Abstract
Study Objectives
Disturbed overnight sleep is a prominent feature of advanced stage Huntington’s disease (HD). Several polysomnography (PSG) studies have reported significant changes of sleep in HD patients, but the findings are not unequivocal. To date, no meta-analysis has investigated the PSG changes in HD patients. The present study meta-analyzed results from studies examining the PSG changes in HD patients compared with controls.
Methods
A literature search performed in MEDLINE, EMBASE, All EBM databases, PsycINFO, and CINAHL databases identified seven studies involving 152 HD patients and 144 controls which were included in our meta-analysis.
Results
Pooled results indicated decreased sleep efficiency, percentage of slow wave sleep and rapid eye movement sleep, and increased percentage of N1 sleep, wake time after sleep onset, and rapid eye movement sleep latency in HD patients compared with controls. We found high heterogeneity in the effect sizes and no indication of systematic publication biases across studies. Meta-regression analyses showed that some of the heterogeneity was explained by age, body mass index (BMI), CAG repeat length, and disease severity of HD patients.
Conclusions
Our study showed that polysomnographic abnormalities are present in HD. Our findings also underscore the need for a comprehensive PSG assessment of sleep changes in patients with HD. Furthermore, the effects of age, BMI and CAG repeat length on sleep changes should be carefully considered and closely monitored in the management of HD.
Keywords: Huntington’s disease, polysomongraphy, meta-analysis
Statement of Significance.
Studies exploring PSG changes in Huntington’s disease (HD) have not established the extent and type of changes in sleep associated with the disease. We performed a meta-analysis which included a relatively large sample size to investigate the polysomongraphic changes in HD compared with controls. This study showed that polysomnographic abnormalities (i.e. decreased sleep efficiency, slow wave sleep, rapid eye movement sleep, and increased wake time after sleep onset) are present in HD. Our findings underscore the need for a comprehensive PSG assessment of sleep changes in patients with HD.
Introduction
Huntington’s disease (HD), a progressive neurodegenerative disorder caused by an abnormal expansion of a CAG repeat sequence on chromosome 4p16.3 [1], affects approximately 4–8 individuals per 100,000 in the general population [2]. HD is characterized by progressively worsening motor dysfunction, cognitive abnormalities, psychiatric symptoms, weight loss, autonomic nervous system dysfunction, and sleep and circadian rhythm disturbances.
Sleep disturbances are prevalent in HD patients with a reported rate that is higher than that in controls (58.1% vs. 34.9%) [3]. Disturbed overnight sleep also is a prominent feature of advanced disease, and is associated with the impairment in cognitive and functional performance of HD patients [3, 4]. Even in early stage HD, significant sleep disturbances and altered rest-activity patterns may be detected [5]. Previous reports indicate that both pre- and symptomatic HD patients with sleep disturbances have significantly poorer neuropsychiatric outcomes and accelerated thalamic degeneration compared with patients without sleep problems [6]. These results highlight the importance of assessing sleep disturbances in the management of HD, which may offer a potentially important therapeutic target [5, 7]. However, sleep problems in HD tend to go undiagnosed by physicians and underreported by patients. This may result from a lack of insight or a perceived relative unimportance for sleep problems compared with the motor, affective, and cognitive features that are recognized as key features of HD [8].
The assessment of sleep disturbances includes self-report questionnaire, sleep diary, face-to-face interview, actigraphy, and polysomnography (PSG). Among these methods, PSG is required to distinguish rapid eye movement (REM) sleep and non-REM sleep, and stages 1–3 (N1–N3) of non-REM sleep. However, PSG studies in HD patients are rare. This may be due to (1) difficulties in recruiting a large sample size due to relatively low incidence of HD in the general population, which may provide insufficient statistical power; and (2) the methodological challenges in putting patients who exhibit complex combinations of motor, cognitive, and psychiatric features through the relatively intense protocols required for sleep research, which makes PSG difficult to perform in patients with HD.
A few prior studies have examined PSG changes in HD, but findings regarding the exact changes of total sleep time (TST) [7, 9, 10], slow wave sleep (SWS) [11, 12], and REM percentage [7, 12] in HD compared with controls are still not fully established due to inconsistencies among results. Pooled analyses via meta-analytic techniques can be useful for resolving discrepancies across studies as they can provide a relatively large sample size for exploring differences in PSG characteristics between HD patients and controls. They also enable investigation of factors associated with variations of results across studies. To our knowledge, no meta-analysis study on PSG evaluated sleep in HD has been conducted to date. The present review covers case–control studies, and uses a meta-analytic approach to identify the pooled effect size (and range of credible values) for PSG changes in patients with HD compared with controls. We also identify moderators that could explain heterogeneity across studies.
Methods
Standard protocol approvals and registrations
The methodology for this study follows PROSPERO protocol CRD42018096632 registered June 6, 2018 in accordance with the preferred reporting items for systematic reviews and meta-analyses statement [13].
Inclusion criteria
The inclusion criteria were constructed (assembled, put together) using the PICOS acronym: Participants (P) were patients with HD according to clinical symptoms and genetic test/family history. Intervention (I) was not applicable because this is a systematic review and meta-analysis of observational studies. Comparison (C) was healthy controls. Outcomes (O) were PSG parameters of sleep. Study design (S) was limited to case-control studies. Other inclusion criteria required that the reviewed studies had been published in English and were obtained from peer-reviewed journals.
Exclusion criteria
By title and abstract screening, we excluded: (1) animal studies; (2) case reports, case series, guidelines, statements, and comments; (3) reviews unrelated to sleep or psychiatry; (4) studies unrelated to HD; and (5) studies in which it was clearly stated in the abstract that no PSG was done and no control group was investigated. By full text screening, we excluded studies: (1) with diagnosis of HD not based on clinical symptoms and genetic test/family history; (2) not using controls; (3) not having a whole-night PSG record; and (4) containing no information on the outcomes of interest.
Information sources
MEDLINE via OVID (up to September 16, 2018); EMBASE via OVID (up to September 16, 2018); All EBM databases via OVID (up to September 16, 2018); PsycINFO via EBSCO (up to September 16, 2018); CINAHL via EBSCO (up to September 16, 2018).
Search
The search strategies for all databases are included in Supplementary Tables S1–S5. The reference lists of all primary studies and review articles were checked for additional references.
Study selection
Two investigators (Y.Z. and R.R.) selected relevant publications independently according to the eligibility criteria. Any disagreement was resolved by thorough discussion and consultation with the senior author (X.T.). When the same group of authors published more than one article using data from the same group of subjects, we considered it as one set of comparisons and used the most comprehensive dataset that was available.
Data collection process
Two investigators (Y.Z. and R.R.) extracted the data independently using a pre-designed form. Disagreements were resolved by thorough discussion and consultation with the senior author (X.T.). The data were entered by a single author (Y.Z.) and verified by two reviewers (Y.Z. and R.R.). Data were obtained from the original articles and by contacting the authors when necessary. The PSG variables examined in this review include TST, wake after sleep onset (WASO), sleep efficiency (SE), and percentage of stage N1, N2, N3, and REM sleep, and REM latency. In the scoring rules of the American Academy Sleep Medicine (AASM), N3 represents SWS and also replaces stage 3 and stage 4 sleep in the Rechtschaffen and Kales (R&K) nomenclature [14]. Thus, the data for stage 3 and stage 4 sleep in the included studies were also extracted for estimating SWS. Additional PSG variables include periodic limb movements index (PLMI), apnea hypopnea index (AHI), and arousal index (AI). Demographic and clinical variables extracted include the number of subjects and their mean age, sex (female percentage), body mass index (BMI), HD severity scored by validity scales, CAG repeat length, adaptation nights (yes/no), study location (in sleep lab/at home), and PSG scoring rules (AASM/R&K).
Quality assessment
A risk of bias assessment was performed (Y.Z. and R.R.) using an adapted version of the National Institute for Health and Care Excellence (NICE) checklist [15]. This checklist assisted in reviewing studies for internal validity by methodically appraising the selection of case–control studies, confounding factors and statistical methods [15].
Statistical analysis
The meta-analysis was conducted using the Comprehensive Meta-Analysis software program. To estimate the aggregate effect-size (standardized mean difference [SMD]) for the differences in PSG variables between patients with HD and controls, the mean and standard deviation and sample size for each group were entered for calculation. For studies which did not provide measures of SWS, but reported S3 and S4 data, the mean values of S3 and S4 effect sizes were used as the SWS effect size. For each global effect-size estimate, the Q statistic and I2 were calculated to examine the presence and magnitude of heterogeneity, and to inform on the degree of overlap between the 95% confidence intervals (CIs) of different studies. I2 values of 75%, 50%, and 25% are considered to indicate high, moderate, and low heterogeneity [16]. The random effects model was used if significant heterogeneity was found; otherwise, the fixed effects model was applied. Publication bias was tested using the Egger regression method [17], with p values of <0.05 suggesting the presence of bias.
An analysis was carried out to analyze potential factors that could moderate heterogeneity across studies. The following predefined moderators were investigated: age, female percentage, BMI, CAG repeat length, disease severity, adaptation night (yes vs. no), and PSG scorning methods (R&K vs. AASM). A subgroup analysis or meta-regression analysis was performed depending on whether the moderators were categorical or continuous variables. The presence of between-group differences was determined by testing the heterogeneity between groups.
Results
Study selection
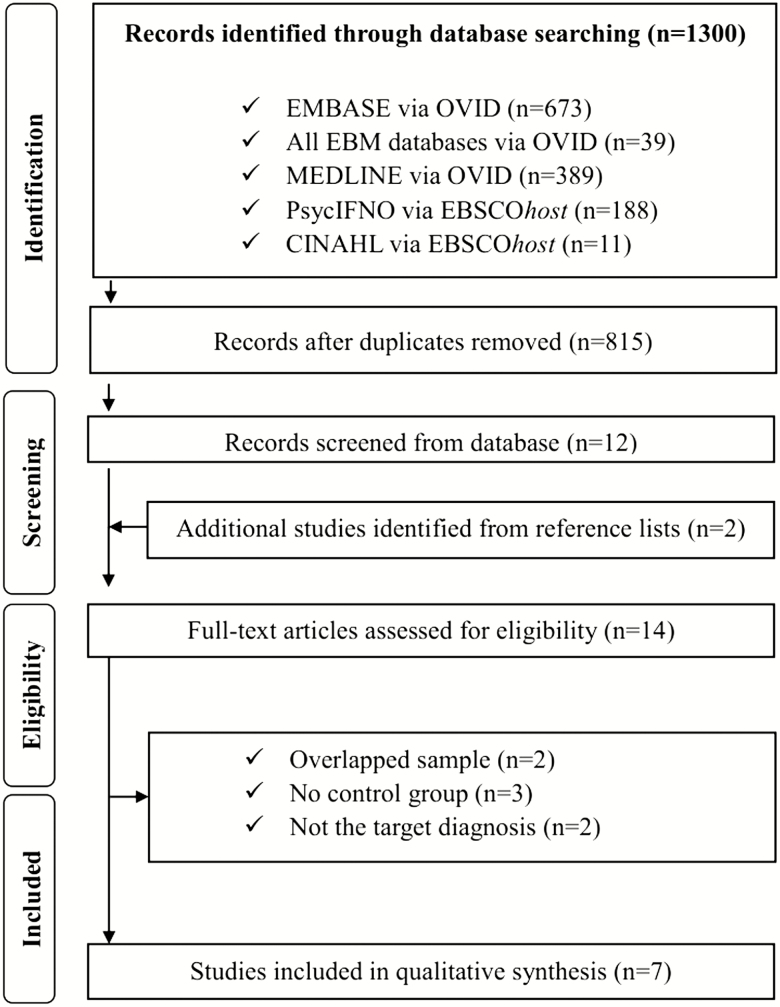
Our search yielded 1,300 publications (Figure 1). After removing the duplicates, we screened the title and abstract of the remaining 815 articles. A total of 14 articles were selected for full paper review. Of these, seven articles [5, 7, 9–12, 18] were found to meet the inclusion criteria in the meta-analysis. The excluded studies and reasons for their exclusion are provided in Supplementary Table S6.
Figure 1.
Flow chart used for the identification of eligible studies.
Description of the included studies
The seven included studies reported on a total of 152 HD patients and 144 controls (Table 1). Mean age (reported in six studies) ranged from 43.5 to 57.3 years for HD patients and from 43.1 to 56.5 years for the control group. Female percentages of HD patients’ and controls ranged from 37.5% to 58.3% (reported in six studies). Mean BMI for HD patients and controls ranged from 21.96 to 27.6 kg/m2 (reported in four studies). All included studies performed PSG in a sleep lab. Four studies [5, 10, 11, 18] used R&K criteria, two studies [7, 9] used AASM criteria, and one study [12] did not specify which criteria was used for scoring sleep. Three studies [5, 10, 12] included adaptation nights for PSG recordings (PSG data from the second night was used for analysis in these three studies), four studies [7, 9, 11, 18] performed only one night of PSG without adaptation.
Table 1.
Study characteristics
| Study | Sample size | Percentage female | Mean age | Mean BMI |
HD duration (year) | CAG repeat expansion | Disease severity | HD diagnosis | ESS (mean) | Adaptation | PSG scoring methods |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Arnulf et al. [18] | 25 HD | 48 | 48.3 ± 12.2 | 23.1 ± 3.5 | NR | 43.0 ± 3.0 | UHDRS motor: 25.4 ± 20.7 UHDRS chorea: 7.2 ± 5.2 UHDRS dystonia: 2.4 ± 3.4 UHDRS psychiatric: 12.9 ± 12.0 |
Genetic diagnosis | 6.8 | N | R&K |
| 25 controls | 48 | 48.2 ± 12.9 | 27.6 ± 7.2 | 5.7 | R&K | ||||||
| Cuturic et al. [11] | 12 HD | 58.3 | 43.5 | 25.8 | NR | NR | UHDRS total score: 18.0 ± 8.8 | Clinical symptoms and family history | 7.2 | N | R&K |
| 9 controls | 55.6 | 43.1 | 26.2 | 6.4 | R&K | ||||||
| Goodman et al. [5] | 9 HD | 44% | 54.7 ± 7.1 | NR | NR | NR | UHDRS motor: 19.9 ± 6.9 UHDRS chorea: 6.8 ± 3.7 |
Genetic diagnosis | 3.0 | Y | R&K |
| 10 controls | 50 | 54.9 ± 9.63 | NR | 5.1 | R&K | ||||||
| Lazar et al. [10] | 31 pre-HD | NR | NR | NR | 41.6 ± 2.4 | NR | Genetic diagnosis | 7.1 | Y | R&K | |
| 25 controls | NR | NR | NR | 5.6 | R&K | ||||||
| Neutel et al. [9] | 29 HD | 52 | 47.9 ± 11.8 | 23.8 ± 3 | NR | 43.8 ± 3.1 | UHDRS total score: 35 (22.3–57.8) UHDRS motor: 18.5 (12.3–35.5) UHDRS chorea: 3.5 (0.3–8.75) UHDRS dystonia: 1(0–3.5) UHDRS psychiatric: 18.5 (7.5–22.3) |
Genetic diagnosis | 7.5 | N | AASM |
| 29 controls | 52 | 47.5 ± 12.3 | 25.4 ± 4 | 5.7 | |||||||
| Piano et al. [7] | 30 HD | 53 | 57.30 ± 12.24 | 21.96 ± 4.01 | 9.43 ± 4.5 | 44.33 ± 4.08 | UHDRS motor: 55.55 ± 23.43 | Clinical symptoms and genetic diagnosis | 6.3 | N | AASM |
| 30 controls | 53 | 56.50 ± 11.85 | 26.27 ± 8.54 | 4.0 | |||||||
| Wiegand et al. [12] | 16 HD | 37.5 | 43.9 ± 8.1 | NR | 4.6 ± 3.4 | NR | TDRS score: 37.5 ± 17.5 | Clinical symptoms and family history | NR | Y | Unspecified |
| 16 controls | 37.5 | 45 ± 9.5 | NR | NR |
AASM, American academy of sleep medicine; BMI, body mass index; ESS, Epworth Sleepiness Scale; HD, Huntington’s disease; R&K, Rechtschaffen and Kales; TDRS, Tardive Dyskinesia Rating Scale; UHDRS, Unified Huntington Disease Rating Scale. For more information, please see Supplementary Table S7.
Patient recruitment methods varied across studies (Supplementary Table S7). Two studies [7, 11] reported that their HD patients were consecutive hospital admissions. One study [9] reported that 11 of 29 HD patients were consecutive hospital admissions. The other four studies [5, 10, 12, 18] did not report how their HD patients were recruited. One study [9] reported that 18 of 29 HD patients were referred for a complaint (mostly from their spouses) of agitation during sleep whereas the other six studies did not report on the clinical complaints of HD patients. Information regarding the recruitment of controls is also provided in Supplementary Table S7.
Risk of bias of individual studies
Supplementary Table S8 shows risk of bias assessment based on the NICE checklist. None of the included studies reported participation rate, compared participants versus nonparticipants or indicated whether measures were taken to prevent knowledge of primary exposure from influencing case ascertainment.
Comparison between HD patients and controls: the whole sample
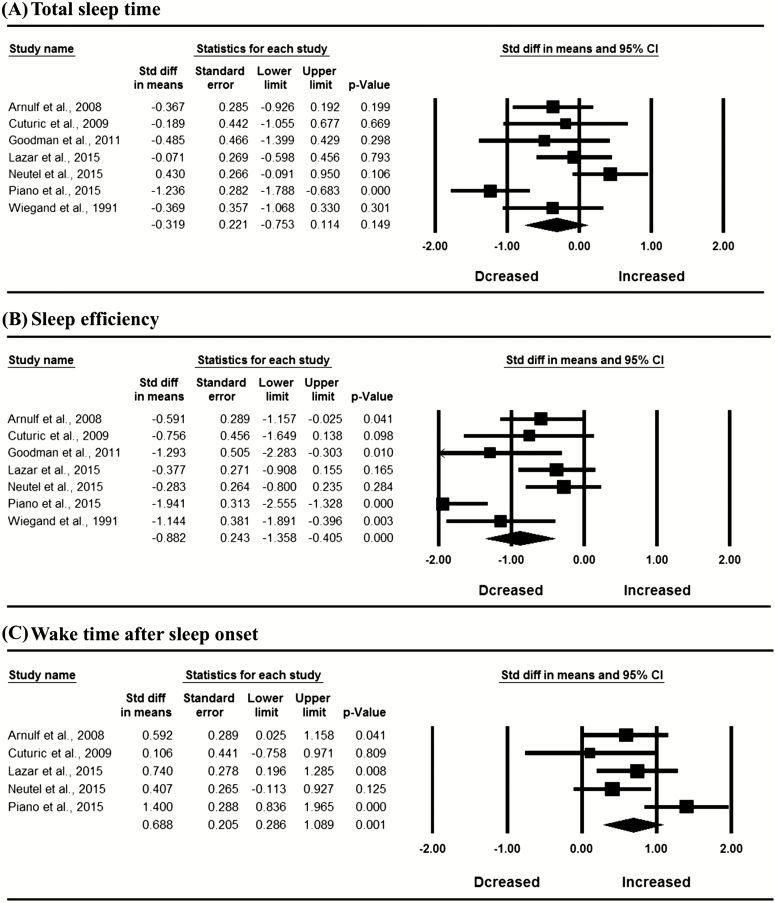
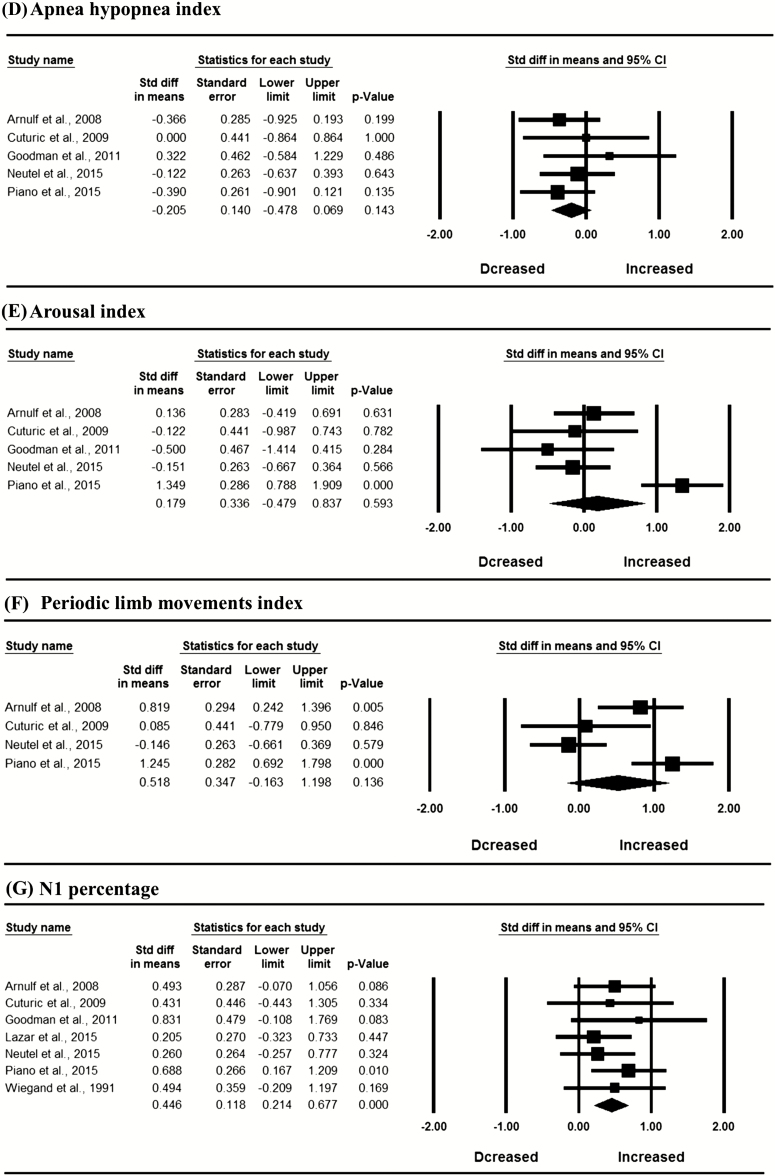
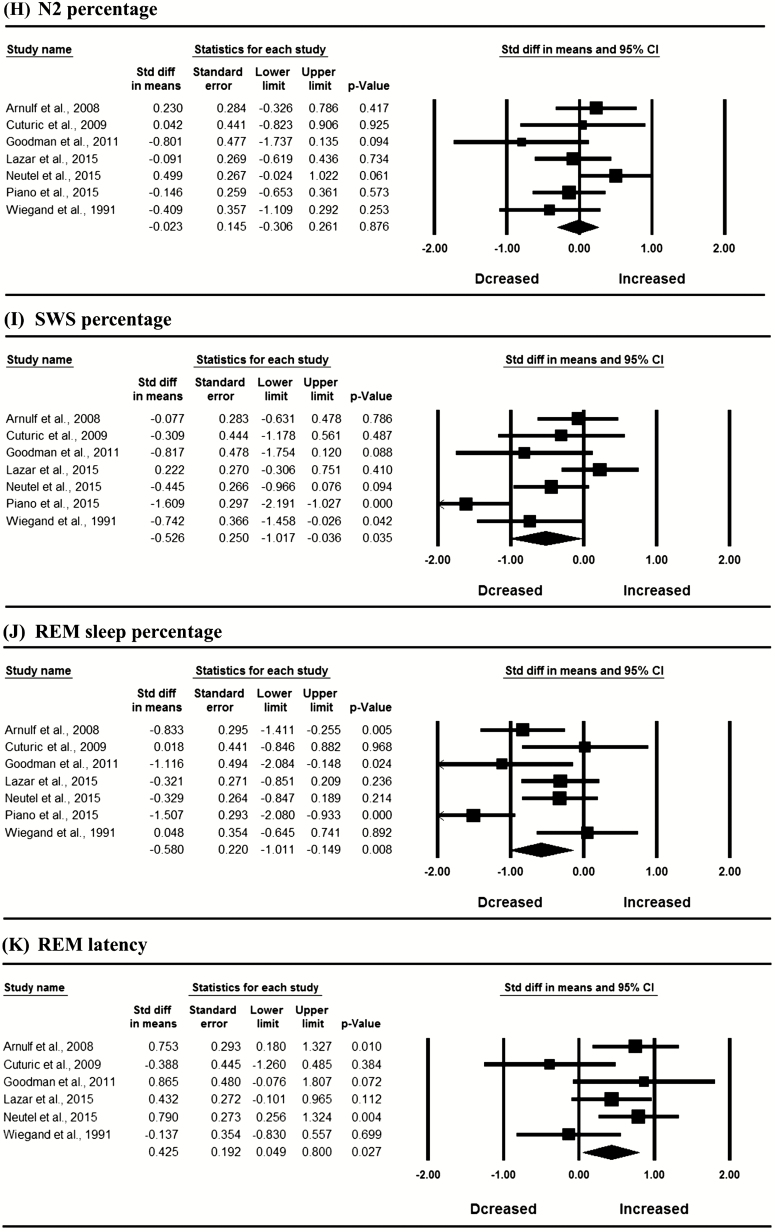
In the whole sample, meta-analysis revealed significantly decreased SE (SMD = −0.882, 95% CI: −1.358 to −0.405, I2 = 72.560%), SWS percentage (SMD = −0.526, 95% CI: −1.017 to −0.036, I2 = 75.422%) and REM percentage (SMD = −0.580, 95% CI: −1.011 to −0.149, I2 = 68.011%), and increased N1 percentage (SMD = 0.446, 95% CI: 0.214 to 0.617, I2 = 0), WASO (SMD = 0.688, 95% CI: 0.286 to 1.089, I2 = 56.044%), and REM latency (SMD = 0.425, 95% CI: 0.049 to 0.800, I2 = 48.235%) in patients with HD compared with controls (Figure 2; p < 0.05). Egger’s test indicated no publication bias in these significant results (p > 0.05). The mean values for PSG variables in HD patients and controls are shown in Supplementary Table S9.
Figure 2.
Meta-analysis of polysomnographic variables in patients with Huntington’s disease compared with controls; REM, rapid eye movement sleep; SWS, slow wave sleep.
Moderator analysis
As given in Table 2, a meta-regression analysis revealed that the increased WASO and REM latency, decreased REM sleep percentage and SWS percentage in HD patients compared with controls were significantly associated with advanced age of HD patients across different studies (p < 0.05). Increased WASO and REM latency, and decreased REM percentage in HD patients compared with controls were significantly associated with lower BMI of HD patients across different studies (p < 0.05). Decreased SWS percentages in HD patients compared with controls were significantly associated with longer CAG repeat length in HD patients (p < 0.05).
Table 2.
Moderator analyses
| Moderators | TST | WASO | SE | N1 percentage | N2 percentage | SWS percentage | REM percentage | REM latency | PLMI | AI | AHI | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Sex (female%) | No. of comparison | 6 | 4 | 6 | 6 | 6 | 6 | 6 | 5 | 4 | 5 | 5 |
| No. of H/C | 121/119 | 96/93 | 121/119 | 121/119 | 121/119 | 121/119 | 121/119 | 91/89 | 96/93 | 105/103 | 105/103 | |
| Point estimate | 0.32 | −3.60 | 1.10 | −0.50 | 3.43 | 0.13 | −1.25 | −0.37 | −5.63 | 4.21 | −1.15 | |
| SE | 4.47 | 9.10 | 4.67 | 2.78 | 2.75 | 4.20 | 4.27 | 4.09 | 11.09 | 7.77 | 3.82 | |
| P | 0.94 | 0.69 | 0.81 | 0.83 | 0.21 | 0.98 | 0.77 | 0.93 | 0.61 | 0.59 | 0.76 | |
| Mean age of HD patients | No. of comparison | 6 | 4 | 6 | 6 | 6 | 6 | 6 | 5 | 4 | 5 | 5 |
| No. of H/C | 121/119 | 96/93 | 121/119 | 121/119 | 121/119 | 121/119 | 121/119 | 91/89 | 96/93 | 105/103 | 105/103 | |
| Point estimate | −0.07 | 0.10 | −0.08 | 0.02 | −0.02 | −0.08 | −0.11 | 0.12 | 0.09 | 0.08 | −0.01 | |
| SE | 0.04 | 0.03 | 0.04 | 0.03 | 0.04 | 0.04 | 0.03 | 0.05 | 0.05 | 0.05 | 0.03 | |
| P | 0.10 | 0.003 | 0.08 | 0.34 | 0.55 | 0.03 | <0.001 | 0.016 | 0.09 | 0.14 | 0.67 | |
| BMI (kg/m2) | No. of comparison | 4 | 4 | 4 | 4 | 4 | 4 | 4 | 3 | 4 | 4 | 4 |
| No. of H/C | 96/93 | 96/93 | 96/93 | 96/93 | 96/93 | 96/93 | 96/93 | 66/63 | 96/93 | 96/93 | 96/93 | |
| Point estimate | 0.28 | −0.36 | 0.28 | −0.10 | 0.10 | 0.28 | 0.44 | −0.43 | −0.34 | −0.37 | 0.11 | |
| SE | 0.25 | 0.13 | 0.26 | 0.13 | 0.14 | 0.24 | 0.13 | 0.20 | 0.20 | 0.20 | 0.13 | |
| P | 0.26 | 0.006 | 0.28 | 0.43 | 0.47 | 0.25 | <0.001 | 0.03 | 0.08 | 0.07 | 0.36 | |
| CAG repeats (n) | No. of comparison | 4 | 4 | 4 | 4 | 4 | 4 | 4 | 3 | 3 | 3 | 3 |
| No. of H/C | 115/109 | 115/109 | 115/109 | 115/109 | 115/109 | 115/109 | 115/109 | 85/79 | 84/84 | 84/84 | 84/84 | |
| Point estimate | −0.23 | 0.13 | −0.39 | 0.13 | 0.05 | −0.57 | −0.29 | 0.17 | 0.20 | 0.81 | −0.003 | |
| SE | 0.39 | 0.24 | 0.36 | 0.13 | 0.18 | 0.24 | 0.26 | 0.17 | 1.05 | 0.88 | 0.29 | |
| P | 0.56 | 0.58 | 0.28 | 0.33 | 0.76 | 0.017 | 0.26 | 0.33 | 0.85 | 0.36 | 0.99 | |
| Disease severity | ||||||||||||
| Excluding Piano study | No. of comparison | 6 | 4 | 6 | 6 | 6 | 6 | 6 | 6 | 3 | 4 | 4 |
| No. of H/C | 122/114 | 97/88 | 122/114 | 122/114 | 122/114 | 122/114 | 122/114 | 122/114 | 66/63 | 75/73 | 75/73 | |
| SMD | −0.109 | 0.520** | −0.615*** | 0.386** | −0.006 | −0.286 | −0.402* | 0.425* | 0.260 | −0.093 | −0.130 | |
| Q | 6.150 | 1.752 | 6.155 | 1.783 | 8.325 | 7.209 | 6.924 | 9.659 | 6.145 | 1.466 | 1.730 | |
| I 2 | 18.696 | 0 | 18.768 | 0 | 39.941 | 30.638 | 27.792 | 48.235 | 67.452* | 0 | 0 | |
| Excluding Piano and Lazar studies | No. of comparison | 5 | 3 | 5 | 5 | 5 | 5 | 5 | 5 | 3 | 4 | 4 |
| No. of H/C | 91/89 | 66/63 | 91/89 | 91/89 | 91/89 | 91/89 | 91/89 | 91/89 | 66/63 | 75/73 | 75/73 | |
| SMD | −0.138 | 0.428* | −0.699*** | 0.443** | −0.003 | −0.412** | −0.426* | 0.410 | 0.260 | −0.093 | −0.130 | |
| Q | 6.139 | 0.859 | 5.327 | 1.189 | 8.027 | 3.055 | 6.807 | 9.641 | 6.145 | 1.466 | 1.730 | |
| I 2 | 34.842 | 0 | 24.913 | 0 | 50.169 | 0 | 41.236 | 58.511* | 67.452* | 0 | 0 | |
| Adaptation | ||||||||||||
| No | No. of comparison | 4 | 4 | 4 | 4 | 4 | 4 | 4 | 3 | 4 | 4 | 4 |
| No. of H/C | 96/93 | 96/93 | 96/93 | 96/93 | 96/93 | 96/93 | 96/93 | 66/63 | 96/93 | 96/93 | 96/93 | |
| SMD | −0.334 | 0.663* | −0.888* | 0.473 | 0.170 | −0.620 | −0.698* | 0.472 | 0.518 | 0.319 | −0.257 | |
| Q | 18.619 | 9.082 | 17.607 | 1.321 | 3.143 | 15.691 | 12.338 | 5.671 | 15.010 | 17.535 | 1.010 | |
| I 2 | 83.888*** | 66.969* | 82.962*** | 0 | 4.555 | 80.880** | 75.685** | 64.734 | 80.013** | 82.891** | 0 | |
| Yes | No. of comparison | 3 | 1 | 3 | 3 | 3 | 3 | 3 | 3 | — | 1 | 1 |
| No. of H/C | 56/51 | 31/25 | 56/51 | 56/51 | 56/51 | 56/51 | 56/51 | 56/51 | — | 9/10 | 9/10 | |
| SMD | −0.232 | 0.740** | −0.847** | 0.397 | −0.306 | −0.384 | −0.375 | 0.337 | — | −0.500 | 0.322 | |
| Q | 0.802 | 0 | 4.117 | 1.402 | 1.792 | 6.282 | 3.679 | 3.119 | — | 0 | 0 | |
| I 2 | 0 | 0 | 51.426 | 0 | 0 | 68.161* | 45.633 | 35.880 | — | 0 | 0 | |
| Between group difference | Q | 0.067 | 0.039 | 0.007 | 0.096 | 3.711 | 0.209 | 0.578 | 0.109 | — | 1.880 | 1.429 |
| P | 0.795 | 0.843 | 0.933 | 0.756 | 0.054 | 0.647 | 0.447 | 0.741 | — | 0.170 | 0.232 | |
| PSG scoring methods | ||||||||||||
| AASM | No. of comparison | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 1 | 2 | 2 | 2 |
| No. of H/C | 59/59 | 59/59 | 59/59 | 59/59 | 59/59 | 59/59 | 59/59 | 29/29 | 59/59 | 59/59 | 59/59 | |
| SMD | −0.400 | 0.897 | −1.103 | 0.473* | 0.173 | −1.019 | −0.911 | 0.790** | 0.546 | 0.595 | −0.257 | |
| Q | 18.489 | 6.438 | 16.406 | 1.307 | 3.013 | 8.528 | 8.922 | 0 | 13.008 | 14.898 | 0.524 | |
| I 2 | 94.591*** | 84.467* | 93.905*** | 23.478 | 66.814 | 88.275** | 88.792** | 0 | 92.312*** | 93.288*** | 0 | |
| R&K | No. of comparison | 4 | 3 | 4 | 4 | 4 | 4 | 4 | 4 | 2 | 3 | 3 |
| No. of H/C | 77/69 | 68/59 | 77/69 | 77/69 | 77/69 | 77/69 | 77/69 | 77/69 | 37/34 | 46/44 | 46/44 | |
| SMD | −0.242 | 0.573** | −0.609*** | 0.414* | −0.059 | −0.117 | −0.543* | 0.449 | 0.526 | −0.055 | −0.135 | |
| Q | 0.884 | 1.485 | 2.677 | 1.437 | 3.513 | 3.897 | 4.608 | 5.346 | 1.914 | 1.386 | 1.728 | |
| I 2 | 0 | 0 | 0 | 0 | 14.604 | 23.008 | 34.893 | 43.882 | 47.755 | 0 | 0 | |
| Between group difference | Q | 0.035 | 0.375 | 0.341 | 0.047 | 0.391 | 2.156 | 0.343 | 0.890 | 0.001 | 0.695 | 0.186 |
| P | 0.852 | 0.540 | 0.559 | 0.828 | 0.532 | 0.142 | 0.558 | 0.346 | 0.980 | 0.404 | 0.666 |
AASM, American academy of sleep medicine; AHI, apnea hypopnea index; AI, arousal index; BMI, body mass index; Q, Cochran’s Q statistic; REM, rapid eye movement sleep, H/C, Huntington disease/control; PLMI, periodic limb movements index; R&K, Rechtschaffen and Kales; SWS, slow wave sleep; SE, sleep efficiency; TST, total sleep time.
*p < 0.05, **p < 0.01, ***p < 0.001.
In the analysis of the contribution of disease severity of HD, meta-analytic calculations were rerun in six studies after excluding the Piano et al. [7] study in which the HD patients appeared to show the greatest disease severity compared with those in other included studies. The decreased SWS percentage in HD patients compared with controls found when considering all seven studies did not reach a statistically significant level (SMD = −0.286, 95% CI: −0.605 to 0.033, I2 = 30.638%). Subsequently, we excluded the Lazar et al. study [10] (also excluding the Piano et al. study [7]) in which pre-HD patients seemingly showed the mildest disease severity and reran the meta-analysis in the remaining five studies. We then detected significantly decreased SWS in HD patients compared with controls again (SMD = −0.412, 95% CI: −0.709 to 0.115, I2 = 0).
Discussion
Summary of findings
This systematic review and meta-analysis included seven case–control studies to examine and compare differences in PSG determined sleep between HD patients and healthy controls. The results found decreased SE, percentage of SWS and REM, and increased N1 percentage, WASO, and REM latency in HD patients compared with healthy controls. Our moderator analyses found that advanced mean age of HD patients was significantly associated with increased WASO and REML, and with decreased SWS and REM sleep percentages. Decreased mean BMI was associated with increased WASO and REM latency, and with decreased REM sleep percentage in HD. Longer CAG repeat length and increased disease severity were associated with decreased SWS percentage in HD.
Sleep abnormalities in HD
Previous studies using subjective evaluations have indicated that sleep disturbance is a common feature in HD patients. The frequency of sleep disturbance in HD patients evaluated by the Pittsburgh sleep quality index is 58.1%, which is higher than that in controls [3, 6]. Objective sleep evaluations indicate that sleep disturbances in HD patients are even worse, which is likely related to the fact that subjective evaluation of sleep has a poor correlation with the findings of objective tools, such as actigraphy and PSG, leading to an underestimation of sleep disturbances in HD patients [19]. Actigraphic data has found a longer time in bed and a significantly increased number of movements during nocturnal bedtime in HD patients [20]. Most importantly, actigraphic data revealed altered rest-activity profiles, suggesting deterioration of circadian timing in HD patients [5]. Additionally, the PSG data we synthesized in the current meta-analysis focused on quantitative sleep parameters and demonstrated disturbed objective sleep continuity and sleep architecture. (i.e. decreased SE, SWS, and REM, increased WASO and REM latency) in patients with HD.
Moderator analysis
CAG repeat length
CAG repeat length makes it possible to predict who will develop HD many years before symptom onset [21]. Significant associations between CAG repeat lengths and HD clinical features such as age of onset, weight loss, cognitive and motor disabilities, decline in living capabilities, brain neurodegeneration and risk of death have also been demonstrated [22]. Our findings also revealed that CAG repeat length is associated with decreased SWS percentage in HD patients compared with controls. Neurochemical and neuroimaging studies have revealed that decreased concentrations of BDNF in plasma and amount of volume decrease in the caudate and total basal ganglia correlates with increased CAG repeat length in HD patients [23, 24]. These changes are involved in the abnormalities observed in sleep [25, 26], and may contribute to the association of CAG repeat length with PSG alternations in HD. However, it should be noted that our results concerning CAG repeat length and sleep were derived from only four included studies [7, 9, 10, 18]. The limited data suggest that our results should be further verified and interpreted with caution. Additionally, it is important to ask whether sleep disturbances are common in other trinucleotide repeat diseases (i.e. myotonic dystrophy (DM) [27] and Friedreich’s ataxia (FA) [28]), and whether sleep disturbances are also related to the trinucleotide repeat length in these diseases. Previous studies found hypersomnia, increased periodic limb movements in sleep (PLMS), sleep apnea, SWS, and REM sleep duration, and decreased N2 in DM1 patients [29, 30]; whereas decreased TST, SE, percentage of SWS and REM sleep, and increased WASO were found in DM2 patients [31]. To our knowledge, how PSG changes in FA patients compare with controls has not been established; however, the presence of sleep apnea correlates with the duration and clinical severity of FA [32]. Additionally, a high prevalence of restless legs syndrome (50%) and PLMS (43.8%) in FA has been reported [33]. By comparison, our findings revealed no significant differences in AHI and PLMI between HD patients and controls, which is different from the findings for FA and DM. Unfortunately, it is unclear whether there are significant associations between the trinucleotide repeat lengths in these diseases and patients’ sleep disturbances. However, these results indicate that studies exploring the relationship between trinucleotide repeat length and PSG changes in these diseases would be useful.
Age
Mean age of HD patients was a significant source of heterogeneity for differences in PSG changes (i.e. increased WASO and REM latency, decreased SWS and REM sleep) between HD patients and controls. Thus, the extent of sleep abnormalities in HD patients appears to be moderated by age. This could involve atrophy of brain areas that is increased with age in HD patients. Previous studies revealed that HD, which typically presents between 35 and 45 years of age [34], could cause progressive atrophy in the striatum [35]. Even in prodromal and early symptomatic HD, putamen atrophy rates of about 2.3% per year and 4.5% per year, and caudate atrophy rates of 1.1%–2.4% per year and 2.9%–4.9% per year, respectively, have been reported in comparisons to controls [36–38]. Furthermore, Stoffers et al. [25] found that neuronal network imbalance between caudate and other brain regions was associated with a continuous hyper-aroused state in insomnia patients. Thus, we speculate that these changes may partly explain the effects of age on sleep in HD patients.
Age is also an important factor that impacts the relationship between longer CAG repeat length and faster clinical progression in HD [22, 39, 40]. Indeed, adjusting for age at the time of disease onset [40], or assessment [39], appears to be necessary to determine the relationship between CAG repeat length and clinical progression. Thus, it is possible that age or duration of disease moderates the association of CAG repeat length with sleep disturbances. However, this speculation cannot be adequately explored in our meta-analysis due to the limited number of included studies.
BMI
The association of sleep with overweight/obesity has been well established in previous studies [41, 42], but little is known about the potential association between underweight conditions and sleep. Our findings showed that the BMI of HD patients was a significant source of heterogeneity for differences in WASO, REM latency, and REM sleep percentage between HD patients and controls. Changes in sleep in HD patients with lower BMI appear more obvious compared with those with higher BMI. Previous studies indicated that weight loss reflected fundamental pathologic mechanisms underlying HD and may serve as a biomarker for monitoring disease progression [43, 44]. Weight loss is already manifest in presymptomatic HD gene carriers [45] and is particularly marked in the final hypokinetic stages of the disease [43]. Weight loss and energy impairment [46, 47] may reflect a wasting status, defined as unintentional weight loss in both fat-free and fat mass [48]. In patients with other wasting diseases such as cancer [49], chronic kidney disease [50], and diabetes [51], objective sleep disturbances evaluated by PSG appear to be a general phenomenon.
Unintentional weight loss might also be considered a core component of “frailty [52],” which can increase health-related vulnerability to even minor stressful events [53, 54]. There is a U-shaped curve relationship between BMI and frailty with the lowest and highest BMI having the strongest association with frailty [55]. Thus, including weight loss as a criterion is consistent with viewing frailty as a wasting disorder [55]. A previous study indicated that there is a bidirectional relationship between sleep disturbances and frailty, in which frailty may result in disruptions of rest activity rhythms with irregular sleep–wake cycles [56]. We therefore considered that lower BMI in HD patients may reflect their frailty status, which could contribute the PSG changes in HD patients compared with controls. It should be noted that the mean age of HD patients in our included studies ranged from 43.5 to 57.3 years, whereas frailty is a condition usually ascribed to elderly people. In general, in the elderly, frailty is linked to aging-related changes in brain and the endocrine system (frail brain and frail endocrine system) [54]. We speculate that the progressively worsening dysfunctions in brain morphology, brain activation, and the endocrine system in HD patients [57, 58] may promote frailty.
Disease severity
Clinically, it is important to determine whether disease severity is associated with sleep changes in HD patients, but findings of previous studies are contradictory. For instance, Wiegand et al. [12] reported that there was no relationship between motor disturbances and sleep variables in HD patients. By comparison, two other studies reported that disease severity was negatively associated with REM percentage in HD patients [7, 18]. Neutel et al. [9] reported that patients with moderate HD had shorter TST, SE, and longer WASO compared with patients with premanifest to mild HD. In our meta-analysis, we performed a sensitivity analysis to explore the effects of disease severity of HD on our pooled effect size of PSG changes, and we found that increased disease severity of HD was associated with decreased SWS. Thus, our findings support a relationship between sleep disturbances and disease severity and highlight the importance of assessing sleep disturbances in the management of HD. However, the association of specific symptoms (i.e. motor, chorea, dystonia, and psychiatric symptoms) of HD with sleep changes could not be explored due to limited available data.
Effects of drugs
It should be noted that none of the seven studies in our meta-analysis excluded patients who had undergone pharmacological therapies (i.e. benzodiazepines, antidepressants, and neuroleptics) which could potentially impact sleep measures. Two included studies did not find any significant difference in any PSG variables between drug-free HD patients and HD patients receiving medication [7, 12]. Another two studies [5, 9], found that PSG changes in untreated patients were similar to those found in all (treated and untreated) HD patients. These findings suggest that PSG changes in HD patients were not impacted by pharmacological treatment. However, three included studies [10, 11, 18] did not specify or did not report whether using pharmacological therapies impacted the PSG findings in HD patients. It is also not possible to perform an analysis exploring the effects of pharmacological therapies on our pooled effect size, due to lack of individual patient data regarding pharmacological therapies. Our findings therefore may be biased by pharmacological effects.
Mechanisms underlying sleep abnormalities in HD
The mechanisms underlying sleep abnormalities in HD patients are still unclear; however, the importance of circadian dysfunction has been emphasized in previous studies [3, 8, 58, 59]. When comparing the sleep schedule data between HD patients and controls, existing evidence revealed altered sleep–wake circadian timing in HD patients [3, 11]. One PSG study also revealed a later time of sleep onset and terminal awakening in patients with HD compared with controls [11]. Actigraphic data further suggests the deterioration of circadian timing in HD patients [5, 20]. In mammals, circadian rhythms are mainly coordinated by the suprachiasmatic nucleus (SCN) [60]. Mechanistically, Bartlett et al. [58] proposed that dysfunction of SCN, which is located in the anterior hypothalamus, plays an important role in sleep abnormalities in HD. Postmortem studies in HD patients also have reported decreases in orexin-releasing neurons which innervate the SCN [61, 62]. Thus, loss of these neurons may be related to dysfunction of SCN, which contribute to circadian disturbances, dysfunctions in the hypothalamic-pituitary-adrenal (HPA) axis, and subsequent altered circadian release of cortisol [61]. Changes in the regulation of circadian rhythms [59, 63] and HPA function [64, 65] may be a potential neurobiologic substrate for sleep abnormalities in HD.
Clinical implications for practice and research
Our study showed that sleep continuity and sleep architecture are abnormal in HD. Sleep disturbances and the underlying circadian rhythm disturbances may be important in relation to outcomes and clinical management in HD patients. Thoroughly defining the extent and nature of sleep abnormalities in HD is not only critical in improving the quality of life in both patients and caregivers, but also in revealing additional clinically relevant information about the disease itself [4]. PSG studies are not routinely performed in patients with HD, but our findings provide useful information to help clinicians understand behavioral sleep problems in HD patients. The findings of this review therefore highlight the need to undertake systematic and routine screening as well as comprehensive assessment of PSG variables in individuals with HD. With respect to implications for research, PSG measures, amongst other neurophysiological parameters, have the potential to aid in better understanding of the neurobiology of HD.
Limitations
First, there are methodological issues that may bias our findings. For instance, four [5, 10, 12, 18] of our seven included studies did not report whether the recruited HD patients were consecutive patients or those with specific sleep complaints referred for a PSG examination. This could potentially result in a selection bias for the participants. In addition, whether the healthy controls were recruited on a voluntary basis for research or recruited from sleep centers was unclear in three studies [9, 10, 12]. Recruiting controls from sleep centers may include individuals with sleep complaints which could potentially bias our pooled effect size of PSG changes in HD patients compared with controls. Second, other methodological factors (i.e. differences in the definition of PSG parameters, and the number of PSGs administered, and variations in bedtime schedule) which may help explain heterogeneity between studies were not analyzed due to lack of individual patient data. Third, as mentioned above, none of the included studies excluded patients who had undergone pharmacological therapies (i.e. benzodiazepines, antidepressants, and neuroleptics) which may impact sleep measures, because the behavioral and motor symptoms of HD patients did not allow the withdrawal of treatment. This may bias the pooled effect size for differences in PSG variables between cases and controls, indicating that our findings should be interpreted with caution. Fourth, although we performed a sensitivity analysis by removing specific studies (Piano et al. [7] and Lazar et al. [10]) to explore the association between HD severity and sleep changes, the associations of motor, chorea, dystonia, and psychiatric symptoms with sleep change in HD patients could not be explored due to limited available data.
Conclusions
In conclusion, the current meta-analysis demonstrates that polysomnographic abnormalities are present in patients with HD. The study findings underscore the need for a comprehensive assessment of sleep changes across PSG in patients with HD. Furthermore, the effects of age, BMI, and CAG repeat length of HD patients on sleep changes should also be carefully considered and closely monitored in the management of HD.
Supplementary Material
Acknowledgments
X.T. designed the study. Y.Z. and R.R. extracted the data and ran the analyses. Y.Z. wrote the first draft of the article. L.D.S., Y.L., J.Z., L.Y., J.S., and L.L. contributed significantly. All authors read and approved the final manuscript. We thank Prof. Isabelle Arnulf for providing additional data that we requested in conducting this meta-analysis.
Funding
This work was supported by the National Natural Science Foundation of China (81530002, 81629002, 81800093), National Basic Research Program of China (2015CB856406), and the National Institutes of Health research grant (MH64827).
Conflict of interest statement. The authors report no disclosures relevant to the article.
References
- 1. A novel gene containing a trinucleotide repeat that is expanded and unstable on Huntington’s disease chromosomes. The Huntington’s Disease Collaborative Research Group. Cell. 1993;72(6):971–983. [DOI] [PubMed] [Google Scholar]
- 2. Harper PS. The epidemiology of Huntington’s disease. Hum Genet. 1992;89(4):365–376. [DOI] [PubMed] [Google Scholar]
- 3. Aziz NA, et al. . Sleep and circadian rhythm alterations correlate with depression and cognitive impairment in Huntington’s disease. Parkinsonism Relat Disord. 2010;16(5):345–350. [DOI] [PubMed] [Google Scholar]
- 4. Goodman AO, et al. . How vital is sleep in Huntington’s disease? J Neurol. 2010;257(6):882–897. [DOI] [PubMed] [Google Scholar]
- 5. Goodman AO, et al. . Asymptomatic sleep abnormalities are a common early feature in patients with Huntington’s disease. Curr Neurol Neurosci Rep. 2011;11(2):211–217. [DOI] [PubMed] [Google Scholar]
- 6. Baker CR, et al. . Subjective sleep problems in Huntington’s disease: a pilot investigation of the relationship to brain structure, neurocognitive, and neuropsychiatric function. J Neurol Sci. 2016;364:148–153. [DOI] [PubMed] [Google Scholar]
- 7. Piano C, et al. . Polysomnographic findings and clinical correlates in huntington disease: a cross-sectional cohort study. Sleep. 2015;38(9):1489–1495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Videnovic A, et al. . ‘The clocks that time us’—circadian rhythms in neurodegenerative disorders. Nat Rev Neurol. 2014;10(12):683–693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Neutel D, et al. . Nocturnal agitation in Huntington disease is caused by arousal-related abnormal movements rather than by rapid eye movement sleep behavior disorder. Sleep Med. 2015;16(6):754–759. [DOI] [PubMed] [Google Scholar]
- 10. Lazar AS, et al. . Sleep deficits but no metabolic deficits in premanifest Huntington’s disease. Ann Neurol. 2015;78(4):630–648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Cuturic M, et al. . Sleep patterns in patients with Huntington’s disease and their unaffected first-degree relatives: a brief report. Behav Sleep Med. 2009;7(4):245–254. [DOI] [PubMed] [Google Scholar]
- 12. Wiegand M, et al. . Nocturnal sleep in Huntington’s disease. J Neurol. 1991;238(4):203–208. [DOI] [PubMed] [Google Scholar]
- 13. Moher D, et al. ; PRISMA Group Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6(7):e1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Iber C, Ancoli-Israel S, Chesson A, et al. . The AASM Manual for Scoring of Sleep and Associated Events: Rules, Terminology and Technical Specifications. 1st ed Westchester, IL: American Academy of Sleep Medicine; 2007. [Google Scholar]
- 15. Excellence NIfHaC. Appendix E: Methodology Checklist: Case–Control Studies. NICE article [PMG6B]. 2012. https://www.nice.org.uk/process/pmg10/chapter/introduction; https://www.nice.org.uk/terms-and-conditions#notice-of-rights. [Google Scholar]
- 16. Higgins JP, et al. . Measuring inconsistency in meta-analyses. BMJ. 2003;327(7414):557–560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Egger M, et al. . Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315(7109):629–634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Arnulf I, et al. . Rapid eye movement sleep disturbances in Huntington disease. Arch Neurol. 2008;65(4):482–488. [DOI] [PubMed] [Google Scholar]
- 19. Piano C, et al. . Subjective assessment of sleep in huntington disease: reliability of sleep questionnaires compared to polysomnography. Neurodegener Dis. 2017;17(6):330–337. [DOI] [PubMed] [Google Scholar]
- 20. Morton AJ, et al. . Disintegration of the sleep–wake cycle and circadian timing in Huntington’s disease. J Neurosci. 2005;25(1):157–163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. McColgan P, et al. . Huntington’s disease: a clinical review. Eur J Neurol. 2018;25(1):24–34. [DOI] [PubMed] [Google Scholar]
- 22. Podvin S, et al. . Multiple clinical features of Huntington’s disease correlate with mutant HTT gene CAG repeat lengths and neurodegeneration. J Neurol. 2019;266(3):551–564. [DOI] [PubMed] [Google Scholar]
- 23. Aylward EH, et al. . Longitudinal change in basal ganglia volume in patients with Huntington’s disease. Neurology. 1997;48(2):394–399. [DOI] [PubMed] [Google Scholar]
- 24. Ciammola A, et al. . Low brain-derived neurotrophic factor (BDNF) levels in serum of Huntington’s disease patients. Am J Med Genet B Neuropsychiatr Genet. 2007;144B(4):574–577. [DOI] [PubMed] [Google Scholar]
- 25. Stoffers D, et al. . The caudate: a key node in the neuronal network imbalance of insomnia? Brain. 2014;137(Pt 2):610–620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Deuschle M, et al. . Serum brain-derived neurotrophic factor (BDNF) in sleep-disordered patients: relation to sleep stage N3 and rapid eye movement (REM) sleep across diagnostic entities. J Sleep Res. 2018;27(1):73–77. [DOI] [PubMed] [Google Scholar]
- 27. Fu YH, et al. . An unstable triplet repeat in a gene related to myotonic muscular dystrophy. Science. 1992;255(5049):1256–1258. [DOI] [PubMed] [Google Scholar]
- 28. Schulz JB, et al. . Diagnosis and treatment of Friedreich ataxia: a European perspective. Nat Rev Neurol. 2009;5(4):222–234. [DOI] [PubMed] [Google Scholar]
- 29. Romigi A, et al. . Comparative sleep disturbances in myotonic dystrophy types 1 and 2. Curr Neurol Neurosci Rep. 2018;18(12):102. [DOI] [PubMed] [Google Scholar]
- 30. Bonanni E, et al. . Disruption of sleep–wake continuum in myotonic dystrophy type 1: beyond conventional sleep staging. Neuromuscul Disord. 2018;28(5):414–421. [DOI] [PubMed] [Google Scholar]
- 31. Romigi A, et al. . Sleep disorders in myotonic dystrophy type 2: a controlled polysomnographic study and self-reported questionnaires. Eur J Neurol. 2014;21(6):929–934. [DOI] [PubMed] [Google Scholar]
- 32. Corben LA, et al. . Increased prevalence of sleep-disordered breathing in Friedreich ataxia. Neurology. 2013;81(1):46–51. [DOI] [PubMed] [Google Scholar]
- 33. Frauscher B, et al. . Restless legs syndrome in Friedreich ataxia: a polysomnographic study. Mov Disord. 2011;26(2):302–306. [DOI] [PubMed] [Google Scholar]
- 34. Foroud T, et al. . Differences in duration of Huntington’s disease based on age at onset. J Neurol Neurosurg Psychiatry. 1999;66(1):52–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Turner LM, et al. . Striatal morphology correlates with frontostriatal electrophysiological motor processing in Huntington’s disease: an IMAGE-HD study. Brain Behav. 2016;6(12):e00511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Tabrizi SJ, et al. ; TRACK-HD Investigators Biological and clinical changes in premanifest and early stage Huntington’s disease in the TRACK-HD study: the 12-month longitudinal analysis. Lancet Neurol. 2011;10(1):31–42. [DOI] [PubMed] [Google Scholar]
- 37. Aylward EH, et al. . Rate of caudate atrophy in presymptomatic and symptomatic stages of Huntington’s disease. Mov Disord. 2000;15(3):552–560. [DOI] [PubMed] [Google Scholar]
- 38. Hobbs NZ, et al. . Automated quantification of caudate atrophy by local registration of serial MRI: evaluation and application in Huntington’s disease. Neuroimage. 2009;47(4):1659–1665. [DOI] [PubMed] [Google Scholar]
- 39. Ravina B, et al. . The relationship between CAG repeat length and clinical progression in Huntington’s disease. Mov Disord. 2008;23(9):1223–1227. [DOI] [PubMed] [Google Scholar]
- 40. Rosenblatt A, et al. . Age, CAG repeat length, and clinical progression in Huntington’s disease. Mov Disord. 2012;27(2):272–276. [DOI] [PubMed] [Google Scholar]
- 41. Lallukka T, et al. . Associations of relative weight with subsequent changes over time in insomnia symptoms: a follow-up study among middle-aged women and men. Sleep Med. 2012;13(10):1271–1279. [DOI] [PubMed] [Google Scholar]
- 42. Chan WS, et al. . A meta-analysis of associations between obesity and insomnia diagnosis and symptoms. Sleep Med Rev. 2018;40:170–182. [DOI] [PubMed] [Google Scholar]
- 43. Myers RH, et al. . Factors associated with slow progression in Huntington’s disease. Arch Neurol. 1991;48(8):800–804. [DOI] [PubMed] [Google Scholar]
- 44. Aziz NA, et al. ; EHDI Study Group Weight loss in Huntington disease increases with higher CAG repeat number. Neurology. 2008;71(19):1506–1513. [DOI] [PubMed] [Google Scholar]
- 45. Mochel F, et al. . Early energy deficit in Huntington disease: identification of a plasma biomarker traceable during disease progression. PLoS One. 2007;2(7):e647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Dubinsky JM. Towards an understanding of energy impairment in Huntington’s disease brain. J Huntingtons Dis. 2017;6(4):267–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Costa de Miranda R, et al. . Body composition and bone mineral density in Huntington’s disease. Nutrition. 2019;59:145–149. [DOI] [PubMed] [Google Scholar]
- 48. Kulstad R, et al. . The energetics of wasting diseases. Curr Opin Clin Nutr Metab Care. 2007;10(4):488–493. [DOI] [PubMed] [Google Scholar]
- 49. Reinsel RA, et al. . Polysomnographic study of sleep in survivors of breast cancer. J Clin Sleep Med. 2015;11(12):1361–1370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Adams RJ, et al. . Chronic kidney disease and sleep apnea association of kidney disease with obstructive sleep apnea in a population study of men. Sleep. 2017;40(1). doi: 10.1093/sleep/zsw015 [DOI] [PubMed] [Google Scholar]
- 51. Whitaker KM, Lutsey PL, Ogilvie RP, et al. . Associations between polysomnography and actigraphy-based sleep indices and glycemic control among those with and without type 2 diabetes: the Multi-Ethnic Study of Atherosclerosis. Sleep. 2018;41(11). doi: 10.1093/sleep/zsy172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Abate M, et al. . Frailty in the elderly: the physical dimension. Eura Medicophys. 2007;43(3):407–415. [PubMed] [Google Scholar]
- 53. Fried LP, et al. ; Cardiovascular Health Study Collaborative Research Group Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci Med Sci. 2001;56(3):M146–M156. [DOI] [PubMed] [Google Scholar]
- 54. Clegg A, et al. . Frailty in elderly people. Lancet. 2013;381(9868):752–762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Hubbard RE, et al. . Frailty, body mass index, and abdominal obesity in older people. J Gerontol A Biol Sci Med Sci. 2010;65(4):377–381. [DOI] [PubMed] [Google Scholar]
- 56. Vaz Fragoso CA, et al. . Sleep–wake disturbances and frailty in community-living older persons. J Am Geriatr Soc. 2009;57(11):2094–2100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Weir DW, et al. . Development of biomarkers for Huntington’s disease. Lancet Neurol. 2011;10(6):573–590. [DOI] [PubMed] [Google Scholar]
- 58. Bartlett DM, et al. . Neuroendocrine and neurotrophic signaling in Huntington’s disease: implications for pathogenic mechanisms and treatment strategies. Neurosci Biobehav Rev. 2016;71:444–454. [DOI] [PubMed] [Google Scholar]
- 59. Logan RW, et al. . Rhythms of life: circadian disruption and brain disorders across the lifespan. Nat Rev Neurosci. 2019;20(1):49–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Nishino H, et al. . The role of suprachiasmatic nuclei of the hypothalamus in the production of circadian rhythm. Brain Res. 1976;112(1):45–59. [DOI] [PubMed] [Google Scholar]
- 61. Petersén A, et al. . Orexin loss in Huntington’s disease. Hum Mol Genet. 2005;14(1):39–47. [DOI] [PubMed] [Google Scholar]
- 62. Aziz A, et al. . Hypocretin and melanin-concentrating hormone in patients with Huntington disease. Brain Pathol. 2008;18(4):474–483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Zee PC, et al. . The brain’s master circadian clock: implications and opportunities for therapy of sleep disorders. Sleep Med Rev. 2007;11(1):59–70. [DOI] [PubMed] [Google Scholar]
- 64. Aziz NA, et al. . Increased hypothalamic-pituitary-adrenal axis activity in Huntington’s disease. J Clin Endocrinol Metab. 2009;94(4):1223–1228. [DOI] [PubMed] [Google Scholar]
- 65. van Wamelen DJ, et al. . Hypothalamic alterations in Huntington’s disease patients: comparison with genetic rodent models. J Neuroendocrinol. 2014;26(11):761–775. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.