Abstract
Aims
The comparative efficacy, safety and tolerability of budesonide‐MMX and oral mesalamine in active, mild‐to‐moderate ulcerative colitis (UC) are unclear. We conducted a network meta‐analysis to fill this evidence gap.
Methods
We searched PubMed, Scopus, Embase, the Cochrane Library, clinical trial registries, regulatory agencies' websites and international conference proceedings, up to July 2018, to identify randomized controlled trials of adult patients with active, mild‐to‐moderate UC, comparing budesonide‐MMX or mesalamine against placebo, or against each other, or different dosing strategies, for induction of remission. Two reviewers independently abstracted study data and outcomes, and assessed each trial's risk‐of‐bias.
Results
We identified and synthesized evidence from 15 eligible trials including 4083 participants. Budesonide‐MMX 9 mg/day and mesalamine >2.4 g/day had similar efficacy for induction of clinical and endoscopic remission (OR = 0.97; 0.59–1.60), both showing superiority over placebo (OR = 2.68; 1.75–4.10, and OR = 2.75; 1.94–3.90, respectively). Furthermore, mesalamine >2.4 g/day was more efficacious than mesalamine 1.6–2.4 g/day (odds ratio = 1.27; 1.03–1.56). Secondary analyses showed that mesalamine >2.4 g/day ranks at the top among comparator treatments regarding safety (serious adverse events; surface under the cumulative ranking area [SUCRA] 79.2%) and tolerability (treatment discontinuations or withdrawals from the study due to adverse events; SUCRA 96.7%). There was no evidence of inconsistency, while heterogeneity between studies and risk of publication bias were low.
Conclusion
Budesonide‐MMX and mesalamine >2.4 g/day had similar efficacy for induction of clinical and endoscopic remission in active, mild‐to‐moderate UC; however, mesalamine >2.4 g/day showed better tolerability. Further high‐quality research is warranted.
Keywords: budesonide‐MMX, mesalamine, ulcerative colitis
1. INTRODUCTION
Ulcerative colitis (UC) is a chronic, idiopathic, immune‐mediated inflammatory disease of the colon and rectum, usually occurring in young adults and resulting in disability.1 It is characterized by intermittent flares of active disease with diarrhoea, abdominal pain and rectal bleeding, alternating with periods of remission.2 Worldwide, the incidence of UC ranges from 0.15 to 57.9 cases per 100,000 persons per year, while its prevalence ranges from 2.42 to 505 per 100,000 population.3
Current clinical guidelines, issued by the European Crohn's and Colitis Organisation4 and the American College of Gastroenterology,5 recommend 5‐aminosalicylic acid (5‐ASA; mesalamine) as first‐line therapy for induction of remission in patients with active, mild‐to‐moderate disease. Systemic corticosteroids are prescribed when symptoms of active colitis do not respond to 5‐ASA. However, the side effects associated with short‐ and long‐term use of systemic steroids prompted the development of a new generation of less toxic corticosteroid drugs such as budesonide, which are characterized by high topical anti‐inflammatory activity and low systemic bioavailability.6 Budesonide‐MMX is an oral formulation of budesonide that uses a colonic release system to pass through the stomach intact and provide targeted drug delivery to the colon.7 Our recent work8 demonstrated that budesonide‐MMX has an advantage over oral systemic steroids for corticosteroid‐related adverse events (nonserious and not leading to drug withdrawal) and a possible slight advantage over standard budesonide.
However, the evidence on comparative efficacy, tolerability and harm of budesonide‐MMX and oral mesalamine for active, mild‐to‐moderate UC is limited. Therefore, we carried out a systematic review of randomized controlled trials (RCTs) evaluating budesonide‐MMX and mesalamine in active UC, and assessed their comparative efficacy, tolerability and harm by means of network meta‐analysis, which allows the assessment of multiple treatments simultaneously by synthesizing data from randomized controlled studies making different comparisons.9, 10 We aimed to generate evidence that can be used to inform clinical decisions.
2. METHODS
We followed the International Society for Pharmacoepidemiology and Outcomes Research (ISPOR) network meta‐analysis guidance,11, 12 and report our findings according to the Preferred Reporting Items for Systematic Reviews and Meta‐Analyses (PRISMA) extension statement for systematic reviews incorporating network meta‐analyses for healthcare interventions.13
2.1. Literature review
We searched the PubMed, Scopus and Embase electronic databases from inception through July 2018. Search algorithms included the terms: budesonide, mesalamine, mesalazine, aminosalicylate, aminosalicylic acid, 5‐ASA, 5ASA, 5‐aminosalicylate, or 5‐aminosalicylic acid, combined with ulcerative colitis. The search was limited to clinical trials. There were no language restrictions.
We also searched the Cochrane Library, the World Health Organization International Clinical Trials Registry Platform, the ClinicalTrials.gov website, and conference proceedings (European Crohn's and Colitis Organisation, United European Gastroenterology Week, and Digestive Disease Week) to ensure identification of all eligible studies.
Two authors (S.B. and M.G.L.) independently screened titles and abstracts, the full texts of the selected articles were examined for eligibility, and reference lists were searched to identify other eligible trials. Finally, we conducted supplemental searches of regulatory agencies' websites (www.ema.europa.eu, www.fda.gov and www.tga.gov.au) to identify drug assessment reports including data of completed but unpublished studies.
2.2. Eligibility criteria
We included parallel‐group RCTs in adults (i.e. the majority of subjects age >18 years) with active mild‐to‐moderate UC that compared budesonide‐MMX or mesalamine against placebo, or against each other, or different dosing strategies, for induction of remission. The duration of induction therapy had to be at least 6 weeks.
To minimize conceptual heterogeneity among the trials (e.g. important differences in study designs, study populations, definitions and measurements of outcomes, previous therapies, or other features), we included in the network only studies published after year 2000. Studies were also excluded if they were observational; had assessed rectal formulations; did not report (or provided insufficient data for) the outcomes of interest; or had enrolled paediatric populations.
2.3. Data extraction and types of outcomes
Two authors (S.B. and M.G.L.) independently abstracted the following data from each study: first author, journal and year of publication, study design and duration, number of randomized participants, population and disease characteristics, outcome definitions, interventions (drug, dosage and schedule), and number of patients with events in intervention and control groups.
For budesonide‐MMX, we considered only the licensed dose for induction of remission (9 mg/day). However, a variety of doses of mesalamine‐based 5‐ASA agents are used in clinical practice. As it is possible that efficacy, tolerability and safety depend on the dose used, different doses could not be ignored in the analysis by representing mesalamine with a single node in the network geometry irrespective of the dose. By contrast, we tried to avoid extreme splitting (i.e. multiple, different nodes for each dose). Therefore, we categorized oral mesalamine use as: 1.6–2.4 g/day and >2.4 g/day. The threshold choice was arbitrary. Different mesalamine formulations of the same dose were considered equivalent on the basis of evidence showing that different mesalamine preparations have similar efficacy and safety.14
We assessed the following outcomes: (i) induction of clinical and endoscopic remission at the last time of assessment in the trial—combined clinical and endoscopic evidence was considered essential; (ii) serious adverse events (SAEs), defined as any untoward medical occurrence that results in death, requires hospital admission or prolongation of existing hospital stay, causes persistent or significant disability/incapacity, or is life threatening15; and (iii) treatment discontinuations or withdrawals from the study due to adverse events (WDAEs).
The quality of individual studies was independently assessed by 2 authors (S.B. and M.G.L.) using the Cochrane risk‐of‐bias (RoB) tool.16, 17 Discrepancies were resolved by consensus.
2.4. Data synthesis and statistical analysis
The odds ratio (OR) was used to measure treatment effects in all comparisons. Study‐level ORs with 95% confidence intervals were calculated according to the intention‐to‐treat principle. Network meta‐analysis was conducted, with a frequentist approach, in Stata software (Stata Corp., College Station, Texas) using the network suite18 and other network‐related commands.19, 20 Multivariate random‐effects meta‐analyses modelled the intervention effects across trials using consistency and inconsistency models.18, 21, 22, 23, 24 The contribution of direct evidence to the mixed estimates was also estimated and plotted.19, 25 Probabilities of each drug being at a specific order, mean ranks of drugs, and surface under the cumulative ranking area (SUCRA) values,18, 19, 21, 26 were estimated with bootstrap resampling (10,000 times). The higher the SUCRA value, the more effective or safe the treatment.
Heterogeneity (within each comparison) was estimated through the restricted maximum likelihood approach, and was assumed to be constant across treatment contrasts (common τ2).18, 22 Predictive intervals, that reflect the level of additional uncertainty anticipated in future studies, were estimated and plotted.19, 20 The τ2, Cochran's Q test and I2 statistics for all direct comparisons were computed. The magnitude of τ2 estimated in every direct synthesis of evidence was compared to quantiles of empirical distributions provided by Turner et al.27
For the inconsistency models, the design‐by‐treatment interaction approach was employed.22, 23, 28 Inconsistency terms were modelled as fixed parameters. Global Wald tests for inconsistency were performed.22, 23 Inconsistency was also explored by node‐splitting using the symmetrical option23, 29 and calculating inconsistency factors between direct and indirect evidence in all closed loops (triangular and quadratic) in the networks.19, 25, 30
Small‐study effects or publication bias were examined with funnel graphs appropriately adjusted for inclusion of studies that compare different pairs of treatments.19, 20, 31
2.5. Nomenclature of targets and ligands
Key protein targets and ligands in this article are hyperlinked to corresponding entries in http://www.guidetopharmacology.org, the common portal for data from the IUPHAR/BPS Guide to PHARMACOLOGY.32
3. RESULTS
3.1. Search results
After duplicates' removal, the database search yielded 1672 literature citations (Figure 1; flow chart). We screened titles and abstracts, and retrieved 72 publications for detailed evaluation. Their full text was carefully read and bibliographies were checked. We initially identified 13 RCTs33, 34, 35, 36, 37, 38, 39, 40, 41, 42, 43, 44, 45 eligible for inclusion in the Network. Two additional eligible studies were identified in ClinicalTrials.gov 46 and regulatory authorities' drug assessment reports,47 for a total of 15 trials (Table 1).
Figure 1.
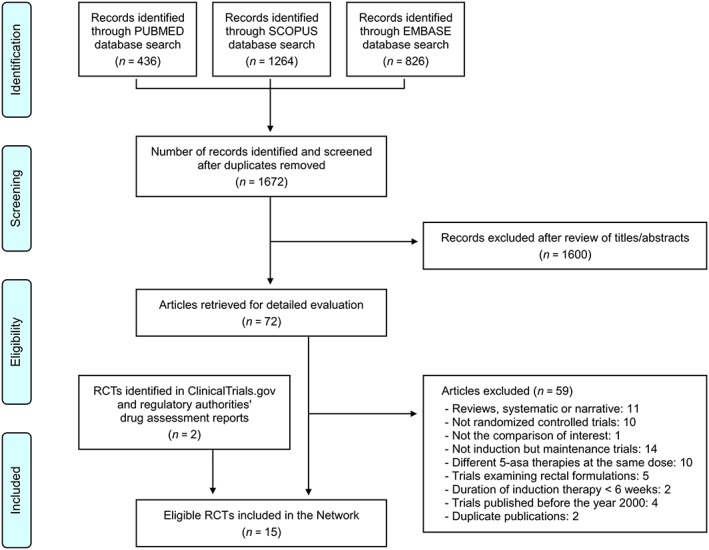
Flow diagram. RCTs, randomized controlled trials
Table 1.
Characteristics of the randomized controlled trials included in the network
| Studyref | Countries and centres | Disease distribution | Study groups and patients randomized | Definition of clinical and endoscopic remission | Duration |
|---|---|---|---|---|---|
| Sandborn et al., 201233 | USA, Canada, Mexico, India: 108 centres | Pancolitis, 40%; left‐sided colitis, 29%; proctosigmoiditis, 29% | Budesonide‐MMX 9 mg/day (n = 127); Mesalamine 2.4 g/day (n = 127); placebo (n = 128) | UCDAI score ≤1 with subscores of 0 for rectal bleeding, stool frequency and mucosal appearance, and a ≥1‐point reduction from baseline in the endoscopic index score | 8 weeks |
| Travis et al., 201434 | Europe, Russia, Israel, Australia: 69 centres | Pancolitis, 21%; left‐sided colitis, 33%; proctosigmoiditis, 45% | Budesonide‐MMX 9 mg/day (n = 127); placebo (n = 128) | UCDAI score ≤1 with subscores of 0 for rectal bleeding, stool frequency and mucosal appearance, and a ≥1‐point reduction from baseline in the endoscopic index score | 8 weeks |
| CB‐01‐02/0547 | Romania: 10 centres | Not reported | Budesonide‐MMX 9 mg/day (n = 15); placebo (n = 17) | UCDAI score ≤1 with subscores of 0 for rectal bleeding and stool frequency, and a ≥1‐point reduction from baseline in the endoscopic index score | 8 weeks |
| Hanauer et al., 200735 | USA, Canada: 41 centres | Pancolitis, 24%; left‐sided colitis, 30%; proctosigmoiditis, 46% | Mesalamine 2.4 g/day (n = 154); Mesalamine 4.8 g/day (n = 147) | Normal stool frequency, no rectal bleeding, a PFA score of 0, normal endoscopy findings, and a PGA score of 0 | 6 weeks |
| Hanauer et al., 200536 | USA, Canada: 55 centres | Pancolitis, 20%; left‐sided colitis, 34%; proctosigmoiditis, 46% | Mesalamine 2.4 g/day (n = 139); Mesalamine 4.8 g/day (n = 129) | Normal stool frequency, no rectal bleeding, a PFA score of 0, normal endoscopy findings, and a PGA score of 0 | 6 weeks |
| Sandborn et al., 200937 | Multinational: 113 centres | Pancolitis, 16%; left‐sided colitis, 36%; proctosigmoiditis, 48% | Mesalamine 2.4 g/day (n = 383); Mesalamine 4.8 g/day (n = 389) | UCDAI score ≤2 with no individual subscore >1 | 6 weeks |
| Feagan et al., 201338 | Belarus, India, Turkey, Ukraine: 26 centres | Pancolitis, 13%; left‐sided colitis, 33%; proctosigmoiditis, 49% | Mesalamine 4.8 g/day (n = 140); placebo (n = 141) | UCDAI score ≤2 with no individual subscore >1 | 6 weeks |
| NCT0103602246 | Europe, Canada: 26 centres | Not reported | Mesalamine 2.4 g/day (n = 16); placebo (n = 15) | Outcome data for clinical and endoscopic remission not reported | 6 weeks |
| D'Haens et al., 200639 | UK, Netherlands, Belgium: 8 centres | Pancolitis, 21%; left‐sided colitis, 74% | Mesalamine‐MMX 2.4 g/day (n = 14); Mesalamine‐MMX 4.8 g/day (n = 11) | UCDAI score ≤1 with subscores of 0 for rectal bleeding and stool frequency, and a ≥1‐point reduction from baseline in the endoscopic index score | 8 weeks |
| Kamm et al., 200740 | Multinational: 49 centres | Pancolitis, 24%; distal colitis, 76% | Mesalamine 2.4 g/day (n = 86); Mesalamine‐MMX 2.4 g/day (n = 86); Mesalamine‐MMX 4.8 g/day (n = 85); placebo (n = 86) | UCDAI score ≤1 with subscores of 0 for rectal bleeding and stool frequency, and a ≥1‐point reduction from baseline in the endoscopic index score | 8 weeks |
| Lichtenstein et al., 200741 | Multinational: 52 centres | Pancolitis, 19%; distal colitis, 81% | Mesalamine‐MMX 2.4 g/day (n = 93); Mesalamine‐MMX 4.8 g/day (n = 94); placebo (n = 93) | UCDAI score ≤1 with subscores of 0 for rectal bleeding and stool frequency, and a ≥1‐point reduction from baseline in the endoscopic index score | 8 weeks |
| Ito et al., 201042 | Japan: 53 centres | Not reported | Mesalamine 2.25 g/day (n = 65); Mesalamine 2.4 g/day (n = 66); Mesalamine 3.6 g/day (n = 65); placebo (n = 33) | UCDAI score ≤2 and a rectal bleeding score of 0 | 8 weeks |
| Hiwatashi et al.43 | Japan: 39 centres | Not reported | Mesalamine 2.25 g/day (n = 63); Mesalamine 4.0 g/day (n = 60) | UCDAI score ≤1 | 8 weeks |
| Ogata et al., 201844 | Japan: 56 centres | Pancolitis, 16%; left‐sided colitis, 46%; proctosigmoiditis, 38% | Mesalamine 2.25 g/day (n = 85); Mesalamine‐MMX 2.4 g/day (n = 85); Mesalamine‐MMX 4.8 g/day (n = 81) | UCDAI score ≤2 and a rectal bleeding score of 0 | 8 weeks |
| Rubin et al., 201745 | USA, Canada, Europe: Multicentre | Pancolitis, 22%; left‐sided colitis, 39%; proctosigmoiditis, 39% | Budesonide‐MMX 9 mg/day (n = 255); placebo (n = 255) *patients continued baseline treatment with oral Pentasa reported at study entry | UCDAI score ≤1 with subscores of 0 for rectal bleeding, stool frequency and mucosal appearance | 8 weeks |
PFA, patient's functional assessment; PGA, physician's global assessment; UCDAI, ulcerative colitis disease activity index,
Four studies compared budesonide‐MMX 9 mg/day to placebo33, 34, 45, 47; 5 studies compared mesalamine 1.6–2.4 g/day to placebo33, 40, 41, 42, 46; 4 studies compared mesalamine >2.4 g/day to placebo38, 40, 41, 42; 9 studies compared mesalamine >2.4 g/day to 1.6–2.4 g/day35, 36, 37, 39, 40, 41, 42, 43, 44; and 1 compared budesonide‐MMX 9 mg/day to mesalamine 1.6–2.4 g/day33 (Figure 2).
Figure 2.
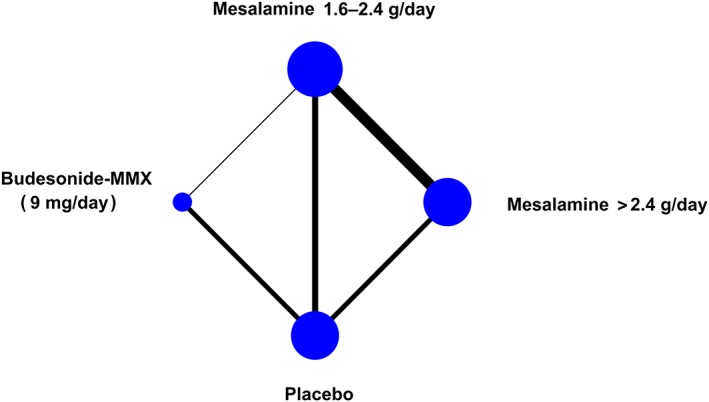
Network geometry. The size of the nodes is proportional to the number of studies evaluating each treatment, while the thickness of the connections is proportional to the number of studies evaluating each direct comparison
In these studies, a total of 4083 patients with active, mild‐to‐moderate UC, were randomized to receive budesonide‐MMX 9 mg/day (n = 524), mesalamine >2.4 g/day (n = 1201), mesalamine 1.6–2.4 g/day (n = 1462) or placebo (n = 896). The mean age of participants ranged from 40 to 46 years, and treatment duration from 6 to 8 weeks. Overall, 790 patients (19.3%) achieved clinical and endoscopic remission, 271 patients (6.6%) discontinued treatment or withdrew from the study due to AEs, while 77 (1.9%) experienced 1 or more SAE. The publication dates of these studies ranged between 2005 and 2018. A summary of the trial characteristics is given in Table 1.
3.2. RoB in included studies
3.2.1. Random sequence generation
Ten trials (67%) reported adequate methods (low RoB). In 5 trials (33%) information was insufficient to permit judgement (unclear RoB).
3.2.2. Allocation concealment
Nine trials (60%) reported adequate methods (low RoB), while in 6 (40%) information was insufficient (unclear risk).
3.2.3. Blinding
All studies were double‐blind.
3.2.4. Incomplete outcome data
Eleven trials (73%) were judged as low‐risk, while risk was unclear in 4 (27%).
3.2.5. Selective outcome reporting
All trials were at low RoB.
3.2.6. Other sources of bias
Two trials (13%) identified in ClinicalTrials.gov and regulatory agencies' websites did not provide information to assess whether an important problem exists (unclear risk).
Overall, our assessment indicated low RoB in 7 studies,33, 34, 37, 38, 42, 43, 45 while the risk was unclear for the remaining 8 studies.35, 36, 39, 40, 41, 44, 46, 47 Quality assessment items (per trial) are presented in Figure 3.
Figure 3.
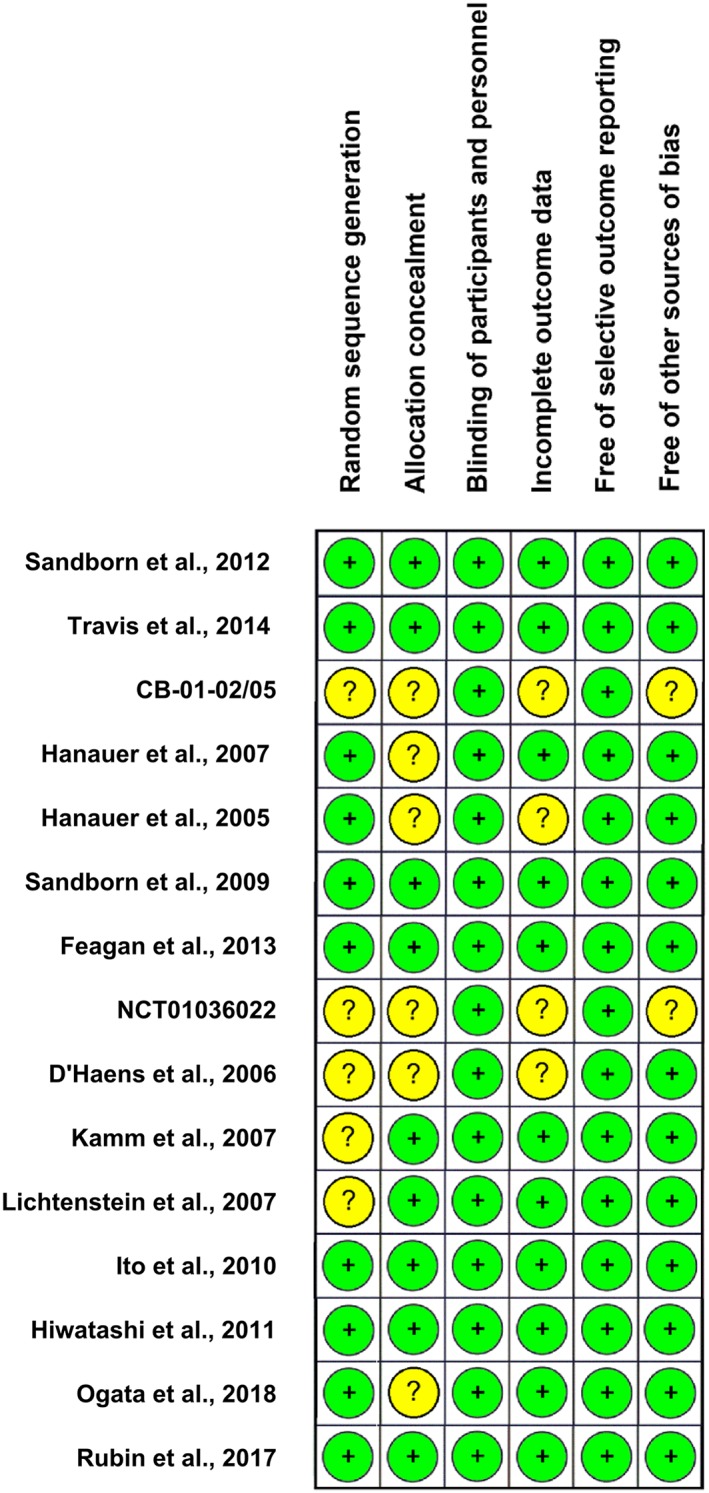
Risk of bias assessment for the studies included in the network. Green (+), low risk of bias; yellow (?), unclear risk of bias
3.3. Results of network meta‐analyses
3.3.1. Induction of clinical and endoscopic remission
Budesonide‐MMX (OR = 2.68; 95% confidence interval: 1.75–4.10), mesalamine >2.4 g/day (OR = 2.75; 1.94–3.90) and mesalamine 1.6–2.4 g/day (OR = 2.17; 1.55–3.05) showed higher efficacy than placebo (Table 2A). Mesalamine >2.4 g/day was also superior to mesalamine 1.6–2.4 g/day (OR = 1.27; 1.03–1.56). None of the comparisons of budesonide‐MMX vs mesalamine >2.4 g/day and mesalamine 1.6–2.4 g/day was statistically significant (Table 2A). In similar, the SUCRA values providing the hierarchy of treatments regarding efficacy, the estimated probabilities of each treatment being the best, as well as the comparative treatment ranks, demonstrated mesalamine >2.4 g/day and budesonide‐MMX ranking at the top and performing almost equally well (Table 3A).
Table 2.
Comparative assessment of budesonide‐MMX and mesalamine in active, mild‐to‐moderate UC
| A. Induction of clinical and endoscopic remission | |||
|---|---|---|---|
| Budesonide‐MMX | |||
| 0.97 (0.59–1.60) | Mesalamine >2.4 g/day | ||
| 1.23 (0.76–2.01) | 1.27 (1.03–1.56) | Mesalamine 1.6–2.4 g/day | |
| 2.68 (1.75–4.10) | 2.75 (1.94–3.90) | 2.17 (1.55–3.05) | Placebo |
| B. Serious adverse events | |||
|---|---|---|---|
| Budesonide‐MMX | |||
| 1.85 (0.59–5.79) | Mesalamine >2.4 g/day | ||
| 1.44 (0.52–3.97) | 0.78 (0.40–1.51) | Mesalamine 1.6–2.4 g/day | |
| 1.35 (0.60–3.04) | 0.73 (0.29–1.82) | 0.94 (0.43–2.04) | Placebo |
| C. Treatment discontinuations or withdrawals from the study due to adverse events | |||
|---|---|---|---|
| Budesonide‐MMX | |||
| 2.22 (1.23–4.02) | Mesalamine >2.4 g/day | ||
| 1.71 (0.98–2.96) | 0.77 (0.52–1.14) | Mesalamine 1.6–2.4 g/day | |
| 0.92 (0.61–1.38) | 0.41 (0.26–0.66) | 0.54 (0.34–0.84) | Placebo |
The column‐defining treatment is compared with the row‐defining treatment. The estimates in the cells are odds ratios (ORs) with 95% confidence intervals. For induction of clinical and endoscopic remission, ORs >1.0 favour the treatment in the left upper square. On the opposite, for safety outcomes (serious adverse events and withdrawals from the study due to adverse events), ORs <1.0 favour the treatment in the left upper square. Statistically significant results are shown in bold.
UC, ulcerative colitis.
Table 3.
Comparative assessment of budesonide‐MMX and mesalamine in active, mild‐to‐moderate ulcerative colitis
| A. Induction of clinical and endoscopic remission | |||
|---|---|---|---|
| SUCRA value (%) | Probability best (%) | Mean rank | |
| Budesonide‐MMX | 75.3 | 45.5 | 1.7 |
| Mesalamine >2.4 g/day | 84.4 | 54.1 | 1.5 |
| Mesalamine 1.6–2.4 g/day | 40.3 | 0.4 | 2.8 |
| Placebo | 0.0 | 0.0 | 4.0 |
| B. Serious adverse events | |||
|---|---|---|---|
| SUCRA value (%) | Probability best (%) | Mean rank | |
| Budesonide‐MMX | 20.5 | 7.6 | 3.4 |
| Mesalamine >2.4 g/day | 79.2 | 61.8 | 1.6 |
| Mesalamine 1.6–2.4 g/day | 51.9 | 13.4 | 2.4 |
| Placebo | 48.4 | 17.2 | 2.5 |
| C. Treatment discontinuations or withdrawals from the study due to adverse events | |||
|---|---|---|---|
| SUCRA value (%) | Probability best (%) | Mean rank | |
| Budesonide‐MMX | 23.0 | 0.3 | 3.3 |
| Mesalamine >2.4 g/day | 96.7 | 90.3 | 1.1 |
| Mesalamine 1.6–2.4 g/day | 68.9 | 9.4 | 1.9 |
| Placebo | 11.5 | 0.0 | 3.7 |
Herein we present: (i) the SUCRA values providing the hierarchy of the competing treatments, (ii) the estimated probabilities of each treatment being the best, and (iii) the mean rank of each treatment using 10,000 draws.
SUCRA, surface under the cumulative ranking area
3.3.2. SAEs
SAE occurrence was not shown to be statistically significantly different between budesonide‐MMX, mesalamine >2.4 g/day, mesalamine 1.6–2.4 g/day, and placebo (Table 2B). On the other hand, the SUCRA values, the mean ranks, and the estimated probabilities of each treatment being the best, demonstrated a trend favouring mesalamine >2.4 g/day (Table 3B).
3.3.3. Treatment discontinuations or WDAEs
The occurrence of WDAEs was statistically significantly lower among patients receiving mesalamine >2.4 g/day (as compared to budesonide‐MMX and placebo). It was also lower in patients receiving mesalamine 1.6–2.4 g/day as compared to placebo. All other comparisons did not reach significance (Table 2C). In agreement, the SUCRA values, the mean ranks, and the estimated probabilities of each treatment being the best, demonstrated mesalamine >2.4 g/day ranking at the top among comparator treatments regarding tolerability (Table 3C).
3.3.4. Assessment of publication bias, homogeneity and consistency of the models
The inspection of funnel plots appropriately adjusted for inclusion of studies comparing different treatments against placebo (Figure 4) suggested a low probability of publication bias for all models.
Figure 4.
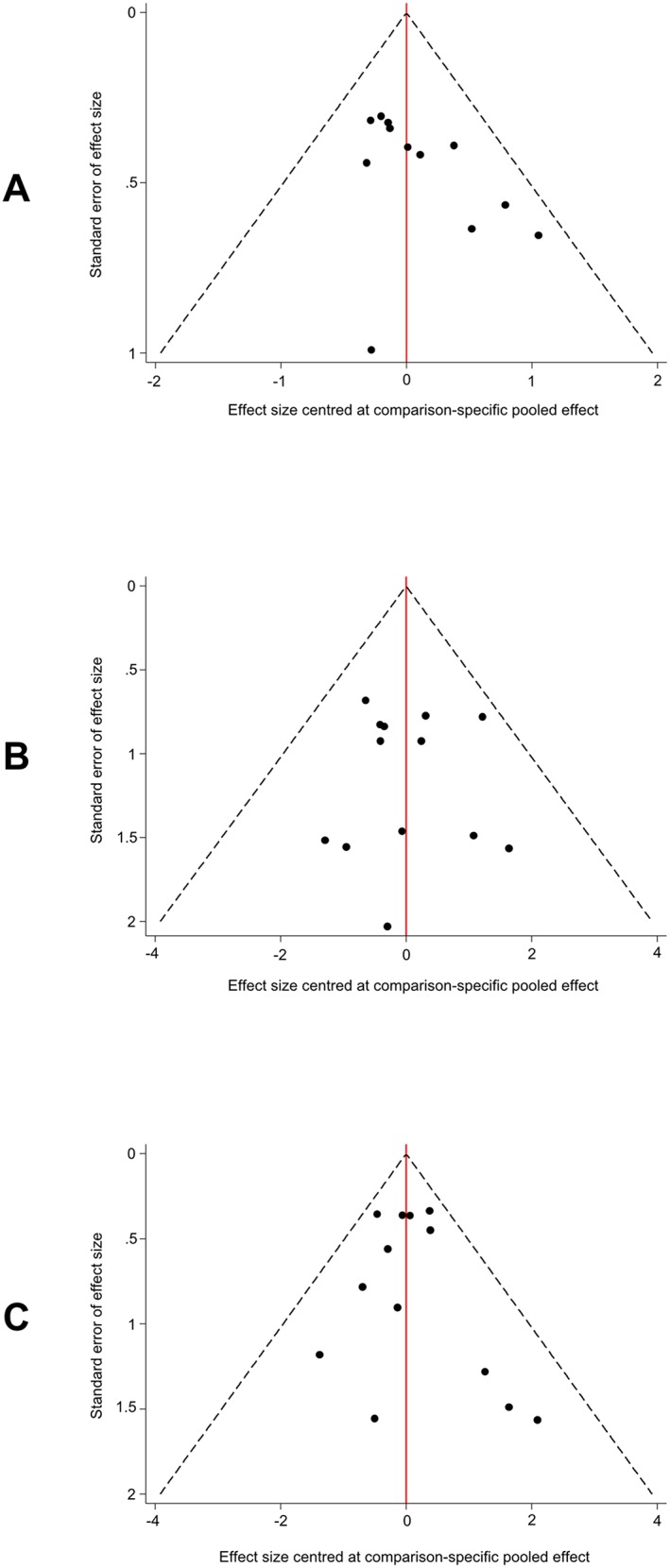
Funnel plots adjusted for inclusion of trials comparing different treatments against placebo. (A) induction of clinical and endoscopic remission. (B) serious adverse events. (C) treatment discontinuations or withdrawals from the study due to adverse events
The conventional statistics (Cochran Q, I2, τ2) calculated for all direct comparisons, and the estimated and plotted predictive intervals reflecting the extent of heterogeneity in network meta‐analytic estimates, indicated very low heterogeneity for all the outcomes (data not shown). This was confirmed by the overall network heterogeneity statistics (i.e. restricted likelihood ratio tests; Table 4).
Table 4.
Networks' assessment for homogeneity and consistency
| Outcome | Heterogeneity (restricted likelihood ratio test) | Inconsistency (global Wald test) |
|---|---|---|
| Induction of clinical and endoscopic remission | P = .22 | P = .91 |
| Serious adverse events | P = .99 | P = .80 |
| Treatment discontinuations or withdrawals from the study due to adverse events | P = .99 | P = .37 |
Finally, there was no evidence of substantial inconsistency when explored either by node splitting, or by calculating the difference between direct and indirect evidence in all closed loops in the networks (data not shown). The global Wald tests for inconsistency were not significant (Table 4).
Nevertheless, given the moderate number of studies included in the analyses, relevant inconsistency or heterogeneity between trials cannot be ruled out.
3.3.5. Additional analysis
In 1 of the included studies,45 patients were randomized to budesonide‐MMX 9 mg or placebo, and continued baseline treatment with oral mesalamine reported at study entry. As a sensitivity analysis, we repeated the network meta‐analysis omitting this study. The results did not materially change (Appendix Tables S1, S2, S3), reinforcing our confidence in the validity of our analyses.
4. DISCUSSION
This systematic review and network meta‐analysis synthesized efficacy, safety and tolerability data from 15 controlled trials (4083 participants) comparing budesonide‐MMX or mesalamine against placebo, or against each other, for induction of remission in adults with active, mild‐to‐moderate UC. It demonstrated that budesonide‐MMX 9 mg/day and mesalamine >2.4 g/day have similar efficacy for induction of clinical and endoscopic remission, both showing superiority over placebo. Furthermore, mesalamine >2.4 g/day was more efficacious than mesalamine 1.6–2.4 g/day. Additional analyses showed that mesalamine >2.4 g/day ranks at the top among comparator treatments regarding safety (i.e. SAEs) and tolerability (i.e. WDAEs).
Randomized evidence comparing budesonide‐MMX vs mesalamine for active mild‐to‐moderate UC was limited and insufficient,33 while previously published meta‐analyses48, 49, 50 have compared oral mesalamine vs placebo, or budesonide‐MMX vs placebo, in a conventional pairwise manner. Our network meta‐analysis employed a broad base of research data, and combined direct evidence (from head‐to‐head trials) and indirect evidence (comparisons of different drugs against a common comparator) to inform clinical decision making.
Our work has strengths: an exhaustive search of multiple databases and grey literature sources was conducted to identify all eligible studies; the search, eligibility assessment and data extraction were undertaken independently by 2 authors; all studies were analysed on an intention‐to‐treat basis, and potential confounding factors—such as age and disease duration—were equally balanced between the groups as patients were randomly allocated; and appropriate frequentist meta‐analysis' methodology was used to synthesize the available data. Finally, there was no evidence of substantial inconsistency, while the heterogeneity between studies and the probability of publication bias were low in all models. Nevertheless, there are limitations: several studies35, 36, 39, 40, 41, 44, 46, 47 had unclear risk of bias; comparator treatments were not evaluated in terms of cost, which is very important in clinical decision‐making; and, finally, the additional limitations of network meta‐analysis should be discussed—in a network meta‐analysis of RCTs, the value of randomization does not hold across studies. Indirect evidence from a network meta‐analysis is considered as of observational nature: results and conclusions may be undermined if substantial clinical or methodological heterogeneity is found.51 Therefore, further high‐quality research (head‐to‐head trials, real‐life studies, and pharmacoeconomic analyses) is needed to verify and extend the current evidence.
5. CONCLUSION
This work confirmed that budesonide‐MMX 9 mg/day and mesalamine >2.4 g/day have similar efficacy for induction of clinical and endoscopic remission in active, mild‐to‐moderate UC; however, mesalamine >2.4 g/day has shown evidence of better tolerability. This information, together with the fact that budesonide‐MMX—differently from mesalamine—is not a maintenance therapy, should help patients and physicians to make clinical decisions regarding the management of active, mild‐to‐moderate UC, that align with their values, preferences, and tolerance of risks and benefits.
COMPETING INTERESTS
S.B. is supported by FIRMAD, and has served as advisor for Ferring. L.P.B. has received consulting fees from Abbvie, Amgen, Biogaran, Biogen, Boerhinger‐Ingelheim, Bristol‐Myers Squibb, Celgene, Celltrion, Ferring, Forward Pharma, Genentech, H.A.C. Pharma, Hospira, Index Pharmaceuticals, Janssen, Lycera, Merck, Lilly, Mitsubishi, Norgine, Pfizer, Pharmacosmos, Pilège, Samsung Bioepis, Sandoz, Takeda, Therakos, Tillots, UCB Pharma and Vifor, and lecture fees from Abbvie, Ferring, H.A.C. Pharma, Janssen, Merck, Mitsubishi, Norgine, Takeda, Therakos, Tillots, Vifor. S.D. has served as a speaker, a consultant and an advisory board member for Abbvie, Allergan, Biogen, Boehringer‐Ingelheim, Celgene, Celltrion, Ferring, Hospira, Johnson & Johnson, Merck, MSD, Takeda, Mundipharma, Pfizer, Sandoz, Tigenix, UCB Pharma, Vifor. All other authors have no competing interests to declare.
CONTRIBUTORS
All authors contributed to the conception and design of the study. S.B., D.P. and M.G.L. contributed to the literature search and data collection. S.B., G.N. and T.L. contributed to the statistical analysis. All authors interpreted the data. S.B. drafted the manuscript. All authors critically revised the manuscript for important intellectual content, approved the final version to be published, and agreed to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Supporting information
Table S1. Comparative assessment of budesonide‐MMX and mesalamine in active, mild‐to‐moderate ulcerative colitis (sensitivity analysis)
Table S2. Comparative assessment of budesonide‐MMX and mesalamine in active, mild‐to‐moderate ulcerative colitis (sensitivity analysis)
Table S3. Networks' assessment for homogeneity and consistency (sensitivity analysis)
Bonovas S, Nikolopoulos GK, Piovani D, et al. Comparative assessment of budesonide‐MMX and mesalamine in active, mild‐to‐moderate ulcerative colitis: A systematic review and network meta‐analysis. Br J Clin Pharmacol. 2019; 85: 2244–2254. 10.1111/bcp.14051
Guarantor of the article: Dr Stefanos Bonovas.
REFERENCES
- 1. Ungaro R, Mehandru S, Allen PB, Peyrin‐Biroulet L, Colombel JF. Ulcerative colitis. Lancet. 2017;389(10080):1756‐1770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Danese S, Fiocchi C. Ulcerative colitis. N Engl J Med. 2011;365(18):1713‐1725. [DOI] [PubMed] [Google Scholar]
- 3. Ng SC, Shi HY, Hamidi N, et al. Worldwide incidence and prevalence of inflammatory bowel disease in the 21st century: a systematic review of population‐based studies. Lancet. 2018;390:2769‐2778. [DOI] [PubMed] [Google Scholar]
- 4. Harbord M, Eliakim R, Bettenworth D, et al. Third European evidence‐based consensus on diagnosis and management of ulcerative colitis. Part 2: current management. J Crohns Colitis. 2017;11(7):769‐784. [DOI] [PubMed] [Google Scholar]
- 5. Kornbluth A, Sachar DB. Ulcerative colitis practice guidelines in adults: American College of Gastroenterology, practice parameters committee. Am J Gastroenterol. 2010;105(3):501‐523. [DOI] [PubMed] [Google Scholar]
- 6. Nunes T, Barreiro‐de Acosta M, Marin‐Jiménez I, Nos P, Sans M. Oral locally active steroids in inflammatory bowel disease. J Crohns Colitis. 2013;7(3):183‐191. [DOI] [PubMed] [Google Scholar]
- 7. Hoy SM. Budesonide MMX(®): a review of its use in patients with mild to moderate ulcerative colitis. Drugs. 2015;75(8):879‐886. [DOI] [PubMed] [Google Scholar]
- 8. Bonovas S, Nikolopoulos GK, Lytras T, Fiorino G, Peyrin‐Biroulet L, Danese S. Comparative safety of systemic and low‐bioavailability steroids in inflammatory bowel disease: systematic review and network meta‐analysis. Br J Clin Pharmacol. 2018;84(2):239‐251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Mills EJ, Thorlund K, Ioannidis JP. Demystifying trial networks and network meta‐analysis. BMJ. 2013;346(may14 2):f2914. [DOI] [PubMed] [Google Scholar]
- 10. Caldwell DM, Ades AE, Higgins JP. Simultaneous comparison of multiple treatments: combining direct and indirect evidence. BMJ. 2005;331(7521):897‐900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Jansen JP, Fleurence R, Devine B, et al. Interpreting indirect treatment comparisons and network meta‐analysis for health‐care decision making: report of the ISPOR task force on indirect treatment comparisons good research practices: part 1. Value Health. 2011;14(4):417‐428. [DOI] [PubMed] [Google Scholar]
- 12. Hoaglin DC, Hawkins N, Jansen JP, et al. Conducting indirect‐treatment‐comparison and network‐meta‐analysis studies: report of the ISPOR task force on indirect treatment comparisons good research practices: part 2. Value Health. 2011;14(4):429‐437. [DOI] [PubMed] [Google Scholar]
- 13. Hutton B, Salanti G, Caldwell DM, et al. The PRISMA extension statement for reporting of systematic reviews incorporating network meta‐analyses of health care interventions: checklist and explanations. Ann Intern Med. 2015;162(11):777‐784. [DOI] [PubMed] [Google Scholar]
- 14. Feagan BG, Chande N, MacDonald JK. Are there any differences in the efficacy and safety of different formulations of oral 5‐ASA used for induction and maintenance of remission in ulcerative colitis? Evidence from Cochrane reviews. Inflamm Bowel Dis. 2013;19:2031‐2040. [DOI] [PubMed] [Google Scholar]
- 15. Edwards IR, Aronson JK. Adverse drug reactions: definitions, diagnosis, and management. Lancet. 2000;356(9237):1255‐1259. [DOI] [PubMed] [Google Scholar]
- 16. Higgins JP, Altman DG, Gøtzsche PC, et al. The Cochrane Collaboration's tool for assessing risk of bias in randomised trials. BMJ. 2011;343(oct18 2):d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Bonovas S, Lytras T, Nikolopoulos G. On the criteria used for assessing the risk of bias in randomized trials included in systematic reviews and meta‐analyses addressing adverse effects. Eur J Epidemiol. 2015;30(3):249‐250. [DOI] [PubMed] [Google Scholar]
- 18. White IR. Network meta‐analysis. Stata J. 2015;15(4):951‐985. [Google Scholar]
- 19. Chaimani A, Salanti G. Visualizing assumptions and results in network meta‐analysis: the network graphs package. Stata J. 2015;15(4):905‐950. [Google Scholar]
- 20. Chaimani A, Higgins JPT, Mavridis D, Spyridonos P, Salanti G. Graphical tools for network meta‐analysis in STATA. PLoS One. 2013;8(10):e76654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. White IR. Multivariate random‐effects meta‐regression: updates to mvmeta. Stata J. 2011;11(2):255‐270. [Google Scholar]
- 22. White IR, Barrett JK, Jackson D, Higgins JPT. Consistency and inconsistency in network meta‐analysis: model estimation using multivariate meta‐regression. Res Synth Methods. 2012;3(2):111‐125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. White IR. Software updates: st0156 2: multivariate random‐effects meta‐analysis. Stata J. 2015;15:1185‐1186. [Google Scholar]
- 24. White IR. Multivariate random‐effects meta‐analysis. Stata J. 2009;9(1):40‐56. [Google Scholar]
- 25. Salanti G, Del Giovane C, Chaimani A, Caldwell DM, Higgins JPT. Evaluating the quality of evidence from a network meta‐analysis. PLoS One. 2014;9(7):e99682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Salanti G, Ades AE, Ioannidis JP. Graphical methods and numerical summaries for presenting results from multiple‐treatment meta‐analysis: an overview and tutorial. J Clin Epidemiol. 2011;64(2):163‐171. [DOI] [PubMed] [Google Scholar]
- 27. Turner RM, Davey J, Clarke MJ, Thompson SG, Higgins JP. Predicting the extent of heterogeneity in meta‐analysis, using empirical data from the Cochrane database of systematic reviews. Int J Epidemiol. 2012;41(3):818‐827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Higgins JPT, Jackson D, Barrett JK, Lu G, Ades AE, White IR. Consistency and inconsistency in network meta‐analysis: concepts and models for multi‐arm studies. Res Synth Methods. 2012;3(2):98‐110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Dias S, Welton NJ, Caldwell DM, Ades AE. Checking consistency in mixed treatment comparison meta‐analysis. Stat Med. 2010;29(7‐8):932‐944. [DOI] [PubMed] [Google Scholar]
- 30. Bucher HC, Guyatt GH, Griffith LE, Walter SD. The results of direct and indirect treatment comparisons in meta‐analysis of randomized controlled trials. J Clin Epidemiol. 1997;50(6):683‐691. [DOI] [PubMed] [Google Scholar]
- 31. Chaimani A, Salanti G. Using network meta‐analysis to evaluate the existence of small‐study effects in a network of interventions. Res Synth Methods. 2012;3(2):161‐176. [DOI] [PubMed] [Google Scholar]
- 32. Harding SD, Sharman JL, Faccenda E, et al. The IUPHAR/BPS Guide to PHARMACOLOGY in 2018: updates and expansion to encompass the new guide to IMMUNOPHARMACOLOGY. Nucl Acids Res. 2018;46:D1091‐D1106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Sandborn WJ, Travis S, Moro L, et al. Once‐daily budesonide MMX® extended‐release tablets induce remission in patients with mild to moderate ulcerative colitis: results from the CORE I study. Gastroenterology. 2012;143(5):1218‐1226.e2 [DOI] [PubMed] [Google Scholar]
- 34. Travis SP, Danese S, Kupcinskas L, et al. Once‐daily budesonide MMX in active, mild‐to‐moderate ulcerative colitis: results from the randomised CORE II study. Gut. 2014;63(3):433‐441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Hanauer SB, Sandborn WJ, Dallaire C, et al. Delayed‐release oral mesalamine 4.8 g/day (800 mg tablets) compared to 2.4 g/day (400 mg tablets) for the treatment of mildly to moderately active ulcerative colitis: the ASCEND I trial. Can J Gastroenterol. 2007;21(12):827‐834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Hanauer SB, Sandborn WJ, Kornbluth A, et al. Delayed‐release oral mesalamine at 4.8 g/day (800 mg tablet) for the treatment of moderately active ulcerative colitis: the ASCEND II trial. Am J Gastroenterol. 2005;100(11):2478‐2485. [DOI] [PubMed] [Google Scholar]
- 37. Sandborn WJ, Regula J, Feagan BG, et al. Delayed‐release oral mesalamine 4.8 g/day (800‐mg tablet) is effective for patients with moderately active ulcerative colitis. Gastroenterology. 2009;137(6):1934‐1943.e1‐3. [DOI] [PubMed] [Google Scholar]
- 38. Feagan BG, Sandborn WJ, D'Haens G, et al. The role of centralized reading of endoscopy in a randomized controlled trial of mesalamine for ulcerative colitis. Gastroenterology. 2013;145(1):149‐157e2. [DOI] [PubMed] [Google Scholar]
- 39. D'Haens G, Hommes D, Engels L, et al. Once daily MMX mesalazine for the treatment of mild‐to‐moderate ulcerative colitis: a phase II, dose‐ranging study. Aliment Pharmacol Ther. 2006;24(7):1087‐1097. [DOI] [PubMed] [Google Scholar]
- 40. Kamm MA, Sandborn WJ, Gassull M, et al. Once‐daily, high‐concentration MMX mesalamine in active ulcerative colitis. Gastroenterology. 2007;132(1):66‐75. [DOI] [PubMed] [Google Scholar]
- 41. Lichtenstein GR, Kamm MA, Boddu P, et al. Effect of once‐ or twice‐daily MMX mesalamine (SPD476) for the induction of remission of mild to moderately active ulcerative colitis. Clin Gastroenterol Hepatol. 2007;5(1):95‐102. [DOI] [PubMed] [Google Scholar]
- 42. Ito H, Iida M, Matsumoto T, et al. Direct comparison of two different mesalamine formulations for the induction of remission in patients with ulcerative colitis: a double‐blind, randomized study. Inflamm Bowel Dis. 2010;16(9):1567‐1574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Hiwatashi N, Suzuki Y, Mitsuyama K, Munakata A, Hibi T. Clinical trial: effects of an oral preparation of mesalazine at 4 g/day on moderately active ulcerative colitis. A phase III parallel‐dosing study. J Gastroenterol. 2011;46(1):46‐56. [DOI] [PubMed] [Google Scholar]
- 44. Ogata H, Yokoyama T, Mizushima S, Hagino A, Hibi T. Comparison of efficacy of once daily multimatrix mesalazine 2.4 g/day and 4.8 g/day with other 5‐aminosalicylic acid preparation in active ulcerative colitis: a randomized, double‐blind study. Intest Res. 2018;16(2):255‐266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Rubin DT, Cohen RD, Sandborn WJ, et al. Budesonide multimatrix is efficacious for mesalamine‐refractory, mild to moderate ulcerative colitis: a randomised, placebo‐controlled trial. J Crohns Colitis. 2017;11(7):785‐791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. A double‐blind, double‐dummy, placebo‐ and active‐controlled dose escalation study to evaluate the safety, tolerability, pharmacokinetics and anti‐inflammatory effects of GSK1399686 in patients with mild to moderately active ulcerative colitis. https://clinicaltrials.gov/ct2/show/study/NCT01036022. Accessed July 27, 2019.
- 47. Australian Government, Department of Health, Therapeutic Goods Administration . AusPAR attachment 2 – extract from the clinical evaluation report for budesonide; November 2014. https://www.tga.gov.au/sites/default/files/auspar‐budesonide‐160111‐cer.pdf. Accessed July 27, 2019.
- 48. Wang Y, Parker CE, Bhanji T, Feagan BG, MacDonald JK. Oral 5‐aminosalicylic acid for induction of remission in ulcerative colitis. Cochrane Database Syst Rev. 2016;(4):CD000543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Sherlock ME, MacDonald JK, Griffiths AM, Steinhart AH, Seow CH. Oral budesonide for induction of remission in ulcerative colitis. Cochrane Database Syst Rev. 2015;(10):CD007698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Ford AC, Achkar JP, Khan KJ, et al. Efficacy of 5‐aminosalicylates in ulcerative colitis: systematic review and meta‐analysis. Am J Gastroenterol. 2011;106(4):601‐616. [DOI] [PubMed] [Google Scholar]
- 51. Bonovas S, Moja L, Danese S. In the presence of conceptual heterogeneity, results of network meta‐analysis comparing therapies in Crohn's disease need to be interpreted with caution. Gastroenterology. 2015;148(7):1483‐1484. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Comparative assessment of budesonide‐MMX and mesalamine in active, mild‐to‐moderate ulcerative colitis (sensitivity analysis)
Table S2. Comparative assessment of budesonide‐MMX and mesalamine in active, mild‐to‐moderate ulcerative colitis (sensitivity analysis)
Table S3. Networks' assessment for homogeneity and consistency (sensitivity analysis)


