Abstract
Grapevine trunk diseases (GTDs) are a major threat to the wine and grape industry. The aim of the study was to investigate the antifungal activity against Neofusicoccum parvum, Diplodia seriata, and Botryosphaeria dothidea of ε-polylysine, chitosan oligomers, their conjugates, Streptomyces rochei and S. lavendofoliae culture filtrates, and their binary mixtures with chitosan oligomers. In vitro mycelial growth inhibition tests suggest that the efficacy of these treatments, in particular those based on ε-polylysine and ε-polylysine:chitosan oligomers 1:1 w/w conjugate, against the three Botryosphaeriaceae species would be comparable to or higher than that of conventional synthetic fungicides. In the case of ε-polylysine, EC90 values as low as 227, 26.9, and 22.5 µg·mL−1 were obtained for N. parvum, D. seriata, and B. dothidea, respectively. Although the efficacy of the conjugate was slightly lower, with EC90 values of 507.5, 580.2, and 497.4 µg·mL−1, respectively, it may represent a more cost-effective option to the utilization of pure ε-polylysine. The proposed treatments may offer a viable and sustainable alternative for controlling GTDs.
Keywords: Botryosphaeria dothidea, conjugate complexes, Diplodia seriata, grapevine trunk diseases, Neofusicoccum parvum
1. Introduction
Grapevine trunk diseases (GTDs) have been reported in most grapevine producing regions worldwide, causing a serious decline and loss of productivity. These diseases include black dead arm, caused by Botryosphaeria dothidea; esca, which includes vascular symptoms and internal white rot in the trunk; eutypiosis, caused by Eutypa lata; Petri disease; black foot; and Phomopsis dieback, being the esca complex the most frequent and increasing syndrome in almost all European countries [1]. A recent International Organization of Vine and Wine (OIV) publication reported that incidence of GTDs was 10% in Spain, 13% in France, and between 8% and 19% in Italy, and that the losses in California were at least 260 M$ per year [2].
A thorough and up-to-date panorama of the state-of-the-art of chemicals (including synthetic organic compounds, inorganic compounds, natural compounds, and plant-defense stimulating compounds) and biocontrol agents that have been tested towards GTDs can be found in the recent review paper by Mondello et al. [2].
Unfortunately, chemical fungicides traditionally used to control aforementioned fungal crop infections, such as sodium arsenite, carbendazim, or tecobunazole, have several drawbacks in terms of toxicity and efficacy, and, in recent years, public pressure to reduce their use has increased. In fact, concerns have been raised about both their environmental impact and the potential associated health risks. In this context, the use of natural antifungals as a feasible alternative is receiving increasing attention.
Among the tested natural compounds, Nascimento et al. [3] reported the antifungal effect of chitosan on several fungal species involved in grapevine decline. Greenhouse experiments using foliar sprays of chitosan on potted grapevine plants growing in a substrate artificially infected with Phaeomoniella chlamydospora or Ilyonectria liriodendri demonstrated that chitosan significantly improved plant growth and decreased disease incidence. More recently, Cobos et al. [4] reported that chitosan oligosaccharides, garlic extract, and vanillin were able to significantly reduce infection in pruning wounds by Diplodia seriata. Galarneau et al. [5] also examined the potential role of antimicrobial phenolic compounds on Neofusicoccum parvum and D. seriata, two causal fungi of Botryospheria dieback.
ε-polylysine (EPL), a natural antimicrobial produced from aerobic bacterial fermentation by Streptomyces albulus, widely used in Japan and USA as an antimicrobial agent in food products, could also be a promising antifungal agent [6]. Although it has been reported to have a strong activity against Escherichia coli, Staphylococcus aureus, and Bacillus subtilis [7], either alone or in chitosan conjugate compounds, its efficacy has not been assayed against GTDs.
In a similar fashion, even though beneficial bacteria inhabiting the rhizosphere and/or the endosphere of plants and their secondary metabolites have been put forward by some authors to reduce grapevine pathogen diseases [6], information reported in the literature is limited [8,9,10,11,12]. These biocontrol agents, such as Streptomyces spp., would affect pathogen performance by antibiosis, competition for niches and nutrients, interference with pathogen signaling, or by stimulation of host plant defenses.
The aim of the study presented herein has been to assess the in vitro antifungal activity of EPL, EPL:chitosan oligomers (EPL:COS) conjugates, and secondary metabolites from two beneficial actinobacteria (Streptomyces rochei and S. lavendofoliae) to control N. parvum, D. seriata, and B. dothidea, three of the most frequently isolated fungal pathogens in GTDs.
2. Results
2.1. Vibrational Analysis of the ε-polylysine: Chitosan Conjugates
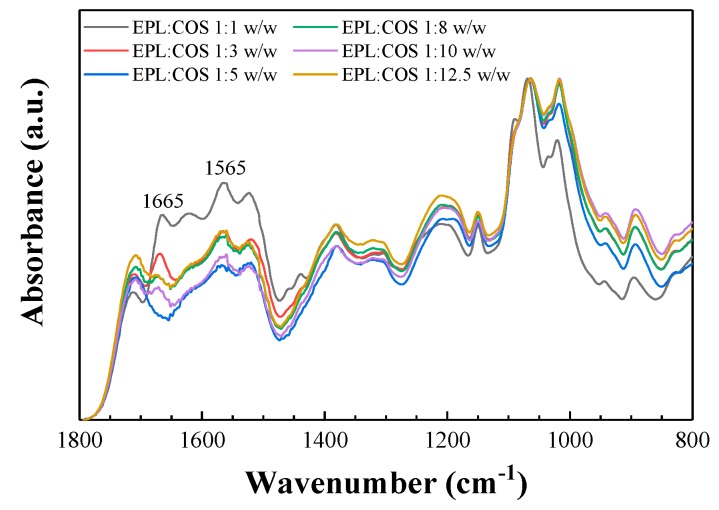
The vibrational spectra of conjugates prepared with six different EPL:COS mass ratios were examined in order to confirm their secondary structure and to determine the most suitable proportion (Figure 1).
Figure 1.
Comparison of the attenuated total reflection (ATR)-Fourier-Transform Infrared (FTIR) spectra of ε-polylysine:chitosan oligomers conjugates prepared with different ε-polylysine:chitosan oligomers mass ratios. Only the fingerprint region is shown.
The absorption bands at 1150 cm−1 and 1018 cm−1 were assigned to asymmetric stretching of the C−O−C bridge and to the skeletal vibration of C−O stretching, respectively [13,14,15]. The absorption band at 895 cm−1 could be assigned to the β-D-configuration. There was a shift of amide/amino bands in the reaction products, indicating the progress of Maillard reaction: the absorption peaks at 1659 cm−1 and 1597 cm−1 (associated with amino groups characteristic of chitosan oligomers) disappeared, and new bands at 1665 cm−1 and 1565 cm−1 were observed. The appearance of these bands suggest that a Schiff base (C=N bond) was formed between the reducing end of chitosan and the amino groups [16]. Thus, the Fourier-Transform Infrared (FTIR) results showed that ε-polylysine had actually attached to chitosan.
An interesting feature was that the absorbance of the bands associated with Schiff base formation were stronger in the 1:1 EPL:COS conjugate than in the spectra of conjugates prepared with other EPL:COS ratios. Thus, the Schiff base for the 1:1 conjugate seems to feature the desired balance of components to undergo the Amadori rearrangement with formation of ketosamines, but avoiding their subsequent decomposition observed in more COS-rich conjugates. This result was in good agreement with the findings of Liang et al. [7] for EPL:chitosan, who concluded that the conjugate with EPL and chitosan ratio of 1:1 exhibited the strongest antibacterial and antifungal activity. Consequently, the 1:1 EPL:COS conjugate was chosen for the mycelial growth inhibition tests in this study.
2.2. Mycelial Growth Inhibition Tests
The in vitro radial growth inhibition attained by each of the treatments against N. parvum is depicted in Figure 2, showing only for one replicate per treatment and dose. Those attained against D. seriata and B. dothidea are depicted in Figures S1 and S2, respectively. The values across the three replicates for the three Botryosphaeriaceae species are summarized in Figure 3.
Figure 2.
N. parvum mycelial growth inhibition assays for: (a) chitosan oligomers; (b) ε-polylysine; (c) S. rochei secondary metabolites; (d) S. lavendofoliae secondary metabolites; (e) ε-polylysine:chitosan (1:1 w/w) conjugates; (f) S. rochei secondary metabolites + chitosan oligomers (1:1 w/w); and (g) S. lavendofoliae secondary metabolites + chitosan oligomers (1:1 w/w). The concentration of the treatments decreases from top to bottom (doses for each treatment are indicated in Table 3). The petri dish in the bottom right corner shows the PDA control. Only one replicate per each treatment and dose is shown.
Figure 3.
Radial growth values of (a) N. parvum; (b) D. seriata; and (c) B. dothidea in the presence of the different treatments under study at different concentrations (in µg·mL−1). COS, EPL, MR, ML and C stand for chitosan oligomers, ε-polylysine, S. rochei secondary metabolites, S. lavendofoliae secondary metabolites and control, respectively. For MR and ML only one column is shown, since no inhibition was detected at any concentration in the 250–1500 µg·mL−1 range. Concentrations labelled with the same uppercase letters are not significantly different at p < 0.05 by Tukey’s test. All values are presented as the average of three repetitions. Error bars represent the standard deviation across three replicates.
The increase in the treatment doses resulted in a reduction in the radial growth of the mycelium in all cases, with statistically significant differences amongst the various concentrations (Figure 3), except for the S. rochei and S. lavendofoliae secondary metabolites-only based treatments (MR and ML, respectively), for which no inhibition was observed.
Doses in the 1000–1500 µg·mL−1 range were required to attain full inhibition of the three Botryosphaeriaceae species for the COS, EPL, and EPL:COS conjugate treatments. As regards the activity of MR+COS and ML+COS treatments, differences were observed as a function of the fungal pathogen species. Full inhibition of D. seriata was attained for both treatments at a dose of 1200 µg·mL−1, whereas it was only observed for ML+COS in the case of B. dothidea. MR+COS treatment led to 89% inhibition at the same dose for this latter pathogen. In the case of N. parvum, the highest doses of MR+COS and ML+COS led to 83% and 89% inhibition, respectively.
The sensitivity tests results may also be expressed in terms of effective concentrations EC50 and EC90, that is, the concentrations that reduce mycelial growth by 50% and 90%, respectively (Table 1). Goodness-of-fit analyses revealed good r2 and low sum of standard errors, showing that parameter fits of sigmoid curves to the dose-response data were significant. In view of the obtained theoretical values, the activity of the treatments—in general terms—would follow the sequence EPL > EPL:COS > ML+COS > COS > MR+COS.
Table 1.
Effective concentrations that inhibited mycelial growth by 50% and 90% (EC50 and EC90, respectively).
| Pathogen | Concentration (µg·mL−1) | Treatment | ||||
|---|---|---|---|---|---|---|
| COS | EPL | EPL:COS | MR + COS | ML + COS | ||
| N. parvum | EC50 | 60.7 | 16.0 | 11.2 | 67.2 | 46.7 |
| EC90 | 1270.0 | 227.0 | 507.5 | 2074.2 | 1101.7 | |
| D. seriata | EC50 | 94.3 | 0.3 | 11.6 | 45.1 | 30.7 |
| EC90 | 1120.7 | 26.9 | 580.2 | 906.9 | 498.2 | |
| B. dothidea | EC50 | 1.8 | 0.4 | 4.2 | 15.8 | 10.7 |
| EC90 | 689.5 | 22.5 | 497.4 | 1019.0 | 490.3 | |
3. Discussion
3.1. Efficacy of the Treatments
In relation to the efficacy of the composites, although the review paper by Mondello et al. [2] provides a qualitative comparison of different treatment against GTDs, specific inhibition rates with their associated concentrations or effective concentrations were not provided. A survey of such values against the three Botryosphaeriaceae species under study is summarized in Table 2 for comparison purposes.
Table 2.
Concentration values and associated inhibition rates, or EC50 values, reported in the literature for other active compounds against the three Botryosphaeriaceae species under study.
| Fungicide | Fungal Species | Concentration (µg·mL−1) | Inhibition rate (%) | EC50(µg·mL−1) | Ref. |
|---|---|---|---|---|---|
| Tebuconazole | N. parvum | 90 | [18] | ||
| D. seriata | 150 | ||||
| Pyraclostrobin | N. parvum | 100 | |||
| D. seriata | 250 | ||||
| Carbendazim, tebuconazole, iprodione, fludioxonil, fluazinam, flusilazole, penconazole, procymidone, myclobutanil, pyraclostrobin | N. parvum | 360–440 * | [19] | ||
| D. seriata | 530–620 * | ||||
| B. dothidea | 450 * | ||||
| Carbendazim | N. parvum | 40 | [20] | ||
| Tebuconazole | 130 | ||||
| Iprodione | 750 | ||||
| Tecobunazole | D. seriata | 300 | [21] | ||
| Fe NPs (FeNPs + neem leaf extract) | D. seriata | 100 (FeNPs / FeNPs+neem 1:1) | 79/80.3 | [22] | |
| B. dothidea | 83/82.5 | ||||
| AgNPs | N. parvum | 40 | 84 | [23] | |
| AgNPs | 30 | 81 | [24] | ||
| Lemon essential oil (limonene, neral, β-pinene, and γ-terpinene) in DMSO | B. dothidea | 2500 | 48.1 | [25] | |
| Chitosan oligosaccharin (mol. wt. <3 kDa) | Botryosphaeria sp. | 1.56 | [3] | ||
| Chitosan oligosaccharides | D. seriata | 1000 | 100 | [4] | |
| Vanillin | 1000 | 89.8 | |||
| Garlic extract | 40000 | 75.3 |
* Data pooled across fungicides to provide mean EC50 values for isolate sensitivity in the original study.
It may be observed that the EC50 values for the treatments presented herein (Table 1), in particular those of EPL and EPL:COS conjugate, were comparable to or better than those of popular synthetic organic compounds used to control GTDs, and only slightly lower than the excellent activities reported for AgNPs.
The results presented for COS were in excellent agreement with those reported by Nascimento et al. [3] and Cobos et al. [4]. However, with regard to this latter study, it should be noted that while the use of polyphenols, such as vanillin or those found in garlic extract, may be suitable against D. seriata and other Botryosphaeriaceae strains [17], it may not be advisable against N. parvum. Galarneau et al. [5] recently found that N. parvum was either uninhibited or promoted by phenolic compounds such as gallic acid, epicatechin, rutin, or piceid. In fact, the authors explained that the ability of N. parvum to tolerate these phenolics or utilize them as carbon sources would contribute to its greater virulence compared to D. seriata.
The Streptomyces spp. secondary metabolites-based treatments showed an unexpected lack of activity when used alone. In fact, the percentage of inhibition of radial growth (PIRG) values, shown in Tables S1–S3, were negative, i.e., the growth of the pathogens was promoted. This was not a case of hormetic response, provided that increasing the concentration did not result in inhibition. The observed mycelial growth promotion may be tentatively ascribed to the presence of molasses and yeast extract in the culture filtrates, together with a poor absorption and bioavailability of the active ingredients in the water-based culture filtrates, resulting from their insolubility or very poor solubility in water.
In relation to one of the active compounds present in the culture filtrates under test, lankacidin, Harada et al. [26] stated that lankacidin-group antibiotics are scarcely soluble in water and that the parts that dissolved are rapidly decomposed to compounds with no antimicrobial activity. To overcome this problem, they prepared inclusion compounds with cyclodextrins. In this study, this solubility problem was solved by forming polyelectrolyte complexes (PECs) with a polycationic polymer, i.e., chitosan oligomers. These chitosan-based PECs have been reported to exhibit favorable physicochemical properties and to preserve chitosan’s biocompatible characteristics [27], which has made this approach very popular in the drug delivery fields [28,29]. In fact, Zhang et al. [30] previously reported that chitosan behaves as an efficient carrier to deliver streptomycin.
3.2. Mechanism of Action
Concerning the mechanism of action (MOA) of the proposed treatments, although the antimicrobial activity of EPL is well documented, its MOA has only been vaguely described. Hyldgaard et al. [31] hypothesized that EPL destabilizes membranes in a carpet-like mechanism by interacting with negatively charged phospholipid head groups, which displace divalent cations and enforce a negative curvature folding on membranes that leads to formation of vesicles/micelles. According to Ye et al. [32], the antimicrobial mechanism of EPL may be attributed not only to disturbances on membrane integrity, but also to oxidative stress by ROS, and to its effects on various gene expressions.
It is worth noting that the fungicidal activity would likely benefit from the substitution of lysine with arginine residues, provided that previous works have demonstrated the superior cell permeability by arginine polymers over lysine-containing ones [33,34]. Mechanistic evidences indicate that arginine can enhance the activity of both translocating and membrane permeabilizing peptides [35,36]. This would be a potential direction for future studies.
Regarding the inhibition mode of chitosan oligomers, Ing et al. [37] proposed several MOAs. The interaction of chitosan’s positive charge with negatively charged phospholipid components would result in an increased permeability and in leakage of cellular contents. Its chelating action would deprive fungi of trace elements essential for their normal growth. Moreover, its binding to fungal DNA would inhibit mRNA synthesis and affect proteins and enzymes production.
Consequently, the activity of EPL:COS conjugates, as noted by Liang et al. [7], should be referred to an enhanced disruption of their cell membranes, leading to damages of structure, function, and permeability, leakage of intracellular components and the ultimate lysis of the cell.
3.3. Applicability to GTDs in vivo
As regards the applicability of the proposed treatments to GTDs in vivo, although it was not covered in this preliminary study, several systems may be envisaged [38]. To reduce symptoms in the field, once the wood is already infected, an approach to be explored would be to apply the products to the soil (injector pole) or to the trunk (trunk injections), mimicking the mechanism activated by winter spraying of sodium arsenite [39]. However, it would be expensive and time-consuming if applied on a large scale [40], and would only be cost-effective when applied in high-value vineyards [41,42].
The proposed antifungal agents may also be administrated by foliar pulverization with minor changes to the formulations (e.g., adding a surfactant as Tween-80). This would be the most practical approach considering the experience of winegrowers. According to Roblin et al. [43], the compounds sprayed on the leaf blades would be able to migrate to the fungal target in the trunk or to trigger the plant defense reaction in distal parts of the plant. In fact, successful use of foliar sprays of chitosan on grapevine plants artificially infested with Phaeomoniella chlamydospora or Neonectria liriodendri have been reported in the literature [3]. However, this application method has the major drawback that the treatments may be easily washed off by rainfall [44]. If this approach was to be chosen, sprays after the period of vintage should be useful since, at this period, the phloem sap begins to be directed in a descending flow towards the roots, assuring the transport of the compounds towards the fungi [43].
Alternatively, as a preventive measure, the active ingredients may also be used to protect pruning wounds to avoid grapevine infection and to limit fungal expansion in the plant, either as painted pastes or as liquid formulations. This application method was evaluated against D. seriata and P. chlamydospora in field trials by Cobos et al. [4], using chitosan oligosaccharides, vanillin, and garlic extract, and resulted in a significant decrease in plant mortality and in the infection rate. Nonetheless, to improve the adherence of the treatments, thickener agents would need to be added to the formulations: e.g., starches, vegetable gums, pectin, or clays such as halloysite.
3.4. Significance of the Reported Findings
Although follow-up studies involving in vivo assays and field tests would be necessary to draw firm conclusions on the effectiveness of the application of the proposed treatments, the fact that they reached higher mycelial growth inhibition than that of commercial fungicides makes them promising candidates for the effective control of botryosphaeriaceous diseases.
It is also worth noting that the three fungal species tested in the present study are not only pathogens of grapevine, but also of other commercially important woody plants. For instance, D. seriata and B. dothidea are phytopathogens of apple [22], N. parvum causes dieback in avocado [45], B. dothidea causes branch dieback of olive [46], and the three of them are associated with branch cankers on almond trees [47]. Consequently, the results of this study may also find application in other pathosystems, resulting in an even higher ecological and economic impact.
4. Materials and Methods
4.1. Reagents, Bacteria and Fungi
High molecular weight chitosan (CAS 9012-76-4; 310000-375000 Da) was purchased from Hangzhou Simit Chemical Technology Co., Ltd. (Hangzhou, China). ε-polylysine (CAS 25104-18-1), phosphate buffer (for microbiology, APHA, pH 7.2), ethyl acetate (CAS 141-78-6; ≥99.5%), and citric acid (CAS 77-92-9; ≥99.5%) were supplied by Sigma-Aldrich Química S.A. (Madrid, Spain). Neutrase® 0.8L enzyme was supplied by Novozymes (Bagsvaerd, Denmark). Potato dextrose agar (PDA), yeast extract, and BactoTM Peptone were purchased from Becton, Dickinson and Company (Franklin Lakes, NJ, USA). Starch casein agar (SCA), Mueller Hinton agar, and malt extract agar (MEA) came from Oxoid Ltd. (Hampshire, UK). Molasses were supplied by ACOR, Sociedad Cooperativa General Agropecuaria (Castilla y León, España).
The three fungal isolates under study, viz. Diplodia seriata (ITACYL_F079), Neofusicoccum parvum (ITACYL_F111), and Botryosphaeria dothidea (ITACYL_F141), were supplied by ITACYL, Instituto Tecnológico Agrario de Castilla y León (Castilla y León, España).
The two Streptomyces spp. strains from which secondary metabolites were produced, Streptomyces lavendofoliae (DSM 40217) and Streptomyces rochei (DSM 41729) were purchased from DSMZ (Deutsche Sammlung von Mikroorganismen und Zellkulturen; Braunschweig, Germany).
4.2. Equipment
A probe-type UIP1000hdT ultrasonicator (Hielscher, Teltow, Germany; 1000 W, 20 kHz) was used for solutions sonication.
To incubate the flasks, controlling the temperature and the stirring speed, an ECOLAN 60 (Labolan; Esparza de Galar, Navarra, Spain) orbital stirrer incubator was used.
Functional groups were identified by Fourier-Transform Infrared spectroscopy with a Nicolet iS50 (Thermo Scientific, Waltham, MA, USA) apparatus equipped with a diamond attenuated total reflection (ATR) module. The spectra were collected in the 400–4000 cm−1 region with a 1 cm−1 spectral resolution; 64 scans were co-added and the resulting interferogram was averaged. The ATR-FTIR spectra were corrected using the advanced ATR correction algorithm [47] available in OMNICTM software suite.
4.3. Preparation of Chitosan Oligomers
Chitosan oligomers were obtained according to the enzymatic procedure described by Santos-Moriano et al. [48], with slight modifications. 20 g of high molecular weight chitosan were dissolved in 1000 mL of Milli-Q water by adding citric acid under constant stirring at 60 °C. Once dissolved, Neutrase® 0.8 L (1.67 g·L−1) was added in order to degrade the polymer chains. The mixture was sonicated for 3 min in cycles of 1 min with sonication and 1 min without sonication to keep the temperature in the 30–60 °C range [14]. At the end of the process, a solution with a pH in the four to six interval with oligomers of molecular weight < 2000 Da was obtained.
4.4. ε-polylysine Treatment
For the preparation of the ε-polylysine treatment, 2 g of EPL were dissolved in 1000 mL of Milli-Q water. The mixture was sonicated for 3 min in cycles of 1 min with sonication and 1 min without sonication so that the temperature remained in the 30–60 °C range.
4.5. Synthesis of ε-polylysine: Chitosan Oligomers Conjugates
Conjugated complexes of ε-polylysine and chitosan oligomers were prepared at different mass ratios, namely 1:1, 1:3, 1:5 1:8, 1:10, and 1:12.5 w/w, respectively. The appropriate amounts of each component were dissolved in Milli-Q water using sonication (5 cycles of 5 min/cycle, taking care not to exceed 60 °C). The resulting solutions were lyophilized, and then heated at 60 °C under 60% relative humidity for 24 h. This synthesis procedure was analogous to other procedures described in the literature for the preparation of EPL:COS conjugates through Maillard reaction [7,49,50]. Only the conjugate with the highest expected activity was assayed in the mycelial growth inhibition tests.
4.6. Secondary Metabolites Production from Streptomyces spp. Strains
Two strains of the genus Streptomyces, viz. Streptomyces lavendofoliae DSM 40217 and Streptomyces rochei DSM 41729 were seeded on starch casein agar medium plates at 28 °C for 10 days. The plates were stored at 4 °C. For long-term storage, lyophilizates from both strains were used.
In order to obtain the secondary metabolites, the method described by Sadigh-Eteghad et al. [51] was followed. Once the fermentation was complete, each final solution of the cultures of both strains was treated with 50 mL of phosphate buffer (pH 6.4) and was sonicated for 5 min. The solutions were then filtered through sterile muslin cloth twice. These solutions (culture filtrates) were used for the mycelial growth inhibition tests.
In order to determine the concentration of bioactive compounds in aforementioned solutions (and the doses used in the inhibition tests), the filtrates were centrifuged, and the supernatant was extracted with 100 mL of ethyl acetate. The solvent with the crude bioactive compounds was concentrated under reduced pressure and then lyophilized. The culture filtrates had a concentration of approx. 2000 μg∙mL−1 (1958 μg∙mL−1 for S. lavendofoliae secondary metabolites and 1877 μg∙mL−1 for S. rochei secondary metabolites), in agreement with Pazhanimurugan et al. [52]. The bioactive compounds in the secondary metabolites of S. lavendofoliae and S. rochei are summarized in Table S4.
4.7. Synthesis of Chitosan Oligomers-secondary Metabolites Inclusion Compounds
Secondary metabolites, either from S. lavendofoliae or from S. rochei, and chitosan oligomers mixtures were prepared by mixing in 1:1 (w/w) ratio of their respective solutions (2000 μg∙mL−1 of bioactive compounds + 2000 μg∙mL−1 COS), followed by sonication. The resulting solutions (ML+COS and MR+COS) were assayed at different concentrations in the inhibition tests.
4.8. In vitro Mycelial Growth Inhibition Tests
The biological activity of the treatments under study was determined by the agar dilution method: aliquots of the original solutions of the various treatments, obtained by dilution of the respective “mother” solutions, were incorporated into the PDA medium to obtain the final concentrations indicated in Table 3. It should be clarified that the tested concentrations were not the same all treatments due to difficulties associated with the estimation of the molecular weights of the polymeric reagents from their viscosities. Petri dishes containing only PDA culture medium (20 mL) were used as the control.
Table 3.
Concentrations assayed for each of the treatments in the mycelial growth inhibition tests. COS, PL, MR and ML stand for chitosan oligomers, ε-polylysine, S. rochei secondary metabolites, and S. lavendofoliae secondary metabolites, respectively.
| Treatment | Concentrations Assayed in the Mycelial Growth Inhibition Tests (μg∙mL−1) |
|---|---|
| COS | 62.5, 125, 250, 500, 750, 1000, 1250, 1500 |
| EPL | 25, 50, 100, 200, 400, 600, 800, 1000 |
| MR | 250, 500, 750, 1000, 1250, 1500 |
| ML | 250, 500, 750, 1000, 1250, 1500 |
| EPL:COS | 250, 500, 750, 1000, 1250, 1500 |
| MR+COS | 200, 400, 600, 800, 1000, 1200 |
| ML+COS | 200, 400, 600, 800, 1000, 1200 |
The mycelial discs of pathogen (5 mm in diameter) were removed from the margins of 7-day-old PDA cultures and transferred to the petri dishes (in triplicate). Plates were incubated at 25 °C. The measurements of fungal growth for D. seriata and N. parvum were taken two, four and five days after inoculation. In contrast, for B. dothidea, measurements were carried out two, four and six days after inoculation, provided that mycelial growth was slower for this later fungus in the control plates.
The inhibition of mycelial growth, or the efficacy of the compound analyzed, for each treatment and concentration, was calculated by the formula:
| Percentage inhibition of radial mycelium growth (%) = ((R1 − R2)/R1) × 100 | (1) |
where R1 and R2 correspond to the average radial growth of the fungal mycelium in the control medium (pure PDA) and in the fungicide-amended medium, respectively.
The results were also expressed as the effective concentrations that reduced mycelial growth by 50% and 90% (EC50 and EC90, respectively), which were determined by the regression of the radial growth inhibition values (%) against the log10 values of the concentrations of antifungal compounds using PROBIT in IBM SPSS Statistics v.25 software. This regression procedure fits the dose-response curve to a sigmoid and calculates the values, with 95% CI, of the dose variable that correspond to a series of probabilities.
4.9. Statistical Analyses
Data were subjected to analysis of variance (ANOVA) in IBM SPSS Statistics v.25 software. Tukey’s HSD test at 0.05 probability level (p < 0.05) was used for the post hoc comparison of means.
5. Conclusions
The efficacy of ε-polylysine, chitosan oligomers, ε-polylysine:chitosan oligomers conjugates, two Streptomyces spp. secondary metabolites, and the combinations of the latter two with chitosan oligomers were examined in vitro against N. parvum, D. seriata and B. dothidea. On the basis of vibrational spectroscopy data, a 1:1 w/w mass ratio was chosen for the EPL:COS conjugate, for which an optimum Schiff base was formed. From the mycelial growth inhibition tests it was found that, in spite of the remarkable contents in bioactive compounds in the culture filtrates, the secondary metabolites of S. rochei and S. lavendofoliae did not inhibit any of the GTD-related fungi, probably due to hydrophobicity reasons. In contrast, upon formation of polyelectrolyte complexes with chitosan oligomers, inhibitions above 80% were attained. In view of the calculated effective concentration values, the antifungal activity of the treatments would follow the sequence EPL > EPL:COS > ML+COS > COS > MR+COS. EC50 values below 100 µg·mL−1 were obtained for all the assayed treatments, suggesting that they could be a viable alternative to conventional synthetic fungicides. In particular, ε-polylysine and ε-polylysine:chitosan oligomers may be put forward as the most promising options, due to the high efficacy of the former and the trade-off between efficacy and cost associated with the latter. In the current context in which the use of synthetic chemical pesticides is more and more restricted, this work constitutes a necessary step for developing efficient treatments that take into account the importance of environmental protection within the scope of sustainable development.
Supplementary Materials
The following are available online at https://www.mdpi.com/2079-6382/8/3/99/s1, Figure S1: D. seriata mycelial growth inhibition assays, Figure S2: B. dothidea mycelial growth inhibition assays, Table S1: Radial growth of mycelium (RG) and percentage of inhibition of radial growth (PIRG) of the different treatments against N. parvum two, four and five days after inoculation, Table S2: Radial growth of mycelium (RG) and percentage of inhibition of radial growth (PIRG) of the different treatments against D. seriata two, four and five days after inoculation, Table S3: Radial growth of mycelium (RG) and percentage of inhibition of radial growth (PIRG) of the different treatments against B. dothidea two, four and six days after inoculation, Table S4: Bioactive secondary metabolites produced by S. lavendofoliae and S. rochei.
Author Contributions
Conceptualization, J.M.G., E.P.L. and J.L.R.; Formal analysis, L.B.-D., M.C.R.-S. and P.M.-R.; Funding acquisition, J.M.-G.; Investigation, L.B.-D. and J.M.-G.; Methodology, J.M.-G., J.C.-G. and M.C.R.-S.; Resources, J.M.-G., E.P.-L., D.R.-R. and J.L.R.; Supervision, J.M.-G. and P.M.-R.; Validation, D.R.-R., J.L.R. and J.C.-G.; Visualization, L.B.-D. and P.M.-R.; Writing—original draft, L.B.-D., J.M.-G. and P.M.-R.; Writing—review & editing, L.B.-D. and P.M.-R.
Funding
This research was funded by Junta de Castilla y León, project number VA258P18.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
References
- 1.Guerin D.L., Fontaine F., Mugnai L. Grapevine trunk disease in European and Mediterranean vineyards: Occurrence, distribution and associated disease-affecting cultural factors. Phytopathol. Mediterr. 2019;58:49–71. [Google Scholar]
- 2.Mondello V., Songy A., Battiston E., Pinto C., Coppin C., Trotel-Aziz P., Clément C., Mugnai L., Fontaine F. Grapevine trunk diseases: A review of fifteen years of trials for their control with chemicals and biocontrol agents. Plant Dis. 2018;102:1189–1217. doi: 10.1094/PDIS-08-17-1181-FE. [DOI] [PubMed] [Google Scholar]
- 3.Nascimento T., Rego C., Oliveira H. Potential use of chitosan in the control of grapevine trunk diseases. Phytopathol. Mediterr. 2007;46:218–224. [Google Scholar]
- 4.Cobos R., Mateos R.M., Álvarez-Pérez J.M., Olego M.A., Sevillano S., González-García S., Garzón-Jimeno E., Coque J.J.R., Brakhage A.A. Effectiveness of natural antifungal compounds in controlling infection by grapevine trunk disease pathogens through pruning wounds. Appl. Environ. Microbiol. 2015;81:6474–6483. doi: 10.1128/AEM.01818-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Galarneau E.R., Wallis C.M., Baumgartner K. Grapevine trunk pathogen Neofusicoccum parvum tolerates host phytoalexin phenolic compounds. Phytopathology. 2018;108:46. [Google Scholar]
- 6.Compant S., Brader G., Muzammil S., Sessitsch A., Lebrihi A., Mathieu F. Use of beneficial bacteria and their secondary metabolites to control grapevine pathogen diseases. BioControl. 2012;58:435–455. doi: 10.1007/s10526-012-9479-6. [DOI] [Google Scholar]
- 7.Liang C., Yuan F., Liu F., Wang Y., Gao Y. Structure and antimicrobial mechanism of ε-polylysine—Chitosan conjugates through Maillard reaction. Int. J. Biol. Macromol. 2014;70:427–434. doi: 10.1016/j.ijbiomac.2014.07.012. [DOI] [PubMed] [Google Scholar]
- 8.Álvarez-Pérez J.M., González-García S., Cobos R., Olego M.Á., Ibañez A., Díez-Galán A., Garzón-Jimeno E., Coque J.J.R., Master E.R. Use of endophytic and rhizosphere actinobacteria from grapevine plants to reduce nursery fungal graft infections that lead to young grapevine decline. Appl. Environ. Microbiol. 2017;83:e01564-17. doi: 10.1128/AEM.01564-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dalmas F., Astarita L., DeFilippis L., Magel E., Fiedler H.P., Bauer R., Hampp R. Growth inhibition of an Araucaria angustifolia (Coniferopsida) fungal seed pathogen, Neofusicoccum parvum, by soil streptomycetes. BMC. Microbiol. 2013;13:168. doi: 10.1186/1471-2180-13-168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Andreolli M., Zapparoli G., Angelini E., Lucchetta G., Lampis S., Vallini G. Pseudomonas protegens MP12: A plant growth-promoting endophytic bacterium with broad-spectrum antifungal activity against grapevine phytopathogens. Microbiol. Res. 2019;219:123–131. doi: 10.1016/j.micres.2018.11.003. [DOI] [PubMed] [Google Scholar]
- 11.Daraignes L., Gerbore J., Yacoub A., Dubois L., Romand C., Zekri O., Roudet J., Chambon P., Fermaud M. Efficacy of P. oligandrum affected by its association with bacterial BCAs and rootstock effect in controlling grapevine trunk diseases. Biol. Control. 2018;119:59–67. doi: 10.1016/j.biocontrol.2018.01.008. [DOI] [Google Scholar]
- 12.Trotel-Aziz P., Abou-Mansour E., Courteaux B., Rabenoelina F., Clément C., Fontaine F., Aziz A. Bacillus subtilis PTA-271 counteracts Botryosphaeria dieback in grapevine, triggering immune responses and detoxification of fungal phytotoxins. Front. Plant Sci. 2019;10:25. doi: 10.3389/fpls.2019.00025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Silva-Castro I., Martín-García J., Diez J.J., Flores-Pacheco J.A., Martín-Gil J., Martín-Ramos P. Potential control of forest diseases by solutions of chitosan oligomers, propolis and nanosilver. Eur. J. Plant Pathol. 2017;150:401–411. doi: 10.1007/s10658-017-1288-4. [DOI] [Google Scholar]
- 14.Matei P.M., Martin-Ramos P., Sanchez-Bascones M., Hernandez-Navarro S., Correa-Guimaraes A., Navas-Gracia L.M., Rufino C.A., Ramos-Sanchez M.C., Martin-Gil J. Synthesis of chitosan oligomers/propolis/silver nanoparticles composite systems and study of their activity against Diplodia seriata. Int. J. Polym. Sci. 2015;2015:1–11. doi: 10.1155/2015/864729. [DOI] [Google Scholar]
- 15.Brugnerotto J., Lizardi J., Goycoolea F.M., Argüelles-Monal W., Desbrières J., Rinaudo M. An infrared investigation in relation with chitin and chitosan characterization. Polymer. 2001;42:3569–3580. doi: 10.1016/S0032-3861(00)00713-8. [DOI] [Google Scholar]
- 16.Umemura K., Kawai S. Modification of chitosan by the Maillard reaction using cellulose model compounds. Carbohydr. Polym. 2007;68:242–248. doi: 10.1016/j.carbpol.2006.12.014. [DOI] [Google Scholar]
- 17.Lambert C., Bisson J., Waffo-Téguo P., Papastamoulis Y., Richard T., Corio-Costet M.-F., Mérillon J.-M., Cluzet S. Phenolics and their antifungal role in grapevine wood decay: Focus on the Botryosphaeriaceae family. J. Agric. Food. Chem. 2012;60:11859–11868. doi: 10.1021/jf303290g. [DOI] [PubMed] [Google Scholar]
- 18.Olmo D., Gramaje D., Armengol J. Evaluation of fungicides to protect pruning wounds from Botryosphaeriaceae species infections on almond trees. Phytopathol. Mediterr. 2017;56:77–86. [Google Scholar]
- 19.Pitt W.M., Sosnowski M.R., Huang R., Qiu Y., Steel C.C., Savocchia S. Evaluation of fungicides for the management of Botryosphaeria canker of grapevines. Plant. Dis. 2012;96:1303–1308. doi: 10.1094/PDIS-11-11-0998-RE. [DOI] [PubMed] [Google Scholar]
- 20.Tennakoon K.M.S., Ridgway H.J., Jaspers M.V., Langford G., Eirian Jones E. Evaluation of fungicide efficacy against Neofusicoccum species causing dieback disease of blueberries in New Zealand. Australas. Plant Pathol. 2018;48:75–84. doi: 10.1007/s13313-018-0565-9. [DOI] [Google Scholar]
- 21.Torres C., Latorre B.A., Undurraga P., Besoain X. Evaluation of DMI fungicides against species of Diplodia and Neofusicoccum associated with Botryosphaeria canker of grapevine. Cienc. Investig. Agrar. 2013;40:131–138. doi: 10.4067/S0718-16202013000100011. [DOI] [Google Scholar]
- 22.Ahmad H., Rajagopal K., Shah A.H., Bhat A.H., Venugopal K. Study of bio-fabrication of iron nanoparticles and their fungicidal property against phytopathogens of apple orchards. IET. Nanobiotechnol. 2017;11:230–235. doi: 10.1049/iet-nbt.2015.0061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Khatami M., Nejad M.S., Almani P.G.N., Salari S. Plant-mediated green synthesis of silver nanoparticles using Trifolium resupinatum seed exudate and their antifungal efficacy on Neofusicoccum parvum and Rhizoctonia solani. IET. Nanobiotechnol. 2016;10:237–243. doi: 10.1049/iet-nbt.2015.0078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Khatami M., Mortazavi S.M., Kishani-Farahani Z., Amini A., Amini E., Heli H. Biosynthesis of silver nanoparticles using pine pollen and evaluation of the antifungal efficiency. Iran. J. Biotechnol. 2017;15:95–101. doi: 10.15171/ijb.1436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ammad F., Moumen O., Gasem A., Othmane S., Hisashi K.N., Zebib B., Merah O. The potency of lemon (Citrus limon L.) essential oil to control some fungal diseases of grapevine wood. C. R. Biol. 2018;341:97–101. doi: 10.1016/j.crvi.2018.01.003. [DOI] [PubMed] [Google Scholar]
- 26.Harada S., Okada J., Takeda M., Yamazaki T. Inclusion compounds of lankacidin-group antibiotics with cyclodextrins. J. Antibiot. 1985;38:877–885. doi: 10.7164/antibiotics.38.877. [DOI] [PubMed] [Google Scholar]
- 27.Hamman J.H. Chitosan based polyelectrolyte complexes as potential carrier materials in drug delivery systems. Mar. Drugs. 2010;8:1305–1322. doi: 10.3390/md8041305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wu Q.X., Lin D.Q., Yao S.J. Design of chitosan and its water soluble derivatives-based drug carriers with polyelectrolyte complexes. Mar. Drugs. 2014;12:6236–6253. doi: 10.3390/md12126236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cheung R., Ng T., Wong J., Chan W. Chitosan: An update on potential biomedical and pharmaceutical applications. Mar. Drugs. 2015;13:5156–5186. doi: 10.3390/md13085156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhang A., Mu H., Zhang W., Cui G., Zhu J., Duan J. Chitosan coupling makes microbial biofilms susceptible to antibiotics. Sci. Rep. 2013;3:3364. doi: 10.1038/srep03364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hyldgaard M., Mygind T., Vad B.S., Stenvang M., Otzen D.E., Meyer R.L. The antimicrobial mechanism of action of epsilon-poly-l-lysine. Appl. Environ. Microbiol. 2014;80:7758–7770. doi: 10.1128/AEM.02204-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ye R., Xu H., Wan C., Peng S., Wang L., Xu H., Aguilar Z.P., Xiong Y., Zeng Z., Wei H. Antibacterial activity and mechanism of action of ε-poly-l-lysine. Biochem. Biophys. Res. Commun. 2013;439:148–153. doi: 10.1016/j.bbrc.2013.08.001. [DOI] [PubMed] [Google Scholar]
- 33.Mitchell D.J., Steinman L., Kim D.T., Fathman C.G., Rothbard J.B. Polyarginine enters cells more efficiently than other polycationic homopolymers. Int. J. Pept. Res. Ther. 2000;56:318–325. doi: 10.1034/j.1399-3011.2000.00723.x. [DOI] [PubMed] [Google Scholar]
- 34.Mutschler A., Tallet L., Rabineau M., Dollinger C., Metz-Boutigue M.H., Schneider F., Senger B., Vrana N.E., Schaaf P., Lavalle P. Unexpected bactericidal activity of poly(arginine)/hyaluronan nanolayered coatings. Chem. Mater. 2016;28:8700–8709. doi: 10.1021/acs.chemmater.6b03872. [DOI] [Google Scholar]
- 35.Andreev K., Bianchi C., Laursen J.S., Citterio L., Hein-Kristensen L., Gram L., Kuzmenko I., Olsen C.A., Gidalevitz D. Guanidino groups greatly enhance the action of antimicrobial peptidomimetics against bacterial cytoplasmic membranes. Biochim. Biophys. Acta. 2014;1838:2492–2502. doi: 10.1016/j.bbamem.2014.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cutrona K.J., Kaufman B.A., Figueroa D.M., Elmore D.E. Role of arginine and lysine in the antimicrobial mechanism of histone-derived antimicrobial peptides. FEBS Lett. 2015;589:3915–3920. doi: 10.1016/j.febslet.2015.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ing L.Y., Zin N.M., Sarwar A., Katas H. Antifungal activity of chitosan nanoparticles and correlation with their physical properties. Int. J. Biomater. 2012;2012:1–9. doi: 10.1155/2012/632698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pertot I., Caffi T., Rossi V., Mugnai L., Hoffmann C., Grando M.S., Gary C., Lafond D., Duso C., Thiery D., et al. A critical review of plant protection tools for reducing pesticide use on grapevine and new perspectives for the implementation of IPM in viticulture. Crop. Protect. 2017;97:70–84. doi: 10.1016/j.cropro.2016.11.025. [DOI] [Google Scholar]
- 39.Di Marco S., Osti F., Mugnai L. First studies on the potential of a copper formulation for the control of leaf stripe disease within esca complex in grapevine. Phytopathol. Mediterr. 2011;50:S300–S309. [Google Scholar]
- 40.Darrieutort G., Lecomte P. Evaluation of a trunk injection technique to control grapevine wood diseases. Phytopathol. Mediterr. 2007;46:50–57. [Google Scholar]
- 41.Di Marco S., Mazzullo A., Calzarano F., Cesari A. The control of esca: Status and perspectives. Phytopathol. Mediterr. 2000;39:232–240. [Google Scholar]
- 42.Rolshausen P.E., Úrbez-Torres J.R., Rooney-Latham S., Eskalen A., Smith R.J., Gubler W.D. Evaluation of pruning wound susceptibility and protection against fungi associated with grapevine trunk diseases. Am. J. Enol. Vitic. 2010;61:113–119. [Google Scholar]
- 43.Roblin G., Luini E., Fleurat-Lessard P., Larignon P., Berjeaud J.M. Towards a preventive and/or curative treatment of esca in grapevine trunk disease: General basis in the elaboration of treatments to control plant pathogen attacks. Crop Protect. 2019;116:156–169. doi: 10.1016/j.cropro.2018.10.016. [DOI] [Google Scholar]
- 44.Bertsch C., Ramírez-Suero M., Magnin-Robert M., Larignon P., Chong J., Abou-Mansour E., Spagnolo A., Clément C., Fontaine F. Grapevine trunk diseases: Complex and still poorly understood. Plant Pathol. 2013;62:243–265. doi: 10.1111/j.1365-3059.2012.02674.x. [DOI] [Google Scholar]
- 45.Arjona-Girona I., Ruano-Rosa D., López-Herrera C.J. Identification, pathogenicity and distribution of the causal agents of dieback in avocado orchards in Spain. Span. J. Agric. Res. 2019;17:e1003. doi: 10.5424/sjar/2019171-13561. [DOI] [Google Scholar]
- 46.Moral J., Agustí-Brisach C., Pérez-Rodríguez M., Xaviér C., Raya M.C., Rhouma A., Trapero A. Identification of fungal species associated with branch dieback of olive and resistance of table cultivars to Neofusicoccum mediterraneum and Botryosphaeria dothidea. Plant. Dis. 2017;101:306–316. doi: 10.1094/PDIS-06-16-0806-RE. [DOI] [PubMed] [Google Scholar]
- 47.Nunn S., Nishikida K. Advanced ATR Correction Algorithm-Application Note 50581. Thermo Scientific; Madison, WI, USA: 2008. p. 4. [Google Scholar]
- 48.Santos-Moriano P., Fernandez-Arrojo L., Mengibar M., Belmonte-Reche E., Peñalver P., Acosta F.N., Ballesteros A.O., Morales J.C., Kidibule P., Fernandez-Lobato M., et al. Enzymatic production of fully deacetylated chitooligosaccharides and their neuroprotective and anti-inflammatory properties. Biocatal. Biotransform. 2017;36:57–67. doi: 10.1080/10242422.2017.1295231. [DOI] [Google Scholar]
- 49.Babiker E.E. Effect of chitosan conjugation on the functional properties and bactericidal activity of gluten peptides. Food. Chem. 2002;79:367–372. doi: 10.1016/S0308-8146(02)00188-7. [DOI] [Google Scholar]
- 50.Song Y., Babiker E.E., Usui M., Saito A., Kato A. Emulsifying properties and bactericidal action of chitosan–lysozyme conjugates. Food. Res. Int. 2002;35:459–466. doi: 10.1016/S0963-9969(01)00144-2. [DOI] [Google Scholar]
- 51.Sadigh-Eteghad S., Dehnad A., Shanebandi D., Khalili I., Razmarayii N., Namvaran A.J.V.R.C. Identification and characterization of a Streptomyces sp. isolate exhibiting activity against multidrug-resistant coagulase-negative Staphylococci. Vet. Res. Commun. 2011;35:477–486. doi: 10.1007/s11259-011-9491-9. [DOI] [PubMed] [Google Scholar]
- 52.Pazhanimurugan R., Radhakrishnan M., Shanmugasundaram T., Gopikrishnan V., Balagurunathan R. Terpenoid bioactive compound from Streptomyces rochei (M32): Taxonomy, fermentation and biological activities. World. J. Microbiol. Biotechnol. 2016;32:161. doi: 10.1007/s11274-016-2121-5. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.