Abstract
Antibodies blocking programmed death 1 (anti-PD-1) or its ligand (anti-PD-L1) are associated with modest response rates as monotherapy in metastatic breast cancer, but are generally well tolerated and capable of generating dramatic and durable benefit in a minority of patients. Anti-PD-1/L1 antibodies are also safe when administered in combination with a variety of systemic therapies (chemotherapy, targeted therapies), as well as with radiotherapy. We summarize preclinical, translational, and preliminary clinical data in support of combination approaches with anti-PD-1/L1 in metastatic breast cancer, focusing on potential mechanisms of synergy, and considerations for clinical practice and future investigation.
Subject terms: Tumour immunology, Breast cancer
Introduction
In the spirit of the Hippocratic dictum to “first, do no harm,” a guiding principle in the management of metastatic breast cancer is to favor less treatment rather than more, unless clear evidence of synergy exists.1 For example, sequential single-agent chemotherapy is favored over multi-agent chemotherapy because it is better tolerated with similar overall survival (OS).2 Recently however, there has been a resurgence of enthusiasm for combination approaches, this time with immune checkpoint antibodies against programmed death 1 (PD-1) or its ligand (PD-L1), based upon preclinical evidence of therapeutic synergy, and recent trials demonstrating acceptable tolerability of these agents with standard-of-care treatment modalities including chemotherapy, radiotherapy, hormone-directed therapies, and targeted therapies (Table 1).3–18
Table 1.
Selected clinical trials demonstrating safety of anti-PD-1/L1 combination therapies
| Therapeutic class | Anti-PD-1/L1 | Secondary agent | Phase | n | Summary | Ref |
|---|---|---|---|---|---|---|
| Chemotherapy | Atezolizumab | Nab-paclitaxel | III | 902 | “IMpassion130”; Improved OS and ORR in PD-L1 + cancers | 3 |
| Pembrolizumab | Paclitaxel, nab-paclitaxel, or gemcitabine/carboplatin | III | 858 | “Keynote-355” | 4 | |
| Pembrolizumab | Capecitabine | Ib | 14 | ORR 43%; 7% grade 3 diarrhea | 5 | |
| Pembrolizumab | Eribulin | II | 104 | “ENHANCE-1”; ORR 15% PD-L1+; Grade >3 19.5% | 6 | |
| Pembrolizumab | Doxorubicin, cyclophosphamide, paclitaxel | III | 69 | “ISPY-2”; Improved Path CR TNBC and ER+; 7% grade 3 | 7 | |
| Durvalumab | Nab-paclitaxel | II | 174 | “GeparNuevo”; 48% Path CR; 27% irSAE | 8 | |
| Radiotherapy | Pembrolizumab | Radiotherapy | II | 9 | 33% ORR, no overlapping toxicities | 9 |
| Pembrolizumab | Radiotherapy (SBRT) | I | 73 | 13% ORR, 9% grade 3, pre/post biopsies | 10 | |
| CDK4/6i | Pembrolizumab | Abemeciclib | II | 28 | “JPCE” ORR 14%; Grade >3 11% | 11 |
| Avelumab | Palbociclib | II | 220 | “PACE” | 12 | |
| HER-2-targeted | Pembrolizumab | T-DM1 | I | 27 | NCT03032107 | |
| Pembrolizumab | Trastuzumab | Ib/II | 58 | “PANACEA” 15% ORR for PD-L1+ (n = 6/40), 0% ORR PD-L1-, 29% grade 3 + AE | 13 | |
| Durvalumab | Trastuzumab | I | 15 | NCT02649686 | ||
| Atezolizumab | T-DM1 | II | 202 | “KATE2” 44% Grade 3+ AE, PFS HR = 0.82 v. T-DM1/placebo (p = NS) | 14 | |
| PARPi | Pembrolizumab | Niraparib | II | 55 | “TOPACIO” 29% ORR, 49% DCR; 22% BRCAmut | 15 |
| Durvalumab | Olaparib | II | 30 | “MEDIOLA” 90% wk12 DCR, no overlapping toxicities | 16 | |
| HDACi | Atezolizumab | Entinostat | Ib/II | 81 | Closed to accrual late 2018 | 17 |
| IDOi | Pembrolizumab | Epacadostat | I/II | 39 | “ECHO-202”; ORR 10% TNBC | 18 |
AE adverse event, CDK4/6i cyclin-dependent kinase 4 and 6 inhibitors, ER+ estrogen receptor-positive, HDACi histone deacetylase inhibitors, HER2 human epidermal growth factor receptor 2, HR hazard ratio, N number, ORR overall response rate, CR complete response, OS overall survival, PARPi poly(ADP ribose) polymerase inhibitors, PD-1 programmed death 1, PD-L1+ programmed death ligand 1-positive, PD-L1- programmed death ligand 1-negative, PFS progression-free survival, NS not significant, DCR disease control rate, BRCAmut germline BRCA gene mutated, T-DM1 trastuzumab emtansine, TNBC triple-negative breast cancer, wk week, IDOi IDO inhibitors
Cytotoxic chemotherapy has pleiotropic immunomodulatory effects that may synergize with anti-PD-1/L1. Recently, the first randomized anti-PD-1/L1 combination trial in metastatic breast cancer, IMpassion130, provided proof-of-concept that anti-PD-1/L1 plus chemotherapy can be safe and more effective than chemotherapy alone. In the trial, atezolizumab (anti-PD-L1) prolonged progression-free survival (PFS) in combination with first-line nab-paclitaxel (7.2 versus 5.5 months, HR 0.80, 95% CI: 0.69–0.92) in the entire population, with a preliminary analysis suggesting prolonged OS in the 41% of subjects with tumors containing at least 1% PD-L1-positive immune cells (25.0 versus 15.5 months, HR 0.62, 95% CI: 0.45–0.86).3 In the second interim analysis, OS was prolonged for the PD-L1-positive population (25.0 versus 18.0 months, HR 0.71, 95% CI: 0.54–0.93) but not the overall population (21.0 versus 18.7 months, HR 0.86, 95% CI: 0.72–1.02, p = 0.077).19 The combination was generally well tolerated without compromising health-related quality of life as reported by patients,20 thereby reducing concerns of harm and increasing enthusiasm for investigation of other anti-PD1/L1 combinations. In addition, the robust negative predictive value of the integral PD-L1 biomarker (SP142 antibody) was promising, allowing for future selection of individuals most likely to derive benefit. Numerous randomized phase III studies combining anti-PD-1/L1 with standard-of-care therapies are ongoing and will be reported over the next several years, potentially increasing the breadth of options for combination immunotherapy in breast cancer.21
However, given the perils of cross-trial comparison, one foreseeable clinical challenge is to ascertain the relative efficacy of dozens of feasible anti-PD-1/L1 combination approaches in metastatic breast cancer. The goals of this review are to describe immunologic mechanisms of synergy of various standard therapeutic approaches with anti-PD-1/L1, summarize available preclinical data, and discuss clinical use and future investigations of anti-PD-1/L1 combination approaches in metastatic breast cancer.
Cytotoxic chemotherapy
Cytotoxic chemotherapy remains a standard-of-care for metastatic breast cancer, with commonly employed agents including microtubule-targeting agents (paclitaxel, nab-paclitaxel, eribulin, docetaxel), anthracyclines (epirubicin, doxorubicin), anti-metabolites (capecitabine, gemcitabine), alkylating agents (cyclophosphamide), and platinums (cisplatin, carboplatin). The immunomodulatory effects of chemotherapy have been the subject of extensive review,22 and include expansion or activation of effector cell populations (including natural killer [NK] cells, dendritic cells [DC], and T cells), depletion or inhibition of suppressor cell populations (tumor-associated macrophages [TAM], myeloid derived suppressor cells [MDSC], Tregs), and induction of immunogenic cell death (ICD), a stress response associated with release of danger-associated molecular patterns (DAMPs) signals and enhanced antigen presentation.22–27 Chemotherapy is also associated with interferon gamma secretion and adaptive PD-L1 upregulation.28,29 For all these reasons, there has been significant interest in evaluating the efficacy of combining chemotherapy with anti-PD-1/L1. On the other hand, patients who have been extensively pretreated with cytotoxic therapy seem less likely to respond to immunotherapy, suggesting immunosuppressive mechanisms may dominate in the context of more extensive therapy.
While there are preclinical models demonstrating the efficacy of anti-PD-1/L1 plus various chemotherapy agents,29,30 there are fewer data comparing the relative efficacy of the various chemotherapy agents plus anti-PD-1/L1, and results across animal models are inconsistent. For example, cyclophosphamide-containing regimens were among the most effective potentiators of anti-PD-1/L1 response in one study,30 whereas cyclophosphamide plus anthracycline failed to enhance anti-PD-1/L1 response in another.31 Because immune effects of chemotherapy are varied, it becomes difficult to compare the effects on the basis of pharmacodynamic activity alone. For example, anti-metabolites (5-FU and gemcitabine) may be superior to anthracycline or cyclophosphamide in depleting MDSCs, doxorubicin may be superior in inducing ICD, whereas cyclophosphamide may be superior in depleting Tregs.
In the phase III IMpassion130 trial, atezolizumab improved PFS when added to first-line nab-paclitaxel in metastatic triple-negative breast cancer (TNBC) in the entire study population, but OS was only prolonged in patients with tumors bearing PD-L1-positive immune cells.3 These results led to regulatory approval in the first-line setting for PD-L1-positive disease. However, an earlier phase I study demonstrated a response rate of 24% and durable responses in the first-line setting with single-agent atezolizumab,32 raising the possibility that the benefits observed with the addition of atezolizumab are additive rather than synergistic. Ongoing studies will address whether benefits of the taxane/atezolizumab combination can be seen in patients with a shorter disease-free interval than 12 months as studied in IMpassion130 and whether alternative chemotherapy backbones could offer similar or greater clinical benefit. The IMpassion131 study is similar to IMpassion130, however evaluating atezolizumab plus paclitaxel rather than nab-paclitaxel (NCT03125902). Importantly, the results of this study may provide clarity on whether prophylactic steroids impair clinical benefit to anti-PD-L1. The multi-arm, non-comparative phase II “TONIC” trial evaluated various induction chemotherapy regimens or radiation followed by anti-PD-1 (nivolumab) in metastatic TNBC. In this small study, highest objective responses were observed following induction cisplatin (23% ORR) and induction doxorubicin (35% ORR), however these findings must be confirmed in a larger study.33 The optimal sequencing of anti-PD-1/L1 with other therapies remains a topic of considerable debate. The KEYNOTE-355 phase III trial will provide additional randomized data of pembrolizumab versus various chemotherapy backbones (NCT02819518). Of note, this trial uses a different PD-L1 IHC assay (DAKO 22c3 antibody) for patient selection, which recently was found to classify more TNBCs as PD-L1-positive, compared to the SP142 assay.34 The impact of PD-L1-discordance may require additional investigation.
Radiotherapy
In the metastatic setting, ionizing radiotherapy is frequently employed to palliate symptoms (for example, to bone metastases or chest wall lesions) or to delay progression of central nervous system metastases using either stereotactic radiosurgery or whole brain radiotherapy. The principal mechanism of radiotherapy is to induce lethal DNA damage to tumor cells or tumor-associated stroma. However, radiotherapy can enhance anti-tumor immunity by engaging both innate and adaptive responses. In some cases, radiotherapy may be associated with regression of non-irradiated tumors, coined the “abscopal effect.” Radiation-induced DNA damage may lead to cell death and serve as a source of antigen and danger signals that facilitate DC maturation and cross-presentation of tumor antigens to prime tumor-specific T cell responses.35,36 However, it has been shown that the vaccine-effect of radiotherapy is modest, and that synergy with checkpoint blockade may depend on pre-existing immunity.37 Similar to chemotherapy, radiotherapy is associated with release of DAMPs such as uric acid, high mobility group box 1 (HMGB1), calreticulin, and double stranded DNA, which act as immunologic adjuvants to activate myeloid cells and facilitate subsequent chemokine release and T-cell recruitment. Radiotherapy may also upregulate MHC class I and FAS adhesion molecules, which may counteract adaptive loss of MHC or beta 2 microglobulin.38 Conversely, radiotherapy can cause immunosuppressive effects, including upregulation of the PD1/PDL1 axis, upregulation of suppressive macrophage receptors including Mertk,39 expansion of Tregs, and possibly apoptosis of tumor infiltrating lymphocytes (TILs).
In preclinical models, suppressive effects of radiotherapy can be mitigated in combination with anti-PD-1/L1. In a melanoma model, anti-CTLA4 plus radiotherapy was associated with PD-L1 upregulation, and the addition of anti-PD-L1 reversed T-cell exhaustion, promoted clonal T-cell expansion within the tumor, and enhanced response.40 It is difficult to ascertain the optimal dose and schedule of radiation plus immune checkpoint inhibitor. Increased dose is associated with more profound release of DAMPs including ATP and HMGB1, but may also promote immunosuppressive effects such as induction of exonucleases that eliminate cytosolic DNA, a key messenger of DC activation and downstream T-cell priming.41,42 In a comparison of various fractionation schedules plus anti-PD-1 using MOC1 and MC38 murine models, higher-dose hypofractionated radiotherapy (8 Gy x 2) was superior to low-dose fractionated radiotherapy (2 Gy× 10) in controlling tumor, enhancing interferon production, and upregulating PD-L1.43 In a breast cancer model, hypofractionated (8 Gy × 3) was superior to high single dose therapy (20–30 Gy).41 Radiation may also cause systemic lymphopenia (with fractionated radiotherapy causing more profound lymphopenia compared to hypofractionated),44 and conversely, systemic immunosuppression may influence efficacy.45 The timing of radiation may also influence response, with one study showing concurrent therapy superior to sequential.46 Radiation combined with anti-PD-1/L1 has been well tolerated in patients with metastatic breast cancer with preliminary reports of tumor response in lesions outside the radiation field.47 Optimizing radiotherapy dose and timing will likely be the subject of future clinical trials. Furthermore, other immune stimulatory agents such as toll-like receptor 3 agonists and fms related tyrosine kinase 3 ligand (Flt3L), may synergize with radiotherapy and may hold unique promise in conjunction with anti-PD-1/L1.48
Endocrine therapy
Estrogen/progesterone modulation remains a cornerstone of palliative therapy of hormone receptor (HR)-positive metastatic breast cancer. FDA-approved estrogen-directed therapies include a selective estrogen receptor modulator (tamoxifen), aromatase inhibitors (exemestane, letrozole, and anastrozole), and a selective estrogen receptor degrader (fulvestrant). These agents may be used as monotherapy (with or without ovarian suppression), or in combination with targeted agents such as mammalian target of rapamycin (mTOR) inhibitors (everolimus) or cyclin-dependent kinase 4/6 (CDK4/6) inhibitors. Most HR-positive breast cancers and about half of TNBCs express the androgen receptor (AR) to some degree, prompting emerging interest in evaluating AR inhibition as a therapeutic strategy.49 Androgen signaling is known to play a negative regulatory role in central (thymic) T-cell production, and androgen ablation/blockade has been shown to facilitate increases in thymus size, lymphocyte count, thymic recombination of the T-cell receptor, and T-cell cytolytic activity.50 In murine breast cancer models, androgen blockade was associated with enhanced T-cell killing via upregulation of the apoptosis ligand, TRAIL.51 In prostate cancer models, AR blockade increased immune responses to vaccination.52 Finally, in a prostate cancer trial, pembrolizumab plus enzalutamide was associated with increased tumor and DC PD-L1 expression, increased circulating PD-1-positive T-cells, and clinical response following enzalutamide progression.53,54 Anti-PD-1/L1 agents combined with androgen blockade are currently being evaluated across a number of clinical trials in the metastatic breast cancer setting (NCT03650894, NCT02971761). Combinations with anti-estrogens are also ongoing, including the multi-arm MORPHEUS trial that combines fulvestrant with atezolizumab +/− other targeted approaches (NCT03280563).
Cyclin-dependent kinase 4/6 inhibitors
Cyclin dependent kinase 4 and 6 inhibitors (CDK4/6i) have dramatically changed the treatment of metastatic HR-positive breast cancer. There are three FDA-approved agents: palbociclib, ribociclib, and abemaciclib. CDK4/6i are thought to work primarily by inducing cytostasis via G1 cell-cycle arrest, but have also been shown to induce apoptosis in vitro.55 Preclinical evidence suggests that CDK4/6i promote anti-tumor immunity by increasing antigen processing and presentation. CDK4/6i also activate tumor cell expression of endogenous retroviral elements and stimulate interferon signaling, resulting in enhanced tumor antigen presentation.56,57 In human epidermal growth factor receptor 2 (HER2)-positive breast cancers, CDK4/6i also increase expression of multiple antigen processing and presentation genes, including MHC Class I and Class II.56 They may also modulate NK cell activity.58 Teo and colleagues observed increased expression of cell-surface calreticulin in TNBC cell lines (HCC1806 and MDA-MB-231) after treatment with ribociclib, suggesting that CDK4/6i can induce ICD.59 In addition, CDK4/6i augment T cell effector function while markedly suppressing proliferation of regulatory T cells. As cell cycle inhibitors, CDK4/6i decrease T cell proliferation; however, CDK4/6i increase the activation of effector T cells and modulate gene expression.57,60 Preclinical and clinical studies have confirmed increased tumor infiltrating T cells61 and decreased Tregs within treated tumors.56,57,60,61
Given their place in standard treatment, a favorable side effect profile, and the documented beneficial immune effects, CDK4/6i may be a promising agent to combine with anti-PD-1/L1. CDK4/6i increase PD-L1 expression in vivo, with mounting preclinical data suggesting synergy with PD-1/PD-L1 blockade.57,59,60,62 For example, in a CT26 model, the clinical activity of abemaciclib was dependent on immunity, and combination anti-PD-L1 plus abemaciclib resulted in superior disease control with complete responses. Of note, concurrent therapy was superior to sequential therapy in this model. A phase Ib study of pembrolizumab plus abemaciclib in heavily pretreated patients with PD-L1-positive estrogen receptor-positive/HER2-negative advanced cancer showed an acceptable safety profile and clinical activity (overall response rate [ORR] 14.3% at 16 weeks with a 75% disease control rate)63 compared to historical controls for single agent pembrolizumab (ORR 12%)64,65 or single agent abemaciclib (ORR 20% with a 42% disease control rate).66
HER2-directed therapy
Overexpression of HER2 is observed in ~20% of breast carcinomas and is associated with an aggressive phenotype. The standard-of-care first-line therapy for metastatic HER2-positive breast cancer is systemic therapy with taxane plus dual anti-HER2 antibody therapy (trastuzumab and pertuzumab), which is associated with impressive gains in OS, and survival correlates with the degree of TILs.67 Both trastuzumab and pertuzumab are capable of eliciting antibody-dependent cellular cytotoxicity (ADCC) via interactions of the antibody fragment crystallizable region (Fc) with Fc receptors found on NK cells and macrophages.68 Trastuzumab emtansine (T-DM1) is an antibody-drug conjugate, approved in the second-line trastuzumab-resistant setting, that augments the cytotoxic effect of trastuzumab via conjugation with the DM1 chemotherapy moiety. DM1 induces DC maturation and stimulates anti-tumor immunity.69 In murine models, T-DM1 therapy is associated with robust increases in T-cell infiltration, Th1 polarization, PD-1/PD-L1 expression, and intratumoral Tregs infiltration. Combination anti-PD-1 plus anti-CTLA-4 plus T-DM-1 was superior to T-DM-1 or anti-PD-1/CTLA-4 in a preclinical model.69 Lapatinib is an oral targeted inhibitor of EGFR and HER2, approved in combination with capecitabine or trastuzumab for metastatic HER2-positive breast cancer. Because lapatinib stabilizes HER2 protein at the cell membrane, it may also enhance the ADCC-effect of trastuzumab.70 Chemotherapeutic agents including taxanes may also enhance trastuzumab-mediated ADCC.71 Additional agents, including margetuximab, are being developed to maximize the ADCC-mediated immunotherapeutic effect of HER2-targeted therapy.72
In addition to modulating ADCC, anti-HER2 antibodies may also interact with adaptive immune responses.73 In a murine model, the activity of anti-HER-2 was dependent on cytotoxic T-cells and interferon secretion, and was improved in combination with anti-PD-1.74 One additional consideration for HER2-positive breast cancer is the antigenic potential of the HER2 protein. The E75 peptide vaccine, derived from an immunodominant epitope of the HER2 extracellular domain, has been shown to induce E75-specific cytotoxic T-cell responses in humans, and is being evaluated for clinical efficacy in the adjuvant setting in a phase III clinical trial.75,76 Trastuzumab was shown to facilitate DC uptake and antigen presentation of HER2, and increase E75-specific T-cell responses.75 HER2 signaling is also associated with downstream activation of the PI3K/mTOR/AKT pathway; therefore blockade may have secondary immune effects including PD-L1 upregulation. However, analysis of the TCGA database found no significant correlation between the mRNA expression levels of HER2 and PD-L1 in 790 available cases of breast cancer.77
In a phase Ib/II trial, a 20% ORR was observed with pembrolizumab plus trastuzumab in trastuzumab-resistant PD-L1-positive tumors.13 In a similar trial, durvalumab plus trastuzumab was safe at standard full doses, but no responses were observed in a heavily pre-treated population.78 Ongoing clinical trials will evaluate whether combination therapy with anti-PD-1/L1 is effective in earlier lines of therapy, including a first-line trial evaluating standard-of-care first-line taxane/trastuzumab/pertuzumab +/− atezolizumab (NCT03199885). Of note, in the randomized phase II KATE2 study, the addition of atezolizumab to second-line T-DM1 only improved PFS, but only in the PD-L1-positive cohort.14
PI3K/AKT/mTOR pathway inhibitors
A recent analysis of 13,349 genomic profiles identified an association of tumor mutational burden with common breast cancer oncogenic driver mutations, including mutations of both the PI3K/AKT/mTOR and RAS/MAPK pathways.79 The mTOR inhibitor, everolimus, is approved in metastatic HR-positive/HER2-negative breast cancer in combination with exemestane,80 and also improves PFS when added to fulvestrant.81 Recently, the PI3K inhibitor, alpelisib, was approved in combination with fulvestrant for tumors bearing an activating PIK3CA mutation.82 Inhibitors of AKT are being developed and show promise in clinical trials. Recent studies have implicated this pathway in promoting an immunosuppressive tumor microenvironment83 via two mechanisms: (1) increased expression of immunosuppressive cytokines and chemokines which promote recruitment of MDSCs and Tregs84 and (2) constitutive expression of PD-L1.85 However, the association with PTEN loss/PIK3CA activation and PD-L1 expression was not observed in a small set of human TNBC specimens.86 Several pre-clinical studies have suggested that inhibition of the PI3K/AKT/mTOR pathway may decrease Tregs and promote CD8+ memory T-cell differentiation.87–89 Preclinical models evaluating the utility of combination therapy are limited, but the addition of anti-PD-1 was found to enhance the benefit of dual blockade of PI3K and CDK4/6 in an AT3OVA in vivo model.59 The PI3K-γ isoform has been specifically implicated in the function of TAM, and inhibitors of PI3K-γ are being clinically evaluated in combination with atezolizumab in TNBC (NCT03961698).
Poly(ADP ribose) polymerase inhibitors
Phase III clinical trials have demonstrated efficacy of PARP inhibitors (PARPi) in metastatic breast cancer patients with a germline BRCA1/2 pathogenic variants (gBRCA). In the Olympiad study, olaparib was associated with an improvement in PFS relative to physician’s choice of non-platinum chemotherapy, showing a median PFS of 7.0 months vs. 4.2 months (HR 0.58, P < 0.001), however, OS was not prolonged.90,91 Similarly the EMBRACA trial evaluated talazoparib in a similar cohort of patients with a median improvement in PFS of 8.6 months vs. 5.6 months (HR 0.54, P < 0.001).92 On the basis of these results, olaparib and talazoparib are now FDA-approved for gBRCA metastatic breast cancer.
In addition to direct antitumor effects, PARP inhibition may have immunomodulatory properties that improve or impair therapeutic efficacy in breast cancer. PARP inhibition has been associated with an increased number and effector function of cytotoxic T cells and NK cells, showing synergy with CTLA-4 inhibition in an immunocompetent BRCA1−/− model of ovarian cancer, with efficacy driven by improved peritoneal T cell effector function and IFNγ production with combination therapy.93 Treatment of human BRCA−/− UWB1.289 cells with IFNγ caused significantly greater cytotoxicity when the cells were treated with a PARP inhibitor,93 suggesting PARP inhibition may prime cells for IFNγ mediated cell death. Recently, PARP inhibition by olaparib was found to induce robust innate and adaptive immune responses in a BRCA-deficient murine ovarian cancer model, as well as enhanced benefit in combination with anti-PD-1, via cytosolic DNA sensing and activation of the stimulator of interferon genes (STING) pathway.94,95 Conversely, PARPi may also interfere with healthy immune function. PARP is known to interact with and activate NF-κB, a master regulator of innate immune function.96 PARP is necessary for optimal DC differentiation, activation, and stimulation of T cells.97 PARP deficiency has been attributed to increases in frequency and function of Tregs, decreased Th1 cytokine/chemokine function, deficiencies in Th2 differentiation, deficiencies in B-cell antibody class switching and somatic immunoglobulin hypermutation.98,99 Given the anticipated pleotropic effects of PARPi on anti-tumor immunity, more mechanistic studies in the context of ongoing clinical trials as well as randomized trials (such as NCT02849496) will be required to evaluate the synergistic potential of PARP inhibition in combination with anti-PD-1/L1. Recently the phase II single arm MEDIOLA trial evaluated olaparib in combination with durvalumab in patients with gBRCA, and demonstrated a disease control rate at 12 weeks of 80%.100
In breast cancer, PARPi have been shown to increase tumor cell expression of PD-L1, thereby suppressing the antitumor T cell response, but also to have a synergistic effect when given with PD-1 inhibition.101 This effect may be due at least in part to inhibition of PARP-mediated CD8+ T cell apoptosis driven by reactive oxygen species produced by tumor cells.102 The Topacio/Keynote-162 trial evaluated niraparib and pembrolizumab in a phase II single arm trial with an ORR of 28% and disease control rate of 50%, with the best responses being demonstrated in patients with a tumor BRCA mutation.15
Emerging therapeutic modalities
Epigenetic modifying agents, including histone deacetylase inhibitors (HDACi), are undergoing phase III evaluation in metastatic breast cancer and may be immunomodulatory.103,104 HDACi target epigenetic pathways inducing transcription modifications associated with growth inhibition, apoptosis, cell differentiation and ultimately anti-tumor effects.105 MDSCs which can suppress T-cell responses, pose an important limitation to immune therapy for breast cancer, but may also serve as a potential target for amplifying host immunity. This has been shown in animal models and in patients with breast cancer.104,106 Preclinical work demonstrates that HDACi may reduce the activity of MDSCs and Tregs,104,107 upregulate MHCI/II, increase sensitivity of breast cancer cells to cytotoxic T-cell mediated lysis, direct NK cell-mediated lysis, and facilitate ADCC.108 Exploratory analyses from the phase II clinical trial ENCORE 301 (exemestane +/− entinostat) demonstrated an increase in HLA-DR-positive monocytes and a decrease in granulocytic and monocytic MDSCs in patients treated with HDACi.109 Immunomodulatory activity was correlated with histone acetylation of peripheral mononuclear cells (suggested biomarker of response) and clinical benefit. Given the immunomodulatory effects of HDACi, it is not surprising that multiple preclinical studies have found synergy with the combination of HDACi and checkpoint blockade in breast cancer and other solid tumors.104,110,111 DNA methyltransferase inhibitors (DNMTi, e.g., azacitidine, decitabine, guadecitabine) and various systemic chemotherapies (gemcitabine, doxorubicin, and others) also increase MHCI and tumor antigen and reduce systemic and intratumoral MDSCs, potentially augmenting anti-PD-1/L1.104
Targeted inhibition of the oncogenic RAS-MAPK pathway, a driver of some breast cancers, may also have immunostimulatory effects. Genomic or transcriptomic activation of the RAS-MAPK pathway has been associated with decreased TIL infiltration in residual disease specimens of patients with TNBC treated with neoadjuvant chemotherapy.112 RAS-MAPK pathway activity has been shown to suppress antigen presentation by decreasing expression of MHC-I and MHC-II. Furthermore, MEK inhibition has been demonstrated to upregulate MHC and PD-L1 expression, suggesting that combining MEK inhibitor plus anti-PD-1/L1 may be a promising therapeutic strategy. Indeed, this combination has yielded preclinical anti-tumor activity and is now being explored in phase I/II clinical trials. However, additional pre-clinical studies suggest that while MEK inhibition may augment TIL infiltration in TNBC, it may also have the unintended consequence of encumbering T cell proliferation, but may extend the survival and fitness of antigen-specific T-cells in the microenvironment.113 MEK signaling occurs downstream of T cell receptor activation. Therefore, inhibition of MEK may also decrease T cell proliferation and cytokine production, which can be overcome by co-administration of T-cell agonists such as anti-OX40.113
Additional immunotherapeutic agents, including agents targeting immune-metabolic pathways (adenosine and indoleamine 2,3-dioxygenase 1 [IDO1]) or T-cell agonists (OX40) are being evaluated in conjunction with anti-PD-1/L1 in breast cancer. Adenosine mediates the pro-tumor effects of the ectoenzyme CD73, which is expressed in TNBC and associated with chemotherapy resistance.114 Activation of adenosine receptors (A2A-R or A2B-R) suppresses T-cell proliferation, cytokine production, and cytotoxicity.115,116 In 4T1 TNBC mouse models, A2A/B inhibition plus anti-PD-l was superior to monotherapy, with the observed benefit dependent on interferon secretion, NK-cells, and CD8+ T-cells.117 The adenosine receptor inhibitor, CPI-44, has been evaluated in conjunction with atezolizumab in early clinical trials, but has not been specifically evaluated in breast cancer patients. IDO1 is induced in DCs and macrophages at sites of inflammation, and degrades tryptophan into immune-suppressive metabolites that are associated with T-cell apoptosis, reduced activation, and Treg phenotype differentiation.118 In 4T1 TNBC orthotopic mouse models, IDO1 knockout results in reduced lung metastasis and improved survival.119 The IDO1 inhibitor, epacadostat, was well tolerated when combined with pembrolizumab, and was associated with a 10% ORR in a small TNBC patient cohort.120 Recently, however, epacadostat failed to improve PFS when combined with pembrolizumab in stage IV melanoma.121 Finally, OX40 is a T-cell agonist molecule which, when stimulated, may reduce the threshold required for initial T-cell activation. In both MMTV-PyMT and 4T1 mammary carcinoma models, combination anti-OX40 plus anti-PD-1/L1 was associated with improved tumor control, but the synergistic effect was demonstrated only when anti-OX40 was administered in sequence with anti-PD-1/L1. Concurrent therapy was more toxic to mice and associated with surges in both Th1 and Th2 cytokines, highlighting the possibility that compensatory feedback mechanisms could modulate efficacy of combination immunotherapy.122
Discussion
Pre-clinical, translational, and early clinical data support ongoing efforts to combine anti-PD-1/L1 with standard-of-care and emerging therapies including chemotherapy, radiotherapy, endocrine therapy, and targeted therapy. A number of putative mechanisms of synergy have been demonstrated, some of which are shared across therapeutic modalities (Fig. 1). An emerging clinical challenge is to determine the optimal combination strategy in the face of a wealth of preclinical and clinical data, as well as to determine whether single-agent anti-PD-1/L1 could be effective in a subset of breast cancers. Summarized below are key considerations in the use of anti-PD-1/L1 combination approaches for metastatic TNBC, HR-positive breast cancer, and HER2-positive breast cancer.
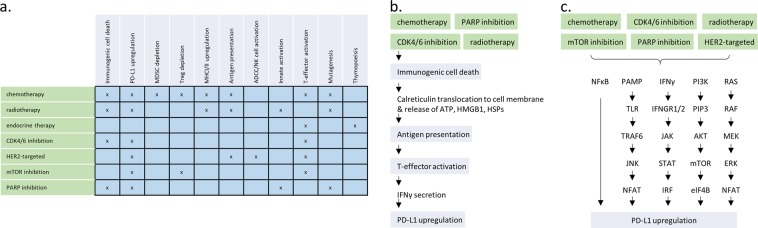
Fig. 1.
Potential Mechanisms of synergy of anti-PD-1/L1 combination therapies. a Standard-of-care therapies in the metastatic breast cancer setting exhibit varied and overlapping immunomodulatory effects that may promote therapeutic synergy with anti-PD-1/L1; b Immunogenic cell death is conserved across a number of anti-neoplastic modalities. A hallmark biologic feature is calreticulin exposure from the endoplasmic reticulum, resulting in downstream antigen presentation, T-cell activation, and adaptive PD-L1 upregulation; c Another common mechanism of synergy is PD-L1 upregulation, which can occur via modulation of a variety of molecular pathways. PD-L1: programmed death ligand 1; MDSC: myeloid derived suppressor cell; Treg: T-regulatory cell; MHC: major histocompatibility complex; ADCC: antibody-dependent cellular cytotoxicity; NK: natural killer; CDK4/6: cyclin-dependent kinase 4/6; HER2: human epidermal growth factor receptor 2; mTOR: mammalian target of rapamycin; PARP; poly(ADP ribose) polymerase; ATP: adenosine triphosphate; HMGD1: high mobility group box 1; HSPs: heat shock proteins; IFNγ: interferon gamma; NFκB: nuclear factor kappa-light-chain enhancer of activated B-cells; PAMP: pathogen-associated molecular pattern; TLR: toll like receptor; TRAF6: TNF receptor associated factor 6; JNK: c-Jun N-terminal kinase; NFAT: nuclear factor of activated T-cells; IFNGR1/2: interferon gamma receptor 1/2; STAT: signal transducer and activator of transcription protein; IRF: interferon regulatory factor; PI3K: phosphoinositide 3-kinase; PIP3: phosphatidylinositol (3,4,5)-triphosphate; AKT: protein kinase B; eIF4B: eukaryotic translation initiation factor 4B
In metastatic PD-L1-positive TNBC, the PFS benefit (and preliminary OS benefit) in IMpassion130 provides level I evidence supporting atezolizumab (anti-PD-L1) plus nab-paclitaxel as a standard approach for first-line therapy for patients with a >12 month distant recurrence free interval and PD-L1-positivity. There are insufficient data to guide whether anti-PD-1/L1 can be effectively combined with alternative chemotherapy regimens. Ongoing randomized trials (including Keynote-355, NCT02819518) are addressing this question. In phase I/II studies of anti-PD-1/L1 monotherapy, efficacy diminishes substantially in later lines of therapy, suggesting that earlier treatment may be more effective. Biomarker assessments from ongoing trials may guide future personalization of chemotherapy plus anti-PD-1/L1 according to patient and/or tumor characteristics. Subjects with PD-L1-negative tumors did not benefit from the addition of atezolizumab, and therefore should be considered for clinical trials evaluating anti-PD-1/L1 in combination with novel agents. A number of existing therapies can induce PD-L1 upregulation, and may be promising for study in the PD-L1-negative TNBC population.
For subjects with HR-positive metastatic breast cancer, tumors are less likely to be PD-L1-positive.64 Several combination strategies have mechanistic basis, including anti-PD-1/L1 plus CDK4/6i (with or without aromatase inhibitor), chemotherapy, mTOR inhibition, HDACi, DNMTi, AR blockade, or radiotherapy. With further research, novel biomarkers including high-throughput genomic/genetic profiling and advanced histologic approaches may be developed to personalize therapy.
Subjects with HER2-positive breast cancer benefit from a multitude of approaches. An ongoing phase III randomized trial will evaluate first-line pertuzumab/trastuzumab/paclitaxel +/− atezolizumab (NCT03199885). An additional combination to be considered is T-DM1 plus anti-PD-1/L1, which in a phase II trial was associated with improved PFS but only in PD-L1-positive tumors (NCT02924883). Combinations with novel agents, such as HER2-directed vaccines, are promising and warrant clinical evaluation.
Despite the relative safety of anti-PD-1/L1 combination therapies, the potential for long-term toxicity exists. A prominent example is immune-related endocrinopathy (thyroid or adrenal dysfunction), which has been observed with anti-PD-1/L1 combination therapy and may require lifelong hormone replacement therapy. Resources should be devoted to evaluate patient reported outcomes and extend the time period for such measures to be assessed. Furthermore, novel phase I statistical designs should be employed to capture late toxicities in dose decision-making. For example, the time-to-event continual reassessment method starts with a target dose limiting toxicity (DLT) rate that the investigators deem acceptable, and the first patient is followed for DLT.123 The toxicity information of previously treated patients is adaptively combined with new patient data using a Bayesian approach, allowing for continuous reassessment of toxicity estimates. Owing to the allowance of staggered enrollment without the need for accrual suspension during DLT follow-up, this design has the potential to substantially shorten the trial duration compared to traditional phase I designs. Furthermore, it has been shown that this design assigns a greater proportion of patients to the target dose.
Conclusion
The IMpassion130 clinical trial serves as proof-of-principle that anti-PD-1/L1 combination approaches can be safe and effective in metastatic breast cancer. A vast body of preclinical, translational, and clinical data supports ongoing efforts to evaluate a variety of combination approaches. As additional clinical trials are completed and various combination approaches are found to be beneficial, careful evaluation must be made to select the optimal combination strategy given unique patient and tumor characteristics. Moreover, robust, systematic, and streamlined biomarker studies are critical if immunotherapy combination strategies are to become applicable for the majority of breast cancer patients.
Acknowledgements
This review effort arose from discussions of the National Cancer Institute’s (NCI) Breast Immuno-Oncology (BIO) Working Group and Task Force, of which the co-authors were members and was supported in edits and data collection by Lynn P. Butler, MS, the NCI Breast Cancer Steering Committee’s Program Coordinator (The EMMES Corporation). Sandra Demaria PhD offered editorial feedback and content recommendations. No compensation was provided outside the normal course of employment for these contributions.
Author contributions
Study concept and design: D.B.P., S.P., M.E.G.-M., E.A.M., and S.A. Acquisition, analysis, or interpretation of data: D.B.P., H.B., S.P., M.E.G.-M., A.T., H.M.A., J.M.B., S.R.G., R.N., K.K., J.W., J.L., S.J.C., M.-Y.P., B.V., J.A.S., E.A.M., and S.A. Drafting of the manuscript: D.B.P., H.B., S.P., M.E.G.-M., A.T., H.M.A., J.M.B., S.R.G., R.N., J.L.G., K.K., J.W., J.L., S.J.C., M.-Y.P., B.V., D.W.C., and J.A.S. Critical revision of the manuscript for important intellectual content: D.B.P., H.B., S.P., M.E.G.-M., A.T., H.M.A., J.M.B., S.R.G., R.N., J.L.G., K.K., J.W., J.L., S.J.C., M.-Y.P., B.V., D.W.C., M.L.D., J.A.S., E.A.M., and S.A. Administrative, technical, or material support: D.B.P., S.P., M.E.G.-M., and A.T. Study supervision: D.B.P., S.P., M.E.G.-M., E.A.M., and S.A.
Data availability
No new datasets were generated or analyzed for this report.
Competing interests
D.B.P.: Research support: BMS, Merck, Brooklyn ImmunoTherapeutics. Speakers Bureau: Genentech, Novartis. Scientific Advisory Board: BMS, Merck, Syndax, Nektar, Puma, Nanostring H.B.: Receives research support from Merck and serves on the Advisory Board for Merck. S.P.: No COI or disclosures Gatti-Mays: No COI or disclosures. A.T.: Research support (to institution): Syndax, Genentech; Stockholder: Bristol-Myers Squibb, Gilead Sciences, Johnson and Johnson, Pfizer; Consultant (DSMB) BeyondSpring Pharmaceuticals (managed by Watermark Research Partners), H.M.A.: Consulting: Merck, Spectrum Pharmaceuticals, Lilly, Amgen, Immunomedics, Pfizer, Genentech, Bristol-Myers Squibb, Genomic Health. Research Funding: Bristol-Myers Squibb, ZIOPHARM Oncology, Lilly, Merck; Travel expenses: Merck, Spectrum Pharmaceuticals, Lilly, Amgen, Puma Biotechnology, Immunomedics, Genentech, Pfizer. Expert panel: Lilly. J.M.B.: Receives research support from Genentech/Roche, Bristol Myers Squibb, and Incyte Corporation, has received consulting/expert witness fees from Novartis, and is an inventor on provisional patents regarding immunotherapy targets and biomarkers in cancer. S.R.G.: No COIs or disclosures. R.N.: Advisory Board: AstraZeneca, Athenex, Celgene, Daiichi Sankyo, Inc, Genentech, MacroGenics, Merck, Novartis, Pfizer, Puma, Syndax. DSMB:G1 Therapeutics Research Funding: AstraZeneca, Celgene, Corcept. Therapeutics, Genentech/Roche, Immunomedics, Merck, Odonate Therapeutics, Pfizer, Seattle Genetics, J.L.G.: National Cancer Institutes has several Cooperative Research and Development Agreements (CRADAs) with various biotech and pharma agencies involved in immunotherapy. Kalinsky: Consulting: Biotheranostics, Eli-Lilly, Pfizer, Amgen, Novartis, Eisai, AstraZeneca, Odonate Therapeutics, Ipsen, Genentech. Speakers’ bureau: Eli-Lilly. Institutional support: Incyte, Genentech, Eli-Lilly, Pfizer, Calithera Biosciences, Acetylon, Seattle Genetics, Amgen, Zeno Pharmaceuticals, CytomX Therapeutics. Spouse: employment at Array Biopharma. J.W.: None. J.L.: Research support: Novartis, Medivation/Pfizer, Genentech, GSK, EMD-Serono, AstraZeneca, Medimmune. Speaker’s Bureau: MedLearning, Physician’s Education Resource, Prime Oncology, Medscape. Honoraria: Uptodate; Advisory committee: AstraZeneca, Pfizer (both uncompensated). Review panels: NCCN, ASCO, NIH PDQ. S.J.C.: Astellas (spouse), RefleXion (Consulting). M.-Y.P.: No COIs or disclosures. B.V.: Research funding: Merck, Phamacyclics, Acerta Pharma; Equity: Select Immunogenomics. D.W.C.: Honoraria (non-accredited CME) Pfizer, and Novartis. Consulting or advisory role: Pfizer, AstraZeneca, Novartis, GlaxoSmithKline, Merck, Roche/Genentech, Agendia, Puma Biotechnology and Dynamo Therapeutics. Research funding (to instituton): Merck, Roche/Genentech, GlaxoSmithKline, and Pfizer. M.L.D.: COI: grants from Epithany, Celgene, EMD Serono, Pfizer, Seattle Genetics, Silverback Therapeutics, Janssen. Stockholder in Epithany. Vendor-sponsored travel: Eli-Lilly, Novartis, Genentech, Ipsen and Amgen. J.A.S.: Receives research support: Deciphera, Inc., Prescient Therapeutics; Paid consultant: Astra Zeneca, Pfizer; Pharmaceutical sponsored research: Genentech. Mittendorf: serves on advisory boards for Merck, SELLAS Lifesciences, AstraZeneca/MedImmune, TapImmune, and Peregrine Pharmaceuticals, and received institutional research funding from Genentech, Astra Zeneca/MedImmune, and SELLAS Lifesciences. S.A.: Advisory Board: BMS, Merck, Genentech (all uncompensated), research funding to institution: Genentech, Merck, Amgen, Novartis, BMS, Celgene.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.D’Abreo N, Adams S. Immune-checkpoint inhibition for metastatic triple-negative breast cancer: safety first? Nat. Rev. Clin. Oncol. 2019;16:399–400. doi: 10.1038/s41571-019-0216-2. [DOI] [PubMed] [Google Scholar]
- 2.Dear, R. F. et al. Combination versus sequential single agent chemotherapy for metastatic breast cancer. Cochrane Database Syst Rev, CD008792, 10.1002/14651858.CD008792.pub2 (2013). [DOI] [PMC free article] [PubMed]
- 3.Schmid P, et al. Atezolizumab and nab-paclitaxel in advanced triple-negative breast cancer. N. Engl. J. Med. 2018;379:2108–2121. doi: 10.1056/NEJMoa1809615. [DOI] [PubMed] [Google Scholar]
- 4.Cortes J, Guo Z, Karantza V, Aktan G. Abstract CT069: KEYNOTE-355: Randomized, double-blind, phase III study of pembrolizumab plus chemotherapy vs placebo plus chemotherapy for previously untreated, locally recurrent, inoperable or metastatic triple-negative breast cancer (mTNBC) Cancer Res. 2017;77:CT069–CT069. [Google Scholar]
- 5.Page D, et al. Abstract P2-09-03: Updated efficacy of first or second-line pembrolizumab (pembro) plus capecitabine (cape) in metastatic triple negative breast cancer (mTNBC) and correlations with baseline lymphocyte and naïve CD4+ T-cell count. Cancer Res. 2019;79:P2-09-03–P02-09-03. [Google Scholar]
- 6.Tolaney S, et al. Abstract PD6-13: Phase 1b/2 study to evaluate eribulin mesylate in combination with pembrolizumab in patients with metastatic triple-negative breast cancer. Cancer Res. 2018;78:PD6-13–PD16-13. [Google Scholar]
- 7.Nanda R, et al. Pembrolizumab plus standard neoadjuvant therapy for high-risk breast cancer (BC): Results from I-SPY 2. J. Clin. Oncol. 2017;35:506–506. doi: 10.1200/JCO.2017.35.15_suppl.506. [DOI] [Google Scholar]
- 8.Loibl S, et al. Randomized phase II neoadjuvant study (GeparNuevo) to investigate the addition of durvalumab to a taxane-anthracycline containing chemotherapy in triple negative breast cancer (TNBC) J. Clin. Oncol. 2018;36:104–104. doi: 10.1200/JCO.2018.36.15_suppl.104. [DOI] [Google Scholar]
- 9.McArthur HL, et al. A single-arm, phase II study assessing the efficacy of pembrolizumab (pembro) plus radiotherapy (RT) in metastatic triple negative breast cancer (mTNBC) J. Clin. Oncol. 2018;36:14–14. doi: 10.1200/JCO.2018.36.5_suppl.14. [DOI] [Google Scholar]
- 10.Luke JJ, et al. Safety and Clinical Activity of Pembrolizumab and Multisite Stereotactic Body Radiotherapy in Patients With Advanced Solid Tumors. J. Clin. Oncol. 2018;36:1611–1618. doi: 10.1200/JCO.2017.76.2229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tolaney SM, et al. Updated efficacy, safety, & PD-L1 status of patients with HR+, HER2− metastatic breast cancer administered abemaciclib plus pembrolizumab. J. Clin. Oncol. 2018;36:1059–1059. doi: 10.1200/JCO.2018.36.15_suppl.1059. [DOI] [Google Scholar]
- 12.Mayer E, et al. Abstract OT3-05-11: Palbociclib after CDK inhibitor and endocrine therapy (PACE): A randomized phase II study of fulvestrant versus palbociclib plus fulvestrant, with and without avelumab, for CDK inhibitor pre-treated HR+/HER2− metastatic breast cancer. Cancer Res. 2018;78:OT3-05-11–OT03-05-11. [Google Scholar]
- 13.Loi S, et al. Pembrolizumab plus trastuzumab in trastuzumab-resistant, advanced, HER2-positive breast cancer (PANACEA): a single-arm, multicentre, phase 1b-2 trial. Lancet Oncol. 2019;20:371–382. doi: 10.1016/S1470-2045(18)30812-X. [DOI] [PubMed] [Google Scholar]
- 14.Emens L, et al. Abstract PD3-01: Results from KATE2, a randomized phase 2 study of atezolizumab (atezo)+trastuzumab emtansine (T-DM1) vs placebo (pbo)+T-DM1 in previously treated HER2+ advanced breast cancer (BC) Cancer Res. 2019;79:PD3-01–PD03-01. doi: 10.1158/0008-5472.CAN-18-1745. [DOI] [Google Scholar]
- 15.Vinayak S, et al. TOPACIO/Keynote-162: Niraparib + pembrolizumab in patients (pts) with metastatic triple-negative breast cancer (TNBC), a phase 2 trial. J. Clin. Oncol. 2018;36:1011–1011. doi: 10.1200/JCO.2018.36.15_suppl.1011. [DOI] [Google Scholar]
- 16.Domchek S, et al. Abstract PD6-11: an open-label, multitumor, phase II basket study of olaparib and durvalumab (MEDIOLA): results in germline BRCA-mutated (gBRCA) HER2-negative metastatic breast cancer (MBC) Cancer Res. 2018;78:PD6-11–PD16-11. [Google Scholar]
- 17.Forero A, et al. Abstract OT2-01-12: ENCORE 602: A randomized, placebo-controlled, double-blind, multicenter phase 2 study (with a phase 1b lead-in) of atezolizumab with or without entinostat in patients with advanced triple negative breast cancer (aTNBC) Cancer Res. 2017;77:OT2-01-12–OT02-01-12. [Google Scholar]
- 18.Spira AI, et al. Efficacy/safety of epacadostat plus pembrolizumab in triple-negative breast cancer and ovarian cancer: Phase I/II ECHO-202 study. J. Clin. Oncol. 2017;35:1103–1103. doi: 10.1200/JCO.2017.35.15_suppl.1103. [DOI] [Google Scholar]
- 19.Schmid P, et al. IMpassion130: updated overall survival (OS) from a global, randomized, double-blind, placebo-controlled, Phase III study of atezolizumab (atezo) + nab-paclitaxel (nP) in previously untreated locally advanced or metastatic triple-negative breast cancer (mTNBC) J. Clin. Oncol. 2019;37:1003–1003. doi: 10.1200/JCO.2019.37.15_suppl.1003. [DOI] [Google Scholar]
- 20.Adams S, et al. Patient-reported outcomes (PROs) from the phase III IMpassion130 trial of atezolizumab (atezo) plus nabpaclitaxel (nP) in metastatic triple-negative breast cancer (mTNBC) J. Clin. Oncol. 2019;37:1067–1067. doi: 10.1200/JCO.2019.37.15_suppl.1067. [DOI] [Google Scholar]
- 21.Adams Sylvia, Gatti-Mays Margaret E., Kalinsky Kevin, Korde Larissa A., Sharon Elad, Amiri-Kordestani Laleh, Bear Harry, McArthur Heather L., Frank Elizabeth, Perlmutter Jane, Page David B., Vincent Benjamin, Hayes Jennifer F., Gulley James L., Litton Jennifer K., Hortobagyi Gabriel N., Chia Stephen, Krop Ian, White Julia, Sparano Joseph, Disis Mary L., Mittendorf Elizabeth A. Current Landscape of Immunotherapy in Breast Cancer. JAMA Oncology. 2019;5(8):1205. doi: 10.1001/jamaoncol.2018.7147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Galluzzi L, Buque A, Kepp O, Zitvogel L, Kroemer G. Immunological effects of conventional chemotherapy and targeted anticancer agents. Cancer Cell. 2015;28:690–714. doi: 10.1016/j.ccell.2015.10.012. [DOI] [PubMed] [Google Scholar]
- 23.Kepp O, et al. Molecular determinants of immunogenic cell death elicited by anticancer chemotherapy. Cancer Metastasis Rev. 2011;30:61–69. doi: 10.1007/s10555-011-9273-4. [DOI] [PubMed] [Google Scholar]
- 24.Obeid M, et al. Calreticulin exposure dictates the immunogenicity of cancer cell death. Nat. Med. 2007;13:54–61. doi: 10.1038/nm1523. [DOI] [PubMed] [Google Scholar]
- 25.Martins I, et al. Molecular mechanisms of ATP secretion during immunogenic cell death. Cell Death Differ. 2014;21:79–91. doi: 10.1038/cdd.2013.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yamazaki T, et al. Defective immunogenic cell death of HMGB1-deficient tumors: compensatory therapy with TLR4 agonists. Cell Death Differ. 2014;21:69–78. doi: 10.1038/cdd.2013.72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Vincent J, et al. 5-Fluorouracil selectively kills tumor-associated myeloid-derived suppressor cells resulting in enhanced T cell-dependent antitumor immunity. Cancer Res. 2010;70:3052–3061. doi: 10.1158/0008-5472.CAN-09-3690. [DOI] [PubMed] [Google Scholar]
- 28.Peng J, et al. Chemotherapy induces programmed cell death-ligand 1 Overexpression via the nuclear factor-kappaB to foster an immunosuppressive tumor microenvironment in ovarian cancer. Cancer Res. 2015;75:5034–5045. doi: 10.1158/0008-5472.CAN-14-3098. [DOI] [PubMed] [Google Scholar]
- 29.Cubas R, et al. Chemotherapy combines effectively with anti-PD-L1 treatment and can augment antitumor responses. J. Immunol. 2018;201:2273–2286. doi: 10.4049/jimmunol.1800275. [DOI] [PubMed] [Google Scholar]
- 30.Pfirschke C, et al. Immunogenic chemotherapy sensitizes tumors to checkpoint blockade therapy. Immunity. 2016;44:343–354. doi: 10.1016/j.immuni.2015.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Grasselly C, et al. The antitumor activity of combinations of cytotoxic chemotherapy and immune checkpoint inhibitors is model-dependent. Front Immunol. 2018;9:2100. doi: 10.3389/fimmu.2018.02100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Emens LA, et al. Long-term clinical outcomes and biomarker analyses of Atezolizumab therapy for patients with metastatic triple-negative breast cancer: a phase 1 study. JAMA Oncol. 2019;5:74–82. doi: 10.1001/jamaoncol.2018.4224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Voorwerk L, et al. Immune induction strategies in metastatic triple-negative breast cancer to enhance the sensitivity to PD-1 blockade: the TONIC trial. Nat. Med. 2019;25:920–928. doi: 10.1038/s41591-019-0432-4. [DOI] [PubMed] [Google Scholar]
- 34.Scott, M., Scorer, P., Barker, C. & Al-Masri, H. Comparison of patient populations identified by different PD-L1 assays in in triple-negative breast cancer (TNBC). Ann. Oncol. 30, 10.1093/annonc/mdz095.009 (2019).
- 35.Demaria S, Golden EB, Formenti SC. Role of local radiation therapy in cancer immunotherapy. JAMA Oncol. 2015;1:1325–1332. doi: 10.1001/jamaoncol.2015.2756. [DOI] [PubMed] [Google Scholar]
- 36.Spiotto Michael, Fu Yang-Xin, Weichselbaum Ralph R. The intersection of radiotherapy and immunotherapy: Mechanisms and clinical implications. Science Immunology. 2016;1(3):eaag1266–eaag1266. doi: 10.1126/sciimmunol.aag1266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Crittenden MR, et al. Tumor cure by radiation therapy and checkpoint inhibitors depends on pre-existing immunity. Sci. Rep. 2018;8:7012. doi: 10.1038/s41598-018-25482-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Paulson KG, et al. Acquired cancer resistance to combination immunotherapy from transcriptional loss of class I HLA. Nat. Commun. 2018;9:3868. doi: 10.1038/s41467-018-06300-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Crittenden MR, et al. Mertk on tumor macrophages is a therapeutic target to prevent tumor recurrence following radiation therapy. Oncotarget. 2016;7:78653–78666. doi: 10.18632/oncotarget.11823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Twyman-Saint Victor C, et al. Radiation and dual checkpoint blockade activate non-redundant immune mechanisms in cancer. Nature. 2015;520:373–377. doi: 10.1038/nature14292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Vanpouille-Box C, et al. DNA exonuclease Trex1 regulates radiotherapy-induced tumour immunogenicity. Nat. Commun. 2017;8:15618. doi: 10.1038/ncomms15618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gameiro SR, et al. Radiation-induced immunogenic modulation of tumor enhances antigen processing and calreticulin exposure, resulting in enhanced T-cell killing. Oncotarget. 2014;5:403–416. doi: 10.18632/oncotarget.1719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Morisada M, et al. PD-1 blockade reverses adaptive immune resistance induced by high-dose hypofractionated but not low-dose daily fractionated radiation. Oncoimmunology. 2018;7:e1395996. doi: 10.1080/2162402X.2017.1395996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Crocenzi T, et al. A hypofractionated radiation regimen avoids the lymphopenia associated with neoadjuvant chemoradiation therapy of borderline resectable and locally advanced pancreatic adenocarcinoma. J. Immunother. Cancer. 2016;4:45. doi: 10.1186/s40425-016-0149-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Manyam BV, et al. Inferior outcomes in immunosuppressed patients with high-risk cutaneous squamous cell carcinoma of the head and neck treated with surgery and radiation therapy. J. Am. Acad. Dermatol. 2015;73:221–227. doi: 10.1016/j.jaad.2015.04.037. [DOI] [PubMed] [Google Scholar]
- 46.Dovedi SJ, et al. Acquired resistance to fractionated radiotherapy can be overcome by concurrent PD-L1 blockade. Cancer Res. 2014;74:5458–5468. doi: 10.1158/0008-5472.CAN-14-1258. [DOI] [PubMed] [Google Scholar]
- 47.Hu ZI, McArthur HL, Ho AY. The Abscopal Effect of Radiation Therapy: What Is It and How Can We Use It in Breast Cancer? Curr. Breast Cancer Rep. 2017;9:45–51. doi: 10.1007/s12609-017-0234-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hammerich L, et al. Systemic clinical tumor regressions and potentiation of PD1 blockade with in situ vaccination. Nat. Med. 2019;25:814–824. doi: 10.1038/s41591-019-0410-x. [DOI] [PubMed] [Google Scholar]
- 49.Traina TA, et al. Enzalutamide for the Treatment of Androgen Receptor-Expressing Triple-Negative Breast Cancer. J. Clin. Oncol. 2018;36:884–890. doi: 10.1200/JCO.2016.71.3495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Velardi E, et al. Sex steroid blockade enhances thymopoiesis by modulating Notch signaling. J. Exp. Med. 2014;211:2341–2349. doi: 10.1084/jem.20131289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kwilas AR, et al. Androgen deprivation therapy sensitizes triple negative breast cancer cells to immune-mediated lysis through androgen receptor independent modulation of osteoprotegerin. Oncotarget. 2016;7:23498–23511. doi: 10.18632/oncotarget.8274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ardiani A, et al. Combination therapy with a second-generation androgen receptor antagonist and a metastasis vaccine improves survival in a spontaneous prostate cancer model. Clin. Cancer Res. 2013;19:6205–6218. doi: 10.1158/1078-0432.CCR-13-1026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Graff JN, et al. Early evidence of anti-PD-1 activity in enzalutamide-resistant prostate cancer. Oncotarget. 2016;7:52810–52817. doi: 10.18632/oncotarget.10547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Bishop JL, et al. PD-L1 is highly expressed in Enzalutamide resistant prostate cancer. Oncotarget. 2015;6:234–242. doi: 10.18632/oncotarget.2703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Torres-Guzman R, et al. Preclinical characterization of abemaciclib in hormone receptor positive breast cancer. Oncotarget. 2017;8:69493–69507. doi: 10.18632/oncotarget.17778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Goel S, et al. CDK4/6 inhibition triggers anti-tumour immunity. Nature. 2017;548:471–475. doi: 10.1038/nature23465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Schaer DA, et al. The CDK4/6 inhibitor Abemaciclib induces a T cell inflamed tumor microenvironment and enhances the efficacy of PD-L1 checkpoint blockade. Cell Rep. 2018;22:2978–2994. doi: 10.1016/j.celrep.2018.02.053. [DOI] [PubMed] [Google Scholar]
- 58.Ruscetti M, et al. NK cell-mediated cytotoxicity contributes to tumor control by a cytostatic drug combination. Science. 2018;362:1416–1422. doi: 10.1126/science.aas9090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Teo ZL, et al. Combined CDK4/6 and PI3Kalpha inhibition is synergistic and immunogenic in triple-negative breast cancer. Cancer Res. 2017;77:6340–6352. doi: 10.1158/0008-5472.CAN-17-2210. [DOI] [PubMed] [Google Scholar]
- 60.Deng J, et al. CDK4/6 inhibition augments antitumor immunity by enhancing T-cell activation. Cancer Disco. 2018;8:216–233. doi: 10.1158/2159-8290.CD-17-0915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Hurvitz S, et al. Abstract S4-06: biological effects of abemaciclib in a phase 2 neoadjuvant study for postmenopausal patients with HR+, HER2- breast cancer. Cancer Res. 2016;77:S4–06. [Google Scholar]
- 62.Chin, D. et al. CDK4/6 inhibitor Abemaciclib exerts intrinsic immunomodulatory effects on human T cells in vitro. Society for the Immunotherapy of Cancer Annual Meeting 2017. (Gaylord Conference Center, National Harbor, MD, 2017).
- 63.Rugo HS, et al. Abstract P1-09-01: A phase 1b study of abemaciclib plus pembrolizumab for patients with hormone receptor-positive (HR+), human epidermal growth factor receptor 2-negative (HER2−) metastatic breast cancer (MBC) Cancer Res. 2018;78:P1-09-01. [Google Scholar]
- 64.Rugo HS, et al. Safety and antitumor activity of Pembrolizumab in patients with estrogen receptor-positive/human epidermal growth factor receptor 2-negative advanced breast cancer. Clin. Cancer Res. 2018;24:2804–2811. doi: 10.1158/1078-0432.CCR-17-3452. [DOI] [PubMed] [Google Scholar]
- 65.Dirix LY, et al. Avelumab, an anti-PD-L1 antibody, in patients with locally advanced or metastatic breast cancer: a phase 1b JAVELIN Solid Tumor study. Breast Cancer Res. Treat. 2018;167:671–686. doi: 10.1007/s10549-017-4537-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Dickler MN, et al. MONARCH 1, A Phase II Study of Abemaciclib, a CDK4 and CDK6 Inhibitor, as a Single Agent, in Patients with Refractory HR(+)/HER2(-) Metastatic Breast Cancer. Clin. Cancer Res. 2017;23:5218–5224. doi: 10.1158/1078-0432.CCR-17-0754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Luen SJ, et al. Tumour-infiltrating lymphocytes in advanced HER2-positive breast cancer treated with pertuzumab or placebo in addition to trastuzumab and docetaxel: a retrospective analysis of the CLEOPATRA study. Lancet Oncol. 2017;18:52–62. doi: 10.1016/S1470-2045(16)30631-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Holgado E, Perez-Garcia J, Gion M, Cortes J. Is there a role for immunotherapy in HER2-positive breast cancer? NPJ Breast Cancer. 2018;4:21. doi: 10.1038/s41523-018-0072-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Muller P, et al. Trastuzumab emtansine (T-DM1) renders HER2+ breast cancer highly susceptible to CTLA-4/PD-1 blockade. Sci. Transl. Med. 2015;7:315ra188. doi: 10.1126/scitranslmed.aac4925. [DOI] [PubMed] [Google Scholar]
- 70.Blackwell KL, et al. Overall survival benefit with lapatinib in combination with trastuzumab for patients with human epidermal growth factor receptor 2-positive metastatic breast cancer: final results from the EGF104900 Study. J. Clin. Oncol. 2012;30:2585–2592. doi: 10.1200/JCO.2011.35.6725. [DOI] [PubMed] [Google Scholar]
- 71.Nami Babak, Maadi Hamid, Wang Zhixiang. Mechanisms Underlying the Action and Synergism of Trastuzumab and Pertuzumab in Targeting HER2-Positive Breast Cancer. Cancers. 2018;10(10):342. doi: 10.3390/cancers10100342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Bang YJ, et al. First-in-human phase 1 study of margetuximab (MGAH22), an Fc-modified chimeric monoclonal antibody, in patients with HER2-positive advanced solid tumors. Ann. Oncol. 2017;28:855–861. doi: 10.1093/annonc/mdx261.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Park S, et al. The therapeutic effect of anti-HER2/neu antibody depends on both innate and adaptive immunity. Cancer Cell. 2010;18:160–170. doi: 10.1016/j.ccr.2010.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Stagg J, et al. Anti-ErbB-2 mAb therapy requires type I and II interferons and synergizes with anti-PD-1 or anti-CD137 mAb therapy. Proc. Natl Acad. Sci. USA. 2011;108:7142–7147. doi: 10.1073/pnas.1016569108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Gall VA, et al. Trastuzumab increases HER2 uptake and cross-presentation by dendritic cells. Cancer Res. 2017;77:5374–5383. doi: 10.1158/0008-5472.CAN-16-2774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Clifton, G. T., Gall, V., Peoples, G. E. & Mittendorf, E. A. Clinical development of the E75 vaccine in breast cancer. Breast Care11, 116–121 (2016). [DOI] [PMC free article] [PubMed]
- 77.Chaganty BKR, et al. Trastuzumab upregulates PD-L1 as a potential mechanism of trastuzumab resistance through engagement of immune effector cells and stimulation of IFNgamma secretion. Cancer Lett. 2018;430:47–56. doi: 10.1016/j.canlet.2018.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Chia SKL, et al. A phase I study of a PD-L1 antibody (Durvalumab) in combination with trastuzumab in HER-2 positive metastatic breast cancer (MBC) progressing on prior anti HER-2 therapies (CCTG IND.229)[ NCT02649686] J. Clin. Oncol. 2018;36:1029–1029. doi: 10.1200/JCO.2018.36.15_suppl.1029. [DOI] [Google Scholar]
- 79.Colli LM, et al. Landscape of combination immunotherapy and targeted therapy to improve cancer management. Cancer Res. 2017;77:3666–3671. doi: 10.1158/0008-5472.CAN-16-3338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Piccart M, et al. Everolimus plus exemestane for hormone-receptor-positive, human epidermal growth factor receptor-2-negative advanced breast cancer: overall survival results from BOLERO-2dagger. Ann. Oncol. 2014;25:2357–2362. doi: 10.1093/annonc/mdu456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Kornblum N, et al. Randomized Phase II Trial of Fulvestrant Plus Everolimus or Placebo in Postmenopausal Women With Hormone Receptor-Positive, Human Epidermal Growth Factor Receptor 2-Negative Metastatic Breast Cancer Resistant to Aromatase Inhibitor Therapy: Results of PrE0102. J. Clin. Oncol. 2018;36:1556–1563. doi: 10.1200/JCO.2017.76.9331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Andre F, et al. Alpelisib for PIK3CA-mutated, hormone receptor-positive advanced breast cancer. N. Engl. J. Med. 2019;380:1929–1940. doi: 10.1056/NEJMoa1813904. [DOI] [PubMed] [Google Scholar]
- 83.Xue G., Zippelius A., Wicki A., Mandala M., Tang F., Massi D., Hemmings B. A. Integrated Akt/PKB Signaling in Immunomodulation and Its Potential Role in Cancer Immunotherapy. JNCI Journal of the National Cancer Institute. 2015;107(7):djv171–djv171. doi: 10.1093/jnci/djv171. [DOI] [PubMed] [Google Scholar]
- 84.Peng W, et al. Loss of PTEN promotes resistance to T cell-mediated immunotherapy. Cancer Disco. 2016;6:202–216. doi: 10.1158/2159-8290.CD-15-0283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Lastwika KJ, et al. Control of PD-L1 expression by oncogenic activation of the AKT-mTOR pathway in non-small cell lung cancer. Cancer Res. 2016;76:227–238. doi: 10.1158/0008-5472.CAN-14-3362. [DOI] [PubMed] [Google Scholar]
- 86.Barrett MT, et al. The association of genomic lesions and PD-1/PD-L1 expression in resected triple-negative breast cancers. Breast Cancer Res. 2018;20:71. doi: 10.1186/s13058-018-1004-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Abu-Eid R, et al. Selective inhibition of regulatory T cells by targeting the PI3K-Akt pathway. Cancer Immunol. Res. 2014;2:1080–1089. doi: 10.1158/2326-6066.CIR-14-0095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Araki K, et al. mTOR regulates memory CD8 T-cell differentiation. Nature. 2009;460:108–112. doi: 10.1038/nature08155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.van der Waart AB, et al. Inhibition of Akt signaling promotes the generation of superior tumor-reactive T cells for adoptive immunotherapy. Blood. 2014;124:3490–3500. doi: 10.1182/blood-2014-05-578583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Robson M, et al. Olaparib for metastatic breast cancer in patients with a germline BRCA mutation. N. Engl. J. Med. 2017;377:523–533. doi: 10.1056/NEJMoa1706450. [DOI] [PubMed] [Google Scholar]
- 91.Robson ME, et al. OlympiAD final overall survival and tolerability results: Olaparib versus chemotherapy treatment of physician’s choice in patients with a germline BRCA mutation and HER2-negative metastatic breast cancer. Ann. Oncol. 2019;30:558–566. doi: 10.1093/annonc/mdz012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Litton JK, et al. Talazoparib in patients with advanced breast cancer and a germline BRCA mutation. N. Engl. J. Med. 2018;379:753–763. doi: 10.1056/NEJMoa1802905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Higuchi T, et al. CTLA-4 blockade synergizes therapeutically with PARP inhibition in BRCA1-deficient ovarian cancer. Cancer Immunol. Res. 2015;3:1257–1268. doi: 10.1158/2326-6066.CIR-15-0044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Ding L, et al. PARP inhibition elicits STING-dependent antitumor immunity in Brca1-deficient ovarian cancer. Cell Rep. 2018;25:2972–2980. doi: 10.1016/j.celrep.2018.11.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Shen J, et al. PARPi triggers the STING-dependent immune response and enhances the therapeutic efficacy of immune checkpoint blockade independent of BRCAness. Cancer Res. 2019;79:311–319. doi: 10.1158/0008-5472.CAN-18-1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Hinz M, et al. A cytoplasmic ATM-TRAF6-cIAP1 module links nuclear DNA damage signaling to ubiquitin-mediated NF-kappaB activation. Mol. Cell. 2010;40:63–74. doi: 10.1016/j.molcel.2010.09.008. [DOI] [PubMed] [Google Scholar]
- 97.Aldinucci A, et al. A key role for poly(ADP-ribose) polymerase-1 activity during human dendritic cell maturation. J. Immunol. 2007;179:305–312. doi: 10.4049/jimmunol.179.1.305. [DOI] [PubMed] [Google Scholar]
- 98.Saenz L, et al. Transcriptional regulation by poly(ADP-ribose) polymerase-1 during T cell activation. BMC Genomics. 2008;9:171. doi: 10.1186/1471-2164-9-171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Ambrose HE, et al. Poly(ADP-ribose) polymerase-1 (Parp-1)-deficient mice demonstrate abnormal antibody responses. Immunology. 2009;127:178–186. doi: 10.1111/j.1365-2567.2008.02921.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Bang Y-J, et al. An open-label, phase II basket study of olaparib and durvalumab (MEDIOLA): Results in patients with relapsed gastric cancer. J. Clin. Oncol. 2019;37:140–140. doi: 10.1200/JCO.2019.37.4_suppl.140. [DOI] [Google Scholar]
- 101.Jiao S, et al. PARP inhibitor upregulates PD-L1 expression and enhances cancer-associated immunosuppression. Clin. Cancer Res. 2017;23:3711–3720. doi: 10.1158/1078-0432.CCR-16-3215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Thoren FB, Romero AI, Hellstrand K. Oxygen radicals induce poly(ADP-ribose) polymerase-dependent cell death in cytotoxic lymphocytes. J. Immunol. 2006;176:7301–7307. doi: 10.4049/jimmunol.176.12.7301. [DOI] [PubMed] [Google Scholar]
- 103.Yardley DA, et al. Randomized phase II, double-blind, placebo-controlled study of exemestane with or without entinostat in postmenopausal women with locally recurrent or metastatic estrogen receptor-positive breast cancer progressing on treatment with a nonsteroidal aromatase inhibitor. J. Clin. Oncol. 2013;31:2128–2135. doi: 10.1200/JCO.2012.43.7251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Kim K, et al. Eradication of metastatic mouse cancers resistant to immune checkpoint blockade by suppression of myeloid-derived cells. Proc. Natl Acad. Sci. USA. 2014;111:11774–11779. doi: 10.1073/pnas.1410626111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Chueh AC, Tse JW, Togel L, Mariadason JM. Mechanisms of histone deacetylase inhibitor-regulated gene expression in cancer cells. Antioxid. Redox. Signal. 2015;23:66–84. doi: 10.1089/ars.2014.5863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Terracina KP, et al. DNA methyltransferase inhibition increases efficacy of adoptive cellular immunotherapy of murine breast cancer. Cancer Immunol. Immunother. 2016;65:1061–1073. doi: 10.1007/s00262-016-1868-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Shen L, et al. Class I histone deacetylase inhibitor entinostat suppresses regulatory T cells and enhances immunotherapies in renal and prostate cancer models. PLoS ONE. 2012;7:e30815. doi: 10.1371/journal.pone.0030815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Gameiro SR, Malamas AS, Tsang KY, Ferrone S, Hodge JW. Inhibitors of histone deacetylase 1 reverse the immune evasion phenotype to enhance T-cell mediated lysis of prostate and breast carcinoma cells. Oncotarget. 2016;7:7390–7402. doi: 10.18632/oncotarget.7180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Tomita Y, et al. The interplay of epigenetic therapy and immunity in locally recurrent or metastatic estrogen receptor-positive breast cancer: correlative analysis of ENCORE 301, a randomized, placebo-controlled phase II trial of exemestane with or without entinostat. Oncoimmunology. 2016;5:e1219008. doi: 10.1080/2162402X.2016.1219008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Orillion A, et al. Entinostat neutralizes myeloid-derived suppressor cells and enhances the antitumor effect of PD-1 inhibition in murine models of lung and renal cell carcinoma. Clin. Cancer Res. 2017;23:5187–5201. doi: 10.1158/1078-0432.CCR-17-0741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Woods DM, et al. HDAC inhibition upregulates PD-1 ligands in melanoma and augments immunotherapy with PD-1 blockade. Cancer Immunol. Res. 2015;3:1375–1385. doi: 10.1158/2326-6066.CIR-15-0077-T. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Loi S, et al. RAS/MAPK activation is associated with reduced tumor-infiltrating lymphocytes in triple-negative breast cancer: therapeutic cooperation between MEK and PD-1/PD-L1 immune checkpoint inhibitors. Clin. Cancer Res. 2016;22:1499–1509. doi: 10.1158/1078-0432.CCR-15-1125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Dushyanthen S, et al. Agonist immunotherapy restores T cell function following MEK inhibition improving efficacy in breast cancer. Nat. Commun. 2017;8:606. doi: 10.1038/s41467-017-00728-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Loi S, et al. CD73 promotes anthracycline resistance and poor prognosis in triple negative breast cancer. Proc. Natl Acad. Sci. USA. 2013;110:11091–11096. doi: 10.1073/pnas.1222251110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Beavis PA, et al. Blockade of A2A receptors potently suppresses the metastasis of CD73+ tumors. Proc. Natl Acad. Sci. USA. 2013;110:14711–14716. doi: 10.1073/pnas.1308209110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Beavis PA, Stagg J, Darcy PK, Smyth MJ. CD73: a potent suppressor of antitumor immune responses. Trends Immunol. 2012;33:231–237. doi: 10.1016/j.it.2012.02.009. [DOI] [PubMed] [Google Scholar]
- 117.Mittal D, et al. Antimetastatic effects of blocking PD-1 and the adenosine A2A receptor. Cancer Res. 2014;74:3652–3658. doi: 10.1158/0008-5472.CAN-14-0957. [DOI] [PubMed] [Google Scholar]
- 118.Muller AJ, Manfredi MG, Zakharia Y, Prendergast GC. Inhibiting IDO pathways to treat cancer: lessons from the ECHO-301 trial and beyond. Semin. Immunopathol. 2019;41:41–48. doi: 10.1007/s00281-018-0702-0. [DOI] [PubMed] [Google Scholar]
- 119.Smith C, et al. IDO is a nodal pathogenic driver of lung cancer and metastasis development. Cancer Disco. 2012;2:722–735. doi: 10.1158/2159-8290.CD-12-0014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Spira Alexander I., Hamid Omid, Bauer Todd Michael, Borges Virginia F., Wasser Jeffrey S., Smith David C., Clark Amy Sanders, Schmidt Emmett V., Zhao Yufan, Maleski Janet E., Gangadhar Tara C. Efficacy/safety of epacadostat plus pembrolizumab in triple-negative breast cancer and ovarian cancer: Phase I/II ECHO-202 study. Journal of Clinical Oncology. 2017;35(15_suppl):1103–1103. doi: 10.1200/JCO.2017.35.15_suppl.1103. [DOI] [Google Scholar]
- 121.Long GV, et al. Epacadostat (E) plus pembrolizumab (P) versus pembrolizumab alone in patients (pts) with unresectable or metastatic melanoma: results of the phase 3 ECHO-301/KEYNOTE-252 study. J. Clin. Oncol. 2018;36:108–108. doi: 10.1200/JCO.2018.36.15_suppl.108. [DOI] [Google Scholar]
- 122.Messenheimer DJ, et al. Timing of PD-1 blockade is critical to effective combination immunotherapy with anti-OX40. Clin. Cancer Res. 2017 doi: 10.1158/1078-0432.CCR-16-2677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Polley MY. Practical modifications to the time-to-event continual reassessment method for phase I cancer trials with fast patient accrual and late-onset toxicities. Stat. Med. 2011;30:2130–2143. doi: 10.1002/sim.4255. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No new datasets were generated or analyzed for this report.