Abstract
New granule neurons are added to the dentate gyrus region of the hippocampus throughout life. Behavioral effects of slowing or stopping this ongoing neurogenesis are generally observed only in complex cognitive tasks involving high levels of cue or memory interference or in emotional tasks presented after stress exposure. We tested the role of new neurons in a one-trial task in naïve rats, a simple orienting task previously shown to be affected by hippocampal lesions. Using a pharmacogenetic method to inhibit adult neurogenesis, we found that loss of new neurons decreased orienting toward a novel auditory cue. Rats lacking new neurons showed this change in orienting only when they were drinking from a water bottle and not when they were exploring the arena, suggesting that the deficit is not in orienting behavior itself but in shifting of attention. Orienting was reduced to the same extent after 4 or 8 weeks of neurogenesis reduction but was not detectably altered after 2 or 3 weeks of treatment, suggesting that new neurons must mature for approximately a month before functioning in this behavior. These findings demonstrate that adult-born neurons affect behavior in a simple attention orienting task in naïve animals with no prior stress or task-related learning.
Keywords: dentate gyrus, granule cells, disengagement, autism, attention, orienting
1. INTRODUCTION
New granule neurons are added to the adult hippocampus throughout life from stem cells within the dentate gyrus. Over the course of days to weeks, the new cells mature, developing axons and dendrites, mature neuronal gene expression, and functional afferent and efferent synapses [1-3]. The adult-born granule neurons are activated by experience, and preventing their birth or survival alters network activity and behavior [4,5] [3]. Although many studies have shown behavioral changes in rodents with increased or decreased adult neurogenesis, it has been difficult to assign a particular function to the new neurons that could underlie all of their behavioral effects or even to define the range of behaviors that are affected by their loss. Changes in behavior related to adult neurogenesis have been observed in a wide variety of tasks, but nearly all require some form of prior learning or aversive experience. Several studies have reported effects of decreasing, or increasing, adult neurogenesis in complex behavior tasks assessing navigation in a difficult version of the radial arm maze [6] or fear-mediated context discrimination task [7-9], both described as pattern separation tasks. These pattern separation tasks, as well as neurogenesis-dependent trace eyeblink conditioning tasks [10,11], are learned in dozens or hundreds of trials over many days, consistent with a suggested role for granule neurons specific to difficult tasks [12,13]. However, behavioral changes related to neurogenesis have also been shown in cognitive tasks that are much easier to learn but incorporate long pre-test delays, focusing on a role in memory [14-16]. Neurogenesis-dependent emotional behaviors, including anxiety- or depressive-like behaviors, are generally tested over much shorter time courses, often in a single session. However, many of these tasks show no role for new neurons at baseline [5,17], reflecting neurogenesis-related changes only after stress [5,18-20]. There are few, if any, behavioral tasks in which naïve animals immediately show effects of changes in adult neurogenesis.
A study published 50 years ago reported that rats with hippocampal lesions showed reduced orienting to a novel stimulus when their attention was focused elsewhere, for example when the rats were drinking from a water bottle [21]. Intriguingly, the orienting behavior of the lesioned rats was normal when the rats were simply exploring the test chamber in the absence of the water bottle, suggesting a specific effect on shifting of attention. This behavioral effect has apparently little in common with the neurogenesis-dependent behavior tests described above, as it requires no prior learning, stress, or other experience. As such, it suggests a very different role for the hippocampus, and possibly new neurons, than generally accepted functions in memory and stress response – and one that could potentially underlie the more well-known effects. Moreover, because the entire task occurs in one brief session, it may be useful for narrowing down the age at which new neurons become behaviorally significant.
We therefore tested the role of adult neurogenesis in orienting to a novel auditory stimulus in the presence or absence of a water bottle. To inhibit adult neurogenesis, we used a pharmacogenetic model in which herpes virus thymidine kinase is expressed under the glial fibrillary acidic protein in transgenic rats (TK rats). When these TK animals are fed the antiviral drug valganciclovir (VGCV), neurogenesis is decreased by 85% within one week and completely eliminated within two weeks, without affecting neurogenesis in the wild type (WT) littermates or affecting astrocyte number in either genotype [22].
2. Materials and methods
2.1. Animals
Mixed litters of heterozygous transgenic rats expressing HSV-TK under the GFAP promoter (GFAP-TK rats) and wild type littermate controls (WT rats) [22] were weaned at three weeks of age, housed with siblings, and genotyped by PCR. Rats were maintained on a 12 h: 12 h reversed light/dark cycle with lights on at 9am. Each rat received approximately 15 g of rat chow per day beginning at weaning and had unlimited access to water prior to testing (see below). Beginning at eight weeks of age, all rats were given 4 mg of valganciclovir, p.o. (mixed into peanut butter and rat chow), twice per week. This treatment is known from previous work to completely inhibit neurogenesis in TK rats without affecting neurogenesis or astrocytes in the WT animals [22]. All procedures followed the Institute of Laboratory Animal Research guidelines and were approved by the Animal Care and Use Committee of the National Institute of Mental Health.
2.2. Apparatus
All experiments occurred in a single testing room with dimmable incandescent lighting that was adjusted to 38 lux. Rats were individually placed inside a small open field (50 × 50 × 50 cm) constructed of white Plexiglas with a black Plexiglas insert on the bottom. A water bottle was positioned in the north-east corner of the arena in some tests but was absent in others. An opaque black curtain separated the experimenter from the testing arena. Rats were temporarily held in an adjacent room immediately prior to testing and had no prior exposure to either the testing or holding rooms.
2.3. Novel auditory stimulus testing
2.3.1. Experiment 1: Eight-week depletion.
After 8 weeks of treatment with VGCV, WT and TK rats were deprived of water for 24 h and randomly assigned to one of two testing conditions: water bottle present (N = 12 per genotype) or absent (N = 10 per genotype). Each rat was individually transferred from the holding room to the testing room in a cage lined with clean bedding and covered with black polyvinyl to minimize light exposure. In tests with the water bottle present, the rat was placed in the arena and allowed to freely explore until it discovered the water bottle and began to drink, and latency to drink was recorded. After 5 sec of water consumption, a brief, novel auditory stimulus (a finger snap by the investigator) was repeated 10 times at intervals of 10 to 45 sec from outside the curtain by the southern wall of the arena. Cues were delivered only when rats were actively consuming water. For rats in the no bottle condition, procedures were similar, except that an auditory stimulus was initiated after 120 sec of free exploration, as pilot studies indicated this to be the average time of drinking onset. Cues were delivered when rats were oriented in any direction except toward the southern wall of the arena, the direction of auditory cue source. An observer blind to the rat’s genotype visually scored drinking just prior to each stimulus presentation (drinking or not drinking) as well as orienting just after each stimulus presentation (present or absent) for each of the ten trials. Orienting was defined as pausing ongoing activity and turning the head toward the sound.
2.3.2. Experiment 2: Recorded stimulus
To test orienting to a stimulus that was entirely consistent across trials and sessions, Experiment 1 was repeated using a recorded auditory stimulus in a new cohort of WT (N = 5) and TK (N = 10) rats treated with VGCV for 8 weeks. The stimulus, consisting of five rapid clicks (totaling one second in length) from a Ruconla pet-training clicker, was recorded and repeated 24 times, with an 8-12s pseudorandom intertrial interval, in an audio file. During testing, the stimulus train was played on a wireless Bluetooth speaker placed just outside the arena, with a volume of 74dB at the center of the arena. All rats were water deprived, and a water bottle was available in the arena. Five seconds after the rats began drinking, the recording was started, and an observer blind to the rat’s genotype visually scored orienting behavior as in Experiment 1.
2.3.3. Experiment 3: Neurogenesis depletion time course
To investigate the time course of behavioral changes following inhibition of neurogenesis, rats were tested either two weeks (N = 11 WT, 15 TK without water bottle, 16 WT, 11 TK with water bottle), three weeks (N = 16 WT, 19 TK without water bottle, 12 WT, 13 TK with water bottle), or four weeks (N = 13 WT, 14 TK without water bottle, 13 WT, 14 TK with water bottle) after the start of VGCV treatment (i.e., at 10, 11, or 12 weeks of age). All rats were tested at only one time point and were naïve at the time of orientation testing, although some rats were later used in Experiment 3 (below). The test sessions were identical to those in Experiment 1, with two exceptions. First, rats were water deprived for only 12 hrs, instead of 24 hrs, because the longer period of deprivation prevented orienting to cues in WT rats at this younger age. Second, although cue presentation did not begin until rats started drinking, as above, cues were delivered in a pre-determined pseudorandomized sequence at 7 – 11 sec intervals over 120 sec without regard to whether rats were actively drinking.
2.3.4. Experiment 4: Home cage water consumption
A subset (N = 8 WT, 16 TK) of the 3-week treatment group from Experiment 2 was maintained on VGCV for another week, and tested for water consumption in the home cage within the housing room. Dividers were placed in cages containing two rats during the week prior to testing. Water bottles were removed from cages 12 h prior to testing. At the time of testing, clean water bottles were provided, and rats were allowed to drink freely for 10 mins. Water bottle weights were determined prior to testing and at five min and ten min time points. Dividers were then removed from the cages and rats were returned to normal water and food regimens.
2.4. Immunohistochemical Validation
Upon completion of behavioral tests, rats were perfused with 4% paraformaldehyde, and brains were postfixed in 4% paraformaldehyde, cryoprotected with glycerol, and coronally sectioned on a freezing microtome (40 μm). A few sections from each brain were immunostained with goat anti-doublecortin (1:200; Santa Cruz) and donkey anti-goat Alexa 488 secondary antibody (1:200; Invitrogen), counterstained with bisbenzimide (1:1000), and coverslipped under PermaFluor. At least 2 sections per brain were examined to qualitatively determine the presence or absence of doublecortin-labeled neurons in the subgranular zone of the dentate gyrus. Brains from TK rats were essentially devoid of DCX+ cells, while those from WT rats had significant numbers of DCX+ cells, as expected [22]. Deviation from this expected pattern, indicating misidentification of genotype or incomplete ablation, was not observed in any rats.
2.5. Statistical Analyses
In tests with a water bottle present, orienting was only analyzed in valid trials, defined as those trials in which the rat was actively drinking. In tests without a water bottle present, all trials were considered valid. Orienting responses were expressed as percentages (100 * # of orienting trials /# valid trials). Data were compared using an unpaired t test, Analysis of Variance (ANOVA) with Holm-Sidak post hoc testing, or linear regression analysis with Holm-Sidak post hoc testing (all in Prism, Graphpad), as appropriate.
3. RESULTS
3.1. Experiment 1: Eight-week depletion
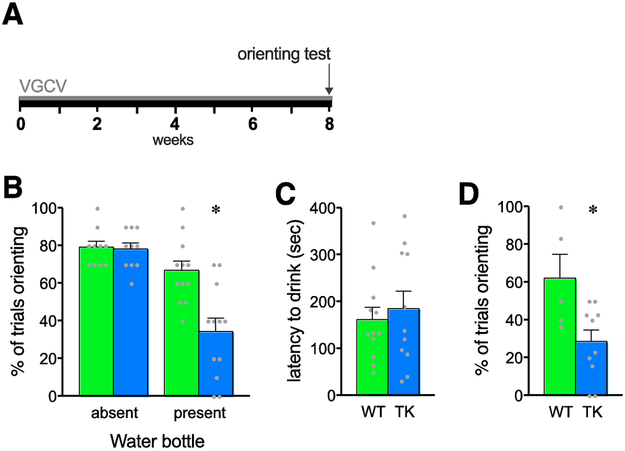
Following 8 weeks of VGCV treatment to inhibit adult neurogenesis, orienting to a novel auditory cue was tested in TK rats and WT controls (Fig 1A). Tests were conducted while rats were drinking from a water bottle or with no bottle in the arena. When no water bottle was present, rats of both genotypes oriented toward the novel stimulus approximately 80% of the time (Fig 1B). However, when rats were drinking, TK rats oriented only half as often as WT rats (Fig 1B). The latency to drink during the test session showed no significant differences across genotype (Fig 1C), suggesting that decreased orienting in TK rats was not due to increased thirst or decreased exploratory drive. Similar orienting in WT and TK rats in the absence of the water bottle suggests that sensory and motor abilities are normal in TK rats. Rats lacking adult neurogenesis, then, show decreased reorienting, or shifting of attention, away from an ongoing behavior toward a novel auditory cue.
Figure 1.
Orienting response rate in rats lacking adult neurogenesis. A. Experimental time course showing 8 weeks of valganciclovir (VGCV) treatment prior to the one-day test. B. The proportion of trials in which rats oriented was decreased in GFAP-TK rats only in the presence of a water bottle (genotype × condition interaction: F1,40 = 9.17, p = .0043, main effect of genotype, F1,40 = 10.37, p = .0025; main effect of water condition, F1,40 = 29.14, p < .0001 by two way ANOVA; * indicates p < 0.05 versus WT in the same condition by Sidak’s multiple comparisons test). C. There was no effect of genotype on drinking onset in the presence of the water bottle (t21 = 0.51, p = .61 by Student’s t-test) D. Decreased orienting in TK rats while drinking from a water bottle was replicated in a second cohort using a pre-recorded auditory stimulus train (t13 = 2.727, p = .02 by Student’s t-test).
3.2. Experiment 2: Eight-week depletion with a recorded stimulus
Following 8 weeks of VGCV treatment to inhibit adult neurogenesis, orienting to a novel auditory cue was tested in TK rats and WT controls using a recorded stimulus. This test was conducted only in rats given access to a water bottle in the arena, and orienting was only analyzed on trials in which rats were drinking when the stimulus began. As in the previous experiment, TK rats that were drinking water oriented only half as often as WT rats while drinking, in 30% of trials compared with 60% (Fig 1D). This finding indicates that the effect of inhibiting adult neurogenesis on orienting is robust, occurring in a second cohort tested using a new recorded stimulus and fixed inter-trial interval by a different investigator.
3.3. Experiment 3: Shorter Periods of Ablation
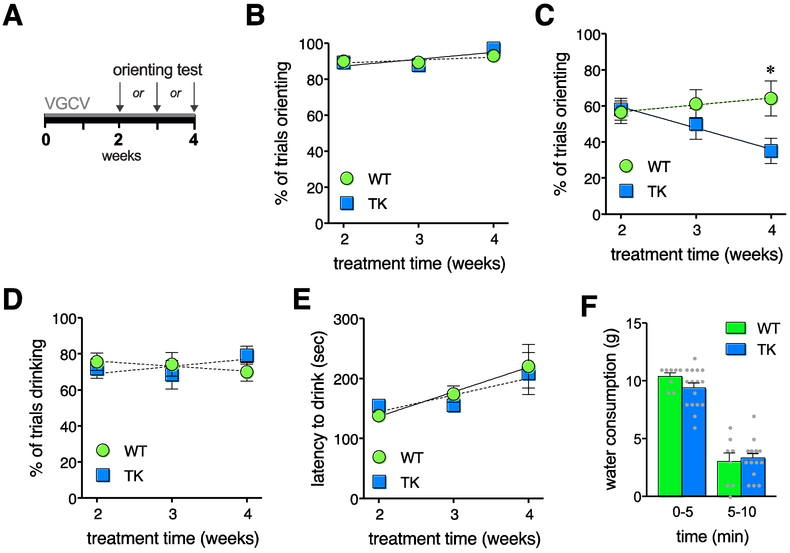
TK rats in Experiments 1 and 2 showed behavior changes in the orienting after receiving VGCV treatment to prevent neurogenesis for 8 weeks. Because the orienting task requires no habituation or training, it is an ideal task for testing the functionality of new neurons at specific ages, e.g., comparing different periods of ablation in order to determine when young neurons become behaviorally functional. Experiment 2 tested orienting after 2 weeks, 3 weeks, or 4 weeks of ablation (Fig 2A).
Figure 2.
Orienting response rate in rats treated to stop neurogenesis for different periods of time. A. Experimental time course showing 2, 3, or 4 weeks of VGCV treatment prior to the one-day test. B. Orienting in the absence of a water bottle was unaffected by genotype (F1,84 = 1.036, p = .31 for slope; F1,85 = 0.084, p = .77 for elevation). C. When animals were drinking, WT and TK rats showed significantly different changes over treatment time (slope: F1, 75 = 4.218, p = .0435), with TK rats showing decreased orienting over time (deviation from zero slope, F1,36 = 4.976, p = .0320; slope = −11.71±5.249) and WT rats showed no detectable effect of treatment time (F1,39 = 0.508, p = .4802; * indicates p = .0072 versus WT at the same time point by Sidak multiple comparisons test. D. The percentage of trials in which rats were drinking did not differ across genotype (slope F1,75 = 1.383, p = .2432; elevation F1,76 = .000, p = .992). E. The latency to begin drinking water during the exploration session showed no effect of genotype (slope F1,75 = 0.3437, p = .5594; elevation F1,76 = 0.085, p = .771). F.Water consumption in the home cage did not differ by genotype (main effect of genotype, F1,22 = 1.003, p = .3276; main effect of time period, F1,22 = 115.9, p < .0001; interaction, F1,22 = 1.106, p = .3044), suggesting that motivation to drink is unaffected by loss of adult neurogenesis.
When no water bottle was present, linear regression analysis showed no effect of genotype or treatment time (Fig 2B), suggesting that TK rats show normal orienting during exploration in the absence of a water bottle. The orienting data were therefore pooled with respect to genotype and showed a small but significant increase in orienting with increasing time of VGCV treatment, which also corresponds with age of the animals (non-zero slope, F1,86 = 6.797, p = .0108).
In the presence of a water bottle (Fig 2C), linear regression analysis comparing the change in orienting over time showed a significant difference in slope between the two genotypes. A negative slope in the TK rats indicated that orienting decreased with increasing treatment duration in this group, while WT rats showed no change in orienting over time. WT and TK rats showed differential orienting after 4 weeks of treatment but not with shorter treatment duration (p = .9199 at 2 weeks, p = 0.5867 at 3 weeks). After 4 weeks of treatment, TK rats showed 45% less orienting than WT rats, similar in magnitude to the 49% decrease observed after 8 weeks. Taken together these data suggest that adult neurogenesis must be inhibited for more than 3 weeks to affect behavior in this task.
A comparison of the proportion of trials in which rats were drinking showed no significant differences across genotype, and data pooled across genotype showed no change in drinking trials over time (F1,77 = 0.017, p = .897). Rats in all groups had the same proportion of valid trials, actively drinking in approximately 80% of the trials (Fig 2D). The latency to begin drinking during the exploration session (Fig 2E) showed no effect of genotype, suggesting that the genotype effect on orienting was not driven by differences in thirst, anxiety-like behavior toward the water bottle, or the timing of the initial auditory cue. When the latency data were pooled across genotype, they showed an increase across time (deviation from zero slope, F1,77 = 9.819, p = .0024), suggesting an increase in drinking latency as animals grow older and/or larger.
3.4. Experiment 4: Home cage drinking
Possible differences in thirst or drinking behavior across genotype at the 4-week treatment time point were also investigated by measuring water consumption in the home cage following 12 hours of water deprivation (Fig 2F). This test showed a decrease in drinking between the first and second halves of the 10-minute test period but showed no effect of genotype or genotype × time interaction (main effect of genotype, F1,22 = 1.003, p = .3276; interaction, F1,22 = 1.106, p = .3044), providing further evidence that differences in orienting in TK rats observed in other experiments are not driven by differences in thirst or drinking behavior.
4. DISCUSSION
Our results show that inhibition of adult neurogenesis decreases orienting toward a novel auditory cue by nearly 50 percent. Importantly, decreased orienting was observed when rats were engaged in drinking from a water bottle but was not observed when rats were simply exploring the empty arena. Testing at different time points after the start of treatment to prevent neurogenesis showed that the change in orienting is not apparent after 2 weeks of neurogenesis inhibition but is fully present after four weeks of ablation. Control data suggest that the effects of new neurons are not driven by changes in thirst, motivation to drink, hearing, or ability to orient in the TK rats. Taken together, these findings demonstrate that adult-born neurons affect behavior in a simple attention orienting task in naïve animals with no prior stress or task-specific learning.
4.1. Functional maturation of new neurons
The time required for new neurons to become functional and participate in hippocampal circuits to affect behavior is not known. The time course of maturation of new neurons has been extensively characterized using molecular markers of mature neurons, growth of dendrites, electrophysiological properties, and expression of activation markers by pharmacological and physiological stimuli [2,3,23-26]. These studies have suggested that new neurons in rats begin to show some features of mature neurons within 2-4 weeks after birth and that most or all new neurons, if they survive the period of developmental cell death, become mature within 4-6 weeks. This time course is faster in rats than in mice and also varies somewhat depending on which specific marker of maturation is used [3]. Some measures suggest special properties of new neurons, including enhanced activation by experience and enhanced synaptic plasticity, which increase over several weeks but then decrease to a mature level [3,27], though it is unclear whether these properties enable new neurons to perform special functions or whether they simply reflect developmental stages of young neurons that are not yet capable altering circuits or behavior in meaningful ways.
Determining when new neurons begin to play a role in behavior requires eliminating or inactivating cohorts of neurons of different ages and testing the effects of these manipulations on neurogenesis-dependent tasks. Here, we show that decreased orienting to novel auditory cues occurs after 4 weeks of inhibition of adult neurogenesis but not after 2 or 3 weeks. Inhibition of adult neurogenesis in this model is rapid and complete [22], suggesting that this delay is not due to slow action of valganciclovir on cell proliferation. Instead, this delay most likely indicates that it takes 4 weeks for new neurons to mature enough to play a role in orienting behavior. However, an alternative possibility is that continued loss of new neurons over this period of time reaches some threshold at which the number of missing new neurons is large enough to affect behavior. For example, neurons may only require 2 weeks of maturation to be functional, but there may be enough 3- or 4-week-old neurons to take their place if they are ablated. Several other studies have shown behavioral effects at the 4 week time point after inhibiting adult neurogenesis [3,28,29], and no effect with loss of neurons for shorter periods of time [3,30,31], both consistent with the current study. However, a few studies have found changes in behavior 2-3 weeks after halting adult neurogenesis[10,11,32], suggesting that the time required for functional maturation of new neurons may be task-dependent. Environmental conditions, which may be related to training in particular tasks, might also alter the course of maturation. For example, the behavioral paradigms showing the most rapid effects of inhibiting adult neurogenesis, trace eyeblink conditioning and contextual fear conditioning [10,11,32], use shock as an unconditioned stimulus, suggesting the possibility that this training-related stressor might accelerate the maturation of the new neurons in the control animals [33], leading to a more rapid behavioral effect of new neurons.
4.2. New neurons in the dentate gyrus are likely responsible for the observed behavioral changes
Neurogenesis in our pharmacogenetic model is inhibited not only in the dentate gyrus but also in the olfactory bulb granule cells and a subset of the periglomerular cells [22], and possibly other populations [34,35]. However, it is likely that the loss of new neurons in the dentate gyrus is responsible for the decrease in orienting, because a similar decrease has been observed in rats with hippocampal lesions [21]. This earlier study found that bilateral aspiration lesions of the hippocampus in adult rats inhibited orienting to a novel auditory cue if attention was focused on a water bottle or on a novel, neutral visual cue. This effect is somewhat surprising, as the hippocampus is not generally considered to be an important node in attention circuits [36]. However, other early studies also observed effects of hippocampal lesions on distractability in operant and runway tasks [36-38]. More recent findings from human fMRI and electrophysiology studies also point to a role for the hippocampus in automatic orienting to novel stimuli [39,40].
4.3. Decreased distraction could impair or improve behavioral performance in different tasks
A role for adult neurogenesis in this particular behavior is noteworthy because it is a simple, unlearned behavioral response, very much at odds with many of the complex behavioral discrimination paradigms shown to rely on new neurons [7,9]. This orienting task has no specific learning or memory component, and involves no discrimination of similar stimuli, suggesting that the behavioral effects are unlikely to be caused by impairment of learning, memory, or pattern separation. Impaired orienting, or disengagement of attention, however, could potentially impact these and other behaviors. Declarative memory encoding and retrieval are both strongly dependent on attentional processes [41,42]. While decreased disengagement of attention might be expected to impair behavior in some cases, it would likely have no effect on or even improve behavior in other tasks, depending on the specific task demands. Tasks without multiple cues presented simultaneously should be unaffected by the specific attentional changes observed here, because rats lacking adult neurogenesis shifted attention to a novel cue normally in the absence of salient pre-potent behaviors. This is consistent with the normal behavior of rodents lacking adult neurogenesis in many cued learning tasks. Tasks that require disengagement from one behavior and switching to another could be impaired by this effect of hippocampal or new neuron ablation, consistent with deficits in behavioral inhibition, or braking of prepotent behaviors, described in early studies of hippocampal function [4,38,43-46]. Enhanced focus on a single cue or prepotent behavior could also potentially produce deficits similar to those seen in mice with a hippocampus-specific NMDA receptor deletion, which swim toward the incorrect beacon when they are placed in the pool closer to it but make the correct choice when they start closer to the correct beacon or equidistant from both [47]. In contrast, tasks that benefit from focusing on a particular stimulus in the presence of potential distractors might be expected to improve following hippocampal lesion or inhibition of adult neurogenesis. This may be related the more rapid learning of shuttle box and visual discrimination tasks after hippocampal lesions [47,48], as well as the improved performance of mice and rats lacking adult neurogenesis in pattern separation tasks [48], in a maze task with distracting odorants [49], and on a radial arm maze working memory task containing distractors that were correct in previous trials [50].
4.4. Orienting and emotion
One important question that has not yet been addressed is the extent to which emotion plays a role in the decreased orienting in the absence of adult neurogenesis. In the current experiments, rats failed to attend to a seemingly neutral novel stimulus when they are occupied by a rewarding behavior (drinking when thirsty). Whether the normal animals attend to the new stimulus because it is potentially frightening, as many unexpected noises can be, or simply because it is novel is currently unknown. The study that originally showed a re-orienting deficit in rats with hippocampal lesions showed a similar effect with an auditory cue of no obvious motivational significance as the initial stimulus and a light as the distractor stimulus, but it is difficult to judge the emotional content of apparently neutral stimuli for lab animals without additional tests. Anxiety can enhance startle to a loud noise, suggesting that differences in emotional baseline might affect orienting or other responses to novel noises as well. However, naïve WT and TK rats show no differences in baseline anxiety levels [17,22], and TK rats show the same rate of orienting to the novel stimulus as WT rats in the absence of a water bottle. Work in monkeys has shown that hippocampal lesions result in a general lack of interest in innately fear-provoking objects and decreased distraction by these objects when reaching over them for rewards [51,52]. Hippocampus-lesioned monkeys showed decreased latencies in trials with neutral objects as well, though the lesion effect was greater in the fearful object trials, primarily due to the large increase in latency shown by the control monkeys [52]. Together, these findings indicate that hippocampal lesions, and by extension possibly new granule neurons, alter distraction by both neutral and emotionally charged (reinforcing or aversive) cues across multiple sensory modalities, with greater behavioral differences in response to emotional cues due to the enhanced attention paid to these stimuli by intact animals.
4.5. Distraction and interactions with the environment throughout life
Difficulty in disengagement of attention has been suggested as a key feature of autism spectrum disorder (ASD), potentially underlying several other symptoms [53]. One study [54] found that children with an ASD diagnosis showed longer latencies to attend to a new visual stimulus if an earlier one remained, in some trials failing to look at the second stimulus for the entire 8-second trial. This effect, along with the finding that these children shifted attention normally if the first visual stimulus disappeared before the other appeared, seems to parallel the changes observed here in animals lacking adult neurogenesis. Structural changes in the hippocampus have been observed in ASD [55,56], but the dentate gyrus granule cells have not previously been identified as a key population. A role for dentate gyrus granule neurons in disengagement of attention could provide a novel functional link between structural changes in the hippocampus and ASD symptoms. As ASD is a developmental disorder, changes in adult neurogenesis, specifically, are clearly not relevant, but the dentate gyrus generates new granule neurons at an even higher rate throughout development than it does in adulthood [57,58].
Our results suggest that throughout adulthood, changes in the rate of neurogenesis may alter the balance of focus on the current task versus distractibility, or alertness toward unexpected changes in the environment. The optimal balance of focus and distractibility is likely to differ depending on the environment, and control by ongoing neurogenesis may provide a mechanism for biasing this balance over periods of several weeks. The addition of new neurons may always increase attention to environmental change as directly indicated by the current findings, or alternatively, new neurons may increase an organism’s ability to adaptively shift between being focused and being distractible by outside stimuli, similar to the role suggested for new neurons in fear generalization [5]. By biasing which stimuli are attended to, adult neurogenesis could alter learning and memory and have very broad effects on interactions with the environment.
Highlights:
Adult neurogenesis increases orienting toward a novel stimulus during focused activity
Rats without ongoing neurogenesis orient normally when they are exploring
Four-week-old neurons affect orienting behavior, but younger neurons do not
Changes in refocusing of attention, or distraction, could affect memory tasks
Acknowledgements
This research was supported by the Intramural Research Program of the NIMH (ZIAMH002784).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Jahn HM, Bergami M, Critical periods regulating the circuit integration of adult-born hippocampal neurons, Cell Tissue Res. 371 (2018) 23–32. doi: 10.1007/s00441-017-2677-x. [DOI] [PubMed] [Google Scholar]
- [2].Toni N, Schinder AF, Maturation and Functional Integration of New Granule Cells into the Adult Hippocampus, Cold Spring Harb Perspect Biol 8 (2016) a018903. doi: 10.1101/cshperspect.a018903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Snyder JS, Choe JS, Clifford MA, Jeurling S.l., Hurley P, Brown A, et al. , Adult-born hippocampal neurons are more numerous, faster maturing, and more involved in behavior in rats than in mice, J Neurosci. 29 (2009) 14484–14495. doi: 10.1523/JNEUROSCI.1768-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Cameron HA, Glover LR, Adult neurogenesis: beyond learning and memory, Annual Review of Psychology. 66 (2015) 53–81. doi: 10.1146/annurev-psych-010814-015006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Glover LR, Schoenfeld TJ, Karlsson R-M, Bannerman DM, Cameron HA, Ongoing neurogenesis in the adult dentate gyrus mediates behavioral responses to ambiguous threat cues, PLoS Biol. 15 (2017) e2001154. doi: 10.1371/journal.pbio.2001154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Clelland CD, Choi M, Romberg C, Clemenson GD, Fragniere A, Tyers P, et al. , A functional role for adult hippocampal neurogenesis in spatial pattern separation, Science. 325 (2009) 210–213. doi: 10.1126/science.1173215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Nakashiba T, Cushman JD, Pelkey KA, Renaudineau S, Buhl DL, McHugh TJ, et al. , Young dentate granule cells mediate pattern separation, whereas old granule cells facilitate pattern completion, Cell. 149 (2012) 188–201. doi: 10.1016/j.cell.2012.01.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Tronel S, Fabre A, Charrier V, Oliet SHR, Gage FH, Abrous DN, Spatial learning sculpts the dendritic arbor of adult-born hippocampal neurons, Proc Natl Acad Sci USA. 107 (2010) 7963–7968. doi: 10.1073/pnas.0914613107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Sahay A, Scobie KN, Hill AS, O'Carroll CM, Kheirbek MA, Burghardt NS, et al. , Increasing adult hippocampal neurogenesis is sufficient to improve pattern separation, Nature. 472 (2011) 466–470. doi: 10.1038/nature09817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Shors TJ, Miesegaes G, Beylin A, Zhao M, Rydel T, Gould E, Neurogenesis in the adult is involved in the formation of trace memories, Nature. 410 (2001) 372–376. doi: 10.1038/35066584. [DOI] [PubMed] [Google Scholar]
- [11].Shors TJ, Townsend DA, Zhao M, Kozorovitskiy Y, Gould E, Neurogenesis may relate to some but not all types of hippocampal-dependent learning, Hippocampus. 12 (2002) 578–584. doi: 10.1002/hipo.10103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Gazzara RA, Altman J, Early postnatal x-irradiation of the hippocampus and discrimination learning in adult rats, J Comp Physiol Psychol. 95 (1981) 484–495. [DOI] [PubMed] [Google Scholar]
- [13].Beylin AV, Gandhi CC, Wood GE, Talk AC, Matzel LD, Shors TJ, The role of the hippocampus in trace conditioning: temporal discontinuity or task difficulty? Neurobiol Learn Mem. 76 (2001) 447–461. doi: 10.1006/nlme.2001.4039. [DOI] [PubMed] [Google Scholar]
- [14].Gao A, Xia F, Guskjolen AJ, Ramsaran AI, Santoro A, Josselyn SA, et al. , Elevation of Hippocampal Neurogenesis Induces a Temporally Graded Pattern of Forgetting of Contextual Fear Memories, J Neurosci. 38 (2018) 3190–3198. doi: 10.1523/JNEUROSCI.3126-17.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Snyder JS, Hong NS, McDonald RJ, Wojtowicz JM, A role for adult neurogenesis in spatial long-term memory, Neuroscience. 130 (2005) 843–852. doi: 10.1016/j.neuroscience.2004.10.009. [DOI] [PubMed] [Google Scholar]
- [16].Akers KG, Martinez-Canabal A, Restivo L, Yiu AP, De Cristofaro A, Hsiang H-LL, et al. , Hippocampal neurogenesis regulates forgetting during adulthood and infancy, Science. 344 (2014) 598–602. doi: 10.1126/science.1248903. [DOI] [PubMed] [Google Scholar]
- [17].Groves JO, Leslie I, Huang G-J, Mchugh SB, Taylor A, Mott R, et al. , Ablating adult neurogenesis in the rat has no effect on spatial processing: evidence from a novel pharmacogenetic model, PLoS Genet. 9 (2013) el003718. doi: 10.1371/journal.pgen.1003718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Snyder JS, Soumier A, Brewer M, Pickel J, Cameron HA, Adult hippocampal neurogenesis buffers stress responses and depressive behaviour, Nature. 476 (2011) 458–461. doi: 10.1038/nature10287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].David DJ, Samuels BA, Rainer Q, Wang J-W, Marsteller D, Mendez I, et al. , Neurogenesis-dependent and -independent effects of fluoxetine in an animal model of anxiety/depression, Neuron. 62 (2009) 479–493. doi: 10.1016/j.neuron.2009.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Schoenfeld TJ, Rhee D, Martin L, Smith J, Sonti A, Padmanaban V, et al. New Neurons Restore Structural and Behavioral Abnormalities in a Rat Model of PTSD, Hippocampus. (n.d.). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Hendrickson CW, Kimble RJ, Kimble DP, Hippocampal lesions and the orienting response, J Comp Physiol Psychol. 67 (1969) 220–227. [DOI] [PubMed] [Google Scholar]
- [22].Snyder JS, Grigereit L, Russo A, Seib D, Brewer M, Pickel J, et al. , A transgenic rat for specifically inhibiting adult neurogenesis, Eneuro. (2016) 1–13. doi: 10.1523/ENEURO.0064-16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Cameron HA, Woolley CS, McEwen BS, Gould E, Differentiation of newly born neurons and glia in the dentate gyrus of the adult rat, Neuroscience. 56 (1993)337–344. [DOI] [PubMed] [Google Scholar]
- [24].Song H, Kempermann G, Overstreet Wadiche L, Zhao C, Schinder AF, Bischofberger J, New neurons in the adult mammalian brain: synaptogenesis and functional integration, J Neurosci. 25 (2005) 10366–10368. doi: 10.1523/JNEUROSCI.3452-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Bergami M, Masserdotti G, Temprana SG, Motori E, Eriksson TM, Göbel J, et al. , A critical period for experience-dependent remodeling of adult-born neuron connectivity, Neuron. 85 (2015) 710–717. doi: 10.1016/j.neuron.2015.01.001. [DOI] [PubMed] [Google Scholar]
- [26].Temprana SG, Mongiat LA, Yang SM, Trinchero MF, Alvarez DD, Kropff E, et al. , Delayed coupling to feedback inhibition during a critical period for the integration of adult-born granule cells, Neuron. 85 (2015) 116–130. doi: 10.1016/j.neuron.2014.11.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Ge S, Yang C-H, Hsu K-S, Ming G-L, Song H, A critical period for enhanced synaptic plasticity in newly generated neurons of the adult brain, Neuron. 54 (2007) 559–566. doi: 10.1016/j.neuron.2007.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Deng W, Saxe MD, Gallina IS, Gage FH, Adult-born hippocampal dentate granule cells undergoing maturation modulate learning and memory in the brain, J Neurosci. 29 (2009) 13532–13542. doi: 10.1523/JNEUROSCI.3362-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Winocur G, Wojtowicz JM, Sekeres M, Snyder JS, Wang S, Inhibition of neurogenesis interferes with hippocampus-dependent memory function, Hippocampus. 16 (2006) 296–304. doi: 10.1002/hipo.20163. [DOI] [PubMed] [Google Scholar]
- [30].Gu Y, Arruda-Carvalho M, Wang J, Janoschka SR, Josselyn SA, Frankland PW, et al. , Optical controlling reveals time-dependent roles for adult-born dentate granule cells, Nat Neurosci. 15 (2012) 1700–1706. doi: 10.1038/nn.3260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Denny CA, Burghardt NS, Schachter DM, Hen R, Drew MR, 4- to 6-week-old adult-born hippocampal neurons influence novelty-evoked exploration and contextual fear conditioning, Hippocampus. 22 (2012) 1188–1201. doi: 10.1002/hipo.20964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Seo D-O, Carillo MA, Chih-Hsiung Lim S, Tanaka KF, Drew MR, Adult Hippocampal Neurogenesis Modulates Fear Learning through Associative and Nonassociative Mechanisms, J Neurosci. 35 (2015) 11330–11345. doi: 10.1523/JNEUROSCI.0483-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Snyder JS, Glover LR, Sanzone KM, Kamhi JF, Cameron HA, The effects of exercise and stress on the survival and maturation of adult-generated granule cells, Hippocampus. 19 (2009) 898–906. doi: 10.1002/hipo.20552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Dayer AG, Cleaver KM, Abouantoun T, Cameron HA, New GABAergic interneurons in the adult neocortex and striatum are generated from different precursors, J Cell Biol. 168 (2005) 415–427. doi: 10.1083/jcb.200407053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Inta D, Cameron HA, Gass P, New neurons in the adult striatum: from rodents to humans, Trends Neurosci. 38 (2015) 517–523. doi: 10.1016/j.tins.2015.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Gustafson JW, Distractibility and reactivity under different response conditions following hippocampal lesions in rats, Behav Biol. 15 (1975) 479–484. doi: 10.1016/S0091-6773(75)92280-4. [DOI] [PubMed] [Google Scholar]
- [37].Crowne DP, W.I. Riddell, Hippocampal lesions and the cardiac component of the orienting response in the rat, J Comp Physiol Psychol. 69 (1969) 748–755. [DOI] [PubMed] [Google Scholar]
- [38].Wickelgren WO, Isaacson RL, Effect of the introduction of an irrelevant stimulus on runway performance of the hippocampectomized rat, Nature. 200 (1963) 48–50. [DOI] [PubMed] [Google Scholar]
- [39].Knight R, Contribution of human hippocampal region to novelty detection, Nature. 383 (1996) 256–259. doi: 10.1038/383256a0. [DOI] [PubMed] [Google Scholar]
- [40].Yamaguchi S, Hale LA, D'Esposito M, Knight RT, Rapid prefrontal-hippocampal habituation to novel events, J Neurosci. 24 (2004) 5356–5363. doi: 10.1523/JNEUROSCI.4587-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Muzzio IA, Kentros C, Kandel E, What is remembered? Role of attention on the encoding and retrieval of hippocampal representations, J Physiol (Lond). 587 (2009) 2837–2854. doi: 10.1113/jphysiol.2009.172445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Chun MM, Turk-Browne NB, Interactions between attention and memory, Curr Opin Neurobiol. 17 (2007) 177–184. doi: 10.1016/j.conb.2007.03.005. [DOI] [PubMed] [Google Scholar]
- [43].Douglas RJ, The hippocampus and behavior, Psychol Bull. 67 (1967) 416–422. [DOI] [PubMed] [Google Scholar]
- [44].Altman J, Brunner RL, Bayer SA, The hippocampus and behavioral maturation, Behav Biol. 8 (1973) 557–596. [DOI] [PubMed] [Google Scholar]
- [45].Takahashi LK, Glucocorticoids, the hippocampus, and behavioral inhibition in the preweanling rat, J Neurosci. 15 (1995) 6023–6034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Isaacson RL, Kimble DP, Lesions of the limbic system: their effects upon hypotheses and frustration, Behav Biol. 7 (1972) 767–793. [DOI] [PubMed] [Google Scholar]
- [47].Bannerman DM, Bus T, Taylor A, Sanderson DJ, Schwarz I, Jensen V, et al. , Dissecting spatial knowledge from spatial choice by hippocampal NMDA receptor deletion, Nat Neurosci. 15 (2012) 1153–1159. doi: 10.1038/nn.3166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Reynolds RP, Whoolery CW, Lucero MJ, Ito N, Redfield RL, Richardson DR, et al. , Exposure to space radiation reduces neurogenesis but enhances pattern separation in both aversive and appetitive testing platforms in mature mice, in: SFN Abstract, 2017: p. 115.04. [Google Scholar]
- [49].Schoenfeld TJ, Rhee D, Cameron HA, The impact of neurogenesis on flexible maze training: effects on hippocampal volume, depression, and cognition, in: SFN Abstract, 2016: p. 740.08. [Google Scholar]
- [50].Saxe MD, Malleret G, Vronskaya S, Mendez I, Garcia AD, Sofroniew MV, et al. , Paradoxical influence of hippocampal neurogenesis on working memory, Proc Natl Acad Sci USA. 104 (2007) 4642–4646. doi: 10.1073/pnas.0611718104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Chudasama Y, Wright KS, Murray EA, Hippocampal lesions in rhesus monkeys disrupt emotional responses but not reinforcer devaluation effects, Biol Psychiatry. 63 (2008) 1084–1091. doi: 10.1016/j.biopsych.2007.11.012. [DOI] [PubMed] [Google Scholar]
- [52].Chudasama Y, Izquierdo A, Murray EA, Distinct contributions of the amygdala and hippocampus to fear expression, Eur J Neurosci. 30 (2009) 2327–2337. doi: 10.1111/j.1460-9568.2009.07012.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Keehn B, Müller R-A, Townsend J, Atypical attentional networks and the emergence of autism, Neurosci Biobehav Rev. 37 (2013) 164–183. doi: 10.1016/j.neubiorev.2012.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Landry R, Bryson SE, Impaired disengagement of attention in young children with autism, J Child Psychol Psychiatry. 45 (2004) 1115–1122. doi: 10.1111/j.1469-7610.2004.00304.x. [DOI] [PubMed] [Google Scholar]
- [55].Hoon AH, Reiss AL, The mesial-temporal lobe and autism: case report and review, Dev Med Child Neurol. 34 (1992) 252–259. [DOI] [PubMed] [Google Scholar]
- [56].Bauman ML, Kemper TL, Neuroanatomic observations of the brain in autism: a review and future directions, Int J Dev Neurosci. 23 (2005) 183–187. doi: 10.1016/j.ijdevneu.2004.09.006. [DOI] [PubMed] [Google Scholar]
- [57].Bayer SA, Altman J, Russo RJ, Zhang X, Timetables of neurogenesis in the human brain based on experimentally determined patterns in the rat, Neurotoxicology. 14 (1993) 83–144. [PubMed] [Google Scholar]
- [58].Snyder JS, Cameron HA, Could adult hippocampal neurogenesis be relevant for human behavior? Behav Brain Res. 227 (2012) 384–390. doi: 10.1016/j.bbr.2011.06.024. [DOI] [PMC free article] [PubMed] [Google Scholar]