Abstract
Background
Infliximab is an effective salvage therapy in acute severe ulcerative colitis; however, the optimal dosing strategy is unknown. We performed a systematic review and meta-analysis to examine the impact of infliximab dosage and intensification on colectomy-free survival in acute severe ulcerative colitis.
Methods
Studies reporting outcomes of hospitalized steroid-refractory acute severe ulcerative colitis treated with infliximab salvage were identified. Infliximab use was categorized by dose, dose number, and schedule. The primary outcome was colectomy-free survival at 3 months. Pooled proportions and odds ratios with 95% confidence intervals were reported.
Results
Forty-one cohorts (n = 2158 cases) were included. Overall colectomy-free survival with infliximab salvage was 79.7% (95% confidence interval [CI], 75.48% to 83.6%) at 3 months and 69.8% (95% CI, 65.7% to 73.7%) at 12 months. Colectomy-free survival at 3 months was superior with 5-mg/kg multiple (≥2) doses compared with single-dose induction (odds ratio [OR], 4.24; 95% CI, 2.44 to 7.36; P < 0.001). However, dose intensification with either high-dose or accelerated strategies was not significantly different to 5-mg/kg standard induction at 3 months (OR, 0.70; 95% CI, 0.39 to 1.27; P = 0.24) despite being utilized in patients with a significantly higher mean C-reactive protein and lower albumin levels.
Conclusions
In acute severe ulcerative colitis, multiple 5-mg/kg infliximab doses are superior to single-dose salvage. Dose-intensified induction outcomes were not significantly different compared to standard induction and were more often used in patients with increased disease severity, which may have confounded the results. This meta-analysis highlights the marked variability in the management of infliximab salvage therapy and the need for further studies to determine the optimal dose strategy.
Keywords: acute severe ulcerative colitis, infliximab, colectomy
INTRODUCTION
Acute severe ulcerative colitis (ASUC) is a potentially life-threatening condition that has historically resulted in emergency colectomy in 30% of patients within 3 months of presentation.1 Twenty-five percent of patients with ulcerative colitis develop ASUC during their disease course, and 15% have 2 or more episodes.2 Corticosteroids represent firstline therapy for ASUC; however, approximately one-third of patients do not respond.1 Infliximab (IFX) and cyclosporine have demonstrated equivalent efficacy as medical salvage therapies in ASUC in randomized controlled trials (RCTs); however, nonrandomized studies have suggested a better treatment response and reduced risk of colectomy at 12 months with IFX.3
The standard induction schedule for IFX, which comprises 3 doses at 5 mg/kg given at weeks 0, 2, and 6, has been derived from studies in Crohn’s disease and moderate to severe outpatient ulcerative colitis.4, 5 However, these conditions differ in their biology and inflammatory disease burden from ASUC. New insights into the pharmacokinetics of IFX in the setting of ASUC that have shown increased drug clearance,6 low serum levels,7 and fecal drug loss8 have led to an interest in dose intensification. In a survey of gastroenterologist members of the International Organization for the Study of Inflammatory Bowel Diseases, the majority preferred dose-intensified or accelerated-schedules9 to standard-schedule induction; however, the evidence to support such an approach is conflicting.10–14
Despite conflicting data, we hypothesized that IFX dose intensification either via higher-dose therapy or shorter dose intervals would result in a reduction in colectomy rates. In this meta-analysis, we sought to examine the efficacy of IFX induction in ASUC and the impact of dosage, dose number, and dose intensification on colectomy-free survival (CFS).
METHODS
Search Strategy
A systematic literature search was performed independently by 2 investigators (M.C.C., D.S.) in accordance with the Preferred Reporting Items for Systematic Review and Meta-Analyses (PRISMA) guidelines (Supplementary Appendix 1). A broad search strategy was utilized, using Medical Subject Headings (MeSH) and key words related to ASUC and treatment with IFX therapy (Supplementary Appendix 2).
Studies were identified from the PubMed/MEDLINE, EMBASE, and CENTRAL databases from January 1999 to July 2018. The reference lists of included articles were manually reviewed, and a hand-search of the main gastroenterology conference abstract directories was performed to identify additional studies for inclusion. Relevant abstracts from British Society of Gastroenterology/Digestive Diseases Week/European Crohn's and Colitis Organisation/United European Gastroenterology Week conferences from the 2014 to July 2018 were included. Discrepancies with regards to article inclusion were resolved by consensus in consultation with the senior authors.
Inclusion and Exclusion Criteria
Studies were included if they met the following selection criteria: (1) observational or interventional design; (2) patients were hospitalized or had acute severe flares of UC refractory to oral or intravenous (IV) corticosteroids; and (3) treatment with IFX as rescue therapy was administered. Furthermore, to be eligible for inclusion, criteria for IFX use, dosing, and schedule of IFX administration and CFS had to be reported.
Studies were excluded if patients had been treated previously with a rescue therapy (eg, cyclosporine, tacrolimus) during the same presentation of ASUC. Studies were also excluded if there was concomitant Clostridium difficile infection or cytomegalovirus colitis as these represent distinct clinical entities that have a different clinical course and have traditionally been excluded from both clinical trials and observational studies. Pediatric studies and studies that focused primarily on chronic active colitis were also excluded. Conference abstracts that had not been published as full-text articles within the last 4 years (before 2014) were excluded.
Outcomes of Interest
The primary outcome was CFS at 3 months after commencement of IFX therapy. Secondary outcomes included CFS survival at 1 and 12 months, adverse drug events, mortality, and postoperative complications.
The use of IFX was categorized by dosage (5 mg/kg or 10 mg/kg), dose number (single- or multiple-dose induction), and dose schedule. Dose schedule was defined as follows: (1) standard-schedule induction: 3 IFX doses at weeks 0, 2, and 6; (2) accelerated-schedule induction: 3 doses within 4 weeks; (3) dose-intensified induction: use of either multiple 10-mg/kg doses or an accelerated schedule with 5 mg/kg (incorporating [2]). The IFX schedule was classified on the basis of the reported intention-to-treat (ITT) strategy.
Data Extraction and Quality Assessment
Data were extracted from included studies by 2 reviewers independently (M.C.C., D.S.). In studies with multiple treatment arms, data extraction was performed in IFX-treated populations only. Corresponding authors were contacted to obtain additional data where required. Risk of bias and study quality were evaluated independently by 2 reviewers (M.C.C., D.S.), and any discrepancies were resolved in consultation with senior authors. Single-arm/extracted cohort studies that described proportions of CFS cases were treated as prevalence studies and assessed with a critical appraisal tool designed by the Joanna-Briggs Institute.15 The quality of nonrandomized studies was assessed with the Newcastle Ottawa Scale.16 The quality of randomized studies was assessed with the Cochrane risk of bias table.
Statistical Analysis
Data were analyzed on ITT principles. A random-effects model for these analyses was selected to provide a more conservative estimate than a fixed-effects model. Weighted pooled proportions of CFS were derived from studies by combining individual proportions and 95% confidence intervals (CIs) using the Freeman-Tukey double arcsine transformation method. Subgroups of IFX strategy were determined from studies that contained sufficient discriminatory information. Analysis of comparative studies that contained combinations of individual treatment groups was performed by converting binary data into pooled odds ratios (ORs).
Potential confounding covariates such as age, disease duration, IV steroid therapy, baseline C-reactive protein (CRP), and albumin levels were also examined. Continuous variables were reported as mean ± SD. Reported medians and interquartile ranges or ranges were converted to means and SDs according to formulae provided by Wan et al.17 Where required, means and variances of treatment groups within studies were pooled for analyses.
Analyses were performed with MIX 2.0 Pro (MIX 2.0 – Professional software for meta-analysis in Excel. Version 2.0.1.5. BiostatXL, 2016. https://www.meta-analysis-made-easy.com. Mountain View, California, USA) to derive pooled proportions and RevMan 5.3 (Review Manager [RevMan], version 5.3; The Nordic Cochrane Centre, Copenhagen, Denmark) to determine ORs in comparative studies and mean covariate differences. A 2-tailed P value <0.05 was considered statistically significant.
Heterogeneity and Publication Bias
Heterogeneity was assessed with the I2 test.18 The I2 statistic estimates the percentage of variation across studies that is due to heterogeneity rather than chance. Following Higgins et al.,18 we considered I2 values of 25%, 50%, and 75% to be low, moderate, and high. These categories do not refer to the absolute amount of observed heterogeneity, but rather to the proportion of the observed effect variance that would remain if the sampling error were to be eliminated. Subgroup analyses were performed if there was moderate or high heterogeneity in pooled effect estimates. Publication bias was assessed with Egger’s test.19
RESULTS
Search Results
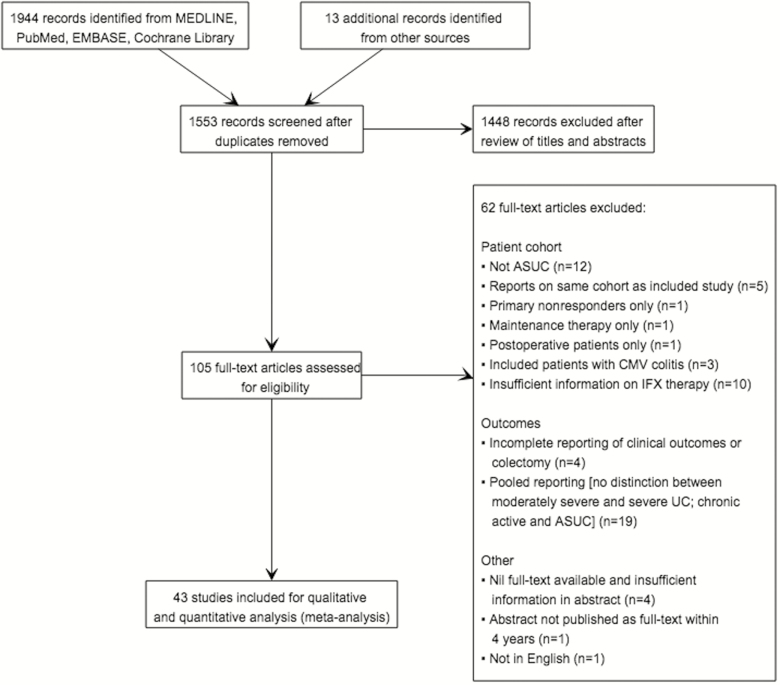
The literature search identified 1944 citations (Fig. 1), of which 105 met the criteria for full-text review. A total of 62 studies were subsequently excluded (Fig. 1): 12 were in non-ASUC cohorts; 5 reported on already included cohorts; 1 examined primary nonresponders to IFX; 1 investigated IFX maintenance therapy; and 1 investigated the postoperative setting. Three studies were excluded due to comorbid CMV colitis. There was insufficient information regarding IFX dosing and/or timing of administration in 10 studies. Four studies did not adequately report clinical outcomes. Nineteen studies were excluded on the basis of pooled outcome reporting without exclusion of patients with moderately severe UC and/or chronic active UC. The full-text versions of 4 studies were not available. One abstract was not published as full text within 4 years, and 1 was not in English.
FIGURE 1.
PRISMA flowchart.
Overall, 43 full-text articles were included for meta-analysis.10–12, 14, 20–58 Two articles published by Laharie et al.37, 38 and similar articles published by Jarnerot et al.33 and Gustavsson et al.29 reported outcomes on the same respective cohorts and were therefore merged for quantitative analysis. Thus, a total of 2158 patients across 41 separate study cohorts were included.
Characteristics of Included Studies
There were 5 RCTs, 30 retrospective and 6 prospective observational cohorts. Study characteristics and considerations for analyses are outlined in Table 1. Of the 5 RCT populations, 3 reported on IFX vs placebo28, 33, 48 and 2 reported on IFX vs cyclosporine.37, 38, 54 Only the IFX-treated arms from these RCTs were extracted for this review. Additional data were obtained from 12 studies by correspondence.10–12, 20, 22, 24, 26, 27, 30, 40, 47, 53 Unadjusted data were utilized for the analysis.
TABLE 1.
Study Characteristics and Considerations for Analysis
| Author | Year | Country | Type of Study | Abstract or Full Text | Definition of Severity | Eligibility for Rescue Therapy | Sample Size | Subgroups | IFX Dose | IFX Dose Number (ITT) | IFX Dose Strategy (ITT) | CFS (N) | Considerations for the Meta-analysis | ||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Month 1 | Month 3 | Month 12 | |||||||||||||
| Al Khoury | 2017 | Canada | Retrospective | Abstract | Mayo severity score 6–12 with Mayo endoscopic score ≥2 | IV steroid-refractory (Oxford criteria) | 72 | 69 | 67 | 64 | |||||
| 37 | 5 mg/kg | 3 | Standard | 36 | 35 | 33 | |||||||||
| 35 | 10 mg/kg | 3 | Standard | 30 | 30 | 29 | |||||||||
| 5 | 10 mg/kg | 3 | Accelerated | 3 | 2 | 2 | |||||||||
| An | 2017 | Australia | Retrospective | Abstract | TLW criteria | IV steroid-refractory | 44 | 5 mg/kg | 38 | 35 | 34 | ||||
| 16 | 3 | Standard | 15 | 13 | 13 | ||||||||||
| 28 | 3 | Accelerated | 23 | 22 | 21 | ||||||||||
| Aratari | 2008 | Italy | Retrospective | Full text | TLW criteria and Powell Tuck | IV steroid-refractory | 11 | 5 mg/kg | 3 | Standard | 11 | 11 | 10 | ||
| Beswick | 2016 | Australia | Prospective observational | Abstract | TLW criteria | IV steroid-refractory | 24 | 5 mg/kg | 22 | 22 | 19 | ||||
| 3 | 5 mg/kg | 1 | Single dose | 3 | 3 | 3 | |||||||||
| 9 | 5 mg/kg | ≥2 | Standard | 9 | 9 | 9 | |||||||||
| 12 | 5 mg/kg | ≥2 | Accelerated | 10 | 10 | 7 | |||||||||
| Bressler | 2008 | Canada | Retrospective | Full text | Hospitalized UC | IV steroid-refractory | 21 | 5 mg/kg | 1 | Single dose | 16 | 13 | NS | ||
| Croft | 2013 | Australia | Prospective observational | Full text | TLW criteria | IV steroid-refractory | 38 | 5 mg/kg | 1 | Single dose | 31 | 28 | 24 | ||
| Dean | 2011 | New Zealand | Retrospective | Full text | Hospitalized UC | IV steroid-refractory | 19 | 5 mg/kg | 1–5 | Single or multiple dose | NS | 15 | 12 | ||
| Duijvis | 2016 | Netherlands | Retrospective | Full text | Hospitalized UC | IV or oral steroid-refractory | 22 | 5 mg/kg | 3 | Standard | 21 | 16 | 12 | Mixture of moderate–severe and severe patients | |
| Fernandes | 2016 | Portugal | Retrospective | Full text | TLW criteria | IV steroid-refractory (Oxford criteria) | 25 | 5 mg/kg | 3 | Standard | 20 | 20 | 19 | ||
| Florhomen | 2011 | Norway | RCT | Full text | TLW criteria | IV steroid-refractory | 13 | 5 mg/kg | 3 | Standard | 13 | 13 | NS | ||
| Gibson | 2015 | Ireland | Retrospective | Full text | Hospitalized UC | IV steroid-refractory | 50 | 36 | 32 | 29 | |||||
| 35 | 5 mg/kg | 3 | Standard | 22 | 20 | 18 | |||||||||
| 15 | 5 mg/kg | 3 | Accelerated | 14 | 12 | 11 | |||||||||
| Gibson | 2018 | Ireland | Retrospective | Abstract | Hospitalized UC | IV steroid-refractory | 145 | ||||||||
| 87 | 5 mg/kg | 3 | Standard | 71 | 66 | 60 | |||||||||
| 58 | 5 mg/kg | 3 | Accelerated | 53 | 49 | 44 | |||||||||
| Govani | 2016 | USA | Retrospective | Abstract | Hospitalized UC | IV steroid-refractory | 55 | 44 | 42 | 33 | Mixture of 5 mg/kg and 10 mg/kg given to patients in both accelerated and high-dose cohorts, unable to include into the meta-analysis | ||||
| 17 | 10 mg/kg starting dose | 3 | NA | 10 | 9 | 9 | |||||||||
| 38 | 5 mg/kg starting dose | 3 | NA | 34 | 33 | 24 | |||||||||
| Jarnerot/ Gustavsson | 2005/ 2010 | Sweden | RCT/retrospective | Full text | Seo index | IV steroid-refractory (failure to improve according to Seo index) | 24 | 4-5 mg/kg | 1 | Single dose | 17 | 17 | 14 | Jarnerot and Gustavsson cohorts merged; mixture of moderate–severe and severe patients | |
| Halpin | 2013 | UK | Retrospective | Full text | TLW criteria | IV steroid-refractory | 44 | 5 mg/kg | 3 | Standard | 34 | 34 | 31 | IV steroid-refractory | |
| Ho | 2009 | UK/Scotland | Prospective observational | Full text | TLW criteria | IV steroid-refractory (Oxford criteria or Ho index) | 21 | 5 mg/kg | 1 | Single dose | 10 | NS | NS | ||
| Hulkower | 2016 | USA | Prospective observational | Abstract | Hospitalized UC/Mayo score >9 | IV steroid-refractory | 4 | 10 mg/kg | 2–3 | Accelerated | 4 | 4 | NS | ||
| Kaser | 2001 | Austria | Prospective observational | Full text | Hospitalized UC | IV steroid-refractory | 6 | 5 mg/kg | 1 | Single dose | 6 | 6 | NS | ||
| Kim | 2015 | South Korea | Retrospective | Full text | Hospitalized UC | IV steroid-refractory | 33 | 5 mg/kg | 3 | Standard | 33 | 33 | 32 | ||
| Kohn | 2007 | Italy | Retrospective | Full text | TLW criteria | IV steroid-refractory | 83 | 5 mg/kg | NS | 71 | NS | 2-mo analysed as 3-mo outcomes; mixture of moderate–severe and severe patients | |||
| 26 | 1 | Single dose | NS | 17 | NS | ||||||||||
| 57 | ≥2 | Weeks 0, 2, 4, or 0, 2, 6 | NS | 54 | NS | ||||||||||
| Laharie | 2012/ 2017 | France | RCT | Full text | Lichtiger score >10 | IV steroid-refractory | 55 | 5 mg/kg | 3 | Standard | NS | 45 | 38 | Laharie 2012/2017 cohorts merged; 2 patients excluded as received CyA; 12-mo outcome derived % estimate | |
| Lees | 2007 | UK | Retrospective | Full text | TLW criteria | IV steroid-refractory | 39 | 5 mg/kg | 1–3 | Single or multiple dose | 26 | 26 | 24 | ||
| Llao | 2016 | Spain | Retrospective | Full text | Montreal classification/TLW | IV steroid-refractory | 14 | 5 mg/kg | 3 | Standard | 14 | 14 | 11 | ||
| Lowenberg | 2014 | Netherlands | Retrospective | Full text | TLW criteria | IV steroid-refractory (Oxford criteria) | 16 | 5 mg/kg | 3 | Standard | 15 | 12 | 10 | ||
| Mocciaro | 2012 | Italy | Retrospective | Full text | TLW criteria | IV steroid-refractory | 30 | 5 mg/kg | 3 | Standard | 25 | 25 | 25 | ||
| Monterubbianesi | 2014 | Italy | Retrospective | Full text | TLW criteria (modified by Chapman) | IV steroid-refractory | 113 | 5 mg/kg | 3 | Standard | 96 | 91 | 83 | ||
| Mortensen | 2011 | Denmark | Retrospective | Full text | Hospitalized UC/SCCAI | IV or oral steroid-refractory | 56 | 5 mg/kg | 1–9 | Single or standard | 46 | 39 | NS | ||
| Nalagatla | 2018 | USA | Retrospective | Full text | Hospitalized UC | IV steroid-refractory | 213 | ||||||||
| 132 | 5 mg/kg | >2 | Standard | 121 | 113 | 96 | |||||||||
| 81 | 5–10 mg/kg | >2 | Accelerated/intensified | 74 | 65 | 58 | |||||||||
| Ordas | 2017 | Spain | Retrospective | Full text | Hospitalized UC | IV steroid-refractory | 131 | 5 mg/kg | 1 or 3 | Single or standard | NS | 112 | 100 | ||
| Regueiro | 2006 | USA | Retrospective | Full text | Partial Mayo score ≥9 | IV steroid-refractory | 11 | 5 mg/kg | 3 | Standard | 7 | 4 | 2 | ||
| Ribaldone | 2017 | Italy | Retrospective | Full text | TLW criteria | IV steroid-refractory | 20 | 5 mg/kg | 3 | Standard | 19 | 19 | 15 | ||
| Sands | 2001 | USA | RCT | Full text | TLW criteria/Lichtiger score | IV steroid-refractory | 11 | 7 | 4 | NS | |||||
| 3 | 5 mg/kg | 1 | Single dose | 3 | 1 | NS | |||||||||
| 3 | 10 mg/kg | 1 | Single dose | 2 | 1 | NS | |||||||||
| 2 | 20 mg/kg | 1 | Single dose | 2 | 2 | NS | |||||||||
| Seah | 2017 | Australia | Retrospective | Full text | TLW criteria | IV steroid-refractory | 41 | 5 mg/kg | 3 | 37 | 36 | 30 | |||
| 30 | Standard | 28 | 28 | 24 | |||||||||||
| 10 | Accelerated | 9 | 8 | 6 | |||||||||||
| Shah | 2018 | USA | Retrospective | Full text | Hospitalized UC | IV or oral steroid-refractory | 126 | 3 | 106 | 97 | 89 | ||||
| 89 | 5 mg/kg | Standard | 78 | 72 | 65 | ||||||||||
| 23 | 5 mg/kg | Accelerated | 16 | 14 | 14 | ||||||||||
| 8 | 10 mg/kg | Standard | 6 | 5 | 4 | ||||||||||
| 6 | 10 mg/kg | Accelerated | 6 | 6 | 6 | ||||||||||
| Shepherd | 2014 | Australia | Retrospective | Abstract | TLW criteria | IV steroid-refractory | 15 | 5 mg/kg | 1–3 | 12 | 10 | 6 | |||
| 11 | 1 | Single dose | 8 | 6 | 4 | ||||||||||
| 4 | ≥2 | Multiple dose | 4 | 4 | 2 | ||||||||||
| Sjoberg | 2013 | Sweden | Retrospective | Full text | TLW criteria | IV steroid-refractory (fulminant colitis index–Lindgren 1998 or Seo index) | 211 | 5 mg/kg | 153 | 149 | 133 | ||||
| 124 | 1 | Single dose | NS | 76 | NS | ||||||||||
| 87 | 2–3 | Standard | NS | 73 | NS | ||||||||||
| Sly | 2017 | USA | Retrospective | Abstract | Hospitalized UC | IV steroid-refractory | 41 | ||||||||
| 18 | 5 mg/kg | 3 | Standard | 16 | 16 | 13 | |||||||||
| 23 | 5–10 mg/kg | 3 | Accelerated | 16 | 14 | 11 | |||||||||
| Sood | 2014 | India | Retrospective | Full text | Lichtiger score | IV steroid-refractory | 28 | 5 mg/kg | 3 | Standard | 25 | 19 | |||
| Van Langenberg | 2015 | Australia | Retrospective | Abstract | TLW criteria | IV steroid-refractory | 88 | 5 mg/kg | 80 | 76 | 67 | ||||
| 41 | 1 | Single dose | 33 | 31 | 28 | ||||||||||
| 47 | ≥2 | Standard | 47 | 45 | 39 | ||||||||||
| Williams | 2016 | UK | RCT | Full text | TLW criteria or clinical judgment | IV steroid-refractory | 135 | 5 mg/kg | 3 | Standard | 106 | 96 | 88 | Moderate-severity TLW in 27% | |
| Yamamoto-Furusho | 2008 | Mexico | Prospective observational | Full text | TLW criteria | IV steroid-refractory | 10 | 5 mg/kg | 1 | Single dose | NS | 2 | 2 |
Abbreviations: NS, not stated; TLW, Truelove and Witt’s.
Twelve study populations reported on single-dose induction,22–24, 29, 31, 33, 34, 36, 48, 50, 51, 53 and 35 studies reported on multiple-dose IFX induction.10–12, 14, 20–22, 25–28, 30, 32, 35–47, 49–54, 56–58 Dose-intensified induction strategies were employed in 11 studies.10–12, 14, 20, 22, 32, 49, 56–58 Of these, 10 studies utilized an accelerated dosing schedule,10–12, 20, 22, 32, 49, 56–58 4 utilized 10-mg/kg dose induction therapy,11, 12, 14, 32 and 4 studies investigated accelerated induction in conjunction with high-dose IFX.11, 12, 32, 58 One study was a single dose finding RCT.48 One abstract assessed standard vs accelerated-schedule induction.14 However, as both arms contained patients who were treated with a combination of 5- and 10-mg/kg dosing, this study was excluded from the comparative meta-analysis. Extracted data for the analysis are detailed in Table 1 and Supplementary Appendix 3.
Pooled Colectomy-Free Survival
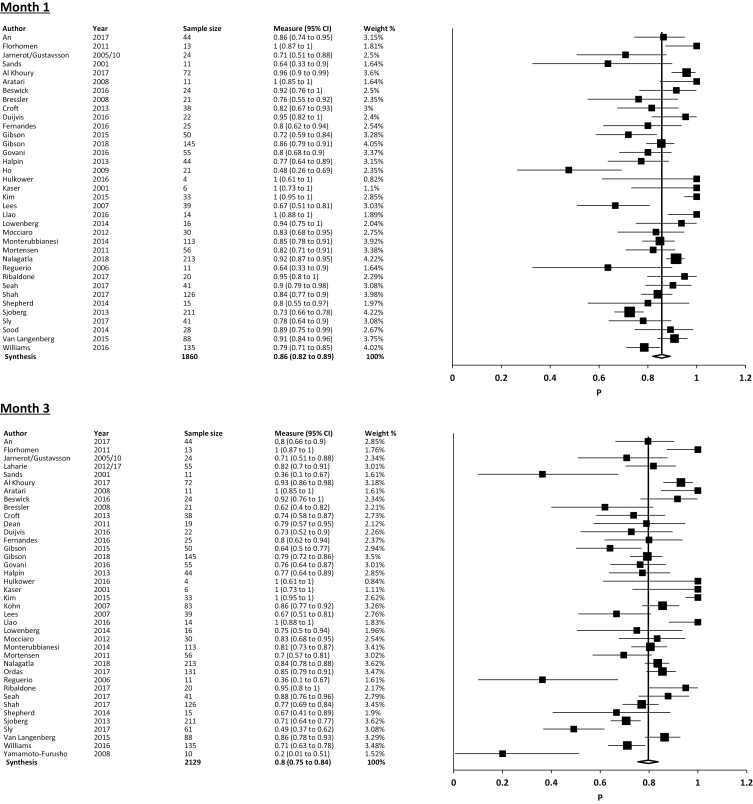
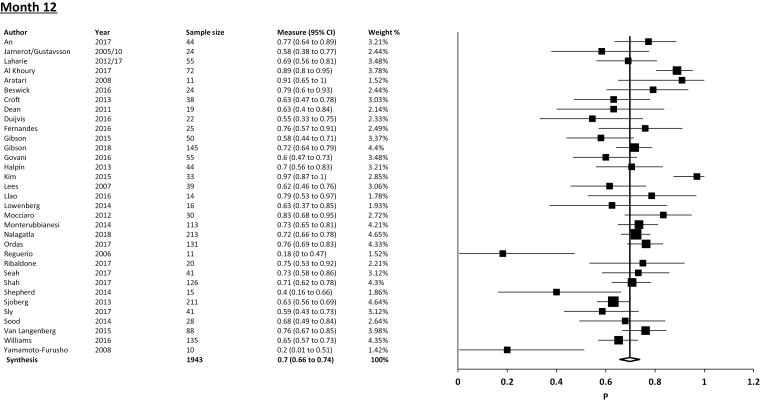
The overall pooled colectomy-free survival following IFX therapy for ASUC from all included studies was 79.7% (95% CI, 75.5% to 83.6%; I2 = 77%; 36 studies, 1659/2129 cases) at 3 months. Pooled CFS at 1 month was 85.7% (95% CI, 82.0% to 89.0%; I2 = 70.6%; 36 studies, 1550/1860 cases), and 69.8% (95% CI, 65.7% to 73.7%; I2 = 67%; 33 studies, 1357/1943 cases) at 12 months (Fig. 2).
FIGURE 2.
Forest plot using random-effects model for overall pooled colectomy-free survival (proportions).
Pooled CFS with 5-mg/kg single-dose induction was 67.3% (95% CI, 57.1% to 76.8%; I2 = 55.1%; 10 studies, 200/307 cases) at 3 months, 78.8% (95% CI, 68.4% to 88.0%; I2 = 40.2%; 9 studies, 127/168 cases) at 1 month, and 57.0% (95% CI, 40.7% to 72.7%; I2 = 60.2%; 6 studies, 75/127 cases) at 12 months.
Pooled CFS with 5-mg/kg standard week 0, 2, and 6 induction was 84.0% (95% CI, 78.3% to 89.1%; I2 = 80.5%; 25 studies, 923/1152 cases) at 3 months, 89.4% (95% CI, 83.9% to 93.9%; I2 = 81.5%; 24 studies, 882/1038 cases) at 1 month, and 73.8% (95% CI, 67.9% to 79.4%; I2 = 74.6%; 24 studies, 772/1080 cases) at 12 months.
Pooled CFS with dose-intensified induction was 78.5% (95% CI, 70.8% to 85.4%; I2 = 49.2%; 11 studies, 254/325 cases) at 3 months, 84.8% (95% CI, 78.0% to 90.6%; I2 = 46.1%; 11 studies, 274/325 cases) at 1 month, and 70.1% (95% CI, 60.2% to 79.2%; I2 = 65.9%; 10 studies, 231/321 cases) at 12 months.
CFS proportions by IFX strategy are described in Table 2.
TABLE 2.
Pooled Colectomy-Free Survival (Random-Effects Model), Expressed as N% (95% CI)
| Month 1 | Month 3 | Month 12 | |
|---|---|---|---|
| Overall colectomy free-survival | 85.7% (82.0%–89.0%; I2 = 70.6%; 36 studies, 1550/1860 cases) |
79.7% (75.48%–83.6%; I2 = 77%; 36 studies, 1659/2129 cases) |
69.8% (65.7%–73.7%; I2 = 67%; 33 studies, 1357/1943 cases) |
| 5-mg/kg single dose | 78.8% (68.4%–88.0%; I2 = 40.2%; 9 studies, 127/168 cases) |
67.3% (57.1%–76.8%; I2 = 55.1%; 10 studies, 200/307 cases) |
57.0% (40.7%–72.7%; I2 = 60.2; 6 studies, 75/127 cases) |
| 5-mg/kg multiple dose | 90.0% (86.1%–93.3%; I2 = 67.7%; 25 studies, 1027/1189 cases) |
85.1% (80.9%–89.0%; I2 = 71.7%; 28 studies, 1125/1379 cases) |
72.8% (68.2%–77.2%; I2 = 60.2%; 25 studies, 881/1231 cases |
| 5-mg/kg standard 026 induction | 89.4% (83.9%–93.9%; I2 = 81.5%; 24 studies, 882/1038 cases) |
84.0% (78.3%–89.1%; I2 = 80.5%; 25 studies, 923/1152 cases) |
73.8% (67.9%–79.4%; I2 = 74.6%; 24 studies, 772/1080 cases) |
| 5-mg/kg accelerated induction | 86.3% (78.5%–92.8%; I2 = 21.7%; 6 studies, 125/145 cases) |
79.7% (72.3%–86.2%; I2 = 0%; 6 studies, 115/145 cases) |
71.2% (63.1%–78.6%; I2 = 0%; 5 studies, 103/145 cases) |
| Dose-intensified induction | 84.8% (78.0%–90.6%; I2 = 46.1%; 11 studies, 274/325 cases) |
78.5% (70.8%–85.4%; I2 = 49.2%; 11 studies, 254/325 cases) |
70.1% (60.2%–79.2%; I2 = 65.9%; 10 studies, 231/321 cases) |
| 10-mg/kg multiple-dose induction | 81.0% (65.4%–93.2%; I2 = 39.9%; 4 studies, 59/75 cases) |
76.7% (59.1%–91.1%; I2 = 48.3%; 4 studies, 56/75 cases) |
69.6% (54.0%–83.3%; I2 = 37.3%; 3 studies, 50/71 cases) |
| 10-mg/kg standard schedule | 84.9% (71.6%–95.0%; I2 = 0%; 2 studies, 36/43 cases) |
79.4% (53.9%–97.1%; I2 = 50.1%; 2 studies, 35/43 cases) |
71.5% (36.4%–96.9%; I2 = 69.7%; 2 studies, 33/43 cases) |
| 10-mg/kg accelerated schedule | 92.7% (60.3%–100%; I2 = 43.7%; 3 studies, 13/15 cases) |
88.3% (63.5%–100%; I2 = 68.9%; 3 studies, 12/15 cases) |
78.8% (8.3%–100%; I2 = 81.7%; 2 studies, 8/11 cases) |
Comparative Cohort Meta-analysis
5-mg/kg multiple-dose induction vs 5-mg/kg single-dose induction
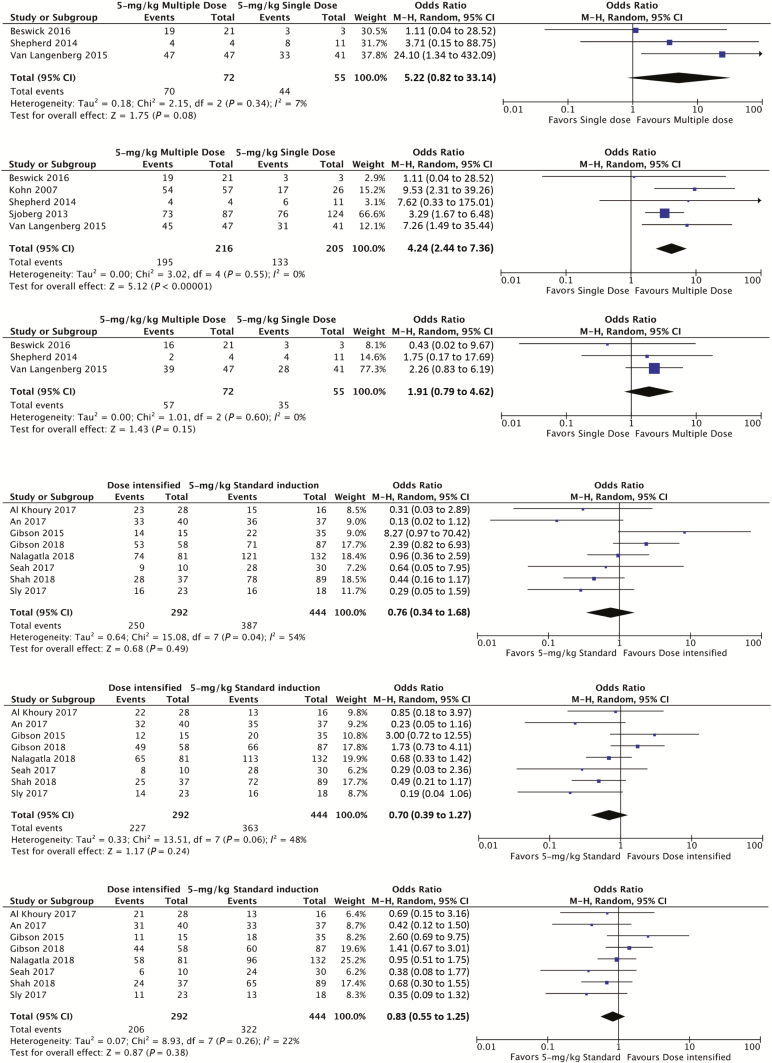
Among comparative studies, 5-mg/kg multiple-dose induction was superior to 5-mg/kg single-dose induction with respect to CFS at 3 months (OR, 4.24; 95% CI, 2.44 to 7.36; P < 0.001; I2 = 0%; 5 studies) (Fig. 3A).22, 50, 51, 53, 59 Multiple-dose induction was numerically superior at 1 and 12 months, but this did not reach statistical significance.
FIGURE 3.
Forest plot using random-effects models assessing CFS at month 1, 3, and 12 for (A) 5-mg/kg multiple-dose vs 5-mg/kg single-dose induction and (B) dose-intensified vs 5-mg/kg standard-schedule induction.
Dose-intensified induction vs standard induction
Dose intensification was not found to be significantly different than standard induction with CFS at 3 months (OR, 0.70; 95% CI, 0.39 to 1.27; P = 0.24; I2 = 48%; 8 studies, 736 cases) (Fig. 3B).10, 12, 20, 49, 56–58, 60 CFS was also not significantly different at 1 month (OR, 0.76; 95% CI, 0.34 to 1.68; P = 0.49; I2 = 54%) or 12 months (OR, 0.83; 95% CI, 0.55 to 1.25; P = 0.31; I2 = 20%).
Subanalyses
Subanalyses were performed to examine 5-mg/kg standard induction compared with individual treatment strategies of 5-mg/kg accelerated, 10-mg/kg standard, and 10-mg/kg accelerated induction.
5-mg/kg standard vs 5-mg/kg accelerated induction
Five studies (391 patients)10, 20, 49, 56, 60 reported the outcomes of patients treated with 5-mg/kg standard-schedule and 5-mg/kg accelerated-schedule induction. Colectomy-free survival was not statistically different between the 2 groups at 1 month (OR, 1.04; 95% CI, 0.29 to 3.69; P = 0.96; I2 = 66%), 3 months (OR, 0.93; 95% CI, 0.39 to 2.22; P = 0.87; I2 = 56%), or 12 months (OR, 0.96; 95% CI, 0.52 to 1.78; P = 0.89; I2 = 32%).
5-mg/kg standard vs 10-mg/kg standard induction dose
Two studies (169 patients)12, 60 reported the outcomes of 5-mg/kg standard vs 10-mg/kg standard induction. Colectomy-free survival was not statistically different between the 2 groups at 1 month (OR, 0.30; 95% CI, 0.08 to 1.15; P = 0.08; I2 = 0%), 3 months (OR, 0.37; 95% CI, 0.12 to 1.16; P = 0.09; I2 = 0%), or 12 months (OR, 0.53; 95% CI, 0.19 to 1.45; P = 0.21; I2 = 0%), favoring 5-mg/kg standard induction.
5-mg/kg standard induction vs 10-mg/kg accelerated dose
Two studies (137 patients)12, 60 reported the outcomes of 5-mg/kg standard vs 10-mg/kg accelerated induction. Colectomy-free survival was not statistically different between the 2 groups at 1 month (OR, 0.27; 95% CI, 0.01 to 13.07; P = 0.51; I2 = 74%), 3 months (OR, 0.32; 95% CI, 0.00 to 31.34; P = 0.62; I2 = 84%), or 12 months (OR, 0.56; 95% CI, 0.01 to 41.34; P = 0.79; I2 = 83%), favoring 5-mg/kg standard induction.
Influence of Covariates and Confounders
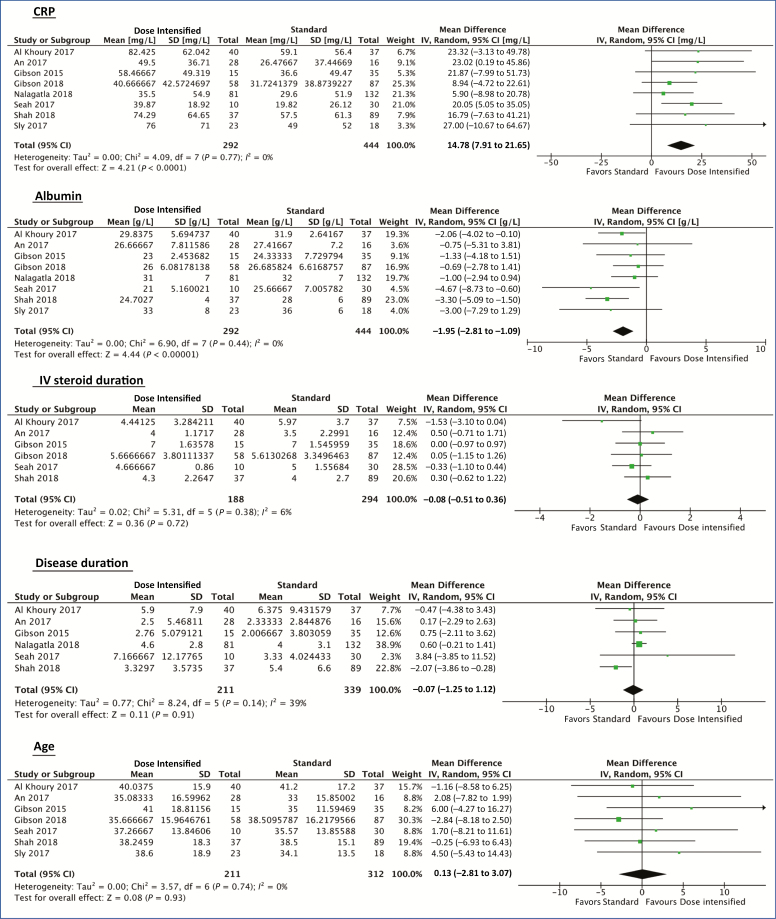
Covariate analysis was performed to assess the relationship of demographic and biochemical factors to outcomes between dose-intensified induction vs standard induction. A metaregression was not performed due to the small number of studies available. Dose-intensified induction patients had a higher mean CRP compared with standard induction (mean difference, 14.78 mg/L; 95% CI, 7.91 to 21.65; P < 0.001) and lower serum albumin (mean difference, –1.95 g/L; 95% CI, –2.81 to –1.09; P < 0.001). There was no significant difference in age, disease duration, or IV steroid duration between the 2 groups (Fig. 4).
FIGURE 4.
Forest plot using random-effects model to assess mean differences in covariates between dose-intensified and 5-mg/kg standard-schedule cohorts.
A narrative synthesis was performed on other studies reporting on the impact of confounders. Hypoalbuminemia was noted to be an independent poor prognostic factor and was associated with colectomy risk.10, 23, 39, 51, 60 Elevated CRP at baseline was associated with risk of colectomy22, 30, 43, 44, 60 and a lower likelihood of achieving mucosal healing.20 Fecal calprotectin was predictive of poor outcome, with a level of >1922.5 mcg/g associated with an 87% risk of colectomy at 1 year.61 Endoscopic features were also prognostic, with the presence of severe endoscopic lesions found to be associated with a higher risk of colectomy by Monterubbianesi et al. (RR, 7.0; 95% CI, 1.09 to 44.7).43 Conversely, achievement of mucosal healing with induction therapy was associated with increased long-term CFS.29 These risk factors were not addressed with dose intensification in these studies.
Multiple studies analyzed outcomes according to IFX strategy. In studies that reported on IFX dose number, single induction was found to have an increased risk of colectomy in 2 studies,36, 53 with a relative risk of 5.76 (95% CI, 1.54 to 21.62; P = 0.005) reported by Kohn et al.,36 although no significant difference was found in a third study by Sjoberg et al.51 Although the study by Govani et al. was not included in our formal analysis due to mixed 5-mg/kg and 10-mg/kg dosing within standard-schedule and accelerated-schedule cohorts, they found that an accelerated-schedule induction had higher 90-day colectomy rates compared with standard-schedule induction (47.1% vs 12.5%; P = 0.01).14 However, accelerated-schedule patients also had a higher baseline CRP (58 mg/L ± 39 vs 37 mg/L ± 3.0; P = 0.06).
Of the studies that reported dose intensification, none documented a strategy of a priori dose intensification for all patients. Seven of these studies had reported that the decision for dose acceleration was based on insufficient clinical or biochemical response to the first infliximab dose.10, 14, 20, 32, 49, 58, 62 The reason for dose escalation was not reported in the remaining 4 studies.12, 56, 57, 60 In the study by Nalagatla et al., an initial dose of 10 mg/kg was selected in patients with more severe clinical, biochemical, or endoscopic disease activity, and among the subgroup of patients who were dose accelerated, an upfront dose of 10 mg/kg was associated with a lower risk of colectomy compared with those who first received 5 mg/kg.58
In individual studies, the use of maintenance therapy with IFX43 and/or immunomodulators28 after induction was associated with reduced colectomy compared with no maintenance (hazard ratio, 0.26; 95% CI, 0.09 to 0.85; P = 0.02).43 Subanalysis to assess the effect of maintenance therapy among our included cohorts could not be performed due to the highly variable combinations of aminosalicylates, thiopurines, and infliximab (Supplementary Appendix 3).
Adverse Events, Postoperative Complications, and Mortality
The pooled adverse drug event rate was 26.1% (344/1319) from 24 studies, the pooled postoperative complication rate was 42.2% (155/367) from 13 studies, and the mortality rate was 1.0% (13/1342) from 22 studies. There were insufficient data to make meaningful comparisons on adverse events, postoperative complications, and mortality between dose-intensified and standard-dose induction across studies. Only 1 study provided data on adverse drug event rates and postoperative complication rates between 5-mg/kg and 10-mg/kg patients.11 The adverse drug event rate was 42.9% (48/112) in those treated with 5-mg/kg induction vs 28.6% (4/14; P = 0.394) in those treated with 10-mg/kg induction. The postoperative complication rate was 78.8% (26/33) among those treated with 5-mg/kg induction vs 0% (0/4) in those treated with 10-mg/kg induction (P = 0.005).
Study Quality, Heterogeneity, and Publication Bias
In all studies, cases were representative of hospitalized steroid-refractory ASUC, and colectomy was utilized as an objective outcome measure. However, the majority of studies were uncontrolled with respect to case selection and disease severity on admission. There were recurrent issues of incomplete outcome reporting and inconsistency in reporting of relevant data (demographics/biochemistry and complication rates). A quality assessment utilizing the Newcastle Ottawa Scale and the Cochrane risk of bias table demonstrated that the majority of included studies in the meta-analysis were of poor quality. Details of study quality assessment can be found in Supplementary Appendix 4.
In our heterogeneity assessment, we identified variability regarding the definitions of disease severity and steroid failure. Among all pooled studies, the I2 test was 67.0%–77.0%, indicating a high proportion of variation across studies due to heterogeneity rather than chance. This was subsequently investigated with subgroup analyses of different IFX strategies. There was no significant publication bias (3 month outcomes: Egger’s intercept = 0.26; P = 0.74). In the comparative cohort meta-analysis: 5-mg/kg single-dose vs 5-mg/kg multiple-dose induction comparisons; there was a low level of heterogeneity between the 5 studies at 3 months (I2 = 0.0%). Among dose-intensified vs standard induction comparisons, the I2 test was 48%, indicating a moderate amount of heterogeneity.
DISCUSSION
In this systematic review and meta-analysis, we summarize the published experience of IFX induction and CFS in ASUC under different induction strategies. Despite being used for more than 15 years, the optimal IFX dose strategy in ASUC is unknown, due to the infrequency of this life-threatening condition and the difficulty of performing well-constructed RCTs. IFX salvage in ASUC has evolved from 5-mg/kg single-dose induction to high-dose and short-interval therapy based on studies with vastly different clinical settings and clinician experiences. Apart from a single RCT by Sands and colleagues exploring different IFX doses in ASUC that was terminated due to slow recruitment,48 no published RCTs have investigated dose induction strategies in ASUC. The lack of strong evidence guiding the optimal use of IFX in ASUC has consequently led to marked variability in clinical management.
In this study, 5-mg/kg multiple-dose IFX induction was superior to 5-mg/kg single-dose rescue therapy for CFS at 3 months. This supports current consensus statements on multiple IFX 5-mg/kg salvage therapy dosing in ASUC63 and provides evidence to avoid the use of 5-mg/kg single-dose induction, which was proposed in older guidelines64. 5-mg/kg multiple-dose induction CFS was favored at 1 and 12 months; however, efficacy at these time points did not reach statistical significance, likely due to the small number of studies that have compared these strategies over time.
Contrary to current trends in clinical practice, dose intensification to 10 mg/kg or dose acceleration with 5 mg/kg was not associated with improved outcomes over 5-mg/kg standard-dose induction. However, we found that dose-intensified strategies were used in patient groups with an overall higher CRP and lower albumin, biochemical profiles indicating greater disease severity and associated with an increased likelihood of colectomy. Although these biochemical differences should be interpreted with caution due to the risk of aggregation bias of mean data, this may mask the true benefit of dose intensification and its potential effect of attenuating the rate of colectomy in high-risk patients. This indicates the need for clinical trials to control for these parameters of disease severity in the future.
Although a recent meta-analysis by Nalagatla and colleagues58 also concluded no difference between dose-intensified and standard induction, our systematic review has, for the first time, quantified the differences in existing cohort severity with respect to CRP and albumin, includes a larger cohort, and demonstrates the poor quality of current source data. Although we recognize that performing a meta-analysis with these available studies of variable quality may be controversial, our paper draws together the currently available evidence and highlights that the optimal dosing regimen for infliximab salvage therapy for ASUC remains unclear. It is also important to note that these findings may be confounded by patient selection and provider bias with respect to how dose intensification strategies were adopted in the included observational cohorts.
The basis on which to apply IFX dose intensification is unknown. Elevated CRP,65 low albumin, antidrug antibodies, and increased body mass index66 are factors that have been associated with increased IFX drug clearance. Although increased IFX drug clearance and a reduced serum half-life have recently been shown to be associated with therapeutic failure in ASUC, it is unclear if dose intensification in this circumstance will improve therapeutic success.67 Higher IFX drug exposure in the ASUC induction phase has not presently been shown to be associated with treatment success,67, 68 with 1 study in fact finding that lower IFX drug exposure within the first week in ASUC was associated with clinical response.69 Although this counterintuitive finding may be explained by responders needing less drug overall, there are likely to be differences in the pharmacodynamic and immunological effects of IFX in individuals that may not be explained by pharmacokinetics alone. Hence, as clinicians increasingly turn to dose escalation, timely clinical assessment of response to rescue therapy is imperative. Although signals exist and algorithms have been proposed regarding dose escalation of IFX based on baseline biochemical profiles70, 71 or CRP and albumin response after induction,13, 72 they have either not been validated or not been shown to improve outcomes.14
Emergent colectomy is associated with a significantly higher mortality rate in comparison with elective surgical management.73 Although perioperative IFX therapy was not shown to increase UC surgical complications in a recent meta-analysis,74 the impact of high-dose therapy is unknown. Decisions regarding dose-escalated salvage therapy vs colectomy in ASUC require careful consideration, particularly with regard to adverse events associated with intensive immunosuppression vs the risk of postoperative complications. Failure to make appropriate decisions on treatment futility and delayed surgical intervention can lead to increased morbidity, mortality, and health care costs.75 Although the overall pooled mortality rate of 1% in our present study is in line with published data,3 the studies examined in this analysis did not provide sufficient information to robustly ascertain complication or mortality rates of dose intensification vs standard induction. Although dose intensification in outpatient UC has not been associated with increased complications,5 it is important that future studies assess adverse events and postoperative complications carefully in ASUC.
There were several limitations of our meta-analysis. Of all the eligible studies, only 11 assessed outcomes prospectively. Infliximab levels were not reported in these cohorts, which represents an important potential confounder of the analysis. Although 2 cohorts11, 58 were analyzed by propensity scoring methodology to adjust for increased biochemical severity in the dose-intensified cohort compared with standard-dose patients, no differences in colectomy rate were observed between dose-intensified and standard-dose induction with matched and unmatched cohorts; hence, unadjusted data were utilized for the analysis. Accelerated induction and high-dose induction were grouped as a single category, owing to the limited number of studies. Additionally, 2 studies by Gibson and colleagues10, 56 may have included patients who overlapped between the cohorts; however, we were unable to obtain this information from the authors. As this likely affected <10% of the Gibson cohort, the studies were included; exclusion of either study did not affect the meta-analysis findings. A high degree of heterogeneity, as measured by the I2 test, also relates to how the use of IFX has evolved over time. Although we assessed for baseline covariates, we were unable to control for all potential confounding factors due to variable study quality and data.
Though this analysis only included hospitalized, steroid-refractory UC, the definition of UC severity and steroid failure was variable and may have resulted in clinical heterogeneity between studies. Clinical response and remission were not examined in this study, given the variable definition of these clinical entities and lack of reporting. Although we attempted to address potential outcome bias for those treated with a single dose of IFX by applying an ITT analysis, the outcomes of single-dose induction may have been adversely impacted, as those who proceeded to colectomy may not have had an opportunity to receive more than 1 dose. Maintenance therapy was also variable between the cohorts and may have affected long-term colectomy rates. Despite these limitations, these data provide confident estimates of CFS with IFX salvage therapy under different strategies in real-world practice.
This meta-analysis highlights the challenges associated with performing controlled trials in ASUC. In particular, the variance in clinical practice and IFX induction permutations presented here underscore the complexity of interpreting data in this setting. Given that placebo-controlled trials of IFX are no longer ethically feasible when exploring optimal IFX dose induction, it is likely that future trials of IFX will require an active control. Although standard-schedule arms may be utilized as comparators to dose-intensified strategies, current practice in patients who are not responding to a first dose is generally to dose-escalate, rather than proceed directly to colectomy. This calls into question whether trials in ASUC should use colectomy as a primary end point, or instead, utilize clinical response or need for further rescue dosing as a pragmatic outcome. Estimates of colectomy rate in this study with standard-schedule dose induction may therefore serve as a useful historical comparator for future studies.
In conclusion, IFX 5-mg/kg multiple-dose induction is effective as medical salvage therapy for ASUC. Although our data do not presently demonstrate the superiority of dose intensification over standard induction, it remains to be seen whether a dose-intensified strategy can further reduce the risk of colectomy when applied uniformly to all patients. However, this approach risks overtreating patients who are destined for a favorable outcome at the expense of increased costs and potential morbidity. Prospective RCTs comparing dose-intensified with standard-dose therapy in ASUC are both planned71 and underway (PREDICT UC; Clinicaltrials.gov: NCT02770040), which may provide more clarity, allow the generation of precise risk profiles, and facilitate prediction of outcome for patients who present with this highly challenging clinical condition.
Supplementary Material
ACKNOWLEDGMENTS
We would like to acknowledge the following investigators for providing additional study data for our analysis: Alex Al-Khoury, Lauren Beswick, Anthony Croft, Eugeni Domenech, David Faleck, Alexander Ford, Samuel Fernandes, David Gibson, Davide Ribaldone, Anne Ten Hove, and Abhinav Vasudevan.
Conflicts of interest: M.C.C. has received travel and educational grants from Abbvie, Ferring, Shire, Orphan, and Takeda; has served as a speaker for DiaSorin; and received research support from Janssen. G.R.S. has served as a consultant, an advisory board member, or a speaker for AbbVie, Ferring, Janssen, Shire, Protagonist, Pfizer, and Takeda Pharmaceuticals. T.B. has served as a speaker for Janssen, Abbvie, Takeda, Pendopharm, Shire, and Ferring; as a consultant for Janssen, Abbvie, Takeda, and Pfizer; and received research support from Janssen, Pentax, and Abbvie. M.C.D. has served as a consultant for Janssen, Abbvie, Pfizer, Takeda, Prometheus labs, Celgene, Merck, and Amgen; and received research support from Janssen, Abbvie, and Prometheus Labs. P.D.C. has served as a consultant, an advisory board member, or a speaker for AbbVie, Baxter, Ferring, Janssen, Shire, and Takeda; and received research support from Ferring, Janssen, and Shire. N.D.Y. has served as an advisory board member for Pfizer. D.S., D.M.F., S.C.S., C.Y.C., Y.K.A., A.C.F., and L.C. have no relevant disclosures.
Supported by: This work was funded in part by an Australian Research Training Scholarship from the University of Melbourne (M.C.C.), the Gandel Philanthropy Grant (M.C.C.), the David Bickart Clinician Research Award from the University of Melbourne (P.D.C.), the Bushell Postdoctoral Award from the Gastroenterological Society of Australia (P.D.C.), and a National Health & Medical Research Council Early Career Fellowship (P.D.C.).
Prior presentation: Preliminary results of this study were presented at ECCO 2018 in Vienna and DDW 2018 in Washington.
REFERENCES
- 1. Kaplan GG, Seow CH, Ghosh S, et al. . Decreasing colectomy rates for ulcerative colitis: a population-based time trend study. Am J Gastroenterol. 2012;107:1879–1887. [DOI] [PubMed] [Google Scholar]
- 2. Dinesen LC, Walsh AJ, Protic MN, et al. . The pattern and outcome of acute severe colitis. J Crohns Colitis. 2010;4:431–437. [DOI] [PubMed] [Google Scholar]
- 3. Narula N, Marshall JK, Colombel JF, et al. . Systematic review and meta-analysis: infliximab or cyclosporine as rescue therapy in patients with severe ulcerative colitis refractory to steroids. Am J Gastroenterol. 2016;111:477–491. [DOI] [PubMed] [Google Scholar]
- 4. Hanauer SB, Feagan BG, Lichtenstein GR, et al. ; ACCENT I Study Group Maintenance infliximab for Crohn’s disease: the ACCENT I randomised trial. Lancet. 2002;359:1541–1549. [DOI] [PubMed] [Google Scholar]
- 5. Rutgeerts P, Sandborn WJ, Feagan BG, et al. . Infliximab for induction and maintenance therapy for ulcerative colitis. N Engl J Med. 2005;353:2462–2476. [DOI] [PubMed] [Google Scholar]
- 6. Kevans D, Murthy S, Iacono A, et al. . Sa2031 accelerated clearance of serum infliximab during induction therapy for acute ulcerative colitis is associated with treatment failure. Gastroenterol. 2012;142:S384–S385 [Google Scholar]
- 7. Seow CH, Newman A, Irwin SP, et al. . Trough serum infliximab: a predictive factor of clinical outcome for infliximab treatment in acute ulcerative colitis. Gut. 2010;59:49–54. [DOI] [PubMed] [Google Scholar]
- 8. Brandse JF, van den Brink GR, Wildenberg ME, et al. . Loss of infliximab into feces is associated with lack of response to therapy in patients with severe ulcerative colitis. Gastroenterol. 2015;149:350–355.e352 [DOI] [PubMed] [Google Scholar]
- 9. Herfarth HH, Rogler G, Higgins PD. Pushing the pedal to the metal: should we accelerate infliximab therapy for patients with severe ulcerative colitis? Clin Gastroenterol Hepatol. 2015;13:336–338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Gibson DJ, Heetun ZS, Redmond CE, et al. . An accelerated infliximab induction regimen reduces the need for early colectomy in patients with acute severe ulcerative colitis. Clin Gastroenterol Hepatol. 2015;13:330–335.e1. [DOI] [PubMed] [Google Scholar]
- 11. Shah SC, Naymagon S, Panchal HJ, et al. . Accelerated infliximab dosing increases 30-day colectomy in hospitalized ulcerative colitis patients: a propensity score analysis. Inflamm Bowel Dis. 2018;24:651–659. [DOI] [PubMed] [Google Scholar]
- 12. Al Khoury A, Chao Cy, Aruljothy A, et al. . P495 intensified infliximab rescue therapy for acute severe ulcerative colitis does not improve long term colectomy-free survival. J Crohns Colitis. 2017;11:S330–S331 [Google Scholar]
- 13. Choy MC, Seah D, Gorelik A, et al. . Predicting response after infliximab salvage in acute severe ulcerative colitis. J Gastroenterol Hepatol. 2018;33:1347–1352. [DOI] [PubMed] [Google Scholar]
- 14. Govani SM, Waljee AK, Stidham RW, et al. . Accelerated dosing of infliximab prevents colectomy within 90 days in only half of patients with severe ulcerative colitis. Gastroenterol. 2016;150:S106–S106. [Google Scholar]
- 15. Munn Z, Moola S, Lisy K, et al. . Methodological guidance for systematic reviews of observational epidemiological studies reporting prevalence and cumulative incidence data. Int J Evid Based Healthc. 2015;13:147–153. [DOI] [PubMed] [Google Scholar]
- 16. Wells G, Shea B, O’Connell D. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses 2011. http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp Accessed October 20, 2017.
- 17. Wan X, Wang W, Liu J, Tong T. Estimating the sample mean and standard deviation from the sample size, median, range and/or interquartile range. BMC Med Res Methodol. 2014;14:135 https://bmcmedresmethodol.biomedcentral.com/articles/10.1186/1471-2288-14-135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327:557–560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315:629–634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. An Y, Chen C, White L, et al. . Accelerated Dosing of Infliximab Induction and Endoscopic Mucosal Healing in Patients With Acute Severe Ulcerative Colitis. Gold Coast, Australia: Australian Gastroenterology Week; 2017. [Google Scholar]
- 21. Aratari A, Papi C, Clemente V, et al. . Colectomy rate in acute severe ulcerative colitis in the infliximab era. Dig Liver Dis. 2008;40:821–826. [DOI] [PubMed] [Google Scholar]
- 22. Beswick L, van Langenberg DR, Rosella O, et al. . Tu1288 the predictive value of early serum infliximab, CRP and faecal calprotectin levels post-first infliximab rescue dose for acute severe colitis: day 1 to 3 is key. Gastroenterol. 2015;148:S-848–S-849. [Google Scholar]
- 23. Bressler B, Law JK, Al Nahdi Sheraisher N, et al. . The use of infliximab for treatment of hospitalized patients with acute severe ulcerative colitis. Can J Gastroenterol. 2008;22:937–940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Croft A, Walsh A, Doecke J, et al. . Outcomes of salvage therapy for steroid-refractory acute severe ulcerative colitis: ciclosporin vs. Infliximab. Aliment Pharmacol Ther. 2013;38:294–302. [DOI] [PubMed] [Google Scholar]
- 25. Dean KE, Hikaka J, Huakau JT, Walmsley RS. Infliximab or cyclosporine for acute severe ulcerative colitis: a retrospective analysis. J Gastroenterol Hepatol. 2012;27:487–492. [DOI] [PubMed] [Google Scholar]
- 26. Duijvis NW, Ten Hove AS, Ponsioen CI, et al. . Similar short- and long-term colectomy rates with ciclosporin and infliximab treatment in hospitalised ulcerative colitis patients. J Crohns Colitis. 2016;10:821–827. [DOI] [PubMed] [Google Scholar]
- 27. Fernandes SR, Santos P, Miguel Moura C, et al. . The use of a segmental endoscopic score may improve the prediction of clinical outcomes in acute severe ulcerative colitis. Rev Esp Enferm Dig. 2016;108:697–702. [DOI] [PubMed] [Google Scholar]
- 28. Florholmen J, Overland G, Olsen T, et al. . Short-and long-term clinical outcomes of infliximab in fulminant ulcerative colitis. Ulcers. 2011;156407. [Google Scholar]
- 29. Gustavsson A, Järnerot G, Hertervig E, et al. . Clinical trial: colectomy after rescue therapy in ulcerative colitis - 3-year follow-up of the Swedish-Danish controlled infliximab study. Aliment Pharmacol Ther. 2010;32:984–989. [DOI] [PubMed] [Google Scholar]
- 30. Halpin SJ, Hamlin PJ, Greer DP, et al. . Efficacy of infliximab in acute severe ulcerative colitis: a single-centre experience. World J Gastroenterol. 2013;19:1091–1097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Ho GT, Lee HM, Brydon G, et al. . Fecal calprotectin predicts the clinical course of acute severe ulcerative colitis. Am J Gastroenterol. 2009;104:673–678. [DOI] [PubMed] [Google Scholar]
- 32. Hulkower B, Fischer M, Sagi S, et al. . Severe corticosteroid-refractory ulcerative colitis successfully treated with serial high-dose infliximab during hospitalization. Am J Gastroenterol. 2016;111:S810–S846. doi: 10.1038/ajg.2016.370.27685309 [Google Scholar]
- 33. Järnerot G, Hertervig E, Friis-Liby I, et al. . Infliximab as rescue therapy in severe to moderately severe ulcerative colitis: a randomized, placebo-controlled study. Gastroenterol. 2005;128:1805–1811. [DOI] [PubMed] [Google Scholar]
- 34. Kaser A, Mairinger T, Vogel W, Tilg H. Infliximab in severe steroid-refractory ulcerative colitis: a pilot study. Wien Klin Wochenschr. 2001;113:930–933. [PubMed] [Google Scholar]
- 35. Kim EH, Kim DH, Park SJ, et al. . Infliximab versus cyclosporine treatment for severe corticosteroid-refractory ulcerative colitis: a Korean, retrospective, single center study. Gut Liver. 2015;9:601–606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Kohn A, Daperno M, Armuzzi A, et al. . Infliximab in severe ulcerative colitis: short-term results of different infusion regimens and long-term follow-up. Aliment Pharmacol Ther. 2007;26:747–756. [DOI] [PubMed] [Google Scholar]
- 37. Laharie D, Bourreille A, Branche J, et al. ; Groupe d’Etudes Thérapeutiques des Affections Inflammatoires Digestives Ciclosporin versus infliximab in patients with severe ulcerative colitis refractory to intravenous steroids: a parallel, open-label randomised controlled trial. Lancet. 2012;380:1909–1915. [DOI] [PubMed] [Google Scholar]
- 38. Laharie D, Bourreille A, Branche J, et al. ; Groupe d’Etudes Thérapeutiques des Affections Inflammatoires Digestives Long-term outcome of patients with steroid-refractory acute severe UC treated with ciclosporin or infliximab. Gut. 2018;67:237–243. [DOI] [PubMed] [Google Scholar]
- 39. Lees CW, Heys D, Ho GT, et al. ; Scottish Society of Gastroenterology Infliximab Group A retrospective analysis of the efficacy and safety of infliximab as rescue therapy in acute severe ulcerative colitis. Aliment Pharmacol Ther. 2007;26:411–419. [DOI] [PubMed] [Google Scholar]
- 40. Llaó J, Naves JE, Ruiz-Cerulla A, et al. . Improved outcome of acute severe ulcerative colitis while using early predictors of corticosteroid failure and rescue therapies. Dig Liver Dis. 2016;48:608–612. [DOI] [PubMed] [Google Scholar]
- 41. Löwenberg M, Duijvis NW, Ponsioen C, et al. . Length of hospital stay and associated hospital costs with infliximab versus cyclosporine in severe ulcerative colitis. Eur J Gastroenterol Hepatol. 2014;26:1240–1246. [DOI] [PubMed] [Google Scholar]
- 42. Mocciaro F, Renna S, Orlando A, et al. . Cyclosporine or infliximab as rescue therapy in severe refractory ulcerative colitis: early and long-term data from a retrospective observational study. J Crohns Colitis. 2012;6:681–686. [DOI] [PubMed] [Google Scholar]
- 43. Monterubbianesi R, Aratari A, Armuzzi A, et al. ; Italian Group for the Study of Inflammatory Bowel Disease (IG-IBD) Infliximab three-dose induction regimen in severe corticosteroid-refractory ulcerative colitis: early and late outcome and predictors of colectomy. J Crohns Colitis. 2014;8:852–858. [DOI] [PubMed] [Google Scholar]
- 44. Mortensen C, Caspersen S, Christensen NL, et al. . Treatment of acute ulcerative colitis with infliximab, a retrospective study from three Danish hospitals. J Crohns Colitis. 2011;5:28–33. [DOI] [PubMed] [Google Scholar]
- 45. Ordas I, Domenech E, Manosa M, et al. . Long-term efficacy and safety of cyclosporine in a cohort of steroid-refractory acute severe ulcerative colitis patients from the ENEIDA registry (1989–2013): a nationwide multicenter study. Am J Gastroenterol. 2017;112(11):1709–1718. [DOI] [PubMed] [Google Scholar]
- 46. Regueiro M, Curtis J, Plevy S. Infliximab for hospitalized patients with severe ulcerative colitis. J Clin Gastroenterol. 2006;40:476–481. [DOI] [PubMed] [Google Scholar]
- 47. Ribaldone DG, Dileo I, Pellicano R, et al. . Severe ulcerative colitis: predictors of response and algorithm proposal for rescue therapy. Ir J Med Sci. 2018;187:385–392. [DOI] [PubMed] [Google Scholar]
- 48. Sands BE, Tremaine WJ, Sandborn WJ, et al. . Infliximab in the treatment of severe, steroid-refractory ulcerative colitis: a pilot study. Inflamm Bowel Dis. 2001;7:83–88. [DOI] [PubMed] [Google Scholar]
- 49. Seah D, Choy MC, Gorelik A, et al. . Examining maintenance care following infliximab salvage therapy for acute severe ulcerative colitis. J Gastroenterol Hepatol. 2018;33:226–231. [DOI] [PubMed] [Google Scholar]
- 50. Shepherd S, Wright EK, Holmes JA, et al. . Outcomes of salvage therapy for acute severe colitis treatment in a single tertiary center: infliximab v. cyclosporine. J Gastroenterol Hepatol. 2014;29(Suppl 2):102–123.23829453 [Google Scholar]
- 51. Sjöberg M, Magnuson A, Björk J, et al. ; Swedish Organization for the Study of Inflammatory Bowel Disease (SOIBD) Infliximab as rescue therapy in hospitalised patients with steroid-refractory acute ulcerative colitis: a long-term follow-up of 211 Swedish patients. Aliment Pharmacol Ther. 2013;38:377–387. [DOI] [PubMed] [Google Scholar]
- 52. Sood A, Midha V, Sharma S, et al. . Infliximab in patients with severe steroid-refractory ulcerative colitis: Indian experience. Indian J Gastroenterol. 2014;33:31–34. [DOI] [PubMed] [Google Scholar]
- 53. Van Langenberg DR, Vasudevan A. Infliximab salvage outcomes in a single australian inflammatory bowel disease centre: highly efficacious and significantly reduces future healthcare utilization in patients with acute severe colitis. J Gastroenterol Hepatol. 2015;30(Suppl 3):117–148.25088839 [Google Scholar]
- 54. Williams JG, Alam MF, Alrubaiy L, et al. . Infliximab versus ciclosporin for steroid-resistant acute severe ulcerative colitis (CONSTRUCT): a mixed methods, open-label, pragmatic randomised trial. Lancet Gastroenterol Hepatol. 2016;1:15–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Yamamoto-Furusho JK, Uzcanga LF. Infliximab as a rescue therapy for hospitalized patients with severe ulcerative colitis refractory to systemic corticosteroids. Dig Surg. 2008;25:383–386. [DOI] [PubMed] [Google Scholar]
- 56. Gibson D, McNally M, Doherty J, et al. . Medium to long-term outcomes in patients receiving accelerated dose infliximab induction for acute severe ulcerative colitis (ASUC) in a multi-centre cohort. J Crohns Colitis. 2018;12(Suppl 1):S332–S333. [Google Scholar]
- 57. Sly N, Werner S, Pitt R, et al. . Accelerated dosing of infliximab is not associated with improved colectomy rates in hospitalized severe ulcerative colitis patients. Am J Gastroenterol. 2017;112(Suppl 1):S382–S383. [Google Scholar]
- 58. Nalagatla N, Falloon K, Tran G, et al. . Effect of accelerated infliximab induction on short- and long-term outcomes of acute severe ulcerative colitis: a retrospective multi-center study and meta-analysis. Clin Gastroenterol Hepatol. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Kohn A, Daperno M, Armuzzi A, et al. . Infliximab in severe ulcerative colitis: short-term results of different infusion regimens and long-term follow-up. Aliment Pharmacol Ther. 2007;26:747–756. [DOI] [PubMed] [Google Scholar]
- 60. Shah S, Naymagon S, Panchal H, et al. . PD-007 YI high-dose infliximab lowers 30-day colectomy rates in hospitalized ulcerative colitis patients: a propensity-score weighted analysis. Inflamm Bowel Dis. 2017;23:S7–S8. [DOI] [PubMed] [Google Scholar]
- 61. Sandborn WJ, Rutgeerts P, Feagan BG, et al. . Colectomy rate comparison after treatment of ulcerative colitis with placebo or infliximab. Gastroenterol. 2009;137:1250–1260; quiz 1520 [DOI] [PubMed] [Google Scholar]
- 62. Beswick L, Van Langenberg DR, Rosella O, et al. . The predictive value of early serum infliximab, CRP and faecal calprotectin levels post-first infliximab rescue dose for acute severe colitis: ‘day 1 to 3 is key’. J Gastroenterol Hepatol. 2015;30(Suppl. 3):117–148.25088839 [Google Scholar]
- 63. Chen JH, Andrews JM, Kariyawasam V, et al. ; IBD Sydney Organisation and the Australian Inflammatory Bowel Diseases Consensus Working Group Review article: acute severe ulcerative colitis - evidence-based consensus statements. Aliment Pharmacol Ther. 2016;44:127–144. [DOI] [PubMed] [Google Scholar]
- 64. Kedia S, Ahuja V, Tandon R. Management of acute severe ulcerative colitis. World J Gastrointest Pathophysiol. 2014;5:579–588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Brandse JF, Jansen JM, Baars PA, et al. . Serum CRP is a better early marker for response to infliximab induction therapy than fecal calprotectin in patients with moderate to severe ulcerative colitis. Gastroenterol. 2014;146:S55–S56. [Google Scholar]
- 66. Dotan I, Ron Y, Yanai H, et al. . Patient factors that increase infliximab clearance and shorten half-life in inflammatory bowel disease: a population pharmacokinetic study. Inflamm Bowel Dis. 2014;20:2247–2259. [DOI] [PubMed] [Google Scholar]
- 67. Kevans D, Murthy S, Mould DR, Silverberg MS. Accelerated clearance of infliximab is associated with treatment failure in patients with corticosteroid-refractory acute ulcerative colitis. J Crohns Colitis. 2018;12:662–669. [DOI] [PubMed] [Google Scholar]
- 68. Ungar B, Mazor Y, Weisshof R, et al. . Induction infliximab levels among patients with acute severe ulcerative colitis compared with patients with moderately severe ulcerative colitis. Aliment Pharmacol Ther. 2016;43(12): 1293–1299. [DOI] [PubMed] [Google Scholar]
- 69. Beswick L, Rosella O, Rosella G, et al. . Exploration of predictive biomarkers of early infliximab response in acute severe colitis: a prospective pilot study. J Crohns Colitis. 2018;12:289–297. [DOI] [PubMed] [Google Scholar]
- 70. Brandse JF, Mathôt RA, van der Kleij D, et al. . Pharmacokinetic features and presence of antidrug antibodies associate with response to infliximab induction therapy in patients with moderate to severe ulcerative colitis. Clin Gastroenterol Hepatol. 2016;14:251–258.e1. [DOI] [PubMed] [Google Scholar]
- 71. Hindryckx P, Novak G, Vande Casteele N, et al. . Review article: dose optimisation of infliximab for acute severe ulcerative colitis. Aliment Pharmacol Ther. 2017;45:617–630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. University of Michigan Severe Ulcerative Colitis Protocol 2016. http://www.med.umich.edu/ibd/docs/severeucprotocol.pdf. Accessed September 10, 2017.
- 73. Singh S, Al-Darmaki A, Frolkis AD, et al. . Postoperative mortality among patients with inflammatory bowel diseases: a systematic review and meta-analysis of population-based studies. Gastroenterol. 2015;149:928–937. [DOI] [PubMed] [Google Scholar]
- 74. Lau C, Dubinsky M, Melmed G, et al. . The impact of preoperative serum anti-tnfα therapy levels on early postoperative outcomes in inflammatory bowel disease surgery. Ann Surg. 2015;261:487–496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Randall J, Singh B, Warren BF, et al. . Delayed surgery for acute severe colitis is associated with increased risk of postoperative complications. Br J Surg. 2010;97:404–409. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.