Brief overview of trials about positive-end expiratory pressure in acute respiratory distress patients before the EPVENT 2 study
The acute respiratory distress syndrome (ARDS) is an acute inflammatory lung edema. The current definition includes three severity stages based on PaO2/FiO2 ratio measured at positive end-expiratory pressure (PEEP) of 5 cmH2O or more (1). ARDS was described for the first time by Ashbaugh et al. in 1967; it was suggested that PEEP could have beneficial effects (2). More than 50 years after its initial description ARDS is still of great concern for the intensivist because it accounts for 10% of ICU admissions and still supports a rough 40% mortality rate (3). Furthermore, ARDS can only be treated in the ICU environment because it very often requires invasive mechanical ventilation and because the mechanical ventilation settings can impact patient outcome. Indeed, the use of low tidal volume (VT) is strongly recommended as it has been demonstrated to decrease absolute mortality by 9% in the landmark ARMA randomized control trial (RCT) (4). It is worth mentioning that this trial was innovative in many aspects, like the titration of VT according predicted (PBW), not measured, body weight, the monitoring of plateau pressure (Pplat), the adjustment of respiratory rate up to 35 breaths/minute, and a pragmatic way to set PEEP. The oxygenation target (PaO2 55–80 mmHg) was managed via a table that modulated FiO2 and PEEP together. However, the main target was to protect the lung from overdistension. The ARMA trial compared VT of 12 mL/kg PBW and Pplat targeted to 50 cmH2O (control group) to VT of 6 mL/kg PBW and Pplat targeted to 30 cmH2O (intervention group). It confirmed that excessive strain was the most important determinant of ventilator-induced lung injury (VILI). The other component of VILI is the atelectrauma resulting from the shear stress imposed by the repeated opening and closing of peripheral lung units to their neighboring lung areas. This component may be prevented by setting PEEP. Therefore, three large trials were done comparing higher to lower PEEP at same low VT. However, none of them was associated with a better outcome from using one strategy over the other. The ALVEOLI trial (5) enrolled a total of 549 ARDS patients at PaO2/FiO2 <300 mmHg between a Low FiO2/High PEEP and a High FiO2/Low PEEP table. The LOVS trial (6) included 985 ARDS patients with PaO2/FiO2 ratio lower than 250 mmHg and tested similar PEEP/FiO2 table as that described in the ALVEOLI trial. Pplat in the high PEEP group was targeted to 40 cmH2O, while in the low PEEP group it was kept under 30 cmH2O. There was no difference between the two groups in terms of mortality and barotrauma. The EXPRESS trial (7) involved 768 patients with ARDS (PaO2/FiO2 <300 mmHg) and used another strategy to set PEEP from the two previous trials. Were compared, at similar VT of 6 mL/kg PBW, PEEP set up to reach a Pplat of 28 to 30 cmH2O (increased recruitment strategy group) to a control group in which PEEP was set up between 5 and 9 cmH2O. The oxygenation target was the same as in the previous trials but was managed by using FiO2 alone. No statistically significant difference was found between the two groups in terms of mortality. However, with the increased recruitment strategy the number of ventilator-free days and organ failure–free days were significantly higher than in the control group. These three trials were meta-analyzed at the individual patient level and it came out a slight but statistically significantly better survival by using higher PEEP than lower PEEP in ARDS patients with PaO2/FiO2 <200 mmHg (8). Finally, the ART trial (9) compared a lung maximal recruitment strategy mostly based on high PEEP to a control group in which PEEP averaged 13 cmH2O, in ARDS patients (PaO2/FiO2 <200 mmHg). Of notice VT was 5 mL/kg PBW in both groups. The mortality was significantly higher by 10% in the lung recruitment group than in the control group. This trial actually strongly suggests that very high PEEP is associated with worst outcome as compared to high PEEP. Surprisingly, the driving pressure (DP), which is the difference between Pplat and PEEP (or better Pplat and total PEEP), was lower in the experimental group than in the controls. This result was therefore in contrast to the findings of a previous landmark study that showed DP as the strongest predictor of death and moreover the mediator of the effect of Pplat, PEEP and VT on mortality in ARDS patients.
In all these trials the PEEP set at the ventilator was based on airway pressure (Paw) and not on transpulmonary pressure (PL). Talmor et al. found out that PL at end-expiration (PL,ee), computed as the difference between Paw and absolute esophageal pressure (Pes), was frequently negative in ARDS patients, due to very positive values of Pes,ee (10). This finding was interpreted as resulting from a prevalent loss of lung volume in the dependent lung parts, close to the Pes sensor location. Their idea was to propose to set PEEP up to the point at which PL,ee was equal to 0 cmH2O or more. They hypothesized that this would promote some recruitment in the dependent lung regions and furthermore provide a personalized approach in tailoring PEEP. They tested this hypothesis in a single center randomized controlled trial, EPVENT 1, over 61 patients (PaO2/FiO2 <300 mmHg) (11). PL was also measured at the end of inspiration (PL,ei) in EPVENT1 to provide some safety because it was expected that Pplat would be very much greater than 30 cmH2O with the higher PEEP likely generated by the Pes-guided strategy. They used a threshold of 25 cmH2O PL,ei as the upper safety limit, which should prompt VT to be reduced down to 4 mL/kg PBW. However, it is worth mentioning that PL,ei can be measured by another method, ie the elastance ratio method (12):
| PL,ei = Pplat × (EL/Ers) | [1] |
where EL and Ers are elastance of the lung and of the respiratory system, respectively, given by:
| EL = [(Pplat – Pes,ei) – (PEEPTOT – Pes,ee)]/VT | [2] |
| Ers = (Pplat –PEEPTOT)/VT | [3] |
where, Pes,ei is Pes measured during an inspiratory hold, PEEPTOT is total PEEP and Pes,ee Pes both measured during an expiratory hold. It is worth mentioning that recent data suggest that absolute PL,ei reflects dependent PL and elastance-related PL,ei method non-dependent PL and PL,ee lung regions in-between (13).
The EPVENT 1 study also included a specific PL,ee/FiO2 table. In the control group they used a PEEP/FiO2 table, as used in the trials led by the ARDSnet work mentioned above, with a slight different oxygenation target. The results showed significant better oxygenation, better compliance and a strong trend towards better survival in the intervention group.
The results of EPVENT1 logically prompted the authors to perform a large multicenter RCT to try to confirm these promising, though preliminary, findings.
The EPVENT 2 trial
EPVENT 2 was a multi-centered RCT conducted between 2012 and 2017 in 14 intensive care units in North America (14). Patients enrolled had moderate-to-severe ARDS criteria according to the Berlin definition. In the patients belonging to the intervention group an esophageal catheter was placed, in order to obtain PL. In this group, PEEP was titrated according a PL,ee/FiO2 table, which was slightly different from the one used in the EPVENT 1 trial. In particular, the range of PL,ee in this table was 0–6 instead 0–10 cmH2O in EPVENT 1. To limit the risk of overdistension in the intervention group, when PL,ei was above 20 cmH2O, VT could be reduced down to 4 mL/kg PBW. The assessment was done once a day. In the control group, the authors used the same PEEP/FiO2 table as in the control group in the OSCILLATE trial. Furthermore, in this group Pplat had to be maintained below 35 cmH2O and not 30 cmH2O. The primary end point was a composite score incorporating death and days free from mechanical ventilation at day 28. There was no difference between the two groups regarding the primary end-point: a more favorable outcome was observed in 49.6% among the 102 patients in the experimental group and in 50.4% among the 98 patients in the control group (P=0.92). Mortality at day 28 was 32.4% vs. 30.6% (P=0.88) and the median days free of mechanical ventilation were 22 and 21 (P=0.85), in experimental and control groups, respectively. There were no other statistical difference between other end-points, like barotrauma, shock-free days, acute kidney injury, PaO2/FiO2 ratio, PEEP and DP between the two groups.
Differences between EPVENT 1 and EPVENT 2 trials
In the EPVENT 2 vs. EPVENT 1 trial, the range of PL,ee used in the PL,ee/FiO2 table was narrower. As an example, for FiO2 0.6 PL,ee should be 4 cmH2O in EPVENT 1 and 2 cmH2O in EPVENT 2. The Pplat upper safety limit in the control group was 35 cmH2O in the EPVENT2 but unspecified in the EPVENT1 and that of PL,ei of 25 cmH2O in EPVENT1 and 20 cmH2O in EPVENT2. These findings would increase the risk of overdistension in the control group in EPVENT2 and result in setting lower PEEP in the experimental group in EPVENT2. ARDS patients were different between the two trials: ARDS was mostly from a secondary or indirect lung injury in EPVENT1 and mostly from pneumonia in EPVENT2 (Figure 1). Gattinoni et al. showed that secondary ARDS has a higher incidence of increased chest wall elastance compared to primary ARDS (19). In this kind of patients, largely prevalent in the EPVENT 1 study, it is reasonable to think that the measure of the PL has an impact on clinical outcome. It could be interesting to focus the Pes-guided PEEP in obese patients without ARDS, representing a population characterized by an increased chest wall elastance, and for which it is difficult to predict the elastance of the chest wall without using the esophageal pressure (20-22). It could also be helpful to test the benefits of PL in a selected population of secondary ARDS, since also this group of patients is likely to have increased chest wall elastance.
Figure 1.
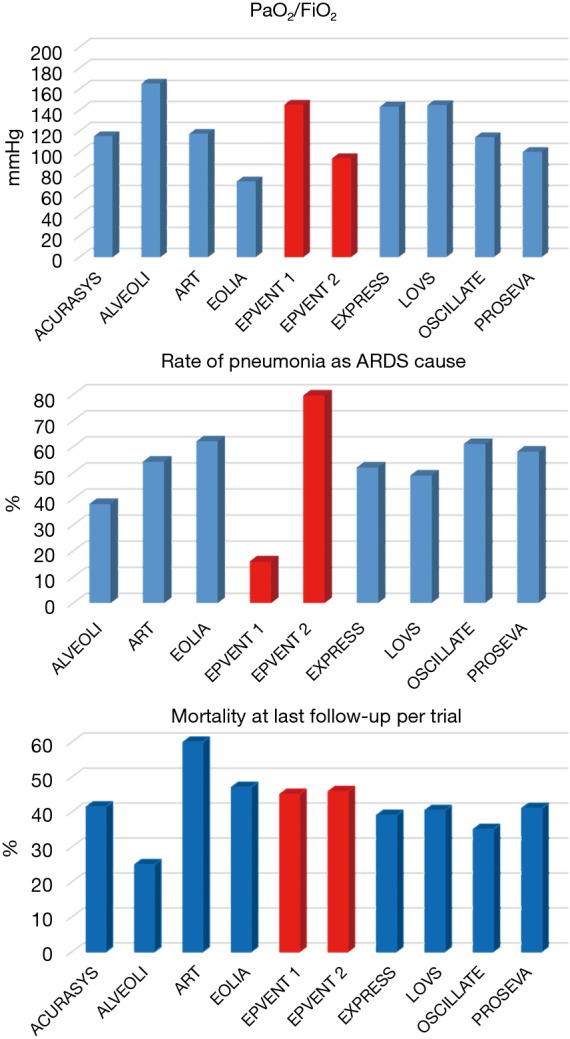
Mean values of partial pressure of arterial oxygen (PaO2) to fraction of inspired oxygen in air (FiO2) ratio, prevalence of pneumonia, and mortality at the last follow-up in the trial in the control groups of recent randomized control trials in acute respiratory distress patients. The studies are shown in alphabetic order in each plot and the references are (5-7,9,11,15-18).
The rate of pneumonia was even higher in EPVENT2 than in any other recent trial in ARDS (Figure 1). Zampieri et al. went back to the ART trial using a machine-learning approach. They found that experimental strategy was more detrimental in patients with pneumonia (23). This could explain the EPVENT 1 positive results, due to the low number of pneumonia ARDS patients, where the high PEEP strategy obtained using PL, could be dangerous.
In EPVENT 1 the intervention treatment was applied for only three days vs 28 days in the EPVENT 2.
Finally, oxygenation was more severely impaired in EPVENT2 than in EPVENT1 at baseline (Figure 1).
Despite of these differences the mortality rate was similar in the control groups of EPVENT2 as in the ones of other recent trials in ARDS patients (Figure 1) (5-7,9,11,15-18). In the experimental group the mortality at day 28 was 17% in EPVENT1, ie twice lower than in EPVENT2.
Reasons for the negative results of the EPVENT 2 trial
Apart from the differences between EPVENT 1 and EPVENT2, other specific reasons may explain the negative results observed in EPVENT2.
A first reason may be a lack of power. The strategy used to power the trial with a composite score minimizes the number of patients to include (24). For instance, in the EPVENT 2 the absolute difference of rescue therapy prevalence between the groups was 8.3% and didn’t reach the statistically significance contrary to the rate of 4.2%, which was statistically significant in the LOVS trial.
Another reason could have been that the control group of EPVENT 2 had a better outcome as compared with the control groups of other RCTs. This was not the case as discussed above (Figure 1).
A third reason is that the PEEP set was the same between control and experimental groups in EPVENT2 contrary to EPVENT1 where PEEP was significantly higher in the Pes-guided group. This can be explained by the case mix as discussed above. However, since the hypothesis for the trial to be beneficial is based on PEEP difference it is not surprising that at same PEEP, same VT and same DP in both groups no difference in mortality was found. We recently set PEEP according to a Pes-guided strategy and a PEEP/FiO2 table as that used in the ARMA trial in 32 ARDS patients, most of them from pneumonia, in supine and prone positions (25). On average the Pes-guided strategy resulted in a 2 cmH2O higher PEEP than with the other strategy whatever the position.
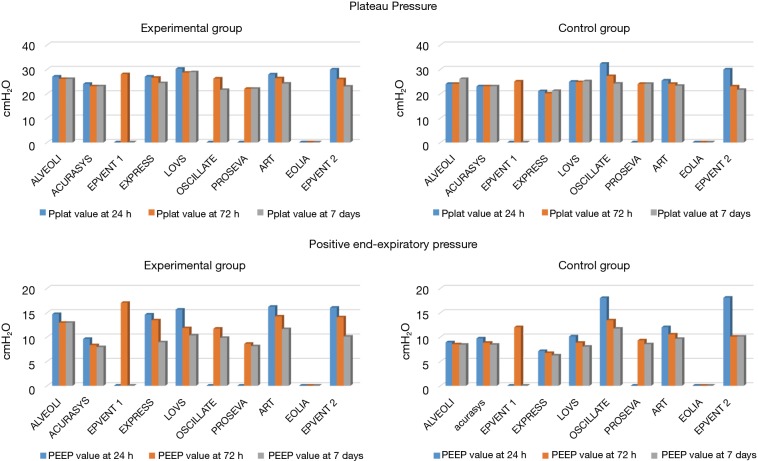
EPVENT2 protocol resulted in higher PEEP in both groups than in the recent ARDS trials (Figure 2).
Figure 2.
Mean values of positive end expiratory pressure (PEEP) in the lower panels and Plateau pressure (Pplat) in the upper panels for control (right panels) and intervention groups (left panels) recent randomized control trials in acute respiratory distress patients. The studies are shown in alphabetic order in each plot and the references are (5-7,9,11,15-18).
Finally, it could be that the concerns about the use of absolute Pes to measure PL,ee were valid (26,27).
Conclusions
EPVENT 2 is a methodologically correct trial with some minor limitations. Overall, this study supports the evidence that the routine use of the Pes-guided PEEP titration doesn’t change the mortality rate. This finding is probably associated with the homogeneity of the chest wall elastance distribution within an ARDS population with a high prevalence of pneumonia.
Acknowledgments
None.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Provenance: This is an invited article commissioned by the Section Editor Xue-Zhong Xing [National Cancer Center (NCC)/Cancer Hospital, Chinese Academy of Medical Sciences (CAMS) and Peking Union Medical College (PUMC), Beijing, China].
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- 1.Ranieri VM, Rubenfeld GD, Thompson BT, et al. Acute respiratory distress syndrome: the Berlin Definition. JAMA 2012;307:2526-33. [DOI] [PubMed] [Google Scholar]
- 2.Ashbaugh DG, Bigelow DB, Petty TL, et al. Acute respiratory distress in adults. Lancet 1967;2:319-23. 10.1016/S0140-6736(67)90168-7 [DOI] [PubMed] [Google Scholar]
- 3.Bellani G, Laffey JG, Pham T, et al. Epidemiology, Patterns of Care, and Mortality for Patients With Acute Respiratory Distress Syndrome in Intensive Care Units in 50 Countries. JAMA 2016;315:788-800. 10.1001/jama.2016.0291 [DOI] [PubMed] [Google Scholar]
- 4.Acute Respiratory Distress Syndrome Network , Brower RG, Matthay MA, et al. Ventilation with lower tidal volumes as compared with traditional tidal volumes for acute lung injury and the acute respiratory distress syndrome. The Acute Respiratory Distress Syndrome Network. N Engl J Med 2000;342:1301-8. 10.1056/NEJM200005043421801 [DOI] [PubMed] [Google Scholar]
- 5.Brower RG, Lanken PN, MacIntyre N, et al. Higher versus lower positive end-expiratory pressures in patients with the acute respiratory distress syndrome. N Engl J Med 2004;351:327-36. 10.1056/NEJMoa032193 [DOI] [PubMed] [Google Scholar]
- 6.Meade MO, Cook DJ, Guyatt GH, et al. Ventilation strategy using low tidal volumes, recruitment maneuvers, and high positive end-expiratory pressure for acute lung injury and acute respiratory distress syndrome: a randomized controlled trial. JAMA 2008;299:637-45. 10.1001/jama.299.6.637 [DOI] [PubMed] [Google Scholar]
- 7.Mercat A, Richard JC, Vielle B, et al. Positive end-expiratory pressure setting in adults with acute lung injury and acute respiratory distress syndrome: a randomized controlled trial. JAMA 2008;299:646-55. 10.1001/jama.299.6.646 [DOI] [PubMed] [Google Scholar]
- 8.Briel M, Meade M, Mercat A, et al. Higher vs lower positive end-expiratory pressure in patients with acute lung injury and acute respiratory distress syndrome: systematic review and meta-analysis. JAMA 2010;303:865-73. 10.1001/jama.2010.218 [DOI] [PubMed] [Google Scholar]
- 9.Cavalcanti AB, Suzumura EA, Laranjeira LN, et al. Effect of Lung Recruitment and Titrated Positive End-Expiratory Pressure (PEEP) vs Low PEEP on Mortality in Patients With Acute Respiratory Distress Syndrome: A Randomized Clinical Trial. JAMA 2017;318:1335-45. 10.1001/jama.2017.14171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Talmor D, Sarge T, O'Donnell CR, et al. Esophageal and transpulmonary pressures in acute respiratory failure. Crit Care Med 2006;34:1389-94. 10.1097/01.CCM.0000215515.49001.A2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Talmor D, Sarge T, Malhotra A, et al. Mechanical ventilation guided by esophageal pressure in acute lung injury. N Engl J Med 2008;359:2095-104. 10.1056/NEJMoa0708638 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Akoumianaki E, Maggiore SM, Valenza F, et al. The application of esophageal pressure measurement in patients with respiratory failure. Am J Respir Crit Care Med 2014;189:520-31. 10.1164/rccm.201312-2193CI [DOI] [PubMed] [Google Scholar]
- 13.Yoshida T, Amato MBP, Grieco DL, et al. Esophageal Manometry and Regional Transpulmonary Pressure in Lung Injury. Am J Respir Crit Care Med. 2018;197:1018-26. 10.1164/rccm.201709-1806OC [DOI] [PubMed] [Google Scholar]
- 14.Beitler JR, Sarge T, Banner-Goodspeed VM, et al. Effect of Titrating Positive End-Expiratory Pressure (PEEP) With an Esophageal Pressure-Guided Strategy vs an Empirical High PEEP-Fio2 Strategy on Death and Days Free From Mechanical Ventilation Among Patients With Acute Respiratory Distress Syndrome: A Randomized Clinical Trial. JAMA 2019. [Epub ahead of print]. 10.1001/jama.2019.0555 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Papazian L, Forel J, Gacouin A, et al. ACURASYS Study Investigators Neuromuscular blockers in early acute respiratory distress syndrome. N Engl J Med 2010;363:1107-16. 10.1056/NEJMoa1005372 [DOI] [PubMed] [Google Scholar]
- 16.Combes A, Hajage D, Capellier G, et al. Extracorporeal Membrane Oxygenation for Severe Acute Respiratory Distress Syndrome. N Engl J Med 2018;378:1965-75. 10.1056/NEJMoa1800385 [DOI] [PubMed] [Google Scholar]
- 17.Ferguson ND, Cook DJ, Guyatt GH, et al. High-frequency oscillation in early acute respiratory distress syndrome. N Engl J Med 2013;368:795-805. 10.1056/NEJMoa1215554 [DOI] [PubMed] [Google Scholar]
- 18.Guérin C, Reignier J, Richard JC, et al. Prone positioning in severe acute respiratory distress syndrome. N Engl J Med 2013;368:2159-68. 10.1056/NEJMoa1214103 [DOI] [PubMed] [Google Scholar]
- 19.Gattinoni L, Pelosi P, Suter PM, et al. Acute respiratory distress syndrome caused by pulmonary and extrapulmonary disease. Different syndromes? Am J Respir Crit Care Med 1998;158:3-11. 10.1164/ajrccm.158.1.9708031 [DOI] [PubMed] [Google Scholar]
- 20.Pelosi P, Croci M, Ravagnan I, et al. The effects of body mass on lung volumes, respiratory mechanics, and gas exchange during general anesthesia. Anesth Analg 1998;87:654-60. [DOI] [PubMed] [Google Scholar]
- 21.Pelosi P, Croci M, Ravagnan I, et al. Total respiratory system, lung, and chest wall mechanics in sedated-paralyzed postoperative morbidly obese patients. Chest 1996;109:144-51. 10.1378/chest.109.1.144 [DOI] [PubMed] [Google Scholar]
- 22.Bein T. Driving pressure in obese ventilated patients: another brick in the (chest) wall. Intensive Care Med 2018;44:1349-51. 10.1007/s00134-018-5288-4 [DOI] [PubMed] [Google Scholar]
- 23.Zampieri FG, Costa EL, Iwashyna TJ, et al. Heterogeneous effects of alveolar recruitment in acute respiratory distress syndrome: a machine learning reanalysis of the Alveolar Recruitment for Acute Respiratory Distress Syndrome Trial. Br J Anaesth 2019;123:88-95. 10.1016/j.bja.2019.02.026 [DOI] [PubMed] [Google Scholar]
- 24.Ferguson ND, Scales DC, Pinto R, et al. Integrating mortality and morbidity outcomes: using quality-adjusted life years in critical care trials. Am J Respir Crit Care Med 2013;187:256-61. 10.1164/rccm.201206-1057OC [DOI] [PubMed] [Google Scholar]
- 25.Mezidi M, Parrilla FJ, Yonis H, et al. Effects of positive end-expiratory pressure strategy in supine and prone position on lung and chest wall mechanics in acute respiratory distress syndrome. Ann Intensive Care 2018;8:86. 10.1186/s13613-018-0434-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hubmayr RD. Is there a place for esophageal manometry in the care of patients with injured lungs? J Appl Physiol 2010;108:481-2. 10.1152/japplphysiol.00027.2010 [DOI] [PubMed] [Google Scholar]
- 27.Bernard GR. PEEP guided by esophageal pressure--any added value? N Engl J Med 2008;359:2166-8. 10.1056/NEJMe0806637 [DOI] [PubMed] [Google Scholar]