Abstract
Objective:
Given controversy over use of contact precautions (CP), this study evaluates the impact of discontinuing CP for methicillin-resistant Staphylococcus aureus (MRSA) and vancomycin-resistant Enterococcus (VRE) and expansion of chlorhexidine gluconate (CHG) use on the health system.
Design:
Retrospective, nonrandomized, observational, quasi-experimental study.
Setting:
2 California hospitals.
Participants:
Inpatients.
Methods:
We compared hospital-wide LabID clinical culture rates (as a marker of healthcare associated infections) 1 year before and after routine CP for endemic MRSA and VRE were discontinued and CHG bathing was expanded to all units. Culture data from patients and cost data on material utilization were collected. Nursing time spent donning personal protective equipment (PPE) was assessed and quantified using time-driven activity-based costing.
Results:
Average positive culture rates before and after discontinuing CP were 0.40 and 0.32 cultures/100 admissions for MRSA (p=0.09), and 0.48 and 0.40 cultures/100 admissions for VRE (p=0.14). When combining isolation gown and CHG costs, the health system saved $643,776 in one year. Prior to the change, 28.5% ICU and 19% Medicine/Surgery beds were on CP for MRSA/VRE. Based on average room entries and donning time, estimated nursing time spent donning PPE for MRSA/VRE before the change was 45,277 hours/year (estimated cost: $4.6 million).
Conclusion:
Discontinuing routine CP for endemic MRSA and VRE did not result in increased rates of MRSA or VRE after one year. With cost savings on materials, increased healthcare worker time, and no concomitant increase in possible infections, elimination of routine CP may add substantial value to inpatient care delivery.
Keywords: Contact precautions, MRSA, VRE, Healthcare associated infections, Cost analysis
Introduction:
The Centers for Disease Control and Prevention and Society for Healthcare Epidemiology of America recommend contact precautions (CP) to decrease transmission of multidrug resistant organisms (MDROs) in acute care hospitals, including methicillin-resistant Staphylococcus aureus (MRSA) and vancomycin-resistant Enterococcus (VRE).1,2 Although common practice, CP for endemic MRSA and VRE have become increasingly controversial given associations with patient harms.3–5
Data demonstrating that CP (gown and gloves) decrease transmission of endemic MRSA and VRE are limited.3 Most studies on the effectiveness of CP include horizontal infection prevention strategies, including improved hand hygiene (HH), decolonization, and/or active surveillance cultures (ASC), not just organism specific vertical prevention strategies.3 Although combination strategies have shown decreases in MDRO acquisition, colonization, and invasive disease, there is no strong evidence supporting use of CP in absence of additional strategies for endemic MRSA or VRE.3,6–17
CP have been associated with patient harms, including fewer healthcare workers (HCW) bedside visits, shorter HCW contact time, and less documentation compared to patients not on CP.18–23 Patients experience delays in admission from the emergency room and discharge to skilled nursing facilities.23–26 CP were also associated with increased preventable adverse events, including falls, pressure ulcers, and medication administration errors.23,27 Patients on CP had increased anxiety and depression, and lower satisfaction.23,28–30 The results of newer studies, however, have conflicting findings and do not show increased adverse events.31
92% of hospitals recently surveyed still use CP for MRSA and VRE (n=87), but at least 30 US hospitals are no longer doing so and instead only employ horizontal infection prevention strategies.3 One study showed no increase in device-associated HAI rates after discontinuing CP for MRSA/VRE.32
The purpose of this study was to determine the impact of discontinuing routine CP for endemic MRSA and VRE on LabID clinical culture rates (marker of HAI rates) in 2 California hospitals and overall health system costs.
Methods:
Hospital Setting:
This study was conducted at Ronald Reagan UCLA Medical Center (Hospital A), a 540-bed tertiary, academic hospital, with 154 intensive care unit (ICU) beds, large transplant population, and level 1 trauma center, and Santa Monica UCLA Medical Center (Hospital B), a 265-bed community, teaching hospital with 22 ICU beds. All beds at hospital A and the vast majority at hospital B are single occupant, private rooms. All rooms have alcohol-based hand rubs and sinks available for HH. CP rooms are equipped with signage, isolation gowns, and gloves.
Study Design and Policy Changes:
We performed a retrospective, nonrandomized, observational, quasi-experimental study comparing clinical culture rates at both hospitals before and after the CP policy change and near universal Chlorhexidine gluconate (CHG) bathing. This study was exempt by the UCLA Institutional Review Board as nonhuman subjects research given the policy was changed for quality improvement purposes.
Routine CP for endemic MRSA and VRE were discontinued on 7/1/14 per the Infection Control Committee recommendation after literature review and concern for harms associated with CP. Data were collected for 1 year before the change at hospital A and 6 months before at hospital B. Prior to 7/1/14, all patients with active disease, history of, or positive surveillance screening for MRSA and/or VRE were placed in CP, requiring gown and glove use upon room entry. An alert flag was placed in the electronic health record, and patients were placed on CP for all subsequent hospitalizations. After 7/1/14, CP were not required for MRSA or VRE, unless draining wounds were present. CP were still required for MDRO gram-negative infections and spore precautions for Clostridium difficile (C. difficile). Policies for droplet and airborne precautions were unchanged. Data were collected for 1 year after the policy change at both hospitals.
CHG bathing was required in ICUs since 2012, except neonatal. Starting in 5/2014, daily 2% CHG bathing was implemented in all units. All patients over 2 months of age undergo CHG bathing, except neonatal ICU, newborn nursery, and perinatal patients without a central line or cesarean section.
HAI Data Collection and Rate Calculations:
Surveillance for MRSA, VRE and C. difficile were performed monthly by Infection Preventionists using the National Healthcare Safety Network (NHSN) Lab ID Event method.33 Hospital A reported all clinical specimens to NHSN and rate data for each culture is available for the entire study period. Hospital B only reported MRSA and VRE bloodstream infections to NHSN prior to 1/2014, and all clinical specimens from 1/2014 to 6/2015. Hospital B collected C. difficile data for the entire study period. C. difficile rates were calculated monthly using the NHSN Facility C. difficile Infection Healthcare Facility-Onset Incidence Rate. C. difficile toxin B gene PCR was used for laboratory identification. MRSA and VRE rates were calculated monthly using the NHSN Overall MDRO Infection/Colonization Incidence Rate.
HH and Personal Protective Equipment (PPE) Compliance:
Trained volunteers directly observed opportunities for HH and PPE, and documented observed and correctly completed opportunities (See appendix for details). PPE compliance requires gloves and a gown tied behind the head and back.
Change in Resistant Isolates:
All Staphylococcus aureus and Enterococcus isolated from specimens submitted for culture (blood, respiratory, skin/soft tissue, wound, or other) were tested for susceptibility to oxacillin/cefoxitin and vancomycin using broth microdilution, if clinically warranted. Active surveillance tests were not included. The percentages of resistant isolates were compared before and after the intervention.
MRSA and VRE Screening:
California law requires MRSA ASC nasal swab testing on all high-risk patients.34,35 High-risk patients include ICU admissions, transfers from outside hospitals or skilled nursing facilities, 30-day readmissions, orthopedic or spine surgery patients receiving prosthetic material, and hemodialysis patients. VRE surveillance testing by rectal swab was performed on patients deemed clinically high-risk by their treating physician’s judgment. Testing was performed using chromogenic media.
Hospital Outcomes:
Pre and post data on average length of stay, 30-day readmissions, and in-hospital mortality were collected. Analyses included all length of stay data and excluded hospice, readmissions for chemotherapy, radiation, rehabilitation, death on first admission, dialysis, delivery, birth, mental diseases, and drug/alcohol abuse treatment.
Cost Data:
Gown and CHG costs were based on total purchasing of materials. UCLA began using washable gowns in some units in 2012 and house wide in hospital A in 8/2013. Washable gowns were phased in at hospital B throughout the study period.
Healthcare Worker Time:
To estimate HCW time spent donning PPE, donning time and average number of room entries were collected. HCW were randomly selected by unit and presence of CP rooms, and timed donning PPE during routine patient care on multiple units. Timing was started when they reached for PPE and stopped after gloves/gown were completely donned.
Randomly selected patient rooms were observed for 30 minutes to 1 hour (total of 26 hours) to assess nursing entries. The average entries per hour was calculated and broken down by ICU or medicine/surgery floor.
Time-driven activity-based costing (TDABC) was used to estimate costs associated with nursing time spent donning PPE, using average PPE donning time, average entries per hour, and nursing capacity time costs.36,37 The capacity cost calculated using TDABC was $1.75 per minute for floor nurses and $1.66 per minute for ICU nurses (internal financial data).
Statistical Analysis:
Pre and post clinical culture rates were compared using Poisson regression models with monthly rates as the unit of analysis. To account for patient days per month (C. difficile) or admissions per month (MRSA, VRE), all models included a (log) offset term. We assessed intervention effect two ways for each infection. The first set of models included a binary term for pre verses post intervention period, with separate analyses for each hospital alone and both hospitals combined, producing 3 sets of results. Based on these models, we computed rate ratios and associated 95% confidence intervals. Next, we constructed a set of models with additional terms for hospital and intervention by hospital interaction. Statistical analyses for clinical culture rates were carried out using SAS 9.4 (SAS institute, Cary, NC).
Pre versus post intervention comparisons were made for resistant isolates, MRSA ASC, VRE surveillance, HH compliance, PPE compliance, length of stay, 30-day readmissions, and in-hospital mortality using chi-square tests for categorical variables and t-tests for continuous variables. These analyses were carried out using Stata 14.0 (StataCorp LP, College Station, TX). P-values <0.05 were considered statistically significant.
Results:
Impact On Infections:
Throughout the study, admissions and patient days were relatively constant (Supplementary Table 1).
There was no increase in LabID clinical culture rates for MRSA, VRE, or C. difficile at either hospital or in combined data after CP were discontinued for endemic MRSA and VRE (Table 1). There were monthly fluctuations in both the pre and post periods (Figure 1). All rates were lower in the post period, except VRE in hospital B and C. difficile in hospital A, although not statistically significant. The rate ratios for the combined data trended toward favoring discontinuation of CP with rate ratios of 0.80 (95% CI:0.62–1.04, p=0.09) for MRSA and 0.83 (95% CI: 0.66–1.06, p=0.14) for VRE.
Table 1:
Mean MRSA, VRE, and C. difficile LabID clinical culture rates (marker of healthcare associated infections) before and after discontinuing routine contact precautions for endemic MRSA and VRE.
| Hospital | Rate Before* | Rate After* | Rate Ratio | P-value | |
|---|---|---|---|---|---|
| MRSA | A | 0.43 (0.35–0.54) | 0.38 (0.31–0.48) | 0.88 (0.64–1.20) | 0.41 |
| B | 0.33 (0.23–0.48) | 0.25 (0.18–0.34) | 0.74 (0.46–1.21) | 0.23 | |
| Combined | 0.40 (0.33–0.48) | 0.32 (0.27–0.38) | 0.80 (0.62–1.04) | 0.09 | |
| VRE | A | 0.62 (0.52–0.74) | 0.58 (0.48–0.69) | 0.93 (0.72–1.20) | 0.58 |
| B | 0.17 (0.10–0.28) | 0.17 (0.12–0.25) | 1.04 (0.55–1.98) | 0.90 | |
| Combined | 0.48 (0.40–0.57) | 0.40 (0.34–0.47) | 0.83 (0.66–1.06) | 0.14 | |
| C. difficile | A | 11.53 (9.88–13.47) | 11.83 (10.18–13.76) | 1.03 (0.83–1.27) | 0.82 |
| B | 10.87 (8.70–13.60) | 9.51 (7.48–12.08) | 0.87 (0.63–1.21) | 0.42 | |
| Combined | 11.31 (9.96–12.85) | 11.06 (9.74–12.57) | 0.98 (0.82–1.17) | 0.81 |
Rates displayed with 95% confidence intervals.
Hospital A = Ronald Reagan UCLA Medical Center.
Hospital B = Santa Monica UCLA Medical Center.
Before = Before contact precautions were discontinued at each site.
After = After contact precautions were discontinued at each site.
Combined = Aggregated data from Ronald Reagan UCLA Medical Center and Santa Monica UCLA Medical Center.
Rates for MRSA and VRE are LabID clinical cultures per 100 admissions. Rate for C. difficile is LabID clinical cultures per 10,000 patient days.
Figure 1:
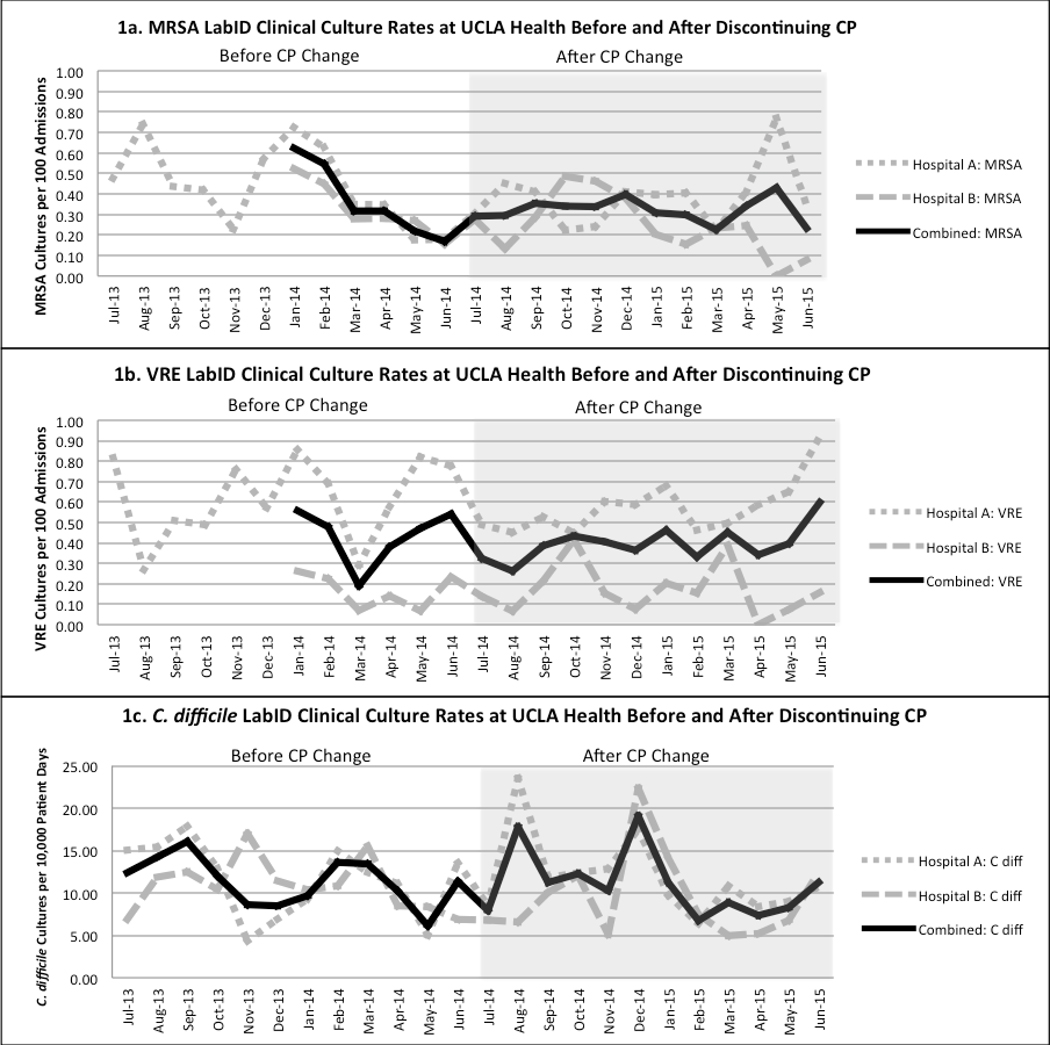
Graphs of the MRSA, VRE, and C. difficile LabID clinical culture rates (marker of healthcare associated infections) before and after discontinuing routine contact precautions for endemic MRSA and VRE*. Hospital A = Ronald Reagan UCLA Medical Center Hospital B = Santa Monica UCLA Medical Center Combined = Aggregated data from Ronald Reagan UCLA Medical Center and Santa Monica UCLA Medical Center. * Data not available from 7/2013 to 12/2013 for SMH for MRSA or VRE cultures.
There were higher overall rates in hospital A compared to B for both MRSA (p=0.015) and VRE (p<0.0001), but not C. difficile (p=0.17). An evaluation for interaction between hospital and before/after period was performed and was not statistically significant for any culture (data not show).
To evaluate the impact on microbial resistance, the percentage of Staphylococcus aureus clinical isolates resistant to methicillin (determined by oxacillin/cefoxitin resistance) and Enterococcus isolates resistant to vancomycin were compared from before and after CP were discontinued. There were no differences found (Table 2).
Table 2:
Comparison of percentage of all isolates positive for MRSA and VRE 1 year before and after the CP policy change.
| Before CP were discontinued |
After CP were discontinued |
P value | ||
|---|---|---|---|---|
| Staphylococcus aureus | % MRSA* | 37.0% | 40.0% | 0.26 |
| n | 699 | 672 | ||
| Enterococcus | % VRE † | 37.7% | 39.1% | 0.62 |
| n | 596 | 567 |
% MRSA = Percent of all Staphylococcus aureus clinical isolates found to be MRSA
% VRE = Percent of all Enterococcus clinical isolates found to be VRE
Data above is combined from both hospitals.
There was no change in percent positive MRSA screening in high-risk patients after CP per discontinued (Table 3). There was a trend toward fewer VRE positive screening tests in the post period, but this was based on a small number of tests and not statistically significant.
Table 3:
Comparison of percentages of positive surveillance screening for MRSA and VRE before and after the CP policy change.
| Before CP were discontinued | After CP were discontinued | P value | ||
|---|---|---|---|---|
| MRSA Nasal Swabs | % Positive | 4.5% | 4.9% | 0.255 |
| n | 11641 | 11543 | ||
| VRE Rectal Swabs | % Positive | 31.7% | 22.6% | 0.084 |
| n | 1045 | 84 |
There was a small increase in HH compliance in hospital A and decrease in HH compliance in hospital B after the policy change (Table 4). PPE compliance improved after CP were no longer required in hospital A from 64% to 74% (p<0.001), but did not change in hospital B.
Table 4:
Hand hygiene rates before and after CP policy change.
| Compliance Rate Before | Compliance Rate After | P value | |
|---|---|---|---|
| Hand Hygiene | |||
| Hospital A | 94% n=22,890 |
96% n=46,589 |
<0.001 |
| Hospital B | 88% n=1,772 |
84% n=2,013 |
<0.001 |
| PPE | |||
| Hospital A | 64% n=1,078 |
74% n=1,540 |
<0.001 |
| Hospital B | 56% n=185 |
50% n=151 |
0.33 |
Hospital A = Ronald Reagan UCLA Medical Center
Hospital B = Santa Monica UCLA Medical Center
PPE = Personal protective equipment (gown and gloves)
There was no change in 30-day readmissions or in-hospital mortality at either hospital (Supplementary Table 2). The combined length of stay was also unchanged, with an average of 5.71 days before and 5.85 days after (p=0.09).
Impact on Costs:
After MRSA/VRE CP were discontinued, isolation gown usage decreased, leading to cost savings of $729,572 (Table 5). CHG bathing was expanded to all units for additional cost of $85,796 per year. This led to overall cost savings of $643,776 per year.
Table 5:
Cost Analysis before and after the CP policy change.
| Cost Savings: | Monthly Cost Before: |
Monthly Cost After: |
Monthly Cost Difference: |
Yearly Cost Difference: |
|---|---|---|---|---|
| Gowns | $106,476 | $45,679 | $60,798 | $729,572 |
| Total Savings | $729,572 | |||
| Additional Costs: | ||||
| CHG Bathing | $16,476 | $23,626 | $7,150 | $85,796 |
| Additional Costs | $7,150 | $85,796 | ||
| Total Cost Savings: | $643,776 |
CHG = Chlorhexidine gluconate
In the ICU, nurses entered patient rooms on average 5.68 times per hour and 1.71 times per hour on medicine/surgery floors. Average PPE donning time was 38 (SD±11) seconds. Before the policy change, approximately 28.5% of ICU patients and 19% of medicine/surgery floor patients were on CP for MRSA and/or VRE (not including C. difficile or MDRO gram-negative infections).
Assuming a constant rate of room entries per hour by nurses and no difference in number of entries whether a patient is on CP or not, total nursing time spent in one year donning PPE for MRSA and VRE was over 45,000 hours. Using TDABC, the capacity cost per minute of nursing time was calculated, and used to estimate the value of time saved by reduction of nursing time donning PPE. This time was worth approximately $4.6 million (Table 6). While this is a sunk cost, and a reduction of labor expenses is not actually recorded, nursing time is freed to focus that quantity of effort on direct patient care.
Table 6:
Nursing time analysis before and after CP policy change.
| Total Beds |
% on CP Before * |
% on CP After * |
Nursing Room Entries per Hour |
Average Entry Time (sec) |
Total Hours per year |
Nursing Cost per Hour |
Total Sunk Cost |
|
|---|---|---|---|---|---|---|---|---|
| ICU | 176 | 28.5% | 0% | 5.68 | 38 | 26,333 | $99.60 | $2,622,727 |
| Med/Surg Floors | 629 | 19% | 0% | 1.71 | 38 | 18,944 | $105.00 | $1,989,124 |
| Total | 805 | 45,277 | $4,611,851 |
For MRSA and VRE only. Does not include C. difficile or multidrug resistant gram-negative organisms.
Discussion:
Although recent data suggest patient harms associated with CP, they remain common practice for MRSA and VRE.3–5 Widespread elimination of CP for MRSA and VRE has been hampered by the absence of published data on the impact this has on HAI rates.
Our study shows that following discontinuation of routine CP for endemic MRSA and VRE and expansion of CHG bathing to nearly all patients, there was no change in the marker of HAIs (LabID clinical culture rates) for MRSA and VRE after one year. Further, the 95% confidence intervals for the rate ratios are narrow and based on the upper limit of the interval, it is unlikely that the true effects could be an increase of more than 4% and 6% respectively.
One concern with our intervention is the impact on other HAIs that require CP to decrease transmission. Even though patients were still on spore precautions for C. difficile, there were overall fewer patients in the hospital on CP and a theoretical concern that this may lead to increases in C. difficile. This was not seen in our study.
There was also concern that not placing patients on CP for MRSA/VRE could lead to changes in resistance profiles of clinical isolates and higher percentages of MRSA and VRE relative to methicillin and vancomycin susceptible isolates. There was no change in percentages of resistant isolates after the policy change. Similarly, our study did not find a difference in MRSA colonization in high-risk patients, which is important given colonization is a risk factor for invasive MRSA infection.38
Although this study does not show an increase in possible HAI rates or surveillance cultures, it does not explain why and it may be due to several factors. First, our MRSA and VRE rates are low and may have decreased the transmission risk. It is unclear if these results are reproducible in hospitals with higher rates. Additionally, UCLA has single occupant patient rooms and near universal CHG bathing. These factors may have also decreased transmission risk. Given the increase in CHG bathing shortly before discontinuing CP, it is not possible to separate the impact of these two interventions. Further data are needed to determine which, if any, of these additional factors are required for success.
Numerous studies have shown that HH is a key factor in decreasing transmission of MDROs and our documented HH compliance rates are relatively high.39,40 Assuming the rates are accurate, the high compliance rates may have also decreased transmission risk and CP may not have provided any marginal benefit. Given discontinuing CP has not been tested at a hospital with a lower HH rate, the critical rate of HH compliance required to prevent a rise in HAI is unknown and further research is necessary. It is also possible that these rates are falsely elevated given the HCW were being observed and the true rates may actually be lower. While our data did not show a clear change in compliance, the new policy relies heavily on good HH and further data are necessary on whether compliance improves after HCW are not required to wear PPE for MRSA/VRE.
Another limitation of this study is that all of the analyses on impacts to cultures and burden of resistant organisms are at the population level. It was not possible to determine the impact on a single patient or hospital unit given not all patients are cultured for resistant organisms.
While these initial finding are encouraging, the data are limited to 2 institutions in a single health system and only one year of post data. Follow up data after one year and data from other hospitals are needed to ensure that MRSA and VRE rates do not creep up over time and to identify additional infection prevention strategies necessary for this to be successful and sustainable.
Another important impact of this policy change is on HCW time. Numerous studies have shown that HCW spend less time directly caring for patients on CP, likely due to the burden of donning PPE.18–23 Although it only took 38 seconds to don PPE correctly, this adds up to a substantial amount of time given how often patients are visited by HCW each day in an 805-bed health system. We estimated nursing time donning PPE over 1 year in our health system at approximately 45,000 hours, time worth an estimated $4.6 million. This time is now freed to provide other services including direct patient care.
There are limitations with the estimation for nursing time spent donning PPE. First, it assumes nurses are compliant with PPE every time, even though our PPE compliance rate was only 50–74%. The total donning time also assumes nurses enter rooms at a constant rate. This seems less likely given data that HCW enter CP rooms less frequently and rates likely differ depending on time of day.21 There may also be an observation bias. These factors could lead to an over-estimation of the donning time. This number, however, does not reflect all of the other providers that spend time donning gowns, including physicians, allied health workers, housekeeping, etc. Although data on total donning time is only an estimate, it does highlight that a significant amount of time is spent donning PPE, time perhaps better spent on other activities that can provide more benefit to patients.
This study showed that one year after discontinuing routine contact precautions for endemic MRSA and VRE and initiation of near universal CHG bathing, there was no increase in LabID clinical culture rates for MRSA or VRE, and the policy change provided significant cost savings on materials and HCW time. Given concerning data on patient harms and no clear benefit, discontinuing routine CP for MRSA and VRE may provide substantial benefit to patients and the health system in terms of cost savings and increased time for direct patient care.18–29 Further data are needed on the optimal hospital settings and horizontal infection prevention strategies needed for the discontinuation of CP to be successful. If CP are effective at preventing transmission of MRSA and VRE in hospitals, further data on which patient populations benefit most from the intervention would help limit universal use. Hospitals that continue to use CP for MRSA and VRE should implement strategies to mitigate the negative impact of CP on patients.
Supplementary Material
Key Points:
One year after discontinuing routine contact precautions for endemic MRSA and VRE and expanding CHG bathing, there was no increase in LabID clinical cultures for MRSA or VRE (marker of healthcare associated infections), and there was considerable savings on materials and healthcare worker time.
Acknowledgments:
Statistical collaboration supported by NIH/National Center for Advancing Translational Science (NCATS) UCLA CTSI Grant Number UL1TR000124. Michael Burke and Douglas Niedzwiecki assisted with TDABC analysis.
Acknowledgments/Funding:
The study was self-funded. Statistical collaboration was supported by NIH/National Center for Advancing Translational Science (NCATS) UCLA CTSI Grant Number UL1TR000124.
The data in this manuscript were presented in part at SHEA Spring 2015: Science Guiding Prevention, Orlando, FL, May 14–17, 2015 (Abstract #6820). The data presented at SHEA was limited to 6 months of post data from 1 hospital (Ronald Reagan UCLA Medical Center).
Appendix:
Hand Hygiene Observation Protocol:
UCLA Health has a volunteer based patient safety program that performs audits of both hand hygiene (HH) and use of personal protective equipment (PPE) in our hospitals. Each volunteer undergoes an application process and then training by a senior member of the team on the HH and PPE policies. Next, the volunteer performs audits under the supervision of a senior member of the team and then they are able to preform audits on their own. The two program leads preform interrater reliability to make sure training is consistent. HH compliance is washing ones hands with soap and water for 15 seconds or use of an alcohol based hand rub. PPE compliance is wearing both gloves and a gown tied behind the head and back. Observations are performed on all shifts, including nights and weekends. They are performed in all units in hospital A and primarily in the emergency room and the intensive care unit in hospital B. Each volunteer collects data for approximately 4 hours per week and collects data on 2 units per shift.
Footnotes
None of the authors have a conflict of interest.
References:
- 1.Calfee DP, Salgado CD, Milstone AM, et al. Strategies to prevent methicillin-resistant Staphylococcus aureus transmission and infection in acute care hospitals: 2014 update. Infect Control Hosp Epidemiol 2014;35:772–796. [DOI] [PubMed] [Google Scholar]
- 2.Muto CA, Jernigan JA, Ostrowsky BE, et al. SHEA guideline for preventing nosocomial transmission of multidrug-resistant strains of Staphylococcus aureus and enterococcus. Infect Control Hosp Epidemiol 2003;24:362–386. [DOI] [PubMed] [Google Scholar]
- 3.Morgan DJ, Murthy R, Munoz-Price LS, et al. Reconsidering Contact Precautions for Endemic Methicillin-Resistant Staphylococcus aureus and Vancomycin-Resistant Enterococcus. Infect Control Hosp Epidemiol 2015;36:1163–1172. [DOI] [PubMed] [Google Scholar]
- 4.Fatkenheuer G, Hirschel B, Harbarth S. Screening and isolation to control meticillin-resistant Staphylococcus aureus: sense, nonsense, and evidence. Lancet 2015;385:1146–1149. [DOI] [PubMed] [Google Scholar]
- 5.Morgan DJ, Kaye KS, Diekema DJ. Reconsidering isolation precautions for endemic methicillin-resistant Staphylococcus aureus and vancomycin-resistant Enterococcus. JAMA 2014;312:1395–1396. [DOI] [PubMed] [Google Scholar]
- 6.Bearman GM, Marra AR, Sessler CN, et al. A controlled trial of universal gloving versus contact precautions for preventing the transmission of multidrug-resistant organisms. Am J Infect Control 2007;35:650–655. [DOI] [PubMed] [Google Scholar]
- 7.Derde LP, Cooper BS, Goossens H, et al. Interventions to reduce colonisation and transmission of antimicrobial-resistant bacteria in intensive care units: an interrupted time series study and cluster randomised trial. Lancet Infect Dis 2014;14:31–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Harbarth S, Fankhauser C, Schrenzel J, et al. Universal screening for methicillin-resistant Staphylococcus aureus at hospital admission and nosocomial infection in surgical patients. JAMA 2008;299:1149–1157. [DOI] [PubMed] [Google Scholar]
- 9.Harris AD, Pineles L, Belton B, et al. Universal glove and gown use and acquisition of antibiotic-resistant bacteria in the ICU: a randomized trial. JAMA 2013;310:1571–1580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Huang SS, Yokoe DS, Hinrichsen VL, et al. Impact of routine intensive care unit surveillance cultures and resultant barrier precautions on hospital-wide methicillin-resistant Staphylococcus aureus bacteremia. Clin Infect Dis 2006;43:971–978. [DOI] [PubMed] [Google Scholar]
- 11.Huskins WC, Huckabee CM, O’Grady NP, et al. Intervention to reduce transmission of resistant bacteria in intensive care. N Engl J Med 2011;364:1407–1418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jain R, Kralovic SM, Evans ME, et al. Veterans Affairs initiative to prevent methicillin-resistant Staphylococcus aureus infections. N Engl J Med 2011;364:1419–1430. [DOI] [PubMed] [Google Scholar]
- 13.Lucet JC, Paoletti X, Lolom I, et al. Successful long-term program for controlling methicillin-resistant Staphylococcus aureus in intensive care units. Intensive Care Med 2005;31:1051–1057. [DOI] [PubMed] [Google Scholar]
- 14.Marshall C, Richards M, McBryde E. Do active surveillance and contact precautions reduce MRSA acquisition? A prospective interrupted time series. PLoS One 2013;8:e58112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Robicsek A, Beaumont JL, Paule SM, et al. Universal surveillance for methicillin-resistant Staphylococcus aureus in 3 affiliated hospitals. Ann Intern Med 2008;148:409–418. [DOI] [PubMed] [Google Scholar]
- 16.Safdar N, Marx J, Meyer NA, Maki DG. Effectiveness of preemptive barrier precautions in controlling nosocomial colonization and infection by methicillin-resistant Staphylococcus aureus in a burn unit. Am J Infect Control 2006;34:476–483. [DOI] [PubMed] [Google Scholar]
- 17.De Angelis G, Cataldo MA, De Waure C, et al. Infection control and prevention measures to reduce the spread of vancomycin-resistant enterococci in hospitalized patients: a systematic review and meta-analysis. J Antimicrob Chemother 2014;69:1185–1192. [DOI] [PubMed] [Google Scholar]
- 18.Dashiell-Earp CN, Bell DS, Ang AO, Uslan DZ. Do physicians spend less time with patients in contact isolation?: a time-motion study of internal medicine interns. JAMA Intern Med 2014;174:814–815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Evans HL, Shaffer MM, Hughes MG, et al. Contact isolation in surgical patients: a barrier to care? Surgery 2003;134:180–188. [DOI] [PubMed] [Google Scholar]
- 20.Masse V, Valiquette L, Boukhoudmi S, et al. Impact of methicillin resistant Staphylococcus aureus contact isolation units on medical care. PLoS One 2013;8:e57057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Morgan DJ, Pineles L, Shardell M, et al. The effect of contact precautions on healthcare worker activity in acute care hospitals. Infect Control Hosp Epidemiol 2013;34:69–73. [DOI] [PubMed] [Google Scholar]
- 22.Saint S, Higgins LA, Nallamothu BK, Chenoweth C. Do physicians examine patients in contact isolation less frequently? A brief report. Am J Infect Control 2003;31:354–356. [DOI] [PubMed] [Google Scholar]
- 23.Stelfox HT, Bates DW, Redelmeier DA. Safety of patients isolated for infection control. JAMA 2003;290:1899–1905. [DOI] [PubMed] [Google Scholar]
- 24.Gilligan P, Quirke M, Winder S, Humphreys H. Impact of admission screening for methicillin-resistant Staphylococcus aureus on the length of stay in an emergency department. J Hosp Infect 2010;75:99–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.McLemore A, Bearman G, Edmond MB. Effect of contact precautions on wait time from emergency room disposition to inpatient admission. Infect Control Hosp Epidemiol 2011;32:298–299. [DOI] [PubMed] [Google Scholar]
- 26.Goldszer RC TE, Yokoe DS, Shadick N, Bardon CG, Johnson PA, Hogan J, Kahlert T, Whittermore A. A program to remove patients from unnecessary contact precautions. Journal of Clinical Outcomes Management 2002;9:553–556. [Google Scholar]
- 27.Karki S, Leder K, Cheng AC. Patients under contact precautions have an increased risk of injuries and medication errors: a retrospective cohort study. Infect Control Hosp Epidemiol 2013;34:1118–1120. [DOI] [PubMed] [Google Scholar]
- 28.Catalano G, Houston SH, Catalano MC, et al. Anxiety and depression in hospitalized patients in resistant organism isolation. South Med J 2003;96:141–145. [DOI] [PubMed] [Google Scholar]
- 29.Day HR, Morgan DJ, Himelhoch S, Young A, Perencevich EN. Association between depression and contact precautions in veterans at hospital admission. Am J Infect Control 2011;39:163–165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mehrotra P, Croft L, Day HR, et al. Effects of contact precautions on patient perception of care and satisfaction: a prospective cohort study. Infect Control Hosp Epidemiol 2013;34:1087–1093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Croft LD, Liquori M, Ladd J, et al. The Effect of Contact Precautions on Frequency of Hospital Adverse Events. Infect Control Hosp Epidemiol 2015;36:1268–1274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Edmond MB, Masroor N, Stevens MP, Ober J, Bearman G. The Impact of Discontinuing Contact Precautions for VRE and MRSA on Device-Associated Infections. Infect Control Hosp Epidemiol 2015;36:978–980. [DOI] [PubMed] [Google Scholar]
- 33.Surveillance for C. difficile, MRSA and other drug-resistant infections. Multidrug-resistant organism and Clostridium difficile infection (MDRO/CDI) module protocol. 2015. http://www.cdc.gov/nhsn/PDFs/pscManual/12pscMDRO_CDADcurrent.pdf Accessed July 29, 2015, 2015.
- 34.California Senate Bill No. 158. An act to amend Sections 1288.5 and 1288.8 of, and to add Sections 1279.6, 1279.7, 1288.45 and 1288.95 to, the Health and Safety Code, relating to health facilities. In: Senate C, ed. 1582008. [Google Scholar]
- 35.California Senate Bill No. 1058. An act to add Sections 1255.8 and 1288.55 to the Health and Safety Code, relating to health. In: Senate C, ed. 10582008. [Google Scholar]
- 36.Kaplan RS, Anderson SR. Time-driven activity-based costing. Harv Bus Rev 2004;82:131–138, 150. [PubMed] [Google Scholar]
- 37.Kaplan RS, Porter ME. How to solve the cost crisis in health care. Harv Bus Rev 2011;89:46–52, 54, 56–61 passim. [PubMed] [Google Scholar]
- 38.Huang SS, Platt R. Risk of methicillin-resistant Staphylococcus aureus infection after previous infection or colonization. Clin Infect Dis 2003;36:281–285. [DOI] [PubMed] [Google Scholar]
- 39.Larson E A causal link between handwashing and risk of infection? Examination of the evidence. Infect Control Hosp Epidemiol 1988;9:28–36. [DOI] [PubMed] [Google Scholar]
- 40.WHO Guidelines on Hand Hygiene in Health Care: First Global Patient Safety Challenge Clean Care Is Safer Care. Geneva2009. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


