Abstract
Background:
Tobacco use is a global pandemic, affecting an estimated 1.2 billion people and resulting in substantial health burdens and associated costs.
Objectives:
The aim of this study was to estimate the efficacy of several treatments for smoking cessation in a real-life setting and to evaluate predictors of smoking abstinence.
Methods:
This research was designed with a sample of 593 cases recorded over the period between 2015 and 2016. Six treatment groups were included: (1) bupropion and motivational interviewing (MI); (2) bupropion, nicotine replacement therapy (NRT), and MI; (3) NRT and MI; (4) varenicline and MI; (5) personal vaporizer electronic cigarette and MI; and (6) electronic cigarette, cigarette like “cigalike,” and MI.
Results:
Results support the efficacy of all treatment groups when used in a real-life setting. The predictors of smoking abstinence were sex, partner smoking status, previous quit attempts, daily consumption, self-efficacy, and level of nicotine dependence.
Conclusions:
The use of different therapeutic strategies in clinical practice, including pharmacotherapy and nonstandard electronic nicotine delivery systems, such as an electronic cigarette, ensures a greater chance of cessation success and the possibility of tailoring interventions according to patients’ resources.
Keywords: Smoking cessation, NRT, bupropion, varenicline, counseling, electronic cigarette
Introduction
Tobacco use is a global pandemic, affecting an estimated 1.2 billion people and resulting in substantial health burdens and associated costs. With approximately 5 million tobacco-related deaths annually, cigarette smoking is the leading cause of preventable premature mortality in the world.1 Death is mainly caused by lung cancer, coronary heart disease, chronic obstructive pulmonary disease, and stroke.2,3 The risk of serious disease diminishes rapidly after quitting and permanent abstinence is known to reduce the risk of lung and other cancers, heart disease, chronic lung disease, and stroke.4,5
“Offer help to quit tobacco use” is 1 of the 6 evidence-based measures recommended by the World Health Organization (WHO) Framework Convention on Tobacco Control (FCTC) in response to the tobacco epidemic.6 The FCTC has been endorsed by over 160 countries. In keeping with FCTC recommendations, state governments are obligated to address and treat tobacco dependence in their primary health care services. Treatments for smoking cessation vary, from medical advice to pharmacotherapy and psychological interventions such as counseling. Evidence-based recommendations indicate that counseling and medication used independently are helpful for treating tobacco dependence and are more effective when used concurrently.7 Moreover, treatments aimed at smoking cessation are among the most cost-effective interventions in the Health Care System.8,9
Unfortunately, the powerful addictive qualities of nicotine create a huge hurdle, even for those who have a sincere desire to quit. Once established, the addiction is very difficult to break and smoking behavior becomes entrenched. Approximately 80% of smokers who attempt to quit on their own relapse within the first month of abstinence and only 3% to 5% remain abstinent at 6 months.10 The pharmacologic effects of nicotine establish tobacco addiction.11 Therefore, it is important to consider pharmacotherapy to improve cessation success rates.
Decreasing the prevalence of traditional cigarette smoking will take a multimodal approach at different social levels: individuals, groups, social and cultural level, national health care systems, and government. Prior to providing advice about how to quit smoking, it is critical to assess patients’ readiness to change, help resolve indecisions about quitting and establish motivation for cessation.12 Motivational interviewing (MI) is an evidence-based counseling method focused on smokers’ concerns about behavior change and cessation.13 The goal of MI is to resolve ambivalence about behavior change and help patients establish internal motivations to change their behaviors.13 Recent meta-analyses indicate that MI has a small but significant effect on quitting smoking and long-term abstinence; an effect that was greatly increased among those who were initially not motivated to quit and among those who received advice to quit from their doctors.14 Another recent meta-analysis found a large effect size among minority smokers, and those who were not ready to quit.15
Moreover, in recent years, there has been an increase in the number of smokers at “Centro per la Prevenzione e Cura del Tabagismo,” Center of Excellence for the Acceleration of Harm Reduction (COEHAR) University of Catania Smoking Cessation Center requesting an e-cigarette for cessation without our prompting. Many of these patients reported that they felt encouraged by their friends and relatives when using an electronic cigarette and were able to quit or reduce smoking. The electronic cigarette is an emerging phenomenon that has become increasingly popular in recent years.16 Users often refer to themselves as “vapers,” because they inhale vapor rather than smoke. “Vaping” communities, both online and in person, advocate for the use of electronic cigarettes,17 and some of these communities are sponsored by manufacturers of the devices. Use of the electronic cigarette has generated considerable global debate, with some authorities wanting to ban or regulate the device. Unlike standard cessation interventions, e-cigarettes are designed to look like traditional cigarettes and simulate the visual, sensory, and behavioral aspects of smoking traditional cigarettes.18,19 Evaluating the efficacy of these devices in a real-life setting is of the utmost importance. Population-based cross-sectional studies have demonstrated improved smoking cessation rates among e-cigarette users.20-22
The aims of this study were to estimate the efficacy of counseling combined with standard pharmacotherapy treatments for smoking cessation (bupropion, nicotine replacement therapy (NRT) and varenicline) or the electronic cigarette in actual clinical practice. Several predictors are associated with successfully sustaining smoking cessation, including late age of initiation of cigarette smoking, longer duration of previous quit attempts, lack of depression, low-to-moderate nicotine dependence, absence of alcohol problems, sustained level of motivation, being married, and not living in a home with other smokers.23 Identification of individual characteristics that predict success in smoking cessation can help to match smokers with a strategy that is more likely to help them quit, to identify smokers who might need more intensive treatment, and to make the most of health care resources.24 Predictors of abstinence were also examined.
Method
This study took place at the COEHAR University of Catania Smoking Cessation Center. The Center has been in operation since January 2003 (II level Outpatient Clinic of the Department of Experimental and Clinical Medicine, Unit of Internal Medicine A.O.U. “Policlinico—Vittorio Emanuele,” University of Catania, Italy). Our multidisciplinary team includes pneumologists, clinical psychologists, specialists in pathological dependence, and professional nurses. The aims of the Center are as follows:
To provide assessments and a variety of interventions to help smokers abstain or at least reduce their tobacco use.
To research tobacco addiction.
To promote health care, to protect the health of nonsmokers, and to prevent young people from becoming addicted to tobacco.
To train health care professionals to provide smoking cessation interventions.
Participants were enrolled in the Smoking Cessation Center’s program that consists of 8 visits. Each smoker participated for 1 year of this 2-year observational study. It was conducted in a real-life setting, with all smokers enrolled from January 2015 to December 2015 and followed up by December 2016. The local ethics committee approved this study.
During the first visit (week 1), detailed sociodemographic factors (age, sex, level of education, and marital status), and smoking history (number of cigarettes smoked daily, years of smoking, smoking status of other members of the household, previous quit attempts) were recorded. Nicotine dependence was calculated by the Fagerström Test for Nicotine Dependence (FTND), a standardized questionnaire.25 Subjective ratings of depression and anxiety were assessed with the Beck Depression Inventory–II (BDI-II)26 and the Beck Anxiety Inventory (BAI).27 Level of self-efficacy was assessed with the Visual Analogue Scale (VAS).28
The BDI-II is a 21-item, self-report rating inventory that measures characteristic attitudes and symptoms of depression.26 Internal consistency for the BDI-II ranges from 0.73 to 0.92 with a mean of .86. Similar reliability has been found for the 13-item short form. The BDI-II demonstrates high internal consistency, with alpha coefficients of .86 and .81 for psychiatric and nonpsychiatric populations, respectively. The BAI evaluates physiological and cognitive symptoms of anxiety, with item overlap with other self-report depression inventories minimized. Each of the 21 BAI items is descriptive of a symptom of anxiety and is rated on a scale of 0 to 3. The BAI can be administered verbally by a trained interviewer or can be self-administered. The BAI has been found to discriminate well between anxious and nonanxious diagnostic groups and, as a result, is useful as a screening measure for anxiety in a variety of clinical populations. It has an average reliability coefficient of 0.92 and a test–retest reliability of 0.75. Self-efficacy can be defined as a person’s belief in his or her capability to organize and execute a course of action needed to produce particular outcomes.29 The VAS measures self-efficacy as a task-specific predictor of behavior, and is quick and easy to use in clinical settings, and validity has been established.28 Exhaled CO was measured by Micro CO (Micro Medical, Rochester, UK).
At their first clinic visit, participants provided demographic information and completed the FTCD. All participants were offered smoking cessation counseling based on the principle of motivational interviewing (10-20 minutes). Typically occurring on the second visit (week 2), they were instructed on how to prepare to stop smoking and to decide on a target quit date (TQD). At that time, they were also typically prescribed medications for nicotine dependence and cravings tailored to their individual needs and preferences.
Evidence-based recommendations indicate that although counseling and medication used separately are helpful for treating tobacco dependence, when used together, cessation outcomes improve.7 The pharmacologic effect of nicotine plays a crucial role in tobacco addiction.11 Therefore, it is important to consider pharmacotherapy to improve cessation success rates.
First selection drugs approved and indicated by the US Public Health Service Clinical Practice Guidelines7 are NRT, bupropion, and varenicline. For these reasons, in our study, we created 6 treatment groups: (1) bupropion and MI; (2) bupropion, NRT, and MI; (3) NRT and MI; (4) varenicline and MI; (5) personal vaporizer electronic cigarette and MI; and (6) electronic cigarette, cigarette like “cigalike,” and MI. Smokers interested in using an electronic cigarette to quit smoking were encouraged to choose an approved smoking cessation treatment. They were also informed that the electronic cigarette is not an approved cessation intervention in Italy despite emerging research suggesting the potential role for the electronic cigarette as a smoking cessation tool. The participants were allocated to treatment groups based on their preferences and the results of FTCD.
Pharmacologic therapies were prescribed over a 12-week period according to manufacturer guidelines. Participants were prescribed varenicline at 1 mg twice daily and bupropion SR at 150 mg twice daily. Participants who smoked ⩾10 cigarettes daily were given a 25-mg NRT patch daily for the first 8 weeks, a 15-mg patch daily for the next 2 weeks, and a 10-mg patch daily for the remaining 2 weeks. Participants who smoked <10 cigarettes daily were given a 15-mg NRT patch once daily for the first 10 weeks and a 10-mg patch daily for the remaining 2 weeks.
E-liquids/cartridges were given over a 12-week period. Participants who smoked ⩾10 cigarettes daily used 18 mg/mL e-liquids/cartridges for the first 8 weeks, 12 mg/mL e-liquids/cartridges for the next 2 weeks, and 6 mg/mL e-liquids/cartridges for the remaining 2 weeks. Participants who smoked <10 cigarettes daily used 12 mg/mL e-liquid/cartridges for the first 8 weeks, 9 mg/mL e-liquids/cartridges for the next 2 weeks, and 6 mg/mL e-liquids/cartridges for the remaining 2 weeks.
Follow-up visits were scheduled 7 to 8 days after the first visit and then at 1-, 2-, 3-, 6-, 9-, and 12-month intervals. During each follow-up visit, smoking cessation counseling was offered, treatment compliance was evaluated, possible side effects and withdrawal symptoms were recorded, and the exhaled single-breath CO was measured. We used a counseling style incorporating the concepts and “spirit” of MI identified by Miller and Rollnick.13
MI is described as a directive, client-centered counseling style used to elicit motivation to change by helping clients explore and resolve ambivalence. Emphasis is placed on creating a collaborative relationship and affirming the client’s autonomy regarding change. Therapists evoke motivation for change by drawing on client’s goals and values rather than by imposing motivation onto the client.13 Table 1 identifies the principles of MI compared with standard counseling approaches.
Table 1.
Principles of motivational interviewing compared with standard counseling approaches.
| Standard approaches | Motivational |
|---|---|
| • Focused on fixing the problem • Directly confront a smoker about the risks and consequences of their behavior |
• Focused on patient’s concerns and perspectives • Ask strategic questions so that a smoker generates their own reasons for quitting |
| • Provide solutions for change | • Egalitarian partnership—collaboratively generate solutions |
| • Practitioner is the expert | • Patient is the expert at what works for him or her |
| • Assumes patient is motivated and ready for change | • Match intervention to patient’s level of readiness to change |
| • Advise, warn, persuade | • Emphasize personal choice |
| • Ambivalence means that the patient is in denial | • Ambivalence is a normal part of the change process |
| • Goals are prescribed | • Goals are collaboratively set. The patient is given choices |
| • Resistance is met with argumentation and correction | • Resistance is an interpersonal pattern influenced by provider behavior |
The 3 components that embody the “spirit” of MI are collaboration, autonomy, and evocation. The 4 general principles of MI are expressing empathy, developing discrepancy between current behavior and important goals or values, rolling with or avoiding struggling with resistance, and supporting self-efficacy for change. Four commonly used techniques are asking open-ended questions, affirming, listening reflectively, and summarizing. Motivational interviewing capitalizes on the idea that if people can talk themselves out of change, they can also talk themselves into change. The primary aim of MI is to help patients develop their own “change talk” by asking key questions. Change talk refers to patients’ positive statements about change and reasons and arguments for change.13 Research indicates that the more patients hear themselves argue for change, the more committed they become to making the change.
Study Outcome Measures
Study outcome measures were the 52nd week success rate (52WSR) (calculated as the ratio between eCO results verified 52-week quitters divided by the number of smokers setting a firm quit date). The co-primary efficacy measures were: the 4-week success rate (4WSR) (calculated as the ratio between eCO results verified 4-week quitters divided by the number of smokers setting a firm quit date), 12th week success rate (12WSR) (calculated as the ratio between eCO results verified 12-week quitters divided by the number of smokers setting a firm quit date), and the 24th week success rate (24WSR) (calculated as the ratio between eCO results verified 24-week quitters divided by the number of smokers setting a firm quit date).30 We followed the Russell Standard, and those lost to follow-up were included as smokers.30 According to this standard a smoker who undergoes at least 1 treatment session on or prior to the quit date and sets a firm quit date is considered a treated smoker (TS). Smokers who attend an assessment session but fail to attend thereafter would not be counted.
Participants who self-reported giving up smoking and had an eCO concentration of ⩽10 ppm at the final follow-up visit were defined as quitters. Smokers who failed to meet this criterion were categorized as smoking cessation failures (ie, relapsers). Participants were counted as lost to follow-up if they were not available to verify their quitting status after setting a quit date.
The regression modeling involved 2 dependent variables: the General Mean Testing (the aggregate mean along interval intervention 1, 2, 3, 4, 5 in each group of treatment about the reported number of cigarettes smoked), and the Successful Treatment Index (the aggregate mean about the number of the reduction unsmoked cigarettes for each smoker in each group of treatment, reported at each intervention planned, divided by the mean of the maximum total cigarettes smoked for each smoker linked at each group of treatment without intervention, where the negative values describe us the grouped reduction).
Results
The number of contacts made to the University of Catania Smoking Cessation Center from January 2015 to December 2015 was 759. The total number of smokers who participated in the treatment program was 593 (78.2% of the 759), with slightly more men (59%) than women and an average age of 47.5 (±12.07) years. As can be seen in Table 2, on average, participants began smoking when they were 17.7 (±5.3) years, had long smoking tenures of 30 (±12.2) years, were heavy smokers with an average daily cigarette consumption of 24.1 (±10.3) cigarettes, and the majority previously attempted to quit smoking (71%) and identified their partner (eg, spouse and significant other) as a smoker (60%). TS demonstrated strong nicotine dependence with an average score of 5.98 (±2.20) on the FTCD. Most of the participants had a high school education or less (82%). The mean score for Self-Efficacy was 5.6 (±2.0) out of 10. The average score of 9.6 in the BDI was below the threshold for depression, and the BAI scores averaged 6.9 in the sample, suggesting a low state of anxiety. The 593 TS were all assigned to MI combined with either bupropion 9% (51), NRT 14% (81), varenicline 19% (112), bupropion, and NRT 9% (119), a “cigalike” electronic cigarette 21% (127), or a personal vaporizer electronic cigarette 17% (103).
Table 2.
Distribution of background variables and predictors of smoking cessation and treatment group (N = 593).
| N | % | Mean | Standard deviation | Standard error of mean | |
|---|---|---|---|---|---|
| Sex | |||||
| Male | 349 | 58.9 | |||
| Female | 244 | 41.1 | |||
| Education level | |||||
| Elementary school | 39 | 6.6 | |||
| Middle school | 162 | 27.3 | |||
| High school | 287 | 48.4 | |||
| Degree/PhD/Master | 105 | 17.7 | |||
| Age of patient (years) | 47.5 | 12.1 | 0.5 | ||
| Smoking initiation age (years) | 17.7 | 5.3 | 0.2 | ||
| Patient smoking history (years) | 29.8 | 12.2 | 0.5 | ||
| Daily cigarette consumption | 24.1 | 10.3 | 0.4 | ||
| Smoker presence partner | |||||
| Yes | 354 | 59.9 | |||
| No | 237 | 40.1 | |||
| Previous quit attempts | |||||
| Yes | 425 | 71.7 | |||
| No | 168 | 28.3 | |||
| Self-efficacy | 5.6 | 2.0 | 0.1 | ||
| Fagerström test for nicotine dependence | 6.0 | 2.2 | 0.1 | ||
| Beck Depression Inventory | 9.6 | 11.1 | 0.5 | ||
| Beck Anxiety Inventory | 6.9 | 8.7 | 0.4 | ||
| Treatment category | |||||
| Varenicline + MI | 112 | 18.9 | |||
| NRT + MI | 81 | 13.7 | |||
| BPR + MI | 51 | 8.6 | |||
| BPR + NRT + MI | 119 | 20.1 | |||
| Personal vaporizer eCig + MI | 103 | 17.4 | |||
| Cigalike + MI | 127 | 21.4 | |||
Abbreviations: MI, motivational interviewing; NRT, nicotine replacement therapy; BPR, bupropion.
Consistent with manufacturer guidelines, all participants in the varenicline, bupropion, and NRT groups stopped using their treatments completely by the end of the 12 weeks of usage. In the groups using personal vaporizers and cigalike, all participants were daily electronic-cigarette users at the end of week 12. The average daily e-liquid consumption was approximately 4 mL for personal vaporizer users and 1 cartridge daily for cigalike users. In the cigalike electronic cigarette and MI group, 4 (3.2%) participants stopped using their cigalike at the end of week 12, and in the personal vaporizer electronic cigarette and MI group, 3 (2.9%) participants stopped using their personal vaporizer completely by the end week 12. All participants who stopped using their cigalike or personal vaporizers relapsed to smoking, suggesting a possible role of electronic-cigarettes as a tool to avoid smoking relapse.
In Table 3, we provide the results of an analysis of our 2 outcome variables (General Mean Testing and Successful Treatment Index) for our sample according to treatment group. As can be seen, the treatment groups differed in their effectiveness on our 2 outcome variables.
Table 3.
ANOVA analysis by 6 treatment groups for General Mean Testing and Successful Treatment Index (sample of N = 593).
| Sum of squares | df | Mean square | F | Significance | |
|---|---|---|---|---|---|
| General Mean Testing | |||||
| Between groups | 1773.214 | 5 | 354.643 | 3.864 | .002 |
| Within groups | 53 879.919 | 587 | 91.789 | ||
| Total | 55 653.134 | 592 | |||
| Successful Treatment Index | |||||
| Between groups | 5351.704 | 5 | 1070.341 | 7.020 | .000 |
| Within groups | 89 493.933 | 587 | 152.460 | ||
| Total | 94 845.637 | 592 | |||
Abbreviations: ANOVA, analysis of variance.
Table 4 demonstrates the efficacy of treatments to reduce and stop cigarette use. In the table, there are 2 pooled data divided into 6 treatment groups. These data reflect the weighted average of the decrease in tobacco consumption, and the Successful Treatment Index (STI), to which we added the category ordinal of the same STI to better understand the reduction of critical mass consumption. The general weighted average column demonstrates that the largest reduction is for the varenicline + MI treatment group with 10.70 fewer cigarettes daily in 12 months. This is followed by treatment group bupropion + MI (13.28 fewer cigarettes); cigalike e-Cig + MI (13.71 fewer cigarettes); bupropion + NRT + MI (14.93 fewer cigarettes); personal vaporizer e-Cig + MI (15.50 fewer cigarettes); and NRT + MI (15.53 fewer cigarettes). Overall, on average across treatment groups, cigarette consumption was reduced by nearly 14 cigarettes daily over the 12 months of treatment.
Table 4.
Distribution of General Testing Mean and Treatment Successful Index along the 6 treatment groups (N = 593).
| General Mean Testinga |
Successful Treatment Index |
Successful Treatment Indexb |
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean | Standard deviation | Standard error of mean | Mean | Standard deviation | Standard error of mean | Failure |
Low quit |
Medium quit |
High quit |
|||||
| N | % | N | % | N | % | N | % | |||||||
| Treatment category | ||||||||||||||
| Varenicline + MI | 10.70 | 9.05 | 0.86 | −19.72 | 14.44 | 1.36 | 9 | 13.0 | 46 | 13.3 | 50 | 32.5 | 7 | 28.0 |
| NRT + MI | 15.53 | 9.06 | 1.01 | −12.72 | 12.08 | 1.34 | 16 | 23.2 | 46 | 13.3 | 16 | 10.4 | 3 | 12.0 |
| BPR + MI | 13.28 | 9.87 | 1.38 | −17.33 | 12.16 | 1.70 | 1 | 1.4 | 32 | 9.3 | 14 | 9.1 | 4 | 16.0 |
| BPR + NRT + MI | 14.93 | 10.77 | 0.99 | −16.46 | 12.49 | 1.14 | 12 | 17.4 | 66 | 19.1 | 36 | 23.4 | 5 | 20.0 |
| Personal vaporizer eCig + MI | 15.50 | 10.68 | 1.05 | −12.36 | 14.00 | 1.38 | 25 | 36.2 | 53 | 15.4 | 20 | 13.0 | 5 | 20.0 |
| Cigalike + MI | 13.71 | 7.98 | 0.71 | −11.95 | 8.39 | 0.74 | 6 | 8.7 | 102 | 29.6 | 18 | 11.7 | 1 | 4.0 |
Abbreviations: MI, motivational interviewing; BPR, bupropion; NRT, nicotine replacement therapy.
12 months.
STI recorded in ordinal categorical variables.
Unfortunately, 69 participants (11%) had not stopped smoking, but 89% of the cases reduced on average about 20% consumption of tobacco.
Table 4 also presents results for the Successful Treatment Index. The treatment category with the greatest success was varenicline + MI with a weighted average reduction of −19.72, followed by bupropion + MI (−17.33); bupropion + NRT + MI (−16.46); NRT + MI (−12.72); personal vaporizer eCig + MI (−12.36); and cigalike eCig + MI with (−11.95). The “Successful Treatment Index” demonstrates reduction and cessation outcomes by treatment groups categorized as “failure, low quit, medium quit, and high quit.” The category “failure” represents about 12% (n = 69) participants who failed to stop smoking; “low quit” of 58.2% (n = 345) of participants who had an average reduction of 20% from their baseline cigarette consumption; “medium quit” of 26% (n = 154) of participants with an average reduction of 40% from their baseline cigarette consumption; and “high quitting” of 4.2% (n = 25) representing a reduction of at least 80% from their baseline cigarette consumption.
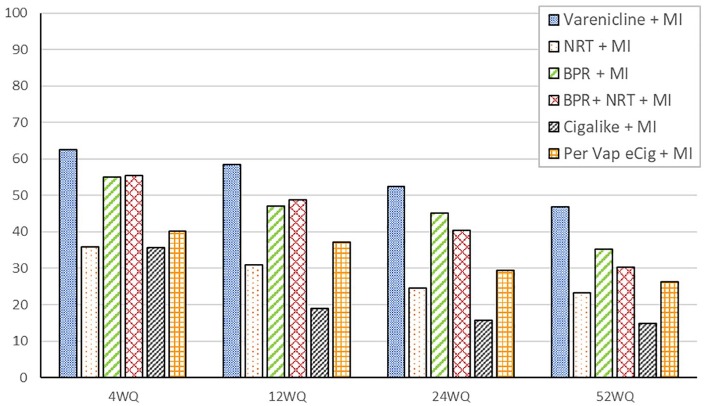
Figure 1 shows the distribution of quit rates across treatment groups at weeks 4, 12, 24, and 52. Among participants treated with MI and Bupropion/NRT, complete cessation rates were as follows: 30.2% (n = 36) at week 52 (52WSR) with a relapse rate of 22.6% (n = 27); 40.3% (n = 49) at week 24 (24WSR); 48.7% (n = 59) at week 12 (12WSR); and 55.4% (n = 63) at week 4 (4WSR). For those treated with bupropion and MI, complete cessation rates were 35.2% (n = 18) at week 52 (52WSR) with a relapse rate of 19.6% (n = 10); 45% (n = 23) at week 24 (24WSR); 47% (n = 24) at week 12 (12WSR); and 54.9% (n = 28) at week 4 (4WSR). For participants in the treatment category nicotine replacement therapy and MI complete cessation rates were 23.4% (n = 19) at week 52 (52WSR) with a relapse rate of 12.5% (n = 10); 24.7% (n = 20 cases) at week 24 (24WSR); 30.8% (n = 2) at week 12 (12WSR); and 35.9% (n = 29) at week 4 (4WSR). For subjects treated with varenicline and MI complete cessation rates were 46.8% (n = 52 cases) at week 52 (52WSR) with a relapse rate of 20.5% (n = 23); 52.2% (n = 58) at week 24 (24WSR); 58.5% (n = 65) at week 12 (12WSR); and 67.5% (n = 75) at week 4 (4WSR). Those treated with cigalike electronic cigarette and MI complete cessation rates were 15.8% (n = 20) at week 52 (52WSR) with a relapse rate of 19.6% (n = 25); 15.8% (n = 20) at week 24 (24WSR); 19% (n = 24) at week 12 (12WSR); and 35.7% (n = 45) at week 4 (4WSR). Participants treated with a personal vaporizer electronic cigarette and MI, the percentage of complete cessation was 26.4% (n = 27) at week 52 (52WSR) with a relapse rate of 13.5% (n = 14); 29.4% (n = 30) at week 24 (24WSR); 37.2% (n = 38) at week 12 (12WSR); and 40.1% (n = 41) at week 4 (4WSR). The Russell Standard was used to verify complete smoking cessation across treatment groups.
Figure 1.
Quit rates across treatment groups (sample of N = 593). MI indicates motivational interviewing; BPR, bupropion; NRT, nicotine replacement therapy.
The study outcomes at 12 months were tested by linear regression analysis. As can be seen in Table 5, treatment resulted in a significant reduction in tobacco consumption.
Table 5.
Predictors of Smoking cessation regression coefficients modeling along General Mean Testing and Successful Treatment Indexa (N = 593).
| Model | Unstandardized coefficients |
Standardized coefficientsb |
t | Significance | Unstandardized coefficients |
Standardized coefficientsc |
t | Significance | ||
|---|---|---|---|---|---|---|---|---|---|---|
| B | Standard error | Beta | B | Standard error | Beta | |||||
| Sex | 0.600 | .635 | .031 | 0.945 | .345 | 0.850 | .875 | .035 | 0.971 | .332 |
| Education level | −0.468 | .403 | −.037 | −1.160 | .246 | −0.582 | .555 | −.037 | −1.048 | .295 |
| Age of patient (year) | −0.049 | .026 | −.063 | −1.912 | .056 | −0.067 | .035 | −.069 | −1.886 | .060 |
| Patient smoking history (year) | −0.043 | .024 | −.079 | −1.823 | .069 | −0.068 | .036 | −.072 | −1.917 | .056 |
| Smoker presence partner | 0.860 | .651 | .044 | 1.320 | .187 | 1.150 | .897 | .047 | 1.282 | .200 |
| Previous quit attempts | 0.078 | .683 | .004 | 0.114 | .910 | −0.068 | .940 | −.003 | −0.072 | .943 |
| Smoking initiation age (year) | 0.025 | .063 | .013 | 0.396 | .692 | 0.038 | .086 | .016 | 0.439 | .661 |
| Daily cigarette consumption | 0.519 | .035 | .550 | 14.938 | .000 | −0.655 | .048 | −.561 | −13.708 | .000 |
| Self-efficacy | −0.431 | .153 | −.090 | −2.810 | .005 | −0.568 | .211 | −.096 | −2.688 | .007 |
| Fagerström test for nicotine dependence | 0.629 | .160 | .145 | 3.937 | .000 | 0.863 | .220 | .161 | 3.920 | .000 |
| Beck Depression Inventory | −0.067 | .038 | −.076 | −1.757 | .079 | −0.117 | .053 | −.107 | −2.219 | .027 |
| Beck Anxiety Inventory | 0.101 | .048 | .089 | 2.075 | .038 | 0.119 | .067 | .085 | 1.777 | .076 |
Weighted least squares regression: weighted by treatment category.
Dependent variable: General Mean Testing.
Dependent variable: Successful Treatment Index.
As can be seen in Table 5, “Daily Consumption Cigarettes,” “Self-Efficacy,” and “Fagerström Test for Nicotine Dependence” were statistically significant predictors. The BAI was a significant predictor for the STI, whereas the BDI was a significant predictor for the General Mean Testing. The variables “education,” “age,” and “smoking initiation age” were not significant predictor variables for either outcome. Significant predictors of smoking cessation are: “Male Gender,” “No Smoker Partner Presence,” “Previous Quit Attempt,” “Low Cigarettes Daily Consumption,” “High Self-Efficacy,” and “Low scores at Fagerström Test for Nicotine Dependence.” This regression was tested at a significance level of 95%. The significant predictors did not change when the significance level was at 99%.
We also monitored for the presence and absence of adverse events during the study. No significant side effects were reported. Only about 5% of electronic cigarettes users and 8% of varenicline users reported transient mouth and throat irritation (e-cigarettes users) and nausea without vomiting (varenicline users).
Discussion and Conclusions
Consistent with meta-analyses, the results of our study confirm the effectiveness of pharmacotherapy (bupropion, nicotine replacement therapy, and varenicline)31 combined with counseling32 for achieving smoking cessation.
In a recent meta-analysis of 49 studies,33 counseling (with and without pharmacotherapy) increased the likelihood of quitting by 40% to 80% compared with minimal support. Our study examined the MI counseling approach for smoking cessation, “a collaborative conversation style for strengthening a person’s own motivation and commitment to change.”34 A meta-analysis of 28 studies demonstrated a significant increase in cessation with MI.35 The US Department of Health and Human Services Clinical Practice Guidelines for treating Tobacco Use and Dependence: Updated 2008, recommends MI as an intervention.7
In addition, our findings demonstrate reductions in cigarettes per day (CPD) with the various interventions. In other research, reductions in CPD by at least 50% were demonstrated with NRT in a pooled analysis of 8 studies.36 In this same analysis, there were too few trials to determine conclusively whether or not other interventions (bupropion, varenicline, electronic cigarettes, snus) increased the likelihood of smoking reduction or cessation.
In our cohort, varenicline combined with MI was the most effective combined therapy in reducing cigarette smoking or achieving smoking cessation, and bupropion combined with MI was the second most effective method. The superiority of varenicline for cessation is consistent with findings of a pooled analysis when outcomes were compared with bupropion (N = 5) and NRT (N = 8).37 The benefit of combining pharmacotherapy with counseling is well-established. Chances for cessation success increased from 70% to 100% when pharmacotherapy was combined with counseling, compared with brief advice or support in a meta-analysis of 53 studies.32 Increasing amounts of behavioral support is estimated to increase cessation success by 10% to 25%, based on 47 pooled trials.32 Interestingly, in our study, adding nicotine replacement therapy did not increase the success rate and demonstrated less effective outcomes. In contrast, NRT was demonstrated to increase cessation by 50% to 60% in a meta-analysis of 133 studies.38
Participants in our study who used the personal vaporizer electronic cigarette had smoking cessation outcomes comparable with nicotine replacement therapy, consistent with other research.39 Less effective for cessation than the personal vaporizer electronic cigarette was the cigalike electronic cigarette and MI treatment group in our study. Other research supports the usefulness of an elctronic-cigarette for cessation. A prospective 12-month randomized controlled trial evaluated smoking reduction and abstinence in 300 smokers who experimented with a first generation cigalike without an intention to quit.40 Smoking reduction was documented at 22.3% and 10.3% at week 12 and week 52, respectively. Complete abstinence from tobacco smoking was documented at 10.7% and 8.7% at week 12 and week 52, respectively. In a prospective 12-month pilot study, e-cigarettes were shown to substantially decrease cigarette consumption without causing significant side effects in smokers with schizophrenia not intending to quit.41 Findings from a 24-month retrospective chart review42 of smokers with chronic obstructive pulmonary disease (COPD) who tried an e-cigarette demonstrated complete abstinence from CTC smoking (54.2%), and dual usage was reported by 45.8%. A systematic review found that elctronic-cigarettes were superior to the nicotine patch in reducing CTC consumption. However, these results were limited to small sample sizes and low-quality studies making it impossible to determine if elctronic-cigarettes help people quit. However, findings demonstrated short-term safety with e-cigarette use.39
Consistent with our population, sociodemographic disparities exist in cigarette smoking.43,44 Most of the patients enrolled in our study had a low level of education. This may be explained by the geographical location of the University of Catania Smoking Cessation Center where the study took place. This is an historical area of the city with a high poverty index and a low educational index, and to the role of “word spread” within the neighborhood. Similarly, the Centers for Disease Control report the National average for smoking in United States differed drastically among those with a graduate degree (4.5%) compared with a General Education Diploma (GED) (40.6%) as well as those living above the poverty level (14.3%) compared with living below (25.3%).43,44 In our study, level of education was not a significant predictor of poor smoking cessation outcomes.
Not surprising, aligned with other research, variables such as “No Smoker Partner Presence,”45 “Fewer Previous Quit Attempts,”46 “Low Daily Cigarette Consumption,”46 “High Self-Efficacy,”46,47 “low scores at Fagerström Test for Nicotine Dependence,”48 and “Male Gender”49 were all significant predictors of successful reduction/smoking cessation.
Smoking cessation yielded considerable savings among all treatment groups. On average, participants in our sample smoked 20 cigarettes a day. Based on the average cost of cigarettes in Italy, our participants would have spent €5.50 daily and €2000 annually on these cigarettes. In contrast, those who quit smoking traditional cigarettes, and used a cigalike electronic-cigarette for 12 months spent approximately €1200, an annual saving of €800. Those who quit and used a personal vaporizer elctronic-cigarette for 12 months spent about €950, an annual savings of €1150. Twelve-week courses of the pharmacologic interventions cost considerably less when compared with the cost of cigarette smoking for 1 year (€2000). The estimated costs are varenicline (€380), bupropion (€280), NRT (€350), bupropion and NRT (€630), with annual savings estimated at €1620, €1720, €1650, and €1370, respectively.
Limitations to this study are its lack of control groups and controlled randomization. The lack of a control group treated exclusively with MI makes it impossible to determine what percentage of reduction/quitting could be attributed to MI. In addition, we did not compare the standard approach with the MI approach alone and, therefore, cannot confirm if the latter is more effective in a real-life setting. In addition, there was no measure of lung function over time, a measure that should be included in future studies.
In conclusion, a multimodal integrated approach appears to be effective to help smokers quit or reduce their smoking. In addition to the standardized therapies to achieve smoking cessation, the use of an electronic cigarette, especially a recently developed personal vaporizer electronic cigarette, has been demonstrated to be an effective and cost-effective strategy for some smokers. However, longitudinal research with long-term follow-up is needed to confirm this finding. The effectiveness could be attributed to the efficiency of the personal vaporizers. Personal vaporizers are equipped with higher capacity lithium batteries, better vaporizing systems, and cartridges that can be refilled with liquid solutions mainly consisting of propylene glycol (PG), glycerol, distilled water, flavors, and nicotine (ie, e-Liquid). Consequently, personal vaporizers provide a more satisfying vaping experience. Users have the choice of an extensive number of puffs and e-liquid aromas. The thicker vapor appears to be more appealing to smokers,50 and its use maintains gestures and rituals that have an important role in smoking addiction. Moreover, nicotine delivery to the bloodstream using second-generation devices is consistently superior compared with cigalikes,51 which appears to contribute to improved cessation outcomes.
Our study suggests the importance of offering an integrated multimodal smoking cessation approach. Combining MI with existing therapies and new tools such as the electronic cigarette has helped participants in real-life settings to quit or reduce their smoking without showing significant side effects.
Although e-cigarettes have only become widely used in the last few years, research evaluating these devices is growing rapidly. The evidence-base is not always consistent and sometimes contradictory, but there is a growing consensus that these products are significantly less harmful than traditional cigarettes.52,53 That said, important research questions remain regarding any potential harms and potential benefits from their use. These questions include, for example, whether these products promote use by nontobacco users; sustain nicotine dependency when dually used; slow intentions to quit in dual users; or encourage relapse to cigarette smoking among former smokers.54
Findings of our study suggest the need for randomized and controlled studies to validate or demonstrate differences in our findings.
Footnotes
Funding:The author(s) received no financial support for the research, authorship, and/or publication of this article.
Declaration of conflicting interests:R.P. has received lecture fees from Pfizer and GSK; he has also served as a consultant to Pfizer and Arbi Group. The remaining authors have no conflicts of interest or declarations regarding the contents of this paper.
Author Contributions: All authors listed have made substantial, direct, and intellectual contribution to the work, and approved it for publication.
ORCID iD: Pasquale Caponnetto  https://orcid.org/0000-0002-9368-5867
https://orcid.org/0000-0002-9368-5867
References
- 1. World Health Organization (WHO). Tobacco or Health: A Global Status Report. Geneva, Switzerland: WHO; 1997:1-32. [Google Scholar]
- 2. Doll R, Pet R, Boreham J, Sutherland I. Mortality in relation to smoking: 50 years’ observation on male British doctors. BMJ. 2004;328:1519-1528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. U.S. Department of Health and Human Service. The Health Consequences of Smoking: A Report of the Surgeon General. Atlanta, GA: Office on Smoking and Health, National Center for Chronic Disease Prevention and Health Promotion, Center for Disease Control and Prevention, U.S. Department of Health and Human Service; https://www.cdc.gov/tobacco/data_statistics/fact_sheets/adult_data/cig_smoking/index.htm. Updated 2004. [Google Scholar]
- 4. U.S. Department of Health and Human Service. The Health Benefits of Smoking Cessation (DHHS Publication No. (CDC) 90-8516). Rockville, MD: Office on Smoking and Health, National Center for Chronic Disease Prevention and Health Promotion, Center for Disease Control and Prevention, U.S. Department of Health and Human Service; 1990. [Google Scholar]
- 5. Lightwood JM, Glantz SA. Short-term economic and health benefits of smoking cessation. Circulation. 1997;96:1089-1096. [DOI] [PubMed] [Google Scholar]
- 6. World Health Organization (WHO). Global Report on the Global Tobacco Epidemic: Implementing Smoke-Free Environments. Geneva, Switzerland: WHO; 2009. [Google Scholar]
- 7. Fiore MC, Jaen CR, Baker TB, et al. Treating Tobacco Use and Dependence: 2008 Update. Rockville, MD: Public Health Service, U.S. Department of Health and Human Services; 2008. [Google Scholar]
- 8. Parrott S, Godfrey C, Raw M, West R, McNeill A. Guidance for commissioners on the cost effectiveness of smoking cessation interventions. Health Educational Authority. Thorax. 1998;53:S1-S38. [PMC free article] [PubMed] [Google Scholar]
- 9. West R. The clinical significance of “small” effects of smoking cessation treatments. Addiction. 2007;102:506-509. [DOI] [PubMed] [Google Scholar]
- 10. Hughes JR, Keely J, Naud S. Shape of the relapse curve and long-term abstinence among untreated smokers. Addiction. 2004;99:29-38. [DOI] [PubMed] [Google Scholar]
- 11. Benowitz NL. Neurobiology of nicotine addiction: implications for smoking cessation treatment. Am J Med. 2008;121:S3-S10. [DOI] [PubMed] [Google Scholar]
- 12. Rosen MI, Ryan C, Rigsby M. Motivational enhancement and MEMS: review to improve medication adherence. Behav Change. 2002;19:183-190. [Google Scholar]
- 13. Miller WR, Rollnick S. Motivational Interviewing: Preparing People for Change. 2nd ed. New York, NY: Guilford Press; 2002. [Google Scholar]
- 14. Lai DT, Cahill K, Qin Y, Tang JL. Motivational interviewing for smoking cessation. Cochrane Database Syst Rev. 2010;1:CD006936. [DOI] [PubMed] [Google Scholar]
- 15. Hettema JE, Hendricks PS. Motivational interviewing for smoking cessation: a meta-analytic review. J Consult Clin Psychol. 2010;78:868-884. [DOI] [PubMed] [Google Scholar]
- 16. Ayers JW, Ribisl KM, Brownstein JS. Tracking the rise in popularity of electronic nicotine delivery systems (electronic cigarettes) using search query surveillance. Am J Prev Med. 2011;40:448-453. [DOI] [PubMed] [Google Scholar]
- 17. McQueen A, Tower S, Sumner W. Interviews with “vapers”: implications for future research with electronic cigarettes. Nicotine Tob Res. 2011;13:860-867. [DOI] [PubMed] [Google Scholar]
- 18. Caponnetto P, Campagna D, Cibella F, et al. Efficiency and safety of an electronic cigarette (ECLAT) as tobacco cigarettes substitute: a prospective 12-month randomized control design study. PLoS ONE. 2013;8:e66317. doi: 10.1371/journal.pone.0066317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Caponnetto P, Maglia M, Cannella MC, et al. Impact of different e-cigarette generation and models on cognitive performances, craving and gesture: a randomized cross-over trial (CogEcig). Front Psychol. 2017;8:127. doi: 10.3389/fpsyg.2017.00127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Giovenco DP, Delnevo CD. Prevalence of population smoking cessation by electronic cigarette use status in a national sample of recent smokers. Addict Behav. 2018;76:129-134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Levy DT, Cummings KM, Villanti AC, et al. A framework for evaluating the public health impact of e-cigarettes and other vaporized nicotine products. Addiction. 2017;112:8-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Zhu SH, Zhuang YL, Wong S, Cummins SE, Tedeschi GJ. E-cigarette use and associated changes in population smoking cessation: evidence from US current population surveys. BMJ. 2017;358:j3262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Caponnetto P, Polosa R. Common predictors of smoking cessation in clinical practice. Respir Med. 2008;102:1182-1192. [DOI] [PubMed] [Google Scholar]
- 24. Caponnetto P, Keller E, Bruno CM, Polosa R. Handling relapse in smoking cessation: strategies and recommendations. Intern Emerg Med. 2013;8:7-12. doi: 10.1007/s11739-012-0864-z. [DOI] [PubMed] [Google Scholar]
- 25. Heatherton TF, Kozlowski LT, Frecker RC, Fagerström KO. The Fagerström test for nicotine dependence: a revision of the Fagerström Tolerance Questionnaire. Br J Addict. 1991;86:1119-1127. [DOI] [PubMed] [Google Scholar]
- 26. Beck AT, Steer RA, Brown G. Beck Depression Inventory II. Florence, Italy: Giunti O.S.; 2006. [Google Scholar]
- 27. Beck AT, Steer RA. Beck Anxiety Inventory. Florence, Italy: Giunti O.S.; 2006. [Google Scholar]
- 28. Turner NM, van de Leemput AJ, Draaisma JM, Oosterveld P, ten Cate OT. Validity of the visual analogue scale as an instrument to measure self-efficacy in resuscitation skills. Med Educ. 2008;42:503-511. [DOI] [PubMed] [Google Scholar]
- 29. Bandura A. Self-Efficacy: The Exercise of Control. New York, NY: WH Freeman & Co; 1997. [Google Scholar]
- 30. West R, Hajek P, Stead L, Stapleton J. Outcome criteria in smoking cessation trials: proposal for a common standard. Addiction. 2005;100:299-303. [DOI] [PubMed] [Google Scholar]
- 31. Cahill K, Stevens S, Perera R, Lancaster T. Pharmacological interventions for smoking cessation: an overview and network meta-analysis. Cochrane Database Syst Rev. 2013;5:CD009329. doi: 10.1002/14651858.CD009329.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Stead LF, Koilpillai P, Fanshawe TR, Lancaster T. Combined pharmacotherapy and behavioural interventions for smoking cessation. Cochrane Database Syst Rev. 2016;3:CD008286. doi: 10.1002/14651858.CD008286.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Lancaster T, Stead LF. Individual behavioural counselling for smoking cessation. Cochrane Database Syst Rev. 2017;3:CD001292. doi: 10.1002/14651858.CD001292.pub3. [DOI] [PubMed] [Google Scholar]
- 34. Miller S, Rollnick WR. Helping People Change. 3rd ed. New York, NY: Guilford Press; 2013. [Google Scholar]
- 35. Lindson-Hawley N, Thompson TP, Begh R. Motivational interviewing for smoking cessation. Cochrane Database Syst Rev. 2015;3:CD006936. [DOI] [PubMed] [Google Scholar]
- 36. Lindson-Hawley N, Hartmann-Boyce J, Fanshawe TR, Begh R, Farley A, Lancaster T. Interventions to reduce harm from continued tobacco use. Cochrane Database Syst Rev. 2016;10:CD005231. doi: 10.1002/14651858.CD005231.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Cahill K, Lindson-Hawley N, Thomas KH, Fanshawe TR, Lancaster T. Nicotine receptor partial agonists for smoking cessation. Cochrane Database Syst Rev. 2016;5:CD006103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Hartmann-Boyce J, Chepkin SC, Ye W, Bullen C, Lancaster T. Nicotine replacement therapy versus control for smoking cessation. Cochrane Database Syst Rev. 2018;5:CD000146. doi: 10.1002/14651858.CD000146.pub5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Hartmann-Boyce J, McRobbie H, Bullen C, Begh R, Stead LF, Hajek P. Electronic cigarettes for smoking cessation. Cochrane Database Syst Rev. 2016;9:CD010216. doi: 10.1002/14651858.CD010216.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Caponnetto P, Russo C, Bruno CM, Alamo A, Amaradio MD, Polosa R. Electronic cigarette: a possible substitute for cigarette dependence. Monaldi Arch Chest Dis. 2013;79:12-19. doi: 10.4081/monaldi.2013.104. [DOI] [PubMed] [Google Scholar]
- 41. Caponnetto P, Auditore R, Russo C, Cappello GC, Polosa R. Impact of an electronic cigarette on smoking reduction and cessation in schizophrenic smokers: a prospective 12-month pilot study. Int J Environ Res Public Health. 2013;10:446-461. doi: 10.3390/ijerph10020446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Polosa R, Morjaria JB, Caponnetto P, Prosperini U, Russo C, Pennisi A. Evidence for harm reduction in COPD smokers who switch to electronic cigarettes. Respir Res. 2016;17:166. doi: 10.1186/s12931-016-0481-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. CDC. Current cigarette smoking among adults in the United States. https://www.cdc.gov/tobacco/data_statistics/fact_sheets/adult_data/cig_smoking/index.htm. Accessed August, 2018.
- 44. Jamal A, Phillips E, Gentzke AS. Current cigarette smoking among adults—United States, 2016. MMWR Morb Mortal Wkly Rep. 2018;67:53-59. doi: 10.15585/mmwr.mm6702a1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Jackson SE, Steptoe A, Wardle J. The influence of partner’s behavior on health behavior change. JAMA Intern Med. 2015;175:385-392. [DOI] [PubMed] [Google Scholar]
- 46. Li L, Borland R, Yong HH, et al. Predictors of smoking cessation among adult smokers in Malaysia and Thailand: findings from the International Tobacco Control Southeast Asia Survey. Nicotine Tob Res. 2010;12:S34-S44. doi: 10.1093/ntr/ntq030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Schnoll RA, Martinez E, Tatum KL, et al. Increased self-efficacy to quit and perceived control over withdrawal symptoms predict smoking cessation following nicotine dependence treatment. Addict Behav. 2011;36:144-147. doi: 10.1016/j.addbeh.2010.08.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Fagerström K, Russ C, Yu CR, Yunis C, Foulds J. The Fagerström test for nicotine dependence as a predictor of smoking abstinence: a pooled analysis of varenicline clinical trial data. Nicotine Tob Res. 2012;14:1467-1473. doi: 10.1093/ntr/nts018. [DOI] [PubMed] [Google Scholar]
- 49. Smith PH, Bessette AJ, Weinberger AH, Sheffer CE, McKee SA. Sex/gender differences in smoking cessation: a review. Prev Med. 2016;92:135-140. http://doi.org.proxy.wexler.hunter.cuny.edu/10.1016/j.ypmed.2016.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Dawkins L, Turner J, Roberts A, Soar K. “Vaping” profiles and preferences: an online survey of electronic cigarette users. Addiction. 2013;108:1115-1125. [DOI] [PubMed] [Google Scholar]
- 51. Farsalinos KE, Spyrou A, Tsimopoulou K, Stefopoulos C, Romagna G, Voudris V. Nicotine absorption from electronic cigarette use: comparison between first and new-generation devices. Sci Rep. 2014;4:4133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Farsalinos KE, Polosa R. Safety evaluation and risk assessment of electronic cigarettes as tobacco cigarette substitutes: a systematic review. Ther Adv Drug Saf. 2014;5:67-86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Nutt DJ, Phillips LD, Balfour D, et al. E-cigarettes are less harmful than smoking. Lancet. 2016;387:1160-1162. [DOI] [PubMed] [Google Scholar]
- 54. Glasser AM, Collins L, Pearson JL, et al. Overview of electronic nicotine delivery systems: a systematic review. Am J Prev Med. 2017;52:e33-e66. [DOI] [PMC free article] [PubMed] [Google Scholar]