Summary
We previously identified a unique population of interstitial muscle progenitors, marked by expression of the Twist2 transcription factor, which fuse specifically to type IIb/x fast-twitch myofibers. Tw2+ progenitors are distinct from satellite cells, a muscle progenitor that expresses Pax7 and contributes to all myofiber types. Through RNA-sequencing and immunofluorescence, we identify the membrane receptor, Nrp1, as a marker of Tw2+ cells but not Pax7+ cells. We also found that Sema3a, a chemorepellent ligand for Nrp1, is expressed by type I and IIa myofibers, but not IIb myofibers. Using stripe migration assays, chimeric cell-cell fusion assays, and a Sema3a transgenic mouse model, we identify Sema3a-Nrp1 signaling as a major mechanism for Tw2+ cell fiber-type specificity. Our findings reveal an extracellular signaling mechanism whereby a cell surface receptor for a chemorepellent confers specificity of intercellular fusion of a specific muscle progenitor with its target tissue.
Graphical Abstract
In Brief
Tw2+ cells are a interstitial muscle progenitor population that fuses specifically with fast-twitch myofibers. Li et al. show that the mechanism of fiber-type specificity revolves around a chemorepulsion system mediated by Sema3a and Nrp1 signaling.
Introduction
Skeletal muscles display a range of metabolic and contractile phenotypes that enable the diversity of muscle functions required for life. Slow-twitch or type I myofibers exhibit oxidative metabolism and are fatigue-resistant, whereas fast-twitch or type II myofibers are more glycolytic, display rapid bursts of contraction, and fatigue rapidly (Schiaffino and Reggiani, 2011). Myofibers can be further classified as type I, IIa, IIx/d or IIb depending on the myosin isoforms they express (Schiaffino and Reggiani, 2011). Fiber type specificity is initially established during fetal life, but can be modulated after birth by activity, hormonal influences and disease (Schiaffino and Reggiani, 2011).
In response to injury and aging, skeletal muscle is among the most regenerative tissues, owing to the presence of satellite cells, a resident stem cell population that resides beneath the muscle basal lamina (Lepper et al., 2009; Sambasivan et al., 2011). Satellite cells contribute to all myofiber types without fiber type specificity (Liu et al., 2017; Pawlikowski et al., 2015). Recently, through lineage tracing of cells expressing the basic-Helix-Loop-Helix (bHLH) transcription factor Twist2, we discovered a distinct skeletal muscle progenitor (Liu et al., 2017). Tw2+ progenitors reside in the muscle interstitium and are anatomically and transcriptionally distinct from Pax7+ satellite cells, which reside beneath the muscle basal lamina (Liu et al., 2017). Moreover, in contrast to satellite cells, Tw2+ cells fuse only with type IIb/x myofibers in vivo and genetic ablation of Tw2+ cells in mice results in specific atrophy of type IIb myofibers (Liu et al., 2017). Tw2+ cells represent the only known fiber-type specific muscle progenitor (Liu et al., 2017). Twist2 expression maintains these cells in an undifferentiated, mesenchymal state and, when cultured in vitro, Tw2+ cells rapidly down-regulate Twist2 expression and differentiate into multi-nucleated myotubes (Liu et al., 2017). Ectopic expression of Twist2 globally represses the myogenic program and activates genes involved in cellular migration and matrix degradation (Liu et al., 2017). The findings highlight the unique roles of Twist2 in regulating myogenesis and tissue invasiveness. The invasiveness of Tw2+ cells is further highlighted by our recent discovery that elevated Twist2 expression marks rhabdomyosarcoma, a highly invasive tumor type of muscle origin (Li et al., 2019).
In this study, we investigated the mechanistic basis of the specificity of Tw2+ progenitors in the formation of type IIb myofibers. Through RNA-Sequencing and ChIP-sequencing, we identified the Neuropilin1 (Nrp1) gene as a direct transcriptional target gene of Twist2 and its expression is enriched in Tw2+ cells compared to Pax7+ satellite cells. Neuropilins act as cell surface receptors for Semaphorins, a class of membrane-bound and secreted proteins that regulate cellular migration, differentiation, and function (Raper, 2000; Sharma et al., 2012). There are 5 classes of semaphorins: classes 4–7 are membrane-bound, while class 3 semaphorins are secreted. Semaphorin3a (Sema3a) belongs to the class 3 semaphorin family and has well-known roles in cancer where it regulates angiogenesis by inhibiting integrin activity (Alto and Terman, 2017; Raper, 2000; Sharma et al., 2012; Tamagnone and Comoglio, 2000). The interaction of Sema3a with Nrp1 and a Plexin co-receptor triggers a G-protein coupled cascade, resulting in activation of cytoskeletal modifying proteins such as Rac and collapsin response mediator proteins (CRMP) (Koncina et al., 2007; Nasarre et al., 2014). This results in destabilization and depolymerization of microtubule networks and chemorepulsion from the Sema3a source (Kumanogoh and Kikutani, 2013). We show that Sema3a is expressed only on type I and IIa, but not type IIb myofibers. In vitro assays reveal selective avoidance of Sema3a by Tw2-derived myoblasts (Tw2-MB) but not Pax7-derived myoblasts (Pax7-MB), which is due to the differential expression of Nrp1. Employing a chimeric fusion assay, we show that the Sema3a/Nrp1 interaction is sufficient to prevent cell-cell fusion between cells expressing the Sema3a ligand and the Nrp1 receptor, respectively. Finally, over-expression of Sema3a in type II myofibers in mice is sufficient to prevent Tw2+ cells from fusing to type IIb myofibers in vivo. These findings uncover an intercellular signaling mechanism whereby Twist2 controls expression of the Nrp1 receptor in Tw2+ cells, which confers myofiber specificity of Tw2+ cell fusion by sensing the presence or absence of its ligand Sema3a.
Results
Nrp1 is enriched in Tw2+ cells and is a Twist2 target gene.
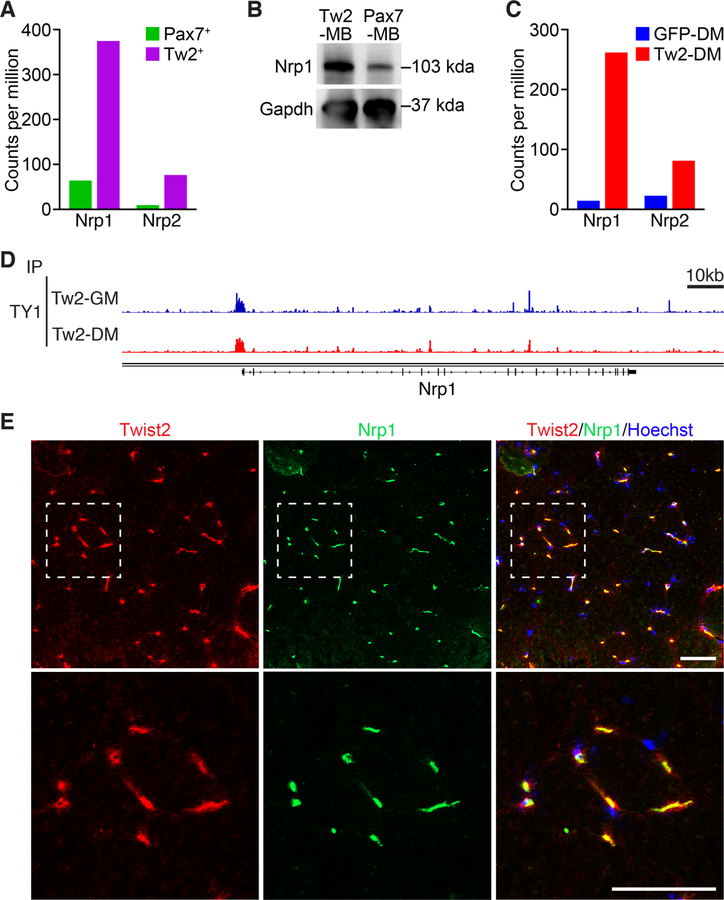
To explore the mechanism underlying the fiber-type specificity of Tw2+ cells, we compared gene expression profiles of Tw2+ and Pax7+ cells freshly isolated from adult mouse skeletal muscle (Liu et al., 2017). Among various genes that were differentially expressed between these two cell populations, we found that Nrp1 mRNA was highly enriched in Tw2+ cells compared to Pax7+ satellite cells (Figure 1A). Analysis of single-cell RNA sequencing data from the Tabula Muris Consortium also revealed that Nrp1 was expressed by Tw2+ cells but not by satellite cells (Figure S1A and B) (Tabula Muris Consortium et al., 2018). We also confirmed by western blot that NRP1 protein was enriched in Tw2-derived myoblasts (Tw2-MB) compared to Pax7-derived myoblasts (Pax7-MB) (Figure 1B).
Figure 1. Nrp1 is expressed in Tw2+ cells and is a target of Twist2.
(A) Levels of Nrp1 and Nrp2 mRNA expression in freshly isolated Tw2+ cells vs Pax7+ cells, as determined by RNA-seq. (B) Western blot of NRP1 protein levels in cultured Tw2-derived myoblasts (Tw2-MB) and Pax7-derived myoblasts (Pax7-MB). GAPDH serves as a loading control. (C) Levels of Nrp1 and Nrp2 mRNA expression in Twist2-overexpressing Tw2-MB (Tw2-DM) and GFP-infected Tw2-MB (GFP-DM) after 4 days in differentiation medium, as determined by RNA-seq. (D) Genome browser shot of Twist2 binding at the Nrp1 promoter in both growth media (GM) and differentiation media (DM). (E) (Top panel) Co-immunostaining of Nrp1 (green), Twist2 (red), and Hoechst (blue) in adult mouse transverse section of quadriceps muscle. (Bottom panel) Zoomed in image of top panel. Scale: 50 µm. See also Figure S1
Because Twist2 expression is rapidly extinguished when Tw2+ cells are isolated and cultured in vitro, we over-expressed Twist2 in Tw2-MB with a retroviral expression vector. Overexpression of Twist2 in Tw2-MB resulted in upregulation of Nrp1 expression, suggesting that Nrp1 may be a transcriptional target of Twist2 (Fig. 1C). Consistent with this notion, ChIP-Seq analysis of Twist2 binding in Tw2-MB showed enrichment at the Nrp1 promoter in both growth media (GM) and differentiation media (DM) (Figure 1D). The Nrp1 family member Nrp2 is also highly enriched in Tw2-MB and upregulated upon Twist2-overexpression (Figures 1A and C).
To determine the localization of Nrp1-expressing cells within muscle tissue, we performed immunofluorescent staining of mouse gastrocnemius muscle with an anti-Nrp1 antibody. Co-staining of Nrp1 with wheat germ agglutinin (WGA) revealed that the majority of Nrp1-expressing cells are interstitial cells (Figure S1C). This is similar to the location of Tw2+ cells, but not that of Pax7+ cells, which are located beneath the muscle basal lamina. Co-staining of Nrp1 and Twist2 revealed near complete co-localization (Figure 1E). Together, these results show that Nrp1 is preferentially expressed in Tw2+ cells but not in Pax7+ cells, likely due to direct regulation by Twist2.
Sema3a is enriched in Type I and IIa myofibers.
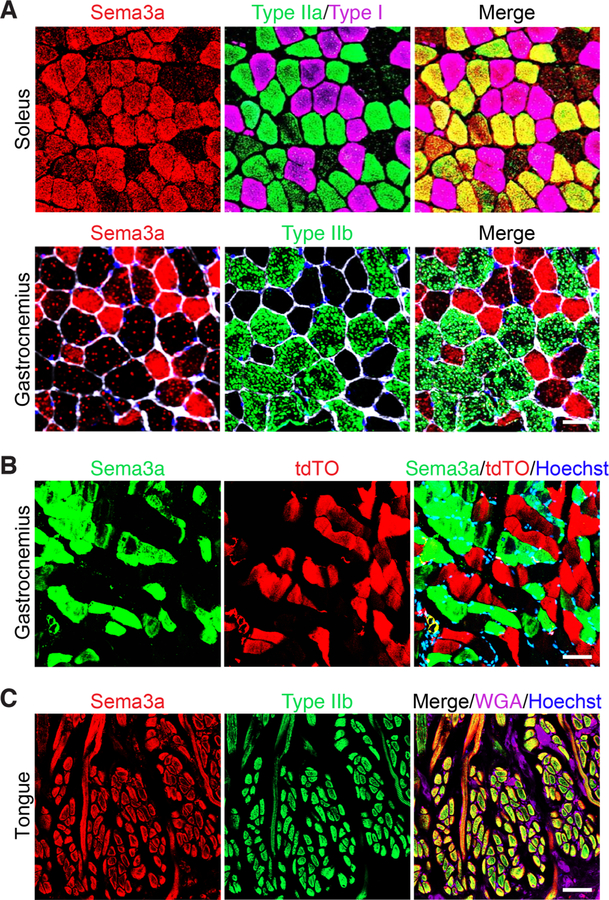
Given that Tw2+ cells are enriched for Nrp1, we sought to determine where its chemorepellent ligand, Sema3a, was expressed in muscle. Co-immunostaining for Sema3a and markers of various muscle fiber types in soleus and gastrocnemius muscles, which are comprised predominantly of slow and mixed myofiber types, respectively, revealed Sema3a expression exclusively on type I and IIa myofibers, but not type IIb myofibers (Figure 2A). Additionally, Sema3a expression and tdTO expression labeled by Twist2-lineage tracing were mutually exclusive in gastrocnemius muscle, with no co-localization (Figure 2B). These findings suggest that Nrp1-expressing Tw2+ cells are repelled from Sema3a-expressing type I and IIa fibers. Interestingly, Tw2+ progenitors do not fuse to tongue muscle, despite the presence of type IIb fibers (Liu et al., 2017). Co-staining of type IIb or fast myosin (My32) with Sema3a in the tongue revealed that type IIb fibers of tongue muscle express Sema3a, which may explain the lack of Tw2+ cell fusion (Figure 2C and S2). Together, these results suggest that the Nrp1 and Sema3a signaling axis may confer type IIb fiber-type specificity to Tw2+ cells.
Figure 2. Sema3a is expressed by type I and IIa myofibers but not type IIb myofibers.
(A) (Top) Transverse sections of soleus muscle from 3-month-old C57Bl6 mice were co-immunostained for Sema3a (red), type IIa myosin (green), and type I myosin (magenta). (Bottom) Transverse sections of gastrocnemius muscles were co-immunostained for Sema3a (red), Type IIb myosin (green), wheat-germ agglutinin (white), and Hoechst (blue). Scale bar: 50 µm. (B) Transverse gastrocnemius muscle sections of Tw2-CreERT2; R26-tdTO mice at 4 months post-TMX were fixed and immunostained for Sema3a (green). Sema3a staining was over-layed with tdTO signal from myofibers receiving Tw2+ cell contribution (red) and counterstained with Hoechst (blue). Scale bar: 50 µm. (C) Transverse sections of tongue muscle from 3-month-old C57Bl6 mice were co-immunostained for Sema3a (red), type IIb myosin (green), wheat-germ agglutinin (magenta), and Hoechst (blue). Scale: 50 µm. See also Figure S2
Tw2-MB and Pax7-MB differentially respond to Sema3a.
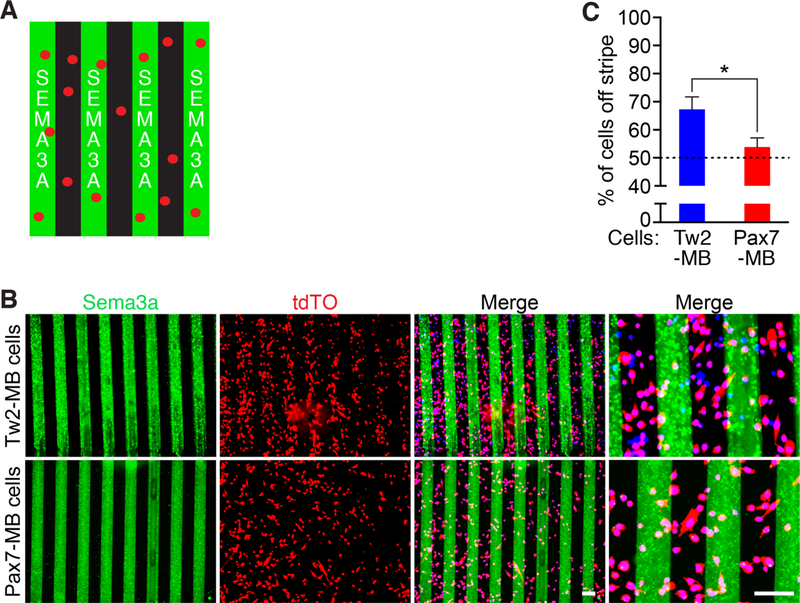
In order to validate that Nrp1 expression in Tw2+ cells was capable of mediating repulsion from Sema3a, we performed Sema3a stripe migration assays. We generated stripes of recombinant SEMA3A on tissue culture dishes and seeded various cell types onto the stripes overnight before fixing and imaging (Figure 3A)(Yamagishi et al., 2016). When we seeded Tw2-MB onto the stripes, we found robust avoidance of Sema3a stripes (Figure 3B and C). However, Pax7-MB showed no avoidance of Sema3a stripes, indicating that Tw2-MB and Pax7-MB differentially respond to Sema3a (Figure 3B and C). We also performed the stripe assay with other cell types that were reported not to express Nrp1, including C2C12 myoblasts, 10T1/2 fibroblasts, mouse embryonic fibroblasts (MEFs), and Cos-7 cells, and found no avoidance of these cells from Sema3a stripes (Figure S3A and B) (Soker et al., 1998; Takagi et al., 1995). These results suggest that Tw2+ cells are highly responsive to Sema3a-mediated chemorepulsion in both growth and differentiation conditions.
Figure 3. Tw2-MB and Pax7-MB are differentially responsive to Sema3a stripes.
(A) Schematic of Sema3a stripe assay. Sema3 stripes are shown in green and cells are shown in red. (B) (Top) Tw2-MB (red) were seeded onto Sema3a stripes (green) and analyzed one day after seeding. (Bottom) Pax7-MB (red) were seeded onto on Sema3a stripes (green) and analyzed one day after seeding. Cells were co-stained with Hoechst (blue). Scale bar: 100 µm. (C) Quantification of Sema3a avoidance as the percent of cells residing off the stripe over total cells in the field. The dashed line represents the baseline for cells unresponsive to Sema3a stripes. Three separate fields were quantified for each sample with a total of 3 samples per cell type. *: p < 0.05. (D) Tw2-MB (red) were differentiated 1-day after seeding onto Sema3a stripes (green). Cells were fixed after 4 days in DM and co-stained with Hoechst (blue). See also Figure S3. Source data for 3C is provided in Supplementary Table 1.
Nrp1 is necessary and sufficient for repulsion from Sema3a stripes.
In order to validate the role of Nrp1 in mediating cellular avoidance of Sema3a stripes, we altered levels of Nrp1 expression in Tw2-MB and Pax7-MB. Using a retroviral construct, we overexpressed Nrp1 in Tw2-MB and seeded those cells onto Sema3a stripes (Figure 4A and S4). We found that overexpression of Nrp1 in Tw2-MB resulted in an ~10% increase of Sema3a avoidance compared to control infected Tw2-MB (Figure 4A and B). When we overexpressed Nrp1 in Pax7-MB, we found that Pax7-MB acquired repulsiveness to the Sema3a stripes to a similar extent to Tw2-MB (Figure 4C and D). This suggests that Nrp1 expression is sufficient to drive the repulsion from Sema3a.
Figure 4. Nrp1 is sufficient to drive repulsion of Tw2-MB and Pax7-MB from Sema3a stripes.
(A) Overexpression of Nrp1 increases repulsion of Tw2-MB from Sema3a stripes. (Top) Control Tw2-MB (red) were seeded on Sema3a stripes (green) and analyzed 1-day after seeding. (Bottom) Nrp1-overexpressing Tw2-MB (red) were seeded on Sema3a stripes (green) and analyzed 1-day after seeding. Cells were co-stained with Hoechst (blue). Scale bar: 100 µm. (B) Quantification of Sema3a avoidance as the percent of cells residing off the stripe in Fig. 4A. The dashed line represents the baseline for cells unresponsive to Sema3a. Three separate fields were quantified for each sample with a total of 3 samples per cell type. *: p < 0.05. (C) Overexpression of Nrp1 resulted in repulsion of Pax7-MB from Sema3a stripes. (Top) Control-infected Pax7-MB (red) were seeded on Sema3a stripes (green) and analyzed 1 day after seeding. (Bottom) Nrp1-overexpresssing Pax7-MB (red) were seeded on Sema3a stripes (green). Cells were analyzed 1 day after seeding and co-stained with Hoechst (blue). Scale bar: 100 µm. (D) Quantification of Sema3a avoidance as the percent of cells residing off the stripe in Fig. 4C. The dashed line represents the baseline for cells unresponsive to Sema3a. Three separate fields were quantified for each sample with a total of 3 samples per cell type. **: p <0.005. See also Figure S4. Source data for 4B and 4C are provided in Supplementary Table 1.
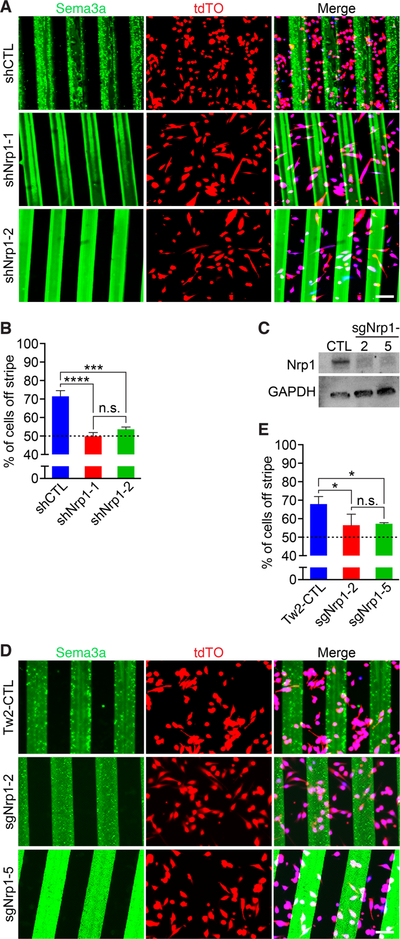
Next, we investigated whether Nrp1 was required for Tw2-MB to avoid Sema3a stripes by reducing Nrp1 expression through shRNA-mediated knockdown and CRISPR/Cas9-mediated knockout. We first tested knockdown of Nrp1 mRNA in Tw2-MB using 4 different retroviral shRNA constructs. Two candidates, shNrp1–1 and shNrp1–2, yielded the greatest degree of knockdown and were used for the stripe assay (Figure S5A). We infected Tw2-MB with retroviral vectors containing shNrp1–1, shNrp1–2, or a control shRNA and seeded them onto Sema3a stripes (Figure 5A). We found that knockdown of Nrp1 significantly decreased Sema3a avoidance, suggesting that Nrp1 is necessary for Sema3a avoidance in Tw2-MB (Figure 5A and B).
Figure 5. Nrp1 is necessary to drive repulsion of Tw2-MB from Sema3a stripes.
(A) Knockdown of Nrp1 by shRNA in Tw2-MB (red) abolished Sema3a avoidance. (Top) Control shRNA (shCtrl) infected Tw2-MB 1 day after seeding on Sema3a stripes (green). (Middle, Bottom) Tw2-MB overexpressing either shNrp1–1 or shNrp1–2 1-day after seeding on Sema3a stripes (green). Cells were co-stained with Hoechst (blue). Scale bar: 100 µm. (B) Quantification of Sema3a avoidance as the percent of cells residing off the stripe in Fig. 5A. The dashed line represents the baseline for cells unresponsive to Sema3a. Three separate fields were quantified for each sample with a total of 3 samples per cell type. ***: p < 0.0005, ****: p < 0.00005. (C) Western blot showing loss of NRP1 protein in Tw2-MB infected with sgRNAs targeting Nrp1. GAPDH was used as a loading control. (D) (Top) Control pLentiCrisprV2-infected Tw2-MB (red) 1 day after seeding on Sema3a stripes (green). (Middle, Bottom) Two separate Nrp1 sgRNA-infected Tw2-MB (sgNrp1–2 and sgNrp1–5) 1-day after seeding on Sema3a stripes (green). Cells were co-stained with Hoechst (blue). Scale bar: 100 µm. (E) Quantification of Sema3a avoidance as the percent of cells residing off the stripe in Fig. 5D. The dashed line represents the baseline for cells unresponsive to Sema3a. Three separate fields were quantified for each sample with a total of 3 samples per cell type. *: p < 0.05. See also Figure S5. Source data for 5B and E are provided in Supplementary Table 1.
In order to corroborate the knockdown data, we deleted the Nrp1 gene in Tw2-MB using CRISPR/Cas9. To target the Nrp1 gene, we used two short guide RNAs (sgRNAs) targeting exon 2 (Figure S5B). We packaged these guides in lentivirus and infected Tw2-MB in order to knockout Nrp1. We verified the correct targeting of sgRNAs in Tw2-MB using a T7E1 assay, as well as TOPO cloning and sequencing (Figure S5C and D). For both guide RNAs (sgNrp1–2 and sgNrp1–5), we identified indels resulting in frameshift mutations (Figure S5D). Western blot of Nrp1 revealed that the majority of NRP1 protein was eliminated by sgNrp1–2 and sgNrp1–5, confirming efficient deletion of Nrp1 by CRISPR/Cas9 (Figure 5C).
Next, we seeded sgNrp1-infected Tw2-MB onto Sema3a stripes and performed a stripe migration assay (Figure 5D). We found that single guide-mediated deletion of Nrp1 in Tw2-MB was sufficient to abolish the majority of Sema3a avoidance, providing further evidence that Nrp1 is required for Tw2-MB to avoid Sema3a (Figure 5E).
The Sema3a/Nrp1 signaling axis represses chimeric myotube formation.
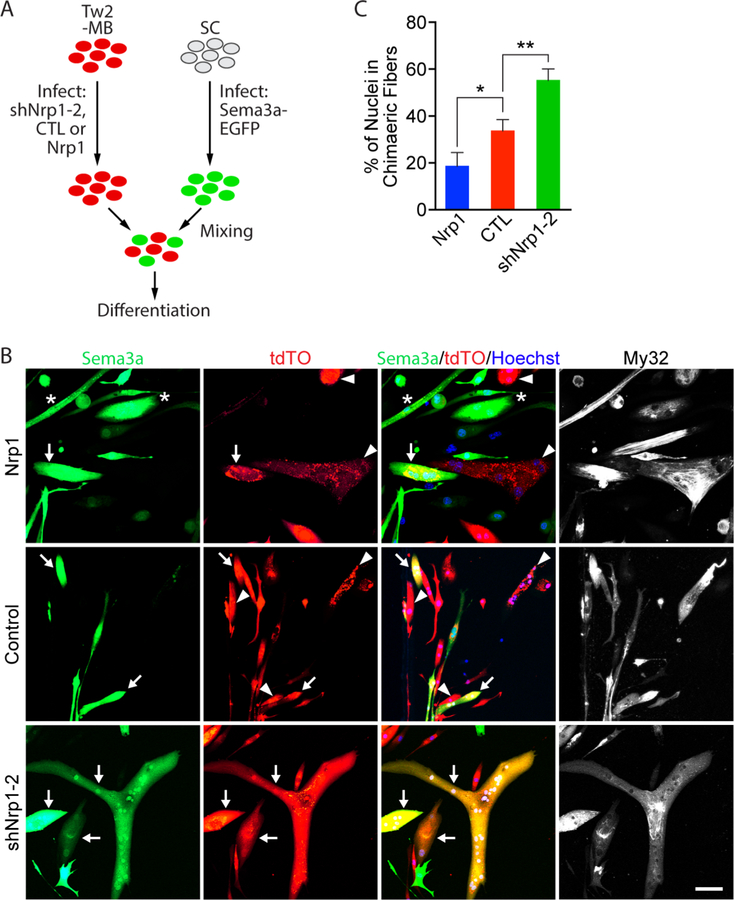
We previously showed that Tw2-MB could fuse to Pax7-MB to form chimeric myotubes (Liu et al., 2017). To explore whether the Sema3a/Nrp1 signaling pathway could keep Tw2+ cells from fusing to Sema3a-expressing fibers, we performed a chimeric fusion assay between Tw2-MB and primary myoblasts (SCs) (Figure 6A). Tw2+ cells were infected with retroviruses expressing either Nrp1, shNrp1–2, or a control empty vector, and SCs were infected with retroviruses expressing Sema3a and an EGFP reporter (Figure 6A and S6A). We first differentiated these cells individually and found that alteration of Nrp1 or Sema3a levels did not impair myotube formation (Figure S6B and C). We then mixed each population of Tw2-MB with Sema3a-overexpressing SCs and allowed them to differentiate for 7 days (Figure 6A). Our results showed that overexpression of Nrp1 significantly decreased the number of nuclei within chimeric myofibers, whereas knockdown of Nrp1 significantly increased the number of chimeric myotube nuclei between Tw2-MB and SCs (Figure 6B, 6C, and S6D). These data suggest that Sema3a-Nrp1 signaling is sufficient to impair Tw2+ cell fusion into Sema3a-expressing myofibers.
Figure 6. The Nrp1-Sema3a signaling axis represses chimeric fusion between Tw2-MB and Sema3a over-expressing primary myoblasts.
(A) Experimental scheme for chimeric fusion assay. Tw2-MB were infected with retroviruses expressing shNrp1–2, control empty vector, or Nrp1, and mixed with primary myoblasts (SCs) infected with retroviruses expressing Sema3a-EGFP in equal numbers. Cells were then differentiated for 7 days. (B) SCs over-expressing Sema3a (green) were mixed with Tw2-MB (tdTO+) infected with Nrp1 (top), control empty vector (middle), or shNrp1–2 (bottom) retroviruses and differentiated for 7 days. Cells were fixed and stained with Hoechst (blue) and an antibody against fast myosin (MY32; white). Arrow indicates chimeric myotubes that are both Sema3a+ and tdTO+; * represents myotubes that Sema3a+ only; and arrowhead represents myotubes that are tdTO+ only. Scale bar: 50 µm. (C) Quantification of percent of nuclei in chimeric fibers for Figure 6B. Percent of nuclei in chimeric fibers was calculated as the percent of the number of nuclei in chimeric fibers over the total number of nuclei (red-only, green-only, and chimeric myofibers). Three fields per sample per experiment were quantified. Three separate experiments were performed. *: p < 0.05, **: p < 0.005. See also Figure S6. Source data for 6C is provided in Supplementary Table 1.
Overexpression of Sema3a in muscle prevents Tw2+ cell contribution to type IIb fibers.
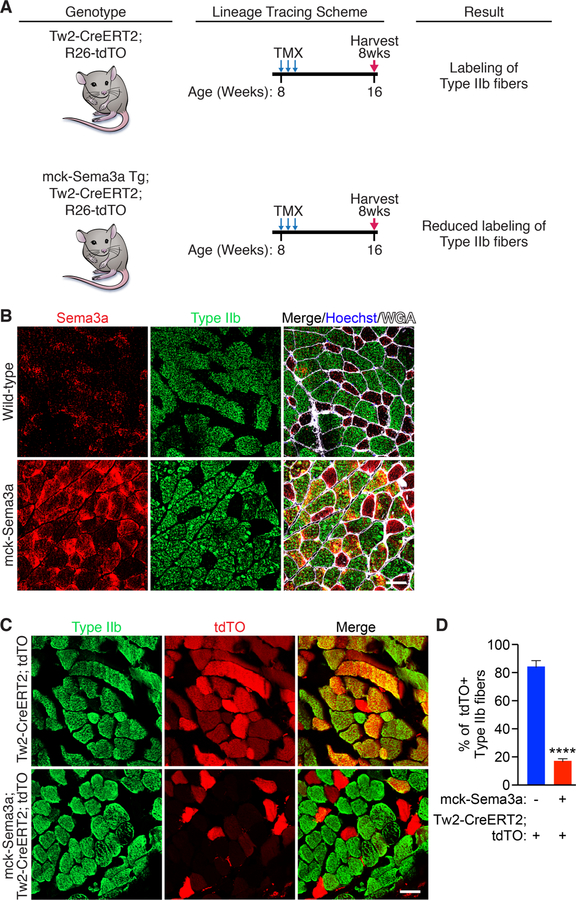
To study the contribution of Nrp1-Sema3a signaling to Tw2+ cell fiber type specificity in vivo, we generated a Sema3a transgenic mouse using the muscle creatine kinase (mck) promoter (Figure 7A). This promoter is preferentially active in fast-twitch myofibers relative to slow-twitch fibers, enabling the expression of Sema3a in fibers where it is normally absent (Dunant et al., 2003; Tai et al., 2011; Yamashita and Yoshioka, 1991). We verified Sema3a overexpression by immunostaining and real-time PCR of gastrocnemius muscle (Figure 7B and S7A). We then crossed the mck-Sema3a transgenic line with a Tw2-CreERT2; tdTO mouse to generate mck-Sema3a; Tw2-CreERT2; tdTO mice (Figure 7A). These mice were injected with 3 doses of tamoxifen starting at 8 weeks of age to begin lineage tracing of Tw2+ cells (Figure 7A). Eight weeks after the first injection, we observed a significant decrease in the number of tdTO+ type IIb myofibers in the mck-Sema3a transgenic mice compared to controls, indicating that Sema3a can prevent Tw2+ cells from fusing to type IIb myofibers in vivo (Figure 7C and D).
Figure 7. Mck-Sema3a Transgenic mice prevent Tw2+ cells from contributing to type IIb fibers.
(A) Schematic of Tw2+ cell lineage tracing in mck-Sema3a transgenic mice. Tw2-CreERT2; tdTO and mck-Sema3a Tg; Tw2-CreERT2; R26-tdTO mice were injected with 3 doses of TMX on 3 alternating days starting at 8 weeks of age. Muscles from these mice were then harvested 8 weeks later. (B) Transverse gastrocnemius muscle sections of wild-type C57Bl6 and mck-Sema3a transgenic littermates were immunostained for Sem3a (red), type IIb myofibers (green), wheat-germ agglutinin (white), and Hoechst (blue). Scale: 50 µm C) Immunostaining of gastrocnemius muscle from Tw2-CreERT2; tdTO mice and mck-Sema3a; Tw2-CreERT2; tdTO mice for type IIb fibers (green) and tdTO (red). Scale: 50µm. (D) Quantification of the percent of tdTO+ type IIb fibers over total type IIb fibers. 500 type IIb myofibers were quantified per mouse (Tw2-CreERT2; tdTO, n = 3 mice; mck-Sema3a; Tw2-CreERT2; tdTO, n = 4 mice). ****: p < 0.00005. See also Figure S7. Source data for 7D is provided in Supplementary Table 1.
Discussion
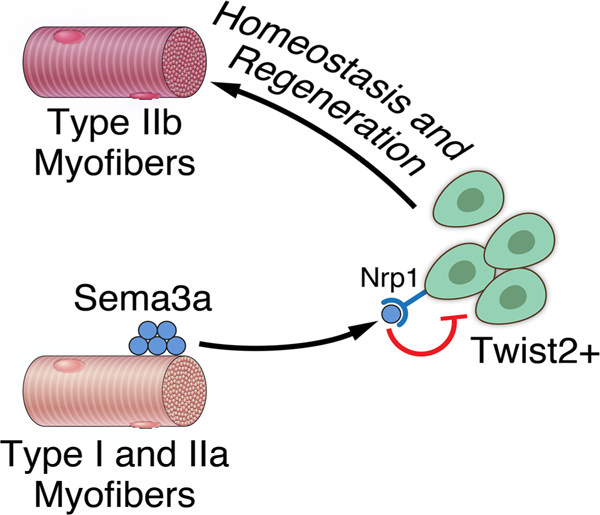
We previously identified a population of Tw2+ progenitors in the muscle interstitium and found that they fuse exclusively to type IIb/x myofibers (Liu et al., 2017). The results of the present study reveal a unique mechanism for fiber-type specificity of these Tw2+ progenitors. Through transcriptional profiling, we found that the membrane receptor Nrp1 is differentially enriched in Tw2+ cells compared to Pax7+ satellite cells, reflecting the direct activation of the Nrp1 gene by Twist2. Sema3a, the chemorepulsive ligand for the Nrp1 receptor, is localized exclusively to type I and IIa myofibers, which do not fuse with Tw2+ cells, and is absent from type IIb fibers, which recruit Tw2+ cells. These findings suggest that Tw2+ cells are repelled from the type I and IIa myofibers that are enriched for Sema3a expression, but are able to fuse to type IIb fibers lacking Sema3a. Since Pax7+ satellite cells are not enriched for Nrp1, they are not repelled from type I and IIa fibers and therefore show no fiber-type specificity.
In vitro stripe migration assays confirmed that manipulating Nrp1 expression altered the ability of Tw2-MB and Pax7-MB to respond to Sema3a. Increasing Nrp1 expression enhanced Sema3a avoidance of both Tw2- and Pax7-MB. Additionally, ablation of Nrp1 expression in Tw2-MB in vitro by either RNAi-mediated knockdown or CRISPR-mediated knockout resulted in loss of Sema3a avoidance. These findings suggest that Nrp1 is both necessary and sufficient for this interaction. It is important to note that in these studies, the percent of cells on or off the stripe is a function of both the chemorepellent concentration and the time cells are allowed to migrate. In this case we chose conditions that enabled us to see both increases and decreases in repulsion upon manipulation of Nrp1 expression. In our chimeric fusion studies we also noticed that Tw2-MB overexpressing Nrp1 were often more compact and smaller in the presence of Sema3a-expressing SCs. This is likely a manifestation of a Sema3a signaling gradient surrounding Tw2-MB causing cytoskeletal reorganization on all sides. In vivo, the formation of a muscle secreted-Sema3a gradient may explain why Tw2+ cells are present in slow-twitch muscles such as the soleus but are unable to contribute to the myofibers (Cioni et al., 2013). Whether Sema3a secreted from type I and IIa myofibers traverses across the muscle basement membrane or whether Tw2+ cells must first reach the muscle compartment before being repelled remains to be answered. However, responsiveness of myofibers and satellite cells to vascular cues suggests that Sema3a is likely capable of diffusing across the basal lamina (Wosczyna and Rando, 2018). The repulsive effects of Sema3a on Nrp1-expressing Tw2+ cells are reminiscent of the role of the Sema3a-Nrp1 signaling axis in the control of motor neuron migration during development and endothelial cell signaling during angiogenesis (Acevedo et al., 2008; Saller et al., 2016).
The Nrp1 family member Nrp2 is also highly enriched in Tw2+ cells and upregulated upon Twist2-overexpression. Nrp1 and 2 confer specificity of Sema3a binding and signaling with Plexin co-receptors. Binding of Sema3a to this co-receptor complex triggers a G-protein coupled cascade, resulting in cytoskeletal and growth cone collapse (Tamagnone and Comoglio, 2000). While Neuropilins are the primary determinants of Sema3a recognition, other Nrp1 co-receptors, such Lcam1, Chl1, and Robo1 have also been shown to interact with Nrp1 to mediate downstream cytoskeletal reorganization (Alto and Terman, 2017). Which of these co-receptors are key for Nrp1 signaling in Tw2+ cells and whether their differential expression may have important biological function in muscle remain to be explored.
The specification of different muscle fiber types and specialization of function is an important aspect of vertebrate evolution. The specific atrophy of type IIb fibers during aging and other disease suggests differential signaling mechanisms maintain structure and function of individual fiber-types (Lee et al., 2016). Our identification of Tw2+ cells and their unique ability to fuse with type IIb/x fibers positions them as potential regulators of fast-twitch fiber homeostasis (Liu et al., 2017). In particular, the large size of type IIb fibers suggests additional mechanisms are required to help maintain their cross-sectional area throughout life (Schiaffino and Reggiani, 2011). During aging, atrophy of fast-twitch fibers could be partially due to impaired Tw2+ cell function or a loss in Tw2+ cell number. This is supported by our finding that type IIb fiber-specific atrophy is seen after ablation of Tw2+ cells (Liu et al., 2017). Whether Tw2+ cell number or function are impaired over time is an area of future study. Additionally, it is interesting to consider why a mechanism of fiber-type specificity revolving around the repulsion of Tw2+ cell fusion would be evolutionarily maintained. It would be logical to conclude that additional fusion of myogenic cells to type I and IIa fibers would actually be beneficial. A likely explanation is that Sema3a secreted by type I and IIa fibers may play additional important roles in regulating fiber-type specific motor neuron innervation or vascular remodeling (Anderson et al., 2016). In this context, the gain of Tw2+ cell fusion may not compensate for other benefits imparted by Sema3a signaling. It is also possible that Tw2+ cell fusion may actually alter the transcriptional or metabolic profile of myofibers. The evolution of Tw2+ cells and their fiber-type specificity will be an exciting area of future study.
In addition to its role in contributing to muscle fiber-type specificity, we recently discovered that Twist2 acts as a regulator of myogenesis in both physiologic and pathologic contexts, such as rhabdomyosarcoma (RMS), a tumor expressing hallmarks of the skeletal muscle lineage (Li et al., 2019). Indeed, the majority of fusion-negative rhabdomyosarcomas contain amplification of the TWIST2 allele, and that Twist2 promotes oncogenesis by redirection of MyoD DNA binding (Li et al., 2019). These functions position Twist2 as not only a marker for Tw2+ progenitors, but a master regulator of their function and differentiation. Interestingly, Sema3a is often dysregulated in many tumor microenvironments where it serves to regulate vascularization and angiogenesis (Acevedo et al., 2008). It will be interesting to see if Twist2-mediated regulation of Nrp1 may play a role in cellular cross-talk amongst cells in the microenvironment of rhabdomyosarcoma and to understand how Twist2 distinguishes between transcriptional targets involved in fiber-type specificity, myogenesis, and oncogenesis.
Overall, our study not only reiterates our findings of Tw2+ cells as fiber-type specific muscle progenitors, but also provides a mechanistic basis for this fiber-type specificity. The idea of muscle progenitor cells adopting pathways traditionally involved in the nervous and vascular systems offers a new class of signaling molecules to analyze in the context of muscle progenitor biology. Given the shared developmental origin of muscle and nervous tissues, it is unsurprising that shared pathways exist that regulate key aspects of their function. Recent studies have shown that Sema3a secreted from satellite cells may regulate fiber-type specification during muscle injury22–26. Additionally, other guidance molecules such as ephrins have also been implicated in muscle fiber identity, motor neuron innervation, and satellite cell fusion (Stark et al., 2015; 2011). It will be interesting to see whether these systems are differentially expressed within satellite cells or other muscle progenitors where they may mark additional fiber-type specific populations or sub-populations.
STAR Methods
LEAD CONTACT AND MATERIALS AVAILABILITY
Further information and requests for data, resources, and reagents should be directed to and will be fulfilled by the Lead Contact, Eric Olson (eric.olson@utsouthwestern.edu).
EXPERIMENTAL MODEL AND SUBJECT DETAILS
Mouse Models
Mck-Sema3a transgenic mice were crossed with Tw2-CreERT2; R26-tdTO (Liu et al., 2017) mice to obtain mck-Sema3a; Tw2-CreERT2; R26-tdTO mice. Age-matched male and female littermates were used in the studies. Mice were maintained on a mixed genetic background. All animal procedures were approved by the Institutional Animal Care and Use Committee at the University of Texas Southwestern Medical Center.
Cell Lines
Tw2-MB, Pax7-MB, and SCs were isolated from mouse hind limb muscle and cultured on Matrigel-coated plates in satellite cell growth medium (SCGM; F10 Ham’s, 20% FBS, 0.2% Primocin, and 2.5 ng µl−1 basic Fibroblast Growth Factor (Gibco)). For myoblast differentiation, we used differentiation media (DM) containing: DMEM, 2% horse serum, 0.2% Primocin. For the culture of all other cells, we used standard growth media (GM) containing: DMEM, 10% FBS, Pen/Strep. Cell lines are from a mixed sex background due to due the larger number of mice required to obtain sufficient numbers of cells.
METHOD DETAILS
RNA and ChIP sequencing
Bulk RNA-seq data was obtained from our previously published study (Liu et al., 2017) and is available in the Gene Expression Omnibus (GEO) under accession codes GSE84377, GSE84378, GSE84379, and GSE 84380.
Single Cell RNA-Sequencing data was pulled from Tabula Muris (Tabula Muris Consortium et al., 2018) (https://tabula-muris.ds.czbiohub.org).
ChIP-Sequencing data was obtained from our previously published study (Li et al., 2019) and is available in the Gene Expression Omnibus (GEO) under accession codes (GSE127988).
Western Blot
Tw2-MB and Pax7-MB were harvested in RIPA buffer (Sigma) supplemented with cOmplete™, Mini, EDTA-free Protease Inhibitor Cocktail (Roche) and PhosSTOP (Sigma) phosphatase inhibitor. Western blot was performed as previously described (Makarewich et al., 2018). Primary antibodies used were: Nrp1 (Abcam, ab81321, 1:1000) and GAPDH (Millipore, CB1001, 1:10,000). Western blots were washed in TBST, incubated with HRP-conjugated secondary antibodies (Bio-Rad), and then developed using a ChemiDoc MP Imaging System (Bio-Rad).
Immunohistochemistry
Immunohistochemistry of skeletal muscle was performed as previously described (Liu et al., 2017). For immunohistochemistry of fixed frozen sections, the following antibodies were used: Twist2 (Abcam, ab66031, 1:200), Sema3a (Abcam, ab23393, 1:100), Nrp1 (Abcam, ab81321, 1:100), and Myh4 (Invitrogen, 14-6503-82, 1:100). For immunohistochemistry of raw embedded frozen sections, the following antibodies were used: Twist2 (Abcam, ab66031, 1:200), Sema3a (Abcam, ab23393, 1:100), Nrp1 (Abcam, ab81321, 1:100), myosin IIa (Developmental Studies Hybridoma Bank, SC-71, 1:10), myosin IIb (Developmental Studies Hybridoma Bank, BF-F3, 1:10), Slow myosin (Sigma-Aldrich, NOQ7.5D, 1:250), and Fast myosin (Sigma-Aldrich, clone My32, 1:250). Alexa Fluor secondary antibodies were used according to the manufacturer’s instructions. TdTO signals were detected by direct fluorescent imaging. Wheat germ agglutinin staining was performed on both frozen and paraffin-embedded sections, using WGA-Alexa Fluor 555 (W32464) or WGA-Alexa 647 conjugate (W32466) (Life Technologies, 50 mg ml−1). Images were taken on a Zeiss LSM700 confocal microscope. Muscle fiber numbers were quantified by ImageJ.
Immunostaining of cultured cells
Immunostaining of cultured cells was performed as previously described (Liu et al., 2017). Primary antibodies include: fast myosin (Sigma-Aldrich, My32, 1:250). Cells were counterstained with Hoechst (ThermoFisher, Hoechst, 3342, 1:1000). Alexa Fluor secondary antibodies were used according to the manufacturer’s instructions.
Stripe Migration Assay
The protocol used for the in vitro stripe migration assay was adapted from a previous study (Yamagishi et al., 2016). In summary, 2.5 µg of recombinant Sema3a-Fc (R&D Systems, 5926-S3-025) was mixed with 7.5 µg of goat anti-mouse IgG Alexa 488 secondary antibody (Abcam, ab150113) in 50 µl PBS and incubated at room temperature for 30 minutes. The mixture was then injected into silicon matrices (Purchased from Martin Bastmeyer) attached to tissue culture dish using an insulin needle. The dishes were then incubated at 37 °C in a cell culture incubator. The stripes were then washed three times with PBS followed by addition of 10 µg of control Fc fragments (Invitrogen, 31205) in 200 µl of PBS on top of the stripes as a counter coating for 30 mins at 37 °C. The dish was washed three times with PBS and then coated with Matrigel (Corning, 356234) for 1 hr at 37 °C. The dish was then washed three times with PBS followed by seeding of cells on top. After 24 hrs, dishes were fixed and counter-stained with Hoechst in order to count the number of cells on and off the stripes.
Real-time RT–PCR analysis
Total RNA was extracted from cultured cells and muscle tissue with Trizol (Invitrogen, 15596026) following the manufacturer’s instructions. From this RNA, cDNA was synthesized using iScript Reverse Transcriptase Supermix (Bio-Rad, 1708840). Selected gene expression was analysed by real-time RT–PCR. For Nrp1 expression, Taqman probe was used (Mm00435379_m1). For Sema3a expression SYBR green was used with the following primers: Fwd – 5′ -GAAGAGCCCTTATGATCCCAAAC-3′; Rev – 5′- AGATAGCGAAGTCCCGTCCC-3′.
Retroviral Infection
The open reading frame of Nrp1 was cloned into the pBabe vector. The open reading frame of Sema3a was cloned into pBabe-EGFP (Parker et al., 2012) (addgene, 36999). shNrp1 plasmids were purchased from Origene (OriGene, TG513573A). 15 µg of retroviral plasmid DNA was transfected using FuGENE HD (Roche, E2311) into Platinum E cells (Cell Biolabs), which were plated on a 10-cm tissue culture dish at a density of 5 × 106 cells per dish, 24 h before transfection. After 48 h of transfection, viral medium was harvested and filtered through a 0.45 μm cellulose filter. The viral supernatant was concentrated overnight using Retro-X concentrator (Clontech, 631456) according to manufacturer’s protocol. The virus was then resuspended in growth medium mixed with Polybrene (Sigma) to a final concentration of 6 μg ml−1.
Tw2-MB and Pax7-MB were plated at a density of 200,000 cells per 35-mm plate in growth medium. After 24 h, the growth medium was replaced with freshly made viral mixture containing Polybrene and bFGF (5 ng ml−1). 24 hrs later, viral medium was replaced with growth medium with bFGF for recovery.
CRISPR/Cas9 Deletion of Nrp1
In order to delete Nrp1 from Tw2-MB, we selected 2 different Nrp1 short guide RNAs (sgRNA) and cloned them into a pLentiV2 vector for lentiviral infection. Lenti-X 293T cells (Clontech, 632180) were transfected with pLentiV2-sgNrp1, psPax2 (addgene, 12260), and pMD2.G (addgene, 12259) using FUGENE HD reagent (Promega, E2311). 48 hours after transfection virus was harvested and concentrated using Lenti-X concentrator (Clontech, 631232) according to manufacturer’s protocol. Tw2-MB were then infected with the lentivirus. Infected Tw2-MB were sorted and subsequently harvested for DNA. The sgRNA targeted region was PCR amplified using the following primers: Fwd – 5′-GAGGGTTTATGGGGGACACT-3′, Rev – 5′-CAGGACATCTGGGGCTACAT-3′. Amplified PCR product was denatured and re-hybridized, and subsequently subjected to treatment with or without T7E1 endonuclease for 15 minutes at room temperature. The treated products were run on a 1.5% agarose gel alongside T7E1 untreated control samples. The T7E1 untreated bands were gel-isolated, cloned into pCRII-TOPO vector (ThermoFisher, 45–0640), and individual colonies were sequenced to reveal the indels generated by CRISPR/Cas9.
Chimeric Fusion Assay
Tw2-MB were infected with retroviruses expressing shNrp1, control empty vector, or Nrp1 and mixed with SCs overexpressing Sema3a-EGFP or EGFP control. The next day, cells were placed into DM and differentiated for 7 days. Cells were then fixed and counterstained with Hoechst (ThermoFisher, Hoechst, 3342, 1:1000). The percent of chimeric fusion was calculated as the number of nuclei within chimeric myofibers vs the number of total nuclei.
Generation of Mck-Sema3a Transgenic Mice
The open reading frame of Sema3a was cloned downstream of the muscle creatine kinase (mck) promoter (Shield et al., 1996). Transgenic mice were generated as previously described (Naya et al., 2000; Papizan et al., 2017). Mice were genotyped using primers specific to the mck promoter and the proximal coding region of Sema3a: Fwd – 5′-CCTGTAGGCTCCTCTATATAACC-3′; Rev – 5′- AGATAGCGAAGTCCCGTC-3′.
Tamoxifen Treatment
Tamoxifen treatment was performed as previously described (Liu et al., 2017). Tamoxifen was administered by intraperitoneal injection to 8-week-old mck-Sema3a;Tw2-CreERT2; R26-tdTO/+ and Tw2-CreERT2; R26-tdTO/+ mice as schematized in Fig. 7A. Hind limb muscles were harvested at 8 weeks after the first injection.
QUANTIFICATION AND STATISTICAL ANALYSIS
Quantification and Source Data
Source data for Figures 3C, 4B-C, 5B, 5E, 6C, and 7D, and Supplemental Figures S3B, S4, S5A, S6A, and S7 have been provided as Supplementary Table 1.
Statistical Analysis
All statistical analyses were performed using GraphPad Prism 8 (GraphPadSoftware). Data are presented as mean ± s.e.m. Comparisons between two groups were tested for statistical significance by using two-tailed student’s t-test. Comparisons of three groups were tested for statistical significance using one-way anova with Tukey post-hoc. P-value definitions and number of biological replicates for each experiment is indicated in the figure legends. Randomization was not used in most of the animal studies. However, images of type IIb and Sema3a staining were taken by an investigator who was blinded to the group allocation. All immunofluorescence images are representative of at least three independent experiments or mice of the same genotypes. No statistical method was used to predetermine sample size.
DATA AND CODE AVAILABILITY
RNA-seq data used in this study is deposited in the Gene Expression Omnibus (GEO) under accession codes GSE84377, GSE84378, GSE84379, GSE84380 (Liu et al., 2017). ChIP-seq data used in this study is deposited in the Gene Expression Omnibus (GEO) under accession code GSE127988 (Li et al., 2019). All other data supporting the findings of this study are available from the corresponding authors on request.
Supplementary Material
KEY RESOURCE TABLE
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Antibodies | ||
| Rabbit Polyclonal anti-Twist2 | Abcam | ab66031 |
| Rabbit Polyclonal anti-Sema3a | Abcam | ab23393 |
| Rabbit Monoclonal anti-Nrp1 | Abcam | ab81321 |
| Mouse Monoclonal anti-Myh4 | Invitrogen | 14–6503-82 |
| Mouse Monoclonal anti-Myh2 | DSHB | SC-71 |
| Mouse Monoclonal anti-Myh4 | DHSB | BF-F3 |
| Mouse Monoclonal anti-Myh7 | Sigma-Aldrich | NOQ7.5D |
| Mouse Monoclonal anti-myosin skeletal, fast (MY32) | Sigma-Aldrich | M4276 |
| WGA-Alexa Fluor 555 | Life Technologies | W32464 |
| WGA-Alexa Fluor 647 | Life Technologies | W32466 |
| Mouse Monoclonal anti-GAPDH | Millipore | CB1001 |
| Chemicals, Peptides, and Recombinant Proteins | ||
| Recombinant Sema3a-Fc | R&D Systems | 5926-S3–025 |
| Recombinant Fc Fragments | Invitrogen | 31205 |
| Matrigel | Corning | 356234 |
| Critical Commercial Assays | ||
| iScript Reverse Transcriptase Supermix | Bio-Rad | 1708840 |
| Nrp1 Taqman Probe | Applied Biosciences | Mm00435379_m1 |
| KAPA SYBR Fast ABI Prism | KAPA Biosciences | 07959435001 |
| Deposited Data | ||
| RNA-Sequencing of isolated Tw2+ and Pax7+ cells | Liu et al. 2017 | GSE84377, GSE84378, GSE84379, and GSE 84380. |
| RNA-Sequencing of Twist2-overexpressing myoblasts vs GFP-expressing myoblasts | Liu et al. 2017 | GSE84377, GSE84378, GSE84379, and GSE 84380. |
| Twist2 ChIP-Sequencing | Li et al. 2019 | GSE127998 |
| Experimental Models: Cell Lines | ||
| Twist2-derived myoblasts | Liu et al. 2017 | N/A |
| Pax7-derived myoblasts | Liu et al. 2017 | N/A |
| Primary myoblasts | This Paper | N/A |
| Experimental Models: Organisms/Strains | ||
| Mck-Sema3a transgenic mice | This paper | N/A |
| Tw2-CreERT2; R26-tdTomato | Liu et al. 2017 | N/A |
| Mck-Sema3a; Tw2-CreERT2; R26-tdTomato | This paper | N/A |
| Oligonucleotides | ||
| Sema3a qPCR Forward Probe - GAAGAGCCCTTATGATCCCAAAC | This paper | N/A |
| Sema3a qPCR Reverse Probe - AGATAGCGCAAGTCCCGTCCC | This paper | N/A |
| Nrp1 T7E1 Forward Primer - GAGGGTTTATGGGGGACACT | This paper | |
| Nrp1 T7E1 Reverse Primer - CAGGACATCTGGGGCTACAT | This paper | |
| Recombinant DNA | ||
| pBabe-Nrp1 | This paper | N/A |
| pBabe-Sema3a-EGFP | This paper | N/A |
| shNrp1 #1–4 | Origene | TG513573A |
| pLentiV2-sgNrp1 #1–8 | This paper | N/A |
| psPax2 | Addgene; http://n2t.net/addgene:12260 | 12260 |
| pMD2.G | Addgene; http://n2t.net/addgene:12259 | 12259 |
| pCRII-TOPO | ThermoFisher | 45-0640 |
| pBabe-EGFP | Addgene; Parker et al. 2012 | 36999 |
| Software and Algorithms | ||
| ImageJ | Schneider et al., 2012 | https://imagej.nih.gov/ij/ |
| GraphPad Prism 8 | GraphPadSoftware | https://www.graphpad.com/scientific-software/prism/ |
| Other | ||
| Silicon Matrix | Martin Bastmeyer, Karlsruhe Institute of Technology | https://znbio.zoo.kit.edu/Mitarbeiter_66.php |
Highlights.
Tw2+ cells are a type IIb/x fiber-specific muscle progenitor.
Nrp1 is a membrane receptor enriched in Tw2+ cells compared to Pax7+ cells.
Sema3a is a type I and IIa-myofiber specific chemorepellent that binds Nrp1.
Overexpression of Sema3a in type IIb myofibers prevents Tw2+ cell fusion.
Acknowledgements
We are grateful to UT Southwestern Flow Cytometry Core Facility, the Moody Foundation Flow Cytometry Facility, and UTSW Genomics and Microarray Core Facility for technical help and service. We thank C. Nolen for technical assistance and Jose Cabrera for graphics assistance. This work was supported by grants from the NIH (AR-067294, HL-130253, HL-138426, HD-087351) and the Robert A. Welch Foundation (grant 1–0025 to E.N.O.). S.L. was supported by a F30 fellowship from the NIH (F30AG056075). N.L was supported by a grant from the NIH (R01AR071980–01A1).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Competing Interests
The authors declare no competing financial interests.
References
- Acevedo LM, Barillas S, Weis SM, Göthert JR, Cheresh DA, 2008. Semaphorin 3A suppresses VEGF-mediated angiogenesis yet acts as a vascular permeability factor. Blood 111, 2674–2680. 10.1182/blood-2007-08-110205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alto LT, Terman JR, 2017. Semaphorins and their Signaling Mechanisms. Methods Mol. Biol 1493, 1–25. 10.1007/978-1-4939-6448-2_1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson JE, Do M-KQ, Daneshvar N, Suzuki T, Dort J, Mizunoya W, Tatsumi R, 2016. The role of semaphorin3A in myogenic regeneration and the formation of functional neuromuscular junctions on new fibres. Biol Rev Camb Philos Soc. 10.1111/brv.12286 [DOI] [PubMed]
- Cioni J-M, Telley L, Saywell V, Cadilhac C, Jourdan C, Huber AB, Huang JZ, Jahannault-Talignani C, Ango F, 2013. SEMA3A signaling controls layer-specific interneuron branching in the cerebellum. Curr. Biol 23, 850–861. 10.1016/j.cub.2013.04.007 [DOI] [PubMed] [Google Scholar]
- Dunant P, Larochelle N, Thirion C, Stucka R, Ursu D, Petrof BJ, Wolf E, Lochmüller H, 2003. Expression of dystrophin driven by the 1.35-kb MCK promoter ameliorates muscular dystrophy in fast, but not in slow muscles of transgenic mdx mice. Mol. Ther 8, 80–89. [DOI] [PubMed] [Google Scholar]
- Koncina E, Roth L, Gonthier B, Bagnard D, 2007. Role of Semaphorins during Axon Growth and Guidance, in: Axon Growth and Guidance, Advances in Experimental Medicine and Biology. Springer New York, New York, NY, pp. 50–64. 10.1007/978-0-387-76715-4_4 [DOI] [PubMed] [Google Scholar]
- Kumanogoh A, Kikutani H, 2013. Immunological functions of the neuropilins and plexins as receptors for semaphorins. Nat. Rev. Immunol 13, 802–814. [DOI] [PubMed] [Google Scholar]
- Lee JD, Fry CS, Mula J, Kirby TJ, Jackson JR, Liu F, Yang L, Dupont-Versteegden EE, McCarthy JJ, Peterson CA, 2016. Aged Muscle Demonstrates Fiber-Type Adaptations in Response to Mechanical Overload, in the Absence of Myofiber Hypertrophy, Independent of Satellite Cell Abundance. J. Gerontol. A Biol. Sci. Med. Sci 71, 461–467. 10.1093/gerona/glv033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lepper C, Conway SJ, Fan C-M, 2009. Adult satellite cells and embryonic muscle progenitors have distinct genetic requirements. Nature 460, 627–631. 10.1038/nature08209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li S, Chen K, Zhang Y, Barnes SD, Jaichander P, Zheng Y, Hassan M, Malladi VS, Skapek SX, Xu L, Bassel-Duby R, Olson EN, Liu N, 2019. Twist2 amplification in rhabdomyosarcoma represses myogenesis and promotes oncogenesis by redirecting MyoD DNA binding. Genes Dev 33, 626–640. 10.1101/gad.324467.119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu N, Garry GA, Li S, Bezprozvannaya S, Sanchez-Ortiz E, Chen B, Shelton JM, Jaichander P, Bassel-Duby R, Olson EN, 2017. A Twist2-dependent progenitor cell contributes to adult skeletal muscle. Nat. Cell Biol 19, 202–213. 10.1038/ncb3477 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makarewich CA, Baskin KK, Munir AZ, Bezprozvannaya S, Sharma G, Khemtong C, Shah AM, McAnally JR, Malloy CR, Szweda LI, Bassel-Duby R, Olson EN, 2018. MOXI Is a Mitochondrial Micropeptide That Enhances Fatty Acid β-Oxidation. Cell Rep 23, 3701–3709. 10.1016/j.celrep.2018.05.058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nasarre P, Gemmill RM, Drabkin HA, 2014. The emerging role of class-3 semaphorins and their neuropilin receptors in oncology. Onco Targets Ther 7, 1663–1687. 10.2147/OTT.S37744 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naya FJ, Mercer B, Shelton J, Richardson JA, Williams RS, Olson EN, 2000. Stimulation of slow skeletal muscle fiber gene expression by calcineurin in vivo. J. Biol. Chem 275, 4545–4548. [DOI] [PubMed] [Google Scholar]
- Papizan JB, Garry GA, Brezprozvannaya S, McAnally JR, Bassel-Duby R, Liu N, Olson EN, 2017. Deficiency in Kelch protein Klhl31 causes congenital myopathy in mice. J. Clin. Invest 127, 3730–3740. 10.1172/JCI93445 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker JB, Palchaudhuri S, Yin H, Wei J, Chakravarti D, 2012. A transcriptional regulatory role of the THAP11-HCF-1 complex in colon cancer cell function. Mol. Cell. Biol 32, 1654–1670. 10.1128/MCB.06033-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pawlikowski B, Pulliam C, Betta ND, Kardon G, Olwin BB, 2015. Pervasive satellite cell contribution to uninjured adult muscle fibers. Skelet Muscle 5, 42 10.1186/s13395-015-0067-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raper JA, 2000. Semaphorins and their receptors in vertebrates and invertebrates. Curr. Opin. Neurobiol 10, 88–94. [DOI] [PubMed] [Google Scholar]
- Saller MM, Huettl R-E, Hanuschick P, Amend A-L, Alberton P, Aszodi A, Huber AB, 2016. The role of Sema3-Npn-1 signaling during diaphragm innervation and muscle development. J. Cell. Sci 129, 3295–3308. 10.1242/jcs.186015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sambasivan R, Yao R, Kissenpfennig A, Van Wittenberghe L, Paldi A, Gayraud-Morel B, Guenou H, Malissen B, Tajbakhsh S, Galy A, 2011. Pax7-expressing satellite cells are indispensable for adult skeletal muscle regeneration. Development 138, 3647–3656. 10.1242/dev.067587 [DOI] [PubMed] [Google Scholar]
- Schiaffino S, Reggiani C, 2011. Fiber types in mammalian skeletal muscles. Physiol. Rev 91, 1447–1531. 10.1152/physrev.00031.2010 [DOI] [PubMed] [Google Scholar]
- Sharma A, Verhaagen J, Harvey AR, 2012. Receptor complexes for each of the Class 3 Semaphorins. Front Cell Neurosci 6, 28 10.3389/fncel.2012.00028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shield MA, Haugen HS, Clegg CH, Hauschka SD, 1996. E-box sites and a proximal regulatory region of the muscle creatine kinase gene differentially regulate expression in diverse skeletal muscles and cardiac muscle of transgenic mice. Mol. Cell. Biol 16, 5058–5068. 10.1128/MCB.16.9.5058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soker S, Takashima S, Miao HQ, Neufeld G, Klagsbrun M, 1998. Neuropilin-1 Is Expressed by Endothelial and Tumor Cells as an Isoform-Specific Receptor for Vascular Endothelial Growth Factor. Cell 92, 735–45. 10.1016/S0092-8674(00)81402-6 [DOI] [PubMed] [Google Scholar]
- Stark DA, Coffey NJ, Pancoast HR, Arnold LL, Walker JPD, Vallée J, Robitaille R, Garcia ML, Cornelison DDW, 2015. Ephrin-A3 promotes and maintains slow muscle fiber identity during postnatal development and reinnervation. J. Cell Biol 211, 1077–1091. 10.1083/jcb.201502036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stark DA, Karvas RM, Siegel AL, Cornelison DDW, 2011. Eph/ephrin interactions modulate muscle satellite cell motility and patterning. Development 138, 5279–5289. 10.1242/dev.068411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabula Muris Consortium, Overall coordination, Logistical coordination, Organ collection and processing, Library preparation and sequencing, Computational data analysis, Cell type annotation, Writing group, Supplemental text writing group, Principal investigators, 2018. Single-cell transcriptomics of 20 mouse organs creates a Tabula Muris. Nature 562, 367–372. 10.1038/s41586-018-0590-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tai PW, Fisher-Aylor KI, Himeda CL, Smith CL, Mackenzie AP, Helterline DL, Angello JC, Welikson RE, Wold BJ, Hauschka SD, 2011. Differentiation and fiber type-specific activity of a muscle creatine kinase intronic enhancer. Skelet Muscle 1, 25 10.1186/2044-5040-1-25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takagi S, Kasuya Y, Shimizu M, Matsuura T, Tsuboi M, Kawakami A, Fujisawa H, 1995. Expression of a Cell Adhesion Molecule, Neuropilin, in the Developing Chick Nervous System. Dev Biol 170, 207–222. 10.1006/dbio.1995.1208 [DOI] [PubMed] [Google Scholar]
- Tamagnone L, Comoglio PM, 2000. Signalling by semaphorin receptors: cell guidance and beyond. Trends Cell Biol 10, 377–383. [DOI] [PubMed] [Google Scholar]
- Wosczyna MN, Rando TA, 2018. A Muscle Stem Cell Support Group: Coordinated Cellular Responses in Muscle Regeneration. Dev. Cell 46, 135–143. 10.1016/j.devcel.2018.06.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamagishi S, Kesavamoorthy G, Bastmeyer M, Sato K, 2016. Stripe Assay to Study the Attractive or Repulsive Activity of a Protein Substrate Using Dissociated Hippocampal Neurons. J Vis Exp 10.3791/54096 [DOI] [PMC free article] [PubMed]
- Yamashita K, Yoshioka T, 1991. Profiles of creatine kinase isoenzyme compositions in single muscle fibres of different types. J. Muscle Res. Cell. Motil 12, 37–44. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
RNA-seq data used in this study is deposited in the Gene Expression Omnibus (GEO) under accession codes GSE84377, GSE84378, GSE84379, GSE84380 (Liu et al., 2017). ChIP-seq data used in this study is deposited in the Gene Expression Omnibus (GEO) under accession code GSE127988 (Li et al., 2019). All other data supporting the findings of this study are available from the corresponding authors on request.