Abstract
Mycotoxins are ubiquitous and unavoidable harmful fungal products with the ability to cause disease in both animals and humans, and are found in almost all types of foods, with a greater prevalence in hot humid environments. These mycotoxins vary greatly in structure and biochemical effects; therefore, by better understanding the toxicological and pathological aspects of mycotoxins, we can be better equipped to fight the diseases, as well as the biological and economic devastations, they induce. Multiple studies point to the association between a recent increase in male infertility and the increased occurrence of these mycotoxins in the environment. Furthermore, understanding how mycotoxins may induce an accumulation of epimutations during parental lifetimes can shed light on their implications with respect to fertility and reproductive efficiency. By acknowledging the diversity of mycotoxin molecular function and mode of action, this review aims to address the current limited knowledge on the effects of these chemicals on spermatogenesis and the various endocrine and epigenetics patterns associated with their disruptions.
Keywords: mycotoxins, spermatogenesis, spermiogenomics, infertility, epigenetics
1. Underestimated Potent Environmental Disruptor: Mycotoxins
Mycotoxins are a heterogeneous group of low molecular weight toxic fungal products with the ability to cause disease in humans and other vertebrates [1,2,3,4]. They are ubiquitous and unavoidable harmful agents [5] found in almost all types of foods, including cereals [6] and wheat derivatives [7], animal [8] and dairy [9] products, fruits [10], and even wine [11,12]. Owing to the vast differences in their structure and biochemical effects [1], some mycotoxins are more prevalent in certain countries and continents than others. In fact, African countries tend to have higher levels of mycotoxin contamination [13,14] due to the optimal conditions for fungal growth such as high temperatures, elevated moisture levels, and lack of proper hygienic measures [13]. A 2012 study reports that 96% of Tunisian and 50% of Moroccan staple foods were contaminated with mycotoxins [15], most commonly by nivalenol and beauvericin. Similarly alarming, a large percentage of processed feeds in Asia and the Americas, feeds in Europe, wheat from Australia [16], and edibles in Lebanon [17] all tested positive for mycotoxins. Furthermore, the average dietary exposure levels to ochratoxin A (OTA) and deoxynivalenol (DON) in a Lebanese urban population far exceeded the toxicological reference values (TRVs) [18]. By better understanding the toxicological and pathological aspects of mycotoxins, we can be better equipped to fight these diseases, and the biological and economic devastation they might induce.
Mycotoxins pose a major health hazard to both animals and humans in general, and a recent increase in male infertility has been associated with the increased occurrence of these mycotoxins in the environment [19]. Infertility rates have worsened from 42 to 48.5 million couples worldwide between 1990 and 2010 [20], affecting 1 out of 7 couples trying to conceive [21]. In fact, a 1% yearly average decline of sperm concentration was recorded in semen quality studies between 1938 and 1991 [20,22,23], alongside decreased sperm parameters and total motile sperm count (TMSC), and increased morphological abnormalities [22,23]. This alarmingly continuous decline of sperm count and human fertility worldwide are attributed to many factors, among which, the significant role of endocrine-disrupting chemicals (EDCs), such as mycotoxins and pesticides [24,25], are not well established.
Although an international scientific consensus on sperm count rate was not achieved throughout the 1970s, a comprehensive review [20] covering a 50-year longitudinal study reported irrefutable evidence of declining semen quality. As several studies attempted to tackle the potential causes of this decline, Bahadur et al. [26] suggested environmental pollution and lifestyle factors as decisive influences on reproductive health with a possible endocrine underlying cause, a possible intimation of epigenetics. Since then, an accumulating body of evidence suggests that pre-conceptional exposure to lifestyle and environmental factors impact the phenotype of the current and subsequent generations through epigenetic mechanisms and developmental plasticity [27]. Dietary habits [28], starvation [29], psychological traumas [30], alcohol consumption [31], smoking [32], toxins [33], physical activity [34], and other emerging factors have all been implicated in influencing the phenotype of organisms and their progeny. These non-genetic interventions, specifically though regulatory mechanisms known as epigenetics, regulate gene expression rather than induce gene mutations [35]. Evidence is leaning toward these mechanisms in compromising the phenotype of the next generation through the remodeling of the epigenetic blueprint of spermatozoa [36]. Cells respond to environmental stressors through increased epigenetic responsiveness and variations providing an adaptive capacity for living things and a developmental plasticity for their future progeny [37]. Further, under the influence of parental or congenital environments, chromatin dynamics can fluctuate between permissive and repressive states to control gene expression transiently or heritably [38]. The sources capable of inducing such epigenetic deregulation can be traced back to internal factors, such as inflammation [39], the microbiome [40], and aging [41], and less widely studied lifestyle and external exposures such as cannabis [42]; pollution and chemical agents [43]; nutrition [44]; fungal toxins, such as Aflatoxins [45,46,47,48,49,50]; ochratoxin [51,52,53] and DON [54]; and endocrine disruptors [55]. In fact, aberrant epigenetic modifications could be the source of many serious human diseases, syndromes, and developmental complications [56]; dysregulated methylation of promoters may silence critical genes involved in tumor suppression, as in the case of hepatocellular carcinoma [57], breast cancer [58], and leukemia [59], or it may disrupt important signaling pathways leading to psychiatric and mental disorders, such as schizophrenia [60] and autism [61], or cardiovascular and metabolic diseases, and even contribute to male infertility [62]. Although the causes of male infertility still remain elusive despite ongoing and extensive investigations, recent studies associate it to epigenetic abnormalities in chromatin states [63], sperm-borne miRNAs [64], and methylation levels of PIWIL1/2 alleles [65], and insinuate possible transgenerational implications [66]. Therefore, both genetic and epigenetic toxicology studies can help in revealing the causes behind decreasing semen quality, and possibly establish the effect of mycotoxins on reproductive health. With the diversity of mycotoxin molecular function and mode of action, this review aims at addressing the current limited knowledge on the effects of these chemicals on spermatogenesis and the various endocrine patterns associated with their disruptions.
2. Spermatogenesis: A Complex and Delicate Process
In mammals, spermatogenesis is a complex process involving the division and differentiation of spermatogonial stem cells into mature spermatozoa [67,68,69,70,71] that takes place in the convoluted seminiferous tubules of the testis [70,71]. The seminiferous tubules are pipe-like structures containing both germ and supporting somatic cells [72,73], which are surrounded by testosterone-producing Leydig cells and blood vessels [74]. Leydig cells are responsible for the production of testosterone [75], without which, spermatogenesis would not advance beyond meiosis [76], while Sertoli cells control the environmental milieu of tubules and facilitate differentiation of spermatozoa via direct contact [77]. With the proper support and signaling from the Sertoli and Leydig cells, germ cells undergo a stepwise differentiation and maturation process from the basement membrane of the seminiferous tubules to the lumen where the differentiated germ cells [72,73] are released into the rete testes as spermatozoa, then travel through the epididymis in preparation for capacitation and ejaculation as mature sperm cells [68,74,77,78]. This process occurs in three major phases, namely spermatocytogenesis, meiosis, and spermiogenesis [68,72]. During spermatocytogenesis, germ cells undergo a cycle of numerous mitotic divisions, generating a cell population from which some contribute to the renewal and maintenance of the stem cell population, while others differentiate to produce spermatogonia and primary spermatocytes [67,68,69] in the meiosis phase. This stage is marked with the duplication and exchange of genetic material crucial for genetic diversity; two successive cell divisions reduce the chromosome number in half and yield four haploid round spermatids [67,68,69]. Subsequently, the last phase of spermiogenesis induces the differentiation of these round spermatids into completely mature, though non-fertilizing, spermatozoa [67,68,69]. The intricate nature of this entire process makes it prone to several types of disruptions at multiple time points, which can jeopardize the quantity and/or quality of spermatogenic yield, leading to reproductive complications [79].
The hypothalamo–pituitary–gonadal (HPG) axis acts in concert through feedback loops to orchestrate the crucial events of spermatogenesis. Gonadotropin releasing hormone (GnRH), a hormone periodically released by the hypothalamus, stimulates the pituitary to release Luteinizing (LH) and Follicle Stimulation (FSH) hormones [80] in a pulsatile fashion [81]. LH stimulates the Leydig cells to produce testosterone [80], which subsequently acts as a negative feedback loop on GnRH production [82]. Altogether, these hormones control steroidogenesis, the complex, multi-enzymatic process through which cholesterol is converted into biologically active steroid hormones [83] such as testosterone secreted by the testicular cells [84]. This process is mediated by the steroidogenic Sertoli and Leydig cells, which express the cholesterol side-chain cleavage enzyme (P450scc) [85]. Steroidogenesis starts with the conversion of cholesterol to pregnenolone by P450scc [83], a slow-acting, rate-limiting enzyme [86], followed by its conversion to progesterone by 3β-Hydroxysteroid dehydrogenase (3βHSD) or to 17α-hydroxypregnenolone by P450c17 [87,88,89]. 3βHSD is also involved in the conversion of 17α-hydroxypregnenolone to 17α-hydroxyprogesterone (17OHP), dehydroepiandrosterone (DHEA) to androstenedione, and androstenediol to testosterone [90]. Other reactions include the conversion of testosterone to dihydrotestosterone by 5α-reductases or to estrogens by aromatases (P450aro) [91,92].
Highlighting the multi-enzymatic and hormonal aspects of spermatogenesis is important to understand the environmental disturbances affecting male infertility, where mycotoxins have been shown to interfere at various levels and disrupt the activity of P450scc, 3βHSDs, 5α-reductases, and/or P450aro in both males and females.
2.1. Effect of Mycotoxins on Fertility
With a clearly complex yet delicate balance of enzymatic and hormonal control of steroidogenesis, it is important to understand the minute impact of mycotoxins on such processes. Unfortunately, research on the effect of mycotoxin in males, specifically on spermatogenesis [93,94,95], steroidogenesis [96], or even the HPG axis [97,98,99,100], is scarcely available in the literature. Few clues drawn from the effect of various mycotoxins on steroidogenesis from female studies [101,102,103,104] portray the biological threat mycotoxins can engender [105]. DON can reduce epididymal, seminal vesicle, and prostate weights; spermatid count; and serum testosterone concentrations, while inducing sperm tail and nuclear morphology abnormalities, in a dose dependent fashion in rats [106,107]. In females, DON inhibited estradiol and progesterone secretion in bovine granulosa cells, increased oocyte and granulosa cell apoptosis, reduced porcine oocyte maturation capacity via autophagy, and induced aberrant epigenetic modifications [96,108]. Furthermore, zearalenone (ZEA) has been found to induce a dose-dependent reduction of aromatase, P450scc, and 3βHSD transcripts in cultured porcine granulosa cells from porcine ovaries [104,109], while beauvericin inhibited estradiol and progesterone synthesis in bovine granulosa cells [101], and showed antagonism toward progesterone cell lines, where it decreased the binding of progesterone to its receptor [110]. In addition, beauvericin exerted potent cytotoxic effects on lung cell surrogates [111] and ovarian hamster cells [112]. Furthermore, in vitro cultured porcine oocytes and embryos exposed to physiological levels of beauvericin showed damaged development [113]. Altogether, the few studies on mycotoxin’s effect on the female reproductive systems establish mycotoxins as possible endocrine disruptors; similar negative reproductive effects in the male reproductive system are anticipated, as reviewed in the following sections.
2.2. Effect of Mycotoxins on Sertoli Cells
The research linking mycotoxins specifically to Sertoli cells is scarce and mostly involves the mycotoxins as summarized in Table 1. Furthermore, the diversity of models and dosage used does not allow for a clear determination of the specific mycotoxin effect on Sertoli cell physiology, gene expression, and dynamics.
Table 1.
Effects of mycotoxins on Sertoli cells.
| Mycotoxin | Species | Dose | Exposure | Main Findings with Respect to Sertoli Cells | Ref. |
|---|---|---|---|---|---|
| CTN | Mouse | 0–200 μM | 6–72 h | Decreased cell viability and proliferation Increased apoptosis, and necrosis in a dose-dependent manner |
[114] |
| DON | Mice | 10 ppm | 90 days | No effect on relative testis weight and testicular spermatid counts No effect on the number of Sertoli cells in the seminiferous tubules |
[107] |
| FB1 | Rabbit | 0.13–10 mg/kg diet * | 196 days | Degeneration of Sertoli cell | [115] |
| OTA | Mice TM4 | 0–5 μM | 24 h | Decreased proliferation Dose-dependent phosphorylation of PI3K (Akt, P70S6K, and S6) and MAPK (ERK1/2 and JNK) pathways |
[94] |
| T-2 | SerW3 cells | 0.012–1.2 μg/mL (0.025–25.72 μM) | 24–48 h | Increased cytotoxicity in a dose-dependent manner Targets blood-testis barrier in vitro |
[116] |
| ZEA | Rat | 0–10 nM | 48 h | Negatively influenced spermatogenesis and male fertility ZEA effect inhibited by in vitro addition of anti-estrogen (ICI 182.780) → ZEA estrogenic activity |
[117] |
| 0–20 g/mL | 24 h | Damages the cytoskeletal structure Disrupts specific secretory functions |
[118] | ||
| 0–200 μM | 6–36 h | Induces apoptosis and necrosis via extrinsic and intrinsic apoptotic pathways | [119] | ||
| 0–20 μmol/L (0–62.3 μM) | Induces apoptosis Activates the Fas-Fas ligand signaling pathway Regulates mitochondrial apoptosis pathway |
[120] | |||
| 20 mg/kg BW * | 5 weeks | Increased serum prolactin No effect on testis weights, serum luteinizing hormone, and follicle-stimulating hormone |
[121] | ||
| 4 or 40 μg | 16 days | Weak estrogen effect on Sertoli cell development in pre-pubertal rats | [122] | ||
| Mice TM4 | 0–100 μM | 24 h | TM4 cell cycle G2/M arrest Apoptosis through ROS- and ER-stress and the ATP/AMPK pathway |
[123] |
* In vivo studies fed a set amount per Kg of Body weight; CTN—citrinin; DON—deoxynivalenol; FB1—fumonisin B1, OTA—ochratoxin A; T-2 = trichothecene-2; ZEA—zearalenone. In parentheses, measures converted to μM.
For example, zearalanone (ZEA) is an estrogenic mycotoxin found to damage Sertoli cells and potentially induce apoptosis [117,118,119] in both mice and rat. The induction of apoptosis and necrosis of rat Sertoli cells by ZEA via extrinsic and intrinsic apoptotic pathways suggest that its reproductive toxicity may be navigated by multiple pathways [119]. The addition of the anti-estrogen ICI 182.780 [117] or other antioxidant enzymes [124] inhibited the effects of ZEA in adult rats [117] and mice [124] neonatally exposed to ZEA, suggesting that its effects are at least partially modulated by its estrogenic activity. Moreover, ZEA treatment can damage the cytoskeletal structure and affect the specific secretory functions of Sertoli cells, which may be an underlying cause of ZEA-induced reproductive toxicity [118].
2.3. Effect of Mycotoxins on Leydig Cells
Mycotoxins also act directly on Leydig cells, disrupting crucial enzymatic and hormonal activities. Table 2 shows the potent steroidogenic and cytotoxic effects of various mycotoxins on Leydig cells. Specifically, aflatoxin B1 (AFB1) can directly reduce testosterone concentration in a dose-dependent manner and inhibit the expression of 3βHSD and 17β-hydroxysteroid dehydrogenase enzymes (HSD17B3) [125]. In mice, AFB1 upregulated renin mRNA along with 193 extracellular matrix and signaling genes, 49 signal transduction genes, 45 immune regulation genes, and 230 cell differentiation genes in the testis [126]. Thus, AFB1 exhibits wide-ranging effects on mRNA expression, but whether this translates into anything meaningful in terms of protein expression remains to be elucidated. Citrinin (CTN) also reduced testosterone levels [127] by inducing the apoptosis of Leydig cells, possibly via p53 expression and activation of multiple caspases. Furthermore, T-2 decreased testosterone levels in mice in a dose-dependent manner [128]. Similarly, ZEA caused a dose- and time-dependent inhibition of testosterone stimulated both by suppression of hCG (10 ng/mL) [128,129] and cAMP [130], while DON exhibited the most cytotoxicity out of seven other tested mycotoxins [131] in MA-10 murine Leydig cell lines.
Table 2.
Effects of mycotoxins on Leydig cells.
| Mycotoxin | Species | Dose | Exposure | Main Findings with Respect to Leydig Cells | Ref. |
|---|---|---|---|---|---|
| AFB1 | Mouse | 50 μg/kg BW * | 45 days | Upregulation of genes involved in cell differentiation, extracellular space, and immunity | [126] |
| Rat | 0–10 μM | 35 days | Extra-hepatic toxicity by inhibition of proteins involved in androgen biosynthesis such as StAR, HSDB3, and HSD17B3 | [125] | |
| CTN | 50 and 100 μM | 36 h | Reduced testosterone secretion Induced apoptosis |
[127] | |
| T-2 | Mouse | 1–102 μM | 24 h | Dose-dependent decrease in testosterone levels | [128] |
| ZEA | 0–20 μg/mL (0–62.3 μM) | 1–24 h | Dose- and time-dependent inhibition of testosterone stimulated by both hCG and cAMP | [130] | |
| 0.01–100 μM | 24 h | Suppressed hCG-induced testosterone secretion | [129] | ||
| 5 μM | 24 h | Modified mitochondrial lipid metabolism Increased energy production Inhibited steroidogenesis and esterification |
[132] | ||
| 0–200 μg/mL (0–623 μM) | 24 h | ER stress pathway activated in ZEA-induced apoptosis | [133] | ||
| Rat | 2.5–20 μg/mL (7.8–62.3 μM) | 12 h | Investigation of anti-ZEA compounds | [134] |
* In vivo study; AFB1—aflatoxin B1; CTN—citrinin; T-2—trichothecene-2; ZEA—zearalenone. In parentheses, measures converted to μM.
2.4. Effect of Mycotoxins on Spermatogenesis
Spermatogonial stem cells (SSCs) are at the foundation of spermatogenesis and male fertility. Similar to other tissue-specific stem cell niches, SSCs are rare, representing only 0.03% of all germ cells in rodent testes [135]. Thus, any cytotoxic effect of mycotoxins on this small percentage of cells can compromise all consecutive processes. An exhaustive search of the literature on the effect of various mycotoxins on SSCs are summarized in Table 3, and show a potent dose and time dependent effect of mycotoxins on these SSCs.
Table 3.
Effect of mycotoxins on spermatogenesis in vivo.
| Toxin | Species | Exposure | Daily Dose * | Effect on Spermatogenesis | Ref. |
|---|---|---|---|---|---|
| AFB1 | Rats | 60 days | 10–50 µg | Reduction of reproductive organ weights and sperm quantity and quality Decreased steroidogenesis |
[138] |
| 48 days | 0.8–3.2 ppm | Dose-dependent decrease of developing spermatozoa in seminiferous tubules | [139] | ||
| CTN | Mice | 7 days | 0.0625–6.25 mg | Increased abnormal spermatozoa Decreased live spermatozoa number and count, and serum testosterone |
[137] |
| DON | Rats | 28 days | 0.5–5 mg | Decreased testicular spermatid numbers Increased germ cell degeneration, sperm retention, and abnormal nuclear morphology |
[106] |
| FB1 | Pigs | 6 months | 0.2–15 mg | Reduced testicular and epididymal sperm reserves Reduced daily sperm production No influence on the relative weights and volume of the testes or epididymis |
[140,141] |
| Rabbits | 175 days | 0.13–10 mg | Delayed puberty, impaired semen quality and spermatogenesis, and induced embryo mortality | [142] | |
| OTA | Rats | 8 weeks | 289 µg | Decrease in stages I and VII germ cells Increase in stages XII and XIII germ cells |
[93] |
| Patulin | Rats | 60–90 days | 0.1 mg | Increased sperm counts Decreased sperm counts |
[143] |
| T-2 | Mice | 7 days | 0–15 mg | Increased abnormal spermatozoa Decreased testicular and cauda epididymal sperm counts, efficiency of sperm production, and serum testosterone concentrations |
[144] |
| ZEA | Rats | 48 h | 5 mg | Germ cell degeneration, especially spermatogonia and spermatocytes | [145] |
| Mice | 7 days | 0–75 mg | Dose-dependent reduction of testicular and cauda epididymal sperm counts and serum testosterone | [129] |
AFB1—aflatoxin B1; CTN—citrinin; DON—deoxynivalenol; FB1—fumonisin B1, OTA—ochratoxin A; T-2—trichothecene-2; ZEA—zearalenone; * Per kg body weight, except ppm in water per animal.
The nonsteroidal estrogenic mycotoxin ZEA is known to cause toxicity within the testes of male rats [136]. Histopathology of ZEA-treated mice (5 mg/kg BW (body weight)) revealed that 12 h after treatment, germ cell degeneration occurred in stages I–VI with the damaged germ cells, especially spermatogonia and spermatocytes, gradually undergoing apoptosis. Also, daily DON exposure for 28 days via gastric intubation of male rats reduced body weight, feed consumption, and epididymal and seminal vesicle weights, while increasing germ cell degeneration, sperm retention, and abnormal nuclear morphology [106]. Similar detrimental effects were seen in adult male mice exposed to a daily intraperitoneal injection of CTN for 7 days with an increase in the number of abnormal spermatozoa and a decrease in the number of live spermatozoa in a dose-dependent manner [137].
Furthermore, intramuscular injection of increasing doses of AFB1 (10, 20, or 50 µg/kg BW) in adult rats resulted in the reduction of reproductive organ weights, daily sperm production, epididymal sperm count, viable sperm, and motile sperm [138]. Similarly, the T-2 toxin showed an increase in the number of abnormal spermatozoa and a decrease in spermatozoa count in adult male mice, and these resulted in low pregnancy rates and high fetal resorption after exposure to 10 and 15 mg/kg BW T-2 toxin [144].
3. Epigenetic Implications
As previously discussed, mycotoxins are implicated in negatively affecting male and female reproductive physiology, altering fertility. Furthermore, epigenetics, the recent field of multigenerational outer-chromosomal inheritance, is gaining momentum in the literature. Therefore, introducing epigenetic mechanisms and the effect of environmental contaminants is imperative to setting the stage for our reviewing the available literature on the epigenetic impact of mycotoxins on male infertility, spermatogenesis, and steroidogenesis. Very few studies have addressed the effect of mycotoxins directly on the male reproductive epigenome. Altogether, epigenetic implications in any process, especially a lagging field of mycotoxin effects on male infertility, is essential to gaining a full scope on the topic and further consideration during future research planning.
3.1. Epigenetic Mechanisms and Environmental Exposure
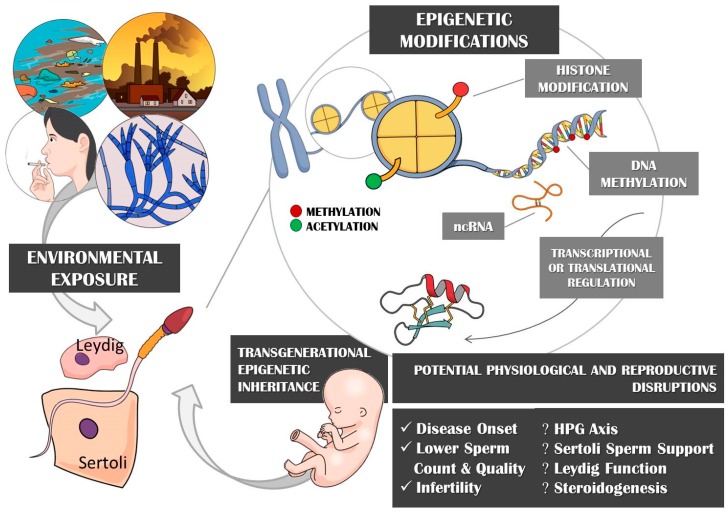
Epigenetics comprise changes in phenotype resulting from differential gene regulation rather than DNA sequence alterations, with these epigenetic regulations playing critical and ubiquitous roles in a wide range of cellular and developmental processes [146]. Recent advances in this field relate epigenetic mechanisms to covalent modifications of DNA (cytosine methylation and hydroxymethylation) [147] and histones (lysine acetylation, lysine and arginine methylation, serine and threonine phosphorylation, and lysine ubiquitination and sumoylation) [148], production of RNA transcripts (miRNA, mRNA, and sRNA) [149], prions [150], and nucleosome positioning [151]. These different control mechanisms, as summarized in Figure 1, can simultaneously be recruited in response to environmental stressors.
Figure 1.
Epigenetic involvement in transgenerational reproductive control.
The most widely studied of the epigenetic modifications is DNA methylation; it plays a critical role in important cellular and developmental processes such as cell differentiation and embryonic development [152]. DNA methyltransferases (DNMTs) are the main components involved in methylating DNA, though other enzymes have also been discovered [153]. The main targets of methylation are the cytosines of CpG islands found near promoters, which can modulate the expression of a given gene, although non-CpG methylation has been observed in embryonic stem cells and neural development [154]. Histone modifications have also been implicated in diverse biological processes such as gene regulation, DNA repair, chromosome condensation (mitosis), and spermatogenesis (meiosis) [155]. Despite the identification of several histone modifications, the functional understanding of these modifications remains unclear. In addition to their control of gene expression, miRNAs received a recent highlight due to their involvement in altering the stress reactivity of the zygote post-fertilization. When sires were exposed to chronic paternal stress, they showed sperm-borne miRNAs alterations with a mechanistic role [156]. In addition to the different regulatory mechanisms, epigenetic patterns can also be altered directly on the DNA by bioactive compounds or indirectly by affecting the enzymes that catalyze DNA methylation and histone modification [157,158], which would explain the diversity of epigenetic responses. Nutrients and bioactive substances, such as retinoic acid, resveratrol, curcumin, sulforphane, and tea polyphenols [159], have been postulated to adopt both strategies. Adding a further complexity to this process suggests that these environmental effects can induce specific or unspecific DNA methylation changes that might affect genetic pathways directly or indirectly. Hence, the multiple mechanisms adopted by epigenetic control can have a global and devastating effect on the organism, which explains the fact that aberrant epigenetic modifications have been correlated with diverse human diseases, syndromes, and developmental defects.
3.2. Contribution of Epigenetics in Environmentally-Induced Disease Predisposition
Although the study of epigenetics initially focused on the role it plays as a regulatory system, recent landmark observations have linked environmental exposure and gene–environment interactions to a significant proportion of human malignancies. The Dutch Hunger Winter remains a classic example of such environmental interactions where pregnant women during the famine period gave birth to children and future generations who exhibited higher rates of obesity, diabetes, schizophrenia, and mortality upon adulthood [144]. The latter set forward the study of transgenerational epigenetic inheritance, where the germline undergoes epigenetic modifications inherited by future generations despite the extensive epigenetic resetting event, with epigenetic tags on some loci associated with metabolic and neurological disorders that can escape this reprogramming [160].
3.3. Epigenetics Involvement in Germline Modulation and Infertility
Investigations of paternal effects demonstrated further that environmental factors are capable of inducing changes in the sperm, and this in turn would affect the organism itself and modulate the developmental programming of the offspring by transgenerational epigenetic inheritance. A high-fat diet given to rats induced modified epigenetic sperm profiles; metabolic dysfunction was apparent throughout two generations [161]. Embryonic male rats exposed to the endocrine disruptor vinclozolin in utero (through maternal administration) manifested adult-onset diseases in the first generation that persisted in four subsequent generations; sperm epigenome modification following treatment at the time of gonadal sex determination enabled this transgenerational transmission. Furthermore, changes in DNA methylation of the male germline resulted in transcriptional changes in several tissues, such as testes, brain, and prostate, which consequently led to adult-onset pathologies including testicular, prostate, and renal abnormalities, and increased incidence of tumors [162,163,164]. The persistence of these epigenetic signatures is contingent with the type and timing of epimutations that could intervene throughout several windows of vulnerability [165,166] and generate different types of epigenetic responses. Furthermore, an array-based DNA methylation profiling in male infertility established the role of allele-specific DNA hypermethylation of PIWIL1/2 involved in RNA-mediated gene silencing [55]. Sperm of mice brought up on a folate-deficient diet showed altered methylation patterns for genes associated with chronic diseases, autism, and development [137]. Moreover, sperm quality and pregnancy rate were improved when males were supplemented with vitamins, such as methyl-group donor folic acid and micronutrients, that might be involved in modifying the epigenome [138]. While alcohol consumption induces a global and unspecific methylation profile change [139], male rats sustained on a high-fructose diet showed modified DNA methylation at peroxisome proliferator-activated receptor α (PPARα) and carnitine palmitoyltransferase 1Acarnitine palmitoyltransferase 1A (CPT1A) promoter regions in their liver [140] and male mice on a low-protein diet had offspring showing modified key lipid and cholesterol biosynthesis genes in the liver [141], which may have resulted from specific modified DNA methylation of the paternal germline.
3.4. Epigenetic Effects of Mycotoxins in Disease and Infertility
The effect of mycotoxins on the modulations of the epigenome as they relate to the male reproductive system and infertility are not well studied. Depending on the timing of the epimutations (germline or zygote), tissues originating from these modified cells would harbor alterations predisposing or leading to a wide range of malignancies [167,168]. For example, long-term exposure to low doses of the carcinogenic AFB1 induces persistent epigenetic changes in primary human hepatocytes, promoting the development of hepatocellular carcinoma [159]. On the other hand, fumonisin B1 exclusively caused DNA hypermethylation in C6 glioma cells associated with human esophageal cancer [143]. Recently, exposure of pregnant women to the mycotoxin Zearalenone/Zearalenol was associated with disease susceptibilities of the progeny [129]. Furthermore, in utero exposure to some mycotoxins severely compromised postnatal development of neonatal rats and delayed testes descent, and it impaired both steroidogenesis and spermatogenesis, inducing a suppressed reproduction at adulthood [160,161]. Additionally, a mycotoxin-induced miRNA modification in various tissues targeted specific genes and increased DNA damage, proliferation, apoptosis, homeostasis, cancer, migration, oxidative stress, and detoxification [132]. Further, OTA revealed deregulated DNA methylation, ncRNA production, and histone modifications associated with cell apoptosis, oxidative stress, cell autophagy, and protein synthesis inhibition [133], whereas histone acetyltransferases regulated OTA toxicity and carcinogenicity [131].
Altogether, these limited studies indicate the possible involvement of mycotoxins in altering the various female and male reproductive cells, be it directly acting on the germline or indirectly via a steroid-like function. However, the targeted role of a given mycotoxin on the various components in male spermatogenesis requires further investigation.
3.5. Transgenerational Epigenetic Inheritance through Imprinted Genes
Both internal and external factors are capable of disturbing the crucial genetic and epigenetic regulation involved in germline development such as the complex process of spermatogenesis. Germline epimutations are capable of inducing drastic implications on an organism compared to epigenetic mosaicism where modifications are contained within specific types of tissues rather than expressed in the whole body [169,170]. During the intricate process of spermatogenesis, germ cells undergo crucial chromatin and step-wise epigenetic changes marked with differential DNA methylation patterns, global shifts in histone post-translational modifications, and production of certain miRNAs that together induce their gradual differentiation into functional sperm cells [171]. The mammalian germline undergoes an extensive remodeling and epigenetic reprogramming that is paramount for imprinting and regulating embryogenesis [172]. Two major reprogramming events underline mammalian development: primordial germ cells (PGCs) go through an almost genome-wide methylation erasure that is hypothesized to wipe out all cellular memory to reach totipotency [173], following which, the fertilized zygote is re-methylated de novo throughout its gradual cleavage and differentiation [174]. Nonetheless, this initial methylation erasure is incomplete to allow some intact and crucial genomic features (escapees) to evade this event [175]; functional analysis of these regions reveals several critical genes involved in brain and neuronal development [176]. However, this event also opens a dangerous developmental time window susceptible to the transfer of environmentally altered epigenetic marks or insults that may impact fertility and embryonic competence [177]. Cannabis (THC) exposure seems to alter methylation patterns of genes in the sperm of rats and humans, causing lower sperm concentrations with a possible transgenerational aftermath [42]. Further, cannabis alters sperm count [178], and even modifies the sperm itself [42], with a significant overlap between THC target genes in rat sperm and aberrantly methylated genes in the brain of rats born to parents exposed to THC during adolescence [42]. During each round of spermatogenesis (SSC to sperm), epigenetic patterns and chromatin states are re-established to generate efficient mature sperm [179]; however, this process is prone to errors and epimutations induced from either internal or environmental sources [180].
A chronic unhealthy lifestyle (lack of exercise, smoking, and drinking) can induce epimutational accumulation in SSCs and contribute to decreased male fertility and poor transgenerational outcomes [21,181,182]. SSCs undergoing clonal amplification before entering meiosis and differentiation can accumulate aberrant epigenetic modifications, which can be carried on to the mature sperm, increasing the chances of these epimutations to be transferred to the next generation [183]. Associations between chemotherapy exposure and aberrant changes in sperm parameters and epigenetic mutations in the spermatogonial stem cell population may compromise human sperm integrity and potentially be transmitted to future generations [184]. Even in vitro processes, such as embryonic stem cell differentiation into SSCs, have been shown to epigenetically alter the germline and promote abnormalities transgenerationally in mice [185,186], further raising a serious question regarding the long-term safety or efficiency of therapeutic stem-cell-based applications. One type of epimutation can transpire through DNA methylation, which in normal circumstances is responsible for important physiological roles such as X inactivation and genomic imprinting [187]. Inheritance and expression of traits associated with imprinted genes is regulated through epigenetic marks; imprinting causes only one copy of the genes to be functional while the other one is silenced in a parent-of-origin manner [188,189]. This monoallelical expression compromised by mutations or epimutations poses more serious implications than biallelically expressed genes; aberrant sperm DNA methylation of imprinted genes is linked to spermatogenic impairments and abnormalities [190,191,192].
Many imprinted genes are clustered and regulated by the single imprinting control region (ICR) [190]. ICRs are part of regulatory regions known as differentially methylated regions (DMRs) that entail discrete DNA elements with a heritable spot useful for distinguishing parental origin [193]. Epigenetic aberrations in imprinted genes have been associated with adverse effects on cancer [194], embryogenesis, nervous system development, and DNA repair [172,195]. Subfertile males harbored dysregulated sperm methylation profiles associated with abnormal sperm parameters [196]; specifically, hypomethylation of H19 and hypermethylation of SNRPN imprint control regions [196,197,198,199], which is exacerbated further by cigarette smoking [200]. Therefore, exposure to reproductive environmental toxins during critical windows of mammalian development can trigger irreversible and heritable epigenetic tags.
Male mice treated with low and high concentrations of ZEA show altered expressions of testicular genes involved in methylation such as Ccnd1, Kdm4a, and Spata2 [95]. Similar effects were seen in human subjects exposed to Bisphenol A (BPA) where sperm DNA hydroxymethylation of several known sperm functional and sperm associated genes were involved in embryonic stem cell differentiation, growth, and early development [201].
4. Challenges to the Study of the Effect of Mycotoxins on Male Spermatogenesis
Challenges to studying the effect of mycotoxins on the male reproductive cells and spermatogenesis are multifaceted, ranging from the established toxicity of these substances to their vast diversity and effects, and to the lack of an easy isolation, purification, and culture system for spermatogenic cells.
First, the toxicity levels of many mycotoxins have not been established in many of the model species, especially farm animals where the majority of the effects are expected as these mycotoxin infections are becoming ubiquitous in farm operations. Many studies [117,124,134,202,203], though not reviewed herein, have focused on antagonizing the effect of these mycotoxins, and many products are marketed for farm animal application to counter the mycotoxin effects. Added to this scarcity of investigations, the majority of the studies observed these mycotoxins as disruptors in females [100,101,102,103,104], and only a few in males [93,94,95,143,204,205], with most of these studies observing the effect of mycotoxins in animal models rather than in cell culture.
Second, isolating Sertoli or Leydig cells is generally a time-consuming and very delicate process that also necessitates the availability of fresh tissue, complex enzymatic digestion steps of the seminiferous tubules, followed by segregation of the various cells using flow cytometry and adhesion purification techniques such as DSA-lectin or gradient centrifugation. Moreover, with spermatogonial stem cells (SSCs) culture being essential to male infertility therapy in humans [206], endangered species preservation, and transgenic animal technology, isolation of these cells have been exhaustively described in the literature [206,207,208,209,210,211,212,213,214], though the survival of these cells beyond 48 to 96 hours in culture is challenging [211,212,215] as they require continuous hormonal or co-culture stimulation. Additionally, identifying the various cells before culture can prove challenging [207,208,210,212,213,216,217], thus many adopt marker-assisted selection following culture, though this technique only reports a percentage of each cell type in the culture.
Therefore, benchmarking of the isolation, purification, and culture of male reproductive cells is essential to determining the effect of individual mycotoxins on spermatogenesis, steroidogenesis, toxicity, and epigenetic marks; this benchmarking will help better decipher the long-term impact farm animals and humans are experiencing when exposed to mycotoxins.
5. Conclusion and Future Directions
Spermatogenesis is a complex process involving a multitude of cells and an interdialogue between neuroendocrine processes. Therefore, any minor disruption of any of the players might lead to dire consequences, including alteration of sperm quality and quantity, infertility, or inconspicuous modifications of the genome or epigenome. With many mycotoxins presenting steroidogenic-like and toxic effects, further focus on male spermatogenesis and the various players and cells involved is needed in order to better understand the immediate reproductive consequences, as well as the risks to future generations. Understanding how epimutations accumulate during parental lifetimes can shed light on their implications regarding fertility, reproductive competence and outcome, and offspring health. Due to the nature of spermatogenesis marked by continuous cycles of mitosis and meiosis, adult males are more prone to accumulating and storing environmentally induced epigenetic alterations than females. Although recent technological advances for epigenetic profiling have been significant, there still remains a need for a systematic understanding of how epigenetics shapes cellular circuitry and disease pathogenesis. These epigenetic modifications may provide possible molecular justifications to understand heritability or predisposing factors observed in some diseases. With the limited amount of research on the effects of mycotoxins and EDCs on male reproduction and fertility, and the challenges associated with culturing the various cells involved, better isolation and culture techniques and further studies are required to determine the crucial time of exposure to environmental toxins and identify factors that result in germline-transmitted adult-onset diseases and those that have an epigenetic basis.
Author Contributions
Conceptualization, D.E.K. and P.Y.A; writing, S.F., M.N., and J.S.; visualization, J.S.; supervision, D.E.K. and P.Y.A.; review, J.S., D.E.K., and P.Y.A.; editing, D.E.K. and P.Y.A.
Funding
This research received no external funding.
Conflicts of Interest
The authors declare no conflict of interest.
Key Contribution
This review presents a new look at the available data on mycotoxins in view of possible epigenetic effects they might cause. It focuses on the available studies and data on the effect of mycotoxins on spermatogenesis, steroidogenesis and the cells involved, and the hypothalamo-pituitary action. Also; this review highlights the gaps in our understanding of the molecular and physiological effects of mycotoxins.
References
- 1.Bennett J.W., Klich M. Mycotoxins. Clin. Microbiol. Rev. 2003;16:497–516. doi: 10.1128/CMR.16.3.497-516.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hope J. A review of the mechanism of injury and treatment approaches for illness resulting from exposure to water-damaged buildings, mold, and mycotoxins. Sci. World J. 2013;2013 doi: 10.1155/2013/767482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Doi K., Uetsuka K. Mechanisms of mycotoxin-induced neurotoxicity through oxidative stress-associated pathways. Int. J. Mol. Sci. 2011;12:5213–5237. doi: 10.3390/ijms12085213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brewer J., Thrasher J., Hooper D. Chronic illness associated with mold and mycotoxins: Is naso-sinus fungal biofilm the culprit? Toxins. 2014;6:66–80. doi: 10.3390/toxins6010066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schatzmayr G., Streit E. Global occurrence of mycotoxins in the food and feed chain: Facts and figures. World Mycotoxin J. 2013;6:213–222. doi: 10.3920/WMJ2013.1572. [DOI] [Google Scholar]
- 6.Bryła M., Waśkiewicz A., Podolska G., Szymczyk K., Jędrzejczak R., Damaziak K., Sułek A. Occurrence of 26 mycotoxins in the grain of cereals cultivated in Poland. Toxins. 2016;8:160. doi: 10.3390/toxins8060160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Assaf H., Betbeder A.-M., Creppy E.E., Pallardy M., Azouri H. Ochratoxin A levels in human plasma and foods in Lebanon. Hum. Exp. Toxicol. 2004;23:495–501. doi: 10.1191/0960327104ht481oa. [DOI] [PubMed] [Google Scholar]
- 8.Marin S., Ramos A., Cano-Sancho G., Sanchis V. Mycotoxins: Occurrence, toxicology, and exposure assessment. Food Chem. Toxicol. 2013;60:218–237. doi: 10.1016/j.fct.2013.07.047. [DOI] [PubMed] [Google Scholar]
- 9.Gallo A., Giuberti G., Frisvad J., Bertuzzi T., Nielsen K. Review on mycotoxin issues in ruminants: Occurrence in forages, effects of mycotoxin ingestion on health status and animal performance and practical strategies to counteract their negative effects. Toxins. 2015;7:3057–3111. doi: 10.3390/toxins7083057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Drusch S., Ragab W. Mycotoxins in fruits, fruit juices, and dried fruits. J. Food Prot. 2003;66:1514–1527. doi: 10.4315/0362-028X-66.8.1514. [DOI] [PubMed] [Google Scholar]
- 11.El Khoury A., Rizk T., Lteif R., Azouri H., Delia M.-L., Lebrihi A. Occurrence of Ochratoxin A- and Aflatoxin B1-Producing Fungi in Lebanese Grapes and Ochratoxin A Content in Musts and Finished Wines during 2004. J. Agric. Food Chem. 2006;54:8977–8982. doi: 10.1021/jf062085e. [DOI] [PubMed] [Google Scholar]
- 12.El Khoury A., Rizk T., Lteif R., Azouri H., Delia M.-L., Lebrihi A. Fungal contamination and Aflatoxin B1 and Ochratoxin A in Lebanese wine–grapes and musts. Food Chem. Toxicol. 2008;46:2244–2250. doi: 10.1016/j.fct.2008.02.026. [DOI] [PubMed] [Google Scholar]
- 13.Darwish W.S., Ikenaka Y., Nakayama S.M., Ishizuka M. An overview on mycotoxin contamination of foods in Africa. J. Vet. Med. Sci. 2014;76:789–797. doi: 10.1292/jvms.13-0563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nleya N., Adetunji M., Mwanza M. Current status of mycotoxin contamination of food commodities in Zimbabwe. Toxins. 2018;10:89. doi: 10.3390/toxins10050089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Serrano A., Font G., Ruiz M., Ferrer E. Co-occurrence and risk assessment of mycotoxins in food and diet from Mediterranean area. Food Chem. 2012;135:423–429. doi: 10.1016/j.foodchem.2012.03.064. [DOI] [PubMed] [Google Scholar]
- 16.Rodrigues I., Naehrer K. A three-year survey on the worldwide occurrence of mycotoxins in feedstuffs and feed. Toxins. 2012;4:663–675. doi: 10.3390/toxins4090663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Joubrane K., Khoury A.E., Lteif R., Rizk T., Kallassy M., Hilan C., Maroun R. Occurrence of aflatoxin B1 and ochratoxin A in Lebanese cultivated wheat. Mycotoxin Res. 2011;27:249–257. doi: 10.1007/s12550-011-0101-z. [DOI] [PubMed] [Google Scholar]
- 18.Raad F., Nasreddine L., Hilan C., Bartosik M., Parent-Massin D. Dietary exposure to aflatoxins, ochratoxin A and deoxynivalenol from a total diet study in an adult urban Lebanese population. Food Chem. Toxicol. 2014;73:35–43. doi: 10.1016/j.fct.2014.07.034. [DOI] [PubMed] [Google Scholar]
- 19.Eze U.A., Okonofua F.E. High Prevalence of Male Infertility in Africa: Are Mycotoxins to Blame? Afr. J. Reprod. Health. 2015;19:9–17. [PubMed] [Google Scholar]
- 20.Carlsen E., Giwercman A., Keiding N., Skakkebaek N.E. Evidence for decreasing quality of semen during past 50 years. Bmj. 1992;305:609–613. doi: 10.1136/bmj.305.6854.609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Stuppia L., Franzago M., Ballerini P., Gatta V., Antonucci I. Epigenetics and male reproduction: The consequences of paternal lifestyle on fertility, embryo development, and children lifetime health. Clin. Epigenet. 2015;7:120. doi: 10.1186/s13148-015-0155-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Swan S.H., Elkin E.P., Fenster L. Have sperm densities declined? A reanalysis of global trend data. Environ. Health Perspect. 1997;105:1228–1232. doi: 10.1289/ehp.971051228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Swan S.H., Elkin E.P., Fenster L. The question of declining sperm density revisited: An analysis of 101 studies published 1934–1996. Environ. Health Perspect. 2000;108:961–966. doi: 10.1289/ehp.00108961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ibeh I.N., Uraih N., Ogonar J.I. Dietary exposure to aflatoxin in human male infertility in Benin City, Nigeria. Int. J. Fertil. Menopausal Stud. 1994;39:208–214. [PubMed] [Google Scholar]
- 25.Martenies S.E., Perry M.J. Environmental and occupational pesticide exposure and human sperm parameters: A systematic review. Toxicology. 2013;307:66–73. doi: 10.1016/j.tox.2013.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bahadur G., Ling K.L.E., Katz M. Andrology: Statistical modelling reveals demography and time are the main contributing factors in global sperm count changes between 1938 and 1996. Hum. Reprod. 1996;11:2635–2639. doi: 10.1093/oxfordjournals.humrep.a019184. [DOI] [PubMed] [Google Scholar]
- 27.Lecoutre S., Petrus P., Ryden M., Breton C. Transgenerational Epigenetic Mechanisms in Adipose Tissue Development. Trends Endocrinol. Metab. Tem. 2018;29:675–685. doi: 10.1016/j.tem.2018.07.004. [DOI] [PubMed] [Google Scholar]
- 28.Mochizuki K., Hariya N., Honma K., Goda T. Relationship between epigenetic regulation, dietary habits, and the developmental origins of health and disease theory. Congenit. Anom. 2017;57:184–190. doi: 10.1111/cga.12213. [DOI] [PubMed] [Google Scholar]
- 29.Jobson M.A., Jordan J.M., Sandrof M.A., Hibshman J.D., Lennox A.L., Baugh L.R. Transgenerational Effects of Early Life Starvation on Growth, Reproduction, and Stress Resistance in Caenorhabditis elegans. Genetics. 2015;201:201–212. doi: 10.1534/genetics.115.178699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Youssef N.A., Lockwood L., Su S., Hao G., Rutten B.P.F. The Effects of Trauma, with or without PTSD, on the Transgenerational DNA Methylation Alterations in Human Offsprings. Brain Sci. 2018;8:83. doi: 10.3390/brainsci8050083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Finegersh A., Homanics G.E. Paternal alcohol exposure reduces alcohol drinking and increases behavioral sensitivity to alcohol selectively in male offspring. PLoS ONE. 2014;9:e99078. doi: 10.1371/journal.pone.0099078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tsai P.C., Glastonbury C.A., Eliot M.N., Bollepalli S., Yet I., Castillo-Fernandez J.E., Carnero-Montoro E., Hardiman T., Martin T.C., Vickers A., et al. Smoking induces coordinated DNA methylation and gene expression changes in adipose tissue with consequences for metabolic health. Clin. Epigenet. 2018;10:126. doi: 10.1186/s13148-018-0558-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Skinner M.K., Ben Maamar M., Sadler-Riggleman I., Beck D., Nilsson E., McBirney M., Klukovich R., Xie Y., Tang C., Yan W. Alterations in sperm DNA methylation, non-coding RNA and histone retention associate with DDT-induced epigenetic transgenerational inheritance of disease. Epigenet. Chromatin. 2018;11:8. doi: 10.1186/s13072-018-0178-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rezapour S., Shiravand M., Mardani M. Epigenetic changes due to physical activity. Biotechnol. Appl. Biochem. 2018;65:761–767. doi: 10.1002/bab.1689. [DOI] [PubMed] [Google Scholar]
- 35.Egger G., Liang G., Aparicio A., Jones P.A. Epigenetics in human disease and prospects for epigenetic therapy. Nature. 2004;429:457–463. doi: 10.1038/nature02625. [DOI] [PubMed] [Google Scholar]
- 36.Donkin I., Barres R. Sperm epigenetics and influence of environmental factors. Mol. Metab. 2018;14:1–11. doi: 10.1016/j.molmet.2018.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lea A.J., Tung J., Archie E.A., Alberts S.C. Developmental plasticity: Bridging research in evolution and human health. Evol. Med. Public Health. 2017;2017:162–175. doi: 10.1093/emph/eox019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Burton A., Torres-Padilla M.E. Epigenetic reprogramming and development: A unique heterochromatin organization in the preimplantation mouse embryo. Brief. Funct. Genom. 2010;9:444–454. doi: 10.1093/bfgp/elq027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Shanmugam M.K., Sethi G. Role of epigenetics in inflammation-associated diseases. Sub-Cell. Biochem. 2013;61:627–657. doi: 10.1007/978-94-007-4525-4_27. [DOI] [PubMed] [Google Scholar]
- 40.Qin Y., Wade P.A. Crosstalk between the microbiome and epigenome: Messages from bugs. J. Biochem. 2018;163:105–112. doi: 10.1093/jb/mvx080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pal S., Tyler J.K. Epigenetics and aging. Sci. Adv. 2016;2:e1600584. doi: 10.1126/sciadv.1600584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Murphy S.K., Itchon-Ramos N., Visco Z., Huang Z., Grenier C., Schrott R., Acharya K., Boudreau M.H., Price T.M., Raburn D.J., et al. Cannabinoid exposure and altered DNA methylation in rat and human sperm. Epigenetics. 2018;13:1208–1221. doi: 10.1080/15592294.2018.1554521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Alfano R., Herceg Z., Nawrot T.S., Chadeau-Hyam M., Ghantous A., Plusquin M. The Impact of Air Pollution on Our Epigenome: How Far Is the Evidence? (A Systematic Review) Curr. Environ. Health Rep. 2018;5:544–578. doi: 10.1007/s40572-018-0218-8. [DOI] [PubMed] [Google Scholar]
- 44.Zhang Y., Kutateladze T.G. Diet and the epigenome. Nat. Commun. 2018;9:3375. doi: 10.1038/s41467-018-05778-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Dai Y., Huang K., Zhang B., Zhu L., Xu W. Aflatoxin B1-induced epigenetic alterations: An overview. Food Chem. Toxicol. Int. J. Publ. Br. Ind. Biol. Res. Assoc. 2017;109:683–689. doi: 10.1016/j.fct.2017.06.034. [DOI] [PubMed] [Google Scholar]
- 46.Hernandez-Vargas H., Castelino J., Silver M.J., Dominguez-Salas P., Cros M.P., Durand G., Le Calvez-Kelm F., Prentice A.M., Wild C.P., Moore S.E., et al. Exposure to aflatoxin B1 in utero is associated with DNA methylation in white blood cells of infants in The Gambia. Int. J. Epidemiol. 2015;44:1238–1248. doi: 10.1093/ije/dyv027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Liu J., Wang Q.C., Han J., Xiong B., Sun S.C. Aflatoxin B1 is toxic to porcine oocyte maturation. Mutagenesis. 2015;30:527–535. doi: 10.1093/mutage/gev015. [DOI] [PubMed] [Google Scholar]
- 48.Rieswijk L., Claessen S.M., Bekers O., van Herwijnen M., Theunissen D.H., Jennen D.G., de Kok T.M., Kleinjans J.C., van Breda S.G. Aflatoxin B1 induces persistent epigenomic effects in primary human hepatocytes associated with hepatocellular carcinoma. Toxicology. 2016;350–352:31–39. doi: 10.1016/j.tox.2016.05.002. [DOI] [PubMed] [Google Scholar]
- 49.Tryndyak V., Kindrat I., Dreval K., Churchwell M.I., Beland F.A., Pogribny I.P. Effect of aflatoxin B1, benzo[a]pyrene, and methapyrilene on transcriptomic and epigenetic alterations in human liver HepaRG cells. Food Chem. Toxicol. Int. J. Publ. Br. Ind. Biol. Res. Assoc. 2018;121:214–223. doi: 10.1016/j.fct.2018.08.034. [DOI] [PubMed] [Google Scholar]
- 50.Wu H.C., Wang Q., Yang H.I., Tsai W.Y., Chen C.J., Santella R.M. Global DNA methylation in a population with aflatoxin B1 exposure. Epigenetics. 2013;8:962–969. doi: 10.4161/epi.25696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Mezzelani A., Raggi M.E., Marabotti A., Milanesi L. Ochratoxin A as possible factor trigging autism and its male prevalence via epigenetic mechanism. Nutr. Neurosci. 2016;19:43–46. doi: 10.1179/1476830515Z.000000000186. [DOI] [PubMed] [Google Scholar]
- 52.Rasic D., Mladinic M., Zeljezic D., Pizent A., Stefanovic S., Milicevic D., Konjevoda P., Peraica M. Effects of combined treatment with ochratoxin A and citrinin on oxidative damage in kidneys and liver of rats. Toxicon. 2018;146:99–105. doi: 10.1016/j.toxicon.2018.03.002. [DOI] [PubMed] [Google Scholar]
- 53.Zhou Y., Gan F., Hou L., Zhou X., Adam Ibrahim Y.A., Huang K. Modulations of DNMT1 and HDAC1 are involved in the OTA-induced cytotoxicity and apoptosis in vitro. Chem.-Biol. Interact. 2017;278:170–178. doi: 10.1016/j.cbi.2017.10.020. [DOI] [PubMed] [Google Scholar]
- 54.Ansari K.I., Hussain I., Das H.K., Mandal S.S. Overexpression of human histone methylase MLL1 upon exposure to a food contaminant mycotoxin, deoxynivalenol. FEBS J. 2009;276:3299–3307. doi: 10.1111/j.1742-4658.2009.07055.x. [DOI] [PubMed] [Google Scholar]
- 55.Baccarelli A., Bollati V. Epigenetics and environmental chemicals. Curr. Opin. Pediatr. 2009;21:243–251. doi: 10.1097/MOP.0b013e32832925cc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Moosavi A., Motevalizadeh Ardekani A. Role of Epigenetics in Biology and Human Diseases. Iran. Biomed. J. 2016;20:246–258. doi: 10.22045/ibj.2016.01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Toh T.B., Lim J.J., Chow E.K. Epigenetics of hepatocellular carcinoma. Clin. Transl. Med. 2019;8:13. doi: 10.1186/s40169-019-0230-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Pasculli B., Barbano R., Parrella P. Epigenetics of breast cancer: Biology and clinical implication in the era of precision medicine. Semin. Cancer Biol. 2018;51:22–35. doi: 10.1016/j.semcancer.2018.01.007. [DOI] [PubMed] [Google Scholar]
- 59.Wouters B.J., Delwel R. Epigenetics and approaches to targeted epigenetic therapy in acute myeloid leukemia. Blood. 2016;127:42–52. doi: 10.1182/blood-2015-07-604512. [DOI] [PubMed] [Google Scholar]
- 60.Alelú-Paz R., Carmona F.J., Sanchez-Mut J.V., Cariaga-Martínez A., González-Corpas A., Ashour N., Orea M.J., Escanilla A., Monje A., Guerrero Márquez C., et al. Epigenetics in Schizophrenia: A Pilot Study of Global DNA Methylation in Different Brain Regions Associated with Higher Cognitive Functions. Front. Psychol. 2016;7:1496. doi: 10.3389/fpsyg.2016.01496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Eshraghi A.A., Liu G., Kay S.-I.S., Eshraghi R.S., Mittal J., Moshiree B., Mittal R. Epigenetics and autism spectrum disorder: Is there a correlation? Front. Cell. Neurosci. 2018;12:78. doi: 10.3389/fncel.2018.00078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Tang Q., Pan F., Yang J., Fu Z., Lu Y., Wu X., Han X., Chen M., Lu C., Xia Y. Idiopathic male infertility is strongly associated with aberrant DNA methylation of imprinted loci in sperm: A case-control study. Clin. Epigenet. 2018;10:134. doi: 10.1186/s13148-018-0568-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Dada R., Kumar M., Jesudasan R., Fernández J.L., Gosálvez J., Agarwal A. Epigenetics and its role in male infertility. J. Assist. Reprod. Genet. 2012;29:213–223. doi: 10.1007/s10815-012-9715-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Jenkins T.G., Aston K.I., James E.R., Carrell D.T. Sperm epigenetics in the study of male fertility, offspring health, and potential clinical applications. Syst. Biol. Reprod. Med. 2017;63:69–76. doi: 10.1080/19396368.2016.1274791. [DOI] [PubMed] [Google Scholar]
- 65.Friemel C., Ammerpohl O., Gutwein J., Schmutzler A.G., Caliebe A., Kautza M., von Otte S., Siebert R., Bens S. Array-based DNA methylation profiling in male infertility reveals allele-specific DNA methylation in PIWIL1 and PIWIL2. Fertil. Steril. 2014;101:1097–1103. doi: 10.1016/j.fertnstert.2013.12.054. [DOI] [PubMed] [Google Scholar]
- 66.Maamar M.B., Sadler-Riggleman I., Beck D., Skinner M.K. Epigenetic transgenerational inheritance of altered sperm histone retention sites. Sci. Rep. 2018;8:5308. doi: 10.1038/s41598-018-23612-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.He Z., Kokkinaki M., Pant D., Gallicano G.I., Dym M. Small RNA molecules in the regulation of spermatogenesis. Reproduction (Camb. Engl.) 2009;137:901–911. doi: 10.1530/REP-08-0494. [DOI] [PubMed] [Google Scholar]
- 68.Clermont Y. Kinetics of spermatogenesis in mammals: Seminiferous epithelium cycle and spermatogonial renewal. Physiol. Rev. 1972;52:198–236. doi: 10.1152/physrev.1972.52.1.198. [DOI] [PubMed] [Google Scholar]
- 69.Staub C., Johnson L. Review: Spermatogenesis in the bull. Anim. Int. J. Anim. Biosci. 2018;12:s27–s35. doi: 10.1017/S1751731118000435. [DOI] [PubMed] [Google Scholar]
- 70.Hamano K.-I., Sugimoto R., Takahashi H., Tsujii H. Spermatogenesis in immature mammals. Reprod. Med. Biol. 2007;6:139–149. doi: 10.1111/j.1447-0578.2007.00177.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Clermont Y., Huckins C. Microscopic anatomy of the sex cords and seminiferous tubules in growing and adult male albino rats. Am. J. Anat. 1961;108:79–97. doi: 10.1002/aja.1001080106. [DOI] [Google Scholar]
- 72.Holstein A.F.E., Knobil J.D. Neill (eds): The Physiology of Reproduction. Andrologia. 1994;26:357. doi: 10.1111/j.1439-0272.1994.tb00816.x. [DOI] [Google Scholar]
- 73.Gwatkin R.B.L. The Sertoli cell, edited by Lonnie, D. Russell and Michael, D. Griswold, Cache River Press, Clearwater, FL, 1993, 826 pp, $137.50. Mol. Reprod. Dev. 1993;36:517. doi: 10.1002/mrd.1080360417. [DOI] [Google Scholar]
- 74.O’Donnell L., Stanton P., de Kretser D.M. Endocrinology of the Male Reproductive System and Spermatogenesis. [(accessed on 11 January 2017)]; Available online: www.endotext.org.
- 75.Neaves W.B. A report prepared for the ford foundation review of research and support in reproductive biology and contraceptive development. Contraception. 1975;11:571–606. doi: 10.1016/0010-7824(75)90111-0. [DOI] [PubMed] [Google Scholar]
- 76.Walker W.H. Testosterone signaling and the regulation of spermatogenesis. Spermatogenesis. 2011;1:116–120. doi: 10.4161/spmg.1.2.16956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Griswold M.D. The central role of Sertoli cells in spermatogenesis. Semin. Cell Dev. Biol. 1998;9:411–416. doi: 10.1006/scdb.1998.0203. [DOI] [PubMed] [Google Scholar]
- 78.Hilscher B., Engemann A. Histological and morphometric studies on the kinetics of germ cells and immature Sertoli cells during human prespermatogenesis. Andrologia. 1992;24:7–10. doi: 10.1111/j.1439-0272.1992.tb02600.x. [DOI] [PubMed] [Google Scholar]
- 79.Esteves S. Male infertility due to spermatogenic failure: Current management and future perspectives. Anim. Reprod. 2015;12:62–80. [Google Scholar]
- 80.Plant T.M. The hypothalamo-pituitary-gonadal axis. J. Endocrinol. 2015;226:T41–T45. doi: 10.1530/JOE-15-0113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Wilson R.C., Kesner J.S., Kaufman J.-M., Uemura T., Akema T., Knobil E. Central electrophysiologic correlates of pulsatile luteinizing hormone secretion in the rhesus monkey. Neuroendocrinology. 1984;39:256–260. doi: 10.1159/000123988. [DOI] [PubMed] [Google Scholar]
- 82.Majumdar S., Mikuma N., Ishwad P., Winters S., Attardi B., Perera A., Plant T. Replacement with recombinant human inhibin immediately after orchidectomy in the hypophysiotropically clamped male rhesus monkey (Macaca mulatta) maintains follicle-stimulating hormone (FSH) secretion and FSH beta messenger ribonucleic acid levels at precastration values. Endocrinology. 1995;136:1969–1977. doi: 10.1210/endo.136.5.7720645. [DOI] [PubMed] [Google Scholar]
- 83.Miller W.L., Auchus R.J. The molecular biology, biochemistry, and physiology of human steroidogenesis and its disorders. Endocr. Rev. 2011;32:81–151. doi: 10.1210/er.2010-0013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Horstman A.M., Dillon E.L., Urban R.J., Sheffield-Moore M. The role of androgens and estrogens on healthy aging and longevity. J. Gerontol. Ser. A Biomed. Sci. Med Sci. 2012;67:1140–1152. doi: 10.1093/gerona/gls068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Halkerston I., Eichhorn J., Hechter O. A requirement for reduced triphosphopyridine nucleotide for cholesterol side-chain cleavage by mitochondrial fractions of bovine adrenal cortex. J. Biol. Chem. 1961;236:374–380. [PubMed] [Google Scholar]
- 86.Tuckey R.C., Cameron K.J. Side-chain specificities of human and bovine cytochromes P-450scc. Eur. J. Biochem. 1993;217:209–215. doi: 10.1111/j.1432-1033.1993.tb18235.x. [DOI] [PubMed] [Google Scholar]
- 87.Thomas J.L., Myers R.P., Strickler R.C. Human placental 3 beta-hydroxy-5-ene-steroid dehydrogenase and steroid 5 → 4-ene-isomerase: Purification from mitochondria and kinetic profiles, biophysical characterization of the purified mitochondrial and microsomal enzymes. J. Steroid Biochem. 1989;33:209–217. doi: 10.1016/0022-4731(89)90296-3. [DOI] [PubMed] [Google Scholar]
- 88.Lachance Y., Labrie C., Simard J., Dumont M., De Launoit Y., Guérin S., Leblanc G., Labrie F. Characterization of human 3 beta-hydroxysteroid dehydrogenase/delta 5-delta 4-isomerase gene and its expression in mammalian cells. J. Biol. Chem. 1990;265:20469–20475. [PubMed] [Google Scholar]
- 89.Lorence M.C., MURRY B.A., TRANT J.M., MASON J.I. Human 3β-hydroxysteroid dehydrogenase/Δ5 → 4isomerase from placenta: Expression in nonsteroidogenic cells of a protein that catalyzes the dehydrogenation/isomerization of C21 and C19 steroids. Endocrinology. 1990;126:2493–2498. doi: 10.1210/endo-126-5-2493. [DOI] [PubMed] [Google Scholar]
- 90.Lee E., Starcevic S., Catlin D. Journal of Investigative Medicine. Slack Inc.; Thorofare, NJ, USA: 1999. Effects of dietary supplements, 19-norandrostenedione, androstenediol and androstenedione on the profile of urine steroids; p. 62A. [Google Scholar]
- 91.Simpson E., Mahendroo M., Nichols J., Bulun S. Aromatase gene expression in adipose tissue: Relationship to breast cancer. Int. J. Fertil. Menopausal Stud. 1994;39:75–83. [PubMed] [Google Scholar]
- 92.Moore R.J., Wilson J.D. Steroid 5alpha-reductase in cultured human fibroblasts. Biochemical and genetic evidence for two distinct enzyme activities. J. Biol. Chem. 1976;251:5895–5900. [PubMed] [Google Scholar]
- 93.Gharbi A., Trillon O., Betbeder A.M., Counord J., Gauret M.F., Pfohl-Leszkowicz A., Dirheimer G., Creppy E.E. Some effects of ochratoxin A, a mycotoxin contaminating feeds and food, on rat testis. Toxicology. 1993;83:9–18. doi: 10.1016/0300-483X(93)90087-9. [DOI] [PubMed] [Google Scholar]
- 94.Park H., Park H.S., Lim W., Song G. Ochratoxin A suppresses proliferation of Sertoli and Leydig cells in mice. Med. Mycol. 2019 doi: 10.1093/mmy/myz016. [DOI] [PubMed] [Google Scholar]
- 95.Zatecka E., Ded L., Elzeinova F., Kubatova A., Dorosh A., Margaryan H., Dostalova P., Korenkova V., Hoskova K., Peknicova J. Effect of zearalenone on reproductive parameters and expression of selected testicular genes in mice. Reprod. Toxicol. (Elmsford N.Y.) 2014;45:20–30. doi: 10.1016/j.reprotox.2014.01.003. [DOI] [PubMed] [Google Scholar]
- 96.Guerrero-Netro H.M., Chorfi Y., Price C.A. Effects of the mycotoxin deoxynivalenol on steroidogenesis and apoptosis in granulosa cells. Reproduction (Camb. Engl.) 2015;149:555–561. doi: 10.1530/REP-15-0018. [DOI] [PubMed] [Google Scholar]
- 97.Gupta M., Bandyopadhyay S., Paul B., Mazumdar S.K. Ovarian steroidogenesis and development of fetuses following ochratoxin A treatment in pregnant rats. Endokrinologie. 1981;77:152–160. [PubMed] [Google Scholar]
- 98.Long G.G., Diekman M.A. Effect of purified zearalenone on early gestation in gilts. J. Anim. Sci. 1984;59:1662–1670. doi: 10.2527/jas1984.5961662x. [DOI] [PubMed] [Google Scholar]
- 99.Parandin R., Behnam-Rassouli M., Mahdavi-Shahri N. Effects of Neonatal Exposure to Zearalenone on Puberty Timing, Hypothalamic Nuclei of AVPV and ARC, and Reproductive Functions in Female Mice. Reprod. Sci. (Thousand Oaks Calif.) 2017;24:1293–1303. doi: 10.1177/1933719116683808. [DOI] [PubMed] [Google Scholar]
- 100.Yegani M., Chowdhury S.R., Oinas N., MacDonald E.J., Smith T.K. Effects of feeding grains naturally contaminated with Fusarium mycotoxins on brain regional neurochemistry of laying hens, turkey poults, and broiler breeder hens. Poult. Sci. 2006;85:2117–2123. doi: 10.1093/ps/85.12.2117. [DOI] [PubMed] [Google Scholar]
- 101.Albonico M., Schutz L.F., Caloni F., Cortinovis C., Spicer L.J. In vitro effects of the Fusarium mycotoxins fumonisin b1 and beauvericin on bovine granulosa cell proliferation and steroid production. Toxicon. 2017;128:38–45. doi: 10.1016/j.toxicon.2017.01.019. [DOI] [PubMed] [Google Scholar]
- 102.Bertero A., Moretti A., Spicer L.J., Caloni F. Fusarium Molds and Mycotoxins: Potential Species-Specific Effects. Toxins. 2018;10:244. doi: 10.3390/toxins10060244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Cortinovis C., Caloni F., Schreiber N.B., Spicer L.J. Effects of fumonisin B1 alone and combined with deoxynivalenol or zearalenone on porcine granulosa cell proliferation and steroid production. Theriogenology. 2014;81:1042–1049. doi: 10.1016/j.theriogenology.2014.01.027. [DOI] [PubMed] [Google Scholar]
- 104.Ranzenigo G., Caloni F., Cremonesi F., Aad P.Y., Spicer L.J. Effects of Fusarium mycotoxins on steroid production by porcine granulosa cells. Anim. Reprod. Sci. 2008;107:115–130. doi: 10.1016/j.anireprosci.2007.06.023. [DOI] [PubMed] [Google Scholar]
- 105.Spensley P. Aflatoxin, the active principle in turkey‘X’disease. Endeavour. 1963;22:75–79. doi: 10.1016/0160-9327(63)90097-8. [DOI] [PubMed] [Google Scholar]
- 106.Sprando R.L., Collins T.F., Black T.N., Olejnik N., Rorie J.I., Eppley R.M., Ruggles D.I. Characterization of the effect of deoxynivalenol on selected male reproductive endpoints. Food Chem. Toxicol. 2005;43:623–635. doi: 10.1016/j.fct.2004.12.017. [DOI] [PubMed] [Google Scholar]
- 107.Sprando R., Pestka J., Collins T., Rorie J., O’donnell M., Hinton D., Chirtel S. The Effect of Vomitoxin (Deoxnivalenol) on Testicular Morphology, Testicular Spermatid Counts and Epididymal Sperm Counts in IL-6KO [B6129-IL6 < tmlKopf > (IL-6 gene deficient)] and WT [B6129F2 (wild type to B6129-IL6 with an intact IL-6 gene)] mice. Food Chem. Toxicol. 1999;37:1073–1079. doi: 10.1016/S0278-6915(99)00103-9. [DOI] [PubMed] [Google Scholar]
- 108.Han J., Wang Q.-C., Zhu C.-C., Liu J., Zhang Y., Cui X.-S., Kim N.-H., Sun S.-C. Deoxynivalenol exposure induces autophagy/apoptosis and epigenetic modification changes during porcine oocyte maturation. Toxicol. Appl. Pharmacol. 2016;300:70–76. doi: 10.1016/j.taap.2016.03.006. [DOI] [PubMed] [Google Scholar]
- 109.Tiemann U., Tomek W., Schneider F., Vanselow J. Effects of the mycotoxins α-and β-zearalenol on regulation of progesterone synthesis in cultured granulosa cells from porcine ovaries. Reprod. Toxicol. 2003;17:673–681. doi: 10.1016/j.reprotox.2003.07.001. [DOI] [PubMed] [Google Scholar]
- 110.Fernández-Blanco C., Frizzell C., Shannon M., Ruiz M.-J., Connolly L. An in vitro investigation on the cytotoxic and nuclear receptor transcriptional activity of the mycotoxins fumonisin B1 and beauvericin. Toxicol. Lett. 2016;257:1–10. doi: 10.1016/j.toxlet.2016.05.021. [DOI] [PubMed] [Google Scholar]
- 111.Behm C., Föllmann W., Degen G.H. Cytotoxic potency of mycotoxins in cultures of V79 lung fibroblast cells. J. Toxicol. Environ. Health Part A. 2012;75:1226–1231. doi: 10.1080/15287394.2012.709170. [DOI] [PubMed] [Google Scholar]
- 112.Mallebrera B., Brandolini V., Font G., Ruiz M. Cytoprotective effect of resveratrol diastereomers in CHO-K1 cells exposed to beauvericin. Food Chem. Toxicol. 2015;80:319–327. doi: 10.1016/j.fct.2015.03.028. [DOI] [PubMed] [Google Scholar]
- 113.Schoevers E.J., Santos R.R., Fink-Gremmels J., Roelen B.A. Toxicity of beauvericin on porcine oocyte maturation and preimplantation embryo development. Reprod. Toxicol. 2016;65:159–169. doi: 10.1016/j.reprotox.2016.07.017. [DOI] [PubMed] [Google Scholar]
- 114.Aydin Y., Orta Yilmaz B., Yildizbayrak N., Korkut A., Arabul Kursun M., Irez T., Erkan M. Evaluation of citrinin-induced toxic effects on mouse Sertoli cells. Drug Chem. Toxicol. 2019:1–7. doi: 10.1080/01480545.2019.1614021. [DOI] [PubMed] [Google Scholar]
- 115.Ewuola E.O. Organ traits and histopathology of rabbits fed varied levels of dietary fumonisin B(1) J. Anim. Physiol. Anim. Nutr. 2009;93:726–731. doi: 10.1111/j.1439-0396.2008.00862.x. [DOI] [PubMed] [Google Scholar]
- 116.Karacaoglu E., Selmanoglu G. T-2 toxin induces cytotoxicity and disrupts tight junction barrier in SerW3 cells. Environ. Toxicol. Pharmacol. 2017;56:259–267. doi: 10.1016/j.etap.2017.10.005. [DOI] [PubMed] [Google Scholar]
- 117.Koraïchi F., Inoubli L., Lakhdari N., Meunier L., Vega A., Mauduit C., Benahmed M., Prouillac C., Lecoeur S. Neonatal exposure to zearalenone induces long term modulation of ABC transporter expression in testis. Toxicology. 2013;310:29–38. doi: 10.1016/j.tox.2013.05.002. [DOI] [PubMed] [Google Scholar]
- 118.Zheng W., Pan S., Wang G., Wang Y.J., Liu Q., Gu J., Yuan Y., Liu X.Z., Liu Z.P., Bian J.C. Zearalenone impairs the male reproductive system functions via inducing structural and functional alterations of sertoli cells. Environ. Toxicol. Pharmacol. 2016;42:146–155. doi: 10.1016/j.etap.2016.01.013. [DOI] [PubMed] [Google Scholar]
- 119.Xu M.L., Hu J., Guo B.P., Niu Y.R., Xiao C., Xu Y.X. Exploration of intrinsic and extrinsic apoptotic pathways in zearalenone-treated rat sertoli cells. Environ. Toxicol. 2016;31:1731–1739. doi: 10.1002/tox.22175. [DOI] [PubMed] [Google Scholar]
- 120.Cai G., Si M., Li X., Zou H., Gu J., Yuan Y., Liu X., Liu Z., Bian J. Zearalenone induces apoptosis of rat Sertoli cells through Fas-Fas ligand and mitochondrial pathway. Environ. Toxicol. 2019;34:424–433. doi: 10.1002/tox.22696. [DOI] [PubMed] [Google Scholar]
- 121.Milano G.D., Becu-Villalobos D., Tapia M.O. Effects of long-term zearalenone administration on spermatogenesis and serum luteinizing hormone, follicle-stimulating hormone, and prolactin values in male rats. Am. J. Vet. Res. 1995;56:954–958. [PubMed] [Google Scholar]
- 122.Filipiak E., Walczak-Jedrzejowska R., Oszukowska E., Guminska A., Marchlewska K., Kula K., Slowikowska-Hilczer J. Xenoestrogens diethylstilbestrol and zearalenone negatively influence pubertal rat’s testis. Folia Histochem. Cytobiol. 2009;47:S113–S120. doi: 10.2478/v10042-009-0049-4. [DOI] [PubMed] [Google Scholar]
- 123.Zhang T.Y., Wu R.Y., Zhao Y., Xu C.S., Zhang W.D., Ge W., Liu J., Sun Z.Y., Zou S.H., Shen W. Ochratoxin A exposure decreased sperm motility via the AMPK and PTEN signaling pathways. Toxicol. Appl. Pharm. 2018;340:49–57. doi: 10.1016/j.taap.2017.12.011. [DOI] [PubMed] [Google Scholar]
- 124.Long M., Yang S.H., Shi W., Li P., Guo Y., Guo J., He J.B., Zhang Y. Protective effect of proanthocyanidin on mice Sertoli cell apoptosis induced by zearalenone via the Nrf2/ARE signalling pathway. Environ. Sci. Pollut. Res. Int. 2017;24:26724–26733. doi: 10.1007/s11356-017-0123-y. [DOI] [PubMed] [Google Scholar]
- 125.Adedara I.A., Nanjappa M.K., Farombi E.O., Akingbemi B.T. Aflatoxin B1 disrupts the androgen biosynthetic pathway in rat Leydig cells. Food Chem. Toxicol. 2014;65:252–259. doi: 10.1016/j.fct.2013.12.027. [DOI] [PubMed] [Google Scholar]
- 126.Austin K., Cockrum R., Jons A., Alexander B., Cammack K. Renin mRNA is upregulated in testes and testicular cells in response to treatment with aflatoxin B1. Theriogenology. 2012;77:331–337. doi: 10.1016/j.theriogenology.2011.08.004. [DOI] [PubMed] [Google Scholar]
- 127.Liu S., Wang D., Zhang J., Zhang D., Gong M., Wang C., Wei N., Liu W., Wang Y., Zhao C. Citrinin reduces testosterone secretion by inducing apoptosis in rat Leydig cells. Toxicol. In Vitro. 2012;26:856–861. doi: 10.1016/j.tiv.2012.04.026. [DOI] [PubMed] [Google Scholar]
- 128.Yang J., Zhang Y., Jing A., Ma K., Gong Q., Qin C. Effects of T-2 toxin on testosterone biosynthesis in mouse Leydig cells. Toxicol. Ind. Health. 2014;30:873–877. doi: 10.1177/0748233712464810. [DOI] [PubMed] [Google Scholar]
- 129.Yang J., Zhang Y., Wang Y., Cui S. Toxic effects of zearalenone and α-zearalenol on the regulation of steroidogenesis and testosterone production in mouse Leydig cells. Toxicol. In Vitro. 2007;21:558–565. doi: 10.1016/j.tiv.2006.10.013. [DOI] [PubMed] [Google Scholar]
- 130.Liu Q., Wang Y., Gu J., Yuan Y., Liu X., Zheng W., Huang Q., Liu Z., Bian J. Zearalenone inhibits testosterone biosynthesis in mouse Leydig cells via the crosstalk of estrogen receptor signaling and orphan nuclear receptor Nur77 expression. Toxicol. In Vitro. 2014;28:647–656. doi: 10.1016/j.tiv.2014.01.013. [DOI] [PubMed] [Google Scholar]
- 131.Eze U., Routledge M., Okonofua F., Huntriss J., Gong Y. Mycotoxin exposure and adverse reproductive health outcomes in Africa: A review. World Mycotoxin J. 2018;11:321–339. doi: 10.3920/WMJ2017.2261. [DOI] [Google Scholar]
- 132.Li Y., Zhang B., Huang K., He X., Luo Y., Liang R., Luo H., Shen X.L., Xu W. Mitochondrial proteomic analysis reveals the molecular mechanisms underlying reproductive toxicity of zearalenone in MLTC-1 cells. Toxicology. 2014;324:55–67. doi: 10.1016/j.tox.2014.07.007. [DOI] [PubMed] [Google Scholar]
- 133.Lin P., Chen F., Sun J., Zhou J., Wang X., Wang N., Li X., Zhang Z., Wang A., Jin Y. Mycotoxin zearalenone induces apoptosis in mouse Leydig cells via an endoplasmic reticulum stress-dependent signalling pathway. Reprod. Toxicol. (Elmsford N.Y.) 2015;52:71–77. doi: 10.1016/j.reprotox.2015.02.007. [DOI] [PubMed] [Google Scholar]
- 134.Wang Y., Zheng W., Bian X., Yuan Y., Gu J., Liu X., Liu Z., Bian J. Zearalenone induces apoptosis and cytoprotective autophagy in primary Leydig cells. Toxicol. Lett. 2014;226:182–191. doi: 10.1016/j.toxlet.2014.02.003. [DOI] [PubMed] [Google Scholar]
- 135.Tegelenbosch R.A., de Rooij D.G. A quantitative study of spermatogonial multiplication and stem cell renewal in the C3H/101 F1 hybrid mouse. Mutat. Res. 1993;290:193–200. doi: 10.1016/0027-5107(93)90159-D. [DOI] [PubMed] [Google Scholar]
- 136.Kuiper-Goodman T., Scott P.M., Watanabe H. Risk assessment of the mycotoxin zearalenone. Regul. Toxicol. Pharmacol. 1987;7:253–306. doi: 10.1016/0273-2300(87)90037-7. [DOI] [PubMed] [Google Scholar]
- 137.Qingqing H., Linbo Y., Yunqian G., Shuqiang L. Toxic effects of citrinin on the male reproductive system in mice. Exp. Toxicol. Pathol. 2012;64:465–469. doi: 10.1016/j.etp.2010.10.015. [DOI] [PubMed] [Google Scholar]
- 138.Supriya C., Girish B.P., Reddy P.S. Aflatoxin B1-Induced Reproductive Toxicity in Male Rats: Possible Mechanism of Action. Int. J. Toxicol. 2014;33:155–161. doi: 10.1177/1091581814530764. [DOI] [PubMed] [Google Scholar]
- 139.Hasanzadeh S., Rezazadeh L. Effects of aflatoxin B1 on the growth processes of spermatogenic cell series in adult male rats. Comp. Clin. Pathol. 2012;22:555–562. doi: 10.1007/s00580-012-1445-2. [DOI] [Google Scholar]
- 140.Gbore F.A. Reproductive organ weights and semen quality of pubertal boars fed dietary fumonisin B1. Anim. Int. J. Anim. Biosci. 2009;3:1133–1137. doi: 10.1017/S1751731109004467. [DOI] [PubMed] [Google Scholar]
- 141.Gbore F.A., Egbunike G.N. Testicular and epididymal sperm reserves and sperm production of pubertal boars fed dietary fumonisin B(1) Anim. Reprod. Sci. 2008;105:392–397. doi: 10.1016/j.anireprosci.2007.11.006. [DOI] [PubMed] [Google Scholar]
- 142.Ewuola E.O., Egbunike G.N. Effects of dietary fumonisin B1 on the onset of puberty, semen quality, fertility rates and testicular morphology in male rabbits. Reproduction (Camb. Engl.) 2010;139:439–445. doi: 10.1530/REP-09-0077. [DOI] [PubMed] [Google Scholar]
- 143.Selmanoglu G. Evaluation of the reproductive toxicity of patulin in growing male rats. Food Chem. Toxicol. Int. J. Publ. Br. Ind. Biol. Res. Assoc. 2006;44:2019–2024. doi: 10.1016/j.fct.2006.06.022. [DOI] [PubMed] [Google Scholar]
- 144.Yang J.Y., Zhang Y.F., Liang A.M., Kong X.F., Li Y.X., Ma K.W., Jing A.H., Feng S.Y., Qiao X.L. Toxic effects of T-2 toxin on reproductive system in male mice. Toxicol. Ind. Health. 2010;26:25–31. doi: 10.1177/0748233709354554. [DOI] [PubMed] [Google Scholar]
- 145.Kim I.H., Son H.Y., Cho S.W., Ha C.S., Kang B.H. Zearalenone induces male germ cell apoptosis in rats. Toxicol. Lett. 2003;138:185–192. doi: 10.1016/S0378-4274(02)00405-8. [DOI] [PubMed] [Google Scholar]
- 146.Dupont C., Armant D.R., Brenner C.A. Seminars in Reproductive Medicine. Thieme Medical Publishers; Stuttgart, Germany: 2009. Epigenetics: Definition, mechanisms and clinical perspective; pp. 351–357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Lister R., Pelizzola M., Dowen R.H., Hawkins R.D., Hon G., Tonti-Filippini J., Nery J.R., Lee L., Ye Z., Ngo Q.-M. Human DNA methylomes at base resolution show widespread epigenomic differences. Nature. 2009;462:315. doi: 10.1038/nature08514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Alhamwe B.A., Khalaila R., Wolf J., von Bülow V., Harb H., Alhamdan F., Hii C.S., Prescott S.L., Ferrante A., Renz H. Histone modifications and their role in epigenetics of atopy and allergic diseases. Allergy Asthma Clin. Immunol. 2018;14:39. doi: 10.1186/s13223-018-0259-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Holoch D., Moazed D. RNA-mediated epigenetic regulation of gene expression. Nat. Rev. Genet. 2015;16:71. doi: 10.1038/nrg3863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Halfmann R., Lindquist S. Epigenetics in the extreme: Prions and the inheritance of environmentally acquired traits. Science. 2010;330:629–632. doi: 10.1126/science.1191081. [DOI] [PubMed] [Google Scholar]
- 151.Becker P.B., Workman J.L. Nucleosome remodeling and epigenetics. Cold Spring Harb. Perspect. Biol. 2013;5:a017905. doi: 10.1101/cshperspect.a017905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Suelves M., Carrió E., Núñez-Álvarez Y., Peinado M.A. DNA methylation dynamics in cellular commitment and differentiation. Brief. Funct. Genom. 2016;15:443–453. doi: 10.1093/bfgp/elw017. [DOI] [PubMed] [Google Scholar]
- 153.Zeng Y., Chen T. DNA methylation reprogramming during mammalian development. Genes. 2019;10:257. doi: 10.3390/genes10040257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Jang H., Shin W., Lee J., Do J. CpG and non-CpG methylation in epigenetic gene regulation and brain function. Genes. 2017;8:148. doi: 10.3390/genes8060148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Rossetto D., Avvakumov N., Côté J. Histone phosphorylation: A chromatin modification involved in diverse nuclear events. Epigenetics. 2012;7:1098–1108. doi: 10.4161/epi.21975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Rodgers A.B., Morgan C.P., Bronson S.L., Revello S., Bale T.L. Paternal stress exposure alters sperm microRNA content and reprograms offspring HPA stress axis regulation. J. Neurosci. 2013;33:9003–9012. doi: 10.1523/JNEUROSCI.0914-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Li Y., Saldanha S.N., Tollefsbol T.O. Impact of epigenetic dietary compounds on transgenerational prevention of human diseases. AAPS J. 2014;16:27–36. doi: 10.1208/s12248-013-9538-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Stefanska B., Karlic H., Varga F., Fabianowska-Majewska K., Haslberger A. Epigenetic mechanisms in anti-cancer actions of bioactive food components—The implications in cancer prevention. Br. J. Pharmacol. 2012;167:279–297. doi: 10.1111/j.1476-5381.2012.02002.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Meeran S.M., Ahmed A., Tollefsbol T.O. Epigenetic targets of bioactive dietary components for cancer prevention and therapy. Clin. Epigenet. 2010;1:101. doi: 10.1007/s13148-010-0011-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Kubota T. Epigenetic alterations induced by environmental stress associated with metabolic and neurodevelopmental disorders. Environ. Epigenet. 2016;2:dvw017. doi: 10.1093/eep/dvw017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Chambers T.J.G., Morgan M.D., Heger A.H., Sharpe R.M., Drake A.J. High-fat diet disrupts metabolism in two generations of rats in a parent-of-origin specific manner. Sci. Rep. 2016;6:31857. doi: 10.1038/srep31857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Nilsson E., King S.E., McBirney M., Kubsad D., Pappalardo M., Beck D., Sadler-Riggleman I., Skinner M.K. Vinclozolin induced epigenetic transgenerational inheritance of pathologies and sperm epimutation biomarkers for specific diseases. PLoS ONE. 2018;13:e0202662. doi: 10.1371/journal.pone.0202662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Skinner M.K., Anway M.D. Epigenetic transgenerational actions of vinclozolin on the development of disease and cancer. Crit. Rev. Oncog. 2007;13:75–82. doi: 10.1615/CritRevOncog.v13.i1.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Anway M.D., Leathers C., Skinner M.K. Endocrine disruptor vinclozolin induced epigenetic transgenerational adult-onset disease. Endocrinology. 2006;147:5515–5523. doi: 10.1210/en.2006-0640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Lacal I., Ventura R. Epigenetic Inheritance: Concepts, Mechanisms and Perspectives. Front. Mol. Neurosci. 2018:11. doi: 10.3389/fnmol.2018.00292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Ly L., Chan D., Trasler J.M. Developmental windows of susceptibility for epigenetic inheritance through the male germline. Semin. Cell Dev. Biol. 2015;43:96–105. doi: 10.1016/j.semcdb.2015.07.006. [DOI] [PubMed] [Google Scholar]
- 167.Skinner M.K., Manikkam M., Guerrero-Bosagna C. Epigenetic transgenerational actions of environmental factors in disease etiology. Trends Endocrinol. Metab. Tem. 2010;21:214–222. doi: 10.1016/j.tem.2009.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Jablonka E., Lamb M.J. The inheritance of acquired epigenetic variations. Int. J. Epidemiol. 2015;44:1103–1105. doi: 10.1093/ije/dyv023. [DOI] [PubMed] [Google Scholar]
- 169.Sloane M.A., Nunez A.C., Packham D., Kwok C.T., Suthers G., Hesson L.B., Ward R.L. Mosaic Epigenetic Inheritance as a Cause of Early-Onset Colorectal Cancer. JAMA Oncol. 2015;1:953–957. doi: 10.1001/jamaoncol.2015.1484. [DOI] [PubMed] [Google Scholar]
- 170.Martin D.I., Ward R., Suter C.M. Germline epimutation: A basis for epigenetic disease in humans. Ann. N. Y. Acad. Sci. 2005;1054:68–77. doi: 10.1196/annals.1345.009. [DOI] [PubMed] [Google Scholar]
- 171.Ge S.-Q., Lin S.-L., Zhao Z.-H., Sun Q.-Y. Epigenetic dynamics and interplay during spermatogenesis and embryogenesis: Implications for male fertility and offspring health. Oncotarget. 2017;8:53804–53818. doi: 10.18632/oncotarget.17479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Migicovsky Z., Kovalchuk I. Epigenetic memory in mammals. Front. Genet. 2011;2:28. doi: 10.3389/fgene.2011.00028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Miyoshi N., Stel J.M., Shioda K., Qu N., Odajima J., Mitsunaga S., Zhang X., Nagano M., Hochedlinger K., Isselbacher K.J., et al. Erasure of DNA methylation, genomic imprints, and epimutations in a primordial germ-cell model derived from mouse pluripotent stem cells. Proc. Natl. Acad. Sci. USA. 2016;113:9545–9550. doi: 10.1073/pnas.1610259113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Seisenberger S., Peat J.R., Hore T.A., Santos F., Dean W., Reik W. Reprogramming DNA methylation in the mammalian life cycle: Building and breaking epigenetic barriers. Philos. Trans. R. Soc. B Biol. Sci. 2013;368:20110330. doi: 10.1098/rstb.2011.0330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Tang W.W., Dietmann S., Irie N., Leitch H.G., Floros V.I., Bradshaw C.R., Hackett J.A., Chinnery P.F., Surani M.A. A Unique Gene Regulatory Network Resets the Human Germline Epigenome for Development. Cell. 2015;161:1453–1467. doi: 10.1016/j.cell.2015.04.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Berletch J.B., Yang F., Xu J., Carrel L., Disteche C.M. Genes that escape from X inactivation. Hum. Genet. 2011;130:237–245. doi: 10.1007/s00439-011-1011-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Nilsson E.E., Sadler-Riggleman I., Skinner M.K. Environmentally induced epigenetic transgenerational inheritance of disease. Environ. Epigenet. 2018;4:dvy016. doi: 10.1093/eep/dvy016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Nassan F.L., Arvizu M., Minguez-Alarcon L., Williams P.L., Attaman J., Petrozza J., Hauser R., Chavarro J. Marijuana smoking and markers of testicular function among men from a fertility centre. Hum. Reprod. (Oxf. Engl.) 2019;34:715–723. doi: 10.1093/humrep/dez002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Jung Y.H., Sauria M.E.G., Lyu X., Cheema M.S., Ausio J., Taylor J., Corces V.G. Chromatin States in Mouse Sperm Correlate with Embryonic and Adult Regulatory Landscapes. Cell Rep. 2017;18:1366–1382. doi: 10.1016/j.celrep.2017.01.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Champroux A., Cocquet J., Henry-Berger J., Drevet J.R., Kocer A. A Decade of Exploring the Mammalian Sperm Epigenome: Paternal Epigenetic and Transgenerational Inheritance. Front. Cell Dev. Biol. 2018;6:50. doi: 10.3389/fcell.2018.00050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Bohacek J., Mansuy I.M. Molecular insights into transgenerational non-genetic inheritance of acquired behaviours. Nat. Rev. Genet. 2015;16:641–652. doi: 10.1038/nrg3964. [DOI] [PubMed] [Google Scholar]
- 182.Kumar N., Singh A.K. Trends of male factor infertility, an important cause of infertility: A review of literature. J. Hum. Reprod. Sci. 2015;8:191–196. doi: 10.4103/0974-1208.170370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Kosan C., Heidel F.H., Godmann M., Bierhoff H. Epigenetic Erosion in Adult Stem Cells: Drivers and Passengers of Aging. Cells. 2018;7:237. doi: 10.3390/cells7120237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Shnorhavorian M.M., Schwartz S., Stansfeld B., Sadler-Riggleman I., Beck D., Skinner M.K. Differential DNA Methylation Regions in Adult Human Sperm following Adolescent Chemotherapy: Potential for Epigenetic Inheritance. PLoS ONE. 2017;12:e0170085. doi: 10.1371/journal.pone.0170085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Lee J., Shinohara T. Epigenetic modifications and self-renewal regulation of mouse germline stem cells. Cell Res. 2011;21:1164–1171. doi: 10.1038/cr.2011.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Sun Y.C., Wang Y.Y., Ge W., Cheng S.F., Dyce P.W., Shen W. Epigenetic regulation during the differentiation of stem cells to germ cells. Oncotarget. 2017;8:57836–57844. doi: 10.18632/oncotarget.18444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Chen T., Li E. DNA Methylation Regulates Genomic Imprinting, X Inactivation, and Gene Expression during Mammalian Development. In: Ma J., editor. Gene Expression and Regulation. Springer New York; New York, NY, USA: 2006. pp. 377–391. [Google Scholar]
- 188.Barlow D.P., Bartolomei M.S. Genomic imprinting in mammals. Cold Spring Harb. Perspect. Biol. 2014:6. doi: 10.1101/cshperspect.a018382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Ferguson-Smith A.C., Bourc’his D. The discovery and importance of genomic imprinting. Elife. 2018;7:e42368. doi: 10.7554/eLife.42368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Marques C.J., Costa P., Vaz B., Carvalho F., Fernandes S., Barros A., Sousa M. Abnormal methylation of imprinted genes in human sperm is associated with oligozoospermia. Mol. Hum. Reprod. 2008;14:67–74. doi: 10.1093/molehr/gam093. [DOI] [PubMed] [Google Scholar]
- 191.Lucifero D., Chaillet J.R., Trasler J.M. Potential significance of genomic imprinting defects for reproduction and assisted reproductive technology. Hum. Reprod. Update. 2004;10:3–18. doi: 10.1093/humupd/dmh002. [DOI] [PubMed] [Google Scholar]
- 192.Nowak K., Stein G., Powell E., He L.M., Naik S., Morris J., Marlow S., Davis T.L. Establishment of paternal allele-specific DNA methylation at the imprinted mouse Gtl2 locus. Epigenetics. 2011;6:1012–1020. doi: 10.4161/epi.6.8.16075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Court F., Tayama C., Romanelli V., Martin-Trujillo A., Iglesias-Platas I., Okamura K., Sugahara N., Simon C., Moore H., Harness J.V., et al. Genome-wide parent-of-origin DNA methylation analysis reveals the intricacies of human imprinting and suggests a germline methylation-independent mechanism of establishment. Genome Res. 2014;24:554–569. doi: 10.1101/gr.164913.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Kim J., Bretz C., Lee S. Epigenetic instability of imprinted genes in human cancers. Nucleic Acids Res. 2015;43:10689–10699. doi: 10.1093/nar/gkv867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.O’Neill C. The epigenetics of embryo development. Anim. Front. 2015;5:42–49. doi: 10.2527/af.2015-0007. [DOI] [Google Scholar]
- 196.Boissonnas C.C., Jouannet P., Jammes H. Epigenetic disorders and male subfertility. Fertil. Steril. 2013;99:624–631. doi: 10.1016/j.fertnstert.2013.01.124. [DOI] [PubMed] [Google Scholar]
- 197.Peng H., Zhao P., Liu J., Zhang J., Zhang J., Wang Y., Wu L., Song M., Wang W. Novel Epigenomic Biomarkers of Male Infertility Identified by Methylation Patterns of CpG Sites Within Imprinting Control Regions of H19 and SNRPN Genes. Omics A J. Integr. Biol. 2018;22:354–364. doi: 10.1089/omi.2018.0019. [DOI] [PubMed] [Google Scholar]
- 198.Rotondo J.C., Selvatici R., Di Domenico M., Marci R., Vesce F., Tognon M., Martini F. Methylation loss at H19 imprinted gene correlates with methylenetetrahydrofolate reductase gene promoter hypermethylation in semen samples from infertile males. Epigenetics. 2013;8:990–997. doi: 10.4161/epi.25798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Li B., Li J.B., Xiao X.F., Ma Y.F., Wang J., Liang X.X., Zhao H.X., Jiang F., Yao Y.Q., Wang X.H. Altered DNA methylation patterns of the H19 differentially methylated region and the DAZL gene promoter are associated with defective human sperm. PLoS ONE. 2013;8:e71215. doi: 10.1371/journal.pone.0071215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Dong H., Wang Y., Zou Z., Chen L., Shen C., Xu S., Zhang J., Zhao F., Ge S., Gao Q., et al. Abnormal Methylation of Imprinted Genes and Cigarette Smoking: Assessment of Their Association With the Risk of Male Infertility. Reprod. Sci. (Thousand Oaks Calif.) 2017;24:114–123. doi: 10.1177/1933719116650755. [DOI] [PubMed] [Google Scholar]
- 201.Zheng H., Zhou X., Li D.K., Yang F., Pan H., Li T., Miao M., Li R., Yuan W. Genome-wide alteration in DNA hydroxymethylation in the sperm from bisphenol A-exposed men. PLoS ONE. 2017;12:e0178535. doi: 10.1371/journal.pone.0178535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.He Q., Riley R.T., Sharma R.P. Pharmacological antagonism of fumonisin B1 cytotoxicity in porcine renal epithelial cells (LLC-PK1): A model for reducing fumonisin-induced nephrotoxicity in vivo. Pharmacol. Toxicol. 2002;90:268–277. doi: 10.1034/j.1600-0773.2002.900507.x. [DOI] [PubMed] [Google Scholar]
- 203.Monge Mdel P., Magnoli A.P., Bergesio M.V., Tancredi N., Magnoli C.E., Chiacchiera S.M. Activated carbons as potentially useful non-nutritive additives to prevent the effect of fumonisin B1 on sodium bentonite activity against chronic aflatoxicosis. Food Addit. Contam. Part A Chem. Anal. Control Expo. Risk Assess. 2016;33:1043–1052. doi: 10.1080/19440049.2016.1185923. [DOI] [PubMed] [Google Scholar]
- 204.Filannino A., Stout T.A., Gadella B.M., Sostaric E., Pizzi F., Colenbrander B., Dell’Aquila M.E., Minervini F. Dose-response effects of estrogenic mycotoxins (zearalenone, alpha- and beta-zearalenol) on motility, hyperactivation and the acrosome reaction of stallion sperm. Reprod. Biol. Endocrinol. 2011;9:134. doi: 10.1186/1477-7827-9-134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Rajkovic A., Uyttendaele M., Debevere J. Computer aided boar semen motility analysis for cereulide detection in different food matrices. Int. J. Food Microbiol. 2007;114:92–99. doi: 10.1016/j.ijfoodmicro.2006.09.031. [DOI] [PubMed] [Google Scholar]
- 206.Galuppo A.G. Spermatogonial stem cells as a therapeutic alternative for fertility preservation of prepubertal boys. Einstein. 2015;13:637–639. doi: 10.1590/S1679-45082015RB3456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Altman E., Yango P., Moustafa R., Smith J.F., Klatsky P.C., Tran N.D. Characterization of human spermatogonial stem cell markers in fetal, pediatric, and adult testicular tissues. Reproduction (Camb. Engl.) 2014;148:417–427. doi: 10.1530/REP-14-0123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Guo J., Cairns B.R. Isolation and Enrichment of Spermatogonial Stem Cells From Human Testis Tissues. Curr. Protoc. Stem Cell Biol. 2019;49:e77. doi: 10.1002/cpsc.77. [DOI] [PubMed] [Google Scholar]
- 209.He Z., Kokkinaki M., Jiang J., Zeng W., Dobrinski I., Dym M. Isolation of human male germ-line stem cells using enzymatic digestion and magnetic-activated cell sorting. Methods Mol. Biol. 2012;825:45–57. doi: 10.1007/978-1-61779-436-0_4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Kossack N., Meneses J., Shefi S., Nguyen H.N., Chavez S., Nicholas C., Gromoll J., Turek P.J., Reijo-Pera R.A. Isolation and characterization of pluripotent human spermatogonial stem cell-derived cells. Stem Cells. 2009;27:138–149. doi: 10.1634/stemcells.2008-0439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Lim J.J., Sung S.Y., Kim H.J., Song S.H., Hong J.Y., Yoon T.K., Kim J.K., Kim K.S., Lee D.R. Long-term proliferation and characterization of human spermatogonial stem cells obtained from obstructive and non-obstructive azoospermia under exogenous feeder-free culture conditions. Cell Prolif. 2010;43:405–417. doi: 10.1111/j.1365-2184.2010.00691.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Liu S., Tang Z., Xiong T., Tang W. Isolation and characterization of human spermatogonial stem cells. Reprod. Biol. Endocrinol. 2011;9:141. doi: 10.1186/1477-7827-9-141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Schmid N., Stockl J.B., Flenkenthaler F., Dietrich K.G., Schwarzer J.U., Kohn F.M., Drummer C., Frohlich T., Arnold G.J., Behr R., et al. Characterization of a non-human primate model for the study of testicular peritubular cells-comparison with human testicular peritubular cells. Mol. Hum. Reprod. 2018;24:401–410. doi: 10.1093/molehr/gay025. [DOI] [PubMed] [Google Scholar]
- 214.Zheng Y., Zhang Y., Qu R., He Y., Tian X., Zeng W. Spermatogonial stem cells from domestic animals: Progress and prospects. Reproduction (Camb. Engl.) 2014;147:R65–R74. doi: 10.1530/REP-13-0466. [DOI] [PubMed] [Google Scholar]
- 215.Guo Y., Liu L., Sun M., Hai Y., Li Z., He Z. Expansion and long-term culture of human spermatogonial stem cells via the activation of SMAD3 and AKT pathways. Exp. Biol. Med. 2015;240:1112–1122. doi: 10.1177/1535370215590822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Guo Y., Hai Y., Gong Y., Li Z., He Z. Characterization, isolation, and culture of mouse and human spermatogonial stem cells. J. Cell. Physiol. 2014;229:407–413. doi: 10.1002/jcp.24471. [DOI] [PubMed] [Google Scholar]
- 217.He Z., Kokkinaki M., Jiang J., Dobrinski I., Dym M. Isolation, characterization, and culture of human spermatogonia. Biol. Reprod. 2010;82:363–372. doi: 10.1095/biolreprod.109.078550. [DOI] [PMC free article] [PubMed] [Google Scholar]