Abstract
Study Objectives
Insomnia is a common precursor to depression; yet, the potential for insomnia treatment to prevent depression has not been demonstrated. Cognitive behavioral therapy for insomnia (CBT-I) effectively reduces concurrent symptoms of insomnia and depression and can be delivered digitally (dCBT-I); however, it remains unclear whether treating insomnia leads to sustained reduction and prevention of depression. This randomized controlled trial examined the efficacy of dCBT-I in reducing and preventing depression over a 1-year follow-up period.
Methods
Patients with Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition (DSM-5) insomnia disorder were randomly assigned to receive dCBT-I or an attentional control. The follow-up sample included 358 patients in the dCBT-I condition and 300 patients in the online sleep education condition. The primary outcome measure was relative rate ratios for depression at 1-year follow-up. Insomnia responses to treatment were also tested as predictors of incident depression at the 1-year follow-up.
Results
At 1-year follow-up, depression severity continued to be significantly lower in the dCBT-I condition relative to control. In addition, the number of individuals who reported no depression at 1-year follow-up was 51% higher in the dCBT-I condition relative to control. In those with minimal to no depression at baseline, the incident rate of moderate-to-severe depression at 1-year follow-up was reduced by half in the dCBT-I condition relative to the control condition.
Conclusion
dCBT-I showed robust effects as an intervention that prevents depression. Future research should examine dose–response requirements and further characterize mechanisms of action of dCBT-I for depression prevention.
Clinical Trial
Sleep to Prevent Evolving Affective Disorders; NCT02988375.
Keywords: depression, insomnia, mobile health, prevention, intervention
Statement of Significance.
Though it is clear that cognitive behavioral therapy for insomnia (CBT-I) can reduce depression concomitant with insomnia, this study demonstrates that CBT-I may also prevent incidence of depression symptoms in those with insomnia. Furthermore, this study delivered CBT-I digitally (dCBT-I), which has significant advantages for accessibility and scalability. Future research should improve the implementation and dissemination of dCBT-I.
Introduction
Despite increasing usage of mental health treatments, rates of depression in the United States have remained largely unchanged in the past decades [1]. By 2030, depression is projected to be among the top leading causes of disability worldwide [2]. Against the backdrop of rising health care costs [3, 4], it is clear that focusing on depression treatment alone is insufficient as a public health strategy. Instead, the prevailing call to action is to prioritize depression prevention [5–7]. In addition to being more cost-effective than depression intervention, prevention of depression is exponentially more impactful in reducing disease burden. However, depression prevention is most efficient and effective when robust and modifiable premorbid risk factors are easily identifiable in a timely manner. Unfortunately, many of the well-established risk factors for depression fall short of these requisites, such as sex (i.e. female), family history of depression, chronic illnesses, childhood trauma, social isolation, and stressful life events. Without easily identifiable and modifiable risk targets, prevention efforts must rely on early detection and early intervention [8]. This is consistent with many existing depression prevention programs such as National Depression Screening Day and routine depression screening in primary care.
One underutilized target for depression prevention is insomnia. Decades of research have established that insomnia is not only highly comorbid with depression but actually contributes to its etiology and trajectory. Indeed, insomnia commonly precedes depression [9–14] and increases the odds of incident depression twofold compared to healthy sleepers [12]. In addition, whereas depression symptoms are alleviated when insomnia is treated [15–19], insomnia symptoms commonly persist following depression treatment [20] and are prognostic of shortened remission and increased relapse [21, 22]. Importantly, insomnia is also a well-defined and modifiable risk factor for depression. Insomnia is highly responsive to cognitive behavioral therapy for insomnia (CBT-I), and evidence of its effectiveness has led to the recommendation of CBT-I as the first-line treatment for chronic insomnia [23, 24]. In addition, multiple studies have confirmed that CBT-I reduces concurrent depression without ostensibly targeting non-sleep depression symptoms [9, 10, 13, 19].
Though insomnia is likely a viable target for depression prevention, few studies have examined depression as a long-term outcome following CBT-I. One barrier has been the accessibility of CBT-I, which has been severely limited by several factors including a scarcity of certified providers, geographic distance to providers, and the requirement of 6–8 weeks of direct patient contact [26, 27]. One response to these limitations is the digital delivery of CBT-I (dCBT-I). dCBT-I is fully automated and accessible via a computer and/or mobile devices with Internet connectivity (e.g. phones and tablets). Recent evidence has supported the short-term effectiveness of dCBT-I for both insomnia [23, 24, 28] and depression [17, 29, 30]. In fact, we have demonstrated that depression severity reduces by 50% acutely following dCBT-I (six weekly sessions), even in vulnerable populations (e.g. racial minorities and low socioeconomic status) [17]
Because of its reach and scalability, dCBT-I is particularly well positioned for depression prevention by targeting insomnia as a risk factor. First, given that depression is highly comorbid with insomnia, dCBT-I may achieve secondary or tertiary prevention simply by increasing widespread access to a treatment that interrupts the progression toward more persistent and recurrent depression [31–33]. This is supported by the GoodNight Study, a randomized controlled trial of dCBT-I in individuals with insomnia and subclinical depression. Results showed that the acute antidepressant effect of dCBT-I was maintained for at least 18 months following treatment [30]. This effect has not yet been replicated. A second way that dCBT-I may prevent depression is to reduce or eliminate insomnia as a premorbid risk factor (i.e. indicated prevention). Though this was also tested in the GoodNight Study [29], no significant differences were found potentially due to low incidence of depression at 6-month follow-up (2% incidence of depression in both the dCBT-I and control conditions).
To further examine the impact of dCBT-I on depression prevention, this randomized controlled trial tested the long-term (i.e. 1-year after treatment completion) rate and incidence of moderate–to-severe depression in a large sample of adults with insomnia. We were interested in (1) the durability of the antidepressant effect of dCBT-I 1-year following treatment (i.e. secondary/tertiary prevention), and (2) the incidence of moderate-to-severe depression at 1-year follow-up in those with minimal depression at baseline (i.e. indicated prevention). Finally, we also tested the degree to which clinical targets for insomnia response and remission might protect against incident depression. We hypothesized that the antidepressant effect of dCBT-I will be maintained at 1-year follow-up and that the incidence of moderate-to-severe depression at 1-year follow-up will be lower in the dCBT-I compared to the control condition. We were also interested in testing whether established clinical targets for insomnia response and remission were predictive of depression prevention.
Methods
Data for this study were obtained from the Sleep to PRevent Evolving Affective Disorders (SPREAD) trial (NCT02988375). Recruitment sampled from 6 hospitals, 38 medical centers, and subscribers of a major health insurance company in southeastern Michigan. Recruitment occurred between 2016 and 2017 and used internet-based methods, including health system-wide e-mail newsletters, existing research databases (e.g. Qualtrics and prior research participants who have consented to future research recruitment), and clinic databases (e.g. health system chart review). Interested participants completed a screening survey via an online questionnaire platform (Qualtrics, Provo, UT) that assessed for study eligibility, including sleep disturbance and sleep disorders, psychiatric difficulties, medical comorbidities, and medication use. Eligible participants met Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition (DSM-5) [34] diagnostic criteria for chronic insomnia disorder. Exclusion criteria (assessed via the screening questionnaire) included diagnosed sleep disorders other than insomnia (e.g. restless legs, narcolepsy) or untreated obstructive sleep apnea, and diagnosed bipolar disorder or seizure disorder. Because the SPREAD trial included a depression prevention aim, individuals with high depression chronicity (self-reported daily or near-daily depressed mood and anhedonia) were excluded. Those who reported suicidality on the screener were further assessed using the Columbia-Suicide Severity Rating Scale (C-SSRS) via telephone within 24 hours by research staff certified in conducting the C-SSRS and referred to psychiatric or emergency services when appropriate.
Study design
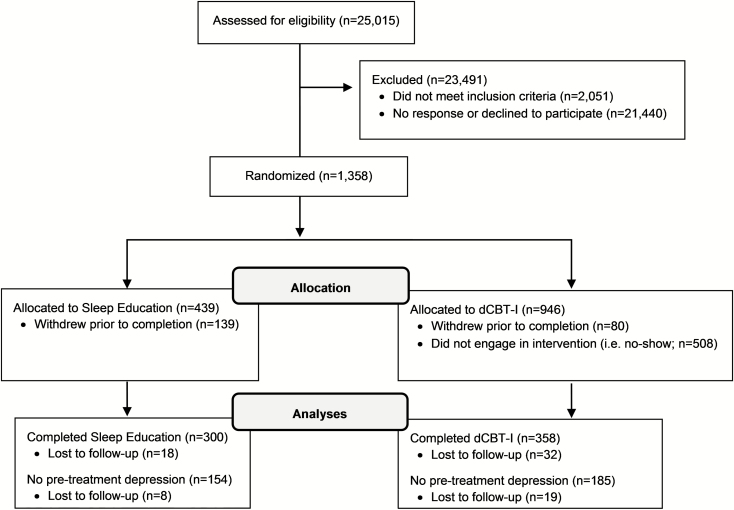
This study used a randomized controlled design with simple randomization into two parallel arms of either dCBT-I or online sleep education (in addition to treatment-as-usual). Simple randomization was computerized and automated centrally through Qualtrics immediately after participants met eligibility criteria. A total of 1385 individuals with insomnia disorder were enrolled and randomized into either the dCBT-I or online sleep education conditions. The research staff was blinded to treatment allocation. Participants were randomized to the dCBT-I condition at a 2:1 ratio due to a higher anticipated attrition rate for an active versus a control condition, as has been previously demonstrated in nearly all internet-based interventions [35]. A total of 358 individuals completed the dCBT-I treatment and 300 completed the online sleep education condition (age range 18–92). See Figure 1 for enrollment flow chart and Table 1 for sample demographics. All procedures were approved by the institutional review board. Informed consent was also given by all participants before any study procedures were executed.
Figure 1.
Flow chart of enrollment. Non-engagement in the intervention (i.e. no-show) was defined as those who did not complete the first session of dCBT-I.
Table 1.
Demographic variables by experimental conditions
| Variables | dCBT-I (N = 358) | Sleep education (N = 300) | ||
|---|---|---|---|---|
| Age | 44.5 | ± 15.8 SD | 45.7 | ± 15.1 SD |
| Sex (female) | 279 | (78.0%) | 240 | (80.0%) |
| Race | ||||
| White | 269 | (75.1%) | 201 | (67.0%) |
| Black | 65 | (18.2%) | 75 | (25.0%) |
| Other | 24 | (3.7 %) | 24 | (8.0%) |
| Education | ||||
| High school or less | 52 | (14.5%) | 44 | (14.7%) |
| Some College | 94 | (26.3%) | 101 | (33.7%) |
| College | 139 | (38.8%) | 88 | (29.3%) |
| Graduate school | 73 | (20.4%) | 67 | (22.3%) |
| Household income | ||||
| Poverty (<15k) | 51 | (14.3%) | 37 | (12.4%) |
| Low (<35k) | 95 | (26.5%) | 96 | (32.0%) |
| Middle (<75k) | 105 | (29.2%) | 85 | (28.3%) |
| Higher (75k +) | 107 | (30.0%) | 82 | (27.3%) |
| Insomnia (ISI) | 17.9 | ± 4.3 SD | 17.7 | ± 4.4 SD |
| Depression (QIDS-SR16) | 10.8 | ± 4.5 SD | 10.8 | ± 4.6 SD |
| None (< 6) | 47 | (13.1%) | 37 | (12.3%) |
| Mild (< 11) | 138 | (38.5%) | 117 | (39.0%) |
| Moderate (< 16) | 116 | (32.4%) | 86 | (28.6%) |
| Severe (<21) | 47 | (13.1%) | 56 | (18.7%) |
| Very Severe (21 +) | 10 | (2.8%) | 4 | (1.3%) |
| QIDS sans sleep items | 8.1 | ± 4.5 SD | 8.0 | ± 4.4 SD |
| Medications for sleep | ||||
| None | 278 | (77.7%) | 239 | (79.7%) |
| Hypnotics | 24 | (6.7%) | 18 | (6.0%) |
| Antidepressants | 31 | (8.7%) | 20 | (6.7%) |
| Benzodiazepines | 15 | (4.2%) | 14 | (4.7%) |
| OTC antihistamines | 41 | (11.5%) | 38 | (12.7%) |
| OTC cold medications | 8 | (2.2%) | 13 | (4.3%) |
| OTC pain medications | 16 | (4.5%) | 20 | (6.7%) |
ISI = Insomnia Severity Index; QIDS-SR16 = Quick Inventory of Depressive Symptomatology; OTC = over the counter.
Measures of interest
Depression was the primary outcome variable and was measured using the 16-item self-report Quick Inventory of Depressive Symptomatology [36] (QIDS-SR16) via the same questionnaire platform as the screening survey. The QIDS-SR16 is a validated and reliable instrument that is used widely in longitudinal clinical trials, including those involving sleep that we and others have conducted [17, 18, 37–39]. The QIDS-SR16 assesses the severity of the nine diagnostic symptom criteria used in Diagnostic and Statistical Manual of Mental Disorders and shows strong consistency with a diagnosis of Major depressive disorder via a structured interview for the DSM [40–42]. Scores range from none (0–5), mild (6–10), moderate (11–15), severe (16–20), and very severe (21–27) [43]. Clinically significant depression was determined using these psychometrically derived severity categories and was operationalized as a score of 11 or higher, corresponding to moderate severity or higher. A “moderate” rather than a “severe” threshold was selected to achieve better balance between sensitivity and specificity; the sensitivity and specificity for a threshold of at least 11 (moderate severity or higher) was 82.4% and 70.3%, respectively, as opposed to 52.9% and 88.4% (severe or very severe) [40]. This threshold can also be considered clinically significant because it is consistent with practices used in common depression screening programs that triage patients for treatment, such as the National Depression Screening Day. Similarly, the threshold for depression remission was determined using a threshold of less than or equal to 6 based on prior psychometric studies and common clinical trial practice [36, 44]. Because the experimental interventions targeted insomnia symptoms, supplemental analyses were completed using only non-sleep items from the QIDS-SR16 (Supplementary materials). Assessments were conducted at pre- and posttreatment, with the final assessment conducted 1 year after concluding treatment (1-year follow-up). Insomnia was measured via the Insomnia Severity Index (ISI) [45, 46].
Covariates
Covariates included sex, age, baseline depression, family history of depression (binary coding based on self-report of paternal or maternal depression), the presence of medical comorbidity (binary coding), season at time of assessment (binary coding: 1 = assessed during Spring/Summer), and baseline anxiety. Anxiety was measured using a single item assessing the frequency of “feeling nervous, anxious, or on edge” within the last 2 weeks, with a four-item Likert response from “Not at all” to “Nearly every day” and coded as an ordinal variable.
dCBT-I condition
Individuals randomized to the dCBT-I condition completed the Sleepio program via the Internet (www.sleepio.com, Big Health Ltd.) Sleepio is among several currently available dCBT-I programs and was selected for this study because it is evidence-based, standardized, fully automated, and has been tested in multiple RCTs comprising almost 7000 participants [17, 19, 47–52]. Participants received access for 12 weeks during which they could take the six core sessions of dCBT-I on a weekly basis. The intervention covered behavioral components (e.g. sleep restriction, stimulus control), cognitive components (e.g. cognitive restructuring, paradoxical intention), relaxation strategies (e.g. progressive muscle relaxation and autogenic training), and sleep hygiene. Sessions were directed by an animated “virtual therapist” who reviews and guides progress with the participant.
Online sleep education
Individuals randomized to the online sleep education condition received six weekly e-mails based on the National Institutes of Health guide to healthy sleep [53] containing information on the following topics: the basics of endogenous sleep regulation; the impact on sleep of health problems such as obesity, diabetes, and hypertension; the effects of sleep disruptive substances, such as caffeine, nicotine, alcohol; and tips on creating a sleep-conducive bedroom environment. Psychoeducation and sleep hygiene were selected because they are common in clinical practice, particularly in primary care [54], and also because they are commonly used as an attention control in clinical trials. Importantly, these are not considered effective standalone treatments for insomnia [55].
Analytical approach
To test the first hypothesis that the antidepressant effect of dCBT-I was maintained 1-year following treatment, improvement in QIDS-SR16 scores (continuous variable) at 1-year follow-up relative to baseline was compared between the dCBT-I and the control conditions via a t-test. These effects were contrasted with the acute antidepressant effect that was previously published. To further characterize the clinical significance of improvements in depression associated with dCBT-I, analysis of depression remission (QIDS-SR16 scores ≤ 6; categorical variable) was also conducted. Depression remission rates between the conditions were compared using a relative rate ratio, and number-needed-to-treat was calculated based on the relative risk ratios. Because the rate of loss to follow-up was higher in the dCBT-I condition (19 lost to follow-up) compared to the control condition (8 lost to follow-up), an intention-to-treat approach (ITT) was used to adjust for potential bias. Data lost to follow-up were handled in the following ways: the depression rate in the control condition was set to zero (i.e. all individuals lost to follow-up in the control condition were assumed to be nondepressed), whereas the depression rate in the dCBT-I group was estimated using maximum likelihood via a generalized linear mixed-effects model1. Supplemental analyses were also conducted using QIDS-SR16 scores with sleep items removed (Supplementary materials).
To test the second hypothesis that incidence of moderate-to-severe depression at 1-year follow-up will be lower in the dCBT-I compared to the control condition, a second ITT analysis was conducted in a subset of individuals who showed minimal to no depression at baseline (QIDS-SR16 ≤ 11; n =339). The incidence of moderate-to-severe depression at 1-year follow-up in the dCBT-I and control conditions were used to compute a relative rate ratio, which was tested for significance at α less than .05. The number-needed-to-treat was also calculated based on the relative rate ratio.
Finally, to further examine and establish potential targets of insomnia improvement to achieve prevention of depression 1 year later, we tested insomnia and its acute response to treatment as predictors of moderate-to-severe depression at 1-year follow-up. This was conducted via generalized linear mixed-effects logistic regression using the full sample. The dependent variable was depression at 1-year follow-up (1 = QIDS-SR16 ≥ 11). The model assessed the significance of the following predictors (coded to represent higher values as worse outcomes): (1) baseline insomnia severity as a continuous variable (ISI at pretreatment); (2) treatment response as a dichotomous variable (0 = improvement in ISI scores ≥ 8 [45]); (3) treatment remission as a dichotomous variable (0 = posttreatment ISI scores ≤ 7 [45]); and (4) maintenance of treatment response as a continuous variable, calculated as a difference score between treatment response at 1-year follow-up and at posttreatment ([follow-up ISI—pretreatment ISI] – [posttreatment ISI—pretreatment ISI]). Age, sex, baseline depression levels, family history of depression, baseline anxiety levels, medical comorbidity, and season at time of assessment were tested as covariates and removed from the final model if nonsignificant (p > .1). Statistical significance was set at p-value less than .05 for all final models.
Results
Durability of antidepressant response of dCBT-I at 1-year follow-up
Baseline sample characteristics suggested that the insomnia severity in this sample was higher compared to other large dCBT-I trials [19, 30] (Table 1). Depression severity at baseline was not different between the two conditions. As expected, high comorbidity between insomnia and depression was also observed, with approximately half the sample reporting moderate-to-severe depression (QIDS-SR16 ≥ 11) at baseline (dCBT-I: 48.3%, 95% CI [43.1 to 53.5], control: 48.7%, 95% CI [43.0 to 54.5], p = .99).
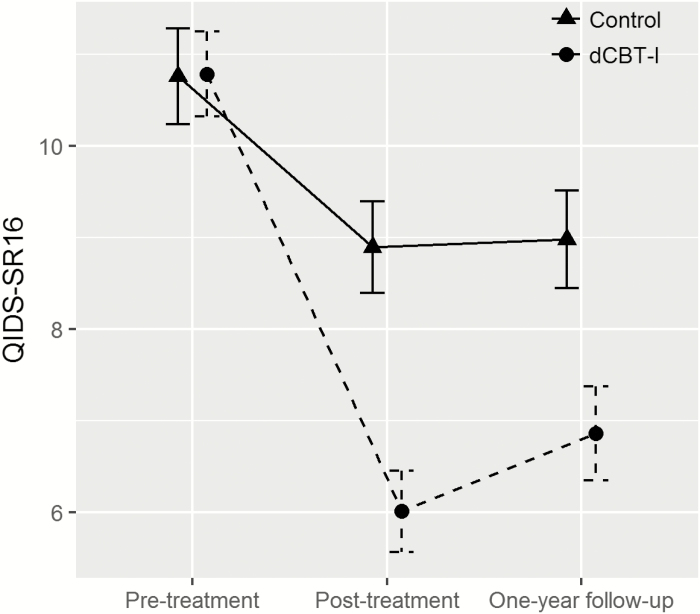
At 1-year follow-up, average improvement in QIDS-SR16 scores in the dCBT-I condition (4.0 ± 5.0 SD point decrease) was over twofold that of the control condition (1.7 ± 4.7 SD point decrease), t(603.28) = –5.98. p < .001. Compared to the previously published acute effects at posttreatment (4.8 ± 5.0 SD point decrease at posttreatment) [17], the antidepressant effect at 1-year follow-up in the dCBT-I condition was only slightly attenuated. In the control condition, change in the antidepressant response at 1-year follow-up was minimal compared to acute effects posttreatment (1.9 ± 3.9 SD point decrease at posttreatment). See Figure 2 for QIDS-SR16 at each time point. To evaluate the clinical significance of the antidepressant effect, analyses also examined the proportion of individuals reporting moderate-to-severe depression 1-year following treatment. Results revealed lower rates of moderate-to-severe depression in the dCBT-I condition (20.9%, 95% CI [16.5 to 25.3]) compared to the control condition (35.5%, 95% CI [29.9 to 41.1]).
Figure 2.
Change in depression severity from pretreatment to 1-year follow-up. Error bars represent 95% confidence intervals. QIDS-SR16 = 16 item self-report Quick Inventory of Depressive Symptomatology.
In examining rates of depression remission (QIDS-SR16 ≤ 6) 1 year after treatment, the ITT analysis also showed higher rates of depression remission in the dCBT-I condition (56.4%, 95% CI [51.3 to 61.6]) compared to the control condition (37.3%, 95% CI [31.9 to 42.8]). On the basis of this, the relative rate ratio for depression remission at 1-year follow-up was 1.51, 95% CI [1.27 to 1.80], p < .001, indicating that remission rates were 51% higher in the dCBT-I compared to the control condition. The number-needed-to-treat indicated that six insomnia patients would need to be treated with dCBT-I to achieve one case of depression remission at 1-year follow-up.
Prevention of depression 1 year later
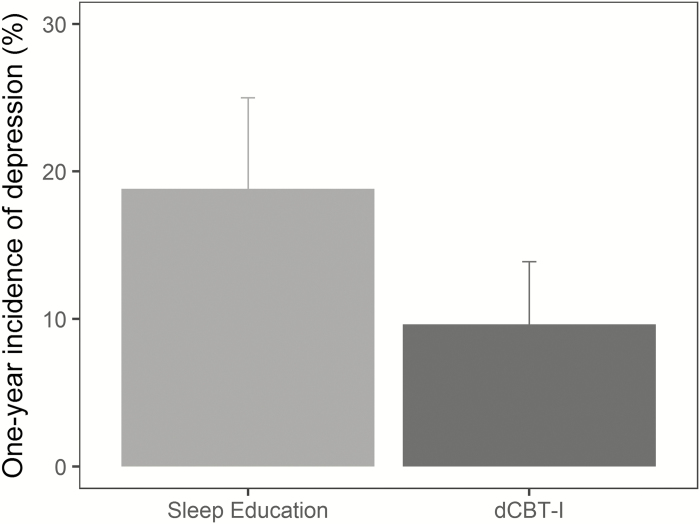
Among individuals with minimal to no depression at baseline, the ITT model suggested that incidence of moderate–to-severe depression at 1-year follow-up was 18.8% in the control condition compared to 9.6% in the dCBT-I condition (Figure 3). The relative rate ratio for incident depression at 1-year follow-up was 0.51, 95% CI [0.26 to 0.81], p < .01, indicating that receiving dCBT-I reduced the risk of developing moderate-to-severe depression by approximately half compared to the control condition.
Figure 3.
One-year incidence of moderate-to-severe depression is lower in the dCBT-I compared to the control condition. Error bars represent 95% confidence intervals.
The number-needed-to-treat based on the ITT results indicated that 11 insomnia patients with minimal to no depression at baseline would need to be treated with dCBT-I to prevent one case of moderate-to-severe depression 1 year later. This suggests that if 100 insomnia patients with minimal to no depression at baseline were provided sleep education, 19 would report moderate-to-severe depression 1-year later; however, half (i.e. 9 or 10) of the 19 cases would have been prevented if they were provided with dCBT-I instead.
How is insomnia response to treatment associated with depression 1 year later?
To examine and establish potential targets of insomnia improvement for depression prevention (e.g. insomnia response and/or remission), a logistic regression tested insomnia and treatment response as predictors of moderate-to-severe depression at 1-year follow-up (Table 2). Model testing of covariates indicated that age, family history of depression, and medical comorbidity were not significant and thus were removed from the final model. Multicollinearity was ruled out based on variance inflation factors, which were all below 2.0. Results revealed that baseline insomnia severity significantly predicted the development of moderate-to-severe depression 1 year later: each additional point on the ISI at baseline increased the odds of depression by 11%, OR = 1.11, 95% CI [1.04 to 1.18], p < .001, above and beyond established risk factors for depression (sex, baseline depression, and baseline anxiety). Acute ISI response to treatment also predicted depression 1 year later. Importantly, odds of developing moderate-to-severe depression were significantly higher for those who did not achieve a clinically significant treatment response (OR = 3.51, 95% CI [1.87 to 6.56], p < .001) or remission (OR = 2.96, 95% CI [1.52 to 5.76], p < .01). Finally, the durability of treatment gains was also an important predictor of developing moderate-to-severe depression: each one-point resurgence on the ISI 1 year after treatment was associated with a 22% increase in odds of depression incidence, OR = 1.22, 95% CI [1.16 to 1.28], p < .001.
Table 2.
Predictors of moderate-to-severe depression 1 year after treatment
| Predictors | B | SE B | P-value | OR | 95% CI OR |
|---|---|---|---|---|---|
| Baseline ISI | 0.10 | 0.03 | <.001 | 1.11 | [1.04 to 1.18] |
| No insomnia response | 1.25 | 0.33 | <.001 | 3.51 | [1.87 to 6.56] |
| No insomnia remission | 1.09 | 0.34 | .001 | 2.96 | [1.52 to 5.76] |
| Maintenance of ISI | 0.20 | 0.03 | <.001 | 1.22 | [1.17 to 1.28] |
No insomnia response was a binary variable (1 = change in ISI at posttreatment < 8). No insomnia remission was a binary variable (1 = posttreatment ISI > 7). Maintenance of ISI was a difference score between the ISI treatment response between posttreatment and 1-year follow-up, with higher value representing recurrence of insomnia severity at 1-year follow-up. This analysis adjusted for baseline depression, sex, baseline anxiety, and season during assessment.
Discussion
This randomized controlled trial examined the impact of digitally delivered insomnia treatment on depression prevention through both maintenance of antidepressant effects and reduced incidence of moderate-to-severe depression 1 year following treatment. Results confirmed that the antidepressant effect of dCBT-I was not only maintained 1 year later but also that the rate of depression remission (QIDS-SR16 ≤ 6) remained higher in the dCBT-I condition relative to the control group. Critically, incidence of moderate-to-severe depression 1 year after treatment was reduced by approximately half (relative risk ratio = 0.51) in the dCBT-I compared to the control condition in those with minimal to no depression at baseline. Moreover, the effect of depression prevention was maintained even when the sleep items were removed (relative risk ratio = 0.36, 95% CI [0.20 to 0.65]; see supplemental analyses for more details), indicating that the results cannot be solely explained by improvements in sleep-related symptoms. Finally, it is noteworthy that the significant improvements in depression were achieved and sustained without any clinician support and were also achieved in a sample that included individuals who reported concurrent and stable use of hypnotics, antidepressants, and other pharmacotherapies (Table 1).
These results add novel information about depression prevention to previous studies that have consistently demonstrated the antidepressant effect of CBT-I, both acutely [15, 16, 19, 25, 56] and with longer-term follow-up [29, 30]. In comparison to prior insomnia and depression studies, this sample had higher representation of racial minorities, individuals with low socioeconomic status (i.e. low income and/or education), and slightly more females. These differences likely explain the higher insomnia severity and depression rates in this sample compared to previous studies, particularly as sex, race, and socioeconomic status are established risk factors for insomnia and depression. In addition, the generalizability of results is supported with a sample recruited from an array of real-world health care settings, including hospitals, primary care clinics, and insurance subscribers.
The magnitude of depression prevention found in this study was also stronger relative to the overall effect of depression prevention programs derived from a meta-analysis of 32 prospective studies (relative risk ratio = 0.79; 21% reduction of depression incidence) [8]. One explanation for the strong effects found in this study may be that insomnia is a robust and modifiable predictor of depression. Without modifiable risk targets, preventive medicine must often rely on early detection (e.g. National Depression Screening Day) and early intervention (e.g. CBT for depression, antidepressants) [8]. In contrast, insomnia (1) is an independent and well-defined risk factor that commonly precedes depression, (2) is frequently and easily identified in primary care settings, and finally, (3) can be effectively treated using an independent, well-defined, and highly effective intervention. As such, the successful reduction of insomnia as either a concurrent or premorbid risk factor could engender larger effects on depression prevention compared to early detection and intervention. Importantly, our results indicate that the clinical targets for insomnia response (i.e. an improvement of over eight points on the ISI [45]) and remission (i.e. posttreatment ISI ≤ 7 [48]) are also relevant targets for depression prevention. This finding is consistent with prior studies showing that similar sleep improvements were associated with acute changes in mental health [19, 48].
Together, results from this study further highlight the importance of working toward dissemination of dCBT-I as a first-line intervention for insomnia. However, one critical area of improvement is to increase the utilization and uptake of dCBT-I. This is particularly relevant for underserved and low-income populations who stand to benefit the most from the increased accessibility of dCBT-I [17]. Factors that may facilitate utilization of dCBT-I include the addition of therapist support and increased tailoring of the intervention to specific circumstances (e.g. nontraditional work schedules). Treatment utilization and adherence is a problem endemic to internet-delivered psychotherapy interventions, with attrition rates commonly falling between 50% and 83% [30, 57, 58] and should be the focus of research in the dissemination and implementation of dCBT-I. This is critical because dCBT-I can have substantial impact due to high scalability, sustainability, and increased equity [17] of using a web-based approach. For example, dCBT-I is well positioned to be integrated into primary care, which is the most common first point of contact for patients with insomnia and comorbid depression [59, 60]. Specifically, a stepped-care approach starting with dCBT-I may be ideal [61] because it elevates underresponders and/or nonresponders to a higher level of treatment with a specialist. In fact, results suggest that patients who do not achieve a clinically significant insomnia response (i.e. reduction of ISI ≥ 8 [45]) or insomnia remission acutely following dCBT-I (i.e. posttreatment ISI ≤ 7) remain at greater risk for developing moderate-to-severe depression, and would likely benefit from more intensive and personalized approaches (e.g. face-to-face clinician administered CBT-I).
Limitations and future directions
Results of this study should be interpreted in light of some limitations. First, cases of depression in this study did not use clinician-evaluated diagnosis, though validation of the QIDS-SR16 against clinical diagnosis indicates adequate sensitivity and specificity at the cutoff selected (82.4% and 70.3%, respectively [40]). In addition, the use of a self-report instrument to screen for depression is generalizable to clinical practice and existing depression prevention programs (e.g. National Depression Screening Day). The threshold used in this study is also clinically relevant because it is consistent with common clinical protocols for triaging patients to depression treatment. Second, this study only examined depression associated with an insomnia disorder. Given the heterogeneity of depression, this is likely a subsample—albeit substantial—of individuals who are at-risk for depression.
Another limitation was the higher lost to follow-up rates in the dCBT-I relative to the control condition; however, both statistical and clinical significance were detected using an intention-to-treat approach, suggesting that the results were robust to potential bias. Furthermore, attrition is an inherent and endemic problem in Internet-based treatments, with dropout rates as high as 81% [62]. Nonetheless, generalizations using the exact parameter estimates should be tempered until they have been replicated. Future implementation research should also seek to better understand how attrition may be improved for dCBT-I, including exploring adaptations or enhancements of dCBT-I for specific populations.
Conclusion
This study provided evidence that digitally delivered cognitive behavioral treatment for insomnia (dCBT-I) is not only an effective treatment for comorbid insomnia and depression but is also highly efficacious intervention for the prevention of depression. Given the wide accessibility and utilization of digital health technology and self-guided health management, dCBT-I has potential for much larger-scale treatment and prevention. Critical next steps include efforts to increase utilization and uptake of dCBT-I and to test the implementation dCBT-I in a stepped-care framework combined with a higher level of care involving face-to-face approaches to fully optimize patient outcomes for insomnia and depression.
Funding
Support for this study was provided through a Pioneer Award to CLD from the Robert Wood Johnson Foundation (Sleep to PRevent Evolving Affective Disorders; SPREAD), and from the National Institute of Mental Health R56MH115150 awarded to C.L.D. Funding for P.C. was provided from the National Heart Lung and Blood Institute (K23HL138166). We would also like to thank David Adler for his continued support of our research program.
Supplementary Material
Acknowledgments
Dr. Espie reports being a co-founder, chief medical officer, and shareholder of and receiving salary from Big Health Ltd and being a developer of Sleepio. No other authors have financial disclosures.
Footnotes
Other methods of data imputation, including last observation carried forward, were tested and yielded similar results.
Disclosure
Dr. Drake reports receiving nonfinancial support from Big Health Ltd (provision of Sleepio for use in clinical trials). No other authors have non-financial disclosures.
References
- 1. Mojtabai R, et al. Trends in psychological distress, depressive episodes and mental health treatment-seeking in the United States: 2001-2012. J Affect Disord. 2015;174:556–561. [DOI] [PubMed] [Google Scholar]
- 2. World Health Organization. The global burden of disease: 2004 update. 2008. [Google Scholar]
- 3. Banthin JS, et al. Financial burden of health care, 2001-2004. Health Aff (Millwood). 2008;27(1):188–195. [DOI] [PubMed] [Google Scholar]
- 4. Bush M. Addressing the root cause: rising health care costs and social determinants of health. N C Med J. 2018;79(1):26–29. [DOI] [PubMed] [Google Scholar]
- 5. Cuijpers P, et al. Preventing depression: a global priority. JAMA. 2012;307(10):1033–1034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Muñoz RF, et al. Prevention of depression worldwide: a wake-up call. Lancet Psychiatry. 2016;3(4):306–307. [DOI] [PubMed] [Google Scholar]
- 7. Ebert DD, et al. It is time to invest in the prevention of depression. JAMA Netw Open. 2018;1(2):e180335. [DOI] [PubMed] [Google Scholar]
- 8. van Zoonen K, et al. Preventing the onset of major depressive disorder: a meta-analytic review of psychological interventions. Int J Epidemiol. 2014;43(2):318–329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Mahowald M. Book review sleep disorders and sleep deprivation: an unmet public health problem by the committee on sleep medicine and research. N Engl J Med. 2007;356(2):199–200. [Google Scholar]
- 10. Li SX, et al. Nocturnal sleep disturbances as a predictor of suicide attempts among psychiatric outpatients: a clinical, epidemiologic, prospective study. J Clin Psychiatry. 2010;71(11):1440–1446. [DOI] [PubMed] [Google Scholar]
- 11. McCall WV, et al. Insomnia severity is an indicator of suicidal ideation during a depression clinical trial. Sleep Med. 2010;11(9):822–827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Baglioni C, et al. Insomnia as a predictor of depression: a meta-analytic evaluation of longitudinal epidemiological studies. J Affect Disord. 2011;135(1-3):10–19. [DOI] [PubMed] [Google Scholar]
- 13. Batterham PJ, et al. Sleep disturbance, personality and the onset of depression and anxiety: prospective cohort study. Aust N Z J Psychiatry. 2012;46(11):1089–1098. [DOI] [PubMed] [Google Scholar]
- 14. Pigeon WR, et al. Meta-analysis of sleep disturbance and suicidal thoughts and behaviors. J Clin Psychiatry. 2012;73(9):e1160–e1167. [DOI] [PubMed] [Google Scholar]
- 15. Manber R, et al. Cognitive behavioral therapy for insomnia enhances depression outcome in patients with comorbid major depressive disorder and insomnia. Sleep. 2008; 31(4): 489–495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Manber R, et al. CBT for insomnia in patients with high and low depressive symptom severity: adherence and clinical outcomes. J Clin Sleep Med. 2011;7(6):645–652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Cheng P, et al. Efficacy of digital CBT for insomnia to reduce depression across demographic groups: a randomized trial. Psychol Med. 2018;49(3):1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Cunningham JEA, et al. Cognitive behavioural therapy for insomnia (CBT-I) to treat depression: a systematic review. J Psychosom Res. 2018;106:1–12. [DOI] [PubMed] [Google Scholar]
- 19. Freeman D, et al. The effects of improving sleep on mental health (OASIS): a randomised controlled trial with mediation analysis. Lancet Psychiatry. 2017;4(10):749–758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Lustberg L, et al. Depression and insomnia: questions of cause and effect. Sleep Med Rev. 2000;4(3):253–262. [DOI] [PubMed] [Google Scholar]
- 21. Dew MA, et al. Temporal profiles of the course of depression during treatment. Predictors of pathways toward recovery in the elderly. Arch Gen Psychiatry. 1997;54(11):1016–1024. [DOI] [PubMed] [Google Scholar]
- 22. Reynolds CF 3rd, et al. Which elderly patients with remitted depression remain well with continued interpersonal psychotherapy after discontinuation of antidepressant medication? Am J Psychiatry. 1997;154(7):958–962. [DOI] [PubMed] [Google Scholar]
- 23. Qaseem A, et al. ; Clinical Guidelines Committee of the American College of Physicians Management of chronic insomnia disorder in adults: a clinical practice guideline from the American College of Physicians. Ann Intern Med. 2016;165(2):125–133. [DOI] [PubMed] [Google Scholar]
- 24. Riemann D, et al. European guideline for the diagnosis and treatment of insomnia. J Sleep Res. 2017;26(6):675–700. [DOI] [PubMed] [Google Scholar]
- 25. Taylor DJ, et al. Cognitive and behavioural therapy for insomnia (CBT-I) in psychiatric populations: a systematic review. Int Rev Psychiatry. 2014;26(2):205–213. [DOI] [PubMed] [Google Scholar]
- 26. Koffel E, et al. Increasing access to and utilization of cognitive behavioral therapy for insomnia (CBT-I): a narrative review. J Gen Intern Med. 2018;33(6):955–962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Thomas A, et al. Where are the behavioral sleep medicine providers and where are they needed? A geographic assessment. Behav Sleep Med. 2016;14(6):687–698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Zachariae R, et al. Efficacy of internet-delivered cognitive-behavioral therapy for insomnia—a systematic review and meta-analysis of randomized controlled trials. Sleep Med Rev. 2016;30:1–10. [DOI] [PubMed] [Google Scholar]
- 29. Christensen H, et al. Effectiveness of an online insomnia program (SHUTi) for prevention of depressive episodes (the GoodNight Study): a randomised controlled trial. Lancet Psychiatry. 2016;3(4):333–341. [DOI] [PubMed] [Google Scholar]
- 30. Batterham PJ, et al. Trajectories of change and long-term outcomes in a randomised controlled trial of internet-based insomnia treatment to prevent depression. BJPsych Open. 2017;3(5):228–235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Keller MB, et al. Predictors of relapse in major depressive disorder. JAMA. 1983;250(24):3299–3304. [PubMed] [Google Scholar]
- 32. Mueller TI, et al. Recurrence after recovery from major depressive disorder during 15 years of observational follow-up. Am J Psychiatry. 1999;156(7):1000–1006. [DOI] [PubMed] [Google Scholar]
- 33. Keller MB, et al. Time to recovery, chronicity, and levels of psychopathology in major depression. A 5-year prospective follow-up of 431 subjects. Arch Gen Psychiatry. 1992;49(10):809–816. [DOI] [PubMed] [Google Scholar]
- 34. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders (DSM-5®). Arlington, VA: American Psychiatric Pub; 2013. [Google Scholar]
- 35. Christensen H, et al. Adherence in internet interventions for anxiety and depression. J Med Internet Res. 2009;11(2):e13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Rush AJ, et al. The 16-item quick inventory of depressive symptomatology (QIDS), clinician rating (QIDS-C), and self-report (QIDS-SR): a psychometric evaluation in patients with chronic major depression. Biol Psychiatry. 2003;54(5):573–583. [DOI] [PubMed] [Google Scholar]
- 37. Landsness EC, et al. Antidepressant effects of selective slow wave sleep deprivation in major depression: a high-density EEG investigation. J Psychiatr Res. 2011;45(8):1019–1026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Kalmbach DA, et al. Shift work disorder, depression, and anxiety in the transition to rotating shifts: the role of sleep reactivity. Sleep Med. 2015;16(12):1532–1538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Taylor DJ, et al. A pilot randomized controlled trial of the effects of cognitive-behavioral therapy for insomnia on sleep and daytime functioning in college students. Behav Ther. 2014;45(3):376–389. [DOI] [PubMed] [Google Scholar]
- 40. Lamoureux BE, et al. Using the QIDS-SR16 to identify major depressive disorder in primary care medical patients. Behav Ther. 2010;41(3):423–431. [DOI] [PubMed] [Google Scholar]
- 41. Yeung A, et al. The Quick Inventory of Depressive Symptomatology, clinician rated and self-report: a psychometric assessment in Chinese Americans with major depressive disorder. J Nerv Ment Dis. 2012;200(8):712–715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Surís A, et al. Psychometric validation of the 16 Item Quick Inventory of depressive symptomatology self-report version (QIDS-SR16) in military veterans with PTSD. J Affect Disord. 2016;202:16–22. [DOI] [PubMed] [Google Scholar]
- 43. Rush AJ, et al. An evaluation of the quick inventory of depressive symptomatology and the Hamilton Rating Scale for depression: a sequenced treatment alternatives to relieve depression trial report. Biol Psychiatry. 2006;59(6):493–501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Trivedi MH, et al. The inventory of depressive symptomatology, clinician rating (IDS-C) and Self-Report (IDS-SR), and the Quick Inventory of Depressive Symptomatology, Clinician Rating (QIDS-C) and Self-Report (QIDS-SR) in public sector patients with mood disorders: a psychometric evaluation. Psychol Med. 2004;34(1):73–82. [DOI] [PubMed] [Google Scholar]
- 45. Morin CM, et al. The Insomnia Severity Index: psychometric indicators to detect insomnia cases and evaluate treatment response. Sleep. 2011;34(5):601–608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Thorndike FP, et al. Validation of the insomnia severity index as a web-based measure. Behav Sleep Med. 2011;9(4):216–223. [DOI] [PubMed] [Google Scholar]
- 47. Espie CA, et al. A randomized, placebo-controlled trial of online cognitive behavioral therapy for chronic insomnia disorder delivered via an automated media-rich web application. Sleep. 2012;35(6):769–781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Espie CA, Emsley R, Kyle SD, et al. Effect of digital cognitive behavioral therapy for insomnia on health, psychological well-being, and sleep-related quality of life: a randomized clinical trial. JAMA Psychiatry. 2019;76(1):21–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Pillai V, et al. The anxiolytic effects of cognitive behavior therapy for insomnia: preliminary results from a web-delivered protocol. 2015;2(2):a–7. [PMC free article] [PubMed] [Google Scholar]
- 50. Bostock S, et al. Sleep and productivity benefits of digital cognitive behavioral therapy for insomnia: a randomized controlled trial conducted in the workplace environment. J Occup Environ Med. 2016;58(7):683–689. [DOI] [PubMed] [Google Scholar]
- 51. Barnes CM, et al. Helping employees sleep well: effects of cognitive behavioral therapy for insomnia on work outcomes. J Appl Psychol. 2017;102(1):104–113. [DOI] [PubMed] [Google Scholar]
- 52. McGrath ER, et al. Sleep to lower elevated blood pressure: a randomized controlled trial (SLEPT). Am J Hypertens. 2017;30(3):319–327. [DOI] [PubMed] [Google Scholar]
- 53. National Institutes of Health. Your guide to healthy sleep. 2011. [Google Scholar]
- 54. Buysse DJ, et al. Effects of diagnosis on treatment recommendations in chronic insomnia–a report from the APA/NIMH DSM-IV field trial. Sleep. 1997;20(7):542–552. [DOI] [PubMed] [Google Scholar]
- 55. Morgenthaler T, et al. ; American Academy of Sleep Medicine Practice parameters for the psychological and behavioral treatment of insomnia: an update. An American Academy of sleep medicine report. Sleep. 2006;29(11):1415–1419. [PubMed] [Google Scholar]
- 56. Ye Y, Zhang Y, Chen J, et al. Internet-based cognitive behavioral therapy for insomnia (ICBT-i) improves comorbid anxiety and depression—a meta-analysis of randomized controlled trials. PLoS One.. 2015;10(11):e0142258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Watson HJ, et al. Predictors of dropout in face-to-face and internet-based cognitive-behavioral therapy for bulimia nervosa in a randomized controlled trial. Int J Eat Disord. 2017;50(5):569–577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Yeung WF, et al. Predictors of dropout from internet-based self-help cognitive behavioral therapy for insomnia. Behav Res Ther. 2015;73:19–24. [DOI] [PubMed] [Google Scholar]
- 59. Aikens JE, et al. Help-seeking for insomnia among adult patients in primary care. J Am Board Fam Pract. 2005;18(4):257–261. [DOI] [PubMed] [Google Scholar]
- 60. Morin CM, et al. Epidemiology of insomnia: prevalence, self-help treatments, consultations, and determinants of help-seeking behaviors. Sleep Med. 2006;7(2):123–130. [DOI] [PubMed] [Google Scholar]
- 61. Manber R, et al. A step towards stepped care: delivery of CBT-I with reduced clinician time. Sleep Med Rev. 2015;19:3–5. [DOI] [PubMed] [Google Scholar]
- 62. Melville KM, et al. Dropout from Internet-based treatment for psychological disorders. Br J Clin Psychol. 2010;49(Pt 4):455–471. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.