Abstract
The light‐sensitive photoreceptors in the retina are extremely metabolically demanding and have the highest density of mitochondria of any cell in the body. Both physiological and pathological retinal vascular growth and regression are controlled by photoreceptor energy demands. It is critical to understand the energy demands of photoreceptors and fuel sources supplying them to understand neurovascular diseases. Retinas are very rich in lipids, which are continuously recycled as lipid‐rich photoreceptor outer segments are shed and reformed and dietary intake of lipids modulates retinal lipid composition. Lipids (as well as glucose) are fuel substrates for photoreceptor mitochondria. Dyslipidemia contributes to the development and progression of retinal dysfunction in many eye diseases. Here, we review photoreceptor energy demands with a focus on lipid metabolism in retinal neurovascular disorders.
Keywords: dyslipidemia, FGF21, photoreceptor, retinal metabolism, β‐oxidation
Subject Categories: Neuroscience, Vascular Biology & Angiogenesis
Glossary
- Autophagy
A process induced under stress to process cellular wastes to reduce toxicity and provide fuel for mitochondria.
- Fatty acid β‐oxidation
The catabolic process that breaks down fatty acid molecules to generate acetyl‐CoA, which in mitochondria enters the citric acid cycle for energy (ATP) production. β‐oxidation occurs in the peroxisomes and mitochondria but in peroxisomes no ATP is produced.
- Glycolysis
The process of breaking down glucose into pyruvic acid for energy production.
- Neovascularization
Uncontrolled blood vessel growth in the eye. New vessels are often fragile and leaky, causing blindness in the late stage of neovascular retinal diseases.
- Oxidative phosphorylation
The process to form ATP through the transfer of electrons from NADH or FADH2 to oxygen by a series of electron carriers in the mitochondrial membrane.
- Photoreceptors
A retinal neuronal cell that is capable of visual phototransduction, converting light signals to electric signals. They possess the highest density of mitochondria in the body.
- Retinal pigment epithelium
The pigmented cell layer provides nutrients and clears wastes for photoreceptors.
Photoreceptor biology and retinal lipid use
Energy demands of the retina
Vertebrate retinas are light‐sensitive neural tissues. Rod and cone photoreceptors of the retina utilize photosensitive pigments to convert photons into electrical impulses (phototransduction; Arshavsky et al, 2002). The retina uses more energy in the dark than in light to maintain the “dark current”. In the light, there is an ongoing outward potassium (K+) current through non‐gated K+‐selective channels, which induces sodium (Na+) ion channel closure and hyperpolarization of photoreceptors. In the light, glutamate release is suppressed and neurons are excited, leading to phototransduction.
By contrast, in the dark, perpetually open (Na+) channels allow a steady flow of ions into the cell, thereby resulting in cellular depolarization (dark current) and glutamate release, which inhibits photoreceptor excitation (Stryer, 1991). More than half of photoreceptor energy (adenosine triphosphate, ATP) is used by Na+/K+ ATPase ion pumps to maintain intracellular ion levels (Hagins et al, 1970; Okawa et al, 2008).
The replacement of shed photoreceptor outer segments is also energy intensive (Du et al, 2015; Ng et al, 2015). Photoreceptors maintain a consistent outer segment length by balancing disk shedding and assembly (Young, 1967; Young & Bok, 1969; LaVail, 1976). Continuous shedding of “used” outer segments containing lipids damaged by light and oxidation is critical for the maintenance of normal retinal function (Fliesler & Anderson, 1983) perhaps as a fuel source scavenged by retinal pigment epithelium (RPE). Other lipid fuel sources may be serum lipids processed by Müller glial cells, and lipids synthesized at a high rate in the inner segments (Wang et al, 2005; Kevany & Palczewski, 2010; Casson et al, 2013). The details of lipid processing in the retina are not fully defined; however, some lipids are used as fuel/energy sources while the others, which cannot be synthesized, are recycled (Chen & Anderson, 1993; Mukherjee et al, 2007).
Fuel sources for the retina to make ATP
ATP, used to transfer energy, is generated via two metabolic pathways: glycolysis in the cytoplasm and oxidative phosphorylation (OXPHOS) in mitochondria. Glycolysis converts one glucose to two pyruvates (yielding 2 ATP). In the presence of oxygen, pyruvate is further converted to acetyl‐CoA, which enters the Krebs cycle (yielding 2 ATP) and forms electron donors for OXPHOS (yielding 34 ATP). However, when there is an oxygen shortage, pyruvate is converted into lactate. In the retina, even without oxygen deficits glucose is mostly metabolized though glycolysis rather than OXPHOS (called aerobic glycolysis or the Warburg effect). It has been shown that in pig retinal explants, only 20% of glucose is oxidized (Wang et al, 1997); despite access to oxygen, 80% is used for glycolysis, which provides intermediates for outer segment synthesis.
The remaining oxidation is of carbons derived from non‐glucose sources such as lipids (Joyal et al, 2016). In rat and rabbit retinas, there is no difference in lactate production in darkness and in light, indicating that aerobic glycolysis is not required for the energy needed to maintain the dark current (Cohen & Noell, 1960; Winkler, 1981). The dark current is maintained with OXPHOS, using over 40% of retinal oxygen (Ames et al, 1992). The fuel source for OXPHOS was unknown for almost 60 years, as most glucose clearly is not metabolized through OXPHOS (Cohen & Noell, 1960). Recently, lipid oxidation (palmitic acid C16:0) was found to be used for energy production in photoreceptor mitochondria (Joyal et al, 2016). The major fatty acids of physiological importance in energy metabolism are those with a chain length ≥ 16 carbons. Fatty acid β‐oxidation (OXPHOS) of one 16‐carbon fatty acid produces 129 ATP, while full oxidation of glucose produces 38 ATP. Knowledge of the potential impact of other fatty acids on retinal metabolism is still limited.
Cones versus rods in energy consumption and production
Cones are more metabolically active than rods (Nikonov et al, 2006; Okawa et al, 2008). In darkness, cones and rods have comparable ATP expenditures, and similar dark currents. However, in the light, rod responses are suppressed, thereby reducing total retinal energy consumption by > 75%. But cones do not saturate in bright light so the energy demand remains high. Even when cones are maximally bleached, they still have a baseline need that is more than 50% of the dark current (Nikonov et al, 2006).
It is essential to coordinately control the synaptic terminal ATP production and Ca2+ concentration to regulate transient exocytosis and ensure recovery for the next action potential (Johnson et al, 2007). Cones increase ATP production by increasing the number of mitochondria (about twofold more than rods) and mitochondrial cristae surface membrane area (about threefold more than rods; Perkins et al, 2003). Cones lower Ca2+ levels during light adaption and increase their response kinetics by utilizing a low affinity/high turnover Na+‐Ca2+ exchanger, while rods use high affinity/low turnover plasma membrane Ca2+ ATPase (Johnson et al, 2007). The knowledge of fuel use in cones and rods is still limited. Loss of hexokinase 2 (a key aerobic glycolysis enzyme) in rods inhibits rod function but not rod survival, while cone hexokinase 2 loss does not affect photoreceptor function (Petit et al, 2018), suggesting that aerobic glycolysis is not necessary for photoreceptor survival, but is a metabolic choice to maintain neuronal function.
Peroxisome fatty acid β‐oxidation
Fatty acid degradation, through β‐oxidation, takes place in both mitochondria and peroxisomes in mammals. Peroxisomal degradation breaks down long‐chain fatty acids (producing no ATP), into shorter chain fatty acids that can be used by mitochondria for further oxidation to acetyl‐CoA, which enters the Krebs cycle to produce ATP (Fig 1; Poirier et al, 2006; Schrader et al, 2015).
Figure 1. β‐oxidation pathway in peroxisome and mitochondria.
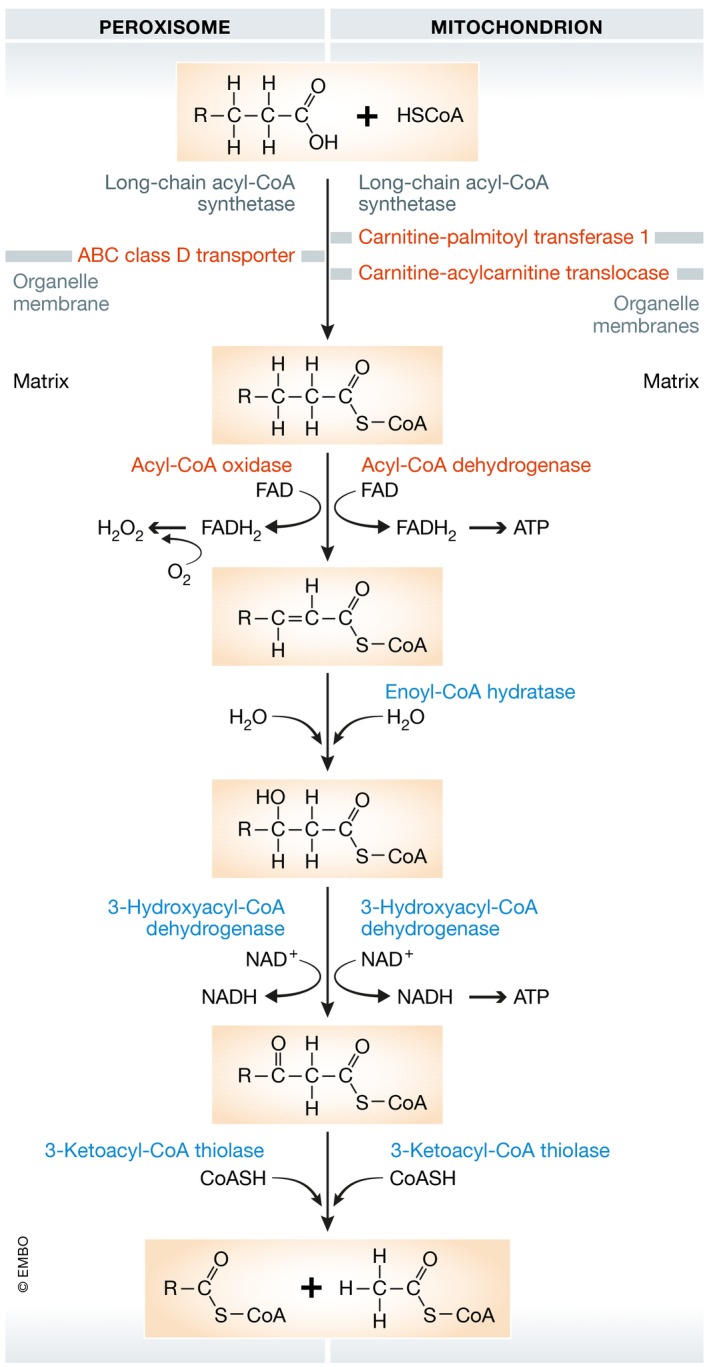
Very long‐chain monocarboxylic (≥22 carbons) and long‐chain dicarboxylic fatty acids are oxidized only in peroxisomes (Poirier et al, 2006). In addition, polyunsaturated fatty acids are also oxidized faster in peroxisomes than in mitochondria (Hiltunen et al, 1986). Long‐chain fatty acids (13–21 carbons) must first be conjugated to either coenzyme A (peroxisomes) or carnitine (mitochondria) outside the organelle and then imported into organelles by ABC class D transporters (peroxisomes) or carnitine‐acylcarnitine translocases (mitochondria). Fatty acids are subsequently degraded by β‐oxidation, which involves four enzymes and leads to the release of acetyl‐CoA, FADH2, and NADH (Fig 1). Acetyl‐CoA enters the Krebs cycle where it is oxidized into CO2 and H2O, and generates additional FADH2 and NADH. FADH2 and NADH from β‐oxidation and the Krebs cycle are then used for ATP production by the mitochondrial electron transport chain.
Retinal lipid composition
A rod photoreceptor has three functional domains: (i) synaptic terminal, (ii) inner segment, and (iii) outer segment (Fig 2A). One retinal lipid source comes from shed photoreceptor outer segments. Rod outer segments consist of stacks of photosensitive disks, which contain proteins (predominantly photosensitive pigments) and lipids (Fliesler & Anderson, 1983), predominantly phospholipids (90–95% of total lipids) and cholesterols (4–6%) (Daemen, 1973).
Figure 2. Schematics of photoreceptor and retinal structure.
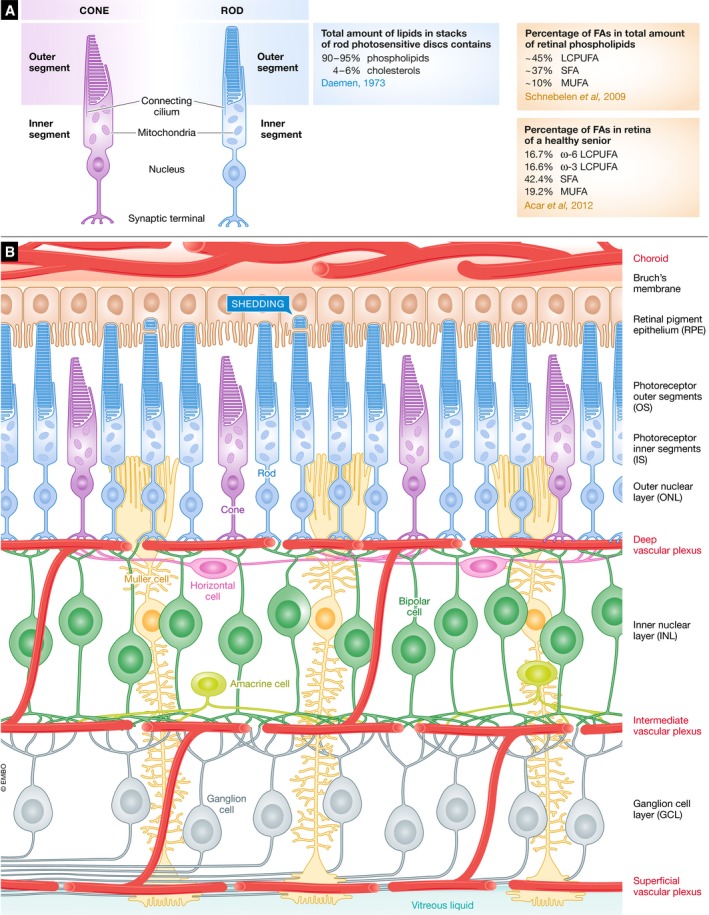
(A) Schematics of rod and cone structure. (B) Schematics of retinal neuronal and vascular arrangement. RPE, retinal pigment epithelium; OS/IS, outer segments/inner segments; ONL, outer nuclear layer; INL, inner nuclear layer; GCL, ganglion cell layer.
Phospholipids consist of a phosphate “head” with fatty acid “tails”, which can be cleaved to provide fatty acids. Retinal phospholipids contain an abundance of long‐chain polyunsaturated fatty acid (LCPUFA, ~45% of total phospholipids), saturated fatty acid (SFA, ~37% of total phospholipids), and monounsaturated fatty acid (MUFA, ~10% of total phospholipids; Schnebelen et al, 2009). While fatty acid composition analysis in the human retina is limited, the retina of a healthy senior is composed of 16.7% ω‐6 LCPUFA, 16.6% ω‐3 LCPUFA, 42.4% SFA, and 19.2% MUFA (Acar et al, 2012).
During postnatal development, rod lipid composition transitions from rich in saturated fatty acids to rich in unsaturated fatty acids (Scott et al, 1988). The increasing unsaturated lipid portion of the maturing retina is biased for selective accretion of docosahexaenoic acid (DHA) while arachidonic acid (AA) levels are reduced (Alessandri & Goustard‐Langelier, 2001). Docosahexaenoic acid accounts for approximately 35% of total phospholipid FA in the retina and 50% in rod outer segments (Stinson et al, 1991), while AA accounts for approximately 8–10% of total phospholipid fatty acid in rod outer segments.
Transcriptional control of retinal cell functions
The retina contains more than 10 different cell types that contribute uniquely to phototransduction (Fig 2B), requiring a highly individualized gene expression pattern. The emergence of single‐cell transcriptomics (scRNAseq) provides insight into the metabolism of individual cells within the retina, which is likely to lead to a greater understanding of the cellular metabolic influences on neovascularization. scRNAseq examines the combinatorial expression of genes, which leads to the clustering of retinal cells according to their gene expression patterns (Fig 3A; Macosko et al, 2015). The scRNAseq approach is very efficient in discovering new retinal cell subtypes (Shekhar et al, 2016; Rheaume et al, 2018). Beside identity markers associated with specialized functions (like phototransduction in photoreceptors; Fig 3B), different retinal cells regulate specific metabolic genes at the transcriptional level to perform certain functions. However, caution is required when analyzing transcriptomic data from rod photoreceptors, as this cell type has low basal gene expression (Macosko et al, 2015), which is correlated with a uniquely closed chromatin architecture compared to cones (Hughes et al, 2017). Moreover, rods are very sensitive to single‐cell dissociation since the end part of rod outer segments is buried in the RPE and may be separated from the main cell body during retinal digestion. Single‐nucleus RNAseq may therefore be more suitable to assess the rod transcriptome in situ (Habib et al, 2017).
Figure 3. Cell‐specific transcriptional regulation of retinal functions.
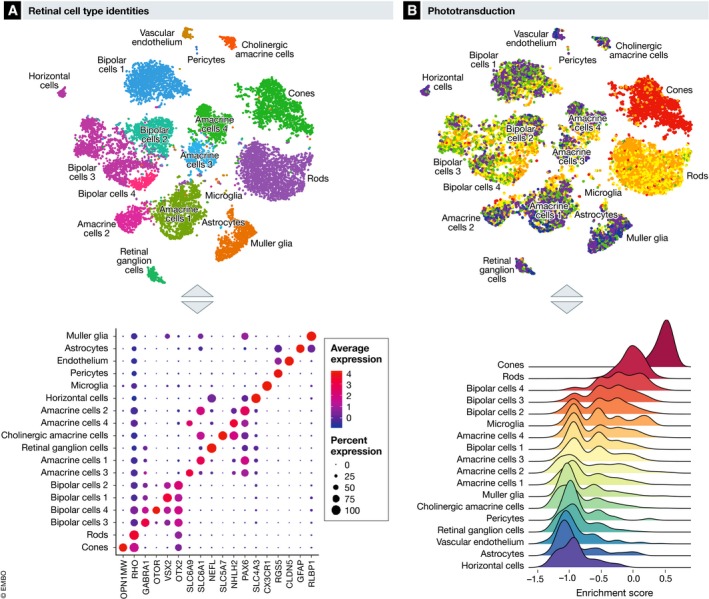
Adapted from Macosko et al, Cell 2015. (A) Retinal cell types can be identified using single‐cell RNAseq based on cell‐specific expression of genes markers. OPN1MW, Opsin 1, medium wave sensitive; RHO, rhodopsin; GABRA1, gamma‐aminobutyric acid type A receptor alpha1 subunit; OTOR, otoraplin; VSX2, visual system homeobox 2; OTX2, orthodenticle homeobox 2; SLC6A9, solute carrier family 6 member 9; SLC6A1, solute carrier family 6 member 1; NEFL, neurofilament light; SLC5A7, solute carrier family 5 member 7; NHLH2, nescient helix‐loop‐helix 2; PAX6, paired box 6; SLC4A3, solute carrier family 4 member 3; CX3CR1, C‐X3‐C motif chemokine receptor 1; RGS5, regulator Of G protein signaling 5; CLDN5, claudin 5; GFAP, glial fibrillary acidic protein; RLBP1, retinaldehyde binding protein 1. (B) Transcriptomic enrichment for specific pathway such as the phototransduction pathways can be scored using gene set variation analysis based on highly variable genes between retinal cell types.
Dyslipidemia in neurovascular retinopathies
Metabolic dysfunction and dyslipidemia produce deleterious effects on the eye (Folz & Trobe, 1991; Chang & Wu, 2013; Yonekawa et al, 2015). Dyslipidemia is characterized by an abnormal circulating lipid profile including triglycerides, cholesterol, low‐density lipoproteins (LDL), high‐density lipoproteins (HDL), or polyunsaturated fatty acids. In premature infants, high triglycerides are associated with increased severity of retinopathy of prematurity (ROP; Sinclair et al, 2018). The ω‐6 LCPUFA, arachidonic acid, level is also significantly lower in severe ROP in premature infants at postmenstrual age of 32 weeks (Lofqvist et al, 2018). Although the results from many studies exploring the associations between diabetic retinopathy (DR) and lipid abnormality are inconsistent, one study found that high circulating LDL cholesterol levels are a significant risk factor for diabetic macular edema and retinal hard exudates (Chang & Wu, 2013). In advanced age‐related macular degeneration (AMD), high HDL cholesterol levels are implicated in the disease pathogenesis in European and Asian populations (Cougnard‐Gregoire et al, 2014; Fan et al, 2017). A recent European Eye Epidemiology consortium study found that HDL is associated with an increased risk of AMD and drusen development, while triglycerides are associated with a decreased risk of AMD and drusen development. Variants in lipid genes and their association with cholesterol levels are unclear. The cholesteryl ester transfer protein risk variant (rs17231506) for AMD was associated with increased HDL cholesterol levels, but lipase C risk variants (rs2043085, rs2070895) were negatively linked with HDL cholesterol levels (Colijn et al, 2019). In retinitis pigmentosa (RP) versus control patients, decreased plasma ω‐3 and ω‐6 LCPUFA are found (Converse et al, 1983; Holman et al, 1994).
Dietary modulation of the lipid supply can positively influence diseases with pathological neovascularization such as ROP, AMD, and DR in patients and in animal models of retinopathy (Gong et al, 2017). Photoreceptor energy demands drive vessel growth (Sapieha, 2012; Joyal et al, 2016, 2018; Fu et al, 2018), while photoreceptor‐derived oxidative stress and inflammation lead to retinal vascular damage or regression (Kern & Berkowitz, 2015; Sun et al, 2017). Retinal disorders such as ROP, DR, AMD, RP, and Zellweger spectrum disorder (ZSSD) are associated with disturbances in photoreceptor activity, which may further affect the blood supply and induce pathological vascular remodeling during disease progression.
Retinopathy of prematurity
Retinopathy of prematurity is a leading cause of blindness in children worldwide (Hellstrom et al, 2013). After preterm birth, the immature retinal vasculature growth is suppressed, secondary to oxygen supplementation, loss of growth factors provided in utero, and metabolic dysregulation. As the neural retina slowly matures, metabolic demand increases, particularly in photoreceptors. The relatively avascular retina becomes hypoxic and deprived of nutrients, driving vascular growth factor expression and subsequent neovascularization. The onset of neovascular ROP at ~32 weeks postmenstrual age coincides with the rapid development and increased metabolic demand of rods (Fulton et al, 1999; Hansen et al, 2017). This observation is supported by rodent studies. In mice, hyperglycemia (a key risk factor for ROP) triggers photoreceptor metabolic alterations and delays retinal vascular development (Fu et al, 2018). In rats, early photoreceptor dysfunction also predicts subsequent neovascularization (Akula et al, 2010).
In premature infants, there is a ~44% decrease in DHA after preterm birth, and serum DHA levels remain low for at least 4 weeks (Lapillonne & Jensen, 2009; Martin et al, 2011). Severe ROP is reduced in premature infants (GA < 32 weeks) receiving ω‐3 LCPUFA versus parenteral soybean and olive oil supplementation (Pawlik et al, 2014). There is also an association between low serum levels of ω‐6 LCPUFA (AA) and later development of ROP (Lofqvist et al, 2018). In mice, dietary ω‐3 versus ω‐6 LCPUFA suppresses retinal neovascularization (Connor et al, 2007; Fu et al, 2017b). Further studies on the impact of DHA and AA and other lipids on photoreceptor function and metabolism are needed.
Diabetic retinopathy
In addition to ROP, DR is also associated with abnormal energy metabolism. DR, a significant complication of diabetes, starts with vascular loss (non‐proliferative DR), followed by neovascularization (proliferative DR). In DR, abnormalities in retinal neural responses occur early before vascular abnormalities are seen, suggesting that neuronal metabolic demands drive vessel growth (De Benedetto et al, 2014; Pescosolido et al, 2015). Mitochondrial dysfunction is accompanied by oxidative stress (Barot et al, 2011), which induces a wide range of microvascular abnormalities throughout the course of DR (Kowluru & Mishra, 2015). In diabetic mice, photoreceptors with their high density of mitochondria contribute to the majority of induced retinal oxidative stress and inflammation, which is associated with retinal vessel loss in DR (Du et al, 2013; Liu et al, 2016; Tonade et al, 2016).
There is clear evidence of neurovascular cross talk in DR. In patients with both proliferative DR and progressive photoreceptor degeneration (RP), spontaneous neovascular regression occurs when photoreceptor loss from RP becomes clinically evident (Lahdenranta et al, 2001). As there is higher retinal energy consumption in darkness versus light, illuminating the retina with 507‐nm light during sleep might reduce the risk of DR progression (Sivaprasad & Arden, 2016). In fact, exposure to a 505‐nm light during sleep leads to the regression of macular edema and improved visual function in early DR patients (Arden et al, 2011). However, a recent multi‐year phase 3 clinical trial (CLEOPATRA) of wearing a light mask at night in DR patients failed to support the hypothesis that decreasing energy needs for photoreceptor “dark current” would inhibit diabetic macular edema (Sivaprasad et al, 2018).
Dyslipidemia is associated with more retinal abnormalities and faster progression of DR (Sacks et al, 2014; Hammer & Busik, 2017). Increasing dietary PUFA versus saturated FA is associated with a reduced incidence and severity of DR (Sasaki et al, 2015). A Mediterranean diet with olive oil or nut supplements showed an additional 48% decrease in incidence of DR in type 2 diabetes when the diet also included ≥ 500 mg/day DHA plus eicosapentaenoic acids, or at least 2 weekly servings of oily fish (Sala‐Vila et al, 2016). In murine models of early DR, fish oil and a ω‐3 LCPUFA‐enriched diet preserve retinal neuronal function (Yee et al, 2010; Sapieha et al, 2012). Linoleic acid‐ versus saturated‐fat‐rich diets inhibit progression of diabetic microangiopathy (Houtsmuller et al, 1980). Not all lipids appear to affect DR. The absence of acid sphingomyelinase (ASM) in ASM−/− mice or inhibition of ASM activity by DHA inhibited the diabetes‐induced degeneration of retinal capillaries (Opreanu et al, 2011). Studies show no association between total cholesterol or high‐density lipoprotein and incidence of DR or macular edema in long‐term type 1 diabetes (Klein et al, 2015). Taken together, these findings suggest a link between some aspects of dyslipidemia and DR progression. As such, dietary modulation of specific lipids may help prevent or treat DR.
Age‐related macular degeneration
The human macula, critical for central vision, consists of a small cone‐dominated fovea surrounded by a rod‐dominated parafovea. AMD particularly affects the macula and is the leading cause of legal blindness in the elderly. Clinically, AMD is classified as either dry or wet (neovascular) based on the absence or presence of pathologic blood vessels invading into the photoreceptor layer (Ambati & Fowler, 2012). Current treatments mainly target neovascular AMD; no drugs are approved by the U.S. Food and Drug Administration for dry AMD.
Mitochondrial morphological changes and dysfunction occur in degenerating macular cones and likely contribute to AMD progression (Barron et al, 2001; Litts et al, 2015). Mitochondrial abnormalities can cause overproduction of superoxide radicals, primarily in the electron transport chain (Fig 4; Selivanov et al, 2011). Oxidative stress causes damage to cell structures, lipids, proteins, and DNA, and particularly affects metabolically active neuronal cells, like photoreceptors. Photoreceptor loss is seen in AMD donor eyes (Curcio et al, 1996). In aging and in early AMD, there is a prominent decline in rod‐mediated ERG sensitivity (Jackson et al, 2002). Additionally, cone dysfunction can predict early AMD and is a reliable measure of AMD progression (Hogg & Chakravarthy, 2006).
Figure 4. Schematics of electron transport chain (ETC).
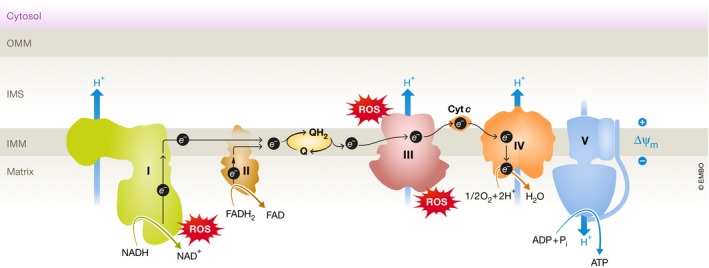
The ETC passes electrons from NADH and FADH 2 to protein complexes (I to V) and mobile electron carriers coenzyme Q (CoQ) and cytochrome c (Cyt c). Oxygen (O2) is the final electron recipient. The transfer of electrons generates energy to pump protons (H+) from the mitochondrial matrix into the intermembrane space. An electrochemical proton gradient is created across the inner mitochondrial membrane, allowing the protons to pass through complex V (ATP synthase) to generate adenosine triphosphate (ATP) from adenosine diphosphate (ADP). Complex I, NADH coenzyme Q reductase, complex II, succinate dehydrogenase, complex III, cytochrome bc 1 complex, complex IV, cytochrome c oxidase. Complex I and complex III are the main sites for superoxide (ROS) formation.
As well as mitochondrial dysfunction, dyslipidemia is also implicated in dry AMD pathogenesis (Gong et al, 2017). The most established clinical hallmark of dry AMD is the formation of subretinal drusen, extracellular deposits rich in lipid and protein (Hageman et al, 2001). Although the exact mechanism of drusen formation is unclear, Bruch's membrane, found between the choriocapillaris and RPE, thickens due to accumulation of oxidized lipids, lipid‐related molecules, and inflammatory debris preceding drusen formation. This slows down nutrient and waste transportation between RPE cells and choroidal vessels, leading to malfunction of RPE cells (Sarks et al, 2007; Curcio, 2018a,b).
Epidemiologic studies link increased HDL levels with AMD across different populations (Fan et al, 2017; Colijn et al, 2019). Genome‐wide association studies also identify several HDL cholesterol genes associated with AMD susceptibility, including genes encoding ATP‐binding cassette transporter A1 (ABCA1), cholesteryl ester transfer protein (CETP), apolipoprotein E (APOE), hepatic lipase C (LIPC), and lipoprotein lipase precursor (LPL) (Chen et al, 2010; Neale et al, 2010; Fritsche et al, 2013, 2016). Impaired ABCA1‐mediated cholesterol efflux in mouse RPE cells or subretinal macrophages induces lipid accumulation and retinal degeneration (Lyssenko et al, 2018; Storti et al, 2019). ApoE is important for the transport of lipids across cell membranes and is highly expressed by RPE cells. ApoE‐null mice exhibit raised serum triglycerides and cholesterol. Thickened Bruch's membrane, and accumulation of lipid deposits in the basal RPE and Bruch's membrane are seen in ApoE‐null and genetically engineered ApoE‐mutated mice (Malek et al, 2005; Edwards & Malek, 2007). However, how CETP, LIPC, and LPL link to AMD pathogenesis is still unclear.
High plasma levels of high‐density lipoprotein cholesterol are associated with an increased risk for advanced AMD (Fan et al, 2017). A 42% decreased incidence of AMD is associated with high plasma ω‐3 LCPUFA levels in a large cohort study of US female health professionals (Christen et al, 2011). A 30% decrease in central geographic atrophy development and a 50% decrease in neovascular AMD development are found in participants with high versus low ω‐3 LCPUFA intake in the Age‐Related Eye Disease Study (AREDS) (Sangiovanni et al, 2009). There was no further reduced risk of progression to advanced AMD in participants with ω‐3 LCPUFA supplementation in the AREDS2 study; however, the participants had a much higher baseline level of circulating ω‐3 LCPUFA in comparison with those in the first AREDS (Souied et al, 2015). It also may be that other fats in fish (alone or in combination with DHA) are required to suppress AMD progression. In a Japanese population with a high baseline intake of fish oil, there was no significant association between serum ω‐3 LCPUFA levels and AMD progression (Kabasawa et al, 2011). Dietary ω‐3 LCPUFA inhibits neovascularization in a laser‐induced wet AMD mouse model and in mice lacking the very low‐density lipoprotein receptor (VLDLR) (Fu et al, 2017b). The lack of VLDLR promotes the development of neovascularization originating from the superficial retinal vasculature similar to some neovascularization seen in AMD. The lack of VLDLR leads to intracellular lipid and glucose insufficiency which drives neovascularization, including retinal angiomatous proliferation and choroidal neovascularization (Joyal et al, 2016). Therefore, both clinical and experimental investigations support the concept that dyslipidemia may be associated with AMD progression.
Retinitis pigmentosa
Retinitis pigmentosa is also associated with abnormal energy metabolism. There are ~60 genes (to date), mostly expressed in rods, which are involved in RP retinal degenerations (Ali et al, 2017). In RP, the initial loss of rods results in night blindness and loss of peripheral vision; central (cone) vision is initially preserved but eventually central vision is also lost, secondary to a bystander effect (Punzo et al, 2009; Ait‐Ali et al, 2015). In mouse models of RP, 34.9% of gene expression changes following cone loss are associated with cellular metabolism (Punzo et al, 2009), suggesting that improving fuel sources (perhaps such as lipids for FA β‐oxidation) may improve cone metabolism. Mathematical models predict that preventing a 1–2% decrease in nutrients can permanently halt cone death even when 90% have already died (Camacho et al, 2016). Therefore, improving nutrient availability is a reasonable general approach to increase cone survival in RP. In RP patients, reduced ocular blood flow is also described as possibly associated with a decreased neuronal demand for nutrient supply (Falsini et al, 2011). Further studies are needed to establish the link between retinal vascular changes at different stages of RP.
Zellweger syndrome spectrum disorders
Zellweger syndrome spectrum disorders (ZSSD), including Zellweger syndrome, neonatal adrenoleukodystrophy, and infantile Refsum disease (Smith et al, 2016), are caused by defects in any of the peroxisomal PEX genes (Crane, 2014), resulting in peroxisomal lipid metabolic dysfunction. As peroxisomes break down long‐chain fatty acids to shorter length chains that can be used in mitochondria, deficits in PEX genes often also result in mitochondrial dysfunction. In ZSSD, the central nervous system is severely affected (Vamecq et al, 2014), producing unique ocular deficits including pigmentary retinopathy and optic atrophy, corneal opacification, cataract, and glaucoma (Folz & Trobe, 1991). In addition, attenuated retinal vasculature and macular edema are reported in infantile Refsum disease (Pakzad‐Vaezi & Maberley, 2014).
Mitochondrial perturbation rapidly occurs following the loss of functional peroxisomes (Salpietro et al, 2015; Schrader et al, 2015). Very long‐chain fatty acids (VLCFAs) accumulate in ZSSD; however, DHA (22:6ω3) is reduced in the plasma and brain (Poulos et al, 1986; Harding et al, 1999). Docosahexaenoic acid treatment maintains visual acuity and retinal function in patients with peroxisome biogenesis disorders (Noguer & Martinez, 2010). Over‐accumulation of VLCFAs is also found in mouse models of peroxisomal biogenesis defects (Baes, 2000; Baes & Van Veldhoven, 2012). VLCFAs may affect membrane properties (Sassa & Kihara, 2014), and defects in the breakdown of VLCFAs may also cause substrate shortage for mitochondrial fatty acid β‐oxidation.
Pathways modifying metabolic lipid use
Peroxisome proliferator‐activated receptor‐alpha (PPARα) and PPARγ are nuclear receptors involved in modulating lipid metabolic homeostasis. PPARα controls lipoprotein lipase expression and triglyceride metabolism, while PPARγ upregulates enzymes involved in steps of fatty acid metabolism like fatty acid entry into mitochondria and peroxisome (Gervois et al, 2000). Genetic deficiency of PPARα in mice leads to a decrease in lipid transporters and retinal degeneration (Pearsall et al, 2017). PPARγ is required for ω‐3 LCPUFA‐induced attenuation of mouse retinal neovascularization (Stahl et al, 2010). PPARγ coactivator‐α (PGC‐1α) regulates mitochondrial biogenesis and respiration (Alaynick, 2008). High‐fat diet‐exposed mice are more likely to develop AMD‐like phenotypes with lack of PGC‐1α (Zhang et al, 2018). PGC‐1α activation increases RPE metabolism and protects against oxidative damage (Satish et al, 2018). Therefore, PPARα, PPARγ, and PGC‐1α may modulate retinal lipid metabolism and therefore be pathways to manipulate in disease treatment.
Cyclooxygenases (COX), lipoxygenases (LOX), and cytochromes P450 (CYP)‐mediated LCPUFA metabolism is important in regulating ocular inflammation, particularly through the LOX and CYP pathways (Gong et al, 2017). Inhibiting COX does not affect proliferative retinopathy (Sapieha et al, 2011). LOX ω‐3 LCPUFA metabolites show anti‐inflammatory and anti‐angiogenic effects, while LOX ω‐6 LCPUFA metabolites are pro‐inflammatory and pro‐angiogenic (Sapieha et al, 2011). However, both CYP2C8 ω‐3 and ω‐6 LCPUFA metabolites are pro‐angiogenic and inhibition of CYP2C8 decreases ocular neovascularization (Shao et al, 2014; Gong et al, 2016a,b).
Genetic association with retinopathies
Understanding genetic susceptibility to ocular disorders may help understand disease mechanisms. In premature infants, gene mutations in vascular endothelial growth factor (VEGF) and insulin growth factor 1 (IGF1) are associated with advanced ROP (Holmstrom et al, 2007). The association of VEGF and IGF1 with ROP is further identified clinically and in animal models (Hellstrom et al, 2013). Genetics in DR has been widely explored. Mutations in metabolic genes such as aldose reductase, endothelial nitric oxide synthase (eNOS), receptor for advanced glycosylation end product (RAGE), adiponectin, peroxisome proliferator‐activated receptor α and γ, and superoxide dismutase 2 (MnSOD), growth factors like VEGF, and erythropoietin (EPO), as well as inflammatory factors like complement factor H (CFH) and CFB, interleukin 6 and interleukin 10, have a positive association with DR (Hampton et al, 2015). Genome‐wide association studies for diabetic macular edema identify a new associate single nucleotide polymorphism in rs1990145 on chromosome 2 (within the second intron of the mitochondrial ribosomal protein L19, MRPL19; Graham et al, 2018). The function of MRPL19 is unclear but other MRP genes including MRPL9 and MRPL23 are associated with retinitis pigmentosa (Kenmochi et al, 2001). Increasing evidence suggests a potential role of noncoding RNAs in regulating retinal inflammation during DR development (Gong & Su, 2017).
Delayed rod‐mediated dark adaption, the first functional biomarker for early AMD, is observed for both the age‐related maculopathy susceptibility 2 (ARMS2) A69S variant and the CFH Y402H variant in AMD patients. In healthy participants with normal macular function, the ARMS2 A69S variant also was associated with delayed rod‐mediated dark adaption (Mullins et al, 2019). In three population‐based studies, the Rotterdam Study, the Beaver Dam Eye Study, and the Blue Mountains Eye Study, single nucleotide polymorphisms in the genes ARMS2, CFH, and complement factor H‐related 5 (CFHR5) significantly increase the risk of late AMD (Buitendijk et al, 2013). In AMD patients with CFH and ARMS2 risk alleles, the treatment response to antioxidants is compromised (Awh et al, 2015). The CFH Y402H variant also seems to limit the effect of dietary DHA supplementation on CNV (Merle et al, 2015).
Potential therapeutic targets
Anti‐vascular endothelial growth factor (VEGF) agents are the primary treatment for pathological retinal vessel growth in eye diseases (Klufas & Chan, 2015; Bakri et al, 2019). However, anti‐VEGF treatment is not always effective (Lux et al, 2007; Nigam et al, 2008) and may remain in systemic circulation up to a few months after a single intravitreal injection (Moorthy & Cheung, 2009; Ueta et al, 2009; Hapani et al, 2010; Bressler et al, 2012; Jalali et al, 2013; Avery et al, 2014). VEGF is an important growth factor to neurons and blood vessels. Therefore, inhibition of VEGF may affect normal neurovascular function.
Since photoreceptor metabolic needs drive neovascularization, improved retinal lipid metabolism might be another strategy to prevent or treat neurovascular retinal diseases. Increasing lipid β‐oxidation by hormonal and transcriptional factor regulation, as well as dietary intervention, may protect retinal function and decrease the demand for neovessels. Targeting dysmetabolism‐induced inflammatory responses may also suppress neovascularization.
Fibroblast growth factor (FGF21), lipid metabolism, and autophagy
Since modulation of retinal metabolism may help restore energy homeostasis to prevent signaling for blood vessel recruitment and therefore prevent neovascularization, the end cause of neurovascular diseases, it is important to assess potential interventions that increase glucose uptake or increase fatty acid oxidation to improve energy homeostasis. A novel candidate for improved lipid metabolism is FGF21. FGF21 is a key metabolic regulator of lipid and glucose use (Kharitonenkov & Larsen, 2011; Lin et al, 2012; Markan et al, 2014). In type 2 diabetes, FGF21 decreases body weight and improves the lipid profile (Gaich et al, 2013; Talukdar et al, 2016). In obese type 2 diabetic mice, FGF21 lowers plasma triglycerides by lipoprotein catabolism in adipose tissue and maintains adipocyte phospholipid homeostasis (Foltz et al, 2012; Schlein et al, 2016; Ye et al, 2016; Stanislaus et al, 2017). FGF21 also increases lipid use in response to amino acid starvation (De Sousa‐Coelho et al, 2013). FGF21 functions through modulating the activities of PPAR and PGC‐1α. FGF21 is crucial for PPARα agonists to ameliorate metabolic disorders in obese mice (Goto et al, 2017). FGF21 regulates PPARγ activity and controls body fat (Dutchak et al, 2012). FGF21 also induces PGC‐1α to modulate glucose and fatty acid metabolism during starvation (Potthoff et al, 2009).
In diabetic mice with insulin deficiency, FGF21 enhances retinal antioxidant defense systems, reduces pro‐inflammatory cytokines, restores disorganized cone photoreceptor segments, and improves retinal function (Fu et al, 2018). FGF21 also regulates adiponectin (APN) production and secretion, and APN is key in mediating FGF21 modulation of glucose and lipid metabolism in mice (Holland et al, 2013; Lin et al, 2013). FGF21, mediated by APN which is associated with a number of metabolic retinal disorders (Fu et al, 2016), inhibits ocular neovascularization in mice (Fu et al, 2017a). FGF21 also increases APN secretion to diminish accumulation of ceramides in obese animals (Holland et al, 2013). Ceramide contributes to the development of DR and thus, modulating ceramide pathway may protect against DR progression (Fox et al, 2006; Opreanu et al, 2011).
Autophagy is induced in under stress (nutrient starvation, infection, or excess reactive oxygen species and recycles cytosolic components to remove damaged and dysfunctional cellular material to maintain cellular homeostasis, provide fuel, and recycle building blocks. In the retina, autophagy‐related proteins are mostly located in cellular layers that are rich in mitochondria and have high energy needs (Mitter et al, 2012). In addition, autophagy also plays an important role in phototransduction and rod integrity (Rodriguez‐Muela et al, 2012; Zhou et al, 2015). Aged mice with mutated autophagy genes have AMD‐like RPE defects (Zhang et al, 2017). Autophagy defects are also reported in human cells from AMD patients (Golestaneh et al, 2017). Some of the defects associated with autophagy deficiency are lipofuscin accumulation, reduced mitochondrial activity, and higher levels of reactive oxygen species—all of which affect angiogenesis. FGF21 influences autophagy. In mice, FGF21 is induced in neurons with mitochondrial dysfunction (Restelli et al, 2018). FGF21, induced with fasting, dephosphorylates transcription factor EB to induce genes involved in autophagy and lipid metabolism (Chen et al, 2017). In monosodium L‐glutamate‐induced obese mice, modeling nonalcoholic fatty liver disease, FGF21 induces autophagy to correct metabolic parameters (decreases triglycerides, improves insulin sensitivity) (Zhu et al, 2016). Further exploration of FGF21 in retinal lipid metabolism and autophagy is of great interest to evaluate its impact on retinal neurovascular stability.
Fenofibrate (PPARα agonist and CYP2C antagonist)
Fenofibrate, a PPARα agonist, increases fatty acid β‐oxidation and improves mitochondrial function. Deficiency of PPARα leads to shortage of retinal energy production and neurodegeneration (Pearsall et al, 2017). In two large‐scale clinical trials, fenofibrate prevents the progression of DR. In the FIELD study, fenofibrate was found to reduce the need for laser‐treatment of DR in type 2 diabetes patients by ~30% (Keech et al, 2007). In the ACCORD Eye study, fenofibrate was found to reduce DR progression by ~40% (ACCORD Study Group et al, 2010). Fenofibrate prevents pathological neovascularization in the rat OIR model by suppressing hypoxia‐inducible factor and VEGF (Chen et al, 2013). Fenofibrate also reduces retinal vascular leakage in a murine diabetic model (Chen et al, 2013). Fenofibrate administration reduces retinal vascular leakage and downregulates VEGF production in the mouse model of type 1 diabetes (Chen et al, 2013). Fenofibrate is also a CYP2C antagonist (Schoonjans et al, 1996; Walsky et al, 2005). CYP2C metabolites from ω‐3 and ω‐6 LCPUFA show pro‐angiogenic effects in mouse models of ROP and AMD (Shao et al, 2014; Gong et al, 2016a). Inhibition of CYP2C with fenofibrate decreases retinal neovascularization (Gong et al, 2016b). Therefore, fenofibrate is a potential candidate to treat neurovascular defects in retinal metabolic disorders.
Dietary ω‐3 LCPUFA intervention
The essential ω‐3 LCPUFA, DHA, influences neovascularization in retinopathy both in human patients and animal models. In AMD, increasing fish intake or ω‐3 LCPUFA supplementation (DHA, EPA) is associated with a decreased risk of AMD progression (Tan et al, 2009; Christen et al, 2011; Pinazo‐Duran et al, 2014). In premature infants and diabetic patients, plasma ω‐3 LCPUFA levels correlate with circulating APN and dietary intake of ω‐3 LCPUFA modulates circulating APN levels (Ito et al, 2014; Fu et al, 2015). In type 2 diabetic patients on a “healthy” Mediterranean diet, additional dietary intake of fish is associated with a 48% decreased incidence of proliferative DR (Sala‐Vila et al, 2016). In mouse models of proliferative ROP, DR, and AMD, dietary ω‐3 LCPUFA, mediated by APN, inhibits ocular neovascularization (Fu et al, 2015, 2017b).
Free fatty acid receptor 1 (FFAR1)
FFAR1, which is activated by medium‐ and long‐chain fatty acids (Briscoe et al, 2003), governs glucose transport by regulating the expression of retinal GLUT1 (Joyal et al, 2016). In mice lacking VLDLR, a genetic deficiency in FFAR1 decreases retinal neovascularization, while FFAR agonist increases retinal neovascularization (Joyal et al, 2016). FFAR1 also mediates actions of nonenzymatically generated nitro‐oxidative products, transarachidonic acids, and induces cerebral microvascular degeneration in rats (Honore et al, 2013). Targeting FFAR1 may prevent pathologic endothelial cell proliferation and degeneration.
Conclusions and perspectives
Generally, photoreceptor metabolism controls retinal neuronal and vascular development. Therefore, maintaining normal photoreceptor function will likely improve retinal vascular abnormalities in disease. As dyslipidemia contributes to disease progression in many retinal metabolic disorders, we may improve photoreceptor energy production by regulating lipid use and increasing lipid fuel sources, including those generated from autophagy. Targeting lipid metabolic modulation may improve neurovascular retinal function and decrease neovascularization.
Conflict of interest
The authors declare that they have no conflict of interest.
Pending issues.
-
•
Anti‐VEGFA therapy affects neuronal function and has systematic effects as VEGFA is an important factor to promote neuronal function.
-
•
Elucidate the link between photoreceptors and retinal vascular function.
-
•
Identify the type of lipids as neuronal energy fuel sources.
-
•
Explore potential therapeutic targets modulating lipid sensors.
Acknowledgements
LEHS is supported by NIH 1R24EY024868, EY017017‐13S1, BCH IDDRC (1U54HD090255); AH is supported by The Swedish Research Council (DNR# #2016‐01131), Government grants under the ALF agreement ALFGBG‐717971 and the Wallenberg Foundations and long‐term support by De Blindas Vänner; ZF is supported by Blind Children's Center, Manton Center for Orphan Disease Research, Boston Children's Hospital OFD/BTREC/CTREC Faculty Career Development Grant; BC is supported by Deutsche Forschungsgemeinschaft (CA 1940/1‐1); QL is supported by Beijing Municipal Natural Science Foundation (#7192039); YS is supported by Boston Children's Hospital OFD/BTREC/CTREC Faculty Career Development Grant, NIH R01EY030140 and BrightFocus Foundation NIH/R01EY029238; YT is supported by Manpei Suzuki Diabetes Foundation; CTC is supported by Intramural Research Program at the National Institute on Alcohol Abuse and Alcoholism; TSK is supported by NIH grants EY022938 and R24 EY024864, and BX003604 from the Department of Veterans Affairs, TSK is the recipient of a Research Career Scientist award from the Department of Veterans Affairs. J‐SJ is supported by Burroughs Wellcome Fund Career Award for Medical Scientists, NSERC RGPIN‐2016‐06743, CIHR 390615, FRQS, and a CIHR New Investigator Award.1
EMBO Mol Med (2019) 11: e10473
See the Glossary for abbreviations used in this article.
Footnotes
Correction added on 9 October 2019, after first online publication: the funding information has been updated.
Contributor Information
Zhongjie Fu, Email: zhongjie.fu@childrens.harvard.edu.
Lois EH Smith, Email: lois.smith@childrens.harvard.edu.
References
- Acar N, Berdeaux O, Gregoire S, Cabaret S, Martine L, Gain P, Thuret G, Creuzot‐Garcher CP, Bron AM, Bretillon L (2012) Lipid composition of the human eye: are red blood cells a good mirror of retinal and optic nerve fatty acids? PLoS One 7: e35102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- ACCORD Study Group , ACCORD Eye Study Group , Chew EY, Ambrosius WT, Davis MD, Danis RP, Gangaputra S, Greven CM, Hubbard L, Esser BA et al (2010) Effects of medical therapies on retinopathy progression in type 2 diabetes. N Engl J Med 363: 233–244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ait‐Ali N, Fridlich R, Millet‐Puel G, Clerin E, Delalande F, Jaillard C, Blond F, Perrocheau L, Reichman S, Byrne LC et al (2015) Rod‐derived cone viability factor promotes cone survival by stimulating aerobic glycolysis. Cell 161: 817–832 [DOI] [PubMed] [Google Scholar]
- Akula JD, Hansen RM, Tzekov R, Favazza TL, Vyhovsky TC, Benador IY, Mocko JA, McGee D, Kubota R, Fulton AB (2010) Visual cycle modulation in neurovascular retinopathy. Exp Eye Res 91: 153–161 [DOI] [PubMed] [Google Scholar]
- Alaynick WA (2008) Nuclear receptors, mitochondria and lipid metabolism. Mitochondrion 8: 329–337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alessandri JM, Goustard‐Langelier B (2001) Alterations in fatty acid composition of tissue phospholipids in the developing retinal dystrophic rat. Lipids 36: 1141–1152 [DOI] [PubMed] [Google Scholar]
- Ali MU, Rahman MSU, Cao J, Yuan PX (2017) Genetic characterization and disease mechanism of retinitis pigmentosa: current scenario. 3 Biotech 7: 251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ambati J, Fowler BJ (2012) Mechanisms of age‐related macular degeneration. Neuron 75: 26–39 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ames A III, Li YY, Heher EC, Kimble CR (1992) Energy metabolism of rabbit retina as related to function: high cost of Na+ transport. J Neurosci 12: 840–853 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arden GB, Jyothi S, Hogg CH, Lee YF, Sivaprasad S (2011) Regression of early diabetic macular oedema is associated with prevention of dark adaptation. Eye (Lond) 25: 1546–1554 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arshavsky VY, Lamb TD, Pugh EN Jr (2002) G proteins and phototransduction. Annu Rev Physiol 64: 153–187 [DOI] [PubMed] [Google Scholar]
- Avery RL, Castellarin AA, Steinle NC, Dhoot DS, Pieramici DJ, See R, Couvillion S, Nasir MA, Rabena MD, Le K et al (2014) Systemic pharmacokinetics following intravitreal injections of ranibizumab, bevacizumab or aflibercept in patients with neovascular AMD. Br J Ophthalmol 98: 1636–1641 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Awh CC, Hawken S, Zanke BW (2015) Treatment response to antioxidants and zinc based on CFH and ARMS2 genetic risk allele number in the Age‐Related Eye Disease Study. Ophthalmology 122: 162–169 [DOI] [PubMed] [Google Scholar]
- Baes M (2000) Mouse models for peroxisome biogenesis disorders. Cell Biochem Biophys 32: 229–237 [DOI] [PubMed] [Google Scholar]
- Baes M, Van Veldhoven PP (2012) Mouse models for peroxisome biogenesis defects and beta‐oxidation enzyme deficiencies. Biochem Biophys Acta 1822: 1489–1500 [DOI] [PubMed] [Google Scholar]
- Bakri SJ, Thorne JE, Ho AC, Ehlers JP, Schoenberger SD, Yeh S, Kim SJ (2019) Safety and efficacy of anti‐vascular endothelial growth factor therapies for neovascular age‐related macular degeneration: a report by the american academy of ophthalmology. Ophthalmology 126: 55–63 [DOI] [PubMed] [Google Scholar]
- Barot M, Gokulgandhi MR, Mitra AK (2011) Mitochondrial dysfunction in retinal diseases. Curr Eye Res 36: 1069–1077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barron MJ, Johnson MA, Andrews RM, Clarke MP, Griffiths PG, Bristow E, He LP, Durham S, Turnbull DM (2001) Mitochondrial abnormalities in ageing macular photoreceptors. Invest Ophthalmol Vis Sci 42: 3016–3022 [PubMed] [Google Scholar]
- Bressler NM, Boyer DS, Williams DF, Butler S, Francom SF, Brown B, Di Nucci F, Cramm T, Tuomi LL, Ianchulev T et al (2012) Cerebrovascular accidents in patients treated for choroidal neovascularization with ranibizumab in randomized controlled trials. Retina 32: 1821–1828 [DOI] [PubMed] [Google Scholar]
- Briscoe CP, Tadayyon M, Andrews JL, Benson WG, Chambers JK, Eilert MM, Ellis C, Elshourbagy NA, Goetz AS, Minnick DT et al (2003) The orphan G protein‐coupled receptor GPR40 is activated by medium and long chain fatty acids. J Biol Chem 278: 11303–11311 [DOI] [PubMed] [Google Scholar]
- Buitendijk GHS, Rochtchina E, Myers C, van Duijn CM, Lee KE, Klein BEK, Meuer SM, de Jong P, Holliday EG, Tan AG et al (2013) Prediction of age‐related macular degeneration in the general population: the Three Continent AMD Consortium. Ophthalmology 120: 2644–2655 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camacho ET, Punzo C, Wirkus SA (2016) Quantifying the metabolic contribution to photoreceptor death in retinitis pigmentosa via a mathematical model. J Theor Biol 408: 75–87 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casson RJ, Chidlow G, Han G, Wood JP (2013) An explanation for the Warburg effect in the adult mammalian retina. Clin Exp Ophthalmol 41: 517 [DOI] [PubMed] [Google Scholar]
- Chang YC, Wu WC (2013) Dyslipidemia and diabetic retinopathy. Rev Diabet Stud 10: 121–132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen H, Anderson RE (1993) Metabolism in frog retinal pigment epithelium of docosahexaenoic and arachidonic acids derived from rod outer segment membranes. Exp Eye Res 57: 369–377 [DOI] [PubMed] [Google Scholar]
- Chen W, Stambolian D, Edwards AO, Branham KE, Othman M, Jakobsdottir J, Tosakulwong N, Pericak‐Vance MA, Campochiaro PA, Klein ML et al (2010) Genetic variants near TIMP3 and high‐density lipoprotein‐associated loci influence susceptibility to age‐related macular degeneration. Proc Natl Acad Sci USA 107: 7401–7406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y, Hu Y, Lin M, Jenkins AJ, Keech AC, Mott R, Lyons TJ, Ma JX (2013) Therapeutic effects of PPARalpha agonists on diabetic retinopathy in type 1 diabetes models. Diabetes 62: 261–272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen L, Wang K, Long A, Jia L, Zhang Y, Deng H, Li Y, Han J, Wang Y (2017) Fasting‐induced hormonal regulation of lysosomal function. Cell Res 27: 748–763 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christen WG, Schaumberg DA, Glynn RJ, Buring JE (2011) Dietary omega‐3 fatty acid and fish intake and incident age‐related macular degeneration in women. Arch Ophthalmol 129: 921–929 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen LH, Noell WK (1960) Glucose catabolism of rabbit retina before and after development of visual function. J Neurochem 5: 253–276 [DOI] [PubMed] [Google Scholar]
- Colijn JM, den Hollander AI, Demirkan A, Cougnard‐Gregoire A, Verzijden T, Kersten E, Meester‐Smoor MA, Merle BMJ, Papageorgiou G, Ahmad S et al (2019) Increased high‐density lipoprotein levels associated with age‐related macular degeneration: evidence from the EYE‐RISK and European eye epidemiology consortia. Ophthalmology 126: 393–406 [DOI] [PubMed] [Google Scholar]
- Connor KM, SanGiovanni JP, Lofqvist C, Aderman CM, Chen J, Higuchi A, Hong S, Pravda EA, Majchrzak S, Carper D et al (2007) Increased dietary intake of omega‐3‐polyunsaturated fatty acids reduces pathological retinal angiogenesis. Nat Med 13: 868–873 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Converse CA, Hammer HM, Packard CJ, Shepherd J (1983) Plasma lipid abnormalities in retinitis pigmentosa and related conditions. Trans Ophthalmol Soc U K 103(Pt 5): 508–512 [PubMed] [Google Scholar]
- Cougnard‐Gregoire A, Delyfer MN, Korobelnik JF, Rougier MB, Le Goff M, Dartigues JF, Barberger‐Gateau P, Delcourt C (2014) Elevated high‐density lipoprotein cholesterol and age‐related macular degeneration: the Alienor study. PLoS One 9: e90973 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crane DI (2014) Revisiting the neuropathogenesis of Zellweger syndrome. Neurochem Int 69: 1‐8 [DOI] [PubMed] [Google Scholar]
- Curcio CA, Medeiros NE, Millican CL (1996) Photoreceptor loss in age‐related macular degeneration. Invest Ophthalmol Vis Sci 37: 1236–1249 [PubMed] [Google Scholar]
- Curcio CA (2018a) Antecedents of soft drusen, the specific deposits of age‐related macular degeneration, in the biology of human macula. Invest Ophthalmol Vis Sci 59: AMD182–AMD194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curcio CA (2018b) Soft drusen in age‐related macular degeneration: biology and targeting via the oil spill strategies. Invest Ophthalmol Vis Sci 59: AMD160–AMD181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daemen FJ (1973) Vertebrate rod outer segment membranes. Biochem Biophys Acta 300: 255–288 [DOI] [PubMed] [Google Scholar]
- De Benedetto U, Querques G, Lattanzio R, Borrelli E, Triolo G, Maestranzi G, Calori G, Querques L, Bandello F (2014) Macular dysfunction is common in both type 1 and type 2 diabetic patients without macular edema. Retina 34: 2171–2177 [DOI] [PubMed] [Google Scholar]
- De Sousa‐Coelho AL, Relat J, Hondares E, Perez‐Marti A, Ribas F, Villarroya F, Marrero PF, Haro D (2013) FGF21 mediates the lipid metabolism response to amino acid starvation. J Lipid Res 54: 1786–1797 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du Y, Veenstra A, Palczewski K, Kern TS (2013) Photoreceptor cells are major contributors to diabetes‐induced oxidative stress and local inflammation in the retina. Proc Natl Acad Sci USA 110: 16586–16591 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du J, Linton JD, Hurley JB (2015) Probing metabolism in the intact retina using stable isotope tracers. Methods Enzymol 561: 149–170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dutchak PA, Katafuchi T, Bookout AL, Choi JH, Yu RT, Mangelsdorf DJ, Kliewer SA (2012) Fibroblast growth factor‐21 regulates PPARgamma activity and the antidiabetic actions of thiazolidinediones. Cell 148: 556–567 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards AO, Malek G (2007) Molecular genetics of AMD and current animal models. Angiogenesis 10: 119–132 [DOI] [PubMed] [Google Scholar]
- Falsini B, Anselmi GM, Marangoni D, D'Esposito F, Fadda A, Di Renzo A, Campos EC, Riva CE (2011) Subfoveal choroidal blood flow and central retinal function in retinitis pigmentosa. Invest Ophthalmol Vis Sci 52: 1064–1069 [DOI] [PubMed] [Google Scholar]
- Fan Q, Maranville JC, Fritsche L, Sim X, Cheung CMG, Chen LJ, Gorski M, Yamashiro K, Ahn J, Laude A et al (2017) HDL‐cholesterol levels and risk of age‐related macular degeneration: a multiethnic genetic study using Mendelian randomization. Int J Epidemiol 46: 1891–1902 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fliesler SJ, Anderson RE (1983) Chemistry and metabolism of lipids in the vertebrate retina. Prog Lipid Res 22: 79–131 [DOI] [PubMed] [Google Scholar]
- Foltz IN, Hu S, King C, Wu X, Yang C, Wang W, Weiszmann J, Stevens J, Chen JS, Nuanmanee N et al (2012) Treating diabetes and obesity with an FGF21‐mimetic antibody activating the betaKlotho/FGFR1c receptor complex. Sci Transl Med 4: 162ra153 [DOI] [PubMed] [Google Scholar]
- Folz SJ, Trobe JD (1991) The peroxisome and the eye. Surv Ophthalmol 35: 353–368 [DOI] [PubMed] [Google Scholar]
- Fox TE, Han X, Kelly S, Merrill AH II, Martin RE, Anderson RE, Gardner TW, Kester M (2006) Diabetes alters sphingolipid metabolism in the retina: a potential mechanism of cell death in diabetic retinopathy. Diabetes 55: 3573–3580 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fritsche LG, Chen W, Schu M, Yaspan BL, Yu Y, Thorleifsson G, Zack DJ, Arakawa S, Cipriani V, Ripke S et al (2013) Seven new loci associated with age‐related macular degeneration. Nat Genet 45: 433–439.e431–432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fritsche LG, Igl W, Bailey JN, Grassmann F, Sengupta S, Bragg‐Gresham JL, Burdon KP, Hebbring SJ, Wen C, Gorski M et al (2016) A large genome‐wide association study of age‐related macular degeneration highlights contributions of rare and common variants. Nat Genet 48: 134–143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu Z, Lofqvist CA, Shao Z, Sun Y, Joyal JS, Hurst CG, Cui RZ, Evans LP, Tian K, SanGiovanni JP et al (2015) Dietary omega‐3 polyunsaturated fatty acids decrease retinal neovascularization by adipose‐endoplasmic reticulum stress reduction to increase adiponectin. Am J Clin Nutr 101: 879–888 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu Z, Gong Y, Liegl R, Wang Z, Liu CH, Meng SS, Burnim SB, Saba NJ, Fredrick TW, Morss PC et al (2017a) FGF21 administration suppresses retinal and choroidal neovascularization in mice. Cell Rep 18: 1606–1613 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu Z, Liegl R, Wang Z, Gong Y, Liu CH, Sun Y, Cakir B, Burnim SB, Meng SS, Lofqvist C et al (2017b) Adiponectin mediates dietary omega‐3 long‐chain polyunsaturated fatty acid protection against choroidal neovascularization in mice. Invest Ophthalmol Vis Sci 58: 3862–3870 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu Z, Lofqvist CA, Liegl R, Wang Z, Sun Y, Gong Y, Liu CH, Meng SS, Burnim SB, Arellano I et al (2018) Photoreceptor glucose metabolism determines normal retinal vascular growth. EMBO Mol Med 10: 76–90 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu Z, Wang Z, Liu CH, Gong Y, Cakir B, Liegl R, Sun Y, Meng SS, Burnim SB, Arellano I et al (2018) Fibroblast growth factor 21 protects photoreceptor function in type 1 diabetic mice. Diabetes 67: 974–985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fulton AB, Dodge J, Hansen RM, Williams TP (1999) The rhodopsin content of human eyes. Invest Ophthalmol Vis Sci 40: 1878–1883 [PubMed] [Google Scholar]
- Gaich G, Chien JY, Fu H, Glass LC, Deeg MA, Holland WL, Kharitonenkov A, Bumol T, Schilske HK, Moller DE (2013) The effects of LY2405319, an FGF21 analog, in obese human subjects with type 2 diabetes. Cell Metab 18: 333–340 [DOI] [PubMed] [Google Scholar]
- Gervois P, Torra IP, Fruchart JC, Staels B (2000) Regulation of lipid and lipoprotein metabolism by PPAR activators. Clin Chem Lab Med 38: 3–11 [DOI] [PubMed] [Google Scholar]
- Golestaneh N, Chu Y, Xiao YY, Stoleru GL, Theos AC (2017) Dysfunctional autophagy in RPE, a contributing factor in age‐related macular degeneration. Cell Death Dis 8: e2537 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gong Y, Fu Z, Edin ML, Liu CH, Wang Z, Shao Z, Fredrick TW, Saba NJ, Morss PC, Burnim SB et al (2016a) Cytochrome P450 oxidase 2C inhibition adds to omega‐3 long‐chain polyunsaturated fatty acids protection against retinal and choroidal neovascularization. Arterioscler Thromb Vasc Biol 36: 1919–1927 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gong Y, Shao Z, Fu Z, Edin ML, Sun Y, Liegl RG, Wang Z, Liu CH, Burnim SB, Meng SS et al (2016b) Fenofibrate inhibits cytochrome P450 epoxygenase 2C activity to suppress pathological ocular angiogenesis. EBioMedicine 13: 201–211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gong Q, Su G (2017) Roles of miRNAs and long noncoding RNAs in the progression of diabetic retinopathy. Biosci Rep 37 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gong Y, Fu Z, Liegl R, Chen J, Hellstrom A, Smith LE (2017) omega‐3 and omega‐6 long‐chain PUFAs and their enzymatic metabolites in neovascular eye diseases. Am J Clin Nutr 106: 16–26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goto T, Hirata M, Aoki Y, Iwase M, Takahashi H, Kim M, Li Y, Jheng HF, Nomura W, Takahashi N et al (2017) The hepatokine FGF21 is crucial for peroxisome proliferator‐activated receptor‐alpha agonist‐induced amelioration of metabolic disorders in obese mice. J Biol Chem 292: 9175–9190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham PS, Kaidonis G, Abhary S, Gillies MC, Daniell M, Essex RW, Chang JH, Lake SR, Pal B, Jenkins AJ et al (2018) Genome‐wide association studies for diabetic macular edema and proliferative diabetic retinopathy. BMC Med Genet 19: 71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Habib N, Avraham‐Davidi I, Basu A, Burks T, Shekhar K, Hofree M, Choudhury SR, Aguet F, Gelfand E, Ardlie K et al (2017) Massively parallel single‐nucleus RNA‐seq with DroNc‐seq. Nat Methods 14: 955–958 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hageman GS, Luthert PJ, Victor Chong NH, Johnson LV, Anderson DH, Mullins RF (2001) An integrated hypothesis that considers drusen as biomarkers of immune‐mediated processes at the RPE‐Bruch's membrane interface in aging and age‐related macular degeneration. Prog Retin Eye Res 20: 705–732 [DOI] [PubMed] [Google Scholar]
- Hagins WA, Penn RD, Yoshikami S (1970) Dark current and photocurrent in retinal rods. Biophys J 10: 380–412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammer SS, Busik JV (2017) The role of dyslipidemia in diabetic retinopathy. Vision Res 139: 228–236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hampton BM, Schwartz SG, Brantley MA Jr, Flynn HW Jr (2015) Update on genetics and diabetic retinopathy. Clin Ophthalmol 9: 2175–2193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen RM, Moskowitz A, Akula JD, Fulton AB (2017) The neural retina in retinopathy of prematurity. Prog Retin Eye Res 56: 32–57 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hapani S, Sher A, Chu D, Wu S (2010) Increased risk of serious hemorrhage with bevacizumab in cancer patients: a meta‐analysis. Oncology 79: 27–38 [DOI] [PubMed] [Google Scholar]
- Harding CO, Gillingham MB, van Calcar SC, Wolff JA, Verhoeve JN, Mills MD (1999) Docosahexaenoic acid and retinal function in children with long‐chain 3‐hydroxyacyl‐CoA dehydrogenase deficiency. J Inherit Metab Dis 22: 276–280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hellstrom A, Smith LE, Dammann O (2013) Retinopathy of prematurity. Lancet 382: 1445–1457 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiltunen JK, Karki T, Hassinen IE, Osmundsen H (1986) beta‐Oxidation of polyunsaturated fatty acids by rat liver peroxisomes. A role for 2,4‐dienoyl‐coenzyme A reductase in peroxisomal beta‐oxidation. J Biol Chem 261: 16484–16493 [PubMed] [Google Scholar]
- Hogg RE, Chakravarthy U (2006) Visual function and dysfunction in early and late age‐related maculopathy. Prog Retin Eye Res 25: 249–276 [DOI] [PubMed] [Google Scholar]
- Holland WL, Adams AC, Brozinick JT, Bui HH, Miyauchi Y, Kusminski CM, Bauer SM, Wade M, Singhal E, Cheng CC et al (2013) An FGF21‐adiponectin‐ceramide axis controls energy expenditure and insulin action in mice. Cell Metab 17: 790–797 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holman RT, Bibus DM, Jeffrey GH, Smethurst P, Crofts JW (1994) Abnormal plasma lipids of patients with Retinitis pigmentosa. Lipids 29: 61–65 [DOI] [PubMed] [Google Scholar]
- Holmstrom G, van Wijngaarden P, Coster DJ, Williams KA (2007) Genetic susceptibility to retinopathy of prematurity: the evidence from clinical and experimental animal studies. Br J Ophthalmol 91: 1704–1708 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honore JC, Kooli A, Hamel D, Alquier T, Rivera JC, Quiniou C, Hou X, Kermorvant‐Duchemin E, Hardy P, Poitout V et al (2013) Fatty acid receptor Gpr40 mediates neuromicrovascular degeneration induced by transarachidonic acids in rodents. Arterioscler Thromb Vasc Biol 33: 954–961 [DOI] [PubMed] [Google Scholar]
- Houtsmuller AJ, Zahn KJ, Henkes HE (1980) Unsaturated fats and progression of diabetic retinopathy. Doc Ophthalmol 48: 363–371 [DOI] [PubMed] [Google Scholar]
- Hughes AE, Enright JM, Myers CA, Shen SQ, Corbo JC (2017) Cell type‐specific epigenomic analysis reveals a uniquely closed chromatin architecture in mouse rod photoreceptors. Sci Rep 7: 43184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito R, Satoh‐Asahara N, Yamakage H, Sasaki Y, Odori S, Kono S, Wada H, Suganami T, Ogawa Y, Hasegawa K et al (2014) An increase in the EPA/AA ratio is associated with improved arterial stiffness in obese patients with dyslipidemia. J Atheroscler Thromb 21: 248–260 [DOI] [PubMed] [Google Scholar]
- Jackson GR, Owsley C, Curcio CA (2002) Photoreceptor degeneration and dysfunction in aging and age‐related maculopathy. Ageing Res Rev 1: 381–396 [DOI] [PubMed] [Google Scholar]
- Jalali S, Balakrishnan D, Zeynalova Z, Padhi TR, Rani PK (2013) Serious adverse events and visual outcomes of rescue therapy using adjunct bevacizumab to laser and surgery for retinopathy of prematurity. The Indian Twin Cities Retinopathy of Prematurity Screening database Report number 5. Arch Dis Child Fetal Neonatal Ed 98: F327–F333 [DOI] [PubMed] [Google Scholar]
- Johnson JE Jr, Perkins GA, Giddabasappa A, Chaney S, Xiao W, White AD, Brown JM, Waggoner J, Ellisman MH, Fox DA (2007) Spatiotemporal regulation of ATP and Ca2+ dynamics in vertebrate rod and cone ribbon synapses. Mol Vis 13: 887–919 [PMC free article] [PubMed] [Google Scholar]
- Joyal JS, Sun Y, Gantner ML, Shao Z, Evans LP, Saba N, Fredrick T, Burnim S, Kim JS, Patel G et al (2016) Retinal lipid and glucose metabolism dictates angiogenesis through the lipid sensor Ffar1. Nat Med 22: 439–445 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joyal JS, Gantner ML, Smith LEH (2018) Retinal energy demands control vascular supply of the retina in development and disease: the role of neuronal lipid and glucose metabolism. Prog Retin Eye Res 64: 131–156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kabasawa S, Mori K, Horie‐Inoue K, Gehlbach PL, Inoue S, Awata T, Katayama S, Yoneya S (2011) Associations of cigarette smoking but not serum fatty acids with age‐related macular degeneration in a Japanese population. Ophthalmology 118: 1082–1088 [DOI] [PubMed] [Google Scholar]
- Keech AC, Mitchell P, Summanen PA, O'Day J, Davis TM, Moffitt MS, Taskinen MR, Simes RJ, Tse D, Williamson E et al (2007) Effect of fenofibrate on the need for laser treatment for diabetic retinopathy (FIELD study): a randomised controlled trial. Lancet 370: 1687–1697 [DOI] [PubMed] [Google Scholar]
- Kenmochi N, Suzuki T, Uechi T, Magoori M, Kuniba M, Higa S, Watanabe K, Tanaka T (2001) The human mitochondrial ribosomal protein genes: mapping of 54 genes to the chromosomes and implications for human disorders. Genomics 77: 65–70 [DOI] [PubMed] [Google Scholar]
- Kern TS, Berkowitz BA (2015) Photoreceptors in diabetic retinopathy. J Diabetes Investig 6: 371–380 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kevany BM, Palczewski K (2010) Phagocytosis of retinal rod and cone photoreceptors. Physiology (Bethesda) 25: 8–15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kharitonenkov A, Larsen P (2011) FGF21 reloaded: challenges of a rapidly growing field. Trends Endocrinol Metab 22: 81–86 [DOI] [PubMed] [Google Scholar]
- Klein BE, Myers CE, Howard KP, Klein R (2015) Serum lipids and proliferative diabetic retinopathy and macular edema in persons with long‐term type 1 diabetes mellitus: the wisconsin epidemiologic study of diabetic retinopathy. JAMA Ophthalmol 133: 503–510 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klufas MA, Chan RV (2015) Intravitreal anti‐VEGF therapy as a treatment for retinopathy of prematurity: what we know after 7 years. J Pediatr Ophthalmol Strabismus 52: 77–84 [DOI] [PubMed] [Google Scholar]
- Kowluru RA, Mishra M (2015) Oxidative stress, mitochondrial damage and diabetic retinopathy. Biochem Biophys Acta 1852: 2474–2483 [DOI] [PubMed] [Google Scholar]
- Lahdenranta J, Pasqualini R, Schlingemann RO, Hagedorn M, Stallcup WB, Bucana CD, Sidman RL, Arap W (2001) An anti‐angiogenic state in mice and humans with retinal photoreceptor cell degeneration. Proc Natl Acad Sci USA 98: 10368–10373 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lapillonne A, Jensen CL (2009) Reevaluation of the DHA requirement for the premature infant. Prostaglandins Leukot Essent Fatty Acids 81: 143–150 [DOI] [PubMed] [Google Scholar]
- LaVail MM (1976) Rod outer segment disk shedding in rat retina: relationship to cyclic lighting. Science 194: 1071–1074 [DOI] [PubMed] [Google Scholar]
- Lin Z, Gong Q, Wu C, Yu J, Lu T, Pan X, Lin S, Li X (2012) Dynamic change of serum FGF21 levels in response to glucose challenge in human. J Clin Endocrinol Metab 97: E1224–E1228 [DOI] [PubMed] [Google Scholar]
- Lin Z, Tian H, Lam KS, Lin S, Hoo RC, Konishi M, Itoh N, Wang Y, Bornstein SR, Xu A et al (2013) Adiponectin mediates the metabolic effects of FGF21 on glucose homeostasis and insulin sensitivity in mice. Cell Metab 17: 779–789 [DOI] [PubMed] [Google Scholar]
- Litts KM, Messinger JD, Freund KB, Zhang Y, Curcio CA (2015) Inner segment remodeling and mitochondrial translocation in cone photoreceptors in age‐related macular degeneration with outer retinal tubulation. Invest Ophthalmol Vis Sci 56: 2243–2253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu H, Tang J, Du Y, Saadane A, Tonade D, Samuels I, Veenstra A, Palczewski K, Kern TS (2016) Photoreceptor cells influence retinal vascular degeneration in mouse models of retinal degeneration and diabetes. Invest Ophthalmol Vis Sci 57: 4272–4281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lofqvist CA, Najm S, Hellgren G, Engstrom E, Savman K, Nilsson AK, Andersson MX, Hard AL, Smith LEH, Hellstrom A (2018) Association of retinopathy of prematurity with low levels of arachidonic acid: a secondary analysis of a randomized clinical trial. JAMA Ophthalmol 136: 271–277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lux A, Llacer H, Heussen FM, Joussen AM (2007) Non‐responders to bevacizumab (Avastin) therapy of choroidal neovascular lesions. Br J Ophthalmol 91: 1318–1322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyssenko NN, Haider N, Picataggi A, Cipollari E, Jiao W, Phillips MC, Rader DJ, Chavali VRM (2018) Directional ABCA1‐mediated cholesterol efflux and apoB‐lipoprotein secretion in the retinal pigment epithelium. J Lipid Res 59: 1927–1939 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macosko EZ, Basu A, Satija R, Nemesh J, Shekhar K, Goldman M, Tirosh I, Bialas AR, Kamitaki N, Martersteck EM et al (2015) Highly parallel genome‐wide expression profiling of individual cells using nanoliter droplets. Cell 161: 1202–1214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malek G, Johnson LV, Mace BE, Saloupis P, Schmechel DE, Rickman DW, Toth CA, Sullivan PM, Bowes Rickman C (2005) Apolipoprotein E allele‐dependent pathogenesis: a model for age‐related retinal degeneration. Proc Natl Acad Sci USA 102: 11900–11905 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markan KR, Naber MC, Ameka MK, Anderegg MD, Mangelsdorf DJ, Kliewer SA, Mohammadi M, Potthoff MJ (2014) Circulating FGF21 is liver derived and enhances glucose uptake during refeeding and overfeeding. Diabetes 63: 4057–4063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin CR, Dasilva DA, Cluette‐Brown JE, Dimonda C, Hamill A, Bhutta AQ, Coronel E, Wilschanski M, Stephens AJ, Driscoll DF et al (2011) Decreased postnatal docosahexaenoic and arachidonic acid blood levels in premature infants are associated with neonatal morbidities. J Pediatr 159: 743–749.e741–742 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merle BM, Richard F, Benlian P, Puche N, Delcourt C, Souied EH (2015) CFH Y402H and ARMS2 A69S polymorphisms and oral supplementation with docosahexaenoic acid in neovascular age‐related macular degeneration patients: the NAT2 study. PLoS One 10: e0130816 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitter SK, Rao HV, Qi X, Cai J, Sugrue A, Dunn WA Jr, Grant MB, Boulton ME (2012) Autophagy in the retina: a potential role in age‐related macular degeneration. Adv Exp Med Biol 723: 83–90 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moorthy S, Cheung N (2009) Cerebrovascular accidents and ranibizumab. Ophthalmology 116: 1834–1835; author reply 1835 [DOI] [PubMed] [Google Scholar]
- Mukherjee PK, Marcheselli VL, de Rivero Vaccari JC, Gordon WC, Jackson FE, Bazan NG (2007) Photoreceptor outer segment phagocytosis attenuates oxidative stress‐induced apoptosis with concomitant neuroprotectin D1 synthesis. Proc Natl Acad Sci USA 104: 13158–13163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mullins RF, McGwin G Jr, Searcey K, Clark ME, Kennedy EL, Curcio CA, Stone EM, Owsley C (2019) The ARMS2 A69S polymorphism is associated with delayed rod‐mediated dark adaptation in eyes at risk for incident age‐related macular degeneration. Ophthalmology 126: 591–600 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neale BM, Fagerness J, Reynolds R, Sobrin L, Parker M, Raychaudhuri S, Tan PL, Oh EC, Merriam JE, Souied E et al (2010) Genome‐wide association study of advanced age‐related macular degeneration identifies a role of the hepatic lipase gene (LIPC). Proc Natl Acad Sci USA 107: 7395–7400 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ng SK, Wood JP, Chidlow G, Han G, Kittipassorn T, Peet DJ, Casson RJ (2015) Cancer‐like metabolism of the mammalian retina. Clin Exp Ophthalmol 43: 367–376 [DOI] [PubMed] [Google Scholar]
- Nigam N, Hedaya J, Freeman WR (2008) Non‐responders to bevacizumab (Avastin) therapy of choroidal neovascular lesions. Br J Ophthalmol 92: 864–865; author reply 865–866 [PMC free article] [PubMed] [Google Scholar]
- Nikonov SS, Kholodenko R, Lem J, Pugh EN Jr (2006) Physiological features of the S‐ and M‐cone photoreceptors of wild‐type mice from single‐cell recordings. J Gen Physiol 127: 359–374 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noguer MT, Martinez M (2010) Visual follow‐up in peroxisomal‐disorder patients treated with docosahexaenoic Acid ethyl ester. Invest Ophthalmol Vis Sci 51: 2277–2285 [DOI] [PubMed] [Google Scholar]
- Okawa H, Sampath AP, Laughlin SB, Fain GL (2008) ATP consumption by mammalian rod photoreceptors in darkness and in light. Curr Biol 18: 1917–1921 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Opreanu M, Tikhonenko M, Bozack S, Lydic TA, Reid GE, McSorley KM, Sochacki A, Perez GI, Esselman WJ, Kern T et al (2011) The unconventional role of acid sphingomyelinase in regulation of retinal microangiopathy in diabetic human and animal models. Diabetes 60: 2370–2378 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pakzad‐Vaezi KL, Maberley DA (2014) Infantile Refsum disease in a young adult: case presentation and brief review. Retin Cases Brief Rep 8: 56–59 [DOI] [PubMed] [Google Scholar]
- Pawlik D, Lauterbach R, Walczak M, Hurkala J, Sherman MP (2014) Fish‐oil fat emulsion supplementation reduces the risk of retinopathy in very low birth weight infants: a prospective, randomized study. JPEN J Parenter Enteral Nutr 38: 711–716 [DOI] [PubMed] [Google Scholar]
- Pearsall EA, Cheng R, Zhou K, Takahashi Y, Matlock HG, Vadvalkar SS, Shin Y, Fredrick TW, Gantner ML, Meng S et al (2017) PPARalpha is essential for retinal lipid metabolism and neuronal survival. BMC Biol 15: 113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perkins GA, Ellisman MH, Fox DA (2003) Three‐dimensional analysis of mouse rod and cone mitochondrial cristae architecture: bioenergetic and functional implications. Mol Vis 9: 60–73 [PubMed] [Google Scholar]
- Pescosolido N, Barbato A, Stefanucci A, Buomprisco G (2015) Role of electrophysiology in the early diagnosis and follow‐up of diabetic retinopathy. J Diabetes Res 2015: 319692 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petit L, Ma S, Cipi J, Cheng SY, Zieger M, Hay N, Punzo C (2018) Aerobic glycolysis is essential for normal rod function and controls secondary cone death in retinitis pigmentosa. Cell Rep 23: 2629–2642 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinazo‐Duran MD, Gomez‐Ulla F, Arias L, Araiz J, Casaroli‐Marano R, Gallego‐Pinazo R, Garcia‐Medina JJ, Lopez‐Galvez MI, Manzanas L, Salas A et al (2014) Do nutritional supplements have a role in age macular degeneration prevention? J Ophthalmol 2014: 901686 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poirier Y, Antonenkov VD, Glumoff T, Hiltunen JK (2006) Peroxisomal beta‐oxidation–a metabolic pathway with multiple functions. Biochem Biophys Acta 1763: 1413–1426 [DOI] [PubMed] [Google Scholar]
- Potthoff MJ, Inagaki T, Satapati S, Ding X, He T, Goetz R, Mohammadi M, Finck BN, Mangelsdorf DJ, Kliewer SA et al (2009) FGF21 induces PGC‐1alpha and regulates carbohydrate and fatty acid metabolism during the adaptive starvation response. Proc Natl Acad Sci USA 106: 10853–10858 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poulos A, Singh H, Paton B, Sharp P, Derwas N (1986) Accumulation and defective beta‐oxidation of very long chain fatty acids in Zellweger's syndrome, adrenoleukodystrophy and Refsum's disease variants. Clin Genet 29: 397–408 [DOI] [PubMed] [Google Scholar]
- Punzo C, Kornacker K, Cepko CL (2009) Stimulation of the insulin/mTOR pathway delays cone death in a mouse model of retinitis pigmentosa. Nat Neurosci 12: 44–52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Restelli LM, Oettinghaus B, Halliday M, Agca C, Licci M, Sironi L, Savoia C, Hench J, Tolnay M, Neutzner A et al (2018) Neuronal mitochondrial dysfunction activates the integrated stress response to induce fibroblast growth factor 21. Cell Rep 24: 1407–1414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rheaume BA, Jereen A, Bolisetty M, Sajid MS, Yang Y, Renna K, Sun L, Robson P, Trakhtenberg EF (2018) Single cell transcriptome profiling of retinal ganglion cells identifies cellular subtypes. Nat Commun 9: 2759 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez‐Muela N, Germain F, Marino G, Fitze PS, Boya P (2012) Autophagy promotes survival of retinal ganglion cells after optic nerve axotomy in mice. Cell Death Differ 19: 162–169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sacks FM, Hermans MP, Fioretto P, Valensi P, Davis T, Horton E, Wanner C, Al‐Rubeaan K, Aronson R, Barzon I et al (2014) Association between plasma triglycerides and high‐density lipoprotein cholesterol and microvascular kidney disease and retinopathy in type 2 diabetes mellitus: a global case‐control study in 13 countries. Circulation 129: 999–1008 [DOI] [PubMed] [Google Scholar]
- Sala‐Vila A, Diaz‐Lopez A, Valls‐Pedret C, Cofan M, Garcia‐Layana A, Lamuela‐Raventos RM, Castaner O, Zanon‐Moreno V, Martinez‐Gonzalez MA, Toledo E et al (2016) Dietary marine omega‐3 fatty acids and incident sight‐threatening retinopathy in middle‐aged and older individuals with type 2 diabetes: prospective investigation from the PREDIMED trial. JAMA Ophthalmol 134: 1142–1149 [DOI] [PubMed] [Google Scholar]
- Salpietro V, Phadke R, Saggar A, Hargreaves IP, Yates R, Fokoloros C, Mankad K, Hertecant J, Ruggieri M, McCormick D et al (2015) Zellweger syndrome and secondary mitochondrial myopathy. Eur J Pediatr 174: 557–563 [DOI] [PubMed] [Google Scholar]
- Sangiovanni JP, Agron E, Meleth AD, Reed GF, Sperduto RD, Clemons TE, Chew EY (2009) {omega}‐3 Long‐chain polyunsaturated fatty acid intake and 12‐y incidence of neovascular age‐related macular degeneration and central geographic atrophy: AREDS report 30, a prospective cohort study from the Age‐Related Eye Disease Study. Am J Clin Nutr 90: 1601–1607 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sapieha P, Stahl A, Chen J, Seaward MR, Willett KL, Krah NM, Dennison RJ, Connor KM, Aderman CM, Liclican E et al (2011) 5‐Lipoxygenase metabolite 4‐HDHA is a mediator of the antiangiogenic effect of omega‐3 polyunsaturated fatty acids. Sci Transl Med 3: 69ra12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sapieha P (2012) Eyeing central neurons in vascular growth and reparative angiogenesis. Blood 120: 2182–2194 [DOI] [PubMed] [Google Scholar]
- Sapieha P, Chen J, Stahl A, Seaward MR, Favazza TL, Juan AM, Hatton CJ, Joyal JS, Krah NM, Dennison RJ et al (2012) Omega‐3 polyunsaturated fatty acids preserve retinal function in type 2 diabetic mice. Nutr Diabetes 2: e36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarks S, Cherepanoff S, Killingsworth M, Sarks J (2007) Relationship of Basal laminar deposit and membranous debris to the clinical presentation of early age‐related macular degeneration. Invest Ophthalmol Vis Sci 48: 968–977 [DOI] [PubMed] [Google Scholar]
- Sasaki M, Kawasaki R, Rogers S, Man RE, Itakura K, Xie J, Flood V, Tsubota K, Lamoureux E, Wang JJ (2015) The associations of dietary intake of polyunsaturated fatty acids with diabetic retinopathy in well‐controlled diabetes. Invest Ophthalmol Vis Sci 56: 7473–7479 [DOI] [PubMed] [Google Scholar]
- Sassa T, Kihara A (2014) Metabolism of very long‐chain Fatty acids: genes and pathophysiology. Biomol Ther (Seoul) 22: 83–92 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satish S, Philipose H, Rosales MAB, Saint‐Geniez M (2018) Pharmaceutical induction of PGC‐1alpha promotes retinal pigment epithelial cell metabolism and protects against oxidative damage. Oxid Med Cell Longev 2018: 9248640 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlein C, Talukdar S, Heine M, Fischer AW, Krott LM, Nilsson SK, Brenner MB, Heeren J, Scheja L (2016) FGF21 lowers plasma triglycerides by accelerating lipoprotein catabolism in white and brown adipose tissues. Cell Metab 23: 441–453 [DOI] [PubMed] [Google Scholar]
- Schnebelen C, Viau S, Gregoire S, Joffre C, Creuzot‐Garcher CP, Bron AM, Bretillon L, Acar N (2009) Nutrition for the eye: different susceptibility of the retina and the lacrimal gland to dietary omega‐6 and omega‐3 polyunsaturated fatty acid incorporation. Ophthalmic Res 41: 216–224 [DOI] [PubMed] [Google Scholar]
- Schoonjans K, Peinado‐Onsurbe J, Lefebvre AM, Heyman RA, Briggs M, Deeb S, Staels B, Auwerx J (1996) PPARalpha and PPARgamma activators direct a distinct tissue‐specific transcriptional response via a PPRE in the lipoprotein lipase gene. EMBO J 15: 5336–5348 [PMC free article] [PubMed] [Google Scholar]
- Schrader M, Costello J, Godinho LF, Islinger M (2015) Peroxisome‐mitochondria interplay and disease. J Inherit Metab Dis 38: 681–702 [DOI] [PubMed] [Google Scholar]
- Scott BL, Racz E, Lolley RN, Bazan NG (1988) Developing rod photoreceptors from normal and mutant Rd mouse retinas: altered fatty acid composition early in development of the mutant. J Neurosci Res 20: 202–211 [DOI] [PubMed] [Google Scholar]
- Selivanov VA, Votyakova TV, Pivtoraiko VN, Zeak J, Sukhomlin T, Trucco M, Roca J, Cascante M (2011) Reactive oxygen species production by forward and reverse electron fluxes in the mitochondrial respiratory chain. PLoS Comput Biol 7: e1001115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shao Z, Fu Z, Stahl A, Joyal JS, Hatton C, Juan A, Hurst C, Evans L, Cui Z, Pei D et al (2014) Cytochrome P450 2C8 omega3‐long‐chain polyunsaturated fatty acid metabolites increase mouse retinal pathologic neovascularization–brief report. Arterioscler Thromb Vasc Biol 34: 581–586 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shekhar K, Lapan SW, Whitney IE, Tran NM, Macosko EZ, Kowalczyk M, Adiconis X, Levin JZ, Nemesh J, Goldman M et al (2016) Comprehensive classification of retinal bipolar neurons by single‐cell transcriptomics. Cell 166: 1308–1323.e1330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinclair R, Schindler T, Lui K, Bolisetty S (2018) Hypertriglyceridaemia in extremely preterm infants receiving parenteral lipid emulsions. BMC Pediatr 18: 348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sivaprasad S, Arden G (2016) Spare the rods and spoil the retina: revisited. Eye (Lond) 30: 189–192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sivaprasad S, Vasconcelos JC, Prevost AT, Holmes H, Hykin P, George S, Murphy C, Kelly J, Arden GB, CLEOPATRA Study Group (2018) Clinical efficacy and safety of a light mask for prevention of dark adaptation in treating and preventing progression of early diabetic macular oedema at 24 months (CLEOPATRA): a multicentre, phase 3, randomised controlled trial. Lancet Diabetes Endocrinol 6: 382–391 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith CE, Poulter JA, Levin AV, Capasso JE, Price S, Ben‐Yosef T, Sharony R, Newman WG, Shore RC, Brookes SJ et al (2016) Spectrum of PEX1 and PEX6 variants in Heimler syndrome. Eur J Hum Genet 24: 1565‐1571 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Souied EH, Aslam T, Garcia‐Layana A, Holz FG, Leys A, Silva R, Delcourt C (2015) Omega‐3 fatty acids and age‐related macular degeneration. Ophthalmic Res 55: 62–69 [DOI] [PubMed] [Google Scholar]
- Stahl A, Sapieha P, Connor KM, Sangiovanni JP, Chen J, Aderman CM, Willett KL, Krah NM, Dennison RJ, Seaward MR et al (2010) Short communication: PPAR gamma mediates a direct antiangiogenic effect of omega 3‐PUFAs in proliferative retinopathy. Circ Res 107: 495–500 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanislaus S, Hecht R, Yie J, Hager T, Hall M, Spahr C, Wang W, Weiszmann J, Li Y, Deng L et al (2017) A novel Fc‐FGF21 with improved resistance to proteolysis, increased affinity toward beta‐klotho, and enhanced efficacy in mice and cynomolgus monkeys. Endocrinology 158: 1314–1327 [DOI] [PubMed] [Google Scholar]
- Stinson AM, Wiegand RD, Anderson RE (1991) Fatty acid and molecular species compositions of phospholipids and diacylglycerols from rat retinal membranes. Exp Eye Res 52: 213–218 [DOI] [PubMed] [Google Scholar]
- Storti F, Klee K, Todorova V, Steiner R, Othman A, van der Velde‐Visser S, Samardzija M, Meneau I, Barben M, Karademir D et al (2019) Impaired ABCA1/ABCG1‐mediated lipid efflux in the mouse retinal pigment epithelium (RPE) leads to retinal degeneration. eLife 8: e45100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stryer L (1991) Visual excitation and recovery. J Biol Chem 266: 10711–10714 [PubMed] [Google Scholar]
- Sun Y, Lin Z, Liu CH, Gong Y, Liegl R, Fredrick TW, Meng SS, Burnim SB, Wang Z, Akula JD et al (2017) Inflammatory signals from photoreceptor modulate pathological retinal angiogenesis via c‐Fos. J Exp Med 214: 1753–1767 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talukdar S, Zhou Y, Li D, Rossulek M, Dong J, Somayaji V, Weng Y, Clark R, Lanba A, Owen BM et al (2016) A long‐acting FGF21 molecule, PF‐05231023, decreases body weight and improves lipid profile in non‐human primates and type 2 diabetic subjects. Cell Metab 23: 427–440 [DOI] [PubMed] [Google Scholar]
- Tan JS, Wang JJ, Flood V, Mitchell P (2009) Dietary fatty acids and the 10‐year incidence of age‐related macular degeneration: the Blue Mountains Eye Study. Arch Ophthalmol 127: 656–665 [DOI] [PubMed] [Google Scholar]
- Tonade D, Liu H, Kern TS (2016) Photoreceptor cells produce inflammatory mediators that contribute to endothelial cell death in diabetes. Invest Ophthalmol Vis Sci 57: 4264–4271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ueta T, Yanagi Y, Tamaki Y, Yamaguchi T (2009) Cerebrovascular accidents in ranibizumab. Ophthalmology 116: 362 [DOI] [PubMed] [Google Scholar]
- Vamecq J, Cherkaoui‐Malki M, Andreoletti P, Latruffe N (2014) The human peroxisome in health and disease: the story of an oddity becoming a vital organelle. Biochimie 98: 4–15 [DOI] [PubMed] [Google Scholar]
- Walsky RL, Gaman EA, Obach RS (2005) Examination of 209 drugs for inhibition of cytochrome P450 2C8. J Clin Pharmacol 45: 68–78 [DOI] [PubMed] [Google Scholar]
- Wang L, Tornquist P, Bill A (1997) Glucose metabolism in pig outer retina in light and darkness. Acta Physiol Scand 160: 75–81 [DOI] [PubMed] [Google Scholar]
- Wang X, Iannaccone A, Jablonski MM (2005) Contribution of Muller cells toward the regulation of photoreceptor outer segment assembly. Neuron Glia Biol 1: 1–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winkler BS (1981) Glycolytic and oxidative metabolism in relation to retinal function. J Gen Physiol 77: 667–692 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye M, Lu W, Wang X, Wang C, Abbruzzese JL, Liang G, Li X, Luo Y (2016) FGF21‐FGFR1 coordinates phospholipid homeostasis, lipid droplet function, and ER stress in obesity. Endocrinology 157: 4754–4769 [DOI] [PubMed] [Google Scholar]
- Yee P, Weymouth AE, Fletcher EL, Vingrys AJ (2010) A role for omega‐3 polyunsaturated fatty acid supplements in diabetic neuropathy. Invest Ophthalmol Vis Sci 51: 1755–1764 [DOI] [PubMed] [Google Scholar]
- Yonekawa Y, Miller JW, Kim IK (2015) Age‐related macular degeneration: advances in management and diagnosis. J Clin Med 4: 343–359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young RW (1967) The renewal of photoreceptor cell outer segments. J Cell Biol 33: 61–72 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young RW, Bok D (1969) Participation of the retinal pigment epithelium in the rod outer segment renewal process. J Cell Biol 42: 392–403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Cross SD, Stanton JB, Marmorstein AD, Le YZ, Marmorstein LY (2017) Early AMD‐like defects in the RPE and retinal degeneration in aged mice with RPE‐specific deletion of Atg5 or Atg7. Mol Vis 23: 228–241 [PMC free article] [PubMed] [Google Scholar]
- Zhang M, Chu Y, Mowery J, Konkel B, Galli S, Theos AC, Golestaneh N (2018) Pgc‐1alpha repression and high‐fat diet induce age‐related macular degeneration‐like phenotypes in mice. Dis Model Mech 11: dmm032698 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Z, Doggett TA, Sene A, Apte RS, Ferguson TA (2015) Autophagy supports survival and phototransduction protein levels in rod photoreceptors. Cell Death Differ 22: 488–498 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu S, Wu Y, Ye X, Ma L, Qi J, Yu D, Wei Y, Lin G, Ren G, Li D (2016) FGF21 ameliorates nonalcoholic fatty liver disease by inducing autophagy. Mol Cell Biochem 420: 107–119 [DOI] [PubMed] [Google Scholar]


