Abstract
We recently described the persistence of detectable serum proinsulin in a large majority of individuals with longstanding type 1 diabetes (T1D), including individuals with undetectable serum C-peptide. Here, we sought to further explore the mechanistic etiologies of persistent proinsulin secretion in T1D at the level of the islet, using tissues obtained from human donors. Immunostaining for proinsulin and insulin was performed on human pancreatic sections from the Network for Pancreatic Organ Donors with Diabetes (nPOD) collection (n = 24). Differential proinsulin processing enzyme expression was analyzed using mass spectrometry analysis of human islets isolated from pancreatic sections with laser capture microdissection (n = 6). Proinsulin processing enzyme mRNA levels were assessed using quantitative real-time PCR in isolated human islets (n = 10) treated with or without inflammatory cytokines. Compared to nondiabetic controls, immunostaining among a subset (4/9) of insulin positive T1D donor islets revealed increased numbers of cells with proinsulin-enriched, insulin-poor staining. T1D donor islets also exhibited increased proinsulin fluorescence intensity relative to insulin fluorescence intensity. Laser capture microdissection followed by mass spectrometry revealed reductions in the proinsulin processing enzymes prohormone convertase 1/3 (PC1/3) and carboxypeptidase E (CPE) in T1D donors. Twenty-four hour treatment of human islets with inflammatory cytokines reduced mRNA expression of the processing enzymes PC1/3, PC2, and CPE. Taken together, these data provide new mechanistic insight into altered proinsulin processing in long-duration T1D and suggest that reduced β cell prohormone processing is associated with proinflammatory cytokine-induced reductions in proinsulin processing enzyme expression.
INTRODUCTION
Type 1 diabetes (T1D) is a chronic autoimmune disease that results from immune-mediated destruction of pancreatic β cells, leading to a lifelong dependence on exogenous insulin therapy.1 Classic models of T1D pathogenesis have suggested near complete destruction of pancreatic β cells by the time of diagnosis.1 However, recent data from clinical cohorts of individuals with long-duration disease have shown that a significant proportion of individuals with T1D >3 years duration have detectable C-peptide, and that C-peptide secretion is meal-responsive.2-5 In parallel, histologic analysis of pancreata from organ donors with diabetes has demonstrated the presence of insulin-containing islets, many years after diagnosis.6,7 Together, these findings have challenged the notion that all β cells are destroyed in diabetes, while also raising a number of questions regarding the molecular and functional phenotype of β cells that persist in long-duration disease.
The hallmark of a healthy β cell is efficient and robust processing of preproinsulin into mature insulin protein, which is secreted in response to nutrient stimulation. Under normal conditions, preproinsulin is converted to proinsulin following cleavage of its signal peptide by signal peptidase within the lumen of the endoplasmic reticulum (ER).8 Proinsulin disulfide bond formation and terminal protein folding occur in the ER and Golgi apparatus, and intact proinsulin is eventually cleaved into mature insulin and C-peptide by the enzymes prohormone convertase 1/3 (PC1/3), PC2, and carboxypeptidase E (CPE) in secretory granules.8 In response to inflammatory, oxidative, and endoplasmic reticulum stress in diabetes, protein processing capacity within the secretory compartment of the β cell can become overwhelmed. This leads to the accumulation of inadequately processed proinsulin9 that can be detected noninvasively in the circulation by measurement of the ratio of proinsulin relative to circulating mature insulin or C-peptide, or the PI:C ratio.10
We recently described the persistence of detectable serum proinsulin in a majority (95.9%) of individuals with longstanding T1D (≥3 years) who were followed as part of the T1D Exchange Registry.11 Remarkably, this finding extended to individuals who had undetectable serum C-peptide; specifically, 89.9% of individuals without detectable stimulated serum C-peptide had readily detectable serum proinsulin.11 Moreover, even in those with detectable C-peptide, PI:C ratios were significantly increased compared to controls, and higher fasting PI:C ratios were found in individuals with the worst-stimulated β cell function.11 Intriguingly, serum proinsulin levels remained steady over 4 years of follow-up, despite continued loss of C-peptide.11 These findings suggested that in long-duration T1D, the ability to secrete proinsulin persists, even in individuals who no longer secrete detectable levels of serum C-peptide.
To elucidate the mechanistic etiologies behind these findings, we analyzed pancreatic sections from donors with established T1D and performed in vitro analyses of cytokine-treated human islets. Immunostaining analysis of pancreatic sections from T1D donors confirmed discrepancies in proinsulin and insulin expression and localization at the level of the islet in some donors with longstanding T1D, while mass spectroscopy analysis of the laser-captured β cells from organ donors with diabetes suggested that defects in hormone processing may arise from impaired expression of proinsulin processing enzymes. Consistent with this, cytokine treatment of human islets led to a reduction in mRNA levels of proinsulin processing enzymes. Taken together, these data highlight a prominent role for defective prohormone processing in T1D and provide novel insights into the molecular phenotype of the β cell in long-duration T1D.
MATERIALS AND METHODS
Human islet sections were obtained through the Network for Pancreatic Organ Donors with Diabetes (nPOD).12 Sections for immunostaining were obtained from 7 nondiabetic controls and 17 donors with T1D. Sections from donors with T1D were selected to include a range of diabetes durations, individuals with and without detectable random C-peptide, and documented presence or absence of insulin-positive islets. Three of the donors with T1D had detectable random serum C-peptide and 13 were classified by nPOD as C-peptide negative (random serum C-peptide <0.017 nmol/L via TOSOH immunoassay).12 One donor with T1D did not have an available serum C-peptide value. Sections for laser capture microdissection (LCM) were obtained from 3 nondiabetic controls and 3 donors with T1D who were known to have positive islet autoantibodies and documented presence of residual insulin positive islets (Case IDs 6234, 6238, 6271, 6212, 6245, and 6247).
Isolated human islets from 10 nondiabetic donors were obtained from the Integrated Islet Distribution Program and the Clinical Islet Cell Laboratory in the University of Louisville Cardiovascular Innovation Institute. Donor characteristics are listed in the Supplemental donor characteristic attachment. Islets from each donor were treated with or without a cytokine cocktail of IL-1β (50 U/ml) and INF-γ (1000 U/ml) (R&D Systems, USA) for 24 hours as previously described.13,14 Total RNA was recovered using RNeasy mini kits (Qiagen, USA), reverse transcribed, and subjected to qRT-PCR using SensiFASTSYBR Lo-ROX kit (Bioline, USA). Data were analyzed in triplicates, normalized to glyceraldehyde-3-phosphate dehydrogenase GAPDH, and presented as fold change in expression relative to untreated islets from the same individual using the 2^-ΔΔCT method. Primer sequences used are listed in the Supplemental Table.
Immunostaining.
Tissue sections were deparaffinized through graded xylene and ethanol and permeabilized in PBS containing 0.1% triton-X 100 (Fisher Scientific). Sections were costained with primary antibodies against insulin (guinea pig; 1:500 and 1:1000; Millipore) and proinsulin (mouse; 1:50 and 1:200; Developmental Studies Hybridoma Bank; detects the B-C junction of human proinsulin) as previously described and validated.15,16 Anti-guinea pig Ig Alexa 647 and anti-mouse Ig Alexa 488 secondary antibodies were used (1:400; Jackson ImmunoResearch). Images were acquired using a LSM 700 confocal microscope (Zeiss). The fluorescence intensity of the images was quantified by using FIJI-Image J (NIH) by a researcher blinded to the donor T1D status, and as described previously.17 Briefly, from each pancreatic section, 3–10 islets were randomly selected, and the fluorescence intensity of insulin and proinsulin (number of pixels) was calculated by measuring their integrated density (ID) and total islet area. The background fluorescence intensity was measured for each channel, and the corrected total islet cell fluorescence intensity was calculated by the following formula: Total Islet Cell Fluorescence (CTCF) = Integrated density - (area of selected cell x mean fluorescence of background).
Mass spectrometry analysis of laser capture microdissection (LCM) islets.
As previously described, high resolution high mass accuracy label-free quantitative mass spectrometry analysis was applied to islets isolated by laser capture microdissection from nPOD pancreatic sections from 3 donors with T1D, and 3 nondiabetic controls.18 To select insulin positive islets for analysis, islets in unstained tissue sections were identified based on intrinsic autofluorescence, which has been shown to correlate with insulin staining.18
Serial 10-μm-thick tissue sections were prepared from nPOD pancreas blocks and attached onto polyethylene naphthalate membrane slides. The tissue sections were dehydrated with graded ethanol solutions before isolating and collecting islets on cups using an ArcturusXT LCM instrument (Thermo Fisher Scientific), which is equipped with dual ultraviolet (UV) and infrared (IR) lasers. The IR laser captures cells of interest, while the UV laser microdissects cells of interest and prevents any significant contamination of the captured material with adjacent acinar tissue. Islets in the unstained tissue sections were identified by their unique and specific intrinsic florescence behavior, which correlates with insulin staining, and were detected using triple filter upon the UV illumination of the specimen.19,20 Protein extraction was performed using the Liquid Tissue MS Protein Prep Kit (Expression Pathology, Rockville, MD) according to the manufacturer’s protocol. Briefly, the LCM cap films containing approximately 3 × 104 cell equivalents (estimates based on the thickness and area of the captured islet tissue) were transferred into 20 μl of liquid tissue buffer in a 1.5 ml low protein binding reaction tube and centrifuged at 10,000 × g for 2 minutes to pellet the film. The islet proteins were extracted by heating the mixture at 95°C for 90 minutes with intermittent mixing at 20-minute intervals. After 90 minutes, the samples were centrifuged at 10,000 × g for 1 minute before cooling in ice for 2 minutes. The equivalent of 1:50 trypsin was added to the extracted protein and incubated at 37°C for 18 hours to generate tryptic peptides. After the trypsin digestion step, an aliquot of the generated peptides was used in a Micro BCA assay to determine the peptide concentrations. The remaining peptides were reduced (10 mM DDT) and alkylated (35 mM iodoacetamide) and stored at −80°C prior to use for mass spectrometry analysis. LC-MS analysis of digested samples was conducted as previously described.21 Tryptic peptides were solubilized in normalized volumes of 0.1% formic acid (FA)/H2O and the concentrations of the digested peptides were determined using a micro BCA assay. The final concentration of the samples was adjusted to 0.5 μg/μl using the same buffer. Identical concentrations (2 μg) of the peptides were analyzed on a Q-Exactive Orbitrap mass spectrometer coupled to an Easy NanoLC-1000 system (Thermo Fisher Scientific). Each sample was analyzed in triplicate. Protein identification and label-free quantification (LFQ) were performed with MaxQuant software package.21,22 Analysis of MS spectra was performed using the following parameters: the acetylation of the protein N-terminus and the oxidation of methionine as variable modifications and carbamido-methylation of cysteine as fixed modification. UniProt-SwissProt human canonical database (version 2016, canonical proteome; 20 198 identifiers) was selected as FASTA file. Seven amino acids were selected as minimum peptide length. Mass accuracy thresholds for the analysis were as follows: 15 ppm for MS and 0.8 Da for MS/MS. Match between runs option was kept as default (match time window: 0.7 minutes; alignment time window: 4 minutes). LFQ was enabled and LFQ minimum ratio count was set to 1. Remaining options were kept as default. Only unique and razor peptides were used for identification and quantitation. Mascot was also used for database searches and identification (Matrix Science). Tandem mass MS2 spectra identifying and validating the peptides used for the validation of the quantitation of each protein are displayed in the Supplemental Figure 1.
Statistics.
Analyses of cytokine treated islet data were performed using GraphPad Prism 7.0. A student’s t test was used to compare the expression of proinsulin processing enzymes between control and cytokine-treated islets.
For proteomic analyses, Perseus (version 1.5.2.6) was used to perform statistical analysis of LFQ proteomic data after Log2 transformation, data imputation, and filtration. Two-sample t tests were used together with permutation-based false discovery rate calculations for comparative quantitative analysis of protein expression in the 2 sample cohorts (T-test, FDR = 0.05, P < 0.05). This analysis takes into consideration all the peptides that are identified from the protein. Pinnacle, a quantitative proteomic analysis software (Optys Tech Corporation), was used for visualization and validation of the LFQ data using the areas under the curve from the extracted ion chromatograms of the precursor ions of unique peptides from targeted proteins. Pinnacle utilizes extracted ion chromatograms generated from the mass spectrometry MS1 data feature (peptides) and their corresponding MS2 fragmentation data to confirm the identity of the signal for each replicate sample. Peptides with the best ionization efficiencies, peak shape, and isotopic fidelity were used for comparative analysis. Actin was used as an internal control. Unpaired t tests were used to compare mean area under the curve values for each subject group. For all analyses, 2-tailed P values of ≤0.05 were considered significant.
RESULTS
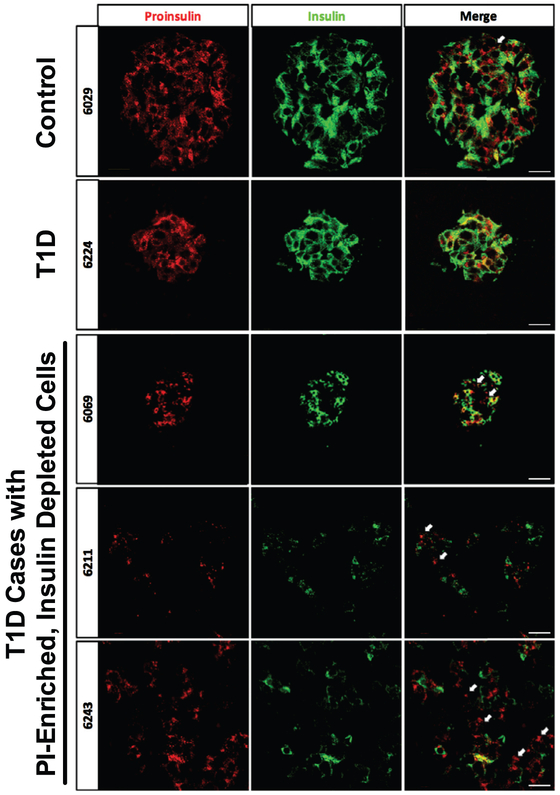
To explore whether abnormalities in proinsulin processing were present in pancreatic tissue sections from 17 donors with T1D from the nPOD collection, immunostaining was performed for insulin and proinsulin in pancreatic sections from nondiabetic control donors and donors characterized as having clinical T1D (duration 1.5–28 years) with a wide range of demographic and clinical characteristics (Table 1). In control donors, the majority of the β cells exhibited both proinsulin and insulin staining with limited overlap observed between regions of proinsulin and insulin staining. Within our cohort of donors with T1D, 47% (n = 8) lacked any detectable insulin and proinsulin staining. Among sections from donors with T1D and insulin positive cells, many sections exhibited scattered insulin positive cells or islets lacking central insulin positive cells. Although 55% (n = 5) of these insulin-positive sections exhibited a staining pattern where the majority of insulin+ β-cells also stained positive for proinsulin, in 4 donors (Table 1, Fig 1; Case IDs 6040, 6069, 6243, and 6211), a different phenotype was seen where multiple proinsulin-enriched but insulin poor β-cells were observed. Quantification of proinsulin fluorescence intensity relative to insulin intensity was suggestive of higher ratios in donors with T1D, although significant variability was present. For nondiabetic controls the median ratio was 0.37 (range of 0.21–1.1), while for donors with T1D, the median ratio was 1.2 (range of 0.4–13.6).
Table 1.
Characteristics of nPOD donor staining
| Case ID | Age | T1D duration (years) |
Sex | BMI | Positive aAbs | Detectable fasting serum C-peptide |
Insulin + cells present |
Proinsulin (PI) staining phenotype |
Ratio of PI /insulin fluorescence |
|---|---|---|---|---|---|---|---|---|---|
| 6015 | 39 | N/A (control) | F | 32.2 | N/A | N/A | + | Multiple costaining cells | 0.365 |
| 6029 | 24 | N/A (control) | F | 22.6 | N/A | N/A | + | Multiple costaining cells | 1.13 |
| 6034 | 32 | N/A (control) | F | 25.2 | N/A | N/A | + | Multiple costaining cells | 0.283 |
| 6055 | 27 | N/A (control) | M | 22.7 | N/A | N/A | + | Multiple costaining cells | 0.420 |
| 6104 | 41 | N/A (control) | M | 20.5 | N/A | N/A | + | Multiple costaining cells | 0.340 |
| 6179 | 20 | N/A (control) | F | 21.8 | N/A | N/A | + | Multiple costaining cells | 0.207 |
| 6229 | 31 | N/A (control) | F | 26.9 | N/A | N/A | + | Multiple costaining cells | 0.534 |
| 6038 | 37.2 | 20 | F | 30.9 | − | + | + | Multiple costaining cells | 0.498 |
| 6041 | 26.3 | 23 | M | 28.4 | − | − | − | None | N/A |
| 6054 | 35.1 | 30 | F | 30.4 | mIAA | − | − | None | N/A |
| 6077 | 32.9 | 19 | F | 22 | mIAA | − | − | None | N/A |
| 6141 | 36.7 | 28 | M | 26 | mIAA, IA-2, GAD, ZnT8 | − | − | None | N/A |
| 6143 | 32.6 | 7 | F | 26.1 | mIAA, IA-2 | − | − | None | N/A |
| 6173 | 44.1 | 15 | M | 23.9 | − | − | − | None | N/A |
| 6180 | 27.1 | 11 | M | 25.9 | mIAA, IA-2, GAD, ZnT8 | − | − | None | N/A |
| 6208 | 32.6 | 16 | F | 23.4 | − | − | − | None | N/A |
| 6040 | 50 | 20 | F | 31.6 | mIAA | − | + | Pseudoatrophic islets with rare insulin+ cells which were mostly PI-enriched, insulin poor cells | 7.15 |
| 6069 | 22.9 | 7 | M | 28.8 | Unknown | Unknown | + | Mixed beta cell phenotype with some islets displaying mostly costaining cells and some islets with numerous PI-enriched, insulin poor cells | 2.10 |
| 6070 | 22.6 | 7 | F | 21.6 | mIAA, IA-2 | − | + | Multiple costaining cells | 0.423 |
| 6121 | 33.9 | 4 | F | 18 | − | + | + | Multiple costaining cells | 13.6 |
| 6211 | 24 | 4 | F | 24.4 | mIAA, IA-2, GAD, ZnT8 | − | + | Occasional PI-enriched, insulin-poor cells | 0.762 |
| 6224 | 21 | 1.5 | F | 22.8 | − | − | + | Multiple costaining cells | 1.22 |
| 6237 | 18 | 12 | F | 26 | mIAA, GAD | − | + | Rare isolated insulin+ cells, all costain | 1.55 |
| 6243 | 13 | 5 | M | 21.3 | miAA | + | + | Clusters of PI-enriched insulin-poor cells | 1.01 |
Abbreviations: aAbs, autoantibodies; BMI, body mass index; GAD, glutamic acid decarboxylase antibody; IA-2, islet antigen 2 antibody; miAA, microinsulin autoantibody; nPOD, Network for Pancreatic Organ Donors with Diabetes; PI, proinsulin; T1D, type 1 diabetes mellitus; ZnT8, zinc transporter 8 antibody.
Fig 1.
Examples of proinsulin (PI) and insulin immunostaining patterns in type 1 diabetes (T1D) donor islets. Immunostaining of proinsulin (green) and insulin (red) was performed on pancreata from nondiabetic control donors and 16 T1D donors. Staining from representative donors exhibiting multiple PI-enriched, and insulin depleted cells are shown (indicated by white arrows; case IDs 6069, 6243, 6211). These cells were rare in nondiabetic control donors. Scale bars represent 200 μm. (For interpretation of the references to color in this figure legend, the reader is referred to the Web version of this article.)
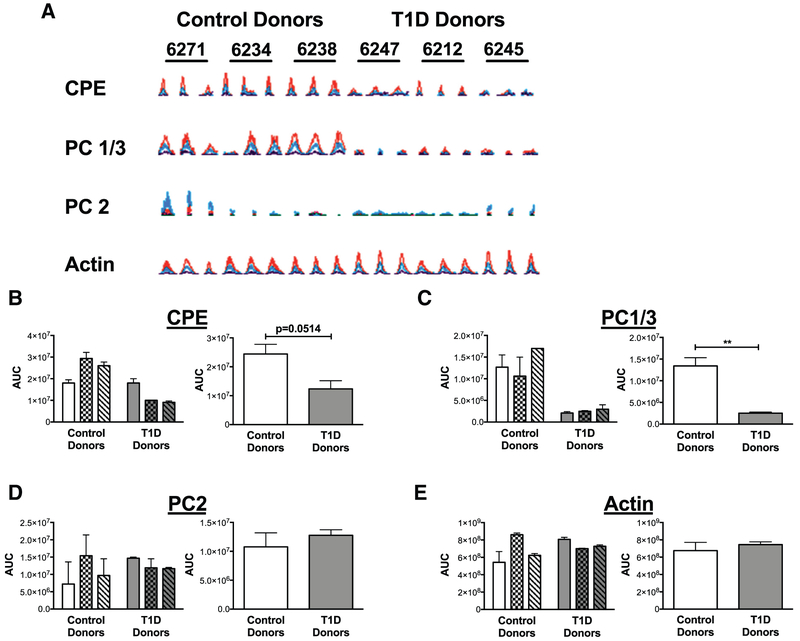
To explore potential mechanisms of impaired prohormone processing, LCM followed by quantitative mass spectrometry analysis of proinsulin processing enzymes was performed on pancreatic sections from nPOD donors with T1D compared to nondiabetic controls. Consistent with a reduction in proinsulin processing, LFQ analysis demonstrated significant reductions in CPE (−3.20 fold change, P < 0.001), as well as PC1/3 levels (−1.66 fold change, P < 0.001) in islets from donors with T1D compared to controls. In contrast, there were no significant differences in the expression levels of PC2 (−0.442 fold change; P = 0.368) or the control protein, actin (−0.007 fold change; P=0.939), between the 2 groups. The area under the curve values from the extracted ion chromatograms of the MS1 features of the representative peptides from the target proteins are quantified in Fig 2, A-E. Peptide scores for each of the 4 peptides were beyond the significance threshold in the Mascot searches. Similar to the LFQ analysis, a comparison of the mean area under the curve values for control donors compared to donors with T1D revealed a clear trend toward a reduction in CPE (P = 0.0514) and a significant reduction in PC1/3 (P = 0.0046) (Fig 2, B and C), but no differences in PC2 or actin expression (Fig 2, D and E).
Fig 2.
Expression of CPE, PC1/3, and PC2 in LCM islets from nondiabetic controls and donors with type 1 diabetes (T1D). A, Extracted ion chromatograms of peptides from nondiabetic individuals (n = 3) compared to T1D donors (n = 3) with sequences AASQPGELK, ALVDLADPR, EELEEELDEAVER and AGFAGDDAPR from CPE, PC1/3, PC2, and actin, respectively. The Mascot identification ion and expect scores were significant for each of the 4 peptides. Expression of the individual peptides in each nPOD case (run as triplicates) is shown. nPOD case IDs are indicated above each set of triplicates. B-E, Quantitation of area under the curve (AUC) for chromatograms from each peptide is shown for each donor on the left, as well as in aggregate form on the right, with means and standard errors indicated. Aggregate means of control donors were compared to donors with type 1 diabetes using an unpaired t-test. (n = 3); **P <0.01 CPE, carboxypeptidase E; LCM, laser capture microdissection; nPOD, Network for Pancreatic Organ Donors with Diabetes; PC1/3, prohormone convertase 1/3; PC2, prohormone convertase 2.
Finally, to test whether reductions in islet proinsulin processing enzyme expression were associated with islet inflammatory stress, we assayed the expression of CPE, PC1/3, and PC2 in human islets from 10 nondiabetic donors that were treated with 24 hours of a cytokine cocktail (50 U/ml IL-1β and 1000 U/ml INF-γ). As shown in Fig 3, cytokine treatment was associated with significant reductions in mRNA expression levels for each of the processing enzymes.
Fig 3.
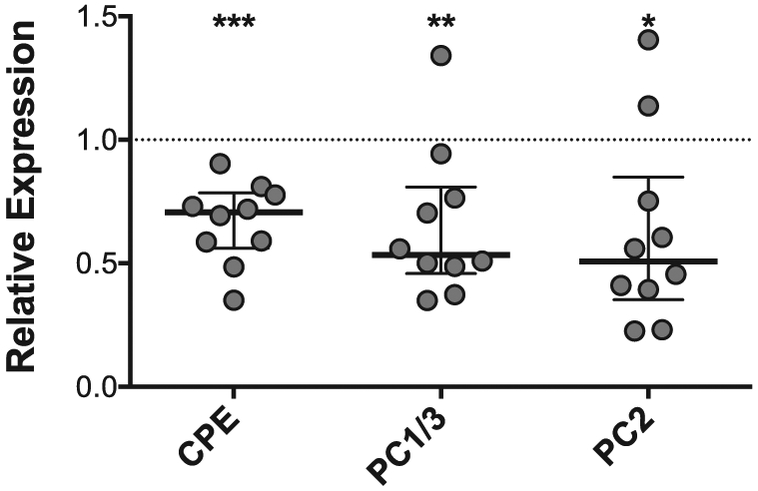
Islet inflammatory stress is associated with reductions in proinsulin processing enzyme expression. qRT-PCR for carboxypeptidase E (CPE), prohormone convertase 1/3 (PC1/3), and prohormone convertase 2 (PC2) was performed in human islets from nondiabetic donors treated with or without an inflammatory cytokine cocktail consisting of IL-1β (50 U/ml) and INF-γ (1000 U/ml) for 24 hours. Bars shown represent median +/− interquartile range of mRNA expression relative to untreated islets from the same donor (indicated by the dashed line). N = 10; *P <0.05; **P <0.01; ***P <0.001.
DISCUSSION
We recently reported that the ability to initiate preproinsulin production and secrete proinsulin persists in most individuals with longstanding T1D, even in those who were functionally C-peptide negative. Moreover, the analysis of longitudinal data from >90 individuals with T1D of ≥3 years duration showed that serum proinsulin levels remained fairly stable over 4 years of follow-up.11 Here, we show for the first-time immunostaining data that identifies a discordance between proinsulin production and conversion to mature insulin within the islets of a subset of individuals with established T1D, as well as proteomic analysis suggesting that this discordance is correlated with a reduction in the protein expression levels of PC1/3 and CPE in islets from donors with T1D. These data may help to explain at a mechanistic level, the deficiency of C-peptide (and insulin) from individuals who are otherwise proinsulin-positive.
The accumulation of incompletely processed proinsulin in longstanding T1D demonstrates a clearly disturbed environment for proinsulin maturation, a process normally regulated by the processing enzymes, PC1/3, 2, and CPE.8 A previous proteomic analysis of whole pancreas tissue lysates from diabetic donors reported a 10-fold decrease in CPE expression compared to nondiabetic donors.23 Our LCM analysis extends this observation by showing that reductions in the expression of proinsulin processing enzymes are specific to islets in individuals with T1D. Although the loss of β-cell mass itself could lead to an absolute reduction in proinsulin processing enzymes, in our study, LCM analysis was only performed on islets that contained insulin. Moreover, our observation that cytokine stress directly lowers the expression of mRNAs encoding prohormone-processing enzymes in human islets supports the notion of a processing defect that is independent of the loss of β-cell mass. Prohormone convertase and CPE activities are also involved in proamylin processing, and evidence of altered pro islet amyloid polypeptide processing has been identified in persons with T1D, lending further credence to our observations.24,25
Regarding our in vitro data suggesting that diminished proinsulin processing enzyme expression may be, at least in part, associated with islet inflammatory stress during T1D, we recognize that the etiology of these observations could be multifactorial. For one, prior reports have described increased proinsulin release and decreased PC1/3 and PC2 protein in human islets treated with inflammatory cytokines.26 Enzyme expression and/or activity could be also impacted by hyperglycemia or other extrinsic factors, as well as cell intrinsic factors such as activation of ER stress, a pathway increasingly implicated in T1D pathogenesis.27,28 Along these lines, reduced expression, translation, and post-translational processing of CPE and PC1/3 have been previously been reported in ex vivo models of ER stress in human islets.29-31 Alternatively, CPE has been identified as an islet autoantigen,32 and we cannot exclude that autoimmune targeting of processing enzymes could lead to altered expression and/or activity of these enzymes. Altered prohormone processing could also represent an inherited phenotype that is present at baseline in some subjects who go on to develop T1D, potentially exacerbating the progression of disease. Consistent with this model, polymorphisms near the gene locus encoding CPE have been linked to T1D susceptibility.33 We observed no difference in PC2 protein levels in the laser-captured β cells from pancreatic sections, but reduced PC2 mRNA levels were observed in cytokine-treated islets. These differences could reflect an effect of cytokines on alpha cells in intact islets, which typically exhibit high relative expression of PC2 compared to β cells.34
The increasing identification of persistent β cells in long-duration T1D raises a number of questions regarding the source of these cells and how their molecular phenotype intersects with the ambient states of immune activation. To date, analysis of persistent β cells in sections from donors with long-duration T1D using confocal microscopy has not provided strong evidence for β cell replication or neogenesis.7 Key unanswered questions are whether these persistent β cells represent dedifferentiated β cells and whether the process of dedifferentiation may enable β cells to escape immune recognition. In this regard, a subset of β cells with reduced immunogenicity was identified in the nonobese diabetic mouse model of T1D. These cells had reduced insulin content and reduced expression of genes associated with β cell identity. In parallel, genes associated with immune modulation and “stemness” were increased.35 Interestingly, β-cell dedifferentiation due to deletion of the transcription factor FoxO1 deletion in mouse models has been linked also to dramatic reductions in the expression of proinsulin processing enzymes.36 The recent observation that many islets in pancreatic sections from individuals with longstanding T1D harbor very low level insulin content in association with identity markers for both β cells and other islet cells also supports the possibility of dedifferentiated islet cells in longstanding T1D.37 Taken together, our results and the above published findings suggest potential associations between altered proinsulin processing, loss of prohormone-processing enzyme expression, and β-cell identity.36 However, additional work is needed in human samples to fully elucidate these relationships.
Donors with T1D exhibited significant heterogeneity with regards to histologic phenotype. Additionally, although we detected circulating proinsulin in 96% of individuals tested, proinsulin or insulin-positive staining was noted in sections from <50% of individuals tested. This may have been in part related to the limited sample size of T1D pancreas donor samples. Also, testing of proinsulin in serum likely provides a more sensitive assay of whole pancreas proinsulin production. By contrast, immunostaining is limited by antibody sensitivity and is limited to analysis of a subset of islets included in the sections. Consistent with our analysis in serum, a recent ELISA-based analysis of pancreas extracts identified detectable proinsulin in 24 donors with T1D, irrespective of the presence of detectable insulin or C-peptide.38 Our observed heterogeneity also likely in part reflects the known heterogeneity of the histologic phenotype and clinical course in T1D.6,39,40 Along these lines, certain individuals with or at-risk for T1D may exhibit a clinical phenotype marked by more profound alterations in prohormone processing. For example, we previously observed that amongst at-risk relatives of individuals with T1D, increased circulating proinsulin/C-peptide ratios were most pronounced amongst young children who eventually progressed to diabetes.41 Identification of such clinical phenotypes associated with abnormal proinsulin processing could potentially allow for targeted treatment with agents improving β cell health.40,42
In summary, our findings demonstrate that persistent circulating proinsulin in certain individuals with long-standing T1D could be related to altered proinsulin processing at the level of the islet, which is exacerbated by islet inflammatory stress. These findings present the tantalizing proposition that such residual proinsulin-producing cells are “sleeping” and could potentially be amenable to a therapeutic strategy repairing the processing deficit and “waking” the cells. Along these lines, our data suggest that in longstanding T1D measurement of proinsulin in addition to C-peptide may provide a more sensitive indicator of persistent β cell mass than the measurement of C-peptide alone. Future work is needed to fully characterize the molecular phenotype of these persistent β cells and to delineate whether therapeutics targeting proinsulin processing could increase endogenous insulin production in certain individuals with T1D.
Supplementary Material
Brief Commentary.
Background
Persistent proinsulin secretion has been described in individuals with longstanding type 1 diabetes (T1D) who no longer secrete C-peptide, but molecular etiologies are not well-understood.
Translational Significance
Analyses of human cadaveric donor islets and pancreatic sections suggest that, compared to nondiabetic controls, a large subset of T1D donor islets revealed increased proinsulin-enriched, insulin-poor cells. Islet proinsulin processing enzyme expression was reduced in donors with T1D and in human islets treated with inflammatory cytokines. These data provide new mechanistic insight into persistent proinsulin secretion in human T1D and suggest that agents targeting proinsulin processing may have therapeutic benefit in some individuals with T1D.
ACKNOWLEDGMENTS
CJG is a signatory of the concerned academics’ letter of complaint to Elsevier, publishers of the Lancet journals. All authors acknowledge the journal’s policy on disclosure of potential conflicts of interest and none of the other authors have any relevant conflicts of interest to disclose. All authors have read the journal’s authorship statement and the manuscript has been reviewed by and approved by all named authors. No editorial support was provided for preparation of the manuscript. E.K.S. and C.E.M serve as guarantors of this work.
A portion of this research was performed with the support of the Network for Pancreatic Organ Donors with Diabetes (nPOD), a collaborative type 1 diabetes research project sponsored by JDRF. Organ Procurement Organizations (OPO) partnering with nPOD to provide research resources are listed at http://www.jdrfnpod.org/for-partners/npod-partners/. We also acknowledge Wojciech Grzesik for technical assistance with proinsulin measurement and LCM capture.
Other Funding Sources: This manuscript was supported by funding from NIDDK K08DK103983 and R03 DK117253 to E.K.S., JDRF 2-SRA-2017-498-M-B to E. K.S., and NIH grants R01 DK093954 (to C.E-M.) R01 DK48280 (to P.A) and UC4 DK 104166 (to C.E-M. and R.G.M.), VA Merit Award I01BX001733 (to C.E-M.), JDRF Pioneer Award and Strategic Research Agreement (to C.E-M.), and JDRF 47-2014-299-Q-R (C.E-M.), JDRF grant number 2-SRA-2017-498-M-B (to E.K.S) JDRF Career Development Award (5-CDA-2016-194-A-N) to TLM
Abbreviations:
- CPE
carboxypeptidase E
- ER
endoplasmic reticulum
- IR
infrared
- LCM
laser capture microdissection
- LFQ
label-free quantification
- nPOD
network for pancreatic organ donors with type 1 diabetes
- PC1/3
prohormone convertase 1/3
- PC2
prohormone convertase 2
- T1D
type 1 diabetes
- UV
ultraviolet
Footnotes
SUPPLEMENTARY MATERIALS
Supplementary material associated with this article can be found in the online version at doi:10.1016/j.trsl.2019.08.001.
REFERENCES
- 1.DiMeglio LA, Evans-Molina C, Oram RA. Type 1 diabetes. Lancet 2018;391:2449–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Davis AK, DuBose SN, Haller MJ, et al. Prevalence of detectable C-peptide according to age at diagnosis and duration of type 1 diabetes. Diabetes Care 2015;38:476–81. [DOI] [PubMed] [Google Scholar]
- 3.Oram RA, Jones AG, Besser RE, et al. The majority of patients with long-duration type 1 diabetes are insulin microsecretors and have functioning beta cells. Diabetologia 2014;57:187–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Oram RA, McDonald TJ, Shields BM, et al. Most people with long-duration type 1 diabetes in a large population-based study are insulin microsecretors. Diabetes Care 2015;38:323–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Keenan HA, Sun JK, Levine J, et al. Residual insulin production and pancreatic ss-cell turnover after 50 years of diabetes: Joslin Medalist Study. Diabetes 2010;59:2846–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Campbell-Thompson M, Fu A, Kaddis JS, et al. Insulitis and beta-cell mass in the natural history of type 1 diabetes. Diabetes 2016;65:719–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lam CJ, Jacobson DR, Rankin MM, Cox AR, Kushner JA. Beta cells persist in T1D pancreata without evidence of ongoing beta-cell turnover or neogenesis. J Clin Endocrinol Metab 2017;102:2647–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Liu M, Wright J, Guo H, Xiong Y, Arvan P. Proinsulin entry and transit through the endoplasmic reticulum in pancreatic beta cells. Vitam Horm 2014;95:35–62. [DOI] [PubMed] [Google Scholar]
- 9.Eizirik DL, Miani M, Cardozo AK. Signalling danger: endoplasmic reticulum stress and the unfolded protein response in pancreatic islet inflammation. Diabetologia 2013;56:234–41. [DOI] [PubMed] [Google Scholar]
- 10.Tersey SA, Nishiki Y, Templin AT, et al. Islet beta-cell endoplasmic reticulum stress precedes the onset of type 1 diabetes in the nonobese diabetic mouse model. Diabetes 2012;61:818–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sims EK, Bahnson HT, Nyalwidhe J, et al. Proinsulin secretion is a persistent feature of type 1 diabetes. Diabetes Care 2018;42:258–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Campbell-Thompson M, Wasserfall C, Kaddis J, et al. Network for Pancreatic Organ Donors with Diabetes (nPOD): developing a tissue biobank for type 1 diabetes. Diabetes Metab Res Rev 2012;28:608–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Juan-Mateu J, Alvelos MI, Turatsinze JV, et al. SRp55 regulates a splicing network that controls human pancreatic beta-cell function and survival. Diabetes 2018;67:423–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Eizirik DL, Sammeth M, Bouckenooghe T, et al. The human pancreatic islet transcriptome: expression of candidate genes for type 1 diabetes and the impact of pro-inflammatory cytokines. PLoS Genet 2012;8:e1002552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Asadi A, Bruin JE, Kieffer TJ. Characterization of antibodies to products of proinsulin processing using immunofluorescence staining of pancreas in multiple species. J Histochem Cytochem 2015;63:646–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mastracci TL, Anderson KR, Papizan JB, Sussel L. Regulation of Neurod1 contributes to the lineage potential of Neurogenin3+ endocrine precursor cells in the pancreas. PLoS Genet 2013;9:e1003278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sun Y, Ikrar T, Davis MF, et al. Neuregulin-1/ErbB4 signaling regulates visual cortical plasticity. Neuron 2016;92:160–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nyalwidhe JO, Grzesik WJ, Burch TC, et al. Comparative quantitative proteomic analysis of disease stratified laser captured microdissected human islets identifies proteins and pathways potentially related to type 1 diabetes. PLoS One 2017;12:e0183908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Van De Winkel M, Pipeleers D. Autofluorescence-activated cell sorting of pancreatic islet cells: purification of insulin-containing B-cells according to glucose-induced changes in cellular redox state. Biochem Biophys Res Commun 1983;114:835–42. [DOI] [PubMed] [Google Scholar]
- 20.Croce A, Bottiroli G. New light in flavin autofluorescence. Eur J Histochem 2015;59:2576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cox J, Mann M. MaxQuant enables high peptide identification rates, individualized p.p.b.-range mass accuracies and proteome-wide protein quantification. Nat Biotechnol 2008;26:1367–72. [DOI] [PubMed] [Google Scholar]
- 22.Luber CA, Cox J, Lauterbach H, et al. Quantitative proteomics reveals subset-specific viral recognition in dendritic cells. Immunity 2010;32:279–89. [DOI] [PubMed] [Google Scholar]
- 23.Burch TC, Morris MA, Campbell-Thompson M, et al. Proteomic analysis of disease stratified human pancreas tissue indicates unique signature of type 1 diabetes. PLoS One 2015;10: e0135663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yonemoto IT, Kroon GJ, Dyson HJ, Balch WE, Kelly JW. Amylin proprotein processing generates progressively more amyloidogenic peptides that initially sample the helical state. Biochemistry 2008;47:9900–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Courtade JA, Klimek-Abercrombie AM, Chen YC, et al. Measurement of pro-islet amyloid polypeptide (1-48) in diabetes and islet transplants. J Clin Endocrinol Metab 2017;102:2595–603. [DOI] [PubMed] [Google Scholar]
- 26.Hostens K, Pavlovic D, Zambre Y, et al. Exposure of human islets to cytokines can result in disproportionately elevated proinsulin release. J Clin Invest 1999;104:67–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Stull ND, Breite A, McCarthy R, Tersey SA, Mirmira RG. Mouse islet of Langerhans isolation using a combination of purified collagenase and neutral protease. J Vis Exp 2012:e4137., e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Marhfour I, Lopez XM, Lefkaditis D, et al. Expression of endoplasmic reticulum stress markers in the islets of patients with type 1 diabetes. Diabetologia 2012;55:2417–20. [DOI] [PubMed] [Google Scholar]
- 29.Jeffrey KD, Alejandro EU, Luciani DS, et al. Carboxypeptidase E mediates palmitate-induced beta-cell ER stress and apoptosis. Proc Natl Acad Sci U S A 2008;105:8452–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pirot P, Naamane N, Libert F, et al. Global profiling of genes modified by endoplasmic reticulum stress in pancreatic beta cells reveals the early degradation of insulin mRNAs. Diabetologia 2007;50:1006–14. [DOI] [PubMed] [Google Scholar]
- 31.Furukawa H, Carroll RJ, Swift HH, Steiner DF. Long-term elevation of free fatty acids leads to delayed processing of proinsulin and prohormone convertases 2 and 3 in the pancreatic beta-cell line MIN6. Diabetes 1999;48:1395–401. [DOI] [PubMed] [Google Scholar]
- 32.Castano L, Russo E, Zhou L, Lipes MA, Eisenbarth GS. Identification and cloning of a granule autoantigen (carboxypeptidase-H) associated with type I diabetes. J Clin Endocrinol Metab 1991;73:1197–201. [DOI] [PubMed] [Google Scholar]
- 33.Onengut-Gumuscu S, Chen WM, Burren O, et al. Fine mapping of type 1 diabetes susceptibility loci and evidence for colocalization of causal variants with lymphoid gene enhancers. Nat Genet 2015;47:381–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Marchetti P, Lupi R, Bugliani M, et al. A local glucagon-like peptide 1 (GLP-1) system in human pancreatic islets. Diabetologia 2012;55:3262–72. [DOI] [PubMed] [Google Scholar]
- 35.Rui J, Deng S, Arazi A, et al. Beta cells that resist immunological attack develop during progression of autoimmune diabetes in NOD mice. Cell Metab 2017;25:727–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Talchai C, Xuan S, Lin HV, Sussel L, Accili D. Pancreatic beta cell dedifferentiation as a mechanism of diabetic beta cell failure. Cell 2012; 150: 1223–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lam CJ, Chatterjee A, Shen E, Cox AR, Kushner JA. Low level insulin content within abundant non-beta islet endocrine cells in long-standing type 1 diabetes. Diabetes 2019;68:598–608. [DOI] [PubMed] [Google Scholar]
- 38.Wasserfall C, Nick HS, Campbell-Thompson M, et al. Persistence of pancreatic insulin mRNA expression and proinsulin protein in type 1 diabetes pancreata. Cell Metab 2017;26:568–75, e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bowman P, Flanagan SE, Hattersley AT. Future roadmaps for precision medicine applied to diabetes: rising to the challenge of heterogeneity. J Diabetes Res 2018;2018:3061620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sims EK, Evans-Molina C, Tersey SA, Eizirik DL, Mirmira RG. Biomarkers of islet beta cell stress and death in type 1 diabetes. Diabetologia 2018;61:2259–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sims EK, Chaudhry Z, Watkins R, et al. Elevations in the fasting serum proinsulin-to-C-peptide ratio precede the onset of type 1 diabetes. Diabetes Care 2016;39:1519–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Oram RA, Sims EK, Evans-Molina C. Beta cells in type 1 diabetes: mass and function; sleeping or dead? Diabetologia 2019;62:567–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.