Abstract
The pharmacological effects of a drug depend on its concentration at the site of action, and therefore on the concentration in blood and on the dose. The relationship between the concentration or dose and the corresponding effect can usually be represented mathematically as a rectangular hyperbola; when effect is plotted against log concentration or log dose, the curve is sigmoidal.
Inevitably, the effect size and the doses causing benefit and harm will differ among individuals, since they are biological phenomena: some individuals are more likely than others to suffer harm at any given dose. Some harmful effects can occur at much lower doses than those used in therapeutics; that is, the log dose–response curve for harm lies far to the left of the log dose–response curve for benefit. Those who suffer such reactions are hypersusceptible. When the dose–response curves for harm and therapeutic effect are in the same range, dose cannot separate the harmful effects from the therapeutic effects, and adverse reactions are collateral. Toxic effects occur when harmful doses are above the doses needed for benefit.
In this review we consider factors that influence a subject's susceptibility to adverse drug reactions. Determinants of susceptibility include Immunological, Genetic, demographic (Age and Sex), Physiological and Exogenous factors (drug–drug interactions, for example), and Diseases and disorders such as renal failure, giving the mnemonic I GASPED. Some susceptibility factors are discrete (for example, all‐or‐none) and some are continuous; susceptibility can therefore be discrete or continuous; and the factors can interact to determine a person's overall susceptibility to harm.
Keywords: adverse drug reactions, pharmacodynamics, pharmacokinetics, genetic polymorphisms, prescribing
1. INTRODUCTION
Some patients become ill from a dose of a drug that in other patients has no discernible effect; some patients die from exposure to drugs that are safe and effective in other patients.
There are in effect two distinct types of adverse drug reaction (ADR): those that will affect all patients, but which occur at different doses in different patients; and those that will affect some patients, but not all, however large a dose is administered. The response in affected patients will necessarily depend on dose; it is a misconception that immunological reactions such as anaphylaxis1 are unrelated to dose, although the dose‐dependence may not be evident within the therapeutic dose range.
In this review we consider the factors that influence the susceptibility of subjects to ADRs. Our review derives primarily from a lecture at the British Pharmacological Society's Pharmacology 2018 meeting and updates a review of the harms from medicines.2
2. THE DOSE OF THE DRUG
The premise on which this review is based is that all pharmacological effects are related to the concentration of a pharmacological agent at its site or sites of action, whether the action is beneficial or detrimental. We have discussed the reasons for this, and its consequences, elsewhere.3, 4 The concentration at the site of action is related, in turn, to the dose administered. Since the dose is usually known, while the concentration at the site of action may be difficult or impossible to measure, it is often convenient to discuss dose–response rather than concentration–response.
The key developments in the history of ideas about dose‐responsiveness date from early in the 20th century, although there is a prehistory.5 The cumulative dose of salicylate at which patients with rheumatic disease demonstrated symptoms and signs of toxicity was established before the First World War.6 A.J. Clark used the data to draw a sigmoid log dose–response curve (Figures 1 and 2).7
Figure 1.

Cumulative percentage of patients who have become salicylate‐toxic plotted against log dose of salicylate (in grains; 1 grain ~ 65 mg) [after references 6, 7]
Figure 2.
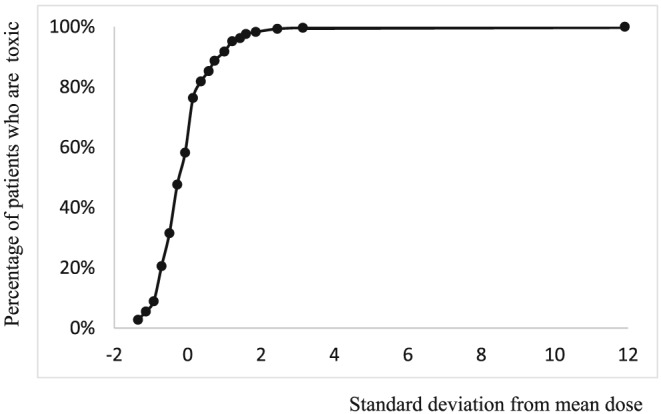
Hanzlik's data6 plotted as a cumulative distribution curve (cumulative percentage vs standard deviation from a mean dose of 186 grains)
The therapeutic dose of salicylate was limited by toxicity. In Hanzlik's practice, ‘the salicylate is given in doses of from 10 to 20 grains every hour until symptoms of intoxication begin to appear.’6 Dosing to toxicity has been largely abandoned outside oncology.8 While toxic ADRs are important, harm can occur with therapeutic doses rather than toxic doses.9 For example, constipation is a collateral adverse reaction to opioid analgesia, and is expected to accompany the therapeutic action. Where the dose–response curve for a significant ADR approaches a maximum at concentrations lower than those used in treating disease, we have characterized it as indicating hypersusceptibility to the adverse effect in comparison with the therapeutic effect. Most such ADRs affect only a small subset of the treated population—the drug would be of limited clinical value if it often caused significant harm before any benefit was realized.
3. THE TIME‐COURSE OF ADMINISTRATION
A second factor that influences the risk of ADRs is the time‐course of exposure in relation to the reaction. This is partly because cumulative dose is a function of time, and the cumulative dose determines the risk of some ADRs. For example, the risk of delayed anthracycline‐induced cardiomyopathy increases with cumulative dose.10
The rate of change of drug concentration can also be important. An example is the development of flushing and wheeze with rapid infusion of acetylcysteine.11
4. SUSCEPTIBILITY FACTORS RELATED TO THE PATIENT
Dose–response and time‐course represent aspects of the drug and its administration. Susceptibility characterizes the contribution of patient factors to the risk of an ADR. The interactions between dose, time‐course, and susceptibility can help clinicians understand, predict, and mitigate ADRs. “The major interacting factors influencing the response of the host to the drug” were set out a 1958 review of untoward reactions to penicillin. The factors in the responding system included, for example, age, sex, hereditary factors, and the “presence or stage of pathological conditions.”12
4.1. Immunological factors
Gell and Coombs classified immunological reactions into 4 types, each of which can be associated with ADRs. Immediate (Type I) hypersensitivity reactions, in which antigen binds to specific immunoglobulin E, result in the degranulation of mast cells and the release of histamine, bradykinin and other mediators that cause the potentially fatal clinical syndrome of airways compromise, hypovolaemia and cardiovascular collapse. Anaphylaxis to β‐lactam antibiotics is an example. In the earliest reported case, a reaction to 15 000 units benzylpenicillin injected intramuscularly in a soldier previously sensitized by dermal application, the reaction was milder and of shorter duration after a further injection of 100 units subcutaneously; oral administration of benzylpenicillin produced desensitization.13 As far back as 1909, Anderson and Rosenau demonstrated that there was a minimum sensitizing dose of horse serum globulins in the guinea pig14; recent studies in patients sensitized to trinitrophenol showed a clear relation between the dose of trinitrophenol–bovine serum albumin and the intensity of the anaphylactic response.15
Haemolytic anaemia provides an example of a drug‐induced Type II immunological reaction mediated by immunoglobulin G. The drug can act as a hapten covalently bound to proteins on the red cell membrane, as happens with penicillin; or can induce Coombs' test‐positive haemolytic anaemia by suppressing immune regulation, as happens with the checkpoint inhibitors nivolumab, pembrolizumab and ipilimumab.16 The proportion of patients who develop a positive Coombs' test with the antihypertensive drug α‐methyldopa increases with increasing dose.17
Serum sickness—fever, urticaria and joint pain following injection of foreign protein—was first delineated in 1905 in patients treated with horse serum containing antibody against diphtheria, used for passive immunization.18 This Type III immunological reaction is the consequence of circulating immune complexes. It can occur with modern biological therapy such as rituximab, a murine–human chimeric monoclonal antibody directed against the B cell surface marker CD20.19
Delayed‐type (Type IV) immunological reactions are cell‐mediated. The proportion of subjects sensitized by the chemical dinitrochlorobenzene, which induces delayed‐type hypersensitivity, increases as the sensitizing dose increases (Figure a); and in sensitized individuals, the size of dermal response depends on the dose used to elicit it (Figure b).20 There appears to be a predisposition to being sensitized by topical allergens: patients with positive patch tests to many allergens are more easily sensitized to dinitrochlorobenzene than patients with no positive patch tests.21
Figure 3.

(A) Percentage of subjects sensitized vs dose of dinitrochlorobenzene on a logarithmic scale [after reference 19]. (B) Wheal thickness response to topical dinitrochlorobenzene vs dose of dinitrochlorobenzene on a logarithmic scale in subjects sensitized to DNCB [after reference 20]; note that the dose required to provoke a response is 2 orders of magnitude less than the dose required to sensitize a subject
4.2. Genetic factors
Genetic factors can determine the pharmacokinetics of drugs. It was established in the 1940s that the hydrolysis of atropine by rabbit serum was determined by a gene called As; hydrolysis was faster in homozygotes than heterozygotes, and absent if the As gene was absent.22 Prolonged apnoea from respiratory muscle paralysis in patients given the muscle relaxant succinylcholine (suxamethonium) is also genetically determined. In homozygous normal subjects, the drug is rapidly metabolized by an enzyme, butyrylcholinesterase, whose activity is impaired in those with prolonged apnoea and in their families.23 The duration of apnoea depends on the dose in both normal and abnormal subjects, but in the latter the dose–response curve is shifted far to the left (Figure 4).24 The ClinVar database now lists 118 genetic variants of butyrylcholinesterase, of which 3/4 are pathogenic or likely to be pathogenic.25
Figure 4.
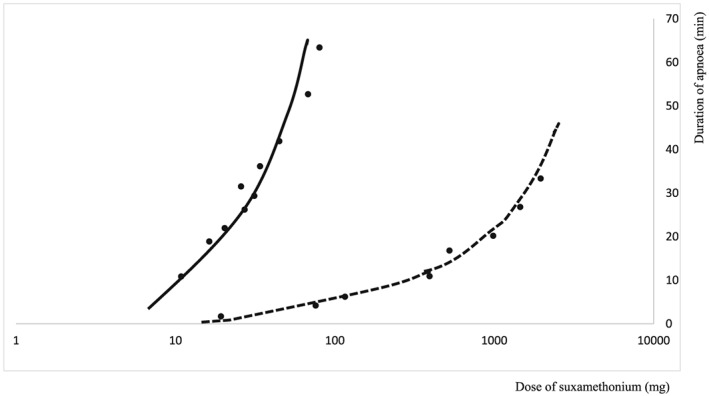
Duration of apnoea (minutes) vs dose of suxamethonium in mg (log scale) for normal subjects (UU, dashed line) and those with 2 abnormal alleles (AA, solid line) [after reference 24]
Pharmacodynamic differences can also be genetically determined. For example, aspirin‐exacerbated respiratory disease (asthma, nasal polyps and aspirin sensitivity: Samter's triad) was recognized to be familial in the 1950s; it is associated with genetic abnormalities, usually in the production or action of cysteinyl leukotrienes.26 Another pharmacodynamic susceptibility recognized in the 1950s was haemolytic anaemia with oxidizing agents. The resistance of haemoglobin in red blood cells to oxidation depends on the function of glucose‐6‐phospate dehydrogenase (G6PD), the key enzyme in the generation of reduced glutathione.27 Over 300 enzyme variants from G6PD AACHEN to G6PD ZHITOMIR are now recognized.28 The extent of haemolysis depends on both the enzyme variant and the dose of oxidizing agent.
Genetic and immunological susceptibility interact through human lymphocyte‐associated antigens. For example, the observation that abacavir binds to and alters the antigen coded for by HLA‐B*5701, so that it alters the repertoire of peptides recognized by the receptor, which now reacts to peptides previously recognized as self, and causes an immune response and tissue damage. This helps to explain the observation that serious cutaneous adverse reactions to abacavir are much commoner in subjects with that genotype.29, 30
4.3. Age
Some ADRs are more common in infants and children, who have immature physiological systems, and others are increased in the elderly with failing physiological systems and increasing frailty and co‐morbidity. The classical example in neonates is grey baby syndrome. In this syndrome, a high concentration of chloramphenicol accumulates as a result of poorly developed hepatic metabolism.31 The high chloramphenicol concentration causes cardiovascular collapse.
A further difficulty in children is that the range of agents is smaller than the range licensed for use in adults, and it is therefore common to use preparations untested in children. A study of children admitted to hospital showed that adverse reactions were more likely to occur with unlicensed and off‐label medicines than licensed medicines (relative risk 1.67; 95% confidence interval 1.38, 2.02; P < .001).32
Some ADRs occur more often in older people than younger adults. For example, in cross‐sectional studies of French and Icelandic populations, the risk of drug‐induced liver injury increased 4‐fold from ages 15–29 years to age >70 years.33 Part of the explanation could be that different populations are exposed to causative agents, such as co‐amoxiclav, to different extents. However, difference in exposure is not the entire explanation: the risk of liver injury with flucloxacillin was 25 times higher in those aged 70–79 years than in those aged 18–49 years34; and age and dosage were independent risk factors for statin‐induced liver injury in a Chinese cohort.35 More generally, a model based on data from 1408 inpatients identified age as a major predictor of preventable harm from medicines,36 as did several other models.37, 38
Responses to drugs can differ qualitatively with age. A good example is the difference in ADRs to the dopamine antagonist metoclopramide. The risk of acute dystonic–dyskinetic reactions was >30 times greater in those younger than 20 years than those older than 65 years; parkinsonism was significantly more common in those aged >65 years than in those <65 years.39 The difference may be due to the change in the balance between dopamine D1 and D2 receptors with age.
Reduced renal function and altered body composition in older people can cause marked changes in drug disposition, which probably contribute to higher rates of hospital admission for ADRs.40 Two important sequelae to physical and mental frailty are falls (and their consequence—femoral fractures) and delirium. Falls are associated with prescription of hypnotics and sedatives41; although benzodiazepines are particularly incriminated, the regular use of a non‐benzodiazepine hypnotic (z‐drug) increased the relative risk of falls 4‐fold in a longitudinal study of nursing home residents.42 In another study, older adults who continued taking drugs that were believed to increase the risk of falls were 10 times more likely to suffer falls than older adults who stopped taking such drugs.43 Opioid analgesics, benzodiazepines, anticholinergic drugs and other commonly used medicines often cause delirium in older people.40, 44
Multi‐morbidity, the simultaneous occurrence of several morbid conditions, increases the risk that the pharmacokinetics or effect of 1 or more medicines is altered by the presence of disease. It also makes polypharmacy more likely, and that increases the risk of drug–drug interactions. The number of possible 2‐way interactions increases from 1 with 2 drugs to 10 with 5 drugs, to 45 with 10 drugs, to 105 with 15 drugs (Figure 5).
Figure 5.
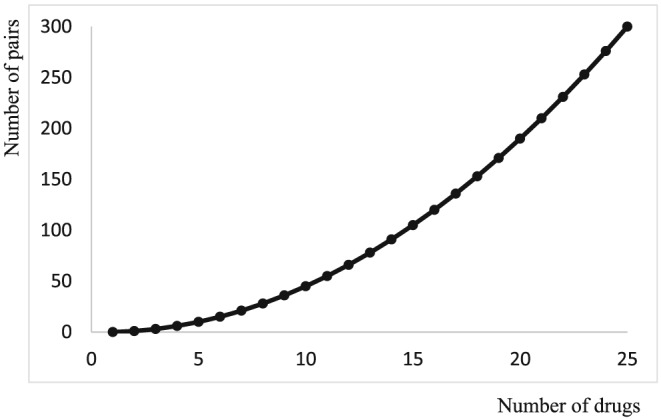
The number of pair‐wise interactions of n drugs, two at a time
4.4. Sex
Some ADRs are limited to one sex for biological reasons. For example, clear‐cell carcinoma of the vagina, a delayed consequence of exposure to diethylstilboestrol in utero,45 can only occur in women. A contemporary example is the risk of abnormal vaginal bleeding in women treated with anticoagulants. This is a particular problem with direct‐acting oral anticoagulants.46 In 1 analysis, this “occurred frequently (9–15/100 [patient‐years]) and significantly more often in women of reproductive age receiving edoxaban compared with women receiving warfarin.”47
Many studies show that, for ADRs that occur in both men and women, the risk is generally higher in women. For example, in a review of studies of ADRs causing or occurring during admission to hospital, 8/15 studies identified female sex as a risk factor, and no trial identified an increased risk in males.38 In an analysis of 48 cohort studies of newly marketed drugs used in general practice the overall age‐standardized relative risk of an ADR being recorded was 1.6 (95% confidence interval 1.5–1.7) in women.48 This may be partly due to the use of standard doses unrelated to body size, since women are on average smaller than men. Some ADRs are twice as common in women as in men. In the case of the potentially fatal arrhythmia torsade de pointes, 1 factor is the sex difference in repolarization of heart muscle, reflected in a longer corrected QT interval in women at baseline.49 Database studies suggest that cough with angiotensin‐converting enzyme inhibitors is approximately twice as common in women as in men, although angioedema from angiotensin‐converting enzyme inhibitors, whose pathogenesis is likely to be similar, is reported 30% more frequently in men.50
4.5. Physiological changes
Pregnancy has a marked influence on body composition and physiological function, and hence on drug disposition. It also exposes the unborn fetus to potentially harmful concentrations of maternal drugs. Wilson noted that the susceptibility of the conceptus to teratogens depended on genotype, the developmental stage at the time of exposure, and the dose of the teratogen.51 The relationship between dose and response was clearly shown in Himalayan rabbits exposed to thalidomide, where the incidence of defects in leg bones, malformation of the digits and sternal synostosis were all dose‐related.52 The degradation of a transcription factor called SALL4 has been implicated in the harm caused by thalidomide, which resembles the Duane Radial Ray Syndrome that results from loss‐of‐function mutations in the gene coding for SALL4.53 There are mutations in the zinc‐finger domain of murine SALL4 that protect it from the action of thalidomide, and explain why mice are not susceptible to thalidomide embryopathy.
Circadian rhythm influences both the disposition of drugs and their effects. In 1 study, unfractionated heparin was given at constant rate by infusion pump. The activated partial thromboplastin time and Factor Xa inhibition assay nevertheless showed peak values (towards midnight) 40% higher than trough values (towards 07.00 hours).54 In healthy male volunteers, the clearance of an intravenous dose of 20 mg methylprednisolone given at 08.00 hours was substantially slower than the clearance of an identical dose given at 16.00 hours.55 Both sex and genetics substantially influence chronopharmacology, at least in mice.56 It is also possible that the menstrual cycle influences drug metabolism and hence the risk of ADRs. The activity of drug‐metabolizing enzymes CYP1A2, CYP2A6 and NAT2 differed significantly between the early and late follicular phase in women of childbearing age, as assessed by caffeine metabolism.57 However, since caffeine metabolite ratios were used to determine enzyme activity, the results for the 3 enzymes were not independent.
4.6. Exogenous factors
Environmental factors and exposure to foodstuffs and interacting medicines are likely to influence the risk of ADRs to drugs.
For example, patch test responses to piperazine depended on environmental temperature.58 The authors of a Swedish study concluded that warm weather increased the risk of drug‐induced hyponatraemia,59 and heat‐related adverse effects of diuretics and some other drugs were twice as frequent in summers affected by heat‐waves as in control summers.60
Foodstuffs can provoke reactions analogous to those caused by medicines, as is the case with tartrazine in patients with aspirin‐exacerbated respiratory disease.61 Foodstuffs and medicines can have pharmacodynamic interactions, as do ethanol and diazepam62; or pharmacokinetic interactions, as happens when mono‐amine oxidase inhibitors prevent the breakdown of tyramine from foods such as blue cheese.63 They can also display pharmaceutical interactions, as when the absorption of tetracycline is reduced by binding to calcium in milk.64
Drug–drug interactions are well established, and an important cause of avoidable ADRs.65 However, there is the serious difficulty, both in general and in individual patients, that while potential ADRs are very numerous, serious adverse reactions are rare, even when they are known to occur. For example, in 1 study of elderly Italian patients, the number of potentially important ADRs was 12 578, but only 464 (4%) of these were observed, and even for the most serious potential reactions, only 5% resulted in clinically significant effects.66 This divergence between theory and clinical observation suggests that the theory needs refinement.
4.7. Disease
Disease can affect the absorption, distribution, metabolism, and elimination of drugs.
The effects of renal and hepatic impairment are well known, and Summaries of Product Characteristics give advice on dosage adjustment, although not all such advice may be based on good evidence. The effects in liver and renal disease are, for the most part, caused by higher drug concentrations. In the case of liver disease, this can be a result of porta‐systemic shunting, which allows orally administered agents to be absorbed without undergoing first‐pass hepatic metabolism; or reduced hepatic elimination as a consequence of reduced metabolism or diminished biliary excretion. In kidney disease, the major effect is on renal drug elimination, but some drugs—notably insulin and 25‐hydroxycholecalciferol—are affected by a reduction in renal metabolism. Pharmacodynamic effects of liver failure are most obvious in patients with cirrhosis, who are especially sensitive to sedative drugs. It is postulated that the sensitivity is related to an increase in GABA‐ergic tone, perhaps because of circulating endogenous benzodiazepines; this is in keeping with the observation that flumazenil can sometimes lighten hepatic coma.67
The influence of other conditions is less well explored. For example, the effects of obesity on drug distribution are of increasing importance as the average body mass index increases. A recent review of vancomycin dosing noted that no recommendations make adjustment for obesity, despite adjustments for actual body weight, renal function, and other relevant parameters.68
The interactions between diseases and the actions of medicines are also important, as is evident, for example, in the hyperglycaemic action of corticosteroids in patients with diabetes; even local corticosteroid injections cause a transient increase in blood glucose concentration.69
5. CONCLUSIONS
The many factors that influence the occurrence of ADRs can be summarized as Dose, Time, and Susceptibility (DoTS), reflecting properties of the drug, the reaction, and the patient. The factors that alter an individual's susceptibility include Immunological and Genetic factors, Age, Sex, Physiological changes, Exogenous influences, and Disease conditions; that is, I GASPED. Interactions between these factors help to explain why some patients suffer serious adverse reactions while others are unaffected; and all depend on dose of the drug.
COMPETING INTERESTS
This work was unfunded. Both R.E.F. and J.K.A. have provided expert advice on ADRs and have sometimes received payment for this advice.
ACKNOWLEDGEMENTS
R.E.F. is very grateful to Professor Simon Dimmitt and Dr John Warren for the invitation to speak at the British Pharmacological Society symposium on ED50 at Pharmacology 2019.
Ferner R, Aronson J. Susceptibility to adverse drug reactions. Br J Clin Pharmacol. 2019; 85: 2205–2212. 10.1111/bcp.14015
Footnotes
nC2 = n!/(n‐2)!2! = n(n‐1)/2
REFERENCES
- 1. Stone SF, Phillips EJ, Wiese MD, Heddle RJ, Brown SGA. Immediate‐type hypersensitivity drug reactions. Br J Clin Pharmacol. 2014;78(1):1‐13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Ferner RE. Harms from medicines: inevitable, in error or intentional. Br J Clin Pharmacol. 2014;77(3):403‐409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Ferner RE, Aronson JK. Cato Guldberg and Peter Waage, the history of the law of mass action, and its relevance to clinical pharmacology. Br J Clin Pharmacol. 2016;81(1):52‐55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Aronson JK, Ferner RE. The law of mass action and the pharmacological concentration‐effect curve: resolving the paradox of apparently non‐dose‐related adverse drug reactions. Br J Clin Pharmacol. 2016;81(1):56‐61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Aronson JK, Ferner RE. It does all depend on the dose. Understanding beneficial and adverse drug effects since 1864: clinical and experimental attitudes to the law of mass action and concentration–effect curves In: Grell O, Cunningham A, Arrizabalaga J, eds. It All Depends on the Dose': Poisons and Medicines in European History. Taylor & Francis Ltd. London: Imprint Routledge; 2018:210‐239. [Google Scholar]
- 6. Hanzlik PJ. A study of the toxicity of the salicylates based on clinical statistics. JAMA. 1913;60(13):957‐962. [Google Scholar]
- 7. Clark AJ. Individual variation in the response to drugs. Trans Edin Med‐Chir Soc. 1935;42(1):1‐16. [PMC free article] [PubMed] [Google Scholar]
- 8. Martin JH, Dimmitt S. The rationale of dose-response curves in selecting cancer drug dosing. Br J Clin Pharmacol. 2019. 10.1111/bcp.13979 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Aronson JK, Ferner RE. Joining the DoTS: new approach to classifying adverse drug reactions. BMJ. 2003;327(7425):1222‐1225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Armenian S, Bhatia S. Predicting and preventing anthracycline‐related cardiotoxicity Am Soc Clin Oncol Educ Book. 2018;38:3‐12. 10.1200/EDBK_100015 [DOI] [PubMed] [Google Scholar]
- 11. Sandilands EA, Bateman DN. Adverse reactions associated with acetylcysteine. Clin Toxicol (Phila). 2009;47(2):81‐88. [DOI] [PubMed] [Google Scholar]
- 12. Guthe T, Idsöe O, Willcox RR. Untoward penicillin reactions. Bull World Health Organ. 1958;19(3):427‐501. [PMC free article] [PubMed] [Google Scholar]
- 13. O'Donovan WJ, Klorfajn I. Sensitivity to penicillin. Anaphylaxis and desensitisation. Lancet. 1946;248(6411):444‐446. [DOI] [PubMed] [Google Scholar]
- 14. Anderson JF, Rosenau MJ. Further studies upon the phenomenon of anaphylaxis. J Med Res. 1909;21(1):1‐19. [PMC free article] [PubMed] [Google Scholar]
- 15. Nassiri M, Babina M, Dölle S, Edenharter G, Ruëff F, Worm M. Ramipril and metoprolol intake aggravate human and murine anaphylaxis: evidence for direct mast cell priming. J Allergy Clin Immunol. 2015;135(2):491‐499. [DOI] [PubMed] [Google Scholar]
- 16. Tanios GE, Doley PB, Munker R. Autoimmune hemolytic anemia associated with the use of immune checkpoint inhibitors for cancer: 68 cases from the Food and Drug Administration database and review. Eur J Haematol. 2019;102(2):157‐162. [DOI] [PubMed] [Google Scholar]
- 17. Carstairs KC, Breckenridge A, Dollery CT, Worlledge SM. Incidence of a positive direct coombs test in patients on alpha‐methyldopa. Lancet. 1966;288(7455):133‐135. [DOI] [PubMed] [Google Scholar]
- 18. Goodall EW. A clinical address on serum sickness. Lancet. 1918;191(4932):361‐365. [Google Scholar]
- 19. D'Arcy CA, Mannik M. Serum sickness secondary to treatment with the murine‐human chimeric antibody IDEC‐C2B8 (rituximab). Arthritis Rheum. 2001;44(7):1717‐1718. [DOI] [PubMed] [Google Scholar]
- 20. Friedmann PS, Moss C, Shuster S, Simpson JM. Quantitative relationships between sensitizing dose of DNCB and reactivity in normal subjects. Clin Exp Immunol. 1983;53(3):709‐715. [PMC free article] [PubMed] [Google Scholar]
- 21. Moss C, Friedmann PS, Shuster S, Simpson JM. Susceptibility and amplification of sensitivity in contact dermatitis. Clin Exp Immunol. 1985;61(2):232‐241. [PMC free article] [PubMed] [Google Scholar]
- 22. Sawin PB, Glick D. Atropinesterase, a genetically determined enzyme in the rabbit. Proc Natl Acad Sci U S A. 1943;29(2):55‐59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Lehmann H, Ryan E. The familial incidence of low pseudocholinesterase level. Lancet. 1956;268(6944):681‐682. [DOI] [PubMed] [Google Scholar]
- 24. Lockridge O. Review of human butyrylcholinesterase structure, function, genetic variants, history of use in the clinic, and potential therapeutic uses. Pharmacol Ther. 2015;148:34‐46. [DOI] [PubMed] [Google Scholar]
- 25. ClinVar database of National Center for Biotechnology Information , US National Library of Medicine . Butyrylcholinesterase. Available at https://www.ncbi.nlm.nih.gov/clinvar. Accessed 20 May 2019.
- 26. Pavón‐Romero GF, Ramírez‐Jiménez F, Roldán‐Alvarez MA, Terán LM, Falfán‐Valencia R. Physiopathology and genetics in aspirin‐exacerbated respiratory disease. Exp Lung Res. 2017;43(8):327‐335. [DOI] [PubMed] [Google Scholar]
- 27. Beutler E, Yeh M, Fairbanks VF. The normal female as a mosaic of X‐chromosome activity: studies using the gene for G‐6‐PD‐deficiency as a marker. Proc Natl Acad Sci U S A. 1962;48(1):9‐16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Online Mendelian Inheritance in Man. Glucose‐6‐phosphate dehydrogenase. Available at http://omim.org/entry/305900. Accessed 1 January 2019.
- 29. Illing PT, Vivian JP, Dudek NL, et al. Immune self‐reactivity triggered by drug‐modified HLA‐peptide repertoire. Nature. 2012;486(7404):554‐558. [DOI] [PubMed] [Google Scholar]
- 30. Mallal S, Nolan D, Witt C, et al. Association between presence of HLA‐B*5701, HLA‐DR7, and HLA‐DQ3 and hypersensitivity to HIV‐1 reverse‐transcriptase inhibitor abacavir. Lancet. 2002;359(9308):727‐732. [DOI] [PubMed] [Google Scholar]
- 31. Mulhall A, de Louvois J, Hurley R. Chloramphenicol toxicity in neonates: its incidence and prevention. Br Med J (Clin Res Ed). 1983;287(6403):1424‐1427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Bellis JR, Kirkham JJ, Nunn AJ, Pirmohamed M. Adverse drug reactions and off‐label and unlicensed medicines in children: a prospective cohort study of unplanned admissions to a paediatric hospital. Br J Clin Pharmacol. 2014;77(3):545‐553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Fontana RJ. Pathogenesis of idiosyncratic drug‐induced liver injury and clinical perspectives. Gastroenterology. 2014;146(4):914‐928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Wing K, Bhaskaran K, Pealing L, et al. Quantification of the risk of liver injury associated with flucloxacillin: a UK population‐based cohort study. J Antimicrob Chemother. 2017;72(9):2636‐2646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Wang LY, Huang YS, Perng CL, Huang B, Lin HC. Statin‐induced liver injury in an area endemic for hepatitis B virus infection: risk factors and outcome analysis. Br J Clin Pharmacol. 2016;82(3):823‐830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Nguyen TL, Leguelinel‐Blache G, Kinowski JM, et al. Improving medication safety: development and impact of a multivariate model‐based strategy to target high‐risk patients. PLoS ONE. 2017;12(2):e0171995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Falconer N, Barras M, Cottrell N. Systematic review of predictive risk models for adverse drug events in hospitalized patients. Br J Clin Pharmacol. 2018;84(5):846‐864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Saedder EA, Lisby M, Nielsen LP, Bonnerup DK, Brock B. Number of drugs most frequently found to be independent risk factors for serious adverse reactions: a systematic literature review. Br J Clin Pharmacol. 2015;80(4):808‐817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Bateman DN, Rawlins MD, Simpson JM. Extrapyramidal reactions with metoclopramide. Br Med J (Clin Res Ed). 1985;291(6500):930‐932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Davies EA, OMahony MS. Adverse drug reactions in special populations – the elderly. Br J Clin Pharmacol. 2015;80(4):796‐807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Hartikainen S, Lönnroos E, Louhivuori K. Medication as a risk factor for falls: critical systematic review. J Gerontol A Biol Sci Med Sci. 2007;62(10):1172‐1181. [DOI] [PubMed] [Google Scholar]
- 42. Westerlind B, Östgren CJ, Mölstad S, Midlöv P, Hägg S. Use of non‐benzodiazepine hypnotics is associated with falls in nursing home residents: a longitudinal cohort study. Aging Clin Exp Res. 2018. 10.1007/s40520-018-1056-0 [DOI] [PubMed] [Google Scholar]
- 43. van der Velde N, Stricker BH, Pols HA, van der Cammen TJ. Risk of falls after withdrawal of fall‐risk‐increasing drugs: a prospective cohort study. Br J Clin Pharmacol. 2007;63(2):232‐237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Collamati A, Martone AM, Poscia A, et al. Anticholinergic drugs and negative outcomes in the older population: from biological plausibility to clinical evidence. Aging Clin Exp Res. 2016;28(1):25‐35. [DOI] [PubMed] [Google Scholar]
- 45. Huo D, Anderson D, Herbst AL. Follow‐up of patients with clear‐cell adenocarcinoma of the vagina and cervix. N Engl J Med. 2018;378(18):1746‐1748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Beyer‐Westendorf J, Michalski F, Tittl L, Hauswald‐Dörschel S, Marten S. Management and outcomes of vaginal bleeding and heavy menstrual bleeding in women of reproductive age on direct oral anti‐factor Xa inhibitor therapy: a case series. Lancet Haematol. 2016;3(10):e480‐e488. [DOI] [PubMed] [Google Scholar]
- 47. Scheres L, Brekelmans M, Ageno W, et al. Abnormal vaginal bleeding in women of reproductive age treated with edoxaban or warfarin for venous thromboembolism: a post hoc analysis of the Hokusai‐VTE study. Br J Obstet Gynaecol. 2018;125(12):1581‐1589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Martin RM, Biswas PN, Freemantle SN, Pearce GL, Mann RD. Age and sex distribution of suspected adverse drug reactions to newly marketed drugs in general practice in England: analysis of 48 cohort studies. Br J Clin Pharmacol. 1998;46(5):505‐511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Drici MD, Clément N. Is gender a risk factor for adverse drug reactions? The example of drug‐induced long QT syndrome. Drug Saf. 2001;24(8):575‐585. [DOI] [PubMed] [Google Scholar]
- 50. Alharbi FF, Kholod AAV, Souverein PC, et al. The impact of age and sex on the reporting of cough and angioedema with renin‐angiotensin system inhibitors: a case/noncase study in VigiBase. Fundam Clin Pharmacol. 2017;31(6):676‐684. [DOI] [PubMed] [Google Scholar]
- 51. Wilson JG. Current status of teratology In: Wilson JG, Fraser FC, eds. Handbook of teratology. New York: Plenum Press; 1977. [Google Scholar]
- 52. Lehmann H, Niggeschulze A. The teratologic effects of thalidomide in Himalayan rabbits. Toxicol Appl Pharmacol. 1971;l8(1):208‐219. [DOI] [PubMed] [Google Scholar]
- 53. Donovan KA, An J, Nowak RP, et al. Thalidomide promotes degradation of SALL4, a transcription factor implicated in Duane radial ray syndrome. Elife. 2018;7:e38430 10.7554/eLife.38430 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Decousus HA, Croze M, Levi FA, et al. Circadian changes in anticoagulant effect of heparin infused at a constant rate. Br Med J (Clin Res Ed). 1985;290(6465):341‐344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Fisher LE, Ludwig EA, Wald JA, Sloan RR, Middleton E, Jusko WJ. Pharmacokinetics and pharmacodynamics of methylprednisolone when administered at 8 am versus 4 pm. Clin Pharmacol Ther. 1992;51(6):677‐688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Ballesta A, Innominato PF, Dallmann R, Rand DA, Lévi FA. Systems Chronotherapeutics. Pharmacol Rev. 2017;69(2):161‐199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Asprodini EK, Tsiokou V, Begas E, et al. Alterations in xenobiotic‐metabolizing enzyme activities across menstrual cycle in healthy volunteers. J Pharmacol Exp Ther. 2019;368(2):262‐271. [DOI] [PubMed] [Google Scholar]
- 58. McCullagh SF. Allergenicity of piperazine: a study in environmental aetiology. Br J Ind Med. 1968;25(4):319‐325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Jönsson AK, Lövborg H, Lohr W, Ekman B, Rocklöv J. Increased risk of drug‐induced hyponatremia during high temperatures. Int J Environ Res Public Health. 2017;14(7):pii: E827 10.3390/ijerph14070827 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Sommet A, Durrieu G, Lapeyre‐Mestre M, Montastruc JL. Association of French PharmacoVigilance Centres. A comparative study of adverse drug reactions during two heat waves that occurred in France in 2003 and 2006. Pharmacoepidemiol Drug Saf. 2012;21(3):285‐288. [DOI] [PubMed] [Google Scholar]
- 61. Juhlin L, Michaëlsson G, Zetterström O. Urticaria and asthma induced by food‐and‐drug additives in patients with aspirin hypersensitivity. J Allergy Clin Immunol. 1972;50(2):92‐98. [DOI] [PubMed] [Google Scholar]
- 62. Haffner JF, Morland J, Setekleiv J, et al. Mental and psychomotor effects of diazepam and ethanol. Acta Pharmacol Toxicol (Copenh). 1973;32(3):161‐178. [DOI] [PubMed] [Google Scholar]
- 63. Tedeschi DH, Fellows EJ. Monoamine oxidase inhibitors: augmentation of pressor effects of peroral tyramine. Science. 1964;144(3623):1225‐1226. [DOI] [PubMed] [Google Scholar]
- 64. Neuvonen PJ. Interactions with the absorption of tetracyclines. Drugs. 1976;11(1):45‐54. [DOI] [PubMed] [Google Scholar]
- 65. Preston CL. (Ed). Stockleys Interactions Checker. [online]. London: Pharmaceutical Press; Available at http: //www.medicinescomplete.com. Accessed 2 January 2019. [Google Scholar]
- 66. Marino A, Capogrosso‐Sansone A, Tuccori M, et al. ANCESTRAL‐ED study group. Expected and actual adverse drug‐drug interactions in elderly patients accessing the emergency department: data from the ANCESTRAL‐ED study. Expert Opin Drug Saf. 2016;15(Suppl 2):45‐50. [DOI] [PubMed] [Google Scholar]
- 67. Goulenok C, Bernard B, Cadranel JF, et al. Flumazenil vs. placebo in hepatic encephalopathy in patients with cirrhosis: a meta‐analysis. Aliment Pharmacol Ther. 2002;16(3):361‐372. [DOI] [PubMed] [Google Scholar]
- 68. Monteiro JF, Hahn SR, Gonçalves J, Fresco P. Vancomycin therapeutic drug monitoring and population pharmacokinetic models in special patient subpopulations. Pharmacol Res Perspect. 2018;6(4):e00420 10.1002/prp2.420 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Stepan JG, London DA, Boyer MI, Calfee RP. Blood glucose levels in diabetic patients following corticosteroid injections into the hand and wrist. J Hand Surg Am. 2014;39(4):706‐712. [DOI] [PMC free article] [PubMed] [Google Scholar]


