct”/”incorrect”). Stroop stimuli were displayed for 3 seconds, with an inter-stimulus interval of 3 seconds. Color name and font color were randomized, such that each of the four possible name and color combinations appeared with equal probability (e.g. 25% of trials displayed the word “red” in the color red; 25% of trials displayed the word “red” in the color green). Dual 1-back stimuli were displayed for 2 seconds, with an inter-stimulus interval of 5 seconds. The stimulus set consisted of 21 letters of the alphabet, with 15 different possible locations, both of which were randomized for each trial. Probabilities for positive “match” and negative “nonmatch” trials were set at 0.65 and 0.35, respectively.
The two gambling tasks were IGT and BART. During IGT, participants were presented with four virtual decks of cards (A, B, C, and D) and were instructed to select one deck at a time, which either awarded or deducted a specified amount of money from their total. Decks yielded either high immediate gains with large future losses (risky decks, A and B) or low immediate gains with small future losses (safe decks, C and D). During BART, participants were presented with a virtual balloon in different colors (red, yellow, or blue) and instructed to “pump” up the balloon to earn monetary rewards. Greater inflation of a balloon increased the participants’ profit but also the probability of “popping” the balloon and losing earnings for that balloon. Balloon color corresponded to maximum inflation capacity and potential earnings (red<yellow<blue). Participants sought to maximize income during each trial while avoiding losses.
IGT trials had a 4-second response window, during which participants were required to play or pass a given deck. Feedback displaying the amount of money awarded or deducted was presented for 2 seconds. BART trials had a 3-second response window, during which participants were required to begin pumping the balloon. Feedback after balloon earnings were collected or lost was displayed for 2 seconds. An additional 2-second fixation (white dot) was presented in BART before stimulus onset to prompt participants for the subsequent trial. The inter-stimulus interval in both gambling tasks was set at 3 seconds.
Imaging data:
Imaging data were collected at the Rochester Center for Brain Imaging using a 3T Siemens TrioTIM scanner (Erlangen, Germany) equipped with a 32-channel receive-only head coil. The fMRI scan began with a MPRAGE scan (TR/TE = 2530/3.44 ms, TI = 1100 ms, FA = 7, matrix = 256×256, resolution 1×1×1mm, slice thickness = 1 mm, 192 slices). For all tasks fMRI data were collected using a gradient echo-planar imaging (EPI) sequence (TR/TE = 2500ms/30ms, FA = 90, slice thickness = 4 mm, matrix = 64 × 64, 4×4 mm in-plane resolution, 42 axial slices, number of volumes = 360).
fMRI Preprocessing:
The fMRI data were preprocessed using the Data Processing Assistant for Resting-State fMRI (DPARSFA) (Yan and Zang 2010) based on SPM8 (http://www.fil.ion.ucl.ac.uk/spm/). For each participant, the first 6 volumes of each fMRI scan were excluded to obtain steady-state tissue magnetization. The remaining 354 volumes were slice timing and head-motion corrected, co-registered to their own structural image, and normalized to Montreal Neurological Institute (MNI) standard space, resampling (3×3×3 mm). After that, all of the data were smoothed using Gaussian kernel (FWHM 4 mm). After removing the linear trend, data were filtered using a band pass of (.01–.08 Hz) for functional connectivity analysis. Before calculating functional connectivity, nuisance covariates were regressed out, including 6 head motion parameters, white matter signal, and cerebrospinal fluid signal. Paired t-tests revealed that there were no differences in average head motion for each of the 6 individual parameters (n=51 participants) between tasks (minimum t=1.79, p=0.08; maximum t=0.095, p=0.93). Our main analyses were conducted using this preprocessing set up. We conducted a further post-hoc analysis in which mean signal in all grey matter voxels is regressed out from each voxel’s time line.
Analytic procedure overview:
An overview of our main analysis is illustrated in Figure 1.
Functional connectivity:
Functional connectivity (FC) measures were computed from each participant’s fMRI data separately for each task as follows. First the time series of each voxel’s activation (across the full 15mins task period) was transformed to z-scores by subtracting the overall mean of that voxel’s activation (across the entire time series for that task) from each time point and then dividing by the overall standard deviation. Z-scoring was undertaken to assign each voxel within a brain region equal weight in the forthcoming analysis. The z-scored fMRI data was then parcellated into anatomical regions of interest (ROIs), n = 45 each hemisphere, defined according to a standardized neuroanatomical template (automated anatomical labeling, Tzourio-Mazoyer et al. 2002). The cerebellum and vermis were excluded.
For each participant, the z-scored fMRI data within each ROI was reduced to a single mean time series and then FC was estimated treating each ROI in turn as a “seed region”. FC with respect to the seed region was measured as Pearson’s correlation between the (mean) activity time series of the seed region and the (mean) time series of each other region in the same neural hemisphere. In a post hoc analysis we recompute FC based on the first principal component of fMRI activation computed within each ROI. Hemispheric connectivity was computed to isolate possible hemispheric effects that are commonly observed in behavior regulation (Stephan et al. 2007), and in particular because of lateralized functionality of candidate CF regions (e.g. Craig et al. 2009 and the Discussion section). However, our key results remained the same in a reanalysis when full brain connectivity was computed instead. Correlation coefficients between ROI pairs were then r to z transformed (i.e., arctanh) as is a customary step in analyzing correlation coefficients.
We then represented each region in terms of its functional connectivity to each other region. As there were 45 regions in each hemisphere, this meant that each region was represented as a vector of 44 r-to-z transformed correlation values. Consequently, the fMRI data for each participant for each task was transformed into a set of number-of-hemispheric-ROIs (45) FC vectors for each hemisphere. Following processing there were 4 such datasets per participant corresponding to the four different cognitive tasks. In total 360 connectivity vectors (each with 44 dimensions) were derived for each of the 51 participants.
Representational similarity analysis correlating individual differences in region-based connectivity to individual differences in self-rated CF
Our primary analysis treated CF-ratings in their original form with each participant represented as a pattern of ratings across 18 questions. To anticipate the concern that ratings of the different questions redundantly recapitulate the same information, in the first section of the results we examine inter-question correlation structure which suggests that this is not the case. To test for an association between participants’ (hemispheric) functional connectivity vectors for a particular seed region and participants’ self-rated CF (measured before and after that specific scanning session) we used RSA. RSA places both participants’ questionnaire data and fMRI data into a common representational space (inter-participant similarity space), enabling a test of the correlation between self-rated CF and fMRI. Specifically, we first computed inter-participant correlation matrices for the “before” and “after” CF questionnaire data separately. This yielded separate “before” and “after” correlation matrices, where each matrix entry corresponds to Pearson’s correlation between the 18 questionnaire item scores given by one participant and another. The matrix entry for a pair of participants who answered the questionnaire differently will be a low correlation coefficient and vice versa. The same process was repeated on the functional connectivity data for each seed region, yielding correlation matrices where each entry corresponds to Pearson’s correlation between two participants’ seed region connectivity vectors. Consequently, a pair of participants with different seed region connectivity patterns will receive a low correlation coefficient (and vice versa). This process is illustrated for both before/after questionnaire data and a single seed region in Figure 1.
The transformation of each participant’s questionnaire-based and seed region connectivity data into common “representational similarity spaces” enabled us to test for a relationship across the different data types. Specifically, we test whether the pattern of individual differences observed for the questionnaire data is also present in the seed region’s connectivity data. If a brain region is associated with self-rated CF in a particular task (whether by cause or effect) we would expect there to be a statistically significant correlation between the inter-participant similarity structure in corresponding correlation matrices. As is customary in RSA, we extracted the below diagonal matrix triangle from each correlation matrix (the correlation matrix is symmetric with ones along the diagonal) and vectorized this to produce a single vector (1275 dimensions). The relationship between questionnaire-based data and functional connectivity data (for each seed region) was quantified by correlating the respective vectors. Because we were principally interested in isolating the effects of CF arising during the fMRI experiments (rather than fatigue associated with the participant’s state before the task) we computed the partial (Spearman) correlation between the “after” CF vector and the vector of functional connectivity pairwise correlations, whilst controlling for the “before” CF vector. This is illustrated in Figure 1 (however in the actual analysis each of the three vectors has 1275 dimensions). This was repeated for each seed region, and the four tasks.
To test the statistical significance of the RSA correlation between self-rated CF and region-based functional connectivity we applied permutation testing as is customary in RSA (Kriegeskorte et al. 2008). Specifically, we randomly shuffled the list of participants’ IDs, and then re-arranged both rows and columns of only the functional connectivity correlation matrix according to this shuffled order. The triangle of the shuffled functional connectivity matrix was extracted, vectorized (1275 dimensions) and partially correlated (Spearman) with the original (unshuffled) “after” vector whilst controlling for the original (unshuffled) “before” vector. This process was repeated 10,000 times and yielded a null distribution of partial correlation coefficients reflecting results arising at chance. The p-value associated with the original (unshuffled) partial correlation was calculated as the proportion of chance (shuffled) partial correlations greater than or equal to the original (unshuffled) correlation coefficient. This entire permutation test was repeated independently (each time with 10,000 permutations) for each seed region and task, yielding number-of-ROIs (90) * number-of-tasks (4) p-values in total.
Data driven search for region-based networks reflecting cognitive fatigue across all tasks
Finally, to identify region-based connectivity networks that are sensitive to CF irrespective of the CF-manipulation task undertaken (see Figure 2) we searched for regions yielding significant RSA correlations across all tasks. To statistically evaluate this, we treated each RSA performed on each ROI as a binomial variable with the probability of a success (in this case a statistically significant result) arising at chance of p=0.05 (the standard significance threshold). We then computed the cumulative binomial probability of achieving ≥ n significant RSA results at chance, over all four tasks on that ROI (n is between 0 and 4). For instance, if n was 3 for a particular ROI, the p-value for that ROI would be computed as the binomial probability of achieving 3 successes summed with the binomial probability of getting 4 successes (as can be computed using the matlab function binopdf). This could be considered as analogous to throwing a twenty-sided die four times, checking for the number twenty each time, and evaluating the chance of throwing that number of twenties in four tests.
Figure 2.
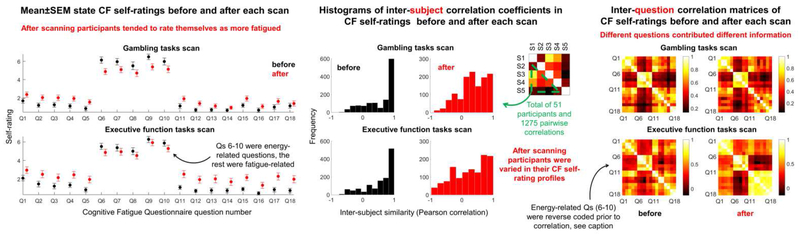
Left, Mean+/−SEM questionnaire ratings across participants, taken before scanning and then after scanning, for the two scan sessions (see Supplementary Table 1 for questions). Middle, histograms of inter-participant correlation coefficients in CF-self rating profiles (across questions, n=18 for each inter-subject correlation) taken before and after each scan. Right, matrices of inter-question correlation coefficients (across subjects, n=51 for each question pair correlation) taken before and after each scan. Ratings for energy-related questions were reverse coded prior to computing inter-question correlation matrices (rating=10-rating), so that they were likely to positively correlate with fatigue-related questions, and ease visual comparison of color-coded magnitudes.
The full complement of binomial tests yielded number-of-ROIs (90) p-values. We then applied False Discovery Rate (FDR, q=.05) to correct these 90 p-values (for 45 seed-based FC matrix per hemisphere) for multiple comparisons.
Pre-registration
No part of the study procedures or analyses was pre-registered prior to the research being conducted.
Data sharing statement
De-identified functional connectivity data, cognitive fatigue rating profiles, demographic information and analysis code necessary to regenerate results figures in the manuscript is available for download at https://osf.io/mt3du/.
Raw MRI data is Participant Health Information which will be available subject to an IRB amendment approved by the University of Rochester’s Institutional Review Board for R21 AG053193. This request can be initiated by a written correspondence to the senior author (Feng Lin) clearly stating the objectives and proposed analyses that will be undertaken on the data. The stimulus presentation code is the intellectual property of the University of Rochester. It will be made available through a signed agreement between the applicant’s University/company and the University of Rochester, which can be initiated by a written request to the senior author (Feng Lin).
Results
Inter-participant and inter-question differences in CF self-ratings before and after scanning
All subsequent analyses are based on relating inter-participant differences in behavioral CF rating profiles to inter-participant differences in neural connectivity profiles. For these analyses to be meaningful there must be diversity amongst participants in their CF-profiles. Additionally, for RSA to be warranted, the different CF-questions should contribute different information to each other, otherwise it would be more appropriate to reduce all 18 questions into a single CF-score and perform a univariate correlation analysis instead of the current pattern-based analysis. We first present qualitative evidence that both of the above were the case.
Figure 2 displays mean+/−SEM CF self-ratings for each of the 18 questions in the questionnaire before and after scanning (gambling top, executive function bottom), alongside histograms of inter-participant similarities in CF-profiles computed before and after scanning. This firstly serves to illustrate that participants tended to rate themselves as being more fatigued / less energetic after the scan than before. However, more important are the “after” histograms which provide evidence that scanning introduced diversity amongst participants in their CF profiles which was not there prior to scanning. Specifically, before scanning, both gambling and executive function histograms of inter-participant correlation coefficients are heavily left skewed, with the largest bin of coefficients (>500/1275) spanning r=0.8 to 1. This suggests that the vast majority of participants began the experiment with very similar levels of CF. After scanning, the degree of left skewness in corresponding histograms is visibly reduced, and uniformity increased, consistent with inter-participant diversity in CF profiles. Specifically, in both histograms (gambling/executive function) the largest bin of coefficients is no longer 0.8 to 1 (which has ~200 values), and most bins across the entire range [−1 to 1] contain upwards of 50 coefficients.
Also displayed in the rightmost panel of Figure 2 are inter-question correlation matrices quantifying the correlation in CF-ratings between each question pair (with each inter-question correlation being across n=51 participants). Correlation matrices are displayed for CF-ratings taken before and after both gambling and executive function tasks (4 matrices in total). Prior to computing correlations, we reverse coded energy-related question ratings (reverse rating=10-rating) to make them likely to correlate positively with fatigue-related ratings. This was to ease viewing of color-coded correlation magnitudes in Figure 2. If all 18 questions redundantly obtained very similar ratings (consistent with the different questions measuring the same quantity) then each correlation coefficient in the matrix would have a high value close to one (discounting the diagonal of ones). This was not the case, and complex structure was visually apparent in the matrices. Although many question pairs were correlated, and some to a high degree (maximum r=0.96 across all four matrices, discounting the matrix diagonal) many others were not (minimum r=−0.15 across all four matrices). The mean correlation coefficient across all four matrices was r=0.51 and the standard deviation was 0.35 with both computed after extracting the below diagonal triangle from each matrix, r-to-z-transforming (arctanh) values prior to computing descriptive statistics and then z-to-r (tanh) back transforming the statistic. The least correlated question pairs tended to be between energy-related and fatigue-related questions, however other patterning is apparent. For instance, in all four matrices questions 13 to 16 tend to have relatively low correlations to questions 1 to 6. Additionally, after the executive function tasks questions 11 to 18 form a visibly clear cluster. The complexities of these differences are beyond the scope of the current study. Suffice it to say we believe these complexities justify treating CF-ratings as a pattern across 18 questions in subsequent analyses, rather than reducing all 18 ratings to a single composite metric (e.g. by averaging them). However, in post hoc tests we explore how composite fatigue-related scores and composite energy-related scores correlate with functional connectivity in specific region pairs.
Individual differences in self-rated CF correlate with individual differences in functional connectivity across multiple tasks
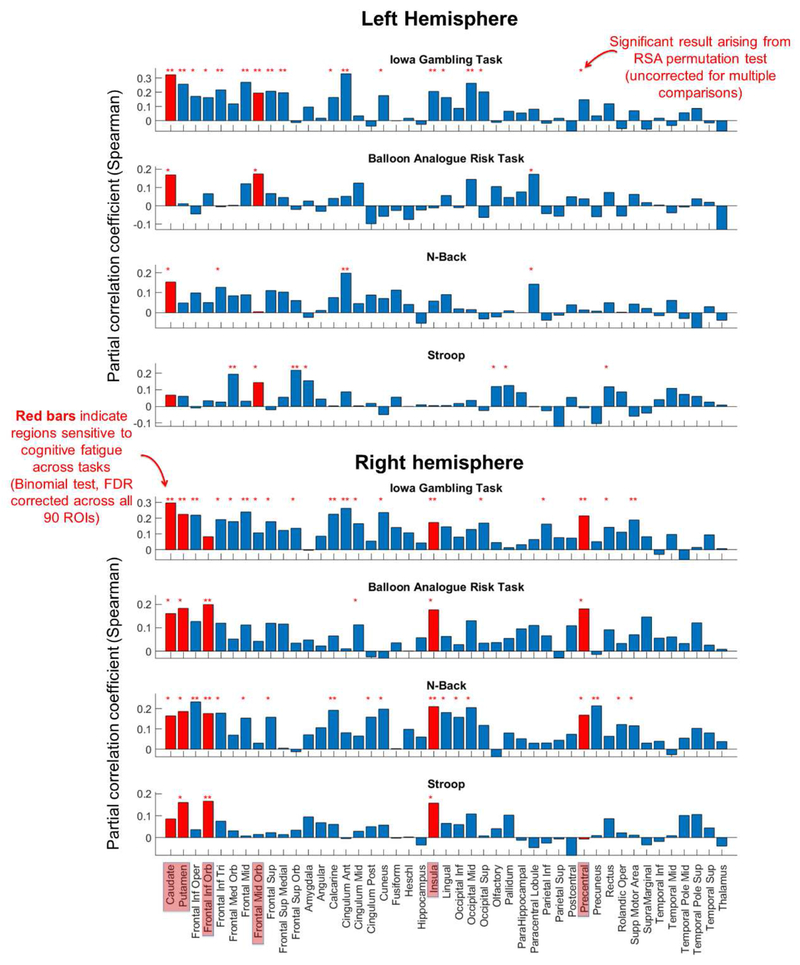
RSA results arising from the comparison of inter-participant differences in self-rated CF with region-based functional connectivity patterns are illustrated for each task and region in Figure 3. Red bars on Figure 3 indicate regions for which significant RSA correlations were detected across more tasks than would be expected at chance (cumulative binomial probability, where chance is given by p=0.05, and the number of tests is four). To recap, we consider such region-based networks as candidates for indicating CF in a cognitive task general fashion. Following FDR correction of the 90 cumulative binomial probabilities associated with the ROIs, seven seed regions were found to be significant.
Figure 3. RSA partial correlation between seed region connectivity patterns and self-rated CF after scanning controlling for CF before scanning (as illustrated in Figure 1).
Statistically significant correlational results that arose from the RSA permutation tests are indicated as red stars. ROIs that yielded more significant RSA results across the four tasks than expected at chance (Binomial test) are highlighted with red bars.
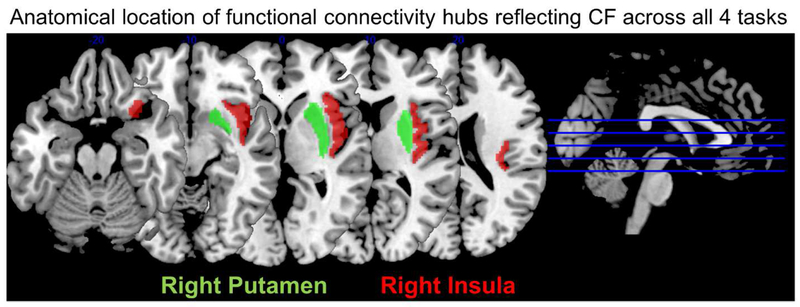
Our key result is that right insula and right putamen-based networks returned significant RSA correlations (p<0.05) in all four tasks (in each case Binomial p=0.0003, FDR corrected). This implicates these two regions as network hubs, whose inter-connectivity profile reflects CF in a task general fashion. The anatomical locations of right insula and putamen ROIs are illustrated in Figure 4.
Figure 4.
Anatomical location of right insula and putamen ROIs.
In addition, the left and right caudate, left middle frontal gyrus (MFG, frontal_mid_orbital in AAL), right inferior frontal gyrus (IFG, frontal_inferior_orbital in AAL), and right precentral gyrus (PG) yielded significant RSA results in 3/4 tasks (in each case Binomial p=0.006, FDR corrected). We take this as tentative evidence that connectivity patterns centered on these regions may reflect CF in a task general fashion.
In Supplementary Figures 1 and 2, we include companion results for when the RSA was performed on CF-self-ratings taken after scanning (without using partial correlation to control for before scanning CF) and CF self-ratings taken before scanning. RSA results derived from after-scan only CF-ratings identify 6 of the 7 ROIs of Figure 3 as reflecting CF (all but Left Caudate). RSA results derived from before-scan only CF-rating detected 0 regions, which is consistent with the low diversity amongst participants CF-profiles observed before scanning (Figure 2).
Post hoc tests of whether connectivity between particular ROI pairs correlated with self-rated CF across tasks
To test whether the binomial test results for the seven CF-related ROIs identified in the previous section were underpinned by (drops in) functional connectivity between specific pairs of brain regions, we conducted a mass univariate partial-correlation analysis.
To simplify interpretation of the analysis and reduce the number of tests computed, we first coarsely reduced the self-rated CF data for every single individual questionnaire by averaging together each individual’s answers for the 13 fatigue-related questions, and then doing the same for the 5 energy-related questions. Rather than being formed from 18 ratings, each questionnaire was thus reduced to a single composite “fatigue” score and a single composite “energy” score. As outlined in the behavioral results and Figure 2, although this data-reduction step simplifies interpretation it likely comes at the expense of losing information and results should be interpreted accordingly.
Then, function connectivity coefficients corresponding to a specific pair of ROIs for a specific task were selected for all participants. Participants’ functional connectivity values (n=51) were partially correlated first with participants’ composite-fatigue scores after scanning, whilst controlling for the corresponding scores before scanning, and second for composite-energy scores. Partial correlations were computed for each functional connectivity coefficient associated with the right insula, putamen, and the other five candidate CF-related ROIs. As each ROI’s connectivity vector was represented by 44 values, and there were 7 ROIs, 2 composite scores (energy/fatigue), and 4 tasks, a total of 2464 partial correlations were computed.
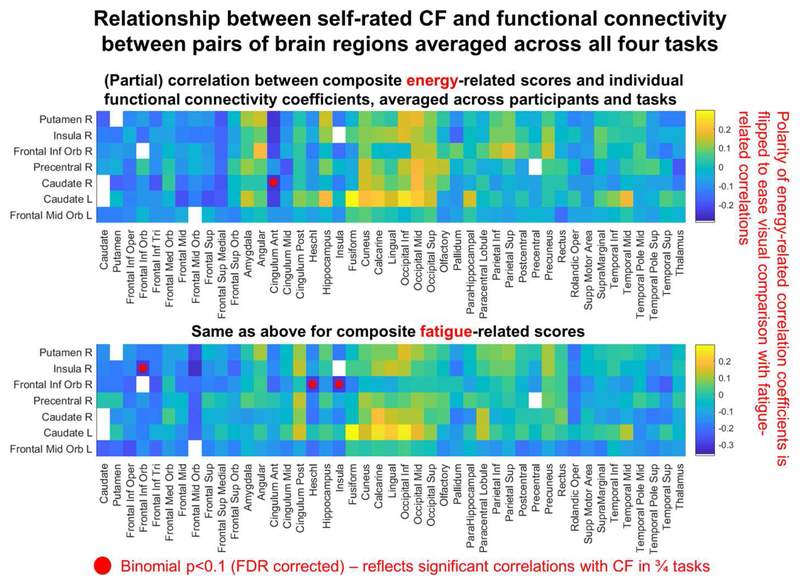
To test whether any ROI pair yielded significant correlations across all four tasks, we tested the cumulative binomial probability of achieving n or more significant results across the four tasks (the same as for the RSA). The resulting binomial p-values were then FDR corrected. The results are illustrated in Figure 5 (note that partial correlation coefficients associated with energy questions are flipped in polarity to ease visual comparison with the fatigue questions). Although no binomial p-value survived FDR correction, three ROI pairs yielded FDR corrected p-values <0.1 (corresponding to significant results in ¾ tasks). For fatigue questions ROI pairs were the right insula and right MFG (r= −0.32, −0.26, −0.31 and−0.31 for IGT, BART, N-Back and Stroop respectively) and also right MFG and right Heschl’s gyrus (r= −0.28, −0.29, −0.33 and −0.24 for IGT, BART, N-Back and Stroop). NB It is possible that the effect for Heschl’s gyrus (best known for containing primary auditory cortex), might have arisen out of imperfect automated anatomical parcellation from the anatomically neighboring insula. Otherwise auditory cortex is likely to have been activated by background scanning noise, but not the visually driven cognitive tasks. Negative correlations indicate that high fatigue-ratings were associated with low connectivity. For energy questions, p<0.1 was detected between right caudate and right anterior cingulate (r=0.34, −0.12, 0.35, 0.37 for IGT, BART, N-Back and Stroop). Positive correlations indicate that high energy-ratings were associated with high connectivity.
Figure 5.
Visualization of participants’ functional-connectivity coefficients for specific region pairs, partially correlated with participants’ CF self-rated after scanning whilst controlling for CF-ratings before scanning. In the top row each cell corresponds to a grand average taken across all 5 “energy” questions, 4 tasks, and 51 participants. In the bottom row each cell corresponds to a grand average taken across all 13 “fatigue” questions, 4 tasks and 51 participants. Correlation coefficients were all r-to-z transformed prior to averaging and then each average was z-to-r back transformed afterwards (computed using arctanh, then tanh respectively).
In sum, the results provide tentative evidence that low connectivity between the selection of ROI pairs identified in the previous paragraph reflects CF in a task general fashion (in 3/4 tasks at least). However, these tests on specific ROI pairs were not sufficient to account for the pattern of CF detected in the insula and putamen across all four tasks in the previous RSA analysis. To be sure about this for the insula (given that insula vs MFG connectivity was implicated in previous ROI pair analysis), we repeated the insula-based RSA analysis after deleting right insula vs right MFG connectivity values from the connectivity pattern vector and still detected a significant outcome across all four tasks. This outcome is consistent with theories that the experience of CF is underpinned by network-level interactions between task general CF systems and other neural circuitry.
Overview of supplemental secondary analyses including controlling for global signal and comparing data to metrics of task performance
Because our functional connectivity analyses could have been parameterized in different ways, and because there were a number of covariates that could have correlated with CF-ratings we conducted a battery of secondary analyses which verified the previous results were not accountable to these choices/factors. These tests are fully documented in Supplementary Materials and include: repeating the RSA after global grey matter signal has been regressed out from the fMRI data (Supplementary Figure 3); repeating the RSA on connectivity computed on signal estimated to be homogenous within each ROI using Principal Components Analysis (Supplementary Figure 4); repeating the RSA on right handers only (Supplementary Figure 5); comparing functional connectivity and CF data to objective task performance metrics; testing for correlations between participants’ ages and connectivity.
Discussion
The current study has revealed evidence that right hemispheric functional connectivity patterns centered on the insula and putamen reflect CF across four cognitive tasks. This is consistent with the hypothesis that domain general neural systems exist that monitor state-fatigue in task specific circuits (Muller and Apps, 2018), and pinpoints the insula and putamen as likely loci for these systems. These systems could therefore underpin the subjective experience of CF. Results also implicated left and right caudate, left MFG, right IFG, and right PG-based networks in % tasks. At the current stage we do not know whether the fMRI data was too noisy, or our analytic measures were not sensitive enough to spot CF-related patterns in these networks, or simply that these networks do not reflect CF for certain tasks.
Because we did not exhaustively test all cognitive tasks, we cannot conclude that right putamen and insula-based connectivity patterns are truly task general indicators of CF and because we did not test physically exerting tasks we cannot conclude our results generalize to the exercise domain (though this would be consistent with Müller and Apps, 2018, see also McMorris et al. 2018 for a related exercise-oriented review). However, the breadth of tasks we have investigated in the context of CF is greater than any other study we are aware of, as is also the consistency in brain regions we have associated with CF across tasks. The closest study to the current that we know of is Wylie et al. (2017), who correlated behaviorally measured CF with fMRI activity associated with an N-back and a speed of processing task and linked caudate nucleus activity to state-fatigue in both tasks for a traumatic brain injury group, but only the N-back task for healthy controls. We anticipate that the current results will generalize to new tasks that rely upon similar cognitive faculties and similar brain regions to those engaged by this study: the N-back task taxes working memory and primarily engages the frontal-parietal circuit (Rottschy et al. 2012); the Stroop test taxes cognitive control and engages the anterior cingulate cortex (Barch et al. 2001); IGT and BART are reliant on risk related decision-making and primarily engage the frontal-parietal circuit and insula (Barch et al. 2001). Future work will ultimately be necessary to ascertain whether insula/putamen-based networks truly reflect fatigue in a domain general fashion.
The brain regions linked to CF in the current study simultaneously encompass those implicated by Müller and Apps (2018) in their review article, as well those implicated by Wylie et al. (2017) (see previous paragraph). Muller and Apps (2018) hypothesize that the anterior insula, dorsal anterior cingulate cortex and dorsolateral prefrontal cortex monitor fatigue in task specific systems including (but not limited to) sensorimotor systems, and then motivate (or not) continued effortful behavior. This account can be sanctioned to explain the CF-related patterns associated with right insula, IFG, PG and left MFG in this study, where the involvement of PG may be related to fatigue in hand related motor functions (King et al. 2014). Whilst the current RSA (Figure 3) did not uncover a link between the anterior cingulate cortex and CF, we did detect a relationship specific to connectivity with the right caudate in Figure 5. Müller and Apps (2018) did not incorporate the striatal regions we detected (putamen and caudate) within their framework, however Wylie et al. (2017) hypothesize activity in the caudate nucleus (which neighbors the putamen) to be a common feature of the experience of CF, no matter the cause.
What roles might insula and putamen play in representing CF? Previous studies have proposed that the insula represents the interoceptive condition of the body, with the anterior insula providing the basis for self-awareness of emotions, sensations and movements (Craig, 2003, Craig, 2009). In particular right insula activation has been linked to the awareness of heat pain, subjective cooling, heartbeat, and self-recognition (Craig, 2009) and left insula activity has been linked to self-rated experience of mental effort Otto et al. (2014). A data-driven “reverse inference” analysis of resting state fMRI data identified the insula as integrating disparate systems associated with affect, sensory-motor processing, and general cognition (Chang et al., 2012). Collectively, these studies would appear to implicate the right insula as representing the self-awareness of fatigue across multiple neural systems.
Turning to address the role of the putamen in CF, the striatum (which contains the putamen) plays a central role in selecting, planning, and executing motor behavior (e.g. DeLong et al. 1984, Jankowski et al. 2009). The putamen is known to contribute to reward-related processes (reviewed by Haber and Knutson, 2010). In particular, putamen activation has been reported to be modulated by anticipated reward effort (Croxson et al. 2009), probability of gaining a reward (Preuschoff et al. 2006), and reward-related prediction error (O’Doherty et al. 2003). In primates, neurons in the putamen encode instructed motivational outcomes of action (Yamada et al. 2004). A meta-analytic review of the neural basis of emotion associated the right putamen with the experience of sadness (Lindquist et al. 2012). Mean diffusivity of the right putamen has been linked to self-reported ratings related to fatigue (Nakagawa et al. 2016). Finally, a recent data driven reverse inference analysis linked posterior putamen to sensorimotor functions, including aspects of their affective qualities (Pauli et al. 2016). Collectively these studies would appear to be consistent with the right putamen playing a motivational role in determining whether to continue expending effort in the face of fatigue in neural systems.
In future work, it will be interesting to conduct more detailed anatomical analyses testing for modularity in CF-related subsystems within the broad regions of interest tested in this study, and how they relate to different components of task-related processing. In addition, the current analysis coarsely measured functional connectivity across the entire task duration, and consequently is not informative of within task dynamic changes in connectivity. We conducted the analysis in this fashion (e.g. as opposed to computing the change in connectivity between the start and end of the task) for simplicity and to avoid the need to select an arbitrary break point(s) for the start and end intervals. However, having now detected that right insula and putamen centered networks reflect CF, it will be valuable to scrutinize within task dynamics of connectivity in more detail. Relatedly, we have thus far only considered our results as being consistent with the hypothesis that insula/putamen monitor and act on fatigue in other neural systems. However, the current study is correlational, and does not establish cause and effect. Other interpretations are possible, including that weak insula connections lead to less efficient processing and thus more fatigue. More detailed future analyses of activity within and between CF-related systems may help shed light on this.
It will also be valuable to test different participant populations, including younger healthy adults and clinical conditions. We have no reason to suppose that results would be radically different in a younger population, however we might expect effect sizes to be smaller for younger participants who tire less rapidly. Tests on clinical populations will be valuable to ascertain whether characteristics of CF-related networks could serve as a warning sign for aging associated cognitive decline (see also Kluger et al. 2017).
In conclusion, whilst mental exhaustion is a daily occurrence to many, as well as being a common symptom of aging and multiple neurological disorders, scientific understanding of the neural bases of CF remains basic. The current study has contributed by revealing that inter-participant differences in self-rated CF correlate with inter-participant differences in insula/putamen-based functional connectivity networks across four diverse cognitive tasks. This is consistent with our working hypothesis that task (and possibly domain general) general CF systems exist in the brain and supports previous work that has implicated the similar regions in representing CF and possibly underpinning self-awareness of CF. We have also identified behavioral and analytic methods that are suitable for detecting neural correlates of CF across varied tasks. We hope that this will provide a foundation for future work, in particular that which aims to explicate the role CF plays in clinical conditions, and especially in neurodegenerative diseases.
Supplementary Material
Acknowledgement:
No conflict of interest to be disclosed. Data collection was funded by NIH R21 AG053193 to F. Lin. We thank three reviewers for their insightful comments.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Alexander NB, Taffet GE, Horne FM, Eldadah BA, Ferrucci L, Nayfield S, Studenski S. 2011. Bedside-to-Bench conference: research agenda for idiopathic fatigue and aging. J Am Geriatr Soc. 58:967–975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnedt JT, Owens J, Crouch M, Stahl J, Carskadon MA. Neurobehavioral performance of residents after heavy night call vs after alcohol ingestion. Jama. 2005. September 7;294(9):1025–33. [DOI] [PubMed] [Google Scholar]
- Barch DM, Braver TS, Akbudak E, Conturo T, Ollinger J, Snyder A. 2001. Anterior cingulate cortex and response conflict: effects of response modality and processing domain. Cereb Cortex. 11:837–848. [DOI] [PubMed] [Google Scholar]
- Blain B, Hollard G, Pessiglione M. 2016. Neural mechanisms underlying the impact of daylong cognitive work on economic decisions. Proceedings of the National Academy of Sciences, 113(25), 6967–6972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bushnik T, Englander J, Wright J. The experience of fatigue in the first 2 years after moderate-to-severe traumatic brain injury: a preliminary report. The Journal of head trauma rehabilitation. 2008. January 1;23(1):17–24. [DOI] [PubMed] [Google Scholar]
- Calabrese M, Rinaldi F, Grossi P, Mattisi I, Bernardi V, Favaretto A, Perini P, Gallo P. 2010. Basal ganglia and frontal/parietal cortical atrophy is associated with fatigue in relapsing-remitting multiple sclerosis. Mult Scler. 16:1220–1228. [DOI] [PubMed] [Google Scholar]
- Chao-Gan Y, Yu-Feng Z. 2010. DPARSF: A MATLAB Toolbox for “Pipeline” Data Analysis of Resting-State fMRI. Frontiers in systems neuroscience. 4:13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang LJ, Yarkoni T, Khaw MW, Sanfey AG. 2012. Decoding the role of the insula in human cognition: functional parcellation and large-scale reverse inference. Cerebral cortex. 20;23(3):739–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaudhuri A, Behan PO. 2004. Fatigue in neurological disorders. Lancet. 363:978–988. [DOI] [PubMed] [Google Scholar]
- Ciric R, Wolf DH, Power JD, Roalf DR, Baum GL, Ruparel K, Shinohara RT, Elliott MA, Eickhoff SB, Davatzikos C, Gur RC. 2017. Benchmarking of participant-level confound regression strategies for the control of motion artifact in studies of functional connectivity. Neuroimage. 154:174–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colangeli S, Boccia M, Verde P, Guariglia P, Bianchini F, Piccardi L. 2016. Cognitive Reserve in Healthy Aging and Alzheimer’s Disease: A Meta-Analysis of fMRI Studies. Am J Alzheimers Dis Other Demen. 31:443–449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook DB, O’Connor PJ, Lange G, Steffener J. 2007. Functional neuroimaging correlates of mental fatigue induced by cognition among chronic fatigue syndrome patients and controls. Neuroimage 36, 108–122. [DOI] [PubMed] [Google Scholar]
- Craig AD. 2003. Interoception: the sense of the physiological condition of the body. Current opinion in neurobiology, 13(4), 500–505. [DOI] [PubMed] [Google Scholar]
- Craig AD. 2009. How do you feel--now? The anterior insula and human awareness. Nature reviews neuroscience, 10(1). [DOI] [PubMed] [Google Scholar]
- Croxson PL, Walton ME, O’Reilly JX, Behrens TEJ, Rushworth MFS. 2009. Effort based cost-benefit valuation and the human brain. J Neurosci. 29:4531–4541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeLong MR, Alexander GE, Georgopoulos AP, Crutcher MD, Mitchell SJ, Richardson RT. 1984. Role of basal ganglia in limb movements. Human neurobiology. ;2(4):235–44. [PubMed] [Google Scholar]
- DeLong MR, Wichmann T. 2007. Circuits and circuit disorders of the basal ganglia. Archives of neurology. 64(1):20–4. [DOI] [PubMed] [Google Scholar]
- Dimitrakopoulos GN, Kakkos I, Dai Z, Wang H, Sgarbas K, Thakor N, Bezerianos A, Sun Y. 2018. Functional connectivity analysis of mental fatigue reveals different network topological alterations between driving and vigilance tasks. IEEE transactions on neural systems and rehabilitation engineering. (4):740–9. [DOI] [PubMed] [Google Scholar]
- Friedman JH, Brown RG, Comella C, Garber CE, Krupp LB, Lou JS, Marsh L, Nail L, Shulman L, Taylor CB. 2007. Fatigue in Parkinson’s disease: a review. Movement disorders: official journal of the Movement Disorder Society. 15;22(3):297–308. [DOI] [PubMed] [Google Scholar]
- Dobryakova E, DeLuca J, Genova HM, Wylie GR. 2013. Neural correlates of cognitive fatigue: cortico-striatal circuitry and effort-reward imbalance. J Int Neuropsychol Soc. 19:849–853. [DOI] [PubMed] [Google Scholar]
- Dobryakova E, Hulst HE, Spirou A, Chiaravalloti ND, Genova HM, Wylie GR, DeLuca J. 2017. Fronto-striatal network activation leads to less fatigue in multiple sclerosis. Mult Scler.1352458517717087. [DOI] [PubMed] [Google Scholar]
- Eldadah BA. 2011. Fatigue and fatigability in older adults. PM R. 2:406–413. [DOI] [PubMed] [Google Scholar]
- Hampson JP, Zick SM, Khabir T, Wright BD, Harris RE. 2015. Altered resting brain connectivity in persistent cancer related fatigue. Neuroimage Clin. 8:305–313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haber SN, Knutson B. 2010. The reward circuit: Linking primate anatomy and human imaging. Neuropsychopharmacology. 3 5(1):4–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inzlicht M, Schmeichel BJ. 2012. What is ego depletion? Toward a mechanistic revision of the resource model of self-control. Perspectives on Psychological Science. 7(5):450–63. [DOI] [PubMed] [Google Scholar]
- Jankowski J, Scheef L, Huppe C, Boecker H. 2009. Distinct striatal regions for planning and executing novel and automated movement sequences. Neuroimage. 44(4):1369–79. [DOI] [PubMed] [Google Scholar]
- King M, Rauch HG, Stein DJ, Brooks SJ. 2014. The handyman’s brain: a neuroimaging meta-analysis describing the similarities and differences between grip type and pattern in humans. Neuroimage. 102 Pt 2:923–937. [DOI] [PubMed] [Google Scholar]
- Kluger BM, Krupp LB, Enoka RM. 2013. Fatigue and fatigability in neurologic illnesses: proposal for a unified taxonomy. Neurology. 80(4):409–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kluger BM, Herlofson K, Chou KL, Lou JS, Goetz CG, Lang AE, Weintraub D, Friedman J. 2016. Parkinson’s disease-related fatigue: A case definition and recommendations for clinical research. Movement Disorders. (5):625–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kluger BM, Pedersen KF, Tysnes OB, Ongre SO, Øygarden B, Herlofson K. 2017. Is fatigue associated with cognitive dysfunction in early Parkinson’s disease?. Parkinsonism & related disorders. 37:87–91. [DOI] [PubMed] [Google Scholar]
- Kriegeskorte N, Mur M, Bandettini P. 2008. Representational similarity analysis - connecting the branches of systems neuroscience. Front Syst Neurosci. 2:4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krupp LB, LaRocca NG, Muir-Nash J, Steinberg AD. 1989. The fatigue severity scale: application to patients with multiple sclerosis and systemic lupus erythematosus. Archives of neurology (10):1121–3. [DOI] [PubMed] [Google Scholar]
- Krupp L Fatigue is intrinsic to multiple sclerosis (MS) andis the most commonly reported symptom of the disease. Mult Scler 2006;12:367–368. [DOI] [PubMed] [Google Scholar]
- Lee KA, Hicks G, Nino-Murcia G. 1991. Validity and reliability of a scale to assess fatigue. Psychiatry Res. 36:291–298. [DOI] [PubMed] [Google Scholar]
- Lerdal A, Bakken LN, Kouwenhoven SE, Pedersen G, Kirkevold M, Finset A, Kim HS. 2009. Poststroke fatigue—a review. Journal of pain and symptom management. 38(6):928–49. [DOI] [PubMed] [Google Scholar]
- Lin F, Roiland R, Heffner K, Johnson M, Chen DG, Mapstone M. Evaluation of objective and perceived mental fatigability in older adults with vascular risk. Journal of psychosomatic research. 2014. June 1;76(6):458–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin F, Ren P, Cotton K, Porsteinsson A, Mapstone M, Heffner KL. 2016. Mental Fatigability and Heart Rate Variability in Mild Cognitive Impairment. Am J Geriatr Psychiatry. 24:374–378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin F, Roiland R, Polesskaya O, Chapman B, Johnson M, Brasch J, Chen DG, Mapstone M. 2013. Fatigability Disrupts Cognitive Processes’ Regulation of Inflammatory Reactivity in Old Age. Am J Geriatr Psychiatry. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindquist KA, Wager TD, Kober H, Bliss-Moreau E, Barrett LF. 2012. The brain basis of emotion: A meta-analytic review. Behav Brain Sci. 35(3): 121–143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim J, Wu WC, Wang J, Detre JA, Dinges DF, Rao H. 2010. Imaging brain fatigue from sustained mental workload: an ASL perfusion study of the time-on-task effect. Neuroimage, 49(4), 3426–3435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMorris T, Barwood M, Corbett J. 2018. Central fatigue theory and endurance exercise: toward an interoceptive model. Neuroscience & Biobehavioral Reviews. 10.1016/j.neubiorev.2018.03.024 [DOI] [PubMed] [Google Scholar]
- Müller T, Apps MA. 2018. Motivational fatigue: A neurocognitive framework for the impact of effortful exertion on subsequent motivation. Neuropsychologia. doi: 10.1016/j.neuropsychologia.2018.04.030 [DOI] [PubMed] [Google Scholar]
- Murphy K, Birn RM, Handwerker DA, Jones TB, Bandettini PA. 2009. The impact of global signal regression on resting state correlations: are anti-correlated networks introduced?. Neuroimage. 44(3):893–905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otto T, Zijlstra FR, Goebel R. 2013. Neural correlates of mental effort evaluation—involvement of structures related to self-awareness. Social cognitive and affective neuroscience, 9(3), 307–315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Doherty JP, Dayan P, Friston K, Critchley H, Dolan RJ. 2003. Temporal difference models and reward-related learning in the human brain. Neuron. 38:329–337 [DOI] [PubMed] [Google Scholar]
- Pauli WM, O’Reilly RC, Yarkoni T, Wager TD. 2016. Regional specialization within the human striatum for diverse psychological functions. Proceedings of the National Academy of Sciences. 113(7):1907–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Persson J, Larsson A, Reuter-Lorenz PA. 2013. Imaging fatigue of interference control reveals the neural basis of executive resource depletion. J Cogn Neurosci. 25:338–351. [DOI] [PubMed] [Google Scholar]
- Preuschoff K, Bossaerts P, Quartz SR. 2006. Neural differentiation of expected reward and risk in human subcortical structures. Neuron. 51:381–390. [DOI] [PubMed] [Google Scholar]
- Rocca MA, Meani A, Riccitelli GC, Colombo B, Rodegher M, Falini A, Comi G, Filippi M. 2016. Abnormal adaptation over time of motor network recruitment in multiple sclerosis patients with fatigue. Mult Scler. 22:1144–1153. [DOI] [PubMed] [Google Scholar]
- Rottschy C, Langner R, Dogan I, Reetz K, Laird AR, Schulz JB, Fox PT, Eickhoff SB. 2012. Modelling neural correlates of working memory: a coordinate-based meta-analysis. Neuroimage. 60:830–846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spiteri S, Hassa T, Claros-Salinas D, Dettmers C, Schoenfeld MA. 2017. Neural correlates of effort-dependent and effort-independent cognitive fatigue components in patients with multiple sclerosis. Mult Scler J. DOI: . [DOI] [PubMed] [Google Scholar]
- Stephan KE, Fink GR, Marshall JC. 2007. Mechanisms of hemispheric specialization: insights from analyses of connectivity. Neuropsychologia. 45:209–228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tajima S et al. 2010. Medial orbitofrontal cortex is associated with fatigue sensation. Neurol Res Int 2010, 671421 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thayer JF, Ahs F, Fredrikson M, Sollers JJ 3rd, Wager TD. 2012. A meta-analysis of heart rate variability and neuroimaging studies: implications for heart rate variability as a marker of stress and health. Neurosci Biobehav Rev. 36:747–756. [DOI] [PubMed] [Google Scholar]
- Tzourio-Mazoyer N, Landeau B, Papathanassiou D, Crivello F, Etard O, Delcroix N, Mazoyer B, Joliot M. 2002. Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single-subject brain. Neuroimage. 15:273–289. [DOI] [PubMed] [Google Scholar]
- Wang C, Trongnetrpunya A, Samuel IB, Ding M, Kluger BM. 2016. Compensatory Neural Activity in Response to Cognitive Fatigue. J Neurosci. 36:3919–3924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wylie GR, Genova HM, DeLuca J, Dobryakova E. 2017. The relationship between outcome prediction and cognitive fatigue: A convergence of paradigms. Cogn Affect Behav Neurosci. 17:838–849. [DOI] [PubMed] [Google Scholar]
- Wylie GR, Dobryakova E, DeLuca J, Chiaravalloti N, Essad K, Genova H. 2017. Cognitive fatigue in individuals with traumatic brain injury is associated with caudate activation. Scientific reports. August 21;7(1):8973. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.