Abstract
Background
Surgical site infections (SSI) are among the most common healthcare-associated infections. The aim of our explorative study was to determine how selected climatic factors are associated with SSI rates.
Methods
SSI rates were calculated for operative procedures included in the surgical site infection surveillance component (OP-KISS) of the German Nosocomial Infection Surveillance System (Krankenhaus-Infektions-Surveillance-System, KISS) during the years from 2000 to 2016. The surgeries were associated with department-related and patient-related data. Data of the German Meteorological Service (Deutscher Wetterdienst, DWD), including outdoor temperature and rainfall, were used to analyze the association between climatic factors and rates of SSI. Analyses focused on temperature which showed strong correlations with other climatic parameters. A descriptive analysis was performed, using the chi-squared test. Adjusted odds ratios (AORs) were calculated for SSI rates in relation to temperature, using a multivariable logistic regression model.
Results
For the altogether 2 004 793 included operative procedures, 32 118 SSIs were documented. Temperatures = 20 °C were associated with a significantly higher occurrence of SSI compared to temperatures <5 °C (AOR: 1.13; 95% confidence intervals [1.06; 1.20]). This increase was found for gram-positive pathogens (AOR: 1.13 [1.03; 1.23]) and, even more pronounced, for gram-negative pathogens (AOR: 1.20 [1.07; 1.35]). The association was strongest for superficial SSI caused by gram-negative pathogens (AOR: 1.38 [1.16; 1.64]).
Conclusion
An association between climatic factors and SSI rates was demonstrated. The predicted rise in global temperatures by up to 4 °C by the end of this century compared to preindustrial levels may increase the likelihood of SSI and should be taken into consideration in future preventive strategies.
Surgical site infections (SSI) are amongst the most common healthcare-associated infections (HAI) in German hospitals (1). This is in line with the results of comparable studies conducted in other countries. The European point prevalence survey on HAI and antimicrobial use in acute care hospitals, in which 28 EU countries and Serbia participated, found that SSI account for approximately 18% of all HAI (2).
Based on the extrapolations of previous point prevalence surveys on HAI, SSI episodes in the EU per year were estimated at about 800 000. Thus, based on the number of cases per year, SSI is one of the HAI with the highest burden on population health (3). With regard to how many of the HAI and SSI are preventable, only estimates are available which vary widely. In most studies, the portion of preventable cases is estimated to be between 30–50% (4– 8).
The prevention of SSI is based on the identification of factors contributing to the occurrence of wound complications which may lead to SSI. Besides known patient-related risk factors, such as male sex, obesity, diabetes mellitus, and old age, as well as surgery-related risk factors, such as implantation of foreign material, high degree of wound contamination, and hypothermia, “other” risk factors have attracted increasing attention in recent years (9). Here, various studies have shown the impact of season and outdoor temperatures on the incidence of SSI. Generally, higher temperatures/warmer weather are associated with an increased incidence of SSI (10– 13). This phenomenon has especially been reported for arthroplasties. Besides SSI, other HAI are also promoted by higher temperatures. For bloodstream infections, for example, this association has already been demonstrated in several studies (14– 16).
Given the increase in temperatures observed worldwide in recent decades and the projections of climate scientists for the future (17), this observation becomes even more relevant from the perspective of infection prevention. Without any significant changes in climate protection measures, the most likely scenario for the future is an increase in the average temperature in Germany by 4 °C by the end of this century (2071–2100 versus 1971–2000). This would go along with a threefold or even fourfold increase in days with maximum temperatures >30 °C (18).
The traditional seasonal fluctuations in the weather are largely determined by stable climatic conditions, for example by the composition of the atmosphere. If the natural greenhouse effect is augmented by additional emissions of, for example, carbon dioxide from the combustion of fossil fuels, the lower layers of the atmosphere will gradually increase in temperature. Today’s global average temperature is approximately 1.1 °C higher compared to preindustrial levels (19) and most of the warming has occurred in the last decades.
Consequently, the number of record temperature and record rainfall events has increased worldwide (20, 21). If this trend continues, today’s extreme weather episodes will occur considerably more frequently in the future or even become the norm. The climatic conditions in Germany would then be similar to the current conditions along the northern coast of the Mediterranean or Southeast Europe. Prolonged dry periods and more intense rainfall also have an impact on many aspects of our accustomed everyday life.
However, research into the impact of climate change on human health has been limited to date. The aim of our analyses was to determine whether an association between selected climatic factors and SSI rates exists and whether any differences can be identified between specific groups of pathogens or depths of infiltration.
Methods
Our analyses are based, on the one hand, on database information about surgical procedures and SSI from hospital departments participating in the “OP-KISS“ module of the German Nosocomial Infection Surveillance System (KISS, Krankenhaus-Infektions-Surveillance-System), and, on the other hand, on meteorological monitoring station data of the German Meteorological Service (DWD, Deutscher Wetterdienst). OP-KISS collects information about selected indicator operative procedures, using the consistent methods of KISS. SSI are classified by depth of infiltration as:
superficial SSI (A1),
deep SSI (A2)
infections with involvement of organs/body cavities (A3).
During the years from 2000 to 2016, information about SSI after operative procedures without placement of an implant, i.e. a non-human foreign body, could be collected up to postoperative day 30 and after operative procedures with implant placement up to postoperative day 365. For each SSI, up to 4 different pathogens could be documented. The methods of KISS and of collection of SSI data have already been described in detail in other publications (22, 23).
We conducted an explorative study in which we calculated SSI rates for included operative procedures performed during the years from 2000 to 2016 as the number of SSI per 1000 operative procedures. The surgeries were associated with certain department-related and patient-related parameters. For the analysis of the association between climatic factors and SSI rates, we used data from the meteorological monitoring stations of the German Meteorological Service (DWD). In the first processing step, the daily values at the measuring points were interpolated to a regular 12 × 12 km grid (24). Next, the daily grid data were aggregated to monthly climatic parameters and assigned to the post code coordinates (centers) from OP-KISS.
The calculation of Spearman’s correlation coefficient showed a moderate to strong correlation between temperature and the variables “relative humidity, “vapor pressure”, “hours of sunshine”, and “number of heat days and frost days” (etable 1). Therefore, the focus of our SSI rate analyses was on the parameter “temperature”—more specifically, on outdoor temperature.
eTable 1. Spread and Spearman’s correlation coefficient for included climatic factors.
| Parameter | Unit | Spread | Spearman’s correlation coefficient | ||||||||||||
| Median | IQR | 10.pct–90.pct | Min–Max | dTemp | Rainf | RelHum | VapP | Sunh | HD (> 30 °C) | ID (<0 °c) | HeRa | Lon | Lat | ||
| dTemp | °C | 9.3 | (4.5–15.6) | (1.5–18.4) | (–7.5–25.8) | 1.000 | 0.166 | –0.647 | 0.960 | 0.814 | 0.673 | –0.673 | 0.256 | –0.044 | –0.009 |
| Rainf | mm | 54.5 | (34.8–81) | (21.2–112.8) | (0–415.4) | 0.166 | 1.000 | 0.153 | 0.267 | –0.116 | 0.093 | –0.072 | 0.585 | –0.161 | –0.089 |
| RelHum | % | 78.8 | (72–84.9) | (66.8–88.5) | (48.7–99.8) | –0.647 | 0.153 | 1.000 | –0.435 | –0.855 | –0.494 | 0.442 | –0.054 | –0.062 | 0.137 |
| VapP | hPa | 9.4 | (7–12.9) | (6–15) | (3.5–19.6) | 0.960 | 0.267 | –0.435 | 1.000 | 0.672 | 0.619 | –0.640 | 0.299 | –0.075 | 0.041 |
| Sunh | Number | 132 | (68–200) | (41–239) | (2–395) | 0.814 | –0.116 | –0.855 | 0.672 | 1.000 | 0.578 | –0.548 | 0.113 | 0.050 | –0.032 |
| HD (> 30 °C) | Number | 0 | (0–0) | (0–3) | (0–25) | 0.673 | 0.093 | –0.494 | 0.619 | 0.578 | 1.000 | –0.257 | 0.157 | 0.027 | –0.077 |
| ID (<0 °c) | Number | 0 | (0–0) | (0–4) | (0–27) | –0.673 | –0.072 | 0.442 | –0.640 | –0.548 | –0.257 | 1.000 | –0.137 | 0.073 | –0.005 |
| HeRa | Number | 0 | (0–0) | (0–1) | (0–10) | 0.256 | 0.585 | –0.054 | 0.299 | 0.113 | 0.157 | –0.137 | 1.000 | –0.053 | –0.126 |
| Lon | Longitude | 9.4 | (8.1–11.9) | (7–13.3) | (6–15) | –0.044 | –0.161 | –0.062 | –0.075 | 0.050 | 0.027 | 0.073 | –0.053 | 1.000 | 0.180 |
| Lat | Latitude | 51.2 | (49.6–52.6) | (48.8–53.4) | (47.4–54.9) | –0.009 | –0.089 | 0.137 | 0.041 | –0.032 | –0.077 | –0.005 | –0.126 | 0.180 | 1.000 |
Data source: 2 004 793 operative procedures from 1 455 OP-KISS departments during the period from January 2000 to December 2016.
10.pct, 10% percentile; 90.pct, 90% percentile; VapP, vapor pressure; mTemp, monthly mean temperature; ID (<0°C), ice days (maximum temperature <0°C); hPa, hectopascal; HD (>30°C), heat days (maximum temperature >30°C); IQR, interquartile range; Lat, latitude; Lon, longitude; Max, maximum; Min, minimum; Rainf, rainfall; RelHum, relative humidity; Sunh, sun hours; HeRa, heavy rain days
In our analyses we present results which are based on a monthly aggregation of meteorological data. The background to this approach is that it is impossible to make a more precise statement on the exact time of onset of an SSI. An addition analysis for the parameter “daily mean temperature” at the day of surgery showed similar results.
Besides descriptive statistics regarding SSI rates, we performed a univariable and multivariable logistic regression analysis to investigate the association between SSI incidence and temperature as well as other department-specific and patient-related parameters.
All analyses were performed using the SPSS (IBM SPSS Statistics, Somer, NY, USA) and SAS (SAS Institute, Cary, NC, USA) statistical software packages. A detailed description of the methodology used is provided in the eMethods section.
The German Protection against Infection Act regulates in section 23 the prevention and handling of infectious diseases in humans. It obliges all hospitals to continuously record data on HAI. Hospital participating in KISS can voluntarily submit these data to the German National Reference Center for the Surveillance of Nosocomial Infections (NRZ, Nationales Referenzzentrum für Surveillance von nosokomialen Infektionen). All data were documented and analyzed in an anonymized form and in accordance with the guidelines and recommendations for Good Epidemiological Practice (GEP) (25). Thus, a positive vote of an ethics committee or informed consent were not required.
Results
Altogether, 2 004 793 operative procedures from 1455 departments, which participated in OP-KISS from January 2000 up to and including December 2016, were included. The structural characteristics of these departments are summarized in eTable 2. For all recorded operative procedures, altogether 32 118 SSI were documented; of these, 13 811 were classified as superficial SSI (A1) and 18 307 as deeper SSI (A2/A3).This corresponds to a crude SSI rate of 16 per 1000 operations. By means of stratification of the SSI rate by the temperature ranges defined above, it was found that the number of SSI increased in the presence of warmer temperatures in the month of surgery. In particular, SSI occurred more frequently after surgical procedures performed during a month with recorded temperatures of = 20 °C compared to all other temperature ranges. A similar trend was observed in a separate analysis of superficial and deep SSI (table 1). For 23 317 SSI, at least one pathogen was reported. For 8801 SSI, no pathogen was documented. The rate of SSI with identification of the pathogen (both gram-positive and gram-negative pathogens) increased with higher temperatures. This applied to both the group of superficial (A1) and the group of deeper (A2/A3) SSI. However, this increase for each temperature group was not linear, but showed fluctuations (table 1).
eTable 2. Structural characteristics of included surgical departments.
| Parameter | Group | Number | Percent |
| Hospital type | Specialized hospital | 89 | 6.1 |
| Primary care | 179 | 12.3 | |
| Maximum care – not a university hospital | 181 | 12.4 | |
| Maximum care – university hospital | 97 | 6.7 | |
| Secondary care | 568 | 39.0 | |
| Tertiary care | 290 | 19.9 | |
| Other/not stated | 51 | 3.5 | |
| Land (German Federal State) | Baden-Wuerttemberg | 140 | 9.6 |
| Bavaria | 179 | 12.3 | |
| Berlin | 67 | 4.6 | |
| Brandenburg | 40 | 2.7 | |
| Bremen | 30 | 2.1 | |
| Hamburg | 59 | 4.1 | |
| Hesse | 95 | 6.5 | |
| Mecklenburg-Western Pomerania | 29 | 2.0 | |
| North Rhine-Westphalia | 344 | 23.6 | |
| Lower Saxony | 139 | 9.6 | |
| Rhineland-Palatinate | 113 | 7.8 | |
| Saarland | 26 | 1.8 | |
| Saxony-Anhalt | 55 | 3.8 | |
| Saxony | 46 | 3.2 | |
| Schleswig-Holstein | 44 | 3.0 | |
| Thuringia | 49 | 3.4 | |
| Hospital operator | Independent/ not-for-profit | 529 | 36.4 |
| Public | 452 | 31.1 | |
| Private | 386 | 26.5 | |
| Other/not stated | 88 | 6.0 |
Data from 1 455 surgical departments that participated in OP-KISS during the period from January 2000 to December 2016.
Table 1. Surgical site infections and surgical site infection rates per 1000 operative procedures by temperature intervals of the monthly mean temperature in the month of surgery.
| Parameter | Total | Monthly mean temperature in the month of surgery | ||||
| Total | <5 °c | 5 to <10 °c | 10 to <15 °c | 15 to <20 °c | ≥ 20 °C | |
| Number of surgeries | 2 004 793 | 557 102 | 500 914 | 385 436 | 493 205 | 68 136 |
| Number of SSI (overall) | 32 118 | 8397 | 7667 | 6177 | 8622 | 1255 |
| Number of SSI (A1) | 13 811 | 3614 | 3188 | 2650 | 3802 | 557 |
| Number of SSI (A2/A3) | 18 307 | 4783 | 4479 | 3527 | 4820 | 698 |
| SSI rate per 1000 surgeries (95% CI) | ||||||
| All SSI | 16.02 [15.85; 16.20] |
15.07 [14.75; 15.40] |
15.31 [14.97; 15.65] |
16.03 [15.63; 16.43] |
17.48 [17.12; 17.85] |
18.42 [17.42; 19.46] |
| All SSI without pathogen | 4.39 [4.30; 4.48] |
4.32 [4.15; 4.50] |
4.08 [3.91; 4.27] |
4.35 [4.15; 4.57] |
4.73 [4.54; 4.92] |
4.95 [4.43; 5.50] |
| All SSI with pathogens | 11.63 [11.48; 11.78] |
10.75 [10.48; 11.02] |
11.22 [10.93; 11.52] |
11.67 [11.34; 12.02] |
12.76 [12.44; 13.07] |
13.47 [12.62; 14.37] |
| All SSI with gram-positive pathogen | 7.91 [7.79; 8.04] |
7.40 [7.18; 7.63] |
7.59 [7.35; 7.84] |
8.08 [7.80; 8.37] |
8.52 [8.27; 8.78] |
9.13 [8.43; 9.87] |
| All SSI with gram-negative pathogen | 4.46 [4.37; 4.56] |
3.99 [3.82; 4.16] |
4.17 [3.99; 4.35] |
4.49 [4.28; 4.70] |
5.16 [4.97; 5.37] |
5.31 [4.78; 5.89] |
| SSI rate per 1000 surgeries (95% CI) | ||||||
| All A1 | 6.89 [6.77; 7.00] |
6.49 [6.28; 6.70] |
6.36 [6.15; 6.59] |
6.88 [6.62; 7.14] |
7.71 [7.47; 7.96] |
8.17 [7.51; 8.88] |
| A1 without pathogen | 2.73 [2.66; 2.81] |
2.76 [2.62; 2.90] |
2.49 [2.35; 2.63] |
2.71 [2.55; 2.88] |
2.92 [2.77; 3.08] |
3.07 [2.67; 3.51] |
| A1 with pathogen | 4.16 [4.07; 4.25] |
3.73 [3.57; 3.89] |
3.87 [3.70; 4.05] |
4.16 [3.96; 4.37] |
4.79 [4.60; 4.98] |
5.11 [4.59; 5.67] |
| A1 with gram-positive pathogen | 2.68 [2.61; 2.75] |
2.39 [2.26; 2.52] |
2.44 [2.31; 2.58] |
2.79 [2.62; 2.96] |
3.08 [2.93; 3.24] |
3.33 [2.91; 3.79] |
| A1 with gram-negative pathogen | 1.69 [1.64; 1.75] |
1.45 [1.35; 1.56] |
1.61 [1.50; 1.72] |
1.68 [1.55; 1.81] |
1.98 [1.86; 2.11] |
2.26 [1.92; 2.65] |
| SSI rate per 1000 surgeries (95% CI) | ||||||
| All A2/A3 | 9.13 [9.00; 9.26] |
8.59 [8.34; 8.83] |
8.94 [8.68; 9.21] |
9.15 [8.85; 9.46] |
9.77 [9.50; 10.05] |
10.24 [9.50; 11.03] |
| A2/A3 without pathogen | 1.66 [1.60; 1.71] |
1.57 [1.46; 1.67] |
1.60 [1.49; 1.71] |
1.64 [1.51; 1.77] |
1.80 [1.69; 1.93] |
1.88 [1.57; 2.23] |
| A2/A3 with pathogen | 7.48 [7.36; 7.60] |
7.02 [6.80; 7.24] |
7.35 [7.11; 7.59] |
7.51 [7.24; 7.79] |
7.97 [7.72; 8.22] |
8.37 [7.70; 9.08] |
| A2/A3 with gram-positive pathogen | 5.23 [5.13; 5.33] |
5.02 [4.83; 5.20] |
5.15 [4.95; 5.35] |
5.30 [5.07; 5.53] |
5.44 [5.23; 5.65] |
5.80 [5.24; 6.40] |
| A2/A3 with gram-negative pathogen | 2.77 [2.70; 2.85] |
2.53 [2.40; 2.67] |
2.56 [2.42; 2.71] |
2.81 [2.65; 2.98] |
3.18 [3.03; 3.34] |
3.05 [2.65; 3.50] |
Data source: 2 004 793 operative procedures from 1455 OP-KISS departments in the period from January 2000 to December 2016 A1 = superficial surgical site infection; A2 = deep surgical site infection; A3 = surgical site infection with organ/body cavity involvement; CI = confidence interval; SSI = surgical site infection
Using a multivariable logistic regression analysis, we calculated adjusted odds ratios (AOR) for the incidence of SSI in relation to the temperature in the month of surgery. The results of the underlying descriptive, univariable and multivariable analyses are listed in the eTables 3– 5. The multivariable logistic regression analysis confirmed the above mentioned result that SSI occur more frequently in the presence of warmer temperatures. In the presence of temperatures = 20 °C, SSI were significantly more likely to occur compared to temperatures <5 °C (AOR: 1.13 [1.06–1.20]). This was primarily attributed to SSI with identification of the pathogen, but it was also found that SSI without pathogen identification occurred more frequently if temperatures were = 20 °C in the month of surgery. However, compared to SSI with pathogen identification, this difference was not significant. SSI with pathogen identification showed an increase in the presence of temperatures = 20 °C in the month of surgery, both in gram-positive (AOR: 1.13 [1.03–1.23]) and—even more pronounced—in gram-negative (AOR: 1.20 [1.07–1.35]) pathogens. Stratified according to SSI depth, the association with temperature was strongest for superficial SSI with gram-negative pathogens. The occurrence of superficial SSI with gram-negative pathogens was up to 38% more likely in the presence of temperatures = 20 °C (AOR: 1,38 [1,16; 1,64]) in the month of surgery compared to temperatures <5 °C.
eTable 3. Descriptive analysis of surgical site infections (all depths).
| Parameter | Category | Number of OP | Number of SSI | SSI rate per 1000 OP | p-value |
| Total | 2 004 793 | 32 118 | 16.0 | ||
| Sex | Male | 789 014 | 15 432 | 19.6 | <0.001 |
| Female | 1 215 779 | 16 686 | 13.7 | ||
| Age in years | <30 | 153 043 | 1308 | 8.5 | <0.001 |
| 30–39 | 183 503 | 1454 | 7.9 | ||
| 40–49 | 175 056 | 1928 | 11.0 | ||
| 50–59 | 286 723 | 4177 | 14.6 | ||
| 60–69 | 438 264 | 7483 | 17.1 | ||
| 70–79 | 522 091 | 10 114 | 19.4 | ||
| ≥ 80 | 246 113 | 5654 | 23.0 | ||
| NNIS risk index (score) | 0 | 932 758 | 7019 | 7.5 | <0.001 |
| 1 | 834 106 | 15 747 | 18.9 | ||
| 2 | 224 382 | 8281 | 36.9 | ||
| 3 | 13 547 | 1071 | 79.1 | ||
| Laparoscopy | Yes | 296 348 | 3123 | 10.5 | <0.001 |
| No | 1 708 445 | 28 995 | 17.0 | ||
| Hospital type | Not specified | 36 144 | 425 | 11.8 | <0.001 |
| Other | 1 215 055 | 15 916 | 13.1 | ||
| Maximum care, university hospital, advanced care | 753 594 | 15 777 | 20.9 | ||
| Type of OP (classification into 7 groups) | Not specified | 30 | 0 | 0.0 | <0.001 |
| Abdominal surgery | 330 305 | 10 839 | 32.8 | ||
| General and other surgery* | 153 427 | 1104 | 7.2 | ||
| Vascular surgery | 97 204 | 2024 | 20.8 | ||
| Gynecology | 358 923 | 3032 | 8.4 | ||
| Cardiac surgery | 145 789 | 5538 | 38.0 | ||
| Trauma & orthopedic surgery | 883 099 | 8741 | 9.9 | ||
| Urology | 36 016 | 840 | 23.3 | ||
| Year of surgery | 2000 | 41 618 | 834 | 20.0 | <0.001 |
| 2001 | 55 143 | 1089 | 19.7 | ||
| 2002 | 63 722 | 1363 | 21.4 | ||
| 2003 | 70 155 | 1407 | 20.1 | ||
| 2004 | 72 503 | 1353 | 18.7 | ||
| 2005 | 86 487 | 1396 | 16.1 | ||
| 2006 | 90 042 | 1503 | 16.7 | ||
| 2007 | 97 814 | 1600 | 16.4 | ||
| 2008 | 105 472 | 1747 | 16.6 | ||
| 2009 | 112 540 | 1793 | 15.9 | ||
| 2010 | 123 980 | 1892 | 15.3 | ||
| 2011 | 133 202 | 2052 | 15.4 | ||
| 2012 | 141 370 | 2050 | 14.5 | ||
| 2013 | 160 381 | 2358 | 14.7 | ||
| 2014 | 192 768 | 2842 | 14.7 | ||
| 2015 | 221 987 | 3521 | 15.9 | ||
| 2016 | 235 609 | 3318 | 14.1 | ||
| Season (as monthly intervals) | December–February | 487 200 | 7345 | 15.1 | <0.001 |
| March–May | 512 666 | 8272 | 16.1 | ||
| June–August | 475 122 | 8425 | 17.7 | ||
| September–November | 529 805 | 8076 | 15.2 |
Data source: 2 004 793 operative procedures from 1 455 OP-KISS departments during the period from January 2000 to December 2016.
The NNIS risk index includes the following parameters: duration of surgery, wound contamination class, ASA score.
One point is awarded for each of the following: duration of surgery >75% percentile, ASA score =3, wound contamination class =3.
* incl. hernia repair surgeries, thyroid goiter surgery, lobectomy, parotidectomy, craniotomy, neck dissection
ASA, American Society of Anesthesiologists; NNIS, National Nosocomial Infections Surveillance System; OP, operative procedure; SSI, surgical site infection
eTable 5. Results of the multivariable analysis (logistic regression) for the outcome “surgical site infection” (all depths) and the variable monthly mean temperature in the month of surgery.
| Parameter | Category | OR (95% CI) |
| Temperature | Per 1-degree Celsius | 1.007 [1.005; 1.009] |
| Sex | male vs. female | 1.014 [0.989; 1.040] |
| Age in years | 30–39 vs. <30 | 1.088 [1.008; 1.174] |
| 40–49 vs. <30 | 1.359 [1.265; 1.459] | |
| 50–59 vs. <30 | 1.608 [1.508; 1.716] | |
| 60–69 vs. <30 | 1.708 [1.605; 1.817] | |
| 70–79 vs. <30 | 1.845 [1.735; 1.963] | |
| ≥ 80 vs. <30 | 2.059 [1.930; 2.196] | |
| NNIS risk index (score) | 1 vs. 0 | 1.794 [1.741; 1.849] |
| 2 vs. 0 | 2.706 [2.613; 2.803] | |
| 3 vs. 0 | 3.781 [3.521; 4.060] | |
| Surgery group (vs. reference: General and other surgery*) | Abdominal surgery | 5.858 [5.494; 6.245] |
| Trauma & orthopedic surgery | 1.005 [0.943; 1.072] | |
| Urology | 2.917 [2.663; 3.195] | |
| Gynecology | 1.289 [1.198; 1.387] | |
| Cardiac surgery | 2.963 [2.773; 3.166] | |
| Vascular surgery | 1.873 [1.739; 2.018] | |
| Access route | Laparoscopic vs. open | 0.244 [0.234; 0.254] |
| Year of surgery | 2000 vs. 2016 | 1.372 [1.269; 1.483] |
| 2001 vs. 2016 | 1.267 [1.181; 1.359] | |
| 2002 vs. 2016 | 1.326 [1.243; 1.415] | |
| 2003 vs. 2016 | 1.263 [1.185; 1.346] | |
| 2004 vs. 2016 | 1.151 [1.079; 1.228] | |
| 2005 vs. 2016 | 1.035 [0.971; 1.103] | |
| 2006 vs. 2016 | 1.056 [0.993; 1.124] | |
| 2007 vs. 2016 | 1.044 [0.982; 1.109] | |
| 2008 vs. 2016 | 1.054 [0.994; 1.118] | |
| 2009 vs. 2016 | 1.039 [0.980; 1.102] | |
| 2010 vs. 2016 | 1.004 [0.948; 1.063] | |
| 2011 vs. 2016 | 1.014 [0.959; 1.073] | |
| 2012 vs. 2016 | 0.956 [0.904; 1.011] | |
| 2013 vs. 2016 | 0.995 [0.943; 1.050] | |
| 2014 vs. 2016 | 1.026 [0.975; 1.080] | |
| 2015 vs. 2016 | 1.115 [1.062; 1.170] |
Data source: 2 004 793 operative procedures from 1 455 OP-KISS departments during the period from January 2000 to December 2016. The NNIS risk index includes the following parameters: duration of surgery, wound contamination class, ASA score.
One point is awarded for each of the following: duration of surgery >75% percentile, ASA score ≥ 3, wound contamination class ≥ 3.
* incl. hernia repair surgeries, thyroid goiter surgery, lobectomy, parotidectomy, craniotomy, neck dissection
ASA, American Society of Anesthesiologists; CI, confidence interval; NNIS, National Nosocomial Infections Surveillance System; OR, odds ratio
When considering temperature not as a categorical variable, but as a continuous variable, it was found that, after adjustment for the factors used in our model, the likelihood of the occurrence of SSI per 1°C temperature increase increased by about 1%. Again, this association was considerably stronger in the case of A1 and SSI with gram-negative pathogens. The likelihood of A1 with gram-negative pathogens increased with each increase in temperature by 1°C by about 2%.
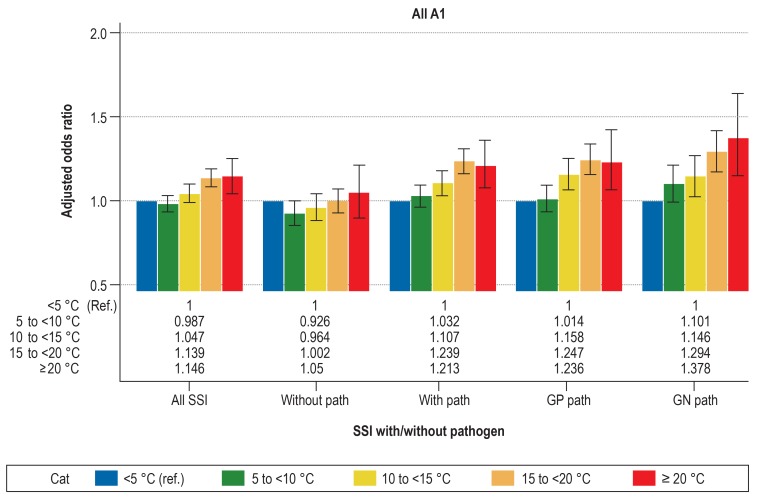
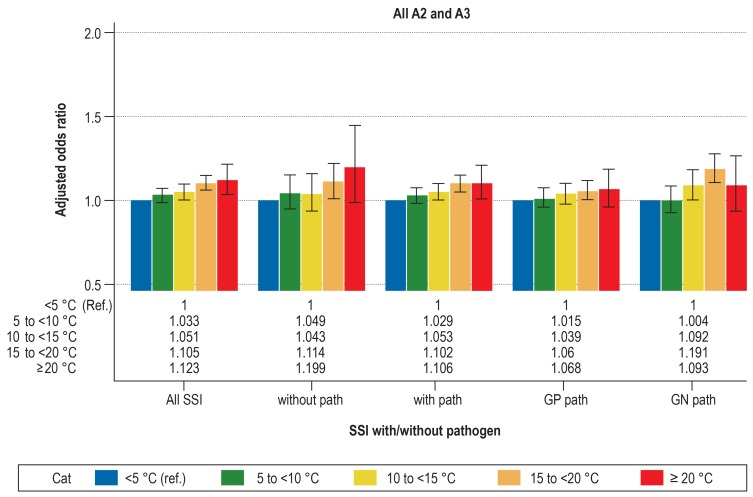
Detailed information about the results of the multivariable logistic regression analysis is presented in Table 2 and Figure 1, about A1 in Figure 2 and about A2/A3 in eFigure 1.
Figure 1.
Adjusted odds ratios with 95% CI from the multivariable logistic regression analysis for SSI by different temperature ranges with the reference <5°C
Data source: 2 004 793 operative procedures from 1455 OP-KISS departments in the period from January 2000 to December 2016. Depicted for various types of SSI of all levels of infiltration depth: all SSI, SSI without pathogens (without path), SSI with pathogens (with path), SSI with gram-positive (GP path) and SSI with gram-negative (GN path) pathogens. All models adjusted for the factors age, sex, NNIS risk index, type of surgery, access route (laparoscopic vs open) and year of surgery. Error bar shows 95% CI. The NNIS risk index includes the following parameters: duration of surgery, wound contamination class, ASA score. One point is awarded for each of the following: duration of surgery >75% percentile, ASA score =3, wound contamination class =3.
ASA, American Society of Anesthesiologists; Cat, category; CI, confidence interval; GN, gram-negative; GP, gram-positive; NNIS, National Nosocomial Infections Surveillance System; Path, pathogen; Ref, reference; SSI, surgical site infection
Figure 2.
Adjusted odds ratios with 95% CI from the multivariable logistic regression analysis for superficial SSI by different temperature ranges with the reference <5°C.
Data source: 2 004 793 operative procedures from 1455 OP-KISS departments in the period from January 2000 to December 2016. Depicted for various types of superficial SSI (A1): all SSI, SSI without pathogen (without path), SSI with pathogen (with path), SSI with gram-positive (GP path) and SSI with gram-negative (GN path) pathogens. All models adjusted for the factors age, sex, NNIS risk index, type of surgery, access route (laparoscopic vs open) and year of surgery. Error bar shows 95% CI. The NNIS risk index includes the following parameters: duration of surgery, wound contamination class, ASA score. One point is awarded for each of the following: duration of surgery >75% percentile, ASA score = 3, wound contamination class = 3.
A1, superficial surgical site infection; ASA, American Society of Anesthesiologists; Cat, category; CI, confidence interval; GN, gram-negative; GP, gram-positive; NNIS, National Nosocomial Infections Surveillance System; Path, pathogen; Ref, reference; SSI, surgical site infection
eFigure 1.
Adjusted odds ratios with 95% CI from the multivariable logistic regression analysis for deep SSI by different temperature ranges with the reference <5°C.
Data source: 2 004 793 operative procedures from 1 455 OP-KISS departments during the period from January 2000 to December 2016.
Depicted for various types of deep SSI (A2 and A3): all SSI, SSI without pathogen (without path), SSI with pathogen (with path), SSI with gram-positive (GP path), and SSI with gram-negative (GN path) pathogens. All models adjusted for the factors age, sex, NNIS risk index, type of surgery, access route (laparoscopic vs open) and year of surgery. Error bar shows 95% CI. The NNIS risk index includes the following parameters: duration of surgery, wound contamination class, ASA score. One point is awarded for each of the following: duration of surgery >75% percentile, ASA score ≥3, wound contamination class ≥3.
A2, deep surgical site infection; A3, surgical site infection with involvement of organs/body cavity; ASA, American Society of Anesthesiologists; Cat, category; CI, confidence interval; GN, gram-negative; GP, gram-positive; NNIS, National Nosocomial Infections Surveillance System; Path, pathogen; Ref, reference; SSI, surgical site infection
Discussion
Our analyses showed that there is a relevant association between temperature and other climatic factors in the month of surgery and the likelihood of SSI. However, when evaluating the strength of the association between SSI risk and temperature, differences were found, depending on the respective focus and perspective.
Both when looking at a temperature increase in increments of 1°C and when using different temperature intervals, a general trend of increasing SSI risk with rising temperatures was observed. On closer examination, it was noted that this increase was largely driven by the temperature range = 20°. Especially with regard to SSI with pathogen identification, it appeared that that this temperature range represents a threshold beyond which the SSI rate rises sharply.
Interestingly, this does not seem to apply in the same way to SSI without pathogen identification. In this case, a temperature increase of 1°C was associated with a higher AOR for SSI, showing a general association between temperature and SSI without documented pathogen, but the comparison of temperatures = 20°C and temperatures <5°C yielded no significant differences.
With regard SSI with pathogen identification, we differentiated between gram-positive and gram-negative pathogens. Independent of the depth of the SSI, we demonstrated that the risk of SSI increased with a rise in temperature, especially when caused by gram-negative pathogens. Surprisingly, the increase in SSI with gram-negative pathogens was not limited to deep SSI, for example after abdominal surgery, but was most obvious in patients with superficial SSI. One possible explanation is that fluctuations in outdoor temperatures have a stronger effect on superficial tissue layers.
Gram-positive pathogens are the most commonly isolated pathogens in SSI (26, 27). This also applies to superficial SSI. Consequently, previous studies on the matter have shown an increase in SSI with gram-positive pathogens during the summer months (28, 29). To the best of our knowledge, the finding that the temperature-associated increase in SSI caused by gram-negative pathogens was, according to our dataset, more pronounced compared to SSI caused by gram-positive pathogens, is a new result which has so far not been demonstrated in comparable studies.
Most SSI are caused by pathogens which belong to the patient’s microbiome. The human microbiome shows seasonal fluctuations (30), but to date these have not been sufficiently studied to explain differences in risk of SSI caused by specific groups of pathogens. The spread of gram-negative pathogens may be more promoted by warmer temperatures than the spread of gram-positive pathogens. It is also conceivable that the cell wall of gram-negative organisms is more resistant to temperature shifts (31), resulting in a selection advantage for these pathogens. These interpretations appear to be justified, especially when taking into account data from studies conducted in intensive-care settings focusing on bloodstream infections which revealed phenomena similar to those demonstrated in our analysis (14).
Using time-series analyses covering a period of 8 years, Perencevich et al. (2008) demonstrated a significant increase in infections caused by gram-negative pathogens during the summer months (32). The authors concluded that this association observed for specific pathogens was largely influenced by temperature. The results of our analyses confirmed the observations of seasonal fluctuations in infections caused by gram-negative pathogens which Perencevich et al. (2008) documented more than 10 years ago.
Since it is very likely that temperatures in Germany will continue to increase in this century, a higher proportion of days and months with mean temperatures = 20°C (so-called “heat days” with maximum temperature of >30°C) can be expected (18). Thus, the described temperature-associated fluctuations of infection rates may be become even more pronounced in the future.
Our study has strengths and limitations which should be taken into account when interpreting the results. A key strength was the large number of included operative procedures, comprising data of more than 1400 departments which participated in OP-KISS during the study period. In addition to the long observation period of 17 years, this helped to reduce the impact of random effects. By adjusting the data for specific risk factors and by exactly matching the climate data with the surgery data using the postcode, reliable results were generated. Given the large number of OP-KISS participants across Germany, our data can, with limitations, be extrapolated to the overall situation in Germany.
The limitations of our study were primarily due to the methodology of KISS in general and of OP-KISS in particular. All data were voluntarily reported by the institutions participating in KISS to the German National Reference Center for the Surveillance of Nosocomial Infections. Here, the heterogeneity of the data collection teams, which differ in sensitivity and specificity in the documentation of SSI, has to be taken into account. OP-KISS is based on a patient-based surveillance principle. Surveillance should be continued after the patient’s discharge from hospital. However, this continued SSI surveillance of patients beyond their inpatient stay (post-discharge surveillance) is implemented differently by the participating departments. Yet, we are not aware that this contributes to any seasonal differences which would reduce the significance of our results.
Furthermore, another limitation that should be mentioned is that no data are available on whether the participating hospital were equipped with air conditioning systems. All temperature data relate to the outdoor temperatures in the month of surgery. These may sometimes have been considerably different from the temperature inside the hospital.
Conclusion
It can be noted that higher monthly mean temperatures, especially in the range = 20°C, are associated with an increased occurrence of SSI. Our analyses showed mostly an association between temperature and superficial SSI caused by gram-negative pathogens. Future analyses should also determine for which pathogen species and which type of surgery this association is particularly strong.
Preventive strategies, developed based on these data, could include, for example, to perform specific elective surgeries in cooler months or to adapt antimicrobial prophylaxis and preoperative decolonization measures to seasonal fluctuations in pathogens. Here, the predicted rise in temperatures caused by climate change should also be taken into account.
Supplementary Material
eMETHODS
Statistical analysis
In our analysis, we evaluated all surgical site infections (SSI) and subgroups of SSI as endpoints. These are defined by identification of pathogens (all SSI, SSI without pathogen identification, SSI with pathogen identification, SSI caused by gram-positive pathogens, SSI caused by gram-negative pathogens), and by depth of the SSI (all depths of infection [A1, A2, A3] combined, superficial SSI [A1] and deep SSI [A2 and A3]).
In the descriptive analysis, we calculated for each of the different endpoints the number of SSI and the SSI rates (per 1000 operative procedures) with 95% confidence intervals. We used the chi-squared test to test for differences between categories of the independent parameters.
Our analyses included the following climate parameters: monthly mean temperature as a continuous and as a categorical variable (<5/5 to <10/10 to <15/15 to <20/= 20°C), rainfall (mm), relative humidity (%), vapor pressure (hectopascal), number of sunshine hours per month, number of heat days (maximum temperature >30°C) per month, number of ice days (maximum temperature <0°C) per month, number of heavy rainfall days (>30 mm per day) as well as longitude and latitude.
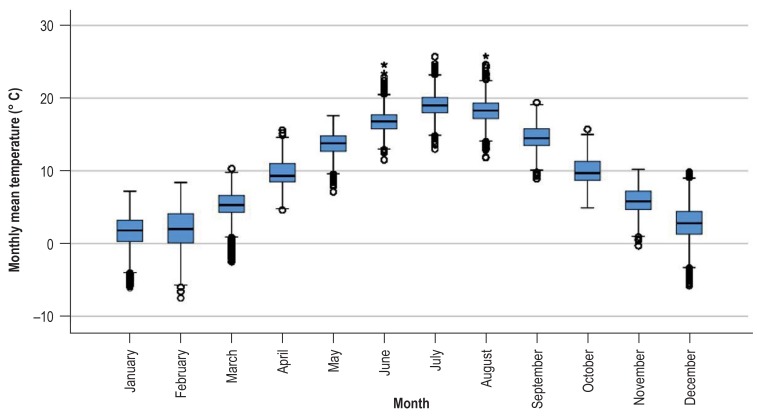
There was an association between temperature and the calendar month. This association is depicted in eFigure 2, showing the variability of temperature within one month at all observation points during the observation period.
Besides the climatic parameters, we included the following independent risk factors and confounders in our analysis:
In the univariable analysis, a logistic regression model was calculated for each endpoint and each independent parameter (climate parameters, department-related parameters, patient-related parameters).
In the multivariable analysis, adjusted odds ratios with 95% confidence intervals were calculated for the mean monthly temperature. Here, we calculated a model with temperature as a continuous parameter (per 1°C) and a model with temperature as a categorical variable with temperature ranges (<5/5 to <10/10 to <15/15 to <20/= 20°C), using temperatures <5°C as a reference. The following parameters were included in all multivariable models: sex, age, type of operative procedure, NNIS risk index, access route of surgery, and year of surgery.
The department-specific parameter “type of hospital care” (maximum-care hospital: yes, no, unknown)
The patient-related factors “sex” and “age group” (<30, 30–39, …, = 80 years)
NNIS risk index: duration of surgery, wound contamination class, ASA score: One point is awarded for each of the following: duration of surgery >75% percentile, ASA score =3, wound contamination class = 3) (NNIS = National Nosocomial Infections Surveillance System; ASA = American Society of Anesthesiologists)
The type of surgery stratified into 7 groups (general and other surgery, abdominal surgery, trauma & orthopedic surgery, urology, gynecology, cardiac surgery, vascular surgery)
The surgical access route (laparoscopic/open)
The season (December–February, March–May, June–August, September–November)
as well as the year of surgery.
Table 2. Results of the multivariable logistic regression analysis for the various forms of surgical site infections in relation to the monthly mean temperature.
| Outcome |
Temperature (continuous) |
Temperature interval (categorical) |
|||
|
per 1 °C OR [95% CI] |
5 to <10 °c vs. <5°c or [95% ci] | 10 to <15°cvs. <5 °c or [95% ci] | 15 to <20 °c vs. <5 °c or [95% ci] | ≥ 20 °C vs. <5 °c or [95% ci] | |
| All SSI | 1.007 [1.005; 1.009] |
1.012 [0.980; 1.044] |
1.048 [1.014; 1.085] |
1.118 [1.084; 1.153] |
1.132 [1.064; 1.204] |
| All SSI without pathogens | 1.003 [1.000; 1.007] |
0.970 [0.913; 1.030] |
0.993 [0.932; 1.057] |
1.043 [0.984; 1.104] |
1.104 [0.982; 1.241] |
| All SSI with pathogens | 1.008 [1.006; 1.011] |
1.030 [0.993; 1.070] |
1.073 [1.031; 1.116] |
1.151 [1.111; 1.194] |
1.146 [1.066; 1.231] |
| All SSI with gram-positive pathogen | 1.007 [1.005; 1.010] |
1.015 [0.970; 1.062] |
1.078 [1.028; 1.131] |
1.121 [1.074; 1.171] |
1.125 [1.031; 1.226] |
| All SSI with gram-negative pathogen | 1.013 [1.010; 1.016] |
1.040 [0.978; 1.105] |
1.113 [1.044; 1.187] |
1.231 [1.162; 1.304] |
1.200 [1.070; 1.346] |
| All A1 | 1.008 [1.006; 1.011] |
0.987 [0.940; 1.036] |
1.047 [0.994; 1.101] |
1.139 [1.087; 1.193] |
1.146 [1.045; 1.256] |
| All A1 without pathogen | 1.001 [0.997; 1.005] |
0.926 [0.858; 0.999] |
0.964 [0.890; 1.044] |
1.002 [0.932; 1.077] |
1.050 [0.906; 1.218] |
| All A1 with pathogen | 1.013 [1.010; 1.017] |
1.032 [0.969; 1.100] |
1.107 [1.037; 1.183] |
1.239 [1.167; 1.315] |
1.213 [1.080; 1.363] |
| All A1 with gram-positive pathogen | 1.015 [1.010; 1.019] |
1.014 [0.937; 1.097] |
1.158 [1.068; 1.256] |
1.247 [1.158; 1.343] |
1.236 [1.071; 1.428] |
| All A1 with gram-negative pathogen | 1.017 [1.012; 1.023] |
1.101 [0.997; 1.216] |
1.146 [1.032; 1.273] |
1.294 [1.178; 1.421] |
1.378 [1.155; 1.644] |
| All A2/A3 | 1.006 [1.004; 1.008] |
1.033 [0.990; 1.077] |
1.051 [1.006; 1.099] |
1.105 [1.061; 1.151] |
1.123 [1.035; 1.218] |
| All A2/A3 without pathogen | 1.007 [1.002; 1.012] |
1.049 [0.951; 1.156] |
1.043 [0.941; 1.157] |
1.114 [1.014; 1.224] |
1.199 [0.992; 1.449] |
| All A2/A3 with pathogen | 1.006 [1.003; 1.008] |
1.029 [0.983; 1.077] |
1.053 [1.002; 1.106] |
1.102 [1.053; 1.152] |
1.106 [1.010; 1.210] |
| All A2/A3 with gram-positive pathogen | 1.003 [1.000; 1.006] |
1.015 [0.961; 1.072] |
1.039 [0.981; 1.101] |
1.060 [1.004; 1.118] |
1.068 [0.959; 1.190] |
| All A2/A3 with gram-negative pathogen | 1.010 [1.006; 1.014] |
1.004 [0.929; 1.084] |
1.092 [1.007; 1.183] |
1.191 [1.108; 1.281] |
1.093 [0.941; 1.268] |
Data source: 2 004 793 operative procedures from 1455 OP-KISS departments during the period from January 2000 to December 2016. The table lists adjusted odds ratios, including 95% CI, for the mean monthly temperature in the month of surgery as a continuous parameter (per 1°C) and for temperature intervals (with the reference <5°C). A1 = superficial surgical site infection; A2 = deep surgical site infection; A3 = surgical site infection with organ/body cavity involvement; CI = confidence interval; OR = odds ratio; SSI = surgical site infection
Key Messages.
The mean temperature in the month of surgery is associated with surgical site infection rates. There is a strong correlation between temperature and other climatic parameters.
Warmer temperatures are associated with an increased likelihood of surgical site infections. This association is particularly strong for temperature ranges above 20°C.
Superficial surgical site infections show a stronger association with the parameter “temperature” than deep surgical site infections.
Surgical site infections caused by gram-negative pathogens were stronger associated with the parameter “temperature” than surgical site infections caused by gram-positive pathogens.
Future prevention strategies could result in certain elective surgeries being performed in cooler months, if possible.
eTable 4. Univariable analysis (logistic regression) for the outcome “surgical site infection“ (all depths).
| Parameter | Category | Odds ratio (95% CI) |
| Mean temperature | Per 1-degree Celsius increase | 1.010 [1.00; 1.011] |
| Rainfall | Per 1-millimeter increase | 1.000 [1.000; 1.000] |
| Relative humidity | Per 1-percent increase | 0.992 [0.991; 0.994] |
| Vapor pressure | Per 1-hectopascal increase | 1.017 [1.014; 1.020] |
| Sunshine hours | Per 1 hour | 1.001 [1.001; 1.001] |
| Heat days (Tmax > 30 °C) | Per 1 heat day | 1.021 [1.016; 1.026] |
| Ice days (Tmax <0 °c) | Per 1 ice day | 0.993 [0.990; 0.997] |
| Heavy rainfall days (> 30 mm/day) | Per 1 heavy rainfall day | 1.026 [1.019; 1.042] |
| Longitude | Per 1-longitude increase | 1.034 [1.029; 1.039] |
| Latitude | Per 1-latitude increase | 0.973 [0.967; 0.979] |
| Age in years | <30 | Reference |
| 30–39 | 0.927 [0.860; 0.999] | |
| 40–49 | 1.292 [1.204; 1.386] | |
| 50–59 | 1.715 [1.611; 1.825] | |
| 60–69 | 2.015 [1.899; 2.137] | |
| 70–79 | 2.291 [2.162; 2.428] | |
| ≥ 80 | 2.727 [2.567; 2.897] | |
| Sex (male vs. female) | male | 1.434 [1.402; 1.466] |
| Temperature in increments of 5 degree (5 groups) | <5 °c | Reference |
| 5 to <10 °c | 1.016 [0.985; 1.048] | |
| 10 to <15 °c | 1.064 [1.030; 1.100] | |
| 15 to <20 °c | 1.163 [1.128; 1.198] | |
| ≥ 20 °C | 1.226 [1.155; 1.302] | |
| Season | December–February | Reference |
| March–May | 1.071 [1.038; 1.106] | |
| June–August | 1.179 [1.143; 1.217] | |
| September–November | 1.011 [0.980; 1.044] | |
| Surgery group (classification into 7 groups) | General and other surgery* | Reference |
| Abdominal surgery | 4.681 [4.399; 4.982] | |
| Trauma & orthopedic surgery | 1.379 [1.295; 1.469] | |
| Urology | 3.295 [3.010; 3.607] | |
| Gynecology | 1.175 [1.097; 1.260] | |
| Cardiac surgery | 5.448 [5.105; 5.814] | |
| Vascular surgery | 2.934 [2.725; 3.159] | |
| Access route (laparoscopic vs. open) |
Laparoscopic | 0.617 [0.594; 0.640] |
| NNIS risk index (score) | 0 | Reference |
| 1 | 2.538 [2.467; 2.611] | |
| 2 | 5.054 [4.894; 5.219] | |
| 3 | 11.323 [10.592; 12.103] | |
| Year of surgery | 2016 | Reference |
| 2000 | 1.432 [1.326; 1.546] | |
| 2001 | 1.410 [1.316; 1.511] | |
| 2002 | 1.530 [1.436; 1.631] | |
| 2003 | 1.433 [1.345; 1.526] | |
| 2004 | 1.331 [1.249; 1.419] | |
| 2005 | 1.149 [1.078; 1.223] | |
| 2006 | 1.189 [1.118; 1.264] | |
| 2007 | 1.164 [1.096; 1.236] | |
| 2008 | 1.179 [1.112; 1.250] | |
| 2009 | 1.133 [1.070; 1.201] | |
| 2010 | 1.085 [1.025; 1.148] | |
| 2011 | 1.095 [1.036; 1.158] | |
| 2012 | 1.030 [0.975; 1.089] | |
| 2013 | 1.045 [0.991; 1.102] | |
| 2014 | 1.048 [0.996; 1.102] | |
| 2015 | 1.128 [1.076; 1.184] | |
| Maximum care hospital | No | Reference |
| Yes | 1.611 [1.576; 1.647] | |
| Unknown | 0.896 [0.814; 0.988] |
Data source: 2 004 793 operative procedures from 1 455 OP-KISS departments during the period from January 2000 to December 2016, listed for all climatic factors and meteorological parameters as well as other risk factors and confounders. The NNIS risk index includes the following parameters: duration of surgery, wound contamination class, ASA score. One point is awarded for each of the following: duration of surgery >75% percentile, ASA score = 3, wound contamination class ≥ 3
* incl. hernia repair surgeries, thyroid goiter surgery, lobectomy, parotidectomy, craniotomy, neck dissection
ASA, American Society of Anesthesiologists; CI, confidence interval; NNIS, National Nosocomial Infections Surveillance System; Tmax, maximum temperature
eFigure 2.
Box plot: Association between temperature and calendar month. The figure shows the variability of the monthly mean temperature within a month at all observation points during the observation period, stratified by calendar months.
Data source: 2 004 793 operative procedures from 1 455 OP-KISS departments during the period from January 2000 to December 2016. The box shows the interquartile range. The median is marked by the horizontal line within the box. The whiskers represent one and a half times the interquartile range.
Acknowledgments
Received on 18 February 2019; revised version accepted on 16 May 2019.
Translated from the original German by Ralf Thoene, MD.
Footnotes
Conflict of interest
The authors declare no conflict of interest.
Funding
The project “Climate and pathogens – Impact of decolonization“ (CLIP-ID) of the Institute of Hygiene and Environmental Medicine at the Charité-University Medicine Berlin was funded by the Federal Ministry of Education and Research within the framework of InfectControl 2020.
References
- 1.Behnke M, Aghdassi SJ, Hansen S, Peña Diaz LA, Gastmeier P, Piening B. The prevalence of nosocomial infection and antibiotic use in German hospitals. Dtsch Arztebl Int. 2017;114:851–857. doi: 10.3238/arztebl.2017.0851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Suetens C, Latour K, Karki T, et al. Prevalence of healthcare-associated infections, estimated incidence and composite antimicrobial resistance index in acute care hospitals and long-term care facilities: results from two European point prevalence surveys, 2016 to 2017. Euro Surveill. 2018;23 doi: 10.2807/1560-7917.ES.2018.23.46.1800516. pii=1800516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cassini A, Plachouras D, Eckmanns T, et al. Burden of six healthcare-associated infections on European population health: estimating incidence-based disability-adjusted life years through a population prevalence-based modelling study. PLoS Med. 2016;13 doi: 10.1371/journal.pmed.1002150. e1002150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.van der Slegt J, van der Laan L, Veen EJ, Hendriks Y, Romme J, Kluytmans J. Implementation of a bundle of care to reduce surgical site infections in patients undergoing vascular surgery. PLoS One. 2013;8 doi: 10.1371/journal.pone.0071566. e71566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Crolla RM, van der Laan L, Veen EJ, Hendriks Y, van Schendel C, Kluytmans J. Reduction of surgical site infections after implementation of a bundle of care. PLoS One. 2012;7 doi: 10.1371/journal.pone.0044599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zywot A, Lau CSM, Stephen Fletcher H, Paul S. Bundles prevent surgical site infections after colorectal surgery: meta-analysis and systematic review. J Gastrointest Surg. 2017;21:1915–1930. doi: 10.1007/s11605-017-3465-3. [DOI] [PubMed] [Google Scholar]
- 7.Ma N, Cameron A, Tivey D, Grae N, Roberts S, Morris A. Systematic review of a patient care bundle in reducing staphylococcal infections in cardiac and orthopaedic surgery. ANZ J Surg. 2017;87:239–246. doi: 10.1111/ans.13879. [DOI] [PubMed] [Google Scholar]
- 8.Schreiber PW, Sax H, Wolfensberger A, Clack L, Kuster SP. Swissnoso: The preventable proportion of healthcare-associated infections 2005-2016: systematic review and meta-analysis. Infect Control Hosp Epidemiol. 2018;39:1277–1295. doi: 10.1017/ice.2018.183. [DOI] [PubMed] [Google Scholar]
- 9.Aghdassi SJS, Gastmeier P. Novel approaches to surgical site infections: what recommendations can be made? Expert Rev Anti Infect Ther. 2017;15:1113–1121. doi: 10.1080/14787210.2017.1404451. [DOI] [PubMed] [Google Scholar]
- 10.Manian FA, Meyer L. Surgical-site infection rates in patients who undergo elective surgery on the same day as their hospital admission. Infect Control Hosp Epidemiol. 1998;19:17–22. doi: 10.1086/647701. [DOI] [PubMed] [Google Scholar]
- 11.Durkin MJ, Dicks KV, Baker AW, et al. Seasonal variation of common surgical site infections: does season matter? Infect Control Hosp Epidemiol. 2015;36:1011–1016. doi: 10.1017/ice.2015.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Reinisch A, Heil J, Woeste G, Bechstein W, Liese J. The meteorological influence on seasonal alterations in the course of acute appendicitis. J Surg Res. 2017;217:137–143. doi: 10.1016/j.jss.2017.05.015. [DOI] [PubMed] [Google Scholar]
- 13.Anthony CA, Peterson RA, Sewell DK, et al. The seasonal variability of surgical site infections in knee and hip arthroplasty. J Arthroplasty. 2018;33:510–514e1. doi: 10.1016/j.arth.2017.10.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schwab F, Gastmeier P, Meyer E. The warmer the weather, the more gram-negative bacteria—impact of temperature on clinical isolates in intensive care units. PLoS One. 2014;9 doi: 10.1371/journal.pone.0091105. e91105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fisman D, Patrozou E, Carmeli Y, et al. Geographical variability in the likelihood of bloodstream infections due to gram-negative bacteria: correlation with proximity to the equator and health care expenditure. PLoS One. 2014;9 doi: 10.1371/journal.pone.0114548. e114548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Eber MR, Shardell M, Schweizer ML, Laxminarayan R, Perencevich EN. Seasonal and temperature-associated increases in gram-negative bacterial bloodstream infections among hospitalized patients. PLoS One. 2011;6 doi: 10.1371/journal.pone.0025298. e25298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Brown PT, Caldeira K. Greater future global warming inferred from Earth‘s recent energy budget. Nature. 2017;552:45–50. doi: 10.1038/nature24672. [DOI] [PubMed] [Google Scholar]
- 18.Hoffmann P, Spekat A. Warum sollten wir eine globale Erwärmung von mehr als 2 Grad vermeiden? In: Lozán JL, Breckle SW, Graßl H, Kasang D, Weisse R, editors. Warnsignal Klima: Extremereignisse: Wissenschaftliche Fakten. Hamburg Wissenschaftliche Auswertungen: 2018. pp. 345–350. [Google Scholar]
- 19.World Meteorological Organization. WMO Statement on the State of the Global Climate in 2018. www.library.wmo.int/doc_num.php?explnum_id=5789 (last accessed on 3 May 2019) [Google Scholar]
- 20.Coumou D, Robinson A, Rahmstorf S. Global increase in record-breaking monthly-mean temperatures. Climatic Change. 2013;118:771–782. [Google Scholar]
- 21.Lehmann J, Coumou D, Frieler K. Increased record-breaking precipitation events under global warming. Climatic Change. 2015;132:501–515. [Google Scholar]
- 22.Brandt C, Hansen S, Sohr D, Daschner F, Ruden H, Gastmeier P. Finding a method for optimizing risk adjustment when comparing surgical-site infection rates. Infect Control Hosp Epidemiol. 2004;25:313–318. doi: 10.1086/502398. [DOI] [PubMed] [Google Scholar]
- 23.Brandt C, Sohr D, Behnke M, Daschner F, Ruden H, Gastmeier P. Reduction of surgical site infection rates associated with active surveillance. Infect Control Hosp Epidemiol. 2006;27:1347–1351. doi: 10.1086/509843. [DOI] [PubMed] [Google Scholar]
- 24.Menz C. Dokumentation des Interpolationsverfahrens. www.swift.dkrz.de/v1/dkrz_a88e3fa5289d4987b4d3b1530c9feb13/ReKliEs-De/Supplement/Info/Interpolationsverfahren_PIK.pdf (last accessed on 3 May 2019) [Google Scholar]
- 25.Deutsche Gesellschaft für Epidemiologie (DGEpi) Leitlinien und Empfehlungen zur Sicherung von guter epidemiologischer Praxis (GEP) Langversion. www.dgepi.de/assets/Leitlinien-und-Empfehlungen/66777155c7/Leitlinien_fuer_Gute_Epidemiologische_Praxis_GEP_vom_September_2018.pdf (last accessed on 3 May 2019) [Google Scholar]
- 26.National Nosocomial Infections Surveillance S. National Nosocomial Infections Surveillance (NNIS) system report, data summary from January 1992 through June 2004, issued October 2004. Am J Infect Control. 2004;32:470–485. doi: 10.1016/S0196655304005425. [DOI] [PubMed] [Google Scholar]
- 27.Weigelt JA, Lipsky BA, Tabak YP, Derby KG, Kim M, Gupta V. Surgical site infections: causative pathogens and associated outcomes. Am J Infect Control. 2010;38:112–120. doi: 10.1016/j.ajic.2009.06.010. [DOI] [PubMed] [Google Scholar]
- 28.Leekha S, Diekema DJ, Perencevich EN. Seasonality of staphylococcal infections. Clin Microbiol Infect. 2012;18:927–933. doi: 10.1111/j.1469-0691.2012.03955.x. [DOI] [PubMed] [Google Scholar]
- 29.Durkin MJ, Dicks KV, Baker AW, et al. Postoperative infection in spine surgery: does the month matter? J Neurosurg Spine. 2015;23:128–134. doi: 10.3171/2014.10.SPINE14559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Smits SA, Leach J, Sonnenburg ED, et al. Seasonal cycling in the gut microbiome of the Hadza hunter-gatherers of Tanzania. Science. 2017;357:802–806. doi: 10.1126/science.aan4834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Beveridge TJ. Structures of gram-negative cell walls and their derived membrane vesicles. J Bacteriol. 1999;181:4725–4733. doi: 10.1128/jb.181.16.4725-4733.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Perencevich EN, McGregor JC, Shardell M, et al. Summer peaks in the incidences of gram-negative bacterial infection among hospitalized patients. Infect Control Hosp Epidemiol. 2008;29:1124–1131. doi: 10.1086/592698. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eMETHODS
Statistical analysis
In our analysis, we evaluated all surgical site infections (SSI) and subgroups of SSI as endpoints. These are defined by identification of pathogens (all SSI, SSI without pathogen identification, SSI with pathogen identification, SSI caused by gram-positive pathogens, SSI caused by gram-negative pathogens), and by depth of the SSI (all depths of infection [A1, A2, A3] combined, superficial SSI [A1] and deep SSI [A2 and A3]).
In the descriptive analysis, we calculated for each of the different endpoints the number of SSI and the SSI rates (per 1000 operative procedures) with 95% confidence intervals. We used the chi-squared test to test for differences between categories of the independent parameters.
Our analyses included the following climate parameters: monthly mean temperature as a continuous and as a categorical variable (<5/5 to <10/10 to <15/15 to <20/= 20°C), rainfall (mm), relative humidity (%), vapor pressure (hectopascal), number of sunshine hours per month, number of heat days (maximum temperature >30°C) per month, number of ice days (maximum temperature <0°C) per month, number of heavy rainfall days (>30 mm per day) as well as longitude and latitude.
There was an association between temperature and the calendar month. This association is depicted in eFigure 2, showing the variability of temperature within one month at all observation points during the observation period.
Besides the climatic parameters, we included the following independent risk factors and confounders in our analysis:
In the univariable analysis, a logistic regression model was calculated for each endpoint and each independent parameter (climate parameters, department-related parameters, patient-related parameters).
In the multivariable analysis, adjusted odds ratios with 95% confidence intervals were calculated for the mean monthly temperature. Here, we calculated a model with temperature as a continuous parameter (per 1°C) and a model with temperature as a categorical variable with temperature ranges (<5/5 to <10/10 to <15/15 to <20/= 20°C), using temperatures <5°C as a reference. The following parameters were included in all multivariable models: sex, age, type of operative procedure, NNIS risk index, access route of surgery, and year of surgery.
The department-specific parameter “type of hospital care” (maximum-care hospital: yes, no, unknown)
The patient-related factors “sex” and “age group” (<30, 30–39, …, = 80 years)
NNIS risk index: duration of surgery, wound contamination class, ASA score: One point is awarded for each of the following: duration of surgery >75% percentile, ASA score =3, wound contamination class = 3) (NNIS = National Nosocomial Infections Surveillance System; ASA = American Society of Anesthesiologists)
The type of surgery stratified into 7 groups (general and other surgery, abdominal surgery, trauma & orthopedic surgery, urology, gynecology, cardiac surgery, vascular surgery)
The surgical access route (laparoscopic/open)
The season (December–February, March–May, June–August, September–November)
as well as the year of surgery.