Abstract
Adolescent alcohol drinking has been linked to increased risk for drug abuse during adulthood. Nicotine microinjected directly into the posterior ventral tegmental area (pVTA) stimulates dopamine (DA) release in the nucleus accumbens (NAc) shell. The α7 nicotinic acetylcholine receptor (nAChR) is a potent regulator of dopaminergic activity in the pVTA. The current experiments examined the effects of peri-adolescent ethanol (EtOH) drinking on the ability of intra-pVTA nicotine to stimulate DA release during adulthood and alterations in α7 nAChR expression within the pVTA. Alcohol-preferring (P) female rats consumed EtOH and/or water during adolescence (post-natal day [PND] 30–60) or adulthood (PND 90–120). Thirty days following removal of EtOH, subjects received microinjections of 1 μM, 10 μM, or 50 μM nicotine into the pVTA concurrently with microdialysis for extracellular DA in the NAc shell. Brains were harvested from an additional cohort after PND 90 for quantification of α7 nAChR within the pVTA. The results indicated that only adolescent EtOH consumption produced a leftward and upward shift in the dose response curve for nicotine to stimulate DA release in the NAc shell. Investigation of α7 nAChR expression within the pVTA revealed a significant increase in animals that consumed EtOH during adolescence compared to naïve animals. The data suggests that peri-adolescent EtOH consumption produced cross-sensitization to the effects of nicotine during adulthood. The interaction between adolescent EtOH consumption and inflated adult risk for drug dependency could be predicated, at least in part, upon alterations in α7 nAChR expression within the mesolimbic reward pathway.
Keywords: α7 nicotinic acetylcholine receptor, adolescent alcohol, ventral tegmental area, dopamine, mesolimbic, nicotine
INTRODUCTION
The prevalence of alcohol (EtOH) drinking in adolescence is high in the United States with 80-90% having consumed EtOH before graduating high school (Johnston et al. 2004). This includes 58% of 12th graders reporting the use of EtOH within the past year and 28% of which engaged in binge drinking within the previous 2 weeks (i.e. > 5 consecutive drinks per drinking episode; Miech et al. 2016; Johnston et al. 2004). Epidemiological data suggests that early initiation of EtOH consumption is associated with a greater risk of developing an alcohol use disorder (AUD) during adulthood (Dawson et al. 2008). Additionally, adolescent EtOH drinking has been linked to increased adulthood use of opioids, cannabis, and other drugs of abuse (Anthony & Petronis 1995). Specifically for nicotine, adolescent binge drinking enhances the likelihood of smoking during adolescence by 88% as well as during adulthood (Best et al. 2000). Conversely, individuals who do not engage in binge drinking have lower rates of smoking during adolescence and adulthood (Bobo & Husten 2000).
Adolescence is a gradual period of transition characterized by widespread neuronal remodeling eventually resulting in the mature adult brain (Spear 2000). Studies of the adolescent brain have consistently demonstrated that numerous regions continue to undergo significant development including the refinement of several neurotransmitter systems. Maturation of cortical and limbic regions during adolescence is marked by excess production of neuronal networks later followed by extensive pruning (Spear 2000; Brenhouse & Andersen 2011).
Dopamine (DA) neurons of the ventral tegmental area (VTA) project to the dorsal striatum, nucleus accumbens (NAc; ventral striatum), amygdala, and prefrontal cortex (PFC). The number of connections, activation of these projections, and firing rate of DA neurons in the VTA reaches its peak during peri-adolescence (McCutcheon et al. 2012; Marinelli & McCutcheon 2014). Moreover, studies in both humans and animals have found mesolimbic and mesocortical DA projections have a heightened sensitivity to reward-related events in the peri-adolescent brain (Hoogendam et al. 2013). Thus, the reorganization and subsequent pruning of connections within this system during adolescence is associated with maturation of the reward pathway with altered excitatory afferents into the VTA. Glutamatergic inputs from the PFC to the VTA are pruned during adolescence, which is associated with a reduction in glutamate receptors (Brenhouse & Andersen 2011; Spear & Swartzwelder 2014). Relevant to the current study, α7 nicotinic acetylcholine receptors (nAChR) are located presynaptically on glutamatergic inputs into the VTA, on GABA interneurons in the VTA, and on VTA DA neurons (Jones & Wonnacot 2004).
Alcohol drinking during adolescence affects development through alterations to ongoing neuronal remodeling processes (Spear & Swartzwelder 2014). Lasting alterations found within the mesolimbic system of the adult brain resulting from adolescent EtOH exposure involves (i) reduced cholinergic regulation, (ii) elevated DA stimulation and release, as well as (iii) epigenetic reprogramming (Vetreno et al. 2014; Sahr et al. 2004; Toalston et al. 2014; Kyzar et al. 2019). A number of studies have investigated how these changes affect the response to ethanol (EtOH) in adulthood. For example, animals given voluntary access to EtOH throughout adolescence were able to acquire operant self-administration of EtOH faster and resisted extinction of self-administration compared to animals without prior EtOH exposure (Gass et al. 2014; Rodd-Henricks et al. 2002a, Rodd-Henricks et al. 2002b; Toalston at el. 2015). Adolescent EtOH drinking was also shown to enhance sensitivity to EtOH reward within the posterior VTA (Toalston et al. 2014). This was in addition to increased stimulated DA release within the NAc shell by either peripheral EtOH administration or direct VTA infusions of EtOH during adulthood (Sahr et al. 2004; Toalston et al. 2014).
Research continues to provide evidence demonstrating the influence of adolescent EtOH drinking on long-term EtOH abuse susceptibility. However, there is little preclinical research examining the consequences of adolescent EtOH consumption on responses to other drugs of abuse during adulthood. Specifically, a single study was found to have examined the effect of adolescent EtOH exposure with intragastric administration of up to 5 g/kg three times per day followed by nicotine self-administration in adulthood (Boutros et al. 2016). The limited research in this area provides the opportunity to investigate the impact of voluntary adolescent EtOH exposure on the response to nicotine within the mesolimbic system during adulthood. Additionally, lasting expression changes and the potential involvement of interaction sites for EtOH and nicotine can be explored.
Previous research has established the activation of neuronal nAChRs in the VTA is one site of action behind the effects of EtOH as well as the principal mechanism behind the psychoactive effects of nicotine (De Biasi & Dani 2011; Doyon et al. 2013). These receptors are part of the Cys-loop ligand-gated ion channel superfamily and composed of five subunits. The subunits assemble in various combinations to make up specific nAChR subtypes with unique channel properties, desensitization, and agonist affinity. The various subunits can assemble into hetero- or homopentamers that are expressed on DA, glutamate, and GABA neurons within the VTA (Morel et al. 2018). The α4β2* nAChR (asterisk indicates other potential nAChR subunits) is the most abundant within the VTA, is highly sensitive to ACh and nicotine, but is rapidly desensitized. The α7-homomeric nAChR has been found to desensitize at a slower rate due to a decreased affinity for ACh and is predominately localized to glutamatergic terminals from the PFC that synapse on DA neurons. Previous work has demonstrated both of these nAChR subtypes have significantly altered expression levels following adolescent EtOH exposure and contribute to increased DA release by alcohol and nicotine (Morel et al. 2018; Hauser et al. 2019). Overall, evidence demonstrates significant perturbations in both the dopaminergic and cholinergic systems following adolescent EtOH exposure (Vetreno et al. 2018). These long-lasting changes within the mesolimbic circuitry likely alter reward processing and response providing some biological explanation for an increased adult risk for nicotine use.
The present study aimed to determine the long-term effects of voluntary peri-adolescent EtOH consumption on the action of nicotine within the mesolimbic DA system during adulthood. The first experiment determined whether a history of adolescent EtOH drinking produced persistent neuroadaptations in VTA DA neurons. This was tested by examining the ability of intra-VTA nicotine to stimulate DA release in the NAc shell. This was followed by an examination of α7 nAChR levels within the posterior VTA as an indicator of significant and lasting changes to the cholinergic system, following adolescent EtOH consumption.
MATERIALS & METHODS
Subjects.
Female adolescent alcohol-preferring (P) rats from the 79th—85th generations at Indiana University School of Medicine (IUSM; Indianapolis, IN) were utilized in the current study. In order to build on previously published work, as well as the ability to maintain body weight throughout the duration of the study, the following experiments were carried out only in female rats (Ding et al. 2009; Toalston et al. 2014, 2015; Hauser et al. 2019; Waeiss et al. 2019). The animals used in these experiments were maintained in facilities fully accredited by the Association for the Assessment and Accreditation of Laboratory Animal Care. All research protocols were approved by the IUSM Institutional Animal Care and Use Committee and followed the National Institutes of Health Guide for Care and Use of Laboratory Animals (NRCC. 2011).
Peri-adolescent & adult EtOH consumption procedure.
The peri-adolescent EtOH drinking model utilized in the present study was carried out in a similar fashion to those published previously (Toalston et al. 2014, 2015; Waeiss et al. 2019). Beginning on PND 28, P female pups were double-housed in plastic shoebox cages. Rats were maintained on a 12-hour reverse light cycle with lights off at 0900. Food and water were available ad libitum to all animals throughout the experiment. Ethyl alcohol (95%; McCormick Distilling Co., Weston, MO) was diluted to 15% and 30% (v/v). Rats were provided 24-hour access to water and both EtOH concentrations in the 3-bottle choice (3BC) paradigm beginning on PND 30 until PND 60. A second cohort of P rats, housed under identical conditions, did not receive access to EtOH during this period. Fluid weights were recorded daily while body weights were measured every other day. After PND 60, all animals remained pair housed in plastic shoebox cages without access to EtOH through at least PND 90.
A third cohort of adult female P rats underwent the same drinking procedure. Briefly, beginning at PND 90 the animals were double-housed in plastic shoebox cages. At this time, animals were provided continuous access to water, 15%, and 30% EtOH (v/v) through PND 120. All adult animals were then maintained in plastic shoebox cages while pair housed until at least PND 150. It would be redundant to have both naïve adolescent and adult water drinking control groups since the animals would have the same experience. Therefore, the total number of animals in the experiments was reduced by utilizing a single water control group.
Microinjection-Microdialysis Protocol.
The microinjection-microdialysis (micro-micro) procedure was carried out as described previously (Ding et al. 2009; Toalston et al. 2014; Deehan et al. 2018). Given the global effects of adolescent EtOH drinking, the micro-micro procedure allows specific investigations of whether EtOH induced neuroadaptations in the pVTA modulate the activity and DA response to nicotine within the mesolimbic system. Following PND 90 for the adolescent cohorts or PND 150 for the adult cohort, rats were stereotaxically implanted with two ipsilateral guide cannulas in the right hemisphere. While under isoflurane anesthesia, a 22-guage microinjection cannula (Plastics One, Inc., Roanoke, VA) was aimed 1.0 mm above the posterior ventral tegmental area (pVTA). An 18-guage microdialysis cannula was also implanted 3.0 mm above the NAc shell. The coordinates for the pVTA were AP −5.6 mm, ML +2.1 mm, DV −8.0 mm and those for the NAc shell were +1.5 mm, ML +2.0 mm, DV −5.3 mm (Paxinos & Watson 1998). Both cannulas were implanted at a 10° angle and protected with stylets until the micro-micro stages of these experiments. Following surgery, animals were single housed in shoebox cages and allowed at least one week of recovery. During this time, the animals were habituated to the experimental housing and handled daily.
Loop-style microdialysis probes were constructed as previously described (Engleman et al. 2000; Ding et al. 2009; Toalston et al. 2014). Probes were manufactured with an active length of 2.0 mm from regenerated cellulose Spectra/Por (Spectrum Laboratories, Inc., Rancho Dominguez, CA) hollow fiber tubing with an inner diameter of 200 μm and molecular weight cut-off of 13 kDa. One day prior to testing, animals were placed under isoflurane anesthesia and the microdialysis probes were inserted into the NAc shell by extending 3.0 mm below the guide cannula. The micro-micro procedure was carried out the following day.
Experiments were performed in awake freely moving animals. Subjects were placed in the experimental chambers and the microdialysis probes were connected to a Harvard pump (Harvard Apparatus, Holliston, MA) used to continuously perfuse the NAc shell with artificial cerebrospinal fluid (aCSF) at a rate of 1 μL/minute throughout the experiment. Microdialysis aCSF was composed of 140.0 mM NaCl, 3.0 mM KCl, 1.2 mM CaCl2, 2.0 mM Na2HPO4 • 7H2O, and 1.0 mM MgCl2 with a pH 7.2 to 7.4. Following a 90-minute washout period, samples were collected in 20-minute intervals beginning with five baseline samples.
Next, rats received a single microinjection challenge directly into the pVTA of either aCSF, 1 μM nicotine, 10 μM nicotine, or 50 μM nicotine solutions. Nicotine HCl (Sigma-Aldrich, St. Louis, MO) concentrations were calculated based on the salt-form. Nicotine concentrations known to be reinforcing and stimulate DA release were selected based on previous intracranial self-administration (ICSA) and micro-micro studies within the pVTA (Hauser et al. 2014a, b; Truitt et al. 2015; Deehan et al. 2018). Importantly, the in vivo concentration of nicotine within the pVTA following microinjection is not known but is expected to be lower than that emitted due to local metabolism and diffusion during the session. Although intra-pVTA injections produce distinct spatial and temporal gradients not experienced in smokers, nicotine concentrations likely approach what was observed in previous experiments investigating peripheral treatment or self-administration in P rats (27 ng/ml; Hauser et al. 2012; Katner et al. 2015). Passive microinjections were carried out with an electrolytic microinfusion transducer (EMIT) system as described previously (Gatto et al. 1994; Rodd-Henricks et al. 2000; Ding et al. 2009). Briefly, subjects received 30 pulse injections over a 10-minute period designed to simulate average intracranial self-administration (ICSA) levels (Rodd-Henricks et al. 2000; Toalston et al 2014). Each infusion injected 100 nl of solution over 5 seconds and was followed by a 15-second timeout period for a total of 3 μl over the 10-minute infusion session. After the microinjection challenge, six additional 20-minute samples of dialysate samples were collected into tubes containing 5 μl of 0.1 N perchloric acid. All samples were immediately frozen on dry ice and stored at −80 °C until analysis for DA content with high performance liquid chromatography (HPLC).
The present microdialysis study utilized 85 female P rats. Subjects were randomly assigned to one of four microinjection conditions with n = 8–9/group of naïve, n = 7–9/group adolescent EtOH drinkers, and n = 4–6/group of adult EtOH drinkers.
Dialysate Analysis.
DA was analyzed using a reversed phase HPLC system with electrochemical detection (Engleman et al. 2000; Ding et al. 2009; Toalston et al. 2014). Samples were loaded into a 10 μl loop and injected onto an analytical column (BDS Hypersil C18, 3 μm, 150 mm × 2.1 mm, Thermo Fisher Scientific, Waltham, MA). Mobile phase consisted of 0.1 mM EDTA, 8 mM KCl, 50 mM phosphoric acid, 100 mg/L OSA, and 10% MeOH with a pH of 6.0. DA detection occurred with a glassy-carbon electrode and an amperometric detector with the oxidation potential set at 350 mV and sensitivity of 100 pA/V (Decade II EC Detector, Antec Scientific, Netherlands). Signal analysis was carried out with a ChromePerfect (Justice Innovations, Inc., Palo Alto, CA) data station. DA content was then determined through comparison to a standard curve generated from 1 nM, 5 nM, and 10 nM DA solutions.
Histology.
Once the experiments were completed, subjects were euthanized and a solution of 1% bromophenol blue was infused into each microdialysis probe and microinjection cannula. Brains were removed, immediately frozen on dry ice, and stored at −80 °C. Forty-micron sections were then collected with a cryostat microtome for verification of microdialysis probe and microinjection placements.
Immunohistochemistry.
On PND 90, naïve rats (n = 6) and adolescent EtOH drinkers (n = 7) were deeply anesthetized with isoflurane and transcardially perfused with phosphate-buffered saline (PBS, pH 7.4) followed by 4% paraformaldehyde (PFA) in PBS. Perfused brains were then removed and post-fixed overnight in 4% PFA at 4 °C. Next, brains were transferred to 30% sucrose in PBS and stored at 4 °C until processed. Serial coronal sections were collected at a thickness of 30 μm using a freezing microtome, immediately placed in a cryoprotectant solution, and stored at −20 °C until processing. Based on results from the micro-micro experiment demonstrating a similar response between adult EtOH drinkers and naïve rats, adult EtOH drinkers were not included in the immunohistochemical analysis to reduce the total number of animals used in the present study.
Immunohistochemistry was carried out as described previously (Johnson et al. 2015). Free-floating sections containing the VTA were initially washed with PBS for 30 minutes at room temperature prior to incubation in 1% H2O2 in PBS for 20 minutes. Sections were then washed in PBS for 10 minutes and PBS with 0.3% Triton X-100 (PBST) for an additional 20 minutes. Sections were then incubated overnight for 12-16 hours in PBST containing mouse anti-α7 nAChR subunit monoclonal antibody (1:100; Catalog # 836701, BioLegend, San Diego, CA) at room temperature. Following a 30-minute wash in PBST, sections were incubated in biotinylated horse anti-mouse IgG secondary antibody (1:500; Catalog # BA-2000, Vector Laboratories, Burlinghame, CA) for 2 hours. Sections were again washed in PBST for 30 minutes and incubated for 1.5 hours with an avidin–biotin–horseradish peroxidase complex provided in a standard Vector Elite kit (1:1000; Catalog # PK-6100, Vector Laboratories). The resulting peroxidase complexes were visualized by exposure for 10 minutes to a chromogen solution containing 0.02% 3, 3’-diaminobenzidine tetrahydrochloride (DAB, Sigma-Aldrich, St. Louis, MO) to produce a dark brown reaction product for α7 nAChR subunit staining. Repeated washing in 0.1 M PB was used to terminate the reaction. Sections were then mounted on clean glass slides, dried overnight, dehydrated, and coverslipped with DPX (Electron Microscopy Sciences, Fort Washington, PA).
Due to known concerns with antibodies directed toward α7 nAChRs, steps were taken to determine specificity (Garg & Loring 2017). An experiment to determine non-specific binding was carried out on separate tissue sections by applying the above-mentioned procedure and substituting the primary antibody with an isotype control (IgG1). Another round of immunohistochemistry was conducted with the omission of the primary antibody to determine the contribution of other components to background staining. These experiments (data not shown), as well as those conducted by other research groups, have determined there was relatively little non-specific binding and likely would not influence the analysis (Dominguez del Toro et al. 1994; Mielke & Mealing 2009; Garg & Loring 2017). Additionally, Western blotting was conducted with a different α7 nAChR antibody as a complementary technique to increase confidence in the immunohistochemical findings. Finally, orthogonal data recently published by our group indicate similar alterations to α7 nAChRs following EtOH exposure with TaqMan low-density array (TLDA) cards for real-time PCR (Hauser et al. 2019).
Sections were analyzed by an observer blind to the treatment conditions and the number of α7 nAChR subunit-immunoreactive (α7-IR) cells were counted. Only tissue sections containing the pVTA, located between −5.2 mm bregma and −6.2 mm bregma, were included in the analysis (Paxinos and Watson, 1998). Overall, four to seven sections per brain were analyzed. The total number of cells counted per section was divided by the total volume of each section. Total volumes from the sections counted for the pVTA were calculated by multiplying the sum of area, determined with ImageJ analysis software (NIH, Bethesda, MD), by a depth of 19 μm. Data are presented as number of cells per mm3.
Protein quantification, SDS-PAGE, and Western blotting.
On PND 90, naïve rats (n = 6) and adolescent EtOH drinkers (n = 6) were deeply anesthetized with isoflurane and rapidly decapitated. Brains were quickly removed, flash frozen in isopentane on dry ice, and stored at −80 °C until processed. Serial coronal sections were then collected at a thickness of 300 μm with a freezing microtome. Micro-punches containing the pVTA were obtained using a 1 mm diameter Harris micro-punch (Electron Microscopy Sciences, Hatfield, PA) as described previously (McBride et al. 2009). The tissue was immediately homogenized in 150 μl of ice-cold N-PER lysis buffer supplemented with Halt Protease inhibitor cocktail (Thermo Scientific, Waltham, MA). At 4 °C, samples were incubated in the lysis buffer on a nutator for 2 hours followed by centrifugation at 10,000 × g for 20 minutes. Supernatants were removed and protein concentrations were measured by the bicinchoninic acid assay (Pierce, Rockford, IL). Protein concentrations were determined with 10 μl of lysate and 200 μl of working reagent at an absorbance of 570 nM with a microplate reader (Bio-Rad, Hercules, CA). All samples were analyzed in duplicates and absorbance values averaged. Adult EtOH drinkers were not included based on neurochemical results from the micro-micro experiment.
Approximately 10 μg of processed tissue protein was loaded per lane and separated on 7.5% polyacrylamide denaturing gels (Bio-Rad, Hercules, CA). This was followed by transfer to nitrocellulose membranes (Amersham, Pittsburgh, PA). Membranes were blocked with 5% milk in TBST buffer (50 mM Tris-Cl, pH 7.6; 150 mM NaCl; 0.1% Tween 20) overnight at 4 °C. Next, membranes were incubated for 2 hours at room temperature with rabbit anti-α7 nAChR subunit (1:10,000; ab10096, Abcam, Cambridge, UK) and mouse anti-GAPDH (1:100,000; Catalog # MA5-15738, Thermo Scientific, Waltham, MA). Secondary antibodies were HRP conjugated goat anti-mouse (1:20,000; Catalog # 31430, Invitrogen, Carlsbad, CA) or HRP-conjugated goat anti-rabbit (1:20,000; ab205718, Abcam, Cambridge, UK). Protein band signals were visualized by adding chemiluminescent HRP substrate reagent (Millipore, Billerica, MA). Films were scanned and densitometry was performed with ImageJ software (NIH, Bethesda, MD).
The second α7 nAChR antibody, ab10096, was also scrutinized for specificity. First, ab10096 was preincubated with a synthetic blocking peptide (ab101467, Abcam, Cambridge, UK) corresponding to a region of the human α7 nAChR subunit, and the ab10096 antigen, in a ratio of 1:5 overnight at 4 °C prior to immunoblotting. Next, in order to exclude the possibility of non-specific interactions with other rat antigens, a comparison was made using liver tissue, which has been determined to express negligible levels of α7 nAChR (Mielke & Mealing 2009; Fagerberg et al. 2014). Finally, omitting ab10096 from the Western blotting procedure was also carried out to substantiate specificity. In each of the three control experiments described above, bands at the expected molecular weight were faint or not detected.
Statistical Analysis.
The average EtOH intake of adolescent drinkers and adult drinkers was calculated based on the last 10 sessions of the 30-day drinking procedure. Contrasting differences between adolescent and adult EtOH consumption was examined with an overall mixed factor ANOVA with between subject factor of ‘Age’ and a within subject factor of ‘Day’. Individual “Day’ differences were determined with an unpaired Student’s t-test (p < 0.05). Microdialysis data are expressed as a percentage of basal DA values to correct for subject-to-subject variability (Engleman et al. 2000). Basal dialysate DA values were calculated as the mean of three baseline samples collected prior to the challenge microinjection. The effects of pVTA nicotine microinjections on extracellular DA were analyzed using a Time × Drinking Condition × Nicotine mixed analysis of variance (ANOVA). Post-hoc comparisons used to determine significance were Tukey’s b for between group differences or Student’s two-tailed t for within group differences. Immunohistochemical data were analyzed with the mean number of α7-IR cells per mm3 calculated from the naïve treatment group and adolescent EtOH drinkers. These values were then compared using a univariate ANOVA (p < 0.05). Western blot densitometric signals were normalized to GAPDH. Analysis of signals were then also compared using a univariate ANOVA (p < 0.05).
RESULTS
Average daily EtOH intake over the final 10 drinking days were 10.2 ± 0.6 g/kg/day for adolescent drinkers and 10.6 ± 1.2 g/kg/day for adult drinkers (Fig. 1). Analysis revealed that the average EtOH consumption was not significantly different between adolescent drinkers and adult drinkers (F1,53 = 0.47, p = 0.50).
Figure 1.
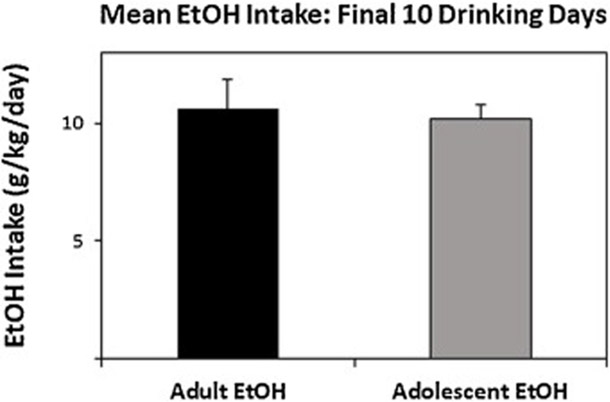
Average daily EtOH consumption for all animals given free-choice access to 15% and 30% EtOH during adolescence (PND 30–60; n = 32) or adulthood (PND 90–120; n = 24). Intake values are calculated as grams per kilogram per day and averaged into a block of the final 10 days. All data shown are mean +SEM.
The mean DA levels in dialysate samples from the NAc shell were 1.22 ± 0.12 nM for the naïve group, 1.28 ± 0.11 nM for adolescent drinkers, and 1.17 ± 0.18 nM for adult drinkers. The basal DA levels in dialysis samples are strongly influenced by the rate of recovery through the microdialysis probe. An accurate assessment of extracellular DA levels with microdialysis can only be determined by a quantitative method such as with the No-Net-Flux protocol.
An initial area under the curve (AUC) analysis of DA levels determined whether there were general effects of Drinking History or a dose-response to nicotine over the duration of the experiment. AUC is a summary analysis that provides a definite integral of a value across time to generate a quantitative value. The analysis indicated a significant Drinking History × Nicotine interaction term (F6,73 = 7.11; p < 0.001; Fig. 2). Reducing the significant 2-way interaction by holding Nicotine constant indicated there were no significant group differences following microinjection of aCSF into the pVTA (F2,18 = 0.56; p = 0.945). However, there were significant group differences in animals treated with 1 μM, 10 μM, or 50 μM nicotine (F2,18 values > 15.77; p values < 0.001; Fig. 2). Post-hoc comparisons with Tukey’s b revealed that at all nicotine concentrations, adolescent EtOH consuming rats had a significantly greater DA response than adult EtOH drinkers or naïve rats.
Figure 2.
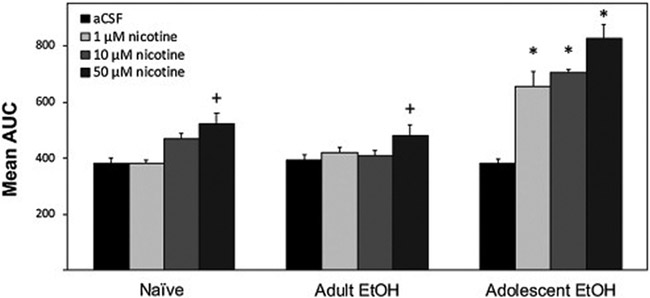
Mean (+SEM) area under the curve (AUC) values to illustrate the general effects of drinking history and dose-response to nicotine. DA response was determined following microinjections of 0 (aCSF), 1, 10, or 50 μM nicotine administered to adult rats previously allowed to consume EtOH during adolescence (Adolescent EtOH; n = 7–9/group), during adulthood (Adult EtOH; n = 4–6/group), or water only (Naïve; n = 8–9/group). Plus sign (+) indicates significantly greater AUC in rats treated with 50 μM nicotine than respective aCSF treatment. Asterisk (*) indicates significantly greater AUC in Adolescent EtOH rats treated with 1, 10, or 50 μM nicotine compared to respective Naïve, Adult EtOH, and aCSF treatment groups.
A more thorough analysis that included individual time points revealed an overall significant Time × Drinking History × Nicotine interaction term (F48,584 = 1.65; p = 0.005; Fig. 3). The 3-way interaction term was initially decomposed by holding Time constant. A significant Drinking History × Nicotine interaction was found for both the first (F6,73 = 2.84; p = 0.015) and second (F6,73= 4.00; p = 0.002) samples collected following nicotine microinjection. To determine the effect of Drinking History on the ability of nicotine microinjected into the pVTA to stimulate DA release in the NAc shell at each time point was derived by reducing the significant 2-way interaction term by holding Nicotine constant. There were no significant group differences during the first and second sample period following treatment with aCSF (F2,18 < 1.69; p values > 0.21). In animals treated with 1 μM, 10 μM, or 50 μM nicotine there were significant group differences during the first and second sample period following microinjection (F2,18 values > 7.24; p values < 0.01). Tukey’s b post-hoc comparisons revealed that all nicotine concentrations and both sample time points, adolescent EtOH consuming rats had significantly higher levels of DA than adult EtOH drinkers or naïve rats.
Figure 3.
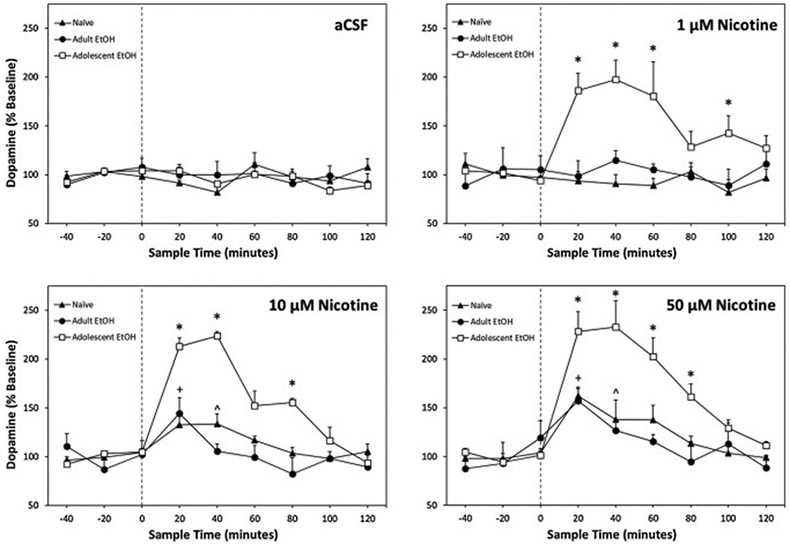
Mean (+SEM) percentage change in extracellular DA in the NAc shell of adult rats microinjected with 0, 1, 10, or 50 μM nicotine directly into the pVTA previously allowed to consume EtOH during adolescence (Adolescent EtOH; n = 7–9/group), during adulthood (Adult EtOH; n = 4–6/group), or water only (Naïve; n = 8–9/group). Asterisk (*) indicates significantly greater DA levels in Adolescent EtOH rats compared to Naïve, Adult EtOH, and respective baseline DA at the specified time points. Plus sign (+) indicates Naïve and Adult EtOH extracellular DA was significantly higher than respective baseline levels. Carrot (^) indicates Naïve DA levels were significanly increased over baseline.
The overall 3-way interaction term was also reduced by holding Drinking History constant. For the naïve animals, individual ANOVAs revealed a significant effect of nicotine concentration during the first three samples following microinjection (F3,28 values > 2.99; p values < 0.05; Fig. 3). During the first and second samples following pVTA microinjection, Tukey’s b post-hoc indicated DA levels in the NAc shell were significantly greater in naïve rats administered 10 and 50 μM nicotine compared to aCSF. During the third sample, DA levels remained significantly elevated in the NAc shell following administration of 50 μM nicotine compared to animals receiving 1 μM nicotine. Within-subject examination of individual nicotine concentrations revealed a significant effect of 50 μM nicotine (F8,56 = 6.19; p < 0.001). Post hoc comparisons indicated DA levels of the first post microinjection sample were significantly higher t7 = 5.71, p = 0.001) at 162% of baseline.
Adult EtOH drinkers also exhibited a significant effect of nicotine concentration during the first sample following microinjection (F3,17 = 5.96; p = 0.006; Fig. 3). Adult drinkers receiving 50 μM nicotine into the pVTA produced significantly elevated DA levels in the NAc shell compared to those receiving 1 μM nicotine or aCSF. Within-subject analysis of individual nicotine concentrations revealed a significant effect of 10 μM (F8,24 = 2.52; p = 0.038) and 50 μM nicotine (F8,40 = 2.23; p = 0.046). Post hoc comparisons indicated DA levels of the first sample following 10 μM nicotine microinjection were significantly higher than baseline (t3 = 3.35, p = 0.044). Additionally, microinjection of 50 μM nicotine resulted in significantly increased extracellular DA during the first sample to 157% compared to baseline (t5 = 3.01, p = 0.027).
Analyses of adolescent EtOH drinkers revealed a significant effect of nicotine concentration during all six samples collected following nicotine treatment (F3,28 values > 3.24; p values < 0.05; Fig. 3). Specifically, 1 μM, 10 μM, and 50 μM nicotine produced significantly higher DA levels during the first and second samples post microinjection compared to aCSF. The third post microinjection sample revealed NAc shell DA following 50 μM nicotine treatment was significantly higher than aCSF controls. During the fourth sample, both 10 μM and 50 μM nicotine treated animals exhibited significantly greater DA levels compared to aCSF controls. Additionally, samples five and six indicated elevated extracellular DA in adolescent drinkers that received 1 μM nicotine over aCSF microinjections. Analyses of individual nicotine concentrations within-subjects revealed a significant effect of 1 μM, 10 μM, and 50 μM nicotine (F8,56 values > 6.98; p < 0.001). Post hoc comparisons demonstrated DA levels of samples 1 - 3 and 5 following 1 μM nicotine microinjection were significantly higher than baseline (t8 values > 2.31, p values < 0.05). Microinjection of 10 μM nicotine resulted in significantly increased extracellular DA during the first four samples compared to baseline (t7 values > 7.72, p values < 0.05). Lastly, adolescent drinkers that received pVTA microinjections of 50 μM nicotine had significantly greater extracellular DA during the first five samples compared to baseline levels (t7 values > 3.80, p values < 0.01). Microinjections of 1 μM, 10 μM, and 50 μM nicotine produced maximal DA response during the second sample reaching 197%, 223%, and 232% compared to baseline levels, respectively.
Immunohistochemistry was used to assess α7-IR cells in the pVTA of adolescent EtOH drinkers and naïve rats in adulthood (>PND 90). In naïve rats and adolescent drinkers, examination of α7-IR cells revealed darkly stained cell bodies and processes (Fig. 4). Within the pVTA, α7-IR cells were significantly increased in adolescent drinkers compared to naïve animals (F1,11 = 16.44, p = 0.002). Adolescent EtOH drinking resulted in an increase of α7-IR cells/mm3 to 193% relative to naïve rats (Fig. 4). Western blot analysis was then utilized to assess α7 nAChR expression in the pVTA. Relative expression levels of α7 nAChR in the pVTA are presented in Fig. 5. Adolescent EtOH consumption significantly increased α7 nAChR expression levels 33% within the pVTA compared to naïve rats (F1,10 = 6.54, p = 0.003).
Figure 4.
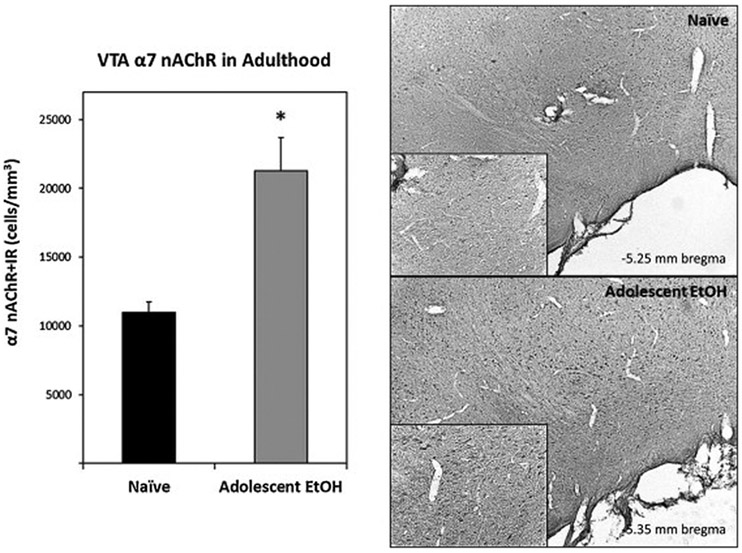
Left: Mean (+SEM) α7-IR cell counts within the pVTA of adult rats allowed to consume water only (Naïve; n = 6) or EtOH during adolescence (Adolescent EtOH; n = 7). Asterisk (*) indicates significantly greater α7-IR cells compared to Naïve rats. Right: Representative photomicrographs (5x magnification, 20x inset) of α7-IR cells in the pVTA of Naïve and Adolescent EtOH during adulthood.
Figure 5.
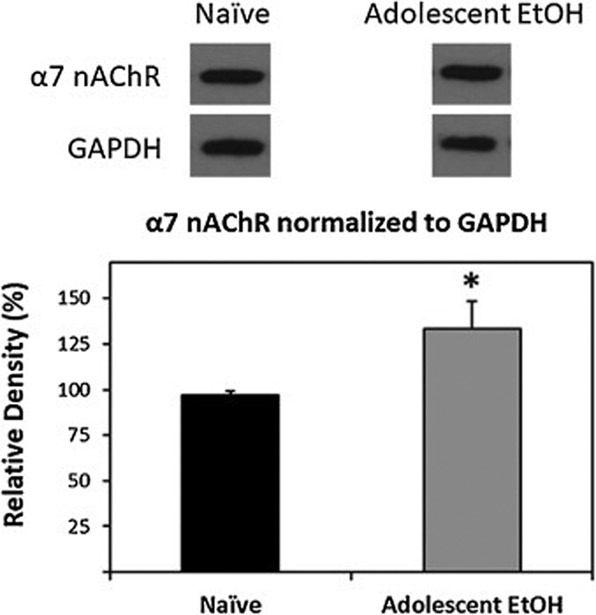
Representative western blots and group data, normalized to GAPDH, illustrating increased α7 nAChR protein levels in the pVTA of rats allowed to consume EtOH during adolescence (Adolescent EtOH; n = 6) compared to water only controls (Naïve; n = 6) in adulthood. Asterisk (*) indicates significantly greater than controls.
DISCUSSION
Overall, the findings of the current study indicate that voluntary adolescent, but not equivalent adult, EtOH consumption resulted in long-lasting functional and neurochemical alterations to the mesolimbic DA system. Specifically, adolescent EtOH drinkers developed a cross-sensitization to nicotine during adulthood. Adolescent EtOH consumption produced a leftward and upward shift in the ability of nicotine microinjected into the pVTA to stimulate DA release in the NAc shell that was not present in naïve animals or adult EtOH drinkers (Figs. 2 and 3). Additionally, rats allowed to consume EtOH during adolescence had significantly elevated α7 nAChR expression in the pVTA compared to naïve rats (Figs. 4 and 5).
Overwhelmingly convergent data have indicated that adolescent EtOH exposure results in persistent alterations to the adult brain that mediates future drug responsivity and self-administration. The rewarding and reinforcing properties of EtOH and nicotine are well known to be associated with increased DA release within the NAc (Di Chiara and Imperato 1988; Wise and Rompre 1989). Furthermore, the associated reinforcing effects of pharmacologically relevant levels of EtOH and nicotine within the pVTA has also been established (Exley et al. 2011; Hauser et al. 2014; Rodd-Henricks et al. 2000; Truitt et al. 2014). The pVTA acts as a neural substrate where EtOH and nicotine are self-administered and interact to enhance DA release in the NAc shell (Tizabi et al. 2002, 2007; Rodd et al. 2010; Corrigall et al. 1994). It has been suggested that the hyperdopaminergic state induced by adolescent EtOH exposure could occur through both independent actions as well as through shared neurotransmitter systems of EtOH and nicotine. Repeated observations have identified neuroadaptations in the dopaminergic and cholinergic systems in adulthood following adolescent EtOH exposure (Crews et al. 2016). Alterations in DA function in adulthood is observed following voluntary consumption or experimenter administered EtOH during adolescence (Toalston et al., 2014; Sahr et al. 2004; Pascual et al. 2009).
A critical question of adolescent research is whether any observed consequence of EtOH exposure is specific to adolescence. Multiple reports now indicate that adolescent EtOH exposure produces unique adaptations in the dopaminergic system that are not observed following comparable adult consumption. Rats exposed to EtOH during adolescence expressed alterations in DRD1 and DRD2 during adulthood but adult rats treated with EtOH did not display comparable effects (Pascual et al. 2009; Coleman et al. 2011). In addition, an increased sensitivity of neuronal activity within a subset of VTA DA neurons to EtOH is observed specifically following adolescent EtOH self-administration (Avegno et al. 2016).
Importantly, it has also been demonstrated that after almost complete denervation of the NAc and the mesolimbic DA system, voluntary responding for EtOH failed to change (Rassnick et al. 1993). This undoubtedly suggests there are DA-independent neurochemical systems that contribute critically to mediating the reinforcing actions of EtOH and nicotine. The cholinergic system has been shown to be especially susceptible to the effects of adolescent binge-like EtOH exposure. Numerous studies demonstrated long-lasting and global reductions in neurons expressing choline acetyltransferase (ChAT), a marker for cholinergic neurons and enzyme responsible for acetylcholine (ACh) biosynthesis, following an adolescent intermittent EtOH (AIE) binge protocol (Coleman et al. 2011; Ehlers et al. 2011; Vetreno et al. 2014). In addition to an overall reduction in neurons expressing ChAT, chronic EtOH reduced the endogenous function of ChAT, ACh uptake, and expression of acetylcholinesterase (Arendt et al. 1989; Floyd et al. 1997). Alterations in the expression of nAChRs in multiple brain regions have also been reported (Figs. 4 and 5; McClintick et al. 2015, 2016; Hauser et al. 2019). These data add to the current findings indicating that the persistent reduction of ChAT produced by adolescent EtOH exposure results in a compensatory upregulation of nAChRs, specifically α7, in adult brains.
Voluntary EtOH consumption in adolescence, but not adulthood, produced lasting upregulation of α7 nAChRs in the pVTA as well as alterations to the actions of EtOH and nicotine at the α7 nAChR (Doyon et al. 2013; Hauser et al. 2019). The homomeric α7 nAChRs are strongly expressed presynaptically on glutamatergic afferents to the VTA and have been found to contribute to N-methyl-D-aspartate (NMDA) receptor dependent long-term potentiation (LTP) through a 20-fold greater permeability for calcium than the other nAChRs (Mansvelder & McGehee 2000; Mansvelder et al. 2002; Gao et al. 2010). The involvement of glutamate and plasticity have been implicated in several drugs of abuse including EtOH and nicotine (Gipson et al. 2013; Berglind et al. 2009; Campbell et al. 2009; Wang et al. 2008). Specifically, glutamate signaling from the medial prefrontal cortex (mPFC) has been shown to play a significant role in both EtOH and nicotine addiction. Several studies have demonstrated that experience with EtOH increases α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) receptor mediated synaptic transmission and can induce LTP of glutamatergic synaptic afferents onto VTA DA neurons (Stuber et al. 2008; Oliva and Wanat 2016). This has also been associated with increased NMDA receptor expression as well as elevated phosphorylation of GluN2B receptor subtype (Pascual et al. 2009). Additional research found increased levels of NR1 and GluR1 subunits, indicating higher levels of both NMDA and AMPA receptors, following long-term exposure to EtOH (Ortiz et al. 1995). Activation of nAChRs by nicotine induces rapid desensitization and internalization, of which α4β2* nAChRs are particularly susceptible (Fenster et al. 1997; Pidoplichko et al. 1997). Despite the rapid transition of α7 nAChRs to a desensitized state, signaling can be spatially and temporally extended through interaction with heterotrimeric GTP-binding proteins (G proteins) via a G protein-binding cluster (GPBC) located on the receptor (Kabbani & Nichols 2018). Specifically, evidence suggests a nicotine induced α7 nAChR interaction with Gαq at the GPBC activates a signaling pathway resulting in prolonged calcium transients and increased neurotransmitter release (Kabbani & Nichols 2018). This unique feature of α7 nAChRs could explain the enhanced dopaminergic response to nicotine by the associated increase in expression levels and subsequent glutamatergic signaling onto VTA DA neurons (Figs. 2-5) following voluntary adolescent EtOH consumption. Collectively, enhanced DA release by nicotine during adulthood may be partly driven by increased levels of α7 nAChRs and subsequent prolonged excitatory signaling of glutamatergic inputs within the VTA. Future experiments hope to establish a causal link between α7 nAChR expression and altered responsivity to nicotine through pharmacological manipulations.
The ability of EtOH and nicotine to cross sensitize is bidirectional. Adolescent, but not adult rats, administered nicotine consumed more EtOH during adulthood than saline treated controls as well as an alteration in GABAa signaling (Thomas et al. 2018). Similarly, exposure to cigarette smoke enhances EtOH consumption during adolescence and adulthood (Burns & Procter 2013). The current data are first to indicate a persistent alteration in the neurochemical response to nicotine within the mesolimbic DA reward system following voluntary adolescent EtOH consumption. The research described above also suggests EtOH and nicotine actions can interact at specific sites, in addition to independent actions, to increase DA release within the NAc. Thus, for both nicotine and EtOH, adolescent exposure to drugs of abuse produce unique neuroadaptations that cross-sensitizes to others during adulthood. The upregulation of α7 nAChRs in the pVTA may be part of the biological basis for reports in humans that heavy adolescent EtOH use greatly enhances adult nicotine use (Dierker et al. 2013).
Highlights.
Adolescent EtOH drinking increased the sensitivity to nicotine during adulthood.
Adolescent drinking enhanced the dopaminergic response to nicotine during adulthood.
VTA α7 nicotinic receptors were elevated in adolescent drinkers compared to controls.
Adolescent, not adult, drinking produced nicotine cross-sensitization in adulthood.
Acknowledgements
This study was supported by NIAAA grants AA007462, AA007611, AA013522, AA019366, AA010721, and AA020396. We thank Eric Brown and Erin Jarvis for their excellent technical assistance. I also thank Penny Peck whose tenacity inspired much needed perseverance while always providing an excuse to leave lab early. The views expressed in this manuscript do not necessarily reflect the views of the NIAAA or those of the NIH.
Footnotes
Publisher's Disclaimer: This is a PDF file of an article that has undergone enhancements after acceptance, such as the addition of a cover page and metadata, and formatting for readability, but it is not yet the definitive version of record. This version will undergo additional copyediting, typesetting and review before it is published in its final form, but we are providing this version to give early visibility of the article. Please note that, during the production process, errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest: The authors declare that they have no conflicts of interest.
References
- Anthony JC, Petronis KR (1995). Early-onset drug use and risk of later drug problems. Drug Alcohol Depend 40:9–15. [DOI] [PubMed] [Google Scholar]
- Arendt T, Allen Y, Marchbanks RM, Schugens MM, Sinden J, Lantos PL, Gray JA (1989) Cholinergic system and memory in the rat: effects of chronic ethanol, embryonic basal forebrain brain transplants and excitotoxic lesions of cholinergic basal forebrain projection system. Neuroscience 33:435–462. [DOI] [PubMed] [Google Scholar]
- Avegno EM, Salling MC, Borgkvist A, Mrejeru A, Whitebirch AC, Margolis EB, Sulzer D, Harrison NL (2016) Voluntary adolescent drinking enhances excitation by low levels of alcohol in a subset of dopaminergic neurons in the ventral tegmental area. Neuropharmacology, 110(Part A), 386–395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berglind WJ, Whitfield TW Jr, LaLumiere RT, Kalivas PW, McGinty JF (2009) A single intra-PFC infusion of BDNF prevents cocaine-induced alterations in extracellular glutamate within the nucleus accumbens. J Neurosci 29(12):3715–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Best D, Rawaf S, Rowley J, Floyd K, Manning V, Strang J (2000) Drinking and smoking as concurrent predictors of illicit drug use and positive drug attitudes in adolescents. Drug and Alcohol Dependence. 60(3) 319–321. [DOI] [PubMed] [Google Scholar]
- Bobo JK, Husten C (2000) Sociocultural influences on smoking and drinking. Alcohol Res Health 24(4) 225–232. [PMC free article] [PubMed] [Google Scholar]
- Boutros N, Semenova S, Markou A (2016) Adolescent alcohol exposure decreased sensitivity to nicotine in adult Wistar rats. Addict Biol 21(4):826–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brenhouse HC, Andersen SL (2011) Developmental trajectories during adoles-cence in males and females: a cross-species understanding of underlying brainchanges. Neurosci. Biobehav. Rev. 35 (8), 1687–1703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burns BE, Proctor WR (2013) Cigarette smoke exposure greatly increases alcohol consumption in adolescent C57BL/6 mice. Alcohol Clin Exp Res 37 Suppl 1E364–E372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell JC, Szumlinski KK, Kippin TE (2009) Contribution of early environmental stress to alcoholism vulnerability. Alcohol 43(7), 547–554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coleman LG Jr, He J, Lee J, Styner M, Crews FT (2011) Adolescent binge drinking alters adult brain neurotransmitter gene expression, behavior, brain regional volumes, and neurochemistry in mice. Alcohol Clin Exp Res 35(4), 671–688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corrigall WA, Coen KM, Adamson KL (1994) Self-administered nicotine activates the mesolimbic dopamine system through the ventral tegmental area. Brain Res 653(1–2):278–284. [DOI] [PubMed] [Google Scholar]
- Crews FT, Vetreno RP, Broadwater MA, Robinson DL (2016) Adolescent Alcohol Exposure Persistently Impacts Adult Neurobiology and Behavior. Pharmacol Rev 68(4) 1074–1109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawson DA, Goldstein RB, Chou SP, Ruan WJ, Grant BF (2008) Age at first drink and the first incidence of adult-onset DSM-IV alcohol use disorders. Alcohol Clin Exp Res 32:2149–2160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Biasi M, Dani JA (2011) Reward, addiction, withdrawal to nicotine. Annu Rev Neurosci 34:105–130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deehan GA Jr, Hauser SR, Getachew B, Waeiss RA, Engleman EA, Knight CP, McBride WJ, Truitt WA, Bell RL, Rodd ZA (2018) Selective breeding for high alcohol consumption and response to nicotine: locomotor activity, dopaminergic in the mesolimbic system, and innate genetic differences in male and female alcohol-preferring, non-preferring, and replicate lines of high-alcohol drinking and low-alcohol drinking rats. Psychopharmacology 235(9) 2755–2769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Chiara G, Imperato A (1988) Drugs abused by humans preferentially increase synaptic dopamine concentrations in the mesolimbic system of freely moving rats. Proc Natl Acad Sci U S A 85:5274–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dierker L, Selya A, Piasecki T, Rose J, Mermelstein R (2013) Alcohol problems as a signal for sensitivity to nicotine dependence and future smoking. Drug Alcohol Depend 132:688–693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding ZM, Rodd ZA, Engleman EA, McBride WJ (2009) Sensitization of ventral tegmental area dopamine neurons to the stimulating effects of ethanol. Alcohol Clin Exp Res 33: 1571–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dominguez del Toro E, Juiz JM, Peng X, Lindstrom J, Criado M (1994) Immunocytochemical localization of the alpha 7 subunit of the nicotinic acetylcholine receptor in the rat central nervous system. J Comp Neurol 349(3):325–42. [DOI] [PubMed] [Google Scholar]
- Doyon WM, Thomas AM, Ostroumov A, Dong Y, Dani JA (2013) Potential substrates for nicotine and alcohol interactions: a focus on the mesocorticolimbic dopamine system. Biochem Pharmacol 86(8) 1181–1193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehlers CL, Criado JR, Wills DN, Liu W, Crews FT (2011) Periadolescent ethanol exposure reduces adult forebrain ChAT+IR neurons: correlation with behavioral pathology. Neuroscience 199, 333–345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engleman EA, McBride WJ, Wilber AA, Shaikh SR, Eha RD, Lumeng L, Li TK, Murphy JM (2000) Reverse microdialysis of a dopamine uptake inhibitor in the nucleus accumbens of alcohol-preferring rats: effects on dialysate dopamine levels and ethanol intake. Alcohol Clin Exp Res 24(6), 795–801. [PubMed] [Google Scholar]
- Exley R, Maubourguet N, David V, Eddine R, Evrard A, Pons S, Marti F, Threlfell S, Cazala P, McIntosh JM, Changeux JP, Maskos U, Cragg SJ, Faure P (2011) Distinct contributions of nicotinic acetylcholine receptor subunit alpha4 and subunit alpha6 to the reinforcing effects of nicotine. Proc Natl Acad Sci U S A 108(18):7577–7582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fagerberg L, Hallström BM, Oksvold P, Kampf C, Djureinovic D, Odeberg J, Habuka M, Tahmasebpoor S, Danielsson A, Edlund K, Asplund A, Sjöstedt E, Lundberg E, Szigyarto CA, Skogs M, Takanen JO, Berling H, Tegel H, Mulder J, Nilsson P, Schwenk JM, Lindskog C, Danielsson F, Mardinoglu A, Sivertsson A, von Feilitzen K, Forsberg M, Zwahlen M, Olsson I, Navani S, Huss M, Nielsen J, Ponten F, Uhlén M (2014) Analysis of the human tissue-specific expression by genome-wide integration of transcriptomics and antibody-based proteomics. Mol Cell Proteomics 13(2):397–406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fenster CP, Rains MF, Noerager B, Quick MW, Lester RAJ. Influence of subunit composition on desensitization of neuronal acetylcholine receptors at low concentrations of nicotine. J Neurosci 17(15):5747–5759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Floyd EA, Young-Seigler AC, Ford BD, Reasor JD, Moore EL, Townsel JG, Rucker HK (1997) Chronic ethanol ingestion produces cholinergic hypofunction in rat brain. Alcohol 14(1), 93–98. [DOI] [PubMed] [Google Scholar]
- Gao M, Jin Y, Yang K, Zhang D, Lukas RJ, Wu J (2010) Mechanisms involved in systemic nicotine-induced glutamatergic synaptic plasticity on dopamine neurons in the ventral tegmental area. J Neurosci 30, 13814–13825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garg BK, Loring RH (2017) Evaluating Commercially Available Antibodies for Rat α7 Nicotinic Acetylcholine Receptors. J Histochem Cytochem 65(9):499–512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gass JT, Glen WB Jr, McGonigal JT, Trantham-Davidson H, Lopez MF, Randall PK, Yaxley R, Floresco SB, Chandler LJ (2014) Adolescent alcohol exposure reduces behavioral flexibility, promotes disinhibition, and increases resistance to extinction of ethanol self-administration in adulthood. Neuropsychopharmacology 39 (11), 2570–2583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gatto GJ, McBride WJ, Murphy JM, Lumeng L, Li T-K (1994) Ethanol self infusion into the ventral tegmental area by alcohol-preferring rats. Alcohol 11:557–564. [DOI] [PubMed] [Google Scholar]
- Gipson CD, Reissner KJ, Kupchik YM, Smith AC, Stankeviciute N, Hensley-Simon ME, Kalivas PW (2013) Reinstatement of nicotine seeking is mediated by glutamatergic plasticity. Proc Natl Acad Sci U S A 110(22):9124–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hauser SR, Katner SN, Deehan GA Jr, Ding ZM, Toalston JE, Scott BJ, Bell RL, McBride WJ, Rodd ZA (2012) Development of an oral operant nicotine/ethanol co-use model in alcohol-preferring (P) rats. Alcohol Clin Exp Res 36:1963–1972 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hauser SR, Bracken AL, Deehan GA Jr, Toalston JE, Ding ZM, Truitt WA, Bell RL, McBride WJ, Rodd ZA (2014a) Selective breeding for high alcohol preference increases the sensitivity of the posterior VTA to the reinforcing effects of nicotine. Addict Biol 19(5), 800–811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hauser SR, Deehan GA Jr, Toalston JE, Bell RL, McBride WJ, Rodd ZA (2014b) Enhanced alcohol-seeking behavior by nicotine in the posterior ventral tegmental area of female alcohol-preferring (P) rats: modulation by serotonin-3 and nicotinic cholinergic receptors. Psychopharmacology (Berl) 231(18), 3745–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hauser SR, Knight CP, Truitt WA, Waeiss RA, Holt IS, Carvajal GB, Bell RL, Rodd ZA (2019) Adolescent intermittent ethanol (AIE) increases the sensitivity to the reinforcing properties of ethanol and the expression of select cholinergic and dopaminergic genes within the posterior ventral tegmental area. Alcohol Clin Exp Res doi: 10.1111/acer.14150 [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- Hoogendam JM, Kahn RS, Hillegers MHJ, van Buuren M, Vink M (2013) Different developmental trajectories for anticipation and receipt of reward during adolescence. Dev Cogn Neurosci 6:113–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson PL, Molosh A, Fitz SD, Arendt D, Deehan GA, Federici LM, Bernabe C, Engleman EA, Rodd ZA, Lowry CA, Shekhar A (2015) Pharmacological depletion of serotonin in the basolateral amygdala complex reduces anxiety and disrupts fear conditioning. Pharmacol Biochem Behav 138, 174–179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston LD, O’Malley PM, Bachman JG, and Schulenberg JE (2004) Monitoring the future national survey results on drug use, 1975-2004 Secondary School Students (NIH Publication No. 04-5507), vol. I National Institute on Drug Abuse, Bethesda, MD. [Google Scholar]
- Jones IW, Wonnacott S (2004) Precise localization of alpha7 nicotinic acetylcholine receptors on glutamatergic axon terminals in the rat ventral tegmental area. J Neurosci 24(50) 11244–11252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kabbani N, Nichols RA (2018) Beyond the Channel: Metabotropic Signaling by Nicotinic Receptors. Trends Pharmacol Sci. 39(4):354–366. [DOI] [PubMed] [Google Scholar]
- Katner SN, Toalston JE, Smoker MP, Rodd ZA, McBride WJ, Engleman EA (2015) Time-course of extracellular nicotine and cotinine levels in rat brain following administration of nicotine: effects of route and ethanol coadministration. Psychopharmacology (Berl) 232(3):551–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kyzar EJ, Zhang H, Pandey SC (2019) Adolescent alcohol exposure epigenetically suppresses amygdala arc enhancer rna expression to confer adult anxiety susceptibility. Biol Psychiatry doi: 10.1016/j.biopsych.2018.12.021 [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mansvelder HD, McGehee DS (2000) Long-term potentiation of excitatory inputs to brain reward areas by nicotine. Neuron, 27, 349–357. [DOI] [PubMed] [Google Scholar]
- Mansvelder HD, Keath JR, McGehee DS (2002) Synaptic mechanisms underlie nicotine-induced excitability of brain reward areas. Neuron 33, 905–919. [DOI] [PubMed] [Google Scholar]
- Marinelli M, McCutcheon JE (2014) Heterogeneity of dopamine neuron activity across traits and states. Neuroscience 282:176–197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McBride WJ, Schultz JA, Kimpel MW, McClintick JN, Wang M, You J, Rodd ZA (2009) Differential effects of ethanol in the nucleus accumbens shell of alcohol-preferring (P), alcohol-non-preferring (NP) and Wistar rats: A proteomics study. Pharmacol Biochem Behav 92(2), 304–313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClintick JN, McBride WJ, Bell RL, Ding Z-M, Liu Y, Xuei X, Edenberg HJ (2015) Gene expression changes in serotonin, GABA-A receptors, neuropeptides and ion channels in the dorsal raphe nucleus of adolescent alcohol-preferring (P) rats following binge-like alcohol drinking. Pharmacol Biochem Beh 129:87–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClintick JN, McBride WJ, Bell RL, Ding Z-M, Liu Y, Xuei X, Edenberg HJ (2016) Gene Expression Changes in Glutamate and GABA-A Receptors, Neuropeptides, Ion Channels, and Cholesterol Synthesis in the Periaqueductal Gray Following Binge-Like Alcohol Drinking by Adolescent Alcohol-Preferring (P) Rats. Alcohol Clin Exp Res 40(5):955–968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCutcheon JE, Conrad KL, Carr SB, Ford KA, McGehee DS, Marinelli M (2012) Dopamine neurons in the ventral tegmental area fire faster in adolescent rats than in adults. J Neurophysiol 108(6), 1620–1630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miech RA, Johnston LD, O’Malley PM, Bachman JG, Schulenberg JE (2016) Monitoring the Future national survey results on drug use, 1975- 2015: Volume I, Secondary school students (Institute for Social Research, The University of Michigan; ). [Google Scholar]
- Mielke JG, Mealing GA (2009) Cellular distribution of the nicotinic acetylcholine receptor alpha7 subunit in rat hippocampus. Neurosci Res 65(3):296–306. [DOI] [PubMed] [Google Scholar]
- Morel C, Montgomery S, Han MH (2018) Nicotine and alcohol: the role of midbrain dopaminergic neurons in drug reinforcement. Eur J Neurosci doi: 10.1111/ejn.14160. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Research Council Committee for the Update of the Guide for the Care & Use of Laboratory Animals (2011) The National Academies Collection: Reports funded by National Institutes of Health In Guide for the Care and Use of Laboratory Animals. Washington (DC): National Academies Press (US) National Academy of Sciences. [Google Scholar]
- Oliva I, Wanat MJ (2016) Ventral Tegmental Area Afferents and Drug-Dependent Behaviors. Front Psychiatry 7:30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ortiz J, Fitzgerald LW, Charlton M, Lane S, Trevisan L, Guitart X, Shoemaker W, Duman RS, Nestler EJ (1995) Biochemical actions of chronic ethanol exposure in the mesolimbic dopamine system. Synapse 21(4):289–98. [DOI] [PubMed] [Google Scholar]
- Pascual M, Boix J, Felipo V, Guerri C (2009) Repeated alcohol administration during adolescence causes changes in the mesolimbic dopaminergic and glutamatergic systems and promotes alcohol intake in the adult rat. J Neurochem 108(4), 920–931. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C (1998) The Rat Brain in Stereotaxic Coordinates, 4th ed. Academic Press, New York. [Google Scholar]
- Pidoplichko VI, DeBiasi M, Wiliams JT, Dani JA (1997) Nicotine activates and desensitizes midbrain dopamine neurons. Nature, 390, 401–404. [DOI] [PubMed] [Google Scholar]
- Rassnick S, Stinus L, Koob GF (1993) The effects of 6-hydroxydopamine lesions of the nucleus accumbens and the mesolimbic dopamine system on oral self-administration of ethanol in the rat. Brain Res 623(1):16–24. [DOI] [PubMed] [Google Scholar]
- Rodd-Henricks ZA, McKinzie DL, Crile RS, Murphy JM, McBride WJ (2000) Regional heterogeneity for the intracranial self-administration of ethanol within the ventral tegmental area of female Wistar rats. Psychopharmacology 149:217–224. [DOI] [PubMed] [Google Scholar]
- Rodd-Henricks ZA, Bell RL, Kuc KA, Murphy JM, McBride WJ, Lumeng L, Li TK (2002a) Effects of ethanol exposure on subsequent acquisition and extinction of ethanol self-administration and expression of alcohol-seeking behavior in adult alcohol-preferring (P) rats: I. Periadolescent exposure. Alcohol Clin Exp Res 26: 1632–41. [DOI] [PubMed] [Google Scholar]
- Rodd-Henricks ZA, Bell RL, Kuc KA, Murphy JM, McBride WJ, Lumeng L, Li TK (2002b) Effects of ethanol exposure on subsequent acquisition and extinction of ethanol self-administration and expression of alcohol-seeking behavior in adult alcohol-preferring (P) rats: II. Adult exposure. Alcohol Clin Exp Res 26: 1642–52. [DOI] [PubMed] [Google Scholar]
- Sahr AE, Thielen RJ, Lumeng L, Li T-K, McBride WJ (2004) Long-Lasting Alterations of the Mesolimbic Dopamine System After Periadolescent Ethanol Drinking by Alcohol-Preferring Rats. Alcohol Clin Exp Res 28(5):702–711. [DOI] [PubMed] [Google Scholar]
- Spear LP (2000) The adolescent brain and age-related behavioral manifestations. Neurosci Biobehav Rev 24:417–463. [DOI] [PubMed] [Google Scholar]
- Spear LP, Swartzwelder HS (2014) Adolescent alcohol exposure and persistence of adolescent-typical phenotypes into adulthood: A mini-review. Neurosci Biobehav Rev 45:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stuber GD, Hopf FW, Hahn J, Cho SL, Guillory A, Bonci A (2008) Voluntary ethanol intake enhances excitatory synaptic strength in the ventral tegmental area. Alcohol Clin Exp Res 32(10):1714–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas AM, Ostroumov A, Kimmey BA, Taormina MB, Holden WM, Kim K, Brown-Mangum T, Dani JA (2018) Adolescent Nicotine Exposure Alters GABAA Receptor Signaling in the Ventral Tegmental Area and Increases Adult Ethanol Self-Administration. Cell Rep 23(1) 68–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tizabi Y, Copeland RJ, Louis VA, Taylor RE (2002) Effects of combined systemic alcohol and central nicotine administration into ventral tegmental area on dopamine release in the nucleus accumbens. Alcohol Clin Exp Res 26(3), 394–399. [PubMed] [Google Scholar]
- Tizabi Y, Bai L, Copeland RJ, Taylor RE (2007) Combined effects of systemic alcohol and nicotine on dopamine release in the nucleus accumbens shell. Alcohol Alcohol 42(5), 413–416. [DOI] [PubMed] [Google Scholar]
- Toalston JE, Deehan GA Jr., Hauser SR, Engleman EA, Bell RL, Murphy JM, Truitt WA, McBride WJ, Rodd ZA. (2014) Reinforcing properties and neurochemical response of ethanol within the posterior ventral tegmental area are enhanced in adulthood by periadolescent ethanol consumption. J Pharmacol Exp Ther 351: 317–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toalston JE, Deehan GA Jr, Hauser SR, Engleman EA, Bell RL, Murphy JM, McBride WJ, Rodd ZA (2015) The reinforcing properties of ethanol are quantitatively enhanced in adulthood by periadolescent ethanol, but not saccharin, consumption in female alcohol-preferring (P) rats. Alcohol 49(5) 513–518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Truitt WA, Hauser SR, Deehan GJ, Toalston JE, Wilden JA, Bell RL, McBride WJ, Rodd ZA (2015) Ethanol and nicotine interaction within the posterior ventral tegmental area in male and female alcohol-preferring rats: evidence of synergy and differential gene activation in the nucleus accumbens shell. Psychopharmacology 232(3), 639–649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vetreno RP, Broadwater M, Liu W, Spear LP, Crews FT. (2014) Adolescent, but Not Adult, Binge Ethanol Exposure Leads to Persistent Global Reductions of Choline Acetyltransferase Expressing Neurons in Brain. PLoS ONE 9(11):1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waeiss RA, Knight CP, Hauser SR, Pratt LA, McBride WJ, Rodd ZA (2019) Therapeutic challenges for concurrent ethanol and nicotine consumption: naltrexone and varenicline fail to alter simultaneous ethanol and nicotine intake by female alcohol-preferring (P) rats. Psychopharmacology (Berl) doi: 10.1007/S00213-019-5174-y. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang F, Chen H, Sharp BM (2008) Neuroadaptive changes in the mesocortical glutamatergic system during chronic nicotine self-administration and after extinction in rats. J Neurochem 106(2):943–56 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wise RA, Rompre PP (1989) Brain dopamine and reward. Annu Rev Psychol 40:191–225 [DOI] [PubMed] [Google Scholar]


