Abstract
House dust mites (HDM) extract is a common trigger of asthma in humans. Chronic exposure to HDM also induces asthma-like pathology in mice. Allergic responses to HDM and other allergens are linked to release of IL-25, IL-33 and TSLP by epithelial cells; these cytokines, especially IL-33, target innate lymphoid cells-2 (ILC2s) to produce type-2 cytokines. To what extent and by what mechanisms IL-25 contributes to chronic HDM-induced pathology is not well understood. In humans, elevated levels of IL-25 appear to be associated with cases of uncontrolled asthma and exacerbated attacks. Here we demonstrate that blockade of IL-25 signaling in either lung conventional dendritic cells (cDCs) or in T cells resulted in similar decreases in production of IL-13 and IL-9 by T cells, reduced mast cell accumulation and tissue remodeling, and improved lung function, but had only modest effects on eosinophilia. Stimulation of cDCs by IL-25 promoted proximal accumulation of T helper cells (Th) and stimulation of Th cells by IL-25 locally promoted IL-13 and IL-9 production. IL-25 made notable contributions to chronic HDM-induced allergic asthma pathology by facilitating clustering and cross-stimulation of different cell types in tissue. Therapeutic targeting of IL-25 in combination with other treatments may be beneficial.
INTRODUCTION
Allergic asthma is a chronic remitting/relapsing disease of the airways. It affects more than 8% of the US population and its incidence is on the rise worldwide (Center for Disease Control and Prevention (CDC) 2016 NHIS data). Allergic asthma is triggered by various allergens/insults and is characterized by pulmonary inflammation and remodeling of tissue, culminating in significantly impaired lung function. This complex disease is thought to consist of various endotypes underlying distinct phenotypes (1). CDC data show that most asthmatic patients are allergic to house dust mites (HDM) (2). HDM contains multiple components that trigger various, at least partially overlapping pathways to initiate inflammation (3, 4). Allergic asthma typically skews towards type-2 responses, though not exclusively. Type-2 responses are thought to be favored by the alarmins/cytokines IL-33, IL-25 and TSLP, which can be rapidly released by epithelial cells in response to an allergic trigger or insult (5, 6). The functions of these cytokines partially overlap, as they can target innate lymphoid cells (ILC2) to quickly produce effector type-2 cytokines such as IL-13, IL-5 and IL-9. These cytokines are also produced by differentiated T helper cells (primarily Th2 and Th9) after adaptive responses have been generated. In addition to activating ILC2s, TSLP may directly stimulate dendritic cells to migrate to lymph nodes (7), and IL-25 and IL-33 may directly target various T cells (8, 9), but to what extent these and potentially other cells targeted by these cytokines ultimately shape asthmatic pathology is not well understood. HDM causes the release of all three alarmins/cytokines, and since IL-33 is the most potent stimulator of ILC2s and type-2 responses among these, IL-25 might well be redundant during the development of chronic HDM-induced asthma pathologies (5, 10). Nevertheless, in humans, some, albeit not all reports have suggested a correlation between elevated IL-25 levels with disease severity, uncontrolled asthma, exacerbations in asthma and rhinosinusitis in patients and strength of allergic responses (11–19). In mice, IL-25 has been reported to make critical contributions in the Ova-asthma model, although mechanisms remain largely unknown (20–22). The Ova-asthma model involves sensitization to Ova via i.p. injections with alum. HDM models are considered to more physiological, because sensitization to allergens occurs in the lung. One group employing HDM challenges in mice reported a role for IL-25 in lung remodeling, most evident in mice over-expressing Smad2 in lung cells (23, 24). By contrast, IL-25 was found to have no notable role in a study involving an acute HDM model (25), or to have only a minor role in a study involving a chronic model with a cocktail of several allergens (26); in these studies, IL-33 or IL-33 and TSLP were found to be critical, respectively. However, these HDM studies involved Balb/c mice, a strain that is strongly biased towards Th2 responses, potentially obscuring IL-25 contributions; in addition, asthma phenotypes were not comprehensively investigated. Therefore, potentially relevant contributions in chronic HDM-induced asthma in mice remain unsettled and possible mechanisms of IL-25 in lung inflammation/remodeling in general remain to be explored, especially given the human data implicating IL-25.
Chronic exposure to HDM in mice is a physiologically relevant model, as it recapitulates many of the pathologies of the human disease, including type-2 inflammation, tissue remodeling and impairment of lung function (27). Pathology does not clearly develop in acute models, in which innate responses predominate. Nevertheless, innate responses do set the stage for development of adaptive immunity and likely continue to play a role throughout following each exposure to allergens (28–30). Among major type-2 effector cytokines elicited with HDM exposure, IL-5 appears most critical for eosinophilia and mucus production, while IL-13 may be critical for the development of many other aspects of this disease, including tissue remodeling (31–33). IL-9 has been implicated in the latter process as well, possibly in part due to its recognized role in mast cell accumulation (34, 35).
IL-25 is a member of the IL-17 cytokine family and the only one associated with type-2 responses (36). All members, including IL-25, signal via the adaptor protein CIKS (Act1) (37). Previously we found that direct repeated administration of IL-25 into lungs induced inflammation (38). This acute inflammation model involved limited production of IL-9 by T cells, which in turn was partly dependent on IL-25-stimulated CD11c+ cells; a similar process may occur during acute HDM-challenges. However, these acute models could not address whether IL-25 and its noted effects ultimately played important roles in driving asthma pathology in a chronic HDM-induced model. Here we demonstrate that IL-25 made critical contributions to overall inflammation, tissue remodeling and lung function impairment in the setting of chronic HDM-induced allergic asthma. IL-25’s impact was selective, as not all phenotypes were affected. We also now demonstrate that IL-25 notably promoted the functional interaction of specifically the conventional dendritic cells (cDCs) subpopulation of CD11c+ cells with differentiated T helper (Th) cells in lung tissue. Stimulation of both cell types by IL-25 was necessary for increased production of IL-13 and especially of IL-9 by Th cells; ultimately this translated into increased tissue remodeling and exacerbated impairment of lung function.
MATERIALS and METHODS
Mice
Sources of mice (C587BL/6J): CIKSflox/-, IL-17RB−/− and IL-17RBflox/- mice were generated in our laboratory (39); IL33−/− (UC Davis KOMP repository, Oakland CA); CCL17−/− reporters gift from Dr. I. Forster (40); RosamT/mG, Itgax(CD11c)-cre(1–1Reiz/J); CD4-cre(Cwi/BfluJ) (Jackson Laboratories, Bar Harbor ME); Zbtb46-cre gift from Dr. M. Nussenzweig (41). Mice were bred and housed in NIAID Institute facilities and all experiments were done with approval of the NIAID Animal Care and Use Committee and in accordance with all relevant institutional guidelines. All control animals were littermates. Females and males were used randomly with the same numbers of males and females in each group.
Chronic HDM-induced asthma model
Mice received house dust mites extracts i.n. (Dermatophagoides pteronyssinus in saline; Greer Laboratories, Lenoir NC) or saline as a control at d0 (200 μg), d7, d12 (100 μg doses) and twice weekly for the next 5 weeks (50 μg doses) (total of 7-weeks) (Supplementary Fig. 1A). Mice were harvested for analysis 24h after the last HDM administration. Unlike HDM-treated mice, untreated mice did not develop HDM-specific antibody responses in our facility (Supplementary Fig. 1B).
Airway hypersensitivity
Airway responsiveness was analyzed by direct measurements of resistance in anaesthetized and tracheostomized mice in response to methacholine at end of 7-week HDM challenge, using a Flexivent system (SCIREQ Co, Montreal, Canada).
Bronchoalveolar lavage fluid (BALF)
BALFs were collected as described (37) Briefly, the lungs were lavaged with 0.5ml of PBS. BALFs were centrifuged at 300× g for 5 min and supernatants stored at −70C for further cytokine analysis. Cells were used for flow cytometry.
Immunohistochemistry
Lungs were prepared as described (37). For collagen deposition, sections were stained with Picro red (Abcam, Cambridge MA) following manufacturers’ directions. For mast cell counts, sections were stained with Toluidine blue or with anti-Mast Cell Tryptase antibody (Abcam, Cambridge MA).
Flow cytometric analysis and intracellular staining
Lung cells were prepared as described (38). Single cell lung suspensions and BALF cells were stained with the following antibodies: AF488-Ly6C(HK1.4), PE-SiglecF(E50–2440), PercP-CD11b(Mac-1), Pe-c7-Ly6G(1A8), APC-CD11c(HL3), APC-cy7-CD4(GK1.5), PB-TCRβ(H57–597). For intracellular staining, cells were cultured for 4h with cell stimulation cocktail (Thermo fisher, Waltham, MA), followed by surface staining. Cells were then permeabilized with BD cytofix/cytoperm kit (BD Biosciences, San Jose, CA) and stained with PE-IL-9(RM9A) and APC-IL-13(ebio13A). Flow cytometry was performed with FACSCanto II or FACS Celesta (BD Biosciences, San Jose CA)) and data were analyzed using FlowJo software (Tree star Inc., Ashland OR). All antibodies were purchased from BD Biosciences (San Jose CA), ebioscience (Thermo fisher, Waltham MA) and Biolegend (Dedham MA).
Lung homogenates for ELISA
Lungs were homogenized in PBS with complete protease cocktail inhibitor (Sigma, Burlington, MA), then centrifugated at high speed for 15 min and supernatants stored at −80C. Total protein was determined using Bradford reagents (BioRad, Hercules, CA)
Real Time PCR (RT-PCR)
cDNA was synthesized with a kit (Qiagen, Germantown MD). Gene expression was quantified with the Taqman Gene Expression Assay kits (Thermo Fisher, Walkersville MD).
Th9 differentiation
Splenic CD4 magnetic bead-sorted cells were isolated form RosamT/mG mice. TdTomato-marked CD4 T-cells were cultured in complete IMDM (Life Technologies -Thermo-Fisher Walkersville MD) with plate-bound anti-CD3 (2μg/ml), anti-CD28 (2μg/ml), anti-IFNγ (10μg/ml) (BioXCell, West Lebanon, NH), TGFβ (4ng/ml) (R&D systems, Minneapolis, MN) and IL-4 (10ng/ml) (Peprotech, Rocky Hill NJ) for 3d, followed by a second round of culture with these reagents. We confirmed that these differentiated cells responded to IL-25 by specifically producing IL-9 mRNA and protein (Supplemental Figure 1C).
Adoptive transfer of Th9 T cells and live cell imaging in the lung
5×106 TdTomato Th9 cells were injected i.v. into WT and CIKS−/− or CCL17+/− and CCL17−/− GFP-reporter mice. 24h later mice were exposed to rIL-25 i.n. (500ng/mouse/day, Biolegend, San Diego CA) for two consecutive days. 24h later mice were given a lethal overdose of avertin and exsanguinated. Lung sections were prepared for live cell imaging as described (42). Briefly, 1.5% of low-melt agarose inflated tissues were sliced into 300–350 μm sections using Leica VT1000-S-Vibrating-Blade-Microtome (Leica Microsystems, Richmond Hill ON, Canada) on ice-cold PBS. Tissue sections were stained with fluorescently labeled anti-CD31 antibody (eBioscience, San Diego, CA) for 2h on ice. Tissues were imaged using Leica DMi8-inverted-5-channel confocal microscope equipped with an Environmental Chamber (NIH Division of Scientific Equipment and Instrumentation Services) to maintain 37°C and 5% CO2. Post-acquisition mages were processed using Imaris (Bitplane, Concord MA).
Flt3L bone-marrow-derived dendritic cells (BMDCs)
1×106 BM cells were incubated in complete RPMI media (Life Technologies-Thermo-Fisher, Walkersville MD), 1/5 of which from supernatants of B16 cells-expressing Flt3L (gift from Dr. Mora, Milennium Pharmaceuticals). Fresh media was added to the cultures every two-three days. On day 8, 5×105 Flt3L-BMDCs were incubated with/without IL-25 (Biolegend, San Diego CA) and IL-13 (Peprotech, Rocky Hill NJ).
Statistical analyses
Statistical significance of data was determined with the unpaired t-test, and if variances between groups were unequal, Welch’s correction was applied. F-test was used to compare variances between two group. In Figure 8C paired t-test was used. All statistical analyses were performed with GraphPad Prism v7.0 (La Jolla CA).
FIG 8.
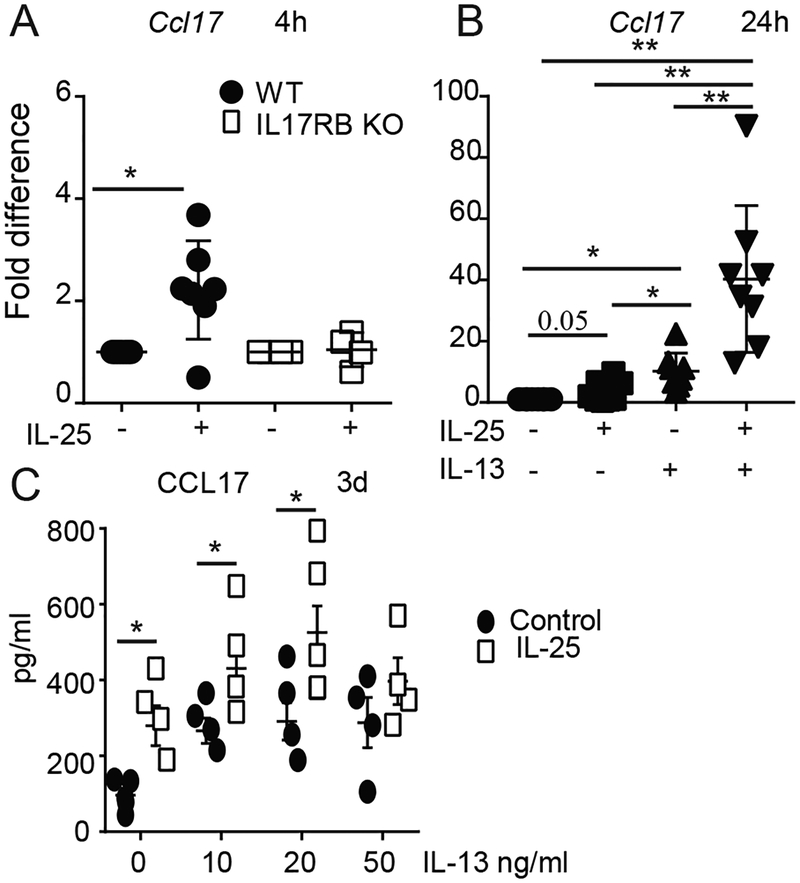
IL-25 synergizes with IL-13. (A-C) Flt3L-induced BMDCs were incubated with IL-25 (500ng/ml) without or with IL-13 (25ug/ml, except in C) as indicated, and relative mRNA levels (A,B) or protein levels (C) of CCL17 are shown. Cells were stimulated for 4h (A), 24h (B) or 3d (C), with increasing concentrations of IL-13 in (C)). Data are the mean ± std of n=6 experiments/mice for (A); n=7 for (B) and n=4 for (C). *p<0.05.
RESULTS
IL-25/IL-17RB signaling shapes immune responses in chronic HDM-induced asthma
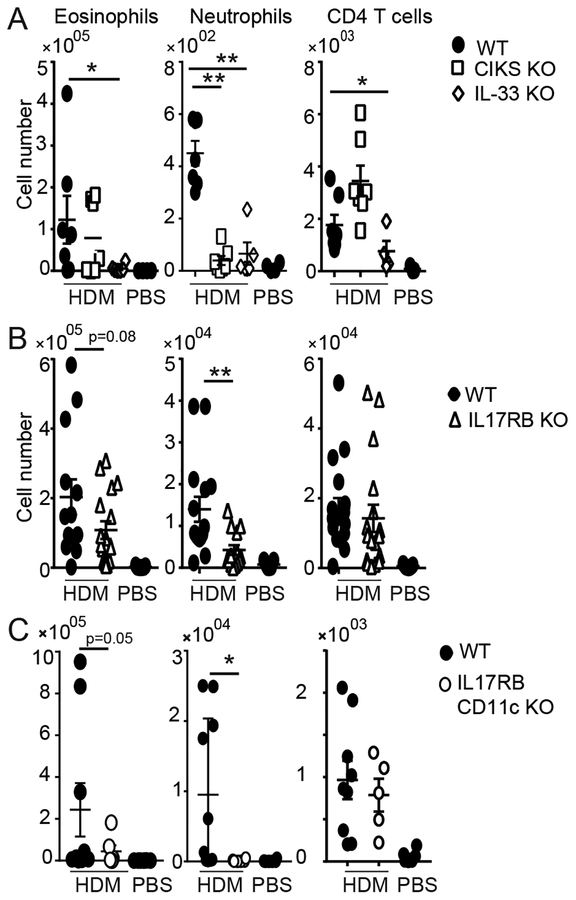
IL-25 and IL-33 levels were notably increased and TSLP levels trended upwards in bronchial alveolar lavage fluid (BALF) of mice 24h after the last HDM exposure in the chronic 7 weeks-long HDM allergic asthma model (Supplementary Fig. 1A,D). To investigate for critical consequences of IL-25 in this model, we characterized immune responses in wild-type (WT) and in mice deficient (KO) in CIKS, IL-33 (Fig. 1A) or IL-17RB (Fig. 1B). CIKS and IL-17RB are essential for IL-25 signaling, and potentially IL-17B; however, since IL-17B antagonizes IL-25 in mucosal inflammation (21), IL-17RB reports only on functional IL-25 signaling. Compared to HDM-treated WT mice, loss of CIKS and loss of IL-17RB led to a reduction of neutrophils infiltrating BALFs, but only marginal reduction of eosinophils and no reduction of CD4+ T cells. By contrast, loss of IL-33 blunted all cellular infiltrations. Mice deficient in CIKS, IL-33 or IL-17RB all exhibited clearly reduced expression of mRNAs for IL-13, IL-9, and the chemokine CCL17 in lungs (Supplementary Fig 2A). Thus IL-25/IL-17RB signaling had a notable impact on the inflammatory phenotype in this model. Its impact was selective however, whereas IL-33 may be critical during the initiation of all responses (28).
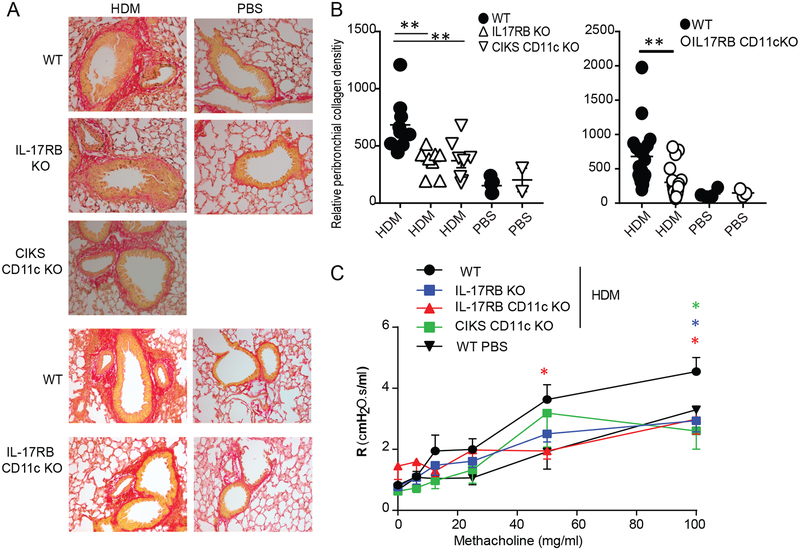
FIG 1.
IL-25 signaling contributes to chronic HDM-induced lung inflammation. Cellular counts in BALFs of WT, IL-33 KO and CIKS KO mice (A); WT and IL-17RB KO mice (B) and WT and IL-17RB CD11c KO mice (C) after chronic exposure to HDM (and PBS for WT; similar to PBS for KO mice). Eosinophils (SiglecF+ CD11c+ CD11b+); neutrophils (Ly6G+, CD11b+, CD11c−); T cells (CD4+ TCRβ+). Significance indicated for HDM-treated WT vs KO mice. Data are the mean ± sem of (A) two independent experiments with n=6–7 mice per group; (B) three independent experiments with n= 7–14; (C) two independent experiments with n=5–9. * p<0.05; **p<0.01
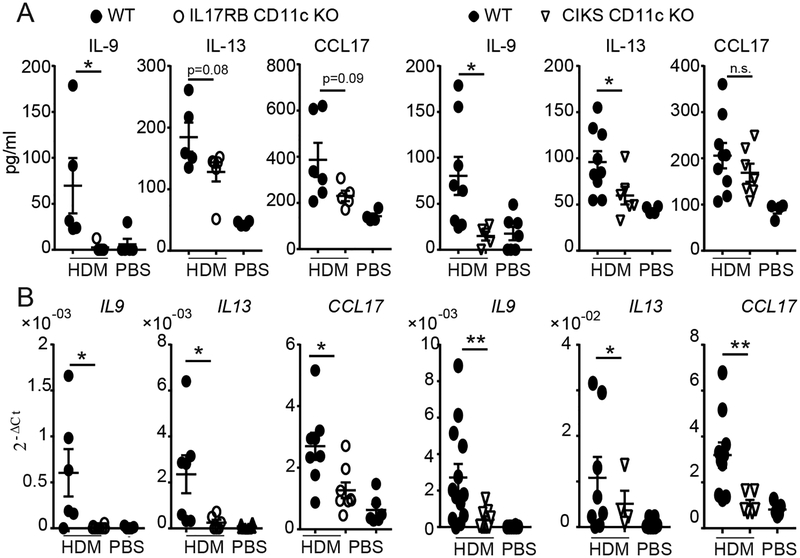
IL-25 stimulates ILC2’s, but in addition may target CD11c+ cells, based on analyses of acute inflammation initiated by direct administration of IL-25 into lungs (38). To determine whether targeting of CD11c+ cells was relevant in the chronic HDM model, we used mice lacking IL-17RB selectively in CD11c+ cells (IL-17RB CD11c KO). Compared to WT, IL-17RB CD11c KO mice showed reduced infiltration of neutrophils in BALFs, moderate loss of eosinophils and essentially no reduction of CD4 T cells (Fig. 1C). These findings mirror what we observed in mice with global loss of CIKS or IL-17RB (Fig. 1A,B). Mice lacking IL-17RB or CIKS specifically in CD11c+ cells also contained less IL-13 and especially less IL-9 protein in BALFs, while CCL17 protein in lungs only trended lower (Fig. 2A; in the case of IL-17RB cCD11c KOs IL-13 levels also trended lower, not quite reaching significance). Lung mRNAs for all three mediators were however notably reduced in both CD11c-specific KO mice (Fig. 2B), similar what we noted for mice with global loss of CIKS or IL-17RB (Supplementary Figure 2A). Therefore, targeting of CD11c+ cells by IL-25 critically contributed to specific aspects of chronic HDM-induced asthma.
FIG 2.
IL-25 signaling in CD11c+ cells promotes HDM-induced type-2 inflammatory mediators. (A) Protein levels of IL-9, IL-13 in BALF and CCL17 in lungs of WT and mutant mice as indicated after HDM (or PBS) exposures. (B) Relative mRNA levels of IL9, IL13 and CCL17 in lungs as in (A). Significance indicated for HDM-treated WT vs KO mice. Data are the mean ± sem of two independent experiments with a total of n=6–8 mice per group for WT and IL-17RB CD11c KO and n=6–12 mice per group for WT and CIKS CD11c KO. *p<0.05; **p<0.01.
IL-25/IL-17RB contributes to tissue remodeling and impaired lung function
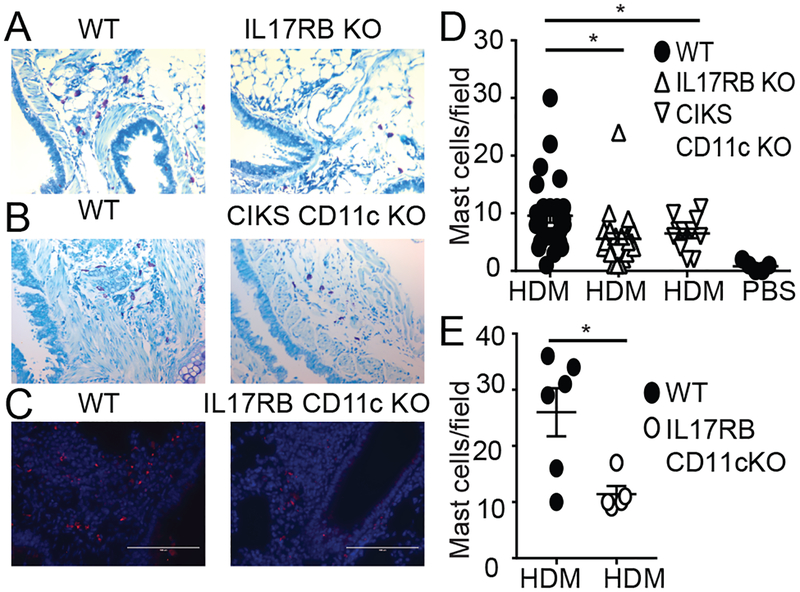
Chronic HDM exposure leads to tissue remodeling. Mast cells have been implicated in such processes (43). Mice impaired in IL-25 signaling – globally (IL-17RB KO) or selectively in CD11c+ cells (IL-17RB CD11c KO and CIKS CD11c KO) - had notably fewer mast cells relative to WT mice after HDM treatments, although numbers were still increased relative to PBS controls (Fig. 3A–E). The IL-25 signaling-impaired mutant mice also presented with much reduced mRNA expression of the mast cell protease Mcpt1 (Supplementary Fig. 2B). By contrast, mucus production appeared largely unaffected by loss of IL-25 signaling (Supplementary Fig. 2C,D).
FIG 3.
IL-25 signaling in CD11c+ cells promotes HDM-induced accumulation of mast cells. (A,B) Toluidine blue staining (C) and α-mast cell tryptase staining of HDM treated WT and mutant mice, as indicated. (D,E) Quantification of mast cells per field based on A,B (D) or C (E), including background in PBS treated WT mice. Significance indicated for HDM-treated WT vs KO mice. Data are the mean ± sem of two independent experiments with two fields per mouse counted with n=6–12 mice per group (A,B,D) and (C,E) one experiment with two fields per mouse with a total of n=3 mice per group. *p<0.05.
Increased collagen deposition is a critical hallmark of human asthma. Deposition of collagen in HDM-treated WT mice was most evident in peri-bronchial areas and this was reduced in mice blocked in IL-25 signaling universally (IL-17RB KO) or only in CD11c+ cells (CIKS CD11c KO, IL-17RB CD11c KO) (Fig. 4A,B). Consistent with these findings, HDM-induced airway hyper-responsiveness to methacholine was reduced in these mutant mice compared to WT mice (Fig. 4C). Thus, IL-25/IL-17RB-mediated signaling notably contributed to tissue remodeling and impaired lung function in the present model, dependent on induced activities in CD11c+ cells.
FIG 4.
IL-25 signaling in CD11c+ cells increases HDM-induced collagen deposition and airway resistance. (A) Representative lung sections of HDM or PBS treated WT and mutant mice, as indicated, stained with Picro red for collagen deposition. (B) Quantification of collagen deposition. Area, integrated density and background values were obtained using Image J software (Corrected intensity= integrated density-(area × mean of the background)). All data were normalized against WT PBS control. Significance indicated for HDM-treated WT vs KO mice. (C) Airway hyper-resistance response to methacholine in HDM or PBS treated WT or mutant mice as indicated. Data are the mean ± sem of two independent experiments with two or three fields per mouse counted with total of n=4–6 mice per group (A,B) and (C) three independent experiments with a total of n=7–8 mice per group, except one experiment for CIKS CD11c KO with n=3 mice. *p<0.05; **p<0.01.
IL-25 signaling controls T helper cell functions via two mechanisms
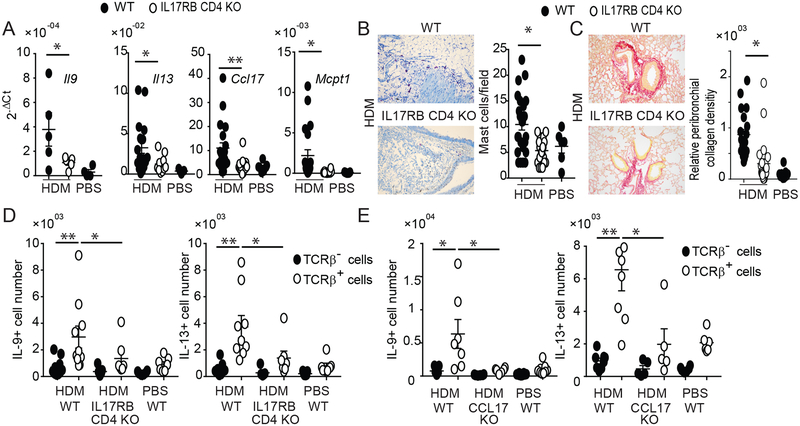
IL-25 and IL-33 target ILC2s to produce IL-13 and IL-9, drivers of type-2 lung inflammation. Since IL-33 is a more potent stimulator of ILC2s, IL-25 should be dispensable. However, in the chronic phase, αβT cells (TCRβ+) Th2 and Th9 are thought to be the major source of both IL-13 and IL-9 in lung. We confirmed this and furthermore demonstrated that global or CD11c+-specific loss of IL-17RB specifically reduced the numbers of these IL-13- and IL-9-producing TCRβ+ cells (Supplementary Fig. 3). (TCRβ− producers included ILC2s; among the TCRβ+ cell populations, only CD4+ Th cells produced notable levels of these cytokines (Supplementary Fig. 4). Thus IL-25/IL-17RB-mediated signaling is important for the HDM treatment-dependent increases in IL-13- and IL-9-producing Th2 and Th9 cells, respectively.
IL-25 can stimulate differentiated Th2 and Th9 to produce IL-13 and IL-9, respectively. We therefore selectively deleted IL-17RB in T cells via CD4-Cre-mediated deletion (IL17RB CD4 KO). These mutant mice exhibited markedly reduced expression of IL-13 and IL-9 mRNA in lungs of HDM-treated mice; in addition, CCL17 and MCPT1 mRNAs were reduced, suggesting that Th2/Th9 mediators induced by IL-25 promoted expression of all of these inflammatory markers (Fig. 5A). As a consequence, mast cells accumulation and peribronchial collagen deposition were also reduced in mice with IL-17RB deletion in T cells (Fig. 5B, C respectively). Also, IL17RB CD4 KO mice presented with reduced numbers of TCRβ+ producers of IL-13 and IL-9 (Fig. 5D). Therefore, IL-25 directly stimulated Th2/Th9 cells to produce IL-13 and IL-9, while IL-25 signaling in CD11c+ cells was critical as well, as it promoted the appearance of Th2/Th9 cells in lung.
FIG 5.
IL-25 signaling in T cells drives HDM-induced type-2 inflammation in lungs. (A) Relative mRNA expression of genes as shown in HDM or PBS treated WT and IL-17RB CD4 KO mice. (B) Representative toluidine blue staining of lung (left) and total mast cell count (right) for mice and treatment as shown. (C) Representative Picro red staining of lung (left) and total relative peribronchial collagen density (right). For (A,B,C) data are the mean± sem of three independent experiments and a total of n=5–12 mice per group. For the mast cells and peribronchial collagen densisity, two fields per mouse were counted. (D, E) Numbers of IL-9- and IL-13-producing TCRβ+ and TCRβ- cells in lungs of HDM or PBS treated WT and IL-17RB-CD4 KO (D) and CCL17 KO mice (E), as indicated. Significance indicated for all TCRβ+ counts from HDM-treated mice and for TCRβ+/TCRβ- counts from WT mice. Data are the mean ± sem of two independent experiments with a total of n=7–9 (D) or n=5–7 (E) mice per group. *p<0.05; **p<0.01.
How might IL-25-stimulated CD11c+ cells accomplish this? CCL17 is a chemokine produced by CD11c+ DCs that potently attracts Th2 and Th9 cells, due to high expression of the CCR4 receptor on these cells. Administration of IL-25 into lungs rapidly increased expression of this chemokine (38). Mice lacking CCL17 largely failed to accumulate TCRβ+ cells in lung capable of producing IL-13 and especially IL-9 (Fig. 5E). The results suggest that IL-25 signaling in CD11c+ cells increased production of CCL17 and likely other mediators needed to attract/promote accumulation of Th2 and Th9 cells, setting them up for direct local stimulation by IL-25.
IL-25 promotes clustering of Th9 cells adjacent to CD11c+ cells
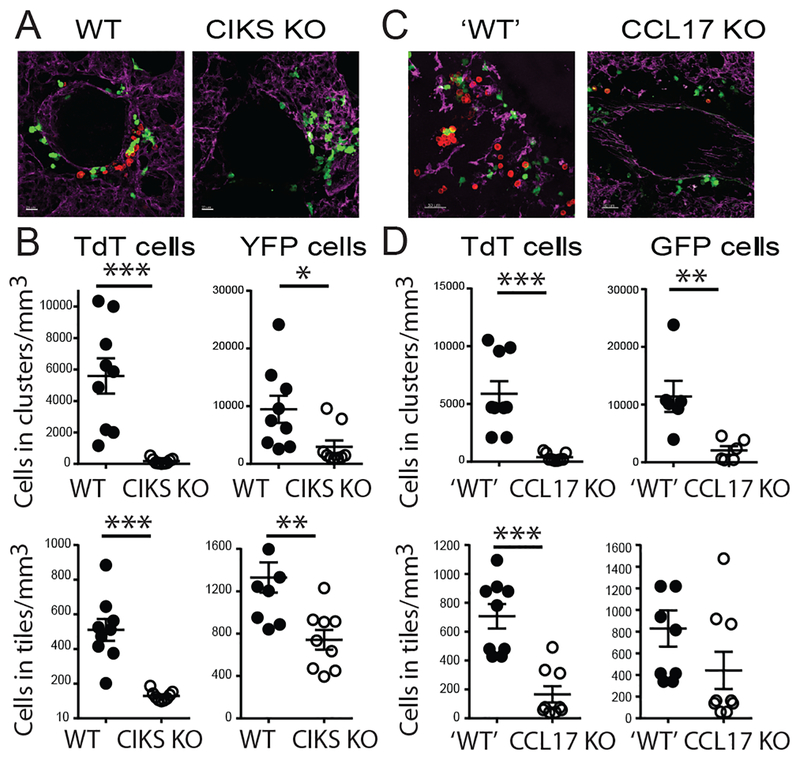
Based on these findings we hypothesized that IL-25 controls localization of Th2/Th9 cells in lung via CD11c+ cells. IL-25 or PBS were administered i.n. into lungs of WT or CIKS KO mice after i.v. injection of in vitro-differentiated Th9 cells. Th9s were marked by TdTomato and CD11c+ cells of recipient mice were marked by YFP. Lung explants were analyzed with confocal microscopy. IL-25 induced clustering of Th9 cells near CD11c+ cells around the airways, especially near blood vessels; this essentially did not occur in recipient mice lacking CIKS. Total levels of Th9 cells present in lung were also reduced, but dramatically less so than the reduction in clustering (Fig. 6A,B; clustering was reduced over 70-fold while total levels of Th9 cells were reduced about 5-fold). We also made use of CCL17 reporter mice, in which a GFP insertion inactivated CCL17 expression. In recipient mice heterozygous for the reporter allele, we again noted clustering of Th9 cells near CCL17+ cells; by contrast, essentially no clustering was seen in homozygous mice deficient in CCL17 (Fig. 6C,D). Lack of CCL17 also reduced the total numbers of Th9 cells in lung, as expected, but this reduction was again much less pronounced than the reduction of clustering. These data indicate that IL-25/IL-17RB signaling promoted some recruitment of Th9s into lungs but was essential for their clustering next to CD11c+ and CCL17+ cells.
FIG 6.
IL-25 induces clustering of Th9 cells proximal to lung dendritic cells. (A,C) Representative lung sections of CD11c-YFP expressing WT and CIKS KO mice (A) or CCL17-GFP reporter ‘WT’ (CCL17+/−) and KO (CCL17−/−) mice (C) after i.v. injection of in vitro differentiated, TdTomato-marked Th9 cells, followed 24h later by two i.n. administrations of IL-25 on successive days, analyzed the following day. (B,D) Quantification of TdTomato T cells and YFP (B) or GFP (D) cell densities in clusters, and total numbers of these cells in lung tiles for data from (A) and (C), respectively, based on counting cells in three sections of left lobule of the lung per mouse from three independent mice/experiments. Clusters defined as ≥ 3 T cells in close proximity to each other, which only occurred very close to (or ostensibly touching) dendritic cells. *p<0.05; **p<0.01; ***p<0.005.
CD11c+ conventional DCs (cDCs) are targeted by IL-25
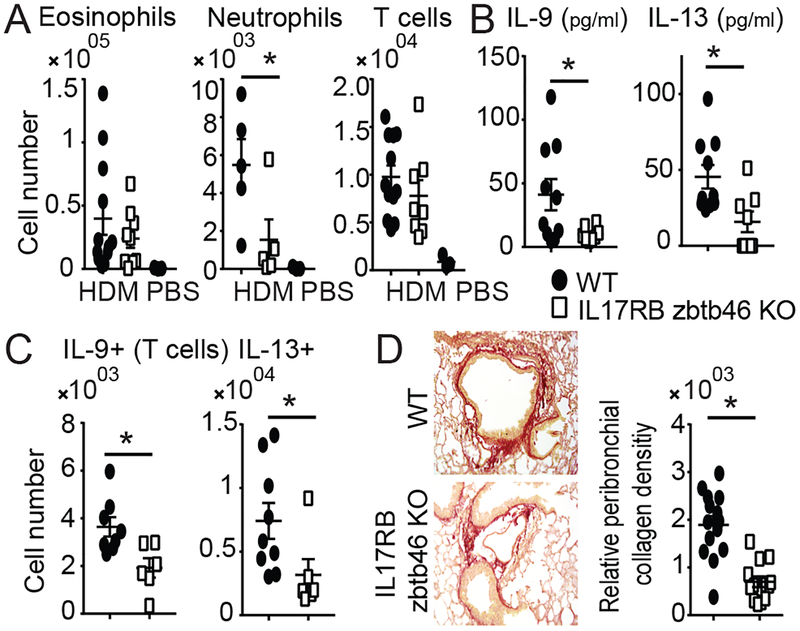
CD11c is expressed by cDCs, but also by certain myeloid cells and smaller subsets of other cells. We generated mice selectively deleted for CIKS in cDCs via Zbtb46-driven Cre (41). Chronic HDM-induced phenotypes in these mice largely mimicked those in mice lacking IL-17RB globally or specifically in CD11c+ cells (Fig. 7; cf. Figs. 1,2,5, Supplementary Fig. 3). In both, HDM-induced recruitment of neutrophils in BALF was reduced relative to WT, but not that of T cells, and eosinophils were only marginally reduced (Fig. 7A). IL-9 and IL-13 protein levels in BALF were reduced in both mutant mice (Fig. 7B), as were numbers of IL-9+ and IL-13+ TCRβ+ cells in lungs (Fig. 7C). Finally, as observed above for mice impaired in IL-25 signaling in CD11c+ cells (Fig. 4A–C), mice impaired in this signaling pathway in cDCs also exhibited reduced collagen deposition (Fig. 7D). These findings identify cDCs among CD11c+ cells as critical targets of IL-25/IL-17RB/CIKS signaling in HDM-induced pathology.
FIG 7.
Conventional lung dendritic cells (cDCs) are critical targets of IL-25 (A,B) Cellular counts (A) and protein levels of IL-9 and IL-13 (B) in BALFs of HDM (or PBS) treated WT and IL-17RB Zbtb46 KO mice. (C) Numbers of IL-9 and IL-13 producing TCRβ+ T cells in lungs of HDM treated WT and mutant mice, as indicated. (D) Representative sections and quantification of collagen deposition around airways of WT and mutant mice, as indicated and as described in Fig. 4. Data are the mean± sem of two independent experiments, n=5–12 mice per group, except one experiment with n=3 mice for WT PBS group in (A). Significance indicated for HDM treated mice. *p<0.05; **p<0.01.
We generated cDCs ex vivo with Flt3L-stimulation in bone marrow-derived cultures (44). IL-25 induced the expression of CCL17 mRNA in such cDC cultures within 4h if derived from WT, but not from IL-17RB-deficicent mice (Fig. 8A). IL-25-induced CCL17 expression was transient, but IL-25 notably boosted CCL17 expression induced by IL-13, even after 24h (Fig. 8B); IL-13 had previously been reported to induce CCL17 in DCs to recruit memory Th2 cells (30). IL-25 also boosted CCL17 protein levels induced by IL-13 alone, especially at lower concentrations of IL-13 (Fig. 8C). Therefore, IL-25 directly targets Flt3L-induced BM-derived cDCs and synergizes with IL-13 to induce CCL17.
DISCUSSION
The findings presented demonstrate that the IL-25/IL-17RB/CIKS signaling pathway is a key contributor to full chronic HDM-induced allergic asthma-like pathology. IL-25 does so by targeting several distinct cell types and coordinating their interactions. Our research revealed that the full chronic HDM-induced pathology depends on direct stimulation of cDCs by IL-25 in the airways, a cell type not previously identified as a physiologically relevant target of IL-25. Evidence is presented that once cDCs are activated in this way, these cells in turn attract differentiated T helper cells to cluster near them. In this way Th9 and Th2 cells localize not only to cells capable of presenting antigens in the airways, but also to the local source of IL-25. We further demonstrate that full chronic HDM-induced pathology also depends on direct activation of the T helper cells by IL-25; this leads to increased production of IL-13 and especially of IL-9.
Chronic exposure to HDM is a physiologically relevant mouse model of asthma. HDM is a common trigger for human asthma and the chronic mouse model recapitulates many aspects of human asthma. HDM contains multiple components able to activate distinct and overlapping pathways leading to the release of various initiators of inflammation, including the alarmins/cytokines IL-33, IL-25 and TSLP, mediators that skew towards type-2 responses. Since IL-33 is a more potent stimulator of ILC2s and type-2 responses than IL-25 and since they also share other target cells, IL-25 might be expected to serve a redundant role in the chronic HDM model; instead, the data presented reveal important novel contributions of this cytokine. The contributions were selective: IL-25 did impact tissue remodeling and airway hyper-responsiveness, but not eosinophilia and mucus production. Mechanistically, the IL-25 pathway notably promoted the functional interaction between cDCs with Th2 and Th9 cells, and presumably facilitated antigen-mediated activation of the Th cells. In addition, and importantly, IL-25 also targeted these cells directly, which in turn was required to boost production of IL-13, and especially of IL-9, ultimately driving pathology. IL-25 enabled physical association of cDCs and Th cells by enhancing production of CCL17 in cDCs, and it then directly stimulated Th2 and Th9 to develop into full effectors. Loss of this signaling pathway in either cDCs or in T cells ameliorated pathologic phenotypes similarly, reducing tissue remodeling and thus improving lung function.
The recruitment of eosinophils was largely unaffected by loss of the IL-25 signaling pathway, which correlated with minimal effects on IL-5 production (Supplementary Figure 2E), a major regulator of eosinophil accumulation in tissue. On the other hand, neutrophils, whose accumulation is generally associated with IL-17 and severe asthma, were notably reduced in mice impaired in IL-25 signaling. Interestingly, cases of severe asthma have also been correlated with higher levels of IL-25 and rhinovirus-provoked asthma exacerbations have been linked to increases in IL-25 (13). How IL-25 regulated neutrophil accumulation in this model and why IL-5 and eosinophil accumulation was much less affected remains to be explored.
IL-25 is produced by so-called chemosensory Brush cells in lungs, which are related to Tuft cells in the intestines. Brush cells may be found primarily in the upper respiratory tract, especially at branch points, intertwined with nerve fibers (45). They appear to constitutively express IL-25, although the cytokine is likely only released with appropriate stimulations (46). A recent study additionally shows that exposure to aeroallergens expands these IL-25-producing cells, dependent on the LTE4/CysLT3R (Leukotriene E4/ cysteinyl leukotriene E4 receptor) pathway and on IL-25 itself; this process appears to be critical for e.g. Alternaria-induced lung inflammation (47). The findings presented in the present study reveal a novel IL-25-initiated process involving both cDCs and Th cells to enhance production of IL-13 and especially IL-9. We propose a model wherein the local release of IL-25 by Brush cells at sites of insult stimulates cDCs to increase CCL17 production and possibly other mediators, which attract and potentially also maintain Th2 and especially Th9 cells; such a scenario then also allows for the direct local stimulation of these Th cells by IL-25 and antigens. This model is supported by our findings that adoptively-transferred Th9 cells clustered near CD11c+ and CCL17+ cells in airways in response to i.n. administration of IL-25 and that the phenotypes resulting from loss of IL-25 signaling in T cells mimicked those resulting from loss of this pathway in cDCs. Blocking IL-25 signaling in either of these cell types led to reduced mast cell accumulation, which is partly controlled by IL-9, and also diminished collagen deposition, which is partly controlled by IL-13, pathologic features that herald impaired lung function.
Our findings further suggest that IL-25 may initiate a feed-forward mechanism between cDCs and Th cells, as Th2-produced IL-13 synergizes with IL-25 to maintain high levels of CCL17 and possibly other mediators, assuring sustained attraction of Th cells. This is supported by the observation that IL-25 markedly boosted IL-13-induced production of CCL17 in Flt3L-dependent BM-derived cDC cultures. It is possible that low homeostatic expression of IL-13 or its rapid release by ILC2’s in acute allergic responses is required to help jump start the feed forward mechanism. In support of this notion, an initial burst of IL-13 produced by alarmin-stimulated ILC2s has been reported to induce CCL17 in cDCs and thereby allow for potent memory Th2 activation (30) or terminal effector differentiation of Th2s in tissue (48, 49). We suggest that IL-25 produced by allergen-stimulated Brush cells may not only stimulate ILC2’s, but can directly activate cDCs and Th cells to enforce the clustering and collaborative interaction of these cells, which may be required to allow for a sufficiently strong activation/terminal differentiation of Th2 and especially Th9 cells at allergen-challenged critical locations in the airways. In sum, IL-25 is a cytokine that not only functions as an alarmin in innate responses, but that has long-term effects on adaptive immune responses in tissue.
Asthma is a disease with different endotypes, suggesting that targeted therapies will depend on classifying patients beforehand; some endotypes may be particularly sensitive to interference with the IL-25 pathway, potentially in combination with specific allergens. But, as shown here, IL-25 signaling does have a notable impact on pathology even in the chronic HDM model, in which multiple inflammatory pathways are activated; this may be due to unique locations of Brush cells and the feed-forward amplifying circuit IL-25 triggers. These findings suggest that therapeutic targeting of the IL-25 pathway may be more generally beneficially, especially if combined with targeting other pathways.
Supplementary Material
Key Points:
IL-25 contributes to specific disease phenotypes in chronic HDM-induced asthma
Lung remodeling is partly dependent on IL-25 signaling in cDCs and in Th cells
IL-25 induces clustering of Th9 T cells next to IL-25-activated cDCs in airways
ACKNOWLEDGEMENTS
We acknowledge use of the NIH tetramer core facility and the KOMP repository for IL-33−/− mice.
This work was supported by the Intramural Research Program of the National Institute of Allergy and Infectious Diseases, National Institutes of Health.
Abbreviations used in this article:
- CIKS
Connection to IκB Kinase and Stress-activated protein kinases
- HDM
House dust mites
- ILC2
Innate lymphoid cells type 2
- cDC
conventional dendritic cells
- Th
T helper
Footnotes
DISCLOSURES
The authors have no financial conflicts of interests.
REFERENCES
- 1.Svenningsen S, and Nair P. 2017. Asthma Endotypes and an Overview of Targeted Therapy for Asthma. Frontiers in medicine 4: 158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Calderon MA, Linneberg A, Kleine-Tebbe J, De Blay F, Hernandez Fernandez de Rojas D, Virchow JC, and Demoly P. 2015. Respiratory allergy caused by house dust mites: What do we really know? The Journal of allergy and clinical immunology 136: 38–48. [DOI] [PubMed] [Google Scholar]
- 3.Post S, Nawijn MC, Hackett TL, Baranowska M, Gras R, van Oosterhout AJ, and Heijink IH. 2012. The composition of house dust mite is critical for mucosal barrier dysfunction and allergic sensitisation. Thorax 67: 488–495. [DOI] [PubMed] [Google Scholar]
- 4.Gregory LG, and Lloyd CM. 2011. Orchestrating house dust mite-associated allergy in the lung. Trends in immunology 32: 402–411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lambrecht BN, and Hammad H. 2014. Allergens and the airway epithelium response: gateway to allergic sensitization. The Journal of allergy and clinical immunology 134: 499–507. [DOI] [PubMed] [Google Scholar]
- 6.Lambrecht BN, and Hammad H. 2012. The airway epithelium in asthma. Nature medicine 18: 684–692. [DOI] [PubMed] [Google Scholar]
- 7.Kitajima M, and Ziegler SF. 2013. Cutting edge: identification of the thymic stromal lymphopoietin-responsive dendritic cell subset critical for initiation of type 2 contact hypersensitivity. Journal of immunology (Baltimore, Md. : 1950) 191: 4903–4907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Peine M, Marek RM, and Lohning M. 2016. IL-33 in T Cell Differentiation, Function, and Immune Homeostasis. Trends in immunology 37: 321–333. [DOI] [PubMed] [Google Scholar]
- 9.Angkasekwinai P, Chang SH, Thapa M, Watarai H, and Dong C. 2010. Regulation of IL-9 expression by IL-25 signaling. Nature immunology 11: 250–256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Barlow JL, Peel S, Fox J, Panova V, Hardman CS, Camelo A, Bucks C, Wu X, Kane CM, Neill DR, Flynn RJ, Sayers I, Hall IP, and McKenzie AN. 2013. IL-33 is more potent than IL-25 in provoking IL-13-producing nuocytes (type 2 innate lymphoid cells) and airway contraction. The Journal of allergy and clinical immunology 132: 933–941. [DOI] [PubMed] [Google Scholar]
- 11.Herberth G, Daegelmann C, Roder S, Behrendt H, Kramer U, Borte M, Heinrich J, Herbarth O, Lehmann I, and L. I. s. group. 2010. IL-17E but not IL-17A is associated with allergic sensitization: results from the LISA study. Pediatr Allergy Immunol 21: 1086–1090. [DOI] [PubMed] [Google Scholar]
- 12.Seys SF, Grabowski M, Adriaensen W, Decraene A, Dilissen E, Vanoirbeek JA, Dupont LJ, Ceuppens JL, and Bullens DM. 2013. Sputum cytokine mapping reveals an ‘IL-5, IL-17A, IL-25-high’ pattern associated with poorly controlled asthma. Clin Exp Allergy 43: 1009–1017. [DOI] [PubMed] [Google Scholar]
- 13.Beale J, Jayaraman A, Jackson DJ, Macintyre JDR, Edwards MR, Walton RP, Zhu J, Man Ching Y, Shamji B, Edwards M, Westwick J, Cousins DJ, Yi Hwang Y, McKenzie A, Johnston SL, and Bartlett NW. 2014. Rhinovirus-induced IL-25 in asthma exacerbation drives type 2 immunity and allergic pulmonary inflammation. Science translational medicine 6: 256ra134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shin HW, Kim DK, Park MH, Eun KM, Lee M, So D, Kong IG, Mo JH, Yang MS, Jin HR, Park JW, and Kim DW. 2015. IL-25 as a novel therapeutic target in nasal polyps of patients with chronic rhinosinusitis. J Allergy Clin Immunol 135: 1476–1485 e1477. [DOI] [PubMed] [Google Scholar]
- 15.Cheng D, Xue Z, Yi L, Shi H, Zhang K, Huo X, Bonser LR, Zhao J, Xu Y, Erle DJ, and Zhen G. 2014. Epithelial interleukin-25 is a key mediator in Th2-high, corticosteroid-responsive asthma. Am J Respir Crit Care Med 190: 639–648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hasegawa T, Uga H, Mori A, and Kurata H. 2017. Increased serum IL-17A and Th2 cytokine levels in patients with severe uncontrolled asthma. Eur Cytokine Netw 28: 8–18. [DOI] [PubMed] [Google Scholar]
- 17.Chen F, Hong H, Sun Y, Hu X, Zhang J, Xu G, Zhao W, Li H, and Shi J. 2017. Nasal interleukin 25 as a novel biomarker for patients with chronic rhinosinusitis with nasal polyps and airway hypersensitiveness: A pilot study. Ann Allergy Asthma Immunol 119: 310–316 e312. [DOI] [PubMed] [Google Scholar]
- 18.Kohanski MA, Workman AD, Patel NN, Hung LY, Shtraks JP, Chen B, Blasetti M, Doghramji L, Kennedy DW, Adappa ND, Palmer JN, Herbert DR, and Cohen NA. 2018. Solitary chemosensory cells are a primary epithelial source of IL-25 in patients with chronic rhinosinusitis with nasal polyps. J Allergy Clin Immunol 142: 460–469 e467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chairakaki AD, Saridaki MI, Pyrillou K, Mouratis MA, Koltsida O, Walton RP, Bartlett NW, Stavropoulos A, Boon L, Rovina N, Papadopoulos NG, Johnston SL, and Andreakos E. 2018. Plasmacytoid dendritic cells drive acute asthma exacerbations. J Allergy Clin Immunol 142: 542–556 e512. [DOI] [PubMed] [Google Scholar]
- 20.Ballantyne SJ, Barlow JL, Jolin HE, Nath P, Williams AS, Chung KF, Sturton G, Wong SH, and McKenzie AN. 2007. Blocking IL-25 prevents airway hyperresponsiveness in allergic asthma. J Allergy Clin Immunol 120: 1324–1331. [DOI] [PubMed] [Google Scholar]
- 21.Reynolds JM, Lee YH, Shi Y, Wang X, Angkasekwinai P, Nallaparaju KC, Flaherty S, Chang SH, Watarai H, and Dong C. 2015. Interleukin-17B Antagonizes Interleukin-25-Mediated Mucosal Inflammation. Immunity 42: 692–703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhang FQ, Han XP, Zhang F, Ma X, Xiang D, Yang XM, Ou-Yang HF, and Li Z. 2017. Therapeutic efficacy of a co-blockade of IL-13 and IL-25 on airway inflammation and remodeling in a mouse model of asthma. Int Immunopharmacol 46: 133–140. [DOI] [PubMed] [Google Scholar]
- 23.Gregory LG, Mathie SA, Walker SA, Pegorier S, Jones CP, and Lloyd CM. 2010. Overexpression of Smad2 drives house dust mite-mediated airway remodeling and airway hyperresponsiveness via activin and IL-25. Am J Respir Crit Care Med 182: 143–154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gregory LG, Jones CP, Walker SA, Sawant D, Gowers KH, Campbell GA, McKenzie AN, and Lloyd CM. 2013. IL-25 drives remodelling in allergic airways disease induced by house dust mite. Thorax 68: 82–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chu DK, Llop-Guevara A, Walker TD, Flader K, Goncharova S, Boudreau JE, Moore CL, Seunghyun In T, Waserman S, Coyle AJ, Kolbeck R, Humbles AA, and Jordana M. 2013. IL-33, but not thymic stromal lymphopoietin or IL-25, is central to mite and peanut allergic sensitization. J Allergy Clin Immunol 131: 187–200 e181–188. [DOI] [PubMed] [Google Scholar]
- 26.Iijima K, Kobayashi T, Hara K, Kephart GM, Ziegler SF, McKenzie AN, and Kita H. 2014. IL-33 and thymic stromal lymphopoietin mediate immune pathology in response to chronic airborne allergen exposure. J Immunol 193: 1549–1559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Johnson JR, Wiley RE, Fattouh R, Swirski FK, Gajewska BU, Coyle AJ, Gutierrez-Ramos JC, Ellis R, Inman MD, and Jordana M. 2004. Continuous exposure to house dust mite elicits chronic airway inflammation and structural remodeling. American journal of respiratory and critical care medicine 169: 378–385. [DOI] [PubMed] [Google Scholar]
- 28.Halim TY, Steer CA, Matha L, Gold MJ, Martinez-Gonzalez I, McNagny KM, McKenzie AN, and Takei F. 2014. Group 2 innate lymphoid cells are critical for the initiation of adaptive T helper 2 cell-mediated allergic lung inflammation. Immunity 40: 425–435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Martinez-Gonzalez I, Steer CA, and Takei F. 2015. Lung ILC2s link innate and adaptive responses in allergic inflammation. Trends in immunology 36: 189–195. [DOI] [PubMed] [Google Scholar]
- 30.Halim TY, Hwang YY, Scanlon ST, Zaghouani H, Garbi N, Fallon PG, and McKenzie AN. 2016. Group 2 innate lymphoid cells license dendritic cells to potentiate memory TH2 cell responses. Nature immunology 17: 57–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ingram JL, and Kraft M. 2012. IL-13 in asthma and allergic disease: asthma phenotypes and targeted therapies. The Journal of allergy and clinical immunology 130: 829–842; quiz 843–824. [DOI] [PubMed] [Google Scholar]
- 32.Barnes PJ 2018. Targeting cytokines to treat asthma and chronic obstructive pulmonary disease. Nature reviews. Immunology 18: 454–466. [DOI] [PubMed] [Google Scholar]
- 33.Lloyd CM, and Snelgrove RJ. 2018. Type 2 immunity: Expanding our view. Science immunology 3. [DOI] [PubMed] [Google Scholar]
- 34.Kearley J, Erjefalt JS, Andersson C, Benjamin E, Jones CP, Robichaud A, Pegorier S, Brewah Y, Burwell TJ, Bjermer L, Kiener PA, Kolbeck R, Lloyd CM, Coyle AJ, and Humbles AA. 2011. IL-9 governs allergen-induced mast cell numbers in the lung and chronic remodeling of the airways. American journal of respiratory and critical care medicine 183: 865–875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sehra S, Yao W, Nguyen ET, Glosson-Byers NL, Akhtar N, Zhou B, and Kaplan MH. 2015. TH9 cells are required for tissue mast cell accumulation during allergic inflammation. The Journal of allergy and clinical immunology 136: 433–440.e431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Xu M, and Dong C. 2017. IL-25 in allergic inflammation. Immunological reviews 278: 185–191. [DOI] [PubMed] [Google Scholar]
- 37.Claudio E, Sonder SU, Saret S, Carvalho G, Ramalingam TR, Wynn TA, Chariot A, Garcia-Perganeda A, Leonardi A, Paun A, Chen A, Ren NY, Wang H, and Siebenlist U. 2009. The adaptor protein CIKS/Act1 is essential for IL-25-mediated allergic airway inflammation. Journal of immunology (Baltimore, Md. : 1950) 182: 1617–1630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Claudio E, Tassi I, Wang H, Tang W, Ha HL, and Siebenlist U. 2015. Cutting Edge: IL-25 Targets Dendritic Cells To Attract IL-9-Producing T Cells in Acute Allergic Lung Inflammation. Journal of immunology (Baltimore, Md. : 1950) 195: 3525–3529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pisitkun P, Ha HL, Wang H, Claudio E, Tivy CC, Zhou H, Mayadas TN, Illei GG, and Siebenlist U. 2012. Interleukin-17 cytokines are critical in development of fatal lupus glomerulonephritis. Immunity 37: 1104–1115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Alferink J, Lieberam I, Reindl W, Behrens A, Weiss S, Huser N, Gerauer K, Ross R, Reske-Kunz AB, Ahmad-Nejad P, Wagner H, and Forster I. 2003. Compartmentalized production of CCL17 in vivo: strong inducibility in peripheral dendritic cells contrasts selective absence from the spleen. The Journal of experimental medicine 197: 585–599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Loschko J, Schreiber HA, Rieke GJ, Esterhazy D, Meredith MM, Pedicord VA, Yao KH, Caballero S, Pamer EG, Mucida D, and Nussenzweig MC. 2016. Absence of MHC class II on cDCs results in microbial-dependent intestinal inflammation. The Journal of experimental medicine 213: 517–534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Thornton EE, Looney MR, Bose O, Sen D, Sheppard D, Locksley R, Huang X, and Krummel MF. 2012. Spatiotemporally separated antigen uptake by alveolar dendritic cells and airway presentation to T cells in the lung. The Journal of experimental medicine 209: 1183–1199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Moretti S, Renga G, Oikonomou V, Galosi C, Pariano M, Iannitti RG, Borghi M, Puccetti M, De Zuani M, Pucillo CE, Paolicelli G, Zelante T, Renauld JC, Bereshchenko O, Sportoletti P, Lucidi V, Russo MC, Colombo C, Fiscarelli E, Lass-Florl C, Majo F, Ricciotti G, Ellemunter H, Ratclif L, Talesa VN, Napolioni V, and Romani L. 2017. A mast cell-ILC2-Th9 pathway promotes lung inflammation in cystic fibrosis. Nature communications 8: 14017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Brasel K, De Smedt T, Smith JL, and Maliszewski CR. 2000. Generation of murine dendritic cells from flt3-ligand-supplemented bone marrow cultures. Blood 96: 3029–3039. [PubMed] [Google Scholar]
- 45.Mindt BC, Fritz JH, and Duerr CU. 2018. Group 2 Innate Lymphoid Cells in Pulmonary Immunity and Tissue Homeostasis. Front Immunol 9: 840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.von Moltke J, Ji M, Liang HE, and Locksley RM. 2016. Tuft-cell-derived IL-25 regulates an intestinal ILC2-epithelial response circuit. Nature 529: 221–225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bankova LG, Lai J, Yoshimoto E, Boyce JA, Austen KF, Kanaoka Y, and Barrett NA. 2016. Leukotriene E4 elicits respiratory epithelial cell mucin release through the G-protein-coupled receptor, GPR99. Proc Natl Acad Sci U S A 113: 6242–6247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Liu B, Lee JB, Chen CY, Hershey GK, and Wang YH. 2015. Collaborative interactions between type 2 innate lymphoid cells and antigen-specific CD4+ Th2 cells exacerbate murine allergic airway diseases with prominent eosinophilia. J Immunol 194: 3583–3593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Van Dyken SJ, Nussbaum JC, Lee J, Molofsky AB, Liang HE, Pollack JL, Gate RE, Haliburton GE, Ye CJ, Marson A, Erle DJ, and Locksley RM. 2016. A tissue checkpoint regulates type 2 immunity. Nat Immunol 17: 1381–1387. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.