Abstract
Staphylococcus aureus is a major human pathogen causing a wide range of nosocomial infections including pulmonary, urinary, and skin infections. Notably, the emergence of bacterial strains resistant to conventional antibiotics has prompted researchers to find new compounds capable of killing these pathogens. Nature is undoubtedly an invaluable source of bioactive molecules characterized by an ample chemical diversity. They can act as unique platform providing new scaffolds for further chemical modifications in order to obtain compounds with optimized biological activity. A class of natural compounds with a variety of biological activities is represented by alkaloids, important secondary metabolites produced by a large number of organisms including bacteria, fungi, plants, and animals. In this work, starting from the screening of 39 alkaloids retrieved from a unique in-house library, we identified a heterodimer β-carboline alkaloid, nigritanine, with a potent anti-Staphylococcus action. Nigritanine, isolated from Strychnos nigritana, was characterized for its antimicrobial activity against a reference and three clinical isolates of S. aureus. Its potential cytotoxicity was also evaluated at short and long term against mammalian red blood cells and human keratinocytes, respectively. Nigritanine showed a remarkable antimicrobial activity (minimum inhibitory concentration of 128 µM) without being toxic in vitro to both tested cells. The analysis of the antibacterial activity related to the nigritanine scaffold furnished new insights in the structure–activity relationships (SARs) of β-carboline, confirming that dimerization improves its antibacterial activity. Taking into account these interesting results, nigritanine can be considered as a promising candidate for the development of new antimicrobial molecules for the treatment of S. aureus-induced infections.
Keywords: natural products, alkaloids, plant secondary metabolites, β-carboline, Staphylococcus aureus, antimicrobial activity, cytotoxicity
This manuscript is dedicated to the memory of Professor Maurizio Botta (University of Siena, Department of Biotechnology, Chemistry and Pharmacy) who prematurely passed away on 2 August 2019. During his successfully scientific career, he synthesized a huge number of small bioactive molecules for the development of new pharmaceutical agents for cancer therapy and/or treatment of microbial infections, thus providing an invaluable contribution in the field of medicinal chemistry and drug discovery, worldwide.
1. Introduction
The discovery of antibiotics in the 1900s led to a medical revolution in the fight against bacterial infections. However, during the years, bacteria have developed different mechanisms to resist the killing activity of antibiotics [1]. The human pathogen Staphylococcus aureus is a microorganism with high adaptability and tenacity, as highlighted by its abundance in the environment and in the normal flora, the variety of virulence factors that it produces, and the capability to colonize various human organs such as nose, pharynx, and skin [2,3,4]. Furthermore, multidrug-resistant S. aureus is one of the major microorganisms causing bloodstream infections associated with high levels of morbidity and mortality worldwide [5]. Considering that S. aureus has successfully evolved numerous strategies to resist the activity of practically all antibiotics, new alternative compounds able to defeat S. aureus-induced infections are urgently needed [6]. Notably, a significant portion of the commercial drugs occurs in nature or is derived from natural products by means of chemical transformations or de novo synthesis [7]. Alkaloids are a group of important secondary metabolites which are produced by a wide variety of organisms including bacteria, fungi, plants, and animals. Chemically, alkaloids are a large and structurally diverse group of nitrogen-containing compounds (one or more nitrogen atoms within a heterocycle ring) [8]. Alkaloids can occur as monomers, dimers (bisalkaloids), trimers, or tetramers. According to their chemical structure, alkaloids are classified in heterocyclic alkaloids (also known as typical alkaloids), containing nitrogen in the heterocycle and originating from amino acids, and nonheterocyclic alkaloids (also known as atypical or proto-alkaloids), containing a nitrogen atom derived from an amino acid which is not a part of the heterocyclic ring [9]. Heterocyclic alkaloids are divided according to their ring structure in several classes of monomeric alkaloids (e.g., pyrrole, pyrrolidine, pyridine, piperidine, indole, quinoline, isoquinoline alkaloids). Since 1940, large-scale efforts have been made to evaluate the antibacterial effects of naturally occurring alkaloids. Several potent monomer and dimer alkaloids were identified, and synthetic modifications were investigated to improve their biological activity [8,9,10,11]. However, a tremendously wide discrepancy between their historical significance and their occurrence in modern drug development exists, and no alkaloids are available in the market as antibacterial drugs [12]. In this work, an in-house library of about 1000 natural products and their derivatives was used as a unique source of lead compounds to identify new potential antibacterial alkaloids. From the screening of all the alkaloids present in this library, the rare β-carboline heterodimer nigritanine was identified and showed a potent antistaphylococcal activity. Therefore, it was thoroughly characterized for its antimicrobial and cytotoxic activities.
2. Results and Discussion
2.1. Alkaloids Collection
Natural products remain the most productive source of leads in antibacterial drug discovery, often providing novel mechanism(s) and chemical structures as useful platforms for the development of drugs. A unique in-house library of about 1000 natural compounds, mostly isolated from several plants used in traditional medicine of South America and collected over the years, is available at the Organic Chemistry Laboratory of the Department of Chemistry and Technology of Drugs (Sapienza University of Rome, Italy). This library consists of natural products belonging to different classes of organic compounds which were previously published and fully characterized [13,14]. It was then enlarged by the addition of other natural small molecules from commercially available sources and synthetic or semi-synthetic derivatives. Currently, all components of our collection are incorporated into a virtual library, and their chemical and physicochemical features are analyzed by means of cheminformatics tools, showing a satisfactory chemical diversity. Therefore, our in-house library is a valid source of chemotypes for the modulation of biomolecular targets, and it was successfully screened in silico and in vitro for the identification of hit and lead compounds in previous early-stage drug discovery projects [15]. One of the largest and most intriguing classes of natural occurring compounds within the library are the alkaloids, which consist of isoquinoline (1–11), quinoline (12–15), and indole (16–39) alkaloids (Table 1).
Table 1.
List of alkaloids tested in this study.
| Mol. | Common Name | Chemical Structure | M.W. | Molecular Formula | Source | Ref. |
|---|---|---|---|---|---|---|
 Isoquinoline Alkaloids | ||||||
| 1 | Dihydroberberine·HCl | 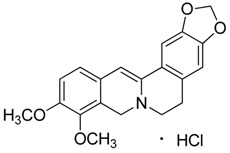 |
337.37 373.83 (+ HCl) |
C20H19NO4·HCl |
Berberis species: Berberis aristata, Berberis lyceum, Berberis petiolaris, Berberis tinctoria (Berberidaceae family) |
[16] |
| 2 | Bulbocapnine·HCl | 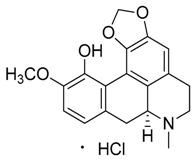 |
325.36 361.82 (+ HCl) |
C19H19NO4·HCl | Species: Corydalis cava (Papaveraceae family) |
[17] |
| 3 | Boldine | 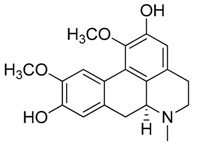 |
327.37 | C19H21NO4 | Species: Peumus boldus (Monimiaceae family) |
[18] |
| 4 | Cotarmine·HCl | 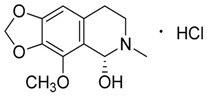 |
237.25 273.71 |
C12H15NO4·HCl | Synthetic | [19] |
| 5 | Chelidonine | 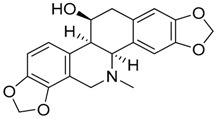 |
353.37 | C20H19NO5 | Species: Chelidonium majus L. (Papaveraceae family) |
[20] |
| 6 | Emetine·HCl | 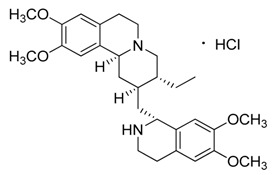 |
480.64 517.10 (+HCl) |
C29H40N2O4 | Species: Psychotria ipecacuanha Stokes (Rubiaceae family) |
[21] |
| 7 | (S)-Glaucine |  |
355.43 | C21H25NO4 | Species: Glaucium luteum L. (Papaveraceae family) |
[22] |
| 8 | Hydrastine |  |
383.39 | C21H21NO6 | Species: Hydrastis canadensis L. (Ranunculaceae family) |
[23] |
| 9 | Noscapine (Narcotine) |  |
413.42 | C22H23NO7 | Species: Papaver somniferum (Papaveraceae family) |
[24] |
| 10 | Papaverine |  |
339.39 | C20H21NO4 | Species: P. somniferum (Papaveraceae family) |
[24] |
| 11 | Tubocurarine Chloride·HCl | 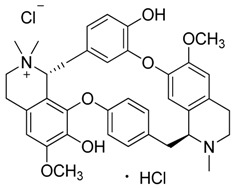 |
609.73 681.65 (+Cl-+HCl) |
C37H41N2O6·HCl + Cl- | Species: Liana Chondrodendron (Menispermaceae family) |
[25] |
 Quinoline Alkaloids | ||||||
| 12 | Cinchonine | 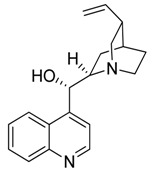 |
294.39 | C19H22N2O | Species: Cinchona ledgeriana, Remijia peruviana (Rubiaceae family) |
[26] |
| 13 | Kokusaginine | 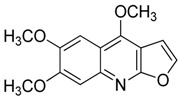 |
259.26 | C14H13NO4 | Species: Esenbeckia leiocarpa (Rutaceae family) |
[27] |
| 14 | Maculine | 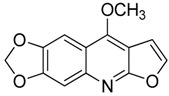 |
243.21 | C13H9NO4 | Species: E. leiocarpa (Rutaceae family) |
[27] |
| 15 | 4-methoxy-2-(1-ethylpropyl)-quinoline | 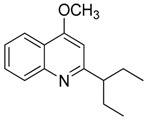 |
229.32 | C15H19NO | Species: E. leiocarpa (Rutaceae family) |
[27] |
 Indole Alkaloids | ||||||
| 16 | Aspidospermine | 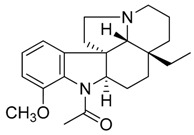 |
354.49 | C22H30N2O2 |
Aspidosperma species: Aspidosperma album, Aspidosperma australe, Aspidosperma exalatum, Aspidosperma peroba, Aspidosperma polyneuron, Aspidosperma pyricollum, Aspidosperma pyrifolium, Aspidosperma quebracho-blanco, Aspidosperma quirandy, Aspidosperma sessiflorum, Aspidosperma rhombeosignatum (Apocynaceae family) |
[28] |
| 17 | Brucine |  |
394.47 | C23H26N2O4 | Species: Strychnos nux-vomica (Apocynaceae family) |
[29] |
| 18 | Diaboline |  |
353.41 | C21H23NO4 | Species: Strychnos castelneana (Loganiaceae family) |
[27] |
| 19 | Physostigmine (Eserine) |  |
275.35 | C15H21N3O2 |
Physostigma venenosum (Fabaceae family) |
[30] |
| 20 | Holstiine |  |
382.45 | C22H26N2O4 | Species: Strychnos henningsii Gilg (Loganiaceae family) |
[31] |
| 21 | Pseudobrucine |  |
410.46 | C23H26N2O5 | Species: S. nux-vomica (Loganiaceae family) |
[29] |
| 22 | Retuline |  |
338.44 | C21H26N2O2 |
Strychnos species: Strychnos camptoneura, S. henningsii (Loganiaceae family) |
[31] |
| 23 | Serotonin |  |
176.22 | C10H12N2O | Species: Laphophora williamsii (Cactaceae family) |
[32] |
| 24 | Triptamine·HCl |  |
160.22 196.68 (+HCl) |
C10H12N2 HCl |
Acacia species (Fabacee family) |
[33] |
| 25 | Vomicine·HC1 | 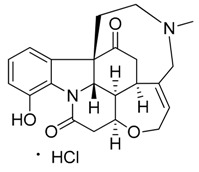 |
380.44 416.90 (+HCl) |
C22H24N2O4 HCl |
Strychnos icaja (Loganiaceae family) |
[27,31] |
| 26 | Vindoline | 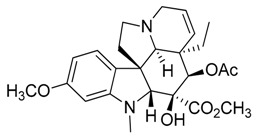 |
456.53 | C25H32N2O6 |
Catharanthus roseus (Apocynaceae family) |
[34] |
 Carboline Alkaloids (Indole Subclass) | ||||||
| 27 | Akagerine | 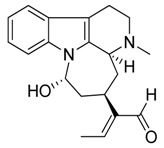 |
324.42 | C20H24N2O2 |
Strychnos species: Strychnos barteri Solered, S. camptoneurine, Strychnos nigritana Bak (Loganiaceae family) |
[31] |
| 28 | Canthin-6-one | 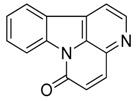 |
220.23 | C14H8N2O | Species: Simaba ferruginea (Simaroubaceae family) |
[35] |
| 29 | α-Carboline | 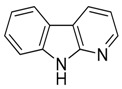 |
168.19 | C11H8N2 | Synthetic | [36] |
| 30 | Harmane | 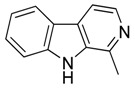 |
182.22 | C12H10N2 | Species: Chimarrhis turbinata, Ophiorrhiza communis, Ophiorrhiza liukiuensis, Ophiorrhiza tomentosa, Psychotria barbiflora (Rubiaceae family) |
[26] |
| 31 | Norharmane | 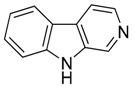 |
168.19 | C11H8N2 | Species: Hygrophorus eburneus (Tricholomataceae family) |
[37] |
| 32 | Harmine | 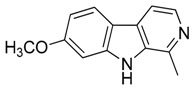 |
212.25 | C13H12N2O | Species: Banisteriopsis caapi (Malpighiaceae family), Grewia bicolor (Malvaceae family), Passiflora edulis f. flavicarpa O. Deg., Passiflora incarnata L. (Passifloraceae family), Tribulus terrestris L., Peganum harmala L. (Zygophyllaceae family) |
[37] |
| 33 | Ibogaine | 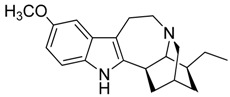 |
310.43 | C20H26N2O | Species: Tabernanthe iboga (Apocynaceae family) |
[38] |
| 34 | Mitragynine | 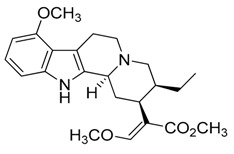 |
398.50 | C23H30N2O4 | Species: Mitragyna speciosa (Rubiaceae family) |
[39] |
| 35 | Nigritanine | 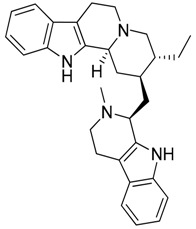 |
452.63 | C30H36N4 |
Strychnos species: Strychnos borteri, S. nigritana Bak. (Loganiaceae family) |
[31] |
| 36 | Paynantheine | 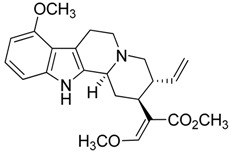 |
396.48 | C23H28N2O4 | Species: M. speciosa (Rubiaceae family) |
[40] |
| 37 | Rhynchophylline | 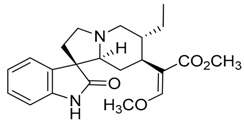 |
384.47 | C22H28N2O4 | Species: M. speciosa, Uncaria rhynchophylla (Rubiaceae family) |
[40] |
| 38 | Speciociliatine | 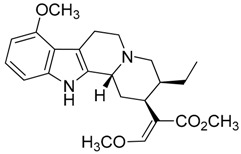 |
398.50 | C23H30N2O4 | Species: M. speciosa (Rubiaceae family) |
[41] |
| 39 | Yohimbine·HCl | 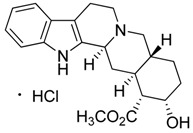 |
354.44 390.90 (+HCl) |
C21H26N2O3 HCl |
Apocynaceae species: Aspidosperma discolor A. DC., Aspidosperma excelsum Benth, Aspidosperma eburneum F. Allem, Aspidosperma marcgravianum Woodson, Aspidosperma oblongum A. DC. |
[37] |
Mol.: molecule number; M.W.: molecular weight; Ref.: references.
Considering the chemical structures of isoquinoline alkaloids, this group can be divided in two major categories: simple isoquinolines, which are composed of a benzene ring fused to a pyridine ring, and benzylisoquinolines, which contain a second aromatic ring [42]. In contrast, indole alkaloids are bicyclic structures consisting of a six-membered benzene ring fused to a five-membered nitrogen-containing pyrrole ring and are among the most numerous (at least 4100 known molecules) and complex alkaloids. In our library, the group of indole alkaloids covers several subclasses, the largest of which is represented by β-carbolines featuring a common tricyclic pyrido[3,4-b]indole ring structure (Table 1). According to the saturation of the N-containing six-membered ring, β-carbolines are categorized in fully aromatic (βCs), dihydro- (DHβCs), and tetrahydro- (THβCs) β-carbolines [43]. Alkaloids from the in-house library belonging to more than 10 plant families (e.g., Apocynaceae, Loganiaceae, Berberidaceae, Papaveraceae, Rubiaceae) are known to occur in several species (Table 1). With the aim to identify new potential antistaphylococcal agents, the in-house library of alkaloids was initially screened towards Gram(+) and Gram(-) reference bacterial strains. For the biological characterization, all the compounds were dissolved in dimethyl sulfoxide (DMSO).
2.2. Antimicrobial Activity
2.2.1. Inhibition Zone Assay
The antimicrobial activity of all collected compounds was initially tested on a reference strain of the Gram(+) S. aureus (S. aureus ATCC 25923) by the inhibition zone assay. The Gram(-) bacterial strain Escherichia coli ATCC 25922 was also included for comparison (Table 2). Most of the compounds resulted to be inactive against both classes of bacteria (data not shown). The only exception was the already characterized methylated derivative of β-carboline, i.e., harmane, which was able to inhibit the growth of both S. aureus and E. coli strains in an agar diffusion assay [44,45,46], with diameters of the inhibition zone of 4.36 and 8.46 mm, respectively (Table 2).
Table 2.
Diameters of the inhibition zone of all the active tested compounds against the reference Gram(+) and Gram(-) bacterial strains.
| Inhibition Zone Assay | Diameter of Inhibition Zone (mm) 1 | |
|---|---|---|
| Compound | Gram-Positive Staphylococcus aureus ATCC 25923 |
Gram-Negative Escherichia coli ATCC 25922 |
| Dihydroberberine·HCl (1) | 7.800 | n.a. |
| (S)-Glaucine (7) | 7.600 | n.a. |
| Canthin-6-one (28) | 6.100 | n.a. |
| Harmane (30) | 4.360 | 8.640 |
| Harmine (32) | n.a. | 6.250 |
| Mytragine (34) | 5.420 | n.a. |
| Nigritanine (35) | 10.39 | n.a. |
| Paynantheine (36) | 8.440 | n.a. |
| Speciociliatine (38) | 8.240 | n.a. |
1 Data represent the mean of three independent experiments with standard deviation (SD) not exceeding 0.2; n.a.: not active.
Interestingly, a greater selectivity towards the human pathogen S. aureus was noted, especially for the β-carboline alkaloids. In fact, the rare heterodimer alkaloid nigritanine (compound 35) as well as some of its analogues (i.e., speciociliatine, mytragine, and paynantheine) showed a powerful activity against the reference strain of S. aureus ATCC 25923, with a diameter of growth inhibition zone ranging from 8.24 to 10.39 mm. Mytragine was previously characterized for its selective anti-Gram(+) efficacy [47]; in contrast, no microbiological data have been provided so far for speciociliatine, paynantheine, and rhyncophylline. Because of these reasons, we decided to examine the activity of nigritanine, mytragine, and the other three abovementioned molecules against three multidrug-resistant clinical isolates of S. aureus. As reported in Table 3, nigritanine was the sole compound that retained a potent activity against the clinical isolates of S. aureus (i.e., S. aureus 1a, 1b, 1c). This was indicated by the similar diameters of inhibition zones. In contrast, all the others molecules completely or almost completely lost their activity towards S. aureus 1a, 1b, and 1c strains.
Table 3.
Diameters of the inhibition zone of some active alkaloids against the reference and clinical isolates of S. aureus strains.
| Inhibition Zone Assay | Diameter of Inhibition Zone (mm) 1 | |||
|---|---|---|---|---|
| Compound | S. aureus ATCC 25923 | S. aureus 1a | S. aureus 1b | S. aureus 1c |
| Mytragine (34) | 5.420 | 4.000 | n.a. | n.a. |
| Nigritanine (35) | 10.39 | 11.20 | 8.440 | 9.100 |
| Paynantheine (36) | 8.440 | 3.800 | n.a. | n.a. |
| Speciociliatine (38) | 8.240 | 4.520 | 4.340 | 4.580 |
1 Data represent the mean of three independent experiments with SD not exceeding 0.2.
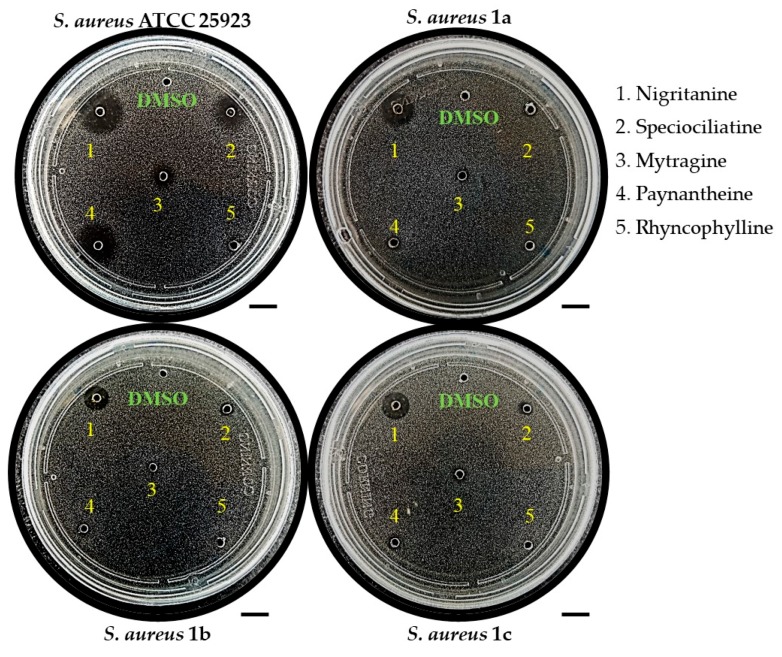
A representative image of the antibacterial activity of these compounds is shown in Figure 1. The growth inhibition zone of nigritanine (zone #1) is clearly evident compared to that of the other alkaloids tested. These results are in line with other published data of alkaloids extracted from Anabasis articulata, showing a potent anti-Gram(+) activity when evaluated by the inhibition zone assay [48].
Figure 1.
Representative image of the inhibition zone assays of nigritanine (35) and some other alkaloids against the reference strain and the three clinical isolates of S. aureus. Scale bars represent 1 cm.
2.2.2. Determination of the Minimum Inhibitory Concentration
The antibacterial activity of nigritanine, speciociliatine, mytragine, paynantheine, and rhyncophylline was also evaluated by the microdilution broth assay to determine the minimum inhibitory concentration (MIC) against the reference strain of S. aureus and the three clinical isolates after 16 hours of treatment (Table 4). Remarkably, although the MIC of nigritanine against the reference strain of S. aureus was higher than the MIC of other alkaloids reported in the literature (i.e., 128 μM, 56.5 μg/mL versus 2–16 μg/mL for vincamine, atropine, allantoin, or trigonelline [49]), the ability to inhibit the growth of the clinical isolates was maintained at the same concentration of 128 μM. This is in contrast with what observed for the aforementioned compounds which completely lost activity when tested against clinical isolates [49]. Similar MIC values were also obtained with alkaloids from leaves of Eclipta alba [50].
Table 4.
Minimum inhibitory concentration (MIC) (μM) of nigritanine, speciociliatine, mytragine, paynantheine, and rhyncophylline against the reference and clinical isolates of S. aureus strains. MICs are the values obtained from three identical readings out of four independent experiments.
| Strains | Nigritanine | Speciociliatine | Mytragine | Paynantheine | Rhyncophylline |
|---|---|---|---|---|---|
| S. aureus ATCC 25923 | 128 μM | > 256 μM | > 256 μM | > 256 μM | > 256 μM |
| S. aureus 1a | 128 μM | > 256 μM | > 256 μM | > 256 μM | > 256 μM |
| S. aureus 1b | 128 μM | > 256 μM | > 256 μM | > 256 μM | > 256 μM |
| S. aureus 1c | 128 μM | > 256 μM | > 256 μM | > 256 μM | > 256 μM |
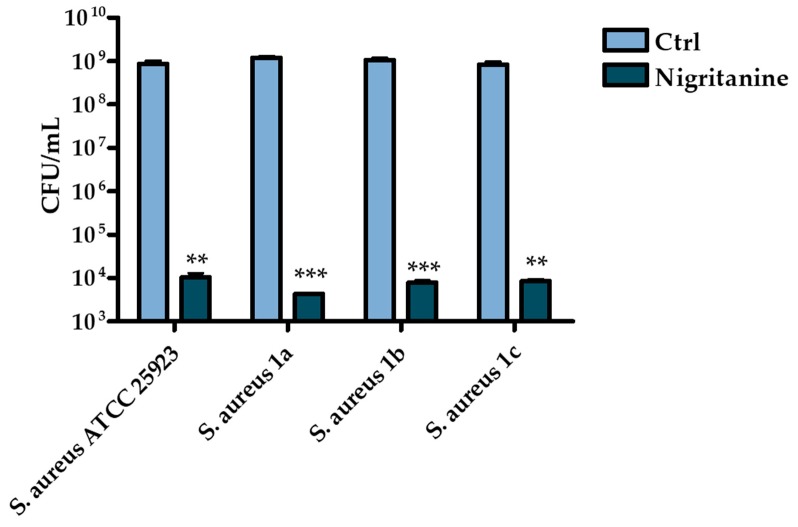
Notably, the MICs of nigritanine were found to correspond to the minimum bactericidal concentration (MBC) which is defined as the minimum concentration of drug causing ≥3 log killing of bacteria after 16 hours of incubation. Indeed, about five log reduction in the number of viable cells of the reference and clinical isolates of S. aureus were detected after treatment with nigritanine at its MIC (i.e., 128 μM) (Figure 2). Note that other plant alkaloid extracts had similar or even higher MIC and MBC values against S. aureus and other Gram(+) bacterial strains. For example, alkaloid extracts from the aerial part of Sida acuta gave MIC and MBC values ranging from 80 to >400 μg/mL against Staphylococcus strains [51], while the MIC of alkaloid extracts of Mahonia aquifolium ranged from 100 to 500 μg/mL against Staphylococcus epidermidis and Staphylococcus hominis strains [52]. Very high MIC values (>500 μg/mL) were obtained with alkaloids isolated from aerial parts of Hypecoum erectum L. (i.e., protopine and allocryptopine) against S. aureus, Bacillus cereus, and Bacillus subtilis strains [53]. Since alkaloids extracts with MICs ranging from 100 to 1000 µg/mL are considered to be compounds endowed with antimicrobial activity [54,55], nigritanine (MIC = 56.5 μg/mL) would represent a highly potential antimicrobial molecule.
Figure 2.
Reduction in the number of viable bacterial cells (evaluated by colony forming unit (CFU) counting) of the reference and clinical isolates of S. aureus strains after 16 hours treatment with nigritanine at the MIC (128 μM) compared to control (Ctrl) samples consisting in vehicle-treated bacterial cells. The data represent the mean of three independent experiments ± SD. The levels of statistical significance versus the Ctrl samples were p < 0.01 (**); p < 0.001 (***).
2.3. Structure–Activity Relationships (SARs) of Nigritanine for Its Antibacterial Activity
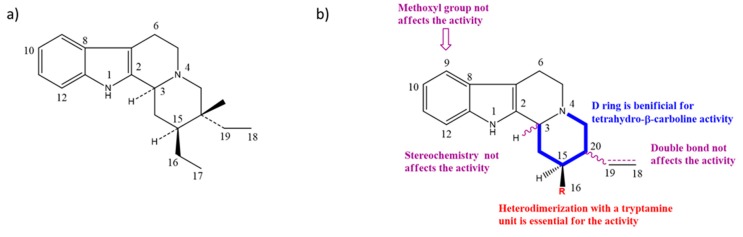
Taking into account all these microbiological data, the new β-carboline alkaloid nigritanine (35) emerged as a promising antibacterial agent against S. aureus. Nigritanine is a rare β-carboline heterodimer from different African Strichnos species. In particular, the interest for the Strichnos genus, due to the large variety of alkaloids and their use in traditional medicine, led Nicoletti et al. to isolate compound 35, along with other alkaloids, from the leaves of Strichnos nigritana Bak and from the stem bark of Strichnos barteri Solered, two species rather common in West Africa. From a chemical standpoint, nigritanine is a heterodimer alkaloid formed by the union of a corynane (Figure 3a) and a tryptamine unit. Interestingly, this corynane heterodimer displays a substantially higher antibacterial activity than the monomeric analogs, confirming the trend observed for the β-carboline homodimer [43,56]. The structure–activity relationships (SARs) were investigated for nigritanine (35) and its monomeric analogs (Figure 3b). Accordingly, the analysis of the antibacterial activity related to the corynane scaffolds indicated that: (1) the tetrahydro-β-carboline scaffold exhibits good activity; (2) the methoxyl group at C9 position, the double bond at C19–C18, and the stereochemistry of C3 and C20 do not affect the activity; (3) the corynane heterodimer shows a substantially higher activity than the monomeric analogs, highlighting that the presence of the tryptamine unit is essential. Notably, for β-carboline indoles, dimerization improves the antibacterial activity possibly because the larger molecule is less susceptible to bacterial efflux [43].
Figure 3.
(a) Chemical structure of corynane. (b) Structure–activity relationship (SARs) analysis of tetrahydro-β-carboline alkaloids with respect to antibacterial activity.
2.4. Cytotoxicity
2.4.1. Hemolytic Assay
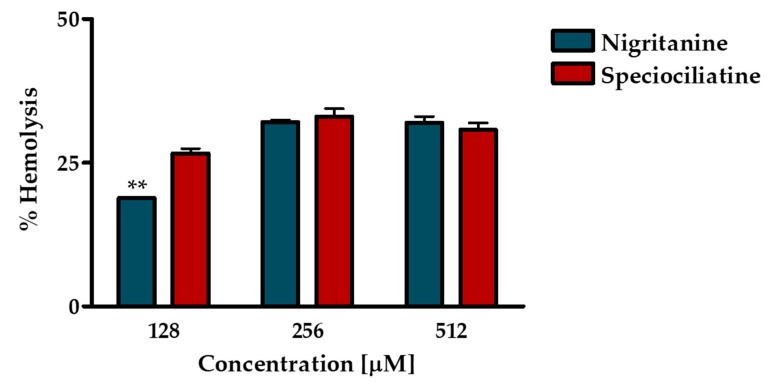
The short-term cytotoxic effect of nigritanine was tested against mammalian red blood cells after 40 minutes treatment at its MIC (128 μM), 2 × MIC (256 μM), and 4 × MIC (512 μM). The least active compound speciociliatine was tested at the same concentrations for comparison. As reported in Figure 4, both compounds displayed a weak toxic effect, causing about 30% hemolysis at the highest concentrations, while nigritanine gave rise to about 20% lysis of erythrocytes at its active concentration (128 μM). These results confirmed the potential safety of nigritanine in mammalian cells at a short term.
Figure 4.
Hemolytic activity of nigritanine at 128 μM (MIC), 256 μM (2 × MIC), and 512 μM (4 × MIC) after 40 minutes of treatment compared to the least active speciociliatine. The data represent the mean ± standard error of the mean (SEM) of three independent experiments. The level of statistical significance between the two compounds was p < 0.01 (**).
2.4.2. Cytotoxic Effect on HaCaT Cells
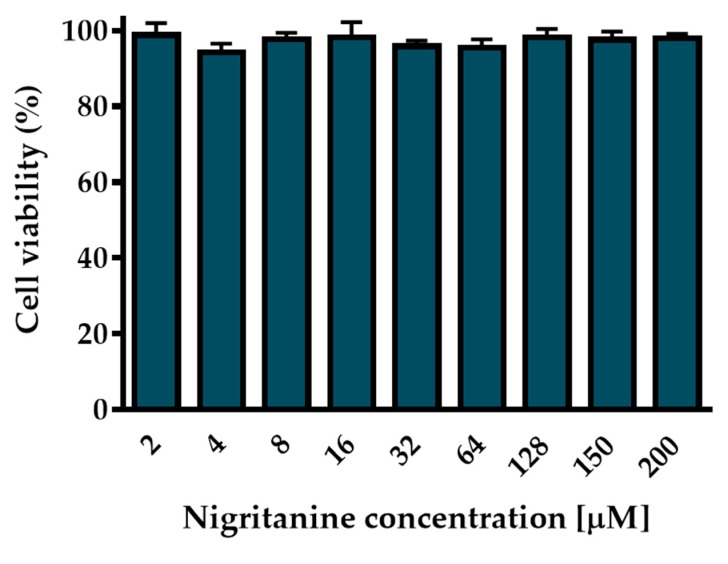
Since the most common site of S. aureus infection is the skin, and keratinocytes represent the major cell type in the epidermis [57], the long-term cytotoxic effect of nigritanine was evaluated by the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay on human immortalized keratinocytes (HaCaT) after 24 h treatment. As indicated in Figure 5, nigritanine did not induce any marked reduction in the percentage of viable keratinocytes after 24 h incubation at a concentration range between 2 μM and 200 μM. Note that other natural alkaloids were cytotoxic at much lower concentrations than 2 μM [49,58,59], which contrasts with the maximum non-toxic concentration tested for nigritanine (200 μM = 88.3 μg/mL). Moreover, the MICs of nigritanine (35) against reference and clinical isolates of S. aureus were equal to 128 μM. These results support the use of this compound as an antibacterial agent harmless to mammalian cells at its active antibacterial concentration.
Figure 5.
Effect of nigritanine on the viability of HaCaT cells determined by the MTT assay. Cell viability is expressed as a percentage with respect to the control. All data are the means of three independent experiments ± SEM.
3. Conclusions
With the increasing occurrence of multidrug-resistant bacterial infections and the low number of new antimicrobial agents on the market, the discovery of new natural compounds with antibiotic action is extremely necessary. In this work, we characterized the antibacterial profile of the heterodimer alkaloid nigritanine (isolated in the 1980s from Strychnos species), against S. aureus strains including clinical isolates. Interestingly, nigritanine resulted to have a potent anti-staphylococcal activity without being toxic to mammalian red blood cells and human keratinocytes at its active concentration. More importantly, it retained its antibacterial activity against three multidrug-resistant clinical isolates of S. aureus, a feature that was not observed for the other tested carboline alkaloids. Thus, the heterodimer alkaloid nigritanine has emerged as a promising scaffold for the design and development of potent and selective antibacterial compounds with low cytotoxicity.
4. Material and Methods
4.1. Chemistry
All the tested compounds (namely, 1–39) are known structures belonging to our in-house library of natural products. The chemical identity of compounds was assessed by re-running Nuclear magnetic resonance spectroscopy (NMR) experiments and proved to be in agreement with the literature data reported below for each compound. The purity of all compounds, checked by reversed-phase High Performance Liquid Chromatography (HPLC), was always higher than 95%.
Compound 1 (dihydroberberine hydrochloride or 9,10-dimethoxy-6,8-dihydro-5H-[1,3]dioxolo[4,5-g]isoquinolino[3,2-a]isoquinoline hydrochloride) was purchased from Fluka (CAS: 483-15-8, St. Louis, MO, USA) and used without further purification.
Compound 2 (bulbocapnine hydrochloride or (S)-11-methoxy-7-methyl-6,7,7a,8-tetrahydro-5H-[1,3]dioxolo[4’,5’:4,5]benzo[1,2,3-de]benzo[g]quinolin-12-ol hydrochloride) was purchased from Sigma-Aldrich (CAS: 632-47-3, St. Louis, MO, USA) and used without further purification.
Compound 3 (boldine or (6aS)-1,10-dimethoxy-6-methyl-5,6,6a,7-tetrahydro-4H-dibenzo[de,g]quinoline-2,9-diol) was purchased from Sigma-Aldrich (CAS: 476-70-0, St. Louis, MO, USA) and used without further purification.
Compound 4 (cotarmine hydrochloride or (R)-4-methoxy-6-methyl-5,6,7,8-tetrahydro-[1,3]dioxolo[4,5-g]isoquinolin-5-ol) was purchased from MolPort (CAS: 82-54-2, Beacon, NY, USA) and used without further purification.
Compound 5 (chelidonine or (5bR,6S,12bS)-13-Methyl-5b,6,7,12b,13,14-hexahydro[1,3]dioxolo[4’,5’:4,5]benzo[1,2-c][1,3]dioxolo[4,5-i]phenanthridin-6-ol) was purchased from Sigma-Aldrich (CAS: 476-32-4, St. Louis, MO, USA) and used without further purification.
Compound 6 (emetine hydrochloride or (2S,3R,11bS)-2-(((R)-6,7-dimethoxy-1,2,3,4-tetrahydroisoquinolin-1-yl)methyl)-3-ethyl-9,10-dimethoxy-2,3,4,6,7,11b-hexahydro-1H-pyrido[2,1-a]isoquinoline hydrochloride) was purchased from MolPort (CAS: 14198-59-5, Beacon, NY, USA) and used without further purification.
Compound 7 ((S)-Glaucine or (6aS)-1,2,9,10-tetramethoxy-6-methyl-5,6,6a,7-tetrahydro-4H-dibenzo[de,g]quinoline) was purchased from MolPort (CAS: 475-81-0, Beacon, NY, USA) and used without further purification.
Compound 8 (hydrastine or (R)-6,7-dimethoxy-3-((R)-6-methyl-5,6,7,8-tetrahydro-[1,3]dioxolo[4,5-g]isoquinolin-5-yl)isobenzofuran-1(3H)-one) was purchased from Sigma-Aldrich (CAS: 118-08-1, St. Louis, Mo., USA) and used without further purification.
Compound 9 (noscapine or narcotine or (3S)-6,7-dimethoxy-3-((5R)-4-methoxy-6-methyl-5,6,7,8,9,9a-hexahydro-[1,3]dioxolo[4,5-g]isoquinolin-5-yl)isobenzofuran-1(3H)-one) was purchased from Sigma-Aldrich (CAS: 128-62-1, St. Louis, MO, USA) and used without further purification.
Compound 10 (papaverine or (6,7-dimethoxyisoquinolin-1-yl)(3,4-dimethoxyphenyl)methanone) was purchased from MolPort (CAS: 58-74-2, Beacon, NY, USA) and used without further purification.
Compound 11 (tubocurarine chloride hydrochloride or (1S,16R)-10,25-dimethoxy-15,15,30-trimethyl-7,23-dioxa-30-aza-15-azoniaheptacyclo[22.6.2.23,6.18,12.118,22.027,31.016,34]hexatriaconta-3(36),4,6(35),8(34),9,11,18(33),19,21,24,26,31-dodecaene-9,21-diol chloride hydrochloride) showed NMR spectra identical to those reported in the literature [25].
Compound 12 (cinchonine or (S)-quinolin-4-yl((1S,2R,4S,5R)-5-vinylquinuclidin-2-yl)methanol) was purchased from Sigma-Aldrich (CAS: 118-10-5, St. Louis, MO, USA) and used without further purification.
Compound 13 (kokusaginine or 4,6,7-trimethoxyfuro[2,3-b]quinoline) showed NMR spectra identical to those reported in the literature [27].
Compound 14 (maculine or 9-methoxy-[1,3]dioxolo[4,5-g]furo[2,3-b]quinoline) showed NMR spectra identical to those reported in the literature [27].
Compound 15 (4-methoxy-2-(1-ethylpropyl)-quinoline) showed NMR spectra identical to those reported in the literature [27].
Compound 16 (aspidospermine or 1-((3aR,5aR,10bR,12bR)-3a-Ethyl-7-methoxy-2,3,3a,5,5a,11,12,12b-octahydro-1H,4H-6,12a-diaza-indeno[7,1-cd]fluoren-6-yl)-ethanone) showed NMR spectra identical to those reported in the literature [28].
Compound 17 (brucine or (4aR,5aS,8aR,15bR)-10,11-dimethoxy-4a,5,5a,7,8,13a,15,15a,15b,16-decahydro-2H-4,6-methanoindolo[3,2,1-ij]oxepino[2,3,4-de]pyrrolo[2,3-h]quinolin-14-one) was purchased from Sigma-Aldrich (CAS: 357-57-3, St. Louis, MO, USA) and used without further purification.
Compound 18 (diaboline or (4bR,7aS,8aR,13R,13aR,13bR)-13-hydroxy-5,6,7a,8,8a,11,13,13a,13b,14-decahydro-7,9-methanoindeno[1,2-d]oxepino[3,4-f]indole-14-carboxylic acid) showed NMR spectra identical to those reported in the literature [60].
Compound 19 (physostigmine or eserine or ((3aR,8bS)-3,4,8b-trimethyl-2,3a-dihydro-1H-pyrrolo[2,3-b]indol-7-yl) N-methylcarbamate) was purchased from MolPort (CAS: 57-47-6, Beacon, NY, USA) and used without further purification.
Compound 20 (holstiine or (15Z)-15-Ethylidene-10-hydroxy-17-methyl-11-oxa-8,17-diazapentacyclo[12.5.2.11,8.02,7.013,22]docosa-2,4,6-triene-9,20-dione) showed NMR spectra identical to those reported in the literature [61].
Compound 21 (pseudobrucine or (4aR,4a1R,5aR,8aS,8a1S,15aS)-5a-hydroxy-10,11-dimethoxy-4a1,5,5a,7,8,8a1,15,15a-octahydro-2H-4,6-methanoindolo[3,2,1-ij]oxepino[2,3,4-de]pyrrolo[2,3-h]quinolin-14(4aH)-one) was purchased from Fisherpharma (CAS: 560-30-5, Beacon, NY, USA) and used without further purification.
Compound 22 (retuline or 1-((3aS,5R,6S,6aS,11bR,E)-12-ethylidene-6-(hydroxymethyl)-1,2,4,5,6,6a-hexahydro-3,5-ethanopyrrolo[2,3-d]carbazol-7(3aH)-yl)ethanone) showed NMR spectra identical to those reported in the literature [62].
Compound 23 (serotonin or 3-(2-aminoethyl)-1H-indol-5-ol) was purchased from Sigma-Aldrich (CAS: 50-67-9, St. Louis, MO, USA) and used without further purification.
Compound 24 (triptamine hydrochloride or 2-(1H-indol-3-yl)ethan-1-amine hydrochloride) was purchased from Sigma-Aldrich (CAS: 343-94-2, St. Louis, MO, USA) and used without further purification.
Compound 25 (vomicine hydrochloride or (4aR,4a1R,6aS,6a1S,13aS)-10-hydroxy-16-methyl-4a,5,13,13a-tetrahydro-2H-6a,4-(ethanoiminomethano)indolo[3,2,1-ij]oxepino[2,3,4-de]quinoline-6,12(4a1H,6a1H)-dione hydrochloride) was purchased from MolPort (5969-84-6, Beacon, NY, USA) and used without further purification.
Compound 26 (vindoline or (3aR,3a1R,4R,5S,5aR,10bR)-methyl 4-acetoxy-3a-ethyl-5-hydroxy-8-methoxy-6-methyl-3a,3a1,4,5,5a,6,11,12-octahydro-1H-indolizino[8,1-cd]carbazole-5-carboxylate) was purchased from MolPort (CAS: 2182-14-1, Beacon, NY, USA) and used without further purification.
Compound 27 (akagerine or (E)-2-((3aS,5R,7S)-7-hydroxy-3-methyl-1,2,3,3a,4,5,6,7-octahydro-3,7a-diazacyclohepta[jk]fluoren-5-yl)but-2-enal) showed NMR spectra identical to those reported in the literature [63].
Compound 28 (canthin-6-one or 6H-indolo[3,2,1-de][1,5]naphthyridin-6-one) was purchased from MolPort (CAS: 479-43-6, Beacon, NY, USA) and used without further purification.
Compound 29 (α-carboline or 9H-pyrido[2,3-b]indole) was purchased from MolPort (CAS: 244-76-8, Beacon, NY, USA) and used without further purification.
Compound 30 (harmane or 1-methyl-9H-pyrido[3,4-b]indole) was purchased from Sigma-Aldrich (CAS: 486-84-0, St. Louis, MO, USA) and used without further purification.
Compound 31 (norharmane or 9H-pyrido[3,4-b]indole) was purchased from Sigma-Aldrich (CAS Number: 244-63-3, St. Louis, MO, USA) and used without further purification.
Compound 32 (harmine or 7-methoxy-1-methyl-9H-pyrido[3,4-b]indole) was purchased from Sigma-Aldrich (CAS: 442-51-3, St. Louis, MO, USA) and used without further purification.
Compound 33 (ibogaine or (6R,7S,11S)-7-ethyl-2-methoxy-6,6a,7,8,9,10,12,13-octahydro-5H-6,9-methanopyrido[1’,2’:1,2]azepino[4,5-b]indole) showed NMR spectra identical to those reported in the literature [38].
Compound 34 (mitragynine or (E)-methyl 2-((2S,3S,12bS)-3-ethyl-8-methoxy-1,2,3,4,6,7,12,12b-octahydroindolo[2,3-a]quinolizin-2-yl)-3-methoxyacrylate) was purchased from BOC Sciences (CAS: 4098-40-2, Shirley, NY, USA) and used without further purification.
Compound 35 (nigritanine or (2S,3R,12bS)-3-ethyl-2-(((S)-2-methyl-2,3,4,9-tetrahydro-1H-pyrido[3,4-b]indol-1-yl)methyl)-1,2,3,4,6,7,12,12b-octahydroindolo[2,3-a]quinolizine) showed NMR spectra identical to those reported in the literature [64]. The chemical characterization of compound 35 is reported below: Brown solid, m.p. 202-204°C; 1H-NMR (CDCl3, 400 MHz): 0.93 (t, J = 7 Hz, 3H, Me-C), 2.46 (s, 3H, Me-N), 3.56 (m, 1H, H-3), 6.40 (s, 1H, H-1 or H-1’), 7.80 (s, 1H, H-1 or H-1’); 13C-NMR (CDCl3, 101 MHz): 11.2 (C18), 20.7 (C6’), 21.5 (C6), 23.7 (C19), 41.9 (C20), 42.7 (N-CH3), 34.9 (C14), 35.0 (C16), 35.8 (C15), 51.3 (C5’), 53.0 (C5), 58.6 (C17), 59.1 (C3), 60.2 (C21), 107.1 (C7), 108.8 (C7’), 110.8 (C12), 110.9 (C12’), 117.5 (C9 or C9’), 117.7 (C9 or C9’), 118.8 (C10 or C10’), 119.3 (C10 or C10’), 120.4 (C11 or C11’), 121.4 (C11 or C11’), 126.7 (C8), 127.0 (C8’), 134.7 (C2), 135.5 (C2’), 135.6 (C13 or C13’), 135.7 (C13 or C13’); m/z (ESI+) 452 (M+, 82%), 437 (6), 408 (10), 267 (8), 253 (12), 199 (27), 185 (100).
Compound 36 (paynantheine or (E)-methyl 3-methoxy-2-((2S,3R,12bS)-8-methoxy-3-vinyl-1,2,3,4,6,7,12,12b-octahydroindolo[2,3-a]quinolizin-2-yl)acrylate) was purchased from BOC Sciences (CAS: 4697-66-9, Shirley, NY, USA) and used without further purification.
Compound 37 (rhynchophylline or (E)-methyl 2-((1’R,6’R,7’S,8a’S)-6’-ethyl-2-oxo-3’,5’,6’,7’,8’,8a’-hexahydro-2’H-spiro[indoline-3,1’-indolizin]-7’-yl)-3-methoxyacrylate) was purchased from BOC Sciences (CAS: 76-66-4, Shirley, NY, USA) and used without further purification.
Compound 38 (speciociliatine or (E)-methyl 2-((2S,3S,12bR)-3-ethyl-8-methoxy-1,2,3,4,6,7,12,12b-octahydroindolo[2,3-a]quinolizin-2-yl)-3-methoxyacrylate) was purchased from BOC Sciences (CAS: 14382-79-7, Shirley, NY, USA) and used without further purification.
Compound 39 (yohimbine hydrochloride or (1R,2S,4aR,13bS,14aS)-methyl 2-hydroxy-1,2,3,4,4a,5,7,8,13,13b,14,14a-dodecahydroindolo[2’,3’:3,4]pyrido[1,2-b]isoquinoline-1-carboxylate hydrochloride) was purchased from Sigma-Aldrich (CAS: 65-19-0, St. Louis, MO, USA) and used without further purification.
4.2. Microorganisms and Cell Line
The reference Gram(+) and Gram(-) strains used for the antimicrobial tests were S. aureus ATCC 25923 and E. coli ATCC 25922, respectively. The multidrug-resistant clinical isolates of S. aureus (1a, 1b, and 1c) were kindly provided by Professor Giammarco Raponi (Sapienza, University of Rome).
HaCaT cells (AddexBio, San Diego, CA, USA) were cultured in Dulbecco’s modified Eagle’s medium supplemented with 4 mM glutamine (DMEMg), 10% heat-inactivated fetal bovine serum (FBS), and 0.1 mg/mL of penicillin and streptomycin at 37 °C and 5% CO2, in 25 cm2 or 75 cm2 flasks.
4.3. Antimicrobial Assays
A bacterial culture inoculum was incubated at 37 °C in Luria–Bertani (LB) broth until reaching an optical density (O.D.) of 0.8 at 590 nm. For the inhibition zone assay, the bacterial culture was diluted 1:2000 and plated in LB-agarose plates. An aliquot (3 μL) of each compound at 5 mM was loaded into holes previously made in the agarose plates [65,66]. Afterwards, the plates were incubated overnight at 30 °C. Afterwards, the diameters of the inhibition zone were measured and reported in Table 2 and Table 3.
For the determination of the MICs, 50 μL of bacterial suspension (2 × 105 cells/mL) in Mueller–Hinton broth (MH) was added to 50 μL MH containing serial dilutions of the compounds (from 256 μM to 2 μM) previously prepared in a 96-well plate. Controls consisted of vehicle-treated bacterial cells [67]. The plate was then incubated for 16 hours at 37 °C, and MIC was defined as the lowest concentration causing 100% visible inhibition of microbial growth. Afterwards, aliquots from the wells corresponding to the MIC of nigritanine (35) and to the control were withdrawn and plated onto LB agar plates for colony forming unit (CFU) counting and MBC determination.
4.4. Cytotoxicity Assays
To evaluate short-term cytotoxicity, selected alkaloids were tested on sheep red blood cells (OXOID, SR0051D). Aliquots of erythrocyte suspension (O.D. of 0.5 at 500 nm) in 0.9 % (w/v) NaCl were incubated for 40 min at 37 °C with three different concentrations (MIC, 2 × MIC, and 4 × MIC) of nigritanine (35) or the same concentrations of speciociliatine (38). The treated samples were then centrifuged for 5 min at 900× g. The amount of hemoglobin released in the supernatant by lysed red blood cells was measured at 415 nm using a microplate reader (Infinite M200; Tecan, Salzburg, Austria). The complete lysis was obtained by suspending erythrocytes in distilled water according to [68,69,70].
To evaluate the in vitro long-term cytotoxicity of nigritanine (35), a colorimetric method was employed. This assay is based on the intracellular reduction of the yellow tetrazolium salt MTT (Sigma-Aldrich, St. Luis, MO, USA) to purple formazan crystals by mitochondrial dehydrogenases of metabolically active cells. Therefore, the amount of purple color is directly proportional to the number of viable cells. About 4 × 10 4 HaCaT cells resuspended in DMEMg supplemented with 2% FBS, without antibiotics, were plated in each well of a 96-well plate. After overnight incubation in a humidified atmosphere containing 5% CO2 at 37 °C, the medium was removed, and fresh serum-free DMEMg containing the compound at different concentration was added in each well. For controls, cells were treated with vehicle. The plate was incubated for 24 h at 37 °C and 5% CO2. Afterwards, the medium was discarded, and 0.5 mg/mL of MTT in Hank’s buffer (136 mM NaCl, 4.2 mM Na2HPO4, 4.4 mM KH2PO4, 5.4 mM KCl, 4.1 mM NaHCO3, pH 7.2, supplemented with 20 mM D-glucose) was added to each well. After 4 h incubation at 37 °C and 5% CO2, 100 μL of acidified isopropanol was added to each well, in order to dissolve the formazan crystals [71,72]. Absorbance was measured by a microplate reader (Infinite M200; Tecan, Salzburg, Austria) at 570 nm, and cell viability was calculated with respect to the control (cells in medium supplemented with vehicle).
4.5. Statistical Analysis
All data are expressed as the mean ± SD or SEM. Statistical analyses were performed using Student’s t-test with the Prism software package (GraphPad, 6.0, San Diego, CA, USA), and the differences were considered to be statistically significant for p < 0.05.
Acknowledgments
We are grateful to Giammarco Raponi, Department of Public Health and Infectious Diseases, Sapienza University of Rome, for providing the clinical isolates.
Author Contributions
B.C. performed the microbiological assays; F.C. performed the cytotoxicity assays, M.R.L. partially contributed to the biological experiments. D.Q. and A.C. performed the acquisition and interpretation of the magnetic resonance spectroscopy (NMR) spectra of bioactive compounds and the determination of purity by High Performance Liquid Chromatography (HPLC). M.M. revised and edited the manuscript. B.B. provided overall guidance. F.G. and M.L.M. conceived the project and wrote the manuscript.
Funding
This work was supported by Italian Ministry of Education, University and Research—Dipartimenti di Eccellenza—L.232/2016 and Italian Institute of Technology. This work was also supported by grants from Sapienza University of Rome (project RM11816436113D8A).
Conflicts of Interest
The authors declare no conflict of interest.
Key Contribution
Starting from the screening of 39 alkaloids, we biologically characterized a heterodimer β-carboline alkaloid, named nigritanine, with potent anti-Staphylococcus activity and non-toxic to mammalian red blood cells and human keratinocytes at its bioactive concentration.
References
- 1.Reygaert W.C. An overview of the antimicrobial resistance mechanisms of bacteria. AIMS Microbiol. 2018;4:482–501. doi: 10.3934/microbiol.2018.3.482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ansari S., Jha R.K., Mishra S.K., Tiwari B.R., Asaad A.M. Recent advances in Staphylococcus aureus infection: Focus on vaccine development. Infect. Drug Resist. 2019;12:1243–1255. doi: 10.2147/IDR.S175014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gajdacs M. The continuing threat of methicillin-resistant Staphylococcus aureus. Antibiotics. 2019;8 doi: 10.3390/antibiotics8020052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mermel L.A., Cartony J.M., Covington P., Maxey G., Morse D. Methicillin-resistant Staphylococcus aureus colonization at different body sites: A prospective, quantitative analysis. J. Clin. Microbiol. 2011;49:1119–1121. doi: 10.1128/JCM.02601-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Keihanian F., Saeidinia A., Abbasi K. Epidemiology of antibiotic resistance of blood culture in educational hospitals in Rasht, North of Iran. Infect. Drug Resist. 2018;11:1723–1728. doi: 10.2147/IDR.S169176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chung P.Y. Novel targets of pentacyclic triterpenoids in Staphylococcus aureus: A systematic review. Phytomedicine Int. J. Phytother. Phytopharm. 2019 doi: 10.1016/j.phymed.2019.152933. [DOI] [PubMed] [Google Scholar]
- 7.Calcaterra A., D’Acquarica I. The market of chiral drugs: Chiral switches versus de novo enantiomerically pure compounds. J. Pharm. Biomed. Anal. 2018;147:323–340. doi: 10.1016/j.jpba.2017.07.008. [DOI] [PubMed] [Google Scholar]
- 8.Debnath B., Singh W.S., Das M., Goswami S., Singh M.K., Maiti D., Manna K. Role of plant alkaloids on human health: A review of biological activities. Mater. Today Chem. 2018;9:56–72. doi: 10.1016/j.mtchem.2018.05.001. [DOI] [Google Scholar]
- 9.Cushnie T.P.T., Cushnie B., Lamb A.J. Alkaloids: An overview of their antibacterial, antibiotic-enhancing and antivirulence activities. Int. J. Antimicrob. Agents. 2014;44:377–386. doi: 10.1016/j.ijantimicag.2014.06.001. [DOI] [PubMed] [Google Scholar]
- 10.Ingallina C., D’Acquarica I., Delle Monache G., Ghirga F., Quaglio D., Ghirga P., Berardozzi S., Markovic V., Botta B. The pictet-spengler reaction still on stage. Curr. Pharm. Des. 2016;22:1808–1850. doi: 10.2174/1381612822666151231100247. [DOI] [PubMed] [Google Scholar]
- 11.Ghirga F., Bonamore A., Calisti L., D’Acquarica I., Mori M., Botta B., Boffi A., Macone A. Green routes for the production of enantiopure benzylisoquinoline alkaloids. Int. J. Mol. Sci. 2017;18 doi: 10.3390/ijms18112464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Amirkia V., Heinrich M. Alkaloids as drug leads—A predictive structural and biodiversity-based analysis. Phytochem. Lett. 2014;10 doi: 10.1016/j.phytol.2014.06.015. [DOI] [Google Scholar]
- 13.Infante P., Alfonsi R., Ingallina C., Quaglio D., Ghirga F., D’Acquarica I., Bernardi F., Di Magno L., Canettieri G., Screpanti I., et al. Inhibition of hedgehog-dependent tumors and cancer stem cells by a newly identified naturally occurring chemotype. Cell Death Dis. 2016;7:E2376. doi: 10.1038/cddis.2016.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mori M., Tottone L., Quaglio D., Zhdanovskaya N., Ingallina C., Fusto M., Ghirga F., Peruzzi G., Crestoni M.E., Simeoni F., et al. Identification of a novel chalcone derivative that inhibits Notch signaling in T-cell acute lymphoblastic leukemia. Sci. Rep. 2017;7:2213. doi: 10.1038/s41598-017-02316-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Infante P., Mori M., Alfonsi R., Ghirga F., Aiello F., Toscano S., Ingallina C., Siler M., Cucchi D., Po A., et al. Gli1/DNA interaction is a druggable target for Hedgehog-dependent tumors. EMBO. J. 2015;34:200–217. doi: 10.15252/embj.201489213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Srivastava S., Srivastava M., Misra A., Pandey G., Rawat A. A review on biological and chemical diversity in Berberis (Berberidaceae) Excli J. 2015;14:247–267. doi: 10.17179/excli2014-399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Meyer A., Imming P. Benzylisoquinoline alkaloids from the Papaveraceae: The heritage of Johannes Gadamer (1867–1928) J. Nat. Prod. 2011;74:2482–2487. doi: 10.1021/np2005049. [DOI] [PubMed] [Google Scholar]
- 18.Urzúa A.P.A. Alkaloids from the bark of Peumus boldus. Fitoterapia. 1983;54:175–177. [Google Scholar]
- 19.Imaki N.M.Y., Shimpuku T., Shirasaka T. A Process for Preparing Cotarnine. U.S. Patent and Trademark Office; Washington, DC, USA: 1986. [Google Scholar]
- 20.Colombo M.L., Bosisio E. Pharmacological activities of Chelidonium majus L. (Papaveraceae) Pharmacol. Res. 1996;33:127–134. doi: 10.1006/phrs.1996.0019. [DOI] [PubMed] [Google Scholar]
- 21.Akinboye E., Bakare O. Biological activities of emetine. Open Nat. Prod. J. 2011;411:8–15. doi: 10.2174/1874848101104010008. [DOI] [Google Scholar]
- 22.Lapa G.P., Sheichenko O., Serezhechkin A.G.N., Tolkachev O. HPLC determination of glaucine in yellow horn poppy grass (Glaucium flavum Crantz) Pharm. Chem. J. 2004;38:441–442. doi: 10.1023/B:PHAC.0000048907.58847.c6. [DOI] [Google Scholar]
- 23.Brown P.N., Roman M.C. Determination of hydrastine and berberine in goldenseal raw materials, extracts, and dietary supplements by high-performance liquid chromatography with UV: Collaborative study. J. AOAC Int. 2008;91:694–701. [PMC free article] [PubMed] [Google Scholar]
- 24.Ramanathan V.S., Chandra P. Recovery, separation and purification of narcotine and papaverine from Indian opium. Bull. Narc. 1981;33:55–64. [PubMed] [Google Scholar]
- 25.Lee M.R. Curare: The South American arrow poison. J. R. Coll. Physicians Edinb. 2005;35:83–92. [PubMed] [Google Scholar]
- 26.Martins D., Nunez C.V. Secondary metabolites from Rubiaceae species. Molecules. 2015;20:13422–13495. doi: 10.3390/molecules200713422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dellemonache F., Dellemonache G., Souza M.A.D., Cavalcanti M.D., Chiappeta A. Isopentenylindole derivatives and other components of Esenbeckia leiocarpa. Gazz. Chim. Ital. 1989;119:435–439. [Google Scholar]
- 28.Guimaraes H.A., Braz-Filho R., Vieira I.J.C. H-1 and C-13-NMR data of the simplest plumeran indole alkaloids isolated from Aspidosperma species. Molecules. 2012;17:3025–3043. doi: 10.3390/molecules17033025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Galeffi C., Ciasca-Rendina M.A., Miranda Delle Monache E., Villar Del Fresno A., Marini Bettòlo G.B. Gradient method for the counter-current separation of alkaloids using a heavy organic phase. J. Chromatogr. 1969;45:407–414. doi: 10.1016/S0021-9673(01)86237-3. [DOI] [Google Scholar]
- 30.Zhao B., Moochhala S.M., Tham S.Y. Biologically active components of Physostigma venenosum. J. Chromatogr. B. 2004;812:183–192. doi: 10.1016/S1570-0232(04)00677-4. [DOI] [PubMed] [Google Scholar]
- 31.Ohiri F.C., Verpoorte R., Baerheim Svendsen A. The African Strychnos species and their alkaloids: A review. J. Ethnopharmacol. 1983;9:167–223. doi: 10.1016/0378-8741(83)90032-6. [DOI] [PubMed] [Google Scholar]
- 32.Mohammad-Zadeh L.F., Moses L., Gwaltney-Brant S.M. Serotonin: A review. J. Vet. Pharm. 2008;31:187–199. doi: 10.1111/j.1365-2885.2008.00944.x. [DOI] [PubMed] [Google Scholar]
- 33.Kousar S., Noreen Anjuma S., Jaleel F., Khana J., Naseema S. Biomedical significance of tryptamine: A review. J. Pharmacovigil. 2017;5 doi: 10.4172/2329-6887.1000239. [DOI] [Google Scholar]
- 34.Song K.M., Park S.W., Hong W.H., Lee H., Kwak S.S., Liu J.R. Isolation of vindoline from Catharanthus roseus by supercritical fluid extraction. Biotechnol. Prog. 1992;8:583–586. doi: 10.1021/bp00018a018. [DOI] [PubMed] [Google Scholar]
- 35.Noldin V.F., de Oliveira Martins D.T., Marcello C.M., da Silva Lima J.C., Delle Monache F., Cechinel Filho V. Phytochemical and antiulcerogenic properties of rhizomes from Simaba ferruginea St. Hill. (Simaroubaceae) Zeitschrift für Naturforschung C. 2005;60:701–706. doi: 10.1515/znc-2005-9-1007. [DOI] [PubMed] [Google Scholar]
- 36.Kumar A.S., Nagarajan R. Synthesis of alpha-carbolines via Pd-catalyzed amidation and Vilsmeier-Haack reaction of 3-acetyl-2-chloroindoles. Org. Lett. 2011;13:1398–1401. doi: 10.1021/ol2000827. [DOI] [PubMed] [Google Scholar]
- 37.Claudia F., Amaral A., Ramos A.D.S., Ferreira J., Santos A.D.S., Cruz J.D.S., De Luna A.V.M., Nery V.V.C., de Lima I.C., Chaves M.H.d.C., et al. LC-HRMS for the Identification of β-carboline and canthinone alkaloids isolated from natural sources. Mass Spectrometry. 2017;187 doi: 10.5772/68075. [DOI] [Google Scholar]
- 38.Alper K.R. Ibogaine: A review. Alkaloids. Chem. Biol. 2001;56:1–38. doi: 10.1016/s0099-9598(01)56005-8. [DOI] [PubMed] [Google Scholar]
- 39.Takayama H. Chemistry and pharmacology of analgesic indole alkaloids from the rubiaceous plant, Mitragyna speciosa. Chem. Pharm. Bull. 2004;52:916–928. doi: 10.1248/cpb.52.916. [DOI] [PubMed] [Google Scholar]
- 40.Leon F., Habib E., Adkins J.E., Furr E.B., McCurdy C.R., Cutler S.J. Phytochemical characterization of the leaves of Mitragyna speciosa grown in USA. Nat. Prod. Commun. 2009;4:907–910. doi: 10.1177/1934578X0900400705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cinosi E., Martinotti G., Simonato P., Singh D., Demetrovics Z., Roman-Urrestarazu A., Bersani F.S., Vicknasingam B., Piazzon G., Li J.H., et al. Following the roots of kratom (Mitragyna speciosa): The evolution of an enhancer from a traditional use to increase work and productivity in Southeast Asia to a recreational psychoactive drug in western countries. Biomed. Res. Int. 2015:968786. doi: 10.1155/2015/968786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Evans W.C. Pharmacopoeial and Related Drugs of Biological Origin. 16th ed. Saunders—Elsevier; Amsterdam, The Netherlands: 2009. pp. 353–416. [Google Scholar]
- 43.Dai J.K., Dan W.J., Schneider U., Wang J.R. β-Carboline alkaloid monomers and dimers: Occurrence, structural diversity, and biological activities. Eur. J. Med. Chem. 2018;157:622–656. doi: 10.1016/j.ejmech.2018.08.027. [DOI] [PubMed] [Google Scholar]
- 44.Dai J.K., Dan W.J., Ren S.Y., Shang C.G., Wang J.R. Design, synthesis and biological evaluations of quaternization harman analogues as potential antibacterial agents. Eur. J. Med. Chem. 2018;160:23–36. doi: 10.1016/j.ejmech.2018.10.012. [DOI] [PubMed] [Google Scholar]
- 45.Nenaah G. Antibacterial and antifungal activities of (β)-carboline alkaloids of Peganum harmala (L) seeds and their combination effects. Fitoterapia. 2010;81:779–782. doi: 10.1016/j.fitote.2010.04.004. [DOI] [PubMed] [Google Scholar]
- 46.Mohammad Reza V.R., Hadjiakhoondi A. Cytotoxicity and antimicrobial activity of harman alkaloids. J. Pharmacol. Toxicol. 2007;2:677–680. doi: 10.3923/jpt.2007.677.680. [DOI] [Google Scholar]
- 47.Parthasarathy S., Bin Azizi J., Ramanathan S., Ismail S., Sasidharan S., Said M.I., Mansor S.M. Evaluation of antioxidant and antibacterial activities of aqueous, methanolic and alkaloid extracts from Mitragyna speciosa (Rubiaceae family) leaves. Molecules. 2009;14:3964–3974. doi: 10.3390/molecules14103964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Benziane Maatalah M., Kambuche Bouzidi N., Bellahouel S., Merah B., Fortas Z., Soulimani R., Saidi S., Derdour A. Antimicrobial activity of the alkaloids and saponin extracts of Anabasis articulate. J. Biotechnol. Pharm. Res. 2012;3:54. [Google Scholar]
- 49.Ozcelik B., Kartal M., Orhan I. Cytotoxicity, antiviral and antimicrobial activities of alkaloids, flavonoids, and phenolic acids. Pharm. Biol. 2011;49:396–402. doi: 10.3109/13880209.2010.519390. [DOI] [PubMed] [Google Scholar]
- 50.Gurrapu S., Estari M. In vitro antibacterial activity of alkaloids isolated from leaves of Eclipta alba against human pathogenic bacteria. Pharmacogn. J. 2017;9:573–577. doi: 10.5530/pj.2017.4.91. [DOI] [Google Scholar]
- 51.Karou D., Savadogo A., Canini A., Yameogo S., Montesano C., Simpore J., Colizzi V., Traore A.S. Antibacterial activity of alkaloids from Sida acuta. Afr. J. Biotechnol. 2006;5:195–200. [Google Scholar]
- 52.Slobodnikova L., Kost’alova D., Labudova D., Kotulova D., Kettman V. Antimicrobial activity of Mahonia aquifolium crude extract and its major isolated alkaloids. Phytother. Res. 2004;18:674–676. doi: 10.1002/ptr.1517. [DOI] [PubMed] [Google Scholar]
- 53.Su Y.F., Li S.K., Li N., Chen L.L., Zhang J.W., Wang J.R. Seven alkaloids and their antibacterial activity from Hypecoum erectum L. J. Med. Plants Res. 2011;5:5428–5432. [Google Scholar]
- 54.Manosalva L., Mutis A., Urzua A., Fajardo V., Quiroz A. Antibacterial activity of alkaloid fractions from Berberis microphylla G. Forst and study of synergism with ampicillin and cephalothin. Molecules. 2016;21:76. doi: 10.3390/molecules21010076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Tegos G., Stermitz F.R., Lomovskaya O., Lewis K. Multidrug pump inhibitors uncover remarkable activity of plant antimicrobials. Antimicrob. Agents Chemother. 2002;46:3133–3141. doi: 10.1128/AAC.46.10.3133-3141.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Locher H.H., Ritz D., Pfaff P., Gaertner M., Knezevic A., Sabato D., Schroeder S., Barbaras D., Gademann K. Dimers of nostocarboline with potent antibacterial activity. Chemotherapy. 2010;56:318–324. doi: 10.1159/000320033. [DOI] [PubMed] [Google Scholar]
- 57.Soong G., Paulino F., Wachtel S., Parker D., Wickersham M., Zhang D., Brown A., Lauren C., Dowd M., West E., et al. Methicillin-resistant Staphylococcus aureus adaptation to human keratinocytes. mBio. 2015;6:e00289-15. doi: 10.1128/mBio.00289-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Malikova J., Zdarilova A., Hlobilkova A., Ulrichova J. The effect of chelerythrine on cell growth, apoptosis, and cell cycle in human normal and cancer cells in comparison with sanguinarine. Cell Biol. Toxicol. 2006;22:439–453. doi: 10.1007/s10565-006-0109-x. [DOI] [PubMed] [Google Scholar]
- 59.Zhou Z.S., Li M., Gao F., Peng J.Y., Xiao H.B., Dai L.X., Lin S.R., Zhang R., Jin L.Y. Arecoline suppresses HaCaT cell proliferation through cell cycle regulatory molecules. Oncol. Rep. 2013;29:2438–2444. doi: 10.3892/or.2013.2360. [DOI] [PubMed] [Google Scholar]
- 60.Delle Monache F.C., Rossi E., Cartoni C., Carpi A., Marini Bettolo G.B. The alkaloids of Strychnos castelneana. Strychnos Alkaloids. 1970;33:279–283. [Google Scholar]
- 61.Martin G.E. Configuration and total assignment of the 1H-and 13C-NMR spectra of the alkaloid holstiine. J. Nat. Prod. 1990;53:793–800. [Google Scholar]
- 62.Tavernier D., Anteunis M., Tits M., Angenot L. The 1H NMR spectra of the strychnos alkaloids retuline isoretuline, and their N-deacetyl compounds. Bull. Des Sociétés Chim. Belg. 2010;87:595–607. doi: 10.1002/bscb.19780870804. [DOI] [Google Scholar]
- 63.Cao M., Muganga R., Nistor I., Tits M., Angenot L., Frederich M. LC–SPE–NMR–MS analysis of Strychnos usambarensis fruits from Rwanda. Phytochem. Lett. 2012;5:170–173. doi: 10.1016/j.phytol.2011.12.003. [DOI] [Google Scholar]
- 64.Nicoletti M., Oguakwa J.U., Messana I. On the alkaloids of two African Strychnos: Strychnos nigritana bak and Strychnos barteri Sol. carbon-13 NMR spectroscopy of nigritanins. Fitoterapia. 1980;87:595–607. [Google Scholar]
- 65.Islas-Rodriguez A.E., Marcellini L., Orioni B., Barra D., Stella L., Mangoni M.L. Esculentin 1-21: A linear antimicrobial peptide from frog skin with inhibitory effect on bovine mastitis-causing bacteria. J. Pept. Sci. Off. Publ. Eur. Pept. Soc. 2009;15:607–614. doi: 10.1002/psc.1148. [DOI] [PubMed] [Google Scholar]
- 66.Mangoni M.L., Fiocco D., Mignogna G., Barra D., Simmaco M. Functional characterisation of the 1–18 fragment of esculentin-1b, an antimicrobial peptide from Rana esculenta. Peptides. 2003;24:1771–1777. doi: 10.1016/j.peptides.2003.07.029. [DOI] [PubMed] [Google Scholar]
- 67.Falciani C., Lozzi L., Pollini S., Luca V., Carnicelli V., Brunetti J., Lelli B., Bindi S., Scali S., Di Giulio A., et al. Isomerization of an antimicrobial peptide broadens antimicrobial spectrum to gram-positive bacterial pathogens. PLoS ONE. 2012;7:e46259. doi: 10.1371/journal.pone.0046259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Buommino E., Carotenuto A., Antignano I., Bellavita R., Casciaro B., Loffredo M.R., Merlino F., Novellino E., Mangoni M.L., Nocera F.P., et al. The outcomes of decorated prolines in the discovery of antimicrobial peptides from Temporin-L. ChemMedChem. 2019;14:1283–1290. doi: 10.1002/cmdc.201900221. [DOI] [PubMed] [Google Scholar]
- 69.Merlino F., Carotenuto A., Casciaro B., Martora F., Loffredo M.R., Di Grazia A., Yousif A.M., Brancaccio D., Palomba L., Novellino E., et al. Glycine-replaced derivatives of [Pro(3),DLeu(9)]TL, a temporin L analogue: Evaluation of antimicrobial, cytotoxic and hemolytic activities. Eur. J. Med. Chem. 2017;139:750–761. doi: 10.1016/j.ejmech.2017.08.040. [DOI] [PubMed] [Google Scholar]
- 70.Grieco P., Carotenuto A., Auriemma L., Saviello M.R., Campiglia P., Gomez-Monterrey I.M., Marcellini L., Luca V., Barra D., Novellino E., et al. The effect of d-amino acid substitution on the selectivity of temporin L towards target cells: Identification of a potent anti-Candida peptide. Biochimica et Biophysica Acta. 2013;1828:652–660. doi: 10.1016/j.bbamem.2012.08.027. [DOI] [PubMed] [Google Scholar]
- 71.Cappiello F., Di Grazia A., Segev-Zarko L.A., Scali S., Ferrera L., Galietta L., Pini A., Shai Y., Di Y.P., Mangoni M.L. Esculentin-1a-derived peptides promote clearance of Pseudomonas aeruginosa internalized in bronchial cells of cystic fibrosis patients and lung cell migration: Biochemical properties and a plausible mode of action. Antimicrob. Agents Chemother. 2016;60:7252–7262. doi: 10.1128/AAC.00904-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Di Grazia A., Luca V., Segev-Zarko L.A., Shai Y., Mangoni M.L. Temporins A and B stimulate migration of HaCaT keratinocytes and kill intracellular Staphylococcus aureus. Antimicrob. Agents Chemother. 2014;58:2520–2527. doi: 10.1128/AAC.02801-13. [DOI] [PMC free article] [PubMed] [Google Scholar]