Abstract
Caveolin-1 (Cav-1) appears to be both a pathophysiological contributor and a target in different inflammatory and hyperproliferative skin conditions as well as in skin aging. Skin fibroblasts demonstrate an up-regulation of Cav-1 expression both in chronological and UV-induced aging, and such an up-regulation was observed both in vitro and in vivo. Typical alterations in aging skin involve a reduction of the dermis thickness, a significant expansion of the dermal white adipose tissue as well as modifications of the content and distribution of hyaluronan, impairment of autophagic flux, a reduction of collagen expression and an increase in tissue inflammation. All of these phenomena can be connected with changes in Cav-1 expression in the aging skin. Modified expression of Cav-1 can also significantly influence the mechanical properties of individual skin layers, thus changing the total mechanical stability of the layered composite skin/WAT, leading to typical structural modifications of the skin surface in the aging skin. Selective reduction of Cav-1 expression has the potential to exert anti-aging effects on the skin.
Keywords: Caveolin-1, skin aging, hyaluronan, collagen, inflammation, TGF-β, dermal-epidermal junction, dermal-hypodermal junction
1. Introduction
Plasma membranes of eukaryotic cells have spatially heterogeneous structures containing lipid clusters enriched in cholesterol and sphingolipids which are referred to as lipid rafts. Lipid rafts can appear in the form of planar structures or caveolae - plasma membrane invaginations forming nanodomains with a typical size of 50–100 nm which are especially prevalent in mechanically stressed cells (Eharri et al., 2015). They are involved in rapid adaptation to cellular volume changes, in various signal transduction processes, and in the processes of endo- and exocytosis (Parton & del Pozo, 2013; Parton, 2018).
Caveolae are enriched for a number of characteristic proteins, such as cavins and caveolins (Cav’s). The presence of caveolin-1 (Cav-1), which is the principal structural component of caveolae, is necessary for their characteristic appearance. Cav-1 is not exclusively localized to the plasma membrane, but was also found in different intracellular compartments (Fridolfsson et al., 2012), and can be transported in extracellular vesicles, providing a long-range mechanism of communication inside of a tissue or even between adjacent tissues (Chang et al., 2017; Crewe et al., 2018). Relative levels and spatial distribution of Cav-1 in extracellular and intracellular compartments are subject to significant changes under pathological conditions as well as over the course of aging and can be quickly affected by different external chemical and physical factors (Sinha et al., 2011). Cav-1 demonstrates a stratified pattern of differential expression in various skin layers (Sando et al., 2003). High expression of Cav-1 is seen at the stratum granulosum / stratum corneum interphase and in the stratum basale as well as in the dermis, especially in association with connective tissue and endothelial cells.
Cav-1 expression correlates with aging both in vitro and in vivo. It is strongly up-regulated in human diploid fibroblasts displaying a senescent phenotype in vitro (Park et al., 2000). Overexpression of Cav-1 leads to higher density of caveolae in plasma membrane (Cho et al., 2003) as well to the morphological modifications of these cells mainly concerning their focal adhesion and formation of actin stress fibres (Cho et al., 2004). Human corneal epithelial cells also demonstrate a continuous increase of Cav-1 levels with aging, and in aged subjects, these cells display almost five times more caveolae than in young subjects [Rhim et al., 2010]. Additionally, oxidative stress leads to an upregulation of Cav-1 expression, which was connected with the development of premature cellular senescence (Zou et al., 2011). Consequently, the overexpression of Cav-1 may be typical not only in chronological but also in photo-induced aging. Very recently, it was reported that in vitro irradiation of melanocytes and keratinocytes with UV-B radiation (10 mJ/cm2) significant increased the Cav-1 expression in these cells (Domingues et al., 2019). Additionally, low-dose UV-C irradiation of mouse embryonic fibroblasts, which is not directly involved in photoaging effects in vivo, also dramatically increased Cav-1 expression in these cells (Volonte et al., 2002). Aging-connected cellular modifications are so heavily pronounced that it was even suggested that Cav-1 could be used as a marker for cellular senescence, at least in fibroblasts and endothelial cells (Hernandez-Segura et al., 2018), and correspondingly, that Cav-1 downregulation could serve as a potential anti-aging approach (Lee et al., 2015). Importantly, development of cellular senescence in vitro can be reversed by the specific reduction of Cav-1 levels in fibroblasts (Cho et al., 2003) indicating that excessive Cav-1 may be causally connected with the aging process.
In contrast, severe Cav-1 deficiency can also produce premature senescence in at least some cell types, and this effect was connected with associated mitochondrial dysfunction (Yu et al., 2017). Therefore, both overexpression and strong suppression of Cav-1 levels can lead to premature cellular senescence, whereas regular cell functions can be restored by normalizing the expression of Cav-1.
Chronologically aged murine and human skin also demonstrate a progressive upregulation of Cav-1 in vivo, which strongly correlates with a reduction of Col1 expression (Lee et al., 2015). In these experiments, the long-term application of methyl-β-cyclodextrin (MβCD), provided anti-aging effects in the skin, causing a significant increase of the dermal thickness and an induction of Col1 expression. MβCD is a cholesterol-depleting agent that disrupts caveolae and demonstrates skin-specific anti-Cav-1 activity inhibiting Cav-1 expression (Ao et al., 2016).
These results collectively demonstrate that the strong modification of Cav-1 expression correlates with progressive skin aging both in vitro and in vivo and may be directly involved in chronological as well as in photo-induced skin aging. In a series of recent articles, we and others have linked local modification of Cav-1 levels in the pathophysiology of a number of disease- and aging-phenomena. This includes, but is not limited to, skin aging (Lee et al., 2015), psoriasis (Kruglikov & Scherer, 2019a), and hypertrophic scarring / fibrosis (Shihata et al., 2017; Kruglikov & Scherer, 2019b). Here we want to highlight how Cav-1 expression levels are connected with well-known macroscopic and mesoscopic structural modifications in the aging skin.
2. Macro- and mesoscopic alterations in the aging skin
Skin aging leads to various macroscopic and mesoscopic alterations within the structures of the individual skin layers as well as in the interactions between these layers, thereby affecting their mechanical stability and resulting in the appearance of typical signs of aging (Kruglikov & Scherer, 2018). These alterations affect both the composition of the skin cell population as well as the interactions between the skin cells and the extracellular matrix (ECM).
2.1. Macroscopic skin alterations in the aging skin
Macroscopically, aging in rodent skin leads to some clear histological alterations, including a reduction of the dermis thickness, a significant expansion of the dermal white adipose tissue (dWAT), and a reduction of the subcutaneous white adipose tissue layer (sWAT) (Rodriguez et al., 2016; Salzer et al., 2018).
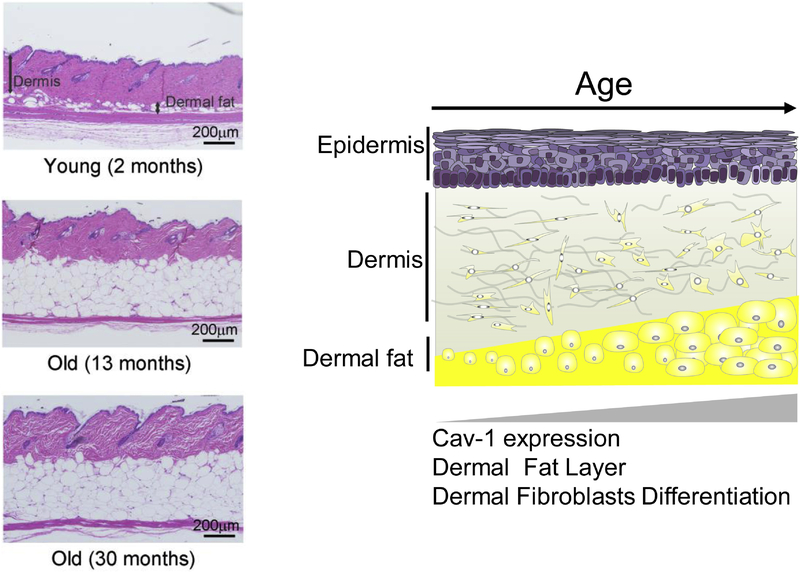
A comparison of the skin samples obtained from the Cav-1−/− mice and control Cav-1+/+ littermates demonstrated that in animals lacking Cav-1, the total dermis thickness is significantly increased, whereas the dWAT layer is strongly reduced (Lee et al., 2015). On the other hand, long-term application of MβCD in aged animals also caused a significant increase of the dermal thickness and an induction of Col1 expression (Lee et al., 2015). Of note, reduction of dWAT can provide an effective increase of dermis thickness through transformation of adipocytes in fibroblast-like cells (Kruglikov et al., 2018; Zhang et al., 2019), which demonstrates a strong cooperation between these two layers (Figure 1).
Fig. 1:
Left: Skin histology (H&E stained) in young (2 months old), middle aged (13 months) and old mice (30 months). Right: Schematic representation of the aging skin.
The volume of dWAT is regulated not only through the processes of lipolysis and lipogenesis, but also through de-differentiation of mature adipocytes into preadipocytes as well as through re-differentiation of these cells into mature adipocytes (Kruglikov et al., 2018). Cav-1 is known to be strongly associated with the differentiation of preadipocytes to mature cells (Scherer et al., 1994) and is also very likely involved in the de-differentiation of mature adipocytes. Very recently, we have demonstrated that these reversible processes are mainly responsible for the evolution of dWAT volume during hair follicle cycling (Zhang et al., 2019). It can be speculated that the significant expansion of dWAT volume in the aging skin may be connected with an impairment of this mechanism; however, this statement must be carefully examined in future research.
These results demonstrate that Cav-1 must be substantially involved in development of macroscopic skin alterations observed in aging skin and that specific reduction of Cav-1 content can provide improvements of macroscopic skin structure, leading to anti-aging effects.
2.2. Mesoscopic skin alterations in aging
Mesoscopically, the dermis of newborn mice contains different types of fibroblasts: papillary fibroblasts, reticular fibroblasts, and pro-adipogenic fibroblasts which exist in two distinct forms and are located near the dermis-hypodermis junction (Driskell et al., 2013). Aging is associated with the accumulation of pro-adipogenic traits in skin fibroblasts (among them, enhanced levels of the master adipogenic factor, PPARγ); these modifications take place at the expense of more traditional fibroblast characteristics (Salzer et al., 2018). These alterations are sensitive to systemic metabolic changes: whereas a high fat diet enhances these modifications, the long-term caloric restriction is able to prevent them (Salzer et al., 2018). Of note also, the density of fibroblasts is higher in the papillary than in the reticular dermis, and the papillar fibroblasts are prone to differentiation into reticular fibroblasts after prolonged cultivation in vitro (Janson et al., 2013). Whether the de-differentiation of mature dermal adipocytes and the appearance of cells with intermediate phenotype near the dermal-hypodermal junction are involved in the aging-associated modulation of the phenotype of the fibroblasts remains to be elucidated.
Skin fibroblasts in vivo are organized in stable clusters whose location is not changed over time despite the loss of some cluster members (Marsh et al., 2018). This spatial stability is provided by a rac1-dependent extension of fibroblast membranes which fill the space of the lost cells. At the same time, rac1 protein levels in mouse fibroblasts are strongly controlled by Cav-1 (Nethe et al., 2010). We can therefore infer that Cav-1 expression is substantially involved in the regulation of the spatial dermal structure.
Cav-1 secreted from WAT adjacent to the dermis-hypodermis junction and transmitted to the dermis through exosomal transport may act as an enhancer of adipogenesis (Chang et al., 2017). This “membrane exchange” is strongly dependent on the metabolic status of adipose tissue, and is significantly higher in hypertrophic WAT. Since dWAT demonstrates pronounced hypertrophy in aging murine skin (Salzer et al., 2018), Cav-1 secretion from adipocytes must be increased with aging. Such a long-range interaction through Cav-1 transport seems to be of general physiological importance. We recently described similar long-range exosomal exchanges of Cav-1 between epithelial cells and adipocytes (Crewe et al., 2018). Related mechanisms are reported for the long-range regulation of the ECM deposition and remodeling in stromal fibroblasts (Albacete-Albacete et al., 2018). Very recently, we argued that exosomal exchange of Cav-1 between the epidermis and WAT must be an important pathophysiological step in psoriasis (Kruglikov & Scherer, 2019a).
As a result, the aged fibroblasts shift towards a more adipogenic phenotype receiving the mixed adipo-epithelial content and thus occupying an intermediate state between “pure” fibroblasts and mature adipocytes. A similar statement concerning varying phenotypes of adipocytes located in dWAT was provided in (Kruglikov & Scherer, 2016) and experimentally proven in (Zhang et al., 2019). Cells with an intermediate adipo-epithelial phenotype are found not only in the skin, but are also clearly apparent in the lung where they are known as “lipofibroblasts” (Rehan et al., 2006), in the mammary gland during pregnancy (Prokesch et al., 2014), and in breast cancer where these cells are placed near the front between the malignant and healthy tissues and are known as cancer-associated fibroblasts (CAFs) (Bochet et al., 2013). Of note, CAFs demonstrate significantly reduced expression of Cav-1 (Qiang et al., 2019).
Cav-1 expression significantly increases with adipogenesis, displaying dramatically higher levels in mature adipocytes than in preadipocytes. Mature adipocytes in subjects demonstrating hypertrophic growth of adipose tissue have higher levels of Cav-1, and its absolute values positively correlate with the change in adipocyte size. In contrast, mature adipocytes in subjects demonstrating hyperplastic growth of adipose tissue do not show such a correlation (Briand et al., 2014). Thus, increased Cav-1 expression in adipocytes is generally associated with their hypertrophic expansion. Whereas the average cell size progressively increases in aging, the surface density of caveolae in adipocytes decreases with increasing age of the mice (Hulstrøm et al., 2013). These processes can significantly affect the spatial structure of dermis and dWAT in aging skin, influencing the de-differentiation of mature dermal adipocytes into adipocyte-derived preadipocytes and the subsequent re-differentiation of these cells into mature adipocytes (Kruglikov et al., 2018; Zhang et al., 2019).
These results demonstrate that the cellular make-up of the skin significantly varies with aging, and this variation can strongly influence the mechanical properties of different skin layers and thus, influences the appearance of skin aging signs. Cav-1 may be critically involved in these processes.
2.3. Collagen expression and inflammation in skin aging
Reduced collagen expression and aging-associated inflammation (sometimes referred to as “inflammaging” are both accepted hallmarks of skin aging (Uitto & Bernstein, 1998; Franceschi et al. 2018).
Cav-1 demonstrates a pronounced negative correlation with expression of collagens, which, among many other settings, was reported in chronologically aged skin (Lee et al., 2015), scleroderma (Castello et al., 2011), keloids (Zhang et al., 2011a; Hsu et al., 2018), and hypertrophic scars (Zhang et al., 2011b). Moreover, suppression of Cav-1 can lead to a pronounced up-regulation of collagen expression in the skin without triggering any degree of skin fibrosis (Lee et al., 2015). This effect was connected with the localization of TGF-β within caveolae in the plasma membrane, as well as with the physical interaction of Cav-1 with TGF-β receptors (Ito et al., 2004): Cav-1 is involved in the internalization of these receptors, which then undergo rapid intracellular degradation (del Galdo et al., 2008).
Cav-1 is also directly and indirectly involved in tissue inflammation. Strong suppression of Cav-1 through application of MβCD caused a dramatic increase of extracellular release of IL-8 (Mathay et al., 2011), which must participate in development of inflammation. Elimination of Cav-1 induces polarization of M2 macrophages in mice (Shivshankar et al., 2014). High levels of Cav-1 expression in the aging skin should prevent this polarization and shift the ratio of M1 vs. M2 macrophages more towards the M1 subtype that can secrete mediators promoting inflammation. Indeed, it was reported that the M1/M2 ratio significantly increases in aging (Gonzalez et al., 2015).
The epidermal lamellar bodies (lamellar granules) are specialized organelles that are secreted by skin keratinocytes. Electron microscopic analysis demonstrates abundant lamellar bodies in the extracellular space of the upper dermis. These lamellar bodies form an impermeable lipid containing membrane that is necessary for skin barrier function. The expression of Cav-1 is differentiation-dependent in keratinocytes. The co-localization of Cav-1 with lamellar bodies, along with their role in the transport of vesicles, suggest that Cav-1 may be involved in the assembly of lamellar bodies, their trafficking, and Cav-1 could therefore be instrumental for their overall function (Feingold, 2012; Sando et al., 2003).
3. Autophagy in aging and its correlation with Cav-1 expression
Autophagy is a catabolic degradation process, eliminating unnecessary or damaged intracellular components (Rudinsztein et al., 2011). Macro-autophagy connected with formation of intracellular vacuoles requires the de novo formation of autophagosomes with the lipidated form of the microtubule-associated protein 1 light chain 3 (LC3-II) as a characteristic marker.
A comparison of the serially passaged human skin fibroblasts undergoing aging and replicative senescence in vitro demonstrates a tremendous increase of the basal level of LC3 secretion near senescent cells, compared to young cells (Demirovic et al., 2015). UV-induced senescence in human dermal fibroblasts is also connected with increased LC3-II expression in these cells (Cavinato et al., 2016; Cavinato et al., 2017). At the same time, lipofuscin, which is a highly cross-linked undegradable aggregate, is also significantly elevated in aged dermal fibroblasts (Tashiro et al., 2014). Together with increased expression of LC3-II in these cells, such an increase of lipofuscin content suggests that the increased number of autophagosomes observed in aged dermal fibroblasts is not connected with an enhanced formation of these structures. Rather it reflects impaired autophagic flux (failure of autophagosomes to fuse with lysosomes) in aged cells (Tashiro et al., 2014). This means that autophagy in senescent cells in the presence of high levels of Cav-1 expression is not really activated but rather defective.
Currently, the function of Cav-1 in autophagy remains not well understood. Cav-1 deficient mice show enhanced autophagy in adipocytes (Le Lay et al., 2010). The deletion of Cav-1 promotes both basal and induced autophagy in primary cultured mouse embryonic fibroblasts. The regulatory role of Cav-1 in autophagy is independent of the formation of caveolae per se, but acts through lipid rafts (Shi et al., 2015). The autophagy factors that form a complex (ATG12, ATG5 and ATG16L) are crucial for autophagosome formation. Cav-1 can not only interact with the ATG12-ATG5 complex, but also regulate the expression of ATG12, ATG5, and ATG16L. The competitive binding of Cav-1 with ATG12-ATG5 complex results in a suppression of autophagosome formation. Therefore, Cav-1 is considered a negative regulator of autophagy (Chen et al., 2014).
Although this point has not yet been investigated properly, we propose that the normalization of Cav-1 expression can re-store autophagy in aging skin cells.
4. Alteration of interfaces between single skin layers in aging
4.1. Dermal-epidermal and dermal-hypodermal junctions
In humans, interfaces between single skin layers in the composite structure epidermis/dermis/WAT are not flat and demonstrate undulations under normal physiological conditions. These undulations at the level of the dermal-epidermal junction are known as rete ridges (downward folds of the epidermis) and dermal papillae (upward projections of the dermis); the corresponding undulations on the dermal-hypodermal junction are known as papillae adiposae. Increased aging correlates with effacement of dermal papillae causing progressive decreases of their height and reduction of dermal papillary projections. The number of dermal papillae per unit skin surface length in the aged human skin decreases to about half of this value in the young skin (Sauermann et al., 2002). Appearance of dermal papillae and papillae adiposae was connected with strongly different mechanical properties in neighboring layers as well as with bi-modular mechanical properties (tension/compression asymmetry) in at least one of these layers (Kruglikov & Scherer, 2018).
The cutaneous basal lamina, which is the ECM layer containing laminin, is significantly contributing to the stability of dermis-epidermis junctions and demonstrates striking age-related modifications. Laminin is progressively degraded in aging, in the process destabilizing the basal lamina and inducing a reduction of undulations at the dermal-epidermal junctions. This degradation is mediated by different MMPs, including MMP-2, MMP-9 and MT1-MMP, as well as by proteases, such as plasmin and cathepsin B. There are some intriguing observations that implicate Cav-1 in this process. First, Cav-1 demonstrates a negative correlation with expression of MMP-2/MMP-9 (Fu et al., 2017). This effect may be related to the fact that MMP-2 and MT1-MMP are co-localized with Cav-1 on the cell surface (Puyraimond et al., 2001). Additionally, at least in some cells, Cav-1 demonstrates correlations with expression and localization of caveolae-associated proteases, such as cathepsin B and pro-urokinase plasminogen activator as well as with their membrane receptors (Cavallo-Medved et al., 2005).
Papillae adiposae are present both in males and females, but they are significantly more pronounced in skin affected by cellulite than in unaffected skin (Querleux et al., 2002). Treatments improving skin appearance by cellulite also reduce these undulations (Lucassen et al., 1997). Taking into account the similar nature of dermal papillae and papillae adiposae, it is not surprising that subjects with cellulite show earlier onset of skin aging (Ortonne et al., 2008).
The structures of the dermal papillae, and very likely also of the papillae adiposae, correlate with Cav-1 content. In psoriatic plaques which are characterized by very low levels of Cav-1 expression (Yamaguchi et al., 2015), the rete ridges demonstrate significant elongation and the dermal papillae show characteristic structures. Treatments improving skin conditions in psoriasis normally increase Cav-1 expression and reduce the undulations within the dermis-epidermis interphase. Previous studies showed that Cav-1 levels are inversely correlated with proliferation of cells (Torres et al., 2006; Fang et al., 2007; Cerezo et al., 2009). Beyond the reduced expression in psoriasis, a significant downregulation of Cav-1 is also detected in other hyper-proliferative skin disorders (Gheida et al., 2018). Accordingly, administration of Cav-1 scaffolding protein peptide in murine psoriasis-like model significantly reduced the proliferation of epidermal cells and the number of infiltrating cells, and resulted in improved skin phenotypes (Yamaguchi et al., 2015). Furthermore, the topography of the dermal-epidermal junctions is more pronounced in proliferative psoriasis skin, but not in the aged skin, which displays a slower proliferation and more flattened rete ridges. As the aged and differentiated cells show increased levels of Cav-1 (Park et al., 2000; Sando et al., 2003), this level may inversely correlate with topography of the dermal-epidermal junctions, and reflect the age of the skin.
4.2. Role of caveolae in cellular adhesion
Proper adhesion is essential for tissue differentiation and remodeling. It is also relevant for the overall mechanical properties of the tissue, and thus plays an important role in skin aging (Kruglikov & Scherer, 2018). The adhesion is provided by anchoring junctions and is complemented by gap junctions, which are responsible for intercellular communication. The main components of anchoring junctions are the cadherins, which build the intercellular connections, as well as the integrins, which provide the attachment of the cells to the ECM.
Chronologically aged human skin demonstrates reduced expression of β1 integrin (Giangreco et al., 2010). Whereas the β1 integrin expressing cells are highly abundant in the young skin and located on the top of rete ridges, such expression almost completely disappears in the aged skin. This modification in expression of proteins involved in attachment of skin cells to the ECM components correlates with a strong reduction of dermal papillae (Giangreco et al., 2010). Similar alterations were observed in UV-induced aging (Bosset et al., 2003). Expressions of cadherin (CDH1) and desmosome (DSC3) genes were reported to be significantly higher in the young compared to aged skin (Kimball et al., 2018). Additionally, cell detachment generally causes the release of Cav-1 from lipid rafts, although this release is differentially affected in young and senescent fibroblasts (Inomata et al., 2006).
The modification of Cav-1 levels affects integrin expression and consequently influences the adhesion capacity of cells (Fu et al., 2010). Mesenchymal stem cells with depleted Cav-1 expression demonstrate decreased cell surface integrin content and lower adhesion rates to different substrates; conversely, induction of Cav-1 in these cells leads to increased expression of different integrins and provides higher adhesion rates to fibronectin and collagen (Sohn et al., 2018). This effect is dependent on the membrane cholesterol content. Furthermore, Cav-1 regulates the endocytosis of integrins, at least β1 integrins, which occurs even in the absence of a fibronectin matrix. A reduction in Cav-1 impairs this process (Shi et al., 2008). In contrast, Cav-1 overexpression was associated with abnormal expression of at least one member of the E-cadherin/α–β catenin complex (Masuelli et al., 2012). Caveolins were also reported to be involved in the generation and modification of gap junctions (Langlois et al., 2008; Defamie et al., 2012).
These results demonstrate that the modification of Cav-1 expression can significantly influence intercellular adhesion processes. As skin aging is strongly dependent on the adhesion between different skin layers as well as between dermis and subcutis (Kruglikov & Scherer, 2017; Kruglikov & Scherer, 2018), this may be an important mechanism by which Cav-1 contributes to the aging process.
5. Alteration of hyaluronan content in aging skin and its interaction with Cav-1
Hyaluronan (HA) is one of the major components of the pericellular coat playing a critical role in skin aging (Papakonstantinou et al., 2012). Macroscopically, aged skin is characterized by the disappearance of HA from the epidermis and its shift towards the reticular dermis (Meyer & Stern, 1994). Progressive reduction of HA size (Longas et al., 1987) and transformation of HA from free to a tissue-bound form (Meyer & Stern, 1994) were also described as the hallmarks of the aging skin. Cav-1 plays an important role in HA homeostasis in the skin, influencing the HA binding capacity and potentially the long-range exosomal transport.
5.1. Binding of HA to the cell surface is Cav-1 dependent
Binding of HA to the cell surface mainly occurs through CD44 receptor and is regulated by MT1-MMP, both of which are spatially co-localized with caveolae (Annabi et al., 2004). Lipid rafts in the plasma membrane are strongly involved in the interaction between CD44 and HA and this effect depends on the molecular weight of HA (Murai, 2015). Additionally, HA binding is negatively correlated with local Cav-1 content: disruption of caveolae leads to a tremendous increase of HA binding capacity (Annabi et al., 2004).
A shift of HA from the epidermis towards the reticular dermis should be connected with a modulation of the binding capacity of HA, which is inversely dependent on the Cav-1 content. Progressive increases of Cav-1 expression with aging, which also results in a flattening of the dermal-epidermal junction, will reduce the HA binding capacity in the epidermis and the papillary dermis, leading to the disappearance of HA from these layers. The progressive reduction of HA size in the aging skin influences not only its binding capacity, but also the long-range exosomal exchange between different skin layers, making their mechanical properties much less flexible. On the other hand, the appearance of tissue-bound HA in the reticular dermis must reflect reduced Cav-1 expression in this layer neighboring the dermal-hypodermal junction.
5.2. HA interacts with TGF-β receptors
In the presence of HA, the majority of the TGF-β receptors are partitioned into Cav-1 lipid raft-associated pools, which significantly attenuates TGF-β1 signaling (Ito et al., 2004a). As a result, collagen synthesis in response to TGF-β1 must be modulated by the presence of HA. It was proposed that the availability of exogenous HA leads to a co-localization of CD44 and TGF-β into Cav-1 lipid raft-associated pools. This effect was observed only with high molecular weight HA (HMW-HA) of 2×106 Da. In contrast, low molecular weight HA (LMW-HA) of 65,000 Da was unable to antagonize the effect of TGF-β1 (Ito et al., 2004b). This demonstrates how not only the content but also the subtype of HA can influence the local collagen production in the tissue.
Vice versa, stimulation with TGF-β1 commonly enhances the total amount of HA in skin fibroblasts through enhanced expression of hyaluronan synthase, whereby the molecular size of newly produced endogenous HA is dependent on the expression levels of hyaluronan-binding protein involved in hyaluronan depolymerization (HYBID) (Nagaoka et al., 2015). In intact human skin, the content of TGF-β1 receptors positively correlates with HAS2 and negatively correlates with HYBID expression. This mechanism works only for HMW-HA, but not for depolymerized HA (as in the case of inflammation or aged skin) (Nagaoka et al., 2015).
5.3. Long-range Cav-1 transport is HA-dependent
Content and release rates of extracellular vesicles in endothelial cells in vitro are dependent on the content and subtype of HA presented in the media (Mirzapoiazova et al., 2015). Both exogenous HMW-HA and LMW-HA significantly increase the release of vesicles containing Cav-1, and this release is spatially connected with caveolae. At the same time, the molecular weight of HA has a strong impact on the content and form of released vesicles. Whereas exogenous LMW-HA causes the release of exosomes, vesicles released by the application of exogenous HMW-HA had a bigger size, different surface structure, and contained neuroblast differentiation associated protein (AHNAK) and annexin A2 (Mirzapoiazova et al., 2015), both of which are known to be involved in intercellular adhesion (Sheppard et al., 2016; Staquicini et al., 2017). AHNAK interacts with annexin 2 and both of the proteins are co-localized in lipid rafts with Cav-1 (Benaud et al., 2003). Recently, AHNAK was shown to potentiate TGF-β signaling and even induce epithelial-mesenchymal transition (Sohn et al., 2018).
From the observations above, we can assume that the long-range exosomal transport of Cav-1 described in (Crewe et al., 2018; Albacete-Albacete et al., 2018) must also be dependent on the content and sub-type of HA in the tissue, which in turn undergoes significant modifications in aging.
In summary, Cav-1 and HA strongly interact with each other. This interaction does not have a simple short-range character; rather, it can also be involved in the long-range re-distribution of Cav-1 in tissue, thereby influencing the global mechanical properties of the skin and contributing to the development of specific structural modifications in the aging skin.
Collectively, these results prompt us to consider Cav-1 not only as a pathophysiological factor, but highlights it as an important target for a variety of different skin conditions, including skin aging. This is supported by a number of different experimental approaches that we summarized in Table 1.
Table 1.
Cav-1 as a target in skin aging
| No | Description | Reference |
|---|---|---|
| 1 | Development of cellular senescence in vitro can be reversed by specific reduction of Cav-1 levels | Cho et al. (2003) |
| 2 | Long-term application of MβCD causes an increase of the dermal thickness and induction of Col1 expression in aged animals | Lee et al. (2015) |
| 3 | Specific re-expression of Cav-1 restores the mechanical sensitivity of cells to extracellular matrix | Lin et al. (2015) |
| 4 | Suppression of Cav-1 leads to a pronounced up-regulation of collagen expression in the skin | Lee et al. (2015) |
| 5 | Disrupting of caveolae provides significant increase of hyaluronan binding capacity | Annabi et al. (2004) |
6. Conclusions
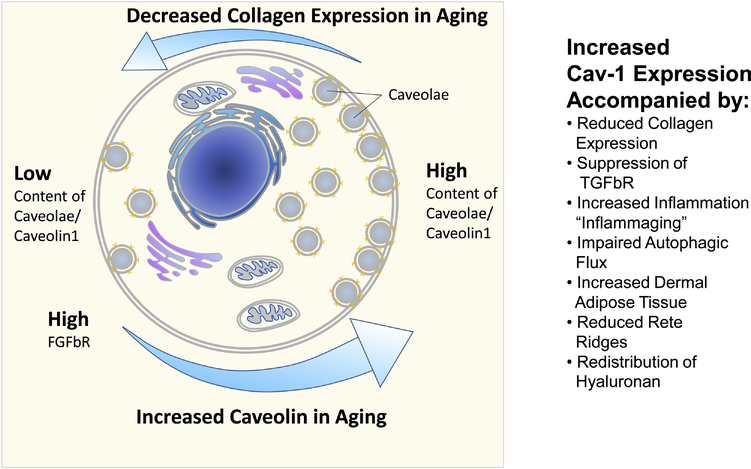
An upregulation of Cav-1 demonstrates strong correlations with aging phenotypes, both in chronological and UV-induced aging in vitro and in vivo (Figure 2). The modification of Cav-1 content significantly affects collagen production and inflammation in the aging process. The age-dependent modulation of Cav-1 also leads to modifications of the content and distribution of hyaluronan in the skin, and contributes to an impairment of the autophagic flux in aging cells. Recent results reported in areas such as skin aging, fibrosis and psoriasis suggest that the above-mentioned interactions are not merely correlations. Rather, Cav-1 is a pathophysiological factor and should be a target for different inflammatory and hyper-proliferative skin conditions, as well as in skin aging. The modulation of Cav-1 expression can significantly influence mechanical properties of single skin layers, as well as the adhesion between adjacent layers. This will affect the mechanical stability of the layered composite skin/WAT. As such, we propose that selective targeting of Cav-1 expression should lead to relevant anti-aging effects in the skin.
Fig. 2:
Summary of age-related changes as a function of cellular caveolin1 content
Highlights.
Caveolin-1 (Cav-1) appears to be both a pathophysiological contributor and a target in different inflammatory and hyperproliferative skin disorders as well as in skin aging.
Typical alterations in aging skin, such as modifications of content and distribution of hyaluronan, impairment of autophagic flux, and a reduction of collagen expression can be connected with modified Cav-1 expression levels.
Cav-1 expression significantly influences the mechanical properties of individual skin layers, enhancing the typical structural modifications of the skin surface in the aging skin.
Acknowledgments
PES is supported by NIH grants RC2DK118620, R01-DK55758, R01-DK099110, P01-DK088761 and P01-AG051459. PES is also supported by an unrestricted grant from the Novo Nordisk Research Foundation.
List of abbreviations
- AHNAK
neuroblast differentiation associated protein
- ATG
autophagy-related protein
- CAFs
cancer associated fibroblasts
- Cav-1
caveolin-1
- Col
collagen
- dWAT
dermal white adipose tissue
- ECM
extracellular matrix
- HA
hyaluronan
- HMW-HA
high molecular weight hyaluronan
- LC3-II
microtubule-associated protein 1 light chain 3
- LMW-HA
low molecular weight hyaluronan
- MβCD
methyl-β-cyclodextrin
- MMP
matrix metalloproteinase
- PPARγ
peroxisome proliferator-activated receptor gamma
- TGF-β
transforming growth factor beta
- WAT
white adipose tissue
Footnotes
Competing interest
ILK is the managing partner of Wellcomet GmbH. Wellcomet GmbH provided support in the form of salaries for ILK, but did not have any additional role in decision to publish or preparation of the manuscript. The commercial affiliation of ILK with Wellcomet GmbH does not alter the adherence to all journal policies on sharing data and materials. PES and ZZ declare no conflict of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Albacete-Albacete L, Navarro-Lerida I, Lopez JA, Martin-Padura I, Astudillo AM, Van-DER-Heyden M, Balsinde J, Orend G, Vazquez J, del Pozo MA, 2018. ECM deposition is driven by caveolin1-dependent regulation of exosomal biogenesis and cargo sorting. bioRxiv, 405506 10.1101/405506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Annabi B, Thibeault S, Moumdjian R, Béliveau R, 2004. Hyaluronan cell surface binding is induced by type I collagen and regulated by caveolae in glioma cells. J. Biol. Chem 279, 21888–21896. [DOI] [PubMed] [Google Scholar]
- Ao M, Wu L, Zhou X, Chen Y, 2016. Methyl-β-cyclodextrin impairs the monocyte-adhering ability of endothelial cells by down-regulating adhesion molecules and caveolae and reorganizing the actin cytoskeleton. Biol. Pharm. Bull 39, 1029–1034. [DOI] [PubMed] [Google Scholar]
- Benaud C, Gentil BJ, Assard N, Garin J, Delphin C, Baudier J, 2004. AHNAK interaction with the annexin 2/S100A10 complex regulates cell membrane cytoarchitecture. J. Cell Biol 164, 133–144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bochet L, Lehuédé C, Dauvillier S, Wang YY, Dirat B, Laurent V, Dray C, Guiet R, Maridonneau-Parini I, Le Gonidec S, Couderc B, Escourrou G, Valet P, Muller C, 2013. Adipocyte-derived fibroblasts promote tumor progression and contribute to desmoplastic reaction in breast cancer. Cancer Res. 73, 5657–5668. [DOI] [PubMed] [Google Scholar]
- Bosset S, Bonnet Duquennoy M, Barre P, Chalon A, Lazou K, Kurfurst R, Bonte F, Schnebert S, Disant F, Le Valet B, Nicolas JF, 2003. Decreased expression of keratinocyte β1 integrins in chronically sun exposed skin in vivo. Br. J. Dermatol 148, 770–778. [DOI] [PubMed] [Google Scholar]
- Briand N, Prado C, Mabilleau G, Lasnier F, Le Lièpvre X, Covington JD, Ravussin E, Le Lay S, Dugail I, 2014. Caveolin-1 expression and cavins stability regulate caveolae dynamics in adipocyte lipid store fluctuation. Diabetes. 63, 4032–4044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavallo-Medved D, Mai J, Dosescu J, Sameni M, Sloane BF, 2005. Caveolin-1 mediates the expression and localization of cathepsin B, pro-urokinase plasminogen activator and their cell-surface receptors in human colorectal carcinoma cells. J. Cell Sci 118, 1493–1503. [DOI] [PubMed] [Google Scholar]
- Cavinato M, Koziel R, Romani N, Weinmüllner R, Jenewein B, Hermann M, Dubrac S, Ratzinger G, Grillari J, Schmuth M, Jansen-Dürr P, 2017. UVB-induced senescence of human dermal fibroblasts involves impairment of proteasome and enhanced autophagic activity. J. Gerontol. Ser. A 72, 632–639. [DOI] [PubMed] [Google Scholar]
- Cavinato M, Jansen-Dürr P, 2017. Molecular mechanisms of UVB-induced senescence of dermal fibroblasts and its relevance for photoaging of the human skin. Exp. Gerontol 94, 78–82. [DOI] [PubMed] [Google Scholar]
- Cerezo A, Guadamillas MC, Goetz JG, Sánchez-Perales S, Klein E, Assoian RK, del Pozo MA, 2009. The absence of caveolin-1 increases proliferation and anchorage-independent growth by a Rac-dependent, Erk-independent mechanism. Mol. Cell. Biol 29, 5046–5059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang CC, Chen CY, Wen HC, Huang CY, Hung MS, Lu HC, Chen WL, Chang CH, 2017. Caveolin- 1 secreted from adipose tissues and adipocytes functions as an adipogenesis enhancer. Obesity. 25, 1932–1940. [DOI] [PubMed] [Google Scholar]
- Chen ZH, Cao JF, Zhou JS, Liu H, Che LQ, Mizumura K, Li W, Choi AM, Shen HH, 2014. Interaction of caveolin-1 with ATG12-ATG5 system suppresses autophagy in lung epithelial cells. Am. J. Physiol. Lung Cell. Mol. Physiol 306, L1016–L1025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho KA, Ryu SJ, Park JS, Jang IS, Ahn JS, Kim KT, Park SC, 2003. Senescent phenotype can be reversed by reduction of caveolin status. J. Biol. Chem 278, 27789–27795. [DOI] [PubMed] [Google Scholar]
- Cho KA, Ryu SJ, Oh YS, Park JH, Lee JW, Kim HP, Kim KT, Jang IS, Park SC, 2004. Morphological adjustment of senescent cells by modulating caveolin-1 status. J. Biol. Chem 279, 42270–42278. [DOI] [PubMed] [Google Scholar]
- Crewe C, Joffin N, Rutkowski JM, Kim M, Zhang F, Towler DA, Gordillo R, Scherer PE, 2018. An endothelial-to-adipocyte extracellular vesicle axis governed by metabolic state. Cell. 175, 695–708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galdo FD, Sotgia F, de Almeida CJ, Jasmin JF, Musick M, Lisanti MP, Jimenez SA, 2008. Decreased expression of caveolin 1 in patients with systemic sclerosis: crucial role in the pathogenesis of tissue fibrosis. Arthritis Rheum. 58, 2854–2865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Defamie N, Mesnil M, 2012. The modulation of gap-junctional intercellular communication by lipid rafts. Biochim. Biophys. Acta (BBA)-Biomembranes 1818, 1866–1869. [DOI] [PubMed] [Google Scholar]
- Demirovic D, Nizard C, Rattan SI, 2015. Basal level of autophagy is increased in aging human skin fibroblasts in vitro, but not in old skin. PloS One. 10, e0126546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Domingues L, Hurbain I, Gilles-Marsens F, Andre N, Dewulf M, Romao M, de Lesegno CV, Blouin C, Guere C, Vie K, Raposo G, Lamaze C, Delevoye C, 2019. Caveolae coupling of melanocytes signaling and mechanics is required for human skin pigmentation. bioRxiv. 666388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Driskell RR, Lichtenberger BM, Hoste E, Kretzschmar K, Simons BD, Charalambous M, Ferron SR, Herault Y, Pavlovic G, Ferguson-Smith AC, Watt FM, 2013. Distinct fibroblast lineages determine dermal architecture in skin development and repair. Nature. 504, 277–281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Echarri A, Del Pozo MA, 2015. Caveolae–mechanosensitive membrane invaginations linked to actin filaments. J. Cell Sci 128, 2747–2758. [DOI] [PubMed] [Google Scholar]
- Fang K, Fu W, Beardsley AR, Sun X, Lisanti MP, & Liu J (2007). Overexpression of caveolin-1 inhibits endothelial cell proliferation by arresting the cell cycle at G0/G1 phase. Cell Cycle. 6, 199–204. [DOI] [PubMed] [Google Scholar]
- Feingold KR, 2012. Lamellar bodies: the key to cutaneous barrier function. J. Invest. Dermatol 132, 1951–1953. [DOI] [PubMed] [Google Scholar]
- Franceschi C, Garagnani P, Parini P, Giuliani C, Santoro A (2018). Inflammaging: a new immune–metabolic viewpoint for age-related diseases. Nat. Rev. Endocrinol 14, 576–590. [DOI] [PubMed] [Google Scholar]
- Fridolfsson HN, Kawaraguchi Y, Ali SS, Panneerselvam M, Niesman IR, Finley JC, Kellerhals SE, Migita MY, Okada H, Moreno AL, Jennings M, Kidd MW, Bonds JA, Balijepalli RC, Ross RS, Patel PM, Miyanohara A, Chen Q, Lesnefsky EJ, Head BP, Roth DM, Insel PA, Patel HH, 2012. Mitochondria-localized caveolin in adaptation to cellular stress and injury. FASEB J. 26, 4637–4649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu C, He J, Li C, Shyy JYJ, Zhu Y, 2010. Cholesterol increases adhesion of monocytes to endothelium by moving adhesion molecules out of caveolae. Biochim. Biophys. Acta (BBA)-Mol. Cell Biol. Lipid 1801, 702–710. [DOI] [PubMed] [Google Scholar]
- Fu P, Chen F, Pan Q, Zhao X, Zhao C, Cho WCS, Chen H, 2017. The different functions and clinical significances of caveolin-1 in human adenocarcinoma and squamous cell carcinoma. Onco Targets Ther. 10, 819–835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gheida SF, Neinaa YMEH, Mohammed DAEA, 2018. Caveolin-1 expression in hyperproliferative skin disorders: A potential predictive marker of disease severity and progression. Dermatol. Sinica 36, 179–184. [Google Scholar]
- Giangreco A, Goldie SJ, Failla V, Saintigny G, Watt FM, 2010. Human skin aging is associated with reduced expression of the stem cell markers beta 1 integrin and MCSP. J. Invest. Dermatol 130, 604–608. [DOI] [PubMed] [Google Scholar]
- Gonzalez OA, Novak MJ, Kirakodu S, Stromberg A, Nagarajan R, Huang CB, Chen KC, Oracca L, Martinez-Gonzalez JM, Ebersole JL, 2015. Differential gene expression profiles reflecting macrophage polarization in aging and periodontitis gingival tissues. Immunol. Invest 44, 643–664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernandez-Segura A, Nehme J, Demaria M, 2018. Hallmarks of cellular senescence. Trend Cell Biol. 28, 436–453. [DOI] [PubMed] [Google Scholar]
- Hsu CK, Lin HH, Harn HI, Ogawa R, Wang YK, Ho YT, Chen WR, Lee YC, Lee JYY, Shieh SJ, Cheng CM, McGrath JA, Tang MJ, 2018. Caveolin-1 controls hyperresponsiveness to mechanical stimuli and fibrogenesis-associated RUNX2 activation in keloid fibroblasts. J. Invest. Dermatol 138, 208–218. [DOI] [PubMed] [Google Scholar]
- Hulstrøm V, Prats C, Vinten J, 2013. Adipocyte size and cellular expression of caveolar proteins analyzed by confocal microscopy. Am. J. Physiol. Cell Physiol 304, C1168–C1175. [DOI] [PubMed] [Google Scholar]
- Inomata M, Shimada Y, Hayashi M, Kondo H, Ohno-Iwashita Y, 2006. Detachment-associated changes in lipid rafts of senescent human fibroblasts. Biochem. Biophys. Res. Com 343, 489–495. [DOI] [PubMed] [Google Scholar]
- Ito T, Williams JD, Fraser DJ, Phillips AO, 2004a. Hyaluronan regulates TGF-ß1 receptor compartmentalisation. J. Biol. Chem 279, 25326–25332. [DOI] [PubMed] [Google Scholar]
- Ito T, Williams JD, Fraser DJ, Phillips AO, 2004b. Hyaluronan attenuates transforming growth factor-β1-mediated signaling in renal proximal tubular epithelial cells. Am. J. Pathol 164, 1979–1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janson D, Saintigny G, Mahé C, Ghalbzouri AE, 2013. Papillary fibroblasts differentiate into reticular fibroblasts after prolonged in vitro culture. Exp. Dermatol 22, 48–53. [DOI] [PubMed] [Google Scholar]
- Kim H, Park SY, Moon S, Lee J, Kim S, 2018. Autophagy in human skin fibroblasts: Impact of age. Int. J. Mol. Sci 19, 2254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimball AB, Alora-Palli MB, Tamura M, Mullins LA, Soh C, Binder RL, Houston NA, Conley ED, Tung JY, Annunziata NE, Bascom CC, Isfort RJ, Jarrold BB, Kainkaryam R, Rocchetta HL, Swift D, Tiesman JP, Toyama K, Xu J, Yan X, Osborne R, 2018. Age-induced and photoinduced changes in gene expression profiles in facial skin of Caucasian females across 6 decades of age. J. Am. Acad. Dermatol 78, 29–39. [DOI] [PubMed] [Google Scholar]
- Kruglikov IL, Scherer PE, 2016. Dermal adipocytes: from irrelevance to metabolic targets? Trend. Endocrinol. Metab 27, 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kruglikov IL, Scherer PE, 2017. General theory of skin reinforcement. PloS One. 12, e0182865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kruglikov IL, Scherer PE, 2018. Skin aging as a mechanical phenomenon: The main weak links. Nutr. Healthy Aging 4, 291–307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kruglikov IL, Zhang Z, Scherer PE, 2018. The role of immature and mature adipocytes in hair cycling. Trends Endocrinol. Metab 30, 93–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kruglikov IL, Scherer PE, 2019a. Caveolin-1 as a pathophysiological factor and target in psoriasis. npj Aging Mech. Dis. 5, 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kruglikov IL, Scherer PE, 2019b. Caveolin-1 as a target in prevention and treatment of hypertrophic scarring. npj Regen. Med. 4, 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langlois S, Cowan KN, Shao Q, Cowan BJ, Laird DW, 2008. Caveolin-1 and-2 interact with connexin43 and regulate gap junctional intercellular communication in keratinocytes. Mol. Biol. Cell 19, 912–928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Lay S, Briand N, Blouin CM, Chateau D, Prado C, Lasnier F, Liepvre X, Hajduch E, Dugail I, 2010. The lipoatrophic caveolin-1 deficient mouse model reveals autophagy in mature adipocytes. Autophagy. 6, 754–763. [DOI] [PubMed] [Google Scholar]
- Lee JA, Choi DI, Choi JY, Kim SO, Cho KA, Lee JB, Yun SJ, Lee SC, 2015. Methyl-β-cyclodextrin up-regulates collagen I expression in chronologically-aged skin via its anti-caveolin-1 activity. Oncotarget. 6, 1942–1953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Longas MO, Russell CS, He XY, 1987. Evidence for structural changes in dermatan sulfate and hyaluronic acid with aging. Carbohydrate Res. 159, 127–136. [DOI] [PubMed] [Google Scholar]
- Lucassen GW, Van der Sluys WLN, Van Herk JJ, Nuijs AM, Wierenga PE, Barel AO, Lambrecht R, 1997. The effectiveness of massage treatment on cellulite as monitored by ultrasound imaging. Skin Res. Technol 3, 154–160. [DOI] [PubMed] [Google Scholar]
- Marsh E, Gonzalez DG, Lathrop EA, Boucher J, Greco V, 2018. Positional stability and membrane occupancy define skin fibroblast homeostasis in vivo. Cell. 175, 1620–1633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masuelli L, Budillon A, Marzocchella L, Mrozek MA, Vitolo D, Di Gennaro E, Losito S, Sale P, Longo F, Ionna F, Lista F, Muraro R, Modesti A, Be R, 2012. Caveolin 1 overexpression is associated with simultaneous abnormal expression of the E cadherin/α–β catenins complex and multiple erbb receptors and with lymph nodes metastasis in head and neck squamous cell carcinomas. J. Cell. Physiol 227, 3344–3353. [DOI] [PubMed] [Google Scholar]
- Mathay C, Pierre M, Pittelkow MR, Depiereux E, Nikkels AF, Colige A, Poumay Y, 2011. Transcriptional profiling after lipid raft disruption in keratinocytes identifies critical mediators of atopic dermatitis pathways. J. Invest. Dermatol 131, 46–58. [DOI] [PubMed] [Google Scholar]
- Meyer LJ, Stern R, 1994. Age-dependent changes of hyaluronan in human skin. J. Invest. Dermatol 102, 385–389. [DOI] [PubMed] [Google Scholar]
- Mirzapoiazova T, Lennon FE, Mambetsariev B, Allen M, Riehm J, Poroyko VA, Singleton PA, 2015. Extracellular vesicles from caveolin-enriched microdomains regulate hyaluronan-mediated sustained vascular integrity. Int. J. Cell Biol 2015, 481491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murai T, 2015. Lipid raft-mediated regulation of hyaluronan–CD44 interactions in inflammation and cancer. Front. Immunol 6, 420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagaoka A, Yoshida H, Nakamura S, Morikawa T, Kawabata K, Kobayashi M, Sakai S, Takahashi Y, Okada Y, Inoue S, 2015. Regulation of hyaluronan (HA) metabolism mediated by HYBID (HYaluronan Binding Protein Involved in HA Depolymerization, KIAA1199) and HA synthases in growth factor-stimulated fibroblasts. J. Biol. Chem 290, 30910–30923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nethe M, Anthony EC, Fernandez-Borja M, Dee R, Geerts D, Hensbergen PJ, Deelder AM, Schmidt G, Hordijk PL, 2010. Focal-adhesion targeting links caveolin-1 to a Rac1-degradation pathway. J. Cell. Sci 123, 1948–1958. [DOI] [PubMed] [Google Scholar]
- Ortonne JP, Zartarian M, Verschoore M, Queille-Roussel C, Duteil L, 2008. Cellulite and skin ageing: is there any interaction? J. Eur. Acad. Dermatol. Venereol 22, 827–834. [DOI] [PubMed] [Google Scholar]
- Papakonstantinou E, Roth M, Karakiulakis G, 2012. Hyaluronic acid: A key molecule in skin aging. Dermatoendocrinol. 4, 253–258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park WY, Park JS, Cho KA, Kim DI, Ko YG, Seo JS, Park SC, 2000. Upregulation of caveolin attenuates epidermal growth factor signaling in senescent cells. J. Biol. Chem 275, 20847–20852. [DOI] [PubMed] [Google Scholar]
- Parton RG, 2018. Caveolae: Structure, function, and relationship to disease. Annu Rev Cell Dev Biol. 34, 111–136. [DOI] [PubMed] [Google Scholar]
- Parton RG, del Pozo MA, 2013. Caveolae as plasma membrane sensors, protectors and organizers. Nat Rev Mol Cell Biol. 14, 98–112. [DOI] [PubMed] [Google Scholar]
- Prokesch A, Smorlesi A, Perugini J, Manieri M, Ciarmela P, Mondini E, Trajanoski Z, Kristiansen K, Giordano A, Bogner-Strauss JG, Cinti S, 2014. Molecular aspects of adipoepithelial transdifferentiation in mouse mammary gland. Stem Cells. 32, 2756–2766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puyraimond A, Fridman R, Lemesle M, Arbeille B, Menashi S, 2001. MMP-2 colocalizes with caveolae on the surface of endothelial cells. Exp. Cell Res 262, 28–36. [DOI] [PubMed] [Google Scholar]
- Qian XL, Pan YH, Huang QY, Shi YB, Huang QY, Hu ZZ, Xiong LX, 2019. Caveolin-1: a multifaceted driver of breast cancer progression and its application in clinical treatment. Onco Targets Ther. 12, 1539–1552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Querleux B, Cornillon C, Jolivet O, Bittoun J, 2002. Anatomy and physiology of subcutaneous adipose tissue by in vivo magnetic resonance imaging and spectroscopy: relationships with sex and presence of cellulite. Skin Res. Technol 8, 118–124. [DOI] [PubMed] [Google Scholar]
- Rehan VK, Sugano S, Wang Y, Santos J, Romero S, Dasgupta C, Keane MP, Stahlman MT, Torday JS, 2006. Evidence for the presence of lipofibroblasts in human lung. Exp. Lung Res 32, 379–393. [DOI] [PubMed] [Google Scholar]
- Rhim JH, Kim JH, Yeo EJ, Kim JC, Park SC, 2010. Caveolin-1 as a novel indicator of wound-healing capacity in aged human corneal epithelium. Mol. Med 16, 527–534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodríguez SA, Grochová D, McKenna T, Borate B, Trivedi NS, Erdos MR, Eriksson M, 2016. Global genome splicing analysis reveals an increased number of alternatively spliced genes with aging. Aging Cell. 15, 267–278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubinsztein DC, Mariño G, Kroemer G, 2011. Autophagy and aging. Cell. 146, 682–695. [DOI] [PubMed] [Google Scholar]
- Salzer MA, Lafzi A, Boenguer-Llegro A, Youssif C, Castellanos A, Solanas G, Peixoto FO, Stephan-Otto A,C, Prats N, Aguilera M, Martín-Caballero J, Heyn H, Benitah SA, 2018. Identity noise and adipogenic traits characterize dermal fibroblast aging. Cell. 175, 1–16. [DOI] [PubMed] [Google Scholar]
- Sando GN, Zhu H, Weis JM, Richman JT, Madison KC, Wertz PW, 2003. Caveolin expression and localization in human keratinocytes suggest a role in lamellar granule biogenesis. J. Invest. Dermatol 120, 531–541. [DOI] [PubMed] [Google Scholar]
- Sauermann K, Clemann S, Jaspers S, Gambichler T, Altmeyer P, Hoffmann K, Ennen J, 2002. Age related changes of human skin investigated with histometric measurements by confocal laser scanning microscopy in vivo. Skin Res. Technol 8, 52–56. [DOI] [PubMed] [Google Scholar]
- Scherer PE, Lisanti MP, Baldini G, Sargiacomo M, Mastick CC, Lodish HF, 1994. Induction of caveolin during adipogenesis and association of GLUT4 with caveolin-rich vesicles. J. Cell Biol 127, 1233–1243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheppard HM, Feisst V, Chen J, Print C, Dunbar PR, 2016. AHNAK is downregulated in melanoma, predicts poor outcome, and may be required for the expression of functional cadherin-1. Melanoma Res. 26, 108–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi F, Sottile J, 2008. Caveolin-1-dependent β1 integrin endocytosis is a critical regulator of fibronectin turnover. J. Cell Sci 121, 2360–2371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi Y, Tan SH, Ng S, Zhou J, Yang ND, Koo GB, McMahon KA, Parton RG, Hill MM, Del Pozzo MA, Kim YS, 2015. Critical role of CAV1/caveolin-1 in cell stress responses in human breast cancer cells via modulation of lysosomal function and autophagy. Autophagy. 11, 769–784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shihata WA, Putra MR, Chin-Dusting JP, 2017. Is there a potential therapeutic role for Caveolin-1 in fibrosis? Front. Pharmacol 8, 567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shivshankar P, Halade GV, Calhoun C, Escobar GP, Mehr AJ, Jimenez F, Martinez C, Bhatnagar H, Mjaatvedt CH, Lindsey ML, Le Saux CJ, 2014. Caveolin-1 deletion exacerbates cardiac interstitial fibrosis by promoting M2 macrophage activation in mice after myocardial infarction. J. Mol. Cell. Cardiol 76, 84–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shoham N, Girshovitz P, Katzengold R, Shaked NT, Benayahu D, Gefen A, 2014. Adipocyte stiffness increases with accumulation of lipid droplets. Biophys. J 106, 1421–1431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinha B, Köster D, Ruez R, Gonnord P, Bastiani M, Abankwa D, Stan RV, Butler-Browne G, Vedie B, Johannes L, Morone N, Parton RG, Raposo G, Sens P, Lamaze C, Nassoy P, 2011. Cells respond to mechanical stress by rapid disassembly of caveolae. Cell. 144, 402–413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sohn M, Shin S, Yoo JY, Goh Y, Lee IH, Bae YS, 2018. Ahnak promotes tumor metastasis through transforming growth factor-β-mediated epithelial-mesenchymal transition. Sci. Rep 8, 14379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sohn J, Lin H, Fritch MR, Tuan RS, 2018. Influence of cholesterol/caveolin-1/caveolae homeostasis on membrane properties and substrate adhesion characteristics of adult human mesenchymal stem cells. Stem Cell Res. Ther 9, 86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staquicini DI, Rangel R, Guzman-Rojas L, Staquicini FI, Dobroff AS, Tarleton CA, Ozbun MA, Kolonin MG, Gelovani JG, Marchiò S, Sidman RL, Hajjar KA, Arap W, Pasqualini R, 2017. Intracellular targeting of annexin A2 inhibits tumor cell adhesion, migration, and in vivo grafting. Sci. Rep 7, 4243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tashiro K, Shishido M, Fujimoto K, Hirota Y, Yo K, Gomi T, Tanaka Y, 2014. Agerelated disruption of autophagy in dermal fibroblasts modulates extracellular matrix components. Biochem. Biophys. Res. Commun 443, 167–172. [DOI] [PubMed] [Google Scholar]
- Torres VA, Tapia JC, Rodríguez DA, Párraga M, Lisboa P, Montoya M, Leyton L, Quest AF, 2006. Caveolin-1 controls cell proliferation and cell death by suppressing expression of the inhibitor of apoptosis protein survivin. J. Cell Sci 119, 1812–1823. [DOI] [PubMed] [Google Scholar]
- Uitto J, Bernstein EF, 1998. Molecular mechanisms of cutaneous aging: connective tissue alterations in the dermis. J. Invest. Dermatol. Symp. Proc 3, 41–44. [PubMed] [Google Scholar]
- Volonte D, Zhang K, Lisanti MP, Galbiati F, 2002. Expression of caveolin-1 induces premature cellular senescence in primary cultures of murine fibroblasts stress-induced premature senescence upregulates the expression of endogenous caveolin-1. Mol. Biol. Cell 13, 2502–2517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaguchi Y, Watanabe Y, Watanabe T, Komitsu N, Aihara M, 2015. Decreased expression of caveolin-1 contributes to the pathogenesis of psoriasiform dermatitis in mice. J. Invest. Dermatol 135, 2764–2774. [DOI] [PubMed] [Google Scholar]
- Yu DM, Jung SH, An HT, Lee S, Hong J, Park JS, Lee H, Lee H, Bahn MS, Lee HC, Han NK, Ko J, Lee JS, Ko YG, 2017. Caveolin- 1 deficiency induces premature senescence with mitochondrial dysfunction. Aging Cell. 16, 773–784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang GY, Yu Q, Cheng T, Liao T, Nie CL, Wang AY, Zheng X, Xie XG, Albers AE, Gao WY, 2011a. Role of caveolin 1 in the pathogenesis of tissue fibrosis by keloid derived fibroblasts in vitro. Br. J. Dermatol 164, 623–627. [DOI] [PubMed] [Google Scholar]
- Zhang GY, He B, Liao T, Luan Q, Tao C, Nie CL, Albers AE, Zheng X, Xie XG, Gao WY, 2011b. Caveolin 1 inhibits transforming growth factor-β1 activity via inhibition of Smad signaling by hypertrophic scar derived fibroblasts in vitro. J. Dermatol. Sci 62, 128–131. [DOI] [PubMed] [Google Scholar]
- Zhang Z, Shao M, Hepler C, Zi Z, Zhao S, An YA, Zhu Y, Ghaben A, Wang MY, Li N, Onodera T, Joffin N, Crewe C, Zhu Q, Kumar A, Xing C, Wang QA, Deng Y, Gordillo R, Kruglikov I, Kusminski CM, Gupta R, Scherer PE, 2019. Dermal adipose tissue – A distinct fat depot with unique functions undergoing reversible de-differentiation. J. Clin. Invest To be published. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zou H, Stoppani E, Volonte D, Galbiati F, 2011. Caveolin-1, cellular senescence and age-related diseases. Mech. Ageing Devel 132, 533–542. [DOI] [PMC free article] [PubMed] [Google Scholar]