Abstract
Background:
Hepatic encephalopathy (HE) is a serious complication of cirrhosis. Decreased serum albumin (ALB) level may facilitate the development of HE and accelerate the death of cirrhotic patients with HE. Recent evidence also suggests that human albumin infusion may reduce the incidence of HE and improve the outcomes of cirrhotic patients. This study aimed to explore the association of serum ALB level with the development of overt HE and HE-associated mortality during hospitalization.
Methods:
Cirrhotic patients admitted to our hospital between January 2010 and February 2019 were screened. Independent predictors for HE were identified by logistic regression analyses. Odds ratio (OR) with 95% confidence interval (95% CI) was calculated. Area under curve (AUC) was calculated by receiver operator characteristic curve analyses.
Results:
Of the 2376 included patients with cirrhosis but without HE at admission, 113 (4.8%) developed overt HE during hospitalizations. ALB level (OR = 0.878, 95% CI = 0.834–0.924) was an independent risk factor for development of overt HE. AUC of ALB level for predicting the development of overt HE was 0.770 (95% CI = 0.752–0.787, p < 0.0001), and the best cut-off value was ⩽31.6 g/l. Of the 183 included patients with cirrhosis and overt HE at admission, 20 (10.9%) died during hospitalizations. ALB level (OR = 0.864, 95% CI = 0.771–0.967) was an independent risk factor for death from overt HE. The AUC of ALB level for predicting death from overt HE was 0.737 (95% CI = 0.667–0.799, p = 0.0001), and the best cut-off value was ⩽22.8 g/l.
Conclusions:
Decreased serum ALB level may be associated with higher risk of overt HE and HE-associated mortality during hospitalizations in cirrhosis.
Keywords: albumin, cirrhosis, hepatic encephalopathy, incidence, mortality
Introduction
Hepatic encephalopathy (HE) is a common and serious complication of end-stage liver disease.1 It is divided into covert and overt HE according to the clinical manifestations and severity of HE. The incidence of overt and covert HE during the clinical course of liver cirrhosis is 30–40% and 20–80%, respectively.1 The risk of death in liver cirrhosis is greatly increased by the occurrence of HE which exerts a huge burden on patients, caregivers, and healthcare systems.2
Except for the well-known drugs, such as lactulose,3 rifaximin,4 L-ornithine-L-aspartate,5 probiotics,6 and zinc,7 the role of human albumin (HA) infusion for the management of HE has been widely and increasingly recognized, but remains controversial.8–10 Recently, an Italian multicenter trial (ANSWER) found that long-term HA administration to patients with decompensated cirrhosis significantly reduced the incidence of grade 3–4 HE.11 The Italian Association for the Study of the Liver (AISF) practice guideline also suggested that HA infusion could decrease the incidence of type C overt HE in cirrhotic patients with ascites.12 By comparison, the AASLD-EASL practice guideline did not recommend HA infusion for the management of HE.1 The inconsistence of recommendations among these societies may be because the potential mechanism of HA infusion in the prevention and treatment HE is elusive and the current evidence is conflicting among the published clinical studies. In the study by Simón-Talero and colleagues,8 which included patients with a mean serum albumin (ALB) level of 30 g/l, the effect of HA infusion was not significant. On the contrary, in the Sharma and coworkers’ study,9 which included patients with a mean serum ALB level of nearly 24 g/l, the effect of HA infusion was significant. Therefore, one may speculate that the difference in patients’ serum ALB levels led to the heterogeneity in the effect of HA infusion on the development and prognosis of HE.
In the present study, we aimed to explore the association of serum ALB level with the incidence and mortality of overt HE in cirrhotic patients during hospitalization.
Methods
Study design
This study protocol was approved by the Medical Ethical Committee of the General Hospital of Northern Theater Command (formerly called as General Hospital of Shenyang Military Area), with approval no. k(2019)18 (ethical approval was obtained on 5 June 2019). The requirement of informed written consent was waived because we only extracted the data from the inpatients’ medical records. The source of our patients comprised two major parts: the first part was a cohort which retrospectively enrolled the patients with liver cirrhosis, without malignancy, consecutively admitted to our hospital from January 2010 to June 2014; the second part was a cohort which prospectively enrolled the patients with liver cirrhosis, without malignancy, consecutively admitted to the Department of Gastroenterology of our hospital and underwent contrast-enhanced computed tomography and upper gastrointestinal endoscopy from December 2014 to February 2019.13 All patients were screened for eligibility. The study protocol conforms to the ethical guidelines of the 1975 Declaration of Helsinki.
As for the association of serum ALB level with development of HE during hospitalizations, the exclusion criteria were as follows: (a) patients with overt HE at admission; (b) patients who received HA infusion before diagnosis of overt HE during hospitalizations; (c) patients who were not diagnosed with overt HE but received HA infusion during hospitalization; and (d) patients in whom serum ALB level was missing.
As for the association of serum ALB level with in-hospital mortality of HE, the exclusion criteria were as follows: (a) patients without overt HE at admission; (b) patients who received HA infusion during hospitalizations; and (c) patients in whom serum ALB level was missing.
Data collection
Data were collected as follows: age, sex, etiology of liver cirrhosis, ascites, acute upper gastrointestinal bleeding (AUGIB), infection, hemoglobin (Hb), white blood cell count (WBC), platelet count (PLT), total bilirubin (TBIL), ALB, alanine aminotransferase (ALT), aspartate aminotransferase (AST), alkaline phosphatase (AKP), gamma-glutamyl transpeptidase (GGT), blood urea nitrogen (BUN), serum creatinine (Scr), potassium (K), sodium (Na), ammonia, prothrombin time (PT), and activated partial thromboplastin time (APTT). Child–Pugh and model for end-stage liver disease (MELD) scores were calculated.14 We recorded the overt HE events at admission or during hospitalization and re-evaluated the diagnosis of overt HE according to the current guideline.1 In-hospital death was also recorded.
Statistical analysis
All statistical analyses were performed using the SPSS software version 20.0 (IBM Corp, Armonk, NY, USA) and MedCalc software version 11.4.2.0 (MedCalc Software, Mariakerke, Belgium). A two-tailed p < 0.05 was considered statistically significant. Logistic regression analysis was performed to identify the independent predictors associated with the development of, and in-hospital death from, overt HE. Receiver operator characteristic (ROC) curve analysis was used to explore the performance of serum ALB level for predicting the development of, and in-hospital death from, overt HE. Area under curve (AUC) was calculated. The best cut-off value with its sensitivity and specificity was also identified. Then, we divided the patients into high- and low-serum-ALB-level groups according to the best cut-off value. Difference between high- and low-serum-ALB-level groups was compared by the nonparametric Mann–Whitney U test and the Chi-square test. The cumulative rates of overt HE and its associated mortality during hospitalization were further assessed with the Kaplan–Meier curve analyses, and the difference between the groups divided according to the best cut-off value was compared by the log-rank test.
Results
Association of serum ALB level with development of overt HE during hospitalizations
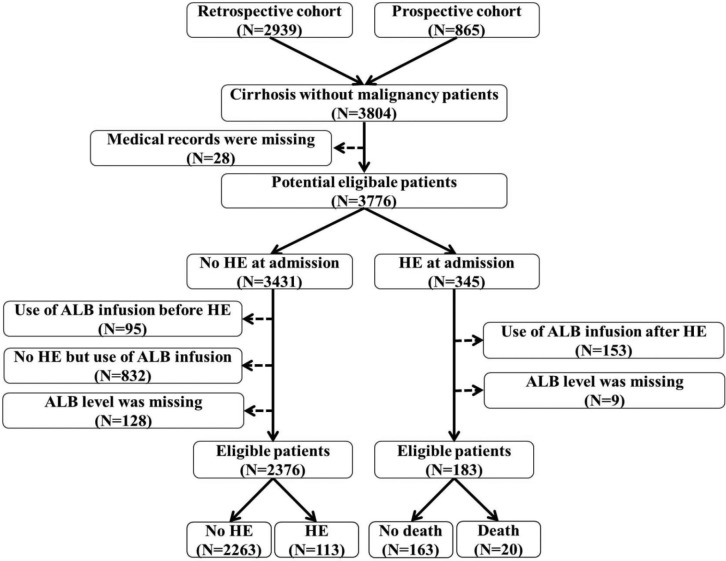
A total of 2376 cirrhotic patients were included (Figure 1). Baseline characteristics were described in Table 1. Median age was 55.26 years (range: 6.20–89.19), and 1619 (68.10%) patients were male. Median serum ALB level was 34.90 g/l (range: 9.60–56.20). Median Child–Pugh and MELD scores were 6 (range: 5–13) and 5.32 (range: –9.67–38.80), respectively. Among them, 113 (4.80%) patients developed overt HE during hospitalizations.
Figure 1.
Flow chart of patient selection.
ALB, albumin; HE, hepatic encephalopathy.
Table 1.
Incidence of overt HE: difference between high-ALB-level (>31.6 g/l) and low-ALB-level (⩽31.6 g/l) groups.
| Variables | Patients, n | Overall* | Patients, n | ALB > 31.6g/l group* | Patients, n | ALB ⩽ 31.6 g/l group* | p value |
|---|---|---|---|---|---|---|---|
| Age, years | 2376 | 55.26 (6.20–89.19) 55.27 ± 12.08 | 1638 | 57.63 (6.20–89.19) 54.80 ± 12.18 | 738 | 55.19 (18.57–89.16) 56.30 ± 11.80 | 0.063 |
| Sex (male %) | 2376 | 1619 (68.1%) | 1638 | 1119 (68.3%) | 738 | 500 (67.8%) | 0.785 |
| Etiology of liver diseases | |||||||
| HBV (%) | 2376 | 920 (38.7%) | 1638 | 651 (39.7%) | 738 | 269 (36.4%) | 0.127 |
| HCV (%) | 2376 | 193 (8.1%) | 1638 | 136 (8.3%) | 738 | 57 (7.7%) | 0.632 |
| Alcohol abuse (%) | 2376 | 904 (38%) | 1638 | 602 (36.8%) | 738 | 302 (40.9%) | 0.053 |
| Autoimmune (%) | 2376 | 147 (6.2%) | 1638 | 83 (5.1%) | 738 | 64 (8.7%) | 0.001 |
| Other etiology (%) | 2376 | 551 (23.2%) | 1638 | 379 (23.1%) | 738 | 172 (23.3%) | 0.928 |
| Ascites (%) | 2376 | 972 (40.9%) | 1638 | 556 (33.9%) | 738 | 416 (56.4%) | <0.001 |
| AUGIB (%) | 2376 | 663 (27.9%) | 1638 | 376 (23%) | 738 | 287 (38.9%) | <0.001 |
| Infection (%) | 2376 | 517 (21.8%) | 1638 | 333 (20.3%) | 738 | 184 (24.9%) | 0.012 |
| Hb (g/l) | 2357 | 98 (23–218) 98.70 ± 30.84 | 1624 | 104 (23–218) 104.04 ± 30.91 | 733 | 84 (27–176) 86.87 ± 27.19 | <0.001 |
| WBC (109/l) | 2359 | 4 (0.5–53.6) 4.76 ± 3.44 | 1626 | 3.8 (0.5–33.5) 4.54 ± 3.08 | 733 | 4.3 (0.5–53.6) 5.24 ± 4.08 | <0.001 |
| PLT (109/l) | 2355 | 80 (3–1278) 105.23 ± 85.80 | 1623 | 81 (3–1278) 107.96 ± 86.92 | 732 | 75 (5–842) 99.18 ± 83.00 | 0.002 |
| TBIL (μmol/l) | 2369 | 19.9 (1.9–809.8) 31.64 ± 47.53 | 1635 | 18.7 (2.4–809.8) 27.61 ± 39.47 | 734 | 22.6 (1.9–679.1) 40.63 ± 60.90 | <0.001 |
| ALB (g/l) | 2376 | 34.9 (9.6–56.2) 34.77 ± 6.22 | 1638 | 37.2 (31.7–56.2) 37.97 ± 4.22 | 738 | 28.75 (9.6–31.6) 27.67 ± 3.49 | <0.001 |
| ALT (U/l) | 2365 | 25.1 (4.23–1460.0) 39.60 ± 71.16 | 1631 | 25 (4.47–1335.0) 39.11 ± 70.96 | 734 | 26 (4.23–1460.0) 40.70 ± 71.65 | 0.138 |
| AST (U/l) | 2366 | 33 (8–1366) 51.82 ± 78.29 | 1632 | 31 (8–1366) 47.83 ± 75.48 | 734 | 39 (8–941) 60.69 ± 83.57 | <0.001 |
| AKP (U/l) | 2362 | 88.83 (1.3–1338.0) 113.0 ± 93.62 | 1629 | 86 (17–1388) 107.34 ± 85.45 | 733 | 92.82 (1.3–980.0) 125.57 ± 108.65 | <0.001 |
| GGT (U/l) | 2361 | 47 (7.54–4562.0) 120.79 ± 236.93 | 1628 | 45.27 (8–4562) 125.99 ± 263.27 | 733 | 51.97 (7.54–1779.18) 109.22 ± 163.49 | 0.369 |
| BUN (mmol/l) | 2321 | 5.39 (1.57–62.45) 6.77 ± 5.16 | 1603 | 5.28 (1.63–51.71) 6.43 ± 4.69 | 718 | 5.74 (1.57–62.45) 7.53 ± 6.01 | <0.001 |
| Scr (μmol/l) | 2321 | 60 (21–1473) 78.83 ± 109.66 | 1603 | 60 (21–1473) 77.31 ± 109.02 | 718 | 59 (23–1353) 82.23 ± 111.07 | 0.868 |
| K (mmol/l) | 2328 | 4 (2.13–7.24) 4.0 ± 0.49 | 1604 | 4 (2.48–7.24) 4.02 ± 0.46 | 724 | 3.95 (2.13–6.00) 3.96 ± 0.55 | 0.001 |
| Na (mmol/l) | 2328 | 139.4 (116.3–160.8) 138.94 ± 3.83 | 1604 | 139.6 (117.9–153.9) 139.27 ± 3.60 | 724 | 138.8 (116.3–160.8) 138.23 ± 4.20 | <0.001 |
| Ammonia (μmol/l) | 966 | 35 (2.2–317.0) 42.89 ± 35.14 | 618 | 33 (2.2–249.0) 38.17 ± 28.07 | 348 | 39 (9–317) 51.26 ± 43.85 | <0.001 |
| PT (seconds) | 2304 | 15.1 (10.3–51.5) 15.59 ± 3.15 | 1594 | 14.6 (10.4–51.0) 15.03 ± 2.74 | 710 | 16.2 (10.3–51.5) 16.83 ± 3.61 | <0.001 |
| APTT (seconds) | 2302 | 40 (19.4–472.4) 41.37 ± 12.63 | 1592 | 39.5 (19.4–472.4) 40.46 ± 12.92 | 710 | 41.5 (23.1–180.0) 43.39 ± 11.72 | <0.001 |
| Child–Pugh score | 2286 | 6 (5–13) 6.72 ± 1.62 | 1581 | 6 (5–12) 6.14 ± 1.27 | 705 | 8 (6–13) 8.02 ± 1.59 | <0.001 |
| MELD score | 2259 | 5.32 (−9.67 to 38.80) 6.08 ± 5.91 | 1638 | 4.63 (−9.67 to 38.80) 5.19 ± 5.43 | 697 | 7.21 (−7.52 to 35.30) 8.06 ± 6.42 | <0.001 |
| Incidence of overt HE (%) | 2376 | 113 (4.8%) | 1638 | 32 (2%) | 738 | 81 (11%) | <0.001 |
Bolded numerals indicate statistical significance.
AKP, alkaline phosphatase; ALB, albumin; ALT, alanine aminotransferase; APTT, activated partial thromboplastin time; AST, aspartate aminotransferase; AUGIB, acute upper gastrointestinal bleeding; BUN, blood urea nitrogen; GGT, gamma-glutamyl transpeptidase; Hb, hemoglobin; HBV, hepatic B virus; HCV, hepatic C virus; HE, hepatic encephalopathy; K, potassium; MELD, model for end-stage liver disease; Na, sodium; PLT, platelet count; PT, prothrombin time; Pts, patients; Scr, serum creatinine; TBIL, total bilirubin; WBC, white blood cell count.
Note: *Continuous variables were reported as median (range) and mean ± standard deviation; categorical variables were reported as frequency (percentage).
In the univariate analysis, the statistically significant risk factors associated with the development of overt HE included age, ascites, AUGIB, infection, Hb, WBC, TBIL, ALB, AST, BUN, Scr, K, Na, ammonia, PT, APTT, Child–Pugh score, and MELD score (Table 2). In the multivariate analysis, the independent risk factors associated with the development of overt HE included age (OR = 1.028, 95% CI: 1.003–1.052, p = 0.025), infection (OR = 2.668, 95%CI: 1.481–4.807, p = 0.001), ALB (OR = 0.878, 95% CI: 0.834–0.924, p < 0.001), ammonia (OR = 1.029, 95%CI: 1.022–1.037, p < 0.001), and PT (OR = 1.098, 95% CI: 1.016–1.187, p = 0.018; Table 2).
Table 2.
Univariate and multivariate analyses of predictors associated with the development of overt HE.
| Variables (n) | Univariate |
Multivariate |
||||
|---|---|---|---|---|---|---|
| OR | 95% CI | p value | OR | 95% CI | p value | |
| Age (years) | 1.021 | 1.004–1.037 | 0.012 | 1.028 | 1.003–1.052 | 0.025 |
| Sex (female versus male) | 1.044 | 0.694–1.571 | 0.836 | |||
| HBV (yes versus no) | 1.134 | 0.773–1.664 | 0.521 | |||
| HCV (yes versus no) | 1.646 | 0.922–2.941 | 0.092 | |||
| Alcohol abuse (yes versus no) | 1.040 | 0.704–1.538 | 0.844 | |||
| Autoimmune (yes versus no) | 1.185 | 0.512–2.744 | 0.692 | |||
| Other etiology (yes versus no) | 1.262 | 0.784–2.031 | 0.338 | |||
| Ascites (yes versus no) | 2.660 | 1.796–3.938 | <0.001 | 1.136 | 0.656–1.967 | 0.650 |
| AUGIB (yes versus no) | 1.694 | 1.148–2.500 | 0.008 | 1.888 | 0.927–3.843 | 0.080 |
| Infection (yes versus no) | 2.318 | 1.565–3.435 | <0.001 | 2.668 | 1.481–4.807 | 0.001 |
| Hb (g/l) | 0.991 | 0.985–0.997 | 0.005 | 0.997 | 0.985–1.009 | 0.621 |
| WBC (109/l) | 1.090 | 1.050–1.132 | <0.001 | 1.032 | 0.962–1.108 | 0.377 |
| PLT (109/l) | 0.997 | 0.995–1.000 | 0.086 | |||
| TBIL (μmol/l) | 1.006 | 1.004–1.009 | <0.001 | 1.002 | 0.999–1.005 | 0.205 |
| ALB (g/l) | 0.853 | 0.826–0.880 | <0.001 | 0.878 | 0.834–0.924 | <0.001 |
| ALT (U/l) | 1.001 | 1.000–1.003 | 0.078 | |||
| AST (U/l) | 1.002 | 1.000–1.003 | 0.016 | 1.000 | 0.998–1.003 | 0.889 |
| AKP (U/l) | 1.000 | 0.998–1.002 | 0.901 | |||
| GGT (U/l) | 1.000 | 0.999–1.001 | 0.893 | |||
| BUN (mmol/l) | 1.072 | 1.049–1.095 | <0.001 | 1.073 | 0.991–1.162 | 0.810 |
| Scr (μmol/l) | 1.001 | 1.000–1.002 | 0.043 | 0.998 | 0.994–1.003 | 0.463 |
| K (mmol/l) | 1.677 | 1.169–2.407 | 0.005 | 1.507 | 0.934–2.433 | 0.093 |
| Na (mmol/l) | 0.938 | 0.897–0.981 | 0.005 | 1.044 | 0.977–1.116 | 0.205 |
| Ammonia (μmol/l) | 1.033 | 1.026–1.039 | <0.001 | 1.029 | 1.022–1.037 | <0.001 |
| PT (seconds) | 1.170 | 1.122–1.220 | <0.001 | 1.098 | 1.016–1.187 | 0.018 |
| APTT (seconds) | 1.013 | 1.001–1.024 | 0.029 | 0.991 | 0.965–1.017 | 0.474 |
| Child–Pugh score* | 1.781 | 1.605–1.977 | <0.001 | |||
| MELD score* | 1.137 | 1.109–1.166 | <0.001 | |||
Bolded numerals indicate statistical significance.
Child–Pugh and MELD scores, which are complex variables comprising many clinically significant variables, were excluded in the multivariate analysis.
AKP, alkaline phosphatase; ALB, albumin; ALT, alanine aminotransferase; APTT, activated partial thromboplastin time; AST, aspartate aminotransferase; AUGIB, acute upper gastrointestinal bleeding; BUN, blood urea nitrogen; CI, confidence interval; DBIL, direct bilirubin; GGT, gamma-glutamyl transpeptidase; Hb, hemoglobin; HBV, hepatic B virus; HCV, hepatic C virus; HE, hepatic encephalopathy; IBIL, indirect bilirubin; INR, international normalization ratio; K, potassium; MELD, model for end-stage liver disease; Na, sodium; OR, odds ratio; PLT, platelet count; PT, prothrombin time; RBC, red blood cell count; Scr, serum creatinine; TBIL, total bilirubin; WBC, white blood cell count.
AUC of serum ALB level for predicting the development of overt HE was 0.770 (95% CI: 0.752–0.787, p < 0.0001; Figure 2). The best cut-off value was ⩽31.6 g/l with a sensitivity of 71.68% and a specificity of 70.97%. According to the cut-off value, 2376 patients were divided into high (n = 1638) and low (n = 738) serum ALB level groups (Table 1). The low-serum-ALB-level group had a significantly higher prevalence of ascites (p < 0.001), AUGIB (p < 0.001), and infection (p = 0.012) than the high-serum-ALB-level group. Hb, WBC, PLT, TBIL, ALB, AST, AKP, BUN, K, Na, ammonia, PT, APTT, Child–Pugh score, and MELD score were significantly different between high- and low-serum-ALB-level groups (p < 0.05, in all comparisons). The low-serum-ALB-level group had a significantly higher incidence of overt HE during hospitalization (11.00% versus 2.00%, p < 0.001; Table 1). Kaplan–Meier curve analysis also found that the low-serum-ALB-level group had a significantly higher cumulative rate of overt HE (p < 0.001; Figure 3).
Figure 2.
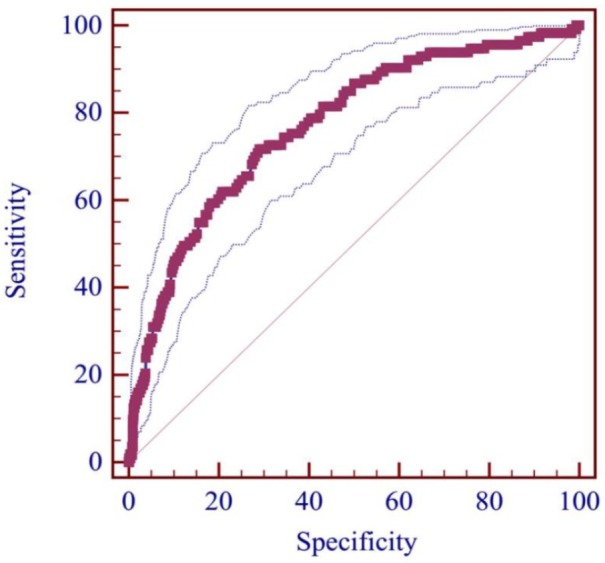
ROC curve of ALB level for predicting the development of overt HE.
ALB, albumin; HE, hepatic encephalopathy; ROC, receiver operating characteristic.
Figure 3.
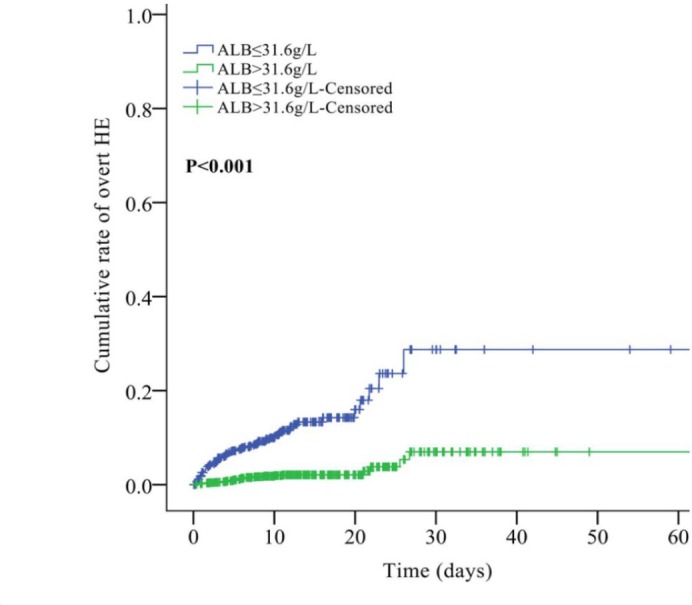
Cumulative rate of overt HE between high and low-ALB-level groups.
ALB, albumin; HE, hepatic encephalopathy.
Association of serum ALB level with mortality of patients with overt HE during hospitalizations
A total of 183 cirrhotic patients with overt HE were included (Figure 1). Baseline characteristics are described in Table 3. Median age was 57.17 years (range: 27.49–95.13), and 134 (73.20%) patients were male. Median ALB level was 30.40 g/l (range: 12.40–48.90). Median Child–Pugh and MELD scores were 9 (range: 6–15) and 9.49 (range: –1.73–51.64), respectively. Among them, in-hospital mortality was 10.9% (20/183).
Table 3.
Mortality of overt HE: difference between high-ALB-level (>22.8 g/l) and low-ALB-level (⩽22.8 g/l) groups.
| Variables | Patients, n | Overall* | Patients, n | ALB > 22.8g/l group* | Patients, n | ALB ⩽ 22.8g/l group* | p value |
|---|---|---|---|---|---|---|---|
| Age, years | 183 | 57.17 (27.49–95.13) 58.21 ± 10.62 | 164 | 57.26 (27.49–95.13) 58.35 ± 10.56 | 19 | 56.35 (30.78–78.81) 56.97 ± 11.32 | 0.605 |
| Sex (male %) | 183 | 134 (73.2%) | 164 | 118 (72%) | 19 | 16 (84.2%) | 0.253 |
| Etiology of liver diseases | |||||||
| HBV (%) | 183 | 58 (31.7%) | 164 | 50 (30.5%) | 19 | 8 (42.1%) | 0.303 |
| HCV (%) | 183 | 15 (8.2%) | 164 | 15 (9.1%) | 19 | 0 (0%) | 0.169 |
| Alcohol abuse (%) | 183 | 86 (47.6%) | 164 | 78 (47.6%) | 19 | 8 (42.1%) | 0.652 |
| Autoimmune (%) | 183 | 8 (4.4%) | 164 | 8 (4.9%) | 19 | 0 (0%) | 0.325 |
| Other etiology (%) | 183 | 35 (19.1%) | 164 | 31 (18.9%) | 19 | 4 (21.1%) | 0.822 |
| Ascites (%) | 183 | 99 (54.1%) | 164 | 83 (50.6%) | 19 | 16 (84.2%) | 0.005 |
| AUGIB (%) | 183 | 60 (32.8%) | 164 | 51 (31.1%) | 19 | 9 (47.4%) | 0.153 |
| Infection (%) | 183 | 37 (20.2%) | 164 | 31 (18.9%) | 19 | 6 (31.6%) | 0.193 |
| Hb (g/l) | 181 | 96 (37–180) 99.47 ± 27.45 | 162 | 98 (37–180) 101.46 ± 27.65 | 19 | 81 (49–116) 82.34 ± 18.67 | 0.003 |
| WBC (109/l) | 181 | 4.7 (1.1–46.1) 6.25 ± 5.09 | 162 | 4.7 (1.1–46.1) 6.05 ± 5.00 | 19 | 6 (2.0–25.3) 7.9 ± 5.62 | 0.088 |
| PLT (109/l) | 180 | 74.5 (16–391) 85.86 ± 57.29 | 161 | 75 (17–391) 87.84 ± 58.85 | 19 | 68 (16–158) 69.05 ± 38.93 | 0.244 |
| TBIL (μmol/l) | 183 | 37.3 (3.6–707.7) 58.83 ± 84.90 | 164 | 36.1 (3.6–707.7) 54.67 ± 85.39 | 19 | 66.5 (24.0–255.5) 94.75 ± 72.99 | 0.001 |
| ALB (g/l) | 183 | 30.4 (12.4–48.9) 30.22 ± 6.11 | 164 | 31.2 (23.0–48.9) 31.47 ± 5.01 | 19 | 21.3 (12.4–22.8) 19.36 ± 3.40 | <0.001 |
| ALT (U/l) | 182 | 28 (8–1064) 45.56 ± 91.53 | 163 | 27 (8–1064) 45.26 ± 96.13 | 19 | 40 (16–131) 48.21 ± 32.72 | 0.027 |
| AST (U/l) | 182 | 42 (9–2454) 94.62 ± 246.32 | 163 | 40 (9–2454) 88.73 ± 241.66 | 19 | 68 (19–1293) 145.11 ± 285.52 | 0.010 |
| AKP (U/l) | 181 | 92 (34–457) 113.80 ± 70.40 | 163 | 93 (34–457) 114.52 ± 72.00 | 18 | 83.5 (47.0–205.4) 107.25 ± 54.96 | 0.763 |
| GGT (U/l) | 181 | 46 (7–1102) 109.02 ± 182.57 | 163 | 47 (7–1055) 107.78 ± 173.49 | 18 | 33.5 (14–1102) 120.28 ± 256.82 | 0.306 |
| BUN (mmol/l) | 176 | 6.56 (1.58–61.01) 8.98 ± 7.99 | 158 | 6.19 (2.32–61.01) 8.82 ± 8.04 | 18 | 8.08 (1.58–29.31) 10.39 ± 7.59 | 0.181 |
| Scr (μmol/l) | 176 | 61 (24–729) 79.29 ± 68.49 | 158 | 60 (24–729) 76.72 ± 68.80 | 18 | 77.5 (36–262) 101.89 ± 63.05 | 0.049 |
| K (mmol/l) | 180 | 4.09 (2.25–7.87) 4.18 ± 0.74 | 162 | 4.05 (2.25–7.87) 4.14 ± 0.72 | 18 | 4.46 (3.24–6.36) 4.51 ± 0.86 | 0.063 |
| Na (mmol/l) | 181 | 138 (116.4–147.2) 137.28 ± 5.35 | 162 | 138.1 (116.4–147.2) 137.52 ± 5.20 | 19 | 136.9 (121.9–144.8) 135.25 ± 6.30 | 0.145 |
| Ammonia (μmol/l) | 175 | 79 (9–480) 88.39 ± 55.73 | 156 | 79.5 (9–480) 88.00 ± 54.70 | 19 | 72 (9–283) 91.57 ± 65.18 | 0.838 |
| PT (seconds) | 178 | 17.3 (12.2–94.6) 18.81 ± 7.15 | 161 | 17.1 (12.2–36.4) 17.86 ± 3.45 | 17 | 22.1 (16.6–94.6) 27.85 ± 18.73 | <0.001 |
| APTT (seconds) | 177 | 44.4 (29.1–180.0) 46.63 ± 14.28 | 160 | 43.5 (29.1–75.4) 44.55 ± 8.27 | 17 | 55 (42.1–180.0) 66.28 ± 33.32 | <0.001 |
| Child–Pugh score | 177 | 9 (6–15) 9.30 ± 2.19 | 160 | 6 (6–15) 9.01 ± 2.01 | 17 | 12 (8–15) 12.06 ± 1.89 | <0.001 |
| MELD score | 173 | 9.49 (−1.73 to 51.64) 11.34 ± 7.75 | 156 | 8.93 (−1.73 to 36.99) 10.30 ± 6.51 | 17 | 22.39 (4.18–51.64) 20.92 ± 11.29 | <0.001 |
| In-hospital death (%) | 183 | 20 (10.9%) | 164 | 11 (6.7%) | 19 | 9 (47.4%) | <0.001 |
Bolded numerals indicate statistical significance.
AKP, alkaline phosphatase; ALB, albumin; ALT, alanine aminotransferase; AST, aspartate aminotransferase; APTT, activated partial thromboplastin time; AUGIB, acute upper gastrointestinal bleeding; BUN, blood urea nitrogen; Hb, hemoglobin; HBV, hepatic B virus; HCV, hepatic C virus; K, potassium; MELD, model for end-stage liver disease; Na, sodium; PLT, platelet count; PT, prothrombin time; Pts, patients; TBIL, total bilirubin; GGT, gamma-glutamyl transpeptidase; Scr, serum creatinine; WBC, white blood cell count.
Note: *Continuous variables were reported as median (range) and mean ± standard deviation; categorical variables were reported as frequency (percentage).
In the univariate analysis, the statistically significant risk factors associated with the in-hospital death of patients with overt HE included WBC, TBIL, ALB, AST, BUN, K, APTT, Child–Pugh score, and MELD score (Table 4). In the multivariate analysis, the independent risk factors associated with the in-hospital death of patients with overt HE included WBC (OR = 1.169, 95% CI: 1.037–1.317, p = 0.011), TBIL (OR = 1.006, 95% CI: 1.001–1.011, p = 0.018), and ALB (OR = 0.864, 95% CI: 0.771–0.967, p < 0.001; Table 4).
Table 4.
Univariate and multivariate analyses of predictors associated with the in-hospital death from overt HE.
| Variables (n) | Univariate |
Multivariate |
||||
|---|---|---|---|---|---|---|
| OR | 95% CI | p value | OR | 95% CI | p value | |
| Age (years) | 1.028 | 0.985–1.073 | 0.197 | |||
| Sex (female versus male) | 1.109 | 0.381–3.232 | 0.849 | |||
| HBV (yes versus no) | 1.445 | 0.499–4.188 | 0.497 | |||
| HCV (yes versus no) | 1.282 | 0.268–6.143 | 0.756 | |||
| Alcohol Abuse (yes versus no) | 1.376 | 0.535–3.545 | 0.508 | |||
| Autoimmune (yes versus no) | 1.173 | 0.137–10.057 | 0.884 | |||
| Other etiology (yes versus no) | 1.478 | 0.498–4.382 | 0.481 | |||
| Ascites (yes versus no) | 2.141 | 0.784–5.847 | 0.1371 | |||
| AUGIB (yes versus no) | 1.797 | 0.701–4.605 | 0.222 | |||
| Infection (yes versus no) | 2.387 | 0.878–6.493 | 0.088 | |||
| Hb (g/l) | 0.985 | 0.967–1.004 | 0.114 | |||
| WBC (109/l) | 1.222 | 1.107–1.348 | <0.001 | 1.169 | 1.037–1.317 | 0.011 |
| PLT (109/l) | 0.999 | 0.990–1.007 | 0.796 | |||
| TBIL (μmol/l) | 1.007 | 1.002–1.012 | 0.007 | 1.006 | 1.001–1.011 | 0.018 |
| ALB (g/l) | 0.852 | 0.782–0.927 | <0.001 | 0.864 | 0.771–0.967 | 0.011 |
| ALT (U/l) | 1.005 | 1.000–1.011 | 0.056 | |||
| AST (U/l) | 1.002 | 1.000–1.003 | 0.027 | 1.000 | 0.998–1.002 | 0.882 |
| AKP (U/l) | 0.994 | 0.985–1.004 | 0.232 | |||
| GGT (U/l) | 1.000 | 0.998–1.003 | 0.705 | |||
| BUN (mmol/l) | 1.060 | 1.014–1.108 | 0.010 | 1.075 | 0.974–1.185 | 0.152 |
| Scr (μmol/l) | 1.005 | 1.000–1.011 | 0.058 | |||
| K (mmol/l) | 1.930 | 1.119–3.328 | 0.018 | 0.620 | 0.223–1.720 | 0.358 |
| Na (mmol/l) | 0.932 | 0.861–1.009 | 0.080 | |||
| Ammonia (μmol/l) | 1.003 | 0.995–1.010 | 0.474 | |||
| PT (seconds) | 1.060 | 0.995–1.128 | 0.071 | |||
| APTT (seconds) | 1.057 | 1.016–1.098 | 0.006 | 1.014 | 0.965–1.067 | 0.576 |
| Child–Pugh score* | 1.459 | 1.171–1.818 | 0.001 | |||
| MELD score* | 1.145 | 1.077–1.217 | <0.001 | |||
Bolded numerals indicate statistical significance.
Child–Pugh and MELD scores, which are complex variables comprising many clinically significant variables, were excluded in the multivariate analysis.
AKP, alkaline phosphatase; ALB, albumin; ALT, alanine aminotransferase; APTT, activated partial thromboplastin time; AST, aspartate aminotransferase; AUGIB, acute upper gastrointestinal bleeding; BUN, blood urea nitrogen; CI, confidence interval; GGT, gamma-glutamyl transpeptidase; Hb, hemoglobin; HBV, hepatic B virus; HCV, hepatic C virus; HE, hepatic encephalopathy; K, potassium; MELD, model for end-stage liver disease; Na, sodium; OR, odds ratio; PLT, platelet count; PT, prothrombin time; Scr, serum creatinine; TBIL, total bilirubin; WBC, white blood cell count.
AUC of serum ALB level for predicting the in-hospital death of patients with overt HE was 0.737 (95% CI: 0.667–0.799, p = 0.0001). The best cut-off value was ⩽22.8 g/l, with a sensitivity of 45.0% and a specificity of 93.9% (Figure 4). According to the cut-off value, 183 patients were divided into high (n = 164) and low (n = 19) serum ALB level groups (Table 3). The low-serum-ALB-level group had a significantly higher prevalence of ascites (p = 0.005) than the high-serum-ALB-level group. Hb, TBIL, ALB, AST, Scr, PT, APTT, Child–Pugh score, and MELD score were significantly different between high- and low-ALB-level groups (p < 0.05, in all comparisons). The low-serum-ALB-level group had a significantly higher mortality of overt HE during hospitalization (47.4% versus 6.7%, p < 0.001; Table 3). Kaplan–Meier curve analysis also found that the low-serum-ALB-level group had a significantly higher cumulative rate of mortality (p < 0.001; Figure 5).
Figure 4.
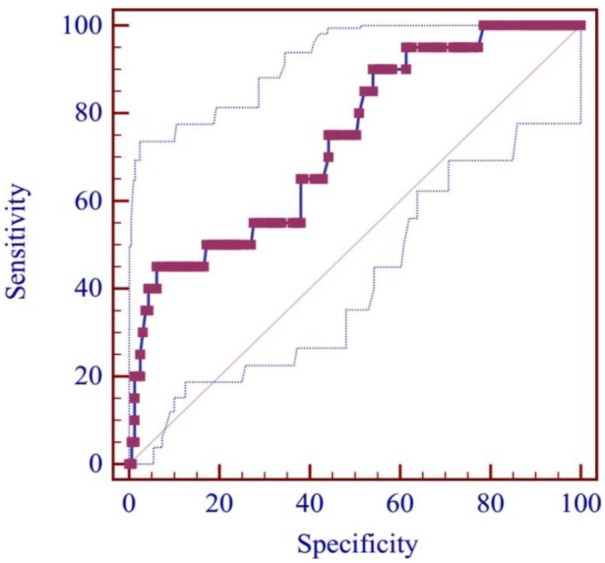
ROC curve of ALB level for predicting the in-hospital death from overt HE.
ALB, albumin; HE, hepatic encephalopathy; ROC, receiver operating characteristic.
Figure 5.
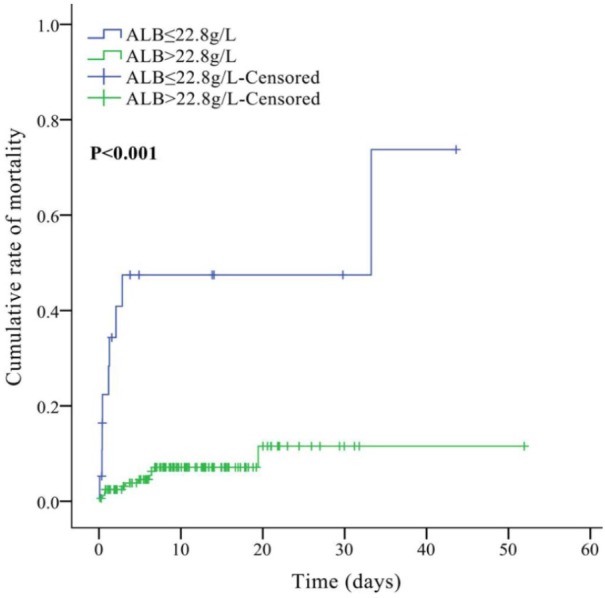
Cumulative rate of mortality between high and low-ALB-level groups.
ALB, albumin.
Discussion
Our study confirmed that serum ALB level was significantly associated with the development of overt HE in liver cirrhosis during hospitalization. As compared with those with serum ALB level > 31.6 g/l, patients with serum ALB level ⩽ 31.6 g/l had a 5.5-fold increased risk of developing overt HE in cirrhotic patients. Our study also found that the serum ALB level was significantly associated with the in-hospital death of cirrhotic patients with overt HE. As compared with those with serum ALB level > 22.8 g/l, patients with serum ALB level ⩽ 22.8 g/l had a nearly 7-fold increased risk of in-hospital death in cirrhotic patients with overt HE.
Serum ALB level and development of HE
Previous studies have explored the epidemiology of HE in cirrhosis and risk factors associated with development of HE. Among the studies we reviewed (Supplementary Table 1), five studies were cross-sectional studies, four were prospective cohort studies, three were retrospective cohort studies, and one was a case-control study. Among them, eight studies explored the association of serum ALB level with development of HE, and six and two studies focused on minimal/covert HE and overt HE, respectively. All of the eight studies performed the univariate analyses and seven of them reported that serum ALB level was a statistically significant risk factor associated with development of HE; six studies performed the multivariate analyses and four of them reported that serum ALB level was an independent risk factor associated with development of HE. Similarly, our study also suggested serum ALB level was a statistically significant risk factor associated with development of HE, regardless of univariate or multivariate analyses.
Compared with previous studies, our study had particular features. First, our study had a larger number of cirrhotic patients than previous studies. Second, Child–Pugh and MELD scores, which are complex variables comprising many clinically significant variables, were excluded in our multivariate analysis. By contrast, the studies by Bhanji and colleagues,15 Bale and colleagues,16 and Nardelli and colleagues17 included Child–Pugh score with or without MELD score in their multivariate analyses. This might affect the statistical results because the two scores had a potential collinearity with many variables for assessing liver dysfunction. Third, the selection of the target population was more appropriate. The patients receiving HA infusion, which would affect serum ALB level, were excluded from our study. Although serum ALB level was also an independent risk factor in the studies of Zhang and colleagues,18 Nardelli and colleagues,17 Tapper and colleagues,19 and Labenz and colleagues,20 none of them had excluded the patients receiving HA infusion. Fourth, the incidence of HE in our study was 4.8% and significantly lower than previous studies in which the incidence of HE was 20.8–45.9% (Supplementary Table 1). This could be explained by the occurrence of HE events observed during hospitalization in our study; by comparison, the occurrence of HE was observed during the long-term follow up in previous studies (length of follow up: 6 months to 5 years). Additionally, we evaluated overt HE, and did not consider minimal HE. Fifth, we performed the ROC curve analysis and identified the best cut-off value of serum ALB level for predicting the development of overt HE.
Serum ALB level and death of cirrhotic patients with HE
We also reviewed previous studies looking at mortality and risk factors associated with death of cirrhotic patients with HE (Supplementary Table 2). Among the five reviewed studies, four studies were retrospective cohort studies and one study was a prospective cohort study. The mortality of HE in the studies of Bustamante and colleagues,21 Fichet and colleagues,22 Cordoba and colleagues,23 and Cui and colleagues24 were 73.9%, 58.6%, 42.8%, and 67.7%, respectively, which was higher than that in our study (10.9%). This could be because the death event during hospitalization was observed in our study; by comparison, the death event during the long-term follow up was observed in previous studies (length of follow up: 3 months to 1 year). Notably, in the study by Jeong and colleagues,25 where the length of follow up was 30 days, the mortality of HE was 6.7%, which was similar to that in our study. All of the five reviewed studies explored the association of serum ALB level with death from overt HE. Four studies reported the statistical results from univariate analyses and two studies showed that serum ALB level was a statistically significant risk factor associated with death from HE. Five studies reported the statistical results from multivariate analyses and one study showed that serum ALB level was an independent risk factor associated with death from HE. Notably, in the study of Cui and colleagues,24 serum ALB level was not associated with death from HE in both univariate and multivariate analyses; this was because all patients with low serum ALB level received HA infusion.
Timing of HA infusion for preventing from development and death from HE in cirrhosis
Early studies explored the role of HA infusion in the prognosis of hospital-admission patients. In 1998, a systematic review of randomized control trials26 showed that HA infusion might increase the mortality of critically ill patients. This conclusion had led to a significant decrease in the application of HA.27 By contrast, in 2011, an updated systematic review did not show that HA infusion increased the risk of death.28 Recently, more and more studies have supported the use of HA infusion in improving the prognosis of patients with cirrhosis and its complications.11,29 However, the timing of HA infusion in cirrhotic patients remained unclear.
Conventionally, hypoalbuminemia in adults is defined by a decreased serum ALB level of <35 g/l, and clinically significant hypoalbuminemia is probably identified by serum ALB level < 25 g/l.30 Current guidelines suggested that the timing of HA infusion should be a serum ALB level of <25 g/l or <20 g/l in various types of patients.31,32 But it should be noted that HA infusion can be commenced earlier in cirrhotic patients with hypovolemia and refractory ascites; even those with a serum ALB level of >25 g/l.31
The cut-off value of serum ALB level for predicting the risk of death was often heterogeneous among the study population, suggesting the heterogeneous timing of HA infusion in different clinical settings. In patients with major abdominal procedures or kidney cancer, serum ALB level < 32 g/l was associated with an increased risk for complications or death.33,34 In patients with hilar cholangiocarcinoma, serum ALB level < 30 g/l was significantly associated with worse outcomes.35 In cirrhotic patients with spontaneous bacterial peritonitis, serum ALB level < 28.5 g/l was associated with worse long-term survival.36 By comparison, our study showed that serum ALB level ⩽ 31.6 g/l might be associated with higher risk of development of HE, and serum ALB level ⩽ 22.8 g/l might be associated with higher risk of in-hospital death from HE. Therefore, we proposed the following assumptions: as for the patients without HE at admission, physicians might consider serum ALB level ⩽ 31.6 g/l as a threshold of HA infusion for prevention of overt HE; as for patients with HE at admission, physicians might consider serum ALB level ⩽ 22.8 g/l as a threshold of HA infusion for preventing the in-hospital death from HE. Certainly, these considerations should be confirmed in future cohort studies regarding albumin infusion for HE.
Limitations
Our study has some limitations. First, our study is a retrospective cohort study in which some patients’ data were missing. Second, we did not consider minimal HE. Third, we focused on the in-hospital outcomes, but not long-term outcomes. Fourth, we cannot explore the mechanism of serum ALB level in predicting the development of, and death from, overt HE.
In conclusion, a decrease of serum ALB level may play an important role in predicting the development of, and death from, overt HE during hospitalization in liver cirrhosis. A serum ALB level of ⩽31.6 g/l may be associated with higher risk for development of overt HE in cirrhosis. A serum ALB level of ⩽22.8 g/l may be associated with higher risk for death from overt HE. However, these findings should be externally validated in different populations. Whether these cut-off values can be considered as the thresholds for HA infusion for preventing the development of, and death from, overt HE in cirrhotic patients should be further explored.
Supplemental Material
Supplemental material, Supplementary_Table_1 for Association of serum albumin level with incidence and mortality of overt hepatic encephalopathy in cirrhosis during hospitalization by Zhaohui Bai, Xiaozhong Guo, Frank Tacke, Yingying Li, Hongyu Li and Xingshun Qi in Therapeutic Advances in Gastroenterology
Supplemental Material
Supplemental material, Supplementary_Table_2 for Association of serum albumin level with incidence and mortality of overt hepatic encephalopathy in cirrhosis during hospitalization by Zhaohui Bai, Xiaozhong Guo, Frank Tacke, Yingying Li, Hongyu Li and Xingshun Qi in Therapeutic Advances in Gastroenterology
Acknowledgments
Zhaohui Bai, Xiaozhong Guo and Frank Tacke are first co-authors.
Zhaohui Bai: reviewed and searched the literature, wrote the protocol, collected the data, performed the statistical analysis, interpreted the data, and drafted the manuscript.
Xiaozhong Guo, Frank Tacke, and Hongyu Li: discussed the findings, and gave critical comments.
Yingying Li: checked the data and performed the statistical analysis.
Xingshun Qi: conceived the work, reviewed and searched the literature, wrote the protocol, checked the data, performed the statistical analysis, interpreted the data, and revised the manuscript.
All authors have made an intellectual contribution to the manuscript and approved the submission.
Footnotes
Funding: The authors received no financial support for the research, authorship, and/or publication of this article.
Conflict of interest statement: The authors declare that there is no conflict of interest.
ORCID iD: Xingshun Qi  https://orcid.org/0000-0002-9448-6739
https://orcid.org/0000-0002-9448-6739
Supplemental material: Supplemental material for this article is available online.
Contributor Information
Zhaohui Bai, Department of Gastroenterology, General Hospital of Northern Theater Command (General Hospital of Shenyang Military Area), Shenyang, PR China; Postgraduate College, Shenyang Pharmaceutical University, Shenyang, PR China.
Xiaozhong Guo, Department of Gastroenterology, General Hospital of Northern Theater Command (General Hospital of Shenyang Military Area), Shenyang, PR China.
Frank Tacke, Department of Gastroenterology and Hepatology, Charité University Medical Center, Berlin, Germany.
Yingying Li, Department of Gastroenterology, General Hospital of Northern Theater Command (General Hospital of Shenyang Military Area), Shenyang, PR China; Postgraduate College, Jinzhou Medical University, Jinzhou, PR China.
Hongyu Li, Department of Gastroenterology, General Hospital of Northern Theater Command (formerly called General Hospital of Shenyang Military Area), Shenyang, PR China.
Xingshun Qi, Department of Gastroenterology, General Hospital of Northern Theater Command (General Hospital of Shenyang Military Area), No. 83 Wenhua Road, Shenyang, Liaoning Province 110840, China.
References
- 1. American Association for the Study of Liver Diseases and European Association for the Study of the Liver. Hepatic encephalopathy in chronic liver disease: 2014 practice guideline by the European association for the study of the liver and the American association for the study of liver diseases. J Hepatol 2014; 61: 642–659. [DOI] [PubMed] [Google Scholar]
- 2. Stepanova M, Mishra A, Venkatesan C, et al. In-hospital mortality and economic burden associated with hepatic encephalopathy in the United States from 2005 to 2009. Clin Gastroenterol Hepatol 2012; 10: 1034–1041. [DOI] [PubMed] [Google Scholar]
- 3. Gluud LL, Vilstrup H, Morgan MY. Nonabsorbable disaccharides for hepatic encephalopathy: a systematic review and meta-analysis. Hepatology 2016; 64: 908–922. [DOI] [PubMed] [Google Scholar]
- 4. Bass NM, Mullen KD, Sanyal A, et al. Rifaximin treatment in hepatic encephalopathy. N Engl J Med 2010; 362: 1071–1081. [DOI] [PubMed] [Google Scholar]
- 5. Kircheis G, Nilius R, Held C, et al. Therapeutic efficacy of L-ornithine-L-aspartate infusions in patients with cirrhosis and hepatic encephalopathy: results of a placebo-controlled, double-blind study. Hepatology 1997; 25: 1351–1360. [DOI] [PubMed] [Google Scholar]
- 6. Cao Q, Yu CB, Yang SG, et al. Effect of probiotic treatment on cirrhotic patients with minimal hepatic encephalopathy: a meta-analysis. Hepatobiliary Pancreat Dis Int 2018; 17: 9–16. [DOI] [PubMed] [Google Scholar]
- 7. Shen YC, Chang YH, Fang CJ, et al. Zinc supplementation in patients with cirrhosis and hepatic encephalopathy: a systematic review and meta-analysis. Nutr J 2019; 18: 34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Simon-Talero M, Garcia-Martinez R, Torrens M, et al. Effects of intravenous albumin in patients with cirrhosis and episodic hepatic encephalopathy: a randomized double-blind study. J Hepatol 2013; 59: 1184–1192. [DOI] [PubMed] [Google Scholar]
- 9. Sharma BC, Singh J, Srivastava S, et al. Randomized controlled trial comparing lactulose plus albumin versus lactulose alone for treatment of hepatic encephalopathy. J Gastroenterol Hepatol 2017; 32: 1234–1239. [DOI] [PubMed] [Google Scholar]
- 10. Sola E, Sole C, Simon-Talero M, et al. Midodrine and albumin for prevention of complications in patients with cirrhosis awaiting liver transplantation. A randomized placebo-controlled trial. J Hepatol 2018; 69: 1250–1259. [DOI] [PubMed] [Google Scholar]
- 11. Caraceni P, Riggio O, Angeli P, et al. Long-term albumin administration in decompensated cirrhosis (ANSWER): an open-label randomised trial. Lancet 2018; 391: 2417–2429. [DOI] [PubMed] [Google Scholar]
- 12. Montagnese S, Russo FP, Amodio P, et al. Hepatic encephalopathy 2018: a clinical practice guideline by the Italian association for the study of the liver (AISF). Dig Liver Dis 2019; 51: 190–205. [DOI] [PubMed] [Google Scholar]
- 13. Li Y, Han B, Li H, et al. Effect of admission time on the outcomes of liver cirrhosis with acute upper gastrointestinal bleeding: regular hours versus off-hours admission. Can J Gastroenterol Hepatol 2018; 2018: 1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Peng Y, Qi X, Dai J, et al. Child-Pugh versus MELD score for predicting the in-hospital mortality of acute upper gastrointestinal bleeding in liver cirrhosis. Int J Clin Exp Med 2015; 8: 751–757. [PMC free article] [PubMed] [Google Scholar]
- 15. Bhanji RA, Moctezuma-Velazquez C, Duarte-Rojo A, et al. Myosteatosis and sarcopenia are associated with hepatic encephalopathy in patients with cirrhosis. Hepatol Int 2018; 12: 377–386. [DOI] [PubMed] [Google Scholar]
- 16. Bale A, Pai CG, Shetty S, et al. Prevalence of and factors associated with minimal hepatic encephalopathy in patients with cirrhosis of liver. J Clin Exp Hepatol 2018; 8: 156–161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Nardelli S, Gioia S, Ridola L, et al. Proton pump inhibitors are associated with minimal and overt hepatic encephalopathy and increased mortality in patients with cirrhosis. Hepatology. Epub ahead of print 4 March 2019. DOI: 10.1002/hep.30304. [DOI] [PubMed] [Google Scholar]
- 18. Zhang Y, Feng Y, Cao B, et al. The effect of small intestinal bacterial overgrowth on minimal hepatic encephalopathy in patients with cirrhosis. Arch Med Sci 2016; 12: 592–596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Tapper EB, Parikh ND, Sengupta N, et al. A risk score to predict the development of hepatic encephalopathy in a population-based cohort of patients with cirrhosis. Hepatology 2018; 68: 1498–1507. [DOI] [PubMed] [Google Scholar]
- 20. Labenz C, Toenges G, Huber Y, et al. Development and validation of a prognostic score to predict covert hepatic encephalopathy in patients with cirrhosis. Am J Gastroenterol 2019; 114: 764–770. [DOI] [PubMed] [Google Scholar]
- 21. Bustamante J, Rimola A, Ventura PJ, et al. Prognostic significance of hepatic encephalopathy in patients with cirrhosis. J Hepatol 1999; 30: 890–895. [DOI] [PubMed] [Google Scholar]
- 22. Fichet J, Mercier E, Genee O, et al. Prognosis and 1-year mortality of intensive care unit patients with severe hepatic encephalopathy. J Crit Care 2009; 24: 364–370. [DOI] [PubMed] [Google Scholar]
- 23. Cordoba J, Ventura-Cots M, Simon-Talero M, et al. Characteristics, risk factors, and mortality of cirrhotic patients hospitalized for hepatic encephalopathy with and without acute-on-chronic liver failure (ACLF). J Hepatol 2014; 60: 275–281. [DOI] [PubMed] [Google Scholar]
- 24. Cui Y, Guan S, Ding J, et al. Establishment and evaluation of a model for predicting 3-month mortality in Chinese patients with hepatic encephalopathy. Metab Brain Dis 2019; 34: 213–221. [DOI] [PubMed] [Google Scholar]
- 25. Jeong JH, Park IS, Kim DH, et al. CLIF-SOFA score and SIRS are independent prognostic factors in patients with hepatic encephalopathy due to alcoholic liver cirrhosis. Medicine (Baltimore) 2016; 95: e3935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Cochrane Injuries Group Albumin Reviewers. Human albumin administration in critically ill patients: systematic review of randomised controlled trials. BMJ 1998; 317: 235–240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Roberts I, Edwards P, McLelland B. More on albumin. Use of human albumin in UK fell substantially when systematic review was published. BMJ 1999; 318: 1214–1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Albumin R. Human albumin solution for resuscitation and volume expansion in critically ill patients. Cochrane Database Syst Rev 2011: CD001208. [DOI] [PubMed] [Google Scholar]
- 29. Di Pascoli M, Fasolato S, Piano S, et al. Long-term administration of human albumin improves survival in patients with cirrhosis and refractory ascites. Liver Int 2019; 39: 98–105. [DOI] [PubMed] [Google Scholar]
- 30. Gatta A, Verardo A, Bolognesi M. Hypoalbuminemia. Intern Emerg Med 2012; 7(Suppl. 3): S193–S199. [DOI] [PubMed] [Google Scholar]
- 31. Liumbruno GM, Bennardello F, Lattanzio A, et al. Recommendations for the use of albumin and immunoglobulins. Blood Transfus 2009; 7: 216–234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Vermeulen LC, Jr, Ratko TA, Erstad BL, et al. A paradigm for consensus. The university hospital consortium guidelines for the use of albumin, nonprotein colloid, and crystalloid solutions. Arch Intern Med 1995; 155: 373–379. [DOI] [PubMed] [Google Scholar]
- 33. Leite JF, Antunes CF, Monteiro JC, et al. Value of nutritional parameters in the prediction of postoperative complications in elective gastrointestinal surgery. Br J Surg 1987; 74: 426–429. [DOI] [PubMed] [Google Scholar]
- 34. Tang Y, Liu Z, Liang J, et al. Early post-operative serum albumin level predicts survival after curative nephrectomy for kidney cancer: a retrospective study. BMC Urol 2018; 18: 111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Waghray A, Sobotka A, Marrero CR, et al. Serum albumin predicts survival in patients with hilar cholangiocarcinoma. Gastroenterol Rep (Oxf) 2017; 5: 62–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Huang CH, Lin CY, Sheen IS, et al. Recurrence of spontaneous bacterial peritonitis in cirrhotic patients non-prophylactically treated with norfloxacin: serum albumin as an easy but reliable predictive factor. Liver Int 2011; 31: 184–191. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, Supplementary_Table_1 for Association of serum albumin level with incidence and mortality of overt hepatic encephalopathy in cirrhosis during hospitalization by Zhaohui Bai, Xiaozhong Guo, Frank Tacke, Yingying Li, Hongyu Li and Xingshun Qi in Therapeutic Advances in Gastroenterology
Supplemental material, Supplementary_Table_2 for Association of serum albumin level with incidence and mortality of overt hepatic encephalopathy in cirrhosis during hospitalization by Zhaohui Bai, Xiaozhong Guo, Frank Tacke, Yingying Li, Hongyu Li and Xingshun Qi in Therapeutic Advances in Gastroenterology