Abstract
BACKGROUND
Anastomotic leaks (AL) and gastric conduit necrosis (CN) are serious complications following oesophagectomy. Some studies have suggested that vascular calcification may be associated with an increased AL rate, but this has not been validated in a United Kingdom population.
AIM
To investigate whether vascular calcification identified on the pre-operative computed tomography (CT) scan is predictive of AL or CN.
METHODS
Routine pre-operative CT scans of 414 patients who underwent oesophagectomy for malignancy with oesophagogastric anastomosis at the Queen Elizabeth Hospital Birmingham between 2006 and 2018 were retrospectively analysed. Calcification of the proximal aorta, distal aorta, coeliac trunk and branches of the coeliac trunk was scored by two reviewers. The relationship between these calcification scores and occurrence of AL and CN was then analysed. The Esophagectomy Complications Consensus Group definition of AL and CN was used.
RESULTS
Complication data were available in n = 411 patients, of whom 16.7% developed either AL (15.8%) or CN (3.4%). Rates of AL were significantly higher in female patients, at 23.0%, compared to 13.9% in males (P = 0.047). CN was significantly more common in females, (8.0% vs 2.2%, P = 0.014), patients with diabetes (10.6% vs 2.5%, P = 0.014), a history of smoking (10.3% vs 2.3%, P = 0.008), and a higher American Society of Anaesthesiologists grade (P = 0.024). Out of the 14 conduit necroses, only 4 occurred without a concomitant AL. No statistically significant association was found between calcification of any of the vessels studied and either of these outcomes. Multivariable analyses were then performed to identify whether a combination of the calcification scores could be identified that would be significantly predictive of any of the outcomes. However, the stepwise approach did not select any factors for inclusion in the final models. The analysis was repeated for composite outcomes of those patients with either AL or CN (n = 69, 16.7%) and for those with both AL and CN (n = 10, 2.4%) and again, no significant associations were detected. In the subset of patients that developed these outcomes, no significant associations were detected between calcification and the severity of the complication.
CONCLUSION
Calcification scoring was not significantly associated with Anastomotic Leak or CN in our study, therefore should not be used to identify patients who are high risk for these complications.
Keywords: Oesophagectomy, Anastomotic leak, Gastric conduit necrosis, Calcification, Computed tomography, Ischaemia
Core tip: Vascular calcification does not predict anastomotic leak (AL) or gastric conduit necrosis (CN) following oesophagectomy for malignancy. There is no association between vascular calcification and severity of AL or CN. AL is significantly more common in female vs male patients. Gastric CN is significantly more common in females, patients with diabetes, a history of smoking and a higher American Society of Anaesthesiologists grade. Inter-rater reliability for calcification scoring of the vessels supplying the gastric tube is excellent.
INTRODUCTION
Oesophagectomy is associated with relatively high incidence of complications[1]. One of the most important complications is anastomotic leak (AL), which has been shown to be associated with post-operative morbidity, subsequent anastomotic stricture and reoperation, and is associated with increased post-operative mortality, extended length of hospital stay and hospital costs[2,3]. Ischaemia of the gastric tube is a key cause of AL[4,5]. Additionally, ischaemia can progress to gastric conduit necrosis (CN), which may result in severe sepsis and death if appropriate interventions are not performed[1]. More minor forms may result in poor perfusion to the gastric tube, particularly the most cranial part, which is used to create the anastomosis[6]. It has been hypothesised that calcification of the arteries supplying the gastric tube, a surrogate marker for atherosclerosis, may contribute to tissue ischaemia and hence be linked to AL and CN.
Several studies have reported a link between vascular calcification on pre-operative computed tomography (CT) scan and subsequent AL[7-12]. Vascular calcification burden has been shown to be strongly correlated with atherosclerotic burden. CT is the gold standard for the measurement of arterial calcification[13]. However, previous studies have been inconsistent in their findings, and there is heterogeneity between study populations.
This single centre retrospective cohort study aims to evaluate the relationship between the extent and location of calcification, as measured on the pre-operative CT scan, and subsequent AL and CN following oesophagectomy with oesophagogastric anastomosis for oesophageal cancer.
MATERIALS AND METHODS
Population
Our institution provides centralised resectional oesophageal cancer services for several hospitals in the West Midlands. The Upper gastrointestinal surgery team maintains a “Tracker” database, which records details of patient demographics, diagnosis, oncological staging, chemotherapy, surgical management, intraoperative details, post-operative outcomes and complications, survival and oncological recurrence. Data are input prospectively by consultant members of the Upper GI Team.
The study inclusion criteria were consecutive patients who had undergone oesophagectomy with oesophagogastric anastomosis for malignancy. Patients were excluded if no pre-operative CT scan was available, or they had insufficient follow up to determine whether outcomes had occurred (i.e., those not discharged before 15th January 2018). Oesophagectomies for benign disease or open and close procedures due to irressectable or metastatic disease were also excluded.
Definition of outcomes
The Esophagectomy Complications Consensus Group (ECCG) consensus definitions of AL and CN were used[1]. These defined AL as a full thickness gastrointestinal surgery defect involving the oesophagus, anastomosis, staple line, or conduit irrespective of presentation or method of identification, and further classified AL as follows: Type I: Local defect requiring no change in therapy or treated medically or with dietary modification; Type II: Localized defect requiring interventional but not surgical therapy, for example, interventional radiology drain, stent or bedside opening, and packing of incision; Type III: Localized defect requiring surgical therapy.
CN was defined as ischaemia or necrosis of the gastric conduit and was classified as follows: Type I: CN focal identified endoscopically. Treatment with additional monitoring or non-surgical therapy; Type II: CN focal identified endoscopically and not associated with free anastomotic or conduit leak. Treatment with surgical therapy not involving oesophageal diversion; Type III: CN extensive. Treatment with conduit resection with diversion.
Image acquisition
Images from pre-operative CT scans of the thorax, abdomen and pelvis were analysed. CT protocols for the referring hospital were broadly similar and were typically enhanced with an iodinated contrast material administered intravenously. Chest and abdominal images were typically acquired in the arterial phase and portal venous phase, respectively. If multiple pre-operative CT scans were available, the scan closest to the date of surgery was used for analysis.
Image evaluation
Two reviewers (BJ and EE) independently evaluated all scans, and disagreements were resolved by consensus. A consultant radiologist acted as an arbitrator in the event that consensus was not reached. Reviewers were blinded to patient demographics, operative characteristics and outcomes whilst analysing the images. Inter-observer consistency was calculated between two reviewers.
The extent of calcification was reported using a visual grading system based on that used by van Rossum et al[7]. It uses simple definitions and can be used in standard CT diagnostic protocols. This is contrasted to other calcium scoring techniques requiring use of special-semi automatic calcium scoring software that are more difficult to integrate into routine practice[14]. The grading system classifies scans as showing no calcification, scoring 0 points, minor calcification (1 point) or major calcification (2 points). Further details of the definitions used are reported in Table 1. Calcification scores were produced for six different vessels, detailed below.
Table 1.
Details of how calcification scores were allocated to each vessel
| Site | Score 1 (Minor calcification) | Score 2 (Major calcification) |
| Proximal aorta | Nine or fewer foci and Three or fewer foci extending over three or more sections | More than nine foci or More than three foci extending over three or more sections |
| Coeliac trunk | Calcifications extending over 3 or fewer sections and Maximal cross- sectional diameter of a single focus less than 10mm | Calcifications extending over three or more sections and maximal cross sectional diameter of a single focus greater than 10mm or Calcifications involving both the proximal and distal parts |
| Right post | One or more calcifications | NA |
| Coeliac arteries | ||
| Left post | One or more calcifications | NA |
| Coeliac arteries | ||
| Distal aorta | Nine or fewer foci and Three or fewer foci extending over three or more sections | More than nine foci or More than three foci extending over three or more sections or Subjectively assessed as having heavy calcifications |
| Aortic bifurcation | Calcifications affecting less than 40% of the circumference of the vessel | Calcifications affecting more than 40% of the circumference of the vessel |
For each site, a score of zero was assigned in cases where there were no calcifications. A focus refers to a distinct area of calcification. Section refers to a single computed tomography image in the axial plane
As the right gastro-epiploic artery is the principal blood supply to the gastric tube and is supplied from the thoracic aorta via the coeliac axis, common hepatic artery and gastroduodenal artery, all of these vessels were included[15]. Although the left gastro-epiploic artery is routinely ligated during oesophagectomy, calcifications of the splenic artery, which supplies it, were still included to allow comparisons with previous studies. Branches of the coeliac axis were grouped together as the right and left post-coeliac arteries.
As there is evidence to suggest that calcification in the abdominal aorta is a general marker of arteriopathy and may be a surrogate marker for coronary artery disease[16], it was decided to include a measurement of calcification in the aorta distal to the origin of the coeliac axis, to determine whether this may also be an independent predictor of AL or CN. When the aorta was so heavily calcified that it was difficult to distinguish distinct calcification foci; a score of 2 was allocated.
As this introduced a qualitative element to the evaluation of the distal aorta, an additional quantitative measure of calcification in the aorta was also considered for comparison. The percentage of the circumference of the aorta that was calcified was measured one axial CT slice superior to the aortic bifurcation. This method has been previously used for measurement of distal aortic calcification in patients with abdominal aortic aneurysms, and was chosen for simplicity as it also used a 0-2 scoring system[17]. This is referred to as the “bifurcation” score.
The Right and Left Post-Coeliac Arteries were scored using a binary 0-1 scale, as calcifications in these smaller vessels were expected to occur relatively infrequently, thus artificially scoring more than two categories may result in imprecise estimates describing random error rather than true associations.
Surgical technique
Oesophagectomies were classified into three operative types. Open surgeries were defined as 2 or 3 stage procedures involving open abdominal incisions with open right thoracotomy. Hybrid approaches used laparoscopic abdominal gastric mobilization (5 port technique) with an open right thoracotomy (hybrid oesophagectomy) plus or minus cervical incision. Finally, minimally invasive oesophagectomies (MIOs) used 5 abdominal ports and thoracoscopic (3 thoracic ports) esophageal mobilization with either intra-thoracic or cervical anastomosis. The decision regarding operative method was at the discretion of the consultant surgeon involved. Ten consultant upper gastrointestinal surgeons were involved in oesophagogastric cancer resections throughout the study period. Before 2006 all procedures were open operations. The first laparoscopic gastric mobilization was performed in the unit in 2006 and fully minimally invasive procedures introduced in 2008.
Statistical analysis
Statistical review was performed by a biomedical statistician. Initially, the inter-rater reliability of the calcification scores were assessed using quadratic weighted Kappa statistics. Analyses were then performed to identify any demographic factors that were associated with AL or CN. Continuous factors that were normally distributed were reported as mean ± SD, and compared between patients with and without the complication using independent samples t-tests. Continuous factors where the distribution was non-normal were reported as medians and interquartile ranges and compared between groups using non-parametric Mann-Whitney tests. Ordinal factors [e.g., American Society of Anaesthesiologists grade (ASA) and T-stage] were also compared between groups using Mann-Whitney tests, whilst nominal factors (e.g., gender and tumour type) were analysed using Fisher’s exact tests.
The predictive accuracy of the calcification scores, with respect to AL and CN, were then assessed using ROC curves. Multivariable binary logistic regression models were then produced, in order to test whether the predictive accuracy could be improved by combining the scores together. These models used a backwards stepwise approach to variable selection, starting with all of the scores in the same model, and iteratively excluding the least predictive scores until those that were significant independent predictors of outcome remained.
Within the subgroup of patients where an outcome occurred, Spearman’s correlation coefficients between the grade of the complication and the calcification scores were calculated, to assess whether there was a tendency for patients with higher score to have more severe complications[18].
Missing data were excluded from the analysis using a pairwise approach. More specifically, where a patient had missing data for one of the factors considered, they would be excluded from the analysis of that factor, but included in the analyses of the other factors for which data were available. A P-value < 0.05 was classed as statistically significant. All analyses were performed using IBM SPSS 22 (IBM Corp. Armonk, NY, United States). Our work has been reported in line with the STROCSS criteria[19].
RESULTS
Patient demographics
Following the exclusions shown in Figure 1, n = 413 patients were included in the final dataset. These patients had a mean age of 64.8 ± 9.5 years at the time of surgery, and the majority (78.9%) were male. More details on the demographics and comorbidities of the cohort are reported in Table 2, whilst Table 3 details disease and treatment related factors.
Figure 1.
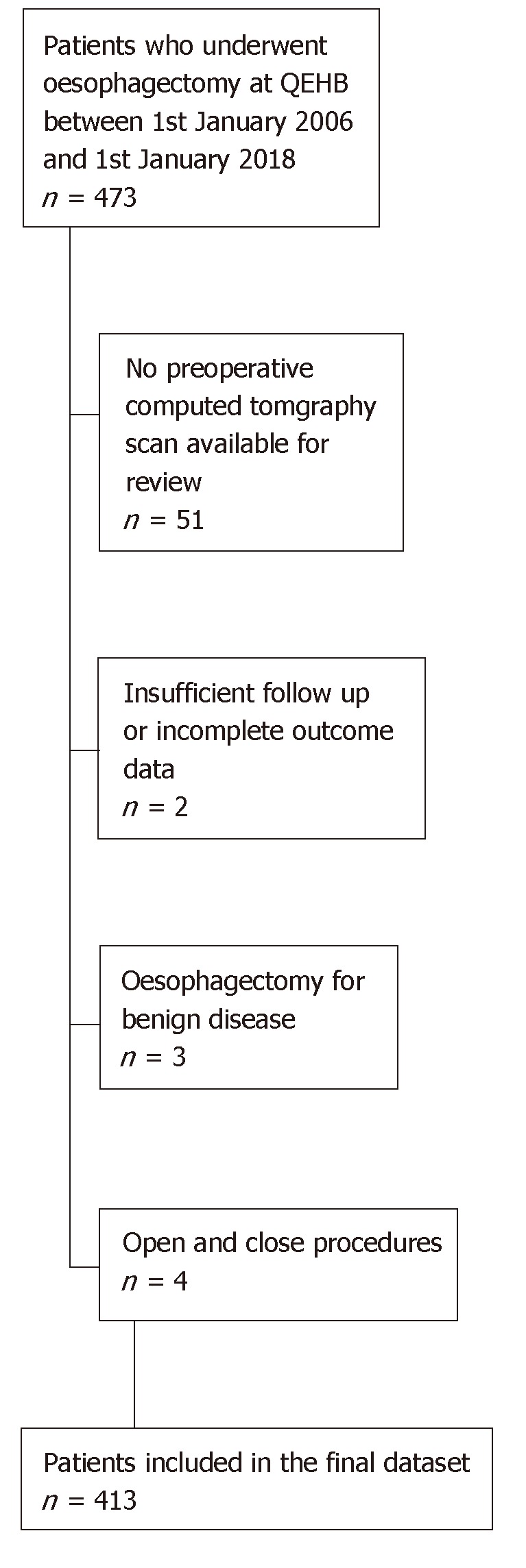
Flow chart showing recruitment and exclusion.
Table 2.
Patient demographics and comorbidities
| n | Statistic | |
| Age at surgery (yr) | 413 | 64.8 ± 9.5 |
| Gender | 413 | |
| Female | 87 (21.1) | |
| Male | 326 (78.9) | |
| BMI (kg/m2) | 402 | 26.8 ± 4.9 |
| ASA | 397 | |
| 1 | 78 (19.6) | |
| 2 | 222 (55.9) | |
| 3 | 89 (22.4) | |
| 4 | 8 (2.0) | |
| ECOG status | 324 | |
| 0 | 146 (45.1) | |
| 1 | 142 (43.8) | |
| 2 | 36 (11.1) | |
| Ischemic heart disease | 412 | |
| No | 360 (87.4) | |
| Yes | 52 (12.6) | |
| Renal impairment | 412 | |
| No | 408 (99.0) | |
| Yes | 4 (1.0) | |
| Diabetes | 412 | |
| No | 364 (88.3) | |
| Yes | 48 (11.7) | |
| COPD | 412 | |
| No | 381 (92.5) | |
| Yes | 31 (7.5) | |
| Previous cancer | 412 | |
| No | 393 (95.4) | |
| Yes | 19 (4.6) | |
| Significant smoking history | 412 | |
| No | 354 (85.9) | |
| Yes | 58 (14.1) | |
| Alcohol misuse/ heavy drinker | 412 | |
| No | 404 (98.1) | |
| Yes | 8 (1.9) |
Data are reported as n (%), mean ± SD, or as median (IQR), as applicable. BMI: Body mass index; ASA: American Society of Anaesthesiologists grade; ECOG: Eastern Cooperative Oncology Group Performance Status; COPD: Chronic obstructive pulmonary disease.
Table 3.
Disease and treatment-related factors
| n | Statistic | |
| Neoadjuvant chemotherapy | 413 | |
| No | 69 (16.7) | |
| Yes | 344 (83.3) | |
| Mandard score | 387 | |
| Mandard 1 (Complete) | 20 (5.2) | |
| Mandard 2 | 26 (6.7) | |
| Mandard 3 | 69 (17.8) | |
| Mandard 4 | 115 (29.7) | |
| Mandard 5 (None) | 88 (22.7) | |
| No Chemo | 69 (17.8) | |
| Operation stages | 413 | |
| Two-stage | 379 (91.8) | |
| Three-stage | 34 (8.2) | |
| Operation type | 413 | |
| Hybrid | 224 (54.2) | |
| MIO | 103 (24.9) | |
| Open | 86 (20.8) | |
| Type of Tumour | 409 | |
| Adenocarcinoma | 322 (78.7) | |
| Adenosquamous | 8 (2.0) | |
| Squamous | 65 (15.9) | |
| Other | 14 (3.4) | |
| T-stage | 410 | |
| T0 | 17 (4.1) | |
| T1 | 43 (10.5) | |
| T2 | 51 (12.4) | |
| T3 | 274 (66.8) | |
| T4 | 25 (6.1) | |
| N-stage | 412 | |
| N0 | 154 (37.4) | |
| N1 | 171 (41.5) | |
| N2 | 54 (13.1) | |
| N3 | 33 (8.0) | |
| M-stage | 405 | |
| M0 | 396 (97.8) | |
| M1 | 9 (2.2) | |
| R-status | 407 | |
| R0 | 255 (62.7) | |
| R1 | 141 (34.6) | |
| R2 | 11 (2.7) | |
| Peri-neural invasion | 314 | |
| No | 207 (65.9) | |
| Yes | 107 (34.1) | |
| Lymph nodes total | 412 | 30.3 ± 10.8 |
| Lymph nodes involved | 412 | 1 (0-4) |
Data are reported as n (%), mean ± SD, or as median (IQR), as applicable. Mandard Score is a measure of tumour regression due to chemotherapy, with a score of 1 being complete regression, and 5 being no regression. MIO: Minimally invasive oesophagectomies.
Data relating to complications were unavailable in n = 2 patients, hence these were excluded from the analyses of outcomes. Of the remaining n = 411, a total of 65 patients (15.8%) developed AL in the post-operative period, consisting of n = 15, n = 16 and n = 34 of grades 1, 2 and 3, respectively. CN occurred in 14 patients (3.4%), with n = 1, n = 5 and n = 8 at grades 1, 2 and 3, respectively. Of those with CN, 10/14 (71%) had an associated AL. Mortality attributed to AL was 6% (4/65) and mortality attributed to CN was 21% (3/14).
Analyses were performed to assess whether any of the factors in Tables 2 or 3 were associated with either of the complication outcomes (Supplementary Tables 1A and B and 2A and B). This found rates of AL to be significantly higher in female patients, at 23.0%, compared to 13.9% in males (P = 0.047). No other demographic or treatment related factors were found to be significantly associated with AL, including operative approach (2 vs 3 stage, 16.4% vs 9.1%, P = 0.330) or neoadjuvant chemotherapy (15.7% vs 16.2%, P = 1.000).
CN was significantly more common in females, (8.0% vs 2.2%, P = 0.014) patients with diabetes (10.6% vs 2.5%, P = 0.014), a history of smoking (10.3% vs 2.3%, P = 0.008), and a higher ASA grade (P = 0.024). There was no significant association with any other demographic or treatment related factor; however, it was noted that patients with CN had significantly fewer involved lymph nodes (median: 0 vs 1, P = 0.034).
Calcification scoring
Analysis of inter-rater reliability found that the two reviewers gave highly consistent calcification scores, with absolute agreement ranging from 95.6% to 99.0% and quadratic weighted Kappa statistics from 0.841 to 0.968 across the six vessels being analysed (Supplementary Table 3). The distribution of the cohort across the final scores is reported in Table 4. The Distal and Bifurcation scores were only recorded in n = 380 cases (92% of the cohort), as the CT scan did not show the full length of the aorta in the remainder. The same was true for n = 1 in the Proximal score.
Table 4.
Predictive accuracy of calcification scores
|
Anastomotic leak |
Conduit necrosis |
||||||
| Distributio n of scores | n/n(%) | AUROC (SE) | P Value | n/n (%) | AUROC (SE) | P value | |
| Proximal | n = 4121 | 0.518 (0.039) | 0.652 | 0.559 (0.067) | 0.454 | ||
| 0 | 159 (38.6) | 24/159 (15.1) | 3/159 (1.9) | ||||
| 1 | 199 (48.3) | 31/197 (15.7) | 10/197 (5.1) | ||||
| 2 | 54 (13.1%) | 10/54 (18.5) | 1/54 (1.9) | ||||
| Coeliac | n = 4131 | 0.514 (0.039) | 0.714 | 0.570 (0.083) | 0.374 | ||
| 0 | 316 (76.5) | 48/315 (15.2) | 9/315 (2.9) | ||||
| 1 | 91 (22.0) | 17/90 (18.9) | 4/90 (4.4) | ||||
| 2 | 6 (1.5) | 0/6 (0.0) | 1/6 (16.7) | ||||
| R Post Coeliac | n = 4131 | 0.502 (0.039) | 0.951 | 0.486 (0.077) | 0.860 | ||
| 0 | 401 (97.1) | 63/400 (15.8) | 14/400 (3.5) | ||||
| 1 | 12 (2.9) | 2/11 (18.2) | 0/11 (0.0) | ||||
| L Post Coeliac | n = 4131 | 0.492 (0.039) | 0.840 | 0.443 (0.072) | 0.465 | ||
| 0 | 337 (81.6) | 54/336 (16.1) | 13/336 (3.9) | ||||
| 1 | 76 (18.4) | 11/75 (14.7) | 1/75 (1.3) | ||||
| Distal | n = 3801 | 0.499 (0.040) | 0.990 | 0.582 (0.076) | 0.297 | ||
| 0 | 50 (13.2) | 6/50 (12.0) | 1/50 (2.0) | ||||
| 1 | 191 (50.3) | 33/191 (17.3) | 6/191 (3.1) | ||||
| 2 | 139 (36.6) | 20/137 (14.6) | 7/137 (5.1) | ||||
| Bifurcation | n = 3801 | 0.545 (0.040) | 0.275 | 0.492 (0.077) | 0.921 | ||
| 0 | 108 (28.4) | 13/108 (12.0) | 4/108 (3.7) | ||||
| 1 | 182 (47.9) | 30/181 (16.6) | 7/181 (3.9) | ||||
| 2 | 90 (23.7) | 16/89 (18.0) | 3/89 (3.4) | ||||
The number of patients for whom the score was recorded. P-values are from the ROC curve analyses. AUROC: Area under the ROC curve. Analyses of outcomes exclude n = 2 patients for whom complication data were not available.
Predictive accuracy of calcification scores
As previously stated, data relating to complications were unavailable in n = 2 patients, hence this analysis was based on the remaining n = 411. None of the calcification scores were found to be significantly associated with either AL or CN (Table 4). The analysis was repeated for composite outcomes of those patients with either AL OR CN (n = 69, 16.7%) and for those with both AL AND CN (n = 10, 2.4%) and, again, no significant associations were detected (Table 5). Multivariable analyses were then performed to identify whether a combination of the calcification scores could be identified that would be significantly predictive of any of the outcomes. However, the stepwise approach did not select any factors for inclusion in the final models.
Table 5.
Predictive accuracy of calcification scores with respect to composite outcomes
| n |
Anastomotic leak or conduit necrosis |
Anastomotic leak and conduit necrosis |
|||||
| n (%) | AUROC (SE) | P value | n (%) | AUROC (SE) | P value | ||
| Proximal | 0.518 (0.038) | 0.634 | 0.574 (0.079) | 0.426 | |||
| 0 | 159 | 25 (15.7) | 2 (1.3) | ||||
| 1 | 197 | 34 (17.3) | 7 (3.6) | ||||
| 2 | 54 | 10 (18.5) | 1 (1.9) | ||||
| Coeliac | 0.525 (0.039) | 0.517 | 0.532 (0.094) | 0.731 | |||
| 0 | 315 | 50 (15.9) | 7 (2.2) | ||||
| 1 | 90 | 18 (20.0) | 3 (3.3) | ||||
| 2 | 6 | 1 (16.7) | 0 (0.0) | ||||
| R Post | 0.501 (0.038) | 0.972 | 0.486 (0.090) | 0.882 | |||
| 0 | 400 | 67 (16.8) | 10 (2.5) | ||||
| 1 | 11 | 2 (18.2) | 0 (0.0) | ||||
| L Post | 0.486 (0.038) | 0.716 | 0.458 (0.087) | 0.648 | |||
| 0 | 336 | 58 (17.3) | 9 (2.7) | ||||
| 1 | 75 | 11 (14.7) | 1 (1.3) | ||||
| Distal | 0.501 (0.039) | 0.976 | 0.605 (0.081) | 0.259 | |||
| 0 | 50 | 7 (14.0) | 0 (0.0) | ||||
| 1 | 191 | 34 (17.8) | 5 (2.6) | ||||
| 2 | 137 | 22 (16.1) | 5 (3.6) | ||||
| Bifurcation | 0.544 (0.039) | 0.272 | 0.481 (0.091) | 0.841 | |||
| 0 | 108 | 14 (13.0) | 3 (2.8) | ||||
| 1 | 181 | 32 (17.7) | 5 (2.8) | ||||
| 2 | 89 | 17 (19.1) | 2 (2.2) | ||||
P values are from the ROC curve analyses. AUROC: Area under the ROC curve.
Within the subset of patients where the outcomes occurred, correlations between the calcification scores and complication grades were then assessed (Supplementary Table 4). However, no significant correlations between were detected between any of the calcification scores and the complication grades, as defined by the ECCG severity grade.
DISCUSSION
An effective method of predicting patients at high risk of AL would be clinically useful in the management of the oesophageal cancer patients, as it would facilitate better pre-operative risk counselling, closer monitoring of high risk patients and perhaps allow more timely intervention should AL occur. Our study found no statistically significant associations between scoring of calcifications of the abdominal arteries and either AL or CN. In addition, for the subgroup of patients with AL or CN, the grade of this complication was not found to be significantly correlated with any of the calcification scores.
Our findings are inconsistent with previous studies on the topic (Table 6). Whilst a small number of studies have found calcification to be associated with AL, the specific arteries implicated have varied between studies. Existing studies have been relatively heterogeneous in terms of operative techniques, ethnicity and other factors, which may account for the variability in results. Additionally, differences in clinical practice, such as different thresholds for investigation of leaks (such as by routine contrast swallow examination) may affect the detection rate of low grade or sub-clinical leaks, and hence be a source of heterogeneity. AL rates differed between studies, probably due to variation in a range of factors, such as cervical location of anastomosis, use of pre-operation chemoradiotherapy, and minimally invasive anastomotic techniques, which have previously been shown to be associated with increased leak rates, despite this not being the case in our cohort[2,3].
Table 6.
Summary of existing literature
| Author (Year) | Type of Oesophagecto-my | n1 | Anastomotic leak rate | Conduit ischaemia rate | Arterial vessels assessed | Association with anastomotic leakage or gastric conduit necrosis | Definition of anastomotic leak |
| van Rossum et al[7], 2015 | 3-stage | 246 | 24% | NA | Aorta, coeliac trunk, right and left post-coeliac arteries | Aorta and right post coeliac calcification associated with leakage | Defined by either extravasation of water-soluble contrast material during a contrast material swallow study or CT scan, visualization of anastomotic dehiscence or fistulae during endoscopy, or visible loss of saliva through the cervical wound |
| Zhao et al[8], 2016 | 3-stage | 709 | 17.20% | NA | Aorta, coeliac trunk, right and left post-coeliac arteries | Aorta and coeliac artery calcifications associated with leakage | Anastomotic leakage was clinically suspected, a CT scan, water-soluble contrast swallow study or endoscopy was performed |
| Goense et al[9], 2016 | 2-stage | 167 | 24% | NA | Aorta, coeliac trunk, right and left post-coeliac arteries | Aortic calcification associated with leakage | Clinical signs of leakage from a thoracic drain, radiologic signs of leakage, including contrast leakage or fluid and air levels surrounding the anastomosis, or signs of anastomotic dehiscence during endoscopy or reoperation |
| Lainas et al[12], 2017 | 2-Stage | 481 | NA | 2.10% | Coeliac Trunk | Extrinsic and intrinsic stenosis of the coeliac artery associated with gastric conduit necrosis | NA |
| Chang et al[10], 2018 | 2-stage | 164 | 8.50% | NA | Aorta, coeliac trunk, right and left post-coeliac arteries | Calcification showed no association with leakage, coeliac trunk stenosis was associated with leakage | Anastomotic dehiscence confirmed during endoscopy or operation |
| Borggreve et al[11], 2018 | 3-stage | 406 | 25.60% | NA | Coronary, supra-aortic, thoracic aorta, coeliac axis, abdominal arota, common iliac external iliac arteries; aortic valve | Calcification of coronary arteries, supra-aortic arteries, and thoracic aorta associated with leakage | Visible loss of saliva through the cervical wound, extravasation of water-soluble contrast material during a contrast swallow study or CT scan, or visualization of anastomotic dehiscence or fistulae during endoscopy or surgical re-intervention |
Number of patients included in the study. CT: Computed tomography; NA: Not reported.
In our institution, contrast studies were only performed on suspicion of AL, as per previous evidence suggesting that routine testing does not improve outcome, can lead to false positive results and risks aspiration pneumonia[20,21]. Additionally, our institution previously had an aggressive policy to re-operate on AL, which probably explains the high rate of Grade 3 leaks. Since 2012, we have favoured endoscopic methods to treat AL, which is in keeping with the current literature[22,23]. The exceptions are if the patient has a severe and life threatening leak or CN, or if endoscopic methods fail.
Although our AL rate is within previously published ranges, it is higher than the 10% audit standard set by the Association of Upper Gastrointestinal Surgeons United Kingdom[24]. This reflects the prospective nature of our complication data and the length of the data collection period, incorporating learning curves for minimal access esophagectomy[25,26] and the increase in leaks due to VEGF inhibitors used in patients during the ST03 trial which our centre recruited to[27].
Studies examining vascular calcification in colorectal anastomotic leakage have produced similarly variable results, which found no association between calcification and AL[28-31].
This is the first study to have evaluated the relationship between vascular calcification and CN in this way. Given the potentially devastating consequences for patients, research into methods of reducing morbidity from CN is highly important. One of the difficulties in investigating CN is that it remains relatively uncommon, meaning that statistical power of analyses is low. This was the case in our study, therefore although we found no statistically significant relationship between calcification and CN, this could be the result of a Type II error.
It is possible that the reason that our study found no association with AL is that examination of vascular abnormalities such as calcification is only a surrogate marker for atherosclerosis, which does not necessarily affect the actual perfusion of the gastric conduit. More complex methods of assessment of gastric conduit perfusion are available but, in general, are not readily available in clinical practice[32]. The use of Indocyanine Green to assess perfusion is a promising development to aid in a more objective assessment intra-operatively, usually after formation of the gastric conduit[33]. Our results suggest that it is micro-perfusion of the gastric conduit that may be more important in anastomotic leakage that the calcification of the main abdomino-thoracic blood vessels. As such a larger, multicentre, prospective study assessing both these variables by pre-operative CT assessment of calcification of the large vessels together with intra-operative micro-perfusion of the gastric conduit by indo-cyanine green perfusion is indicated to definitively answer this important question.
Another possible reason for our negative findings is that other factors could be at play, such as anastomotic tension, surgical technique and other patient factors [34]. A range of risk factors for AL have been identified[2,3,34]. To our knowledge, female gender has not previously been reported as a risk factor. Evidence relating to risk factors for CN is more sparse, although co-morbid conditions and coeliac artery stenosis have been previously implicated[35,36]. Our findings that female gender, diabetes, smoking and higher ASA grade are risk factors in our population will help us consent these patients more carefully and monitor them closely after surgery.
This study has some limitations, such as the inability to obtain all CT scans and the fact this was largely a retrospective study. However, we did utilise an accurate and prospectively maintained database with high quality outcome data.
To overcome the issues of small numbers of patients affected, further research in this area should be performed using large multi-centre datasets. Some multi-centre studies are assessing complications after oesophageal surgery, for example, Esodata (www.esodata.org)[37] and the Oesophagogastric Anastomosis Audit (OGAA; www.ogaa.org.uk) which aims to collect data of anastomotic complications after oesophagectomy, including CN, from a large group of international oesophageal units, to define the accurate incidence and outcome of this problem[38].
It is only with prospective, standardised data from these multi-centre registries that we can help address the void of high quality literature on this important topic.
In conclusion, Calcification scoring scored on pre-operative CT scans was not found to be significantly associated with AL or CN following oesophagectomy in our United Kingdom cohort and therefore cannot be used to identify or predict patients who are high risk for these complications.
ARTICLE HIGHLIGHTS
Research background
Anastomotic leaks (AL) are a serious complication following oesophagectomy, resulting in a reduction in both quality and quantity of life. When severe, AL can lead to conduit necrosis (CN) and complete breakdown of the anastomosis, resulting in pneumonia, sepsis and very poor patient outcomes. The formation and continued integrity of the anastomosis and gastric conduit is reliant on adequate perfusion of the gastric tube by the gastro-epiploic arcades.
Research motivation
One of the factors with the ability to affect perfusion at the anastomosis is calcification of the arteries supplying the gastric conduit and remnant oesophagus. Recent evidence has inconsistently linked calcification of these arteries with AL and CN. Arterial calcification, which can be routinely measured on pre-operative computed tomography (CT) scan, could, therefore, become an important aid in both patient selection and anastomotic risk assessment.
Research objectives
The objectives of this study were therefore to evaluate whether an association exists between calcification of arteries supplying the gastric conduit, namely the proximal aorta, distal aorta, coeliac trunk and branches of the coeliac trunk, and AL.
Research methods
Utilising routine pre-operative CT thorax, abdomen and pelvis scans, two blinded reviewers independently score vessel calcification according to the visual grading system proposed by van Rossum et al. Our prospectively maintained departmental database of patients undergoing oesophagectomy between 2006 and 2017 was examined to identify patients experiencing post-operative AL or CN. Inter-rater reliability of scoring of vessel calcification was statistically assessed using quadratic weighted kappa analyses. Univariable analyses was then performed to identify demographic and operative factors associated with AL. Subsequently, multivariable binary logistic regression models were produced to optimise the accuracy of AL prediction by artery calcification.
Research results
Of 411 patients with available data, 65 (15.8%) developed a AL post-operatively. Additionally, 4 patients had a CN not associated with AL. Rates of AL were higher in female patients (P = 0.047) and rates of CN were higher in female patients (P = 0.014), diabetic patients (P = 0.014), positive smoking history (P = 0.008) and higher ASA grade (P = 0.024). Inter-rater reliability scoring found excellent agreement between the two reviewers (absolute agreement 95.6%-99%). None of the calcification scores were associated with AL or CN on univariable or composite score analysis. Additionally, increasing calcification score was not associated with increasing severity of complications as defined by Esophagectomy Complications Consensus Group criteria.
Research conclusions
This study found no association between vascular calcification and AL or CN. Previous literature is highly heterogenous with regards to the location of calcification assessed, published leak definitions and AL rates. At the time of writing, this is the first study to aim to identify an association between vascular calcification in the aorta and coeliac axis branches within a United Kingdom population.
Research perspectives
This study and others will inform large prospective multi-centre studies currently being conducted, including the Oesophago-Gastric Anastomosis Audit, which aims to provide more definitive data with regards to factors associated with AL. Our results suggest that it is micro-perfusion of the gastric conduit that may be more important in anastomotic leakage that the calcification of the main abdomino-thoracic blood vessels. As such a larger, multicentre, prospective study assessing both these variables by pre-operative CT assessment of calcification of the large vessels together with intra-operative micro-perfusion of the gastric conduit by indo-cyanine green perfusion may well be the best method to definitively answer this research question.
ACKNOWLEDGEMENTS
We would like to thank all Consultant Upper Gastrointestinal Surgeons from University Hospitals Birmingham for allowing their patients to be included in the study. Jefferies BJ received funding from the Arthur Thompson Trust in support of his intercalated BSc Clinical Anatomy studies. In addition, the Upper gastrointestinal surgery Patient Groups of UHB and Sandwell and Walsall have kindly supported the study.
Footnotes
Institutional review board statement: The study was registered No. CARMS-15096 with University Hospitals Birmingham Clinical Audit Registration and Management System (CARMS), who granted ethical approval.
Informed consent statement: Local ethical review was obtained and confirmed that no consent was needed due to the non-interventional nature of the study.
Conflict-of-interest statement: The authors declare no conflicts of interest.
STROBE statement: The authors have read the STROBE checklist, and the manuscript was prepared and revised according to the STOBE statement checklist.
Manuscript source: Unsolicited Manuscript
Peer-review started: May 9, 2019
First decision: June 12, 2019
Article in press: June 24, 2019
Specialty type: Gastroenterology and hepatology
Country of origin: United Kingdom
Peer-review report classification
Grade A (Excellent): A
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Demetrashvili Z, Tao R S-Editor: Cui LJ L-EditorA: E-Editor: Zhou BX
Contributor Information
Benjamin J Jefferies, College of Medical and Dental Sciences, University of Birmingham, Birmingham B15 2TT, United Kingdom.
Emily Evans, Department of Radiology, University Hospitals Birmingham NHS Foundation Trust, Birmingham B15 2WB, United Kingdom.
James Bundred, College of Medical and Dental Sciences, University of Birmingham, Birmingham B15 2TT, United Kingdom.
James Hodson, Institute of Translational Medicine, University Hospitals Birmingham NHS Foundation Trust, Birmingham B15 2TT, United Kingdom.
John L Whiting, Department of Upper Gastrointestinal Surgery, University Hospitals Birmingham NHS Foundation Trust, Birmingham B15 2TH, United Kingdom.
Colm Forde, Department of Radiology, University Hospitals Birmingham NHS Foundation Trust, Birmingham B15 2WB, United Kingdom.
Ewen A Griffiths, Department of Upper Gastrointestinal Surgery, University Hospitals Birmingham NHS Foundation Trust, Birmingham B15 2TH, United Kingdom; Institute of Cancer and Genomic Sciences, University of Birmingham, Birmingham B15 2TT, United Kingdom. ewen.griffiths@uhb.nhs.uk.
References
- 1.Low DE, Alderson D, Cecconello I, Chang AC, Darling GE, DʼJourno XB, Griffin SM, Hölscher AH, Hofstetter WL, Jobe BA, Kitagawa Y, Kucharczuk JC, Law SY, Lerut TE, Maynard N, Pera M, Peters JH, Pramesh CS, Reynolds JV, Smithers BM, van Lanschot JJ. International Consensus on Standardization of Data Collection for Complications Associated With Esophagectomy: Esophagectomy Complications Consensus Group (ECCG) Ann Surg. 2015;262:286–294. doi: 10.1097/SLA.0000000000001098. [DOI] [PubMed] [Google Scholar]
- 2.Aminian A, Panahi N, Mirsharifi R, Karimian F, Meysamie A, Khorgami Z, Alibakhshi A. Predictors and outcome of cervical anastomotic leakage after esophageal cancer surgery. J Cancer Res Ther. 2011;7:448–453. doi: 10.4103/0973-1482.92016. [DOI] [PubMed] [Google Scholar]
- 3.Van Daele E, Van de Putte D, Ceelen W, Van Nieuwenhove Y, Pattyn P. Risk factors and consequences of anastomotic leakage after Ivor Lewis oesophagectomy†. Interact Cardiovasc Thorac Surg. 2016;22:32–37. doi: 10.1093/icvts/ivv276. [DOI] [PubMed] [Google Scholar]
- 4.Zehetner J, DeMeester SR, Alicuben ET, Oh DS, Lipham JC, Hagen JA, DeMeester TR. Intraoperative Assessment of Perfusion of the Gastric Graft and Correlation With Anastomotic Leaks After Esophagectomy. Ann Surg. 2015;262:74–78. doi: 10.1097/SLA.0000000000000811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ikeda Y, Niimi M, Kan S, Shatari T, Takami H, Kodaira S. Clinical significance of tissue blood flow during esophagectomy by laser Doppler flowmetry. J Thorac Cardiovasc Surg. 2001;122:1101–1106. doi: 10.1067/mtc.2001.117835. [DOI] [PubMed] [Google Scholar]
- 6.Linder G, Hedberg J, Björck M, Sundbom M. Perfusion of the gastric conduit during esophagectomy. Dis Esophagus. 2017;30:143–149. doi: 10.1111/dote.12537. [DOI] [PubMed] [Google Scholar]
- 7.van Rossum PS, Haverkamp L, Verkooijen HM, van Leeuwen MS, van Hillegersberg R, Ruurda JP. Calcification of arteries supplying the gastric tube: a new risk factor for anastomotic leakage after esophageal surgery. Radiology. 2015;274:124–132. doi: 10.1148/radiol.14140410. [DOI] [PubMed] [Google Scholar]
- 8.Zhao L, Zhao G, Li J, Qu B, Shi S, Feng X, Feng H, Jiang J, Xue Q, He J. Calcification of arteries supplying the gastric tube increases the risk of anastomotic leakage after esophagectomy with cervical anastomosis. J Thorac Dis. 2016;8:3551–3562. doi: 10.21037/jtd.2016.12.62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Goense L, van Rossum PS, Weijs TJ, van Det MJ, Nieuwenhuijzen GA, Luyer MD, van Leeuwen MS, van Hillegersberg R, Ruurda JP, Kouwenhoven EA. Aortic Calcification Increases the Risk of Anastomotic Leakage After Ivor-Lewis Esophagectomy. Ann Thorac Surg. 2016;102:247–252. doi: 10.1016/j.athoracsur.2016.01.093. [DOI] [PubMed] [Google Scholar]
- 10.Chang DH, Brinkmann S, Smith L, Becker I, Schroeder W, Hoelscher AH, Haneder S, Maintz D, Spiro JE. Calcification score versus arterial stenosis grading: comparison of two CT-based methods for risk assessment of anastomotic leakage after esophagectomy and gastric pull-up. Ther Clin Risk Manag. 2018;14:721–727. doi: 10.2147/TCRM.S157352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Borggreve AS, Goense L, van Rossum PSN, van Hillegersberg R, de Jong PA, Ruurda JP. Generalized cardiovascular disease on a preoperative CT scan is predictive for anastomotic leakage after esophagectomy. Eur J Surg Oncol. 2018;44:587–593. doi: 10.1016/j.ejso.2018.01.225. [DOI] [PubMed] [Google Scholar]
- 12.Lainas P, Fuks D, Gaujoux S, Machroub Z, Fregeville A, Perniceni T, Mal F, Dousset B, Gayet B. Preoperative imaging and prediction of oesophageal conduit necrosis after oesophagectomy for cancer. Br J Surg. 2017;104:1346–1354. doi: 10.1002/bjs.10558. [DOI] [PubMed] [Google Scholar]
- 13.Bailey G, Meadows J, Morrison AR. Imaging Atherosclerotic Plaque Calcification: Translating Biology. Curr Atheroscler Rep. 2016;18:51. doi: 10.1007/s11883-016-0601-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Komen N, Klitsie P, Hermans JJ, Niessen WJ, Kleinrensink GJ, Jeekel J, Lange JF. Calcium scoring in unenhanced and enhanced CT data of the aorta-iliacal arteries: impact of image acquisition, reconstruction, and analysis parameter settings. Acta Radiol. 2011;52:943–950. doi: 10.1258/ar.2011.110189. [DOI] [PubMed] [Google Scholar]
- 15.Liebermann-Meffert DM, Meier R, Siewert JR. Vascular anatomy of the gastric tube used for esophageal reconstruction. Ann Thorac Surg. 1992;54:1110–1115. doi: 10.1016/0003-4975(92)90077-h. [DOI] [PubMed] [Google Scholar]
- 16.An C, Lee HJ, Lee HS, Ahn SS, Choi BW, Kim MJ, Chung YE. CT-based abdominal aortic calcification score as a surrogate marker for predicting the presence of asymptomatic coronary artery disease. Eur Radiol. 2014;24:2491–2498. doi: 10.1007/s00330-014-3298-3. [DOI] [PubMed] [Google Scholar]
- 17.Matsushita M, Nishikimi N, Sakurai T, Nimura Y. Relationship between aortic calcification and atherosclerotic disease in patients with abdominal aortic aneurysm. Int Angiol. 2000;19:276–279. [PubMed] [Google Scholar]
- 18.Dindo D, Demartines N, Clavien PA. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg. 2004;240:205–213. doi: 10.1097/01.sla.0000133083.54934.ae. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Agha RA, Borrelli MR, Vella-Baldacchino M, Thavayogan R, Orgill DP STROCSS Group. The STROCSS statement: Strengthening the Reporting of Cohort Studies in Surgery. Int J Surg. 2017;46:198–202. doi: 10.1016/j.ijsu.2017.08.586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jones CM, Clarke B, Heah R, Griffiths EA. Should routine assessment of anastomotic integrity be undertaken using radiological contrast swallow after oesophagectomy with intra-thoracic anastomosis? Best evidence topic (BET) Int J Surg. 2015;20:158–162. doi: 10.1016/j.ijsu.2015.06.076. [DOI] [PubMed] [Google Scholar]
- 21.Jones CM, Heah R, Clarke B, Griffiths EA. Should routine radiological assessment of anastomotic integrity be performed after oesophagectomy with cervical anastomosis? Best evidence topic (BET) Int J Surg. 2015;15:90–94. doi: 10.1016/j.ijsu.2015.01.034. [DOI] [PubMed] [Google Scholar]
- 22.Rausa E, Asti E, Aiolfi A, Bianco F, Bonitta G, Bonavina L. Comparison of endoscopic vacuum therapy versus endoscopic stenting for esophageal leaks: systematic review and meta-analysis. Dis Esophagus. 2018:31. doi: 10.1093/dote/doy060. [DOI] [PubMed] [Google Scholar]
- 23.Dasari BV, Neely D, Kennedy A, Spence G, Rice P, Mackle E, Epanomeritakis E. The role of esophageal stents in the management of esophageal anastomotic leaks and benign esophageal perforations. Ann Surg. 2014;259:852–860. doi: 10.1097/SLA.0000000000000564. [DOI] [PubMed] [Google Scholar]
- 24.AUGIS. Provision of Services document [Internet] 2016. p. [cited 2019 Jan 28]. Available from: http://www.augis.org/provision-of-services-document/ [Google Scholar]
- 25.Ramage L, Deguara J, Davies A, Hamouda A, Tsigritis K, Forshaw M, Botha AJ. Gastric tube necrosis following minimally invasive oesophagectomy is a learning curve issue. Ann R Coll Surg Engl. 2013;95:329–334. doi: 10.1308/003588413X13629960045751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.van Workum F, Stenstra MHBC, Berkelmans GHK, Slaman AE, van Berge Henegouwen MI, Gisbertz SS, van den Wildenberg FJH, Polat F, Irino T, Nilsson M, Nieuwenhuijzen GAP, Luyer MD, Adang EM, Hannink G, Rovers MM, Rosman C. Learning Curve and Associated Morbidity of Minimally Invasive Esophagectomy: A Retrospective Multicenter Study. Ann Surg. 2019;269:88–94. doi: 10.1097/SLA.0000000000002469. [DOI] [PubMed] [Google Scholar]
- 27.Cunningham D, Stenning SP, Smyth EC, Okines AF, Allum WH, Rowley S, Stevenson L, Grabsch HI, Alderson D, Crosby T, Griffin SM, Mansoor W, Coxon FY, Falk SJ, Darby S, Sumpter KA, Blazeby JM, Langley RE. Peri-operative chemotherapy with or without bevacizumab in operable oesophagogastric adenocarcinoma (UK Medical Research Council ST03): primary analysis results of a multicentre, open-label, randomised phase 2-3 trial. Lancet Oncol. 2017;18:357–370. doi: 10.1016/S1470-2045(17)30043-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Komen N, Klitsie P, Dijk JW, Slieker J, Hermans J, Havenga K, Oudkerk M, Weyler J, Kleinrensink GJ, Lange JF. Calcium score: a new risk factor for colorectal anastomotic leakage. Am J Surg. 2011;201:759–765. doi: 10.1016/j.amjsurg.2010.01.033. [DOI] [PubMed] [Google Scholar]
- 29.Boersema GS, Vakalopoulos KA, Kock MC, van Ooijen PM, Havenga K, Kleinrensink GJ, Jeekel J, Lange JF. Is aortoiliac calcification linked to colorectal anastomotic leakage? A case-control study. Int J Surg. 2016;25:123–127. doi: 10.1016/j.ijsu.2015.12.008. [DOI] [PubMed] [Google Scholar]
- 30.Eveno C, Latrasse V, Gayat É, Lo Dico R, Dohan A, Pocard M. Colorectal anastomotic leakage can be predicted by abdominal aortic calcification on preoperative CT scans: A pilot study. J Visc Surg. 2016;153:253–257. doi: 10.1016/j.jviscsurg.2016.03.007. [DOI] [PubMed] [Google Scholar]
- 31.Pochhammer J, Tröster F, Blumenstock G, Closset J, Lang S, Weller MP, Schäffer M. Calcification of the iliac arteries: a marker for leakage risk in rectal anastomosis-a blinded clinical trial. Int J Colorectal Dis. 2018;33:163–170. doi: 10.1007/s00384-017-2949-7. [DOI] [PubMed] [Google Scholar]
- 32.Jansen SM, de Bruin DM, van Berge Henegouwen MI, Strackee SD, Veelo DP, van Leeuwen TG, Gisbertz SS. Optical techniques for perfusion monitoring of the gastric tube after esophagectomy: a review of technologies and thresholds. Dis Esophagus. 2018:31. doi: 10.1093/dote/dox161. [DOI] [PubMed] [Google Scholar]
- 33.Sarkaria IS, Bains MS, Finley DJ, Adusumilli PS, Huang J, Rusch VW, Jones DR, Rizk NP. Intraoperative near-infrared fluorescence imaging as an adjunct to robotic-assisted minimally invasive esophagectomy. Innovations (Phila) 2014;9:391–393. doi: 10.1097/IMI.0000000000000091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kassis ES, Kosinski AS, Ross P, Jr, Koppes KE, Donahue JM, Daniel VC. Predictors of anastomotic leak after esophagectomy: an analysis of the society of thoracic surgeons general thoracic database. Ann Thorac Surg. 2013;96:1919–1926. doi: 10.1016/j.athoracsur.2013.07.119. [DOI] [PubMed] [Google Scholar]
- 35.Briel JW, Tamhankar AP, Hagen JA, DeMeester SR, Johansson J, Choustoulakis E, Peters JH, Bremner CG, DeMeester TR. Prevalence and risk factors for ischemia, leak, and stricture of esophageal anastomosis: gastric pull-up versus colon interposition. J Am Coll Surg. 2004;198:536–41; discussion 541-542. doi: 10.1016/j.jamcollsurg.2003.11.026. [DOI] [PubMed] [Google Scholar]
- 36.Cassivi SD. Leaks, strictures, and necrosis: a review of anastomotic complications following esophagectomy. Semin Thorac Cardiovasc Surg. 2004;16:124–132. doi: 10.1053/j.semtcvs.2004.03.011. [DOI] [PubMed] [Google Scholar]
- 37.International society for diseases of the Esophagus. International Oesophageal Database. 2018. Available from: https://www.isde.net/ [Google Scholar]
- 38.OGAA Study Group. Oesophago-gastric Anastomosis Audit [Internet] 2018. p. [cited 2018 Oct 20];. Available from: http://www.ogaa.org.uk. [Google Scholar]


