Abstract
Salmonella contamination in foods and their formation of biofilms in food processing facility are the primary bacterial cause of a significant number of foodborne outbreaks and infections. Broad lytic phages are promising alternatives to conventional technologies for pathogen biocontrol in food matrices and reducing biofilms. In this study, 42 Salmonella phages were isolated from environmentally-sourced water samples. We characterized the host range and lytic capacity of phages LPSTLL, LPST94 and LPST153 against Salmonella spp., and all showed a wide host range and broad lytic activity. Electron microscopy analysis indicated that LPSTLL, LPST94, and LPST153 belonged to the family of Siphoviridae, Ackermannviridae and Podoviridae, respectively. We established a phage cocktail containing three phages (LPSTLL, LPST94 and LPST153) that had broad spectrum to lyse diverse Salmonella serovars. A significant decrease was observed in Salmonella with a viable count of 3 log10 CFU in milk and chicken breast at either 25 °C or 4 °C. It was found that treatment with phage cocktail was able to significantly reduced biofilm on a 96-well microplate (44–63%) and on a stainless steel surface (5.23 to 6.42 log10). These findings demonstrated that the phage cocktail described in this study can be potentially used as a biological control agent against Salmonella in food products and also has the effect to reduce Salmonella formed biofilms.
Keywords: phage, cocktail, Salmonella, biological control, foods, biofilms
1. Introduction
Salmonella is gram-negative, rod-shaped bacterium that belongs to the family of Enterobacteriaceae. It is one of the most common food-borne pathogens, and has been considered as a significant public health threat and economic burden. It is a facultative intracellular human pathogen and the causative agent of non-typhoidal salmonellosis [1,2]. The symptoms of the disease are abdominal pain, vomiting, inflammatory diarrhea, nausea, fever, headache and the disease is frequently transmitted via contaminated foods, water and biofilms [3,4]. Salmonella outbreaks by consuming contaminated food products were previously reported, and chicken, pork, beef and dairy products were common vehicles [5,6]. It is estimated that every year Salmonella spp. cause 93.8 million illnesses and 155,000 deaths worldwide [7]. According to USDA-ERS estimation, economically, US $2.5 million was lost due to 1.4 million cases of salmonellosis in 2007 [8]. In the United States alone, Salmonella is annually responsible for 11% of illnesses, 35% of total hospitalizations and 28% of deaths associated with food-borne diseases [9,10]. In addition, over 265 people were sickened owing to consumption of chicken salad that was contaminated by Salmonella in eight states in the USA in 2018. Among them, 94 were hospitalized and one person died [11]. In China, non-typhoidal Salmonella spp. are extremely important. From 2008 to 2012, approximately 70 outbreaks were reported, which led to 4151 hospitalized cases and four deaths with many provinces affected [12,13,14].
Salmonella spp. are frequently described as environmental persisters [15,16] and can form surface-associated complex communities known as biofilms in both food matrices and industrial settings [17,18,19,20]. Salmonella biofilm was reported as bacterial reservoir in a food processing facility, and lead to several food-borne disease outbreaks [21]. For example, Salmonella outbreaks that caused 2138 cases of illness was related to the consumption of Salmonella biofilm-contaminated chicken [22].
Conventional control measures such as chemical disinfectant, biocides, and heat treatment are frequently used against various Salmonella serovars, in food products and to reduce biofilms [23,24,25]. However, they all possess certain disadvantages. Chemical disinfectants such as ascorbic acid, calcium carbonate and diacetyl-tartaric esters of fatty acids have an adverse impact on the taste, aroma, and texture, which are very desirable traits of foods [26]. Chemical preservatives such as sodium benzoate and benzoic acid could also lead to number of side effects such as asthma, allergic contact dermatitis, hives, convulsions and intestinal hemorrhage diarrhea [27,28]. Moreover, general disinfectants (sodium hypochlorite, sodium hydroxide, and benzalkonium chloride) failed to reduce Salmonella enterica biofilms [21] due to high bacterial resistance [29]. The heat treatment can destroy vitamins thus reducing the nutritional value of foods [30,31] and also produce advanced glycation end-products (AGEs) attributed to health-threatening complications [32].
As most conventional methods showed limited effect on Salmonella control, antibiotics once were considered as an effective method to reduce the Salmonella burden in both farms and industries. However, later this was proved as leading to another issue, the prevalence of antimicrobial resistant bacteria. Antibiotics have been banned from Sweden in 1986, the Danish Pig Production Committee (NCPP) in 1995 and the European Union (EU) in 1999 [33]. As a novel strategy, phages were described as a promising approach to control and preserve pathogens in food products and to reduce biofilms [34,35,36,37]. Human and animal bodies are reservoirs for great amounts of phages. As reported, phages can easily be detected from healthy humans, animals, and foods [38,39,40,41]. Phages do not cause harm to humans and animals and there have been no reports so far describing any phage infection in the human body [38,39]. Thus, phage application seems to be a safe prerequisite to biologically control pathogens in food processing line [42].
Bacteriophages are frequently used for inactivation and control of food-borne pathogens, such as Salmonella, Escherichia coli O157:H7, Listeria and Campylobacter in different foods [43,44,45,46,47,48,49,50,51,52,53,54]. Several physical, chemical, and biological approaches such as cold oxygen plasma, ultraviolet irradiation, ultrasound, natural substances, quorum sensing inhibition, antimicrobial coating, and bacteriophages have been proposed as tools to control biofilms in the food industry. However, most of these strategies are either limited with efficacy, not cost-effective, or not practical for implementation in food processing facilities [55]. Among these advanced approaches, bacteriophages are considered as potential candidates to reduce or eliminate biofilms [37]. Few studies have been conducted on the effectiveness of phage against biofilms formed by Salmonella spp. on food or food processing surfaces. This study aims to evaluate the efficacy of phage cocktail as a zoonotic Salmonella control approach in diverse foods and biofilms.
2. Materials and Methods
2.1. Bacterial Strains and Culture Conditions
A selection of 55 different bacterial strains, including 41 Salmonella strains that encompassed in 11 distinct serovars, and a cohort of 14 non-Salmonella strains (including E. coli A. hydrophila, S. aureus and Listeria), were used in this study to screen phage by spot test (Table 1). These strains were stored with 20% (v/v) glycerol at −80 °C. All bacterial strains were streaked on tryptic soy agar (TSA; DifcoTM, BD, Franklin Lakes, NJ, USA) before the experiment, and obtained single colonies were recovered by culturing in tryptic soy broth (TSB, DifcoTM, BD, USA) overnight at 37 °C to ensure the purity of the bacterial stock. Salmonella enterica was used for isolation, propagation, and purification of phages. The phages were isolated and enriched using 2-YT broth medium (1.6 g of peptone, 1.0 g of yeast extract, and 0.5 g NaCl, in 100 mL of distilled water; pH 7.4). To determine the phage titer, a double-layer agar plates method was applied, with an overlay layer containing 0.7% agar and a bottom layer with 1.5% agar [54].
Table 1.
List of bacterial strains used in this study.
| Bacterial Strains | Strain ID Number | Numbers of Strains | Source of Strains |
|---|---|---|---|
| S.enterica serovar Typhimurium | ATCC 14028, ATCC 13311 | 2 | ATCC |
| UK-1, ST8, SGSC 4903, SL 1344, LT2 | 5 | LS | |
| S.enterica serovar Enteritidis | ATCC 13076 | 1 | ATCC |
| SJTUF 10978, SJTUF 10984 | 2 | SJTU | |
| LK5-3820, SGSC 4901 | 2 | LS | |
| S. enterica Serovar Pullorum | CVCC 519 | 1 | LS |
| S.enterica serovar Dublin | 3710,3723 | 2 | LS |
| S. enterica subsp. enterica serovar Anatum | ATCC 9270 | 1 | ATCC |
| S. enterica subsp. arizonae | CDC 346-86 | 1 | CDC |
| S. enterica subsp. enterica serovar Javiana | CVM 35943 | 1 | LS |
| S. enterica subsp. enterica serovar Kentucky | CVM 29188 | 1 | LS |
| S. enterica serovar Newport | E20002725 | 1 | CDC |
| S. enterica serovar Paratyphi B | CMCC 50094 | 1 | CMCC |
| S. enterica serovar Choleraesuls | ATCC 10708 | 1 | ATCC |
| Drug resistant S. enterica serovar Typhimurium | LST10, LST11, LST12, LST13, LST14, LST15, LST16, LST17, LST18, LST19 | 10 | LS |
| Drug resistant S. enterica serovar Enteritidis | LSE6, LSE7, LSE8, LSE9, LSE10, LSE11, LSE12, LSE13, LSE15 | 9 | LS |
| E. coli | BL21, DH5α | 2 | TB |
| ATCC 933 | 1 | ATCC | |
| F18AC, C83715, T10 | 3 | LS | |
| A. hydrophila | ZYAH72, ZYAH75, J1, D4 | 4 | LS |
| S. aureus | ATCC 6538, ATCC 8095, ATCC 29213 | 3 | ATCC |
| Listeria | ATCC 1914 | 1 | ATCC |
Abbreviation: ATCC, American Type Culture Collection; LS, Lab Stock; SJTU, Shanghai Jiao Tong University; CDC, Centers for Disease Control and Prevention; TB, TransGen Biotech; CMCC, National Center for Medical Culture Collection.
2.2. Enrichment, Isolation, Purification, and Preparation of Phages
A total of 42 phages were isolated from environmentally sourced water samples collected in Wuhan, China in accordance with previously described method [56]. For enrichment, isolation and purification of phages, modified methods from previously published articles were used [57,58,59]. In brief, 10 mL of a 0.22-μm-filtered sample was mixed with 40 mL 2-YT broth medium and 10 mL exponential growth phase Salmonella cultures at a ratio of 1:4:1 (v/v/v). After 24 h of incubation at 37 °C with gentle agitation, the cultured was centrifuged (8000× g/15 min) and filtered again using 0.22-μm filters (Millipore, Dublin, Ireland). Then phage activity in the supernatant was detected with spot assay [54,60]. The double layer agar method was used to determine the titer of the phage stock. Dilutions of the phage stock (100 μL each) were made in sterile SM buffer (10 mM NaCl, 10 mM MgSO4, 50 mM Tris:HCl, pH 7.5), mixed with a suspension of exponential phase Salmonella (about 109 CFU/mL, 100 μL) and added to 4 mL of molten (45 °C ≤ temperature ≤ 50 °C) TSB agar (0.7%). The mixtures were then poured onto the surface of TSA agar plates and were allowed to set at room temperature for 5 min. Thereafter, the plates were incubated at 37 °C for 24 h, and resulting plaques were quantified. To purify the phages, picking individual plaque by using a pipette or a wire loop, and then suspended in TSB with exponential phase Salmonella at 37 °C for 24 h. Then centrifuged (8000× g/15 min) and filtered again using 0.22-μm filters used as a single phage culture. The purification process was repeated at least three times, and then confirmed pure individual phage stock. Purified phages were stored at 4 °C and used for different experiments during the whole study.
The phage cocktail was prepared by mixing three phages with a ratio of 1:1:1, each phage at a titer of 9 log10 PFU/mL. The phage cocktail was later diluted in sterile SM buffer to reach the target concentration for treatment of Salmonella in foods and biofilms.
2.3. Screening of Phages Based on Spot Test and Lytic Capacity
2.3.1. Spot Test
Spot testing was applied to measure the ability of phages to infect different serovars of bacteria with a modified method from previous publication [54]. 100 μL of test bacterial culture that are in exponential phase was transferred to 4 mL of molten (45 °C ≤ temperature ≤ 50 °C) TSB agar (0.7% w/v). The mixture was then poured onto surface of TSA agar plates and allowed to dry for 5 min. When the overlay agar was set, 5 μL of each phage solution was spotted onto bacterial lawns and allowed to dry. The plates were then incubated at 37 °C for 20 to 24 h. After incubation, any bacterial lawn with formation of clear spots/plaques were considered as phage sensitive.
2.3.2. Lytic Activity
Phage lytic activity was analyzed in the 96-well microtiter plate by measuring the optical density (OD600nm) every hour with various applied multiplicity of infection (MOI; ratio of phage titers to bacterial counts measured) to determine the efficiency of phage virulence according to previously described method [61]. In brief, the test group with 100 μL of fresh cultured Salmonella (7 log10 CFU/mL) was added to 100 μL of individually diluted phage lysate (6 log10–9 log10 PFU/mL) in wells of 96 well-mirotitre plate. The control group was set up with same volume of fresh overnight cultures of Salmonella (7 log10 CFU/mL) mixed with plain TSB medium instead of phage. Samples were incubated at 37 °C on an orbital shaker at 160 rpm. Optical density (OD600nm) of the mixture was measured with a microplate reader (Infinite M200 Pro, Tecan, 140 Switzerland) at 37 °C, with an interval of 1 h.
2.4. Determination of Host Range by Efficiency of Plating (EOP)
The three phages that revealed widest bactericide host range in spot assays (LPSTLL, LPST94 and LPST153) were analyzed with efficiency of plating (EOP) either individually or assayed as a cocktail with ratio of 1:1:1. To evaluate the host range, EOP was performed as modified methods described in previous reports [61,62]. Each phage was serially diluted and tested in triplicates on sensitive bacterial host. Test bacterial strains were grown overnight at 37 °C. After incubation, 100 μL of bacterial culture was applied in double layer plate assays together with 100 μL of diluted phage lysate. Dilution factors between 106–109 were applied in this study. The plates were incubated overnight at 37 °C and the number of plaque forming units (PFU) was counted. The EOP was calculated (average PFU on test bacteria / average PFU on host bacteria). The average EOP value was classified as EOP 0.5 to 1.0, high efficiency; EOP 0.2 to <0.5, moderate efficiency; 0.001 to <0.2, low efficiency; and <0.001, inefficient [61,62].
2.5. Transmission Electron Microscopy (TEM)
Ten microliter lysate with a high titer (>10 log10 PFU/mL) of purified phage was fixed onto a copper grid and negatively stained with 0.5% phosphotungstic acid (PTA) [56,62,63]. Thereafter, negatively stained copper grids were examined, and the images of phage were captured using a Philips CM12 transmission electron microscope (Hitachi H-7000FA, Tokyo, Japan), at Wuhan Institute of Virology (China Academy of Sciences, Wuhan, China) and analyzed via Digital Micrograph Demo 3.9.1 software (Gatan, Pleasanton, CA, USA).
2.6. Phage Stability in Foods
Stability of phage cocktail in milk and on chicken breast experiments were conducted. Briefly, phage lysates were firstly added in milk to reach a final titer of 6 log10 and 7 log10 PFU/mL. Phages were also applied on chicken breast to a final titer of 6 log10 and 7 log10 PFU/cm2 by pipette transferring the lysate onto the surface of the chicken breast, followed by spreading the lysate with a sterile spreader. Both inoculated samples (milk and chicken breast) were incubated at 25 °C or 4 °C for 48 h. At each time-point (0, 1, 3, 6, 12, 24 and 48 h), aliquoted milk or pre-cut chicken breast were taken to enumerate phage titer using a double-layer agar method.
2.7. Biological Control of Salmonella in Foods Using Phage Cocktail
2.7.1. Food Samples
Pasteurized milk was purchased from a local supermarket. The chicken breast was also obtained from a local supermarket then sliced aseptically in the laboratory. The chicken breast was cut into pieces (1 cm × 1 cm square) using a sterile scalpel blade on the sterile station board. Food samples were general screened with TSA for the presence of any microorganisms and only those ones that are free from background microorganisms were used in this study.
2.7.2. Adding Salmonella and Phage Cocktail for Treatment
Salmonella biocontrol experiments using phage cocktail were conducted at 4 °C (refrigerator temperature) and 25 °C (room temperature) [64]. Study groups were temperature acclimated for 20 min; thereafter S. Typhimurium ATCC 14028 or a mixture of both S. Typhimurium ATCC 14028 and S. Enteritidis ATCC 13076 was added to milk to a final viable count of 3 log10 CFU/mL. The 1 cm × 1 cm square chicken breast sections were placed in the center of the sterile Petri-dishes, 3 log10 CFU/cm2 (final viable count) Salmonella suspension was added and spread over the sample surfaces. The chicken breast samples were dried for 30 to 40 min.
For phage cocktail treatment, phage cocktail was added to MOI of 1000 (add 10 μL of 8 log10 PFU/mL phage to reach a final titer of 6 log10 PFU/mL) or 10000 (add 10 μL of 9 log10 PFU/mL phage to reach a final titer of 7 log10 PFU/mL) in milk. phage cocktail was added with an MOI of 1000 (spot 10 μL of 8 log10 PFU/mL phage to reach a final titer of 6 log10 PFU/cm2) or MOI of 10000 (spot 10 μL of 9 log10 PFU/mL phage for a final titer of 7 log10 PFU/cm2) by pipette transferring the lysate followed by spreading the lysate with a sterile spreader on surface of the chicken breast samples. Then incubated at either 4 °C or 25 °C.
2.7.3. Recovered Bacterial Load from Treated Foods
For the recovered bacterial load from milk, an aliquot of the samples was taken out after 0, 1, 3, 6, 12, 24 and 48 h of incubation at the corresponding temperature, and to avoid plating the bacteriophage, samples were centrifuged at 3000× g for 10 min [65] for bacterial precipitation and the supernatant containing phage was the discarded. Any changes in respective of the bacterial viable count in both control and experimental group were determined by adding 1 mL of PBS, followed by vigorous vortex, serial dilution and plating at each time point.
For the recovered bacterial load from chicken, pre-cut 1 cm2 chicken breast sample was taken out after 0, 1, 3, 6, 12, 24 and 48 h of incubation then sample was transferred to 2 mL Eppendorf tube and 1 mL PBS buffer was added to the sample in a sterile environment. The chicken sample was homogenized with sterile bars and vortexed [50]. To avoid plating the bacteriophage, 1 mL homogenized sample was centrifuged at 3000× g for 10 min [65] for bacterial precipitation and the supernatant containing phage was then discarded. Any changes in respective of the bacterial viable count in both control and experimental group were determined by adding 1 mL of PBS, followed by vigorous vortex, serial dilution and plating at each time point.
2.8. Effect of Phage Cocktail Against Biofilm of Salmonella in 96-Well Microplate and on Stainless Steel Surface
2.8.1. Biofilm Assay in 96-Well Microplate
The colorimetric method was applied to quantitatively determine the effectiveness of the phage cocktail treatment on reducing biofilm of Salmonella according to the previously described method [66] with some adaptation. Overnight culture of Salmonella was prepared. In each well of the 96-well microplate, single Salmonella ATCC 14028 or a mixture of Salmonella enterica (ATCC 13076 and ATCC 14028) were inoculated into LB without NaCl, to reach a final viable count of 4 log10 CFU/mL. The microtiter plate was incubated at 30 °C (favorable temperature for biofilm formation) [67] for 72 h under static condition to allow bacteria to attach on well and further form biofilms. The medium was renewed every 24 h, followed by the phage cocktail treatment at a final titer of 7 log10 and 8 log10 PFU/mL. Phosphate-buffered saline (PBS) was used as control instead of phage. Samples were further incubated at 30 °C for 24 h. After phage cocktail treatment, each well was rinsed with PBS for 5 times and allow to air-dry. After rinse with PBS, 98% methanol was added, and left for 10 min. The methanol was then removed, and plates were air dried again. Samples were then stained with 1% crystal violet solution for 45 min followed by elution with 33% acetic acid. The OD of eluted sample was measured by a spectrometer at a wavelength of 600 nm. The biofilm reduction percentages were calculated according to the following formula [(C − B) − (T − B)]/[(C − B)] × 100 where C = average OD600nm of the control group, B = average OD600nm of blank wells containing test medium and T = average OD600nm of phage-treated wells.
2.8.2. Biofilm Assay on Stainless Steel Surface
The phage cocktail was tested for their ability to reduce biofilm cells from stainless steel (SS) coupons (1 cm × 1 cm square) according to published method [68] with some modification. The overnight bacterial culture was diluted 1:50 and inoculated into 10 mL LB without NaCl in 50 mL Falcon tubes; SS coupons were completely submerged in a Falcon tube containing 10 mL diluted bacteria to enable biofilm formation. The tubes were incubated without shaking at 30 °C for 72 h to enable development of biofilms on these coupons. Following coupons were transferred from the tube and washed five times with PBS to remove planktonic cells. The coupons were submerged in a tube containing 5 mL LB without NaCl and 5 mL phages solution with a final concentration of (7 log10 and 8 log10 CFU/mL) and incubated at 30 °C for 24 h. PBS used as a control instead of phage cocktail. Following incubation, SS coupons were rinsed with PBS 5 times and transferred to a sterile Petri dish that contained 1 mL of PBS, scrubbed, transferred to a test tube, and vortexed for 2 min to disperse the biofilm. The solution was centrifuged for 2 min at 12,000× g to separate bacteria from unabsorbed phage. Cells were diluted in PBS for counting. Salmonella was quantified by direct plating (viable count CFU/cm2). The logarithmic reduction of biofilm cells was calculated according to the following formula [log (untreated viable cell density)−log (treated viable cell density)].
2.9. Statistical Analysis
Food model assays and biofilm assays were done in triplicates and two samples per treatment were tested in each replicate. Results were reported as mean values of the three replicates with error bars suggesting the standard deviation of the mean. The bacterial and phage data were transformed to log10 units. The efficacy of phage cocktail in reducing the number of viable Salmonella in all foods and biofilms examined was evaluated by comparing the data obtained with the PBS-treated control samples to the phage cocktail-treated samples. Statistical analyses were performed by two-way analysis of variance (ANOVA) followed by Bonferroni’s test with 95% confidence interval using Prism 5.03 for Windows (GraphPad software, San Diego, CA, USA). Statistical significance was considered at significance level of p < 0.05.
3. Results
3.1. Isolation and Screening of Phages
A total of 42 different phages were isolated from environmentally sourced water samples using S. enterica as host. All isolated phages showed distinct difference in plaque size and turbidity from each other. All 42 isolated phages were able to lyse their host throughout the purification process. When these phages were screened by spot testing, 17% of isolated phages (7 out of 42) formed clear plaques and were capable to lyse at least two serovars (Table 2), whereas the rest of them were highly specific in infecting only their host. Spot test results showed that phages LPSTLL, LPST94, and LPST153 had broader host range compared to other phages (LPST81, LPST89, LPST109, and LPST115) isolated in this study (Table 2). Phages LPSTLL and LPST94 lysed all 41 (100%) tested strains that belong to 11 Salmonella serovars including drug-resistant Salmonella. Phage LPST153 lysed 50-100% strains of 9 Salmonella serovars (except 2 serovars; Newport and Kentucky). However, none of the phages isolated in this study were capable to lyse E. coli or other tested non-Salmonella bacteria. These results indicated that LPSTLL, LPST94 and LPST153 are Salmonella-specific and they all showed a broad lytic spectrum.
Table 2.
Sensitivity of different Salmonella serovars and other bacterial strains to lyse by selected phages determined by spot testing.
| Bacterial Strains | % of Positive Spot Test against Salmonella Serovars and Other Bacterial Strains | ||||||
|---|---|---|---|---|---|---|---|
| LPST81 | LPSTLL | LPST89 | LPST94 | LPST109 | LPST115 | LPST153 | |
| Salmonella serovars | |||||||
| Typhimurium (N = 7) | 71.4 | 100 | 100 | 100 | 100 | 71.4 | 85.7 |
| Enteritidis (N = 5) | 60 | 100 | 40 | 100 | 60 | 20 | 80 |
| Dublin (N = 2) | 0 | 100 | 0 | 100 | 50 | 0 | 50 |
| Choleraesuls (N = 1) | 0 | 100 | 0 | 100 | 0 | 0 | 100 |
| Newport (N = 1) | 0 | 100 | 100 | 100 | 0 | 0 | 0 |
| Paratyphi B (N = 1) | 100 | 100 | 0 | 100 | 100 | 0 | 100 |
| Anatum (N = 1) | 0 | 100 | 0 | 100 | 0 | 0 | 0 |
| Pullorum (N = 1) | 0 | 100 | 0 | 100 | 0 | 0 | 100 |
| Javiana (N = 1) | 0 | 100 | 100 | 100 | 0 | 0 | 100 |
| Kentucky (N = 1) | 0 | 100 | 0 | 100 | 0 | 0 | 0 |
| S. arizonae (N = 1) | 0 | 100 | 0 | 100 | 0 | 0 | 100 |
| Drug resistant Salmonella serovars | |||||||
| Typhimurium (N = 10) | 60 | 100 | 90 | 100 | 70 | 10 | 90 |
| Enteritidis (N = 9) | 11.1 | 100 | 33.3 | 100 | 22.2 | 11.1 | 88.9 |
| Other bacterial strains | |||||||
| E. coli (N = 6) | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| A. hydrophila (N = 4) | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
|
S. aureus (N = 3) Listeria (N = 1) |
0 0 |
0 0 |
0 0 |
0 0 |
0 0 |
0 0 |
0 0 |
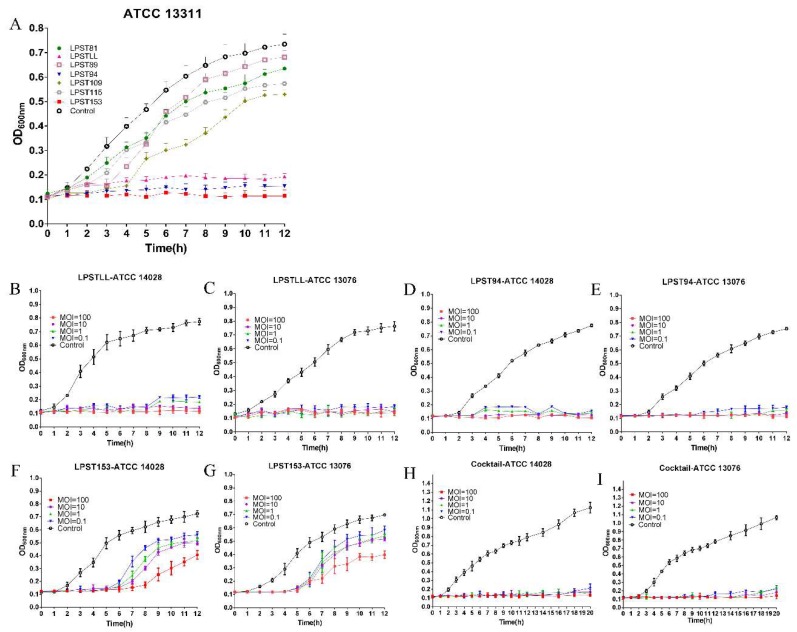
In the process of selecting the most effective phages, further screening was carried out by lytic activity test. Lytic activity assay was conducted for 7 phages that could lyse more the 2 strains of Salmonella (Figure 1A). Inhibited growth of host bacteria (S. Typhimurium ATCC 13311) in 2 h was observed for all 7 tested phages. After 2 h, phages LPSTLL, LPST94 and LPST153 constantly inhibited the growth of host in 12 h whereas other phages (LPST81, LPST89, LPST109, and LPST115) lost the ability to reduce bacterial cell numbers, which resulted in commence of bacterial growth (Figure 1A). Results of lytic activity indicated that LPSTLL, LPST94 and LPST153 had strong lytic capacity. Those phages were selected for further conforming lytic activity and make the cocktail using this three phages. The lytic activity profile of LPSTLL, LPST94, LPST153 and phage cocktail against Salmonella enterica serovar Typhimurium (ATCC 14028) and Salmonella enterica serovar Enteritidis (ATCC 13076) were also generated at MOI of 0.1, 1, 10 and 100 to confirm the findings. Phages LPSTLL and LPST94 could constantly inhibit the growth of both S. Typhimurium and S. Enteritidis in 12 h and LPST153 could in 5 h (Figure 1B–G). Phage cocktail could constantly inhibit the growth of both S. Typhimurium and S. Enteritidis with low counts at MOI ratios of 0.1, 1, 10 and 100 in 20 h (Figure 1H,I). Phage cocktail extended bacterial inhibition and exhibited strong lytic ability; therefore it could be a potential candidate for the control of Salmonella.
Figure 1.
(A) Comparison of the lytic ability of selected phages using S. enterica serovar Typhimurium (ATCC 13311) as a host at MOI of 1 in TSB broth; Lytic ability of phage LPSTLL to lyse S. enterica serovar Typhimurium and S. enterica serovar Enteritidis in TSB medium at different MOIs of 100, 10, 1 and 0.1 at 37 °C in vitro: (B) S. enterica serovar Typhimurium ATCC 14028, (C) S. enterica serovar Enteritidis ATCC 13076; Lytic ability of phage LPST94 to lyse S. enterica serovar Typhimurium and S. enterica serovar Enteritidis in TSB medium at different MOIs of 100, 10, 1 and 0.1 at 37 °C in vitro: (D) S. enterica serovar Typhimurium ATCC 14028, (E) S. enterica serovar Enteritidis ATCC 13076; Lytic ability of phage LPST153 to lyse S. enterica serovar Typhimurium and S. enterica serovar Enteritidis in TSB medium at different MOIs of 100, 10, 1 and 0.1 at 37 °C in vitro: (F) S. enterica serovar Typhimurium ATCC 14028, (G) S. enterica serovar Enteritidis ATCC 13076; and Lytic ability of phage cocktail to lyse S. enterica serovar Typhimurium and S. enterica serovar Enteritidis in TSB medium at different MOIs of 100, 10, 1 and 0.1 at 37 °C in vitro: (H) S. enterica serovar Typhimurium ATCC 14028, (I) S. enterica serovar Enteritidis ATCC 13076. Values represent mean with standard deviation of three replicates of each time point.
3.2. Host Range of Phages by Efficiency of Plating (EOP)
Phages LPSTLL, LPST94, LPST153 and their phage cocktail mix were analyzed by EOP to confirm their host range (Table 3). Phage cocktail revealed the broadest spectrum of lytic activity compared with single phages LPSTLL, LPST94 and LPST153 isolated in our study. Phage cocktail had a moderate to high efficiency (0.2 to 1.0) to infect all (N = 41) of Salmonella strains including drug-resistant Salmonella. For the single phage efficiency, LPST94 showed the broadest spectrum of lytic activity. This phage had a high efficiency (0.5 to 1.0) to infect the majority of S. Typhimurium strains but the EOP values were moderate (0.2 to 0.5) for some S. Enteritidis and drug-resistant Salmonella strains. The LPST94 phage could also lyse all drug-resistance Salmonella strains (N = 19) in our collection, the EOP values ranged from 0.001 to 0.2. LPSTLL lysed all 7 strains of S. Typhimurium with EOP values (0.5 to 1.0) and LPST153 could lyse S. Typhimurium (N = 7), the EOP values (0.1 to 1.0). These two phages also could lyse all S. Enteritidis (N = 5), and EOP values were between 0.001 to <0.2. The EOP values were 0.001 to <0.2 or negative for other serovars. As previous reported, S. Enteritidis and S. Typhimurium are the most common serovars that could cause salmonellosis by contaminated foods [69]. These results suggested that the phage cocktail has a wide host range of lytic activity (Table 3).
Table 3.
Efficiency of plating (EOP) by phages (LPSTLL, LPST94, LPST153 and cocktail) against different Salmonella serovars.
| Bacterial Strains | LPSTLL | LPST94 | LPST153 | Cocktail | Strains | LPSTLL | LPST94 | LPST153 | Cocktail |
|---|---|---|---|---|---|---|---|---|---|
| S.enterica serovar Typhimurium | Drug resistance S. enterica serovar Typhimurium | ||||||||
| ATCC 14028 | 1 | 1 | 0.18 | 1 | LST10 | 0 | 0.1 | 0.004 | 0.26 |
| ATCC 13311 | 1 | 1 | Host | 1 | LST11 | 0.19 | 0.4 | 0.003 | 0.48 |
| UK-1 | Host | Host | 0.17 | 1 | LST12 | 0.1 | 0.3 | 0.007 | 66 |
| ST8 | 1 | 1 | 0.1 | 1 | LST13 | 0.02 | 0.45 | 0.009 | 0.89 |
| SGSC 4903 | 1 | 1 | 1 | 1 | LST14 | 0.1 | 0.1 | 0.006 | 38 |
| SL 1344 | 1 | 1 | 1 | 1 | LST15 | 0.006 | 0.15 | 0.1 | 0.32 |
| LT2 | 1 | 1 | 1 | 1 | LST16 | 0.007 | 0.1 | 0.002 | 0.22 |
| S.enterica serovar Enteritidis | LST17 | 0 | 0.4 | 0.005 | 0.73 | ||||
| ATCC 13076 | 0.1 | 0.4 | 0.1 | 0.6 | LST18 | 0.009 | 0.1 | 0.010 | 0.24 |
| SJTUF 10978 | 0.02 | 0.24 | 0.19 | 0.4 | LST19 | 0 | 0.2 | 0.016 | 0.31 |
| SJTUF 10984 | 0.1 | 0.19 | 0.17 | 0.3 | Drug resistance S. enterica serovar Enteritidis | ||||
| LK5-3820 | 0.005 | 0.4 | 0.003 | 0.35 | LSE6 | 0 | 0.005 | 0 | 0.23 |
| SGSC 4901 | 0.18 | 0.3 | 0.12 | 0.5 | LSE7 | 0.017 | 0.1 | 0.004 | 0.37 |
| S.enterica serovar Dublin | LSE8 | 0.003 | 0.3 | 0.1 | 0.55 | ||||
| 3710 | 0 | 0.19 | 0 | 0.3 | LSE9 | 0.015 | 0.4 | 0 | 0.62 |
| 3723 | 0 | 0.2 | 0 | 0.25 | LSE10 | 0 | 0.1 | 0 | 0.23 |
| S. enterica serovar Choleraesuls | LSE11 | 0.1 | 0.006 | 0 | 0.29 | ||||
| ATCC 10708 | 0.009 | 0.16 | 0 | 0.3 | LSE12 | 0 | 0.2 | 0 | 0.27 |
| S. enterica serovar Newport | LSE13 | 0 | 0.007 | 0 | 0.24 | ||||
| E20002725 | 0 | 0.15 | 0 | 0.23 | LSE15 | 0.14 | 0.1 | 0.013 | 0.51 |
| S. enterica serovar Paratyphi B | |||||||||
| CMCC 50094 | 0.900 | 0.2 | 0.19 | 0.44 | |||||
| S. enterica Serovar Pullorum | |||||||||
| CVCC 519 | 0.017 | 0.2 | 0.1 | 0.67 | |||||
| S. enterica subsp. Enterica serovar Javiana | |||||||||
| CVM 35943 | 0.012 | 0.1 | 0.003 | 0.24 | |||||
| S. enterica subsp. enterica serovar Anatum | |||||||||
| ATCC 9270 | 0 | 0.007 | 0 | 0.21 | |||||
| S. enterica subsp. enterica serovar Kentucky | |||||||||
| CVM 29188 | 0.4 | 0.2 | 0.1 | 0.76 | |||||
| S. enterica subsp. arizonae | |||||||||
| CDC 346-86 | 0.004 | 0.2 | 0 | 0.27 | |||||
EOP 0.5 to 1.0, high efficiency; EOP 0.2 to <0.5, moderate efficiency; 0.001 to <0.2, low efficiency; and <0.001, inefficient.
3.3. Morphology of Phages and Stability of Phage Cocktail in Foods
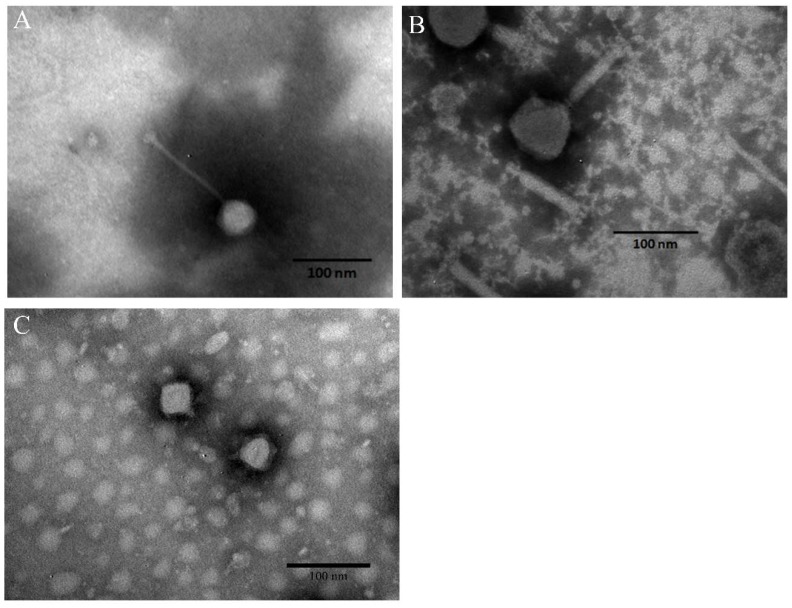
All three selected phages that were as identified tailed phages by transmission electron microscopic (TEM) and appeared to fall into the order of Caudovirales (Figure 2). Transmission electron microscopic examination of phage LPSTLL showed that it has isometric head with 55.27 ± 5.13 nm (n = 3) diameter, and a long non-contractile tail 126.8 ± 4.72 nm (n = 3) long (Figure 2A). These characteristics suggested that LPSTLL belonged to Siphoviridae family. Phage LPST94 had an icosahedral head, and a long, rigid and relatively thick contractile tail terminated in a baseplate with spikes as examined by TEM. The head of phage was 67.53 ± 2.20 nm in diameter (n = 3) and tail was 116.45 ± 4.05 nm long (n = 3) (Figure 2B). The morphology of the phage virion suggested that LPST94 belonged to the Ackermannviridae family. Phage LPST153 was found with short-tailed. The head diameter and tail lengths were 51.54 ± 4.70 nm and 7.33 ± 2.45 nm (n = 3), respectively (Figure 2C). TEM picture suggested that this phage belonged to the Podoviridae family. From the above results, it is suggested that the three selected phages belonged to three different families, Siphoviridae, Ackermannviridae, and Podoviridae.
Figure 2.
Morphological characteristics of phages. (A) TEM image of phage LPSTLL; (B) TEM image of phage LPST94 and (C) TEM image of phage LPST153. Bar 100 nm.
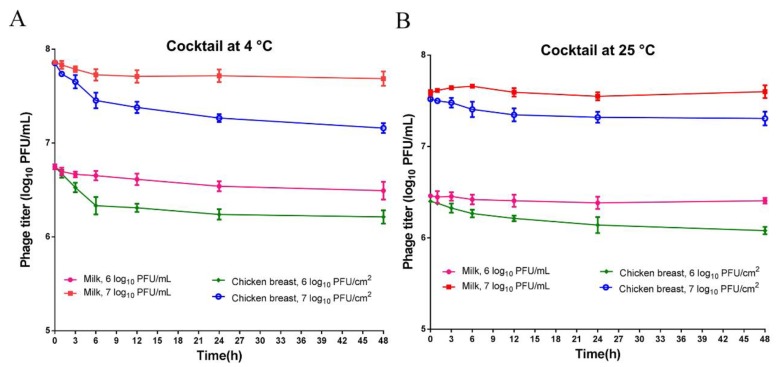
When phage cocktail was added to milk, and chicken breast, the titers remained stable with no inactivation observed at both 4 °C (Figure 3A) and 25 °C (Figure 3B). The results revealed that phage cocktail remained stable under the test conditions and could be a promising candidate to control Salmonella in foods.
Figure 3.
Stability of phage cocktail in foods (milk and chicken breast). Phage cocktail titer of 6 log10 PFU/mL and 7 log10 PFU/mL, incubated for 48 h at 4 °C (A) and 25 °C (B). Values represent mean with standard deviation of three replicates of each time point.
3.4. Application of Phage Cocktail in Controlling Food-borne S. Typhimurium and S. Enteritidis
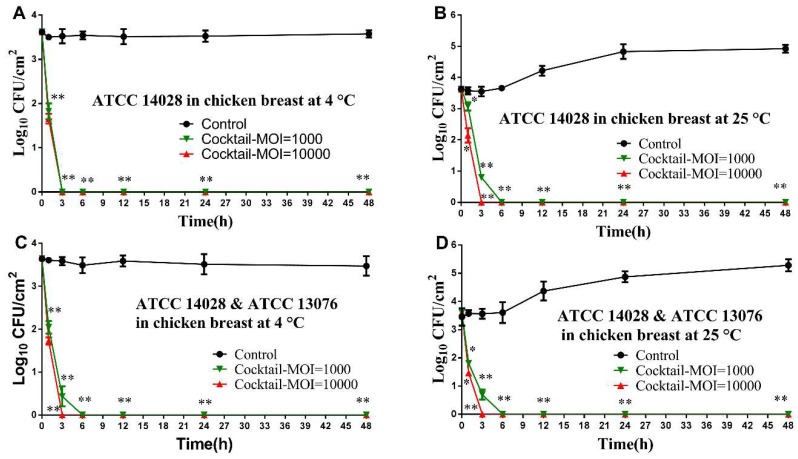
Phage cocktail composed of 1:1:1 mixture of phage LPSTLL, LPST94 and LPST153 was evaluated for biological control of experimentally contaminated milk and chicken breast. Samples were inoculated with either S. Typhimurium (ATCC 14028) at a final concentration 3 log10 CFU/mL alone, or a mixture of Salmonella (S. Typhimurium ATCC 14028 and S. Enteritidis ATCC 13076) culture at same concentration 3 log10 CFU/mL at 4 °C and 25 °C.
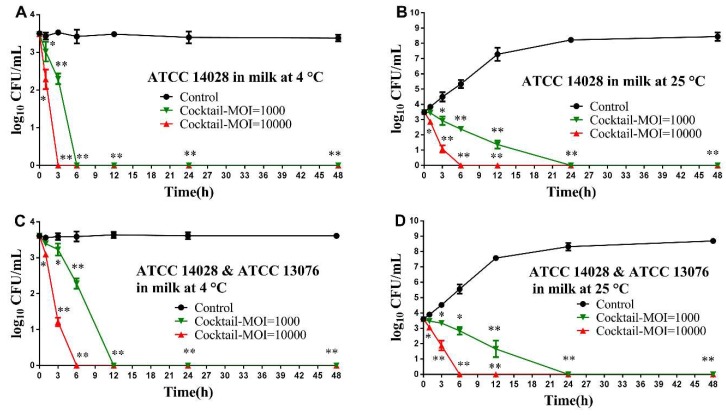
In milk assay, the effectiveness of phage cocktail to reduce Salmonella was remarkable; the viable count of the S. Typhimurium in milk was reduced below detectable limit (<1 CFU/100 μL) after 3 h and 6 h at 4 °C using an MOI of 10000 and 1000, respectively (Figure 4A). While at an MOI of 10000 and 1000 for single Salmonella or a mixture of Salmonella, the viable counts declined completely after 6 h and 24 h, respectively, at 25 °C (Figure 4B & 4D). For treatment against the mixture of Salmonella (S. Typhimurium ATCC 14028 and S. Enteritidis ATCC 13076), there was almost a complete elimination of Salmonella in milk after 6 h and 12 h at 4 °C with an MOI of 10000 and 1000, respectively (Figure 4C).
Figure 4.
Effectiveness of phage cocktail in reducing the S. Typhimurium ATCC 14028 and S. Enteritidis ATCC 13076 in milk. (A) Effect of phage cocktail on growth of S. Typhimurium ATCC 14028 in milk at 4 °C; (B) Effect of phage cocktail on growth of S. Typhimurium ATCC 14028 in milk at 25 °C; (C) Effect of phage cocktail on growth of Salmonella mixture (S. Typhimurium ATCC 14028 and S. Enteritidis ATCC 13076) in milk at 4 °C and (D) Effect of phage cocktail on growth of Salmonella mixture (S. Typhimurium ATCC 14028 and S. Enteritidis ATCC 13076) in milk at 25 °C. Values represent mean with standard deviation of three determinations. ** Significant at p < 0.01; * Significant at p < 0.05.
The ability of phage cocktail to reduce the level of artificially contaminated Salmonella on chicken breast is also demonstrated, there are no viable count could be recovered by direct plating after 3 h incubation using both MOIs of 10000 and 1000 at 4 °C (Figure 5A). Similarly, at 25 °C, the Salmonella counts were eliminated completely after 3 h and 6 h upon application of phage cocktail at an MOI of 10000 and 1000, respectively (Figure 5B). Like the observations of S. Typhimurium ATCC 14028, at both 4 °C and 25 °C, administration of phage cocktail with an MOI of 10000 and 1000 revealed a similar trend of reduction of Salmonella mixture (Figure 5C,D).
Figure 5.
Effectiveness of phage cocktail in reducing the S. Typhimurium ATCC 14028 and S. Enteritidis ATCC 13076 in chicken breast. (A) Effect of phage cocktail on growth of S. Typhimurium ATCC 14028 in chicken breast at 4 °C; (B) Effect of phage cocktail on growth of S. Typhimurium ATCC 14028 in chicken breast at 25 °C; (C) Effect of phage cocktail on growth of Salmonella mixture (S. Typhimurium ATCC 14028 and S. Enteritidis ATCC 13076) in chicken breast at 4 °C; and Effect of phage cocktail on growth of Salmonella mixture (S. Typhimurium ATCC 14028 and (D) S. Enteritidis ATCC 13076) in chicken breast at 25 °C. Values represent mean with standard deviation of three determinations. ** Significant at p < 0.01; * Significant at p < 0.05.
3.5. Effect of Phage Cocktail Against biofilm of Salmonella
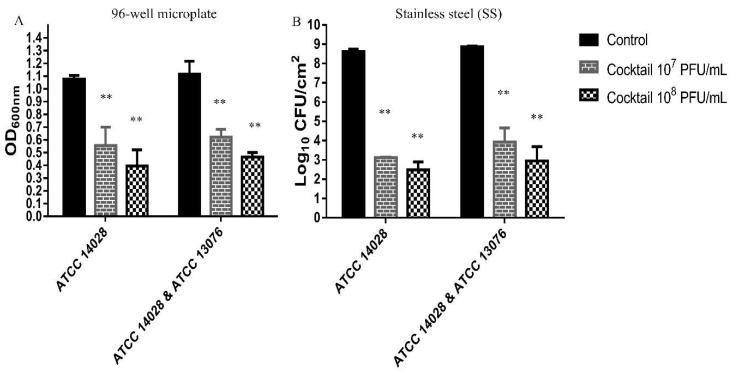
The efficacy of phage cocktail composed of 1:1:1 mixture of the phages LPSTLL, LPST94 and LPST153 against biofilm of S. Typhimurium (ATCC 14028) alone or a mixture of Salmonella (S. Typhimurium ATCC 14028 and S. Enteritidis ATCC 13076) in 96-well microplate or on stainless steel (SS) surface were evaluated at 30 °C (favorable temperature for biofilm formation) [67]. Figure 6 shows the reduction of biofilm after phage cocktail treatment with titers of 7 log10 PFU/mL and 8 log10 PFU/mL for 24 h. Treatment of S. Typhimurium (ATCC 14028) alone or a mixture of Salmonella (S. Typhimurium ATCC 14028 and S. Enteritidis ATCC 13076) with phage cocktail reduced the biofilm in 96-well microplate or on stainless steel (SS) surface significantly (p < 0.01). In 96-well microplate, the single S. Typhimurium (ATCC 14028) biofilm removal activity of 48.3% and 63.25% were respectively observed when phage cocktail was applied to a final titre of 7 log10 and 8 log10 PFU/mL (Figure 6A). For the mixture of Salmonella (S. Typhimurium ATCC 14028 and S. Enteritidis ATCC 13076), the concentration of biofilm decreased to 44.28% and 58.14% when 7 log10 and 8 log10 PFU/mL phage cocktail was applied, respectively (Figure 6A). Phage cocktail achieved average 5.50 log10 and 6.42 log10 reduction of single S. Typhimurium (ATCC 14028) biofilm with respective titers of 7 log10 and 8 log10 PFU/mL on a stainless steel surface (Figure 6B). Similarly, treatment with the mixture of Salmonella (S. Typhimurium ATCC 14028 and S. Enteritidis ATCC 13076), resulted in decreasing biofilms concentration of at least 5.23 log10 CFU/mL and 5.77 log10 CFU/mL with phage cocktail titers 7 log10 and 8 log10 PFU/mL, respectively (Figure 6B).
Figure 6.
Effect of phage cocktail on biofilm. (A) Effect of phage cocktail on 72-h-old biofilm in 96-well microplate and (B) on stainless steel surface at 30 °C after 24-h post-infection. Values represent mean with standard deviation of five determinations. ** Significant at p < 0.01.
4. Discussion
Phages are plentiful through surroundings, with approximately >1031 phages particles on the earth [70]. In this study, 42 phages were isolated from environmentally sourced water samples, which contained a low density of Salmonella strains [71,72]. It has been reported that sampling sites with low bacterial host density appear to have broad range lytic phage [73]. The selection of appropriate phages to be used in phage therapy based on spot test, and lytic activity against various Salmonella serovars [54,74]. Based on spot test results, phage LPSTLL, LPST94 and LPST153 showed broad lytic range activity as they could lyse 50–100% tested Salmonella serovars (S. Typhimurium, S. Enteritidis, S. Dublin, S. Kentucky, S. Paratyphi B etc.) including drug-resistant Salmonella. In the process of selecting the most effective phage, further screening of phages by lytic activity test was carried out [75,76]. Results of lytic activity indicated that LPSTLL, LPST94, and LPST153 had the strongest lytic capacity among all tested samples. The three phages LPSTLL, LPST94 and LPST153 tested in this study represented broad lytic range activity and high efficiency to inactivate the Salmonella.
From the lytic activity results, phage cocktail showed a higher lytic activity against Salmonella in vitro and could constantly inhibit the growth of S. Typhimurium ATCC 14028 and S. Enteritidis ATCC 13076 for up to 20 h compared with single applied phage LPSTLL, LPST94 and LPST153 that suppressed host growth for 12 h at MOIs of 0.1, 1, 10 and 100. Since different groups of phage recognize different receptors on host cell, a phage cocktail mix therefore could potentially delay the development of bacterial resistance or even prevent it. As the consequences of this, large proportion of bacteria will remain sensitive to certain phage cocktail candidates even after being infected, which will lead to a more severe viable count decline compared with singe phage treatment [77,78]. In contrast, single phage FGCSSa1, LPST10, LPST18, and LPST23 isolated by other researchers only revealed a 2–6 h inhibition with respect to host cell growth (S. Typhimurium PT160) at MOIs of 0.3–10 [54,71]. However, previously reported phage cocktail could inhibit their host growth for 12 h [79]. The above results are in accordance with other studies [45,75] that achieved higher inactivation by using phage cocktails, than that obtained with single-phage suspension. Mariann Landsberger and her coworkers investigated that one phage can cooperate to overcome CRISPR resistance of bacteria, leading to immunosuppression and leaving the host vulnerable to future, which allow other phage to infect and further lyse the bacteria [80]. Therefore, the phage cocktails approach has the potential to be one of the most promising choices to control Salmonella.
In this study, the three most virulent phages with the broadest host ranges within our collection were selected for establishment of phage cocktail for biocontrol applications and to reduce biofilm. As examined by TEM, three phages, LPSTLL, LPST94 and LPST153, belonged to the order of Caudovirales and family of Siphoviridae, Ackermannviridae and Podoviridae, respectively. Phages belong to these families all have the potential, as suggested by other literatures, to apply as biocontrol candidates against Salmonella and their biofilms [81,82,83,84,85,86].
In the phage stability study, it showed that phage cocktail effectivity remained stable over the tested periods both in milk and on chicken breast (Figure 3). However, small losses in phage titers in diverse food products (Chinese cabbage, chicken breast, mixed seafood and chocolate milk) were observed by other researches [52,87]. Our results suggested that phage cocktail is a suitable candidate for biological control of Salmonella in foods. Other researchers also showed that the phage cocktail could control pathogenic bacteria in foods, biofilms and food safety quality [88,89].
Application of the phage cocktail was predominantly effective against Salmonella in milk and on the surface of chicken breast, reducing the numbers of Salmonella counts down below the detectable limit (<1 CFU/100 μL) at both 4 °C and 25 °C with an MOI of 10000 and 1000. Because the high stability of phage cocktail could reduce the growth rate of bacteria at that temperature [90,91]. It has been demonstrated that, using high concentrations of phages generally achieved high reduction rates of pathogens [74,88,92]. When high titers are applied, phages are capable to absorb to the bacterial cells causing lysis to the cytoplasmic membrane without replication [10,93], reported as a process known as "lysis from without" [94]. Although very few study used a phage cocktail to control Salmonella in milk, some research applied a phage cocktail to reduce E. coli or Listeria and the bacterial counts dropped below the detectable limit in milk. In our study, phage cocktail application in milk led to the Salmonella count dropping below the detection limit when an MOI of 10000 and 1000 was applied, and this is consistent with the application of cocktails to reduce other bacteria [65,95]. Our results showed Salmonella counts (3 Log10 CFU/cm2) were eliminated completely with an MOI of 10000 and 1000 on chicken breast upon phage cocktail treatment. It was reported that, only 1 log CFU/g Salmonella viable count reduction was found either with single phage treatment at an MOI of 10000–1000000 [90,96] or with phage cocktail at an MOI of 10 [97] on chicken breast. The results demonstrated that the phage cocktail showed promise as biocontrol agents to control Salmonella in milk and on chicken breast compared with other similar studies and thereby could potentially reduce foodborne illness.
The biofilm study has shown that phage cocktail can infect Salmonella biofilm and has the potential to reduce tested S. Typhimurium and S. Enteritidis strains. The results suggested that phage cocktail treatment on two abiotic surfaces can effectively reduce the biofilms. It has been found that treatment with a phage cocktail eradicated post-treated biofilm in 96-well microplate (44–63%) and on stainless steel surface (ranging from 5.23 to 6.42 log10). Many types of research showed that effective biofilm eradications (ranging from 1 to 6 log), depend on the elements of the biofilm, the age of biofilm, phage effectiveness and length of treatment [98,99]. In this study, the data provided the proof of the principle that the application of phage cocktail could reduce the S. Typhimurium and S. Enteritidis in certain food types and to reduce biofilms on food contact surfaces that are important to maintain public health.
5. Conclusions
We isolated broad host lytic phages, LPSTLL, LPST94 and LPST153, prepared and characterized a phage cocktail with a broad spectrum of activity against diverse Salmonella serovars. Our results revealed that treatment of artificially contaminated foods with a phage cocktail completely lysed and eliminated the contaminating Salmonella, as well as eradiated the biofilms on food contact surfaces, indicating that this phage cocktail is a prime candidate for the biological control of Salmonella and can inactivate the biofilms that are resistant to traditional approaches.
Author Contributions
This work was carried out in collaboration with all authors. Conceptualization, M.S.I., Y.Z., and J.L.; Data curation, M.S.I., I.N., Y.Z., K.L., and T.Y.; Formal analysis, M.S.I., and J.L.; Funding acquisition, J.L.; Investigation, M.S.I., Y.Z., J.L. and X.W.; Methodology, M.S.I., Y.Z. and I.N.; Project administration, M.S.I., J.L. and X.W.; Resources, M.S.I., J.L.; Software, M.S.I. and J.L.; Supervision, J.L.; Validation, M.S.I. and J.L.; Visualization, M.S.I., Y.Z., and J.L.; Writing—original draft, M.S.I.; Writing—review & editing, J.L., L.L. and Y.Z. All authors read and approved the final manuscript.
Funding
This research was supported by the National Natural Science Foundation of China (31772083), Special fund for Technology Innovation of Hubei Province (2019AHB074), the National Key Research and Development Program of China (2017YFC1600100), the Fundamental Research Funds for the Central Universities (2662017JC040, 2662016QD010), China Scholarship Council (201806765004) and the National Innovation and Entrepreneurship Training Program for Undergraduates (201810504087, 201710504081). We would like to thank Prof. Ian F. Connerton’s group for their careful revision of the manuscript.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- 1.CDC FoodNet Fast Home Page. [(accessed on 22 March 2018)]; Available online: https://wwwn.cdc.gov/foodnetfast/
- 2.EFSA The European Union summary report on trends and sources of zoonoses, zoonotic agents and food-borne outbreaks in 2016. EFSA J. 2017;15:1–288. doi: 10.2903/j.efsa.2017.5077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Haraga A., Ohlson M.B., Miller S.I. Salmonellae interplay with host cells. Nat. Rev. Microbiol. 2008;6:53–66. doi: 10.1038/nrmicro1788. [DOI] [PubMed] [Google Scholar]
- 4.Pui C.F., Wong W.C., Chai L.C., Tunung R., Jeyaletchumi P., Hidayah M.S.N., Ubong A., Farinazleen M.G., Cheah Y.K. Salmonella: A foodborne pathogen. Int. Food Res. J. 2011;18:465–473. [Google Scholar]
- 5.CDC Salmonella Outbreak Investigations from 2018. [(accessed on 7 February 2019)]; Available online: https://www.cdc.gov/salmonella/outbreaks-2018.html.
- 6.Paudyal N., Anihouvi V., Hounhouigan J., Matsheka M.I., Sekwati-Monang B., Amoa-Awua W., Atter A., Ackah N.B., Mbugua S., Asagbra A. Prevalence of foodborne pathogens in food from selected African countries-A meta-analysis. Int. J. Food Microbiol. 2017;249:35–43. doi: 10.1016/j.ijfoodmicro.2017.03.002. [DOI] [PubMed] [Google Scholar]
- 7.Majowicz S.E., Musto J., Scallan E., Angulo F.J., Kirk M., O’Brien S.J., Jones T.F., Fazil A., Hoekstra R.M. The global burden of nontyphoidal Salmonella gastroenteritis. Clin. Infect. Dis. Off. Publ. Infect. Dis. Soc. Am. 2010;50:882. doi: 10.1086/650733. [DOI] [PubMed] [Google Scholar]
- 8.Hanning I.B., Nutt J.D., Ricke S.C. Salmonellosis outbreaks in the United States due to fresh produce: Sources and potential intervention measures. Foodborne Pathog. Dis. 2009;6:635–648. doi: 10.1089/fpd.2008.0232. [DOI] [PubMed] [Google Scholar]
- 9.Scallan E., Hoekstra R.M., Angulo F.J., Tauxe R.V., Widdowson M.A., Roy S.L., Jones J.L., Griffin P.M. Foodborne illness acquired in the United States-major pathogens. Emerging Infect. Dis. 2011;17:16–22. doi: 10.3201/eid1701.P21101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hooton S.P., Atterbury R.J., Connerton I.F. Application of a bacteriophage cocktail to reduce Salmonella Typhimurium U288 contamination on pig skin. Int. J. Food. Microbiol. 2011;151:157–163. doi: 10.1016/j.ijfoodmicro.2011.08.015. [DOI] [PubMed] [Google Scholar]
- 11.Multistate Outbreak of Salmonella Typhimurium Linked to Chicken Salad. Centers for Disease Control and Prevention. [(accessed on 11 October 2018)];2018 Available online: https://www.cdc.gov/salmonella/typhimurium-02-8/index.html.
- 12.Ke B., Sun J., He D., Li X., Liang Z., Ke C.W. Serovar distribution, antimicrobial resistance profiles, and PFGE typing of Salmonella enterica strains isolated from 2007–2012 in Guangdong, China. BMC Infect. Dis. 2014;14:338. doi: 10.1186/1471-2334-14-338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Huang X., Huang Q., Shi W., Liang J.H., Ling-Ling L.U., Deng X.L., Zhang Y.H. Epidemiological burden of nontyphoidal Salmonella infection in Guangzhou. Chin. J. Food Hyg. 2014;26:217–222. [Google Scholar]
- 14.Luo Q., Li S., Liu S., Tan H. Foodborne illness outbreaks in China, 2000–2014. Int. J. Clin. Exp. Med. 2017;10:5821–5831. [Google Scholar]
- 15.Stapels D.A.C., Hill P.W.S., Westermann A.J., Fisher R.A., Thurston T.L., Saliba A.-E., Blommestein I., Vogel J., Helaine S. Salmonella persisters undermine host immune defenses during antibiotic treatment. Science. 2018;362:1156–1160. doi: 10.1126/science.aat7148. [DOI] [PubMed] [Google Scholar]
- 16.White A.P., Gibson D.L., Kim W., Kay W.W., Surette M.G. Thin aggregative fimbriae and cellulose enhance long-term survival and persistence of Salmonella. J. Bacteriol. 2006;188:3219–3227. doi: 10.1128/JB.188.9.3219-3227.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Steenackers H., Hermans K., Vanderleyden J., Keersmaecker S.C.J.D., Steenackers H., Hermans K., Vanderleyden J., Keersmaecker S.C.J.D. Salmonella biofilms: An overview on occurrence, structure, regulation and eradication. Food Res. Int. 2012;45:502–531. doi: 10.1016/j.foodres.2011.01.038. [DOI] [Google Scholar]
- 18.Cogan T.A., Bloomfield S.F., Humphrey T.J. The effectiveness of hygiene procedures for prevention of cross-contamination from chicken carcases in the domestic kitchen. Lett. Appl. Microbiol. 2010;29:354–358. doi: 10.1046/j.1472-765X.1999.00656.x. [DOI] [PubMed] [Google Scholar]
- 19.Reij M.W., Den Aantrekker E.D., ILSI Europe Risk Analysis in Microbiology Task Force Recontamination as a source of pathogens in processed foods. Int. J. Food Microbiol. 2004;91:1–11. doi: 10.1016/S0168-1605(03)00295-2. [DOI] [PubMed] [Google Scholar]
- 20.Rodrigues D., Teixeira P., Oliveira R., Azeredo J. Salmonella enterica Enteritidis biofilm formation and viability on regular and triclosan-impregnated bench cover materials. J. Food Prot. 2011;74:32. doi: 10.4315/0362-028X.JFP-10-167. [DOI] [PubMed] [Google Scholar]
- 21.Corcoran M., Morris D., De Lappe N., O’Connor J., Lalor P., Dockery P., Cormican M. Commonly used disinfectants fail to eradicate Salmonella enterica biofilms from food contact surface materials. Appl. Environ. Microbiol. 2014;80:1507. doi: 10.1128/AEM.03109-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Perez-Rodriguez F., Valero A., Carrasco E., García R.M.A., Zurera G. Understanding and modelling bacterial transfer to foods: A review. Trends Food Sci. Technol. 2008;19:131–144. doi: 10.1016/j.tifs.2007.08.003. [DOI] [Google Scholar]
- 23.Chylkova T., Cadena M., Ferreiro A., Pitesky M. Susceptibility of Salmonella Biofilm and Planktonic Bacteria to Common Disinfectant Agents Used in Poultry Processing. J. Food Prot. 2017;80:1072. doi: 10.4315/0362-028X.JFP-16-393. [DOI] [PubMed] [Google Scholar]
- 24.Neetoo H., Mahomoodally F. Use of antimicrobial films and edible coatings incorporating chemical and biological preservatives to control growth of Listeria monocytogenes on cold smoked salmon. BioMed Res. Int. 2014;2014:534915. doi: 10.1155/2014/534915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Musyoka J.N., Abong G.O., Mbogo D.M., Fuchs R., Low J., Heck S., Muzhingi T. Effects of Acidification and Preservatives on Microbial Growth during Storage of Orange Fleshed Sweet Potato Puree. Int. J. Food Sci. 2018;2018:7435–7443. doi: 10.1155/2018/8410747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Latou E., Mexis S.F., Badeka A.V., Kontominas M.G. Shelf life extension of sliced wheat bread using either an ethanol emitter or an ethanol emitter combined with an oxygen absorber as alternatives to chemical preservatives. J. Cereal Sci. 2010;52:457–465. doi: 10.1016/j.jcs.2010.07.011. [DOI] [Google Scholar]
- 27.Pawlowska A.M., Zannini E., Coffey A., Arendt E.K. “Green preservatives”: Combating fungi in the food and feed industry by applying antifungal lactic acid bacteria. Adv. Food Nutr. Res. 2012;66:217. doi: 10.1016/B978-0-12-394597-6.00005-7. [DOI] [PubMed] [Google Scholar]
- 28.Khoshnoud M.J., Siavashpour A., Bakhshizadeh M., Rashedinia M. Effects of sodium benzoate, a commonly used food preservative, on learning, memory, and oxidative stress in brain of mice. J. Biochem. Mol. Toxicol. 2017;32:2. doi: 10.1002/jbt.22022. [DOI] [PubMed] [Google Scholar]
- 29.Hoiby N., Bjarnsholt T., Givskov M., Molin S., Ciofu O. Antibiotic resistance of bacterial biofilms. Int. J. Antimicrob. Agents. 2010;35:322–332. doi: 10.1016/j.ijantimicag.2009.12.011. [DOI] [PubMed] [Google Scholar]
- 30.Omer M.T., Syed A.A., Khalid J., Askari B. Study to evaluate the impact of heat treatment on water soluble vitamins in milk. JPMA J. Pak. Med. Assoc. 2010;60:909. [PubMed] [Google Scholar]
- 31.Leskova E., Kubíková J., Kováčiková E., Košická M., Porubská J., Holčíková K. Vitamin losses: Retention during heat treatment and continual changes expressed by mathematical models. J. Food Compos. Anal. 2006;19:252–276. doi: 10.1016/j.jfca.2005.04.014. [DOI] [Google Scholar]
- 32.Uribarri J., Woodruff S., Goodman S., Cai W., Chen X., Pyzik R., Yong A., Striker G.E., Vlassara H. Advanced glycation end products in foods and a practical guide to their reduction in the diet. J. Am. Diet. Assoc. 2010;110:911–916. doi: 10.1016/j.jada.2010.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mark C., Christian F., Enric M., Paul M.M., Ian P. The European ban on growth-promoting antibiotics and emerging consequences for human and animal health. J. Antimicrob. Chemother. 2003;52:159–161. doi: 10.1093/jac/dkg313. [DOI] [PubMed] [Google Scholar]
- 34.Matsuzaki S., Uchiyama J., Takemurauchiyama I., Daibata M. Perspective: The age of the phage. Nature. 2014;509:S9. doi: 10.1038/509S9a. [DOI] [PubMed] [Google Scholar]
- 35.Goodridge L.D., Bisha B. Phage-based biocontrol strategies to reduce foodborne pathogens in foods. Bacteriophage. 2011;1:130–137. doi: 10.4161/bact.1.3.17629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Woolston J., Parks A.R., Abuladze T., Anderson B., Li M., Carter C., Hanna L.F., Heyse S., Charbonneau D., Sulakvelidze A. Bacteriophages lytic for rapidly reduce contamination on glass and stainless steel surfaces. Bacteriophage. 2013;3:e25697. doi: 10.4161/bact.25697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Meng X., Shi Y., Ji W., Meng X., Zhang J., Wang H., Lu C., Sun J., Yan Y. Application of a bacteriophage lysin to disrupt biofilms formed by the animal pathogen Streptococcus suis. Appl. Environ. Microbiol. 2011;77:8272. doi: 10.1128/AEM.05151-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Keen E.C., Adhya S.L. Phage Therapy: Current Research and Applications. Clin. Infect. Dis. 2015;61:141–142. doi: 10.1093/cid/civ257. [DOI] [Google Scholar]
- 39.Kutter E.M., Gvasalia G., Alavidze Z., Brewster E. Phage therapy in clinical practice: treatment of human infections. Curr. Pharm. Biotechnol. 2010;11:69–86. doi: 10.2174/138920110790725401. [DOI] [PubMed] [Google Scholar]
- 40.Ruchi T., Kuldeep D., Amit K., Anu R., Sanjay K. Bacteriophage therapy for safeguarding animal and human health: A review. Pak. J. Biol. Sci. 2014;17:301. doi: 10.3923/pjbs.2014.301.315. [DOI] [PubMed] [Google Scholar]
- 41.Hyman P., Abedon S.T., Hyman P., Abedon S.T. Bacteriophages in Health & Disease. CABI; Wallingford, UK: 2012. Bacteriophages in health and disease. [Google Scholar]
- 42.Mccallin S., Alam S.S., Barretto C., Sultana S., Berger B., Huq S., Krause L., Bibiloni R., Schmitt B., Reuteler G. Safety analysis of a Russian phage cocktail: From metagenomic analysis to oral application in healthy human subjects. Virology. 2013;443:187–196. doi: 10.1016/j.virol.2013.05.022. [DOI] [PubMed] [Google Scholar]
- 43.Carlton R.M., Noordman W.H., Biswas B., Meester E.D.D., Loessner M.J. Bacteriophage P100 for control of Listeria monocytogenes in foods: Genome sequence, bioinformatic analyses, oral toxicity study, and application. Regul. Toxicol. Pharmacol. 2005;43:301–312. doi: 10.1016/j.yrtph.2005.08.005. [DOI] [PubMed] [Google Scholar]
- 44.Greer G.G. Bacteriophage control of foodborne bacteriat. J. Food Prot. 2005;68:1102. doi: 10.4315/0362-028X-68.5.1102. [DOI] [PubMed] [Google Scholar]
- 45.O’Flynn G., Coffey A., Fitzgerald G.F., Ross R.P. The newly isolated lytic bacteriophages st104a and st104b are highly virulent against Salmonella enterica. J. Appl. Microbiol. 2006;101:251–259. doi: 10.1111/j.1365-2672.2005.02792.x. [DOI] [PubMed] [Google Scholar]
- 46.O’Flynn G., Ross R.P., Fitzgerald G.F., Coffey A. Evaluation of a cocktail of three bacteriophages for biocontrol of Escherichia coli O157:H7. Appl. Environ. Microbiol. 2004;70:3417–3424. doi: 10.1128/AEM.70.6.3417-3424.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Abuladze T., Li M., Menetrez M.Y., Dean T., Senecal A., Sulakvelidze A. Bacteriophages reduce experimental contamination of hard surfaces, tomato, spinach, broccoli, and ground beef by Escherichia coli O157:H7. Appl. Environ. Microbiol. 2008;74:6230–6238. doi: 10.1128/AEM.01465-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sparvero L.J., Asafuadjei D., Rui K., Tang D., Amin N., Im J., Rutledge R., Lin B., Amoscato A.A., Zeh H.J. RAGE (Receptor for Advanced Glycation Endproducts), RAGE Ligands, and their role in Cancer and Inflammation. J. Transl. Med. 2009;7:17. doi: 10.1186/1479-5876-7-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Carter C.D., Parks A., Abuladze T., Li M., Woolston J., Magnone J., Senecal A., Kropinski A.M., Sulakvelidze A. Bacteriophage cocktail significantly reduces Escherichia coli O157: H7 contamination of lettuce and beef, but does not protect against recontamination. Bacteriophage. 2012;2:178. doi: 10.4161/bact.22825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Spricigo D.A., Bardina C., Cortés P., Llagostera M. Use of a bacteriophage cocktail to control Salmonella in food and the food industry. Int. J. Food Microbiol. 2013;165:169–174. doi: 10.1016/j.ijfoodmicro.2013.05.009. [DOI] [PubMed] [Google Scholar]
- 51.Albino L.A., Rostagno M.H., Húngaro H.M., Mendonça R.C. Isolation, characterization, and application of bacteriophages for Salmonella spp. biocontrol in pigs. Foodborne Pathog. Dis. 2014;11:602–609. doi: 10.1089/fpd.2013.1600. [DOI] [PubMed] [Google Scholar]
- 52.Bao H., Zhang P., Zhang H., Zhou Y., Zhang L., Wang R. Bio-Control of Salmonella Enteritidis in Foods Using Bacteriophages. Viruses. 2015;7:4836–4853. doi: 10.3390/v7082847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Galarce N., Escobar B., Rojas V., Navarro C., Turra G., Robeson J., Borie C. Application of a virulent bacteriophage cocktail leads to reduction of serovar Enteritidis counts in processed meat products. Biocontrol Sci. Technol. 2016;26:1–26. doi: 10.1080/09583157.2015.1125447. [DOI] [Google Scholar]
- 54.Huang C., Shi J., Ma W., Zhi L., Jia W., Li J., Wang X. Isolation, characterization, and application of a novel specific Salmonella bacteriophage in different food matrices. Food Res. Int. 2018;111:631–641. doi: 10.1016/j.foodres.2018.05.071. [DOI] [PubMed] [Google Scholar]
- 55.Srinivasan S., Harrington G.W., Xagoraraki I., Goel R. Factors affecting bulk to total bacteria ratio in drinking water distribution systems. Water Res. 2008;42:3393–3404. doi: 10.1016/j.watres.2008.04.025. [DOI] [PubMed] [Google Scholar]
- 56.Akhtar M., Viazis S., Diez-Gonzalez F. Isolation, identification and characterization of lytic, wide host range bacteriophages from waste effluents against Salmonella enterica serovars. Food Control. 2014;38:67–74. doi: 10.1016/j.foodcont.2013.09.064. [DOI] [Google Scholar]
- 57.Klieve A.V. Methods in Gut Microbial Ecology for Ruminants. Springer; Dordrecht, The Netherlands: 2005. Bacteriophages; pp. 39–46. [Google Scholar]
- 58.Mullan W.M. Bacteriophage and Food Fermentations. Phage Assay and Enumeration. [(accessed on 29 March 2018)]; Available online: https://slideplayer.com/slide/13011905/
- 59.Van T.R., Kropinski A.M. Bacteriophage enrichment from water and soil. Methods Mol. Biol. 2009;501:15. doi: 10.1007/978-1-60327-164-6_2. [DOI] [PubMed] [Google Scholar]
- 60.Jin W.J., Ji H.K., Sang P.S., Han J.E., Ji Y.C., Park S.C. Protective effects of the Aeromonas phages pAh1-C and pAh6-C against mass mortality of the cyprinid loach (Misgurnus anguillicaudatus) caused by Aeromonas hydrophila. Aquaculture. 2013;2:289–295. doi: 10.1016/j.aquaculture.2013.09.045. [DOI] [Google Scholar]
- 61.Huang C., Virk S.M., Shi J., Zhou Y., Willias S.P., Morsy M.K., Abdelnabby H.E., Liu J., Wang X., Li J. Isolation, Characterization, and Application of Bacteriophage LPSE1 Against Salmonella enterica in Ready to Eat (RTE) Foods. Front. Microbiol. 2018;9:1046. doi: 10.3389/fmicb.2018.01046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Mohammadali Khan M., Nilsson A.S. Correction: Isolation of phages for phage therapy: A comparison of spot tests and efficiency of plating analyses for determination of host range and efficacy. PLoS ONE. 2015;10:e0118557. doi: 10.1371/journal.pone.0118557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ackermann H.W. Phage classification and characterization. Methods Mol. Biol. 2009;501:127. doi: 10.1007/978-1-60327-164-6_13. [DOI] [PubMed] [Google Scholar]
- 64.Laguerre O., Derens E., Palagos B. Study of domestic refrigerator temperature and analysis of factors affecting temperature: A French survey. Int. J. Refrig. 2002;25:653–659. doi: 10.1016/S0140-7007(01)00047-0. [DOI] [Google Scholar]
- 65.Tomat D., Casabonne C., Aquili V., Balagué C., Quiberoni A. Evaluation of a novel cocktail of six lytic bacteriophages against Shiga toxinproducing Escherichia coli in broth, milk and meat. Food Microbiol. 2018;76:434–442. doi: 10.1016/j.fm.2018.07.006. [DOI] [PubMed] [Google Scholar]
- 66.Kostaki M., Chorianopoulos N., Braxou E., Nychas G.J., Giaouris E. Differential biofilm formation and chemical disinfection resistance of sessile cells of Listeria monocytogenes strains under monospecies and dual-species (with Salmonella enterica) conditions. Appl. Environ. Microbiol. 2012;78:2586–2595. doi: 10.1128/AEM.07099-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Else T.A., Pantle C.R., Amy P.S. Boundaries for Biofilm Formation: Humidity and Temperature. Appl. Environ. Microbiol. 2003;69:5006–5010. doi: 10.1128/AEM.69.8.5006-5010.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Shen C., Luo Y., Nou X., Bauchan G., Zhou B., Wang Q., Millner P. Enhanced Inactivation of Salmonella and Pseudomonas Biofilms on Stainless Steel by Use of T-128, a Fresh-Produce Washing Aid, in Chlorinated Wash Solutions. Appl. Environ. Microbiol. 2012;78:6789–6798. doi: 10.1128/AEM.01094-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Tarabees R., Msa E., Shawish R., Basiouni S., Shehata A.A. Isolation and characterization of Salmonella Enteritidis and Salmonella Typhimurium from chicken meat in Egypt. J. Infect. Dev. Ctries. 2017;11:314–319. doi: 10.3855/jidc.8043. [DOI] [PubMed] [Google Scholar]
- 70.Rohwer F., Segall A.M. In retrospect: A century of phage lessons. Nature. 2015;528:46–48. doi: 10.1038/528046a. [DOI] [PubMed] [Google Scholar]
- 71.Careysmith G.V., Billington C., Cornelius A.J., Hudson J.A., Heinemann J.A. Isolation and characterization of bacteriophages infecting Salmonella spp. FEMS Microbiol. Lett. 2006;258:182–186. doi: 10.1111/j.1574-6968.2006.00217.x. [DOI] [PubMed] [Google Scholar]
- 72.Bielke L., Higgins S., Donoghue A., Donoghue D., Hargis B.M. Salmonella Host Range of Bacteriophages That Infect Multiple Genera. Poult. Sci. 2007;86:2536–2540. doi: 10.3382/ps.2007-00250. [DOI] [PubMed] [Google Scholar]
- 73.Wongsuntornpoj S., Switt A.I.M., Bergholz P., Wiedmann M., Chaturongakul S. Salmonella phages isolated from dairy farms in Thailand show wider host range than a comparable set of phages isolated from U.S. dairy farms. Vet. Microbiol. 2014;172:345–352. doi: 10.1016/j.vetmic.2014.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Duc H.M., Son H.M., Honjoh K.I., Miyamoto T. Isolation and application of bacteriophages to reduce Salmonella contamination in raw chicken meat. LWT. 2018;91:353–360. doi: 10.1016/j.lwt.2018.01.072. [DOI] [Google Scholar]
- 75.Mateus L., Costa L., Silva Y.J., Pereira C., Cunha A., Almeida A. Efficiency of phage cocktails in the inactivation of Vibrio in aquaculture. Aquaculture. 2014;424–425:167–173. doi: 10.1016/j.aquaculture.2014.01.001. [DOI] [Google Scholar]
- 76.Pereira C., Silva Y.J., Santos A.L., Cunha Â., Gomes N.C.M., Almeida A. Bacteriophages with Potential for Inactivation of Fish Pathogenic Bacteria: Survival, Host Specificity and Effect on Bacterial Community Structure. Mar. Drugs. 2011;9:2236–2255. doi: 10.3390/md9112236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Tanji Y., Shimada T., Yoichi M., Miyanaga K., Hori K., Unno H. Toward rational control of Escherichia coli O157:H7 by a phage cocktail. Appl. Microbiol. Biotechnol. 2004;64:270–274. doi: 10.1007/s00253-003-1438-9. [DOI] [PubMed] [Google Scholar]
- 78.Jaewoo B., Byeonghwa J., Sangryeol R. Effective inhibition of Salmonella Typhimurium in fresh produce by a phage cocktail targeting multiple host receptors. Food Microbiol. 2019;77:52–60. doi: 10.1016/j.fm.2018.08.011. [DOI] [PubMed] [Google Scholar]
- 79.Pereira C., Moreirinha C., Lewicka M., Almeida P., Clemente C., Romalde J.L., Nunes M.L., Almeida A. Characterization and in vitro evaluation of new bacteriophages for the biocontrol of Escherichia coli. Virus Res. 2017;227:171–182. doi: 10.1016/j.virusres.2016.09.019. [DOI] [PubMed] [Google Scholar]
- 80.Landsberger M., Gandon S., Meaden S., Rollie C., Chevallereau A., Chabas H., Buckling A., Westra E.R., Houte S.V. Anti-CRISPR Phages Cooperate to Overcome CRISPR-Cas Immunity. Cell. 2018;174:908–916. doi: 10.1016/j.cell.2018.05.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Novacek J., Šiborová M., Benešík M., Pantůček R., Doškař J., Plevka P. Structure and genome release of Twort-like Myoviridae phage with a double-layered baseplate. Proc. Natl. Acad. Sci. USA. 2016;113:9351. doi: 10.1073/pnas.1605883113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Hooton S.P., Timms A.R., Rowsell J., Wilson R., Connerton I.F. Salmonella Typhimurium-specific bacteriophage ΦSH19 and the origins of species specificity in the Vi01-like phage family. Virol. J. 2011;8:1–14. doi: 10.1186/1743-422X-8-498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Sylwia P., Magdalena K., Romuald G., Lidia M., Anna M. Bacteriophages as an alternative strategy for fighting biofilm development. Pol. J. Microbiol. 2014;63:137. [PubMed] [Google Scholar]
- 84.Sunderland K., Yang M., Mao C. Phage-Enabled Nanomedicine: From Probes to Therapeutics in Precision Medicine. Angew. Chem. 2016;56:1964. doi: 10.1002/anie.201606181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Yele A.B., Thawal N.D., Sahu P.K., Chopade B.A. Novel lytic bacteriophage AB7-IBB1 of Acinetobacter baumannii: Isolation, characterization and its effect on biofilm. Arch. Virol. 2012;157:1441–1450. doi: 10.1007/s00705-012-1320-0. [DOI] [PubMed] [Google Scholar]
- 86.Islam M.S., Raz A., Liu Y., Elbassiony K.R.A., Dong X., Zhou P., Zhou Y., Li J. Complete Genome Sequence of Aeromonas Phage ZPAH7 with Halo Zones, Isolated in China. Microbiol. Resour. Announc. 2019;8:e01678-18. doi: 10.1128/MRA.01678-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Guenther S., Herzig O., Fieseler L., Klumpp J., Loessner M.J. Biocontrol of Salmonella Typhimurium in RTE foods with the virulent bacteriophage FO1-E2. Int. J. Food Microbiol. 2012;154:66–72. doi: 10.1016/j.ijfoodmicro.2011.12.023. [DOI] [PubMed] [Google Scholar]
- 88.El-Shibiny A., El-Sahhar S., Adel M. Phage applications for improving food safety and infection control in Egypt. J. Appl. Microbiol. 2017;123:556. doi: 10.1111/jam.13500. [DOI] [PubMed] [Google Scholar]
- 89.Moye Z., Woolston J., Sulakvelidze A. Bacteriophage Applications for Food Production and Processing. Viruses. 2018;10:205. doi: 10.3390/v10040205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Sharma C.S., Dhakal J., Nannapaneni R. Efficacy of Lytic Bacteriophage Preparation in Reducing Salmonella in Vitro, on Turkey Breast Cutlets, and on Ground Turkey. J. Food Prot. 2015;78:1357. doi: 10.4315/0362-028X.JFP-14-585. [DOI] [PubMed] [Google Scholar]
- 91.Sharma M., Patel J.R., Conway W.S., Ferguson S., Sulakvelidze A. Effectiveness of bacteriophages in reducing Escherichia coli O157:H7 on fresh-cut cantaloupes and lettucet. J. Food Prot. 2009;72:1481. doi: 10.4315/0362-028X-72.7.1481. [DOI] [PubMed] [Google Scholar]
- 92.Bigwood T., Hudson J.A., Billington C. Influence of host and bacteriophage concentrations on the inactivation of food-borne pathogenic bacteria by two phages. FEMS Microbiol. Lett. 2010;291:59–64. doi: 10.1111/j.1574-6968.2008.01435.x. [DOI] [PubMed] [Google Scholar]
- 93.Abedon S. Phage therapy pharmacology: Calculating phage dosing. Adv. Appl. Microbiol. 2011;77:1. doi: 10.1016/B978-0-12-387044-5.00001-7. [DOI] [PubMed] [Google Scholar]
- 94.Abedon S.T. Lysis from without. Bacteriophage. 2011;1:46–49. doi: 10.4161/bact.1.1.13980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Guenther S., Huwyler D., Richard S., Loessner M. Virulent Bacteriophage for Efficient Biocontrol of Listeria monocytogenes in Ready-To-Eat Foods. Appl. Environ. Microbiol. 2009;75:93–100. doi: 10.1128/AEM.01711-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Sukumaran A.T., Nannapaneni R., Kiess A., Sharma C.S. Reduction of Salmonella on chicken meat and chicken skin by combined or sequential application of lytic bacteriophage with chemical antimicrobials. Int. J. Food Microbiol. 2015;207:8–15. doi: 10.1016/j.ijfoodmicro.2015.04.025. [DOI] [PubMed] [Google Scholar]
- 97.Yeh Y., Purushothaman P., Gupta N., Ragnone M., Verma S.C., Mello A.S.D. Bacteriophage application on red meats and poultry: Effects on Salmonella population in final ground products. Meat Sci. 2017;127:30–34. doi: 10.1016/j.meatsci.2017.01.001. [DOI] [PubMed] [Google Scholar]
- 98.Corbin B.D., Rjc M.L., Aron G.M. Bacteriophage T4 multiplication in a glucose-limited Escherichia coli biofilm. Can. J. Microbiol. 2001;47:680–684. doi: 10.1139/w01-059. [DOI] [PubMed] [Google Scholar]
- 99.Sharma M., Ryu J.H., Beuchat L.R. Inactivation of Escherichia coli O157:H7 in biofilm on stainless steel by treatment with an alkaline cleaner and a bacteriophage. J. Appl. Microbiol. 2010;99:449–459. doi: 10.1111/j.1365-2672.2005.02659.x. [DOI] [PubMed] [Google Scholar]