Abstract
Tinnitus masking patterns have long been known to differ from those used for masking external sound. In the present study, we compared the shape of tinnitus tuning curves (TTCs) to psychophysical tuning curves (PTCs), the latter using as a target, an external sound that mimics the tinnitus characteristics. A secondary goal was to compare sound levels required to mask tinnitus to those required to mask tinnitus-mimicking sounds. The TTC, PTC, audiometric thresholds, tinnitus pitch, and level matching results of 32 tinnitus patients were analyzed. Narrowband noise maskers were used for both PTC and TTC procedures. Patients were categorized into three groups based on a combination of individual PTC–TTC results. Our findings indicate that in 41% of cases, the PTC was sharp (V shape), but the TTC showed a flat configuration, suggesting that the tinnitus-related activity in that subgroup does not behave as a regular stimulus-induced activity. In 30% of cases, V-shape PTC and TTC were found, indicating that the tinnitus-related activity may share common properties with stimulus-induced activity. For a masker centered at the tinnitus frequency, the tinnitus was more difficult to mask than the mimicking tone in 72% of patients; this was particularly true for the subset with V-shape PTCs and flat TTCs. These results may have implications for subtyping tinnitus and acoustic therapies, in particular those targeting the tinnitus frequency.
Keywords: tinnitus, psychophysical tuning curves, tinnitus tuning curve, cochlear dead regions, tinnitus masking, psychoacoustic
Introduction
Chronic tinnitus is defined as a constant perception of sound without any external stimulation. It affects approximately 10% of the general population and can be very debilitating for around 2% of the population, as it can be associated with severe distress, insomnia, and depression (Erlandsson & Hallberg, 2000; Folmer & Griest, 2000; Langguth, Kleinjung, et al., 2007; Langguth, Landgrebe, Kleinjung, Sand, & Hajak, 2011; Lasisi & Gureje, 2011; McCormack et al., 2014; McCormack, Edmondson-Jones, Somerset, & Hall, 2016). Tinnitus is described as a bell ringing, a steam whistle, crickets, and other common everyday sounds (Stouffer & Tyler, 1990). When the loudness of the tinnitus is matched with external sounds, the frequencies composing the tinnitus percept are usually matched with tones only a few decibels above hearing thresholds, and this tinnitus equivalent level (TEL) is only weakly correlated with annoyance or handicap (Andersson, 2003; Basile, Fournier, Hutchins, & Hébert, 2013; Hiller & Goebel, 2006, 2007). For some people suffering from tinnitus, it is possible to render the tinnitus inaudible by using sound stimulation. Over the past few decades, the properties of tinnitus masking have been investigated by numerous research groups using various techniques with the objective of unveiling pathophysiological mechanisms of tinnitus (e.g., cochlear vs. central) and differentiating possible subtypes (Burns, 1984; Cazals & Dauman, 1990; Dauman & Cazals, 1989; Feldmann, 1971; Formby & Gjerdingen, 1980; Fowler, 1940; Mitchell, 1983; Roberts, Moffat, Baumann, Ward, & Bosnyak, 2008; Shailer, Tyler, & Coles, 1981; Tyler & Conrad-Armes, 1984; Wegel, 1931). As such, if tinnitus-related activity arises from the cochlea and is similar to the neural activity evoked by a tone or a narrowband noise, then tinnitus and external sound masking should be similar in terms of frequency resolution and ease of masking. Overall, the studies showed great variability between tinnitus patients: For instance, minimal stimulation at any frequency is sufficient to mask tinnitus in some cases (Cazals & Dauman, 1990; Dauman & Cazals, 1989; Feldmann, 1971; Mitchell, 1983; Tyler & Conrad-Armes, 1984), while for others, only high-level stimulation can mask it (Burns, 1984; Feldmann, 1971; Mitchell, 1983; Tyler & Conrad-Armes, 1984). Surprisingly, tinnitus has been reported to be unmaskable in some rare cases even when reported as a faint signal (Feldmann, 1971; Fournier et al., 2018; Langenbeck, 1953; Mitchell, 1983). From these results, most authors concluded that tinnitus was not arising from the cochlea but rather from the auditory centers.
However, most studies have assessed the masking of tinnitus without assessing the masking of external sounds at the same frequency. They thus inferred that tinnitus masking was different from masking of external sounds. To directly compare masking of external sounds to tinnitus masking, the two procedures should be performed within the same individual using similar targets, that is, the external sound should be similar in pitch and intensity to the perceived tinnitus. A common method to determine the best masker frequency to mask an external sound is called psychophysical tuning curve (PTC). A PTC represents the level of a narrowband masker required to just mask a fixed signal as a function of the masker frequency. For normal-hearing individuals, it is well known that the closer the frequency of the masker is to the target, the lower the masker level required to mask the target: The PTC has a V shape, and this pattern is thought to originate from cochlear mechanisms. A tinnitus tuning curve (TTC) is the level of a narrowband masker required to just mask the tinnitus as a function of the masker frequency (Moore, 2012). Direct comparison of TTCs and PTCs with similar targets (tinnitus for the former and an external sound mimicking tinnitus for the latter) was done in only two studies with a limited number of tinnitus patients (Burns, 1984; Tyler & Conrad-Armes, 1984; 10 and 8 patients, respectively). These researchers first performed a pitch- and a level-matching task to measure the tinnitus characteristics of each patient. They then assessed the TTCs and the PTCs using narrowband maskers with different center frequencies. In both of these studies, the conventional V shape was observed for the PTCs but not for the TTCs (Burns, 1984; Tyler & Conrad-Armes, 1984). From those results, the investigators concluded that tinnitus is unlikely to have a cochlear origin. However, there is some evidence of V-shaped TTCs in some cases (Formby & Gjerdingen, 1980; Fournier et al., 2018; Wegel, 1931). However, PTCs were not measured in those cases.
The main goal of the present study was to investigate and compare TTCs and PTCs for a large group of tinnitus patients. We were also interested in comparing the intensity levels required to mask tinnitus to those required to mask a tinnitus-mimicking sound. Finally, PTCs are often used to detect cochlear dead regions (CDRs; Kluk & Moore, 2006; Moore, 2004; Moore & Alcántara, 2001). These are regions where the cochlear inner hair cells or auditory nerve fibers are poorly functioning or are entirely nonfunctional. In these cases, the tips of the PTCs are shifted away from the target frequency. It is assumed that the target frequency is detected by hair cells and neurons with a characteristic frequency at the boundary of the dead region corresponding to the frequency of the shifted tip (Moore, 2004). CDRs have been shown to be present in many tinnitus patients (Etchelecou, Coulet, Derkenne, Tomasi, & Noreña, 2011; Kiani, Yoganantha, Tan, Meddis, & Schaette, 2013; Tan, Lecluyse, McFerran, & Meddis, 2013). In this context, the PTCs measured in the present study were also used to investigate the prevalence of CDR at the tinnitus frequency in our patient cohort.
Materials and Methods
Patients
The medical records of 38 tinnitus patients of the IMERTA clinic in Marseille were analyzed in this retrospective study. The inclusion criteria were tinnitus as the patient’s primary or secondary complaint after hearing loss. The data files were chosen from patients with chronic tinnitus (mean duration: 7 years, min: 1 month, max: 20 years) who had performed the psychoacoustic tasks during the standard clinical assessment. The records of six patients were excluded from further analysis: Two patients reported significant modification of their tinnitus during the psychoacoustic measurements (either a disappearance [n = 1] or a major tinnitus pitch modification [n = 1]). The records of two other patients were excluded because of too severe hearing loss in the tinnitus ear. Finally, data for two additional patients were excluded because of an inability to ignore the tinnitus percept in the contralateral ear. Thirty-two records, including 13 bilateral and 19 unilateral tinnitus cases, were analyzed. The mean age was 52 years (SD = 14), and there were 18 women and 14 men (see Table 1 for a detailed description of all patients). For two patients, the hearing test results were lost, and for four patients, the hearing test was not performed at the same time as the psychoacoustic measurements. The results of these six patients were included in most of the analyses except for which those that include measurements expressed in sensation level (dB SL). All patients gave oral consent for the use of their medical data. The project was approved by “Ramsay Général de Santé” institutional review board (ethics approval number COS-RGDS-2017-09-001).
Table 1.
Demographics and Tinnitus Characteristics of the Patients.
| #Patient | Age (years) | Sex | Tinnitus matching | Tinnitus width (Hz) | Tinnitus laterality | Tinnitus duration (years) | Tinnitus frequency (Hz) | Tinnitus equivalent level (dB SPL) | TTC type | PTC type | TTC−PTC at F0 (dB) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 8 | Missing | Female | Noise | 800 | Bilateral | 1 | 500 | 30 | Flat | Flat | −12 |
| 16 | 62 | Female | Noise | 800 | Bilateral | 12 | 4000 | 70 | Flat | Flat | 2 |
| 27 | 67 | Male | Noise | 400 | Unilateral | 20 | 8300 | 59 | Flat | Flat | 2 |
| 4 | 65 | Female | Tonal | 1 | Bilateral | 2 | 200 | 49 | Flat | Flat | 6 |
| 18 | 68 | Female | Tonal | 1 | Unilateral | 20 | 6000 | 70 | Flat | Flat | −8 |
| 19 | 74 | Male | Tonal | 1 | Bilateral | 20 | 6400 | 81 | Flat | Flat | −10 |
| 21 | 36 | Female | Tonal | 1 | Unilateral | 1 | 11200 | 51 | Flat | Flat | 3 |
| 23 | 50 | Female | Tonal | 1 | Unilateral | 20 | 8400 | 98 | Flat | Flat | 1 |
| 29 | 61 | Male | Tonal | 1 | Bilateral | 11 | 8900 | 46 | Flat | Flat | −1 |
| 12 | 73 | Male | Noise | 400 | Unilateral | 1 | 6500 | 43 | Flat | U | 21 |
| 6 | 72 | Female | Noise | 785 | Bilateral | 1 | 2000 | 1 | Flat | U | 34 |
| 25 | Missing | Female | Noise | 700 | Missing | Missing | 6800 | 49 | Flat | U | 32 |
| 2 | 44 | Female | Tonal | 1 | Unilateral | 15 | 1575 | 1 | Flat | U | 10 |
| 3 | Missing | Female | Tonal | 1 | Bilateral | 8 | 1300 | 43 | Flat | U | 8 |
| 28 | 39 | Male | Tonal | 1 | Unilateral | 3 | 8000 | 46 | Flat | U | 0 |
| 5 | 42 | Male | Noise | 800 | Bilateral | 5 | 2000 | 28 | Flat | V | 53 |
| 11 | 54 | Female | Noise | 400 | Bilateral | 5 | 6200 | 34 | Flat | V | 24 |
| 13 | 61 | Female | Noise | 300 | Unilateral | 5 | 5000 | 41 | Flat | V | 15 |
| 15 | 19 | Female | Noise | 700 | Unilateral | 2 | 3700 | 61 | Flat | V | 9 |
| 26 | 43 | Female | Noise | 1300 | Unilateral | 1 | 7300 | 48 | Flat | V | 3 |
| 1 | 46 | Female | Tonal | 1 | Unilateral | 2 | 2100 | 26 | Flat | V | 21 |
| 31 | 56 | Male | Tonal | 1 | Unilateral | 0.1 | 7400 | 49 | Flat | V | −4 |
| 22 | 60 | Male | Tonal | 1 | Unilateral | 5 | 6650 | 67 | Shifted V | Flat | 1 |
| 14 | 61 | Male | Noise | 600 | Unilateral | 4 | 6200 | 65 | Shifted V | Shifted V | 9 |
| 9 | 46 | Female | Tonal | 1 | Bilateral | 19 | 4300 | 45 | Shifted V | Shifted V | 16 |
| 20 | 55 | Male | Tonal | 1 | Unilateral | 0.3 | 8200 | 57 | Shifted V | Shifted V | 19 |
| 30 | 54 | Male | Tonal | 1 | Unilateral | 1 | 9100 | 86 | Shifted V | Shifted V | −2 |
| 10 | 40 | Female | Noise | 600 | Unilateral | 14 | 5500 | 17 | Shifted V | V | 46 |
| 7 | 46 | Male | Noise | 300 | Bilateral | Missing | 1150 | 55 | V | V | 8 |
| 32 | 40 | Male | Noise | 160 | Bilateral | 1 | 10600 | 14 | V | V | −3 |
| 17 | 63 | Female | Tonal | 1 | Bilateral | 8 | 2700 | 11 | V | V | −3 |
| 24 | 23 | Male | Tonal | 1 | Unilateral | 2 | 6800 | 35 | V | V | 1 |
Note. PTC = psychophysical tuning curves; TTC = tinnitus tuning curves.
Stimuli and Apparatus
Hearing assessment
A health-care professional assessed hearing thresholds for each ear using a GSI 61 audiometer. Otoscopy was first performed to rule out any earwax impaction or middle ear pathology (otitis media). Hearing thresholds were then measured using the standard Hughson-Westlake procedure (Harrell, 2002) with TDH-39P headphones for frequencies from 250 to 8000 Hz and Sennheiser HDA-280 headphones for higher frequencies (>8000 Hz). The hearing thresholds measured in dB HL were converted into dB sound pressure level (SPL) by using the calibration values of each headphone.
Psychoacoustic tasks
All the psychoacoustic measurements were obtained using Sennheiser HD-600 headphones and a Sound Blaster X-fi HD amplifier (model SB1240). Stimuli were generated using MATLAB. This program allowed the experimenter to manually control stimulation parameters such as the duration, intensity, center frequency, and bandwidth of the presented sounds. All of the psychoacoustic measurements were performed monaurally. The test ear was the tinnitus ear for patients with unilateral tinnitus and the ear with the loudest tinnitus percept for those with bilateral tinnitus. The experimenter adjusted the sounds based on patient responses for each psychoacoustic measurement.
Tinnitus pitch and level matching
For matching tinnitus pitch, a 1-kHz pure tone was first presented at an audible level. The patient was asked to judge the pitch of the target sound relative to that of their tinnitus: If the tinnitus was higher pitched, the frequency of the target was increased (½ octave steps) by the experimenter until the patient reported that it resembled the tinnitus percept. This was followed by a more precise matching procedure using 1/32 octave steps. If the tinnitus was judged to be more low-pitched than the target, the frequency of the target was decreased. The procedure was repeated until the patient reported a satisfactory tinnitus pitch match. The experimenter could also use a narrowband noise of a variable width to establish the best correspondence possible between the target sound and the timbre of the tinnitus. As such, after the best pitch match was obtained using pure tones, a narrowband noise of 1 octave bandwidth centered at the same frequency as the best pure-tone match was presented. The participant was then asked if the tinnitus corresponded more to the pure tone or to the noise. If the noise was chosen, the bandwidth was then increased or decreased until a satisfactory match was obtained. A participant reporting a multifrequency noise-like tinnitus usually reported a better match with a noise band. A satisfactory match was achieved for all the participants. For the tinnitus level matching, a target sound at the tinnitus frequency was presented at a low level (near the threshold), and its level was increased in 3 dB steps until the loudness of the target and the tinnitus was judged to be similar.
PTC
To measure a PTC, the fixed signal (or reference signal) was a pure tone at the tinnitus frequency with a level close to the loudness of the tinnitus (typically about 10 dB SL). The reference signal was always a pure tone even if the tinnitus was matched to a narrowband noise during the pitch-matching procedure. In this case, the pure tone frequency of the reference signal corresponded to the center frequency of the narrowband noise found in the pitch-matching procedure. The masker was a narrowband noise with a bandwidth of 320 Hz chosen to minimize the effect of beats (Kluk & Moore, 2006). A sequence of a 1-s narrowband noise masker followed by a 1-s silence period was presented continuously. A fixed tone signal of 0.1 s was presented three times consecutively during the presentation of the 1-s narrowband masker. The tone signal sequence started 250 ms after the beginning of the noise with an intersignal interval of 100 ms.
The experimenter controlled the stimuli. First, the tinnitus frequency (Hz) and its matching level (dB SPL), as previously measured with the pitch- and level-matching method, were entered into the software. The experimenter verified that the patient was able to distinguish the external tone from the tinnitus. If the patient had difficulty differentiating the two, the signal level was increased by a few decibels. No other adjustments were made to the target. The mean level of the reference signal was 48 dB SPL (SD = 22), and the mean TEL as assessed by the matching procedure was 46 dB SPL (SD = 23). A paired-sample t test comparing those two measures did not reveal a significant difference, t(31) = −1.87, p = .07. All patients were presented with a predetermined order of masker center frequencies: F−1, F−1/2, F−1/4, F−1/8, F0, F+1/8, F+1/4, F+1/2 octave. Each noise was initially presented at a low sensation level. The level was raised in 3 dB steps. The patient was instructed to alert the experimenter when they no longer heard the tone pips in the noise or when the sound level was deemed uncomfortable. Depending on patient reliability, the measurement was repeated two or three times for each center frequency, before testing the subsequent frequency.
TTC
To measure TTCs, the procedure and parameters were the same as described for the PTCs: The same noise maskers were presented in the same predetermined order. The level was also raised in 3 dB steps. The patient was instructed to alert the experimenter when they no longer heard their tinnitus in the noise or when the sound level was deemed uncomfortable. If the tinnitus was not maskable for a specific masker frequency, the final value was the sound limit of the program, that is, 106 dB SPL or the loudest masker level tolerated. Note, this situation was quite rare, with only one patient reaching the sound limit of the program for only the F+1/2 octave masker. The measurement was repeated two or three times, depending on patient reliability, for each center frequency before proceeding to the next.
Procedure
Clinical examination began generally with a case history of the patient. It was usually followed by the hearing test performed in a soundproof audiometric booth. All other measurements were performed in a quiet medical office.
Statistical Analysis
The PTCs or TTCs were first classified as having good frequency resolution if the difference in masker level between F0 and F−1 and F0 and F+1/2 was ≥12 dB for the former and ≥ 9 dB for the latter and were considered as having poor frequency resolution otherwise. Every PTC or TTC with poor resolution was classified as flat. If the PTC or TTC had good frequency resolution, then the shape was defined with more criteria. A V-shaped TTC or PTC was defined as a F0 masker level that was at least 3 dB lower than the masker level for the two neighboring masker frequencies (F−1/8 and F+1/8). A U-shaped PTC or TTC was defined as a curve that did not meet the V-shape criterion but met the less strict criterion of at least 3 dB lower masker level for F0 compared with F−1/4 and F+1/4. The name U shape refers to the absence of a sharp tip but with some frequency selectivity. Shifted V-shaped PTCs or TTCs, consistent with the presence of a CDR for the PTC, were defined as present when the level of the masker at the shifted tip was at least 3 dB lower than that at F0, for both PTCs and TTCs.
The TTCs were first categorized as V shape, U shape, shifted V shape, or flat. Categorization was refined by consideration of the PTC frequency resolution. For simplicity, the U shape and V shape were merged as one subtype (V shape) for the figures, but the two categories are still presented separately in Table 1. To evaluate the frequency selectivity or resolution of the PTCs and TTCs, we normalized the masker values for all masker frequencies by subtracting the value obtained at F0 for each of the different groups.
Noise-like tinnitus and tone-like tinnitus was diagnosed based on the results obtained in the pitch-matching task. Patients were all presented with both pure tones and narrowband noises during the pitch-matching procedure. If the patient reported a more satisfactory tinnitus pitch match while pure tones were presented, tinnitus was considered tone-like. Reversely, if the patient felt more confident of their pitch match after narrowband noises were presented, tinnitus was considered noise-like.
The tinnitus sample was divided into three tinnitus pitch groups: low (n = 8, min: 200 Hz, max: 2100 Hz); medium (n = 11, min: 2700 Hz, max: 6500 Hz); and high (n = 13, min: 6650 Hz, max: 11200 Hz).
Statistical analyses of the data were performed using the Statistical Package for Social Sciences software (v22; SPSS Inc., Chicago, IL).
Results
PTC and TTC Shapes
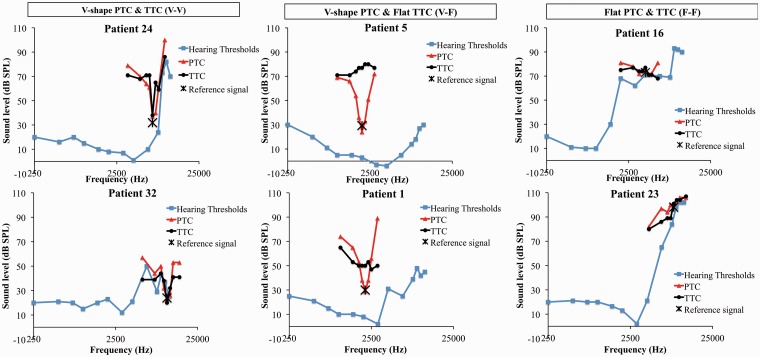
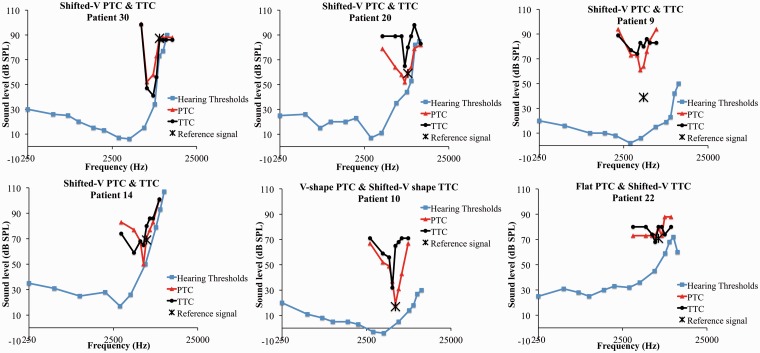
Examples of PTCs and TTCs are presented in Figure 1 (PTC: red triangles, TTC: black circles). The PTCs displayed good/fair frequency selectivity with a V/U shape in 56% (n = 18) of cases (see results for Patients 24, 32, 5, 1 in Figure 1). In 31% (n = 10) of cases, the PTC was flat. Only four shifted V-shaped PTCs (12%) were found (see Figure 2). Note, we did not test at higher frequencies. Most TTCs (69%) were flat (n = 22; see results for Patients 5, 1, 16, 23 in Figure 1). In some cases, the flat TTCs had slightly higher masker levels for the higher neighboring frequencies (F+1/4, F+1/2) probably due to increased hearing loss at high frequencies (Figure 1, Patient 23). Only 4 patients had a V-shaped TTC (see Patients 24, 32 in Figure 1), while 6 had a shifted V-shaped TTC (Figure 2), for a total of 31% of patients displaying some level of frequency resolution for tinnitus masking. When classifying patients on the combination of PTCs and TTCs, the three most prevalent categories were as follows: V/U-shaped PTC–flat TTC (n = 13), V/U-shaped PTCs and TTCs (n = 4), and flat PTCs and TTCs (n = 9; Figure 1, Table 1). For reading clarity, the V/U-shaped PTC and TTC groups are abbreviated by V-V, the V/U shape PTCs–flat TTCs by V-F, and the flat PTCs and TTCs by F-F. Other categories included shifted V-shaped PTCs and TTCs (n = 4), V-shaped PTCs and shifted V-shaped TTCs (n = 1), and flat PTCs and shifted V-shaped TTCs (n = 1; Figure 2). This last category (Patient 22, Figure 2) was not one we expected find. We speculate that its presence in this patient could be due to our permissive criteria for a shifted shape. Three cases of W-shaped PTCs were also found. These are most probably the result of the interaction between the noise masker and the target signal (Etchelecou et al., 2011; Kluk & Moore, 2006).
Figure 1.
Individual examples of PTCs (red triangles) and TTCs (black diamonds) for each TTC and PTC subgroup (V-V, V-F, and F-F). The blue squares show the hearing thresholds.
PTC = psychophysical tuning curve; TTC = tinnitus tuning curve; SPL = sound pressure level.
Figure 2.
PTCs (red triangles) and TTCs (black diamonds) for all patients with at least one shifted V shape. The blue squares show the hearing thresholds.
PTC = psychophysical tuning curve; TTC = tinnitus tuning curve; SPL = sound pressure level.
Frequency Selectivity of PTC and TTC
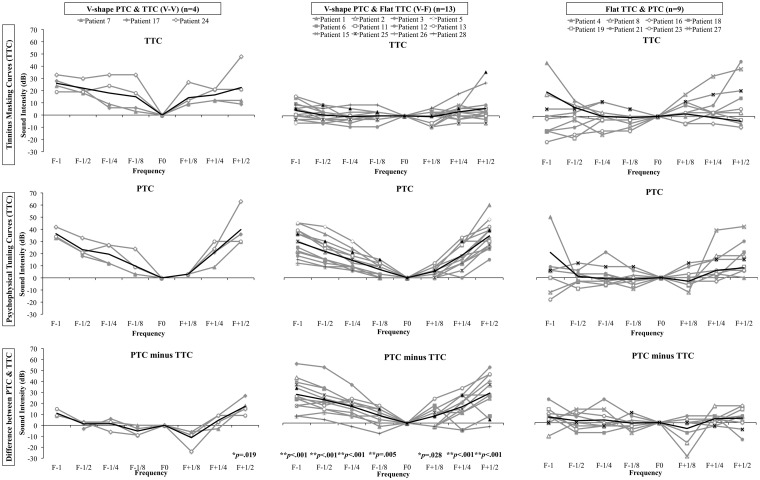
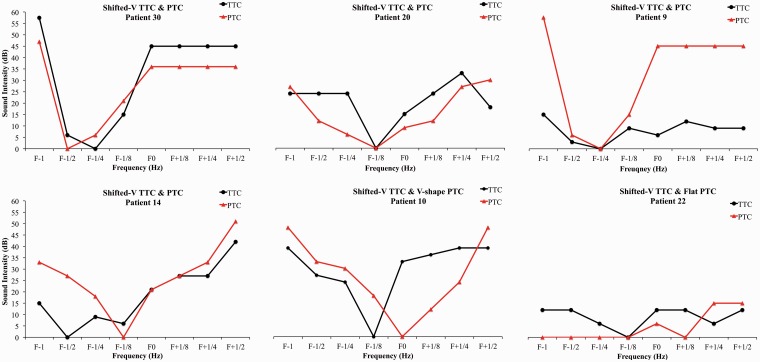
To compare the frequency selectivity of the curves, repeated-measures analysis of variance (ANOVA) was computed on the normalized masker level using Type (PTCs vs. TTCs) and Frequency (F−1, F−1/2, F−1/4, F−1/8, F+1/8, F+1/4, F+1/2) as the within-subject factors and Group (V-V, V-F, and F-F) as between-subject factor. The triple interaction was significant, F(12, 138) = 2.0, p = .03. To follow-up on the triple interaction, a similar ANOVA was run for each group separately. There was a significant interaction for the V-V, F(6, 18) = 6.3, p = .001, and V-F, F(6, 72) = 13.25, p < .001, groups only (Figure 3). To explore these interactions further, paired-sample t tests were run between the normalized masker levels for the TTCs and PTCs for each frequency for the V-V and V-F groups. This allowed us to compare the frequency resolution between the PTCs and the TTCs. We assumed that higher normalized masker values indicated better frequency resolution. As expected, the PTCs had significantly higher frequency resolution than the TTCs for all frequencies for the V-F subgroup (Figure 3). For the V-V group, where the PTCs and the TTCs were both classified V shaped, the PTCs had significant higher frequency resolution at F+1/2 than the TTCs (Figure 3). The normalized TTCs and PTCs for the shifted V shape are presented in Figure 4.
Figure 3.
PTCs (upper graphs) and TTCs (middle graphs) normalized to the masker level at F0 and the difference between the PTCs and the TTCs (lower graphs).
PTC = psychophysical tuning curve; TTC = tinnitus tuning curve.
Figure 4.
PTCs (red triangles) and TTCs (black diamonds) normalized to the masker level at F0 for all the patients with at least one shifted V shape.
PTC = psychophysical tuning curve; TTC = tinnitus tuning curve.
Tone Easier to Mask Than Tinnitus
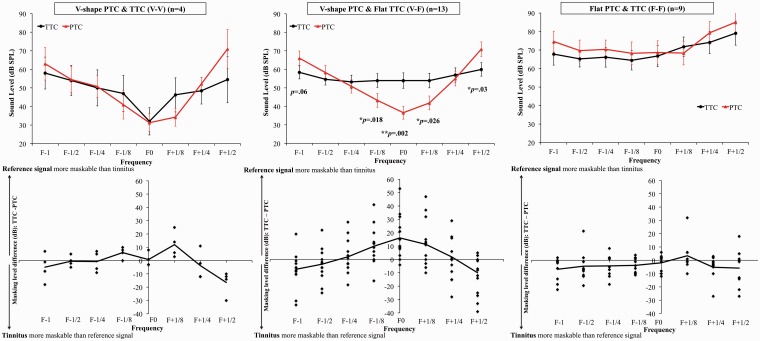
To compare the masker levels required to mask the reference signal to the masker levels required to mask the tinnitus, a repeated-measures ANOVA was computed on maskers levels (dB SPL) using Type (PTCs vs. TTCs) and Frequency (F−1, F−1/2, F−1/4, F−1/8, F0, F+1/8, F+1/4, F+1/2) as the within-subject factors and Group (V-V, V-F and F-F) as between-subject factor. The three-way interaction between Type, Frequency, and Group was significant, F(21, 196) = 2.05, p = .006. To explore the interaction, an ANOVA was run separately for each group. The interaction between Type and Frequency was significant for groups V-V, F(7, 21) = 6.3, p < .001, and V-F, F(7, 84) = 17.66, p < .001. To explore the interaction, paired-sample t tests were run between the two types (PTCs vs. TTCs) for each frequency for these two groups. The tests revealed that tinnitus required a higher masker level than the reference signal at F−1/8, F0, and F+1/8 for the V-F subgroup (Figure 5). Of the 13 patients in this subgroup, 11 required higher masker levels to mask the tinnitus than the reference signal for the F0 masker. The mean masker level difference between the tinnitus and the reference signal at F0 was 17 dB (SD = 15.8) for this group. In comparison, the difference was 0.8 dB (SD = 5) and −2 dB (SD = 6), for the V-V and the F-F groups, respectively. Finally, tinnitus was more easily maskable than the reference signal for the frequency masker F+1/2 for two out of the three subgroups, V-V and V-F (Figure 5).
Figure 5.
The upper graphs represent the average PTCs (red triangles) and TTCs (black diamonds) for each masker frequency for each TTC and PTC group. The lower graphs represent individual differences between the TTC and PTC value for each masker frequency. A positive value suggests that the reference signal was more maskable than the tinnitus, and a negative value suggests that the tinnitus was more maskable than the reference signal.
PTC = psychophysical tuning curve; TTC = tinnitus tuning curve; SPL = sound pressure level.
Overall, at the tinnitus frequency (F0), 23 out of 32 (72%) patients needed a higher masker level to mask their tinnitus than to mask the reference signal (min: 1 dB, max: 53 dB; Table 2). We decided to compare the group of patients for whom tinnitus required a higher masker level than the reference signal (n = 23) with the group of patients for whom the reverse was true (n = 8). The two groups did not differ in age, tinnitus duration, TEL, and hearing threshold at the tinnitus frequency. The difference between the presentation levels of the target for the PTCs and the tinnitus level (estimated from a matching procedure) was 1.2 dB (SD = 1.7) for the group where tinnitus was easier to mask than the reference signal and 1.5 dB (SD = 1) for the group where the tinnitus was more difficult to mask than the reference signal. This suggests that the ease in masking tinnitus in the group where tinnitus was more maskable than the reference signal was not attributable to differences in tinnitus levels or to a difference in the levels of the target between the two groups. The two groups marginally differed for tinnitus bandwidth with a lower bandwidth for the group with more easily maskable tinnitus (107 Hz vs. 352 Hz, p = .05).
Table 2.
Masker Level for Different Maskers Frequencies for Each Tinnitus Frequency Group.
| Mean frequency (Hz) | PTC level (dB SPL) | TTC level (dB SPL) | Mean difference: TTC−PTC at F0 (dB) | |
|---|---|---|---|---|
| All patients (n = 32) | ||||
| F−1 | 2764 | 72.1 | 65.6 | −5.8 |
| F−1/2 | 3866 | 62.3 | 60.1 | −2.2 |
| F−1/4 | 4598 | 58.6 | 59.0 | 0.4 |
| F−1/8 | 5014 | 52.7 | 57.4 | 4.8 |
| F0 | 5468 | 50.0 | 59.4 | 9.4 |
| F+1/8 | 5962 | 53.3 | 63.3 | 10.1 |
| F+1/4 | 6503 | 65.8 | 65.6 | −0.2 |
| F+1/2 | 7686 | 77.8 | 69.1 | −8.7 |
| Low-frequency tinnitus (n = 8) | ||||
| F−1 | 677 | 69.0 | 65.1 | −3.9 |
| F−1/2 | 957 | 55.4 | 56.9 | 1.5 |
| F−1/4 | 1138 | 47.5 | 52.3 | 4.8 |
| F−1/8 | 1241 | 39.9 | 52.3 | 12.4 |
| F0 | 1353 | 35.9 | 51.9 | 16.0 |
| F+1/8 | 1476 | 38.1 | 56.8 | 18.6 |
| F+1/4 | 1609 | 51.6 | 54.1 | 2.5 |
| F+1/2 | 1899 | 67.8 | 53.0 | −14.8 |
| Medium-frequency tinnitus (n = 11) | ||||
| F−1 | 2568 | 78.1 | 68.4 | −7.7 |
| F−1/2 | 3632 | 66.3 | 62.0 | −4.3 |
| F−1/4 | 4319 | 62.2 | 61.7 | −0.5 |
| F−1/8 | 4710 | 55.1 | 59.8 | 4.7 |
| F0 | 5136 | 53.5 | 64.5 | 11.0 |
| F+1/8 | 5601 | 58.6 | 64.2 | 5.6 |
| F+1/4 | 6108 | 67.9 | 69.1 | 1.2 |
| F+1/2 | 7137 | 79.9 | 69.9 | −10.0 |
| High-frequency tinnitus (n = 13) | ||||
| F−1 | 4213 | 69.3 | 63.8 | −5.6 |
| F−1/2 | 5855 | 63.2 | 60.5 | −2.6 |
| F−1/4 | 6963 | 62.5 | 60.9 | −1.5 |
| F−1/8 | 7593 | 58.5 | 58.6 | 0.1 |
| F0 | 8281 | 55.8 | 59.8 | 4.0 |
| F+1/8 | 9030 | 58.1 | 66.7 | 8.6 |
| F+1/4 | 9848 | 72.6 | 69.7 | −2.9 |
| F+1/2 | 11711 | 82.3 | 78.5 | −3.8 |
| Noise-like tinnitus (n = 15) | ||||
| F0 | 5050 | 44.1 | 60.3 | 16.2 |
| Tone-like tinnitus (n = 17) | ||||
| F0 | 5837 | 55.2 | 58.7 | 3.4 |
Note. PTC = psychophysical tuning curves; TTC = tinnitus tuning curves.
The correlations between the individual differences between TTCs and PTCs at F0 and neighboring frequencies were strong for the closest frequencies and declined for the distant ones, suggesting good reliability (F0/F−1/8: r = .63, p < .001; F0/F−1/4: r = .55, p = .001; F0/F−1/2: r = .38, p = .027; F0/F + 1/8: r = .77, p < .001; F0/F + 1/4: r = .66, p < .001; F0/F+1/2: r = .18, n.s.). Overall, at F0, the mean noise level required to mask the tinnitus was around 26 dB SL (n = 26, min: 2, max: 74), while 23 dB SL was perceived to mask the external tone (n = 26, min: −4, max: 55). There was a significant positive correlation between the TEL and the tinnitus masker level at F0, when both were expressed in dB SL (n = 26, r = .59, p = .002). This suggests that lower TELs require lower level maskers than higher TEL, at least when the masker is centered at the tinnitus frequency. The correlation between the level of the reference signal and the masker level required to mask the reference signal at F0 was also significant (n = 26, r = .53, p = .005).
Tonal Versus Noise-Like Tinnitus
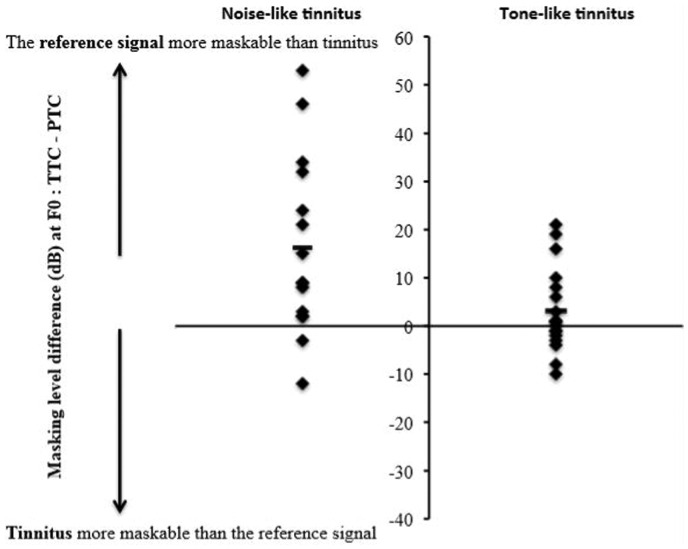
To investigate whether tonal tinnitus was easier or harder to mask than noise-like tinnitus, the level differences of the masker at F0, that is, the TTC minus the PTC level, were compared using an independent sampled t-test. The noise-like tinnitus group had a significantly higher difference than the tone-like tinnitus group, t(19.6) = −2.4, p = .025, with means of 16.2 dB and 3.2 dB, respectively (Figure 6). The mean F0 frequency and the PTC and the TTC levels for each group are shown in Table 2. In the noise-like tinnitus group, only two patients had a negative level difference at F0 compared with six in the tone-like tinnitus group (values below the x axis of Figure 6). The prevalence of noise-like tinnitus did not differ across groups: V-V (tone-like: 2, noise-like: 2), V-F (tone-like: 5, noise-like: 8), and F-F (tone-like: 6, noise-like: 3).
Figure 6.
The figure shows the individual masker level differences between the TTCs and the PTCs at F0 for the noise-like and tone-like tinnitus groups. The black hyphen represents the means for each group.
PTC = psychophysical tuning curve; TTC = tinnitus tuning curve.
PTCs and TTCs for Different Tinnitus Frequencies
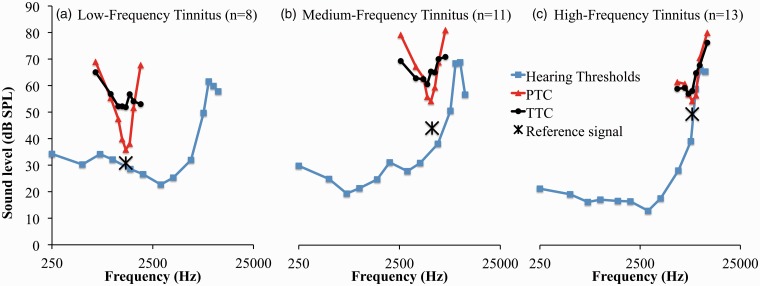
Because it has previously been reported that high-frequency tinnitus is easier to mask than low-frequency tinnitus (Feldmann, 1971; Mitchell, 1983, Tyler & Conrad-Armes, 1984), we categorized patients based on their predominant tinnitus frequency, for example, low, medium and high frequency (Table 2). To test whether the frequency of the tinnitus affected the differences in masker level between PTCs and TTCs, a mixed ANOVA was run using Type (TTC/PTC) and Masker Frequency as within-subject factors and Group (high-, medium- and low-pitch tinnitus) as a between-subject factor. The three-way interaction was significant, F(14, 203) = 2.2, p = .008. However, post hoc tests did not reveal any significant differences. The difference in masker level between the TTC and the PTC at F0 was 16 dB (SD = 20) for the low-frequency tinnitus group, 11 dB (SD = 16) for the medium-frequency group, and 4 dB (SD = 10) for the high-frequency group (see Table 2, Figure 7). The mean masker levels for tinnitus masking at F0 were 38 dB SL (SD = 22), 29 dB SL (SD = 22), and 17 dB SL (SD = 11) for the low-, medium-, and high-frequency groups, respectively. The mean TEL was 16 dB SL (SD = 5), 13 dB SL (SD = 11), and 13 dB SL (SD = 7), for the same groups, respectively.
Figure 7.
PTCs (red triangles) and TTCs (black diamonds) for the three groups based on tinnitus frequency: low-, medium-, and high-frequency. The blue squares show the hearing thresholds.
PTC = psychophysical tuning curve; TTC = tinnitus tuning curve; SPL = sound pressure level.
Discussion
The main goal of the present study was to compare, in a relatively large set of tinnitus patients, TTCs with PTCs when the target was an external tone mimicking the tinnitus characteristics in terms of pitch and loudness. The PTCs provide an estimate of frequency selectivity around the tinnitus frequency and may also suggest the presence of CDRs. The PTCs also provide an estimate of the ease of masking a faint sound with and without the presence of hearing loss. As previously reported in the literature, we found that most TTCs shapes indicated very low levels of frequency selectivity (Burns, 1984; Feldmann, 1971; Mitchell, 1983; Tyler & Conrad-Armes, 1984), while most PTCs showed some frequency selectivity. When combining the PTC and TTC results, 41% of the patients were categorized as V-F, 28% as F-F, and 12% as V-V. Shifted V-shaped TTCs were found in 19% of cases. The V-shaped and shifted V-shaped TTC categories are interesting because the tinnitus behaves similarly to what would be expected from masking a physical sound.
Tinnitus masking occurred at a significantly lower masker level at F−1 and F+1/2 compared with the PTCs. Similar results were obtained by Tyler and Conrad-Armes (1984): They used masker frequencies that extended far away from the tinnitus frequency, ranging from 0.5 to 10 kHz. They found that maskers more than an octave away from the tinnitus frequency required lower SPLs to mask tinnitus than were required to mask an external sound. They concluded that for most tinnitus patients, TTCs required lower masker levels than PTCs for masker frequencies that are around ±1 octave away from the tinnitus frequency. These investigators also reported that for some patients, the masker levels required to mask the tinnitus and the reference signal were similar mostly in the frequency regions of the tinnitus pitch. Finally, not much can be interpreted for the F-F group considering the absence of frequency resolution of the cochlear filter and the presence of cochlear damage as suggested by the flat PTCs. Across groups, in most cases (72%), tinnitus was more difficult to mask (i.e., required a higher level of the masker) than a matching external pure tone for masker centered at F0. This was particularly true for the V-F group, for which 11 out of 13 patients required a higher masker level for the tinnitus than the reference signal. However, for a minority of patients (25%), tinnitus was more easily masked than the reference signal when the masker was centered at the tinnitus frequency. Our results suggest that the type of tinnitus (tone- vs. noise-like) influence tinnitus masking: Noise-like tinnitus requires higher levels of masker to mask the tinnitus than the reference signal compared with tone-like tinnitus for which the tinnitus and the reference signal are masked by closer masker levels. Narrowband noises may not well be adapted to masking noise-like tinnitus, due to a wider pattern of excitation of noise-type tinnitus compared with tone-like tinnitus. Finally, 4 patients out of 32 displayed a shifted PTC tip suggestive of a CDR at the tinnitus frequency.
Tinnitus Classification Derived From TTC and PTC
In contrast to previous studies, the more closely spaced masker frequencies used in the present study (1/8 octave) and by one other group (Formby & Gjerdingen, 1980) might explain why we found V-shaped and shifted V-shaped TTCs for some of our patient population, while others did not (Burns, 1984; Tyler & Conrad-Armes, 1984). The maskers used by Formby and Gjerdingen (1980) were spaced by different values, but all were very close to 200 Hz (mean: 193 Hz ± 51, excluding one separation value of 794 Hz), and they found a V-shaped TTC in a single case study. Close inspection of Figure 1 from Tyler and Conrad-Armes (1984) suggests that Subject 6 had a V-shaped TTC with a lower tinnitus masker level at the tinnitus frequency (6132 Hz, ∼80 dB SPL) than at the two neighboring frequencies (6000 Hz, ∼90 dB SPL; 7000 Hz, ∼85 dB SPL). In the same study, Subject 3 also seemed to display a shifted V-shaped TTC with a lower tinnitus masker level at 5000 Hz (50 dB SPL) than at the tinnitus frequency (6402 Hz, 70 dB SPL). In this particular case, the PTC was also shifted toward 5000 Hz, suggestive of the presence of a CDR near 6000 Hz. Overall, these results suggests that closely space masker frequencies may be more suited at finding V-shaped TTC than higher space maskers and are recommended in future research on TTC.
Tinnitus Harder to Mask Than the Reference signal
The faint sound mimicking tinnitus (the reference signal) was more easily masked than the tinnitus for 72% of patients by a masker centered at F0. This was not directly assessed in the two previous studies comparing PTCs and TTCs (Burns, 1984; Tyler & Conrad-Armes, 1984), but similar results can be derived from their figures. Both studies showed that for some, tinnitus was more maskable at F0 than was a tone mimicking tinnitus (Patients 2, 3, 5 for Tyler & Conrad-Armes, 1984; Patient 6, Burns, 1984), while, for others, the tone was clearly more maskable than the tinnitus (Patients 7, 4 for Tyler & Conrad-Armes, 1984; Patients 2, 5 for Burns, 1984) or very close in levels (Patients 10, 9, 8, 6, 1 for Tyler & Conrad-Armes, 1984; Patient 4, Burns, 1984). Overall, these results suggest that, for some patients, tinnitus masking can be achieved quite easily with minimal stimulation.
Tinnitus Masking in Relation to Tinnitus Characteristics
Many researchers have attempted to link the ease of masking tinnitus with its characteristics such as pitch, TEL, and noisiness (tonal vs. noise; Burns, 1984; Feldmann, 1971; Langenbeck, 1953; Mitchell, 1983; Mitchell, Vernon, & Creedon, 1993; Tyler & Conrad-Armes, 1984). Feldmann (1971) reported that patients who presented high-pitch tinnitus and high-frequency hearing loss displayed a typical TTC called convergent type. This type required a lower masker level for high-frequency maskers than for low- and medium-frequency maskers. He also reported a few reverse cases with a lower masker level for low-frequency maskers than for the high-frequency maskers in cases of normal hearing presenting with a pulsating hum that he categorized as a divergent type. Differences in TTCs for different tinnitus pitches have also been reported: Subject 4 of Tyler and Conrad-Armes (1984), which was the only subject with a low-frequency tinnitus (1910 Hz) as opposed to a high-frequency tinnitus (>5200 Hz), displayed a sharp PTC and a very flat TTC. In line with those findings, in the present study, we also found that the difference in masking between the TTC and the PTC at F0 was higher for the low-frequency tinnitus group than for the medium and the high-frequency groups.
Another tinnitus characteristic that has been assessed in relation to tinnitus masking is TEL. Some researchers reported an absence of relationship between the TEL of the tinnitus and its maskability (Burns, 1984; Mitchell, 1983). However, the conclusion of those two studies relies upon observations of single cases and on overall masking levels over a wide range of frequency maskers. When the relation between masker level and TEL are restricted to the tinnitus pitch region, one study showed a significant relationship between tinnitus masker level and the TEL (r = .78, p = .001, n = 22; Mitchell et al., 1993). We similarly found a moderate significant relationship between the TEL and the masker level when the noise was centered at F0. These results suggest that the higher the TEL, the greater the level of a masker that is required to just mask the tinnitus. A similar relationship was found between the masker level at F0 and the level of the mimicking tone (PTC).
To our knowledge, there are no other reports comparing the ease of masking between noise-like and tone-like tinnitus. We classified the patient as experiencing noise-like tinnitus only if he reported a more satisfactory tinnitus pitch matching when presented with a narrowband noise than a tone. Indeed, we did not use the description of the patient to infer tinnitus type, for example, noise-like or tone-like, but rather used the matching procedure. It is well known that some tinnitus and hearing-impaired patients have difficulty differentiating a noise from a tone (Eggermont, 2012), which could lead to erroneous classification of the internal representation of the tinnitus. We did find, however, that patients with noise-like tinnitus had higher masker level differences between TTCs and PTCs at F0. These results suggest that noise-like tinnitus is more difficult to mask than tone-like tinnitus. It is possible that tinnitus-related activity in noise-like tinnitus displays a more widespread excitation pattern in the tonotopic pathway than tone-like tinnitus. In these cases, broadband noises might be a more effective masker.
Cochlear Dead Regions
The association between tinnitus and CDR has been investigated previously (Etchelecou et al., 2011; Kiani et al., 2013; Moore, Vinay, & Sandhya, 2010; Tan et al., 2013; Weisz, Hartmann, Dohrmann, Schlee, & Norena, 2006). PTCs and the TEN test (Moore, Huss, Vickers, Glasberg, & Alcántara, 2000) evaluations revealed that CDRs can be present in tinnitus patients (between 34 to 55% of cases; Etchelecou et al., 2011; Kiani et al., 2013; Moore et al., 2010; Tan et al., 2013; Weisz et al., 2006). However, CDRs are not sufficient or necessary to induce tinnitus (Etchelecou et al., 2011), and it remains unclear if the prevalence of CDRs is higher in tinnitus patients than in matched controls with hearing loss (Kiani et al., 2013; Tan et al., 2013). Indeed, Kiani et al. (2013) concluded that the prevalence of CDRs in tinnitus patients might not be disproportionately high compared with subjects with hearing loss that do not suffer from tinnitus. Interestingly, they found that, in tinnitus patients with CDRs, the predominant frequency of the tinnitus spectrum was usually associated with a frequency at or above the edge frequency of the CDR. In the current study, the prevalence of CDR at the tinnitus frequency, as defined by a shift of the tip of the PTCs away from F0, was low, with only 4 patients out of the 32 patients investigated. The low prevalence of CDRs is probably related to the fact that we only assessed the PTCs at the tinnitus frequency. A more thorough investigation of the presence of CDRs, in particular at frequencies higher than the tinnitus frequency, might increase the prevalence of CDRs in our sample.
The four patients with shifted V-shaped PTCs, suggestive of the presence of CDRs, also had shifted V-shaped TTCs. Importantly, this result suggests that even in the presence of a CDR at or near the tinnitus frequency, tinnitus masking can occur. For these patients the shifted tip of the TTCs corresponded roughly to the shifted tip of the PTCs. If one assumes that the shifted tip of a PTC reflects off-frequency listening (Moore, 2004), then it seems likely that the shifted tip of the TTC reflects off-frequency masking. If the frequency region of the CDRs encompasses the tinnitus frequency, it should be difficult to mask the tinnitus by using external sounds targeted at or around the tinnitus frequency. One possible method for the acoustic signal to interfere with the tinnitus at more central levels would be by stimulating at the CDR boundary. An alternative possible explanation is that the CDR in those patients is composed of less functional but not completely nonfunctional sensory cells. In this case, a high level of stimulation could be sufficient to produce masking. This could also explain why a predominant tinnitus frequency within a CDR could be pitch matched. A tone presented at a frequency within the CDR should have a similar pitch to a tone presented at the edge frequency even though the percept may be distorted if the CDR is completely nonfunctional (Huss & Moore, 2005). Distorted perception has been shown to occur only when the frequency of the tone falls more than 0.5 octaves away from the edge frequency, which is not the case for the four patients presented here (Huss & Moore, 2005). Alternatively, if pitch is not coded entirely in terms of place, than the tinnitus pitch match in the presence of CDRs could have been made through temporal coding. There is also the possibility that patients might have made errors during the pitch-matching procedure.
Implications for the Mechanisms and Subtyping of Tinnitus
The results obtained in the present study may have implications for the mechanisms of tinnitus. The combination of sharp PTCs with flat TTCs (V-F pattern of masking) is generally interpreted as being consistent with the idea that tinnitus-related activity is generated at central level (Tyler & Conrad-Armes, 1984). Conversely, it was postulated that narrow TTCs and PTCs could reflect tinnitus-related activity generated at the cochlear level (Tyler & Conrad-Armes, 1984). While we believe that this interpretation is too simplistic, we agree that the V-F pattern of masking is indeed not consistent with peripheral tinnitus-related activity. This tinnitus subtype represents an interesting paradox: While tinnitus can be described as having a clear pitch (suggesting that tinnitus-related activity is circumscribed to a relatively narrow tonotopic region), the masking pattern does not show any frequency selectivity. We suggest that this particular pattern of masking (V-F) could result from tinnitus-related activity that is generated in the nonlemniscal (nontonotopic) pathway or in a central region where neurons integrate sensory inputs over a wide frequency range (Møller, 2003, 2006; Møller, Møller, & Yokota, 1992). In that particular tinnitus subtype, any stimulus above a certain threshold may activate the central neurons that integrate sensory inputs over a wide frequency range involved in the representation of tinnitus, thereby interfering with the tinnitus percept. It is unclear, however, how this mechanism can account for the fact that most tinnitus has a clear pitch. On the other hand, V-shaped TTCs may result from tinnitus-related activity that is generated at the cochlear level or at central level within the lemniscal (tonotopic) pathway.
Clinical Implications
The TTC and PTC results might have several clinical implications. First, the current clinical practice of tinnitus pitch matching often uses a forced-choice paradigm that is considered difficult for patients, which requires a substantial amount of time and has poor test–retest reliability (Burns, 1984; Henry, Fausti, Flick, Helt, & Ellingson, 2000; Henry, Flick, Gilbert, Ellingson, & Fausti, 2001, 2004; Mitchell & Creedon, 1995; Nageris, Attias, & Raveh, 2010). The TTC procedure may be used clinically as a complementary validation of the tinnitus pitch obtained by other procedures for some cases. A masking task has been reported to be much easier to do for subjects than the tinnitus pitch-matching task (Formby & Gjerdingen, 1980). In the present study and a previous one (Fournier et al., 2018), TTCs were found, in some cases, to show a minimum at/near the dominant tinnitus frequency. Therefore, TTCs may be useful for confirming the dominant tinnitus pitch in those 30% of cases showing V-shaped masking patterns. Interestingly, TTCs can also reveal or suggest additional tinnitus components that have not been found during the tinnitus pitch-matching procedure where a single dominant pitch is assessed (see Fournier et al., 2018). It is conceivable that the masking curve of a multifrequency tinnitus could result in multiple masking curve tips where each tips would coincide with one predominant frequency component. As an example, we previously showed a case where the hearing loss notch, the tinnitus pitch match, and the tip of the masking curve of a tinnitus patient were all converging at 3 kHz (Figure 3, Fournier et al., 2018). Still, there was another clear tip in the masking curve at 8 kHz that we interpreted as a potential additional tinnitus component (masking values at adjacent frequencies were more than 20 dB higher): The patient would have a tinnitus with a 3- and 8-kHz frequency component. As we did not validate with the patient that his tinnitus had indeed two frequency components, we cannot be sure that the second tip was truly an additional tinnitus component. The importance of a good and reliable tinnitus pitch match has been reported to be critical for some acoustic therapies precisely targeting the tinnitus frequency region (Engineer et al., 2011; Pantev, Okamoto, & Teismann, 2012; Tass, Adamchic, Freund, von Stackelberg, & Hauptmann, 2012; Tyler et al., 2017; Wegger, Ovesen, & Larsen, 2017; Wunderlich et al., 2015). Failure of good pitch matching has been reported as one of several possible factors explaining treatment failure for those therapies (Tass et al., 2012; Tyler et al., 2017; Wunderlich et al., 2015). The corroboration of tinnitus pitch matching using another technique than the classical forced-choice paradigm might be even more important as the tinnitus pitch may be more complex in some patients with potentially more than one component (Fournier et al., 2018; Zagólski & Stręk, 2018). This may also corroborate patients’ report of several tinnitus pitches.
Note, however, that the lack of frequency selectivity of the TTCs in subjects with V-F and F-F masking patterns questions the validity of the acoustic approaches that are based on targeting the tinnitus frequency. Indeed, it is unclear how an acoustic stimulus can specifically target the tinnitus frequency when the TTC is flat (or even showing some degree of selectivity when frequencies further away from the tinnitus frequency are used). Some selectivity of tinnitus masking has been found in subjects with high-frequency hearing loss: The minimum masking level (MML), which is the lowest level of a noise required to just mask the tinnitus, was lower in the high-frequency regions compared with lower frequency regions (Fournier et al., 2018). If the search for a lower MML has some significance for the design of therapeutic acoustic sequences (note, targeting the frequency region where MML is lower may be more efficient than targeting the tinnitus pitch—when the two regions do not coincide; Fournier et al., 2018), this result suggests that the measurement of the TTC should be done at and around the tinnitus frequency and also at frequencies in the hearing loss regions, as the two frequency regions may not always coincide.
In the clinic, tinnitus masking is usually performed by measuring the lowest level of a broadband noise or a narrowband noise required to just mask the tinnitus and is referred to as “minimum masking level (MML)” (Henry, 2016). Specialized audiologists, as part of their tinnitus psychoacoustic assessment, sometimes perform this measure, but it is not routinely performed or even recommended for clinical use (Langguth, Goodey, et al., 2007). This is surprising considering that the MML was once judged essential to evaluate the possible efficacy of tinnitus maskers as a treatment and to guide the fitting of noise generators (Vernon & Meikle, 1981). In this context, the MML was measured using narrowband noise of different center frequencies to determine the noise that provided the most efficient masking at the lowest levels for use in noise generators. This was thought to maximize acceptance and comfort: Acceptance of masking therapies was reported to be higher for low masker levels, and these may interfere less with hearing communication than higher levels of noise. Given the results of the current study and other related research on the topic of tinnitus masking (Fournier et al., 2018; Mitchell et al., 1993; Roberts et al., 2008), we believe that MML, or TTC, should be incorporated in an audiology battery of tests for tinnitus. These results could guide intervention using noise masker as was once recommended (Vernon & Meikle, 1981). However, clinical efficacy of this technique and similar ones has been made solely on the basis of retrospective clinical data of patients treated with tinnitus masking (Henry, Schechter, Nagler, & Fausti, 2002). There is a clear need for more clinical research in this area. To date, the fitting of noise generators provided by almost all hearing aid companies relies mostly on clinician experience and patients’ own impressions of the therapy.
Acknowledgements
The authors thank Victoria Duda, Salima Jiwani, and Marina Siponen for a thorough revision of the article. The authors also thank Brian C. J. Moore for insightful comments, critics, and reviews of the article.
Declaration of Conflicting Interests
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding
The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by COST, BMBS COST Action BM1306: TINNET—Better Understanding the Heterogeneity of Tinnitus to Improve and Develop New Treatments. M. W. was supported through funding provided by TINNET Short-Term Scientific Mission, and P. F. was supported by postdoctoral funding provided by Fonds de recherche Québec – Santé and Canadian Institute of Health Research. This work was conducted with the financial assistance of CNRS, Aix-Marseille Université, B2V, and the supplementary pension institution of Klesia.
References
- Andersson G. (2003) Tinnitus loudness matching in relation to annoyance and grading of severity. Auris, Nasus, Larynx 30(2): 129–133. doi: 10.1016/S0385-8146(03)00008-7. [DOI] [PubMed] [Google Scholar]
- Basile C.-É., Fournier P., Hutchins S., Hébert S. (2013) Psychoacoustic assessment to improve tinnitus diagnosis. PLoS One 8(12): e82995 doi:10.1371/journal.pone.0082995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burns E. M. (1984) A comparison of variability among measurements of subjective tinnitus and objective stimuli. International Journal of Audiology 23(4): 426–440. doi:10.3109/00206098409081535. [DOI] [PubMed] [Google Scholar]
- Cazals Y., Dauman R. (1990) Tinnitus deteriorates frequency selectivity in addition to hearing loss. In: Hoke M. (ed) Cochlear mechanisms and otoacoustic emissions vol. 7 Basel, Switzerland: S. Karger, pp. 201–205. [Google Scholar]
- Dauman R., Cazals Y. (1989) Auditory frequency selectivity and tinnitus. Archives of Oto-Rhino-Laryngology 246(5): 252–255. [DOI] [PubMed] [Google Scholar]
- Eggermont J. J. (2012) The neuroscience of tinnitus, Oxford, England: OUP. [Google Scholar]
- Engineer N. D., Riley J. R., Seale J. D., Vrana W. A., Shetake J. A., Sudanagunta S. P., Kilgard M. P. (2011) Reversing pathological neural activity using targeted plasticity. Nature 470(7332): 101–104. doi:10.1038/nature09656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erlandsson S. I., Hallberg L. R. (2000) Prediction of quality of life in patients with tinnitus. British Journal of Audiology 34(1): 11–20. doi: 10.3109/03005364000000114. [DOI] [PubMed] [Google Scholar]
- Etchelecou M.-C., Coulet O., Derkenne R., Tomasi M., Noreña A. J. (2011) Temporary off-frequency listening after noise trauma. Hearing Research 282(1–2): 81–91. doi:10.1016/j.heares.2011.09.006. [DOI] [PubMed] [Google Scholar]
- Feldmann H. (1971) Homolateral and contralateral masking of tinnitus by noise-bands and by pure tones. Audiology 10(3): 138–144. doi: 10.3109/00206097109072551. [DOI] [PubMed] [Google Scholar]
- Folmer R. L., Griest S. E. (2000) Tinnitus and insomnia. American Journal of Otolaryngology 21(5): 287–293. doi:10.1053/ajot.2000.9871. [DOI] [PubMed] [Google Scholar]
- Formby C., Gjerdingen D. B. (1980) Pure-tone masking of tinnitus. Audiology 19(6): 519–535. doi: 10.3109/00206098009070083. [DOI] [PubMed] [Google Scholar]
- Fournier P., Cuvillier A.-F., Gallego S., Paolino F., Paolino M., Quemar A., Norena A. (2018) A new method for assessing masking and residual inhibition of tinnitus. Trends in Hearing 22: 233121651876999 doi:10.1177/2331216518769996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fowler E. P. (1940) Head noises: Significance, measurements and importance in diagnosis and treatment. Archives of Otolaryngology – Head and Neck Surgery 32(5): 903–914. doi:10.1001/archotol.1940.00660020910007. [Google Scholar]
- Harrell R. W. (2002) Puretone evaluation. In: Katz J. (ed) Handbook of clinical audiology, Baltimore, MD: Lippincott Williams & Wilkins, pp. 71–87. [Google Scholar]
- Henry J. A. (2016) “Measurement” of tinnitus. Otology & Neurotology 37(8): e276–e285. doi:10.1097/MAO.0000000000001070. [DOI] [PubMed] [Google Scholar]
- Henry J. A., Fausti S. A., Flick C. L., Helt W. J., Ellingson R. M. (2000) Computer-automated clinical technique for tinnitus quantification. American Journal of Audiology 9(1): 36–49. doi: 10.1044/1059-0889(2000/002). [DOI] [PubMed] [Google Scholar]
- Henry J. A., Flick C. L., Gilbert A., Ellingson R. M., Fausti S. A. (2001) Comparison of two computer-automated procedures for tinnitus pitch matching. Journal of Rehabilitation Research and Development 38(5): 557–566. [PubMed] [Google Scholar]
- Henry J. A., Flick C. L., Gilbert A., Ellingson R. M., Fausti S. A. (2004) Comparison of manual and computer-automated procedures for tinnitus pitch-matching. Journal of Rehabilitation Research and Development 41(2): 121–138. doi: 10.1682/JRRD.2004.02.0121. [DOI] [PubMed] [Google Scholar]
- Henry J. A., Schechter M. A., Nagler S. M., Fausti S. A. (2002) Comparison of tinnitus masking and tinnitus retraining therapy. Journal of the American Academy of Audiology 13(10): 559–581. doi: 10.1080/03655230600895556. [PubMed] [Google Scholar]
- Hiller W., Goebel G. (2006) Factors influencing tinnitus loudness and annoyance. Archives of Otolaryngology – Head & Neck Surgery 132(12): 1323–1330. doi:10.1001/archotol.132.12.1323. [DOI] [PubMed] [Google Scholar]
- Hiller W., Goebel G. (2007) When tinnitus loudness and annoyance are discrepant: Audiological characteristics and psychological profile. Audiology & Neuro-Otology 12(6): 391–400. doi:10.1159/000106482. [DOI] [PubMed] [Google Scholar]
- Huss M., Moore B. C. J. (2005) Dead regions and pitch perception. Journal of the Acoustical Society of America 117(6): 3841–3852. doi: 10.1121/1.1920167. [DOI] [PubMed] [Google Scholar]
- Kiani F., Yoganantha U., Tan C. M., Meddis R., Schaette R. (2013) Off-frequency listening in subjects with chronic tinnitus. Hearing Research 306: 1–10. doi:10.1016/j.heares.2013.08.012. [DOI] [PubMed] [Google Scholar]
- Kluk K., Moore B. C. J. (2006) Detecting dead regions using psychophysical tuning curves: A comparison of simultaneous and forward masking. International Journal of Audiology 45(8): 463–476. doi:10.1080/14992020600753189. [DOI] [PubMed] [Google Scholar]
- Langenbeck B. (1953) Diagnosis by testing hearing above threshold. Acta Oto-Laryngologica 43(4–5): 439–456. doi:10.3109/00016485309119866. [DOI] [PubMed] [Google Scholar]
- Langguth B., Goodey R., Azevedo A., Bjorne A., Cacace A., Crocetti A., Vergara R. (2007) Consensus for tinnitus patient assessment and treatment outcome measurement: Tinnitus Research Initiative meeting, Regensburg, July 2006. Progress in Brain Research 166: 525–536. doi:10.1016/S0079-6123(07)66050-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langguth B., Kleinjung T., Fischer B., Hajak G., Eichhammer P., Sand P. G. (2007) Tinnitus severity, depression, and the big five personality traits. Progress in Brain Research 166: 221–225. doi:10.1016/S0079-6123(07)66020-8. [DOI] [PubMed] [Google Scholar]
- Langguth B., Landgrebe M., Kleinjung T., Sand G. P., Hajak G. (2011) Tinnitus and depression. The World Journal of Biological Psychiatry 12(7): 489–500. doi:10.3109/15622975.2011.575178. [DOI] [PubMed] [Google Scholar]
- Lasisi A. O., Gureje O. (2011) Prevalence of insomnia and impact on quality of life among community elderly subjects with tinnitus. The Annals of Otology, Rhinology, and Laryngology 120(4): 226–230. doi:10.1177/000348941112000402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCormack A., Edmondson-Jones M., Fortnum H., Dawes P., Middleton H., Munro K. J., Moore D. R. (2014) The prevalence of tinnitus and the relationship with neuroticism in a middle-aged UK population. Journal of Psychosomatic Research 76(1): 56–60. doi:10.1016/j.jpsychores.2013.08.018. [DOI] [PubMed] [Google Scholar]
- McCormack A., Edmondson-Jones M., Somerset S., Hall D. (2016) A systematic review of the reporting of tinnitus prevalence and severity. Hearing Research 337: 70–79. doi:10.1016/j.heares.2016.05.009. [DOI] [PubMed] [Google Scholar]
- Mitchell C. (1983) The masking of tinnitus with pure tones. International Journal of Audiology 22(1): 73–87. doi:10.3109/00206098309072771. [DOI] [PubMed] [Google Scholar]
- Mitchell C. R., Creedon T. A. (1995) Psychophysical tuning curves in subjects with tinnitus suggest outer hair cell lesions. Otolaryngology-Head and Neck Surgery 113(3): 223–233. doi:10.1016/S0194-5998(95)70110-9. [DOI] [PubMed] [Google Scholar]
- Mitchell C. R., Vernon J. A., Creedon T. A. (1993) Measuring tinnitus parameters: Loudness, pitch, and maskability. Journal of the American Academy of Audiology 4(3): 139–151. [PubMed] [Google Scholar]
- Møller A. R. (2003) Pathophysiology of tinnitus. Otolaryngologic Clinics of North America 36(2): 249–266. doi:10.1016/S0030-6665(02)00170-6. [DOI] [PubMed] [Google Scholar]
- Møller A. R. (2006) Neural plasticity in tinnitus. Progress in Brain Research 157: 365–372. doi:10.1016/S0079-6123(06)57022-0. [DOI] [PubMed] [Google Scholar]
- Møller A. R., Møller M. B., Yokota M. (1992) Some forms of tinnitus may involve the extralemniscal auditory pathway. The Laryngoscope 102(10): 1165–1171. doi:10.1288/00005537-199210000-00012. [DOI] [PubMed] [Google Scholar]
- Moore B. C. J. (2004) Dead regions in the cochlea: Conceptual foundations, diagnosis, and clinical applications. Ear and Hearing 25(2): 98–116. doi: 10.1097/01.AUD.0000120359.49711.D7. [DOI] [PubMed] [Google Scholar]
- Moore B. C. J. (2012) The psychophysics of tinnitus. In: Eggermont J. J., Zeng F.-G., Fay R. R., Popper A. N. (eds) Tinnitus, New York, NY: Springer, pp. 187–253. [Google Scholar]
- Moore B. C. J., Alcántara J. I. (2001) The use of psychophysical tuning curves to explore dead regions in the cochlea. Ear and Hearing 22(4): 268–278. doi: 10.1097/00003446-200108000-00002. [DOI] [PubMed] [Google Scholar]
- Moore B. C. J., Huss M., Vickers D. A., Glasberg B. R., Alcántara J. I. (2000) A test for the diagnosis of dead regions in the cochlea. British Journal of Audiology 34: 205–224. doi: 10.3109/03005364000000131. [DOI] [PubMed] [Google Scholar]
- Moore B. C. J., Vinay, Sandhya (2010) The relationship between tinnitus pitch and the edge frequency of the audiogram in individuals with hearing impairment and tonal tinnitus. Hearing Research 261(1–2): 51–56. doi:10.1016/j.heares.2010.01.003. [DOI] [PubMed] [Google Scholar]
- Nageris B. I., Attias J., Raveh E. (2010) Test-retest tinnitus characteristics in patients with noise-induced hearing loss. American Journal of Otolaryngology 31(3): 181–184. doi:10.1016/j.amjoto.2008.12.007. [DOI] [PubMed] [Google Scholar]
- Pantev C., Okamoto H., Teismann H. (2012) Music-induced cortical plasticity and lateral inhibition in the human auditory cortex as foundations for tonal tinnitus treatment. Frontiers in Systems Neuroscience 6: 50 doi:10.3389/fnsys.2012.00050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts L. E., Moffat G., Baumann M., Ward L. M., Bosnyak D. J. (2008) Residual inhibition functions overlap tinnitus spectra and the region of auditory threshold shift. Journal of the Association for Research in Otolaryngology: JARO 9(4): 417–435. doi:10.1007/s10162-008-0136-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shailer M. J., Tyler R. S., Coles R. R. (1981) Critical masking bands for sensorineural tinnitus. Scandinavian Audiology 10(3): 157–162. doi: 10.3109/01050398109076176. [DOI] [PubMed] [Google Scholar]
- Stouffer J. L., Tyler R. S. (1990) Characterization of tinnitus by tinnitus patients. The Journal of Speech and Hearing Disorders 55(3): 439–453. doi: 10.1044/jshd.5503.439. [DOI] [PubMed] [Google Scholar]
- Tan C. M., Lecluyse W., McFerran D., Meddis R. (2013) Tinnitus and patterns of hearing loss. Journal of the Association for Research in Otolaryngology: JARO 14(2): 275–282. doi:10.1007/s10162-013-0371-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tass P. A., Adamchic I., Freund H.-J., von Stackelberg T., Hauptmann C. (2012) Counteracting tinnitus by acoustic coordinated reset neuromodulation. Restorative Neurology and Neuroscience 30(2): 137–159. doi:10.3233/RNN-2012-110218. [DOI] [PubMed] [Google Scholar]
- Tyler R., Cacace A., Stocking C., Tarver B., Engineer N., Martin J., Vanneste S. (2017) Vagus nerve stimulation paired with tones for the treatment of tinnitus: A prospective randomized double-blind controlled pilot study in humans. Scientific Reports 7(1): 11960 doi:10.1038/s41598-017-12178-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyler R. S., Conrad-Armes D. (1984) Masking of tinnitus compared to masking of pure tones. Journal of Speech and Hearing Research 27(1): 106–111. doi: 10.1044/jshr.2701.106. [DOI] [PubMed] [Google Scholar]
- Vernon J. A., Meikle M. B. (1981) Tinnitus masking: Unresolved problems. CIBA Foundation Symposium 85: 239–262. doi: 10.1002/9780470720677.ch14. [DOI] [PubMed] [Google Scholar]
- Wegel R. L. (1931) A study of tinnitus. Archives of Otolaryngology – Head and Neck Surgery 14(2): 158–165. doi:10.1001/archotol.1931.00630020182004. [Google Scholar]
- Wegger M., Ovesen T., Larsen D. G. (2017) Acoustic coordinated reset neuromodulation: A systematic review of a novel therapy for tinnitus. Frontiers in Neurology 8: 36 doi:10.3389/fneur.2017.00036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weisz N., Hartmann T., Dohrmann K., Schlee W., Norena A. (2006) High-frequency tinnitus without hearing loss does not mean absence of deafferentation. Hearing Research 222(1–2): 108–114. doi:10.1016/j.heares.2006.09.003. [DOI] [PubMed] [Google Scholar]
- Wunderlich R., Lau P., Stein A., Engell A., Wollbrink A., Rudack C., Pantev C. (2015) Impact of spectral notch width on neurophysiological plasticity and clinical effectiveness of the tailor-made notched music training. PLoS One 10(9): e0138595 doi:10.1371/journal.pone.0138595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zagólski O., Stręk P. (2018) Double tinnitus in a single ear. International Journal of Audiology 57(3): 236–239. doi:10.1080/14992027.2017.1409439. [DOI] [PubMed] [Google Scholar]