Abstract
Synthetic cathinones are used for their stimulant-like properties. Stimulant-induced neurochemical changes are thought to occur at different times in different brain regions and neurotransmitter systems. This study sought to examine the behavioral and neurochemical effects of α-pyrrolidinopentiophenone (α-PVP) and mephedrone (4MMC) in female rats. Methods probed the chronology of effects of synthetic cathinone exposure. Female rats were trained to self-administer α-PVP, 4MMC, or saline. Drug exposure ceased after 7 days of autoshaping for half of each drug group; the other half self-administered for another 21 days. Amygdala, hippocampus, hypothalamus, PFC, striatum, and thalamus were extracted, and tissue was analyzed with electrochemical detection and liquid chromatography mass spectrometry. Responding was minimal during autoshaping; thus, most infusions were delivered noncontingently in the autoshaping phase. Rats acquired self-administration of α-PVP and 4MMC. Synthetic cathinone administration, and duration of exposure produced several effects on neurotransmitters. α-PVP primarily increased serotonin, 5-hydroxy-3-acetic acid (5-HIAA), norepinephrine, and glutamate in hypothalamus. In contrast, 4MMC decreased serotonin and 5-HIAA in several brain regions. Longer durations of exposure to both synthetic cathinones increased 5-HIAA, norepinephrine, and glutamate in multiple brain regions compared to the short exposure during autoshaping. Notably, both α-PVP and 4MMC produced minimal changes in dopamine levels, suggesting that the dopaminergic effects of these synthetic cathinones are transient. These alterations in neurotransmitter levels indicate that synthetic cathinone use may produce differential neurochemical changes during the transition from use to abuse.
Keywords: alpha-PVP, female, mephedrone, neurotransmitter, self-administration
1.0. Introduction
Synthetic cathinones are a class of psychoactive substances that are used for their stimulant-like properties [1, 2], 4-Methylmethcathinone (4MMC; mephedrone), a ring-substituted cathinone, was one of the first widely-used synthetic cathinones, and is, therefore, the most well-studied synthetic cathinone [1, 3]. α-Pyrrolidinopentiophenone (α-PVP) continues to be used by high school students in the U.S. [4], and its use has led to multiple recent medical emergencies [5]. Prevalence of synthetic cathinone use increased from 2017 to 2018 [6], and identification of synthetic cathinones in drug products continues to rise in some parts of the U.S. [7].
Synthetic cathinones alter the activity of monoamine transporters, similar to classical stimulants of abuse such as cocaine and methamphetamine [8, 9]. 4MMC releases dopamine (DA), norepinephrine (NE), and serotonin (5-HT) [10, 11], while α-PVP blocks DA and NE transport [12, 13]. Microdialysis studies also show that α-PVP activates the reinforcement pathway in the brain by increasing extracellular DA levels in the nucleus accumbens shell [14].
Men are more likely to use synthetic cathinones than women [15–18], and are more likely to experience adverse effects of synthetic cathinones than women [19]. Studies of classical stimulants of abuse using rodent models show results that parallel the sex and gender differences found in humans [20]. Female rats acquire cocaine and methamphetamine self-administration faster than males, and have higher levels of cocaine and methamphetamine intake than males [21, 22]; however, these differences are only apparent when low doses and complex schedules of reinforcement (e.g. progressive ratio) are used [22–24].
Few preclinical studies have examined effects of synthetic cathinones in both sexes under the same conditions. For example, 4MMC has been shown to produce locomotor sensitization in male rats [25, 26] and in female mice [27]. Female rats acquire robust 4MMC self-administration [28], but their experimental conditions were different from those used with males [29–32], making comparison between the sexes difficult. Nonetheless, there were no sex differences in acquisition of 4MMC self-administration when the same training procedure was used for both sexes [28, 33]. Similarly, self-administration of α-PVP has been investigated in male [32, 34–37] and female rats [38]. Although experimental conditions were different for each sex, both males and females acquired self-administration of α-PVP. A few direct comparisons of effects of synthetic cathinones in male and female rodents have been conducted. Male and female rodents showed similar pentedrone-, pentylone-, methylone- and α-PVP-induced locomotor stimulation [39, 40]. α-PVP produced some similarities across sexes in somatic signs, while sex differences were evident for flattened body posture [40]. Male and female rats showed similar place preference for 3,4-methylenedioxypyrovalerone (MDPV), but males were more likely than females to show conditioned taste aversion to MDPV [41]. Furthermore, the vast majority of the research on neurochemical effects of synthetic cathinones has been conducted in males [42, 43]. Thus, given the few direct comparisons between males and females, it is unclear to what extent the behavioral and neurochemical effects of α-PVP and 4MMC may differ between sexes.
Stimulant-induced neurochemical changes are thought to occur at different times during drug exposure for different affected brain regions or neurotransmitter systems [44]. Thus, drug abuse can be divided into stages, with each stage defined by changes in specific brain regions and specific neurotransmitter systems. The binge/intoxication stage is associated with changes in DA in striatum, thalamus, and nucleus accumbens, while the preoccupation/anticipation stage is associated with changes in glutamate (GLU) in hippocampus, PFC, and striatum [44–47]. In male rats, brief α-PVP exposure during autoshaping lowered GLU in hippocampus and lowered NE in thalamus, whereas longer exposure to α-PVP during self-administration altered GLU in amygdala and PFC, and had no effect on NE in thalamus. Similarly, 4MMC lowered 5-HT in hippocampus and prefrontal cortex (PFC), and lowered GLU in thalamus, but only after self-administration [32]. These alterations in neurochemistry suggest that synthetic cathinone use likely produces sequential neurochemical changes during the transition from use to abuse [32]. What remains unknown is if treatment need differs based on the progression of synthetic cathinone abuse, based on sex, or based on both progression and sex.
This study sought to examine the behavioral and neurochemical effects of α-PVP and 4MMC in female rats. Methods probed the chronology of effects of synthetic cathinone exposure, using the same procedures employed in a prior study with male rats [32]. Neurotransmitter profiles were measured in addiction-related brain regions to investigate if neuronal signaling differed based on mechanisms of action, and if signaling changed as a function of duration of synthetic cathinone exposure. Female rats self-administered synthetic cathinones or vehicle for 7 or 28 days in an attempt to produce the neurochemical effects that are thought to align with different stages of drug abuse [44].
2.0. Methods
2.1. Subjects
Adult female Sprague-Dawley rats (Envigo, Frederick, MD, USA) (total n=48), aged approximately 65-70 days at the start of the experiment, were housed individually in polycarbonate cages with hardwood bedding. Rats were housed in temperature-controlled conditions (20-24°C) with a 12 h standard light-dark cycle (lights on at 0700). Rats had free access to water in the home cage, and were fed 17 g of food daily. Experiments were approved by the Institutional Animal Care and Use Committee for RTI, and complied with the ARRIVE guidelines. All research was conducted as humanely as possible, and followed the principles of laboratory animal care [48].
2.2. Drugs
α-PVP and 4MMC were synthesized in house using standard synthetic procedures. They were formulated as recrystalized salt and were > 97% pure. The purity was assessed by carbon, hydrogen, nitrogen (CHN) combustion analysis, and proton nuclear magnetic resonance spectroscopy. Compounds were dissolved in saline (Butler Schein, Dublin, OH, USA). Gentamicin and heparin, used for maintaining catheter patency, were purchased from Butler Schein.
2.3. Apparatus
Experimental sessions were conducted in operant conditioning chambers for rats (MED Associates, St. Albans, VT, USA) housed inside sound-attenuating chambers (MED Associates). Each operant conditioning chamber contained two retractable levers, with a stimulus light above each lever, and a house light. One lever was designated as the active lever and the other lever was designated as inactive. The side of the operant conditioning chamber associated with the active lever was counterbalanced across subjects. Fans provided ventilation for each chamber and speakers provided white noise. Infusion pumps (Med Associates) were located outside the chamber. Experimental events were arranged and recorded by MED-PC software (Med-Associates).
A Thermo Scientific CoulArray Multi-Channel ECD Array system (model 5600A; Thermo Scientific, Waltham, MA, USA) was used to analyze neurotransmitter concentrations. The array detector contained 16 coulometric electrochemical cells that provided quantitation of multiple neurotransmitters and metabolites simultaneously. An Agilent 1100 HPLC System (Santa Clara, CA, USA) and an Applied Biosystems API 4000 Triple Quadrupole liquid chromatography mass spectrometer (LC-MS) with Turbo Ion Spray source (Foster City, CA, USA) were used for quantitation of GLU.
2.4. Surgical Procedures
Rats were surgically implanted with chronic indwelling jugular catheters under general anesthesia as previously described [32]. The external end of the catheter was secured by a quick connect harness. Rats were allowed to recover from surgery for a minimum of 7 days before beginning the experiment. Catheters were flushed daily with saline prior to the session, and with a post-flush solution containing 0.96% gentamicin, 2.88% heparin, and 96.2% saline after the session to maintain patency. All catheters were checked for patency prior to the start of the experiment.
2.5. Drug Self-Administration
Rats were randomly assigned to one of three drug groups: α-PVP (0.1 mg/kg/infusion), 4MMC (0.5 mg/kg/infusion), or saline. Drug doses were chosen based on their prior use in studies demonstrating acquisition of self-administration in rats using the same schedule of reinforcement [28, 31–35]. Within each drug group, rats were randomly assigned to one of two duration groups: autoshaping only (AO), or autoshaping followed by an additional 21 days of self-administration (self-administration; SA) (n=8/group).
All rats were first trained to self-administer through an autoshaping procedure for 7 days [49, 50]. This ensured that rats in the AO groups received a consistent, limited amount of drug exposure, regardless of how quickly they acquired self-administration. This brief drug exposure may be sufficient to produce changes in neurotransmitter levels indicative of the binge/intoxication stage of drug abuse because this stage is associated with acute drug self-administration and acute drug reward [44, 46, 47]. During autoshaping sessions, active lever extension was paired with an infusion (0.1 ml) based on a random time 60 s schedule. Fifteen seconds of lever extension, or a lever press resulted in an infusion (5.9 s) and initiated a 20-s timeout, which was signaled by illumination of both stimulus lights [32, 34]. Throughout training and self-administration sessions, the inactive lever was extended, and presses on this lever were recorded, but had no programmed consequence. Autoshaping sessions delivered 15 infusions within the first 30 min of the session. Rats then remained in the operant conditioning chamber for 15 min with only the inactive lever present and no drug infusions available, which provided additional exposure to the lack of programmed consequences associated with the inactive lever.
Following autoshaping, sessions stopped for rats in AO groups, whereas SA groups continued to self-administer on a fixed ratio 1 schedule of reinforcement (FR1) for an additional 21 days during daily 60 min sessions. The 21 days of self-administration was hypothesized to produce changes in neurotransmitter levels indicative of the preoccupation/anticipation stage of drug abuse. This stage is associated with the positive reinforcing effects and conditioned effects of drugs [44, 46, 51].
2.6. Brain Sample Collection
Rats were euthanized by rapid decapitation one day after their last self-administration session in order to avoid direct drug effects [32, 52]. The hypothalamus was removed from the ventral side of the brain and divided into halves. Then the brain was cut down the mid line and each cortical half was opened, and the hippocampus was removed [53]. Next, each half was cut into three coronal slices using midline anatomical markers moving rostral to caudal. The first cut was made at the beginning of the corpus callosum, the second at the fornix, and the third cut at the end of the corpus callosum. PFC was taken from the first section. Striatum was removed from the second slice. Finally, thalamus and amygdala were removed from the third section [54, 55]. All tissue samples were placed in aluminum foil, inserted into a cryovial, flash frozen in liquid nitrogen, and stored at −80°C.
2.7. Neurotransmitter Quantitation
Brain tissue samples were weighed and placed in cryovials containing stainless steel grinding balls. A tissue buffer solution (pH 3) consisting of 0.05 M Na2HPO4, 0.03 M citric acid, 2 mM ascorbic acid, and an Internal Standard (IS) of 200 ng/ml 3,4-dihydroxybenzylamine (DHBA) was added to each brain tissue sample at a ratio of 20 μl/mg of tissue while samples were homogenated by two 30-s cycles on the geno/grinder (SPEX SamplePrep, Metuchen, NJ), and then centrifuged. Supernatant was then transferred to a 96 well plate for analysis by Electrochemical Detection (ECD).
For the GLU analysis, an additional 10 μl aliquot of supernatant was transferred to a 700 μl deep, 96 well plate, and diluted with 490 μl of 5 mM ammonium acetate and 0.1% formic acid, aqueous (LC-MS/MS mobile phase A). The plate was mixed at 1000 RPM for 4 min. Then 100 μl was transferred to a new 700 μl deep, 96 well plate and diluted with an additional 400 μl of LC-MS/MS mobile phase A (total dilution of 250 fold). A 50 μl aliquot of 10 μg/ml L-glutamic-2,3,3,4,4-d5 acid (d5-GLU) in water was added. The plate was sealed and mixed at 1000 RPM for 4 min prior to analysis.
2.7.1. Electrochemical methods.
Ultra-high pressure liquid chromatography (UPLC) coupled with ECD was used to simultaneously measure DA, dihydroxyphenylacetic acid (DOPAC), homovanillic acid (HVA), 5-HT, 5-hydroxy-3-acetic acid (5-HIAA), and NE. A 10-μl aliquot was injected onto a Luna Omega 2.1 x 150 mm column (Phenomenex, Torrance, CA) coupled to a LPG-3400RS pump, WPS-3000TBRS autosampler, and a CoulArray electrochemical detector. The column was heated to 30°C. The mobile phase consisted of 50 mM sodium phosphate, 47 mM citric acid, 0.14 mM EDTA, 0.64 mM octanesulfonic acid, and 5% methanol, with a flow rate of 0.4 ml/min. The detector was set to sequentially deliver potentials of −150 mV, 150 mV, 400 mV, and 600 mV.
Standards for ECD were prepared by weighing approximately 1 mg of analyte, DA (Sigma-Aldrich, Buchs, Switzerland), DOPAC (Sigma-Aldrich, Buchs, Switzerland), HVA (Sigma-Aldrich, Buchs, Switzerland), 5-HT (Sigma-Aldrich, St. Louis, MO), 5-HIAA (Sigma-Aldrich, St. Louis, MO), and NE (Sigma-Aldrich, St. Louis, MO). Each standard was transferred to a volumetric flask and diluted to volume with tissue buffer to create stock solutions. A stock solution containing approximately 10 μg/ml of analyte was then diluted to encompass a concentration range from 1000 to 0.5 ng/ml.
2.7.2. LC-MS/MS methods.
GLU was analyzed by liquid chromatography-tandem mass spectrometry (LC-MS/MS) using an API 4000 Triple Quadrupole mass spectrometer with a Turbo Ion Spray source (Applied Biosystems/MDS Sciex, Foster City, CA) in positive ion mode, coupled with Agilent 1100 HPLC system (Santa Clara, CA). A XBridge HILIC 4.6 x 150 mm column (Waters, Ireland) was used for analyte separation. Mobile phase A consisted of 5 mM ammonium acetate and 0.1% formic acid (aqueous) and mobile phase B consisted of acetonitrile Mobile phase A was held at 50% for 1 min, then increased linearly to 90% for 1.5 min, and held for 2.5 min before returning to initial conditions. The flow rate was 0.5 ml/min. Ion source temperature and spray voltage were 650 °C and 4000 V, respectively. Transitions monitored for quantitation were: GLU, 148.0→ 83.9; GLUd5 (IS), 152.9→ 134.8.
Standards for GLU analysis were prepared by weighing approximately 25 mg of GLU (Alfa Aesar, Ward Hill, MA). The standard was transferred to a volumetric flask and diluted to volume with mobile phase A (5 mM ammonium acetate and 0.1% formic acid, aqueous) and labeled as stock solution. The stock solution containing approximately 2.5 mg/ml of GLU was then diluted to encompass a concentration range from 2500 to 10 ng/ml.
2.8. Data Analysis
Statistical analyses were conducted using NCSS (Number Cruncher Statistical Systems, Kaysville, Utah, USA). For all analyses, α-PVP and 4MMC were analyzed separately, and compared to saline.
For self-administration data, autoshaping data were analyzed with separate mixed factors ANOVAs (drug x lever x exposure duration) to compare active and inactive lever presses for drug and saline groups, with drug and duration as between-subjects factors, and lever as a within-subjects factor. The additional 21 days of self-administration for SA groups were analyzed with separate mixed factors ANOVAs (drug x lever x session). These analyses compared active and inactive lever presses for drug groups to the saline group, with drug as a between-subjects factor, and lever and session number as within-subjects factors.
Neurotransmitter and metabolite data were analyzed with between-factors duration (AO vs SA) x drug group (cathinone vs saline) ANOVAs. Each brain region and neurotransmitter (or metabolite) combination was analyzed separately. All tests were considered significant at p<0.05, and were followed with Tukey’s post hoc tests as appropriate.
There were two cases in which neurotransmitter data were excluded. First, there were a few cases in which amygdala samples had very elevated levels of DA, DOPAC, and HVA. Due to the proximity of the amygdala to the striatum in the brain, it was determined that a small amount of striatum was likely included as part of the amygdala sample. Second, in some samples of PFC, an unknown peak interfered with the integration of the DOPAC peak. In instances that DOPAC was not integrable due to this interference, the DOPAC data were excluded.
3.0. Results
3.1. Self-administration
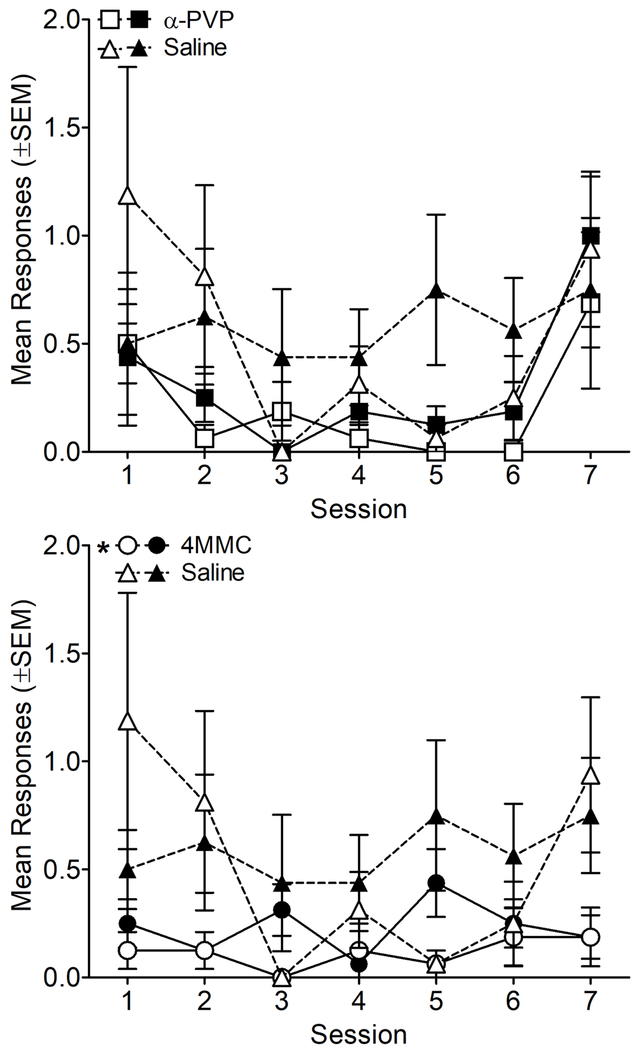
Self-administration data from the autoshaping phase are shown in Figure 1. Lever presses for rats in the AO and SA groups did not significantly differ during autoshaping, nor were there interactions between duration and drug group, or duration and lever during autoshaping (p>0.05). Therefore, data were collapsed across AO and SA groups within drug for graphical representation (Figure 1). Saline rats lever pressed more than 4MMC rats during autoshaping [main effect of drug: F(1, 28)=6.03, p<0.05], whereas responding was similar for α-PVP and saline rats during autoshaping. There were no significant differences between active and inactive responses for any group (p>0.05). These results indicate that during autoshaping, most infusions were delivered noncontingently for all groups.
Figure 1.
Mean responses on the active (filled symbols) and inactive (open symbols) levers as a function of session for rats self-administering α-PVP (top panel), 4MMC (bottom panel), or saline (both panels) during autoshaping. * indicates a significant difference from saline. n=16/group.
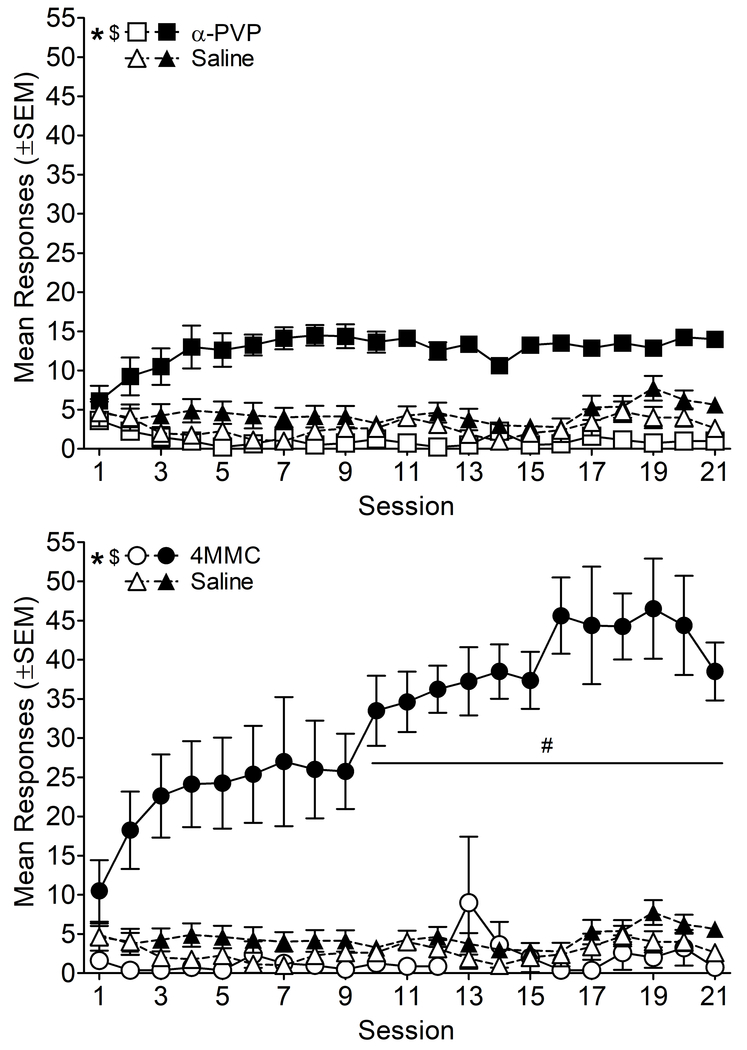
Self-administration data from the FR1 phase for SA groups are shown in Figure 2. α-PVP rats responded more than saline rats (Figure 2, top panel), [main effect of drug: F(1, 14)=12.48, p<0.05], and rats made more α-PVP active responses than both α-PVP inactive responses and saline active responses [main effect of lever: F(1, 14)=129.04, p<0.05; drug x lever interaction: F(1, 14)=70.69, p<0.05]. Active responses increased from session 1 for sessions 4, 7, 8, 9, 11, and 17-21, and active responses were greater than inactive responses during sessions 2-21 [main effect of session: F(20, 280)=1.73, p<0.05; lever x session interaction: F(20, 280)=3.78, p<0.05]. The α-PVP group made more responses than the saline group during sessions 4, 6-10, and 13-16 when data were collapsed across active and inactive levers [drug x session interaction: F(20, 280)=2.00, p<0.05].
Figure 2.
Mean responses on the active (filled symbols) and inactive (open symbols) levers as a function of session for rats self-administering α-PVP (top panel), 4MMC (bottom panel), or saline (both panels) on an FR1 schedule of reinforcement. * indicates a significant difference from saline for active lever presses, and $ indicates a significant difference between active and inactive lever presses within drug group (drug x lever interactions). # indicates a significant difference from session 1 for 4MMC (drug x session interaction). n=8/group.
During the 21 days of self-administration, rats self-administered more 4MMC than saline, responded more on the active than inactive lever, and made more 4MMC active responses than both 4MMC inactive responses and saline active responses (Figure 2, bottom panel) [main effect of drug: F(1, 14)=45.06, p<0.05; main effect of lever: F(1, 14)=105.47, p<0.05; drug x lever interaction: F(1,14)=84.34, p<0.05]. Responding for 4MMC increased from session 1 for sessions 10-21, and responding for 4MMC was greater than responding for saline for sessions 6-21 [main effect of session: F(20, 280)=5.36, p<0.05; drug x session interaction: F(20, 280)=4.69, p<0.05]. Rats responded more on the active than inactive lever during sessions 3-21, and active lever presses were higher than session 1 for sessions 10-21 [lever x session interaction: F(20, 280)=4.34, p<0.05]. Therefore, rats acquired self-administration of α-PVP and 4MMC, and self-administered significantly more of both synthetic cathinones than saline.
3.2. Neurotransmitters
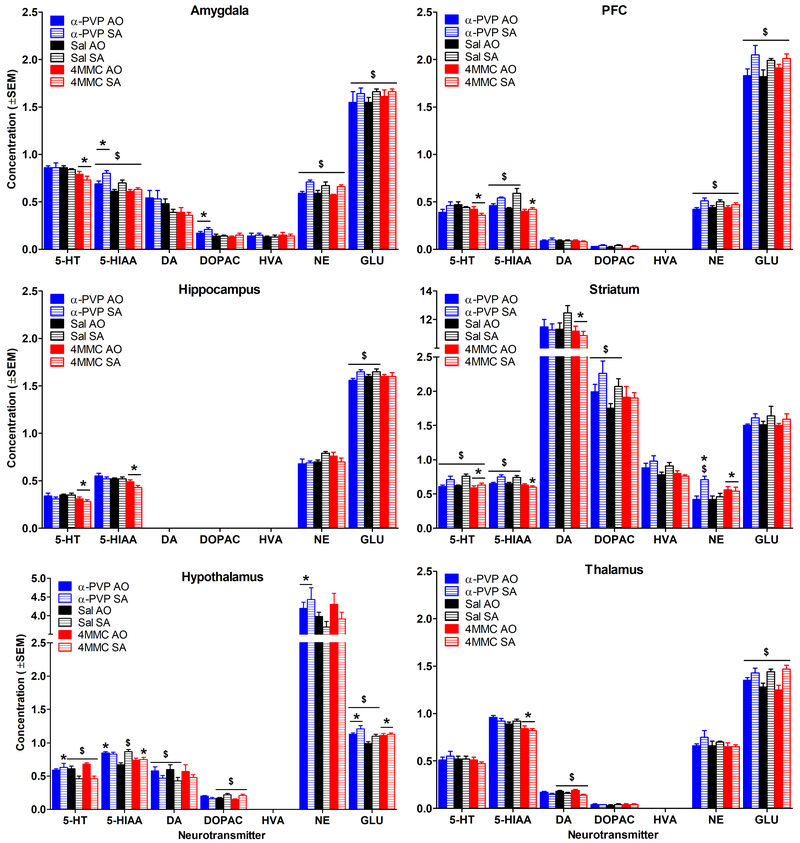
Neurotransmitter and metabolite concentrations for all brain regions and all rat groups are shown in Figure 3. There were several differences between drug groups, between durations (AO vs SA), and there were interactions between drug and duration.
Figure 3.
Mean neurotransmitter and metabolite concentrations as a function of brain region. Concentration units are ng/ml for all analytes except for GLU which is shown in μg/ml. Note different y axis scale for hypothalamus and striatum. * indicates a significant difference from saline for the same duration (main effect or interaction), and $ indicates a significant difference from AO for the same drug/saline (main effect or interaction). Sal = saline. n=5-8/group for DA, DOPAC, and HVA in amygdala; n=3-8/group for DOPAC in PFC; n=7-8/group for all other neurotransmitters and metabolites.
3.2.1. Effects of duration.
All statistically significant effects of duration of exposure to experimental conditions on neurotransmitters are shown in Table 1. Amygdala showed elevated 5-HIAA, NE, and GLU following greater durations of α-PVP, 4MMC, and saline exposure (Table 1 and Figure 3). Hippocampus also showed increased GLU following longer exposure to α-PVP and saline. There were multiple drug- and neurotransmitter-specific effects of duration in hypothalamus. Longer exposure to α-PVP or saline decreased DA, but increased GLU, whereas longer exposure to 4MMC or saline decreased 5-HT, but increased DOPAC. Interestingly, longer exposure to saline increased 5-HIAA in hypothalamus, while duration of exposure to α-PVP or 4MMC had no effect on 5-HIAA in this brain region.
Table 1.
F values for significant main effects of longer duration of exposure on neurotransmitter concentrations, and duration by drug interactions that only showed significant effects of duration for one drug group (p < 0.05). Arrows indicate the direction of effects of duration compared to the AO group. Cells containing group names (α-PVP or Saline) indicate interactions that were only significant for one group. Cells without group names indicate main effects that occurred when data were collapsed across drug and saline groups. Degrees of freedom are 1, 28 for all comparisons except for 5-HIAA in PFC which has 1, 27 degrees of freedom.
| Brain Region | 5-HT | 5-HIAA | DA | DOPAC | NE | GLU |
|---|---|---|---|---|---|---|
| α-PVP Compared to Saline | ||||||
| Amygdala | ↑ 13.88 | ↑ 10.02 | ↑ 4.76 | |||
| Hippocampus | ↑ 10.01 | |||||
| Hypothalamus | ↑ 11.20 | ↓ 6.31 | ↑ 6.77 | |||
| ↑ Saline 14.18 | ||||||
| PFC | ↑ 23.25 | ↑ 11.80 | ↑ 7.50 | |||
| Striatum | ↑ 16.41 | ↑ 15.62 | ↑ 5.64 | ↑ 10.87 | ||
| ↑ α-PVP 6.18 | ||||||
| Thalamus | ↑ 9.94 | |||||
| 4MMC Compared to Saline | ||||||
| Amygdala | ↑ 6.23 | ↑ 7.76 | ↑ 5.49 | |||
| Hypothalamus | ↓ 25.89 | ↑ 13.92 | ↑ 9.26 | |||
| ↑ Saline 11.43 | ||||||
| PFC | ↑ 11.19 | ↑ 7.04 | ↑ 8.47 | |||
| ↑ Saline 6.78 | ||||||
| Striatum | ↑ 10.24 | ↑ Saline 7.39 | ||||
| Thalamus | ↓ 6.71 | ↑ 22.63 | ||||
In PFC, greater duration of exposure to either synthetic cathinone or saline increased NE and GLU, and longer exposure to α-PVP or saline increased 5-HIAA (Figure 3). In striatum, longer exposure to α-PVP, 4MMC, or saline increased 5-HT. α-PVP and saline groups also showed increased 5-HIAA and DOPAC as a function of duration, and NE was elevated with greater duration of exposure in striatum, but only for α-PVP. Longer α-PVP, 4MMC, and saline exposure increased GLU in thalamus, while longer 4MMC or saline exposure decreased DA in thalamus. Duration did not alter HVA for either drug or saline (data not shown in Table 1).
3.2.2. Effects of drug.
All statistically significant effects of drug on neurotransmitters, and interactions between duration and drug are shown in Table 2. Some drug effects only occurred for one duration; these interactions are denoted by the duration names (AO or SA) in the corresponding cells in the table. Despite α-PVP and 4MMC being indirect-acting DA receptor agonists, there were very few drug effects on DA in any brain region for either drug. Only 4MMC altered DA, and only in striatum where it decreased DA compared to saline (Table 2 and Figure 3). In contrast, α-PVP increased DOPAC in amygdala, indicating metabolism of DA in this region.
Table 2.
F values for significant main effects of drug, and significant duration by drug interactions on neurotransmitter concentrations (p < 0.05). Arrows indicate the direction of drug effects compared to the saline group. Cells containing duration names (AO or SA) indicate interactions that were only significant for one duration. Cells without duration names indicate main effects. Degrees of freedom are 1, 28 for all comparisons except for 5-HIAA in PFC and DOPAC in amygdala, which have 1, 27 and 1, 23 degrees of freedom, respectively.
| Brain Region | 5-HT | 5-HIAA | DA | DOPAC | NE | GLU |
|---|---|---|---|---|---|---|
| α-PVP Compared to Saline | ||||||
| Amygdala | ↑ 11.72 | ↑ 6.43 | ||||
| Hypothalamus | ↑ SA 4.63 | ↑ 5.86 | ↑ 5.54 | ↑ 12.07 | ||
| ↑ AO 14.18 | ||||||
| Striatum | ↑ 5.79 | |||||
| ↑ SA 6.18 | ||||||
| 4MMC Compared to Saline | ||||||
| Amygdala | ↓ 9.17 | |||||
| Hippocampus | ↓ 7.13 | ↓ 10.49 | ||||
| Hypothalamus | ↑ SA 11.43 | ↑ 6.44 | ||||
| PFC | ↓ 7.33 | ↓ 12.60 | ||||
| ↓ SA 6.78 | ||||||
| Striatum | ↓ 7.41 | ↓ 14.98 | ↓ 4.29 | ↑ 4.58 | ||
| ↓ SA 7.39 | ||||||
| Thalamus | ↓ 8.80 | |||||
4MMC produced global serotonergic effects. 4MMC decreased 5-HT in amygdala, hippocampus, PFC, and striatum. 4MMC also decreased 5-HIAA in hippocampus and thalamus for both AO and SA groups, and decreased 5-HIAA for SA groups in hypothalamus, PFC, and striatum. In contrast, α-PVP caused localized effects on 5-HT. α-PVP increased 5-HIAA in amygdala for AO and SA groups, and in hypothalamus, α-PVP increased 5-HT for the SA group and increased 5-HIAA for the AO group. Additionally, α-PVP elevated NE in hypothalamus, and exposure to α-PVP SA, 4MMC AO, and 4MMC SA conditions elevated NE in striatum. The only effect of synthetic cathinones compared to saline on GLU was seen in hypothalamus, where exposure to both synthetic cathinones increased GLU.
4.0. Discussion
Female rats in the present study acquired self-administration of α-PVP and 4MMC, and earned a similar amount of these synthetic cathinones as males from a past study that were exposed to identical experimental conditions [32]. (Average infusions/session male 4MMC: 32.8; female 4MMC: 32.6; male α-PVP: 14.3; female α-PVP: 12.7). These findings are consistent with past studies that showed no sex differences in acquisition of 4MMC self-administration [28, 33]. Interestingly, comparison of the present results to those of a past study show that male rats self-administered more saline than female rats (average infusions/session male: 7.2; female: 4.5) [32]. A few prior studies found no sex differences in saline self-administration; however, these studies used sessions that were 6 hrs in length or longer [56, 57], or used a social context during self-administration [58], which complicates comparison to the present study.
Synthetic cathinone administration, and duration of exposure to experimental conditions produced several effects on neurotransmitters in the present study. Compared to saline, effects of α-PVP were primarily localized to hypothalamus where α-PVP increased 5-HT, 5-HIAA, NE, and GLU. In contrast, 4MMC decreased 5-HT and 5-HIAA in several brain regions compared to saline. Longer durations of exposure to both synthetic cathinones and saline increased 5-HIAA, NE, and GLU in multiple brain regions compared to AO groups.
Interestingly, duration of exposure to experimental conditions altered several neurotransmitter levels, and these alterations were not merely a result of drug effects because they were also observed for saline groups (Figure 3). In fact, all instances of effects of duration for synthetic cathinone groups were also observed for the saline group except one (NE in striatum for α-PVP; Figure 3, Table 1). When compared to the respective AO groups, a longer duration of exposure to the present experimental procedures (SA groups) increased GLU in PFC for females, but decreased GLU in PFC for males [32]. Similarly, GLU in thalamus, and NE in amygdala and PFC increased following a longer duration of exposure to drug or saline self-administration for females, but there were no systematic effects of duration on these neurotransmitter levels for males [32].
There also appear to be general sex differences in neurotransmitter levels that were not attributable to duration of exposure or drug. These differences were most notable for NE in amygdala, which was higher for males (group means of 0.88-1.08 ng/ml) than females (group means of 0.57-0.71 ng/ml). In contrast, NE was lower for males than females in hypothalamus, hippocampus, and PFC for most experimental groups. DOPAC was also lower in striatum for males than females for most groups [32].
There are several potential explanations for the effects of duration that were observed across synthetic cathinone groups and saline groups. Learning may be responsible for the effects of duration, since even saline SA rats learned that responding on the active lever illuminated the stimulus light. Another possibility is that the noncontingent drug/saline exposure during AO vs contingent drug/saline exposure during SA produced the effects of duration. Although the duration of contingent and noncontingent exposure were not matched in the present study, this theory is supported by previous research showing that the effects of drugs of abuse on neurotransmitters may differ depending upon whether they are administered contingently or noncontingently [59, 60]. Food restriction may have also played a role in the neurochemical effects because food restriction is known to interact with neurotransmitter levels [61], and SA groups experienced a longer duration of food restriction than AO groups. Future research should confirm if these neurotransmitter changes between AO and SA groups are due to learning, contingent vs. noncontingent drug/saline exposure, cumulative drug exposure, cumulative food restriction, aging, or simply the passage of time. The present study used adult rats, and only 3 weeks of time passed between when AO and SA brain samples were collected. Furthermore, several effects of duration occurred for drug groups and saline groups (see NE and GLU in amygdala and PFC in Figure 3). Therefore, learning may be the most parsimonious explanation for the effects of duration on neurotransmitters.
With respect to effects of synthetic cathinones on neurotransmitters, two prior studies examining 4MMC self-administration in male rats found that 4MMC had no effect on DA or 5-HT in male striatum [30, 32], whereas 4MMC decreased DA and 5-HT in female striatum in the present study. In contrast, 4MMC had no effect on HVA or DOPAC in striatum for males or females [30, 32]. The present 4MMC results are also partially consistent with results from methamphetamine self-administration in male rats, which produced no effect on DA, HVA, DOPAC, and 5-HT [30]. These differences across studies are most likely due to sex differences in effects of 4MMC self-administration on neurotransmitter levels, especially given that one past study in male rats used identical procedures as those used in the present study [32].
Although both α-PVP and 4MMC have potent functional effects on DA [10, 12, 13, 62, 63], these synthetic cathinones produced minimal dopaminergic effects on neurotransmitter levels in the present study, consistent with a previous study using the same procedures in male rats [32]. Compared to saline, α-PVP elevated DOPAC in amygdala in the present study, while 4MMC blunted DA in striatum (Table 2). The minimal dopaminergic effects observed in the present study may be due to brain samples being collected approximately 24 hrs after the last self-administration session, which was done to avoid interference from direct drug effects. Therefore, the α-PVP- and 4MMC-induced increases in DA seen in previous studies [10, 12, 42, 64] may only be observable during the duration of the direct drug effects.
Compared to saline groups, the most prominent effects of α-PVP exposure were broad spectrum increases in neurotransmitter levels in hypothalamus, albeit without dopaminergic effects (Table 2). Furthermore, 4MMC decreased 5-HT and 5-HIAA in several brain regions compared to saline groups (Table 2), which aligns with its action as a relatively potent 5-HT releaser [10]. In contrast, α-PVP does not have functional serotoninergic effects [12]. Thus, it was surprising to find that it increased 5-HT and 5-HIAA levels in amygdala and hypothalamus, respectively. It is unclear if these unexpected serotonergic effects of α-PVP are a result of sex differences in effects of α-PVP on 5-HT transport or basal 5-HT levels since past neurochemical research on α-PVP was conducted in male rodents [12, 42, 65], or if the effects are a result of α-PVP binding to 5-HT1A [11]. The correlation between effects of 5-HT and 5-HIAA, which was most evident in hippocampus, hypothalamus, and striatum, suggests that the drug- and duration-induced serotonergic differences might be related to 5-HT metabolism. In contrast, the lack of correlation between effects of DA and its metabolites, DOPAC and HVA, suggests that the dopaminergic differences may reflect changes in DA synthesis.
One limitation of the present study is that only one dose of each synthetic cathinone was examined. Although it is difficult to compare the potency of α-PVP and 4MMC since one blocks DA and the other releases DA, the doses of α-PVP and 4MMC used in this study produced different levels of responding (Figure 2). Thus, it is possible that dose, and the functional potency of the dose, plays a role in the neurochemical effects of synthetic cathinones.
One primary purpose of the present study was to extend our knowledge about the chronological changes caused by synthetic cathinone abuse, and examine if stimulant-induced neurochemical changes occur simultaneously or in stages [cf. 44, 46, 47], and if the same changes occur for males and females. Some effects of synthetic cathinones in the present study were attributable to the drug alone (Table 2); however, several neurochemical effects were dependent on duration of exposure. This interaction between duration of exposure and drug effects was most evident for 5-HIAA. Seven days of exposure to α-PVP increased 5-HIAA in hypothalamus compared to saline, an effect that was not observed following self-administration. In contrast, longer α-PVP self-administration increased 5-HT in hypothalamus, and increased NE in striatum. There were no effects of 4MMC on neurotransmitters that were isolated to AO groups, but self-administration of 4MMC altered 5-HIAA in hypothalamus, PFC, and striatum compared to saline. Similar to what was observed in male rats [32], the divergent effects on neurotransmitters across brain regions show that duration of synthetic cathinone exposure impacts the same neurotransmitter differently across brain regions.
Some of the neurotransmitter changes observed in specific brain regions in the present study corroborate past theories of the trajectory of neurochemical changes during stimulant abuse. Effects of 4MMC on DA in striatum aligns with the binge/intoxication stage of abuse. Meanwhile, the α-PVP-induced increase in NE in hypothalamus and the 4MMC-induced decrease in 5-HT in amygdala aligns with the negative reinforcement/withdrawal stage of abuse [44, 46]. It was unexpected that neurochemical changes indicative of negative reinforcement appeared in this study, particularly because indicators of negative reinforcement were present for both AO and SA groups, and drug exposure was confined to no more than a total of 28 days during 1 hr sessions. Although the other effects of synthetic cathinones on neurotransmitters in specific brain regions observed in the present study do not completely align with previous theories on the stages of drug abuse, the changes seen here nevertheless suggest that synthetic cathinone use and abuse likely produces some sequential neurochemical changes. Furthermore, neurochemical data from the SA groups suggests that 21 days of self-administration during 1 hr sessions might only model the initial stages of long-term drug effects, as evidenced by striatal disruption of neurotransmitter levels. Longer self-administration sessions (e.g. 6 hrs) or more than 21 self-administration sessions may be needed to model the additional stages of drug abuse.
5.0. Conclusion
In summary, female rats acquired self-administration of α-PVP and 4MMC. Both synthetic cathinones produced regional changes in neurotransmitter levels, particularly in levels of 5-HIAA. Longer durations of exposure to synthetic cathinones also elevated neurotransmitter levels, most notably in amygdala, hypothalamus, and PFC, suggesting that learning may produce changes in addiction-related brain regions. The mechanism of action of synthetic cathinones may play a minor role in changes in neurotransmitter levels in the brain of female rats, as evidenced by the differential effects on 5-HT. Notably, both α-PVP and 4MMC produced minimal changes in DA and DA metabolite levels when measured one day after the last self-administration session, suggesting that the dopaminergic effects of these synthetic cathinones are transient. Lastly, the alterations in neurotransmitter levels observed in the present study indicate that synthetic cathinone use may produce differential neurochemical changes during the transition from use to abuse.
Acknowledgements
Declarations of interest: none. The authors thank Daniel Barrus, Ricardo Cortes, Kimberly Custer, Tony Landavazo, Timothy Lefever, Nikita Pulley, Shanequa Taylor, and Jenny Wiley for technical assistance. Funding: This research was supported by National Institute of Health [grant numbers DA039315 and DA012970]. The funding source had no other role other than financial support.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Madras BK, The Growing Problem of New Psychoactive Substances (NPS), in: Baumann MH, Glennon RA, Wiley JL (Eds.), Neuropharmacology of New Psychoactive Substances (NPS), Springer, Cham, Switzerland, 2017, pp. 1–18. [DOI] [PubMed] [Google Scholar]
- [2].Baumann MH, Awash in a sea of ‘bath salts’: implications for biomedical research and public health, Addiction 109 (2014) 1577–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Baumann MH, Glennon RA, Wiley JL, Neuropharmacology of new psychoactive substances (NPS): The science behind the headlines, Springer, Cham, Switzerland, 2017. [Google Scholar]
- [4].Palamar JJ, Rutherford C, Keyes KM, “Flakka” use among high school seniors in the United States, Drug Alcohol Depend 196 (2019) 86–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].NDEWS, National Drug Early Warning System (NDEWS): Southeastern Florida (Miami Area) Sentinel Community Site (SCS) Drug Use Patterns and Trends, 2018, National Drug Early Warning System, 2018.
- [6].Miech RA, Schulenberg JE, Johnston LD, Bachman JG, O’Malley PM, Patrick ME, National Adolescent Drug Trends in 2018 , Monitoring the Future, Ann Arbor, MI, 2018. [Google Scholar]
- [7].NDEWS, National Drug Early Warning System (NDEWS): Texas Sentinel Community Site (SCS) Drug Use Patterns and Trends, 2018, National Drug Early Warning System, 2018.
- [8].Simmler LD, Liechti ME, Interactions of Cathinone NPS with Human Transporters and Receptors in Transfected Cells, in: Baumann MH, Glennon RA, Wiley JL (Eds.), Neuropharmacology of New Psychoactive Substances (NPS), Springer, Cham, Switzerland, 2017, pp. 49–72. [DOI] [PubMed] [Google Scholar]
- [9].Simmons SJ, Leyrer-Jackson JM, Oliver CF, Hicks C, Muschamp JW, Rawls SM, Olive MF, DARK Classics in Chemical Neuroscience: Cathinone-Derived Psychostimulants, ACS Chem Neurosci 9 (2018) 2379–2394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Baumann MH, Ayestas MA Jr., Partilla JS, Sink JR, Shulgin AT, Daley PF, Brandt SD, Rothman RB, Ruoho AE, Cozzi NV, The designer methcathinone analogs, mephedrone and methylone, are substrates for monoamine transporters in brain tissue, Neuropsychopharmacology 37 (2012) 1192–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Rickli A, Hoener MC, Liechti ME, Monoamine transporter and receptor interaction profiles of novel psychoactive substances: para-halogenated amphetamines and pyrovalerone cathinones, Eur Neuropsychopharmacol 25 (2015) 365–76. [DOI] [PubMed] [Google Scholar]
- [12].Marusich JA, Antonazzo KR, Wiley JL, Blough BE, Partilla JS, Baumann MH, Pharmacology of novel synthetic stimulants structurally related to the “bath salts” constituent 3,4-methylenedioxypyrovalerone (MDPV), Neuropharmacology 87 (2014) 206–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Glennon RA, Young R, Neurobiology of 3,4-methylenedioxypyrovalerone (MDPV) and alpha-pyrrolidinovalerophenone (alpha-PVP), Brain Res Bull 126 (2016) 111–126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Hataoka K, Kaizaki-Mitsumoto A, Numazawa S, Alpha-PVP induces the rewarding effect via activating dopaminergic neuron, J Toxicol Sci 42 (2017) 539–543. [DOI] [PubMed] [Google Scholar]
- [15].Stogner JM, Miller BL, Investigating the ‘bath salt’ panic: the rarity of synthetic cathinone use among students in the United States, Drug Alcohol Rev 32 (2013) 545–9. [DOI] [PubMed] [Google Scholar]
- [16].Froberg BA, Levine M, Beuhler MC, Judge BS, Moore PW, Engebretsen KM, McKeown NJ, Rosenbaum CD, Young AC, Rusyniak DE, Acute Methylenedioxypyrovalerone Toxicity, J Med Toxicol 11 (2015) 185–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Johnson PS, Johnson MW, Investigation of “bath salts” use patterns within an online sample of users in the United States, J Psychoactive Drugs 46 (2014) 369–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Wood KE, Exposure to bath salts and synthetic tetrahydrocannabinol from 2009 to 2012 in the United States, J Pediatr 163 (2013) 213–6. [DOI] [PubMed] [Google Scholar]
- [19].Forrester MB, Synthetic cathinone exposures reported to Texas poison centers, Am J Drug Alcohol Abuse 38 (2012) 609–15. [DOI] [PubMed] [Google Scholar]
- [20].Roth ME, Cosgrove KP, Carroll ME, Sex differences in the vulnerability to drug abuse: a review of preclinical studies, Neurosci Biobehav Rev 28 (2004) 533–46. [DOI] [PubMed] [Google Scholar]
- [21].Lynch WJ, Carroll ME, Sex differences in the acquisition of intravenously self-administered cocaine and heroin in rats, Psychopharmacology (Berl) 144 (1999) 77–82. [DOI] [PubMed] [Google Scholar]
- [22].Roth ME, Carroll ME, Sex differences in the acquisition of IV methamphetamine self-administration and subsequent maintenance under a progressive ratio schedule in rats, Psychopharmacology (Berl) 172 (2004) 443–9. [DOI] [PubMed] [Google Scholar]
- [23].Roberts DC, Bennett SA, Vickers GJ, The estrous cycle affects cocaine self-administration on a progressive ratio schedule in rats, Psychopharmacology (Berl) 98 (1989) 408–11. [DOI] [PubMed] [Google Scholar]
- [24].Carroll ME, Morgan AD, Lynch WJ, Campbell UC, Dess NK, Intravenous cocaine and heroin self-administration in rats selectively bred for differential saccharin intake: phenotype and sex differences, Psychopharmacology (Berl) 161 (2002) 304–13. [DOI] [PubMed] [Google Scholar]
- [25].Lisek R, Xu W, Yuvasheva E, Chiu YT, Reitz AB, Liu-Chen LY, Rawls SM, Mephedrone (‘bath salt’) elicits conditioned place preference and dopamine-sensitive motor activation, Drug Alcohol Depend 126 (2012) 257–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Berquist MD 2nd, Traxler HK, Mahler AM, Baker LE, Sensitization to the locomotor stimulant effects of “bath salt” constituents, 4-methylmethcathinone (4-MMC) and 3,4-methylenedioxypyrovalerone (MDPV), in male Sprague-Dawley rats, Drug Alcohol Depend 164 (2016) 128–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Berquist MD 2nd, Peet MM, Baker LE, Behavioral sensitization following concurrent exposure to mephedrone and D-amphetamine in female mice, Behav Pharmacol 26 (2015) 180–3. [DOI] [PubMed] [Google Scholar]
- [28].Creehan KM, Vandewater SA, Taffe MA, Intravenous self-administration of mephedrone, methylone and MDMA in female rats, Neuropharmacology 92 (2015) 90–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Aarde SM, Angrish D, Barlow DJ, Wright MJ Jr., Vandewater SA, Creehan KM, Houseknecht KL, Dickerson TJ, Taffe MA, Mephedrone (4-methylmethcathinone) supports intravenous self-administration in Sprague-Dawley and Wistar rats, Addict Biol 18 (2013) 786–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Motbey CP, Clemens KJ, Apetz N, Winstock AR, Ramsey J, Li KM, Wyatt N, Callaghan PD, Bowen MT, Cornish JL, McGregor IS, High levels of intravenous mephedrone (4-methylmethcathinone) self-administration in rats: neural consequences and comparison with methamphetamine, J Psychopharmacol 27 (2013) 823–36. [DOI] [PubMed] [Google Scholar]
- [31].Nguyen JD, Grant Y, Creehan KM, Vandewater SA, Taffe MA, Escalation of intravenous self-administration of methylone and mephedrone under extended access conditions, Addict Biol (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Marusich JA, Gay EA, Blough BE, Analysis of neurotransmitter levels in addiction-related brain regions during synthetic cathinone self-administration in male Sprague-Dawley rats, Psychopharmacology (Berl) 236 (2019) 903–914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Vandewater SA, Creehan KM, Taffe MA, Intravenous self-administration of entactogen-class stimulants in male rats, Neuropharmacology 99 (2015) 538–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Nguyen JD, Bremer PT, Ducime A, Creehan KM, Kisby BR, Taffe MA, Janda KD, Active vaccination attenuates the psychostimulant effects of alpha-PVP and MDPV in rats, Neuropharmacology 116 (2017) 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Aarde SM, Creehan KM, Vandewater SA, Dickerson TJ, Taffe MA, In vivo potency and efficacy of the novel cathinone alpha-pyrrolidinopentiophenone and 3,4-methylenedioxypyrovalerone: self-administration and locomotor stimulation in male rats, Psychopharmacology (Berl) 232 (2015) 3045–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Gannon BM, Galindo KI, Mesmin MP, Sulima A, Rice KC, Collins GT, Relative reinforcing effects of second-generation synthetic cathinones: Acquisition of self-administration and fixed ratio dose-response curves in rats, Neuropharmacology 134 (2018) 28–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Gannon BM, Rice KC, Collins GT, Reinforcing effects of abused ‘bath salts’ constituents 3,4-methylenedioxypyrovalerone and alpha-pyrrolidinopentiophenone and their enantiomers, Behav Pharmacol 28 (2017) 578–581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Javadi-Paydar M, Harvey EL, Grant Y, Vandewater SA, Creehan KM, Nguyen JD, Dickerson TJ, Taffe MA, Binge-like Acquisition of α-pyrrolidinopentiophenone (α-PVP) Self-Administration in Female Rats, Psychopharmacology (Berl) [Epub ahead of print] (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Javadi-Paydar M, Nguyen JD, Vandewater SA, Dickerson TJ, Taffe MA, Locomotor and reinforcing effects of pentedrone, pentylone and methylone in rats, Neuropharmacology 134 (2018) 57–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Marusich JA, Lefever TW, Blough BE, Thomas BF, Wiley JL, Pharmacological effects of methamphetamine and alpha-PVP vapor and injection, Neurotoxicology 55 (2016) 83–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].King HE, Wakeford A, Taylor W, Wetzell B, Rice KC, Riley AL, Sex differences in 3,4-methylenedioxypyrovalerone (MDPV)-induced taste avoidance and place preferences, Pharmacol Biochem Behav 137 (2015) 16–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Baumann MH, Bukhari MO, Lehner KR, Anizan S, Rice KC, Concheiro M, Huestis MA, Neuropharmacology of 3,4-Methylenedioxypyrovalerone (MDPV), Its Metabolites, and Related Analogs, Curr Top Behav Neurosci 32 (2017) 93–117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Lopez-Rodriguez AB, Viveros MP, Bath salts and polyconsumption: in search of drug-drug interactions, Psychopharmacology (Berl) (2019). [DOI] [PubMed] [Google Scholar]
- [44].Koob GF, Volkow ND, Neurocircuitry of addiction, Neuropsychopharmacology 35 (2010) 217–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Becker JB, Koob GF, Sex Differences in Animal Models: Focus on Addiction, Pharmacol Rev 68 (2016) 242–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Koob GF, Le Moal M, Addiction and the brain antireward system, Annu Rev Psychol 59 (2008) 29–53. [DOI] [PubMed] [Google Scholar]
- [47].Koob GF, Le Moal M, Plasticity of reward neurocircuitry and the ‘dark side’ of drug addiction, Nat Neurosci 8 (2005) 1442–1444. [DOI] [PubMed] [Google Scholar]
- [48].National Research Council, Guide for the Care and Use of Laboratory Animals, 8th ed., National Academies Press; (US), Washington, D.C., 2011. [Google Scholar]
- [49].Carroll ME, Lac ST, Autoshaping i.v. cocaine self-administration in rats: effects of nondrug alternative reinforcers on acquisition, Psychopharmacology (Berl) 110 (1993) 5–12. [DOI] [PubMed] [Google Scholar]
- [50].Marusich JA, Beckmann JS, Gipson CD, Bardo MT, Methylphenidate as a reinforcer for rats: contingent delivery and intake escalation, Exp Clin Psychopharmacol 18 (2010) 257–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Koob GF, Drug addiction, The Corsini Encyclopedia of Psychology (2010) 1–4. [Google Scholar]
- [52].Wee S, Wang Z, Woolverton WL, Pulvirenti L, Koob GF, Effect of aripiprazole, a partial dopamine D2 receptor agonist, on increased rate of methamphetamine self-administration in rats with prolonged session duration, Neuropsychopharmacology 32 (2007) 2238–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Spijker S, Dissection of Rodent Brain Regions, in: Li KW (Ed.), Neuroproteomics, Humana Press, Totowa, NJ, 2011, pp. 13–26. [Google Scholar]
- [54].Chiu K, Lau WM, Lau HT, So KF, Chang RC, Micro-dissection of rat brain for RNA or protein extraction from specific brain region, Journal of Visualized Experiments (2007) 269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Honkanen A, Modulation of Brain Dopaminergic Neurotransmission in Alcohol-Preferring Rats by Alcohol and Opioids, Department of Pharmacy, University of Helsinki, 1999. [Google Scholar]
- [56].Johansen A, McFadden LM, The neurochemical consequences of methamphetamine self-administration in male and female rats, Drug Alcohol Depend 178 (2017) 70–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Sanchez V, Moore CF, Brunzell DH, Lynch WJ, Sex differences in the effect of wheel running on subsequent nicotine-seeking in a rat adolescent-onset self-administration model, Psychopharmacology (Berl) 231 (2014) 1753–1762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Peartree NA, Hatch KN, Goenaga JG, Dado NR, Molla H, Dufwenberg MA, Campagna A, Mendoza R, Cheung THC, Talboom JS, Neisewander JL, Social context has differential effects on acquisition of nicotine self-administration in male and female rats, Psychopharmacology (Berl) 234 (2017) 1815–1828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Orejarena MJ, Berrendero F, Maldonado R, Robledo P, Differential changes in mesolimbic dopamine following contingent and non-contingent MDMA self-administration in mice, Psychopharmacology (Berl) 205 (2009) 457–66. [DOI] [PubMed] [Google Scholar]
- [60].Lecca D, Cacciapaglia F, Valentini V, Acquas E, Di Chiara G, Differential neurochemical and behavioral adaptation to cocaine after response contingent and noncontingent exposure in the rat, Psychopharmacology (Berl) 191 (2007) 653–67. [DOI] [PubMed] [Google Scholar]
- [61].Pothos EN, Creese I, Hoebel BG, Restricted eating with weight loss selectively decreases extracellular dopamine in the nucleus accumbens and alters dopamine response to amphetamine, morphine, and food intake, J Neurosci 15 (1995) 6640–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Cameron K, Kolanos R, Vekariya R, De Felice L, Glennon RA, Mephedrone and methylenedioxypyrovalerone (MDPV), major constituents of “bath salts,” produce opposite effects at the human dopamine transporter, Psychopharmacology (Berl) 227 (2013) 493–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Simmler LD, Buser TA, Donzelli M, Schramm Y, Dieu LH, Huwyler J, Chaboz S, Hoener MC, Liechti ME, Pharmacological characterization of designer cathinones in vitro, Br J Pharmacol 168 (2013) 458–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Kehr J, Ichinose F, Yoshitake S, Goiny M, Sievertsson T, Nyberg F, Yoshitake T, Mephedrone, compared with MDMA (ecstasy) and amphetamine, rapidly increases both dopamine and 5-HT levels in nucleus accumbens of awake rats, Br J Pharmacol 164 (2011) 1949–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Kolanos R, Sakloth F, Jain AD, Partilla JS, Baumann MH, Glennon RA, Structural Modification of the Designer Stimulant α-Pyrrolidinovalerophenone (α-PVP) Influences Potency at Dopamine Transporters, ACS Chem Neurosci 6 (2015) 1726–1731. [DOI] [PMC free article] [PubMed] [Google Scholar]