We describe trends in high-grade cervical lesions (CIN2+), identified through population-based surveillance in 2008–2015. In addition to changed screening recommendations, observed CIN2+ declines among screened women aged 18–24 years indicate a population-level impact of human papillomavirus vaccination.
Keywords: human papillomavirus, vaccine impact, disease surveillance, cervical intraepithelial neoplasia
Abstract
Background
We describe changes in rates of cervical intraepithelial neoplasia grades 2, 3 and adenocarcinoma in situ (CIN2+) during a period of human papillomavirus (HPV) vaccine uptake and changing cervical cancer screening recommendations.
Methods
We conducted population-based laboratory surveillance for CIN2+ in catchment areas in 5 states, 2008–2015. We calculated age-specific CIN2+ rates per 100000 women by age groups. We estimated incidence rate ratios (IRR) of CIN2+ for 2-year periods among all women and among screened women to evaluate changes over time.
Results
A total of 16572 CIN2+ cases were reported. Among women aged 18–20 and 21–24 years, CIN2+ rates declined in all sites, whereas in women aged 25–29, 30–34, and 35–39 years, trends differed across sites. The percent of women screened annually declined in all sites and age groups. Compared to 2008–2009, rates among screened women were significantly lower for all 3 periods in women aged 18–20 years (2010–2011: IRR 0.82, 95% confidence interval [CI] 0.67–0.99; 2012–2013: IRR 0.63, 95% CI 0.47–0.85; 2014–2015: IRR 0.44, 95% CI 0.28–0.68) and lower for the latter 2 time periods in women aged 21–24 years (2012–2013: IRR 0.86, 95% CI 0.79–0.94; 2014–2015: IRR 0.61, 95% CI 0.55–0.67).
Conclusions
From 2008–2015, both CIN2+ rates and cervical cancer screening declined in women aged 18–24 years. The significant decreases in CIN2+ rates among screened women aged 18–24 years are consistent with a population-level impact of HPV vaccination.
In the United States, human papillomavirus (HPV) vaccination has been recommended for adolescent females aged 11–12 years since 2006 [1]. The vaccination series can be started at 9 years of age, and is also recommended through 26 years of age for those not vaccinated previously. The uptake of the vaccine has been gradual, with regional variation [2–5]. Nationally, the proportion of women aged 19–26 who had ever received at least 1 dose of the HPV vaccine increased from 10.5% in 2008 to 40.2% in 2014 [5]. Through 2014, 99% of all vaccine doses given were the quadrivalent vaccine [6].
The impact of the vaccination program on HPV-associated cancers may not be observed for decades, given the long natural history of HPV infection and carcinogenesis. Several efforts to monitor the vaccine’s impact have evaluated outcomes evident soon after infection occurs that can be used as early endpoints. They include assessing the prevalence of vaccine-type infections at genital sites, either in the general population [7–9] or in women undergoing cervical cancer screening [10], and the monitoring of genital warts diagnoses [11, 12]. Additional monitoring efforts target intermediate outcomes, such as high-grade cervical lesions or precancers that typically take years to develop and that are detected through cervical cancer screening [13–15].
In the years since the HPV vaccine was introduced, the US cervical cancer screening recommendations have changed multiple times [16–18], complicating the interpretation of population trends in cervical precancers. In 2012, most major medical organizations recommended delaying an initial screening to 21 years, regardless of age at sexual debut, and screening at longer intervals (every 3 years or, in women aged at least 30 years, every 5 years if high-risk HPV testing is performed in addition to cytology) [17]. These guidelines were designed to avoid unnecessary procedures in women with lesions that have a high probability of regressing without treatment [17]. As a consequence of fewer women being screened annually, fewer cervical lesions are expected to be detected. Thus, it is difficult to determine whether any decreases in the rates of cervical lesions in the population represent the vaccine’s impact or the results of fewer women receiving annual screenings. Considering that only screened women are at risk of having a lesion detected, using the number of women screened each year as the denominator in incidence rate calculations is a viable approach to controlling for the impact of changing screening guidelines [13, 14, 19–22].
The HPV Vaccine Impact Monitoring Project (HPV-IMPACT) was established to monitor the incidence of high-grade cervical lesions, including cervical intraepithelial neoplasia (CIN) grades 2 and 3 and adenocarcinoma in situ (AIS; together referred to as CIN2+), in catchment areas within 5 geographically-dispersed states. A prior report on HPV-IMPACT cases from 2008–2012 documented decreases in the incidence of CIN2+ in women residing in 4 of the HPV-IMPACT catchment areas, as well as declines in screening [15]. Since that report, a fifth HPV-IMPACT site has established robust reporting for all years of surveillance, and all sites have developed methods to estimate the proportion of women screened each year. The objectives of these analyses are to describe changes in the rates of CIN2+ and cervical cytology screening in the 5 HPV-IMPACT sites from 2008–2015, and to estimate changes in the CIN2+ incidence both in the general population and among screened women.
METHODS
HPV-IMPACT is a network of surveillance sites that consists of partnerships between the Centers for Disease Control and Prevention, state health departments, and academic institutions. Surveillance sites are located in California, Connecticut, New York, Oregon, and Tennessee. Each site conducts surveillance in a catchment area in which approximately 300000 women aged ≥18 years reside. For 3 sites (CT, NY, TN), the catchment area is a single county. For 2 sites, the catchment area is a subset of the population of 1 (CA) or 2 (OR) counties. Each site has conducted population-based laboratory surveillance for CIN2+ since 2008, as described previously [15, 23]. Briefly, all pathology laboratories serving catchment area residents report CIN2+ cases to HPV-IMPACT staff at each site. Because women may have multiple diagnostic and treatment procedures as part of a single CIN2+ case, the incidence date was defined as the date of the earliest qualifying CIN2+ diagnosis for each woman, and the final diagnosis for each case was defined as the highest-grade lesion identified within 6 months of the incidence date.
Annual population totals of women in the age groups of 18–20, 21–24, 25–29, 30–34, and 35–39 years were obtained for each catchment area. Population denominators were based on county-level data from the Centers for Disease Control and Prevention’s National Center for Health Statistics (https://www.cdc.gov/nchs/nvss/bridged_race/data_documentation.htm); for CA and OR, county estimates were adjusted for the specific catchment area using American Community Survey data.
Each site established methodology for estimating the annual cervical cancer screening utilization by age group (Supplemental Table 1). Although particular data sources varied, all estimates were based on counts of cervical screening tests using either insurance claims or laboratory utilization data. To estimate the number of screened women in each year, the proportion screened in each year’s age stratum was multiplied by the total population of women in the corresponding group.
We analyzed the surveillance years in 4 periods of 2 years each: 2008–2009, 2010–2011, 2012–2013, and 2014–2015. We compared the characteristics of cases, including age groups, diagnoses, races/ethnicities, insurance statuses, and vaccination histories, by site and period. For each site and across all sites, we calculated the incidence rates per 100000 women in each age group, using population estimates as the denominator. We also estimated the incidence per 100000 screened women in each age group, using each site’s estimated number of screened women as the denominator.
CIN2+ incidence rate ratios (IRR) were calculated by comparing the incidences in the 3 latter time periods with the earliest period using Poisson regression. All analyses were stratified by age group. Analyses were repeated with the outcome restricted to the highest-grade lesions: CIN3 and AIS (CIN3/AIS). Adjustments for surveillance sites were considered because screening methods varied by site, which could have influenced observed changes in rates over time. In addition, subset analyses were conducted that excluded cases reported during the first year of the system, when some reported cases might have been initially diagnosed in 2007 (prevalent cases), to determine whether any observed declines were attributable to this surveillance artifact.
RESULTS
A total of 16572 CIN2+ cases among women aged 18–39 years were reported in the 5 catchment areas in 2008–2015, including 5399 cases of CIN3/AIS. Characteristics of the reported cases by age group, diagnosis, race/ethnicity, insurance, and vaccination status, both overall and by 2-year surveillance periods, are detailed in Table 1. The total number of cases declined in each 2-year period, and differences in proportions of case characteristics were observed within the reduced numbers. Notable changes over time included a lower number and proportion of cases in women aged 18–20 years (from 7.5% [348/4643] in 2008–2009 to 0.6% [21/3775] in 2014–2015); an increase in the number and proportion of cases diagnosed with CIN2/3 (from 15.9% [740/4643] to 22.1% [833/3775]); and a corresponding decrease in the number and proportion of cases diagnosed with CIN2 (from 51.4% [2388/4643] to 45.9% [1732/3775]). The proportion of cases known to be vaccinated remained similar over time, but the number of vaccinated cases declined (16.9% [786/4643] in 2008–2009; 16.2% [613/3775] in 2014–2015); the proportion and number of cases in women with with unknown vaccination statuses increased.
Table 1.
Characteristics of CIN2+ Cases, by Year of Diagnosis
| Total | 2008–2009 | 2010–2011 | 2012–2013 | 2014–2015 | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Characteristic | n | % | n | % | n | % | n | % | n | % |
| Total | 16572 | 4643 | 4185 | 3969 | 3775 | |||||
| Age, years | ||||||||||
| 18–20 | 558 | 3.4 | 348 | 7.5 | 138 | 3.3 | 51 | 1.3 | 21 | 0.6 |
| 21–24 | 3699 | 22.3 | 1284 | 27.7 | 1059 | 25.3 | 851 | 21.4 | 505 | 13.4 |
| 25–29 | 5771 | 34.8 | 1471 | 31.7 | 1432 | 34.2 | 1475 | 37.2 | 1393 | 36.9 |
| 30–34 | 4163 | 25.1 | 960 | 20.7 | 961 | 23.0 | 1029 | 25.9 | 1213 | 32.1 |
| 35–39 | 2381 | 14.4 | 580 | 12.5 | 595 | 14.2 | 563 | 14.2 | 643 | 17.0 |
| Diagnosis | ||||||||||
| CIN2 | 8221 | 49.6 | 2388 | 51.4 | 2122 | 50.7 | 1979 | 49.9 | 1732 | 45.9 |
| CIN2/3 | 2952 | 17.8 | 740 | 15.9 | 683 | 16.3 | 696 | 17.5 | 833 | 22.1 |
| CIN3/AIS | 5399 | 32.6 | 1515 | 32.6 | 1380 | 33.0 | 1294 | 32.6 | 1210 | 32.1 |
| Race/ethnicity | ||||||||||
| Non-Hispanic White | 8681 | 52.4 | 2311 | 49.8 | 2119 | 50.6 | 2201 | 55.5 | 2050 | 54.3 |
| Non-Hispanic Black | 2324 | 14.0 | 626 | 13.5 | 601 | 14.4 | 562 | 14.2 | 535 | 14.2 |
| Hispanic | 2256 | 13.6 | 585 | 12.6 | 558 | 13.3 | 544 | 13.7 | 569 | 15.1 |
| Asian | 670 | 4.0 | 161 | 3.5 | 144 | 3.4 | 184 | 4.6 | 181 | 4.8 |
| Other | 903 | 5.5 | 386 | 8.3 | 298 | 7.1 | 130 | 3.3 | 89 | 2.4 |
| Missing | 1738 | 10.5 | 574 | 12.4 | 465 | 11.1 | 348 | 8.8 | 351 | 9.3 |
| Insurance | ||||||||||
| Private | 8851 | 53.4 | 2638 | 51.8 | 2170 | 51.9 | 2132 | 53.7 | 1911 | 50.6 |
| Public | 3871 | 23.4 | 1109 | 23.9 | 1097 | 26.2 | 815 | 20.5 | 850 | 22.5 |
| Uninsured | 484 | 2.9 | 170 | 3.7 | 113 | 2.7 | 114 | 2.9 | 87 | 2.3 |
| Other | 631 | 3.8 | 160 | 3.5 | 189 | 4.5 | 156 | 3.9 | 126 | 3.3 |
| Missing | 2735 | 16.5 | 566 | 12.2 | 616 | 14.7 | 752 | 19.0 | 801 | 21.2 |
| Vaccination status | ||||||||||
| Vaccinated | 2974 | 18.0 | 786 | 16.9 | 775 | 18.5 | 800 | 20.2 | 613 | 16.2 |
| Not vaccinated | 3988 | 24.1 | 1584 | 34.1 | 1096 | 26.2 | 811 | 20.4 | 497 | 13.2 |
| Unknown | 9610 | 58.1 | 2273 | 49.0 | 2314 | 55.3 | 2358 | 59.4 | 2665 | 70.6 |
| Site | ||||||||||
| California | 2825 | 17.1 | 701 | 15.1 | 657 | 15.7 | 770 | 19.4 | 697 | 18.5 |
| Connecticut | 4406 | 26.6 | 1289 | 27.8 | 1170 | 28.0 | 999 | 25.2 | 948 | 25.1 |
| New York | 3324 | 20.1 | 1099 | 23.7 | 831 | 19.9 | 702 | 19.7 | 692 | 18.3 |
| Oregon | 3290 | 19.9 | 869 | 18.7 | 834 | 19.9 | 845 | 21.3 | 742 | 19.7 |
| Tennessee | 2727 | 16.5 | 685 | 14.8 | 693 | 16.6 | 653 | 16.5 | 696 | 18.4 |
All data are from the Human Papillomavirus Vaccine Impact Monitoring Project (HPV-IMPACT), 2008–2015.
Abbreviations: AIS, adenocarcinoma in situ; CIN2+, cervical intraepithelial neoplasia grades 2, 3, 2/3, or AIS; CIN, cervical intraepithelial neoplasia; CIN2, CIN grade 2; CIN3, CIN grade 3.
Case characteristics also varied significantly by surveillance site (Table 2). New York and Oregon had the highest proportions of cases occurring in non-Hispanic Whites. Connecticut and Tennessee had the highest proportions with public insurance.
Table 2.
Characteristics of CIN2+ Cases, by Site
| Total | California | Connecticut | New York | Oregon | Tennessee | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Characteristic | n | % | n | % | n | % | n | % | n | % | n | % |
| Age, years | ||||||||||||
| 18–20 | 558 | 3.4 | 38 | 1.4 | 185 | 4.2 | 155 | 4.7 | 63 | 1.9 | 117 | 4.3 |
| 21–24 | 3699 | 22.3 | 431 | 15.3 | 1121 | 25.4 | 928 | 27.9 | 593 | 18.0 | 626 | 23.0 |
| 25–29 | 5771 | 34.8 | 1000 | 35.4 | 1488 | 33.8 | 1076 | 32.4 | 1202 | 36.5 | 1005 | 36.9 |
| 30–34 | 4163 | 25.1 | 856 | 30.3 | 1040 | 23.6 | 729 | 21.9 | 900 | 27.4 | 638 | 23.4 |
| 35–39 | 2381 | 14.4 | 500 | 17.7 | 572 | 13.0 | 436 | 13.1 | 532 | 16.2 | 341 | 12.5 |
| Diagnosis | ||||||||||||
| CIN2 | 8221 | 49.6 | 1184 | 41.9 | 2582 | 58.6 | 1729 | 52.0 | 1499 | 45.6 | 1227 | 45.0 |
| CIN2/3 | 2952 | 17.8 | 623 | 22.1 | 577 | 13.1 | 572 | 17.2 | 694 | 21.1 | 486 | 17.8 |
| CIN3/AIS | 5399 | 32.6 | 1018 | 36.0 | 1247 | 28.3 | 1023 | 30.8 | 1097 | 33.3 | 1014 | 37.2 |
| Race/ethnicity | ||||||||||||
| NH White | 8681 | 52.4 | 861 | 30.5 | 1994 | 45.3 | 2238 | 67.3 | 2018 | 61.3 | 1570 | 57.6 |
| NH Black | 2324 | 14.0 | 420 | 14.9 | 559 | 12.7 | 578 | 17.4 | 104 | 3.2 | 663 | 24.3 |
| Hispanic | 2256 | 13.6 | 588 | 20.8 | 879 | 20.0 | 299 | 9.0 | 284 | 8.6 | 206 | 7.6 |
| Asian | 670 | 4.0 | 359 | 12.7 | 71 | 1.6 | 66 | 2.0 | 132 | 4.0 | 42 | 1.5 |
| Other | 903 | 5.5 | 109 | 14.0 | 616 | 14.0 | 45 | 1.4 | 126 | 3.8 | 7 | 0.3 |
| Missing | 1738 | 10.5 | 488 | 6.5 | 287 | 6.5 | 98 | 3.0 | 626 | 19.0 | 239 | 8.8 |
| Insurance | ||||||||||||
| Private | 8851 | 53.4 | 1718 | 60.8 | 2442 | 55.4 | 1943 | 58.5 | 1412 | 42.9 | 1336 | 49.0 |
| Public | 3871 | 23.4 | 584 | 20.7 | 1497 | 34.0 | 708 | 21.3 | 336 | 10.2 | 746 | 27.4 |
| Uninsured | 484 | 2.9 | 16 | 0.6 | 172 | 3.9 | 79 | 2.4 | 153 | 4.7 | 64 | 2.4 |
| Other | 631 | 3.8 | 48 | 1.7 | 61 | 1.4 | 104 | 3.1 | 186 | 5.7 | 232 | 8.5 |
| Missing | 2735 | 16.5 | 459 | 16.3 | 234 | 5.3 | 490 | 14.7 | 1203 | 36.6 | 349 | 12.8 |
| Vaccination | ||||||||||||
| Vaccinated | 2974 | 18.0 | 279 | 9.9 | 871 | 19.8 | 833 | 25.1 | 540 | 16.4 | 451 | 16.5 |
| Not vaccinated | 3988 | 24.1 | 490 | 17.4 | 1415 | 32.1 | 937 | 28.2 | 635 | 19.3 | 511 | 18.7 |
| Unknown | 9610 | 58.0 | 2056 | 72.8 | 2120 | 48.1 | 1554 | 46.8 | 2115 | 64.3 | 1765 | 64.7 |
All data are from the Human Papillomavirus Vaccine Impact Monitoring Project (HPV-IMPACT), 2008–2015. P-values for chi-square tests all <.001.
Abbreviations: AIS, adenocarcinoma in situ; CIN2+, cervical intraepithelial neoplasia grades 2, 3, 2/3, or AIS; CIN, cervical intraepithelial neoplasia; CIN2, CIN grade 2; CIN3, CIN grade 3; NH, non-Hispanic.
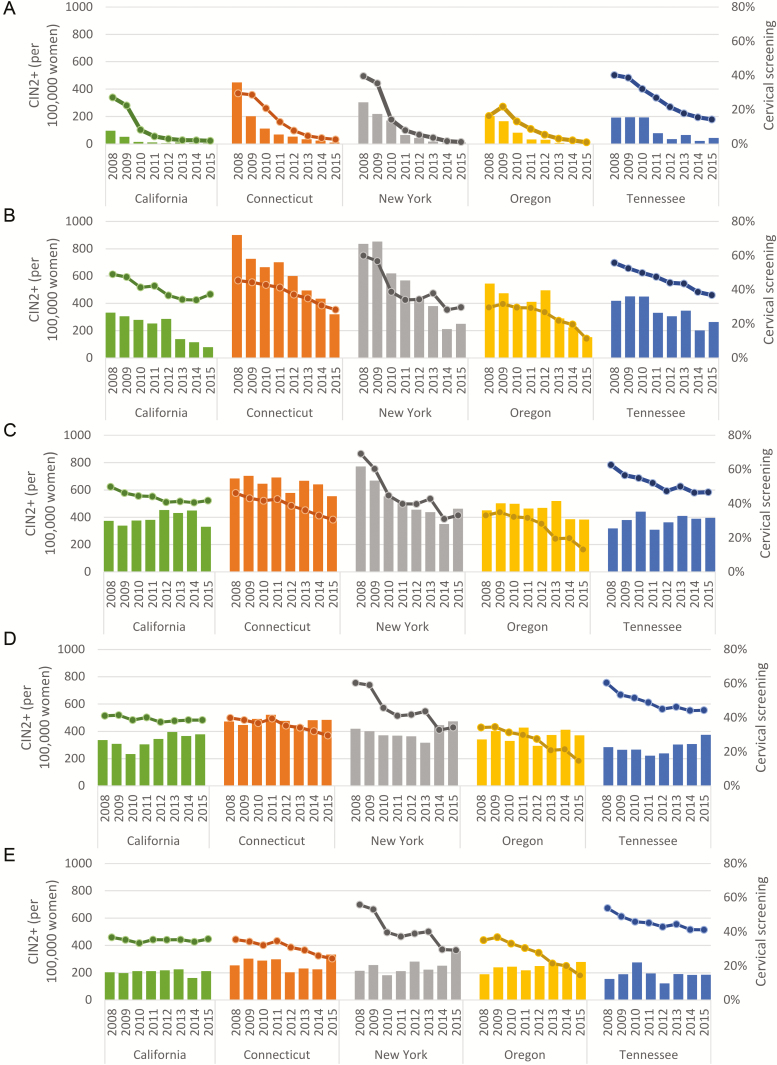
In all sites, the rates of CIN2+ per 100000 women generally declined over time in age groups 18–20 and 21–24 (Figure 1). There was no consistent pattern of rates across all sites in the 3 older age groups. The proportion of women screened also generally declined over time in all sites, although the magnitude of declines varied by site, and declines in the 30–34 and 35–39 year age groups were minimal in California. Figure 2 shows the incidence rates per 100000 women (panel A) and per 100000 screened women (panel B) with all sites combined, stratified by age group. Among all women (Figure 2A), the rates in age groups 18–20, 21–24, and 25–29 declined between 2008 and 2015. In age groups 30–34 and 35–39, the rates were slightly higher in 2015 compared with 2008. In the rates calculated among screened women (Figure 2B), there were declines in the 2 youngest age groups, but in the 3 oldest age groups, the rates were higher in 2015 than in 2008.
Figure 1.
CIN2+ incidence per 100000 women (bars) and estimated proportion of women screened (lines), by age group and site. Participant ages (A) 18–20 years, (B) 21–24 years, (C) 25–29 years, (D) 30–34 years, and (E) 35–39 years. All data are from the Human Papillomavirus Vaccine Impact Monitoring Project (HPV-IMPACT), 2008–2015. Abbreviation: CIN2+, cervical intraepithelial neoplasia grades 2, 3, 2/3 and adenocarcinoma in situ.
Figure 2.
CIN2+ incidence (A) per 100000 women and (B) per 100000 screened women. All data are from the Human Papillomavirus Vaccine Impact Monitoring Project (HPV-IMPACT), 2008–2015. Abbreviation: CIN2+, cervical intraepithelial neoplasia grades 2, 3, 2/3 and adenocarcinoma in situ.
Compared to 2008–2009, population CIN2+ rates in women aged 18–20 years declined sharply and significantly in each 2-year surveillance period, with declines of 61% (95% CI 52–68%) in 2010–2011, 85% (95% CI 80–89%) in 2012–2013, and 94% (95% CI 90–94%) in 2014–2015 (Table 3). In women aged 21–24 years, declines were also significant for all periods, but the magnitude of the declines was smaller (20%, 36%, and 62% declines, respectively). In women aged 25–29 years, the rates in 2010–2011 and 2012–2013 were not significantly different from the rates in 2008–2009, but the rates were significantly lower in 2014–2015 (IRR 0.85, 95% CI 0.79–0.91). There was a significant 10% increase in rates in 30–34 year old women in 2014–2015 compared to 2008–2009. There were no significant decreases or increases among women aged 30–39 years.
Table 3.
CIN2+ per 100000 Women and per 100000 Estimated Screened Women at 5 Sites
| Age Group, Years | Years | CIN2+, n | Population Denominator | CIN2+ per 100000 Women per Year | Screening Denominator | CIN2+ per 100000 Screened Women per Year | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Rate | IRR | 95% CI | aIRR | 95% CI | Rate | IRR | 95% CI | aIRR | 95% CI | |||||
| 18–20 | 2008–2009 | 348 | 165 606 | 210.1 | 1.00 | 1.00 | 50 241 | 692.7 | 1.00 | 1.00 | ||||
| 2010–2011 | 138 | 168 137 | 82.1 | 0.39 | (0.32–0.48) | 0.39 | (0.32–0.47) | 24 431 | 564.9 | 0.82 | (0.67–0.99) | 0.80 | (0.66–0.98) | |
| 2012–2013 | 51 | 165 633 | 30.8 | 0.15 | (0.11–0.20) | 0.14 | (0.11–0.19) | 11 680 | 436.6 | 0.63 | (0.47–0.85) | 0.66 | (0.49–0.88) | |
| 2014–2015 | 21 | 160 356 | 13.1 | 0.06 | (0.04–0.10) | 0.06 | (0.06–0.10) | 6 876 | 305.4 | 0.44 | (0.28–0.68) | 0.50 | (0.32–0.79) | |
| 21–24 | 2008–2009 | 1284 | 219 635 | 584.6 | 1.00 | 1.00 | 104 508 | 1228.6 | 1.00 | 1.00 | ||||
| 2010–2011 | 1059 | 227 592 | 465.3 | 0.80 | (0.73–0.86) | 0.80 | (0.74–0.87) | 90 777 | 1166.6 | 0.95 | (0.88–1.03) | 0.96 | (0.88–1.04) | |
| 2012–2013 | 851 | 228 828 | 371.9 | 0.64 | (0.58–0.69) | 0.64 | (0.59–0.70) | 80 775 | 1053.5 | 0.86 | (0.79–0.94) | 0.87 | (0.80–0.95) | |
| 2014–2015 | 505 | 226 382 | 223.1 | 0.38 | (0.34–0.42) | 0.39 | (0.35–0.43) | 67 882 | 743.9 | 0.61 | (0.55–0.67) | 0.64 | (0.57–0.71) | |
| 25–29 | 2008–2009 | 1471 | 290 829 | 505.8 | 1.00 | 1.00 | 144 416 | 1018.6 | 1.00 | 1.00 | ||||
| 2010–2011 | 1432 | 297 502 | 481.3 | 0.95 | (0.88–1.02) | 0.95 | (0.88–1.02) | 127 612 | 1122.2 | 1.10 | (1.02–1.18) | 1.09 | (1.02–1.17) | |
| 2012–2013 | 1475 | 309 893 | 476.0 | 0.94 | (0.88–1.01) | 0.94 | (0.88–1.01) | 119 124 | 1238.2 | 1.22 | (1.13–1.31) | 1.23 | (1.15–1.32) | |
| 2014–2015 | 1393 | 324 780 | 428.9 | 0.85 | (0.79–0.91) | 0.85 | (0.79–0.92) | 110 344 | 1262.4 | 1.24 | (1.15–1.33) | 1.31 | (1.22–1.42) | |
| 30–34 | 2008–2009 | 960 | 261 881 | 367.0 | 1.00 | 1.00 | 118 701 | 809.6 | 1.00 | 1.00 | ||||
| 2010–2011 | 961 | 273 774 | 351.4 | 0.96 | (0.88–1.05) | 0.96 | (0.88–1.05) | 109 585 | 877.9 | 1.08 | (0.99–1.19) | 1.08 | (0.99–1.18) | |
| 2012–2013 | 1029 | 289 619 | 355.3 | 0.97 | (0.89–1.06) | 0.98 | (0.89–1.07) | 106 319 | 967.8 | 1.20 | (1.10–1.31) | 1.22 | (1.12–1.33) | |
| 2014–2015 | 1213 | 299 793 | 404.6 | 1.10 | (1.01–1.20) | 1.11 | (1.02–1.21) | 99 249 | 1222.2 | 1.51 | (1.39–1.64) | 1.58 | (1.45–1.72) | |
| 35–39 | 2008–2009 | 580 | 261 930 | 221.8 | 1.00 | 1.00 | 109 161 | 532.2 | 1.00 | 1.00 | ||||
| 2010–2011 | 595 | 252 769 | 235.4 | 1.06 | (0.95–1.19) | 1.07 | (0.95–1.20) | 91 635 | 649.3 | 1.22 | (1.09–1.37) | 1.22 | (1.09–1.37) | |
| 2012–2013 | 563 | 254 749 | 221.0 | 1.00 | (0.89–1.12) | 1.00 | (0.89–1.13) | 86 877 | 648.0 | 1.22 | (1.09–1.37) | 1.24 | (1.11–1.40) | |
| 2014–2015 | 643 | 267 513 | 240.0 | 1.09 | (0.97–1.21) | 1.09 | (0.98–1.22) | 78 266 | 820.3 | 1.55 | (1.38–1.73) | 1.62 | (1.45–1.81) | |
All data are from the Human Papillomavirus Vaccine Impact Monitoring Project (HPV-IMPACT), 2008–2015. All aIRRs were adjusted for surveillance site. Bolded values denote statistical significance. Population denominator is the sum of the number of women in the catchment areas for each of the two years. Screening denominator is the sum of the estimated number of screened women in the catchment areas for each of the two years.
Abbreviations: aIRR, adjusted incidence rate ratio; CI, confidence interval; CIN2+, cervical intraepithelial neoplasia grades 2, 3 and adenocarcinoma in situ; IRR, incidence rate ratio.
Declines in the incidence of CIN2+ among young women were still observed, but were less pronounced, in analyses among estimated screened women (Table 3). In women aged 18–20 years, compared to 2008–2009, rates per 100000 screened women declined by 18% (95% CI 1–23%) in 2010–2011, 37% (95% CI 12–53%) in 2012–2013, and 56% (95% CI 32–72%) in 2014–2015. In screened women aged 21–24 years, compared to 2008–2009, the rates did not differ significantly in 2010–2011, but declined significantly in 2012–2013 and 2014–2015, by 24% (95% CI 6–21%) and 39% (95% CI 33–45%), respectively. Rates per 100000 screened women increased significantly over time in women aged 25–29, 30–34, and 35–39 years.
As expected, the baseline rates of CIN3/AIS (ranging from 42.9 per 100000 women in 18–20 year-olds to 181.6 per 100000 in 25–29 year-olds) were lower than the baseline rates of CIN2+ (which ranged from 210.1 per 100000 women in 18–20 year-olds to 584.6 per 100000 in 21–24 year-olds; Table 4). Notably, the highest baseline rate of CIN3/AIS occurred in an older age group (ie, in 25–29-year-olds) than the highest baseline rate of CIN2+ (which occurred in 21–24-year-olds). The pattern of declines and increases in the rates of CIN3/AIS among the estimated screened women were similar to the rates for CIN2+ and occurred in the same age groups, although fewer differences were statistically significant, most likely owing to the smaller sample size of CIN3/AIS cases.
Table 4.
CIN3/AIS per 100000 Women and Per 100000 Estimated Screened Women at 5 Sites
| Age Group (Years) | Years | N CIN3/ AIS | Population Denominator | CIN3/AIS per 100000 Women per Year | Screening Denominator | CIN3/AIS per 100000 Screened Women per Year | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Rate | IRR | 95% CI | aIRR | 95% CI | Rate | IRR | 95% CI | aIRR | 95% CI | |||||
| 18–20 | 2008–2009 | 71 | 165 606 | 42.9 | 1.00 | 1.00 | 50 241 | 141.3 | 1.00 | 1.00 | ||||
| 2010–2011 | 26 | 168 137 | 15.5 | 0.36 | (0.23–0.57) | 0.36 | (0.23–0.56) | 24 431 | 106.4 | 0.75 | (0.48–1.18) | 0.72 | (0.46–1.14) | |
| 2012–2013 | 7 | 165 633 | 4.2 | 0.10 | (0.05–0.21) | 0.10 | (0.04–0.21) | 11 680 | 59.9 | 0.42 | (0.20–0.92) | 0.42 | (0.19–0.92) | |
| 2014–2015 | 6 | 160 356 | 3.7 | 0.09 | (0.04–0.20) | 0.09 | (0.04–0.20) | 6 876 | 87.3 | 0.62 | (0.27–1.42) | 0.67 | (0.29–1.56) | |
| 21–24 | 2008–2009 | 333 | 219 635 | 151.6 | 1.00 | 1.00 | 104 508 | 318.6 | 1.00 | 1.00 | ||||
| 2010–2011 | 253 | 227 592 | 111.2 | 0.73 | (0.62–0.86) | 0.74 | (0.63–0.87) | 90 777 | 278.7 | 0.87 | (0.74–1.03) | 0.88 | (0.75–1.04) | |
| 2012–2013 | 199 | 228 828 | 87.0 | 0.57 | (0.48–0.68) | 0.58 | (0.49–0.69) | 80 775 | 246.4 | 0.77 | (0.65–0.92) | 0.78 | (0.66–0.93) | |
| 2014–2015 | 118 | 226 382 | 52.1 | 0.34 | (0.28–0.42) | 0.35 | (0.28–0.43) | 67 882 | 173.8 | 0.55 | (0.44–0.67) | 0.57 | (0.46–0.70) | |
| 25–29 | 2008–2009 | 528 | 290 829 | 181.6 | 1.00 | 1.00 | 144 416 | 365.6 | 1.00 | 1.00 | ||||
| 2010–2011 | 483 | 297 502 | 162.4 | 0.89 | (0.79–1.01) | 0.89 | (0.79–1.01) | 127 612 | 378.5 | 1.04 | (0.92–1.17) | 1.03 | (0.91–1.16) | |
| 2012–2013 | 510 | 309 893 | 164.6 | 0.91 | (0.80–1.02) | 0.91 | (0.80–1.02) | 119 124 | 428.1 | 1.17 | (1.04–1.32) | 1.19 | (1.05–1.34) | |
| 2014–2015 | 428 | 324 780 | 131.8 | 0.73 | (0.64–0.82) | 0.73 | (0.64–0.83) | 110 344 | 387.9 | 1.06 | (0.93–1.21) | 1.12 | (0.98–1.27) | |
| 30–34 | 2008–2009 | 357 | 261 881 | 136.3 | 1.00 | 1.00 | 118 701 | 301.6 | 1.00 | 1.00 | ||||
| 2010–2011 | 362 | 273 774 | 132.2 | 0.97 | (0.84–1.12) | 0.97 | (0.84–1.12) | 109 585 | 332.2 | 1.10 | (0.95–1.27) | 1.10 | (0.95–1.27) | |
| 2012–2013 | 365 | 289 619 | 126.0 | 0.92 | (0.80–1.07) | 0.93 | (0.80–1.07) | 106 319 | 343.3 | 1.14 | (0.99–1.32) | 1.16 | (1.00–1.34) | |
| 2014–2015 | 446 | 299 793 | 148.8 | 1.09 | (0.95–1.25) | 1.09 | (0.95–1.26) | 99 249 | 449.4 | 1.49 | (1.30–1.72) | 1.55 | (1.35–1.79) | |
| 35–39 | 2008–2009 | 226 | 261 930 | 86.3 | 1.00 | 1.00 | 109 161 | 207.0 | 1.00 | 1.00 | ||||
| 2010–2011 | 256 | 252 769 | 101.3 | 1.17 | (0.98–1.40) | 1.17 | (0.98–1.40) | 91 635 | 279.4 | 1.35 | (1.13–1.61) | 1.33 | (1.11–1.59) | |
| 2012–2013 | 213 | 254 749 | 83.6 | 0.97 | (0.80–1.17) | 0.97 | (0.80–1.17) | 86 877 | 245.2 | 1.18 | (0.98–1.43) | 1.19 | (0.99–1.44) | |
| 2014–2015 | 212 | 267 513 | 79.2 | 0.92 | (0.76–1.11) | 0.92 | (0.76–1.11) | 78 266 | 269.6 | 1.31 | (1.08–1.58) | 1.34 | (1.11–1.61) | |
All data are from the Human Papillomavirus Vaccine Impact Monitoring Project (HPV-IMPACT), 2008–2015. All aIRRs were adjusted for surveillance site. Bolded values denote statistical significance. Population denominator is the sum of the number of women in the catchment areas for each of the two years. Screening denominator is the sum of the estimated number of screened women in the catchment areas for each of the two years.
Abbreviations: aIRR, adjusted incidence rate ratio; AIS, adenocarcinoma in situ; CI, confidence interval; CIN3, cervical intraepithelial neoplasia grade 3; IRR, incidence rate ratio.
Adjusting for surveillance site did not result in any meaningful changes to IRRs in analyses using all women as the denominator (Tables 3 and 4). In the analyses using estimated screened women as the denominator, after adjusting for surveillance site, the declines in the 2 younger age groups were slightly lower in magnitude and the increases in the older age groups became more pronounced, but the direction and significance of differences did not change. Excluding data from 2008 did not meaningfully change the model results (not shown).
DISCUSSION
In this analysis of population-based surveillance data, we found that in young women aged 18–20 and 21–24 years, the incidence of CIN2+ lesions declined significantly and markedly between 2008 and 2015. These declines were broadly consistent across the 5 surveillance sites and were observed in analyses of both all women in the population and estimated screened women, indicating that the declines are not exclusively attributable to reductions in screening and could be attributable, at least in part, to HPV vaccine impact.
The impact of the US female vaccination program on various outcomes has been shown in several studies. Nationally representative survey data have been used to document declines in the prevalence of quadrivalent vaccine-type cervicovaginal HPV infections in adolescents and young women aged 14–19 and 20–24 years [8, 9]. Analyses of claims data have documented decreases in genital warts, abnormal cervical cytology, and high-grade cervical histology lesions [11, 12, 14]. A statewide surveillance system in New Mexico that has tracked CIN diagnoses and maintained a registry of Papanicolaou testing documented declines in CIN1, CIN2, and CIN3 in young women aged 15–19 years and declines in CIN2 in women aged 20–24 years from 2007–2014 [13]. Recently, a report on cancer registry–based surveillance for CIN3 showed a downward trend among young women in Michigan, although no data on screenings were available [24]. A previous analysis of HPV-IMPACT cases from 4 sites that reported through 2012 showed declines in the youngest age groups, as well as declines in screening, although that analysis did not allow determination of the proportion of declines attributable to reductions in screening [15]. Our analysis extends those findings by showing that the incidence of CIN2+ also declined among estimated screened women in the 18–20 and 21–24 year age groups. The decreases among screened women indicate that changing screening practices are not responsible for the entirety of declining CIN2+ rates in these age groups, although performing the analysis among screened women did attenuate the magnitude of declines in young women. However, in 2012, more conservative management of abnormal screening results in 21–24-year-old women was recommended, encouraging observation over colposcopy, biopsy, and treatment, and these additional measures may have contributed to the observed declines in CIN2+ incidences in young women [25].
We also noted an increased incidence of CIN2+ among screened women in the 3 older age groups evaluated in this analysis, particularly in the analysis among screened women. Changes in screening and management recommendations, including a delayed age for a first screening, conservative management of younger women, an increased length of screening intervals, and incorporating clinical HPV testing into screening algorithms, would all be expected to result in an increased yield of CIN2+ per screening episode and an older age at an initial diagnosis [13, 25–28]. Such changes occurring during the surveillance period might, in part, explain our findings of increased CIN2+ incidences among the screened women in some age groups. Continued surveillance is necessary to determine whether the observed increased rates in the largely-unvaccinated older age groups represent a shift in age at diagnosis. An alternative explanation for increased CIN2+ rates is that there has been increased exposure to HPV due to changes in sexual behavior. Some data indicate an increase in other sexually transmitted infections in the United States [29].
A strength of HPV-IMPACT is the inclusion of 5 geographically-dispersed surveillance areas, enabling the documentation of the vaccine’s impact in different regions of the United States that have varying population characteristics. Over time, in 18–20 and 21–24 year olds, declines in CIN2+ rates were evident in all sites, as were declines in the proportion screened. However, we observed heterogeneity across sites in baseline (2008) CIN2+ rates and estimated screening proportions, and some heterogeneity in crude trends over time (such as the timing of declines in case rates in some age groups), which could signal regional variations in true incidences of the disease. At baseline (2008), the CIN2+ rates were highest in Connecticut and New York and lowest in California; the differences in baseline rates were greatest in the 3 youngest age groups. We speculate that the differences in these baseline disease rates might have been impacted by population characteristics in the different catchment areas (as reflected in demographic differences among cases), differences in clinical practices (eg, screening and diagnostic practices), or variations in the sensitivity of the surveillance system by site. While catchment area estimates of vaccinations in the population are not known, national surveys have documented differences in vaccination rates by state and region, and these differences could impact differences in the timing and magnitude of reductions in CIN2+ rates [4].
This analysis has several limitations. The individual HPV-IMPACT sites had some operational differences that could result in differences in case reporting completeness and the accuracy of denominators. These include different numbers of pathology laboratories, out-of-catchment laboratories serving catchment residents, and varying reliance on manual reporting streams. The sites had different locally-available resources for estimating the proportion of women screened for cervical cancer in each year. Inaccurate site-specific screening estimates would result in underestimates or overestimates of CIN2+ rates per 100000 screened women; however, because the methods within sites have been consistent over time, this limitation is unlikely to affect the direction of the trends observed. Adjustments for sites in statistical models did not alter the direction or significance of our findings, although it did modify the magnitude in the analyses conducted among screened women. Finally, the analysis using screened women as the denominator would be affected if there were an association between screening and vaccination in the population. If screened women are more likely than unscreened women to have been vaccinated, the analysis would overestimate vaccine impact; if screened women are less likely to be vaccinated, the analysis would underestimate vaccine impact.
In conclusion, this analysis of 8 years of active, population-based laboratory surveillance data for high-grade cervical lesions is consistent with the vaccine’s expected impact in young women ages 18–20 and 21–24 years. Continued surveillance is important for monitoring the impact of HPV vaccination in the United States as vaccinated women age into older age groups and as women who received the 9-valent vaccine, introduced in 2015 in the United States [30], and those who received a 2-dose vaccination schedule [31] reach the cervical cancer screening age.
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Acknowledgments. The authors thank Tiffanie Markus, PhD, Department of Health Policy, Vanderbilt University Medical Center; Mohamed Mokhtar Desouki, MD, PhD, Department of Pathology, Microbiology and Immunology, Vanderbilt University Medical Center; and Martin Whiteside, PhD, Director, Tennessee Comprehensive Cancer Control Program for their collaboration; and the Division of TennCare, Tennessee Department of Finance and Administration, and Tennessee Blue Cross/Blue Shield for providing screening data.
Additional Human Papillomavirus Vaccine Impact Monitoring Project (HPV-IMPACT) Working Group members include: Sheelah Blankenship, MS, and Stephanie Allen, MPH, Vanderbilt University Medical Center; James Meek, MPH, Kyle Higgins, BS, and James Hadler, MD, MPH, Yale School of Public Health; Lynn Sosa, MD, CT Department of Public Health; Kayla Saadeh, MPH, Ashley Williamson, MPH, and Deanna Fink, BA, California Emerging Infections Program; Michael J. Silverberg, Kaiser Permanente Northern California, Robert Laing, MPH, and Sean Schafer, MD, MPH, Oregon Health Authority; and Marina Oktapodas, MS and Christina Felsen, MPH, University of Rochester.
Financial support. This work was supported by a cooperative agreement through the Centers for Disease Control and Prevention’s Emerging Infections Program (grant numbers U50CK000482 [California], U50CK000488 [Connecticut], U50CK000486 [New York], U50CK000484 [Oregon], and U50CK000491 [Tennessee]). The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control.
Potential conflicts of interest. L. M. N. reports personal fees from Merck, outside the submitted work. All other authors report no potential conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
Contributor Information
HPV-IMPACT Working Group:
Sheelah Blankenship, Stephanie Allen, James Meek, Kyle Higgins, James Hadler, Lynn Sosa, Kayla Saadeh, Ashley Williamson, Deanna Fink, Michael J Silverberg, Robert Laing, Sean Schafer, Marina Oktapodas, and Christina Felsen
References
- 1. Markowitz LE, Dunne EF, Saraiya M, et al. ; Centers for Disease Control and Prevention. Human papillomavirus vaccination: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep 2014; 63:1–30. [PubMed] [Google Scholar]
- 2. Dunne EF, Stokley S, Chen W, Zhou F. Human papillomavirus vaccination of females in a large health claims database in the United States, 2006–2012. J Adolesc Health 2015; 56:408–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Reagan-Steiner S, Yankey D, Jeyarajah J, et al. National, regional, state, and selected local area vaccination coverage among adolescents aged 13–17 years - United States, 2015. MMWR Morb Mortal Wkly Rep 2016; 65:850–8. [DOI] [PubMed] [Google Scholar]
- 4. Walker TY, Elam-Evans LD, Singleton JA, et al. National, regional, state, and selected local area vaccination coverage among adolescents aged 13–17 years - United States, 2016. MMWR Morb Mortal Wkly Rep 2017; 66:874–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Williams WW, Lu PJ, O’Halloran A, et al. Surveillance of vaccination coverage among adult populations - United States, 2015. MMWR Surveill Summ 2017; 66:1–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Stokley S, Jeyarajah J, Yankey D, et al. Human papillomavirus vaccination coverage among adolescents, 2007–2013, and postlicensure vaccine safety monitoring, 2006–2014--United States. MMWR Morb Mortal Wkly Rep 2014; 63:620–4. [PMC free article] [PubMed] [Google Scholar]
- 7. Markowitz LE, Hariri S, Lin C, et al. Reduction in human papillomavirus (HPV) prevalence among young women following HPV vaccine introduction in the United States, National Health and Nutrition Examination Surveys, 2003–2010. J Infect Dis 2013; 208:385–93. [DOI] [PubMed] [Google Scholar]
- 8. Markowitz LE, Liu G, Hariri S, Steinau M, Dunne EF, Unger ER. Prevalence of HPV after introduction of the vaccination program in the United States. Pediatrics 2016; 137:e20151968. [DOI] [PubMed] [Google Scholar]
- 9. Oliver SE, Unger ER, Lewis R, et al. Prevalence of human papillomavirus among females after vaccine introduction-National Health and Nutrition Examination Survey, United States, 2003–2014. J Infect Dis 2017; 216:594–603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Dunne EF, Naleway A, Smith N, et al. Reduction in human papillomavirus vaccine type prevalence among young women screened for cervical cancer in an integrated US Healthcare Delivery System in 2007 and 2012–2013. J Infect Dis 2015; 212:1970–5. [DOI] [PubMed] [Google Scholar]
- 11. Bauer HM, Wright G, Chow J. Evidence of human papillomavirus vaccine effectiveness in reducing genital warts: an analysis of California public family planning administrative claims data, 2007–2010. Am J Public Health 2012; 102:833–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Flagg EW, Schwartz R, Weinstock H. Prevalence of anogenital warts among participants in private health plans in the United States, 2003–2010: potential impact of human papillomavirus vaccination. Am J Public Health 2013; 103:1428–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Benard VB, Castle PE, Jenison SA, et al. ; New Mexico HPV Pap Registry Steering Committee. Population-based incidence rates of cervical intraepithelial neoplasia in the human papillomavirus vaccine era. JAMA Oncol 2017; 3:833–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Flagg EW, Torrone EA, Weinstock H. Ecological Association of human papillomavirus vaccination with cervical dysplasia prevalence in the United States, 2007–2014. Am J Public Health 2016; 106:2211–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Hariri S, Johnson ML, Bennett NM, et al. ; HPV-IMPACT Working Group. Population-based trends in high-grade cervical lesions in the early human papillomavirus vaccine era in the United States. Cancer 2015; 121:2775–81. [DOI] [PubMed] [Google Scholar]
- 16. Bulletins--Gynecology ACoP. ACOG practice Bulletin no. 109: cervical cytology screening. Obstet Gynecol 2009; 114:1409–20. [DOI] [PubMed] [Google Scholar]
- 17. Moyer VA. Screening for cervical cancer: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med 2012; 156:880–91, w312. [DOI] [PubMed] [Google Scholar]
- 18. Sawaya GF, Huchko MJ. Cervical cancer screening. Med Clin North Am 2017; 101:743–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Baldur-Felskov B, Dehlendorff C, Junge J, Munk C, Kjaer SK. Incidence of cervical lesions in Danish women before and after implementation of a national HPV vaccination program. Cancer Causes Control 2014; 25:915–22. [DOI] [PubMed] [Google Scholar]
- 20. Brotherton JM, Fridman M, May CL, Chappell G, Saville AM, Gertig DM. Early effect of the HPV vaccination programme on cervical abnormalities in Victoria, Australia: an ecological study. Lancet 2011; 377:2085–92. [DOI] [PubMed] [Google Scholar]
- 21. Brotherton JM, Gertig DM, May C, Chappell G, Saville M. HPV vaccine impact in Australian women: ready for an HPV-based screening program. Med J Aust 2016; 204:184–184e1. [DOI] [PubMed] [Google Scholar]
- 22. Ogilvie GS, Naus M, Money DM, et al. Reduction in cervical intraepithelial neoplasia in young women in British Columbia after introduction of the HPV vaccine: An ecological analysis. Int J Cancer 2015; 137:1931–7. [DOI] [PubMed] [Google Scholar]
- 23. Hariri S, Markowitz LE, Bennett NM, et al. Monitoring effect of human papillomavirus vaccines in US population, emerging infections program, 2008–2012. Emerg Infect Dis 2015; 21:1557–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Watson M, Soman A, Flagg EW, et al. Surveillance of high-grade cervical cancer precursors (CIN III/AIS) in four population-based cancer registries, United States, 2009–2012. Prev Med 2017; 103:60–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Massad LS, Einstein MH, Huh WK, et al. 2012 updated consensus guidelines for the management of abnormal cervical cancer screening tests and cancer precursors. J Low Genit Tract Dis 2013; 17(Suppl 1):S1–S27. [DOI] [PubMed] [Google Scholar]
- 26. ASCUS-LSIL Triage Study (ALTS) Group. A randomized trial on the management of low-grade squamous intraepithelial lesion cytology interpretations. Am J Obstet Gynecol 2003; 188:1393–400. [DOI] [PubMed] [Google Scholar]
- 27. Huh WK, Ault KA, Chelmow D, et al. Use of primary high-risk human papillomavirus testing for cervical cancer screening: interim clinical guidance. J Low Genit Tract Dis 2015; 19:91–6. [DOI] [PubMed] [Google Scholar]
- 28. United States Preventive Services Task Force. Draft recommendation statement, cervical cancer: Screening Available at: https://www.uspreventiveservicestaskforce.org/Page/Document/draft-recommendation-statement/cervical-cancer-screening2. Accessed 31 January 2018.
- 29. Centers for Disease Control and Prevention. Sexually transmitted disease surveillance 2016. Atlanta, GA: U.S. Department of Health and Human Services, 2017. [Google Scholar]
- 30. Petrosky E, Bocchini JA Jr, Hariri S, et al. ; Centers for Disease Control and Prevention. Use of 9-valent human papillomavirus (HPV) vaccine: updated HPV vaccination recommendations of the advisory committee on immunization practices. MMWR Morb Mortal Wkly Rep 2015; 64:300–4. [PMC free article] [PubMed] [Google Scholar]
- 31. Meites E, Kempe A, Markowitz LE. Use of a 2-dose schedule for human papillomavirus vaccination-updated recommendations of the advisory committee on immunization practices. Am J Transplant 2017; 17:834–7. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.