Abstract
Aims
Sulfonylureas are recommended as second‐line treatment in the management of type 2 diabetes. However, they are still commonly used also as first‐line treatment instead of metformin. Given the controversial cardiovascular safety of sulfonylureas, we aimed to determine if their use as first‐line treatment is associated with adverse cardiovascular events among patients with newly treated type 2 diabetes compared with metformin.
Methods
We conducted a population‐based cohort study of patients with newly treated type 2 diabetes using the UK's Clinical Practice Research Datalink. Initiators of metformin and sulfonylurea monotherapy were matched on high‐dimensional propensity score, and Cox proportional hazards models were used to compare the rate of cardiovascular events (myocardial infarction, ischaemic stroke, cardiovascular death, and all‐cause mortality) with sulfonylureas vs metformin.
Results
Our cohort included 94 750 patients initiating treatment for type 2 diabetes, 17 612 on a sulfonylurea and 77 138 on metformin. After matching, sulfonylurea monotherapy, compared with metformin monotherapy, was not associated with an increased risk of myocardial infarction (hazard ratio [HR]: 1.04, 95% confidence interval [CI]: 0.85–1.25) but was associated with increased risks of ischaemic stroke (HR: 1.25, 95% CI: 1.002–1.56), cardiovascular death (HR: 1.25, 95% CI: 1.06–1.47), and all‐cause mortality (HR: 1.60, 95% CI: 1.45–1.76). This represents an additional 2.0 ischaemic strokes, 3.5 cardiovascular deaths, and 21.4 all‐cause deaths per 1,000 patients per year with sulfonylureas.
Conclusions
Initiating treatment of type 2 diabetes with a sulfonylurea rather than metformin is associated with higher rates of ischaemic stroke, cardiovascular death, and all‐cause mortality.
Keywords: myocardial infarction, pharmacoepidemiology, stroke, sulfonylureas, type 2 diabetes
What is already known about this subject
Sulfonylureas are recommended as second‐line treatment of type 2 diabetes.
Despite recommendations, sulfonylureas are still commonly used also as first‐line treatment instead of metformin.
The cardiovascular safety of sulfonylureas as first‐line treatment is controversial, with previous studies providing heterogeneous results.
What this study adds
Among patients initiating type 2 diabetes treatment, sulfonylurea monotherapy is not associated with an increased risk of myocardial infarction compared with metformin monotherapy.
However, it is associated with increased risks of ischaemic stroke, cardiovascular death, and all‐cause mortality.
In absolute terms, use of sulfonylurea monotherapy is associated with an additional 2.0 ischaemic strokes, 3.5 cardiovascular deaths, and 21.4 overall deaths per 1,000 patients per year relative to the use of metformin monotherapy.
1. INTRODUCTION
Sulfonylureas are recommended as second‐line treatment in the management of type 2 diabetes.1, 2 However, these drugs are still commonly used also as first‐line treatment instead of metformin. Indeed, a recent drug utilization study from the USA showed that, between 2005 and 2016, every 10th patient with type 2 diabetes initiated pharmacotherapy with a sulfonylurea.3 Importantly, this does not appear to be a US‐specific phenomenon, since similar numbers have also been observed in the UK.4
The cardiovascular safety of sulfonylureas is controversial. The controversy initially arose during the 1970s, when the University Group Diabetes Program trial found an increased risk of cardiovascular death with the sulfonylurea tolbutamide compared with diet or insulin.5 Subsequently, the UK Prospective Diabetes Study (UKPDS) found that, relative to diet, the sulfonylureas chlorpropamide, glibenclamide or glipizide were not associated with an increased risk of cardiovascular outcomes, including myocardial infarction (MI), stroke, sudden death, angina and heart failure.6 However, UKPDS was underpowered for individual cardiovascular endpoints.
The cardiovascular effects of sulfonylureas in the specific setting of first‐line treatment with metformin as the comparator have also been examined in several observational studies, which have provided heterogeneous results.7 However, many of these studies had important methodological limitations. Although the studies without major limitations suggest that sulfonylureas increase the risk of cardiovascular events, all examined composite endpoints8, 9, 10 or all‐cause mortality as their primary endpoints.11, 12 Consequently, the effect of sulfonylureas as first‐line treatment on the risk of individual cardiovascular events remains unclear. Given the limitations of this literature, the widespread use of sulfonylureas, and the high underlying cardiovascular risk in this population,13 there remains an urgent need to assess the cardiovascular effects of sulfonylureas. We therefore conducted a matched population‐based cohort study to compare the risk of cardiovascular events among newly treated patients with type 2 diabetes prescribed sulfonylureas vs those prescribed metformin.
2. METHODS
2.1. Data sources
We conducted this population‐based cohort study using data from the UK Clinical Practice Research Datalink (CPRD).14 The CPRD contains the detailed clinical records of more than 14 million patients seen at more than 700 general practitioner practices in the UK. These data include demographic information, lifestyle variables (e.g. smoking), anthropometric variables (e.g. body mass index [BMI]), recorded clinical diagnoses (using the Read coding system), symptoms, prescriptions issued (based on the British National Formulary), clinical measures (e.g. blood pressure), and laboratory test results. CPRD data have been validated extensively.15, 16, 17
Data from the CPRD were linked to the Hospital Episode Statistics (HES) repository, which contains detailed hospitalization data, and to Office for National Statistics (ONS) vital statistics data. Diagnoses in HES are recorded using the International Classification of Diseases (ICD) 10th revision coding system, and procedures are recorded in HES using the Office of Population Censuses and Surveys Classification of Interventions and Procedures version 4 system.14 Diagnoses in ONS are recorded using ICD‐9 (pre‐2001) and ICD‐10 (2001 onwards) codes.
The study protocol was approved by the Independent Scientific Advisory Committee of the CPRD (protocol number 14_019AMnARA, which was made available to journal reviewers) and the Research Ethics Board of the Jewish General Hospital in Montreal, Canada.
The data that support the findings of this study were obtained from the CPRD. Restrictions apply to the availability of these data, which were used under license for this study.
2.2. Study population
We identified a cohort of newly treated patients with type 2 diabetes who were initiating a sulfonylurea or metformin as their first‐ever antidiabetic drug between 1 April 1998 and 31 March 2013, with follow‐up until 31 March 2014. Although sulfonylureas are often used as second‐line treatment, they are recommended as first‐line therapy among patients who are intolerant to or with contraindications to metformin.18 To ensure that all patients were newly treated and to measure baseline characteristics, inclusion was restricted to patients with at least 1 year of observation time in the CPRD prior to this first prescription of an antidiabetic drug. The date of this prescription defined cohort entry. Patients who were prescribed sulfonylureas and metformin in combination or with other antidiabetic drugs at cohort entry were excluded, leaving a cohort of patients who initiated treatment with sulfonylurea or metformin monotherapy. To increase the likelihood of a diagnosis of type 2 diabetes, we also excluded all patients aged <40 years at cohort entry. In addition, we excluded all women with a diagnosis of polycystic ovarian syndrome as this is another indication for metformin. Furthermore, we excluded patients with no recorded follow‐up. Patients were followed until the date of the cardiovascular event (defined below) or censoring due to death (for outcomes other than all‐cause mortality), departure from the CPRD, or end of the study period (31 March 2014), whichever occurred first.
2.3. Exposure assessment
Our primary analysis used an as‐treated approach that assessed the effect of current use of sulfonylurea monotherapy or metformin monotherapy on the risk of cardiovascular events. Exposure was defined by the prescription for a sulfonylurea or metformin that defined cohort entry. Patients were considered continuously exposed until drug discontinuation, defined as a gap of 30 days after the end of a prescription, or a prescription for a new antidiabetic drug, at which point the patient was censored.
2.4. Outcomes
The primary outcome of this study was MI during follow‐up. Secondary outcomes were ischaemic stroke, cardiovascular mortality, and all‐cause mortality. MI (ICD‐10 codes I21.X) and ischaemic stroke (ICD‐10 codes I63.X, I64.X) were defined using HES, with events defined using primary and secondary diagnoses. The diagnostic codes to identify MI in HES have been previously validated (positive predictive value: 91.5%, 95% confidence interval [CI]: 90.8–92.1),15 while a systematic review of the validity of stroke diagnoses in administrative data suggests that these data are valid (positive predictive value of 80% or greater).19 HES data were supplemented by ONS data where MI (ICD‐9 codes 410.X; ICD‐10 codes as above) and ischaemic stroke (ICD‐9 codes 433.X, 434.X, 436.X; ICD‐10 codes as above) were listed as the underlying cause of death. Cardiovascular mortality (ICD‐10 codes I0–99) was defined using ONS data, and all‐cause mortality was defined using all 3 data sources. In the event of discrepancies between databases regarding the date of death, the earliest date was used.
2.5. Potential confounders
Potential confounders were assessed at cohort entry. These confounders included demographic characteristics (age, sex, calendar year), lifestyle variables (smoking [ever, never, unknown], excessive alcohol use), BMI (<25.0 kg/m2, 25.0–29.9 kg/m2, ≥ 30 kg/m2, unknown), previous medical history (atrial fibrillation or flutter, cancer, cerebrovascular disease, chronic obstructive pulmonary disease, coronary heart disease, heart failure, hyperlipidaemia, hypertension, previous MI, previous coronary revascularization, previous stroke, and thyroid disease), complications of diabetes (neuropathy, peripheral arterial or vascular disease, renal disease, and retinal disorder) and glycated haemoglobin level (≤7%, 7.1–8.0%, >8%, unknown). Medical history was assessed using any recorded diagnosis prior to study cohort entry, and lifestyle variables and glycated haemoglobin level were measured using the most recently recorded value prior to cohort entry. In addition, we assessed use of the following medications in the year before cohort entry: angiotensin converting enzyme inhibitors, angiotensin II receptor blockers, β‐blockers, diuretics, digoxin, statins, fibrates, acetylsalicylic acid, clopidogrel, warfarin, nonsteroidal anti‐inflammatory drugs, opioid analgesics and paracetamol. The number of unique nonantidiabetic medications and number of hospitalizations in the prior year, 2 proxies for overall health, were also measured.
2.6. Statistical analysis
To minimize potential confounding, we matched sulfonylurea patients and metformin patients on high‐dimensional propensity score (HDPS).20 Using logistic regression, we constructed a propensity model that included the prespecified potential confounders described above as well 500 empirically identified covariates to estimate the propensity (or probability) of receiving a sulfonylurea for each patient. Separate propensity scores were calculated for each outcome, with the areas of nonoverlap of the HDPS distributions trimmed; the size of the cohort used for each outcome differed slightly due to small differences in the outcome‐specific HDPS distributions. We then used a greedy matching algorithm to match each sulfonylurea user with 1 metformin user (calliper: propensity score of 0.05).
We described the characteristics of patients before and after matching, with categorical data presented as count data with proportions and continuous data presented as means with standard deviations. Differences between groups are described using standardized differences, with a difference greater than 0.1 considered to be important.21 Incidence rates with 95% CIs were estimated using Poisson regression overall and by treatment group, with the timing of events examined by plotting the cumulative incidence proportion.
In our primary analysis, we used Cox proportional hazards models to estimate the hazard ratio (HR) and 95% CI for MI with sulfonylureas vs metformin. Similar models were constructed for the outcomes of ischaemic stroke, cardiovascular mortality, and all‐cause mortality. In prespecified secondary analyses, we examined potential effect modification by a history of previous cardiovascular events by including interaction terms in our models. In additional secondary analyses, we examined potential effect modification by age (≥75 vs <75 years) and sex. Furthermore, we conducted subgroup analyses in which exposure to sulfonylureas was sub‐classified by cohort entry dose (a World Health Organization‐defined daily dose <1 vs ≥1). Finally, in sensitivity analyses, we used an approach analogous to an intention‐to‐treat to examine the potential for informative censoring. In this analysis, exposure was defined at cohort entry, and patients were followed until an event or censoring due to death (for the outcomes other than all‐cause mortality), departure from the CPRD, end of the study period (31 March 2014), or a maximum follow‐up of 1 year, whichever occurred first. Follow‐up was restricted to a maximum of 1 year to minimize the potential dilution of the observed effect typically associated with such analyses.
Key protein targets and ligands in this article are hyperlinked to corresponding entries in http://www.guidetopharmacology.org, the common portal for data from the IUPHAR/BPS Guide to PHARMACOLOGY.
3. RESULTS
3.1. Study cohort
Following the application of our inclusion criteria, 77 138 metformin monotherapy users and 17 612 sulfonylurea monotherapy users were eligible for inclusion (Figure 1). For our analysis of MI, 496 metformin users and 6 sulfonylurea users were trimmed due to nonoverlap of the HDPS distributions, resulting in a final study cohort of 76 642 metformin users and 17 606 sulfonylurea users. The sizes of the final cohorts for the other 3 outcomes were similar (Figure 1).
Figure 1.
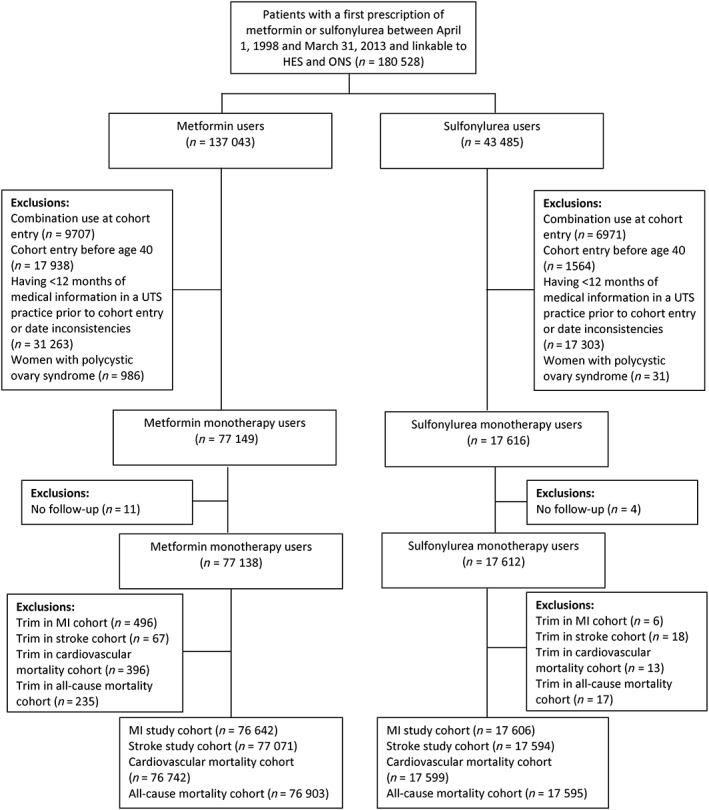
Flow diagram describing cohort construction
The trimmed cohort for our analysis of MI included 94 248 patients who were followed for a mean of 1.5 (standard deviation: 1.8) years (Table S1). During this follow‐up, 890 MIs occurred, resulting in an overall incidence rate of 6.4 per 1000 person‐years (95% CI: 6.0–6.8). The incidence rates per 1000 person‐years for ischaemic stroke, cardiovascular death and all‐cause mortality were 5.4 (95% CI: 5.0–5.8), 8.9 (95% CI: 8.4–9.4) and 24.4 (95% CI: 23.6–25.2), respectively.
3.2. Patient characteristics
There were several notable differences between metformin and sulfonylurea monotherapy users before matching (Table 1; Figure S1). Compared with metformin users, sulfonylurea users were more likely to be older and to have a BMI < 25 kg/m2. In addition, they were more likely to have a history of cancer and most cardiovascular comorbidities but were less likely to have a history of hypertension or dyslipidaemia. Sulfonylurea users had a greater prevalence of peripheral arterial or vascular disease and renal disease but a lower prevalence of neuropathy. Furthermore, sulfonylurea users had a greater number of hospitalized episodes of care in the prior year than metformin users. After matching on HDPS (13,999 matched pairs in the cohort for MI), no important differences in baseline demographic or clinical characteristics were present (Table 1, Tables S2–S4, Figure S2). The mean age of the cohort for MI was 67.1 years, and 57.4% were men. Similar proportions of metformin and sulfonylurea patients had histories of coronary artery disease (28.5 vs 28.3%), previous MI (9.9 vs 9.5%), hypertension (58.0 vs 58.0%) and BMI ≥ 30 kg/m2 (28.9 vs 28.6%), with all standardized differences <0.1.
Table 1.
Characteristics of sulfonylurea users and metformin users among patients with type 2 diabetes
| Characteristic | Before matching | After matchinga | ||||
|---|---|---|---|---|---|---|
| Metformin | Sulfonylurea | Standardized difference | Metformin | Sulfonylurea | Standardized difference | |
| Total | 77 138 | 17 612 | 13 999 | 13 999 | ||
| Age, y (mean, SD) | 63.1 (11.7) | 68.2 (12.5) | 0.43 | 67.1 (12.3) | 67.0 (12.4) | 0.01 |
| Male | 43 467 (56.4) | 10 121 (57.5) | 0.02 | 8025 (57.3) | 8040 (57.4) | 0.00 |
| Excessive alcohol use, n (%) | 2696 (3.5) | 509 (2.9) | 0.02 | 430 (3.1) | 416 (3.0) | 0.01 |
| Smoking status, n (%) | ||||||
| Ever | 46 168 (59.9) | 9107 (51.7) | 0.16 | 7646 (54.6) | 7537 (53.8) | 0.02 |
| Never | 29 617 (38.4) | 7262 (41.2) | 0.06 | 5581 (39.9) | 5674 (40.5) | 0.01 |
| Missing | 1353 (1.8) | 1243 (7.1) | 0.26 | 772 (5.5) | 788 (5.6) | 0.00 |
| Body mass index, n (%) | ||||||
| < 25.0 kg/m2 | 6646 (8.6) | 5110 (29.0) | 0.54 | 3232 (23.1) | 3228 (23.1) | 0.00 |
| 25.0–29.9 kg/m2 | 23 884 (31.0) | 6232 (35.4) | 0.09 | 5300 (37.9) | 5324 (38.0) | 0.00 |
| ≥ 30.0 kg/m2 | 43 485 (56.4) | 4159 (23.6) | 0.71 | 4043 (28.9) | 4002 (28.6) | 0.01 |
| Missing | 3123 (4.1) | 2111 (12.0) | 0.30 | 1424 (10.2) | 1445 (10.3) | 0.00 |
| Glycated haemoglobin (%), n (%) | ||||||
| ≤ 7 | 10 752 (14.0) | 1601 (9.1) | 0.16 | 1265 (9.1) | 1341 (9.6) | 0.02 |
| 7.1–8.0 | 19 464 (25.4) | 2563 (14.6) | 0.28 | 2298 (16.4) | 2275 (16.3) | 0.00 |
| > 8 | 27 903 (36.4) | 5799 (33.0) | 0.07 | 4971 (35.5) | 4904 (35.0) | 0.01 |
| Unknown | 18 516 (24.2) | 7631 (43.4) | 0.42 | 5465 (39.0) | 5479 (39.1) | 0.00 |
| Medical history, n (%) | ||||||
| HF | 3554 (4.6) | 1945 (11.0) | 0.24 | 1253 (8.9) | 1247 (8.9) | 0.00 |
| Hypertension | 48 652 (63.1) | 9884 (56.1) | 0.14 | 8126 (58.0) | 8117 (58.0) | 0.00 |
| Thyroid disease | 7689 (10.0) | 1527 (8.7) | 0.04 | 1224 (8.7) | 1262 (9.0) | 0.01 |
| Cerebrovascular disease | 5534 (7.2) | 2009 (11.4) | 0.15 | 1452 (10.4) | 1442 (10.3) | 0.00 |
| Atrial fibrillation or flutter | 5884 (7.6) | 2314 (13.1) | 0.18 | 1678 (12.0) | 1634 (11.7) | 0.01 |
| Cancer | 8305 (10.8) | 2729 (15.5) | 0.14 | 2034 (14.5) | 2016 (14.4) | 0.00 |
| COPD | 9062 (11.8) | 2579 (14.6) | 0.09 | 1923 (13.7) | 1924 (13.7) | 0.00 |
| Coronary artery disease | 18 977 (24.6) | 5173 (29.4) | 0.11 | 3988 (28.5) | 3958 (28.3) | 0.00 |
| Hyperlipidaemia | 20 588 (26.7) | 3435 (19.5) | 0.17 | 2931 (20.9) | 2947 (21.0) | 0.01 |
| Previous coronary revascularization | 3265 (4.2) | 804 (4.6) | 0.02 | 641 (4.6) | 641 (4.6) | 0.00 |
| Previous MI | 5723 (7.4) | 1839 (10.4) | 0.11 | 1384 (9.9) | 1336 (9.5) | 0.01 |
| Previous stroke | 3890 (5.0) | 1357 (7.7) | 0.11 | 1016 (7.3) | 988 (7.1) | 0.01 |
| Drugs, n (%) | ||||||
| ACEi | 28 159 (36.5) | 5122 (29.1) | 0.16 | 4266 (30.5) | 4244 (30.3) | 0.00 |
| ARBs | 8688 (11.3) | 1216 (6.9) | 0.15 | 1098 (7.8) | 1064 (7.6) | 0.01 |
| Beta‐blockers | 19 343 (25.1) | 4401 (25.0) | 0.00 | 3681 (26.3) | 3612 (25.8) | 0.01 |
| Diuretics | 26 146 (33.9) | 6884 (39.1) | 0.11 | 5312 (37.9) | 5218 (37.3) | 0.01 |
| Digoxin | 2749 (3.6) | 1645 (9.3) | 0.24 | 1066 (7.6) | 1074 (7.7) | 0.01 |
| Statins | 43 436 (56.3) | 5881 (33.4) | 0.47 | 5231 (37.4) | 5239 (37.4) | 0.00 |
| Fibrates | 1293 (1.7) | 238 (1.4) | 0.03 | 202 (1.4) | 207 (1.5) | 0.00 |
| Clopidogrel | 2289 (3.0) | 546 (3.1) | 0.01 | 483 (3.5) | 460 (3.3) | 0.01 |
| Warfarin | 3561 (4.6) | 1306 (7.4) | 0.12 | 975 (7.0) | 976 (7.0) | 0.00 |
| Acetylsalicylic acid | 25 230 (32.7) | 5558 (31.6) | 0.02 | 4435 (31.7) | 4346 (31.1) | 0.01 |
| NSAIDs | 9105 (11.8) | 2192 (12.5) | 0.02 | 1782 (12.7) | 1770 (12.6) | 0.00 |
| Opioid analgesics | 22 413 (29.1) | 5337 (30.3) | 0.03 | 4203 (30.0) | 4146 (29.6) | 0.01 |
| Paracetamol | 24 571 (31.9) | 6052 (34.4) | 0.05 | 4746 (33.9) | 4682 (33.4) | 0.01 |
| Complications of diabetes, n (%) | ||||||
| Neuropathy | 5199 (6.7) | 676 (3.8) | 0.13 | 595 (4.3) | 599 (4.3) | 0.00 |
| Peripheral arterial or vascular disease | 2989 (3.9) | 1087 (6.2) | 0.11 | 791 (5.7) | 781 (5.6) | 0.00 |
| Renal disease | 6801 (8.8) | 2064 (11.7) | 0.10 | 1647 (11.8) | 1542 (11.0) | 0.02 |
| Retinal disorders | 9601 (12.5) | 1676 (9.5) | 0.09 | 1426 (10.2) | 1404 (10.0) | 0.01 |
| Number of unique nonantidiabetic medications (mean, SD) | 7.53 (5.58) | 7.99 (6.23) | 0.08 | 7.8 (6.1) | 7.8 (6.1) | 0.01 |
| 0, n (%) | 3518 (4.6) | 956 (5.4) | 0.04 | 779 (5.6) | 761 (5.4) | 0.01 |
| 1, n (%) | 4348 (5.6) | 1000 (5.7) | 0.00 | 799 (5.7) | 822 (5.9) | 0.01 |
| 2, n (%) | 5418 (7.0) | 1240 (7.0) | 0.00 | 984 (7.0) | 1023 (7.3) | 0.01 |
| 3, n (%) | 6190 (8.0) | 1418 (8.1) | 0.00 | 1128 (8.1) | 1144 (8.2) | 0.00 |
| 4+, n (%) | 57 664 (74.8) | 12 998 (73.8) | 0.02 | 10 309 (73.6) | 10 249 (73.2) | 0.01 |
| Number of hospitalization episodes of care (mean, SD) | 0.18 (0.56) | 0.47 (1.14) | 0.32 | 0.4 (0.8) | 0.4 (1.1) | 0.00 |
| 0, n (%) | 67 244 (87.2) | 12 616 (71.6) | 0.39 | 10 589 (75.6) | 10 695 (76.4) | 0.02 |
| 1, n (%) | 7512 (9.7) | 3251 (18.5) | 0.25 | 2379 (17.0) | 2272 (16.2) | 0.02 |
| 2, n (%) | 1623 (2.1) | 1039 (5.9) | 0.19 | 658 (4.7) | 636 (4.5) | 0.01 |
| 3, n (%) | 483 (0.6) | 383 (2.2) | 0.13 | 224 (1.6) | 239 (1.7) | 0.01 |
| 4+, n (%) | 276 (0.4) | 323 (1.8) | 0.14 | 149 (1.1) | 157 (1.1) | 0.01 |
ACEi, angiotensin‐converting enzyme inhibitor; ARBs, angiotensin II receptor blockers; COPD, chronic obstructive pulmonary disease; HF, heart failure; MI, myocardial infarction; NSAIDs, nonsteroidal anti‐inflammatory drugs; SD, standard deviation.
Patients characteristics described above are those of the matched analysis of myocardial infarction. Due to modest differences in the high‐dimensional propensity score distributions, minor differences exist between the characteristics of patients included in the analysis of myocardial infarction and those included in the analysis of other outcomes (Tables S2–S4).
3.3. Cardiovascular outcomes
Compared with the use of metformin monotherapy, the use of sulfonylurea monotherapy was not associated with an increased risk of MI in the matched cohort (HR: 1.04, 95% CI: 0.85–1.29; Table 2 and Figure 2). However, use of sulfonylureas was associated with higher risks of ischaemic stroke (HR: 1.25, 95% CI: 1.002–1.56), cardiovascular death (HR: 1.25, 95% CI: 1.06–1.56) and all‐cause mortality (HR: 1.60, 95% CI: 1.45–1.76) in the matched cohorts. In absolute terms, these risks represent an additional 2.0 ischaemic strokes, 3.5 cardiovascular deaths and 21.4 overall deaths per 1,000 patients per year with the use of sulfonylureas. Secondary analyses revealed no difference in risk among patient with and without a previous history of cardiovascular events for any of the cardiovascular outcomes examined (Figure 3; Table S5). There was no evidence of effect modification by sex for MI, ischaemic stroke or cardiovascular death; however, sulfonylureas were associated with a greater increase in all‐cause mortality among men than among women (P‐for‐interaction: .04 Figure 3; Table S6). Furthermore, patients aged <75 years using sulfonylureas were at greater increased risks of ischaemic stroke, cardiovascular death and all‐cause mortality than those aged ≥75 years, in whom no increased risks of stroke and cardiovascular death were observed (P‐for‐interaction: .06, .01 and <.0001, respectively; Figure 3; Table S7). Finally, similar estimates were obtained with lower and higher dose sulfonylureas for all outcomes (Table S8).
Table 2.
Hazard ratios for cardiovascular outcomes and all‐cause mortality associated with sulfonylurea, compared with metformin, in patients with type 2 diabetesa
| Exposure | No. of patients | No. of events | PY | Incidence rate (95% CI) [per 1000 PY] | HR (95% CI) |
|---|---|---|---|---|---|
| Myocardial infarction | |||||
| Metformin | 13 999 | 185 | 21 188 | 8.7 (7.5–10.1) | 1.00 (reference) |
| Sulfonylurea | 13 999 | 166 | 18 008 | 9.2 (7.9–10.7) | 1.04 (0.85–1.29) |
| Ischaemic stroke | |||||
| Metformin | 13 957 | 155 | 21 195 | 7.3 (6.2–8.6) | 1.00 (reference) |
| Sulfonylurea | 13 957 | 166 | 17 924 | 9.3 (7.9–10.8) | 1.25 (1.002–1.56) |
| Cardiovascular death | |||||
| Metformin | 13 984 | 287 | 21 306 | 13.5 (12.0–15.1) | 1.00 (reference) |
| Sulfonylurea | 13 984 | 309 | 18 065 | 17.1 (15.3–19.1) | 1.25 (1.06–1.47) |
| All‐cause mortality | |||||
| Metformin | 13 926 | 706 | 21 167 | 33.4 (30.9–35.9) | 1.00 (reference) |
| Sulfonylurea | 13 926 | 991 | 18 069 | 54.8 (51.5–58.4) | 1.60 (1.45–1.76) |
Abbreviation: CI, confidence interval; HR, hazard ratio; PY, person‐years.
Patients were matched on high‐dimensional propensity score, and analyses used an as‐treated approach.
Figure 2.
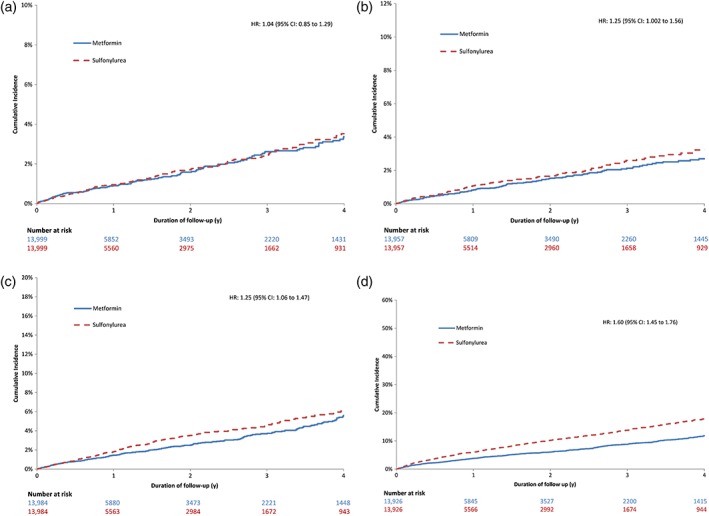
Cumulative incidence of cardiovascular events among users of sulfonylureas and metformin, matched on high‐dimensional propensity score. (a) Myocardial infarction; (b) ischaemic stroke; (c) cardiovascular death; (d) all‐cause mortality
Figure 3.

Hazard ratios for cardiovascular outcomes and all‐cause mortality associated with sulfonylurea, compared with metformin, in patients with type 2 diabetes. *Denotes a history of myocardial infarction; †denotes a history of ischaemic stroke; and ‡denotes a history of myocardial infarction or ischaemic stroke. DDD: defined daily dose; CI, confidence interval; HR, hazard ratio
The sensitivity analysis that used an intention‐to‐treat approach produced similar results for MI (HR: 1.01, 95% CI: 0.79–1.28; Table S9). However, the intention‐to‐treat analyses for ischaemic stroke and cardiovascular death produced attenuated results, with sulfonylurea use no longer associated with an increased risk (ischaemic stroke: HR: 0.96, 95% CI: 0.76–1.22; cardiovascular death: HR: 1.08, 95% CI: 0.90–1.30). Although attenuated, the association between sulfonylurea use and all‐cause mortality remained significant in the intention‐to‐treat analyses (HR: 1.22, 95% CI: 1.11–1.35).
4. DISCUSSION
This population‐based cohort study was designed to examine the association between the use of sulfonylureas as first‐line treatment and adverse cardiovascular events among newly treated patients with type 2 diabetes. We found that, compared with use of metformin monotherapy, use of sulfonylurea monotherapy was not associated with an increased risk of MI but was associated with 25% relative increases in the risks of ischaemic stroke and cardiovascular death and a 60% relative increase in the risk of all‐cause mortality. We also found important effect modification, with increased risks of ischaemic stroke and cardiovascular death with sulfonylureas restricted to younger patients and greater increases in all‐cause mortality with younger patients than with those aged 75 years or more. Our findings are consistent with the American Diabetes Association's recommendation to use metformin as the first‐line therapy among patients with type 2 diabetes,1 a recommendation based on the efficacy, safety and low cost of metformin relative to other antidiabetic drugs. Among those who are intolerant to metformin or with contraindications to its use, the use of antidiabetic drugs with more favourable cardiovascular profiles than sulfonylureas may be considered pending the results of the ongoing Cardiovascular Outcome Study of Linagliptin vs Glimepiride in Patients with Type 2 Diabetes (CAROLINA) trial, which is examining the cardiovascular safety of sulfonylureas.22 Options include the use of glucagon‐like peptide 1 (GLP‐1) receptor agonists23 and sodium glucose cotransporter 2 (SGLT‐2) inhibitors,24 both of which have been shown to decrease cardiovascular outcomes relative to placebo. Although pioglitazone has been shown to reduce cardiovascular events,25 concerns regarding its association with bladder cancer persist.26
Although this study examined the use of sulfonylureas as first‐line therapy, its results may also have implications for the interpretation of recent trials of newly‐marketed antidiabetic drugs such as dipeptidyl peptidase 4 (DPP‐4) inhibitors, GLP‐1 receptor agonists and SGLT‐2 inhibitors, where sulfonylureas are often used as a background or rescue medication. For example, in the randomized, placebo‐controlled Liraglutide Effect and Action in Diabetes: Evaluation of Cardiovascular Outcome Results (LEADER) trial (n = 9340),23 the GLP‐1 receptor agonist liraglutide was associated with a significant reduction in the primary composite endpoint of death from cardiovascular causes, nonfatal MI and nonfatal stroke (HR: 0.87, 95% CI: 0.78–0.97). Although liraglutide appeared to have beneficial effects on nonfatal MI (HR: 0.88, 95% CI: 0.75–1.03) and nonfatal stroke (HR: 0.89, 95% CI: 0.72–1.11), its beneficial effects on the primary composite endpoint were driven by cardiovascular death (HR: 0.78, 95% CI: 0.66–0.93). Patients randomized to placebo had a significantly higher incidence of introduction of sulfonylureas during follow‐up than those randomized to liraglutide (10.8 vs 7.6%, P < .0001). Similar findings were observed in the Empagliflozin, Cardiovascular Outcomes, and Mortality in Type 2 Diabetes trial (EMPA‐REG OUTCOME) of the SGLT‐2 inhibitor empagliflozin.24 Given the observed increase in cardiovascular death with sulfonylureas in the present study, there is a need to assess the impact of postrandomization initiation of sulfonylureas on the results of the LEADER and EMPA‐REG OUTCOME trials.
There are several potential mechanisms to explain our observed results. Some have suggested that the increased cardiovascular risk of sulfonylureas may be caused by impaired ischaemic preconditioning due to the presence of sulfonylurea receptors on the vascular cells and cardiomyocytes.27, 28, 29 However, others have suggested that this is an unlikely explanation as some sulfonylureas such as tolbutamide have low affinity for the sulfonylurea receptors of cardiomyocytes.30 Another hypothesized mechanism involves sulfonylureas' effect on the ATP‐dependent potassium channels on cardiac cell and coronary vessels, which may impair coronary vasodilation and increase myocardial damage if sulfonylureas are present during a MI.31 Furthermore, studies have shown that the sulfonylurea action of inhibiting ATP‐dependent potassium channels may prolong QT intervals, potentially leading to an increased risk of arrhythmias and sudden cardiac death.32, 33 These mechanisms are consistent with our finding of greater cardiovascular mortality with sulfonylureas but no difference in MI. However, it is important to acknowledge that these effects may not be induced by all sulfonylureas. Newer sulfonylureas such as gliclazide have a higher selectivity for pancreatic sulfonylurea receptors over cardiac receptors.31, 34 However, we recently compared the cardiovascular effects of pancreas‐specific, short‐acting sulfonylureas to those of nonspecific, long‐acting sulfonylureas, finding no differences in the rates of MI, ischaemic stroke, cardiovascular death or all‐cause mortality.35
The mechanism behind the observed increase in all‐cause mortality is less clear. It may be attributable to the known increased risks of hypoglycaemia and weight gain associated with sulfonylureas.1 Sulfonylurea acts on the pancreatic β cells, leading to increased insulin secretion, which lowers glycaemia, potentially inducing hypoglycaemia.36 Using the same cohort as the present study, we recently assessed the risk of severe hypoglycaemia with sulfonylureas, finding that they are associated with a substantial increase relative to metformin (HR: 4.53; 95% CI: 2.76–7.45).37 However, with only 94 patients hospitalized for severe hypoglycaemia in this study, there were insufficient data to examine the association between hypoglycaemia and cardiovascular outcomes. Nonetheless, previous studies have shown that hypoglycaemia is associated with increased cardiovascular events and both all‐cause and cardiovascular mortality,38, 39 and this represents a potential mechanism behind the observed differences in the present study. Furthermore, sulfonylurea use increases insulin growth factor (IGF)‐1 levels, which are associated with an increased risk of all‐cause mortality40, 41 and cardiovascular related mortality.40 Although the mechanism involved in the association between elevated IGF‐1 levels and cardiovascular disease is not completely understood, this association is supported by elevated cardiovascular risk among patients with acromegaly, in whom IGF‐1 levels are elevated.42, 43 While ischaemic stroke is unlikely to be a neurological sequela of hypoglycaemia, they share several common symptoms (e.g. confusion, loss of consciousness, seizures), and previous studies have shown that, although uncommon, acute symptomatic hypoglycaemia can cause imaging abnormalities that mimic those of acute ischaemic stroke.44 Our stratified analyses revealed no multiplicative effect modification by previous cardiovascular events, suggesting that the mechanism is potentially not cardiovascular per se but associated with repeated hypoglycaemia. In contrast, our previous comparison of different sulfonylureas found that nonspecific, long‐acting sulfonylureas were associated with a substantially higher rate of severe hypoglycaemia compared with pancreas‐specific, short‐acting sulfonylureas (HR: 2.83; 95% CI 1.64–4.88) but no difference in cardiovascular events, providing some evidence against this hypothesis.
With body weight not routinely collected at all clinic visits and an average follow‐up of 1.5 years, there were too many missing data to assess the association between weight gain and cardiovascular outcomes. However, with a relatively short follow‐up duration, it is unlikely that sulfonylurea‐associated weight gain was sufficient to cause the observed increased risks of cardiovascular and all‐cause mortality over this time. There has also been some evidence linking sulfonylureas to an increased risk of cancer,45 potentially via increased insulin and IGF‐1 levels,46 which may promote tumorigenesis,47 although many of the observational studies comparing the cancer risk of metformin and sulfonylureas are methodologically flawed.48 However, with an average duration of follow‐up of 1.5 years, it is unlikely that the observed increase in all‐cause mortality can be attributable to an increased risk of cancer. Finally, given our observational design, it is possible that the increased mortality with sulfonylureas is the product of residual confounding by indication.
The cardiovascular effects of sulfonylureas have been examined previously in observational studies.7 However, many of these studies had important methodological shortcomings that limit the interpretation of their results, including exposure misclassification, time‐lag bias, and selection bias. Higher quality studies8, 9, 10 have suggested an increased risk, although most used a composite endpoint as their primary endpoint, which may mask the associations with the individual components of the composite endpoint. Our study produced consistent results with previous studies examining the association between sulfonylureas and all‐cause mortality.11, 12 Our study offers several advantages over these previous studies, including matching on HDPS to reduce confounding and our use of an as‐treated approach to assess exposure during the aetiologically relevant time window.
The use of MI as the primary endpoint also warrants some discussion. Given the previously mentioned limitations of composite endpoints, we elected to examine individual clinical endpoints. Given the weight gain associated with the use of sulfonylureas,1 their known association with hypoglycaemia (a known risk factor for myocardial ischaemia),38, 39 and the clinical and public health importance of MI in this patient population, we believed that MI was a relevant primary endpoint for this study. Furthermore, given the presence of previous validation studies of MI in these data sources, it offered important advantages over cardiovascular death, as establishing cause of death in the absence of an autopsy can be quite difficult, resulting in potential misclassification bias.
Our study has some potential limitations. First, despite the use of several approaches to minimize confounding, residual confounding remains a potential limitation. However, under most reasonable circumstances, it is highly unlikely that an unmeasured confounder could explain our observed association between sulfonylurea use and cardiovascular death (Figure S3).49 Second, the results obtained in our sensitivity analysis using an intention‐to‐treat approach were attenuated and no longer statistically significant for cardiovascular death. Such analyses are known to result in a dilution of the observed effect; we restricted follow‐up to 12 months in these analyses to help minimize any potential bias towards the null hypothesis. Nonetheless, we are unable to rule out that some of the observed discordance may be due to the potential presence of informative censoring in our primary analysis. Third, although patients were followed for up to 14 years, the mean follow‐up remained relatively short (1.5 years) in our primary analyses. Additional studies are needed to examine the effects of longer durations of sulfonylurea use. Fourth, our results are generalizable to the use of sulfonylureas as first‐line treatment; additional studies are needed to assess the safety of sulfonylureas in other settings. Fifth, due to the relatively small number of non‐Caucasian patients included in this study, it was not feasible to assess effect modification by race. Sixth, our definition for ischaemic stroke included both thrombotic and embolic stroke; depending on the underlying mechanism, the inclusion of embolic stroke may have introduced some nondifferential outcome misclassification. Finally, the CPRD records prescriptions issued by general practitioners and has no records of drug dispensings or patient adherence.
Among newly treated patients with type 2 diabetes, use of sulfonylureas as first‐line treatment is not associated with an increased risk of MI compared with use of metformin. However, it is associated with significantly higher risks of ischaemic stroke, cardiovascular death, and all‐cause mortality. In absolute terms, use of sulfonylureas is associated with an additional 2.0 ischaemic strokes, 3.5 cardiovascular deaths and 21.4 overall deaths per 1000 patients per year relative to the use of metformin. This increased risk must be considered when deciding the most appropriate choice of antidiabetic drug when initiating medical therapy among patients with type 2 diabetes.
COMPETING INTERESTS
S.S. has consulted for and received speaking fees from Novartis, Boehringer‐Ingelheim, and AstraZeneca and research grants from Bayer Pharma AG, Boehringer‐Ingelheim, Bristol‐Myers Squibb and Novartis. The other authors have competing interests to declare.
CONTRIBUTORS
S.S. conceived of the study idea and obtained funding. K.B.F., L.A., and S.S. contributed to the study design, and H.Y. conducted the statistical analysis. K.B.F. drafted the manuscript. All authors were involved in the interpretation of data and critically reviewed the manuscript for important intellectual content. K.B.F. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Supporting information
Table S1.
Incidence rates of cardiovascular events and all‐cause mortality among users of metformin and sulfonylureas.
Table S2. Characteristics of sulfonylurea users and matched metformin users among patients with type 2 diabetes included in the analysis of stroke.
Table S3. Characteristics of sulfonylurea users and matched metformin users among patients with type 2 diabetes included in the analysis of cardiovascular death.
Table S4. Characteristics of sulfonylurea users and matched metformin users among patients with type 2 diabetes included in the analysis of all‐cause mortality.
Table S5. Hazard ratios for cardiovascular events and all‐cause mortality associated with sulfonylurea, compared with metformin, in patients with type 2 diabetes, by previous history of the outcome of interest.
Table S6. Hazard ratios for cardiovascular events and all‐cause mortality associated with sulfonylurea, compared with metformin, in patients with type 2 diabetes, by sex.
Table S7. Hazard ratios for cardiovascular events and all‐cause mortality associated with sulfonylurea, compared with metformin, in patients with type 2 diabetes, by age.
Table S8. Hazard ratios for cardiovascular events and all‐cause mortality associated with sulfonylurea, compared with metformin, in patients with type 2 diabetes, with sulfonylurea use defined by dose.
Table S9. Hazard ratios for cardiovascular events and all‐cause mortality associated with sulfonylurea, compared with metformin, in patients with type 2 diabetes, using an intention‐to‐treat approach.
Figure S1. High‐dimensional propensity score distribution among users of sulfonylureas and metformin prior to matching.
Figure S2. High‐dimensional propensity score distribution among users of sulfonylureas and metformin after matching.
Figure S3. Rule‐out method to determine how strong an unmeasured confounder is needed to explain the observed association between sulfonylurea use and cardiovascular death.
ACKNOWLEDGEMENTS
K.B.F. and L.A. are recipients of Checheur‐Boursier awards from the Fonds de la recherche du Québec – santé (Quebec Foundation for Health Research) and William Dawson Scholar awards from McGill University. A.D. is the recipient of a Research Fellowship from the German Research Foundation (Deutsche Forschungsgemeinschaft). O.H.Y. holds a Chercheur‐Boursier Clinicien Junior 1 award from the Fonds de la recherche du Québec – santé. S.S. is the recipient of the James McGill Professor award.
This study was funded by Boehringer‐Ingelheim. The study sponsor was not involved in the design, analysis, interpretation or content of this manuscript but was provided the opportunity to provide comments on it.
Filion KB, Douros A, Azoulay L, Yin H, Yu OH, Suissa S. Sulfonylureas as initial treatment for type 2 diabetes and the risk of adverse cardiovascular events: A population‐based cohort study. Br J Clin Pharmacol. 2019;85:2378–2389. 10.1111/bcp.14056
REFERENCES
- 1. American Diabetes Association . Approaches to glycemic treatment. Standards of medical care in diabetes ‐ 2016. Diabetes Care. 2016;39(Supplement 1):S52‐S59. [DOI] [PubMed] [Google Scholar]
- 2. Inzucchi SE, Bergenstal RM, Buse JB, et al. Management of hyperglycaemia in type 2 diabetes, 2015: a patient‐centred approach. Update to a position statement of the American Diabetes Association and the European Association for the Study of Diabetes. Diabetologia. 2015;58(3):429‐442. [DOI] [PubMed] [Google Scholar]
- 3. Montvida O, Shaw J, Atherton JJ, Stringer F, Paul SK. Long‐term trends in antidiabetes drug usage in the U.S.: real‐world evidence in patients newly diagnosed with type 2 diabetes. Diabetes Care. 2018;41(1):69‐78. [DOI] [PubMed] [Google Scholar]
- 4. Sharma M, Nazareth I, Petersen I. Trends in incidence, prevalence and prescribing in type 2 diabetes mellitus between 2000 and 2013 in primary care: a retrospective cohort study. BMJ Open. 2016;6(1):e010210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Meinert CL, Knatterud GL, Prout TE, et al. A study of the effects of hypoglycemic agents on vascular complications in patients with adult‐onset diabetes. II. Mortality results. Diabetes. 1970;19(Suppl):789‐830. [PubMed] [Google Scholar]
- 6. UK Prospective Diabetes Study (UKPDS) Group . Intensive blood‐glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). Lancet. 1998;352(9131):837‐853. [PubMed] [Google Scholar]
- 7. Azoulay L, Suissa S. Sulfonylureas and the risks of cardiovascular events and death: a methodological meta‐regression analysis of the observational studies. Diabetes Care. 2017;40(5):706‐714. [DOI] [PubMed] [Google Scholar]
- 8. Roumie CL, Hung AM, Greevy RA, et al. Comparative effectiveness of sulfonylurea and metformin monotherapy on cardiovascular events in type 2 diabetes mellitus: a cohort study. Ann Intern Med. 2012;157(9):601‐610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. McAfee AT, Koro C, Landon J, et al. Coronary heart disease outcomes in patients receiving antidiabetic agents. Pharmacoepidemiol Drug Saf. 2007;16(7):711‐725. [DOI] [PubMed] [Google Scholar]
- 10. Schramm TK, Gislason GH, Vaag A, et al. Mortality and cardiovascular risk associated with different insulin secretagogues compared with metformin in type 2 diabetes, with or without a previous myocardial infarction: a nationwide study. Eur Heart J. 2011;32(15):1900‐1908. [DOI] [PubMed] [Google Scholar]
- 11. Azoulay L, Schneider‐Lindner V, Dell'aniello S, Schiffrin A, Suissa S. Combination therapy with sulfonylureas and metformin and the prevention of death in type 2 diabetes: a nested case‐control study. Pharmacoepidemiol Drug Saf. 2010;19(4):335‐342. [DOI] [PubMed] [Google Scholar]
- 12. Wheeler S, Moore K, Forsberg CW, et al. Mortality among veterans with type 2 diabetes initiating metformin, sulfonylurea or rosiglitazone monotherapy. Diabetologia. 2013;56(9):1934‐1943. [DOI] [PubMed] [Google Scholar]
- 13. Huxley R, Barzi F, Woodward M. Excess risk of fatal coronary heart disease associated with diabetes in men and women: meta‐analysis of 37 prospective cohort studies. BMJ. 2006;332(7533):73‐78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Herrett E, Gallagher AM, Bhaskaran K, et al. Data resource profile: Clinical Practice Research Datalink (CPRD). Int J Epidemiol. 2015;44(3):827‐836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Herrett E, Shah AD, Boggon R, et al. Completeness and diagnostic validity of recording acute myocardial infarction events in primary care, hospital care, disease registry, and national mortality records: cohort study. BMJ. 2013;346:f2350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Herrett E, Thomas SL, Schoonen WM, Smeeth L, Hall AJ. Validation and validity of diagnoses in the General Practice Research Database: a systematic review. Br J Clin Pharmacol. 2010;69(1):4‐14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Khan NF, Harrison SE, Rose PW. Validity of diagnostic coding within the General Practice Research Database: a systematic review. Br J Gen Pract. 2010;60(572):e128‐e136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. McCulloch DK. Initial management of blood glucose in adults with type 2 diabetes mellitus. UpToDate: UpToDate, Waltham, MA (Accessed July 24, 2016); 2016.
- 19. Andrade SE, Harrold LR, Tjia J, et al. A systematic review of validated methods for identifying cerebrovascular accident or transient ischemic attack using administrative data. Pharmacoepidemiol Drug Saf. 2012;21(Suppl 1):100‐128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Schneeweiss S, Rassen JA, Glynn RJ, Avorn J, Mogun H, Brookhart MA. High‐dimensional propensity score adjustment in studies of treatment effects using health care claims data. Epidemiology. 2009;20(4):512‐522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Normand ST, Landrum MB, Guadagnoli E, et al. Validating recommendations for coronary angiography following acute myocardial infarction in the elderly: a matched analysis using propensity scores. J Clin Epidemiol. 2001;54(4):387‐398. [DOI] [PubMed] [Google Scholar]
- 22. Rosenstock J, Marx N, Kahn SE, et al. Cardiovascular outcome trials in type 2 diabetes and the sulphonylurea controversy: rationale for the active‐comparator CAROLINA trial. Diab Vasc Dis Res. 2013;10(4):289‐301. [DOI] [PubMed] [Google Scholar]
- 23. Marso SP, Daniels GH, Brown‐Frandsen K, et al. Liraglutide and cardiovascular outcomes in type 2 diabetes. New Engl J Med. 2016;375(18):1797‐1798. [DOI] [PubMed] [Google Scholar]
- 24. Zinman B, Wanner C, Lachin JM, et al. Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. New Engl J Med. 2015;373(22):2117‐2128. [DOI] [PubMed] [Google Scholar]
- 25. Kernan WN, Viscoli CM, Furie KL, et al. Pioglitazone after ischemic stroke or transient ischemic attack. New Engl J Med. 2016;374(14):1321‐1331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Tuccori M, Filion KB, Yin H, et al. Pioglitazone use and bladder cancer risk: a population‐based cohort study. BMJ. 2016;352:i1541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Tomai F, Crea F, Gaspardone A, et al. Ischemic preconditioning during coronary angioplasty is prevented by glibenclamide, a selective ATP‐sensitive K+ channel blocker. Circulation. 1994;90(2):700‐705. [DOI] [PubMed] [Google Scholar]
- 28. Toombs CF, Moore TL, Shebuski RJ. Limitation of infarct size in the rabbit by ischaemic preconditioning is reversible with glibenclamide. Cardiovasc Res. 1993;27(4):617‐622. [DOI] [PubMed] [Google Scholar]
- 29. Gross GJ, Auchampach JA. Blockade of ATP‐sensitive potassium channels prevents myocardial preconditioning in dogs. Circ Res. 1992;70(2):223‐233. [DOI] [PubMed] [Google Scholar]
- 30. Meier JJ, Gallwitz B, Schmidt WE, Mügge A, Nauck MA. Is impairment of ischaemic preconditioning by sulfonylurea drugs clinically important? Heart. 2004;90(1):9‐12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Sola D, Rossi L, Schianca GP, et al. Sulfonylureas and their use in clinical practice. Arch Med Sci. 2015;11(4):840‐848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Najeed SA, Khan IA, Molnar J, Somberg JC. Differential effect of glyburide (glibenclamide) and metformin on QT dispersion: a potential adenosine triphosphate sensitive K+ channel effect. Am J Cardiol. 2002;90(10):1103‐1106. [DOI] [PubMed] [Google Scholar]
- 33. Lepran I, Baczko I, Varro A, et al. ATP‐sensitive potassium channel modulators: both pinacidil and glibenclamide produce antiarrhythmic activity during acute myocardial infarction in conscious rats. J Pharmacol Exp Ther. 1996;277(3):1215‐1220. [PubMed] [Google Scholar]
- 34. Miki T, Liss B, Minami K, et al. ATP‐sensitive K+ channels in the hypothalamus are essential for the maintenance of glucose homeostasis. Nat Neurosci. 2001;4(5):507‐512. [DOI] [PubMed] [Google Scholar]
- 35. Douros A, Yin H, Yu OHY, Filion KB, Azoulay L, Suissa S. Pharmacologic differences of sulfonylureas and the risk of adverse cardiovascular and hypoglycemic events. Diabetes Care. 2017;40(11):1506‐1513. [DOI] [PubMed] [Google Scholar]
- 36. Proks P, Reimann F, Green N, Gribble F, Ashcroft F. Sulfonylurea stimulation of insulin secretion. Diabetes. 2002;51(Suppl 3):S368‐S376. [DOI] [PubMed] [Google Scholar]
- 37. Yu O, Azoulay L, Yin H, Filion KB, Suissa S. Sulfonylureas as initial treatment for type 2 diabetes and the risk of severe hypoglycemia. Am J Med. 2018;131(3):317.e11‐317.e22. [DOI] [PubMed] [Google Scholar]
- 38. Cha SA, Yun JS, Lim TS, et al. Severe hypoglycemia and cardiovascular or all‐cause mortality in patients with type 2 diabetes. Diabetes Metab J. 2016;40(3):202‐210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Lee AK, Warren B, Lee CJ, et al. The association of severe hypoglycemia with incident cardiovascular events and mortality in adults with type 2 diabetes. Diabetes Care. 2018;41(1):104‐111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. van Bunderen CC, van Nieuwpoort IC, van Schoor NM, Deeg DJH, Lips P, Drent ML. The association of serum insulin‐like growth factor‐I with mortality, cardiovascular disease, and cancer in the elderly: a population‐based study. J Clin Endocrinol Metab. 2010;95(10):4616‐4624. [DOI] [PubMed] [Google Scholar]
- 41. Andreassen M, Raymond I, Kistorp C, Hildebrandt P, Faber J, Kristensen LØ. IGF1 as predictor of all cause mortality and cardiovascular disease in an elderly population. Eur J Endocrinol. 2009;160(1):25‐31. [DOI] [PubMed] [Google Scholar]
- 42. Mosca S, Paolillo S, Colao A, et al. Cardiovascular involvement in patients affected by acromegaly: an appraisal. Int J Cardiol. 2013;167(5):1712‐1718. [DOI] [PubMed] [Google Scholar]
- 43. Pivonello R, Auriemma RS, Grasso LF, et al. Complications of acromegaly: cardiovascular, respiratory and metabolic comorbidities. Pituitary. 2017;20(1):46‐62. [DOI] [PubMed] [Google Scholar]
- 44. Yong AW, Morris Z, Shuler K, Smith C, Wardlaw J. Acute symptomatic hypoglycaemia mimicking ischaemic stroke on imaging: a systematic review. BMC Neurol. 2012;12(1):139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Chen Y, Du L, Li L, et al. Cancer risk of sulfonylureas in patients with type 2 diabetes mellitus: a systematic review. J Diabetes. 2017;9(5):482‐494. [DOI] [PubMed] [Google Scholar]
- 46. Pasello G, Urso L, Conte P, Favaretto A. Effects of sulfonylureas on tumor growth: a review of the literature. Oncologist. 2013;18(10):1118‐1125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Pollak M. Insulin and insulin‐like growth factor signalling in neoplasia. Nat Rev Cancer. 2008;8(12):915‐928. [DOI] [PubMed] [Google Scholar]
- 48. Suissa S, Azoulay L. Metformin and the risk of cancer: time‐related biases in observational studies. Diabetes Care. 2012;35(12):2665‐2673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Schneeweiss S. Sensitivity analysis and external adjustment for unmeasured confounders in epidemiologic database studies of therapeutics. Pharmacoepidemiol Drug Saf. 2006;15(5):291‐303. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1.
Incidence rates of cardiovascular events and all‐cause mortality among users of metformin and sulfonylureas.
Table S2. Characteristics of sulfonylurea users and matched metformin users among patients with type 2 diabetes included in the analysis of stroke.
Table S3. Characteristics of sulfonylurea users and matched metformin users among patients with type 2 diabetes included in the analysis of cardiovascular death.
Table S4. Characteristics of sulfonylurea users and matched metformin users among patients with type 2 diabetes included in the analysis of all‐cause mortality.
Table S5. Hazard ratios for cardiovascular events and all‐cause mortality associated with sulfonylurea, compared with metformin, in patients with type 2 diabetes, by previous history of the outcome of interest.
Table S6. Hazard ratios for cardiovascular events and all‐cause mortality associated with sulfonylurea, compared with metformin, in patients with type 2 diabetes, by sex.
Table S7. Hazard ratios for cardiovascular events and all‐cause mortality associated with sulfonylurea, compared with metformin, in patients with type 2 diabetes, by age.
Table S8. Hazard ratios for cardiovascular events and all‐cause mortality associated with sulfonylurea, compared with metformin, in patients with type 2 diabetes, with sulfonylurea use defined by dose.
Table S9. Hazard ratios for cardiovascular events and all‐cause mortality associated with sulfonylurea, compared with metformin, in patients with type 2 diabetes, using an intention‐to‐treat approach.
Figure S1. High‐dimensional propensity score distribution among users of sulfonylureas and metformin prior to matching.
Figure S2. High‐dimensional propensity score distribution among users of sulfonylureas and metformin after matching.
Figure S3. Rule‐out method to determine how strong an unmeasured confounder is needed to explain the observed association between sulfonylurea use and cardiovascular death.


