Abstract
Background:
In the biopsychosocial formulation of functional neurological (conversion) disorder (FND), little is known about relationships between social behavior and brain anatomy. We hypothesized that social behavior would relate to brain areas implicated in affiliative behaviors and that social network size would correlate with symptom severity and predisposing vulnerabilities in FND.
Methods:
This neuroimaging pilot probed how social network size, as measured by the Social Network Index, related to structural brain profiles in 23 patients with motor FND (15 woman and 8 men). FreeSurfer cortical thickness and subcortical volumetric analyses were performed correcting for multiple comparisons. Stratified analyses compared FND patients with a low social network size to matched healthy controls. Secondary exploratory analyses in an expanded sample of 38 FND patients investigated relationships between social network size, risk factors and patient-reported symptom severity.
Results:
Adjusting for age and gender, neuroimaging analyses showed that social network size positively correlated with left nucleus accumbens and hippocampal volumes in patients with FND; stratified analyses did not show any group-level differences. In individuals with FND, social network size correlated with health-related quality of life, graduating college, working full-time and a non-epileptic seizure diagnosis; social network size inversely related to lifetime trauma burden, post-traumatic stress disorder severity and age.
Limitations:
Only patient-reported scales were used and social network size information was not collected for healthy subjects.
Conclusions:
This neuroimaging pilot adds to the literature linking affiliation network brain areas to pro-social behaviors and enhances the biopsychosocial conceptualization of FND.
1. Introduction
The biopsychosocial model is a leading framework through which to conceptualize functional neurological (conversion) disorder (FND)(Pick et al., 2018). Social behavior is a health determinant, and evidence suggests that in neuropsychiatric populations enhanced social networks aid recovery and adherence to therapeutic interventions(Dhand et al., 2016; Dhand et al., 2018). Although FND is highly prevalent and disabling, little is known about social functioning in this population(Vaidya-Mathur et al., 2016). Given the importance of the biopsychosocial model for treatment planning, this is an important gap in the literature.
Although brain-social behavior relationships in FND have yet to be investigated, links between social functioning and neural networks have been studied in healthy individuals and other neuropsychiatric disorders. For example, social network size in healthy subjects correlated with amygdalar volume(Bickart et al., 2011), and individual differences in the connectivity strengths of brain networks associated with affiliative and perceptual-related social cognition positively correlated with the ability to develop and maintain social networks(Bickart et al., 2012). Similarly, social network size has been shown to correlate with increased coupling between the anterior cingulate cortex and structures of the default mode network in healthy subjects(Noonan et al., 2018). In frontotemporal dementia, atrophy in mesolimbic, reward-related (affiliation) brain areas showed a strong relationship with socioemotional detachment(Bickart et al., 2014). Predisposing vulnerabilities for FND (e.g. depression, anxiety, post-traumatic stress disorder (PTSD) etc.) are also linked to impaired social functioning(Charuvastra and Cloitre, 2008; Kupferberg et al., 2016), underscoring the importance of characterizing relationships across social and psychiatric domains in this population.
In this neuroimaging pilot study, we investigated associations between structural brain profiles and social network size in 23 patients with motor FND. We hypothesized that individual differences in structural profiles within brain areas implicated in pro-social behavior (affiliation) would correlate with social network size, and that the FND subgroup with low social network size would exhibit group-level structural alternations when compared to matched healthy controls. In secondary exploratory analyses, we examined correlations between social network size, patient-reported symptom severity, and predisposing vulnerabilities in a larger sample of patients with FND with available social network size data (n=38). Symptom severity, impaired physical and mental health, trauma burden, poor stress coping, maladaptive personality traits, and insecure attachment were hypothesized to negatively correlate with social network size. Of note, data from this cohort has been previously used to investigate structural brain-symptom severity and brain-disease risk relationships in FND(Perez et al., 2017a; Perez et al., 2018; Perez et al., 2017b; Williams et al., 2018); none of these prior studies investigated brain-social behavior relationships.
2. Methods
2.1. Subjects
The study was approved by the Institutional Review Board of Partners Healthcare. We recruited 38 patients with motor FND (25 women, 13 men; age: 40.2±14.2 years; average illness duration: 3.1±3.7 years). Sixteen met diagnostic criteria for clinically-established functional movement disorders, 12 had rule-in signs of functional weakness, and 19 had psychogenic nonepileptic seizures (PNES; 17 documented, 1 clinically-established, 1 probable)(American Psychiatric Association, 2013; LaFrance et al., 2013). FND diagnoses were not mutually exclusive with nine exhibiting mixed symptoms. Of 38 subjects with social network size data, 23 (15 women, 8 men; mean age 37.8±14.0) completed brain magnetic resonance imaging (MRI) data collection. Participant details can be found in Supplementary Table 1.
Exclusion criteria included lack of stated fluency in the English language, illiteracy, history of mania or psychosis, intellectual disability, active suicidality, current alcohol dependence, or illicit drug abuse. Seven had major neurological disorders or comorbid epilepsy. All of these subjects were excluded from MRI scans. Eight additional subjects did not participate in the MRI portion of the study for the following reasons: abnormal head movements at rest (n=2), possible pregnancy (n=1), MRI contraindication (n=3), meeting criteria for probable PNES only (n=1), and claustrophobia (n=1). All individuals scanned did not have any comorbid major neurological conditions with known central nervous system effects. 23 matched healthy controls (15 woman, 8 men, age 39.0±13.8 years) were recruited through advertisements as a neuroimaging comparison group. Lifetime psychiatric comorbidities across all subjects were assessed using the Structured Clinical Interview (SCID-I) for DSM-IV-TR.
2.2. Psychometric measures
Social network size was calculated using the Social Network Index (SNI), a 13-item self-report questionnaire that polls the number of persons a subject interacts with at least twice a week across 12 different roles (e.g. spouse, parents, friends, work, other social groups)(Cohen et al., 1997). An example item includes “How many close friends do you have? (meaning people that you feel at ease with, can talk to about private matters, and can call on for help)” and “How many of these friends do you see or talk to at least once every 2 weeks?”. The latter question is scored on a scale ranging from zero to seven or more individuals. We additionally collected a psychometric battery assessing patient-reported symptom severity and the spectrum of predisposing vulnerabilities for the development of FND including(Jalilianhasanpour et al., 2018): Screening for Somatoform Symptoms Conversion Disorder Subscale scale (SOMS:CD), Patient Health Questionnaire-15 (PHQ-15), Somatoform Dissociation Questionnaire-20 (SDQ), Short Form Health Survey (SF-36), RSQ, Relationship Scales Questionnaire, Dissociative Experiences Scale (DES), PTSD Checklist for DSM-5 (PTSD:PCL5), Childhood Trauma Questionnaire (CTQ), Life Events Checklist (LEC), Beck Depression lnventory-II (BDI-II), Spielberger State Trait Anxiety Inventory (STAI), Toronto Alexithymia Score (TAS), NEO Five Factor Inventory (NEO), Relationship Scales Questionnaire (RSQ), and the Connor-Davidson Resilience Scale (CD-RISC).
2.3. Descriptive Psychometric Analyses
We used nonparametric tests (Spearman correlation coefficients or Mann Whitney U) to identify statistically significant univariate relationships between social network size and neuropsychiatric variables. Analyses were performed in IBM SPSSv24 (Chicago).
2.4. MRI acquisition and preprocessing
Subjects were placed in a Siemens 3 T Trio Scanner to acquire a 3D T1-weighted magnetization prepared rapid acquisition gradient echo sequence. MRI sequence acquisition and FreeSurfer 6.0 image preprocessing methods were employed as previously reported (Perez et al., 2018; Williams et al., 2018).
2.5. FreeSurfer analyses
For within-group surface-based structural analyses, a general linear model evaluated the effect of social network size on cortical thickness, adjusting for age and gender. A statistical significance of p<0.05 corrected for multiple comparisons at the cluster-wise level was used. Subcortical volumes were calculated using the FreeSurfer segmentation pipeline and normalized for total intracranial volume at the subject-level. Linear regression analyses evaluated the relationship between social network size and subcortical volumes adjusting for age, gender, and correcting for multiple comparisons using Bonferroni with α=0.05.
Following identification of statistically significant within-group findings, separate post-hoc analyses were performed adjusting for: 1) depression and anxiety (BDI-II and STAI-trait anxiety scores); 2) selective serotonin reuptake inhibitors (SSRI) and/or serotonin-norepinephrine reuptake inhibitors (SNRI) use; and 3) motor FND subtype.
Finally, to contextualize within-group findings, similar to prior approaches by our group(Perez et al., 2018; Perez et al., 2017b), a stratified logistic regression was performed to compare structural differences between FND subjects in the lower 50% of social network size vs. healthy controls.
3. Results
3.1. Relationships between social network size and other predisposing vulnerabilities
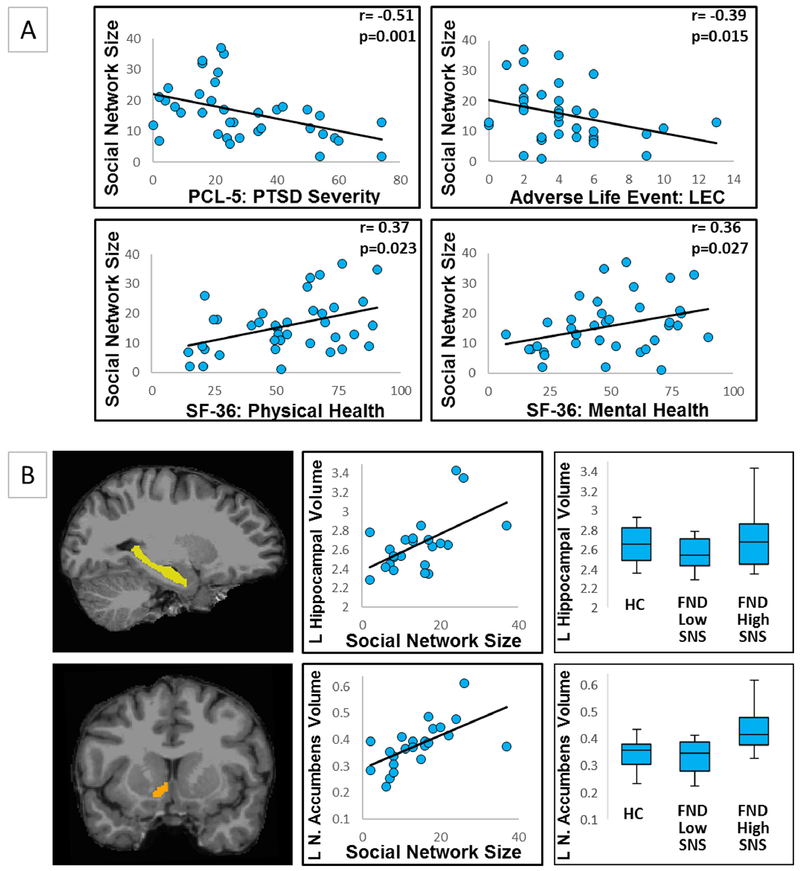
In patients with FND, mean social network size was 15.8±9.1 individuals. In univariate analyses, social network size positively correlated with SF-36 physical health (r=0.37, p=0.023), SF-36 mental health (r=0.36, p=0.027), working/studying full-time (p=0.002), having graduated college (p=0.001), and PNES vs other motor FND subtypes (p=0.034). Social network size negatively correlated with age (r=−0.37, p=0.024), PCL5-PTSD severity (r=−0.51, p=0.001), and lifetime trauma burden (LEC) (r=−0.39, p=0.015) (Figure 1). The psychometric scores were highly inter-related as shown in the Supplementary Table 2 correlation matrix, and thus a second level regression analysis was not performed.
Figure 1.
Panel A - univariate relationships between social network size and other predisposing vulnerabilities in motor functional neurologic disorders (FND). Displayed are the four statistically significant psychometric correlations with social network size (as measured by the Social Network Index) in patients with FND. LEC, Life Events Checklist “Happened to Me” score; SF-36, Short Form Health Survey-36; PCL-5: PTSD, Post-Traumatic Stress Disorder Checklist for DSM-5. Panel B - individual differences in left hippocampal (pcorrected =0.009) and nucleus accumbens (pcorrected =0.046) volumes correlated with social network size in patients with motor FND. For visual display purposes only, scatter plots of normalized hippocampal and nucleus accumbens volumes are plotted against individual social network size scores. Note: normalized volumes were multiplied by 1000 for ease of viewing. Box and whisker plots display normalized left hippocampal and nucleus accumbens volumes for the 12 patients with FND reporting the lowest 50% social network size (FND Low SNS), the other 11 patients with FND (FND High SNS), and 23 healthy controls. SNS indicated social network size; HC, healthy controls; L, left.
3.2. Neuroimaging findings
In MRI analyses, social network size positively correlated with left hippocampal (pcorrected=0.009) and nucleus accumbens (pcorrected=0.046) volumes. In post-hoc analyses, the relationship between social network size and left hippocampal volume remained significant adjusting separately for depression and trait anxiety (pcorrected=0.009), SSRI/SNRI use (pcorrected=0.008), and PNES vs other motor FND subtypes (pcorrected=0.012). The association between social network size and left nucleus accumbens volume remained significant adjusting for PNES vs other motor FND subtype (pcorrected=0.016) but did not hold when controlling for depression and trait anxiety (pcorrected=0.064) or SSRI/SNRI use (pcorrected=0.056).
In a stratified regression analysis performed in the lower 50% (N=12) of FND patients with social network size vs controls, there were no between-group cortical or subcortical differences.
4. Discussion
In this MRI study, individual differences in social network size positively correlated with left hippocampal and nucleus accumbens volumetric profiles in patients with FND. These brain–social behavior relationships are consistent with previous findings from Bickart and colleagues and can be contextualized through the cognitive-affective neuroscience literature(Bickart et al., 2014; Bickart et al., 2012; Bickart et al., 2011). From a network perspective, our findings localize to nodes within the amygdalar-based affiliation network that includes the ventromedial prefrontal cortex, anterior cingulate, medial temporal lobe, dorsal temporal pole, nucleus accumbens and hippocampus. This network is implicated in pro-social behavior, with stronger functional connectivity across these brain areas predicting social network size in healthy patients(Bickart et al., 2012; Bickart et al., 2011). Furthermore, studies in FND populations and related conditions have found alterations in these two areas. For example, individuals with functional dystonia(Tomic et al., 2018) and chronic pain(Geha et al., 2008) showed reduced nucleus accumbens volume compared to controls. In research previously conducted in this FND cohort, hippocampal volumes negatively correlated with trauma burden and maladaptive coping tendencies(Perez et al., 2017a; Williams et al., 2018). Others have shown that patients with FND display reduced hippocampal activity when processing distressing life experiences(Aybek et al., 2014). Moreover, recent research suggests that the hippocampus plays important roles in the ability to integrate information flexibly(Montagrin et al., 2018) and in modulating negative affect, functions that aid positive social interactions. The nucleus accumbens (and ventral striatum more broadly) is linked to value (reward) based decision-making and reduced processing of pleasurable context in major depression(Epstein et al., 2006). Notably, patients with major depression exhibit reduced social networks(Visentini et al., 2018), and social support in patients with PTSD promotes positive clinical outcomes(Ozer et al., 2003). Thus, our study advances our dimensional understanding of brain-social behavior relationships that likely cuts across several neuropsychiatric disorders.
With regards to the psychometric data, social network size correlated with mental health and negatively correlated with lifetime adverse event burden and PTSD severity. Social networks are known to partially mediate the relationship of adverse life events and psychiatric comorbidities(McLafferty et al., 2018). A study of patients with PNES and comorbid PTSD found an increased tendency to use maladaptive, emotion-focused coping strategies(Zeng et al., 2018), which could impair social behavior. Our study also found that patients with PNES had modestly larger social networks compared to other motor FND subtypes, which is consistent with a previous study that examined socialization preferences in individuals with PNES(Vaidya-Mathur et al., 2016). That study found, contrary to common perception, that patients with PNES reported considerable participation in diverse social activities. This highlights possible FND subtype-specific differences in social behaviors, and suggests that relationships between FND, psychiatric comorbidities and social behavior are likely complex and multi-layered. Future research investigating potential links between social behavior, treatment engagement and clinical outcomes may be a fruitful area of inquiry.
Given that physical health is a primary concern for patients with functional motor symptoms, it is notable that patients with larger social networks reported better overall physical health-related quality of life in our study. This is consistent with previous findings in non-clinical populations linking physical health to larger social networks and more robust support systems(Ho et al., 2018; Kauppi et al., 2017). Consistent with the Berkman social network structure model, having a larger social network may provide patients with more opportunities to receive social support which can positively promote physical well-being(Berkman et al., 2000). We speculate that in those FND patients who are relatively socially isolated, social interventions designed to increase one’s support structure, including the use of support groups, could be beneficial in promoting physical health improvements. Examples of such interventions that could be studied include the potential utility of participating in online FND forums as well as mechanisms of efficacy for group psychotherapy. Future large-scale research studies should also be conducted to directly access the relationship between social support and physical health in patients with FND. Furthermore, patients with FND who had graduated college and/or were working or studying full-time reported having a larger social network, while older patients reported smaller networks consistent with findings in healthy adults(Sander et al., 2017).
Limitations include the small sample size, heterogenous motor FND subtypes, lack of SNI data in controls, and a reliance on self-report measures. Detailed neuropsychological testing would have also aided the identification of links between social behavior and cognitive performance. While the use of a transdiagnostic approach to the study of FND remains debated(Kanaan et al., 2017), we have previously shown similarities across motor FND subtypes supporting the utility of this approach(Jalilianhasanpour et al., 2018). Additionally, social network size does not comprehensively account for social support and other important factors, highlighting the utility of other quantitative tools for social network analyses(Dhand et al., 2018). Given the modest sample size, caution should be taken to not over-interpret the negative findings of this study, including that future research should continue to investigate potential associations between illness duration, age of onset, personality factors and other clinical variables as they may relate to social behavior in FND. It also remains unclear if the development of FND promotes subsequent changes in social behavior, or if specific patterns of socializing are a contributing vulnerability. Prospective longitudinal studies are needed to investigate potential links between clinical outcomes and social functioning.
In conclusion, this pilot study provides preliminary evidence that social network size positively correlated with physical and mental health status in patients with FND. We also observed that volumetric profiles in the nucleus accumbens and hippocampus, regions of the affiliation network, related to social behavior in this FND cohort. These results extend findings published in healthy individuals and neurodegenerative disorders. More research is needed to comprehensively characterize social behaviors in FND to aid patient-centered biopsychosocial conceptualizations and interventions.
Supplementary Material
Table 1.
Neuropsychiatric factors associated with social network size in motor functional neurological disorders.
| FND (n=38) | Test Statistics | P-Value | |
|---|---|---|---|
| Age | 40.2±14.2 | −0.37 | 0.024 |
| Female (n=25) vs. Male (n=13) | 14.8 ± 8.1 vs. 17.5 ±10.9 | 150.0 | 0.70 |
| White (n=33) vs. non-white (n=5) | 16.3 ± 9.5 vs. 12.2 ± 5.8 | 60.0 | 0.33 |
| Married (n=17) vs. other (21) | 15.8 ± 9.9 vs. 15.7 ± 8.7 | 169.0 | 0.78 |
| College graduate (n=14) vs. non-college graduate (n=24) | 20.9 ± 7.3 vs. 12.8 ± 8.8 | 61.0 | 0.0011 |
| Working full time or student (n=14) vs. other (n=24) | 20.6 ± 6.8 vs. 12.9 ± 9.2 | 67.5 | 0.0023 |
| PNES (n=19) vs. Other motor FND (n=19) | 18.9 ± 9.0 vs. 12.6 ± 8.3 | 108.0 | 0.034 |
| Illness duration (years) | 3.1 ±3.7 | −0.14 | 0.38 |
| SOMS: CD | 7.0±6.4 | −0.16 | 0.33 |
| PHQ-15 | 12.4±4.6 | 0.12 | 0.49 |
| SDQ | 30.6±8.4 | −0.022 | 0.90 |
| SF-36 mental health | 49.0±21.7 | 0.36 | 0.027 |
| SF-36 physical health | 53.8±22.5 | 0.37 | 0.023 |
| DES | 18.0±12.5 | 0.047 | 0.78 |
| PCL 5:PTSD* | 29.3±20.2 | −0.51 | 0.0014 |
| LEC “happened to me” | 4.2±2.7 | −0.39 | 0.015 |
| CTQ-abuse | 27.8±13.5 | −0.087 | 0.60 |
| CTQ-neglect | 20.0±8.4 | 0.13 | 0.45 |
| BDI-II | 17.6±10.9 | −0.22 | 0.18 |
| STAI-trait anxiety | 46.4±10.1 | −0.16 | 0.33 |
| TAS | 59.2±12.8 | 0.12 | 0.49 |
| NEO-neuroticism | 26.4±7.8 | −0.32 | 0.052 |
| NEO-extraversion | 24.4±6.9 | 0.23 | 0.17 |
| NEO-conscientiousness | 31.1±7.1 | 0.12 | 0.46 |
| NEO-agreeableness | 34.9±5.0 | −0.084 | 0.62 |
| NEO-openness | 25.5±6.0 | 0.08 | 0.63 |
| RSQ secure | 2.9±0.6 | 0.10 | 0.54 |
| RSQ fearful | 2.8±1.1 | −0.09 | 0.59 |
| RSQ preocuppied | 2.6±0.6 | 0.02 | 0.90 |
| RSQ dismissive | 3.3±0.6 | −0.07 | 0.67 |
| CD-RISC | 61.8±16.6 | 0.17 | 0.31 |
Test statistic refers to Spearman correlation for continuous variables or to Mann Whitney U coefficient for dichotomous variables.
Indicates that one subject had missing data for the PTSD: PCL 5. BDI, Beck Depression Inventory-II; CD-RISC, Connor-Davidson Resilience Scale; CTQ, Childhood Trauma Questionnaire; DES, Dissociative Experiences Scale; FND, Functional Neurological Disorder; LEC, Life Events Checklist; NEO, NEO Five Factor Inventory. PHQ-15, Patient Health Questionnaire-15; PNES, Psychogenic Non-Epileptic Seizures; PTSD: PCL 5, Post-Traumatic Stress Disorder Checklist for DSM-5; RSQ, Relationship Scales Questionnaire; SDQ, Somatoform Dissociation Questionnaire-20; SF-36, Short Form Health Survey-36; SOMS: CD, Conversion Disorder Subscale of the Screening for Somatoform Symptoms-7 scale; SSRI/SNRI, selective serotonin reuptake inhibitors and/or serotonin-norepinephrine reuptake inhibitors; STAI, Spielberger State Trait Anxiety Inventory; TAS, Toronto Alexithymia Score.
Highlights.
Social network size in FND correlated with hippocampal and nucleus accumbens volume
Social network size in FND positively correlated with mental and physical health
This pilot study adds to the biopsychosocial understanding of patients with FND
Acknowledgements:
Funding:
D.L.P. was funded by the National Institute of Mental Health Grant K23MH111983-02, Massachusetts General Hospital Physician-Scientist Development Award, and the Sidney R. Baer Jr. Foundation.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts of Interest/Disclosures:
B.C.D., consultant at Merck, Med Learning Group and Haymarket; royalties from Oxford University Press and Cambridge University Press; on the editorial board of Neuroimage: Clinical, Cortex, Hippocampus, Neurodegenerative Disease Management. A.D. received honoraria from Harvard Medical School, Henry Ford Hospital, and Boston College. D.L.P., received honoraria from Harvard Medical School, the American Academy of Neurology, Movement Disorder Society, and Toronto Western Hospital. All other authors report no conflicts of interest.
References
- American Psychiatric Association, 2013. Diagnostic and statistical manual of mental disorders (DSM-5), 5th edition ed. American Psychiatric Pub, Washington, DC. [Google Scholar]
- Aybek S, Nicholson TR, Zelaya F, O’Daly OG, Craig TJ, David AS, Kanaan RA, 2014. Neural correlates of recall of life events in conversion disorder. JAMA Psychiatry 71, 52–60. [DOI] [PubMed] [Google Scholar]
- Berkman LF, Glass T, Brissette I, Seeman TE, 2000. From social integration to health: Durkheim in the new millennium. Social science & medicine (1982) 51, 843–857. [DOI] [PubMed] [Google Scholar]
- Bickart KC, Brickhouse M, Negreira A, Sapolsky D, Barrett LF, Dickerson BC, 2014. Atrophy in distinct corticolimbic networks in frontotemporal dementia relates to social impairments measured using the Social Impairment Rating Scale. J Neurol Neurosurg Psychiatry 85, 438–448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bickart KC, Hollenbeck MC, Barrett LF, Dickerson BC, 2012. Intrinsic amygdala-cortical functional connectivity predicts social network size in humans. The Journal of neuroscience : the official journal of the Society for Neuroscience 32, 14729–14741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bickart KC, Wright CI, Dautoff RJ, Dickerson BC, Barrett LF, 2011. Amygdala volume and social network size in humans. Nature neuroscience 14, 163–164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charuvastra A, Cloitre M, 2008. Social bonds and posttraumatic stress disorder. Annual review of psychology 59, 301–328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen S, Doyle WJ, Skoner DP, Rabin BS, Gwaltney JM Jr., 1997. Social ties and susceptibility to the common cold. Jama 277, 1940–1944. [PubMed] [Google Scholar]
- Dhand A, Luke DA, Lang CE, Lee JM, 2016. Social networks and neurological illness. Nature reviews. Neurology 12, 605–612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhand A, White CC, Johnson C, Xia Z, De Jager PL, 2018. A scalable online tool for quantitative social network assessment reveals potentially modifiable social environmental risks. Nat Commun 9, 3930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epstein J, Pan H, Kocsis JH, Yang Y, Butler T, Chusid J, Hochberg H, Murrough J, Strohmayer E, Stern E, Silbersweig DA, 2006. Lack of ventral striatal response to positive stimuli in depressed versus normal subjects. The American journal of psychiatry 163, 1784–1790. [DOI] [PubMed] [Google Scholar]
- Geha PY, Baliki MN, Harden RN, Bauer WR, Parrish TB, Apkarian AV, 2008. The brain in chronic CRPS pain: abnormal gray-white matter interactions in emotional and autonomic regions. Neuron 60, 570–581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho EC, Hawkley L, Dale W, Waite L, Huisingh-Scheetz M, 2018. Social capital predicts accelerometry-measured physical activity among older adults in the U.S.: a cross-sectional study in the National Social Life, Health, and Aging Project. BMC public health 18, 804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jalilianhasanpour R, Williams B, Gilman I, Burke MJ, Glass S, Fricchione GL, Keshavan MS, LaFrance WC Jr., Perez DL, 2018. Resilience linked to personality dimensions, alexithymia and affective symptoms in motor functional neurological disorders. J Psychosom Res 107, 55–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanaan RAA, Duncan R, Goldstein LH, Jankovic J, Cavanna AE, 2017. Are psychogenic non-epileptic seizures just another symptom of conversion disorder? J Neurol Neurosurg Psychiatry 88, 425–429. [DOI] [PubMed] [Google Scholar]
- Kauppi M, Elovainio M, Stenholm S, Virtanen M, Aalto V, Koskenvuo M, Kivimaki M, Vahtera J, 2017. Social networks and patterns of health risk behaviours over two decades: A multi-cohort study. J Psychosom Res 99, 45–58. [DOI] [PubMed] [Google Scholar]
- Kupferberg A, Bicks L, Hasler G, 2016. Social functioning in major depressive disorder. Neuroscience and biobehavioral reviews 69, 313–332. [DOI] [PubMed] [Google Scholar]
- LaFrance WC Jr., Baker GA, Duncan R, Goldstein LH, Reuber M, 2013. Minimum requirements for the diagnosis of psychogenic nonepileptic seizures: a staged approach: a report from the International League Against Epilepsy Nonepileptic Seizures Task Force. Epilepsia 54, 2005–2018. [DOI] [PubMed] [Google Scholar]
- McLafferty M, O’Neill S, Armour C, Murphy S, Bunting B, 2018. The mediating role of various types of social networks on psychopathology following adverse childhood experiences. Journal of affective disorders 238, 547–553. [DOI] [PubMed] [Google Scholar]
- Montagrin A, Saiote C, Schiller D, 2018. The social hippocampus. Hippocampus 28, 672–679. [DOI] [PubMed] [Google Scholar]
- Noonan MP, Mars RB, Sallet J, Dunbar RIM, Fellows LK, 2018. The structural and functional brain networks that support human social networks. Behav Brain Res 355, 12–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozer EJ, Best SR, Lipsey TL, Weiss DS, 2003. Predictors of posttraumatic stress disorder and symptoms in adults: a meta-analysis. Psychological bulletin 129, 52–73. [DOI] [PubMed] [Google Scholar]
- Perez DL, Matin N, Barsky A, Costumero-Ramos V, Makaretz SJ, Young SS, Sepulcre J, LaFrance WC Jr., Keshavan MS, Dickerson BC, 2017a. Cingulo-insular structural alterations associated with psychogenic symptoms, childhood abuse and PTSD in functional neurological disorders. J Neurol Neurosurg Psychiatry 88, 491–497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez DL, Matin N, Williams B, Tanev K, Makris N, LaFrance WC Jr., Dickerson BC, 2018. Cortical thickness alterations linked to somatoform and psychological dissociation in functional neurological disorders. Human brain mapping 39, 428–439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez DL, Williams B, Matin N, LaFrance WC Jr., Costumero-Ramos V, Fricchione GL, Sepulcre J, Keshavan MS, Dickerson BC, 2017b. Corticolimbic structural alterations linked to health status and trait anxiety in functional neurological disorder. Journal of neurology, neurosurgery, and psychiatry 88, 1052–1059. [DOI] [PubMed] [Google Scholar]
- Pick S, Goldstein LH, Perez DL, Nicholson TR, 2018. Emotional processing in functional neurological disorder: a review, biopsychosocial model and research agenda. J Neurol Neurosurg Psychiatry. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sander J, Schupp J, Richter D, 2017. Getting together: Social contact frequency across the life span. Developmental psychology 53, 1571–1588. [DOI] [PubMed] [Google Scholar]
- Tomic A, Agosta F, Sarasso E, Petrovic I, Basaia S, Pesic D, Kostic M, Fontana A, Kostic VS, Filippi M, 2018. Are there two different forms of functional dystonia? A multimodal brain structural MRI study. Mol Psychiatry. [DOI] [PubMed] [Google Scholar]
- Vaidya-Mathur U, Myers L, Laban-Grant O, Lancman M, Lancman M, Jones J, 2016. Socialization characteristics in patients with psychogenic nonepileptic seizures (PNES). Epilepsy & behavior : E&B 56, 59–65. [DOI] [PubMed] [Google Scholar]
- Visentini C, Cassidy M, Bird VJ, Priebe S, 2018. Social networks of patients with chronic depression: A systematic review. Journal of affective disorders 241, 571–578. [DOI] [PubMed] [Google Scholar]
- Williams B, Jalilianhasanpour R, Matin N, Fricchione GL, Sepulcre J, Keshavan MS, LaFrance WC Jr., Dickerson BC, Perez DL, 2018. Individual differences in corticolimbic structural profiles linked to insecure attachment and coping styles in motor functional neurological disorders. J Psychiatr Res 102, 230–237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng R, Myers L, Lancman M, 2018. Post-traumatic stress and relationships to coping and alexithymia in patients with psychogenic non-epileptic seizures. Seizure 57, 70–75. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.