Abstract
Study Objectives
Report the first prevalence estimates of advanced sleep phase (ASP), familial advanced sleep phase (FASP), and advanced sleep–wake phase disorder (ASWPD). This can guide clinicians on the utility of screening for extreme chronotypes both for clinical decision-making and to flag prospective participants in the study of the genetics and biology of FASP.
Methods
Data on morning or evening sleep schedule preference (chronotype) were collected from 2422 new patients presenting to a North American sleep center over 9.8 years. FASP was determined using a severity criterion that has previously identified dominant circadian mutations in humans. All patients were personally seen and evaluated by one of the authors (C.R.J.).
Results
Our results demonstrate an ASP prevalence of 0.33%, an FASP prevalence of 0.21%, and an ASWPD prevalence of at least 0.04%. Most cases of young-onset ASP were familial.
Conclusions
Among patients presenting to a sleep clinic, conservatively 1 out of every 300 patients will have ASP, 1 out of every 475 will have FASP, and 1 out of every 2500 will have ASWPD. This supports obtaining a routine circadian history and, for those with extreme chronotypes, obtaining a family history of circadian preference. This can optimize treatment for evening sleepiness and early morning awakening and lead to additional circadian gene discovery. We hope these findings will lead to improved treatment options for a wide range of sleep and medical disorders in the future.
Keywords: advanced sleep phase, advanced sleep-wake phase disorder, familial advanced sleep phase, circadian, prevalence
Statement of Significance.
There are currently no prevalence estimates of advanced sleep phase (ASP), familial advanced sleep phase (FASP), and advanced sleep–wake phase disorder (ASWPD) to assist clinicians in deciding whether to screen for this sleep pattern in routine clinic visits and to assist researchers in identifying FASP individuals and their families. Our sleep clinic, which routinely screens for circadian phenotype, provides a good forum from which to determine prevalence of FASP. Our results demonstrate an ASP prevalence of 0.33%, an FASP prevalence of 0.21%, and an ASWPD prevalence of at least 0.04%. Most cases of young-onset ASP were familial. This suggests that approximately 1 out of every 475 new patients presenting to a sleep clinic will also have FASP, making every sleep center an ideal location to further our understanding of circadian clock gene influences on human health and disease.
Introduction
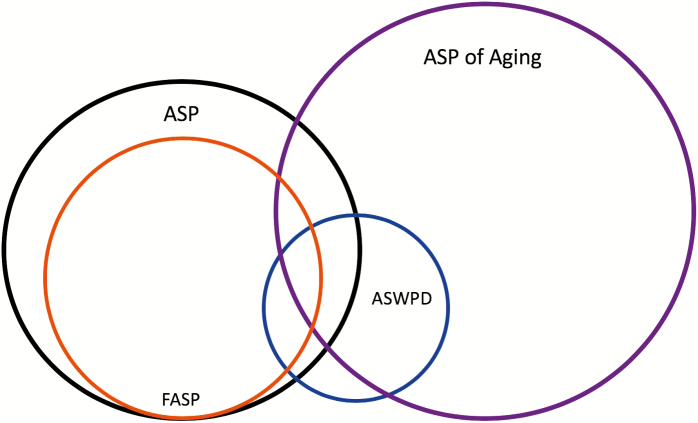
Advanced sleep phase (ASP) is characterized by a stable and unusually phase-advanced circadian sleep–wake rhythm relative to local solar time. We refer to familial advanced sleep phase (FASP) when ASP is demonstrated in multiple biologically related family members [1]. Advanced sleep–wake phase disorder (ASWPD) is defined as a marked phase advance of the sleep–wake cycle accompanied by a sleep related complaint [2]. FASP is a subtype of ASP and overlaps with ASWPD (Figure 1). Identifying these individuals in a clinical setting appears challenging as they are presumed to be very rare. As the third edition of the International Classification of Sleep Disorders (ICSD-3) observes, “The existing literature suggests that clinicians are unlikely to encounter patients with stringently defined ASWPD.” [2] The rarity of encountering patients with stringently defined ASWPD is likely a result of the diagnostic requirement for a chronic or recurrent complaint related to the patient’s advanced sleep–wake schedule [2]. Indeed, in patients with young-onset ASP, their early morning awakening may not be perceived as a school or work problem, but rather as an advantage. This may explain why ASP was thought to be exceedingly rare in the general population [3]. Many such individuals do not seek medical attention for their ASP. Prior to 1999, the authors are aware of only two convincing case reports of severe, young-onset ASWPD [4, 5]. Young-onset ASWPD was initially thought to be sporadic, but one of these case reports alluded to a family history [4]. Two other case reports are difficult to interpret due to conventional morning wake time and difficulty maintaining sleep in one report [6] and late age of onset in another [7].
Figure 1.
Venn diagram of relationship between ASP, FASP, ASWPD, and ASP of aging. The relative size of the circles does not reflect the relative prevalence of ASP versus ASP of aging, as the prevalence of ASP of aging is not known.
Another potential obstacle to the clinical identification of patients with ASP, FASP, and ASWPD is a lack of recommended cutoff scores on widely used circadian questionnaires to reliably suggest unusual circadian phase advance. Two questionnaires commonly used for this purpose are the Munich Chronotype Questionnaire (MCTQ) [8] and the Horne–Ostberg Morningness/Eveningness Questionnaire (MEQ) [9]. Data from the growing MCTQ database of Roenneberg et al. [8] suggest that individual sleep schedule on free days, a marker of chronotype, is approximately normally distributed and varies with latitude, geographical region, and urban or rural lifestyle [10]. Using midpoint of sleep on work-free days (MSF) calculations, chronotype has been shown to delay by 4 minutes for each degree of longitude one moves from east to west, even within the same time zone [10]. The average MSF corrected for extra catch-up sleep on the weekends (MSFsc) also has a latitudinal dependence. In the northerly latitudes of central Europe, MSFsc is centered on 04:00, whereas MSF distribution in India centers on 03:00. Therefore, “intermediate” chronotypes in central Europe would be “late types” in India [11]. Chronotype also has a strong age dependence, with rapidly increasing phase delay through adolescence that peaks sharply at age 20 years and is followed by a gradually slowing but persistent trend back toward typical childhood chronotype by the eighth decade [12]. Therefore, ideal criteria for ASP might incorporate age, latitude, and longitude. However, to allow for routine clinical use, our experience identifying and phenotyping FASP kindreds in North America suggests that individuals and families with an MSF less than or equal to 01:30, MEQ scores at least 71, and onset of these sleep schedules occurring prior to the age of 30 years likely have a familial form of ASP [1, 13–19].
In 1999, three families containing a total of 29 strikingly phase-advanced individuals were reported by one of the authors (C.R.J.) under the descriptive term “Familial Advanced Sleep Phase Syndrome (FASPS).” [1] We have modified the term to “Familial Advanced Sleep Phase (FASP)” as “syndrome” may connote a disorder to some whereas many FASP individuals have no complaint about their sleep schedule. One of these families was used to genetically map and clone the first reported human circadian clock gene mutation in the PER2 gene [1, 13]. The ASP probands in this study were discovered by C.R.J. because they came to clinical attention with complaints suggestive of obstructive sleep apnea (OSA). Owing to the absence of circadian complaints, most ASP probands would not meet ICSD-3 diagnostic criteria for ASWPD [20]. For this reason, we refer to individuals and families with the onset of extreme sleep phase advance by age 30 years as ASP and FASP, respectively.
The ASP participants reported here were living urban lifestyles within the North American state of Utah, comparable with the MCTQ central European database. The initial MCTQ database (N = 500) suggested that no more than 2% of the general population in Germany and Switzerland were self-described “extreme early types,” with only 0.8% having MSF less than or equal to 02:00 [8]. Expansion of the MCTQ database (N ≈ 25 000) found estimates of approximately 2% of the general population with MSF less than or equal to 02:00 and approximately 0.67% with MSF less than or equal to 01:30 [12]. A more recent expansion of the MCTQ database of central European responders (N = 92 567) found a mean MSF (not corrected for workweek sleep deprivation) of 04:58 (±1.56), 2.89% with MSF less than or equal to 02:00, and 0.97% with MSF less than or equal to 01:30 (personal communication, T. Roenneberg in 2012). Correcting for latitude predicts a mean Utah MSF of 04:37. If our clinic population is representative of the local population, this would represent the mean MSF for those presenting to the clinic.
Comparing the MCTQ with another widely used tool to assess chronotype, the MEQ [9], revealed reasonable correlations between MEQ and MSF scores (r = −0.73) in a predominantly young adult Dutch population (N = 2481) [21]. Pooling MEQ data from 741 young adults, 484 adults, and 40 elderly participants in London and the United States yielded a weighted prevalence estimate of extreme morningness (i.e. MEQ scores ≥71) of approximately 0.51% [22–25]. Taken together, the MCTQ and MEQ databases would predict a prevalence of individuals with extreme morning chronotypes resembling ASP (i.e. MSF ≤ 01:30 and MEQ ≥ 71) to range between 0.50% and 1.00% in the North American general population. Whether a similar prevalence range accurately reflects the prevalence of individuals with young-onset ASP and FASP is currently unknown.
To address this question, we report here the first estimated prevalence of young-onset ASP, FASP, and ASWPD obtained from a sleep clinic population. Data on self-report and objective methods used to characterize chronotype and circadian rhythms in young-onset ASP, FASP, and ASWPD participants are provided. Next, differences in self-reported markers of circadian rhythms, excessive daytime sleepiness (EDS), and depressive affect in young-onset ASP participants and their conventional chronotype family members were examined. Finally, we explore whether apnea–hypopnea index (AHI) predicts MEQ-determined chronotype to gauge whether using a population presenting with symptoms of OSA has potential for generalizability of the results.
Methods
Research approval for this study was obtained from the University of Utah Institutional Review Board.
Participants
As in many general sleep disorder clinics, most participants presented for signs and symptoms concerning for OSA. This is not surprising, given the high prevalence of OSA in men and women and the availability of clinical treatment options [2]. Name, hospital medical record number, date of first visit, new versus return physician evaluation code, and diagnosis code numbers for all patients seen by C.R.J. from January 1994 to October 2003 were imported from our hospital’s scheduling and billing computer record system into an Excel spreadsheet. The spreadsheet was then edited to delete patient encounters other than the initial patient visit. Computer data entry from January 1994 to August 1995 did not use sufficiently specific sleep disorder diagnosis codes or discriminate nighttime sleep laboratory procedures performed by technologists from daytime clinic visits with the physician. However, of the 637 encounters from this period, 571 complete paper charts were available for review and the appropriate diagnosis and procedure codes were manually entered into the edited spreadsheet. The total number of patients seen was 2422, of whom 1748 (72.2%) had suspected or proven OSA.
Screening for ASP
Careful screening for ASP began following identification of the first FASP proband seen by C.R.J. in November of 1992. This 72-year-old female was first described in 1999 [1]. Habitual workday and free day sleep schedules were routinely discussed with patients during their initial clinic visit, given the relevance of sleep timing to all sleep disorders. Patients describing MSF less than or equal to 01:30 with onset of this sleep schedule prior to age 30 years were informed of our research following their clinic visit. Willing participants signed a consent form approved by the University of Utah Institutional Review Board. Initial contact and enrollment of patient’s family members was accomplished by telephone, email, or US Postal Service.
Expanded phenotyping of patients screening positive for ASP and their family members
Enrolled participants were asked to complete six questionnaires: (1) Participant Information Form detailing demographic information and family medical history; (2) Epworth Sleepiness Scale (ESS) [26]; (3) A “General Sleep Questionnaire” (GSQ) designed to assess habitual workday and free day sleep schedules, sleep disorders, medical history, and psychosocial influences on sleep (Supplementary Figure 1); (4) A visual analog “Wake and Sleep Questionnaire” developed to assess latencies from lights out to initial sleep onset and from final sleep offset to getting out of bed (Supplementary Figure 2); (5) Morningness/Eveningness Questionnaire [9]; and (6) Beck Depression Inventory II (BDI-II) [27]. Participants also completed a 45–60 minute-structured telephone interview conducted by C.R.J. or a trained research coordinator. Interview questions included habitual workday and free day sleep schedules, alarm use, daytime napping, daily rhythms of mood and alertness, effects of seasonal and time zone changes on sleep, confounding influences on sleep (e.g. caffeine, alcohol, medications), age-related changes in sleep schedule, and age-related history of chronotype from childhood to the present (Supplementary Figure 3). This detailed screening included evaluation for alternative causes of early morning awakening, such as depression, daytime obligations, insomnia, and OSA.
When possible, questionnaires and structured interview results were compared with in-laboratory polysomnography (PSG), home sleep-stage recordings, and 10-day ambulatory wrist actigraphy with coincident sleep logs, and one evening of salivary dim light melatonin onset (DLMO). The phase angle between melatonin onset and the sleep–wake cycle has been correlated with endogenous circadian period [28] and the DLMO itself estimates the magnitude of circadian rhythm advance or delay. PSG was recorded and scored according to standard procedures [29, 30]. Home sleep-stage recordings were performed using the Zeo automated wireless system (Zeo, Inc., Newton, MA) that has shown reliability with in-laboratory PSG [31] and outpatient actigraphy and sleep logs [32]. Actigraphy was performed using the MicroMini-Motionlogger (Ambulatory Monitoring, Inc., Ardsley, NY) and Actiwatch-L (Respironics, Murrysville, PA) actigraphs. Actigraphy data were downloaded using Action-W 2.4.17 and Actiware-Sleep 3.4 software and scored automatically using the Cole-Kripke sleep algorithm. Saliva samples to determine DLMO were collected every 30 minutes for 5–6 hours before habitual bedtime on nonworkdays by participants in their homes using “Salivette” saliva collection tubes (Sarstedt, Inc., Newton, NC). SolidPhase, Inc. (Portland, ME) analyzed saliva melatonin content using the BUHLMANN Direct Saliva Melatonin Radio Immunoassay (ALPCO Diagnostics, Salem, NH). Participants were instructed to remain in less than or equal to 30 lux of dim lighting throughout their saliva collection protocol. Lux values were obtained using the Sinometer LX1010B Mini Digital Lux Meter (ShenZhen, China) and recorded on a saliva collection log at the time of each saliva sample.
ASP criteria included the following: (1) the ability to fall asleep before 20:30 and to wake before 05:30 throughout the year in the absence of professional or psychosocial demands or environmental influences (e.g. early morning work start time, self-imposed morning bright light); (2) the presence of only one major sleep period each day; (3) onset of this stable sleep–wake schedule before the age of 30 years; (4) the sleep pattern is not maintained by morning stimulants or evening sedative use; (5) the sleep pattern did not develop within 3 months of a traumatic brain injury; and (6) the sleep pattern is not due to another medical, neurological, or mental disorder (e.g. chronic insomnia disorder, major depressive disorder).
AHI predicting MEQ-determined chronotype
To gauge whether an OSA population is skewed toward morningness or eveningness, we assessed whether AHI selects for MEQ using the Wisconsin Sleep Cohort. Repeated measures linear regression analysis (PROC MIXED, SAS) was performed on MEQ and the log of the AHI scores from 3188 studies on 1324 participants from the Wisconsin Sleep Cohort. The Wisconsin Sleep Cohort’s methods for collection and interpretation of PSG recordings and AHI scores have been described [33].
Results
Estimated North American prevalence of early-onset ASP and FASP
Eight patients with ASP (three females) were identified and enrolled from January 1994 to October 2003 (9.8 consecutive years). Seven ASP subjects presented with EDS as their primary complaint. The eighth ASP subject presented with a previous diagnosis of OSA, complaining of nasal continuous positive airway pressure mask discomfort. Diagnostic PSG demonstrated OSA in all five male ASP subjects. The first female ASP proband had suspected upper airway resistance syndrome and declined PSG and multiple sleep latency testing (MSLT), anticipating a change in her condition after completion of her pregnancy. Subsequently, she had upper airway surgery with self-reported resolution of her hypersomnia. The second female ASP proband presented with worsening EDS secondary to a previous diagnosis of narcolepsy without cataplexy. Diagnostic PSG demonstrated obstructive hypopneas of mild degree (AHI = 11) plus respiratory effort–related arousals of approximately five per hour in supine and lateral positions. The third female proband was diagnosed with insufficient sleep associated with ASP syndrome and possible idiopathic hypersomnia without long sleep time following PSG and MSLT recordings.
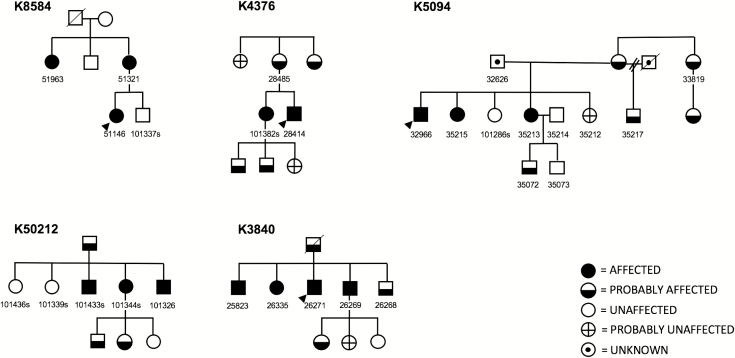
No ASP probands presented with chief complaint of their advanced schedule, and it was thought to contribute to the ultimate diagnosis in one of eight patients. Two probands had ASP onset before the age of 12 years and the remaining six probands had ASP onset before the age of 22 years. All eight ASP probands reported at least one first-degree relative with a similarly advanced sleep–wake schedule, suggesting FASP. At least one relative with ASP from each proband was willing to be interviewed for research purposes. Family member enrollment and circadian phenotyping revealed 15 ASP participants in five families, confirming FASP in five kindreds consistent with an autosomal dominant mode of transmission (Figure 2). Two ASP relatives had age of onset by age 40 years, with the remaining ASP relatives having onset prior to the age of 22 years.
Figure 2.
FASP pedigrees. Circles, females; squares, males; diagonal line through symbols, deceased; numbers underneath symbols, DNA identifier; arrows, probands.
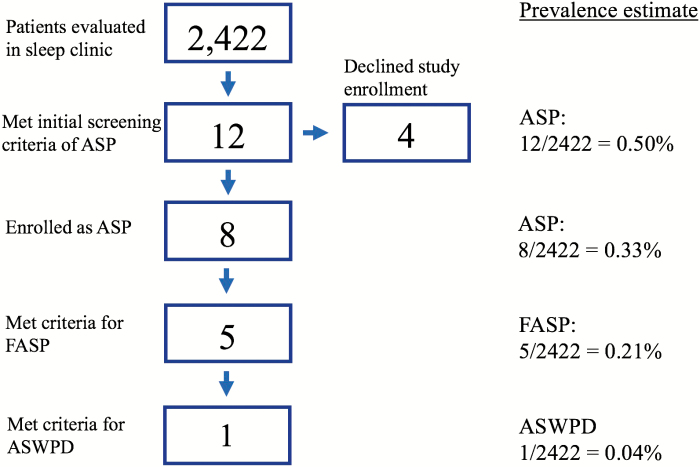
Of the 2422 patients that were seen from January 1994 to October 2003, eight ASP probands and five FASP kindreds were identified who met our ASP criteria. One ASP proband had a sleep complaint related to her advanced sleep schedule. Therefore, the estimated prevalence of early-onset ASP, FASP, and ASWPD among patients pursuing evaluation at a North American academic medical center is 0.33% (8/2422), 0.21% (5/2422), and 0.04% (1/2422), respectively (Figure 3). We consider these estimates to be conservative, as four subjects describing FASP declined research participation and were not included in the above results. If these four individuals do, in fact, have ASP, the ASP prevalence estimate is 0.50% (12/2422). Assuming the four to have FASP, an upper estimate of FASP from the dataset would be 0.37% (9/2422). Further, more mild sleep patterns that did not meet our strict criteria were seen in family members of the three ASP individuals who were not categorized as FASP. Four additional individuals and eight of their relatives were also initially enrolled in our study but ultimately were not included in this data due to not meeting the strict criteria.
Figure 3.
Flow chart of patient enrollment and prevalence estimates.
We also considered the prevalence estimate for ASP and FASP among the population pursuing evaluation for OSA whose ASP was not thought to directly contribute to the diagnosis. Of the 1748 patients with suspected or proven OSA that were seen from January 1994 to October 2003, seven patients met criteria for ASP and four for FASP. Therefore, the estimated prevalence of early-onset ASP and FASP among patients pursuing evaluation at a North American academic medical center for symptoms of OSA is 0.40% (7/1748) and 0.23% (4/1748), respectively.
Chronotype and circadian rhythm markers of young-onset ASP and FASP participants
MEQ scores, MSF times, and saliva DLMO for the ASP probands and their ASP relatives are included in Table 1.
Table 1.
Chronotype and circadian rhythm markers of young-onset ASP and FASP participants
| Kindred; ID | MEQ | GSQ MSF | Intrvw MSF | Earliest MSF | SL MSF | Zeo MSF | ACT MSF | PSG MSF | DLMO |
|---|---|---|---|---|---|---|---|---|---|
| 5094; 32966 | 80 | 1:03 | 0:32 | 23:53 | — | — | 0:53† | 1:19 | — |
| 5094; 35215 | 82 | — | 1:04 | 0:13 | — | — | — | — | — |
| 5094; 35213 | 68 | 1:38 | 1:38 | — | — | — | — | — | — |
| 50212; 101344 | 78 | 1:05 | 2:05 | 0:45 | — | — | — | 1:09† | — |
| 50212; 101326 | 72 | 2:10 | 1:11 | 23:23 | 1:45† | 1:58† | 2:35† | — | — |
| 50212; 101433 | 76 | 23:34 | — | — | — | — | — | — | — |
| 3840; 25823 | 72 | — | 23:53 | 23:53 | — | — | — | — | — |
| 3840; 26271 | — | 0:00 | 0:15 | — | — | — | — | 0:05 | — |
| 3840; 26269 | — | 0:05 | — | — | — | — | — | — | — |
| 3840; 26335 | 75 | — | 0:45 | — | — | — | — | — | — |
| 4376; 28414 | 76 | — | — | — | 1:47† | — | 3:17† | 0:43 | 17:44 |
| 4376; 101382 | 76 | 0:43 | 2:43 | 23:00 | 1:54 | 2:33 | 2:13 | — | 19:50† |
| 8584; 51146 | 82 | 1:25 | 1:50 | 23:30 | 1:44 | 1:50 | 1:54 | — | — |
| 8584; 51321 | 74 | 0:26 | 2:13 | 0:15 | 1:30 | 1:30 | 1:24 | — | — |
| 8584; 51963 | 78 | 0:53 | 1:15 | 0:23 | — | — | — | — | — |
| 50048; 100381 | 77 | 0:00 | 1:08 | 0:15 | — | — | — | 1:23 | — |
| 50187; 101263 | 78 | 1:40 | 1:38 | 22:30 | 1:31† | 0:56† | 1:04† | 01:53 | — |
| 50192; 101311 | 74 | 1:08 | 1:30 | 23:23 | 2:23 | 2:26 | — | — | 17:33 |
| AVG | 76.1 | 0:51 | 01:19 | 23:47 | 01:47 | 01:52 | 01:54 | 01:05 | 18:22 |
| SD | 2.59 | 0.56 | 0.55 | 0.72 | 0.32 | 0.67 | 0.85 | 0.58 | 1.27 |
ID, DNA sample identifier; MEQ, Horne-Ostberg Morningness/Eveningness Questionnaire; GSQ, general sleep questionnaire; MSF, midpoint of sleep on work-free days; Intrvw, structured interview; Earliest, earliest possible sleep onset and final wake up time without prior sleep deprivation; SL, sleep log; Zeo, wireless home sleep recording system; ACT, actigraphy; PSG, polysomnography; DLMO, dim light melatonin onset; Shaded rows, probands; AVG, average; SD, standard deviation; a, data obtained during Daylight Saving Time; -, data not collected.
†Data obtained during Daylight Saving Time.
Fourteen non-advanced and non-delayed (i.e. “conventional”) chronotype family members were also identified and characterized in our ASP and FASP kindreds. Comparison between self-reported markers of circadian rhythms, EDS, and depressive affect in ASP participants and their conventional chronotype family members are included in Table 2. Comparison of MSF and MEQ in the ASP cohort versus the conventional sleeping family members is demonstrated in supplementary Figure 4.
Table 2.
Self-reported markers of circadian rhythms, excessive daytime sleepiness, and depressive affect in young-onset ASP and conventional chronotype family members
| Conventional | ASP | ||||
|---|---|---|---|---|---|
| Parameter | n | M ± SD | n | M ± SD | Cohen’s d |
| MEQ | 14 | 57.21 ± 8.71 | 17 | ***76.1 ± 3.88 | 2.80 |
| GSQ MSF | 11 | 03:05 ± 1.01 | 15 | ***0:51 ± 0.74 | 2.87 |
| GSQ MSW | 11 | 02:27 ± 0.90 | 16 | ***0:37 ± 0.55 | 2.55 |
| Intrvw MSF | 14 | 03:06 ± 1.01 | 16 | ***01:19 ± 0.76 | 2.09 |
| Intrvw MSW | 14 | 02:36 ± 0.82 | 15 | ***01:10 ± 0.66 | 1.69 |
| Earliest MSF | 11 | 02:04 ± 0.78 | 13 | ***23:47 ± 0.62 | 3.65 |
| Speed SO | 11 | 43.27 ± 21.18 | 14 | 44.57 ± 16.42 | 0.07 |
| Speed WU | 11 | 55.27 ± 23.55 | 14 | *76.29 ± 19.11 | 0.98 |
| ESS | 11 | 11.36 ± 5.01 | 15 | 9.80 ± 6.36 | 0.27 |
| BDI-II | 13 | 6.31 ± 5.98 | 19 | 5.63 ± 6.99 | 0.10 |
ASP, advanced sleep phase; n, subsample size; M, mean; SD, standard deviation; MEQ, Horne-Ostberg Morningness/Eveningness Questionnaire; GSQ, general sleep questionnaire; MSF, midpoint of sleep on work-free days; MSW, midpoint of sleep on workdays; Intrvw, structured interview; Earliest, earliest possible sleep onset and final wake up time without prior sleep deprivation; Speed SO, speed of evening sleep onset (visual analog scale from 0-100); Speed WU, speed of morning wake up (visual analog scale from 0-100); ESS, Epworth Sleepiness Scale; BDI-II, Beck Depression Inventory-II.
Student’s two-tailed unpaired t-test *p < .05, ***p < .001.
AHI severity does not predict MEQ-determined chronotype
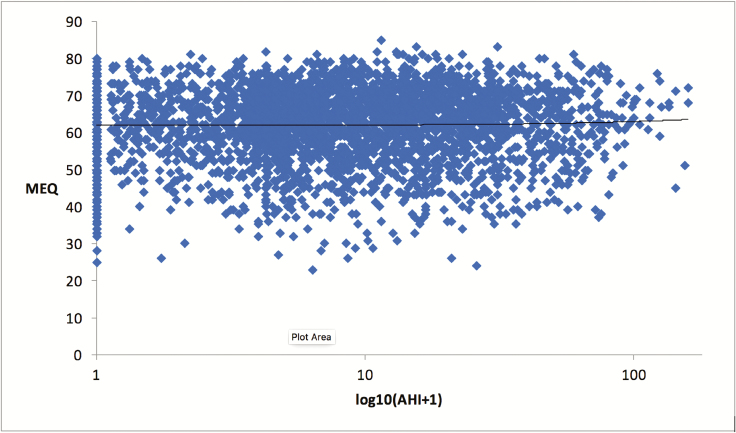
The majority of the ASP and FASP probands in this investigation pursued evaluation at an academic medical center for symptoms of potential OSA. To assess if OSA selects for chronotype, data were obtained MEQ and AHI scores from 3188 studies on 1324 participants from the Wisconsin Sleep Cohort [33]. Repeated measures linear regression analysis revealed no significant predictive relationship between MEQ-determined early morning or late night sleep schedule preference and AHI severity (Figure 4).
Figure 4.
AHI severity does not predict MEQ-determined chronotype. N = 3188. Females, 1491 (mean age = 57.7); males: 1697 (mean age: 59.4). Mean MEQ scores = 62.08 ± 9.71. Mean AHI scores = 14.08 ± 17.78. Trend line reveals no significant relationship between AHI and MEQ (β = 0.08, SE β = 0.13, p = 0.52, R2 = 1.0 e−5).
Discussion
In this study, we provide the first prevalence estimates of ASP, FASP, and ASWPD based on a sleep clinic population. All patients were personally seen and evaluated by one of our authors (C.R.J.). Of the eight young-onset ASP probands, five FASP families were identified who met our strict ASP criteria. Estimates show an ASP prevalence of 0.33% (8/2422), FASP prevalence of 0.21% (5/2422), and ASWPD prevalence of 0.04% (1/2422) (Figure 3). Of the 2422 total patients, 1748 presented for OSA. Therefore, the estimated prevalence in an OSA population is 0.40% (7/1748) for ASP and 0.23% (4/1748) for FASP. As the eighth proband did not have OSA and instead had a complaint related to FASP, she meets criteria for ASWPD and is not included in the estimate from an OSA population.
Data characterizing chronotype and circadian rhythms using subjective and objective methods for the eight young-onset ASP probands and their ASP family members in this study are given in Table 1. Clock times are reported in participants’ local time zone and not solar time. MCTQ data from 21 600 German participants strongly suggests that the human circadian clock is predominantly entrained by solar time rather than local legal (“social”) time [10]. Therefore, we consider our phenotyping to be conservative, as four ASP probands and one ASP relative performed recordings during Daylight Saving Time, effectively delaying these clock times by 1 hour in reference to solar time. Three ASP probands (32966, 101344, and 51146) reported routinely delaying their evening sleep onset to avoid early morning awakening, making their advanced MSF times all the more impressive. Proband 101263 denied delaying evening sleep onset, and we were unable to collect this information from probands 25823, 28414, 100381, and 101311. Salivary DLMOs collected on 170 North American adult participants (85 females, average MEQ = 52.11 ± 9.10) revealed a mean DLMO of 20:50 (±1:12) [34]. Using the same method of DLMO calculation, ASP proband 101311 is within the earliest 0.21% of this normative database. Similarly, ASP proband 28414 and ASP family member 101382 are within the earliest 0.50% and 20.5% of this normative database, respectively. As 101382 performed her DLMO during Daylight Saving Time, she would be predicted to be within the earliest 4.7% of this normative database using solar (i.e. nonsocial) time. Owing to financial constraints, sleep logs, actigraphy, Zeo, DLMO, and PSG data were collected on fewer conventional chronotypes than ASP participants, limiting statistical comparisons on these measures.
Significant differences in MSF clock times and MEQ scores between ASP participants and their conventional chronotype family members were expected given our ASP classification criteria (Table 2, Supplementary Figure 4). The tendency toward rigid sleep schedules on workdays (midpoint of sleep on workdays) and nonworkdays (MSF) in “extreme early types” has been reported in a large North American FASP kindred [1] and a European study using the MCTQ [8]. Our data confirm this tendency. Young-onset ASP participants sleep an average of 5–10 minutes later on weekends compared to an average of 30–38 minutes for their conventional chronotype family members (Table 2). Latency of final sleep offset (speed of morning wake up) was determined using a visual analog scale we developed that ranges from 0 to 100: “On awakening most mornings, how long does it take you to become fully alert and active?” (0 = several hours; 100 = seconds; Supplementary Figure 2). Our findings suggest that ASP individuals feel alert more quickly in the morning, i.e. have less sleep inertia, compared to their conventional chronotype family members (Table 2). The tendency for morning types to be more subjectively alert upon awakening may be explained, in part, by core body temperature rhythms. Previous research suggests that morning types sleep at a later portion of their temperature rhythm compared to conventional and evening chronotypes [24].
No significant differences were observed in Beck Depression Inventory scores between ASP participants and their family members with conventional chronotypes (Table 2). This supports that early morning awakenings observed in ASP and FASP probands are not the result of comorbid depression. No significant differences were observed between ASP participants and their conventional chronotype family members in self-reported daytime sleepiness (Table 2). Elevated ESS scores may be expected for the eight ASP probands who each presented with clinical symptoms of EDS related to OSA (n = 7) or idiopathic hypersomnia (n = 1). However, ESS scores from these eight ASP probands were combined with ESS scores from seven FASP family members (total n = 15), which may account for these nonsignificant results. Sleep quality and quantity are typically reported as normal for age when ASWPD patients without comorbidities allow themselves to fall asleep and awaken without regard to social constraints [2].
Our method of screening for FASP began with habitual sleep time and thus does not account for individuals who may be advanced but can easily overcome their circadian clock and sleep at a more conventional time. In addition, our criteria rely on mid-sleep time on free days and responses to the MEQ, which may have excluded some individuals who appear advanced by other measures. Although this may lead to an underestimate of ASP, our rigorous screening method has proven effective in identifying FASP. These strict criteria avoid false positives and increase the yield for genetic screening in families with a dominant pattern of inheritance. This method has led to successful identification of multiple clock genes that co-segregate in the family and are then recapitulated in mouse models [1, 13, 15, 17, 18]. Therefore, this screening method can translate simple questions about sleep timing on an entry sleep clinic questionnaire into identification of families with an autosomal dominant sleep trait and lead to identification of genes controlling our circadian clock.
Our ASWPD prevalence estimate of 0.04% includes only those individuals presenting with ASP prior to age 30 years. This estimate does not account for individuals with ASP of aging, whose schedule advance with aging prompts complaint. Therefore, the present estimate likely underestimates the true prevalence of ASWPD, even within a sleep clinic population. Figure 1 illustrates the relationship between ASP, FASP, ASWPD, and ASP of aging. FASP represents the majority of young-onset ASP, and a small portion of this group will complain of their advanced phase, thus falling into ASWPD. However, some with ASWPD may have ASP only due to aging or other unidentified causes. Although it is known that chronotype advances with age, the formal definition and prevalence of ASP of aging is not defined [12]. Therefore, the relative size of the circles in Figure 1 does not reflect the relative prevalence of ASP versus ASP of aging, as the prevalence of ASP of aging is not known.
The young-onset ASP and FASP prevalence estimates reported here were based on a clinical population presenting to an academic medical center. We have also calculated the prevalence from the population presenting only for OSA, resulting in an ASP prevalence of 0.40% (7/1748) and FASP prevalence of 0.23% (4/1748). This limited sample was used because we can assess whether this sample selects for chronotype and thus can consider whether the estimate may have a potential for generalizability. We conducted a linear regression analysis of MEQ and AHI scores from 1324 participants using the Wisconsin Sleep Cohort database (Figure 4). Results of this analysis found no significant predictive relationship between these variables, indicating that using a sleep clinic population presenting for symptoms of OSA does not appear to bias the chronotype of the sample. Whether our ASP and FASP prevalence estimates can be further generalized to the population merits additional investigation. In addition, as our team published research on FASP beginning in 1999, it is possible this altered the patient group seeking care in the clinic, making this clinic estimate less representative of other sleep clinics. This is not a population-based sample.
We aim to raise awareness about ASP and FASP with the hope this prompts routine screening, enhancing clinical care for those affected. It is notable that many with ASP and FASP are not troubled by this sleep pattern. Increased awareness will improve screening for these phenotypes and enrollment in related research. Taken together, our findings suggest that ASP and FASP subjects routinely present to sleep centers with primary complaints that do not appear directly related to sleep–wake schedule advance. Our ASP and FASP prevalence estimates are higher than one would predict based on the prevailing understanding of ASP from ASWPD. Our data support that ASWPD is very rare at one out of every 2500. Milder versions or those who develop ASP of aging are not accounted for in the dataset.
In our clinical experience, a six-bed sleep clinic running five nights per week may see 1500 patients and perform 450 new diagnostic PSGs annually. Our FASP prevalence estimate of 0.21% would predict approximately one incidental FASP PSG recording per six-bed sleep center per year. The 2016–2017 American Academy of Sleep Medicine (AASM) Membership Directory (www.aasmnet.org) lists 1512 AASM-accredited sleep centers currently operating within the United States. Therefore, approximately 1000 diagnostic PSG recordings may be performed on potential OSA patients who also have FASP in the United States annually. This number is likely conservative, as 1 615 135 PSG recordings were estimated to have been performed in the United States in the year 2001 [35]. Assuming only 50% of these recordings were performed for new patient OSA evaluations, our FASP prevalence in an OSA population estimate of 0.23% predicts 1857 PSG recordings were performed on potential OSA patients with FASP in 2001 alone. To the best of our knowledge, only 11 FASP families have been reported to date [1, 15–18, 36, 37], suggesting that there is potential to identify far more FASP individuals during routine clinic evaluation.
Individuals with ASP often complain of a longstanding pattern of evening sleepiness and early morning awakening with trouble returning to sleep. They may have shorter sleep hours if attempting to stay up for social, work, or family obligations without the ability to awaken later. There is a growing body of literature linking fewer hours of sleep to multiple negative health outcomes, including increased mortality, diabetes mellitus, hypertension, cardiovascular disease, and obesity [38]. It is essential for clinicians to appreciate the circadian explanation for shorter sleep hours for this patient population to provide appropriate counseling and treatment options.
Therefore, we recommend routine screening for circadian phenotypes in all sleep clinics with two questions:
(1) On a long weekend or vacation with few or no responsibilities or obligations, when would you go to bed?
(2) On a long weekend or vacation with few or no responsibilities or obligations, when is your final awakening for the night?
These simple questions included in new patient packets can allow the clinician to screen for both ASP and delayed sleep phase, with follow-up questions if atypical timing is noted. Asking about long weekend and vacation rhythms is important to remove the extrinsic demands of school, work, and family and rebound sleep during weekends.
Recognizing that extreme sleep–wake schedule advance is not exceedingly rare may assist clinicians in separating the early morning awakenings of ASP from the early morning awakenings of insomnia, nocturia, environmental sleep disruptions, and major depressive disorder. Identifying FASP subjects will require clinicians to routinely ask about habitual workday and free day sleep–wake schedules and explore familial patterns of ASP if extreme sleep–wake schedule advances are described. Our findings further suggest that ASP individuals with onset prior to age 30 years should be considered likely FASP probands.
Importantly, the clinical identification of young-onset FASP individuals and their family members has facilitated the discovery of autosomal dominant circadian clock gene mutations in PER2 [13, 14], CKIδ [15, 16], PERIOD3 [17], CRY2 [18], TIM [39], and DEC2 [19]. FASP clock gene mutations have been found to co-segregate with familial migraine (cK1δ) [15] and depressive affect (PER3) [17], and regulatory roles for core clock genes in tumor suppression [40], sugar metabolism [41], behavioral activation [19], and the age of onset of bipolar disorder [42] have been reported. Therefore, functional characterization of newly discovered clock gene mutations may lead to improved treatment options for a wide range of sleep and medical disorders in the future. Our findings provide an indication and mechanism for clinicians to routinely screen for ASP and FASP subjects.
Funding
The work was supported by NIH grants and by the William Bowes Neurogenetics Fund.
Conflict of interest statement. None declared.
Supplementary Material
Acknowledgments
We thank Jian Ying, Alun William, and Ken Boucher from the University of Utah Department of Epidemiology for advice on the calculation of random chance familial aggregation. We are also grateful to the families that participated in this work. The authors are grateful to Dr. Andrew Krystal for helpful discussions and comments on the manuscript.
References
- 1. Jones CR, et al. Familial advanced sleep-phase syndrome: a short-period circadian rhythm variant in humans. Nat Med. 1999;5(9):1062–1065. [DOI] [PubMed] [Google Scholar]
- 2. American Academy of Sleep Medicine. International Classification of Sleep Disorders–Third Edition (ICSD-3). Darien, IL: American Academy of Sleep Medicine; 2014. [Google Scholar]
- 3. Schrader, et al. The prevalence of delayed and advanced sleep phase syndromes. J Sleep Res.. 1993;2(1):51–55. [DOI] [PubMed] [Google Scholar]
- 4. Billiard M, et al. A case of advanced-sleep phase syndrome. Sleep Res. 1993;22: 109. [Google Scholar]
- 5. Singer C, et al. Case report: use of the dim light melatonin onset in the treatment of ASPS with bright light. Sleep Res. 1989;18: 445. [Google Scholar]
- 6. Kamei R, et al. Advanced-sleep phase syndrome studied in a time isolation facility. J Sleep Res.. 1979;6: 115. [Google Scholar]
- 7. Moldofsky H, et al. Treatment of a case of advanced sleep phase syndrome by phase advance chronotherapy. Sleep. 1986;9(1):61–65. [DOI] [PubMed] [Google Scholar]
- 8. Roenneberg T, et al. Life between clocks: daily temporal patterns of human chronotypes. J Biol Rhythms. 2003;18(1):80–90. [DOI] [PubMed] [Google Scholar]
- 9. Horne JA, et al. A self-assessment questionnaire to determine morningness-eveningness in human circadian rhythms. Int J Chronobiol. 1976;4(2):97–110. [PubMed] [Google Scholar]
- 10. Roenneberg T, et al. The human circadian clock entrains to sun time. Curr Biol. 2007;17(2):R44–R45. [DOI] [PubMed] [Google Scholar]
- 11. Roenneberg T. What is chronotype? Sleep Biol Rhythm. 2012;10(2):75–76. [Google Scholar]
- 12. Roenneberg T, et al. A marker for the end of adolescence. Curr Biol. 2004;14(24):R1038–R1039. [DOI] [PubMed] [Google Scholar]
- 13. Toh KL, et al. An hPer2 phosphorylation site mutation in familial advanced sleep phase syndrome. Science. 2001;291(5506):1040–1043. [DOI] [PubMed] [Google Scholar]
- 14. Xu Y, et al. Modeling of a human circadian mutation yields insights into clock regulation by PER2. Cell. 2007;128(1):59–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Xu Y, et al. Functional consequences of a CKIdelta mutation causing familial advanced sleep phase syndrome. Nature. 2005;434(7033):640–644. [DOI] [PubMed] [Google Scholar]
- 16. Brennan KC, et al. Casein kinase iδ mutations in familial migraine and advanced sleep phase. Sci Transl Med. 2013;5(183):183ra56, 1–183ra56, 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Zhang L, et al. A PERIOD3 variant causes a circadian phenotype and is associated with a seasonal mood trait. Proc Natl Acad Sci U S A. 2016;113(11):E1536–E1544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Hirano A, et al. A Cryptochrome 2 mutation yields advanced sleep phase in humans. Elife. 2016;5: e16695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. He Y, et al. The transcriptional repressor DEC2 regulates sleep length in mammals. Science. 2009;325(5942):866–870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Ohayon MM, et al. DSM-IV and ICSD-90 insomnia symptoms and sleep dissatisfaction. Br J Psychiatry. 1997;171:382–388. [DOI] [PubMed] [Google Scholar]
- 21. Zavada A, et al. Comparison of the Munich chronotype Questionnaire with the Horne-Ostberg’s Morningness-Eveningness Score. Chronobiol Int. 2005;22(2):267–278. [DOI] [PubMed] [Google Scholar]
- 22. Smith CS, et al. Evaluation of three circadian rhythm questionnaires with suggestions for an improved measure of morningness. J Appl Psychol. 1989;74(5):728–738. [DOI] [PubMed] [Google Scholar]
- 23. Robilliard DL, et al. The 3111 Clock gene polymorphism is not associated with sleep and circadian rhythmicity in phenotypically characterized human subjects. J Sleep Res. 2002;11(4):305–312. [DOI] [PubMed] [Google Scholar]
- 24. Baehr EK, et al. Individual differences in the phase and amplitude of the human circadian temperature rhythm: with an emphasis on morningness-eveningness. J Sleep Res. 2000;9(2):117–127. [DOI] [PubMed] [Google Scholar]
- 25. Duffy JF, et al. Relationship of endogenous circadian melatonin and temperature rhythms to self-reported preference for morning or evening activity in young and older people. J Investig Med. 1999;47(3):141–150. [PMC free article] [PubMed] [Google Scholar]
- 26. Johns MW. A new method for measuring daytime sleepiness: the Epworth sleepiness scale. Sleep. 1991;14(6):540–545. [DOI] [PubMed] [Google Scholar]
- 27. Beck A. The Beck Depression Inventory (BDI-II) 1–2. San Antonio, TX: Harcourt Brace & Company, 1978. [Google Scholar]
- 28. Duffy JF, et al. Association of intrinsic circadian period with morningness-eveningness, usual wake time, and circadian phase. Behav Neurosci. 2001;115(4):895–899. [DOI] [PubMed] [Google Scholar]
- 29. Hobson JA.In: Rechtschaffen A and Kales A, ed. A Manual of Standardized Terminology, Techniques and Scoring System for Sleep Stages of Human Subjects. 1– 12. Los Angeles: UCLA: Brain Information Service/Brain Research Institute, 1968. [Google Scholar]
- 30. Keenan S.In: Sudhansu C, ed. Sleep Disorders Medicine : Basic Science, Technical Considerations, and Clinical Aspects. Polysomnographic technique: an overview. Boston, MA: Elsevier Science; 1994. [Google Scholar]
- 31. Shambroom JR, et al. Validation of an automated wireless system to monitor sleep in healthy adults. J Sleep Res. 2012;21(2):221–230. [DOI] [PubMed] [Google Scholar]
- 32. Curtis B, et al. Comparison of an ambulatory sleep-stage recorder with outpatient actigraphy and sleep logs across a wide range of sleep phenotypes. Sleep. 2011;34:A323. [Google Scholar]
- 33. Young T, et al. The occurrence of sleep-disordered breathing among middle-aged adults. N Engl J Med. 1993;328(17):1230–1235. [DOI] [PubMed] [Google Scholar]
- 34. Burgess HJ, et al. Individual differences in the amount and timing of salivary melatonin secretion. PLoS One. 2008;3(8):e3055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Tachibana N, et al. A quantitative assessment of sleep laboratory activity in the United States. J Clin Sleep Med. 2005;1(1):23–26. [PubMed] [Google Scholar]
- 36. Reid KJ, et al. Familial advanced sleep phase syndrome. Arch Neurol. 2001;58(7):1089–1094. [DOI] [PubMed] [Google Scholar]
- 37. Satoh K, et al. Two pedigrees of familial advanced sleep phase syndrome in Japan. Sleep. 2003;26(4):416–417. [DOI] [PubMed] [Google Scholar]
- 38. Itani O, et al. Short sleep duration and health outcomes: a systematic review, meta-analysis, and meta-regression. Sleep Med. 2017;32:246–256. [DOI] [PubMed] [Google Scholar]
- 39. Kurien P, et al.. TIMELESS mutation alters phase responsiveness and causes advanced sleep phase. Proc Natl Acad Sci U.S.A. 2019;116( 24):12045–12053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Fu L, et al. The circadian gene Period2 plays an important role in tumor suppression and DNA damage response in vivo. Cell. 2002;111(1):41–50. [DOI] [PubMed] [Google Scholar]
- 41. Zhang EE, et al. Cryptochrome mediates circadian regulation of cAMP signaling and hepatic gluconeogenesis. Nat Med. 2010;16(10):1152–1156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Benedetti F, et al. A length polymorphism in the circadian clock gene Per3 influences age at onset of bipolar disorder. Neurosci Lett. 2008;445(2):184–187. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.